Abstract
Objective
This study was undertaken to evaluate benzodiazepine (BZD) administration patterns before transitioning to non‐BZD antiseizure medication (ASM) in pediatric patients with refractory convulsive status epilepticus (rSE).
Methods
This retrospective multicenter study in the United States and Canada used prospectively collected observational data from children admitted with rSE between 2011 and 2020. Outcome variables were the number of BZDs given before the first non‐BZD ASM, and the number of BZDs administered after 30 and 45 min from seizure onset and before escalating to non‐BZD ASM.
Results
We included 293 patients with a median (interquartile range) age of 3.8 (1.3–9.3) years. Thirty‐six percent received more than two BZDs before escalating, and the later the treatment initiation was after seizure onset, the less likely patients were to receive multiple BZD doses before transitioning (incidence rate ratio [IRR] = .998, 95% confidence interval [CI] = .997–.999 per minute, p = .01). Patients received BZDs beyond 30 and 45 min in 57.3% and 44.0% of cases, respectively. Patients with out‐of‐hospital seizure onset were more likely to receive more doses of BZDs beyond 30 min (IRR = 2.43, 95% CI = 1.73–3.46, p < .0001) and beyond 45 min (IRR = 3.75, 95% CI = 2.40–6.03, p < .0001) compared to patients with in‐hospital seizure onset. Intermittent SE was a risk factor for more BZDs administered beyond 45 min compared to continuous SE (IRR = 1.44, 95% CI = 1.01–2.06, p = .04). Forty‐seven percent of patients (n = 94) with out‐of‐hospital onset did not receive treatment before hospital arrival. Among patients with out‐of‐hospital onset who received at least two BZDs before hospital arrival (n = 54), 48.1% received additional BZDs at hospital arrival.
Significance
Failure to escalate from BZDs to non‐BZD ASMs occurs mainly in out‐of‐hospital rSE onset. Delays in the implementation of medical guidelines may be reduced by initiating treatment before hospital arrival and facilitating a transition to non‐BZD ASMs after two BZD doses during handoffs between prehospital and in‐hospital settings.
Keywords: benzodiazepine, epilepsy, pediatric, seizure, status epilepticus, treatment
Key Points.
We evaluated BZD administration patterns before transitioning to second‐line medication in pediatric refractory convulsive status epilepticus
More than one third of patients received more than two BZDs before escalating to a non‐BZD ASM
More than half of patients received BZD doses after 30 min from seizure onset
Out‐of‐hospital seizure onset and intermittent SE were associated with the failure to escalate from BZDs to non‐BZD ASMs
In approximately half of patients who received two or more BZDs before hospital arrival, the rescue algorithm was restarted following patient handoffs
1. INTRODUCTION
Status epilepticus (SE) affects 17–23/100,000 children per year, 1 , 2 , 3 , 4 with short‐term mortality of 0%–3% 1 , 3 , 5 , 6 , 7 , 8 and frequent, related neurocognitive sequelae. 9 Refractory SE (rSE) ensues when SE does not respond to initial antiseizure medications (ASMs), and the outcome is often worse in rSE. 10
Major prognostic factors for SE outcome are age, etiology, and time to treatment. 6 , 9 , 11 , 12 Among these factors, time to treatment, including treatment escalation, is most amenable to modification. There is no evidence‐based timeline on when to administer each ASM, although animal models suggest that benzodiazepines (BZDs) may progressively become less efficacious after 30 min of ongoing seizure activity. 13 , 14 Current SE guidelines recommend administration of the first BZD within 5–10 min from seizure onset and a transition to non‐BZD ASMs at 10–20 min from seizure onset. 15 , 16 However, in clinical practice, treatment often occurs more slowly than recommended by guidelines. 17 , 18 Slower treatment is independently associated with longer seizure duration, increased need for continuous infusions, more frequent hypotension, and increased mortality. 11 , 19 No studies have focused on BZD administration patterns before escalating to non‐BZD ASMs in rSE.
We aimed to describe the escalation from BZDs to non‐BZD ASMs in patients with rSE in clinical practice, and analyze potential risk factors related to deviations from treatment guideline recommendations at this initial stage. We hypothesized that patients received a higher number of BZD doses than recommended by guidelines before transitioning to non‐BZD ASMs, with applications frequently outside the most efficacious timeframe.
2. MATERIALS AND METHODS
2.1. Standard protocol approvals, registrations, and patient consents
The study was approved by the institutional review board at each participating institution. Written informed consent was obtained from parents or guardians.
2.2. Study design
We retrospectively analyzed data from a prospective, observational study at 19 pediatric hospitals in the United States and Canada within the Pediatric Status Epilepticus Research Group (pSERG). 20 This multicenter study collects data prospectively on patients with rSE, with the overall goal of delineating strategies for improving the management and eventually the outcome of children with SE. 20 Data regarding time to treatment in the first 81 patients, 21 the influence of a prior diagnosis of epilepsy and of a prior episode of SE on time to treatment, 22 the association of delays to treatment with short‐term outcomes, 11 and the factors associated with treatment delays 23 have been published previously. Information on the cumulative first‐line BZD dosing within a range of 10 min after treatment initiation in patients who underwent rSE, different types of BZDs used, routes of administration, and the effect on outcome were presented in a different article. 24 However, the pattern of separate BZD dose administration from SE onset in the setting of the delayed transition to non‐BZD ASM, irrespective of the cumulative BZD dosing within the first 10 min of treatment initiation, have not been analyzed.
