Abstract
In Western populations, the incidence of oesophageal squamous cell carcinoma (OSCC) has been declining, whereas the incidence of oesophageal adenocarcinoma (OAC) has been increasing. Our study examines temporal trends in the incidence of oesophageal cancer in the Netherlands between 1989 and 2016, in addition to predicting future trends through 2041. Data from the Netherlands Cancer Registry and Statistics Netherlands were collected to obtain incidence trends of OSCC and OAC for the period 1989 to 2016. Age‐period‐cohort (APC) modelling was used to estimate the contribution of age, calendar period and birth cohort on the observed incidence trends. To predict the future numbers of new cases of both OSCC and OAC from 2017 to 2041, log‐linear APC models were fitted to the trends of 1989 to 2016. The age‐standardised incidence rates of OSCC have decreased slightly for men and increased slightly for women. In contrast, a marked increase in the incidence of OAC was observed, ranging from 2.8 per 100 000 persons in 1989 to 10.1 in 2016. This increase in OAC incidence was more prominent in men, and it will result in an increased risk of OAC for successive generations. Future projections indicate that the incidence of OAC will further increase to 13.1 per 100 000 persons in 2037 to 2041, meaning that there will be 13 259 cases of OAC in 2037 to 2041, as compared to 9386 diagnoses in 2017 to 2021. The changing epidemiologic trends in oesophageal cancer in the Netherlands should be reflected in the development of prevention, early detection and treatment strategies.
Keywords: age‐period‐cohort modelling, incidence, oesophageal adenocarcinoma, oesophageal squamous cell carcinoma, trends
Short abstract
What's new?
The incidence of esophageal adenocarcinoma (OAC) is on the rise in Western countries. Better understanding of this trend could facilitate critical improvements in OAC prevention, early detection, and treatment strategies. Here, the authors investigated trends in OAC incidence from 1989 to 2016 for successive birth cohorts in the Netherlands. OAC incidence was found to have increased significantly since 1989, with risk rising most noticeably in men. Analyses through 2037‐2041 predict continued growth in OAC cases. The findings highlight the importance of promoting measures to prevent esophageal cancer, particularly those aimed at controlling modifiable risk factors, such as obesity and smoking.
Abbreviations
- AAPC
average annual percent change
- APC
age‐period‐cohort
- ASR
age‐standardised incidence rate
- BO
Barrett's oesophagus
- CI
confidence interval
- EAPC
estimated annual percent change
- ESP
European Standard Population
- GORD
gastro‐oesophageal reflux disease
- ICD‐O
International Classification of Diseases for Oncology
- IKNL
Netherlands Comprehensive Cancer Organisation
- Log
logarithmic
- NCR
Netherlands Cancer Registry
- OAC
oesophageal adenocarcinoma
- OSCC
oesophageal squamous cell carcinoma
- TNM
tumour‐nodes‐metastasis
- UICC
Union for International Cancer Control
1. INTRODUCTION
Oesophageal cancer is the eighth most commonly diagnosed cancer worldwide, and it is the sixth most common cause of cancer fatality. 1 The incidence of oesophageal cancer, and particularly oesophageal adenocarcinoma (OAC), has risen in Western populations in recent decades. By 2030, the Netherlands is projected to have the largest incidence rate of OAC of all countries worldwide. 2 The prognosis of oesophageal cancer is related mainly to stage at diagnosis. Unfortunately, oesophageal cancer is often diagnosed at a locally advanced or metastatic stage. With a 5‐year survival rate of 22% in the Netherlands, the prognosis of oesophageal cancer remains poor, despite improvements in neo‐adjuvant therapy and surgery. 3
The two most common histological subtypes of oesophageal cancer are oesophageal squamous cell carcinoma (OSCC) and adenocarcinoma (OAC). In most cases, OSCC arises in the epithelial cells in the lining of the oesophagus, and it is more common in males than it is in females. It is most prevalent in developing regions (eg, South‐East and Central Asia). In contrast, OAC occurs mostly in developed regions (eg, Western Europe and Northern America), and it usually develops in metaplastic intestinal metaplasia in the lower third of the oesophagus. Male predominance is even stronger for this subtype, with an overall male to female ratio of 4.4. 4 , 5
The main risk factors for OSCC are smoking and alcohol consumption. 6 Gastro‐oesophageal reflux disease (GORD), Barrett's oesophagus (BO) and obesity are the main factors associated with OAC. 7 , 8 , 9 Changes in the prevalence of these risk factors over time may affect the incidence trends in oesophageal cancer. The development of new diagnostic tools and treatment options for oesophageal cancer can also influence the incidence rates of oesophageal cancer over time. Age‐period‐cohort (APC) modelling can enhance understanding of incidence trends by disentangling age, calendar‐period and birth‐cohort effects. Age effects result from biological and social processes of ageing that are internal to individuals. Calendar‐period effects arise from variations in external factors that affect all ages equally at the same time (eg, changes in population exposures, diagnostic practices and disease classifications). In contrast, birth‐cohort effects result from experiences or exposures of a group of people as they move through life. They thus do not affect all ages equally (eg, changes in the prevalence of individual risk factors). 10 , 11 The understanding oesophageal cancer trends can be enhanced by APC models, which may also provide clues to the origins and development of oesophageal cancer for future research.
