Abstract
Background and aims
Pharmaceutical opioids are a significant contributor to the global ‘opioid crisis’, yet few studies have comprehensively distinguished between opioid types. We measured whether a range of common pharmaceutical opioids varied in their contribution to the rates and characteristics of harm in a population‐wide indicator of non‐fatal overdose.
Design
Retrospective observational study of emergency department (ED) patient care records in the Victorian Emergency Minimum Data set (VEMD), July 2009 to June 2019.
Setting
Victoria, Australia.
Cases
ED presentations for non‐fatal overdose related to pharmaceutical opioid use (n = 5403), where the specific pharmaceutical opioid was documented.
Measurements
We compared harms across the nine individual pharmaceutical opioids most commonly sold, and considered where multiple opioids contributed to the overdose. We calculated supply‐adjusted rates of ED presentations using Poisson regression and used multinomial logistic regression to compare demographic and clinical characteristics of presentations among nine distinct pharmaceutical opioids and a 10th category where multiple opioids were documented for the presentation.
Findings
There were wide differences, up to 27‐fold, between supply‐adjusted rates of overdose. When considering presentations with sole opioids, the highest supply‐adjusted overdose rates [per 100 000 oral morphine equivalents (OME); 95% confidence interval (CI)] were for codeine (OME = 0.078, 95% CI = 0.073–0.08) and oxycodone (OME =0.029, 95% CI = 0.027–0.030) and the lowest were for tapentadol (OME = 0.004, 95% CI = 0.003–0.006) and fentanyl (OME = 0.003, 95% CI = 0.002–0.004). These rates appeared related to availability rather than opioid potency. Most (62%) poisonings involved females. Codeine, oxycodone and tramadol were associated with younger presentations (respectively, 59.5%, 41.7% and 49.8% of presentations were 12‐34 years old), and intentional self‐harm (respectively 65.2%, 50.6%, and 52.8% of presentations). Relative to morphine, fentanyl [ 0.32 relative risk ratio (RRR)] and methadone ( 0.58 RRR) presentations were less likely to be coded as self‐harm. Relative to morphine–buprenorphine, codeine, oxycodone and tramadol presentations were significantly more likely to be associated with the less urgent triage categories (respectively 2.18, 1.80, 1.52, 1.65 RRR).
Conclusions
In Victoria, Australia, rates and characteristics of emergency department presentations for pharmaceutical opioids show distinct variations by opioid type.
Keywords: Codeine, emergency department, fentanyl, opioids, overdose, oxycodone, oxycodone–naloxone, pharmaceutical opioids, Tapentadol, tramadol
INTRODUCTION
There has been a dramatic rise of opioid‐related deaths during the past two decades throughout a range of high‐income countries [1]. In the United States there are more than 300 000 emergency department (ED) visits for non‐fatal opioid overdoses per year [2], and in Australia there are almost 150 hospitalizations for opioid harms each day [3]. A substantial proportion of opioid‐related mortality is attributed to pharmaceutical opioids—35% of deaths in the United States [4] and 70% in Australia [5]. Further, almost two‐thirds (61%) of Victorian ED presentations for opioid overdoses are related to pharmaceutical opioids [6]. Despite the substantial burden of harm and some indications that this harm varies among opioid types [7, 8, 9], there are few comprehensive investigations of how specific pharmaceutical opioids relate to real‐world harms.
Pharmaceutical opioids are often either represented as a single category or with various pre‐determined groupings, which can make understanding harms with individual opioids difficult. For example, the Centers for Disease Control and Prevention (CDC) presents overdose deaths involving pharmaceutical opioids under three categories: (i) ‘natural and semisynthetic opioids’, which includes morphine, codeine, hydrocodone and oxycodone, (ii) methadone and (iii) ‘synthetic opioids other than methadone’, which includes fentanyl, fentanyl analogues and tramadol [10]. The International Classification of Diseases (ICD) coding categories for pharmaceutical opioids also vary throughout countries. This lack of specificity is potentially problematic to inform interventions, as risk profiles appear to vary by opioid. One US study examining serious adverse events with different opioids found a positive correlation between serious adverse drug events and opioid potency [7]. Another adverse drug event study set in Australia compared oxycodone–naloxone and tapentadol, and found the organ system classifications and the most frequently reported event (respectively, ‘drug withdrawal syndrome’ and serotonin syndrome) varied between the opioids [8]. Analysis of Australian ambulance data similarly found supply‐adjusted rates of harm varied across seven pharmaceutical opioids—the highest rate was accounted for by the lowest potency opioid (possibly reflecting greater availability), and characteristics such as suicidal intent also varied by opioid [9].
Although forensic analyses of fatal overdoses typically comprehensively describe which opioids are implicated in the death there are relatively small case numbers, and delays up to multiple years to access confirmed results. In contrast, the analysis of non‐fatal overdose data available in routinely collected ED data are suited for more timely surveillance of opioid‐related harms [2]. ED data contain information on patient characteristics, poisoning intent and outcomes of the ED which are important in order to understand the nature opioid poisoning by different opioid types [2, 11].
Opioid overdose is an acute condition which can result from excessive consumption of opioids or other factors that increase the effects of opioids (e.g. loss of tolerance to opioids or consumption of combinations of substances that increase the effects of opioids). In toxicology, ‘poisoning’ results from the excessive administration of any pharmacological agent, psychoactive or not, so the term ‘opioid poisoning’ is largely synonymous with the lay term ‘opioid overdose’ [12]. Non‐fatal overdose is estimated to occur 13–30 times more frequently than fatal overdose and is predictive of later fatal overdose [13, 14]. Although fatal overdose is routinely tracked [15], studies of non‐fatal overdose are less common.
We previously compared pharmaceutical opioids using the harm indicator of ambulance callouts related to extramedical use of prescription opioids [9]. This study seeks to extend this work using ED data, and a more specific focus on opioid poisoning.
This study has two research questions:
Do rates of ED presentations for non‐fatal opioid poisoning in Victoria differ among nine pharmaceutical opioids, and do these rates vary over time?
Do non‐fatal opioid poisoning ED presentation characteristics vary by opioid type and over time?
METHODS
The research questions and detailed methods were published a priori [16]. The study is reported according to the REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) Statement (Supporting information, Table S1).
Study design
This was a retrospective analysis of administrative data from Emergency Department (ED) presentations for non‐fatal overdose related to pharmaceutical opioid use.
Participants
This study is set in Australia, which has the eighth highest per‐capita licit pharmaceutical opioid consumption in the world [3]. The catchment population is the state of Victoria, who comprise 26% of the national population [17], and whose pattern of pharmaceutical opioid related deaths [15] and ambulance attendances [9] are broadly comparable with other Australian jurisdictions. Consistent with other studies, we excluded individuals under the age of 12 years, as almost all the pharmaceutical opioid poisonings under this age are classed as unintentional ingestion [18, 19, 20].
Data sources
Emergency department data
Australia has a universal health‐care scheme which covers the cost of emergency treatment within public hospitals [21]. Data were obtained from the Victorian Emergency Minimum Dataset (VEMD) which aggregates ED presentation data from all 38 public hospitals with a 24‐hour ED in Victoria. The VEMD database has comprehensive data quality systems and is maintained by the state government [11]. We extracted all VEMD records from July 2009 to June 2019 pertaining to pharmaceutical opioid poisoning (n = 5403). Relevant presentations were identified using a combination of searching (1) narrative data on pharmaceutical opioid drug names and poisoning terms (including common misspellings of such terms) and (2) relevant International Classification of Diseases 10th revision, Australian modification (ICD‐10‐AM) poisoning diagnosis codes (‘poisoning by narcotics and psychodysleptics’, code T40). As the ICD‐10‐AM does not provide a comprehensive breakdown of poisoning types by opioid type, the data extraction relied mainly upon narrative (free text) searches.
