Abstract
Objectives
Maintenance therapy is one strategy to prolong survival in patients with acute myeloid leukemia (AML) following hematopoietic stem cell transplantation (HSCT). We evaluated real‐world treatment patterns and outcomes in patients with newly diagnosed FLT3‐mutated AML receiving HSCT after complete remission with first‐line chemotherapy.
Methods
A global, retrospective chart review to evaluate maintenance therapy and outcomes in patients with FLT3‐mutated AML after HSCT.
Results
Data from 1208 charts from eight countries showed that most patients (n = 765 [63.3%]) received no maintenance therapy after HSCT, 219 (18.1%) received FLT3 inhibitor maintenance therapy, and 224 (18.5%) received other types of maintenance therapy. No systematic differences were observed in healthcare resource utilization across the three groups. Clinical benefit was observed with FLT3 inhibitor maintenance over no maintenance therapy with relapse‐free survival (adjusted hazard ratio [HR] 0.57 [95% CI 0.34‐0.94], P < .05). FLT3 inhibitor and other maintenance also demonstrated overall survival benefit over no maintenance (adjusted HR 0.50 [95% CI 0.28‐0.89] and 0.46 [95% CI 0.23‐0.91], respectively; both P < .05).
Conclusions
Real‐world maintenance therapies after HSCT in patients with FLT3‐mutated AML were heterogeneous. While overall use of healthcare resources was not significantly increased in patients receiving maintenance therapy versus those who did not, clinical outcomes were improved.
Keywords: hematopoietic stem cell transplantation, leukemia, myeloid, acute, outcome assessment, health care
1. INTRODUCTION
Acute myeloid leukemia (AML) is a heterogeneous disease involving a clonal population of myeloid stem cells with aberrant differentiation and proliferation, wherein molecular mutations and cytogenetic abnormalities can impact the overall prognosis. 1 Among genetic risk factors, activating mutations in fms‐like tyrosine kinase 3 (FLT3) are present in up to 35% of patients with AML. 2 FLT3 mutations confer a worsened prognosis, characterized by lower complete remission (CR) rates and shorter disease‐free or event‐free survival, particularly in patients with internal tandem duplications (ITD) compared with patients with wild‐type FLT3. 1 , 2 , 3 , 4 The reported impact on prognosis for patients with FLT3 tyrosine kinase domain (TKD) point mutations, which occur in about 10% of patients, is less consistent. 2 , 5
Many patients with high‐risk AML undergo hematopoietic stem cell transplantation (HSCT) as part of their therapy. 6 , 7 , 8 However, patients with higher‐risk AML experience higher post‐HSCT relapse rates 2 , 9 , 10 and increased risk of early relapse after transplant compared with standard‐risk patients. 11 In those with FLT3‐ITD AML, after allogeneic HSCT, the relapse incidence 2 years post‐HSCT was approximately 30% compared with approximately 16% in patients without FLT3‐ITD, 9 and relapse was associated with a significantly shorter overall survival (OS) in these patients. 12
In some populations, maintenance therapies in AML have been found to be feasible and beneficial in sustaining remission. 13 , 14 , 15 , 16 There is growing interest in providing maintenance therapy after HSCT for patients with AML in an attempt to reduce relapses, yet the treatment approaches that clinicians currently use are not clearly understood. 17 Recently completed or ongoing studies evaluating the safety and efficacy of post‐transplant maintenance therapies in patients with FLT3‐mutated AML include gilteritinib (NCT02997202), crenolanib (NCT02400255, NCT01522469, NCT03250338), sorafenib (NCT01398501, NCT01578109), and others (NCT01477606, NCT01468467). In addition to weighing the benefits and risks of preventative intervention on patients’ health, additional therapies have the potential to affect overall healthcare resource utilization (HRU). The use of healthcare resources associated with various aspects of treatment—such as hospitalizations, clinic visits, or transfusions—is an important component to understand when developing any new treatment modality due to the impact on a patient's family life as well as healthcare costs. 18 , 19 , 20
Despite the known risks and burden of relapse, recommendations and guidelines for maintenance therapy after HSCT are lacking. 6 , 7 , 21 In this study, we obtained real‐world data collected from the medical charts of patients with newly diagnosed FLT3‐mutated (FLT3 mut+) AML who received HSCT after achieving CR with first‐line chemotherapy. Given the lack of standard guidelines and recommendations for maintenance therapy after HSCT in this population, our objective was to characterize real‐world maintenance therapy patterns and compare clinical outcomes and HRU among patients treated with various maintenance approaches.
2. METHODS
2.1. Study design and population
This study was a retrospective chart review of adult patients with FLT3‐mutated AML who had achieved CR with first‐line chemotherapy and then undergone HSCT. Physicians from eight countries—Canada, France, Germany, Italy, Japan, Spain, the United Kingdom, and the United States—extracted data from eligible patient charts. This research was granted exemption from full review by the New England Institutional Review Board, given that no personally identifiable information would be collected. Data‐privacy regulations were adhered to in all countries with participating physicians.
Participating physicians used a prespecified rule to randomly select the medical records of up to 10 eligible patients. To be eligible for chart abstraction, adult patients (aged 18 years or older) were required to have a confirmed AML diagnosis between January 1, 2015, and October 31, 2018, and be under the responding physician's care from the initial diagnosis. Patients were required to have a confirmed FLT3 mutation, have achieved CR with first‐line chemotherapy, have subsequently undergone HSCT, and have at least 1 year of follow‐up data after the first CR or confirmed date of death within 1 year after the first CR. Patients with acute promyelocytic leukemia or AML secondary to prior treatment for other neoplasms were excluded. The baseline period was defined as the date that AML was diagnosed to the date of HSCT, which was considered the index date, and the study period was defined as the index date to the most recent follow‐up visit or date of death.
Data on baseline characteristics, treatment patterns, treatment outcomes, and AML‐related HRU were collected. A standardized case report form, which was pilot‐tested by physicians from different countries, was used by individual physicians to maintain consistency in data abstraction. Additionally, real‐time error and logic checking were implemented to ensure data quality; automatic data validation was incorporated in the case report form which was hosted online. For instance, when appropriate, some responses were validated by responses to previous questions (eg, the date of treatment initiation must be after the date of initial AML diagnosis). If there were any logical inconsistencies with these responses, physicians would be requested to double‐check their responses. Baseline characteristics included demographics, comorbidities, and performance status, as well as AML‐related characteristics (eg, genetic mutations, molecular and cytogenetic abnormalities, extramedullary involvement). Data on treatment patterns consisting of first‐line chemotherapy regimens (induction/re‐induction and consolidation therapies), HSCT type, and maintenance therapies after HSCT were also obtained. Maintenance therapies were defined on the case report form as non‐myelosuppressive treatment with chemotherapy and/or targeted therapeutic agents that are administered over a period of months to years to sustain remission after first‐line chemotherapy and/or HSCT (if applicable). Healthcare resource utilization was characterized using data abstracted on services related to AML care, including hospitalizations, emergency department visits, outpatient visits, and AML‐related management and monitoring.
