Abstract
Objectives
Uncontrolled vulvar lichen sclerosus (VLS) is often associated with distressful symptoms of genital itch, irritation, and pain and can lead to a pathological process including anatomical changes, scarring, and an elevated risk of cancer in the genital area. First‐line topical corticosteroid as monotherapy is frequently not adequate to fully suppress disease activity and control symptoms. This study evaluated the efficacy of fractional CO2 laser treatments as adjunctive therapy where recalcitrant VLS had been improved, but not adequately controlled, with topical corticosteroid treatment. Outcomes were evaluated up to 12 months after a series of CO2 laser treatments delivered via a fractional handpiece.
Materials and Methods
Women with a diagnosis of VLS supported by histologic findings on biopsy and/or clinical signs on physical examination received up to five monthly laser treatments. Subjects maintained existing topical corticosteroid and any exogenous hormone treatment during the study. Investigators assessed severity (0 = not present, 1 = mild, 2 = moderate, or 3 = severe) of clinical signs and architectural changes present before adjunctive study interventions and at follow‐up visits. Subjects reported the presence of clinical symptoms and impact on quality of life on 4‐ or 5‐point Likert scales. The validated Female Sexual Function Index (FSFI) was used to assess changes in sexual function. Four subjects were biopsied before adjunctive laser treatment and at follow‐up.
Results
Twelve females, 11 postmenopausal, with a mean age of 57 ± 10 years received three to five monthly CO2 laser treatments. Significant improvement in all prominent clinical signs and architectural changes were reported at the 3‐ and 6‐month follow‐ups after the treatment series. Significant improvement was maintained at the 12‐month follow‐up, with 89% of subjects showing at least one‐point improvement in elasticity compared to baseline; 86% in lichenification; 88% in sclerosis; and 80% in whitening and parchment‐like skin. Labial fusion and the extent of disease improved in 50% of patients. Ulcerations present in three subjects at baseline resolved after treatment. Subjects reported 86% improvement in dyspareunia and 83% in skin tearing. Quality of life improved significantly after treatment (p < 0.01). The 6‐month follow‐up FSFI showed significant improvement in sexual function compared to baseline (p < 0.05), with a mean point improvement of 4.5. Histology findings after treatment showed some positive improvement, as a decrease in dermal hyalinized zone thickness. There were no treatment complications or adverse events related to the treatment.
Conclusions
Fractional CO2 laser treatment outcomes showed improvement in predominant clinical signs and architectural changes in VLS recalcitrant to topical corticosteroid treatment. Adjunctive laser treatment relieved symptoms and improved quality of life as well as sexual function. Fractional CO2 laser treatment may provide an advanced treatment modality for the management of recalcitrant VLS with improved patient care and sustainable outcomes. Further study in a larger population and with CO2 laser treatment to both vulvar tissue and the vaginal canal should be explored.
Keywords: fractional CO2 laser, sexual dysfunction, topical corticosteroids, vulvar dermatoses, vulvar lichen sclerosus
INTRODUCTION
Vulvar lichen sclerosus (VLS) is a chronic, progressive, inflammatory, epithelial disorder commonly affecting the vulva, perineum, and perianal areas. The term “VLS” is preferred to the previous nomenclature, “kraurosis vulvae,” “vulvar dystrophy,” “white spot disease,” “lichen sclerosus et atrophicus,” or “guttate scleroderma.” 1 Although the disease affects women of all ages, most cases are diagnosed in postmenopausal women. 2 Patchy, thin, glistening, porcelain‐white plaques may be present, accompanied by purpura, hyperkeratosis, fissures, erosions, or ulcerations. 3 Disease progression may lead to architectural changes, including reabsorption of the labia minora, clitoral fusion, stenosis of the introitus, and posterior fourchette adhesions, as well as the development of vulvar intraepithelial neoplasia/squamous cell carcinoma. 1 , 4 VLS etiology remains largely unknown. Several studies suggest that its origin is multifactorial with various etiologies including genetic, autoimmune, hormonal, local, and infectious. 4 VLS is known to be a debilitating disease having significant negative impact on quality of life due to symptoms of itch, pain, tearing of the vulvar skin, dyspareunia and, in some cases, dysuria and restriction of micturition. VLS patients often also suffer from significantly reduced sexual activity and satisfaction. 1 , 5 , 6
Thus, the goals of VLS treatment are to alleviate symptoms, prevent or possibly reverse architectural change, minimize the risk of developing vulvar intraepithelial neoplasia/squamous cell carcinoma, and prevent or improve sexual dysfunction. 1 , 7 , 8 Topical application of potent or ultrapotent topical corticosteroids, most commonly clobetasol propionate ointment, is currently the mainstay of medical treatment, followed by a prolonged management phase. 9 , 10 Topical corticosteroid as monotherapy is typically successful in patients who are diagnosed in the early stages of VLS and receive adequate long‐term management. 1 , 9
Notwithstanding the efficacy of first‐line corticosteroid treatment, particularly in early or mild disease, management of VLS remains complex. Challenges arise for a variety of reasons, including the following:
-
–
In certain patients, VLS symptoms are not adequately controlled, and clinical signs of active VLS are not optimally suppressed, despite adequate treatment with topical corticosteroids.
-
–
Throughout the chronic disease course of VLS, symptoms frequently relapse, often becoming refractory to corticosteroid therapy.
-
–
Ongoing application of topical corticosteroids to the anogenital area requires long‐term patient compliance and close monitoring by a medical provider to balance symptom control and suppression of the underlying disease with the risks of topical corticosteroid overuse. 11
-
–
Partially compliant or noncompliant patients may suffer from a higher relapse rate and increased rate of complications. 12
-
–
Many patients are diagnosed at later stages with architectural changes that do not improve with medical therapies.
-
–
VLS is associated with sexual dysfunction and a significant burden on quality of life. 5
The need for second‐line or adjunctive therapies to augment topical corticosteroid standard‐of‐care is well recognized. Medical providers have explored various therapies to address symptoms and active disease. Published accounts reflect variable success in treating recalcitrant VLS with medical therapies, such as intralesional corticosteroids, topical calcineurin inhibitors, oral or topical retinoids, and immunosuppressors. 1 , 2 , 10 Surgical modalities are currently the main treatment of architectural changes arising from chronic VLS. 2
An ideal adjunctive therapy for VLS would augment topical corticosteroid therapy to achieve and maintain remission in recalcitrant cases and address architectural change as a means to improve sexual dysfunction. Studies on fractional carbon dioxide (CO2) laser as a treatment modality for other vulvovaginal conditions suggest its potential efficacy in treating recalcitrant VLS. In these studies, vaginal treatment with fractional CO2 laser has been shown to reconstitute normal vaginal flora, 13 drive collagenogenesis and tissue remodeling, 14 and alleviate symptoms of vaginal atrophy. 15 Fractional CO2 resurfacing has also been shown to be successful in achieving remission in patients with severe hyperkeratotic VLS that is unresponsive to topical corticosteroids. Following CO2 laser treatment, these patients' VLS became manageable with topical corticosteroid treatment. 16 Significantly, CO2 laser has also been successful in treating clitoral phimosis caused by VLS. 17
This study assesses the long‐term outcome and safety profile of fractional CO2 laser therapy as adjunctive treatment in patients with VLS whose disease was recalcitrant to standard topical corticosteroids. It provides a comprehensive evaluation of patient symptoms, clinical disease activity, architectural change, and psychosexual well‐being.