An experienced data analyst (J.H.) manually checked all extracted records to ensure that the presentation contained information on specific opioids and to confirm the presentation related to a pharmaceutical opioid poisoning (and not, for example, instances where there was a non‐opioid drug poisoning and an opioid was used for analgesia as a part of clinical care). A detailed description of the presentation identification and coding are available in the protocol [16], and search terms used and an extraction process flow‐chart are available in Supporting information, Fig. S1.
There were fewer than five deaths relating to opioid poisoning recorded in the VEMD during the study period, so deaths were not included in our analyses due to small cell sizes.
Eight presentation characteristics were examined, with response categories aggregated where necessary to preserve cell sizes for analysis:
Age (12–34, 35–54, 55–65, and > 65 years)
Sex (male, female)
Geographical region (metro, regional/rural, interstate/overseas/unknown; home postcode‐based)
Country of birth [Oceania and Antarctica (including Australia)], Europe and the Americas, Asia and Middle East and Africa)
Patient SEIFA (a proxy for socio‐economic status where higher numbers indicate greater advantage; home postcode‐based; split to reflect approximately evenly sized quintiles for multinomial regression analysis [22]
Intent (intentional self‐harm versus other intention)
Admission outcome (hospital admission for further treatment or ED presentation only)
Triage category [presentations identified as Australasian Triage Scale (ATS) categories 1 or 2 are the most acute and should be taken immediately to an assessment and treatment area; ATS categories 3–5 have a longer waiting time [23])
Opioids of interest
This study examined 10 opioid categories: (1) buprenorphine, (2) codeine, (3) fentanyl, (4) methadone, (5) morphine, (6) oxycodone, (7) oxycodone–naloxone, (8) tapentadol, (9) tramadol and (10) multiple opioids. The first nine categories are the pharmaceutical opioids most commonly used for analgesia in outpatient settings in Australia, with our previous work indicating that less common opioids were captured in too few numbers to report [9]. The 10th category captured ED presentations that involved multiple opioids, at least one of which was listed in the first nine categories.
Sales data
Data concerning individual pharmaceutical opioid products sold to Victorian community pharmacies were acquired through the health information company IQVIA (iqvia.com). These population‐level data were supplied per month, and included strength and unit information to allow the calculation of oral morphine equivalents (OME) in milligrams. As opioids vary in strength, the representation of supply in OME is a method previously used [5, 7] to represent the analgesic effect of different opioids on the same scale [24].
Analysis
Aim 1: supply‐adjusted rates of harm
Using sales data, we calculated supply per month in OME for the nine opioids to represent harms as a proportion of supply. We used Poisson regression to generate incident rate ratios (IRRs) to estimate the supply‐adjusted rate of ED presentation of each opioid (the outcome variable) and how the rate changed over time (the predictor). The primary analysis considered each of the opioid categories as mutually exclusive—that is, a presentation was included in the rate calculation where the specific opioid was the only opioid involved in the presentation (Table 1). A sensitivity analysis explored the effect of selecting single‐opioid poisonings versus multiple opioid poisonings. To this effect, rates were calculated using presentations where the specific opioid was the only opioid involved or was one of multiple opioids involved in the presentation (Supporting information, Table S3). Supply‐adjusted rates were not calculated for the ‘multiple opioids’ category due to the heterogeneity of the OME denominator for each presentation. Rates were aggregated over 6‐month periods for analyses to enable minimum cell sizes of five, and were presented by year for ease of interpretation.
TABLE 1.
Supply adjusted rates and trends for ED presentations by opioid type, Victoria, Australia, July 2009–June 2019.
| Opioid a | Frequency | Supply‐adjusted rate b (per 100 000 mg OME per year over entire 2009–19 study period) | Time trend c (per 100 000 OME per 6‐month increase) | |||
|---|---|---|---|---|---|---|
| n (%) | Rate | 95% CI | IRR | 95% CI | P‐value | |
| Codeine | 2008 (37.2%) | 0.076 | 0.073–0.080 | 0.99 | 0.98–1.00 | 0.3 |
| Oxycodone | 1437 (26.6%) | 0.029 | 0.027–0.030 | 1.05 | 1.04–1.06 | <0.0001 |
| Tramadol | 542 (10.0%) | 0.015 | 0.014–0.016 | 1.00 | 0.98–1.02 | 0.96 |
| Morphine | 201 (3.7%) | 0.010 | 0.009–0.011 | 0.98 | 0.95–1.01 | 0.19 |
| Oxycodone–naloxone | 146 (2.7%) | 0.008 | 0.007–0.010 | 0.97 | 0.93–1.01 | 0.14 |
| Methadone | 580 (10.7%) | 0.007 | 0.006–0.007 | 1.01 | 0.99–1.03 | 0.36 |
| Buprenorphine | 90 (1.7%) | 0.006 | 0.004–0.007 | 1.00 | 0.97–1.04 | 0.87 |
| Tapentadol | 36 (1.2%) | 0.004 | 0.003–0.006 | 1.12 | 1.01–1.24 | 0.03 |
| Tapentadol (2014–2019 only) d | 36 (0.7%) | 0.004 | 0.003–0.006 | 1.12 | 1.00–1.25 | 0.04 |
| Fentanyl | 71 (1.3%) | 0.003 | 0.002–0.004 | 1.04 | 0.99–1.10 | 0.11 |
| Multiple opioids e | 289 (5.4%) | |||||
Opioid category represents 10 mutually exclusive groups (the first nine specific opioids listed were the only pharmaceutical opioids involved in the presentation).
Supply‐adjusted rate of emergency department (ED) presentations is per 100 000 mg oral morphine equivalents (OME) per year during the entire study period (i.e. a yearly average from July 2009–June 2019).
Time trend incident rate ratio (IRR) is calculated on 6‐month intervals (time as a continuous variable) using Poisson regression per 100 000 mg OME. CI = confidence interval.
Due to low sales volume and no attendances related to tapentadol prior 2014, overall trends are also presented for January 2014–June 2019.
Supply‐adjusted rates not calculated for multiple opioids due to the heterogeneity of the OME denominator for each presentation.
Aim 2: characteristics of presentations
We used multinomial logistic regression to analyze opioid‐poisoning characteristics to explore how opioid type varies by presentation characteristic. Opioid type was the outcome variable in all regressions, with morphine serving as the reference category (the standard reference opioid for calculating opioid doses [24]). Separate regressions were run, with each of the eight characteristics serving as the primary independent variable. Year was included in all models as a secondary independent variable to assess whether the opioid type variation by presentation characteristic varied over time. The regressions also controlled for age and sex (see Supporting information, Figs S3–S10).
Categories were aggregated where necessary to ensure that all analyses reported cell sizes of at least five. All quantitative analyses were conducted in SAS or Stata, with P‐values less than 0.05 considered significant with no correction for multiple testing [25].
Missing data
There were minimal missing data (< 5%), with fewer than five presentations missing data for sex, 75 with a missing birth country and 59 with unknown geographical region (which was grouped with overseas and interstate presentations for the purpose of analysis). Presentations with unknown geographical region were a subset of the 118 presentations with missing data for SEIFA, which is calculated based on postcode. With the exception of country of geographical region, all missing data were handled via list‐wise deletion in the multinomial regression.
We could not locate codeine content information on ‘Chemists Own Cough Suppression Linctus 200 ml’ from the previous or current brand owners (Aspen and Arrow). Available data states 6858 units were sold January–August 2009 to August 2010 (of more than 63 million total codeine units supplied in the study period, or approximately 0.1% of all codeine sales). These units did not contribute to the total volume of codeine used to calculate supply‐adjusted rates.
Ethics
The Monash University Human Research Ethics Committee has approved the Victorian Injury Surveillance Unit (VISU) at Monash University to analyze the VEMD for injury surveillance purposes (21427). Cells fewer than five were not reported, as per approval requirements, although zeros are preserved.