2.2. Endpoints
The primary endpoints included post‐HSCT maintenance therapy patterns and AML‐related HRU during the study period. Given the large number of different individual treatments, a hierarchy of treatments was used to create mutually exclusive regimen categories. For example, within the post‐HSCT maintenance therapies, FLT3 inhibitors (forming the “FLT3 inhibitor maintenance therapy” cohort) were assigned the highest level, followed by hypomethylating agents (HMAs), other targeted therapies, and cytotoxic chemotherapy without targeted agents. Treatment regimens could only be assigned to one category. Patients who had their post‐HSCT maintenance therapy regimen assigned to the HMA, other targeted therapies, or cytotoxic chemotherapy without targeted agents category were grouped together to form the “other maintenance therapy” cohort. Ultimately, patients were included in one of three cohorts: FLT3 inhibitor maintenance therapy, other maintenance therapy, and no maintenance therapy. Healthcare resources evaluated included service‐related resources such as visit type and duration, as well as treatment and monitoring procedures such as transfusions, medications, and lab testing or imaging. Exploratory clinical outcomes included evaluation of relapse‐free survival (RFS) and OS during the study period, both of which were measured from the time of HSCT (index date) to the time of event or censoring at the date of the last visit.
2.3. Statistical analyses
Baseline characteristics, treatment patterns, and AML‐related HRU during the study period were summarized using descriptive statistics for each study cohort. Additionally, AML‐related HRU was compared between study cohorts using generalized linear models adjusting for the following covariates: age at index date, sex, race, time from diagnosis to index date, body mass index, Eastern Cooperative Oncology Group (ECOG) grade, measurable residual disease status, extramedullary involvement, risk status, and HSCT type. For survival outcomes, time‐to‐event analyses were conducted beginning at the index date, with censoring at the end of data availability for patients without clinical events. Kaplan‐Meier curves and Cox proportional hazards models were used to describe and compare RFS and OS between cohorts. The following baseline covariates were adjusted in the Cox regression models: age at index date, sex, race, country, time from diagnosis to index date, body mass index, ECOG grade, measurable residual disease status, extramedullary involvement, risk status, and HSCT type. All analyses were conducted using R Statistical Software, version 3.6.1.
3. RESULTS
A total of 311 hematologists and oncologists (Table S1) from eight countries contributed data from 1208 patients with FLT3‐mutated AML who underwent HSCT. Among these patients, 219 received a FLT3 inhibitor as post‐HSCT maintenance therapy, 224 received other types of post‐HSCT maintenance therapy, and 765 did not receive post‐HSCT maintenance therapy. Follow‐up duration (mean ± SD) was comparable between patient cohorts (FLT3 inhibitor maintenance therapy, 15.4 ± 9.7 months; other maintenance therapy, 16.1 ± 10.6 months; no maintenance therapy, 16.6 ± 11.6 months). The median (interquartile range) duration of maintenance therapy after HSCT was 198.00 (99.00, 349.00) days in patients receiving FLT3 inhibitor maintenance therapy and 127.00 (58.00, 201.00) days in patients receiving other maintenance therapy; median durations for specific maintenance therapies are shown in Table S2. The median time from HSCT to initiation of maintenance therapy was 1.08 months (FLT3 inhibitor maintenance therapy, 1.18 months; other maintenance therapy, 0.95 months).
Mean age of patients was 53.5 years at the time of HSCT; 62.8% of patients were male, and 80.4% were white (Table 1). Common comorbidities included hypertension (33.0%), diabetes (15.2%), and coronary heart disease (6.8%). Most patients had good‐to‐moderate performance status with 84.7% having an ECOG grade 0 or 1. More patients who received other maintenance therapy had known extramedullary involvement compared with patients who received FLT3 inhibitors or no maintenance therapy. Across the three cohorts, more patients in the FLT3 inhibitor cohort had poor cytogenetic or molecular risk status while more patients in the other cohort were minimal residual disease–positive, despite being in CR. Over 70% of patients had an ITD, with or without a TKD mutation. Normal cytogenetics was higher among patients who had received FLT3 inhibitor maintenance therapy (31.5%) compared with those who received other (14.3%) or no (28.1%) maintenance therapy. Additional AML‐related acquired mutations and cytogenetic or molecular abnormalities at HSCT are shown in Table S3.
TABLE 1.
Patient baseline characteristics
| Characteristics | Post‐HSCT maintenance therapy | ||
|---|---|---|---|
| FLT3 Inhibitor n = 219 | Other n = 224 | None n = 765 | |
| Age at HSCT date (years), mean ±SD | 55.1 ± 13.5 | 52.7 ± 13.8 | 53.3 ± 12.9 |
| Diagnosis to HSCT date (months), mean ±SD | 6.7 ± 4.5 | 9.2 ± 6.6 | 6.1 ± 5.4 |
| Male | 145 (66.2) | 154 (68.8) | 460 (60.1) |
| BMI (kg/m2), mean ± SD | 24.7 ± 3.8 | 23.8 ± 2.7 | 24.6 ± 3.6 |
| Country | |||
| United States | 23 (10.5) | 40 (17.9) | 116 (15.2) |
| Canada | 0 | 0 | 30 (3.9) |
| France | 62 (28.3) | 29 (13.0) | 109 (14.3) |
| Germany | 26 (11.9) | 11 (4.9) | 83 (10.9) |
| Italy | 44 (20.1) | 60 (26.8) | 103 (13.5) |
| Japan | 14 (6.4) | 54 (24.1) | 12 (1.6) |
| Spain | 27 (12.3) | 24 (10.7) | 153 (20.0) |
| United Kingdom | 23 (10.5) | 6 (2.7) | 159 (20.8) |
| Race | |||
| White | 187 (85.4) | 153 (68.3) | 631 (82.5) |
| Asian or Pacific Islander | 18 (8.2) | 47 (21.0) | 46 (6.0) |
| Black or African | 10 (4.6) | 24 (10.7) | 84 (11.0) |
| Other | 1 (0.5) | 0 | 2 (0.3) |
| Unknown | 3 (1.4) | 0 | 2 (0.3) |
