Abstract
Subcutaneous semaglutide, at a 2.4 mg once‐weekly maintenance dose, is approved in the United States for weight management in individuals with a body mass index (BMI) of 30 kg/m2 or higher, or with a BMI of 27 kg/m2 or higher and at least one obesity‐related co‐morbidity. To investigate the usability of the semaglutide pen‐injector in individuals who met these criteria, we report post hoc analysis of the summative (human factors validation) usability testing and safety analysis involving patients with type 2 diabetes (an obesity‐related co‐morbidity) with the same pen‐injector, limited to the 26 out of 30 patients with a BMI of 27 kg/m2 or higher (11 pen‐injector–naïve, 15 pen‐injector–experienced) and 15 non‐pharmacist healthcare professionals (HCPs). Participants performed two simulated injections into an injection pad. No potentially serious use errors occurred. Mean subjective ease‐of‐use rating on a seven‐point scale, where 1 = difficult and 7 = easy, was 6.9 for the second injection in all three groups. These results suggest that the semaglutide pen‐injector is easy to use and not associated with serious use errors when used by pen‐injector–naïve or pen‐injector–experienced patients meeting the requirement for weight management with semaglutide treatment, and by non‐pharmacist HCPs.
Keywords: antiobesity drug, GLP‐1 analogue, type 2 diabetes
1. INTRODUCTION
Subcutaneous semaglutide (Wegovy; Novo Nordisk A/S), delivered by a single‐dose pen‐injector (Figure S1 ) at a maintenance dose of 2.4 mg once weekly, is approved in the United States for weight management in individuals with a body mass index (BMI) of 30 kg/m2 or higher, or with a BMI of 27 kg/m2 or higher and at least one obesity‐related co‐morbidity. 1
The semaglutide single‐dose pen‐injector is a shield‐activated autoinjector. Autoinjectors were initially developed for emergency use but have been used for chronic conditions for decades and for glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) treatment for type 2 diabetes (T2D) since 2014. 2 Shield activation entails the release of the injection by pushing the pen‐injector against the patient's skin, as opposed to activation via a button.
The semaglutide pen‐injector will be the first autoinjector for weight management, calling for an examination of its usability in the target population.
Semaglutide is also approved (under the name Ozempic; Novo Nordisk A/S) for the treatment of T2D at doses of up to 1 mg once weekly in several regions, including Europe, the United States, and Japan. 3 , 4 In most markets, semaglutide for the treatment of T2D is delivered in a multidose pen‐injector, whereas it is delivered in the semaglutide single‐dose pen‐injector in Japan. The semaglutide pen‐injectors for weight management and T2D differ from each other only in dose delivered, colour scheme, and labelling.
As a step in the development of the semaglutide pen‐injector, a summative (human factors validation) usability test and safety analysis of the semaglutide pen‐injector in the context of T2D management was conducted in the United States and reported previously. 5 Four groups were tested (n = 15 per group): patients with T2D with/without pen‐injector experience, non‐pharmacist healthcare professionals (HCPs), and pharmacists. As a non‐interventional summative usability study, it did not entail medical treatment. Four tasks were assessed: (a) pen‐injector carton retrieval, (b) first simulated injection, (c) pen‐injector retrieval, and (d) second simulated injection. The pharmacists completed only the first task because it was the only task that a pharmacist would conduct, whereas the other three groups completed all four tasks. The participants rated the ease of each task on an integer scale, upon which 1 = difficult and 7 = easy. The study found that the semaglutide single‐dose pen‐injector was easy to use and was not associated with any serious use errors. 5 Mean ease‐of‐use ratings were 6.7 (task 1), 5.9 (task 2), 6.6 (task 3), and 6.9 (task 4).
The aim of this post hoc analysis was to examine the usability of the semaglutide pen‐injector in the subgroup of participants with T2D who met the requirements for weight management. In addition, we wanted to examine differences in ease‐of‐use ratings between pen‐injector–naïve and pen‐injector–experienced patients in this subgroup. Of the four tasks originally studied, only the tasks representing the two simulated injections were relevant for weight management and were evaluated in this analysis. The two product‐retrieval (differentiation) tasks were not included in this post hoc analysis because they were based on labelling for the product for treatment of T2D. The selected participants included patients with a BMI of 27 kg/m2 or higher (i.e. the threshold for weight management in the presence of one or more co‐morbid conditions, such as T2D) and all non‐pharmacist HCPs; pharmacists did not perform simulated injections.