RESULTS
In the 10‐year period between July 2009 and June 2019, there were 5403 public Victorian ED attendances for poisonings able to be associated with specific pharmaceutical opioids.
Supply‐adjusted rates of poisoning
The raw ED rates and raw opioid supply data used to calculate the supply‐adjusted rates are shown in Supporting information, Fig. S2a,Sb. There was a 27‐fold difference between the highest and lowest supply‐adjusted overdose rates for the opioids examined (Fig. 1 and Table 1). When considering presentations with sole opioids, the highest supply‐adjusted overdose rates [per 100 000 OME; 95% confidence interval (CI)] were for codeine (0.078, 95% CI = 0.073–0.08) and oxycodone (0.029, 95% CI = 0.027–0.030) and the lowest two were for tapentadol (0.004, 95% CI = 0.003–0.006) and fentanyl (0.003, 95% CI = 0.002–0.004).
FIGURE 1.
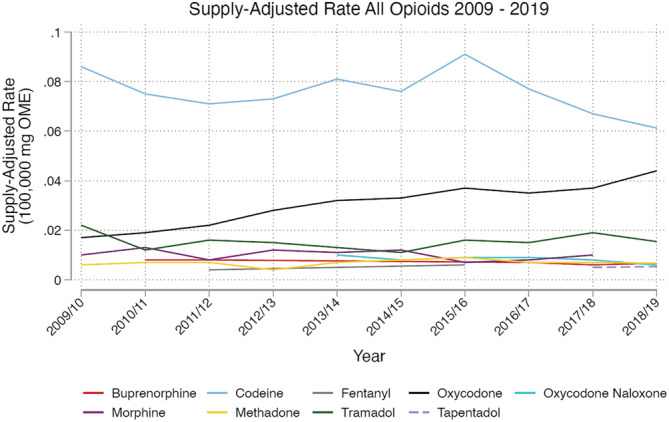
Emergency department presentations for nine opioids, per 100 000 mg oral morphine equivalents of opioids supplied to the community. Rates not calculated for when emergency department (ED) presentations were < 10 per year for that opioid, or drug was not available on the Victorian market‐place for that period of time. Search criteria for ED presentations was a free‐text search for all pharmaceutical opioid drug names, including variations that include generic and brand names for the opioids of interest together with common misspellings, combined with overdose/poisoning terms, and/or the ICD‐10‐AM code T40.3 (methadone). See bottom of Supporting information, Fig. S1 for further detail on the search strategy
Rates appeared relatively stable over the study period for seven of the nine opioids (Table 1, Fig. 1 and Supporting information, Table S2). There was a significant increase in rate for oxycodone (IRR = 1.05, 95% CI = 1.04–1.06, P < 0.0001) and tapentadol (IRR = 1.12, 95% CI = 1.00–1.02, P = 0.03). A pattern of reducing supply‐adjusted rates of ED presentations associated with codeine appeared in the final 2 years of the study period (Fig. 1), but was not significant during the 10 years of the study.
Similar results were observed in the planned sensitivity analysis where the opioid of interest could be one of multiple opioids involved (Supporting information, Table S3). The only differences were that morphine and oxycodone–naloxone swapped ranking between the 4th and 5th highest rate, and tapentadol no longer had a statistically significant time trend.
Presentation characteristics
Presentation characteristics are presented for each opioid in Table 2. We analyzed characteristics relative to our reference opioid, morphine, while controlling for age and sex (Table 3), and report significant differences below.
TABLE 2.
Patient and presentation characteristics for pharmaceutical opioid‐related overdoses in Victoria, 2009–19.
| Opioid type | All | Buprenorphine | Codeine | Fentanyl | Methadone | Morphine | Oxycodone | Oxycodone–naloxone | Tapentadol | Tramadol | Multiple opioids | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size for opioid type (%) | 5403 (100) | 90 (1.7) | 2008 (37.2) | 71 (1.3) | 580 (10.7) | 201 (3.7) | 1436 (26.6) | 146 (2.7) | 36 (0.7) | 542 (10.0) | 289 (5.4) | ||||||||||||
| Characteristic | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Age | 12–34 years | 2589 | 47.9 | 31 | 34.4 | 1195 | 59.5 | 24 | 33.8 | 242 | 41.7 | 52 | 25.9 | 599 | 41.7 | 40 | 27.4 | 12 | 33.3 | 270 | 49.8 | 124 | 42.9 |
| 35–54 years | 2080 | 38.5 | 37 | 41.1 | 650 | 32.4 | 35 | 49.3 | 299 | 51.6 | 90 | 44.8 | 551 | 38.4 | 65 | 44.5 | 17 | 47.2 | 206 | 38.0 | 129 | 44.6 | |
| 55–65 years | 408 | 7.6 | 7 | 7.8 | 106 | 5.3 | † | † | 30 | 5.2 | 27 | 13.4 | 142 | 9.9 | 27 | 18.5 | † | † | 36 | 6.6 | 25 | 8.7 | |
| > 65 years | 326 | 6 | 15 | 16.7 | 57 | 2.8 | † | † | 9 | 1.6 | 32 | 15.9 | 144 | 10.0 | 14 | 9.6 | † | † | 30 | 5.5 | 11 | 3.8 | |
| Sex | Male | 2056 | 38.1 | 54 | 60 | 572 | 28.5 | 41 | 57.8 | 350 | 60.3 | 107 | 53.2 | 553 | 38.5 | 59 | 40.4 | 13 | 36.1 | 200 | 36.9 | 104 | 36 |
| Female | 3347 | 62 | 36 | 40 | 1436 | 71.5 | 30 | 42.3 | 230 | 39.7 | 94 | 46.8 | 883 | 61.5 | 87 | 59.6 | 23 | 63.9 | 342 | 63.1 | 185 | 64 | |
| Region a | Metropolitan Melbourne | 3779 | 69.9 | 72 | 80 | 1490 | 74.2 | 33 | 46.5 | 446 | 76.9 | 121 | 60.2 | 926 | 64.5 | 93 | 63.7 | 21 | 58.3 | 372 | 68.6 | 202 | 69.9 |
| Non‐metropolitan | 1624 | 30.1 | 18 | 20 | 518 | 25.8 | 38 | 53.5 | 134 | 23.1 | 80 | 39.8 | 510 | 35.5 | 53 | 36.3 | 15 | 41.7 | 170 | 31.4 | 87 | 30.1 | |
| Country of birth | Oceania | 4568 | 84.6 | 70 | 77.8 | 1711 | 85.2 | 59 | 83.1 | 506 | 87.2 | 169 | 84.1 | 1222 | 85.1 | 122 | 83.6 | 27 | 75 | 437 | 80.6 | 242 | 83.7 |
| EU and Americas | 412 | 7.6 | 7 | 7.8 | 137 | 6.8 | 7 | 9.9 | 29 | 5 | 21 | 10.5 | 121 | 8.4 | 16 | 11 | † | † | 38 | 7 | 31 | 10.7 | |
| Middle East and Africa | 206 | 3.8 | 6 | 6.7 | 82 | 4.1 | † | † | 12 | 2.1 | † | † | 48 | 3.3 | † | † | † | † | 43 | 7.9 | 7 | 2.4 | |
| Asia | 142 | 2.6 | † | † | 62 | 3.1 | † | † | 9 | 1.6 | † | † | 34 | 2.4 | † | † | † | † | 15 | 2.8 | † | † | |
