Abstract
Background
Dogs with atopic dermatitis are often immunoglobulin (Ig)E‐sensitised to Dermatophagoides farinae (Df) house dust mites, yet limited data exist on the sensitisation rates to the individual Df allergens, Der f 2 and Zen 1.
Objectives
To determine the IgE sensitisation rates to Df, Der f 2 and Zen 1 in atopic dogs from geographically diverse countries.
Animals
Serum was collected from 32 laboratory dogs in Japan, and 837 atopic dogs from 11 countries from five continents: Asia (Japan, Thailand, Taiwan), Europe (Italy, Latvia, the Netherlands, UK), North America (USA), South America (Argentina, Brazil) and Africa (South Africa).
Methods and materials
We determined Df‐, Der f 2‐ and Zen 1‐specific IgE levels by ELISA. Correlations between the IgE values for these three allergens were calculated.
Results
The IgE seropositivity rates for Df varied between 74% (Argentina) and 100% (the Netherlands, Thailand, South Africa), those for Der f 2 between 12% (Argentina) and 88% (South Africa), and for Zen 1 between 70% (Argentina) and 100% (the Netherlands). Apart from the especially low seropositivity rate for Der f 2‐specific IgE in Argentina, the percentage of IgE sensitisation varied little between countries. There was significant correlation between the IgE levels to these three allergens which was highest between Df and Zen 1, and lowest between Zen 1 and Der f 2.
Conclusions and clinical relevance
The IgE sensitisation to Df is geographically widespread. Der f 2 and Zen 1 are major allergens for dogs in almost all countries where this was evaluated.
Background – Dogs with atopic dermatitis are often immunoglobulin (Ig)E‐sensitised to Dermatophagoides farinae (Df) house dust mites, yet limited data exist on the sensitisation rates to the individual Df allergens, Der f 2 and Zen 1. Objectives – To determine the IgE sensitisation rates to Df, Der f 2 and Zen 1 in atopic dogs from geographically diverse countries. Conclusions and clinical relevance – The IgE sensitisation to Df is geographically widespread. Der f 2 and Zen 1 are major allergens for dogs in almost all countries where this was evaluated.
Résumé
Contexte
Les chiens atopiques ont souvent des immunoglobulines (Ig)E dirigés contre les acariens de poussière de maison Dermatophagoides farinae (Df) alors que des données limitées existent sur les taux de sensibilisation aux allergènes Df individuels, Derf 2 et Zen 1.
Objectifs
Déterminer les taux de sensibilisation d'IgE à Df, Derf 2 et Zen 1 chez les chiens atopiques issus de pays de différentes régions.
Sujets
Le serum a été collecté chez 32 chiens de laboratoires au Japon et 837 chiens atopiques de 11 pays des cinq continents: Asie (Japon, Thailand et Taiwan), Europe (Italie, Latvia, Pays Bas, Royaume Uni), Amérique du Bord (USA), Amérique du Sud (Argentine, Brésil) et Afrique (Afrique du Sud).
Matériels et méthodes
Nous déterminons les taux d'IgE spécifiques de Df, Derf 3 et Zen 1 par ELISA. Les corrélations entre les valeurs d'IgE pour ces trois allergènes ont été calculées.
Résultats
Les taux de séropositivité d'IgE pour Df variaient entre 74% (Argentine) et 100% (Pays Bas, Thaïlande, Afrique du Sud), ceux pour Derf 2 entre 12% (Argentine) et 88% (Afrique du Sud) et ceux pour Zen 1 entre 70% (Argentine) et 100% (Pays Bas). A part pour le taux de séropositivité particulièrement faible pour les IgE spécifiques de Derf 2 en Argentine, le pourcentage de sensibilisation des IgE variait peu entre les pays Il y avait une corrélation significative entre les taux d'IgE à ces trois allergènes qui était plus élevée entre Df et zen 1, et plus faible entre Zen 1 et Derf 2.
Conclusions et importance clinique
La sensibilisation des IgE à Df est géographiquement étendue. Der f 2 et zen 1 sont des allergènes majeurs pour le chien dans presque tous les pays évalués.
Resumen
Introducción
los perros con dermatitis atópica suelen estar sensibilizados con inmunoglobulina (Ig) E a los ácaros del polvo doméstico Dermatophagoides farinae (Df), aunque existen datos limitados sobre las tasas de sensibilización a los alérgenos Df individuales, Der f 2 y Zen 1.
Objetivos
determinar las tasas de sensibilización de IgE a Df, Der f 2 y Zen 1 en perros atópicos de países geográficamente diversos.
Animales
se recogió suero de 32 perros de laboratorio en Japón y 837 perros atópicos de 11 países de los cinco continentes: Asia (Japón, Tailandia, Taiwán), Europa (Italia, Letonia, Países Bajos, Reino Unido), América del Norte (USA.), América del Sur (Argentina, Brasil) y África (Sudáfrica).
Métodos y materiales
determinamos los niveles de IgE específicos de Df‐, Der f 2‐ y Zen 1 mediante ELISA. Se calcularon las correlaciones entre los valores de IgE para estos tres alérgenos.
Resultados
las tasas de seropositividad de IgE para Df variaron entre 74% (Argentina) y 100% (Países Bajos, Tailandia, Sudáfrica), las de Der f 2 entre 12% (Argentina) y 88% (Sudáfrica), y para Zen 1 entre el 70% (Argentina) y el 100% (Holanda). Aparte de la tasa de seropositividad especialmente baja para la IgE específica de Der f 2 en Argentina, el porcentaje de sensibilización a la IgE varió poco entre los países. Hubo una correlación significativa entre los niveles de IgE con estos tres alérgenos, que fue más alta entre Df y Zen 1, y más baja entre Zen 1 y Der f 2.
Conclusiones y relevancia clínica
la sensibilización por IgE a Df está muy extendida geográficamente. Der f 2 y Zen 1 son alérgenos importantes para los perros en casi todos los países en los que se evaluó.
