Abstract
Gut microbiota are essential to nutrient metabolism and the maintenance of hindgut health. The characterization of faecal bacterial communities from healthy individuals is important for the establishment of baseline data that can be compared to periods of gut dysbiosis. Diet is a key determinant of the faecal microbial community structure and generation of volatile fatty acids, a main energy source for the host. While rhinoceroses are herbivores, black rhinoceroses are browsers and white rhinoceroses are grazers. The objective of our study was to characterize and compare diets, faecal bacterial communities, nutrients and metabolites between and amongst Southern white rhinoceroses and Southern black rhinoceroses (n = 3 rhinos/species) managed at Disney's Animal Kingdom®. Faecal bacterial communities were similar between individual white rhinos and dissimilar between species and individual black rhinos. Faecal butyrate and propionate molar proportions and concentrations were greater in black rhinos than white rhinos, whereas lactate was greater in white rhinos. The Shannon diversity, total operational taxonomic units, and relative abundance of Firmicutes were greater in white than black rhinos. The relative abundance of Proteobacteria in faeces from black rhinos was 3‐fold greater than from white rhinos. One black rhino had a greater relative abundance of Verrucomicrobia (7.45 ± 1.31%) than all other individual rhinos (0.01–1.37%). White rhinoceroses demonstrated similar abundances of bacterial phyla and communities between one another and by individual, while black rhinoceroses were more dissimilar by individual. The dissimilarities between black rhinos were suspected to be due to total diet consumption variability, including browse diversity, and lack of direct contact. In contrast, the white rhinos commingled (i.e. nose‐to‐nose contact) and consumed similar amounts of hay, pellets and training items. These results suggest that species‐specific diets and the individual contribute to differences in faecal bacterial communities, nutrients and metabolites between black and white rhinos housed at the same institution.
Keywords: diversity, inter‐animal, microbiota, rhinoceroses, rhinos, VFA
1. INTRODUCTION
Rhinoceroses are herbivores that convert feed into energy through hindgut microbial fermentation and individual species differ in their specialization for plant groups (Steuer et al., 2010). White rhinoceroses (Ceratotherium simum simum) are grazers that consume leaves and stems of grasses/monocots, while black rhinoceroses (Diceros bicornis minor) are browsers that consume trees, shrubs, forbs, herbs—mainly dicotyledonous plants. Microbiota (bacteria, fungi, archaea) are essential to nutrient metabolism and hindgut health. Gut microbiota convert non‐digestible plant cell wall components into volatile fatty acids (VFA) which are the major end‐products of fermentation and a main energy source of the host (Julliand & Grimm, 2017). The VFA composition is dependent on the gut microbial composition and has been shown to vary by diet type and health status in the horse, the closest domestic model for rhinoceroses hindgut fermentation (Hussein et al., 2004; Murray et al., 2009). Microbial enzymes break down complex polysaccharides and simple sugars (Julliand & Grimm, 2017). Therefore, quantification of fermentation end‐products and identification of the different bacterial functional groups (i.e. fibre‐ vs. starch‐degrading) can be used to understand gut microbial activity and health.
There are few studies describing the gut bacterial community structure of white rhinoceroses (WR) (Bian et al., 2013; Williams et al., 2019) and eastern black rhinoceros (BR) (Antwis et al., 2019; Gibson et al., 2019) under human care. Spot faecal microbial communities and metabolites from four rhinoceros species housed at different institutions were described and compared with as‐fed diet amount differences within and across species reported (Roth et al., 2019). Additionally, one study compared the faecal bacterial communities from one Indian rhinoceros (San Diego Zoo) and one BR (St. Louis Zoo) with other managed and wild mammals; however, this study used techniques with a low number of bacterial DNA sequences and did not provide dietary details (Ley et al., 2008). A metagenomic approach demonstrated differences between the faecal microbiomes of zoo managed and wild black rhinoceroses (Gibson et al., 2019). Faecal VFA from zoo managed black and greater one‐horned rhinoceroses were previously reported in relation to diet (Clauss, Castell, Kienzle, Dierenfeld, et al., 2007; Clauss et al., 2005) and faecal lactate concentrations from white rhinoceroses were increased in comparison with black, greater one‐horned, and Sumatran rhinoceroses under human care (Roth et al., 2019). To date, no published studies have collectively characterized the faecal bacteria and fermentation end‐products via repeat faecal sampling from Southern BR or compared these measures between black and white rhinoceroses managed at the same institution over multiple time points. This aforementioned approach is suspected to determine if the faecal bacteria from an individual over multiple timepoints is static or variable from faecal to faecal sample, and removes zoological institution as an influencing factor.
Equine fermentation has been used as an adequate model for BR microbial fermentation (Huntley et al., 2017). Previous work in horses demonstrated an increase in cecal bacterial diversity from horses consuming hay versus those diets with increased starch content (Hansen et al., 2015). Julliand and Grimm (2016) initially demonstrated that faecal microbiota are not representative of the proximal hindgut (i.e. cecum and ventral colon); however, both GIT regions and faeces are susceptible to dietary variations and changes (Julliand & Grimm, 2016). A correlation between horse faecal and cecum bacterial groups was identified, indicating the validity of using faecal samples as a non‐invasive sampling technique for assessing the hindgut microbial ecosystem under dietary changes (Julliand & Grimm, 2017).
Provided the differences in dietary intakes on a BW‐basis between species and species‐specific diets offered to rhinoceroses and considering the previous work by Roth et al. (2019), we hypothesized that the faecal bacterial communities and fermentation end‐products are distinct between the two species and similar within the same species. The objectives of our study were to characterize and compare the faecal bacterial communities and fermentation end‐products between and amongst white and black rhinoceroses over a 4‐week period. The research presented is novel as no study has compared the faecal bacterial communities between and amongst two rhinoceros species housed at the same institution, especially in the context of measured dietary intake. Initial research findings from this study will contribute knowledge to the zoo field about the relationship amongst diet, faecal bacterial, and fermentation end‐products in healthy rhinos so that in the future we can potentially identify markers of gut dysbiosis and rhinoceros health status.
2. MATERIALS AND METHODS
2.1. Animal information
Two male and one female Southern BR and three female Southern WR were enrolled into the study at Disney's Animal Kingdom® in December 2018 and February 2019 respectively (Table 1). Individual body weights were measured at the start of the study. Rhinoceroses were previously trained to walk onto the scale located within their housing location. None of the female rhinoceroses were pregnant or lactating and none of the rhinoceroses received antibiotics or displayed signs of gut dysbiosis during the study. BR were housed individually and allowed access to individual, outside yards on a rotating basis from 0800–1600 h daily, while WR were housed together on a rotating basis during the day and individually overnight. The rotation for BR indicates that on a given day, all 3 rhinoceroses would be outside in separate yards or on another day two rhinoceroses would be outside and one would remain inside. The rotation for WR indicates that from day to day, there was a different combination of which rhinoceroses were housed outside together. WR enrolled in the study were also housed in yards on a rotating basis with non‐study, adult WR due to management needs.
TABLE 1.
Description of individual rhinoceroses
| Rhino | Species | Sex | Age (yr) | Bodyweight (kg) |
|---|---|---|---|---|
| BR1 | Southern black rhinoceros | M | 17 | 1307 |
| BR2 | Southern black rhinoceros | M | 16 | 1144 |
| BR3 | Southern black rhinoceros | F | 21 | 1384 |
| WR1 | Southern white rhinoceros | F | 15 | 1829 |
| WR2 | Southern white rhinoceros | F | 19 | 1884 |
| WR3 | Southern white rhinoceros | F | 8 | 1855 |
2.2. Diets, dry matter intake and feed sampling
BR were offered a daily ration of Browser Rhino Cube 5Z1P (Mazuri® Exotic Animal Nutrition), timothy and Bermuda grass hays, wheat bran, browse and a variety of enrichment/training items. Amounts offered are in agreement with what is described in Sullivan and Valdes (2019). BR differed between individuals in specific feeds consumed due to their own preferences. Available browse provided as 28.7 ± 7.6% of the total as‐fed diet (mean ± SD) included: ear leaf acacia (Acacia auriculiformis), golden wattle (Acacia longifolia), banana leaves and stalks (Musa spp.), Japanese blueberry (Elaeocarpus), silverberry (Elaeagnus), cactus pads (Opuntia) and hydroponic barley (Hordeum vulgare). Enrichment and training items were offered three times per day and included LS Primate biscuit (Mazuri® Exotic Animal Nutrition), sweet potato, green beans, apple, carrot, green and romaine lettuces, cucumber, zucchini and cauliflower. These items were rotated on a set schedule but consisted of less than 10% of the total as‐fed diet per day. All BR were supplemented daily with Emcelle® Tocopherol Vitamin E Supplement (Stuart Products Inc.) and Sodium Phosphate Monobasic (Medisca). Feed intake was measured via total collection of diet items offered and orts per individual rhino. This was done over a continuous 72 h period during week 2 for BR1, week 3 for BR2 and week 4 for BR3 due to management preference.