| At sea/not stated/other | 75 | 1.4 | † | † | 16 | 0.8 | † | † | 24 | 4.1 | † | † | 11 | 0.8 | † | † | † | † | 9 | 1.7 | † | † | |
| Patient SEIFA decile | 1–2 (least advantaged) | 602 | 11.4 | † | † | 213 | 10.8 | 18 | 25.7 | 57 | 10.5 | 26 | 13.2 | 163 | 11.6 | 22 | 15.6 | † | † | 53 | 10 | 39 | 13.7 |
| 3–4 | 981 | 18.6 | † | † | 355 | 17.9 | 13 | 18.6 | 54 | 9.9 | 34 | 17.3 | 309 | 21.9 | 26 | 18.4 | † | † | 115 | 21.7 | 57 | 20 | |
| 5–6 | 949 | 18 | † | † | 368 | 18.6 | 12 | 17.1 | 88 | 16.2 | 41 | 20.8 | 246 | 17.4 | 28 | 19.9 | † | † | 103 | 19.5 | 42 | 14.7 | |
| 7–8 | 1674 | 31.7 | 26 | 29.2 | 672 | 33.9 | 9 | 12.9 | 162 | 29.8 | 51 | 25.9 | 439 | 31.1 | 48 | 34 | 10 | 29.4 | 163 | 30.8 | 94 | 33 | |
| 9–10 (greatest advantage) | 1079 | 20.4 | 28 | 31.5 | 373 | 18.8 | 18 | 25.7 | 183 | 33.6 | 45 | 22.8 | 254 | 18 | 17 | 12.1 | 11 | 32.4 | 95 | 18 | 53 | 18.6 | |
| Intent | Other | 2558 | 47.3 | 69 | 76.7 | 698 | 34.8 | 60 | 84.5 | 430 | 74.1 | 133 | 66.2 | 710 | 49.4 | 68 | 46.6 | 16 | 44.4 | 256 | 47.2 | 114 | 39.5 |
| Intentional self‐harm | 2845 | 52.7 | 21 | 23.3 | 1310 | 65.2 | 11 | 15.5 | 150 | 25.9 | 68 | 33.8 | 726 | 50.6 | 78 | 53.4 | 20 | 55.6 | 286 | 52.8 | 175 | 60.6 | |
| Admission outcome b | Presentation only | 2774 | 51.3 | 47 | 52.2 | 1073 | 53.4 | 43 | 60.6 | 253 | 43.6 | 89 | 44.3 | 779 | 54.3 | 76 | 52.1 | 15 | 41.7 | 258 | 47.6 | 138 | 47.8 |
| Admission | 2629 | 48.7 | 43 | 47.8 | 935 | 46.6 | 28 | 39.4 | 327 | 56.4 | 112 | 55.7 | 657 | 45.8 | 70 | 48 | 21 | 58.3 | 284 | 52.4 | 151 | 52.3 | |
| Triage Category | Requires immediate assessment | 1454 | 26.9 | 18 | 20 | 447 | 22.3 | 31 | 43.7 | 236 | 40.7 | 70 | 34.8 | 370 | 25.8 | 40 | 27.4 | 7 | 19.4 | 131 | 24.2 | 104 | 36 |
| Does not require immediate assessment | 3949 | 73.1 | 72 | 80 | 1561 | 77.7 | 40 | 56.3 | 344 | 59.3 | 131 | 65.2 | 1066 | 74.2 | 106 | 72.6 | 29 | 80.6 | 411 | 75.8 | 185 | 64 | |
Suppressed cells (n < 5).
Non‐metropolitan region category combines the regional/rural (28%) and the interstate/overseas (OS)/unknown (2%) presentations to allow presentation of small cell sizes.
Emergency department (ED) presentation only includes discharge to home or left without treatment completed; admission can be at the same hospital or another hospital campus.
TABLE 3.
Regression estimates from multinomial logistic regression for each characteristic.
| Opioid type | Buprenorphine | Codeine | Fentanyl | Methadone | Morphine | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | |||||
| Age | |||||||||||||||
| ≤ 34 years | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| 35–54 years | 0.67 | 0.37 | 1.21 | 0.33 | 0.23 | 0.47 | 0.83 | 0.44 | 1.54 | 0.70 | 0.48 | 1.02 | |||
| 55–64 years | 0.38 | 0.15 | 0.99 | 0.17 | 0.10 | 0.28 | 0.43 | 0.16 | 1.19 | 0.21 | 0.12 | 0.39 | |||
| ≥ 65 years | 0.73 | 0.34 | 1.57 | 0.08 | 0.05 | 0.13 | 0.38 | 0.14 | 1.04 | 0.06 | 0.03 | 0.13 | |||
| Year | 1.24 | 1.13 | 1.36 | 1.13 | 1.07 | 1.19 | 1.22 | 1.10 | 1.34 | 1.21 | 1.14 | 1.29 | |||
| Sex | |||||||||||||||
| Female | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| Male | 1.35 | 0.81 | 2.24 | 0.37 | 0.28 | 0.50 | 1.22 | 0.70 | 2.11 | 1.38 | 0.99 | 1.91 | |||
| Year | 1.24 | 1.13 | 1.36 | 1.13 | 1.07 | 1.19 | 1.22 | 1.10 | 1.34 | 1.21 | 1.14 | 1.29 | |||
| Region | |||||||||||||||
| Metropolitan Melbourne | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| Interstate/OS/unknown | 0.34 | 0.04 | 3.10 | 0.52 | 0.18 | 1.54 | 0.71 | 0.08 | 6.65 | 1.75 | 0.60 | 5.07 | |||
| Regional/rural | 0.36 | 0.20 | 0.66 | 0.50 | 0.37 | 0.68 | 1.71 | 0.98 | 2.97 | 0.33 | 0.23 | 0.48 | |||
| Year | 1.25 | 1.14 | 1.37 | 1.13 | 1.07 | 1.20 | 1.21 | 1.10 | 1.34 | 1.21 | 1.14 | 1.29 | |||
| Country of birth | |||||||||||||||
| Oceania | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| EU and Americas | 0.92 | 0.36 | 2.36 | 1.60 | 0.95 | 2.70 | 1.41 | 0.54 | 3.67 | 0.94 | 0.50 | 1.75 | |||
| Asia | 2.13 | 0.51 | 8.83 | 1.56 | 0.55 | 4.43 | 1.42 | 0.25 | 7.99 | 0.77 | 0.23 | 2.58 | |||
| Middle East and Africa | 3.63 | 0.99 | 13.36 | 2.71 | 0.97 | 7.55 | 0.70 | 0.08 | 6.45 | 0.97 | 0.31 | 3.07 | |||
| Year | 1.24 | 1.13 | 1.36 | 1.13 | 1.07 | 1.19 | 1.22 | 1.10 | 1.35 | 1.21 | 1.14 | 1.29 | |||
| SEIFA quintile a | |||||||||||||||
| 1–3 (least advantaged) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| 4–5 | 0.59 | 0.22 | 1.58 | 0.67 | 0.41 | 1.12 | 0.74 | 0.33 | 1.67 | 0.65 | 0.36 | 1.18 | |||
| 6–7 | 0.93 | 0.42 | 2.07 | 0.89 | 0.57 | 1.41 | 0.25 | 0.10 | 0.62 | 1.06 | 0.63 | 1.77 | |||
| 8 | 1.53 | 0.65 | 3.59 | 1.27 | 0.75 | 2.17 | 0.42 | 0.15 | 1.13 | 1.61 | 0.89 | 2.91 | |||
| 9–10 (most advantaged) | 1.58 | 0.73 | 3.42 | 0.76 | 0.47 | 1.23 | 0.70 | 0.32 | 1.50 | 1.91 | 1.13 | 3.22 | |||
| Year | 1.25 | 1.14 | 1.37 | 1.12 | 1.06 | 1.19 | 1.21 | 1.09 | 1.34 | 1.21 | 1.14 | 1.29 | |||
| Intent | |||||||||||||||
| Intentional self‐harm | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| Other | 1.74 | 0.98 | 3.10 | 0.35 | 0.25 | 0.48 | 3.11 | 1.53 | 6.34 | 1.71 | 1.20 | 2.44 | |||