| ECOG performance status a | |||
| Grade 0 or 1 | 194 (88.6) | 183 (81.7) | 646 (84.4) |
| Grade 2 or higher | 25 (11.4) | 40 (17.9) | 117 (15.3) |
| Unknown | 0 | 1 (0.5) | 2 (0.3) |
| Comorbidities a | |||
| Hypertension | 75 (34.3) | 72 (32.1) | 252 (32.9) |
| Diabetes | 31 (14.2) | 35 (15.6) | 117 (15.3) |
| Mental health condition | 15 (6.9) | 21 (9.4) | 43 (5.6) |
| COPD | 9 (4.1) | 15 (6.7) | 23 (3.0) |
| Renal disease | 9 (4.1) | 10 (4.5) | 32 (4.2) |
| Peripheral artery disease | 9 (4.1) | 9 (4.0) | 19 (2.5) |
| Coronary heart disease | 7 (3.2) | 15 (6.7) | 60 (7.8) |
| Congestive heart failure | 7 (2.3) | 5 (2.2) | 16 (2.1) |
| Stroke | 5 (2.3) | 7 (3.1) | 14 (1.8) |
| WHO classification a | |||
| Myelodysplasia‐related | 16 (7.3) | 31 (13.8) | 129 (16.9) |
| Recurrent genetic abnormalities | 111 (50.7) | 119 (53.1) | 328 (42.9) |
| Unknown | 11 (5.0) | 15 (6.7) | 28 (3.7) |
| Unspecified | 81 (37.0) | 59 (26.3) | 280 (36.6) |
| Risk status a | |||
| Favorable risk | 30 (13.7) | 54 (24.1) | 158 (20.7) |
| Intermediate risk | 83 (37.9) | 109 (48.7) | 332 (43.4) |
| Poor risk | 104 (47.5) | 59 (26.3) | 262 (34.3) |
| Unknown | 2 (0.9) | 2 (0.9) | 13 (1.7) |
| HSCT type | |||
| Allogeneic | 183 (83.6) | 143 (63.8) | 605 (79.1) |
| Reduced intensity allogeneic | 17 (7.7) | 45 (20.1) | 58 (7.6) |
| Autologous | 19 (8.7) | 36 (16.1) | 102 (13.3) |
| Disease status at HSCT | |||
| Complete remission | 208 (95.0) | 211 (94.2) | 745 (97.4) |
| Relapse | 11 (5.0) | 13 (5.8) | 20 (2.6) |
| Response to first‐line chemotherapy | |||
| CR | 155 (70.8) | 157 (70.1) | 579 (75.7) |
| CR with partial hematologic recovery | 42 (19.2) | 42 (18.8) | 122 (16.0) |
| CR with incomplete hematologic recovery | 18 (8.2) | 19 (8.5) | 46 (6.0) |
| CR with incomplete platelet recovery | 4 (1.8) | 6 (2.7) | 18 (2.4) |
| MRD status at complete remission | |||
| MRD negative | 111 (50.7) | 110 (49.1) | 434 (56.7) |
| MRD positive | 84 (38.4) | 100 (44.6) | 203 (26.5) |
| Unknown | 24 (11.0) | 14 (6.3) | 128 (16.7) |
| Known extramedullary involvement a | 54 (24.7) | 139 (62.1) | 208 (27.2) |
| Mutations and cytogenetic abnormalities | |||
| FLT3 status b | |||
| ITD | 135 (61.6) | 122 (54.5) | 460 (60.1) |
| ITD & TKD | 22 (10.1) | 27 (12.1) | 84 (11.0) |
| TKD | 62 (28.3) | 75 (33.5) | 221 (28.9) |
| Allelic frequency of the ITD mutation (%), mean ± SD | 41.2 ± 20.2 | 41.5 ± 23.0 | 43.3 ± 22.5 |
| Normal cytogenetics | 69 (31.5) | 32 (14.3) | 215 (28.1) |
Values are presented as n (%), unless otherwise noted.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CR, complete remission; ECOG, Eastern Cooperative Oncology Group; FLT3, Fms‐like tyrosine kinase 3; HSCT, hematopoietic stem cell transplantation; ITD, internal tandem duplication; MRD, measurable residual disease; SD, standard deviation; TKD, tyrosine kinase domain; WHO, World Health Organization.
Data obtained at the index date (date of HSCT). As assessed by the physician according to risk stratification by the National Comprehensive Cancer Network Acute Myeloid Leukemia guidelines.
FLT3 mutation status reported at initial diagnosis.
Across all three cohorts, allogeneic transplantation (including reduced intensity and standard allogeneic transplantation) accounted for the majority of HSCT (FLT3 inhibitor, 91.3%; other, 83.9%; none, 86.7%) (Table 1). Autologous transplants were received by 8.7% of patients on FLT3 inhibitor maintenance therapy, 16.1% of patients on other maintenance therapy, and 13.3% of patients on no therapy after HSCT. Approximately 96% of patients were in CR at the time of HSCT. Over 90% of the patients used cytotoxic chemotherapy for induction and/or consolidation therapy prior to HSCT (Table S4). FLT3 inhibitors were used by 22.1% of patients in combination with cytotoxic chemotherapy and 6.1% of patients without chemotherapy during induction therapy; 16.1% and 5.9% of patients used FLT3 inhibitors, respectively, during consolidation therapy.
After HSCT, 63.3% of patients did not receive maintenance therapy. Those receiving post‐HSCT maintenance therapy included 219 (18.1%) patients who received FLT3 inhibitor therapy and 224 (18.5%) patients receiving other types of maintenance therapy (Table 2). The most commonly used FLT3 inhibitor in post‐HSCT maintenance therapy among the FLT3 inhibitor maintenance therapy group was midostaurin (63.9%) followed by sorafenib (26.5%). Cytotoxic chemotherapy, consisting of predominantly cytarabine‐based regimens, accounted for 70.5% of the other non‐FLT3 inhibitor maintenance therapies, while HMAs and other targeted therapies accounted for the remaining treatments, at 14.7% for each therapy.
TABLE 2.
Agents used for post‐HSCT maintenance therapy
| N = 1,208 n (%) | |
|---|---|
| FLT3 inhibitors | 219 (18.1) a |
| Midostaurin | 140 (63.9) b |
| Sorafenib | 58 (26.5) b |
| Gilteritinib | 14 (6.4) b |
| Quizartinib | 9 (4.1) b |
| Other maintenance therapy | 224 (18.5) a |
| Hypomethylating agents | 33 (14.7) b |
| Azacitidine | 22 (9.8) b |
| Decitabine | 11 (4.9) b |
| Other targeted therapies | 33 (14.7) b |
| Enasidenib | 10 (4.5) b |
| Gemtuzumab ozogamicin | 9 (4.0) b |
| Ivosidenib | 8 (3.6) b |
| Venetoclax | 6 (2.7) b |
| Glasdegib | 1 (0.5) b |
| Cytotoxic chemotherapy | 158 (70.5) b |
| Cytarabine‐based chemotherapy | 98 (43.8) b |
| Low‐dose cytarabine | 24 (10.7) b |
| Standard‐dose cytarabine | 42 (18.8) b |
| High‐dose cytarabine | 12 (5.4) b |
| Other chemotherapies | 60 (26.8) b |
| None | 765 (63.3) a |
Abbreviations: FLT3, Fms‐like tyrosine kinase 3; HSCT, hematopoietic stem cell transplantation; SD, standard deviation.
Percentages are based on total number of patients in sample; agents within specific therapy types are not mutually exclusive.
Percentages are based on the number of patients within each post‐HSCT maintenance therapy type as the denominator.