2.3. Patients
Inclusion criteria were (1) admission to a pSERG center between June 2011 and February 2020, (2) age from 1 month to 21 years, and (3) focal or generalized convulsive seizures at the onset that evolved into rSE. In our study, rSE was considered when seizures continued after administration of at least two different ASM types, including at least one BZD and one non‐BZD ASM. Exclusion criteria were (1) nonconvulsive SE detected on electroencephalogram without convulsive seizures at onset or limited to infrequent myoclonic jerks; (2) no data on relevant variables such as age, sex, location of rSE onset, type of SE, convulsive duration, time to administration of the first BZD, dose of the first BZD, and time to administration of the first non‐BZD ASM; and (3) unconventional transition strategies, including first non‐BZD ASM received before or at the same time as first BZD, first continuous infusion received before or at the same time as first BZD, or first continuous infusion received before or at the same time as first non‐BZD ASM. 15 An inclusion diagram is detailed in Figure S1 (https://github.com/tsheehan01/BZD_Transition). If a patient had more than one episode of rSE during the study period, only the first episode was included to meet the assumption of independence of observations.
Data were collected with a standardized data acquisition tool and were based on caregivers' interviews, care provider documentation in the medical records, and emergency medical services (EMS) documentation, if applicable. Subsequently, data were entered into an electronic database hosted by Cincinnati Children's Hospital Medical Center. Details of the pSERG consortium are available in the original pSERG research plan. 20
2.4. Variables
The primary outcome variable was the number of BZD doses administered from the beginning of SE treatment and before escalating to the first non‐BZD ASM. The variables analyzed in the multivariate model were age (continuous in years), sex, type of SE (intermittent, defined as multiple seizures without return to baseline; or continuous, considered a single prolonged seizure), location of SE onset (in hospital/out of hospital), prior epilepsy (yes/no), prior SE (yes/no), time to treatment initiation from seizure onset (continuous in minutes), and inadequate first BZD dosing (yes/no; first BZD dose was defined as inadequate if a patient received less than 100% of the minimum recommended dose based on current medical guidelines for the treatment of SE, 15 , 16 detailed in the methodology of Vasquez et al. 24 ). We did not include the ASM as chronic medication as a variable in the model because it is highly correlated with the variable prior epilepsy.
The secondary outcome variables were (1) the number of BZD doses administered beyond 30 min from seizure onset and before non‐BZD ASM, and (2) the number of BZD doses administered beyond 45 min from seizure onset and before non‐BZD ASM. The variables analyzed in these multivariate models were age (continuous in years), sex, type of SE (intermittent/continuous), location of SE onset (in hospital/out of hospital), prior epilepsy (yes/no), and prior SE (yes/no). The threshold of 30 min onward after seizure onset was chosen because it aligns with the classical definition of SE 25 and because responsiveness to BZDs in animal models is markedly lower after that time point. 26 , 27 , 28 However, limited animal and human series with prolonged SE presented complete resolution after a single BZD dose, suggesting a potential involvement of other factors in BZD response besides seizure duration, or the need for longer seizure duration to develop BZD resistance in some cases. 29 , 30 As responsiveness to BZDs may still be present later, we added an additional threshold of 45 min.
2.5. Statistical analysis
Demographic and clinical characteristics were summarized using descriptive statistics. We initially used multivariate negative binomial regression models in all cases, because its dispersion parameter allows unequal mean and variance. A large number for theta obtained when modeling the primary outcome with the negative binomial regression and the likelihood ratio test supported the use of Poisson regression in this specific case. Unless stated otherwise, continuous variables are presented as median (interquartile range [IQR]) and categorical variables are presented as number (percentage). A conventional alpha level of .05 was considered statistically significant for all analyses. All statistical analyses were performed with R (v3.4.1), 31 RStudio, 32 and the packages gmodels, 33 gdata, 34 tableone, 35 dplyr, 36 survival, 37 ggplot2, 38 and MASS. 39
2.6. Data availability statement
All relevant data, statistical analyses, and results can be found in File S2 (https://github.com/tsheehan01/BZD_Transition).
3. RESULTS
3.1. Study population
We included 293 patients (55% males) with a median (IQR) age of 3.8 (1.3–9.3) years. rSE started out of hospital in 201 (69%) patients and in hospital in 92 (31%) patients. Table 1 summarizes the main demographic and clinical features. Among patients with out‐of‐hospital onset, the first BZD was administered by caregivers in 56 (27.9%) patients, EMS in 51 (25.4%) patients, a secondary hospital in 47 (23.4%) patients, and a referral hospital in 47 (23.4%) patients. Ninety‐four (46.8%) patients with out‐of‐hospital onset did not receive any rescue medication until hospital arrival. Among 54 patients who received two or more BZDs in the prehospital setting by caregivers or EMS, 26 (48.1%) received at least one additional BZD at hospital arrival as the first in‐hospital ASM.
TABLE 1.