| Year | 1.24 | 1.13 | 1.36 | 1.12 | 1.06 | 1.19 | 1.22 | 1.10 | 1.34 | 1.21 | 1.14 | 1.28 | |||
| Admission outcomeb | |||||||||||||||
| Admission | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| Presentation only | 1.51 | 0.91 | 2.53 | 1.27 | 0.93 | 1.71 | 2.01 | 1.14 | 3.54 | 0.89 | 0.64 | 1.25 | |||
| Year | 1.25 | 1.14 | 1.37 | 1.13 | 1.07 | 1.20 | 1.23 | 1.12 | 1.36 | 1.21 | 1.13 | 1.28 | |||
| Triage category | |||||||||||||||
| Requires immediate assessment | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| Does not require immediate assessment | 2.18 | 1.20 | 3.96 | 1.80 | 1.31 | 2.47 | 0.71 | 0.41 | 1.24 | 0.81 | 0.58 | 1.14 | |||
| Year | 1.24 | 1.13 | 1.36 | 1.13 | 1.07 | 1.19 | 1.21 | 1.10 | 1.34 | 1.21 | 1.14 | 1.28 | |||
| Opioid type | Oxycodone | Oxycodone–naloxone | Tapentadol | Tramadol | Multiple opioids | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | |||||
| Age | |||||||||||||||
| ≤ 34 years | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| 35–54 years | 0.55 | 0.38 | 0.79 | 0.94 | 0.55 | 1.59 | 0.79 | 0.35 | 1.79 | 0.46 | 0.31 | 0.67 | 0.62 | 0.41 | 0.95 |
| 55–64 years | 0.43 | 0.26 | 0.72 | 1.11 | 0.56 | 2.21 | 0.26 | 0.05 | 1.27 | 0.25 | 0.14 | 0.44 | 0.37 | 0.20 | 0.70 |
| ≥ 65 years | 0.38 | 0.23 | 0.62 | 0.52 | 0.24 | 1.12 | 0.59 | 0.19 | 1.88 | 0.18 | 0.10 | 0.32 | 0.14 | 0.07 | 0.30 |
| Year | 1.20 | 1.13 | 1.27 | 1.55 | 1.42 | 1.69 | 2.23 | 1.80 | 2.77 | 1.18 | 1.11 | 1.26 | 1.19 | 1.11 | 1.27 |
| Sex | |||||||||||||||
| Female | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| Male | 0.57 | 0.42 | 0.77 | 0.59 | 0.38 | 0.92 | 0.51 | 0.24 | 1.07 | 0.54 | 0.39 | 0.75 | 0.51 | 0.35 | 0.74 |
| Year | 1.20 | 1.13 | 1.27 | 1.55 | 1.42 | 1.69 | 2.23 | 1.80 | 2.77 | 1.18 | 1.11 | 1.26 | 1.19 | 1.11 | 1.27 |
| Region | |||||||||||||||
| Metropolitan Melbourne | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| Interstate/OS/unknown | 0.75 | 0.26 | 2.23 | 1.41 | 0.36 | 5.52 | 2.15 | 0.35 | 13.03 | 0.94 | 0.30 | 2.98 | 0.53 | 0.13 | 2.18 |
| Regional/rural | 0.80 | 0.59 | 1.09 | 0.74 | 0.47 | 1.18 | 0.84 | 0.39 | 1.79 | 0.64 | 0.45 | 0.91 | 0.62 | 0.42 | 0.91 |
| Year | 1.20 | 1.13 | 1.27 | 1.55 | 1.42 | 1.69 | 2.23 | 1.80 | 2.76 | 1.18 | 1.11 | 1.26 | 1.19 | 1.11 | 1.27 |
| Country of birth | |||||||||||||||
| Oceania | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| EU and Americas | 1.19 | 0.71 | 2.01 | 1.47 | 0.70 | 3.08 | 1.86 | 0.54 | 6.36 | 1.36 | 0.75 | 2.46 | 2.02 | 1.08 | 3.78 |
| Asia | 1.15 | 0.40 | 3.31 | 1.68 | 0.43 | 6.54 | 2.49 | 0.42 | 14.95 | 1.45 | 0.47 | 4.48 | 0.91 | 0.24 | 3.48 |
| Middle East and Africa | 1.95 | 0.69 | 5.52 | 0.80 | 0.14 | 4.51 | 1.79 | 0.19 | 16.94 | 5.02 | 1.76 | 14.30 | 1.41 | 0.40 | 4.93 |
| Year | 1.20 | 1.13 | 1.27 | 1.55 | 1.42 | 1.70 | 2.24 | 1.80 | 2.77 | 1.18 | 1.11 | 1.26 | 1.19 | 1.11 | 1.27 |
| SEIFA quintile a | |||||||||||||||
| 1–3 (least advantaged) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| 4–5 | 1.02 | 0.61 | 1.69 | 0.73 | 0.35 | 1.52 | 0.38 | 0.07 | 1.97 | 0.89 | 0.51 | 1.54 | 0.70 | 0.38 | 1.31 |
| 6–7 | 0.77 | 0.49 | 1.23 | 0.81 | 0.43 | 1.54 | 0.86 | 0.27 | 2.71 | 0.90 | 0.55 | 1.49 | 0.79 | 0.46 | 1.37 |
| 8 | 1.35 | 0.79 | 2.31 | 1.06 | 0.52 | 2.16 | 1.27 | 0.39 | 4.12 | 0.92 | 0.51 | 1.66 | 1.03 | 0.55 | 1.95 |
| 9–10 (most advantaged) | 0.76 | 0.47 | 1.24 | 0.45 | 0.21 | 0.94 | 1.67 | 0.58 | 4.86 | 0.72 | 0.42 | 1.22 | 0.71 | 0.39 | 1.27 |
| Year | 1.19 | 1.13 | 1.26 | 1.55 | 1.42 | 1.69 | 2.19 | 1.76 | 2.73 | 1.18 | 1.11 | 1.25 | 1.19 | 1.11 | 1.27 |
| Intent | |||||||||||||||
| Intentional self‐harm | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| Other | 0.57 | 0.41 | 0.78 | 0.49 | 0.32 | 0.77 | 0.43 | 0.21 | 0.91 | 0.55 | 0.39 | 0.78 | 0.40 | 0.27 | 0.59 |
| Year | 1.19 | 1.13 | 1.26 | 1.55 | 1.42 | 1.69 | 2.23 | 1.80 | 2.76 | 1.18 | 1.11 | 1.25 | 1.18 | 1.11 | 1.26 |
| Admission outcomeb | |||||||||||||||
| Admission | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| Presentation only | 1.53 | 1.13 | 2.08 | 1.74 | 1.12 | 2.70 | 1.22 | 0.58 | 2.54 | 1.07 | 0.77 | 1.50 | 1.11 | 0.77 | 1.61 |
| Year | 1.21 | 1.14 | 1.28 | 1.57 | 1.44 | 1.71 | 2.24 | 1.81 | 2.78 | 1.18 | 1.11 | 1.26 | 1.19 | 1.11 | 1.27 |
| Triage category | |||||||||||||||
| Requires immediate assessment | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| Does not require immediate assessment | 1.52 | 1.11 | 2.10 | 1.51 | 0.94 | 2.42 | 2.34 | 0.97 | 5.68 | 1.65 | 1.16 | 2.36 | 0.96 | 0.65 | 1.40 |
| Year | 1.20 | 1.14 | 1.27 | 1.55 | 1.42 | 1.70 | 2.24 | 1.81 | 2.78 | 1.18 | 1.11 | 1.26 | 1.19 | 1.11 | 1.27 |
Abbreviations: RRR = relative risk ratio; CI = confidence interval; OS = overseas
Proportion of cells which contained fewer than five observations for the regression: age (56%), sex (56%), admission (68%), country of birth (74%), intent (67%), region (71%), SEIFA quintile (82%) and triage (68%). All models controlled for age and sex.