AML patients across all study cohorts had frequent HRU during the study period. Overall, there were no systematic differences in HRU across the three cohorts, though small numeric differences were observed in monthly means depending on the HRU type. While no differences were observed in the average number of monthly inpatient or emergency department visits, differences were observed in outpatient visits across the three cohorts (Figure 1A). However, after adjustment for baseline covariates, the incidence rates of inpatient visits, emergency department visits, and outpatient visits did not differ significantly between patients who received maintenance therapy (FLT3 inhibitor or other) and patients who did not receive maintenance therapy (Table 3). Compared to patients with no maintenance therapy, patients with other maintenance therapy had significantly shorter inpatient stays (adjusted IRR, 0.40; P < .01) but longer palliative care stays (adjusted IRR, 5.17; P < .05) (Table 3). Approximately 50% of patients received a blood transfusion during the study period, and no differences were observed in the number of blood transfusions received by patients across cohorts (Figure 1B). Regarding monitoring and treatment procedures, adjusted models suggested fewer imaging examinations in patients on either FLT3 inhibitor or other maintenance therapy compared with patients on no maintenance therapy and fewer bone marrow biopsies and antibiotic courses in those on other versus no maintenance therapy (Table 3).
FIGURE 1.
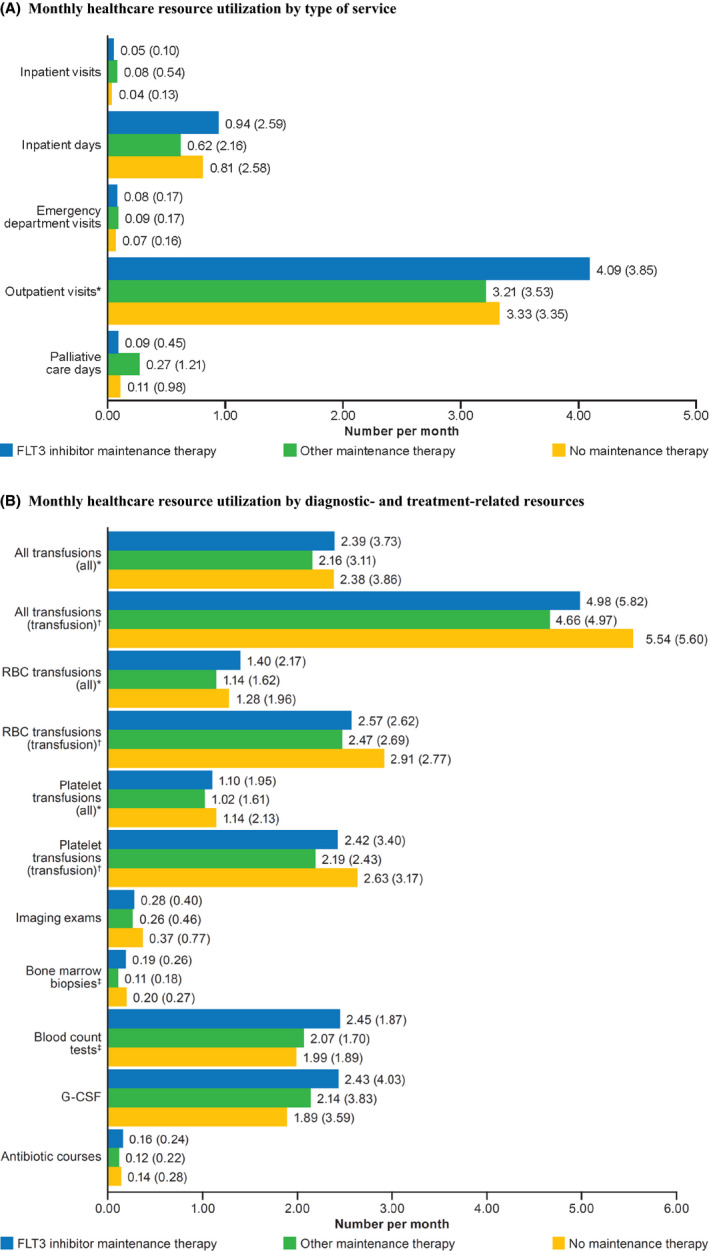
Monthly healthcare resource utilization for patients post‐HSCT receiving FLT3 maintenance therapy, other maintenance therapy, or no maintenance therapy. A, The monthly healthcare resource utilization by type of service (inpatient, outpatient, emergency department, or palliative care) identified using number of days or visits is shown in the bar graph. Abbreviations: CI, confidence interval; ED, emergency department; FLT3, Fms‐like tyrosine kinase 3; G‐CSF, granulocyte colony‐stimulating factor; HRU; healthcare resource utilization; IRR, incidence rate ratio; RBC, red blood cell. Numbers at the end of bars indicate means (standard deviations), representing continuous variables. Bolded numbers in inset table indicate statistical significance at P < .05. *Significantly different between maintenance therapy groups, P < .05. B, The healthcare resource utilization by diagnostic‐ and treatment‐related resources identified using the number of each resource used per month is shown in the bar graph. Numbers at the end of bars indicate means (standard deviations), representing continuous variables. Bolded numbers in inset table indicate statistical significance at P < .05. †Analysis of all patients receiving transfusions on a monthly basis for the entire study period. ‡Analysis of only patients receiving transfusions on a monthly basis for the entire study period. §Significantly different between maintenance therapy groups, P < .05
TABLE 3.
Adjusted incidence rate ratios of healthcare resource utilization between post‐HSCT maintenance therapy and no maintenance therapy
| HRU | Adjusted a IRR (95% CI), per person‐month | |||
|---|---|---|---|---|
| FLT3 inhibitor vs None | P‐Value | Other vs None | P‐Value | |
| Inpatient visits | 1.12 (0.81‐1.56) | .50 | 0.76 (0.51‐1.14) | .19 |
| Inpatient days | 0.99 (0.55‐1.77) | .97 | 0.40 (0.21‐0.76) | <.01 |
| Emergency department visits | 0.82 (0.59‐1.14) | .24 | 0.76 (0.53‐1.08) | .13 |
| Outpatient | 1.15 (0.92‐1.45) | .23 | 0.94 (0.73‐1.20) | .60 |
| Palliative care days b | 1.32 (0.40‐4.38) | .65 | 5.17 (1.46‐18.28) | <.05 |
| Blood transfusion c | 0.71 (0.48‐1.04) | .08 | 0.84 (0.56‐1.28) | .43 |
| Red blood cell transfusion c | 0.73 (0.51‐1.05) | .09 | 0.81 (0.55‐1.20) | .29 |
| Platelet transfusion c | 0.71 (0.48‐1.07) | .10 | 0.93 (0.60‐1.44) | .74 |
| Imaging exams | 0.70 (0.55‐0.91) | <.01 | 0.66 (0.50‐0.87) | <.01 |
| Bone marrow biopsies | 0.93 (0.76‐1.15) | .51 | 0.76 (0.60‐0.96) | <.05 |
| Blood count tests | 1.06 (0.89‐1.26) | .50 | 1.04 (0.87‐1.25) | .67 |
| Granulocyte colony‐stimulating factor | 1.35 (0.82‐2.21) | .24 | 1.14 (0.67‐1.95) | .62 |
| Antibiotic courses | 0.83 (0.63‐1.09) | .17 | 0.72 (0.53‐0.97) | <.05 |
Bolded numbers in table indicate statistical significance at P < .05.