Knowledge about current and future trends in oesophageal cancer in the Netherlands is needed in order to enable the development of prevention, early detection and treatment strategies, which are crucial to improving survival and quality of life for patients. The trends may alert decision‐makers to the public‐health impact of oesophageal cancer and the urgency of prioritising resources in cancer services. 12 , 13
In our study, we describe the incidence of OSCC and OAC for men and women in the Netherlands between 1989 and 2016. We also assess the influence of age, birth cohort and period of diagnosis on these trends to identify factors underlying the changing trends. The combined age, period and cohort effects were used to calculate future trends in the incidence of OSCC and OAC in the Netherlands through 2041.
2. MATERIALS AND METHODS
2.1. Study population and population data
The study population consisted of all cases of oesophageal cancer that were newly diagnosed between 1989 and 2016 in the Netherlands. Data on the calendar year of diagnosis, age at diagnosis, sex, topography, morphology, degree of differentiation and tumour‐nodes‐metastasis (TNM) classification were provided by the Netherlands Comprehensive Cancer Organisation (IKNL), 14 which has been responsible for the Netherlands Cancer Registry (NCR) since 1989. Registration employees of IKNL extract cancer data from medical records in hospitals in the Netherlands, focusing on patient and tumour characteristics.
Topography and morphology were coded as C15 (malignant neoplasm of oesophagus), in accordance with the International Classification of Diseases for Oncology, third edition (ICD‐O‐3). 15 Morphology types were defined as follows: OSCCs: 8051 to 8052, 8070 to 8076, 8078, 8083 to 8084 and 8094; OACs: 8140 to 8141, 8144 to 8145, 8201, 8210 to 8211, 8230, 8255, 8260 to 8263, 8310, 8320, 8480 to 8481, 8490, 8570 to 8571, 8573 to 8574 and 8576. The morphological subtypes within the category of “total oesophageal cancer” include both OSCC and OAC, as well as other histological subtypes and cases with unspecified histology. In addition, tumours were staged according to the Union for International Cancer Control (UICC) TNM classification, which is an anatomically based classification that describes the extent of the primary tumour and the regional lymph‐node involvement, in addition to the presence or absence of distant metastases. Data through 1998 were coded according to the fourth edition of the UICC, 16 with data from 1999 to 2002 coded according to the fifth edition, 17 data from 2003 to 2009 according to the sixth edition 18 and data from 2010 to 2016 according to the seventh edition. 19 The corresponding population estimates for 1989 to 2016 and the population predictions for 2017 to 2041 were obtained from Statistics Netherlands, 20 by calendar year, age and sex. The population figures for each calendar year were based on the population structure of the Netherlands on 1 January.
2.2. Statistical analysis
2.2.1. Patient and tumour characteristics
Patient and tumour characteristics were summarised according to descriptive analyses. Categorical data were presented as frequencies and proportions, and continuous data were presented as medians and 25th and 75th percentiles. Data were stratified by sex and the two histological subgroups OSCC and OAC. For categorical variables, the frequencies refer to complete cases.
2.2.2. Trend analyses
Trend analyses were performed for the total number of oesophageal cancer cases and stratified by histological subtype for the total population, as well as for men and women separately. The age‐specific incidence rates across the years were calculated as the number of new patients per 100 000 person‐years, using 5‐year age groups (0‐4 to 95+). Age‐standardised incidence rates (ASRs) were calculated using the revised European Standard Population (ESP). 21 Microsoft Office Excel 2010 was used for calculating and visualising the ASRs.