2. METHODS
2.1. Procedures
Novo Nordisk A/S (Søborg, Denmark) contracted Emergo by UL (Chicago, Illinois, and Concord, Massachusetts) to carry out usability testing for the semaglutide pen‐injector. Detailed information about the testing process has been published previously. 5 The summative (human factors validation) usability testing of the semaglutide single‐dose pen‐injectors included the pen‐injector four‐pack cartons and their instructions for use (IFU). Injections were simulated using an injection pad. For patients the injection pad was attached to their abdomen or thigh using a Velcro strap, and, for HCPs, the injection pad was attached to a chair to simulate a seated patient. A use error was defined as an action or lack of action that was different from that described in the IFU and caused a result that (a) was different from the result expected, (b) was not caused solely by device failure, and (c) did or could result in harm. There were three categories of use errors: serious use errors, defined as those that could be associated with a serious adverse event; a non‐serious use error, which could potentially be associated with a non‐serious adverse event; and a use error with no potential for harm. No participants were trained on the use of the pen‐injector. Participants used the IFU, as necessary, to learn to inject correctly.
2.2. Statistical analysis
Group comparisons in subjective ease‐of‐use ratings for each injection were made using a non‐parametric Kruskal‐Wallis test for the difference of mean ranks of scores in the three groups. The level of statistical significance was P less than .05.
2.3. Study conduct
The protocol was approved by the Allendale Investigational Review Board (Old Lyme, Connecticut), and fulfilled the provisions of the Declaration of Helsinki. All participants provided informed consent and were compensated for their participation in the study. In addition, they could end their participation at any point without losing compensation. The fact that Novo Nordisk was the sponsor of the study was not disclosed in recruitment materials or when requesting informed consent to avoid biasing participants based on their opinion of the company.
3. RESULTS
3.1. Baseline characteristics
In total, 41 of the 60 participants included in the original analysis were eligible for this post hoc analysis. These participants consisted of 26 patients meeting the anticipated criteria for weight management (11 pen‐injector–naïve and 15 pen‐injector–experienced), as well as 15 non‐pharmacist HCPs. Baseline characteristics of these 41 participants are shown in Table 1.
TABLE 1.
Baseline characteristics
| Characteristic | Group | ||
|---|---|---|---|
| Pen‐injector–naïve patients (n = 11) | Pen‐injector–experienced patients (n = 15) | Non‐pharmacist HCPs (n = 15) | |
| Age, y, mean (range) | 66 (39‐82) | 58 (32‐73) | 49 (31‐68) |
| Female, n (%) | 4 (36) | 9 (60) | 10 (67) |
| BMI, kg/m2, mean | 32.3 | 37.3 | NA |
| ≥30 kg/m2, n (%) | 8 (72.7) | 15 (100) | NA |
| ≥27‐<30 kg/m2, n (%) | 3 (27.3) | 0 (0) | NA |
| Vision a | |||
| Normal | 1 | 1 | 6 |
| Corrected with contacts/glasses for reading | 7 | 8 | 3 |
| Corrected with contacts/glasses for distance | 0 | 4 | 3 |
| Corrected with contacts/glasses for distance and reading | 3 | 2 | 3 |
| Hearing | |||
| Normal | 9 | 14 | 15 |
| Corrected with hearing aids in both ears b | 2 | 1 | 0 |
| Dexterity | |||
| Normal | 7 | 11 | 13 |
| Arthritis, both hands | 2 | 1 | 1 |
| Arthritis, right hand c | 1 | 1 | 0 |
| Neuropathy, both hands | 0 | 1 | 0 |
| Neuropathy, right hand | 0 | 1 | 0 |
| Numbness, right index finger | 1 | 0 | 0 |
| Tremor in both hands | 0 | 0 | 1 |
| Highest education level completed | NA | ||
| High school | 0 | 3 | |
| Some college/college | 11 | 7 | |
| Associates degree | 0 | 4 | |
| Advanced degree | 0 | 1 | |
| Occupation | NA | NA | |
| Registered nurse | 6 | ||
| Physician | 5 | ||
| Certified diabetes educator | 4 | ||
| Year in professional practice, mean (range) | NA | NA | 19 (5‐47) |
| Work environment d | NA | NA | |
| Hospital | 11 | ||
| Primary care clinic | 2 | ||
| Long‐term care clinic | — | ||
| Endocrinology clinic | — | ||
| Family medicine clinic | 1 | ||
| Outpatient clinic | 1 | ||
Note: All values are counts, unless otherwise specified.
Abbreviations: BMI, body mass index; HCP, healthcare professional; NA, not applicable.
One patient reported more than one vision correction/impairment.
There were no patients who had hearing impairments that were corrected in one ear only.
There were no patients who had arthritis in their left hand only.
Some HCPs reported more than one work environment.