Abbreviations: CI, confidence interval; FLT3, Fms‐like tyrosine kinase 3; HRU, healthcare resource utilization; IRR, incidence rate ratio.
Models were adjusted for the following covariates, unless otherwise specified: age at index date, sex, race, time from diagnosis to index date, body mass index, Eastern Cooperative Oncology Group grade, measurable residual disease status, extramedullary involvement, risk status, and HSCT type.
The following covariates were removed from the list of overall covariates for adjustment for model stability: measurable residual disease status, extramedullary involvement, and risk status.
Analysis of only patients receiving transfusions on a monthly basis for the entire study period.
Over the course of the study period, a total of 191 patients experienced AML relapse or death. Patients in the FLT3 inhibitor maintenance group appeared to have longer RFS than those in the other two groups, though the difference was not statistically significant (log‐rank P = .10); the median RFS was not reached in any of the cohorts (Figure 2A). In the adjusted Cox regression models, the risk for relapse or death in the “FLT3 inhibitor” cohort compared with the “no maintenance therapy” cohort was lower (adjusted HR of RFS 0.57 [95% CI 0.34‐0.94], P < .05) (Table 4). RFS was no difference between patients on other maintenance therapy compared with no maintenance therapy.
FIGURE 2.
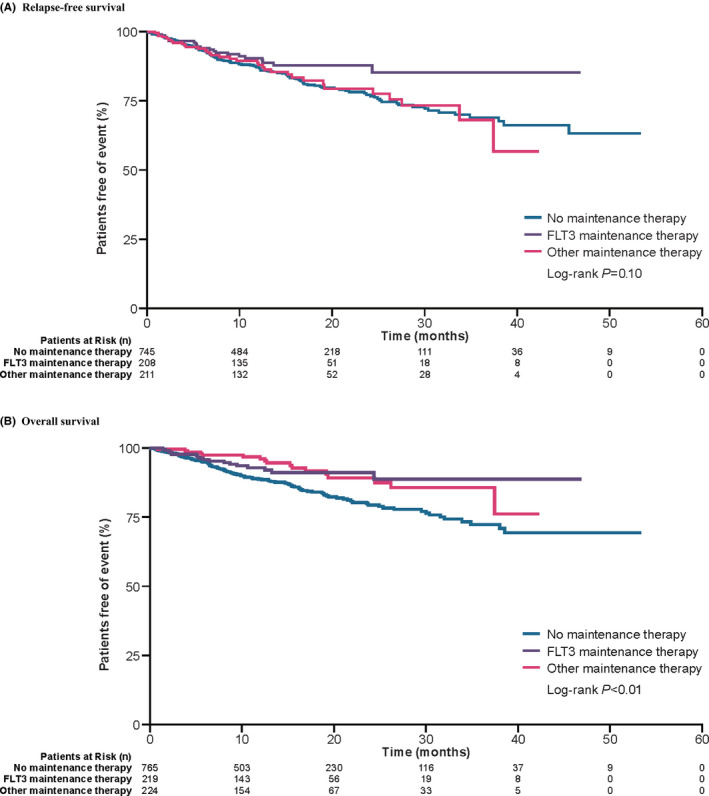
Relapse‐free and overall survival curves for patients post‐HSCT receiving FLT3 maintenance therapy, other maintenance therapy, or no maintenance therapy. A, Relapse‐free survival for the different types of maintenance therapies is estimated using Kaplan‐Meier curves. B, Overall survival for the different types of maintenance therapies is estimated using Kaplan‐Meier curves. Abbreviations: FLT3, Fms‐like tyrosine kinase 3; HSCT, hematopoietic stem cell transplantation
TABLE 4.
Cox regression analyses for relapse‐free survival and overall survival
| Relapse‐free survival | Overall survival | |||
|---|---|---|---|---|
| Unadjusted | Adjusted a | Unadjusted | Adjusted a | |
| FLT3 inhibitor vs none | ||||
| HR (95% CI) | 0.61 (0.39‐0.96) | 0.57 (0.34‐0.94) | 0.53 (0.32‐0.89) | 0.50 (0.28‐0.89) |
| P‐Value | <.05 | <.05 | <.05 | <.05 |
| Other vs none | ||||
| HR (95% CI) | 0.97 (0.67‐1.42) | 0.91 (0.55‐1.52) | 0.50 (0.30‐0.84) | 0.46 (0.23‐0.91) |
| P‐Value | .88 | .72 | <.01 | <.05 |
Abbreviations: CI, confidence interval; FLT3, Fms‐like tyrosine kinase 3; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation.
Adjusted for age at index date, sex, race, country, time from diagnosis to index date, body mass index, Eastern Cooperative Oncology Group grade, measurable residual disease status, extramedullary involvement, risk status, and HSCT type.
Patients on any type of maintenance therapy were found to have longer OS compared with patients not on any maintenance therapy post‐HSCT (log‐rank P < .01) (Figure 2B). Results from Cox regression models were consistent with those seen in the Kaplan‐Meier analysis. When comparing individual maintenance therapies, the risk of death was lower in the FLT3 inhibitor cohort (adjusted HR 0.50 [95% CI 0.28‐0.89], P < .05) and the other maintenance therapy cohort (adjusted HR 0.46 [95% CI 0.23‐0.91], P < .05) compared with the no maintenance therapy cohort (Table 4).
Additional analyses were conducted to determine the impact of the following factors on survival: use of FLT3 inhibitors prior to HSCT, FLT3 mutation type, allelic frequency, specific FLT3 inhibitor uses, and transplant type. Results of the additional analyses indicate that most of these factors did not significantly affect the post‐HSCT OS and RFS among patients who used FLT3 inhibitors for maintenance therapy except for patients receiving allogeneic HSCT and patients with an allelic frequency ≥0.5. A sensitivity analysis with FLT3 inhibitor use in induction therapy as an additional covariate in the adjusted Cox regression models showed no impact on OS and RFS.
4. DISCUSSION
Best practices and recommendations from treatment guidelines on maintenance therapy after HSCT are limited. The AML treatment guidelines do not address routine maintenance strategies. 7 , 21 The European Society for Medical Oncology recognizes several therapeutic options and acknowledges that results from larger studies are needed 6 ; meanwhile, for patients with FLT3‐ITD AML after allogeneic HSCT, the European Society of Blood and Marrow Transplantation recommends post‐transplant FLT3 inhibitor maintenance therapy for a minimum of 2 years. 8 Several small or retrospective studies have identified potential benefits of post‐HSCT maintenance therapy with various FLT3 inhibitors. 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 A recently published phase 2 study evaluating sorafenib found promising results, 30 and larger studies, including phase 3 trials evaluating gilteritinib (EudraCT 2016‐001061‐83, NCT02997202), are ongoing.