In order to examine trends in ASRs and assess the significance of changes in these trends, joinpoint analyses were performed. 22 The Joinpoint Regression Program was used to estimate joinpoints at which a statistically significant change occurred in a trend. In the results, the estimated annual percent change (EAPC) in time segments before and after joinpoints and the average annual percent change (AAPC) over the entire period of 1989 to 2016 were used to depict the trends. P values less than .05 were regarded as statistically significant.
2.2.3. APC modelling
To estimate the effects of age, calendar period and birth cohort on time trends in the incidence of oesophageal cancer, age‐specific incidence rates of OSCC and OAC in various calendar periods and birth cohorts were visually displayed in Excel. The analyses were carried out using incidence rates of oesophageal cancer arranged in 5‐year age groups and 5‐year calendar periods. Data were classified into 10 age groups (from 40‐44 to 85‐89) and six calendar periods (from 1989‐1993 to 2014‐2016). The last calendar period (2014‐2016) was truncated to 3 years, as data on 2017 and 2018 were not yet available at the time of analysis. People younger than 40 years and older than 89 years at the date of diagnosis were excluded, as the incidence of oesophageal cancer in these groups was very low. Given the age and calendar year groups, 15 birth cohorts (born in 1900‐1908 to 1970‐1976) could be defined.
Following descriptive data analysis in Excel, APC modelling was performed to arrive at a quantitative description of the changing incidence of oesophageal cancer. Log‐linear Poisson regression models were fitted to estimate age, calendar‐period and birth‐cohort effects, based on the assumption that age‐specific incidence counts of oesophageal cancer followed a Poisson distribution. 23 Three different submodels (age, age‐period and age‐cohort) were fitted first, after which the full APC models were fitted. 24 The Genmod procedure in SAS version 9.2 was used to perform APC modelling.
2.2.4. Future predictions
To predict the future numbers of new cases and the ASRs of both squamous cell carcinoma and adenocarcinoma of the oesophagus from 2017 to 2041, log‐linear APC models were fitted to recent trends (1989‐2016). Corresponding population estimates and predictions for the period 2017 to 2041 were obtained from Statistics Netherlands, 20 by calendar year, age and sex. The APC models and future predictions were fitted in R, using the NORDPRED package. The four to six most recent 5‐year periods observed (depending on goodness of fit) were extrapolated using a power function to level off the growth, with a projection of the recent linear trend for the last 10 years, attenuated (or accentuated, in the case of negative trends) by 25%, 50%, 75% and 75% in the second, third, fourth and fifth periods, respectively. 25 , 26
3. RESULTS
3.1. Patient and tumour characteristics
Between 1989 and 2016, 40 691 cases of oesophageal carcinomas were diagnosed in the Netherlands. Of these cases, 14 709 were defined as OSCC (8734 in men; 5975 in women), and 23 771 were defined as OAC (18 986 in men; 4785 in women). Other histological subtypes and cases with unspecified histology were rare (only 2211 cases), accounting for only 5.4% of all oesophageal carcinomas. Median age at OSCC diagnosis was 66 years (25th‐75th percentile, 59‐73 years) for men and 69 years (60‐77 years) for women. Median age at OAC diagnosis was 68 years (60‐75 years) for men and 74 years (64‐82 years) for women. Most OACs were located in the lower oesophagus, while OSCCs were more evenly distributed throughout the oesophagus. More than 90% of the oesophageal carcinomas with known differentiation grade were diagnosed at differentiation grades 2 or 3. In addition, most oesophageal carcinomas were diagnosed in advanced TNM stages 3 or 4 (70% for men and 64% for women). Information on differentiation grade and TNM stages was not available for 14%‐36% of the oesophageal carcinoma diagnoses (Table 1). The TNM stadia for all oesophageal carcinomas diagnosed between 1989 and 2016 are displayed in Figure S1.
TABLE 1.