MATERIALS AND METHODS
Adult females with a diagnosis of VLS, supported by histologic changes on biopsy (hyperkeratosis, epidermal atrophy, lichenoid dermal inflammation, and elastic fiber loss thickness) and/or clinical signs on physical examination, and recalcitrant to topical corticosteroid treatment were eligible for enrollment at four clinics in the United States. Recalcitrant VLS was defined as reflecting inadequate symptomatic and/or clinical improvement despite treatment with topical corticosteroids of an adequate potency (e.g., clobetasol propionate 0.05% ointment or other ultrapotent corticosteroids) and a sufficient duration (12 weeks of use) or lack of disease control with corticosteroid maintenance therapy. Subjects maintained existing topical corticosteroid and any exogenous hormone treatment during the study. Topical corticosteroid treatment was not with held before or after the laser treatment. History of vulvar or gynecological malignancy, unexplained vaginal bleeding, pelvic organ prolapse > Stage 2, and pregnancy excluded subjects from participation. Study participants received up to five monthly fractional CO2 laser treatments and were evaluated at the 6‐week and 3‐, 6‐, and 12‐month follow‐ups after the treatment series. The study was approved by an Institutional Review Board, and all study participants provided written informed consent.
Device description
Subjects were treated with a fractional CO2 laser (CO2RE® laser system; Candela Corporation) emitting light at a wavelength of 10.6 µm, which is readily absorbed by water in the tissue. The CO2 gas tube is radiofrequency‐excited and air‐cooled. The system has a programmable two‐axis scanning laser beam that allows the physician to select the density coverage from a selection of predetermined patterns of various sizes, based on the skin area to be treated and clinical indication. The laser system has six different treatment modes, including a non‐fractionated resurfacing mode (classic mode), a cutting mode (surgical mode), and four different ablative treatment modes (mid, light, deep, and fusion modes), coagulating at depths of up to 900 µm with deep mode. 18 A disposable fractional handpiece specifically designed for vulvar treatment (CO2RE Intima external handpiece) was used with the ablative deep mode and/or fusion mode. Fusion mode is a combination of mid mode and deep mode, with deep mode targeting the mid dermis and intending to remove aged tissue and stimulate new collagen, while mid mode targets the epidermis and uppermost dermis and is intended to increase coverage for treating pigment. Deep mode delivers a core 150 µm beam having 30–70 mJ per channel. This yields fluence from 170 to 396 J/cm2. Fusion mid mode delivers energy in an annulus (ring) having an area of 0.518 mm2, which surrounds the core beam. The energy in the ring ranges from 45–128 mJ, yielding a fluence range between 8.1 and 24.8 J/cm2. Within the operating range of fusion mode, the depth of ablation correlates almost linearly with fluence.
Study procedure
Before the first treatment, the anogenital region was examined for evidence of any signs of active infection, atypical nevi, tears, or lesions. Affected treatment sites included labia majora, interlabial sulci, labia minora, vestibule, clitoral hood, perineum, and perianal region. Topical anesthetic gel (compound tetracaine/lidocaine or BLT cream [compound benzocaine, lidocaine, tetracaine]) was applied to the vulvar area for 15–30 minutes before treatment. The external handpiece was used to administer a single‐pass laser exposure with the ablative deep mode and/or fusion mode. As treatment discomfort can last for several days, the use of cold compresses applied to the area and an over‐the‐counter pain medication, such as acetaminophen, were recommended to relieve discomfort. Subjects were advised against sexual activities for 7 days following treatment. There was not a washout period before CO2 treatment or a window immediately after treatment in which the existing topical corticosteroid therapy was withheld.
Punch biopsies of affected anogenital skin were taken before adjunctive laser treatment and at posttreatment follow‐up appointments to assess histological findings of VLS, only in enrolled subjects who voluntarily consented. The tissue specimens were evaluated for the following histologic findings: hyperkeratosis (scale of 1–4), atrophy (present or not present), acanthosis (present or not present), lichenoid dermal inflammation (scale of 1–4), dermal hyalinized zone thickness (measured in mm), elastic fiber loss thickness by elastin stain (measured in mm), and basement membrane thickness by periodic acid–Schiff stain (scale of 1–4).
Investigator assessments
Investigators assessed severity (0 = not present, 1 = mild, 2 = moderate, or 3 = severe) of clinical signs and architectural changes at all study visits. The list of clinical signs and architectural changes was generated through international expert consensus for inclusion in an adult vulvar lichen sclerosus severity scale. 19 Clinical signs included fissures, whitening, parchment‐like skin, extent of the disease, erosion, ulceration, hyperkeratosis, excoriations, lichenification, loss of elasticity, sclerosis, and telangiectasia. Architectural changes included clitoral hood fusion, labial fusion, narrowing of the introitus, anterior changes/fusion anteriorly below the clitoris, and formation of posterior commissure bands.
Subject assessments
VLS symptoms (itch, pain unrelated to intercourse, superficial dyspareunia, skin tearing, or external bleeding with intercourse) and impact on quality of life and sexual function (desire to have sexual intercourse decreased due to physical appearance or self‐esteem) were self‐reported, using 4‐ or 5‐point Likert scales, 19 at all study visits.
The validated Female Sexual Function Index (FSFI) was also used to assess changes in sexual function. 20 The FSFI assesses domains of sexual function, including desire, arousal, lubrication, orgasm, satisfaction, and pain, in addition to providing an overall score regarding sexual function. The aggregated FSFI score is a sum of weighted answers for each of the 19 items in the questionnaire, with a maximum score of 36.0 (high level of sexual functional) and a minimum score of 2.0 (low level of sexual functional). Subjects completed the questionnaire at baseline or before first treatment, and at the 3‐ and 6‐month follow‐up visits.
Subjects also completed Satisfaction Questionnaires, according to a pre‐defined scale (2 = very satisfied, 1 = satisfied, 0 = uncertain, −1 = dissatisfied, −2 = very dissatisfied) at the 6‐week and 3‐, 6‐, and 12‐month follow‐up visits.