Zusammenfassung
Hintergrund
Hunde mit atopischer Dermatitis haben oft sensibilisierte Immunglobulin (Ig) E auf die Hausstaubmilbe Dermatophagoides farinae (Df); allerdings gibt es nur limitierte Daten über die Sensibilisierungsraten auf die individuellen Df Allergene, Der f 2 und Zen 1.
Ziele
Eine Feststellung der Sensibilisierungsraten auf Df, Der f 2 und Zen 1 bei atopischen Hunden aus geografisch unterschiedlichen Ländern.
Tiere
Es wurde Serum von 32 Laborhunden aus Japan sowie von 837 atopischen Hunden aus 11 Ländern und fünf Kontinenten genommen: Asien (Japan, Thailand, Taiwan), Europa (Italien, Lettland, Niederlande, UK), Nordamerika (USA), Südamerika (Argentinien, Brasilien) und Afrika (Südafrika).
Methoden und Materialien
Wir bestimmten Df‐, Der f 2‐ und Zen 1‐spezifische IgE Werte mittels ELISA. Es wurden die Korrelationen zwischen den IgE Werten für diese drei Allergene kalkuliert.
Ergebnisse
Die IgE Seropositiviätsraten für Df variierten zwischen 74% (Argentinien) und 100% (Niederlande, Thailand, Südafrika), jene für Der f 2 zwischen 12% (Argentinien) und 88% (Südafrika), und für Zen 1 zwischen 70% (Argentinien) und 100% (Niederlande). Außer der sehr niedrigen Seropositivität für Der f 2‐spezifisches IgE in Argentinien, variierte der Prozentsatz der IgE Sensibilisierung zwischen den Ländern wenig. Es bestanden signifikante Korrelationen zwischen den IgE Werten gegenüber diesen drei Allergenen, wobei die Korrelation zwischen Df und Zen 1 am höchsten und zwischen Zen 1 und Der f 2 am niedrigsten war.
Schlussfolgerungen und klinische Bedeutung
Die IgE Sensibilisierung auf Df ist geografisch weit verbreitet. Der f 2 und Zen 1 stellen Hauptallergene für Hunde in fast allen Ländern dar, wo diese evaluiert wurden.
要約
背景
アトピー性皮膚炎の犬は、Dermatophagoides farinae (Df) イエダニに対して免疫グロブリン (Ig) E感作していることが多いが、個々のDfアレルゲンであるDer f 2およびZen 1に対する感作率のデータは限られている。
目的
本研究の目的は、地理的に多様な国のアトピー性犬におけるDf、Der f 2およびZen 1に対するIgE感作率を測定することであった。
供試動物
日本の32頭の実験犬および5大陸11カ国の837頭のアトピー犬から血清を採取した。アジア (日本、タイ、台湾) 、ヨーロッパ (イタリア、ラトビア、オランダ、イギリス) 、北米 (アメリカ) 、南米 (アルゼンチン、ブラジル) 、アフリカ (南アフリカ) の5大陸11カ国のアトピー犬837頭から血清を採取した。
材料と方法
ELISA法により、Df‐、Der f 2‐、Zen 1‐特異的IgE値を測定した。これら3つのアレルゲンに対するIgE値の相関関係を算出した。
結果
DfのIgE陽性率は74% (アルゼンチン) から100% (オランダ、タイ、南アフリカ) 、Der f 2のIgE陽性率は12% (アルゼンチン) から88% (南アフリカ) 、Zen 1のIgE陽性率は70% (アルゼンチン) から100% (オランダ) とばらつきがあった。アルゼンチンのDer f 2特異的IgEの陽性率が特に低かったことを除けば、IgE感作の割合は国による違いはほとんどなかった。これら3つのアレルゲンに対するIgEレベルには有意な相関関係があり、DfおよびZen 1間で最も高く、Zen 1およびDer f 2間で最も低かった。
結論と臨床的妥当性
Dfに対するIgE感作は地理的に広範囲に及んでいる。Der f 2およびZen 1は、評価対象となったほとんどすべての国の犬にとって主要なアレルゲンである。
摘要
背景
特应性皮炎犬通常对称为粉尘螨的美洲尘螨(Df)过敏, 但对单个Df过敏原、Der f 2和Zen 1的致敏率数据有限。
目的
确定来自不同地理国家的特应性犬对Df、Der f 2和Zen 1的IgE致敏率。
动物
从日本的32只实验犬和来自5大洲11个国家的837只特应性犬中采集血清: 亚洲 (日本、泰国、台湾) 、欧洲 (意大利、拉脱维亚、荷兰、英国) 、北美 (美国) 、南美 (阿根廷、巴西) 和非洲 (南非) 。
方法和材料
我们通过ELISA测定了Df‐、Der f 2‐和Zen 1‐特异性IgE水平。计算这三种过敏原的IgE值之间的相关性。
结果
Df的IgE血清阳性率在74% (阿根廷) 和100% (荷兰、泰国、南非) 之间变化, Der f 2在12% (阿根廷) 和88% (南非) 之间变化, Zen 1在70% (阿根廷) 和100% (荷兰) 之间变化。除阿根廷Der f 2特异性IgE的血清阳性率特别低外, 各国之间IgE致敏百分比差异很小。这三种过敏原的IgE水平之间存在显著相关性, Df和Zen 1之间最高, Zen 1和Der f 2之间最低。
结论和临床相关性
IgE对Df的致敏作用在地理上广泛存在。在几乎所有进行评价的国家中, Der f 2和Zen 1是犬的主要过敏原。
Resumo
Contexto
Cães com dermatite atópica apresentam comumente sensibilização mediada por IgE para os ácaros da poeira doméstica Dermatophagoides farinae (Df). Entretanto, poucos dados estão disponíveis a respeito da frequência de sensibilização aos alérgenos individuais de Df, Der f 2 e Zen 1.