Individual WR were offered a daily ration of Browser Rhino Cube 5Z1P, Bermudagrass hay and supplemented with Emcelle® Tocopherol Vitamin E in amounts described by (Sullivan & Valdes, 2019). One of the primary ingredients of the Browser Rhino Cube is timothy hay and was designed to be low in iron for browsing species, but otherwise appropriate for wild herbivores in that it is low in starch, high in soluble and total fibres, with a complete vitamin/mineral composition. This pellet has been used for successful health maintenance for the last ten years at our institution and other AZA and international zoological institutions. Bermudagrass was divided into two feedings; one to the individual WR overnight and the second as a proportion of the total WR group amount offered in the yard during the day. Enrichment and training items offered three times per day included timothy alfalfa cubes, alfalfa hay and Petting Zoo 5MJZ pellets (Mazuri® Exotic Animal Nutrition) and comprised less than 5% of the total as‐fed diet per day. Feed intakes were measured over a continuous 72 h period during week 2 of the study for all three animals due to management preference. The individual intakes of Bermudagrass hay were estimated by calculating the proportion of hay offered to an individual WR within the group and multiplying that proportion by the total intake for the group. Total collections of all other diet items were measured for each individual WR, instead of estimating from a group amount.
All feed items offered to both BR and WR were sampled as per Disney's quality control protocols. This was done once during the study, with samples pulled from the centralized Animal Nutrition Center, where all feed originates before reaching the animals. These representative samples were sent on ice within 24 h of sampling for dry matter and nutrient analyses including crude protein, ADF, NDF, crude fat, starch and minerals at Dairy One Laboratories. Sampling included 8 bales of each hay type being cored by a Penn State hay probe with drill adapter (Nasco), and combined to contribute to a representative sample of each type for send out and analysis. Pellet samples were taken from a minimum of 5 fresh unopened bags, cut and pulled with gloved hands, and then combined for a representative sample for laboratory analysis. Produce and all other feed samples were taken from the Nutrition Center, included multiple items of each product sampled with gloved hands. Browse samples were taken from the 4°C cooler where they are stored for no more than 3 days post‐harvest prior to feeding. Whole branch (leaves and stem) samples using multiple branches were analysed as the BR consume all of the plant offered.
2.3. Faecal sampling
Freshly voided faecal samples (500 g) were collected between 0500–0900 h. A total of 12 faecal samples were collected from each animal over a 4‐week period with a minimum of two samples collected per animal per week. Each sample was collected from the centre of the faecal bolus and placed in sterile bags. A total of 2 g of fresh faeces were mixed with 30 g double distilled water into a slurry. Faecal pH was measured with an Orion Versa StarPro (Thermo Scientific) pH meter immediately after collection. Faecal samples for VFA and nutrient analyses were immediately frozen with liquid nitrogen and those for DNA extractions stored frozen at −20°C. VFA and nutrient analyses were performed at Dairy One Laboratories.
2.4. Bacterial 16S rRNA analyses
Microbial DNA was isolated from faecal samples using the QIAamp PowerFecal DNA kit and protocol (Qiagen). The Qiagen vortex adapter with the Vortex‐Genie Shaker (Scientific Industries) were used for the initial cell lysis step. DNA was quantified using the Qubit 4 fluorometer (Invitrogen) before sending DNA extracts for amplification and 16S rDNA sequencing at the Microbial Systems Molecular Biology Laboratory at the University of Michigan. Briefly, the V4 hypervariable region of the 16S rRNA gene was amplified and sequenced using the Illumina MiSeq 2 × 250 platform with 500 cycles and the previously outlined methods (Kozich et al., 2013). Demultiplexed sequences were analysed with the bioinformatics program, Mothur v1.43, using the MiSeq SOP (https://www.mothur.org/wiki/MiSeq_SOP, accessed 12/14/20) and data was visualized with the Tidyverse package of R Studio.
These sequence data have been submitted to the Sequence Read Archive of GenBank at NCBI under accession number PRJNA599136. All paired‐end sequence reads were assembled and screened for sequences with ambiguous bases and a maximum length of 250 base pairs. A total of 2,497,635 sequences remained after screening. A total of 294,578 unique sequences were aligned with the silva.v4.fasta reference file. Chimeras were identified by VSEARCH and subsequently removed in Mothur. Non‐chimeric sequences were classified with the SILVA v123 reference files and sequences that were identified as Archaea, Chloroplast, Cyanobacteria, Eukaryota, Mitochondria and unknown were removed (3.4% of total sequences). Uncorrected pairwise distances were calculated between the aligned sequence reads with a cut‐off of 0.03 to denote 97% sequence similarity. Individual samples were normalized to 13,699 sequence reads. Measurements of alpha diversity included ACE richness, Chao 1 richness, Good's Coverage, Shannon Diversity index and Inverse Simpson Index. Bray–Curtis dissimilarity indices were calculated to compare community structures and compositions within and across rhino species. A non‐metric multidimensional scaling (nMDS) was calculated with Mothur and visualized with R Studio to compare the faecal bacterial community structures of the rhinoceros species. Shared OTUs between and within rhino species were calculated. An analysis of similarity (ANOSIM) in MOTHUR was used to identify bacterial community differences between individuals, species and within a species (Clarke, 1993).
2.5. Statistics
Means of rhinoceros species for faecal nutrients, VFA and pH were evaluated with PROC MIXED in SAS (v9.4, SAS Institute) with the random effect of individual rhinoceros and the fixed effects of species and the interaction of species by sample number. Differences by rhinoceros species and the interaction between species and sample number were declared at p < 0.05 and trends at 0.05 ≤ p ≤ 0.10. The Shapiro‐Wilk normality test was used in R to test for normality of the relative abundances of bacterial phyla and alpha diversity measures. An Analysis of Variance test followed by a Tukey's Honest Significant Difference test was performed on the normally distributed data. The Kruskal‐Wallis Rank Sum test in R was performed to determine differences between means by rhinoceros species and the Wilcoxon Rank Sum test was used for pairwise comparison of the means between individual rhinos and species.
3. RESULTS
3.1. Dry matter intake
Mean DMI was 21.8 ± 2.6 kg/d (mean ± standard deviation) for BR with DMI as 1.7 ± 0.3% BW and 23.6 ± 2.4 kg/d for WR with DMI as 1.3 ± 0.1% BW. NDF (% total DMI) was 53.4 ± 2.8% for BR and 65.4 ± 2.4% for WR (Table 2). Crude protein (% total DMI) was 14.6 ± 0.6% for BR and 9.2 ± 0.9% for WR. Average browse, pellet, hay and training item intakes of BR as a percentage of total DMI were 18.3 ± 9.3%, 30.4 ± 11.3%, 47.3 ± 11.5% and 4.0 ± 1.3% respectively. Average pellet, hay and training item intakes of WR as a percentage of total DMI were 10.3 ± 4.3%, 86.8 ± 4.9% and 2.9 ± 0.9% respectively. Individual nutrient and DMI values from each rhinoceros are located in (File S1).
TABLE 2.