In addition to the scarcity of best practice recommendations, real‐world data on the use and outcomes associated with maintenance therapies after transplant for patients with FLT3‐mutated AML are also lacking. Despite the high risk of adverse clinical outcomes after transplantation, including disease progression and relapse, 31 the potential benefits of using maintenance therapies to prolong remission and survival remain to be determined in ongoing studies. In this retrospective chart review, we found that the approaches to post‐HSCT maintenance therapy in patients with FLT3 mut+ AML across various countries were heterogeneous. About 37% of patients received any post‐HSCT maintenance therapy, and only about half of those patients (18% of all patients) received a FLT3 tyrosine kinase inhibitor. It is important to note that the choice of maintenance therapy reported in this study—particularly FLT3 inhibitor therapy—was likely influenced by the availability of evidence for FLT3‐mutated AML in addition to the accessibility to the general population (including through market availability and/or insurance coverage) of the agent in different countries. 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 To date, gilteritinib, midostaurin, and sorafenib have approval in multiple countries, whereas quizartinib is only approved in Japan. 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 , 45
Substantial or systematic differences in HRU for patients receiving maintenance therapies were not observed. In this study, the use of hospitals, emergency departments, and several other resources was similar across cohorts and did not exceed the use observed in the patients not receiving any maintenance therapy. Only palliative care use was significantly greater in patients on other non‐FLT3 inhibitor maintenance therapies compared with no maintenance therapy. Thus, it should not be expected that patients would require additional healthcare resources to support maintenance therapies, though further study would assist in clarifying resource utilization and costs. Relapsed and refractory episodes have been associated with increased HRU 46 and high healthcare costs, particularly related to hospitalization. 19 , 20 , 47 Lessening the economic burden associated with relapsed and refractory episodes is therefore a secondary advantage to the clinical benefits for patients of achieving and maintaining CR.
Although the rate of AML‐related HRU was similar, clinical outcomes were improved in patients who were given any maintenance therapy compared with those not receiving any maintenance therapy. Of note, the use of FLT3 inhibitors prior to HSCT in some patients could have influenced the physicians’ decision to continue treatment post‐HSCT and the subsequent outcomes. However, the finding of improved survival in patients with FLT3‐mutated AML receiving post‐HSCT maintenance therapy is encouraging and consistent with other studies. Notably, patients receiving FLT3 inhibitors as maintenance therapy had a lower hazard of relapse and/or death compared with patients not receiving any maintenance therapy. In small or retrospective comparative studies of maintenance therapy, OS rates were improved in patients receiving sorafenib 25 , 27 and midostaurin 28 compared with those not receiving FLT3 inhibitor maintenance therapy. In a recently published phase 2, randomized, double‐blind study of post‐HSCT patients with FLT3‐ITD–positive AML, sorafenib maintenance therapy was found to reduce the risk of relapse and death compared with placebo (ie, no maintenance therapy). 30 As mentioned previously, studies evaluating the safety and efficacy of several different agents (including gilteritinib, sorafenib, and crenolanib) as maintenance therapies in patients with FLT3‐mutated AML post‐transplant have recently been completed or are ongoing.
As a retrospective chart review, this study does have several limitations. First, only data available and accessible to physicians could be abstracted, and, as a result, HRU may be underestimated if patients received care elsewhere or if care was not documented. Additionally, the accuracy of physician‐determined risk status or sensitivity of measurable residual disease is difficult to confirm in this type of study even though all physicians were hematologists and oncologists with multiple years of practice. Survivor bias may exist in these data wherein physicians selected patients that survived rather than died. To mitigate potential bias, physicians were specifically informed to consider patients that were both alive or deceased and to avoid selecting patients based on treatment response. A randomization scheme was also used to select patients. The finding of improvements in OS but not RFS could point to differences in non‐relapse mortality, which may indicate a selection bias based upon post‐transplant fitness and/or transplant‐related toxicity. Finally, potential confounders, such as differences in proportions of patients that received autologous versus allogeneic HSCT (although regression models for OS and RFS were adjusted for HSCT type) or broad variability in maintenance treatments among patients, as well as selection bias, wherein certain patients are more likely to be prescribed maintenance therapy by their providers, may be inherent to a retrospective study. Additional analyses conducted to explore potential impacts show a minimal effect of the use of FLT3 inhibitor prior to HSCT, type of FLT3 inhibitor, FLT3 mutation, allelic frequency, and type of HSCT, although many cohorts had relatively smaller sample sizes and thus lower statistical power. The findings of our study may help providers identify current global practices and generate data or hypotheses for future investigation.
5. CONCLUSIONS
Using data from medical charts of patients with FLT3‐mutated AML in multiple countries, we observed that the use of post‐HSCT maintenance therapy, including FLT3 inhibitors, was heterogeneous, and its use may significantly reduce the risk of clinical events without substantial increases in HRU. The results of this study support continued evaluation of post‐HSCT maintenance therapies to prevent relapse and prolong survival for patients with AML and FLT3 mutations.
CONFLICT OF INTEREST
JDG has received consulting revenue from Astellas, Daiichi‐Sankyo, and Novartis; research funding from Novartis; and postmarket royalties from Novartis through the Dana‐Farber Cancer Institute. YS, HY, and JF are employees of Analysis Group, which received consulting fees from Astellas. MVS is an employee of Astellas.
AUTHOR CONTRIBUTIONS
All authors were involved in the conception of the study design; and contributed to the acquisition, analysis, and interpretation of the study data. All authors participated in the drafting of the manuscript, provided the final approval of the submitted version, and agree to be held accountable for all aspects of the work.
Supporting information
Tables S1‐S4
ACKNOWLEDGEMENTS
Medical writing/editorial support was provided by Stephanie Phan, PharmD, and Elizabeth Hermans, PhD, from Peloton Advantage, LLC, an OPEN Health company (Parsippany, NJ), and funded by the study sponsor.
Griffin JD, Song Y, Yang H, Freimark J, Shah MV. Post‐transplant maintenance therapy in patients with FLT3‐mutated acute myeloid leukemia: Real‐world treatment patterns and outcomes. Eur J Haematol. 2021;107:553–565. 10.1111/ejh.13692
Funding information
Astellas Pharma, Inc
Novelty Statements
This work describes the real‐world use of different maintenance therapies after transplantation, which is important given the lack of standard guidelines/recommendations for strategies to manage patients with FLT3‐mutated acute myeloid leukemia (AML) with complete remission after first‐line chemotherapy and transplantation. The real‐world use of maintenance therapy was strikingly heterogeneous; the use of any type of maintenance therapy appeared beneficial for improving clinical outcomes compared with no maintenance therapy. Overall, maintenance therapy to prevent relapse significantly improved survival without substantially increasing healthcare resource use in patients with FLT3‐mutated AML achieving complete remission with first‐line chemotherapy followed by hematopoietic stem cell transplantation.