The 15 pen‐injector–experienced patients all had experience with multidose pen‐injectors for injection of insulin, a GLP‐1 RA, or both insulin and a GLP‐1 RA, for the treatment of diabetes, but no experience with single‐dose pen‐injectors for diabetes or any other indication.
3.2. Ease‐of‐use ratings
The mean ease‐of‐use ratings among the three groups of participants were measured on a seven‐point scale, where 1 = difficult and 7 = easy. Mean scores for the first injection task were 6.3, 6.1, and 5.5 in the pen‐injector–naïve patients, pen‐injector–experienced patients, and non‐pharmacist HCPs, respectively. However, the differences between the groups were not statistically significant (P = .16). All participants whose rating for the first simulated injection was not 7 gave a higher rating for the second simulated injection. The mean ease‐of‐use rating was 6.9 in all three groups. Paired profiles of the ease‐of‐use ratings for the three groups are shown in Figure 1.
FIGURE 1.
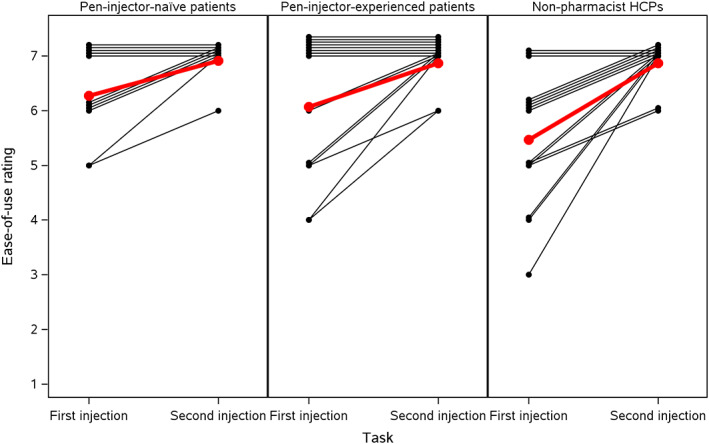
Paired profiles of ease‐of‐use* ratings between pen‐injector–naïve and pen‐injector–experienced patients, and non‐pharmacist HCPs; *rated using a 1‐7 scale where 1 = difficult and 7 = easy. Red lines are group means. HCP, healthcare professional
3.3. Performance of use
Of the use errors reported in the main analysis related to the two simulated injection tasks, 5 none were made by the four patients who were excluded from the current analysis because of their BMI (<27 kg/m2). The use‐error findings for the main analysis therefore apply directly to this analysis of pen‐injector–naïve and pen‐injector–experienced patients (BMI ≥27 kg/m2 and at least one obesity‐related co‐morbidity) and non‐pharmacist HCPs. There were no potentially serious use errors in any of the three groups tested. Of the use errors that did occur, none had the potential to cause harm. All participants completed the second injection correctly without any use errors. The root cause of each error was assessed to be prior experience with other pen‐injectors or syringes, the requirement to read the IFU completely, or misinterpretations of the IFU. The IFU were not amended in response to these misinterpretations because the errors were limited to first‐time use and the development team assessed that attempts at further improvement of the IFU would not be helpful.
4. DISCUSSION
This post hoc analysis examined the usability of the semaglutide single‐dose pen‐injector by patients suitable for treatment with semaglutide for weight management and non‐pharmacist HCPs. No serious use error was made by a member of any group. The non‐serious errors were all made on the first injection and none of these had the potential to cause harm. Ease‐of‐use ratings were generally at the high end of the 1‐7 scale for all three groups for the first injection, and all participants whose rating was below 7 on the first injection increased their rating on the second injection, with a mean ease‐of‐use rating of 6.9 in all groups. All pen‐injector–experienced patients had used traditional, push‐button–activated pen‐injectors but not shield‐activated pen‐injectors (as used for semaglutide). It is therefore possible that, in some cases, reliance on existing knowledge might have hampered the immediate understanding of the working principle of the novel pen‐injector. Similarly, some HCPs might have been unfamiliar with shield‐activated pen‐injectors. However, ease‐of‐use ratings did not differ significantly between groups on the first injection. The lack of serious use errors and high ease‐of‐use scores were achieved without any face‐to‐face training. We inferred from this outcome that the semaglutide pen‐injector does not compromise patient safety and is easy to use. However, it is still important that HCPs counsel their patients to read the IFU thoroughly to avoid use errors.
Similar analysis of the single‐dose pen‐injector for dulaglutide, a GLP‐1 RA used exclusively for treatment of T2D, has also suggested that patients find single‐dose pen‐injectors easy to use. 6 However, the study protocol included patients with a BMI of 23 kg/m2 or higher, and the applicability of these results to those with overweight or obesity is unknown. Therefore, it is not possible to directly compare these results with our findings.