Demographic and clinical characteristics
| Characteristic | Patients in the entire cohort, N = 293 | Patients with out‐of‐hospital onset, n = 201 | Patients with in‐hospital onset, n = 92 |
|---|---|---|---|
| Age at SE in years | |||
| Median (IQR) | 3.8 (1.3–9.3) | 3.2 (1.2–8.9) | 4.8 (2.0–10.2) |
| Sex, n (%) | |||
| Male | 162 (55.3%) | 120 (59.7%) | 42 (45.7%) |
| Female | 131 (44.7%) | 81 (40.3%) | 50 (54.3%) |
| Race, n (%) | |||
| White | 187 (63.8%) | 126 (62.7%) | 61 (66.3%) |
| African American | 57 (19.5%) | 42 (20.9%) | 15 (16.3%) |
| American Indian/Alaska Native | 1 (.3%) | 0 (0%) | 1 (1.1%) |
| Asian | 10 (3.4%) | 5 (2.5%) | 5 (5.4%) |
| Arabic | 8 (2.7%) | 5 (2.5%) | 3 (3.3%) |
| Hawaiian/Pacific Islander | 2 (.7%) | 2 (1.0%) | 0 (0%) |
| Unknown/not reported | 28 (9.6%) | 21 (10.4%) | 7 (7.6%) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 43 (14.7%) | 32 (15.9%) | 11 (12.0%) |
| Not Hispanic or Latino | 225 (76.8%) | 150 (74.6%) | 75 (81.5%) |
| Unknown/not reported | 25 (8.5%) | 19 (9.5%) | 6 (6.5%) |
| Medical history, n (%) a | |||
| DD/ID | 154 (52.6%) | 108 (53.7%) | 46 (50.0%) |
| Cerebral palsy | 31 (10.6%) | 19 (9.5%) | 12 (13.0%) |
| History of epilepsy | 146 (49.8%) | 102 (50.7%) | 44 (47.8%) |
| History of SE | 63 (21.5%) | 40 (19.9%) | 23 (25.0%) |
| No past neurological history | 96 (32.8%) | 60 (29.9%) | 36 (39.1%) |
| Duration of convulsive SE, min | |||
| Median (IQR) | 127 (60–286) | 140 (75–300) | 108 (48–182) |
| Type of SE, n (%) | |||
| Continuous | 102 (34.8%) | 75 (37.3%) | 27 (29.3%) |
| Intermittent | 191 (65.2%) | 126 (62.7%) | 65 (70.7%) |
| Etiology of SE, n (%) | |||
| Unknown | 104 (35.5%) | 71 (35.3%) | 33 (35.9%) |
| Structural | 70 (23.9%) | 44 (21.9%) | 26 (28.3%) |
| Genetic | 57 (19.5%) | 44 (21.9%) | 13 (14.1%) |
| Metabolic | 15 (5.1%) | 9 (4.5%) | 6 (6.5%) |
| Other | 47 (16.0%) | 33 (16.4%) | 14 (15.2%) |
| Time to first BZD from seizure onset, min | |||
| Median (IQR) | 15 (5–37) | 20 (5–50) | 8 (4–20) |
| Inadequate first BZD dosing, n (%) | |||
| Yes | 166 (56.7%) | 106 (52.7%) | 60 (65.2%) |
| No | 127 (43.3%) | 95 (47.3%) | 32 (34.8%) |
| Time to first non‐BZD ASM from seizure onset, min | |||
| Median (IQR) | 63 (35–126) | 76 (45–155) | 39 (24–72) |
Abbreviations: ASM, antiseizure medication; BZD, benzodiazepine; DD, developmental delay; ID, intellectual disability; IQR, interquartile range (first quartile Q1 to third quartile Q3); SE, status epilepticus.
Percentages do not add up to 100% because patients may belong to more than one category.
3.2. Primary outcome
The number of BZD doses administered from the beginning of SE treatment and before escalating to the first non‐BZD ASM is depicted in Figure 1 (data table in File S3, https://github.com/tsheehan01/BZD_Transition). One hundred six (36.2%) patients received more than two BZDs before escalating to a non‐BZD ASM; 111 (37.9%) patients received two BZDs before a non‐BZD ASM. In the multivariate model, out‐of‐hospital SE onset presented a trend toward a higher number of BZDs compared to in‐hospital SE onset (incidence rate ratio [IRR] = 1.17, 95% confidence interval [CI] = .99–1.40, p = .06). The later the treatment initiation was from seizure onset, the less likely a patient was to receive multiple doses of BZD before escalating to a non‐BZD ASM (IRR = .998, 95% CI = .997–.999 per min, p = .01). An underdosed first BZD did not affect the final number of BZDs administered before transitioning (IRR = 1.00, 95% CI = .85–1.17, p = .99). All statistical results are summarized in File S4 (https://github.com/tsheehan01/BZD_Transition).
FIGURE 1.
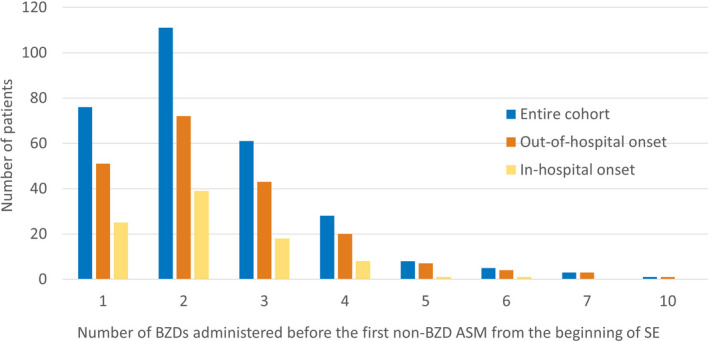
Bar graph representing the number of benzodiazepines (BZDs) administered before the first non‐BZD antiseizure medication (ASM) from the beginning of status epilepticus (SE), in the entire cohort (blue), subpopulation of patients with out‐of‐hospital SE onset (orange), and subpopulation of patients with in‐hospital SE onset (yellow)
3.3. Secondary outcomes
The number of BZD doses administered beyond 30 and 45 min from seizure onset and before non‐BZD ASM are shown in Figures 2 and 3, respectively (data tables in File S5, https://github.com/tsheehan01/BZD_Transition). The percentages of patients who received at least one BZD beyond 30 and 45 min were 57.3% and 44%, respectively. In the multivariate models, patients who presented with out‐of‐hospital SE onset were more likely to receive more doses of BZDs compared to in‐hospital SE onset beyond 30 min (IRR = 2.43, 95% CI = 1.73–3.46, p < .0001) and 45 min (IRR = 3.75, 95% CI = 2.40–6.03, p < .0001). Also, patients who presented with intermittent SE were more likely to receive more doses of BZDs compared to continuous SE beyond 45 min (IRR = 1.44, 95% CI = 1.01–2.06, p = .04). We included an exploratory interaction term between SE location onset and SE type that was not statistically significant. All statistical results are summarized in File S4 (https://github.com/tsheehan01/BZD_Transition).
FIGURE 2.