SEIFA is a proxy for socio‐economic status; categories were split to reflect approximately evenly sized quintiles for the analysis.
Presentation only includes discharge to home or left without treatment completed; admission can be at the same hospital or another hospital campus.
During the study period, morphine and codeine presentations appeared to reduce over time in contrast to the temporal pattern for most other opioid presentations, which increased or remained stable (Fig. 1, Supporting information, Figs S3–S10 with probabilities estimated from the model). Multinomial logistic regression modelling showed a significant association between time and the probability of presentation [relative risk ratio (RRR) = > 1, P < 0.05] for all opioids relative to morphine, holding the primary characteristic and age and sex constant (Table 3). This implies that the likelihood of a presentation involving other opioids relative to morphine increased during the study period. The relative distribution of response options appeared similar across time for all characteristics except for minor shifts across the variable of country of birth.
A quarter (26%) of morphine presentations were for patients aged < 35 years. For presentations involving codeine, methadone, oxycodone, tramadol and multiple opioids, patients aged < 35 years were more likely to present compared to morphine.
Just over half (53%) of the morphine presentations were male. For presentations involving codeine, oxycodone, oxycodone–naloxone, tramadol and the multiple opioid category, females were more likely to present compared to morphine presentations.
Sixty per cent of the morphine presentations were from the metropolitan region (compared with approximately 75% of the study state’s general population [26]). Presentations from regional areas were less likely to present for buprenorphine, codeine, methadone, tramadol and multiple opioids compared with morphine.
Most (84%) of the morphine presentations reported a country of birth within Oceania (which includes Australia). In contrast, tramadol presentations were more likely to report a Middle Eastern or African origin compared to Oceania and multiple opioids were more likely to involve patients from Europe and the Americas.
There were fewer morphine poisoning presentations in the most disadvantaged of socio‐economic status quintiles. Relative to morphine, fentanyl and oxycodone–naloxone presentations skewed towards disadvantage and methadone towards advantage.
A third (34%) of the morphine presentations were classed as intentional self‐harm. Codeine, oxycodone, oxycodone–naloxone, tapentadol, tramadol and multiple‐opioid presentations were more likely to be related to intentional self‐harm relative to morphine. In contrast, fentanyl and methadone presentations were less likely to be coded as self‐harm relative to morphine.
Just over half (56%) of morphine presentations involved hospital admission. Relative to morphine, fentanyl, oxycodone and oxycodone–naloxone presentations were more likely to be ‘presentation only’ (including presentations which left prior to treatment completion) rather than hospital admissions.
A third (35%) of the morphine presentations were triaged as requiring immediate assessment and treatment. Relative to morphine–buprenorphine, codeine, oxycodone and tramadol presentations were more likely to be associated with the less urgent triage categories.
DISCUSSION
This study examined a decade of ED presentations for pharmaceutical opioids and found that both rates and characteristics of presentations varied by opioid type. Overall, there were large differences in the supply‐adjusted rates of harm and diverging trends between opioids in terms of characteristics such as age, gender and intent. The least restricted opioid, codeine, appeared most commonly in intentional self‐harm with younger females. In contrast to most opioids, fentanyl and methadone were relatively more likely to be involved in non‐intentional poisonings. The patterns observed here are broadly consistent with previous work examining broader harms with extramedical use of prescription opioids [9], despite the more specific focus on opioid poisoning in this study.
These findings related to opioid poisoning raise a number of important issues. First, there are clear differences between pharmaceutical opioids. As a result, rather than considering pharmaceutical opioids as a single category of drugs, the unique patterns of poisoning for different opioids suggest targeted prevention strategies for different opioids are warranted, particularly where there are large differences in populations or intent. When analyses consider these opioids as a larger group much of this detail may be lost, preventing the identification of the need for more targeted responses to address harms with different opioids.
Secondly, the rank order of harms appeared to be linked more clearly with access—the highest rates were for the two most widely used opioids in Australia (codeine and oxycodone). In contrast, methadone, when represented as OMEs, was supplied in the largest volume, yet there were relatively fewer poisonings associated with its supply, which may reflect the tight control over methadone which is provided predominantly in daily supervised doses in community pharmacies under the framework of opioid agonist treatment [27]. Similarly, following the removal of sale of codeine as an over‐the‐counter medicine in Australia in 2018 [28], we observed clear reductions in the rates of harm, consistent with reductions in codeine‐related hospitalizations [29] and poisons information centre calls [30] reported elsewhere. Tapentadol and oxycodone–naloxone are of interest, given their relatively large supply volumes in the later years of observation yet relatively low rates of poisonings. Further work is needed to understand why the rates of harm appear lower for these strong opioids relative to others, and to observe if the pattern continues.
Thirdly, there were marked differences in intent by opioid. Opioids including buprenorphine, methadone and fentanyl and morphine have a minority of presentations classed as intentional poisonings, representing a stark difference from codeine where two‐thirds of presentations were classed as intentional self‐harm. The overall proportion of intentional harm seen with these pharmaceutical opioids in this study (53%) was almost double that seen in an earlier US study of ED visits (27%). This finding highlights that, in Australia, targeted responses tailored for younger women are needed to address intentional poisonings, while different responses such as naloxone provision and overdose prevention education may remain appropriate to address harms with opioids such as fentanyl, buprenorphine and methadone.
Fourthly, in contrast to other opioids, we observed that people born in the Middle East and Africa were associated with tramadol presentations. This is perhaps reflective of distinct patterns of opioid use from these original regions [31], and may reflect that within some communities specific strategies are needed.
Fifthly, socio‐economic status has been associated with drug‐related deaths [32], and opioid fatalities more specifically have been shown to be associated with lower socio‐economic status [33]. However, the link between prescription opioids poisonings and socio‐economic status appears less clear in this study. We saw diverging trends depending on opioid type. This may be explained by the measure of socio‐economic status being based on the postcode, as opposed to an individual’s income. Some areas postcodes in metropolitan Victoria have extremes of wealth and disadvantage within a single postcode, which may have led to conflicting or unexpected findings.
Overall, the findings raise some broader issues for policy. There are important differences between opioids, but also changing patterns over time. This was most notable with oxycodone, highlighting the importance of ongoing monitoring of harms. This will remain important, given substantial changes in opioid policy and regulation in Australia in the past 24 months [34]. In light of the mixed or unclear impacts of policy changes such as prescription monitoring on opioid‐related harms in the United States [35, 36], understanding how similar policies impact on harms in Australia remains important for future study. Administrative data sets are useful for monitoring changes, although this research may be extended through use of linked data to determine the relationship between changes in access to opioids and any experience of harm.
Strengths
This is one of the largest studies, to our knowledge, of opioid overdose ED presentations which encapsulates over 5000 events from 38 hospitals over 10 years (compared with previous studies with substantially smaller samples of opioid related ED events [37, 38]). This data set is one of the few to have uniquely considered opioids such as tapentadol and oxycodone–naloxone, which are newer, and with rapidly increasing use in the community. A further strength of this work was the manual checking of ED presentation text fields to identify specific opioids and confirms that presentations were related to poisoning. Measurement of opioid poisoning using coding alone can be insensitive with high rates of missed cases [37]. Also, previous studies estimate that 30% of ICD codes for opioid poisoning actually relate to opioid exposure without an opioid poisoning [38]. This study reinforces the utility of using a free‐text search function in combination with ICD‐10‐AM codes and manual checking, and we estimate that ICD codes alone only accounted for 3% of opioid poisoning presentations. Use of supply‐adjusted rates using sales data enables adjustment for availability to determine if lesser‐used opioids may be associated with disproportionate amounts of harm and captures all opioid supply, rather than being limited to those that are supplied on reimbursed prescriptions.