The main limitation of this study is that it was a retrospective post hoc analysis. The results suggest that use errors are rare with the semaglutide single‐dose pen‐injector when patients meet the study's criteria for requiring weight management. A prospective study is needed to test this hypothesis. Other limitations of this study relate to the simulated nature of the injections, which might not exactly replicate the complete experience of use in the intended environment. When injections are performed into an injection pad, it is not known whether patients would have performed differently if they had felt the needle or experienced discomfort because of the drug product. The experience in our experimental setting might also differ from that in the natural setting in other ways 7 ; for example, being observed while performing a task might create performance anxiety. This analysis only included adults aged 18 years or older, which means that the results of this analysis cannot be extrapolated to adolescents or to elderly populations specifically. In addition, usability may be different in individuals with cognitive impairment.
In conclusion, this post hoc analysis found that in patients suitable for treatment with semaglutide for weight management and non‐pharmacist HCPs, regardless of prior pen‐injector experience, the semaglutide pen‐injector was associated with ease of use. A prospective study is warranted to examine the hypothesis that the semaglutide pen‐injector is easy to use.
CONFLICT OF INTEREST
DCK is a consultant for EoFlow, Fractyl, Lifecare, Novo Nordisk, Roche Diagnostics, Samsung, and Thirdwayv. SB is an employee of Emergo by UL, based in Chicago, Illinois. Over the time of this analysis and the development of this report, EE and MM were employees at Emergo by UL, Concord, Massachusetts. Emergo by UL performs various usability testing studies on behalf of Novo Nordisk and is paid by Novo Nordisk. MF, MQ, TS, and SS are employees of Novo Nordisk, Søborg, Denmark, and MQ and TS own stock in the company.
AUTHOR CONTRIBUTIONS
All authors contributed to the analysis and the writing of the manuscript. EE, MM, MQ, SB, SS, and TS also contributed to the design of the summative usability test, and EE, SB, and MM also contributed to the conduct of the summative usability test and to data collection.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14509.
Supporting information
FIGURE S1. Use of the single‐dose, shield‐activated pen‐injector for semaglutide. (a) Pull the pen‐injector cap straight off the pen‐injector and (b) push the pen‐injector firmly against the skin until the yellow bar inside the pen‐injector window has stopped moving
ACKNOWLEDGEMENTS
We thank all the participants who were involved in the summative usability testing process; and Catherine Starling, at AXON Communications, for medical writing and editorial assistance (funded by Novo Nordisk A/S). This study was funded by Novo Nordisk A/S, Denmark. Novo Nordisk A/S contributed to the design and conduct of the trial, the analysis and interpretation of the data, and review and approval of the manuscript.
Klonoff DC, Bassock S, Engels E, et al. Semaglutide single‐dose pen‐injector: Post hoc analysis of summative usability testing for weight management. Diabetes Obes Metab. 2021;23(11):2590-2594. doi: 10.1111/dom.14509
Funding information Novo Nordisk A/S, Denmark
DATA AVAILABILITY STATEMENT
The data sets analysed during the current study are available on reasonable request.
REFERENCES
- 1. U.S. Food and Drug Administration FDA approves new drug treatment for chronic weight management, first since 2014. https://www.fda.gov/news‐events/press‐announcements/fda‐approves‐new‐drug‐treatment‐chronic‐weight‐management‐first‐2014. Accessed August 24, 2021.
- 2. Selam JL. Evolution of diabetes insulin delivery devices. J Diabetes Sci Technol. 2010;4(3):505‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Novo Nordisk Ozempic® (semaglutide) summary of product characteristics. 2018. https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004174/WC500244163.pdf. Accessed August 24, 2021.
- 4. Novo Nordisk Ozempic® (semaglutide) prescribing information. 2019. https://www.novo-pi.com/ozempic.pdf. Accessed August 24, 2021.
- 5. Klonoff DC, Bassock S, Dwyer A, et al. Evaluating the usability and safety of the semaglutide single‐dose pen‐injectors through summative (human factors) usability testing. J Diabetes Investig. 2021;12(6):978‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matfin G, van Brunt K, Zimmermann AG, et al. Safe and effective use of the once weekly dulaglutide single‐dose pen in injection‐naïve patients with type 2 diabetes. J Diabetes Sci Technol. 2015;9(5):1071‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1. Use of the single‐dose, shield‐activated pen‐injector for semaglutide. (a) Pull the pen‐injector cap straight off the pen‐injector and (b) push the pen‐injector firmly against the skin until the yellow bar inside the pen‐injector window has stopped moving
Data Availability Statement
The data sets analysed during the current study are available on reasonable request.