Patient and tumour characteristics according to histological subtype in men and women with oesophageal cancer diagnosed between 1989 and 2016 in the Netherlands
| Total | OSCC | OAC | ||||
|---|---|---|---|---|---|---|
| Male (n = 29 189) | Female (n = 11 502) | Male (n = 8734) | Female (n = 5975) | Male (n = 18 986) | Female (n = 4785) | |
| Age (years), median (25th‐75th percentiles) | 67 (59‐75) | 71 (62‐80) | 66 (59‐73) | 69 (60‐77) | 68 (60‐75) | 74 (64‐82) |
| Topography, n (%) | ||||||
| Oesophagus, upper third | 1299 (4.5) | 1057 (9.2) | 1079 (12.4) | 849 (14.2) | 140 (0.7) | 125 (2.6) |
| Oesophagus, middle third | 3887 (13.3) | 3344 (29.1) | 2737 (31.3) | 2486 (41.6) | 928 (4.9) | 656 (13.7) |
| Oesophagus, lower third | 21 449 (73.5) | 5783 (50.3) | 3714 (42.5) | 1840 (30.8) | 16 799 (88.5) | 3619 (75.6) |
| Other | 2554 (8.7) | 1318 (11.4) | 1204 (13.8) | 800 (13.4) | 1119 (5.9) | 385 (8.1) |
| Differentiation grade, n (%) | ||||||
| Grade 1: low grade | 1049 (3.6) | 411 (3.6) | 328 (3.8) | 232 (3.9) | 721 (3.8) | 179 (3.7) |
| Grade 2: intermediary | 7676 (26.3) | 3192 (27.8) | 2976 (34.1) | 1994 (33.4) | 4654 (24.5) | 1189 (24.8) |
| Grade 3: high grade | 9646 (33.0) | 3599 (31.3) | 2572 (29.5) | 1719 (28.8) | 6670 (35.1) | 1691 (35.5) |
| Grade 4: undifferentiated | 325 (1.1) | 123 (1.1) | 27 (0.3) | 10 (0.2) | 35 (0.2) | 8 (0.2) |
| N.A. | 10 493 (36.0) | 4177 (36.2) | 2831 (32.3) | 2020 (33.7) | 6906 (36.4) | 1718 (35.8) |
| TNM stage, n (%) | ||||||
| Stage 1 | 2470 (8.5) | 944 (8.2) | 436 (5.0) | 427 (7.1) | 1981 (10.4) | 491 (10.3) |
| Stage 2 | 5150 (17.6) | 2156 (18.7) | 1714 (19.6) | 1293 (21.6) | 3277 (17.3) | 792 (16.6) |
| Stage 3 | 7388 (25.3) | 2602 (22.6) | 2562 (29.3) | 1599 (26.8) | 4612 (24.3) | 915 (19.1) |
| Stage 4 | 10 013 (34.3) | 2895 (25.2) | 2459 (28.2) | 1268 (21.2) | 7001 (36.9) | 1434 (30.0) |
| N.A. | 4168 (14.3) | 2905 (25.3) | 1563 (17.9) | 1388 (23.3) | 2115 (11.1) | 1153 (24.0) |
Note: Categorical data are expressed as n (%) and continuous data are expressed as median and 25th and 75th percentiles (interquartile range).
Abbreviations: N.A., not available; OAC, oesophageal adenocarcinoma; OSCC, oesophageal squamous cell carcinoma; TNM, tumour‐nodes‐metastasis.
3.2. Trends in the incidence of oesophageal cancer, 1989 to 2016
The incidence of oesophageal cancer increased between 1989 and 2016 for all histological subgroups. The incidence of OSCC increased from 3116 in 1989 to 1995 to 4496 in 2010 to 2016, while the incidence of OAC increased from 2512 to 9985 in the same periods. The incidence for men was higher than the incidence for women for all histological subgroups (Table 2).
TABLE 2.