Safety assessments
A visual analog scale from 0 (no pain) to 10 (worst possible pain) was used to measure discomfort associated with treatment. 21 Immediate posttreatment tissue responses and adverse events were reported.
Statistical analysis
The Wilcoxon signed‐rank test for paired data was used to analyze the differences between baseline and follow‐up scores. Statistical significance was set at p < 0.05.
RESULTS
Twelve females (mean age 57 ± 10 years, range 39–73 years; mean weight 168 ± 35 lbs; Fitzpatrick skin type I–IV; and 83% Caucasian) were enrolled and treated. Eleven subjects were postmenopausal (mean years post‐menopause 9.8 ± 6.4; range, 2–21 years). The mean FSFI at baseline was 13.9 ± 10.1 (range, 2–33). Before laser treatment, all 12 subjects exhibited clinical signs of whitening, parchment‐like skin, and labial fusion. Most subjects (92%, 11/12) had loss of elasticity as well. Lichenification and sclerosis were present in 75% (9/12) of subjects. Ten subjects exhibited narrowing of the introitus and/or formation of posterior commissure bands/fourchette webs (83%, 10/12). Nine subjects had perianal involvement (75%, 9/12), and eight subjects demonstrated clitoral hood fusion (67%, 8/12). Seven subjects (58%, 7/12) displayed hyperkeratosis and/or telangiectasia. Six subjects (50%, 6/12) presented with anterior changes (fusion anteriorly below the clitoris). Fissures and/or erosions were present in six subjects (50%, 6/12); three subjects (25%, 3/12) demonstrated ulcerations; and two subjects had mild excoriation (17%, 2/12). The extent of disease was primarily moderate (58%, 7/12) or severe (33%, 4/12), with one subject experiencing mild disease (8%, 1/12).
Clinical symptoms of superficial (introital) dyspareunia affected 83% (10/12) and skin tearing 67% (8/12) of the subjects. Itch affected six subjects (50%, 6/12), and seven subjects (58%, 7/12) experienced pain (i.e., burning, soreness, discomfort) unrelated to intercourse. A decrease in sexual function was reported in 58% (7/12) of the subjects. All 12 subjects reported that VLS had negatively impacted their quality of life, with 67% (8/12) reporting that they had been strongly affected.
Treatments
All 12 subjects underwent three to five monthly treatments with fractional CO2 laser using the Intima external fractional handpiece and single‐pass laser pulses with a repetition rate of 0.5–1 second. Treatment parameters were selected according to severity of the condition in the various vulvar areas (Table 1). Various square pattern sizes were tailored to each patient's anatomy, focusing on selected treatment areas, while avoiding areas that were not intended for treatment. Treatment parameters included deep mode with energy level of 50–65 mJ; fractional coverage of 5% and/or fusion mode with energy level of 50–70 mJ; and fractional coverage of 25%–30% and ring energy of 78.5–94.4 mJ. A total of 57 sessions were conducted (10 subjects had five treatments, 1 subject had four treatments, and 1 subject had three treatments). The mean duration was 8.4 ± 3.3 minutes with an average of 120 ± 46 pulses applied at each treatment session.
Table 1.
Treatment parameters administered to the various affected vulvar areas
| Deep mode parameters | Fusion mode parameters | ||||||
|---|---|---|---|---|---|---|---|
| mJ | Fractional coverage (%) | Pattern size (mm) | mJ | Ring (mJ) | Fractional coverage (%) | Pattern size (mm) | |
| Labia majora | 50–65 | 5 | 4.5 × 4.5, 7.8 × 7.8 | 50–70 | 78.5–92.1 | 25, 30 | 2.6 × 2.6, 2.7 × 2.7, 5.2 × 5.2, 7.8 × 7.8 |
| Intralabial sulci | 50–60 | 5 | 4.5 × 4.5, 7.8 × 7.8 | 60–70 | 89.7–92.1 | 30 | 2.6 × 2.6, 5.2 × 5.2 |
| Labia minora | 50–60 | 5 | 4.5 × 4.5, 7.8 × 7.8 | 60–70 | 89.7–94.4 | 30 | 2.6 × 2.6, 5.2 × 5.2 |
| Vestibule | 50–65 | 5 | 4.5 × 4.5, 7.8 × 7.8 | 60–70 | 89.7–92.1 | 30 | 2.6 × 2.6, 5.2 × 5.2 |
| Introitus | 50–65 | 5 | 4.5 × 4.5, 7.8 × 7.8 | 60–70 | 89.7–94.4 | 30 | 2.6 × 2.6, 5.2 × 5.2 |
| Clitoral hood | 50–65 | 5 | 4.5 × 4.5, 7.8 × 7.8, 1.2 × 1.2 | 50–70 | 78.5–92.1 | 30 | 2.6 × 2.6, 5.2 × 5.2, 7.8 × 7.8 |
| Perineum | 50–65 | 5 | 4.5 × 4.5, 7.8 × 7.8 | 60–70 | 89.7–92.1 | 30 | 2.6 × 2.6, 5.2 × 5.2 |
| Hymen | 60 | 5 | 4.5 × 4.5 | 60–70 | 89.7–92.1 | 30 | 2.6 × 2.6, 5.2 × 5.2 |
| Peri‐rectal | 65 | 92 | 30 | 2.6 × 2.6 | |||
| Lateral labia majora (outside) | 65 | 5 | 1.2 × 1.2 | ||||
Subjects reported discomfort associated with treatment on a 10‐cm visual analogue scale of 0 = no pain to 10 = worst possible pain. Mean discomfort/pain level was 2.6 ± 2.5. Subjects reported mostly none to minimal discomfort (range, 0–2.5) during treatment (61%, 35/57 treatments).
Mild to moderate erythema and edema were present immediately following treatment. Side effects were limited to one case of severe erythema and one case of mild pinpoint bleeding immediately following treatment that resolved without intervention. No adverse events related to the treatment were observed during the study.
Investigator assessments
Nine of the 12 treated subjects were assessed at the 6‐week follow‐up, 11 subjects at both the 3‐ and 6‐month follow‐ups, and 10 subjects at the 12‐month follow‐up. One subject missed the 6‐week follow‐up; one subject missed the 12‐month follow‐up; one subject missed the 6‐week and the 3‐ and 12‐month follow‐ups; and one subject missed the 6‐week and 6‐month follow‐ups but provided her self‐assessments remotely at the 6‐month follow‐up.
Investigators assessed the severity of clinical signs and architectural changes at all study visits. The extent of disease was moderate to severe in most subjects (11/12) at baseline and improved in 50% (5/10) of subjects assessed at the 12‐month follow‐up. All prominent VLS clinical signs and architectural changes present at baseline demonstrated significant improvement at the 3‐, 6‐, and 12‐month follow‐ups after the treatment series. Mean severity scores and the percentage improvement for each parameter at each endpoint are shown in Figures 1 and 2, respectively.