Limitations
There are limitations to consider. Free‐text ED data are limited to key information that is clinically relevant, as opposed to being collected for research purposes, and can vary in quality over time and between hospitals as coding procedures change. Where the opioid type was not specifically noted we are unable to capture it in our data. For this reason, the results in terms of the absolute rates of harm are most probably a substantial underestimate of opioid‐related harm in the population. However, we are not aware of any reason why this would systematically affect one opioid over another to reduce our confidence in the estimation of relative rates of harm. A final limitation is that the source of opioids is not captured in the ED presentations data, precluding assessment of diversion. In calculating rates as ED presentations per oral morphine equivalents, per opioid type, ED presentations were captured regardless of the source, whereas the oral morphine equivalents (opioid supply) were based on legal sources only (i.e. opioid products sold to Victorian community pharmacies). However, to date, supply of opioids including fentanyl outside the medical system is rare in Australia [39].
CONCLUSIONS
Rates of harm vary by opioid, and these are not necessarily ordered by opioid potency. Harm metrics should disaggregate pharmaceutical opioids where possible, as clear patterns relating to demographics and intent differ by opioid type that can be used to inform more specific prevention strategies.
DECLARATION OF INTERESTS
During the past 5 years, S.N. has been an investigator on untied education grants from Indivior, unrelated to the current work. S.N. has provided training to health‐care professionals on identifying and treating codeine dependence, for which her institution has received honoraria from Indivior. D.L. has received speaking honoraria from the following: Astra Zeneca, Indivior, Janssen‐Cilag, Lundbeck, Servier and Shire, and has participated on Advisory Boards for Indivior and Lundbeck. T.L., S.N. and D.L. have been investigators on an untied education grant from Seqirus. The Victorian Injury Surveillance Unit (VISU) is supported by the Victorian Government.
AUTHOR CONTRIBUTIONS
Tina Lam: Conceptualization; funding acquisition; investigation; methodology; project administration. Jane Hayman: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; validation. Janneke Berecki‐Gisolf: Conceptualization; data curation; formal analysis; investigation; methodology. Paul Sanfilippo: Conceptualization; data curation; formal analysis; investigation; methodology. Dan Lubman: Conceptualization; funding acquisition; investigation; methodology. Suzanne Nielsen: Conceptualization; formal analysis; funding acquisition; investigation; project administration.
Supporting information
Table S1 The RECORD statement – checklist of items extended from the STROBE statement that should be reported in observational studies using routinely collected health data.
Fig S1 Flowchart of cases identified at each stage of the search
Table S2 Estimated supply‐adjusted rates with confidence intervals for each opioid in Victoria, from July 2009–June 2019
Table S3 Supply adjusted trends for ED presentations by opioid type, Victoria July 2009 – June 2019 (aim 1 sensitivity analysis)
Fig. S2a Raw ED presentation rates by opioid type, July 2009–June 2019
Fig S2b Raw opioid supply, Victorian community pharmacies July 2009–June 2019
Fig S3 Association between Age and probability of ED Attendance by Opioid Type (controlling for sex and year)
Fig S4 Association between Sex and probability of ED Attendance by Opioid Type (controlling for age and year)
Fig S5 Association between Patient Geographic Region and probability of ED Attendance by Opioid Type (controlling for age, sex and year).
Fig S6 Association between Country of Birth and ED Attendance by Opioid Type (controlling for age, sex and year)
Fig S7 Association between SEIFA quintile and ED Attendance by Opioid Type (controlling for age, sex and year)
Fig S8 Association between Intent and probability of ED Attendance by Opioid Type (controlling for age, sex and year).
Fig S9 Association between Admission Outcome and probability of ED Attendance by Opioid Type (controlling for age, sex and year).
Fig S10 Association between Triage Category and probability of ED Attendance by Opioid Type (controlling for age, sex and year).
ACKNOWLEDGEMENTS
This study is supported by an untied educational grant from Seqirus, who are the Australian distributors of Palexia® (tapentadol) and Tramal® (tramadol). The study was funded by an untied educational grant from Seqirus. S.N. is the recipient of an NHMRC Career Development Fellowship (no. 1163961). D.L. is supported by an NHMRC Leadership Fellowship (no. 1196892). The funders have no role in the study design, study conduct, analysis or data interpretation. Prior to publication, Seqirus will have the opportunity to review the manuscript and provide comment on factual inaccuracies, if identified. The Victorian Injury Surveillance Unit (VISU) are supported by the Victorian Government. The authors acknowledge the Victorian Agency for Health Information for providing data from the Victorian Emergency Minimum Dataset (VEMD).
Lam T, Hayman J, Berecki‐Gisolf J, Sanfilippo P, Lubman DI, Nielsen S. Pharmaceutical opioid poisonings in Victoria, Australia: Rates and characteristics of a decade of emergency department presentations among nine pharmaceutical opioids. Addiction. 2022;117:623–636. 10.1111/add.15653
Funding information National Health and Medical Research Council, Grant/Award Numbers: 1163961, 1196892; Seqirus; State Government of Victoria
REFERENCES
- 1. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants ‐ United States, 2015–2016. Morb Mortal Wkly Rep. 2018;67:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vivolo‐Kantor AM, Seth P, Gladden RM, Mattson CL, Baldwin GT, Kite‐Powell A et al. Vital Signs: Trends in Emergency Department Visits for Suspected Opioid Overdoses—United States, July 2016–September 2017. Contract no.: US Department of Health and Human Services/Centers for Disease Control and Prevention 9, 2018, vol. 67, no. 9. Washington, DC: US Department of Health and Human Services/Centers for Disease Control and Prevention; 2018. [DOI] [PMC free article] [PubMed]
- 3. Australian Institute for Health and Welfare (AIHW) . Opioid harm in Australia and comparisons between Australia and Canada. Canberra, Australia: AIHW; 2018. [Google Scholar]
- 4. Scholl LSP, Kariisa M, Wilson N, Baldwin G. Drug and opioid‐involved overdose deaths—United States, 2013–2017. Morb Mortal Wkly Rep. 2019;67:1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roxburgh A, Hall WD, Dobbins T, et al. Trends in heroin and pharmaceutical opioid overdose deaths in Australia. Drug Alcohol Depend. 2017;179:192–8. [DOI] [PubMed] [Google Scholar]
- 6. Lam T, Kuhn L, Hayman J, et al. Recent trends in heroin and pharmaceutical‐opioid related harms in Victoria, Australia up to 2018. Addiction. 2020;115:261–9. [DOI] [PubMed] [Google Scholar]
- 7. Murphy DL, Lebin JA, Severtson SG, Olsen HA, Dasgupta N, Dart RC. Comparative rates of mortality and serious adverse effects among commonly prescribed opioid analgesics. Drug Saf. 2018;41:787–95. [DOI] [PubMed] [Google Scholar]
- 8. Abeyaratne C, Lalic S, Bell JS, Ilomäki J. Spontaneously reported adverse drug events related to tapentadol and oxycodone/naloxone in Australia. Ther Adv Drug Saf. 2018;9:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nielsen S, Crossin R, Middleton M, et al. Comparing rates and characteristics of ambulance attendances related to extramedical use of pharmaceutical opioids in Victoria, Australia from 2013 to 2018. Addiction. 2020;115:1075–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hedegaard H, Miniño AM, Warner M. Drug Overdose Deaths in the United States, 1999–2018. NCHS Data Brief. 2020;356:1–8. [PubMed] [Google Scholar]
- 11. Department of Health and Human Services (DHHS) Victorian Emergency Minimum Dataset (VEMD): State Government of Victoria, Australia; 2019 [cited 2019 18 Feb]. Available at: https://www2.health.vic.gov.au/hospitals-and-health-services/data-reporting/health-data-standards-systems/data-collections/vemd. (Accessed February18, 2019).