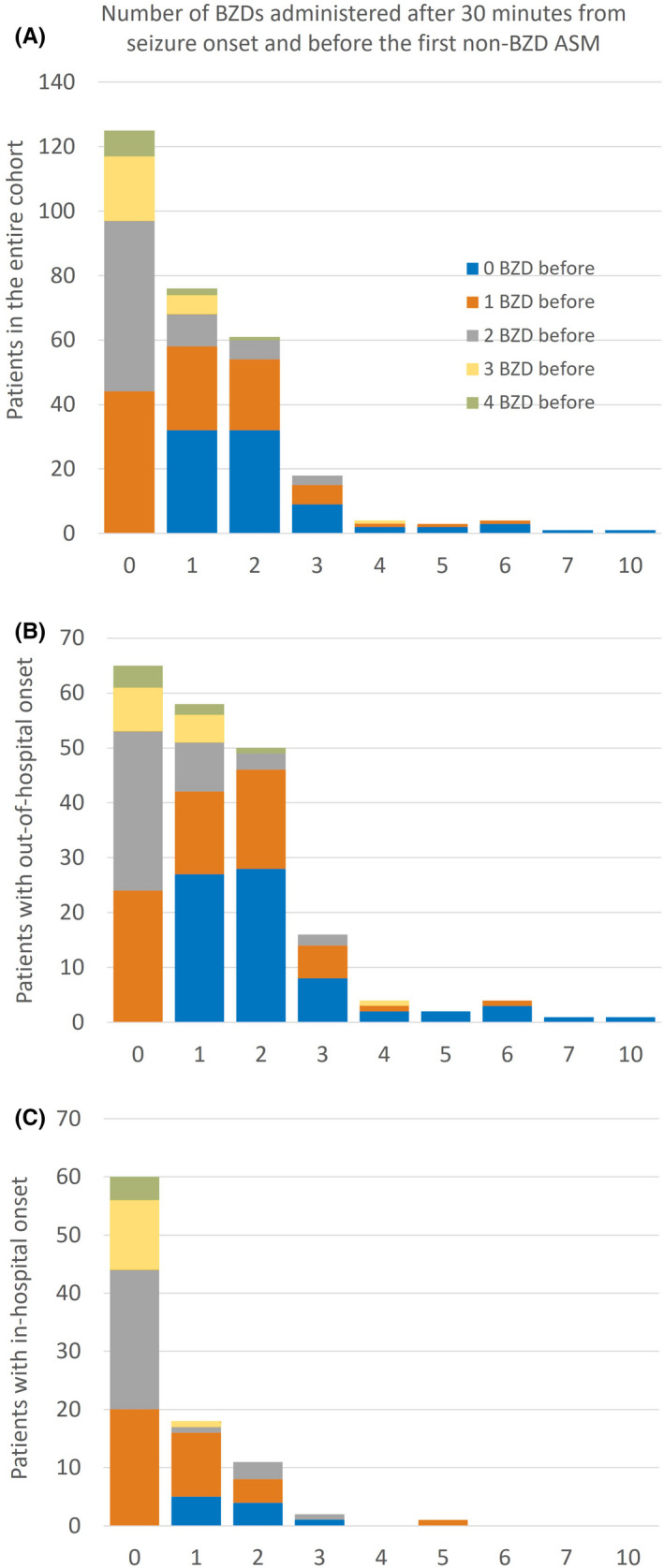
Bar graphs representing the number of benzodiazepines (BZDs) administered after 30 min from seizure onset and before the first non‐BZD antiseizure medication (ASM) in the entire cohort (A), subpopulation of patients with out‐of‐hospital status epilepticus onset (B), and subpopulation of patients with in‐hospital status epilepticus onset (C). The colors of the stacked bars represent the number of BZDs already administered during the first 30 min after seizure onset. For example, in the entire cohort (A), 76 patients received one BZD after the first 30 min from seizure onset and before the first non‐BZD ASM (second bar); of those 76 patients, 32 patients had not received any BZD within the first 30 min (blue section of the second bar), 26 patients had received one BZD (orange section of the second bar), 10 patients had received two BZDs (gray section), et cetera
FIGURE 3.
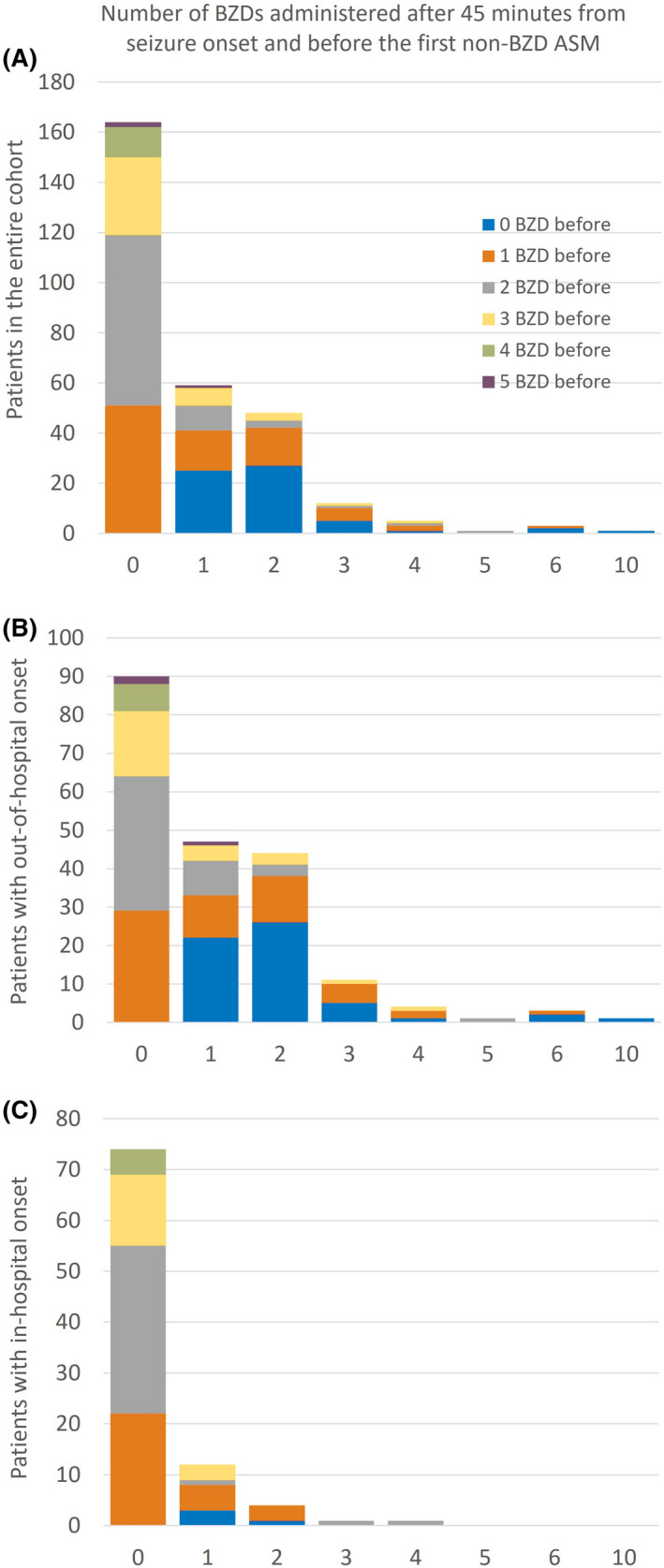
Bar graphs representing the number of benzodiazepines (BZDs) administered after 45 min from seizure onset and before the first non‐BZD antiseizure medication (ASM) in the entire cohort (A), subpopulation of patients with out‐of‐hospital status epilepticus onset (B), and subpopulation of patients with in‐hospital status epilepticus onset (C). The colors of the stacked bars represent the number of BZDs already administered during the first 45 min after seizure onset. For example, in the entire cohort (A), 59 patients received one BZD after the first 45 min from seizure onset and before the first non‐BZD ASM (second bar); of those 59 patients, 25 patients had not received any BZD within the first 45 min (blue section of the second bar), 16 patients had received one BZD (orange section of the second bar), 10 patients had received two BZDs (gray section), et cetera
4. DISCUSSION
In our study population of children with rSE, more than one third of patients received more than two BZDs before escalating to a non‐BZD ASM, and more than half of patients received BZDs after 30 min from seizure onset. Remarkably, in about half of patients who received two or more BZDs before hospital arrival, the rescue algorithm was restarted in patient handoffs at the interface between prehospital and in‐hospital settings. Early treatment initiation for SE—compared to delayed initiation—was associated with a higher likelihood of receiving additional BZD doses before transitioning, and out‐of‐hospital SE onset and intermittent SE were risk factors for receiving BZDs beyond 30 min after seizure onset. Although receiving an underdosed first BZD may hypothetically justify repeated BZD doses, our model, adjusted for potential confounders, showed that the final BZD count was not driven by this factor.
Time to treatment initiation may play a role in the final BZD count based on our multicenter data. If treatment for SE was started later from seizure onset, patients were less likely to receive multiple doses of BZDs before transitioning to a non‐BZD ASM. A potential explanation may be that with clear evidence of evolution into established SE, the care team more readily recognizes the need to escalate to second‐line ASM and to avoid additional trials of BZDs. As there is no prior literature analyzing factors promoting multiple BZD dosing, we cannot compare our results with other studies.