Figure 1.
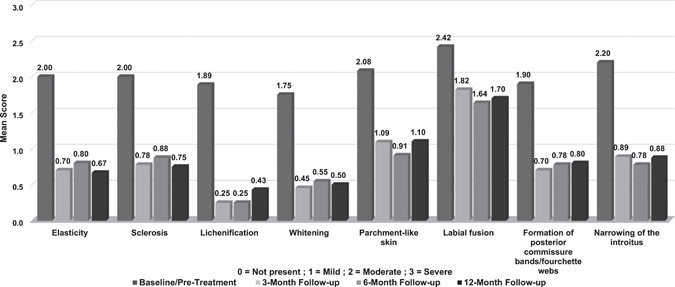
Mean scores for prominent vulvar lichen sclerosus clinical signs and architectural changes at baseline (pretreatment) and at the 3‐, 6‐, and 12‐month follow‐ups, after three to five monthly CO2 laser treatments
Figure 2.
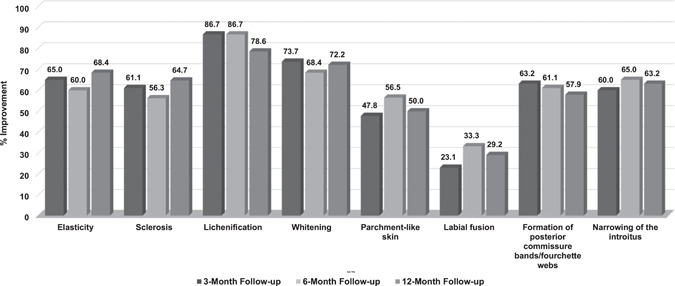
Percentage improvement in prominent vulvar lichen sclerosus clinical signs and architectural changes at the 3‐, 6‐, and 12‐month follow‐ups, after three to five monthly CO2 laser treatments
Subjects showed highly significant improvement (p < 0.01) in elasticity at all follow‐up visits. Improvement of at least 1 point was observed in all subjects at the 6‐week and 3‐month follow‐up visits and in 90% (9/10) and 89% (8/9) of subjects at the 6‐ and 12‐month follow‐up visits, respectively. Whitening and parchment‐like skin showed significant improvement (p < 0.05) at the 6‐week follow‐up visit in 78% (7/9) of subjects and highly significant improvement (p < 0.01) at the 3‐, 6‐, and 12‐month follow‐up visits in 82% (9/11), 82% (9/11), and 80% (8/10) of subjects, respectively. Sclerosis and lichenification demonstrated significant improvement (p < 0.05) at all follow‐up visits, with slightly different rates of improvement. Sclerosis improved in 86% (6/7), 78% (7/9), 75% (6/8), and 88% (7/8) of subjects, while lichenification improved in 83% (5/6), 100% (8/8), 100% (8/8), and 86% (6/7) of the subjects at the 6‐week, and 3‐, 6‐, and 12‐month visits, respectively. One case of worsening in sclerosis and one case of lichenification that were not present at baseline were observed at follow‐ups.
Improvement in labial fusion was seen in 44% (4/9), 36% (4/11), 55% (6/11), and 50% (5/10) of subjects at the 6‐week and 3‐, 6‐, and 12‐month follow‐up visits, respectively, with significant improvement (p < 0.05) at the 3‐, 6‐, and 12‐month follow‐up visits. The severity of formation of posterior commissure bands/fourchette webs showed significant improvement (p < 0.05) at the 6‐week follow‐up and highly significant improvement (p < 0.01) at the 3‐, 6‐, and 12‐month follow‐up visits, with at least 1‐point improvement observed in 75% (6/8), 90% (9/10), 89% (8/9), and 80% (8/10) of subjects, respectively. Improvement in narrowing of the introitus was observed in 57% (4/7) of subjects at the 6‐week follow‐up, 89% (8/9) at both the 3‐ and 6‐month follow‐ups, and 88% (7/8) at the 12‐month follow‐up, with highly significant improvement (p < 0.01) at the 3‐ and 6‐month follow‐ups and significant improvement at the 12‐month follow‐up (p < 0.05). Examples of improvement in prominent VLS clinical signs and architectural changes present at baseline are shown in Figures 3, 4, 5.
Figure 3.
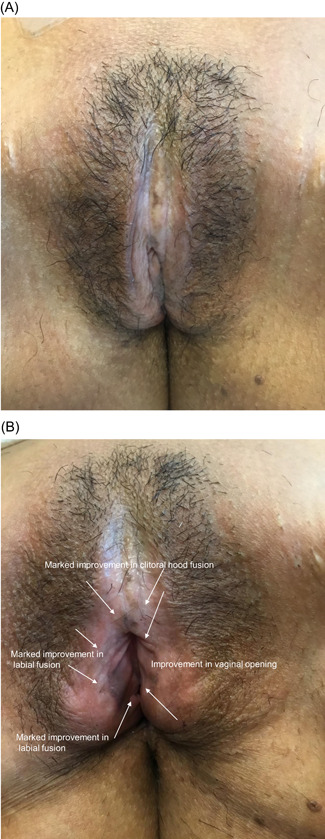
(A) 47‐year‐old female presented with severe labial and clitoral hood fusion, moderate narrowing of the introitus, moderate whitening, parchment‐like skin, and lichenification. Perirectal fissure was observed. (B) At the 6‐month follow‐up after five CO2 laser treatments, there was a marked improvement in labial fusion and clitoral hood fusion, as well as improvement in the vaginal opening and in whitening, parchment‐like skin, and lichenification
Figure 4.
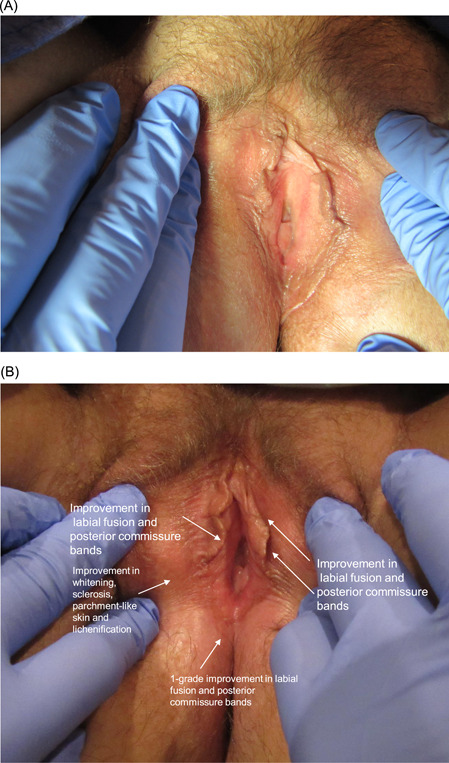
(A) 47‐year‐old female presented with moderate labial fusion and posterior commissure bands along with mild whitening, sclerosis, parchment‐like skin, and lichenification. (B) At the 6‐month follow‐up after four CO2 laser treatments, there was improvement in whitening, sclerosis, parchment‐like skin, and lichenification, as well as a 1‐grade improvement in labial fusion and posterior commissure bands
Figure 5.