Objetivos
Determinar a frequência de sensibilização de IgE para Df, Der f 2 e Zen 1 em cães atópicos de países geograficamente diversos.
Animais
O soro de 32 cães de laboratório no Japão, e 837 cães atópicos de 11 países de cinco continentes: Ásia (Japão, Tailândia, Tawain), Europa (Itália, Latvia, Holanda, Reino Unido), América do Norte (USA), América do Sul (Argentina, Brasil) e África (África do Sul).
Métodos e materiais
Determinamos os níveis de IgE específicos para Df, Der f 2 e Zen 1 por ELISA. Foram calculadas as correlações entre os valores de IgE para esses três alérgenos.
Resultados
As taxas de soropositividade de IgE para Df variaram entre 74% (Argentina) e 100% (Holanda, Tailândia, África do Sul). As taxas para Der f 2 variaram entre 12% (Argentina) e 88% (África do Sul), e para Zen 1 entre 70% (Argentina) e 100% (Holanda). Além da taxa de soropositividade especialmente baixa de IgE específica para Der f 2 na Argentina, houve pouca variação na porcentagem de sensibilização por IgE entre os países. Houve correlação significativa entre os níveis de IgE para esses três alérgenos, que foi maior entre Df e Zen 1 e menor entre Zen 1 e Der f 2.
Conclusões e relevância clínica
A sensibilização por IgE ao Df é geograficamente difundida. Der f 2 e Zen 1 são os principais alérgenos para cães em quase todos os países onde isso foi avaliado.
Introduction
Atopic dermatitis (AD) is a genetically predisposed, pruritic and inflammatory skin disease often associated with immunoglobulin (Ig)E directed against environmental allergens. 1 , 2 As IgE antibodies are thought to play a major role in the pathogenesis of AD, 3 it is important to precisely identify disease‐exacerbating allergens targeted by these reaginic antibodies. In dogs, house dust mites (HDM) allergens, especially those from Dermatophagoides farinae (Df) are considered to be relevant to AD because of the high percentage of positive reactions to intradermal tests with Df, as well as the high level of serum Df‐specific IgE in atopic dogs. 4 Additional arguments in support of the relevance of these HDM allergens for canine AD is that they are present in most households with atopic dogs, and treatment of the dog’s environment with acaricides improves the clinical signs in some dogs sensitised to Df. 5 Furthermore, the isolation of atopic dogs in cleaned cages in a veterinary hospital for two weeks results in a marked clinical improvement in dogs with detectable IgE against allergens (mainly Df) from the environment. 6 Importantly, the long‐documented skin reactivity and highly specific IgE to Df in healthy dogs, 7 , 8 confirms that the mere sensitisation to Df does not always correlate with the presence of atopic signs.
As of early 2021, there were 36 Df proteins considered as allergens for humans (www.allergen.org; page last accessed April 7, 2021). In dogs, the first characterised Df major allergens – that is those recognised by ≥50% of sera from patients sensitised to the parent extract – were the high‐molecular weight (HMW) Der f 15 and Der f 18 chitinases. 9 , 10 The low‐molecular weight (LMW) Der f 2 was initially shown to represent a major Df allergen for dogs in Japan, 11 , 12 and a similar status was later confirmed in Spain, the UK and Malaysia. 13 , 14 , 15 . More recently, Zen 1, another HMW allergen of yet‐unknown function, also was determined to be a major Df allergen in atopic dogs from multiple countries. 13 , 15 , 16 Among these four allergens, only Der f 2 has been confirmed to be of clinical importance, as two studies established the high treatment success rate of a commercially available Der f 2 mono‐allergen immunotherapy (Allermmune HDM, Zenoaq; Koriyama City, Fukushima prefecture, Japan) in dogs with AD. 17 , 18
The goal of this study was to expand the knowledge on the seroprevalence of IgE specific to Df and two of its allergens (Der f 2 and Zen 1) in countries spanning multiple continents. Such a survey is important in preparation for the development of immunotherapy with specific molecular allergens. The data presented herein will clearly establish Der f 2 and Zen 1 as major Df allergens for dogs, worldwide.
Methods and materials
Study population
We assembled sera from 837 dogs in 11 countries: Argentina (50 dogs), Brazil (181), Italy (50), Japan (102), Latvia (30), the Netherlands (52), South Africa (42), Taiwan (100), Thailand (56), the UK (59) and the USA (115). Dogs were selected irrespective of their breed, sex and age, by veterinarians specialised in dermatology. The diagnosis of AD was made after exclusion of ectoparasitic and skin infections, following widely accepted guidelines. 19 Care was taken not to enroll atopic dogs whose signs fully responded to an elimination diet. To harmonise selection, dogs should have fulfilled at least five of eight criteria for the diagnosis of AD. 20
For the establishment of enzyme‐linked immunosorbent assay (ELISA) positive thresholds, we selected 32 healthy, adult laboratory beagles (19 females, 13 males; mean age 24 months), living in a climate‐controlled, mite‐poor, helminth‐free facility in Japan. This cohort was chosen as a way to obtain a “Df‐non‐sensitised” nonatopic control group owing to its limited exposure to HDMs. The rearing environment was tested beforehand to be negative for Df allergens (Mitey Checker, Sumika Environmental Science; Osaka, Japan).
For all dogs, blood was collected with the owners’ consent and after approbation of the local animal care and use committee, where applicable and necessary.
Sample collection and handling
After collection, sera were separated by centrifugation and kept frozen in standard freezers immediately thereafter. Allergen‐specific IgE levels were measured by ELISA as described below. To reduce interoperator fluctuations between measurements of samples from different geographical regions and different collection times, all samples were sent to a central research laboratory in Japan (Zenoaq) by courier at –20°C for IgE levels to be determined using the same method and operator.