DATA AVAILABILITY STATEMENT
Researchers may request access to anonymized participant‐level data, trial‐level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study‐Sponsors/Study‐Sponsors‐Astellas.aspx
REFERENCES
- 1. De Kouchkovsky I, Abdul‐Hay M. ‘Acute myeloid leukemia: a comprehensive review and 2016 update’. Blood Cancer J. 2016;6(7):e441. Epub 2016/07/02. 10.1038/bcj.2016.50 PubMed PMID: 27367478; PubMed Central PMCID: PMCPMC5030376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381(9865):484‐495. 10.1016/s0140-6736(12)61727-9 [DOI] [PubMed] [Google Scholar]
- 3. Ravandi F, Kantarjian H, Faderl S, et al. Outcome of patients with FLT3‐mutated acute myeloid leukemia in first relapse. Leuk Res. 2010;34(6):752‐756. Epub 2009/11/03. 10.1016/j.leukres.2009.10.001 PubMed PMID: 19878996; PubMed Central PMCID: PMCPMC4082769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moreno I, Martin G, Bolufer P, et al. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. 2003;88(1):19‐24. Epub 2003/01/29. PubMed PMID: 12551822. [PubMed] [Google Scholar]
- 5. Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33(2):299‐312. Epub 2019/01/18. 10.1038/s41375-018-0357-9 PubMed PMID: 30651634; PubMed Central PMCID: PMCPMC6365380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heuser M, Ofran Y, Boissel N, et al. Acute myeloid leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2020;31(6):697‐712. Epub 2020/03/17. 10.1016/j.annonc.2020.02.018 PubMed PMID: 32171751. [DOI] [PubMed] [Google Scholar]
- 7. Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424‐447. Epub 2016/11/30. 10.1182/blood-2016-08-733196 PubMed PMID: 27895058; PubMed Central PMCID: PMCPMC5291965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bazarbachi A, Bug G, Baron F, et al. Clinical practice recommendation on hematopoietic stem cell transplantation for acute myeloid leukemia patients with FLT3‐internal tandem duplication: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2020;105(6):1507‐1516. Epub 2020/04/04. 10.3324/haematol.2019.243410 PubMed PMID: 32241850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brunet S, Labopin M, Esteve J, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30(7):735‐741. Epub 2012/02/01. 10.1200/JCO.2011.36.9868 PubMed PMID: 22291086. [DOI] [PubMed] [Google Scholar]
- 10. Li GX, Wang L, Yaghmour B, Ramsingh G, Yaghmour G. The role of FLT3 inhibitors as maintenance therapy following hematopoietic stem cell transplant. Leuk Res Rep. 2018;10:26‐36. Epub 2018/08/17. 10.1016/j.lrr.2018.06.003 PubMed PMID: 30112274; PubMed Central PMCID: PMCPMC6092446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bazarbachi AH, Al Hamed R, Malard F, Mohty M, Bazarbachi A. Allogeneic transplant for FLT3‐ITD mutated AML: a focus on FLT3 inhibitors before, during, and after transplant. Ther Adv Hematol. 2019;10:2040620719882666. Epub 2019/11/09. 10.1177/2040620719882666 PubMed PMID: 31700594; PubMed Central PMCID: PMCPMC6826920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song Y, Magenau J, Li Y, et al. FLT3 mutational status is an independent risk factor for adverse outcomes after allogeneic transplantation in AML. Bone Marrow Transplant. 2016;51(4):511‐520. Epub 2015/07/21. 10.1038/bmt.2015.170 PubMed PMID: 26191952; PubMed Central PMCID: PMCPMC4720584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Griffin PT, Komrokji RS, De Castro CM, et al. A multicenter, phase II study of maintenance azacitidine in older patients with acute myeloid leukemia in complete remission after induction chemotherapy. Am J Hematol. 2015;90(9):796‐799. Epub 2015/06/20. 10.1002/ajh.24087 PubMed PMID: 26089240. [DOI] [PubMed] [Google Scholar]
- 14. Pusic I, Choi J, Fiala MA, et al. Maintenance therapy with decitabine after allogeneic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2015;21(10):1761‐1769. Epub 2015/06/10. 10.1016/j.bbmt.2015.05.026 PubMed PMID: 26055299; PubMed Central PMCID: PMCPMC4568135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wei A, Tan P, Perruzza S, et al. Maintenance lenalidomide in combination with 5‐azacitidine as post‐remission therapy for acute myeloid leukaemia. Br J Haematol. 2015;169(2):199‐210. Epub 2015/02/04. 10.1111/bjh.13281 PubMed PMID: 25643589. [DOI] [PubMed] [Google Scholar]
- 16. Huls G, Chitu DA, Havelange V, et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457‐1464. Epub 2019/01/12. 10.1182/blood-2018-10-879866 PubMed PMID: 30630862. [DOI] [PubMed] [Google Scholar]
- 17. Rashidi A, Walter RB, Tallman MS, Appelbaum FR, DiPersio JF. Maintenance therapy in acute myeloid leukemia: an evidence‐based review of randomized trials. Blood. 2016;128(6):763‐773. Epub 2016/06/30. 10.1182/blood-2016-03-674127 PubMed PMID: 27354720; PubMed Central PMCID: PMCPMC4982451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crawford R, Sully K, Conroy R, et al. Patient‐centered insights on treatment decision making and living with acute myeloid leukemia and other hematologic cancers. Patient. 2020;13(1):83‐102. Epub 2019/08/29. 10.1007/s40271-019-00384-9 PubMed PMID: 31456136; PubMed Central PMCID: PMCPMC6957575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pandya BJ, Chen CC, Medeiros BC, et al. Economic and clinical burden of relapsed and/or refractory active treatment episodes in patients with acute myeloid leukemia (AML) in the USA: a retrospective analysis of a commercial payer database. Adv Ther. 2019;36(8):1922‐1935. Epub 2019/06/22. 10.1007/s12325-019-01003-7 PubMed PMID: 31222713; PubMed Central PMCID: PMCPMC6822861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pandya BJ, Chen CC, Medeiros BC, et al. Economic and clinical burden of acute myeloid leukemia episodes of care in the United States: a retrospective analysis of a commercial payer database. J Manag Care Spec Pharm. 2020;26(7):849‐859. Epub 2020/04/14. 10.18553/jmcp.2020.19220 PubMed PMID: 32281456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Japan Society of Hematology . Hematopoietic tumors clinical practice guidelines, 2018 edition. Chapter 1 Leukemia. 2018: Available from: http://www.jshem.or.jp/gui‐hemali/1_1.html Accessed June 11, 2020.