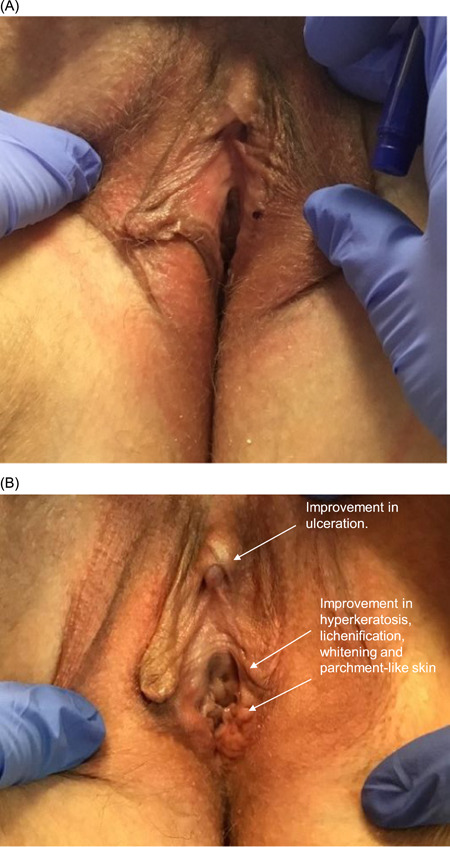
(A) 51‐year‐old female presented with moderate labial fusion and ulceration on the right labium and mild hyperkeratosis, lichenification, whitening, parchment‐like skin, and sclerosis. The left labium minus was reabsorbed and significantly smaller than the right. (B) At the 6‐month follow‐up after four CO2 laser treatments, there was improvement in ulceration, hyperkeratosis, lichenification, whitening, and parchment‐like skin. Labial fusion did not show improvement
Clinical signs and architectural changes that were less prominent at baseline also tended to improve with laser treatment. By the 12‐month follow‐up, telangiectasia improved in all six subjects, and fissures improved in 80% (4/5). One subject developed mild fissures at the 12‐month follow‐up. Erosions improved in 60% (3/5) of subjects, while one subject had 1‐point worsening (mild to moderate). Improvement in hyperkeratosis was observed in 67% (4/6) of subjects, with one case of worsening (mild to moderate). Improvement in anterior changes (fusion anteriorly below the clitoris causing urethral occlusion) was seen in 83% (5/6) of subjects. Perianal involvement demonstrated improvement in 75% (6/8) of subjects. Clitoral hood fusion improved in 57% (4/7) of subjects. Two monthly treatments with the fractional CO2 laser completely resolved mild excoriation present in two subjects at baseline. Likewise, mild to severe ulcerations that were present in three subjects at baseline were not observed after three to four treatments.
Progression of treatment outcome as an average across the clinical signs and the architectural changes present at baseline is shown in Figure 6. For those clinical signs present at baseline (baseline score >0 on severity scale), an average severity grade was calculated at each endpoint to evaluate overall treatment outcome, as follows:
Figure 6.
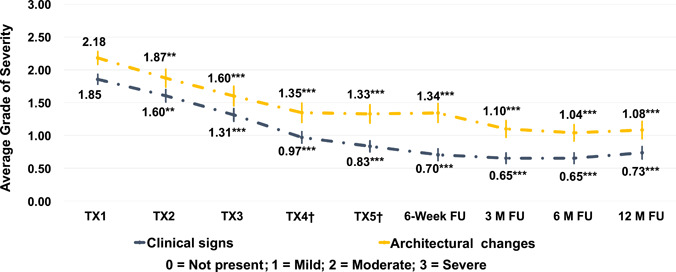
Average severity grades (with standard error of the mean bars) at each endpoint for those architectural changes (top line) and clinical signs (bottom line) that were present at baseline (baseline score >0). †For subjects not treated at study visit TX4 or TX5 (optional), values for assessments were carried over from TX3. **p < 0.01 (Wilcoxon signed‐rank test for paired data); ***p < 0.001 (Wilcoxon signed‐rank test for paired data)
A similar calculation was done for architectural changes present at baseline.
Disease severity gradually decreased during the laser treatment series and was sustained through the 6‐month follow‐up. Improvements decreased slightly between the 6‐ and 12‐month follow‐up intervals.
Subject assessments
Self‐reported symptoms of superficial dyspareunia and skin tearing or bleeding with intercourse showed a marked reduction (p < 0.05) after treatment, with 86% (6/7) and 83% (5/6) of subjects reporting improvement at the 12‐month follow‐up, respectively (Figure 7). At least 1‐point improvement in itching was reported for 75%–83% of subjects over all follow‐up visits, with 75% (3/4) of subjects experiencing improvement at the 12‐month follow‐up. Pain unrelated to intercourse improved in all subjects at the 6‐week (5/5 subjects), 3‐month (6/6), and 6‐month (7/7) follow‐up visits and in 80% (4/5) of subjects at the 12‐month follow‐up. One subject reported 1‐point worsening at the 3‐, 6‐, and 12‐month follow‐up visits, and one subject (previously reported as improved) reported worsening at the 12‐month follow‐up visit.
Figure 7.
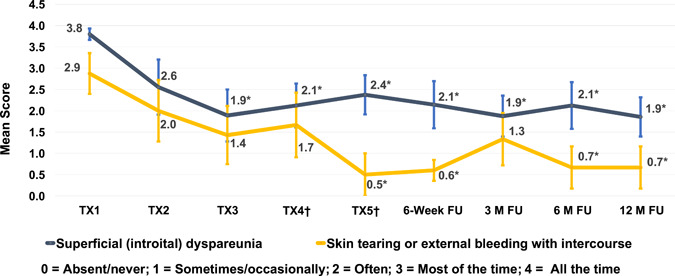
Mean self‐assessment scores (with standard error of the mean bars) of superficial dyspareunia and skin tearing during study. †For subjects not treated at study visit TX4 or TX5 (optional), values for assessments were carried over from TX3. *p < 0.05 (Wilcoxon signed‐rank test for paired data)
Subjects reported that VLS had moderate to strong (negative) impact on both quality of life and sexual function at baseline, with mean scores of 3.4 ± 1.0 and 3.7 ± 0.8, respectively. There was significant improvement in quality of life after one treatment and in sexual function after two treatments. Improvement continued with treatment, with the greatest impact reported at the 12‐month follow‐up, where mean scores improved to 0.8 for quality of life and to 0.5 for sexual function (Figure 8).