Average nutrient content of the total dietary dry matter intake (DMI) consumed by three black and three white rhinos over a 72 h period
| Nutrient (% of DMI) | Species (mean ± SD) | p‐value | |
|---|---|---|---|
| Black rhino | White rhino | ||
| Crude protein | 14.0 ± 1.00 | 9.74 ± 0.43 | <0.01 |
| Neutral detergent fibre | 53.5 ± 2.10 | 69.5 ± 1.49 | <0.001 |
| Acid detergent fibre | 32.7 ± 1.41 | 36.8 ± 0.41 | 0.01 |
| Lignin | 6.82 ± 1.05 | 4.81 ± 0.04 | 0.03 |
| Starch | 3.17 ± 0.80 | 3.76 ± 0.11 | 0.27 |
| Crude fat | 3.38 ± 0.23 | 1.58 ± 0.15 | <0.01 |
| Ash | 7.48 ± 0.39 | 6.07 ± 0.20 | 0.01 |
| Calcium | 0.71 ± 0.10 | 0.48 ± 0.05 | 0.02 |
| Phosphorus | 0.37 ± 0.06 | 0.24 ± 0.02 | 0.02 |
| Magnesium | 0.22 ± 0.03 | 0.17 ± 0.01 | 0.04 |
| Potassium | 1.63 ± 0.05 | 1.31 ± 0.01 | <0.001 |
| Sodium | 0.24 ± 0.07 | 0.08 ± 0.03 | 0.02 |
| Sulphur | 0.31 ± 0.05 | 0.32 ± 0.01 | 0.74 |
| Iron (ppm) | 133 ± 33.4 | 103 ± 10.1 | 0.22 |
| Zinc (ppm) | 60.3 ± 15.3 | 41.6 ± 4.94 | 0.11 |
| Copper (ppm) | 13.9 ± 2.79 | 12.8 ± 0.77 | 0.51 |
| Manganese (ppm) | 82.5 ± 11.2 | 124 ± 0.35 | <0.01 |
| Molybdenum (ppm) | 1.99 ± 0.27 | 1.07 ± 0.10 | 0.01 |
| Selenium (ppm) | 0.26 ± 0.08 | 0.30 ± 0.02 | 0.41 |
| Cobalt (ppm) | 0.63 ± 0.09 | 0.50 ± 0.03 | 0.08 |
3.2. Faecal pH, VFA and nutrients
Dry matter, lignin, copper, iron, cobalt and potassium of faecal contents did not differ by species or the interaction between species and sample week (Table 3). Faecal contents of crude protein, acid detergent fibre, crude fat, calcium, phosphorus, sodium and sulphur were greater in BR than WR. Neutral detergent fibre was greater in WR (69.9%) than BR (66.4%), while zinc tended to be greater in BR (97.3 ppm) than WR (79.9 ppm) faecal samples (p = 0.07). There was a trend for faecal pH to differ between species (p = 0.06) with 6.07 ± 0.10 (mean ± SE) from BR and 6.36 ± 0.10 from WR. The faecal concentration of acetate did not differ by species (Table 3). Faecal molar proportions (mol/100 mol total VFA) of acetate were 67.8 ± 0.70% for BR and 80.9 ± 0.27% for WR (p < 0.001). Mean molar proportions of faecal butyrate were greater in BR (8.67 ± 0.23%) than WR (4.43 ± 0.09%). Faecal isobutyrate and propionate molar proportions and concentrations were greater in BR than WR (p < 0.001). Faecal lactate concentrations were greater in WR (7.56 ± 0.48 mM) than in BR (2.74 ± 0.52 mM) (p < 0.001) (Table 3).
TABLE 3.
The effect of species on faecal metabolites and nutrient contents from three black and 3 white rhinoceroses (n = 12 samples/animal)
| Species | Standard error | p‐value* | ||
|---|---|---|---|---|
| Black rhino | White rhino | |||
| Fermentation by‐products in faeces | ||||
| Acetate (mM) | 146 | 136 | 7.01 | 0.33 |
| Propionate (mM) | 44.5 | 23.3 | 1.53 | <0.001 |
| Butyrate (mM) | 18.4 | 7.50 | 0.76 | <0.001 |
| Isobutyrate (mM) | 4.19 | 1.51 | 0.19 | <0.001 |
| Total VFA (mM) | 213 | 169 | 9.06 | <0.01 |
| Acetate (%a) | 67.8 | 80.9 | 0.53 | <0.001 |
| Propionate (%) | 21.4 | 13.8 | 0.42 | <0.001 |
| Butyrate (%) | 8.67 | 4.43 | 0.17 | <0.001 |
| Isobutyrate (%) | 2.07 | 0.86 | 0.11 | <0.001 |
| Lactate (mM) | 2.74 | 7.56 | 0.64 | <0.001 |
| pH | 6.07 | 6.36 | 0.10 | 0.06 |
| Ammonia (% CPEb) | 0.73 | 0.23 | 0.06 | <0.001 |
| Faecal nutrients (% DM‐basis) | ||||
| Dry matter | 21.1 | 21.1 | 0.56 | 0.98 |
| Crude protein | 10.3 | 8.08 | 0.28 | <0.001 |
| Neutral detergent fibrec | 66.4 | 69.9 | 0.67 | <0.01 |
| Acid detergent fibre | 41.1 | 39.3 | 0.38 | <0.01 |
| Lignin | 8.09 | 7.87 | 0.17 | 0.38 |
| Crude fat | 4.30 | 2.12 | 0.13 | <0.001 |
| Ash | 9.55 | 9.51 | 1.36 | 0.99 |
| Calcium | 0.35 | 0.23 | 0.02 | <0.01 |
| Phosphorus | 0.59 | 0.35 | 0.03 | <0.01 |
| Magnesium | 0.11 | 0.12 | 0.003 | 0.74 |
| Potassium | 1.40 | 1.27 | 0.05 | 0.11 |
| Sodium | 0.26 | 0.11 | 0.02 | <0.001 |
| Sulphur | 0.26 | 0.19 | 0.01 | <0.01 |
| Iron (ppm) | 606 | 531 | 140 | 0.72 |
| Zinc (ppm) | 97.3 | 79.9 | 5.80 | 0.07 |
| Copper (ppm) | 19.0 | 26.2 | 3.20 | 0.16 |
| Manganese (ppm) | 116 | 116 | 4.12 | 0.95 |
| Molybdenum (ppm) | 2.36 | 0.61 | 0.09 | <0.001 |
| Selenium (ppm) | 0.26 | 0.14 | 0.02 | <0.001 |
| Cobalt (ppm) | 1.08 | 3.20 | 0.76 | 0.10 |
Calculated as mol/100 mol total volatile fatty acids.
Crude protein equivalent.
Neutral detergent fibre (cellulose, lignin, and hemicellulose); acid detergent fibre (cellulose and lignin).
Differences between least squares means declared at p < 0.05 and trends at 0.05 ≤ p ≤ 0.10.
BR demonstrated greater intra‐species variation than WR (Table 4). Individual molar proportions of VFA, pH, crude protein, lignin, crude fat, sodium, zinc, copper, manganese and cobalt did not differ amongst individual WR. Faecal lignin, calcium, pH, potassium, sodium, copper and cobalt did not differ amongst individual BR. Molar proportions of acetate differed amongst each individual BR, but were all lower than individual WR. Molar proportions of propionate differed amongst each individual BR but were all greater than individual WR (Table 4). BR3 had the greatest total VFA and acetate concentrations. Faecal butyrate was lower in BR3 than BR1 and BR2, but was greater than all individual WR. Lactate was greater in all individual WR than in all individual BR and no inter‐species differences were observed. Faecal ammonia, ash, crude protein, crude fat, phosphorus, zinc, manganese, sulphur and selenium were greatest in BR3, in comparison with all other individual rhinos. Faecal iron was greatest in WR1 and BR3 with 996 ppm and 880 ppm respectively (Table 4). Individual faecal nutrients, VFA, lactate, pH and ammonia values from each rhinoceros are located in Supporting Information (File S2).
TABLE 4.