ELISA for allergen‐specific IgE levels
ELISA for the determination of serum levels of Df‐, Der f 2‐ and Zen 1‐specific IgE antibodies were carried out as described previously with minor modifications. 12 , 13 Briefly, lyophilised recombinant Der f 2, native Zen 1 and a whole (crude) extract of Df (Greer Laboratories; Lenoir, NC, USA) were respectively reconstituted to concentrations of 50 μg/mL, 0.1 μg/mL and 1 μg/mL with sodium carbonate buffer (pH 9.6) to prepare the solid‐phase antigen, and 100 μL of the resulting solution was added to each well of a 96 well flat‐bottomed microtitre plate (Thermo Fisher Scientific; Tokyo, Japan). Following incubation for 1 h at 37°C, the solid‐phase antigen was removed by a wash buffer and 100 μL ELISA buffer (25% Block Ace, cat. no. UK‐B80, Megmilk Snow Brand; Hokkaido, Japan) was added into each well for another 1 h of incubation as a blocking agent. A negative control and standard sample defined as a positive control (Zenoaq), and test serum samples at 1:10 or 1:50 dilution and ELISA buffer alone (as a blank) were added to the appropriate wells in duplicate, and the plates were incubated for 1 h at 37°C. After washing, a peroxidase‐conjugated goat anti‐dog IgE antibody (A40‐125P, Bethyl Laboratories; Montgomery, TX, USA) diluted up to 10,000‐fold with ELISA buffer was added as the secondary antibody and incubated for 1 h at 37°C. Of importance is that, by immunoelectrophoresis and ELISA, this antiserum is known to specifically recognise canine IgE with <0.01% reactivity with purified dog IgG1, IgG2, IgA and IgM. The colour development was initiated by adding 3,3′,5,5′‐tetramethylbenzidine (TMB) substrate solution (Kirkegaard and Perry Laboratories; Gaithersburg, MD, USA), as instructed by the manufacturer, with the plate protected from light for 30 min. The optical density (OD) was measured at a 450 nm wavelength using a microtitre plate reader (Infinite M200 Pro, Tecan; Mannedorf, Switzerland) after the enzyme reaction had been stopped by the addition of 0.5 M sulfuric acid.
We set the threshold for the seropositivity for each of the three tested allergens as the mean optical density (OD) plus two standard deviations (SD) of the allergen‐specific IgE levels in the sera from the 32 healthy laboratory dogs.
Statistics
In the absence of a predefined hypothesis, and owing to the descriptive nature of our objectives, statistical comparisons of the allergen‐specific IgE seroprevalence between countries and regions were not performed. However, we calculated the correlation between the OD values for the three allergens, two at a time, using the Spearman rank correlation test (Prism 9, Graphpad Software; La Jolla, CA, USA).
Results
Study population
Among the 837 dogs enrolled, 51% were female and 49% male; 7% were <1‐year‐old, 27% 1–3‐year‐old, 43% 3–8‐year‐old and 22% >8‐year‐old. The most frequently represented breeds were shi tzu (48), French bulldog (44), shiba inu (41), Labrador retriever (42), poodle (33), golden retriever (32), Lhasa apso (28), German shepherd dog (22), Maltese (21), boxer (20), dachshund (19), pug (19), beagle (17), toy poodle (17), American Staffordshire terrier (16), Yorkshire terrier (16) and West Highland white terrier (15).
Determination of positivity thresholds
In the 32 healthy laboratory dogs, the mean (SD) OD values for serum IgE specific for Der f 2, Zen 1 and the Df whole extract were 0.243 (0.028), 0.117 (0.039) and 0.129 (0.029), respectively. Setting the seropositivity levels as the means (+2 SD), the calculated ELISA positivity thresholds were 0.299 for Df‐0.196 for Der f 2‐ and 0.187 for Zen 1‐specific IgE,.
Seroprevalence of IgE sensitisation to Dermatophagoides farinae, Der f 2 and Zen 1
In all 837 dogs taken together, the seropositivity rates for Df, Der f 2 and Zen 1 were 93%, 68% and 89%, respectively.
The individual IgE serum levels for all dogs are represented on Figure 1a, b and c. Using the selected thresholds, only one, two and one laboratory dogs had marginally positive IgE levels against Df, Der f 2 and Zen 1, respectively.
Figure 1.

Levels of Dermatophagoides farinae, Der f 2 and Zen 1‐specific immunoglobulin (Ig)E in atopic dogs from multiple countries and from control laboratory dogs.
(a) Optical densities for D. farinae‐specific IgE; (b) optical densities for Der f 2‐specific IgE; (c) optical densities for Zen 1‐specific IgE. Each dot represents a single dog, and the red bars depict the median optical densities.
The seroprevalence of IgE against the three allergens is summarised in Table 1. With the selected thresholds, the overall IgE seropositivity rates for Df in these atopic dogs varied between 74% (Argentina) and 100% (the Netherlands, Thailand, South Africa), that for Der f 2 between 12% (Argentina) and 88% (South Africa), and that for Zen 1 between 70% (Argentina) and 100% (the Netherlands). Outside of the especially low seropositivity rate for Der f 2‐specific IgE in Argentina, the percentages appeared to vary little between countries.
Table 1.