Figure 8.
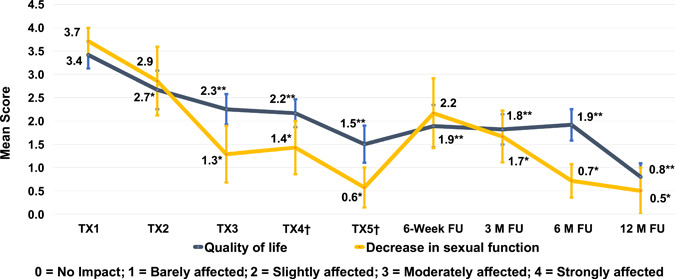
Mean self‐assessment scores (with standard error of the mean bars) for impact of vulvar lichen sclerosus on decrease in sexual function and quality of life during the study. †For subjects who were not treated at study visit TX4 or TX5 (optional), values for assessments were carried over from TX3. *p < 0.05 (Wilcoxon signed‐rank test for paired data); **p < 0.01 (Wilcoxon signed‐rank test for paired data)
Subjects completed the FSFI questionnaire at baseline or before first treatment, and at the 3‐ and 6‐month follow‐up visits. At baseline, the overall FSFI score was low (mean = 13.9 ± 10), with a minimum of 2 and a maximum of 33. Improvement in FSFI was reported by 55% (6/11) of subjects at the 3‐month follow‐up. At the 6‐month follow‐up, there was significant mean improvement (p < 0.05) of 4.5 points compared to the overall FSFI score pretreatment. Most subjects (92%, 11/12) reported improvement by the 6‐month follow‐up visit.
Most subjects reported satisfaction with treatment outcome during the study. At the 12‐month follow‐up, 50% (5/10) of subjects reported “very satisfied,” with an additional 30% (3/10) reporting “satisfied.”
HISTOLOGICAL CHANGES
Punch biopsies were obtained from labium majus, labium minus, or the perianal region of four subjects before treatment and at posttreatment follow‐up appointments. Baseline biopsies of histological markers of VLS showed mild hyperkeratosis, epidermal atrophy, lichenoid dermal inflammation, and elastic fiber loss thickness, as well as mild basement membrane thickening. Posttreatment biopsies were taken from one subject at the 3‐month follow‐up and from two subjects at the 6‐month follow‐up. Histology findings after treatment showed a slight reduction in basement membrane thickness at the 6‐week follow‐up, and a reduced dermal hyalinized zone at the 6‐month follow‐up (Figure 9). The histological findings of the dermal hyalinized zone correlate to clinical signs of scarring and tissue resorption. A decrease in this histologic finding implies clinical improvement. Improvement in elastic fiber loss thickness and hyperkeratosis was not observed.
Figure 9.
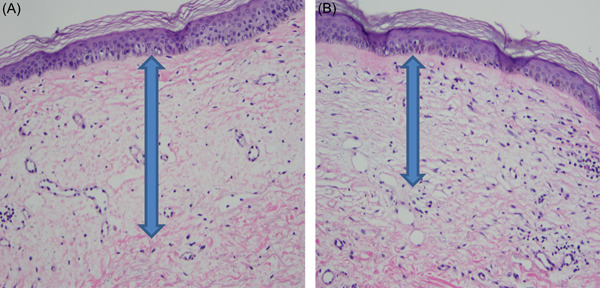
(A) Pretreatment histology of a 73‐year‐old woman showing hyperkeratosis, atrophy, elastic fiber loss, basement membrane thickening, and dermal hyalinized thickness of 0.4 mm. (B) At the 6‐month follow‐up, histology showed a reduced hyalinized zone with a dermal thickness of 0.25 mm. Hyperkeratosis and atrophy were still present, and basement membrane thickening remained unchanged (hematoxylin and eosin stain)
One additional subject was biopsied at the 6‐week follow‐up. This biopsy was taken early due to a significant flare of vulvar irritation and itch. The pathology showed psoriasiform dermatitis with candidiasis. There was no significant improvement or worsening of histologic VLS findings for this subject. The subject's flare of symptoms resolved with nystatin ointment.
DISCUSSION
Management of VLS can be challenging and complicated. It must, over decades of a woman's life, comprehensively address multiple facets of the disease, including symptoms, clinical signs of disease activity, risk for developing vulvar intraepithelial neoplasia/squamous cell carcinoma, architectural changes, and psychosexual well‐being. While the standard treatment of topical corticosteroids is successful in many VLS cases, especially those diagnosed early, there is a subset of patients with recalcitrant VLS who suffer from persistent symptoms, intermittent flares, and complications, notwithstanding diligent treatment. 12 There are a number of factors that may cause VLS to be recalcitrant to standard therapy, including, among others, innate disease extent or degree of severity, late‐stage diagnosis, and noncompliance with treatment. Multiple second‐line and adjunctive treatments have been employed to treat recalcitrant VLS, with varying levels of success. Current second‐line and adjunctive medical treatments may improve persistent symptoms and clinical signs of active lichen sclerosus but will not reverse existing scarring and architectural changes and, thus, are unlikely to improve sexual function and sexual self‐esteem.
One particularly promising adjunctive treatment for recalcitrant VLS is fractional CO2 laser therapy. The CO2 laser is one of the most widely used lasers in the dermatology field, offering treatment for a wide range of indications. 22 Laser irradiation by fractional CO2 laser involves a micro‐ablative action that stimulates the production of heat shock proteins that transform the growth factors involved in the activation of fibroblasts and neocollagenesis. 14 , 23 Previous studies have shown that fractional CO2 laser treatment of vaginal tissue promotes collagen and elastin growth, mucosa remodeling, submucosal vascularity, and clinical improvement in the symptoms of vaginal atrophy and urinary incontinence. 14 , 24 , 25 , 26
Early studies with ablative CO2 laser treatments for the treatment and management of VLS showed some success but were associated with treatment complications and prolonged healing of several weeks. 27 , 28 More recent case studies have shown positive outcomes in achieving remission with fractional CO2 laser and subsequent maintenance with topical corticosteroid treatment. 16 , 29 A 2017 literature review of light and energy‐based devices in gynecology described the impact of fractional CO2 laser on restoring the epithelial structure and included several reports of successful treatment of lichen sclerosus with fractional CO2 laser. 30 The data reviewed showed that subjective symptom resolution of lichen sclerosus was significant, while treatment complications were minimal. Furthermore, histological evaluations before and after the laser treatment in two patients demonstrated curative tissue changes, and tissue appearance was maintained throughout the 6‐month posttreatment follow‐up period.