Inter‐animal variation of faecal metabolites and nutrient contents from three black and 3 white rhinoceroses (n = 12 samples/animal)
| Individual Rhino† | Standard error | ||||||
|---|---|---|---|---|---|---|---|
| BR1 | BR2 | BR3 | WR1 | WR2 | WR3 | ||
| Fermentation by‐products in faeces | |||||||
| Acetate (mM) | 146b | 97.8d | 195a | 137bc | 157b | 114cd | 8.44 |
| Propionate (mM) | 44.7ab | 39.6b | 49.3a | 23.5c | 26.7c | 19.8c | 2.53 |
| Butyrate (mM) | 19.1a | 14.9b | 21.3a | 7.57c | 8.70c | 6.24c | 1.22 |
| Isobutyrate (mM) | 4.54a | 3.59b | 4.45ab | 1.13c | 1.99c | 1.42c | 0.32 |
| Total VFA (mM) | 214b | 156d | 270a | 170cd | 194bc | 142d | 11.9 |
| Acetate (%‡) | 68.1c | 63.1d | 72.2b | 81.0a | 81.0a | 80.7a | 0.52 |
| Propionate (%) | 20.8b | 25.2a | 18.3c | 13.9d | 13.6d | 13.9d | 0.43 |
| Butyrate (%) | 8.87a | 9.26a | 7.87b | 4.49c | 4.38c | 4.42c | 0.28 |
| Isobutyrate (%) | 2.14ab | 2.40a | 1.66b | 0.58c | 0.98c | 1.01c | 0.17 |
| Lactate (mM) | 2.78c | 1.94c | 3.52bc | 6.66ab | 8.33a | 7.68a | 1.12 |
| pH | 6.01ab | 6.39ab | 5.86b | 6.26ab | 6.33ab | 6.48a | 0.18 |
| Ammonia (% CPE§) | 0.65a | 0.63a | 0.87a | 0.39b | 0.13c | 0.16bc | 0.08 |
| Faecal nutrients (% DM‐basis) | |||||||
| Dry matter | 20.2a | 21.6ab | 21.5a | 22.8b | 20.5ac | 19.9ac | 0.46 |
| Crude protein | 9.56b | 9.81b | 11.7a | 8.35c | 8.01c | 7.89c | 0.14 |
| Neutral detergent fibre¶ | 67.5c | 67.4c | 63.6d | 68.2bc | 69.9ab | 71.7a | 0.75 |
| Acid detergent fibre | 42.2a | 40.8ab | 40.4b | 40.0b | 38.0d | 40.0b | 0.52 |
| Lignin | 8.51a | 7.79ab | 8.23ab | 7.32b | 8.15ab | 8.12ab | 0.29 |
| Crude fat | 4.18b | 3.95b | 4.80a | 2.21c | 2.14c | 2.02c | 0.29 |
| Ash | 9.16b | 7.66b | 12.1a | 14.2a | 6.40b | 7.91b | 0.80 |
| Calcium | 0.37a | 0.35a | 0.35a | 0.34a | 0.17b | 0.19b | 0.02 |
| Phosphorus | 0.52b | 0.52b | 0.73a | 0.35cd | 0.39c | 0.31d | 0.02 |
| Magnesium | 0.11b | 0.11b | 0.13a | 0.12a | 0.12ab | 0.10b | 0.01 |
| Potassium | 1.46a | 1.32a | 1.42a | 1.13b | 1.46a | 1.21b | 0.05 |
| Sodium | 0.26a | 0.22a | 0.29a | 0.12b | 0.09b | 0.11b | 0.02 |
| Sulphur | 0.23b | 0.24b | 0.30a | 0.22b | 0.18c | 0.17c | 0.01 |
| Iron (ppm) | 524b | 426b | 880a | 996a | 273b | 356b | 116 |
| Zinc (ppm) | 83.4b | 86.5b | 122a | 84.7b | 82.2b | 72.5b | 4.54 |
| Copper (ppm) | 17.4b | 16.8b | 24.1b | 35.9a | 17.5a | 24.0a | 2.52 |
| Manganese (ppm) | 104c | 111bc | 135a | 120b | 116bc | 112bc | 4.78 |
| Molybdenum (ppm) | 2.32ab | 2.16b | 2.61a | 0.81c | 0.43d | 0.56cd | 0.11 |
| Selenium (ppm) | 0.23b | 0.24b | 0.34a | 0.17c | 0.14cd | 0.12d | 0.01 |
| Cobalt (ppm) | 1.03a | 1.05a | 1.67a | 4.97a | 1.54a | 2.54a | 0.87 |
Least squares means with different letters a,b,c, or d significantly differ (p < 0.05).
Calculated as mol/100 mol total volatile fatty acids
Crude protein equivalent
Neutral detergent fibre (cellulose, lignin, and hemicellulose); acid detergent fibre (cellulose and lignin)
3.3. Faecal bacterial communities between species
The nMDS plots showed differences in the faecal bacterial communities between rhinoceros species (Figure 1). The spread of Bray–Curtis values was increased between species. Distinct faecal bacterial community structures were observed between rhinoceros species and between individual BR and WR (ANOSIM, p < 0.001). The faecal bacterial community of BR3, the only female BR, was more similar to the communities of her male conspecifics than with the female WR. BR and WR shared a total of 21 out of a total of 7,761 OTUs (0.27% OTUs shared, 15.2% of total sequence reads). The following OTUs shared between species were related to the following taxa and consisted of >1% of the total sequence reads: OTU 1 (Spirochaetaceae), OTUs 3 and 5 (unclassified Bacteroidetes), OTU 9 (Prevotellaceae), OTU 14 (Prevotellaceae YAB2003 group) (Table 5). Good's coverage, Chao1 Richness, and ACE Richness did not differ by species. WR had a greater number of total OTUs (1084 ± 19 vs. 929 ± 29 respectively) and an increase in Shannon Diversity (5.23 ± 0.02 vs. 4.91 ± 0.05 respectively) and inverse Simpson index (73.9 ± 1.81 vs. 57.6 ± 3.57 respectively) in comparison to BR (p < 0.001) (Table 6). The relative abundance of Firmicutes was greater in WR (29.0 ± 0.46%) than BR (26.2 ± 0.85%) (p = 0.01) (Table 6). The relative abundance of Bacteroidetes was greater in BR (49.2 ± 1.16%) than WR (41.6 ± 0.67%) (p < 0.001). The relative abundance of Proteobacteria was greater in BR (1.31 ± 0.09%) than WR (0.47 ± 0.04%), while the relative abundance of Spirochaetae was nearly 2‐fold greater in WR (15.6 ± 0.51%) than BR (8.37 ± 0.55%) (p < 0.001). No differences in the relative abundances of Fibrobacteres (BR: 2.01 ± 0.22%, WR: 3.78 ± 0.47%) and Planctomycetes (BR: 0.29 ± 0.04%, WR: 0.30 ± 0.02%) were observed between species.
FIGURE 1.

(a) Non‐metric multidimensional scaling (nMDS) plot of Bray‐Curtis dissimilarity comparing faecal bacterial community structures between rhinoceros species (n = 36 faecal samples/species). (b) nMDS plot of Bray‐Curtis dissimilarity comparing faecal bacterial community structures between individual rhinoceros (BR, black rhinoceros; WR, white rhinoceros) (n = 12 faecal samples/individual). BR1 and BR2 are adult males. BR3 and all WR are adult females. Stress = 0.17, RMSE = 0.90 [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 5.
Shared faecal bacterial operational taxonomic units between rhinoceros species (n = 72 faecal samples)
| OTU # | Bacterial Taxa | Total # of sequence reads | % of total sequences |
|---|---|---|---|
| 1 | Spirochaetaceae | 61,174 | 2.82 |
| 3 | unclassified Bacteroidetes | 51,531 | 2.37 |
| 5 | unclassified Bacteroidetes | 46,973 | 2.16 |
| 9 | Prevotellaceae | 34,423 | 1.58 |
| 14 | Prevotellaceae_YAB2003_group | 23,534 | 1.08 |
| 18 | Prevotellaceae_UCG‐003 | 19,348 | 0.89 |
| 19 | Acidaminococcaceae | 18,744 | 0.86 |
| 27 | Prevotellaceae_unclassified | 14,851 | 0.68 |
| 44 | Spirochaetaceae | 9998 | 0.46 |
| 49 | Lachnospiraceae_unclassified | 8992 | 0.41 |
| 59 | Lentisphaerae_RFP12_gut_group_unclassified | 7828 | 0.36 |
| 63 | Lachnospiraceae_unclassified | 7302 | 0.34 |
| 99 | Lachnospiraceae | 4645 | 0.21 |
| 109 | Spirochaetales | 4171 | 0.19 |
| 120 | Ruminococcaceae_NK4A214_group | 3684 | 0.17 |
| 123 | Rikenellaceae_RC9_gut_group | 3566 | 0.16 |
| 170 | Prevotellaceae_UCG−001 | 2488 | 0.11 |
| 183 | Ruminococcaceae | 2276 | 0.10 |
| 251 | Clostridiales | 1510 | 0.07 |
| 273 | Lachnospiraceae_UCG−006 | 1332 | 0.06 |
| 352 | Clostridiales | 959 | 0.04 |
TABLE 6.