- 22. Battipaglia G, Ruggeri A, Massoud R, et al. Efficacy and feasibility of sorafenib as a maintenance agent after allogeneic hematopoietic stem cell transplantation for Fms‐like tyrosine kinase 3‐mutated acute myeloid leukemia. Cancer. 2017;123(15):2867‐2874. Epub 2017/04/08. 10.1002/cncr.30680 PubMed PMID: 28387928. [DOI] [PubMed] [Google Scholar]
- 23. Xuan L, Wang Y, Huang F, et al. Effect of sorafenib on the outcomes of patients with FLT3‐ITD acute myeloid leukemia undergoing allogeneic hematopoietic stem cell transplantation. Cancer. 2018;124(9):1954‐1963. Epub 2018/03/07. 10.1002/cncr.31295 PubMed PMID: 29509276. [DOI] [PubMed] [Google Scholar]
- 24. Chen YB, Li S, Lane AA, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for Fms‐like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Marrow Transplant. 2014;20(12):2042‐2048. Epub 2014/09/23. 10.1016/j.bbmt.2014.09.007 PubMed PMID: 25239228; PubMed Central PMCID: PMCPMC4253683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brunner AM, Li S, Fathi AT, et al. Haematopoietic cell transplantation with and without sorafenib maintenance for patients with FLT3‐ITD acute myeloid leukaemia in first complete remission. Br J Haematol. 2016;175(3):496‐504. Epub 2016/10/28. 10.1111/bjh.14260 PubMed PMID: 27434660; PubMed Central PMCID: PMCPMC5083189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Battipaglia G, Massoud R, Ahmed SO, et al. Efficacy and feasibility of sorafenib as a maintenance agent after allogeneic hematopoietic stem cell transplantation for Fms‐like tyrosine kinase 3 mutated acute myeloid leukemia: an update. Clin Lymphoma Myeloma Leuk. 2019;19(8):506‐508. Epub 2019/05/28. 10.1016/j.clml.2019.04.004 PubMed PMID: 31122828. [DOI] [PubMed] [Google Scholar]
- 27. Bazarbachi A, Labopin M, Battipaglia G, et al. Allogeneic stem cell transplantation for FLT3‐mutated acute myeloid leukemia: in vivo T‐cell depletion and posttransplant sorafenib maintenance improve survival. A retrospective Acute Leukemia Working Party‐European Society for blood and marrow transplant study. Clin Hematol Int. 2019;1(1):58‐74. 10.2991/chi.d.190310.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schlenk RF, Weber D, Fiedler W, et al. Midostaurin added to chemotherapy and continued single‐agent maintenance therapy in acute myeloid leukemia with FLT3‐ITD. Blood. 2019;133(8):840‐851. Epub 2018/12/20. 10.1182/blood-2018-08-869453 PubMed PMID: 30563875. [DOI] [PubMed] [Google Scholar]
- 29. Sandmaier BM, Khaled S, Oran B, Gammon G, Trone D, Frankfurt O. Results of a phase 1 study of quizartinib as maintenance therapy in subjects with acute myeloid leukemia in remission following allogeneic hematopoietic stem cell transplant. Am J Hematol. 2018;93(2):222‐231. Epub 2017/11/02. 10.1002/ajh.24959 PubMed PMID: 29090473; PubMed Central PMCID: PMCPMC6585789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burchert A, Bug G, Fritz LV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3‐internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020;38(26):2993‐3002. Epub 2020/07/17. 10.1200/JCO.19.03345 PubMed PMID: 32673171. [DOI] [PubMed] [Google Scholar]
- 31. Rautenberg C, Germing U, Haas R, Kobbe G, Schroeder T. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation: prevention, detection, and treatment. Int J Mol Sci. 2019;20(1):228. Epub 2019/01/11. 10.3390/ijms20010228 PubMed PMID: 30626126; PubMed Central PMCID: PMCPMC6337734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Center for Drug Evaluation and Research . Application number: 211349Orig1s000 approval letter. [updated December 20, 2005]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/211349Orig1s000Approv.pdf Accessed June 26, 2020.
- 33. European Medicines Agency . Xospata [updated August 11, 2019]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/xospata Accessed June 26, 2020.
- 34. Astellas announces approval in Japan for Xospata 40 mg tablets for the treatment of FLT3mut+ relapsed or refractory AML [updated September 21, 2018]. Available from: https://www.astellas.com/system/files/news/2018‐09/180921_3_En.pdf Accessed June 26, 2020.
- 35. Daiichi Sankyo's VANFLYTA receives approval in Japan for the treatment of relapsed/refractory FLT3‐ITD AML [updated June 18, 2019]. Available from: https://www.daiichisankyo.com/media_investors/media_relations/press_releases/detail/007030.html Accessed June 26, 2020.
- 36. European Medicines Agency . Nexavar [updated June 18, 2014]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/nexavar Accessed July 6, 2020.
- 37. Center for Drug Evaluation and Research Approval Package For: Application Number NDA 21‐923, Approval Letter(s) [updated November 28, 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021923_s000_Nexavar_Approv.pdf Accessed July 6, 2020.
- 38. Summary basis of decision ‐ Nexavar ‐ Health Canada [updated April 15, 2019]. Available from: https://hpr‐rps.hres.ca/reg‐content/summary‐basis‐decision‐detailOne.php?linkID=SBD00101 Accessed July 6, 2020.
- 39. Nexavar approved for the treatment of kidney cancer in Japan [updated January 28, 2008]. Available from: https://www.investor.bayer.com/securedl/8815 Accessed July 6, 2020.
- 40. Department of Health and Human Services, Food and Drug Administration . NDA Approval: NDA 207997 Original #1, NDA 207997 Original #2 [updated April 28, 2017]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/207997Orig1s000ltr.pdf Accessed July 6, 2020.
- 41. Regulatory decision summary ‐ Rydapt ‐ Health Canada [updated April 15, 2019]. Available from: https://hpr‐rps.hres.ca/reg‐content/regulatory‐decision‐summary‐detail.php?lang=en&linkID=RDS00279 Accessed July 6, 2020.
- 42. European Medicines Agency . Rydapt [updated October 25, 2017]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/rydapt Accessed July 6, 2020.
- 43. Astellas announces acceptance of XOSPATA® (gilteritinib) for regulatory review in China by the National Medical Products Administration [updated April 10, 2020]. Available from: https://www.astellas.com/system/files/news/2020‐04/20200410_en_1.pdf Accessed September 14, 2020.
- 44. Daiichi Sankyo launches FLT3 inhibitor VANFLYTA® in Japan for the treatment of patients with relapsed/refractory FLT3‐ITD AML [updated October 10, 2019]. Available from: https://www.daiichisankyo.com/media_investors/media_relations/press_releases/detail/007063.html Accessed September 14, 2020.
- 45. Novartis receives FDA approval for Rydapt® in newly diagnosed FLT3‐mutated acute myeloid leukemia (AML) and three types of systemic mastocytosis (SM) [updated April 28, 2017]. Available from: https://www.novartis.com/news/media‐releases/novartis‐receives‐fda‐approval‐rydapt‐newly‐diagnosed‐flt3‐mutated‐acute‐myeloid‐leukemia‐aml‐and‐three‐types‐systemic‐mastocytosis‐sm Accessed October 2, 2020.
- 46. Griffin JD, Yang H, Song Y, Kinrich D, Shah MV, Bui CN. Treatment patterns and healthcare resource utilization in patients with FLT3‐mutated and wild‐type acute myeloid leukemia: a medical chart study. Eur J Haematol. 2019;102(4):341‐350. Epub 2018/12/24. 10.1111/ejh.13205 PubMed PMID: 30578743; PubMed Central PMCID: PMCPMC6850763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mahmoud D, Skikne BS, Kucmin‐Bemelmans I, Alleman C, Hensen M. Overall economic burden of total treatment costs in acute myeloid leukemia throughout the course of the disease. Blood. 2012;120(21):3614. 10.1182/blood.V120.21.3614.3614 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S4
Data Availability Statement
Researchers may request access to anonymized participant‐level data, trial‐level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study‐Sponsors/Study‐Sponsors‐Astellas.aspx