Given that long‐term use of topical corticosteroids is considered the standard of care for VLS, this study utilized fractional CO2 laser therapy as an adjunctive treatment for recalcitrant VLS. All subjects maintained existing pre‐study topical corticosteroid and any exogenous hormone treatment throughout the study.
As a threshold matter, the results of this study show that as an adjunct to topical corticosteroids, fractionated CO2 laser treatment was both safe and well tolerated. The studied treatment improved the symptoms and clinical signs of the disease, architectural changes arising over the course of chronic disease, and the subjects' psychosexual well‐being. Symptoms of itch, pain unrelated to sexual intercourse, skin tearing, and superficial dyspareunia improved with treatment. Treatment also resulted in statistically significant improvement in all prominent clinical signs (elasticity, sclerosis, lichenification, whitening, and parchment‐like skin) and architectural changes (labial fusion, formation of posterior commissure bands/fourchette webs, and narrowing of the introitus) at the 3‐, 6‐, and 12‐month follow‐ups. The extent of the disease improved in 50% (5/10) of subjects at the 12‐month follow‐up. Clinical signs (fissures, erosions, hyperkeratosis, and telangiectasia) and architectural changes (clitoral hood fusion and anterior changes) that were less prominent before treatment also improved with laser therapy. There was complete resolution of ulceration and excoriations in some cases. Overall, improvements in most of the standardized clinical signs and symptoms of VLS were maintained out to 12 months after treatment.
In a few cases, however, the improvement in clinical signs of VLS and architectural change waned between the 6‐ and 12‐month follow‐up intervals. Similar diminishment of clinical improvement between the 6‐ and 12‐month follow‐up intervals was also reported in another vulvar laser study. In that study, following a series of three monthly fractional CO2 treatments to the vulvar skin and the vaginal canal of postmenopausal women with vulvovaginal atrophy, there was a slight decrease between the 6‐ and 12‐month follow‐up intervals in mean vaginal health index (VHI) for changes in vaginal elasticity, fluid volume, vaginal pH level, epithelial integrity, and moisture. 24
Quality of life was improved significantly after a single fractional CO2 laser treatment, with greatest improvement reported at the 12‐month follow‐up. There was high satisfaction with treatment outcome, and subjects commented on a return to various life activities, such as horseback riding and the ability to orgasm naturally. The mean FSFI score before treatment was very low, with most subjects (11/12) experiencing sexual dysfunction, indicated by FSFI score ≤26.55. 31 Significant improvement in FSFI (mean, 4.5 points) was reached at the 6‐month follow‐up (p < 0.05), correlating to self‐reported improvement in symptoms of superficial dyspareunia and skin tearing during the same period. Sexual function showed the greatest improvement at the 6‐ and 12‐month follow‐ups compared to baseline. Although FSFI was not assessed at the 12‐month follow‐up in this study, FSFI improvements were sustained in an earlier study at the 12‐month follow‐up following fractional CO2 treatments to the vulvar skin and the vaginal canal of postmenopausal women with vulvovaginal atrophy. 24 The similarities in the two studies support further study of combined CO2 laser treatment of both the vulvar tissue and the vaginal canal in patients with VLS, with the aim of greater impact on sexual function.
In previous studies, fractional CO2 laser treatments have been shown to induce tissue remodeling with neoformation of collagen and elastic fibers on atrophic skin 14 and a more irregular, thicker epidermis, as well as a decrease in the hyalinization zone of lichen sclerosus. 32 In our study, histological assessment also showed a mild decrease in the dermal hyalinized zone and in basement membrane thickness with fractional CO2 laser treatment. Hyperkeratosis and atrophy that were present in baseline and 6‐month posttreatment biopsies had improved in severity on clinical examination.
In contrast to the clearly evident improvements in the areas of symptoms, clinical signs, architectural changes, and resulting psychosexual well‐being in this study, histological evaluation did not reveal substantial changes posttreatment. Baseline pathology showed subtle histologic disease consistent with lichen sclerosus, and histology from biopsies taken after treatment were essentially similar. These results are unsurprising, given that the studied laser treatments were additive to an ongoing regimen of topical corticosteroids. Previous studies have established that treatment with topical corticosteroids can resolve pathognomonic histologic findings. 33 It is expected that the subtle histologic findings before and after treatment in our subjects are a consequence of the ongoing topical corticosteroid treatment. Hence, standardized measurements of clinical signs and symptoms may be a more practical tool to measure treatment outcome over time.
The incidence of VLS is most prevalent in postmenopausal women with a predilection for atrophy resulting from the hypoestrogenic state of vaginal tissue. 31 Combining fractional CO2 treatment to the vaginal canal with that of the external vulvar regions has been shown to restore parameters of vaginal health, particularly for women with a recent postmenopausal status. 24 Although the current study evaluated fractional CO2 treatment solely to vulvar regions as adjunctive treatment to potent topical corticosteroid therapy, VLS patients may also benefit from fractional CO2 treatment to the vaginal canal. Previous clinical findings report a diminishing effect of fractional CO2 laser treatment on atrophied vaginal tissue beyond the 12‐month follow‐up, which suggests that a retreatment interval of 12–18 months may be beneficial to sustain improvement. 15 In this pilot study, patients received up to five monthly treatments, while in an earlier, case study report of four patients with VLS, patients underwent five to seven monthly fractional CO2 sessions and remained free of clinical signs at 6 months after treatment. 32 Two of the subjects had a single maintenance treatment after 6 months, and three subjects had subsequent intravaginal CO2 treatment for atrophy typical of menopause. 32 As VLS usually requires ongoing, long‐term, management to maintain remission, clinical studies with a larger sample size should explore these treatment concepts.
CONCLUSION
Fractional CO2 laser treatment as adjunctive therapy to topical corticosteroids in VLS patients recalcitrant to standard corticosteroid monotherapy resulted in improvement across multiple facets of the disease. Treatment improved patient symptoms, predominant clinical signs, and architectural changes. It also improved quality of life and sexual function with long‐term sustainable results. As such, adjunctive fractional CO2 laser may provide medical providers with a valuable tool to enhance VLS disease management over the course of chronic disease. Further study in a larger population and with combined CO2 laser treatment of both vulvar tissue and the vaginal canal should be explored.
CONFLICT OF INTERESTS
Kristen Stewart will receive an honorarium for this article. Sunbal Javaid receives speaker honoraria. Konika P. Schallen is an employee of Candela; the work for this study was carried out at her private clinic. The remaining authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
We would like to thank Jason Charles Reutter, MD, for his valuable contribution to the histology findings and preparation of the histology figures. The authors acknowledge Doran Rozen and Asaf Mader for critical review of the manuscript. This study was funded by Candela Medical.