- 12. World Health Organization (WHO) . Community Management of Opioid Overdose. Geneva, Switzerland: WHO Document Production Services; 2014. [PubMed] [Google Scholar]
- 13. Caudarella A, Dong H, Milloy MJ, Kerr T, Wood E, Hayashi K. Non‐fatal overdose as a risk factor for subsequent fatal overdose among people who inject drugs. Drug Alcohol Depend. 2016;162:51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stoove MA, Dietze PM, Jolley D. Overdose deaths following previous non‐fatal heroin overdose: record linkage of ambulance attendance and death registry data. Drug Alcohol Rev. 2009;28:347–52. [DOI] [PubMed] [Google Scholar]
- 15. Pennington Institute . Australia’s Annual Overdose Report 2019. Melbourne, Australia: Penington Institute; 2019. [Google Scholar]
- 16. Lam T, Hayman J, Berecki‐Gisolf J, Sanfillippo P, Lubman DI, Nielsen S. Comparing rates and characteristics of emergency department presentations related to pharmaceutical opioid poisoning in Australia: a study protocol for a retrospective observational study. BMJ Open. 2020;10(9). e038979. https://bmjopen.bmj.com/content/10/9/e038979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Australian Bureau of Statistics . 3101.0—Australian Demographic Statistics, Mar 2018. Available at: http://www.abs.gov.au/ausstats/abs@.nsf/mf/3101.0
- 18. Rhodes AE, Bethell J, Spence J, Links PS, Streiner DL, Jaakkimainen RL. Age–sex differences in medicinal self‐poisonings: a population‐based study of deliberate intent and medical severity. Soc Psychiatry Psychiatr Epidemiol. 2008;43:642–52. [DOI] [PubMed] [Google Scholar]
- 19. Gunnell D, Ho D, Murray V. Medical management of deliberate drug overdose: a neglected area for suicide prevention? Emerg Med J. 2004;21:35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tadros A, Layman SM, Davis SM, Bozeman R, Davidov DM. Emergency department visits by pediatric patients for poisoning by prescription opioids. Am J Drug Alcohol Abuse. 2016;42:550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Australian Institute for Health and Welfare (AIHW) . Australia's Health 2018. Canberra, Australia: AIHW. p. 2018. [Google Scholar]
- 22. Australian Bureau of Statistics (ABS) . Measures of Socioeconomic Status. Canberra, Australia: ABS; 2011. [Google Scholar]
- 23. Australasian College for Emergency Medicine . Guidelines on the Implementation of the Australasian Triage Scale in Emergency Departments. Melbourne, Australia: Australasian College for Emergency Medicine; 2016. [Google Scholar]
- 24. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25:733–7. [DOI] [PubMed] [Google Scholar]
- 25. Feise RJ. Do multiple outcome measures require p‐value adjustment? BMC Med Res Methodol. 2002;2:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKenzie F. Population and Housing in Regional Victoria: Trends and Policy Implications. Victoria, Australia: The State of Victoria Department of Environment, Land, Water and Planning; 2020. [Google Scholar]
- 27. Victoria State Government . Pharmacotherapy policy in Victoria 2016. Available at: https://www2.health.vic.gov.au/public-health/drugs-and-poisons/pharmacotherapy/pharmacotherapy-policy-in-victoria
- 28. Roberts DM, Nielsen S. Changes for codeine. Aust Prescr. 2018;41:2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harris K, Jiang A, Knoeckel R, Isoardi KZ. Rescheduling codeine‐containing analgesics reduced codeine‐related hospital presentations. Med J Aust. 2020;212:328. https://www.mja.com.au/journal/2020/212/7/rescheduling-codeine-containing-analgesics-reduced-codeine-related-hospital [DOI] [PubMed] [Google Scholar]
- 30. Cairns R, Brown JA, Buckley NA. The impact of codeine re‐scheduling on misuse: a retrospective review of calls to Australia’s largest poisons Centre. Addiction. 2016;111:1848–53. [DOI] [PubMed] [Google Scholar]
- 31. Klein A, Patwardhan S, Loglo MGA. Divergences and commonalities between the US opioid crisis and prescription medicine mis/use in West Africa. Int J Drug Policy. 2020;76:102640. [DOI] [PubMed] [Google Scholar]
- 32. Heyman GM, McVicar N, Brownell H. Evidence that social‐economic factors play an important role in drug overdose deaths. Int J Drug Policy. 2019;74:274–84. [DOI] [PubMed] [Google Scholar]
- 33. Altekruse SF, Cosgrove CM, Altekruse WC, Jenkins RA, Blanco C. Socioeconomic risk factors for fatal opioid overdoses in the United States: findings from the mortality disparities in American communities study (MDAC). PLOS ONE. 2020;15:e0227966‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haines S, Savic M, Picco L, Nielsen S, Carter A. Unintended consequences of using real time prescription monitoring systems. Med J Aust. 2020;213:142–142.e1. [DOI] [PubMed] [Google Scholar]
- 35. Chen Q, Larochelle MR, Weaver DT, et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Network Open. 2019;2:1–12. e187621‐e. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2723405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fink DS, Schleimer JP, Sarvet A, et al. Association between prescription drug monitoring programs and nonfatal and fatal drug overdoses: a systematic review. Ann Intern Med. 2018;168:783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rowe C, Vittinghoff E, Santos GM, Behar E, Turner C, Coffin PO. Performance measures of diagnostic codes for detecting opioid overdose in the emergency department. Acad Emerg Med Off J Soc Acad Emerg Med. 2017;24:475–83. [DOI] [PubMed] [Google Scholar]
- 38. Reardon JM, Harmon KJ, Schult GC, Staton CA, Waller AE. Use of diagnosis codes for detection of clinically significant opioid poisoning in the emergency department: a retrospective analysis of a surveillance case definition. BMC Emerg Med. 2016;16:1–6. https://bmcemergmed.biomedcentral.com/articles/10.1186/s12873-016-0075-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barratt M, Latiner J, Jauncey M, Tay E, Nielsen S. Urine drug screening for early detection of unwitting use of fentanyl and its analogues among people who inject heroin in Sydney. Australia. Drug Alcohol Rev. 2018;37:847–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 The RECORD statement – checklist of items extended from the STROBE statement that should be reported in observational studies using routinely collected health data.
Fig S1 Flowchart of cases identified at each stage of the search
Table S2 Estimated supply‐adjusted rates with confidence intervals for each opioid in Victoria, from July 2009–June 2019
Table S3 Supply adjusted trends for ED presentations by opioid type, Victoria July 2009 – June 2019 (aim 1 sensitivity analysis)
Fig. S2a Raw ED presentation rates by opioid type, July 2009–June 2019
Fig S2b Raw opioid supply, Victorian community pharmacies July 2009–June 2019
Fig S3 Association between Age and probability of ED Attendance by Opioid Type (controlling for sex and year)
Fig S4 Association between Sex and probability of ED Attendance by Opioid Type (controlling for age and year)
Fig S5 Association between Patient Geographic Region and probability of ED Attendance by Opioid Type (controlling for age, sex and year).
Fig S6 Association between Country of Birth and ED Attendance by Opioid Type (controlling for age, sex and year)
Fig S7 Association between SEIFA quintile and ED Attendance by Opioid Type (controlling for age, sex and year)
Fig S8 Association between Intent and probability of ED Attendance by Opioid Type (controlling for age, sex and year).
Fig S9 Association between Admission Outcome and probability of ED Attendance by Opioid Type (controlling for age, sex and year).
Fig S10 Association between Triage Category and probability of ED Attendance by Opioid Type (controlling for age, sex and year).