The location of SE onset is related to the final BZD count based on our multicenter data. Out‐of‐hospital SE onset showed a trend toward a higher total number of BZD doses compared to in‐hospital onset, and this difference was more prominent with a longer time from seizure onset. Potential explanations of this factor based on our data and prior literature 18 may be related to different series of events. First, a considerable proportion of patients with out‐of‐hospital SE onset are not receiving any BZD before hospital arrival, or some patients receive multiple doses of BZD because most EMS personnel are not authorized to give a second‐line ASM and may not have other options available. 40 , 41 , 42 The prehospital setting is a key area for a timely and optimized management of SE, because most patients start seizing at this location; however, the lack of standardized EMS guidelines that are aligned with medical protocols, given the challenges and limitations of SE care in the field, hinder optimal prehospital care. Second, transportation to the hospital takes time. 43 Third, if the patient did not receive any BZD out of the hospital, the treatment starts late once the patient arrives at the hospital; conversely, if the treatment was started outside the hospital by caregivers or EMS, the medical team typically repeats a trial of BZD in the hospital setting, even if the patient had received two or more BZDs before hospital arrival.
The influence of nodes of care on breakdowns of treatment administration and escalation may play a role. Improved communication between each level of care, continuous documentation and charting that travel with the patient, and confidence in the treatment administered by first responders (caregivers and EMS) or outside emergency departments in primary or secondary medical centers may prevent iterative treatments, thus making transitions between steps in the treatment algorithm smoother. Patient handoffs are a critical source of miscommunication and have the potential to lead to adverse events. 44 , 45 Specifically, a recent literature review raised concerns about clinical handovers at the interface between prehospital and in‐hospital settings, 46 as sign‐outs in emergency conditions, in noisy and stressful environments, in addition to a lack of time, may further complicate information transfer. 46
Furthermore, SE treatment initiation is often delayed, and catch‐up dosing does not consider timing. Many initial BZD doses and additional administration trials occur outside the most efficacious timeframe, whereas expediting or overlapping an escalation to non‐BZD ASMs may increase the likelihood of SE cessation and ultimately reduce poor outcomes associated with this condition. 1 , 41 , 47 , 48 Although human studies on early polytherapy are still scarce, preclinical data support combining a BZD with a second‐line ASM or an N‐methyl‐D‐aspartate receptor antagonist to increase seizure control. 49
Intermittent SE is related to more delayed treatment initiation or access to care 23 , 41 and may also promote new trials with BZDs later in time before escalating to non‐BZD ASMs. A potential explanation may be uncertainty regarding response to BZD by the care provider or clinical difficulties in recognizing recurrent convulsive seizures as intermittent SE to pursue the next lines of ASM. Future research assessing the risk–benefit ratio of treating after a first seizure and subsequent drowsiness, as well as better biomarkers or technology in the field to help predict evolution into SE, may mitigate uncertainty and improve the management of this condition.