Stewart K, Javaid S, Schallen KP, Bartlett S, Carlson NA. Fractional CO2 laser treatment as adjunctive therapy to topical steroids for managing vulvar lichen sclerosus. Lasers Surg Med. 2022;54:138–151. 10.1002/lsm.23476
REFERENCES
- 1. Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14(1):27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pérez‐López FR, Vieira‐Baptista P. Lichen sclerosus in women: a review. Climacteric. 2017;20(4):339–47. [DOI] [PubMed] [Google Scholar]
- 3. Fruchter R, Melnick L, Pomeranz MK. Lichenoid vulvar disease: a review. Int J Womens Dermatol. 2017;3(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh N, Ghatage P. Etiology, clinical features, and diagnosis of vulvar lichen sclerosus: a scoping review. Obstet Gynecol Int. 2020;2020:7480754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haefner HK, Aldrich NZ, Dalton VK, Gagné HM, Marcus SB, Patel DA, et al. The impact of vulvar lichen sclerosus on sexual dysfunction. J Women's Health (Larchmt). 2014;23(9):765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van de Nieuwenhof HP, Meeuwis KA, Nieboer TE, Vergeer MC, Massuger LF, De Hullu JA. The effect of vulvar lichen sclerosus on quality of life and sexual functioning. J Psychosom Obstet Gynaecol. 2010;31(4):279–84. [DOI] [PubMed] [Google Scholar]
- 7. Preti M, Scurry J, Marchitelli CE, Micheletti L. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28(7):1051–62. [DOI] [PubMed] [Google Scholar]
- 8. Halonen P, Jakobsson M, Heikinheimo O, Riska A, Gissler M, Pukkala E. Lichen sclerosus and risk of cancer. Int J Cancer. 2017;140(9):1998–2002. [DOI] [PubMed] [Google Scholar]
- 9. Akel R, Fuller C. Updates in lichen sclerosus: British Association of Dermatologists guidelines for the management of lichen sclerosus 2018. Br J Dermatol. 2018;178(4):823–4. [DOI] [PubMed] [Google Scholar]
- 10. Chi CC, Kirtschig G, Baldo M, Brackenbury F, Lewis F, Wojnarowska F. Topical interventions for genital lichen sclerosus. Cochrane Database Syst Rev. 2011;2011(12):CD008240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coondoo A, Phiske M, Verma S, Lahiri K. Side‐effects of topical corticosteroids: a long overdue revisit. Indian Dermatol Online J. 2014;5(4):416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee A, Bradford J, Fischer G. Long‐term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151(10):1061–7. [DOI] [PubMed] [Google Scholar]
- 13. Athanasiou S, Pitsouni E, Antonopoulou S, Zacharakis D, Salvatore S, Falagas ME, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–8. [DOI] [PubMed] [Google Scholar]
- 14. Salvatore S, Leone Roberti Maggiore U, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22(8):845–9. [DOI] [PubMed] [Google Scholar]
- 15. Samuels JB, Garcia MA. Treatment to external labia and vaginal canal with CO2 laser for symptoms of vulvovaginal atrophy in postmenopausal women. Aesthet Surg J. 2019;39(1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee A, Lim A, Fischer G. Fractional carbon dioxide laser in recalcitrant vulvar lichen sclerosus. Australas J Dermatol. 2016;57(1):39–43. [DOI] [PubMed] [Google Scholar]
- 17. Kroft J, Shier M. A novel approach to the surgical management of clitoral phimosis. J Obstet Gynaecol Can. 2012;34:465–71. [DOI] [PubMed] [Google Scholar]
- 18. Hurliman E, Zelickson B, Kenkel J. In‐vivo histological analysis of a fractional CO2 laser system intended for treatment of soft tissue. J Drugs Dermatol. 2017;16(11):1085–90. [PubMed] [Google Scholar]
- 19. Sheinis M, Selk A. Development of the adult vulvar lichen sclerosus severity scale—a Delphi consensus exercise for item generation. J Low Genit Tract Dis. 2018;22(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self‐report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208. [DOI] [PubMed] [Google Scholar]
- 21. Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, et al. Assessment of pain. Br J Anaesth. 2008;101(1):17–24. [DOI] [PubMed] [Google Scholar]
- 22. Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014;23(1):49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prignano F, Campolmi P, Bonan P, Ricceri F, Cannarozzo G, Troiano M, et al. Fractional CO2 laser: a novel therapeutic device upon photobiomodulation of tissue remodeling and cytokine pathway of tissue repair. Dermatol Ther. 2009;22(suppl 1):S8–15. [DOI] [PubMed] [Google Scholar]
- 24. Alexiades MR. Fractional CO2 laser treatment of the vulva and vagina and the effect of postmenopausal duration on efficacy. Lasers Surg Med. 2021;53(2):185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arroyo C. Fractional CO2 laser treatment for vulvovaginal atrophy symptoms and vaginal rejuvenation in perimenopausal women. Int J Women's Health. 2017;9:591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palacios S, Ramirez M. Efficacy of the use of fractional CO2RE Intima laser treatment in stress and mixed urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 2020;244:95–100. [DOI] [PubMed] [Google Scholar]
- 27. Kartamaa M, Reitamo S. Treatment of lichen sclerosus with carbon dioxide laser vaporization. Br J Dermatol. 1997;136(3):356–9. [PubMed] [Google Scholar]
- 28. Peterson CM, Lane JE, Ratz JL. Successful carbon dioxide laser therapy for recalcitrant anogenital lichen sclerosus. Dermatol Surg. 2004;30(8):1148–51. [DOI] [PubMed] [Google Scholar]
- 29. Pagano T, Conforti A, Buonfantino C, Schettini F, Vallone R, Gallo A, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long‐term use of topic corticocorticosteroid: a prospective longitudinal study. Menopause. 2020;27(4):418–22. [DOI] [PubMed] [Google Scholar]
- 30. Tadir Y, Gaspar A, Lev‐Sagie A, Alexiades M, Alinsod R, Bader A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bond DS, Vithiananthan S, Leahey TM, Thomas JG, Sax HC, Pohl D, et al. Prevalence and degree of sexual dysfunction in a sample of women seeking bariatric surgery. Surg Obes Relat Dis. 2009;5(6):698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mendieta‐Eckert M, Torrontegui Bilbao J, Zabalza Estévez I, Landa Gundin N. Treatment of vulvar lichen sclerosus et atrophicus with fractional carbon dioxide laser therapy: a report of 4 cases. Actas Dermosifiliogr (Engl Ed). 2021;112(1):85–8. [DOI] [PubMed] [Google Scholar]
- 33. Krapf JM, Mitchell L, Holton MA, Goldstein AT. Vulvar lichen sclerosus: current perspectives. Int J Women's Health. 2020;12:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