Immunoglobulin (Ig)E seroprevalence to Dermatophagoides farinae (Df), Der f 2 and Zen 1 allergens.
| As a % of all collected sera | As a % of Df‐positive sera | ||||
|---|---|---|---|---|---|
| Whole Df | Der f 2 | Zen 1 | Der f 2 | Zen 1 | |
| Americas | |||||
| Argentina | 74% | 12% | 70% | 16% | 95% |
| Brazil | 91% | 77% | 90% | 86% | 95% |
| USA | 89% | 81% | 77% | 86% | 83% |
| Europe | |||||
| Italy | 94% | 48% | 94% | 51% | 100% |
| Latvia | 93% | 63% | 97% | 64% | 100% |
| Netherlands | 100% | 85% | 100% | 88% | 100% |
| UK | 98% | 86% | 97% | 89% | 98% |
| Asia | |||||
| Japan | 88% | 56% | 83% | 63% | 91% |
| Taiwan | 94% | 67% | 83% | 71% | 88% |
| Thailand | 100% | 75% | 86% | 75% | 86% |
| Africa | |||||
| South Africa | 100% | 88% | 98% | 88% | 98% |
| Average | 93% | 67% | 89% | 71% | 94% |
| Median | 94% | 75% | 90% | 75% | 95% |
| Minimum | 74% | 12% | 70% | 16% | 83% |
| Maximum | 100% | 88% | 100% | 89% | 100% |
Bold percentages indicate a major allergen status (i.e. ≥50% of sera with IgE to the whole extract of D. farinae also had IgE against this single allergen).
In order to determine if there would be a variation in allergen‐specific IgE seroprevalence between different climates, we selected the USA in which veterinarians from nine clinics spanning different regions with various climates had collected samples. Individual OD values from all dogs are shown on Figure 2a, b and c, while the simplified map of the main continental US climates and clinic locations is depicted on Figure 2d. Overall, we did not find any apparent variations in the OD values and positivity rates between US regions, indicating that the sensitisations to Df and its allergens were fairly uniform and geographically widespread.
Figure 2.
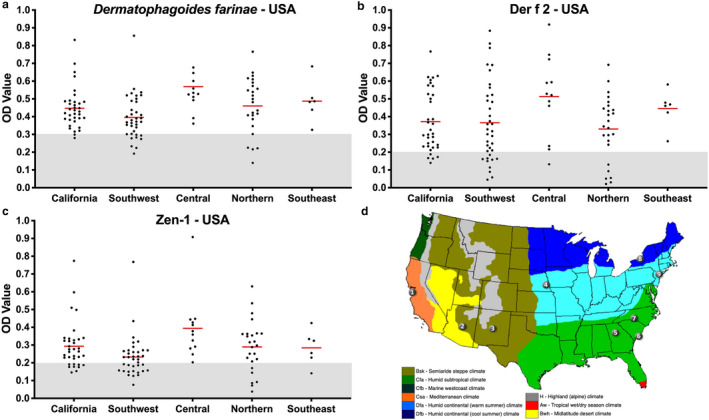
Levels of Dermatophagoides farinae, Der f 2 and Zen 1‐specific immunoglobulin (Ig)E in atopic dogs from the USA.
(a) Optical densities for D. farinae‐specific IgE; (b) optical densities for Der f 2‐specific IgE; (c) optical densities for Zen 1‐specific IgE; (d) simplified map of the climates of the continental USA with the location of clinics that provided atopic dog sera. Colours: orange,California (clinic 1); olive, the Southwest (clinics 2 and 3); light blue, central region (clinics 4 and 9); light green, the Southeast (clinics 5, 6 and 7); and dark blue, northern region (clinic 8). Image courtesy of Wikimedia Commons. Each dot represents a single dog, and the red bars highlight median optical densities.
In an attempt to determine the relationship between the IgE sensitisation to these three allergens, we assessed if they were correlated. The serum IgE values between the allergens, calculated two at a time, were significantly correlated (Figure 3a, b and c). The highest correlation coefficient was between Df and Zen 1 (Spearman r = 0.88), and the lowest was between Der f 2 and Zen 1 (r = 0.34).
Figure 3.

Correlations between Dermatophagoides farinae, Der f 2 and Zen 1.
(a) The correlation between Df and Zen 1 is substantial, and is higher than that observed between Df and Der f 2 (b) or between Der f 2 and Zen 1 (c). Spearman’s rank (r) correlation coefficients are indicated. All correlations were significant at a P < 0.0001.
Finally, we applied to this study the currently accepted benchmark for the definition of major allergens [i.e. ≥50% of dogs sensitised to the parent allergen extract (Df) having detectable IgE against the single allergen]. As shown in Table 1, both Der f 2 and Zen 1 reached a major allergen status in dogs from all countries, except for Der f 2 in Argentina (16%).
Discussion
This study’s results strongly suggest that a majority of atopic dogs from multiple countries and continents are similarly sensitised, not only to a whole‐body Df extract, but also to two of its allergens, Der f 2 and Zen 1.
The results of this geographically widespread survey expand those of local seroprevalence studies in France, Japan, Malaysia, Spain, Switzerland, UK and USA for Der f 2, and France, Malaysia, Switzerland, UK and USA for Zen 1. 11 , 12 , 13 , 14 , 15 , 16
Our results confirm that most atopic dogs worldwide are IgE‐sensitised to the Df HDM, a finding long ago established (and reviewed 4 ), and which was confirmed more recently in the USA 16 and UK; 15 it is surprisingly lower in Malaysia. 13 That an IgE sensitisation to this particular mite species is widespread is remarkable, as it occurs even when another mite species, Dermatophagoides pteronyssinus (Dp), predominates in the environment of the same geographical areas, such as the São Paolo Brazilian metropolis, Malaysia and the UK. 21 In such situations, the detected IgE sensitisation to Df probably represents, a cross‐reactivity to another house dust (Dp or other) or storage mite species, or to the Toxocara canis (Tc) nematode (see below for further discussion). 22 , 23 , 24 In Malaysia, the low rate of IgE seropositivity to Df likely reflects its scarcity in the local environment. 22 , 25
Der f 2, an MD2‐like lipid (ML)‐binding protein is a major allergen in humans sensitised to Df. 26 Although at first it was believed to be of lesser importance in dogs with AD, 27 , 28 a high IgE seroprevalence to this allergen was later confirmed in atopic dogs in Japan, 11 , 12 Spain, 14 Malaysia 13 and the UK. 15 In one of our previous papers, 16 we reported that the rate of positive serum IgE detection to this allergen was low in atopic dogs from the USA, France and Switzerland. In retrospect, we believe that this low seropositivity rate likely resulted from an artificially high positive threshold established for our ELISA to this allergen. Indeed, we had used a mean +3 SD of the Der f 2‐IgE levels in 10 laboratory dogs. As only one of these 10 dogs had any IgE above our assay’s detection limit to this allergen, the established threshold was probably too high to reflect biological reality. Looking back at the original dataset, nine of 17 (53%), eight of 12 (67%) and four of 33 (12%) atopic dogs did, in fact ,have detectable Der f 2‐specific IgE in France, Switzerland and USA (North Carolina), respectively; this would make Der f 2 also a major allergen in Europe and not in North Carolina. 16 Altogether, the results of the present study add to the existing body of evidence that Der f 2 now can be considered a major allergen for dogs sensitised to Df in almost every country where such investigations have been undertaken recently. A notable exception to such status is the very low IgE seroprevalence to Der f 2 in Argentina (12%, this study). Homes in Argentina are known to harbour mixed populations of Df and Dp, 29 so dogs should have been exposed to the Df HDM in their local environment. The presence of detectable IgE to Df and Zen 1 in the same atopic dogs from different Argentinean cities suggests that a degradation of serum IgE had not occurred during shipment of the sera to the laboratory. The reason(s) behind the uniquely low IgE seroprevalence to Der f 2 in Argentine atopic dogs for now will remain an unanswered question.