This study highlights a need for an optimized escalation to non‐BZD ASMs after adequate administration of BZD doses, which is important because nonadherence to SE guidelines may negatively impact patient outcomes. 50 Treatment algorithms are commonly restarted instead of continued in the transfer of care (from caregivers to EMS, emergency department, and referral hospital), leading to further delays in ASM transition. Our findings suggest a need for optimized prehospital care and more effective patient handoffs involving a standardized practice for the management of SE. Our results may also provide baseline data for questioning the prominence of BZD as the best first‐line option when treatment delays are the norm in the field. Future SE guidelines may consider the time from seizure onset when deciding on the first rescue treatment to administer. An example may be starting with early polytherapy (a BZD plus a non‐BZD ASM) in SE that has persisted for longer than 30 min. 49 Improved prehospital management, faster transition to non‐BZD ASM, improved handoff at points of care, and potentially novel treatment approaches such as catch‐up dosing or early polytherapy, among others, may provide future opportunities for care improvements in selected patients with rSE.
Results need to be interpreted in the setting of data acquisition. Our population is not representative of all children with seizures or SE, but only of children with witnessed convulsive SE onset who did not respond to initial ASMs and were treated at large academic centers. We do not have information on the proportion of EMS allowed to administer a non‐BZD ASM, the proportion of patients with intravenous access to administer second‐line ASMs, or the number of nodes patients transitioned through (outside hospital locations such as primary care centers, the number of EMS or hospital transfers, etc.), which could be used to assess whether more nodes of care affect the transition from BZD to non‐BZD ASMs. We have information on the location of ASM administration, but this does not consider other potential transient locations where treatments were not given. We did not include the etiology of SE in the model because it is often unknown in the early stages of seizure occurrence and rarely conditions BZD usage; past medical history, such as prior epilepsy diagnosis and prior SE event, may be a greater determinant of BZD usage and was included in our multivariate models. As factors influencing variability may be very extensive (variability in clinical practice over time and among regions and providers at the same point in time ‐different EMS systems, hospitals, doctors, etc.), our sample size is not sufficiently large to consider all potential effects while maintaining the robustness of our model. Lastly, the 45‐min threshold may be arguable, as we do not have extensive and conclusive literature about the exact cutoff and conditions for BZD resistance in humans. We decided to choose only one additional and acceptable threshold to prevent multiple testing. Despite these challenges, our data provide information on medication escalation patterns in a large dataset as well as opportunities for improvement in SE. BZD dosing, types, routes of administration, and effect on outcome were already addressed in another study from our original cohort. 24
5. CONCLUSIONS
In this multicenter study on pediatric rSE in the United States and Canada, about one third of patients received more than two BZD doses before transitioning to non‐BZD ASMs, and more than half of patients received BZD doses after 30 min from seizure onset. Out‐of‐hospital seizure onset and intermittent SE were associated with the failure to escalate from BZDs to non‐BZD ASMs. Specifically, patient handoffs between prehospital and in‐hospital settings may hinder the escalation of ASM due to restarts of treatment algorithms and may offer an opportunity for future intervention and improvement in care.
CONFLICT OF INTEREST
J.N.B. has served as a consultant for Novartis. T.A.G. is funded by National Institutes of Health (NIH) grants 2U01‐NS045911, U10‐NS077311, R01‐NS053998, R01‐NS062756, R01‐NS043209, R01‐LM011124, and R01‐NS065840; has received consulting fees from Supernus, Sunovion, Eisai, and UCB; serves as an expert consultant for the US Department of Justice and has received compensation for work as an expert on medicolegal cases; and receives royalties from a patent license. E.J.N. is on the professional advisory board of the Epilepsy Foundation of America and served on an advisory board for Zogenix. J.J.R.'s spouse is an editor for UpToDate. D.T. receives research funding from Children's Miracle Network Hospitals and has previously received consultation fees from Gerson Lehrman Group, Guidepoint, IQVIA, and bioStrategies Group. R.S. receives research funding from PCORI, NIH, the Pediatric Epilepsy Research Foundation, and the University of Michigan; receives royalties from UpToDate for authorship of topics related to neonatal seizures, is a consultant for the Epilepsy Study Consortium, and is an associate editor for Neurology. M.S.W. serves as a scientific consultant and on the clinical advisory board for Sage Pharmaceuticals. A.W. receives research funding from Novartis, Eisai, Pfizer, UCB, Acorda, Lundbeck, GW Pharma, Upsher‐Smith, and Zogenix and receives publication royalties from UpToDate. T.L. serves on the Council of the American Clinical Neurophysiology Society, on the American Board of Clinical Neurophysiology, as founder and consortium principal investigator of pSERG, as an associate editor for Wyllie's Treatment of Epilepsy 6th and 7th editions, and as a member of the New Onset Refractory Status Epilepticus Institute, PACS1 Foundation, and CCEMRC; served as associate editor of Seizure and served on the Laboratory Accreditation Board for Long Term (Epilepsy and Intensive Care Unit) Monitoring in the past; is part of patent applications to detect and predict clinical outcomes, and to manage, diagnose, and treat neurological conditions, epilepsy, and seizures; is coinventor of the TriVox Health technology, and T.L. and Boston Children's Hospital might receive financial benefits from this technology in the form of compensation in the future; received research support from the Epilepsy Research Fund, the NIH, the Epilepsy Foundation of America, the Epilepsy Therapy Project, and the Pediatric Epilepsy Research Foundation; received research grants from Lundbeck, Eisai, Upsher‐Smith, Mallinckrodt, Sunovion, Sage, Empatica, and Pfizer, including past device donations from various companies, including Empatica, SmartWatch, and Neuro‐electrics; in the past, served as a consultant for Zogenix, Upsher‐Smith, Amzell, Engage, Elsevier, UCB, Grand Rounds, Advance Medical, and Sunovion; performs video electroencephalography long‐term and intensive care unit (ICU) monitoring, electroencephalography, and other electrophysiological studies at Boston Children's Hospital and affiliated hospitals and bills for these procedures, and evaluates pediatric neurology patients and bills for clinical care; and has received speaker honoraria from national societies including the AAN, AES, and ACNS, and for grand rounds at various academic centers. T.L.'s wife, Dr. Karen Stannard, is a pediatric neurologist, and she performs video electroencephalography long‐term and ICU monitoring, electroencephalography, and other electrophysiological studies and bills for these procedures, and she evaluates pediatric neurology patients and bills for clinical care. None of the other authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTIONS
Theodore Sheehan and Marta Amengual‐Gual participated in drafting and revising the manuscript for content, including medical writing, study concept and design, data acquisition, analysis and interpretation of data, statistical analysis, and study supervision or coordination; Bo Zhang and Kush Kapur participated in revising the manuscript for content, study concept and design, analysis and interpretation of data, and study supervision or coordination. All other authors participated in revising the manuscript for content, including medical writing, study concept and design, data acquisition, interpretation of data, and study supervision or coordination. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
This study and our consortium were supported by the Epilepsy Foundation of America (EF‐ 213583, Targeted Initiative for Health Outcomes), by the American Epilepsy Society/Epilepsy Foundation of America Infrastructure Award, by the Pediatric Epilepsy Research Foundation, and by the Epilepsy Research Fund. M.A.‐G. and C.B.A. were supported by Fundación Alfonso Martín Escudero.