Trends in the incidence of oesophageal cancer, by histological subtype in men and women diagnosed between 1989 and 2016 and future predictions for 2017 to 2041 in the Netherlands
| Total | OSCC | OAC | ||||
|---|---|---|---|---|---|---|
| 1989‐2016 | Male (n = 29 189) | Female (n = 11 502) | Male (n = 8734) | Female (n = 5975) | Male (n = 18 986) | Female (n = 4785) |
| Incidence (calculated), 7‐year categories | ||||||
| Number of cases, n | ||||||
| 1989‐1995 | 3985 | 2034 | 1914 | 1202 | 1847 | 665 |
| 1996‐2002 | 5644 | 2430 | 2006 | 1295 | 3257 | 943 |
| 2003‐2009 | 8414 | 3140 | 2213 | 1583 | 5730 | 1344 |
| 2010‐2016 | 11 146 | 3898 | 2601 | 1895 | 8152 | 1833 |
| Total | OSCC | OAC | ||||
|---|---|---|---|---|---|---|
| 2017‐2041 | Male (n = 60 474) | Female (n = 22 210) | Male (n = 12 295) | Female (n = 10 786) | Male (n = 48 143) | Female (n = 10 816) |
| Incidence (predicted), 5‐year categories | ||||||
| Number of cases, n | ||||||
| 2017‐2021 | 9970 | 3516 | 2243 | 1762 | 7759 | 1627 |
| 2022‐2026 | 11 416 | 4068 | 2479 | 2030 | 9205 | 1912 |
| 2027‐2031 | 12 514 | 4561 | 2578 | 2233 | 10 189 | 2205 |
| 2032‐2036 | 13 108 | 4916 | 2487 | 2347 | 10 353 | 2450 |
| 2037‐2041 | 13 466 | 5149 | 2508 | 2414 | 10 637 | 2622 |
Abbreviations: OAC, oesophageal adenocarcinoma; OSCC, oesophageal squamous cell carcinoma.
The ASRs of oesophageal cancer per 100 000 person‐years are displayed in Figure 1. In men, the ASR of oesophageal cancer increased from 10.5 per 100 000 person‐years in 1989 to 23.9 in 2016, with an AAPC of 2.8% (95% confidence interval [CI] 2.4‐3.2). The increase in ASR was most prominent from 1989 to 2008, with an EAPC of 3.5% (95% CI 3.1‐4.0), and it attenuated after 2008 (EAPC of 1.0%, 95% CI 0.0‐2.0). In women, the ASR of oesophageal cancer also increased, albeit to a lesser extent: from 3.7 per 100 000 person‐years in 1989 to 7.5 in 2016, with an AAPC of 1.7% (95% CI 1.4‐2.1) (Figure 1A).
FIGURE 1.

Age‐standardised incidence rates for all oesophageal cancers combined (A) and OACs and OSCCs separately for men and women (B) in the Netherlands, 1989 to 2016 (log scale). Fitted dashed lines were obtained from joinpoint regression analyses; the black diamonds indicate the significant joinpoint. ESP, European Standard Population; OAC, oesophageal adenocarcinoma; OSCC, oesophageal squamous cell carcinoma [Color figure can be viewed at wileyonlinelibrary.com]
For OSCC in men, the ASR changed from 6.1 per 100 000 person‐years in 1989 to 5.1 in 2016 with an AAPC of −0.4% (95% CI −0.8 to −0.1). In women, the ASR of OSCC increased slightly from 2.1 per 100 000 person‐years in 1989 to 3.9 in 2016 with an AAPC of 0.9% (95% CI 0.5‐1.4). For OAC in men, the ASR increased from 4.9 per 100 000 person‐years in 1989 to 17.8 in 2016, with an AAPC of 5.0% (95% CI 4.4‐5.6) was observed. The ASR has started to level off, with the greatest increase in ASR from 1989 to 2005 (EAPC 6.8%; 95% CI 5.9‐7.7), with a lower ASR increase from 2005 to 2016 (EAPC 2.3%; 95% CI 1.5‐3.2). In women, the ASR of OAC also increased, albeit to a lesser extent: from 1.3 per 100 000 person‐years in 1989 to 3.4 in 2016, with an AAPC of 3.3% (95% CI 3.0‐3.7) (Figure 1B). Significant joinpoints were observed only in men for all types of oesophageal cancer (in 2008) and for OAC (in 2005) (Table S1). These results are depicted by the dashed black regression lines in Figure 1.
3.3. APC modelling in the incidence of oesophageal cancer, 1989 to 2016
The age‐specific incidence rates of OSCC in various calendar periods and birth cohorts are visualised in Figure 2A,B, respectively. Within all calendar periods and birth cohorts, the incidence rates of OSCC rose steeply with age. Incidence rates of OSCC did not exhibit any clear shift either over time or over successive generations. For OAC, the incidence rates were higher for older age groups than they were for younger age groups in all calendar periods and birth cohorts (Figure 2C,D). In contrast to OSCC, the incidence rates of OAC did reveal a clear shift over time, as well as over successive generations. Incidence rates were higher in recent calendar periods than they were in earlier calendar periods, and they were higher for younger generations than they were for older generations, as indicated by the increasing trend.