Our study establishes the unequalled IgE seropositivity rate to the novel Df allergen, Zen 1, in all countries where this had been investigated. This observation is similar to that previously reported for France, Switzerland, the USA 16 and UK. 15 In Malaysia, the positivity rate is substantially lower (30%), 13 possibly reflecting the scarcity of Df in the local environment. 25 Of interest is that Zen 1, a still uncharacterised protein, is not yet a recognised Df allergen for atopic humans. By contrast, in our previous ELISA inhibition study, we found that IgE to Zen 1 represented about half (median 50%; range 22–84%) of the IgE against Df, an unprecedented proportion for any single allergen. 16 This difference in sensitisation rates between the human and canine species is noteworthy and deserving of further study.
As done previously, 15 , 16 we compared the IgE levels between Df, Der f 2 and Zen 1, two at a time. Our results confirmed the significant and uniquely high correlation that exists between the IgE sensitisations to Df and Zen 1, with Spearman’s rank correlation coefficients ranging from 83% 16 to 96%. 15 These results imply that when a dog has detectable serum IgE against the whole‐body extract of Df, it is very likely to have a similar level of anti‐Zen 1 IgE. This implication is further supported by our previous observation that Zen 1‐specific IgE represents a large fraction of that to Df. We also found a similar, yet much lower, correlation between Df‐ and Der f 2‐specific IgE, which varied between 23% (not significant; our unpublished results from canine sera used in 16 ) and 41% (significant; this study). Finally, the correlation between Der f 2‐ and Zen 1‐specific IgE is the weakest, with correlations ranging from 5% (not significant; unpublished results in 16 ) to 34% (significant; this study). The latter comparison suggests a lack of cross‐reactivity between these two Df allergens, which is supported by the absence of notable homology between their amino acid sequences and glycosylation patterns.
In humans, the main immunodominant Df allergens are those belonging to the first and second allergen groups (i.e. Der f 1 and Der p 1, Der f 2 and Der p 2). The importance of the canine HMW major allergens Der f 15 and Der f 18 appears lower, while that of Zen 1 has been largely ignored. 30 , 31 A simple phenomenon could explain such a difference in major allergens between these two species: the rate of endoparasitism with helminths. Indeed, we recently confirmed the existence of a profound cross‐reactivity between Df and the ubiquitous digestive nematode Tc in dogs from Switzerland, 23 and the USA (our unpublished data). Of interest is that the three HMW allergens Der f 15, Der f 18 and Zen 1 (as well as Der f 23) are heavily O‐glycosylated – an unusual pattern among allergens – and this unique glycosylation pattern mirrors the one present on the Tc mucins, which are incorporated in the “fuzzy coat” used as a decoy by their larvae. 32 It is thus logical to suspect that an as‐yet‐unspecified fraction of the Df‐ and Zen 1‐specific IgE detected in this study might, in fact, cross‐react with proteins from Tc helminths. By contrast, Der f 2 only exhibits a few attached N‐glycans that are unlikely to cross‐react with IgE recognising the nematode’s mucins. 33 Such a difference in cross‐reactivity with helminth proteins might explain the differences in sensitisation rates between Der f 2, on the one hand, and the whole‐body Df and Zen 1, on the other.
An important remark is that an IgE sensitisation to a specific allergen does not mean that such an allergen is clinically important – that is, disease‐inducing. In other words, one should not assume that because all atopic dogs in countries, such as the Netherlands, Thailand, Latvia or South‐Africa, are IgE‐sensitised to Df, that these HDM are responsible for the clinical signs of AD in these dogs. Indeed, because of the known cross‐reactivity that exists between Df and Tc, and the presence of mite‐sensitisation in nearly all normal dogs, 7 the clinical relevance of IgE against Df and any of its major allergens remains to be established unequivocally. Notwithstanding the latter statement, Der f 2 is likely to represent an important allergen, as a Der f 2 mono‐immunotherapy has been shown to lead to a rapid and substantial improvement in clinical signs in dogs sensitised to Df. 17 , 18
A limitation of our work is that we also did not study the IgE seropositivity rate for the two other HMW Df proteins considered to be major allergens for dogs in the USA, Der f 15 and Der f 18. 9 , 10 The main reason for this lack of testing was the absence of commercially available recombinant proteins and their notoriously unusual O‐glycosylation pattern that prevents their production in simple bioengineering systems. Nevertheless, the relevance of these HMW allergens could be questioned, as a previous study reported an identical – if not higher – prevalence of IgE directed against HMW Df allergens in healthy and atopic dogs. 34
This study’s results highlight the need for further research studies. As Der f 15 and Der f 18 only have been shown to be major Df allergens in atopic dogs in the USA and Spain, 14 expanding the seroprevalence data beyond these two countries is needed. Furthermore, owing to the high IgE seropositivity to Df observed in normal dogs, 7 determining the IgE seropositivity rates to the four major Df allergens in healthy dogs is indispensable. Research on the IgE cross‐reactivity between Df O‐glycosylated HMW allergens among themselves and those of Tc also is critical. Finally, clinical trials on Zen 1 immunotherapy would help confirm the clinical relevance of this unique allergen.