Sheehan T, Amengual‐Gual M, Vasquez A, Abend NS, Anderson A, Appavu B, et al; the Pediatric Status Epilepticus Research Group . Benzodiazepine administration patterns before escalation to second‐line medications in pediatric refractory convulsive status epilepticus. Epilepsia. 2021;62:2766–2777. 10.1111/epi.17043
Theodore Sheehan and Marta Amengual‐Gual contributed equally.
DATA AVAILABILITY STATEMENT
All relevant data, statistical analyses, and results can be found in File S2 (https://github.com/tsheehan01/BZD_Transition).
REFERENCES
- 1. Chin RF, Neville BG, Peckham C, Bedford H, Wade A, Scott RC, et al. Incidence, cause, and short‐term outcome of convulsive status epilepticus in childhood: prospective population‐based study. Lancet. 2006;368:222–9. [DOI] [PubMed] [Google Scholar]
- 2. Coeytaux A, Jallon P, Galobardes B, Morabia A. Incidence of status epilepticus in French‐speaking Switzerland: (EPISTAR). Neurology. 2000;55:693–7. [DOI] [PubMed] [Google Scholar]
- 3. DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, et al. A prospective, population‐based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–35. [DOI] [PubMed] [Google Scholar]
- 4. Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology. 1998;50:735–41. [DOI] [PubMed] [Google Scholar]
- 5. Loddenkemper T, Syed TU, Ramgopal S, Gulati D, Thanaviratananich S, Kothare SV, et al. Risk factors associated with death in in‐hospital pediatric convulsive status epilepticus. PLoS One. 2012;7:e47474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maytal J, Shinnar S, Moshé SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics. 1989;83:323–31. [PubMed] [Google Scholar]
- 7. Singh RK, Stephens S, Berl MM, Chang T, Brown K, Vezina LG, et al. Prospective study of new‐onset seizures presenting as status epilepticus in childhood. Neurology. 2010;74:636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu YW, Shek DW, Garcia PA, Zhao S, Johnston SC. Incidence and mortality of generalized convulsive status epilepticus in California. Neurology. 2002;58:1070–6. [DOI] [PubMed] [Google Scholar]
- 9. Raspall‐Chaure M, Chin RF, Neville BG, Scott RC. Outcome of paediatric convulsive status epilepticus: a systematic review. Lancet Neurol. 2006;5:769–79. [DOI] [PubMed] [Google Scholar]
- 10. Kravljanac R, Djuric M, Jankovic B, Pekmezovic T. Etiology, clinical course and response to the treatment of status epilepticus in children: a 16‐year single‐center experience based on 602 episodes of status epilepticus. Eur J Paediatr Neurol. 2015;19:584–90. [DOI] [PubMed] [Google Scholar]
- 11. Gaínza‐Lein M, Sánchez Fernández I, Jackson M, Abend NS, Arya R, Brenton JN, et al. Association of time to treatment with short‐term outcomes for pediatric patients with refractory convulsive status epilepticus. JAMA Neurol. 2018;75:410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Logroscino G, Hesdorffer DC, Cascino GD, Annegers JF, Bagiella E, Hauser WA. Long‐term mortality after a first episode of status epilepticus. Neurology. 2002;58:537–41. [DOI] [PubMed] [Google Scholar]
- 13. Feng HJ, Mathews GC, Kao C, Macdonald RL. Alterations of GABA A‐receptor function and allosteric modulation during development of status epilepticus. J Neurophysiol. 2008;99:1285–93. [DOI] [PubMed] [Google Scholar]
- 14. Burman RJ, Selfe JS, Lee JH, van den Berg M, Calin A, Codadu NK, et al. Excitatory GABAergic signalling is associated with benzodiazepine resistance in status epilepticus. Brain. 2019;11(142):3482–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 16. Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence‐based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016;16:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill CE, Parikh AO, Ellis C, Myers JS, Litt B. Timing is everything: where status epilepticus treatment fails. Ann Neurol. 2017;82:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sánchez Fernández I, Abend NS, Amengual‐Gual M, Anderson A, Arya R, Barcia Aguilar C, et al. Association of guideline publication and delays to treatment in pediatric status epilepticus. Neurology. 2020;95:e1222–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng JY. Latency to treatment of status epilepticus is associated with mortality and functional status. J Neurol Sci. 2016;370:290–5. [DOI] [PubMed] [Google Scholar]
- 20. Sánchez Fernández I, Abend NS, Agadi S, An S, Arya R, Carpenter JL, et al. Gaps and opportunities in refractory status epilepticus research in children: a multi‐center approach by the Pediatric Status Epilepticus Research Group (pSERG). Seizure. 2014;23:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanchez Fernandez I, Abend NS, Agadi S, An S, Arya R, Brenton JN, et al. Time from convulsive status epilepticus onset to anticonvulsant administration in children. Neurology. 2015;84:2304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sánchez Fernández I, Jackson MC, Abend NS, Arya R, Brenton JN, Carpenter JL, et al. Refractory status epilepticus in children with and without prior epilepsy or status epilepticus. Neurology. 2017;88:386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sánchez Fernández I, Gaínza‐Lein M, Abend NS, Anderson AE, Arya R, Brenton JN, et al. Factors associated with treatment delays in pediatric refractory convulsive status epilepticus. Neurology. 2018;90(19):e1692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vasquez A, Gaínza‐Lein M, Abend NS, Amengual‐Gual M, Anderson A, Arya R, et al. First‐line medication dosing in pediatric refractory status epilepticus. Neurology. 2020;11(95):e2683–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeLorenzo RJ, Garnett LK, Towne AR, Waterhouse EJ, Boggs JG, Morton L, et al. Comparison of status epilepticus with prolonged seizure episodes lasting from 10 to 29 minutes. Epilepsia. 1999;40:164–9. [DOI] [PubMed] [Google Scholar]