The effect of species on faecal bacterial phyla and alpha diversity from three black and 3 white rhinoceroses (n = 12 samples/animal)
| Species | p‐value* | |||
|---|---|---|---|---|
| Black rhino | White rhino | Standard error | ||
| Bacterial phyla (% relative abundance)a | ||||
| Bacteroidetes | 49.2 | 41.6 | 0.91 | <0.001 |
| Fibrobacteres | 2.01 | 2.85 | 0.35 | 0.01 |
| Firmicutes | 26.2 | 29.0 | 0.65 | 0.01 |
| Lentisphaera | 4.52 | 3.71 | 0.17 | <0.01 |
| Proteobacteria | 1.31 | 0.47 | 0.06 | <0.001 |
| Spirochaetes | 8.37 | 15.6 | 0.53 | <0.001 |
| Tenericutes | 0.67 | 1.43 | 0.07 | <0.001 |
| Verrucomicrobia | 2.94 | 0.09 | 0.34 | 0.01 |
| Unclassified Bacteria | 2.96 | 2.01 | 0.10 | <0.001 |
| Firmicutes: Bacteroidetes | 0.53 | 0.70 | 0.03 | <0.001 |
| Diversity measurements | ||||
| Shannon diversity index | 4.91 | 5.22 | 0.03 | <0.001 |
| Inverse Simpson index | 57.6 | 73.9 | 2.69 | <0.01 |
| Number of OTUsb | 929 | 1084 | 48.0 | <0.01 |
| ACE richness | 1250 | 1408 | 55.8 | 0.89 |
| CHAO richness estimator | 1222 | 1401 | 58.9 | 0.59 |
| Good's coverage | 98.7 | 98.9 | 1.71 × 10−3 | 0.81 |
Mean relative abundances ≥1% are displayed.
Operational taxonomic unit.
Differences between means declared at p < 0.05 and trends at 0.05 ≤ p ≤ 0.10.
3.4. Bacterial communities within a species
Faecal bacterial communities, mean relative abundance of bacterial phyla and alpha diversity measures did not differ by the interaction of individual rhino and sampling week. Faecal bacterial community differences were not observed amongst WR but observed between BR in the nMDS plots (ANOSIM, p < 0.001) (Figure 1). There was an increase in the Bray–Curtis spread between the female BR and the two male BRs. A greater number of OTUs were shared within a rhino species than across species. A total of 79 OTUs were shared amongst BR, whereas 317 OTUs were shared amongst WR (Figure 2). The male BRs shared 185 OTUs with each other, while the female BR shared 87 and 91 OTUs with 1BR and 2BR respectively. A greater number of shared OTUs was observed within individual rhinos (n = 12 samples/rhino) (Figure 2). The mean total number of OTUs did not differ between individual WRs but were different between individual BRs (Figure 3). The female BR3 had greater Shannon Diversity than the two male BRs (p < 0.001) and one of the 0.1 WR (p = 0.02) (Figure 3). The mean relative abundance of the phyla Verrucomicrobia was greater in BR1 (7.45 ± 1.31%) than in all other individual rhinos whose relative mean abundances ranged from 0.01–1.37% (Figure 4). The Firmicutes to Bacteroidetes ratios and mean relative abundances of Bacteroidetes, Fibrobacteres, Firmicutes, Proteobacteria, and unclassified Bacteria did not differ between individual WR. The mean relative abundances of Bacteroidetes was greater in BR3 (54.4 ± 0.74%) than in all other individual rhinos whose mean relative abundances ranged from 40.0–47.2% (p < 0.05). The mean Firmicutes to Bacteroidetes ratio from BR3 was lower (0.46 ± 0.01) than each individual WR (0.70 ± 0.03) (p < 0.01). The mean relative abundances of Fibrobacteres, Proteobacteria and unclassified Bacteria were not different amongst individual BR (Figure 4).
FIGURE 2.
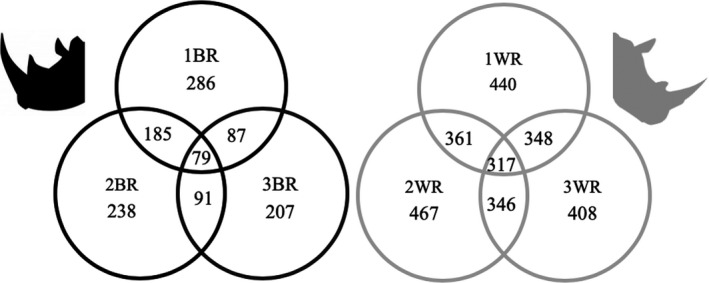
Venn diagram of total shared bacterial operational taxonomic units between rhinoceros species (BR, black rhinoceros; WR, white rhinoceros) (n = 72 total faecal samples, 12 samples/individual)
FIGURE 3.

Boxplots comparing the (a) total operational taxonomic units and (b) Shannon diversity indices between individual rhinoceroses (BR, black rhinoceros; WR, white rhinoceros). Means within boxplots with different letters a,b, or c are significantly different (p < 0.05). Black dots represent the diversity measure from individual faecal samples (n = 12 samples/individual) [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.

Boxplots comparing the mean relative abundances of faecal bacterial phyla between individual rhinoceroses (BR, black rhinoceros; WR, white rhinoceros). Means within boxplots with different letters a,b, or c are significantly different (p < 0.05). Black dots represent the phylum relative abundance of individual faecal samples (n = 12 samples/individual) [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
The present study characterized and compared the dietary nutrients and faecal nutrients, fermentation end‐products and bacteria between species of rhinoceroses and between individual rhinoceroses of the same species at the same institution. Species‐specific diets and the variety offered to the same species at different institutions likely contribute to the faecal bacterial community structures and fermentation by‐products (Sullivan, 2019). It is important to characterize faecal bacterial communities from healthy animals so that we can start to find biomarkers of dysbiosis.
The faecal nutrient and fermentation by‐product profile were different between species and more dissimilar between individual BR and similar between individual WR. Dietary mineral contents of both species of rhinoceros are similar and within the ranges previously reported from horse and BR (Clauss, Castell, Kienzle, Schramel, et al., 2007). Faecal nutrient contents demonstrated similar patterns to dietary nutrients. Potential differences in nutrient digestibility, not measured in the present study, may have also contributed to these findings. For example, both dietary and faecal NDF were greater in WR than in BR, whereas calcium, phosphorus and crude protein were greater in the diet and faeces of BR than in WR. All BR received a previously recommended daily phosphorus supplement that likely contributed to the increased faecal phosphorus content in comparison with WR (Sullivan & Valdes, 2019). BR had greater molar proportions and concentrations of butyrate, isobutyrate and propionate in comparison with WR, while WR had nearly 3‐fold higher concentrations of lactate. These findings are consistent with previous observations of these VFA from BR and WR (Roth et al., 2019). Although lactate is a main end‐product of starch fermentation by amylolytic bacteria, dietary starch DMI from the current study was similar between BR (3.23%) and WR (3.76%). While horses with simple colonic obstruction and distension had greater lactate concentrations in comparison with horses on a grass‐fed diet, they did not demonstrate symptoms of colic or laminitis during the 4‐week period (Daly et al., 2012) suggesting that lactate is not necessarily a marker of horse gut health. Furthermore, horse cecal pH has been shown to decrease with an accumulation in lactate, while lactate producers have been associated with the developmental stage of laminitis and colic (A. Biddle et al., 2013). It is of interest that the present study and Roth et al. (2019) demonstrated greater lactate contents from WR. Given these findings in WR and the negative association between lactate and hindgut health, future studies should report the starch content of diets offered and determine if this lactate proportion is unique to WR under managed care or also observed in faeces from wild WR. As bacteria from the phylum Fibrobacteres ferment cellulose and produce succinic acid and acetate (Neumann et al., 2017), the elevated NDF DMI of WR (69.6%) in comparison with BR (53.5%) may have contributed to the increased relative abundance of the phylum Fibrobacteres and proportion of acetate from WR. Butyrate is a major metabolite for colonocytes that are necessary for the intestinal lumen absorption of water, chloride and sodium and inhibits inflammatory pathways in humans with inflammatory bowel disease (Bedford & Gong, 2018). Butyrate production by the bacterium, Roseburia intestinalis, was greater when cultured under high‐iron than normal in vitro conditions (Dostal et al., 2015). It was suggested that the level of dietary iron affects the gut microbiome and the production of butyrate (Dostal et al., 2015). While butyrate impacts have been extensively studied in pigs (Bedford & Gong, 2018), this would be an inappropriate model considering rhinoceros digestive physiology, as compared to a horse model. Additionally, hindgut butyrate concentrations were increased in the hindguts of horses fed high starch versus high fibre diets, suggesting a potential link between starch intake and butyrate production (De Fombelle et al., 2003). While the iron and starch intakes were low and did not differ between species in the present study, the greater faecal butyrate proportions in BR than in WR may warrant future study of the not yet understood connection amongst inflammation, dietary iron and starch and butyrate in rhinoceroses. This would be especially important in BR species who are subject to iron overload disorder (Olias et al., 2012; Paglia & Tsu, 2012).