In conclusion, this study unequivocally establishes the geographically widespread IgE‐sensitisation to Df, Der f 2 and Zen 1 in atopic dogs from countries on five continents.
Author contributions
Claude Favrot: Conceptualization, Data curation, Formal analysis, Visualization, Writing‐original draft. Thierry Olivry: Data curation, Validation, Visualization, Writing‐original draft. Toshiroh Iwasaki: Data curation, Writing‐original draft.
Acknowledgements
We thank Wei Yee Chan and Gayathri Thevi Selvarajah (University of Putra, Malaysia), Anita Patel (Dermatology Referrals, Warlingham, UK), Cathy Curtis (Dermatology Referral Service, Ware, UK), Rosario Cerundolo (3 Dick White Referrals, Suffolk, UK), Chiara Noli (Servici Dermavet, Italy), Wilfried Basteijns (The Netherlands), Brett Wildermuth (Tierdermatologie Dr. Wildermuth, Germany), Marconi Farias and Lucas Ludwig (Pontifical Catholic University of Parana, Brazil), Luiz Henrique Machado (UNESP São Paulo, Brazil), Lerpen Duangkaew (Kasetsart University, Thailand), Andrew Leisewitz (University of Pretoria, South Africa), Alla Olivr? (Veterinary Dermatology Riga, Latvia), Charles Chen (Asian Veterinary Specialist Referral Center, Taiwan), Gustavo Tartara (Rosario National University, Argentina), Fogel Fernando and Martinez Sofía (National University of the Center of the Buenos Aires Province, Argentina), Grandinetti Jesica (National University of La Plata, Argentina), Alvarez Silvina (ClinDermoVet, Resistencia Chaco, Argentina), Reynes Lisandro (University of Salvador, Argentina), Scarpa Miguel (University of Buenos Aires, Argentina), and Animal Clinical Investigation (USA) for providing the canine sera samples. We are grateful for the technical assistance of Toshihiro Tsukui and Miyuki Kageyama (both of Zenoaq; Koriyama City, Fukushima, Japan). Open Access Funding provided by Universitat Zurich.
Sources of Funding: Zenoaq, Japan. Open Access Funding provided by Universitat Zurich.
Conflicts of Interest: Claude Favrot is a consultant for Zenoaq; Claude Favrot and Thierry Olivry have received research support and lecturing honorarium from this company; Toshiro Iwasaki has received lecturing honorarium from Zenoaq.
References
- 1. Halliwell R. Revised nomenclature for veterinary allergy. Vet Immunol Immunopathol 2006; 114: 207–208. [DOI] [PubMed] [Google Scholar]
- 2. Halliwell RE, Deboer DJ. The ACVD task force on canine atopic dermatitis (III): the role of antibodies in canine atopic dermatitis. Vet Immunol Immunopathol 2001; 81: 159–168. [DOI] [PubMed] [Google Scholar]
- 3. Pucheu‐Haston CM, Bizikova P, Eisenschenk MNC, et al. Review: The role of antibodies, autoantigens and food allergens in canine atopic dermatitis. Vet Dermatol 2015; 26: 115‐e130. [DOI] [PubMed] [Google Scholar]
- 4. Hill PB, Deboer DJ. The ACVD task force on canine atopic dermatitis (IV): environmental allergens. Vet Immunol Immunopathol 2001; 81: 159–168. [DOI] [PubMed] [Google Scholar]
- 5. Swinnen C, Vroom M. The clinical effect of environmental control of house dust mites in 60 house dust mite‐sensitive dogs. Vet Dermatol 2004; 15: 31–36. [DOI] [PubMed] [Google Scholar]
- 6. Fujimura M. The study of canine atopic dermatitis involving the isolation of dogs. Pol J Vet Sci 2011; 14: 273–277. [DOI] [PubMed] [Google Scholar]
- 7. Layne EA, DeBoer DJ. Allergen‐specific IgE in nonatopic dogs. Vet Dermatol 2019; 30: 78–79. [DOI] [PubMed] [Google Scholar]
- 8. Lian TM, Halliwell RE. Allergen‐specific IgE and IgGd antibodies in atopic and normal dogs. Vet Immunol Immunopathol 1998; 66: 203–223. [DOI] [PubMed] [Google Scholar]
- 9. McCall C, Hunter S, Stedman K, et al. Characterization and cloning of a major high molecular weight house dust mite allergen (Der f 15) for dogs. Vet Immunol Immunopathol 2001; 78: 231–247. [DOI] [PubMed] [Google Scholar]
- 10. Weber E, Hunter S, Stedman K, et al. Identification, characterization, and cloning of a complementary DNA encoding a 60‐kd house dust mite allergen (Der f 18) for human beings and dogs. J Allerg Clin Immunol 2003; 112: 79–86. [DOI] [PubMed] [Google Scholar]
- 11. Masuda K, Tsujimoto H, Fujiwara S, et al. IgE sensitivity and cross‐reactivity to crude and purified mite allergens (Der f 1, Der f 2, Der p 1, Der p 2) in atopic dogs sensitive to Dermatophagoides mite allergens. Vet Immunol Immunopathol 1999; 72: 303–313. [DOI] [PubMed] [Google Scholar]
- 12. Yamashita K, Fujiwara C, Azuma R, et al. Determination of antigenic proteins of housedust mites in 90 dogs suffering from atopic dermatitis. J Vet Med Sci 2002; 64: 673–676. [DOI] [PubMed] [Google Scholar]