- 26. Jones DM, Esmaeil N, Maren S, Macdonald RL. Characterization of pharmacoresistance to benzodiazepines in the rat Li‐pilocarpine model of status epilepticus. Epilepsy Res. 2002;50:301–12. [DOI] [PubMed] [Google Scholar]
- 27. Kapur J, Macdonald RL. Rapid seizure‐induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17:7532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walton NY, Treiman DM. Response of status epilepticus induced by lithium and pilocarpine to treatment with diazepam. Exp Neurol. 1988;101:267–75. [DOI] [PubMed] [Google Scholar]
- 29. Joshi S, Rajasekaran K, Hawk KM, Chester SJ, Goodkin HP. Status epilepticus: role for etiology in determining response to benzodiazepines. Ann Neurol. 2018;83:830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen NT, Chamberlain JM, Gaillard WD. Timing and selection of first antiseizure medication in patients with pediatric status epilepticus. Epilepsy Res. 2019;149:21–5. [DOI] [PubMed] [Google Scholar]
- 31. R Foundation for Statistical Computing. R: a language and environment for statistical computing. 2017. www.R‐project.org. [Google Scholar]
- 32. RStudio . RStudio: integrated development for R. Version 1.0.153. 2016. http://www.rstudio.com/.
- 33. gmodels: various R programming tools for model fitting. R package version 2.16.2. Version 2.16.2. 2015. https://CRAN.R‐project.org/package=gmodels.
- 34. gdata: various R programming tools for data manipulation. R package version 2.18.0. Version 2.18.0. 2017. https://CRAN.R‐project.org/package=gdata.
- 35. tableone: create 'table 1' to describe baseline characteristics with or without propensity score weights. R package version 0.8.1. Version 0.8.1. 2017. https://CRAN.R‐project.org/package=tableone.
- 36. dplyr: a grammar of data manipulation. R package 3.2.0. Version 0.8.5. 2016. https://CRAN.R‐project.org/package=dplyr.
- 37. survival: survival analysis. R package version 2.44‐1.1. 2021. https://CRAN.R‐project.org/package=survival.
- 38. ggplot2: create elegant data visualisations using the grammar of graphics. R package version 3.2.0. 2021. https://CRAN.R‐project.org/package=ggplot2.
- 39. MASS: support functions and datasets for Venables and Ripley's MASS. R package version 7.3‐52. Version 7.3‐52. 2020. https://CRAN.R‐project.org/package=MASS.
- 40. Seinfeld S, Shinnar S, Sun S, Hesdorffer DC, Deng X, Shinnar RC, et al. Emergency management of febrile status epilepticus: results of the FEBSTAT study. Epilepsia. 2014;55:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chin RF, Neville BG, Peckham C, Wade A, Bedford H, Scott RC. Treatment of community‐onset, childhood convulsive status epilepticus: a prospective, population‐based study. Lancet Neurol. 2008;7:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee B. Treatment gap for convulsive status epilepticus in resource‐poor countries. Epilepsia. 2018;2:135–9. [DOI] [PubMed] [Google Scholar]
- 43. Gaínza‐Lein M, Fernández IS, Ulate‐Campos A, Loddenkemper T, Ostendorf AP. Timing in the treatment of status epilepticus: from basics to the clinic. Seizure. 2019;68:22–30. [DOI] [PubMed] [Google Scholar]
- 44. Janagama SR, Strehlow M, Gimkala A, Rao GVR, Matheson L, Mahadevan S, et al. Critical communication: a cross‐sectional study of signout at the prehospital and hospital interface. Cureus. 2020;12:e7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168:1755–60. [DOI] [PubMed] [Google Scholar]
- 46. Wood K, Crouch R, Rowland E, Pope C. Clinical handovers between prehospital and hospital staff: literature review. Emerg Med J. 2015;32:577–81. [DOI] [PubMed] [Google Scholar]
- 47. Hassan H, Rajiv KR, Menon R, Menon D, Nair M, Radhakrishnan A. An audit of the predictors of outcome in status epilepticus from a resource‐poor country: a comparison with developed countries. Epileptic Disord. 2016;18:163–72. [DOI] [PubMed] [Google Scholar]
- 48. Tabarki B, Yacoub M, Selmi H, Oubich F, Barsaoui S, Essoussi AS. Infantile status epilepticus in Tunisia. Clinical, etiological and prognostic aspects. Seizure. 2001;10(5):365–9. [DOI] [PubMed] [Google Scholar]
- 49. Amengual‐Gual M, Sánchez Fernández I, Wainwright MS. Novel drugs and early polypharmacotherapy in status epilepticus. Seizure. 2019;68:79–88. [DOI] [PubMed] [Google Scholar]
- 50. Uppal P, Cardamone M, Lawson JA. Outcomes of deviation from treatment guidelines in status epilepticus: a systematic review. Seizure. 2018;58:147–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data, statistical analyses, and results can be found in File S2 (https://github.com/tsheehan01/BZD_Transition).
All relevant data, statistical analyses, and results can be found in File S2 (https://github.com/tsheehan01/BZD_Transition).