Several recent studies have characterized the faecal bacterial community structures of rhinoceroses managed at different zoological institutions, with one study comparing Southern BR from the wild versus managed care (Gibson et al., 2019). Previously reported Firmicutes to Bacteroidetes ratios from Eastern BR under human care (Antwis et al., 2019) and four species of rhinoceroses (Roth et al., 2019) were above one, whereas the mean ratios from the present study were below one. The mean Firmicutes to Bacteroidetes ratios from wild BR was 2.90 and zoo‐held BR was 1.13 (Gibson et al., 2019), whereas we reported 0.53 from BR and 0.70 from WR. One study reported Firmicutes to Bacteroidetes ratios above one in four out of five Southern WR, while a second study reported a mean Firmicutes to Bacteroidetes ratio of 0.60 from Southern WR under human care (Bian et al., 2013; Williams et al., 2019). While elevated ratios of Firmicutes to Bacteroidetes have been associated with obesity in horses and humans, this association has not yet been studied in rhinoceroses and should not be interpreted as a cause of or result of obesity (Biddle et al., 2018; Riva et al., 2017). It is important to consider that the phylum Firmicutes consists of over 200 genera, that also include potential probiotics positively associated with gut health, Lactobacillus and Bacillus spp., while the phylum Bacteroidetes also contains over 200 genera with a diverse set of metabolic functions in the hindgut (Rinninella et al., 2019; Thomas et al., 2011). Previous gut microbiota studies of rhinoceroses not only utilize different sample collection methods, sequencing platforms and downstream analyses but also include a variety of diets offered across institutions that likely contribute to inter‐study differences in these phyla.
The phylum Verrucomicrobia was observed to be more abundant in the guts of zoo‐held versus wild mammals (McKenzie et al., 2017), in low abundance in Southern WR (Bian et al., 2013), not reported in zoo‐held and wild Southern BR (Gibson et al., 2019), and greater than 10% abundance in healthy horses (Steelman et al., 2012) and greater one‐horned rhinoceroses (Borah et al., 2019). Verrucomicrobia was increased in faecal samples from human patients that received broad‐spectrum antibiotics (Dubourg et al., 2013), is ubiquitous in soil samples, and was found on the soil surface (0–6 cm) at 2–20% abundances (Bergmann et al., 2011). Given that the mean relative abundance of Verrucomicrobia was consistently elevated in faecal samples from one male BR, in comparison with all other rhinos, the contribution of the individual through repeat sampling should be considered when evaluating main effects of subsequent studies. This elevation in Verrucomicrobia is of interest as this rhinoceros does not have a history of antibiotic treatment and that faecal boluses were freshly collected from substrate‐free surfaces to minimize sample contamination with soil or debris. While sand consumption by this individual could not be quantified, the faecal ash content—a marker of sand accumulation in horses (Hotwagner & Iben, 2008), was greatest in the female BR and no difference between male BR.
The most abundant and shared OTU between rhinoceroses in this study was related to the family Spirochaetaceae. Bacterial species from this family are motile, found in the environment, utilize soluble sugars released from cellulose by cellulolytic bacteria and ferment pectin into acetate and propionate (Liu et al., 2014; Stanton, 1980). Relative abundances of the phyla Spirochete were 2‐fold greater in WR than BR from the present study, similar to reported abundances from wild and domestic equids (Edwards et al., 2020), and greater than what was previously reported in rhinoceroses (Gibson et al., 2019; Roth et al., 2019). Although Roth et al. (2019) targeted the V4 hypervariable region of the 16S rRNA gene as the present study did, downstream sequence analyses differed, along with the taxonomic reference file used. A shotgun sequencing approach was used to capture the taxonomic profile of the faecal bacteria in wild versus zoo‐held rhinos (Gibson et al., 2019). While similar relative abundances of bacterial phyla were previously observed between these two sequencing methods, other variables such as DNA extraction and sequence analysis methods, individual animal, diet and environment are also potential contributors to the differences between studies (Brumfield et al., 2020).
Alpha diversity is a measure of richness and evenness in a sample and a platform to better understand the outcomes of ecological processes of the hindgut. Both alpha and beta diversity measures differed by species and differences in diversity were more pronounced between individual BR than individual WR. Alpha diversity measures have varied across rhinoceros studies. The faecal bacterial diversity was previously lower in iron overload susceptible versus resistant rhinoceros species (Roth et al., 2019), no difference in alpha diversity was seen between BR from the wild or under human care (Gibson et al., 2019), and both BR and WR had greater alpha diversities under human care than from the wild (McKenzie et al., 2017). While microbial diversity was increased in horses without equine metabolic syndrome (EMS) than with EMS, this was suspected to demonstrate a broader bacterial functional potential in healthier horses (Elzinga et al., 2016). The female BR had similar OTU richness and Shannon diversity to WR than to the male BR, but the cause is not known. She historically was a finicky eater, who consumed a greater amount of Browser Rhino Cubes daily (8.2 kg DM), in comparison with the male BR (5.5 kg DM) and all WR (2.4 kg DM). A lower diversity, as observed in BR in comparison with WR, does not imply a less stable or healthy bacterial community, and a higher diversity is not necessarily desirable (Shade, 2017).
The faecal bacterial community structures, as evaluated by beta diversity, were dissimilar by species and more similar within species, and members of the same species shared more OTUs than not. This is suspected to be a result of typical, species‐specific diets (Williams et al., 2019), socialization preferences and housing environment (Caruso et al., 2019; Ley et al., 2008). WR commingle and have direct contact (e.g. nose‐to‐nose) with one another during the day, whereas the BR, solitary by nature, do not have direct contact and receive a variety of browse species and produce enrichment. Our findings of low intra‐animal variation and greater inter‐animal variation coincide with previous findings in other herbivores (Bian et al., 2013; Daly et al., 2012; Jami et al., 2014).
Roth et al. (2019) reported distinct diets by host and reported the as‐fed diets at different institutions. The institutions offered different pellets that included ADF‐25 (6.2% starch, 652 ppm iron), ADF‐16 (19.8% starch, 490 ppm iron) or Moose Breeder (2.2% starch, 724 ppm iron) (Mazuri®, St. Louis,MO) (Roth et al., 2019), whereas the present study fed Mazuri® Browser Rhino Cube (6.5% starch, 317 ppm iron). Note that listed starch and iron contents were obtained from in‐house pellet samples submitted for analysis at Dairy One Laboratories (Ithaca, NY). One institution fed 0%, a second fed 18%, and the present study fed 28.7 ± 7.6% browse on an as‐fed basis to BR, while WR were fed an assortment of hay types by institution which can vary in nutrient content (Roth et al., 2019). WR were fed a smaller proportion of pellet as compared to total DM (7.5–15%), than the BR (23–40%) in the present study. This reflects long‐term challenges in maintaining body weight and condition with the appropriate feed options available, specific to each individual (File S1). WR used in the present study historically struggled with over conditioning, and BW’s are optimized with a high grass hay proportion. Our institution opts to not offer high‐iron, calcium and protein alfalfa hay to BR (Sullivan et al., 2020), and though a high proportion of browse is offered as previously described, high fibre pellet feeding was increased due to caloric maintenance need for the male BR, as well as adapting to a picky eater in our female BR with a lower BCS. With this discrepancy in diets offered and nutrient contents, it is important for future research to better describe the diets and nutrients, such as starch, iron, and fibre consumed. This would provide a better understanding of the relationship amongst specific nutrients and the hindgut fermentation as seen in previous studies of the domestic horse (Daly et al., 2012; Dougal et al., 2017).