- 13. Chan WY, Selvarajah GT, Ajat M, et al. The detection of house dust mite Dermatophagoides farinae, Der f 2 and Zen‐1 allergen‐specific immunoglobulin E antibodies in dogs with atopic Dermatitis in Malaysia. Vet Immunol Immunopathol 2019; 212: 43–49. [DOI] [PubMed] [Google Scholar]
- 14. Moya R, Carnes J, Sinovas N, et al. Immunoproteomic characterization of a Dermatophagoides farinae extract used in the treatment of canine atopic dermatitis. Vet Immunol Immunopathol 2016; 180: 1–8. [DOI] [PubMed] [Google Scholar]
- 15. Patel A, Curtis CF, Cerundolo R. Incidence of anti‐Der f 2and anti‐Zen1‐specific immunoglobulin E antibodies in atopic dogs from South‐East England. Vet Rec 2019; 184: 317–322. [DOI] [PubMed] [Google Scholar]
- 16. Olivry T, Dunston SM, Favrot C, et al. The novel high molecular weight Dermatophagoides farinae protein Zen‐1 is a major allergen in North American and European mite allergic dogs with atopic dermatitis. Vet Dermatol 2017; 28: 177‐e38. [DOI] [PubMed] [Google Scholar]
- 17. Fischer N, Tarpataki N, Leidi F, et al. An open study on the efficacy of a recombinant Der f 2 (Dermatophagoides farinae) immunotherapy in atopic dogs in Hungary and Switzerland. Vet Dermatol 2018; 29: 337‐e118. [DOI] [PubMed] [Google Scholar]
- 18. Kawano K, Mizuno T. A pilot study of the effect of pullulan‐conjugated Der f 2 allergen‐specific immunotherapy on canine atopic dermatitis. Vet Dermatol 2017; 28: 583‐e141. [DOI] [PubMed] [Google Scholar]
- 19. Hensel P, Santoro D, Favrot C, et al. Canine atopic dermatitis: detailed guidelines for diagnosis and allergen identification. BMC Vet Res 2015; 11: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Favrot C, Steffan J, Seewald W, et al. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet Dermatol 2010; 21: 23–31. [DOI] [PubMed] [Google Scholar]
- 21. Thomas WR. Geography of house dust mite allergens. Asian Pac J Allergy Immunol 2010; 28: 211–224. [PubMed] [Google Scholar]
- 22. Saridomichelakis MN, Marsella R, Lee KW, et al. Assessment of cross‐reactivity among five species of house dust and storage mites. Vet Dermatol 2008; 19: 67–76. [DOI] [PubMed] [Google Scholar]
- 23. Fischer N, Rostaher A, Zwickl L, et al. A Toxocara canis infection influences the immune response to house dust mite allergens in dogs. Vet Immunol Immunopathol 2018; 202: 11–17. [DOI] [PubMed] [Google Scholar]
- 24. Zwickl LLMN, Joekel DE, Fischer NM, et al. Total and Toxocara canis larval excretory/secretory antigen‐ and allergen‐specific IgE in atopic and non‐atopic dogs. Vet Dermatol 2018; 29: 222‐e80. [DOI] [PubMed] [Google Scholar]
- 25. Mariana A, Ho TM, Gendeh BS, et al. First report on sensitization to allergens of a house dust mite, Suidasia pontifica (Acari: Saproglyphidae). Southeast Asian J Trop Med Public Health 2000; 31: 722–723. [PubMed] [Google Scholar]
- 26. Batard T, Baron‐Bodo V, Martelet A, et al. Patterns of IgE sensitization in house dust mite‐allergic patients: implications for allergen immunotherapy. Allergy 2016; 71: 220–229. [DOI] [PubMed] [Google Scholar]
- 27. Noli C, Bernadina WE, Willemse T. The significance of reactions to purified fractions of Dermatophagoides pteronyssinus and Dermatophagoides farinae in canine atopic dermatitis. Vet Immunol Immunopathol 1996; 52: 147–157. [DOI] [PubMed] [Google Scholar]
- 28. Nuttall TJ, Hill PB, Bensignor E, et al. House dust and forage mite allergens and their role in human and canine atopic dermatitis. Vet Dermatol 2006; 17: 223–235. [DOI] [PubMed] [Google Scholar]
- 29. Neffen HE, Fernández‐Caldas E, Predolini N, et al. Mite sensitivity and exposure in the city of Santa Fe, Argentina. J Investig Allergol Clin Immunol 1996; 6: 278–282. [PubMed] [Google Scholar]
- 30. Thomas WR. House dust mite allergens: new discoveries and relevance to the allergic patient. Curr Allergy Asthma Rep 2016; 16: 69. [DOI] [PubMed] [Google Scholar]
- 31. Thomas WR, Smith W‐A, Hales BJ, et al. Characterization and immunobiology of house dust mite allergens. Int Arch Allergy Immunol 2002; 129: 1–18. [DOI] [PubMed] [Google Scholar]
- 32. Maizels RM. Toxocara canis: molecular basis of immune recognition and evasion. Vet Parasitol 2013; 193: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Halim SA, Carlsson MC, Madsen CB, et al. Glycoproteomic analysis of seven major allergenic proteins reveals novel post‐translational modifications. Mol Cell Proteomics 2015; 14: 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nuttall TJ, Lamb JR, Hill PB. Characterisation of major and minor Dermatophagoides allergens in canine atopic dermatitis. Res Vet Sci 2001; 71: 51–57. [DOI] [PubMed] [Google Scholar]