In conclusion, faecal bacterial communities and VFA were dissimilar by species and more similar amongst individual WR than between individual BR. As WR socialize, have direct contact and have less variety in diet items offered, in comparison with BR, there was less inter‐species variation. Although alpha diversity was lower in BR than WR, this did not indicate that their faecal bacterial communities were less stable than those of WR, and need not indicate compromised health. The relative abundance of Bacteroidetes from both species was greater than what has previously been observed from other rhinoceros gut microbiology studies, indicating potential differences in diet, sample processing methodologies, and location amongst other variables. Additionally, this study demonstrates inter‐animal variation in faecal bacteria and metabolite composition. Lastly, it highlights the importance of describing complete diets and nutrient content offered to animals in zoological institutions so that we can better understand the relationship amongst diet composition, faecal bacterial communities and rhinoceros gut health.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ANIMAL WELFARE STATEMENT
The authors confirm adherence to the ethical policies of the journal and that all animal procedures were approved by the University of Florida’s Institutional Animal Care and Use Committee under protocol 201810435 and by Disney’s Animal Care and Welfare Committee under internal review 1812.
Supporting information
File S1
File S2
ACKNOWLEDGEMENTS
This research was supported by work performed by the University of Michigan Microbial Systems Molecular Biology Laboratory. This research was done within and supported by Disney's Animal Kingdom®. We extend our gratitude to the rhinoceros keepers and managers at Disney’s Animal Kingdom® for assisting with the successful execution of the study. Thank you to Shannon Livingston, Assistant Nutritionist and the Animal Nutrition Center interns, Shannon Finet, Joshua Figg, Selena Heard, Jenn Larsen, Megan Pearce, Delaney Ruf, Savannah Smith, and Jessica Thomas for assisting with sample collections and preparation of shipments.
REFERENCES
- Antwis, R. E. , Edwards, K. L. , Unwin, B. , Walker, S. L. , & Shultz, S. (2019). Rare gut microbiota associated with breeding success, hormone metabolites and ovarian cycle phase in the critically endangered eastern black rhino. Microbiome, 7, 1–12. 10.1186/s40168-019-0639-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford, A. , & Gong, J. (2018). Implications of butyrate and its derivatives for gut health and animal production. Animal Nutrition, 4, 151–159. 10.1016/j.aninu.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann, G. T. , Bates, S. T. , Eilers, K. G. , Lauber, C. L. , Caporaso, J. G. , Walters, W. A. , Knight, R. , & Fierer, N. (2011). The under‐recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biology and Biochemistry, 43, 1450–1455. 10.1016/j.soilbio.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian, G. , Ma, L. , Su, Y. , & Zhu, W. (2013). The microbial community in the feces of the white rhinoceros (Ceratotherium simum) as determined by barcoded pyrosequencing analysis. PLoS One, 8, 1–9. 10.1371/journal.pone.0070103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddle, A. , Stewart, L. , Blanchard, J. , & Leschine, S. (2013). Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity, 5, 627–640. 10.3390/d5030627 [DOI] [Google Scholar]
- Biddle, A. S. , Tomb, J. F. , & Fan, Z. (2018). Microbiome and blood analyte differences point to community and metabolic signatures in lean and obese horses. Frontiers in Veterinary Science, 5, 12–14. 10.3389/fvets.2018.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borah, P. , Dutta, R. , Moni Barkalita, L. , Buragohain, L. , Deka, P. , Ali, S. , Choudhury, B. , & Basumatary, P. (2019). Deciphering the fecal microbiome of Indian rhinoceros (Rhinoceros unicornis) by metagenomic approach. Asian Journal of Conservation Biology, 8, 135–141. [Google Scholar]
- Brumfield, K. D. , Huq, A. , Colwell, R. R. , Olds, J. L. , & Leddy, M. B. (2020). Microbial resolution of whole genome shotgun and 16S amplicon metagenomic sequencing using publicly available NEON data. PLoS One, 15, 1–21. 10.1371/journal.pone.0228899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso, R. , Ono, M. , Bunker, M. E. , Núñez, G. , & Inohara, N. (2019). Dynamic and asymmetric changes of the microbial communities after cohousing in laboratory mice. Cell Reports, 27, 3401–3412. 10.1016/j.celrep.2019.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, K. (1993). Non‐parametric multivariate analyses of changes in community structure. Austrailian Ecology, 18, 117–143. 10.1111/j.1442-9993.1993.tb00438.x [DOI] [Google Scholar]
- Clauss, M. , Castell, J. C. , Kienzle, E. , Dierenfeld, E. S. , Flach, E. J. , Behlert, O. , Ortmann, S. , Streich, W. J. , Hummel, J. , & Hatt, J. M. (2007). The influence of dietary tannin supplementation on digestive performance in captive black rhinoceros (Diceros bicornis). Journal of Animal Physiology and Animal Nutrition, 91, 449–458. 10.1111/j.1439-0396.2006.00673.x [DOI] [PubMed] [Google Scholar]
- Clauss, M. , Castell, J. C. , Kienzle, E. , Schramel, P. , Dierenfeld, E. S. , Flach, E. J. , Behlert, O. , Streich, W. J. , Hummel, J. , & Hatt, J. M. (2007). Mineral absorption in the black rhinoceros (Diceros bicornis) as compared with the domestic horse. Journal of Animal Physiology and Animal Nutrition, 91, 193–204. 10.1111/j.1439-0396.2007.00692.x [DOI] [PubMed] [Google Scholar]
- Clauss, M. , Polster, C. , Kienzle, E. , Wiesner, H. , Baumgartner, K. , Von Houwald, F. , Ortmann, S. , Streich, W. , & Dierenfeld, E. (2005). Studies on digestive physiology and feed digestibilities in captive Indian rhinoceros (Rhinoceros unicornis). Journal of Animal Physiology and Animal Nutrition, 89, 229–237. 10.1111/j.1439-0396.2005.00546.x [DOI] [PubMed] [Google Scholar]
- Daly, K. , Proudman, C. J. , Duncan, S. H. , Flint, H. J. , Dyer, J. , & Shirazi‐Beechey, S. P. (2012). Alterations in microbiota and fermentation products in equine large intestine in response to dietary variation and intestinal disease. British Journal of Nutrition, 107, 989–995. 10.1017/S0007114511003825 [DOI] [PubMed] [Google Scholar]
- De Fombelle, A. , Varloud, M. , Goachet, A. G. , Jacotot, E. , Philippeau, C. , Drogoul, C. , & Julliand, V. (2003). Characterization of the microbial and biochemical profile of the different segments of the digestive tract in horses given two distinct diets. Animal Science, 77, 293–304. 10.1017/S1357729800059038 [DOI] [Google Scholar]
- Dostal, A. , Lacroix, C. , Bircher, L. , Pham, V. T. , Follador, R. , Zimmermann, M. B. , & Chassard, C. (2015). Iron modulates butyrate production by a child gut microbiota in vitro. Mbio, 6(6), 1–12. 10.1128/mBio.01453-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougal, K. , Harris, P. A. , Girdwood, S. E. , Creevey, C. J. , Curtis, G. C. , Barfoot, C. F. , Argo, C. M. , & Newbold, C. J. (2017). Changes in the total fecal bacterial population in individual horses maintained on a restricted diet over 6 weeks. Frontiers in Microbiology, 8, 1–11. 10.3389/fmicb.2017.01502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubourg, G. , Lagier, J. C. , Armougom, F. , Robert, C. , Audoly, G. , Papazian, L. , & Raoult, D. (2013). High‐level colonisation of the human gut by Verrucomicrobia following broad‐spectrum antibiotic treatment. International Journal of Antimicrobial Agents, 41, 149–155. 10.1016/j.ijantimicag.2012.10.012 [DOI] [PubMed] [Google Scholar]
- Edwards, J. E. , Schennink, A. , Burden, F. , Long, S. , van Doorn, D. A. , Pellikaan, W. F. , Dijkstra, J. , Saccenti, E. , & Smidt, H. (2020). Domesticated equine species and their derived hybrids differ in their fecal microbiota. Animal Microbiome, 2, 1–13. 10.1186/s42523-020-00027-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga, S. E. , Weese, J. S. , & Adams, A. A. (2016). Comparison of the fecal microbiota in horses with equine metabolic syndrome and metabolically normal controls fed a similar all‐forage diet. Journal of Equine Veterinary Science, 44, 9–16. 10.1016/j.jevs.2016.05.010 [DOI] [Google Scholar]
- Gibson, K. M. , Nguyen, B. N. , Neumann, L. M. , Miller, M. , Buss, P. , Daniels, S. , Ahn, M. J. , Crandall, K. A. , & Pukazhenthi, B. (2019). Gut microbiome differences between wild and captive black rhinoceros – Implications for rhino health. Scientific Reports, 9, 1–11. 10.1038/s41598-019-43875-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, N. C. K. , Avershina, E. , Mydland, L. T. , Næsset, J. A. , Austbø, D. , Moen, B. , Måge, I. , & Rudi, K. (2015). High nutrient availability reduces the diversity and stability of the equine caecal microbiota. Microbial Ecology in Health & Disease, 26, 1–8. 10.3402/mehd.v26.27216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotwagner, K. , & Iben, C. (2008). Evacuation of sand from the equine intestine with mineral oil, with and without psyllium. Journal of Animal Physiology and Animal Nutrition, 92, 86–91. [DOI] [PubMed] [Google Scholar]
- Huntley, N. F. , Naumann, H. D. , Kenny, A. L. , & Kerley, M. S. (2017). Black rhinoceros (Diceros bicornis) and domestic horse (Equus caballus) hindgut microflora demonstrate similar fermentation responses to grape seed extract supplementation in vitro. Journal of Animal Physiology and Animal Nutrition, 101, 195–209. [DOI] [PubMed] [Google Scholar]
- Hussein, H. S. , Vogedes, L. A. , Fernandez, G. C. J. , & Frankeny, R. L. (2004). Effects of cereal grain supplementation on apparent digestibility of nutrients and concentrations of fermentation end‐products in the feces and serum of horses consuming alfalfa cubes. Journal of Animal Science, 82, 1986–1996. [DOI] [PubMed] [Google Scholar]
- Jami, E. , White, B. A. , & Mizrahi, I. (2014). Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. PLoS One, 9, e85423. 10.1371/journal.pone.0085423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julliand, V. , & Grimm, P. (2016). HORSE SPECIES SYMPOSIUM: The microbiome of the horse: History and current knowledge. Journal of Animal Science, 94, 2262–2274. [DOI] [PubMed] [Google Scholar]
- Julliand, V. , & Grimm, P. (2017). The impact of diet on the hindgut microbiome. Journal of Equine Veterinary Science, 52, 23–28. 10.1016/j.jevs.2017.03.002 [DOI] [Google Scholar]
- Kozich, J. J. , Westcott, S. L. , Baxter, N. T. , Highlander, S. K. , & Schloss, P. D. (2013). Development of a dual‐index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Applied and Environment Microbiology, 79, 5112–5120. 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley, R. E. , Hamady, M. , Lozupone, C. , Turnbaugh, P. , Ramey, R. R. , Bircher, J. S. , Schlegel, M. L. , Tucker, T. A. , Schrenzel, M. D. , Knight, R. , & Gordon, J. I. (2008). Evolution of mammals and their gut microbes. Science, 320, 1647–1651. 10.1126/science.1155725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Wang, J. K. , Zhu, W. , Pu, Y. Y. , Guan, L. L. , & Liu, J. X. (2014). Monitoring the rumen pectinolytic bacteria Treponema saccharophilum using real‐time PCR. FEMS Microbiology Ecology, 87, 576–585. [DOI] [PubMed] [Google Scholar]
- McKenzie, V. J. , Song, S. J. , Delsuc, F. , Prest, T. L. , Oliverio, A. M. , Korpita, T. M. , Alexiev, A. , Amato, K. R. , Metcalf, J. L. , Kowalewski, M. , Avenant, N. L. , Link, A. , Di Fiore, A. , Seguin‐Orlando, A. , Feh, C. , Orlando, L. , Mendelson, J. R. , Sanders, J. , & Knight, R. (2017). The effects of captivity on the mammalian gut microbiome. Integrative and Comparative Biology, 57, 690–704. 10.1093/icb/icx090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, J. A. M. D. , Scott, B. , & Hastie, P. M. (2009). Fermentative capacity of equine faecal inocula obtained from clinically normal horses and those predisposed to laminitis. Animal Feed Science and Technology, 151, 306–311. 10.1016/j.anifeedsci.2009.01.011 [DOI] [Google Scholar]
- Neumann, A. P. , McCormick, C. , & Suen, G. (2017). Fibrobacter communities in the gastrointestinal tracts of diverse hindgut‐fermenting herbivores are distinct from those of the rumen. Environmental Microbiology, 19, 3768–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olias, P. , Mundhenk, L. , Bothe, M. , Ochs, A. , Gruber, A. D. , & Klopfleisch, R. (2012). Iron overload syndrome in the black rhinoceros (Diceros bicornis): Microscopical lesions and comparison with other rhinoceros species. Journal of Comparative Pathology, 147, 542–549. 10.1016/j.jcpa.2012.07.005 [DOI] [PubMed] [Google Scholar]
- Paglia, D. E. , & Tsu, I. H. (2012). Review of laboratory and necropsy evidence for iron storage disease acquired by browser rhinoceroses. Journal of Zoo and Wildlife Medicine, 43, S92–S104. 10.1638/2011-0177.1 [DOI] [PubMed] [Google Scholar]
- Rinninella, E. , Raoul, P. , Cintoni, M. , Franceschi, F. , Miggiano, G. A. D. , Gasbarrini, A. , & Mele, M. C. (2019). What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms, 7, 1–22. 10.3390/microorganisms7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva, A. , Borgo, F. , Lassandro, C. , Verduci, E. , Morace, G. , Borghi, E. , & Berry, D. (2017). Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environmental Microbiology, 19, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, T. L. , Switzer, A. , Watanabe‐Chailland, M. , Bik, E. M. , Relman, D. A. , Romick‐Rosendale, L. E. , & Ollberding, N. J. (2019). Reduced Gut microbiome diversity and metabolome differences in rhinoceros species at risk for iron overload disorder. Frontiers in Microbiology, 10, 1–15. 10.3389/fmicb.2019.02291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shade, A. (2017). Diversity is the question, not the answer. ISME Journal, 11, 1–6. 10.1038/ismej.2016.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton, T. B. , & Canale‐Parola, E. (1980). Treponema bryantii sp. nov., a rumen spirochete that interacts with cellulolytic bacteria. Archives of Microbiology, 127, 145–156. 10.1007/BF00428018 [DOI] [PubMed] [Google Scholar]
- Steelman, S. M. , Chowdhary, B. P. , Dowd, S. , Suchodolski, J. , & Janečka, J. E. (2012). Pyrosequencing of 16S rRNA genes in fecal samples reveals high diversity of hindgut microflora in horses and potential links to chronic laminitis. BMC Veterinary Research, 8, 1–11. 10.1186/1746-6148-8-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer, P. , Clauss, M. , Südekum, K. H. , Hatt, J. M. , Silinski, S. , Klomburg, S. , Zimmermann, W. , Fickel, J. , Streich, W. J. , & Hummel, J. (2010). Comparative investigations on digestion in grazing (Ceratotherium simum) and browsing (Diceros bicornis) rhinoceroses. Comparative Biochemistry and Physiology ‐ A Molecular and Integrative Physiology, 156, 380–388. 10.1016/j.cbpa.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Sullivan, K. (2019). Rhinoceros nutrition TAG update: Wellness and prevention. Association of Zoos and Aquaria Midyear Meeting. [Google Scholar]
- Sullivan, K. E. , Mylniczenko, N. D. , Nelson, S. E. , Coffin, B. , & Lavin, S. R. (2020). Practical management of iron overload disorder (IOD) in Black rhinoceros (BR; Diceros bicornis). Animals, 10, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, K. E. , Valdes, E. V. , , & (2019). Update on rhinoceros nutrition. In Miller R. E., & Lamberski N.& Calle W. (eds.), Fowler’s zoo and wild animal medicine current therapy (Volume 9, pp. 699–706). W.B. Saunders. [Google Scholar]
- Thomas, F. , Hehemann, J. H. , Rebuffet, E. , Czjzek, M. , & Michel, G. (2011). Environmental and gut bacteroidetes: The food connection. Frontiers in Microbiology, 2, 1–16. 10.3389/fmicb.2011.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, C. , Ybarra, A. , Meredith, A. , Durrant, B. , & Tubbs, C. (2019). Gut microbiota and phytoestrogen‐associated infertility in southern white rhinoceros. Mbio, 10(2), 1–13. 10.1128/mBio.00311-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1
File S2