FIGURE 2.

Age‐specific incidence rates of OSCC and OAC for men and women combined, Netherlands 1989 to 2016 (log scale). (A) Age‐specific incidence rates of OSCC in various calendar periods. (B) Age‐specific incidence rates of OSCC in various birth cohorts. (C) Age‐specific incidence rates of OAC in various calendar periods. (D) Age‐specific incidence rates of OAC in various birth cohorts. OAC, oesophageal adenocarcinoma; OSCC, oesophageal squamous cell carcinoma [Color figure can be viewed at wileyonlinelibrary.com]
The calendar‐period and birth‐cohort trends in the incidence of OSCC and OAC for men and women are displayed in Figure 3. For OSCC, the calendar‐period effect was not quantitatively relevant for either men or women (Figure 3A,B). The birth‐cohort effect showed a relatively stable pattern in the risk of OSCC for both men and women born between 1904 and 1944. For men and women born after 1944, the risk of OSCC decreased. The risk of OSCC declined more rapidly for men than it did for women.
FIGURE 3.
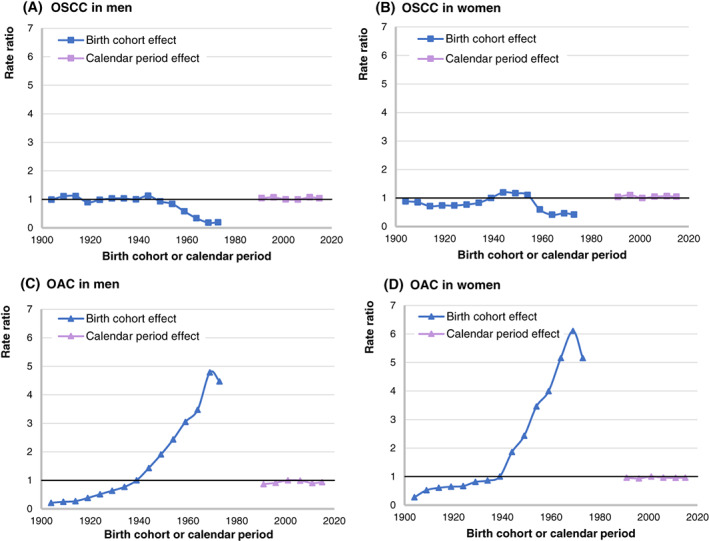
Calendar‐period trend (1989‐2016) and birth‐cohort trend (1900‐1976) in the incidence of OSCC and OAC in the Netherlands, expressed as age‐adjusted risk relative to the diagnosis period 1999 to 2003 and birth cohorts 1935 to 1943. Calendar periods and birth cohorts are denoted by the central year. (A) Calendar‐period and birth‐cohort trends for OSCC in men. (B) Calendar‐period and birth‐cohort trends for OSCC in women. (C) Calendar‐period and birth‐cohort trends for OAC in men. (D) Calendar‐period and birth‐cohort trends for OAC in women. OAC, oesophageal adenocarcinoma; OSCC, oesophageal squamous cell carcinoma [Color figure can be viewed at wileyonlinelibrary.com]
The calendar‐period effect for OAC was also not quantitatively relevant for either men or women (Figure 3C,D). The birth‐cohort effect showed a continuous increase in the risk of OAC for both men and women born between 1904 and 1969. The relative risk of OAC for men born around 1969 was more than 400% that of men born around 1939. The relative risk of OAC for women born around 1969 was more than 600% that of women born around 1939. A drop in the risk of OAC was observed for both men and women born after 1969.
3.4. Future trends in the incidence of oesophageal cancer, 2015 to 2040
The predicted incidence of oesophageal cancer through 2041 is presented in 5‐year categories in the lower part of Table 2. For men, the total incidence of oesophageal cancer is expected to increase to from 9970 diagnoses in 2017 to 2021 to 13 466 in 2037 to 2041. For women, these figures are 3516 and 5149, respectively. The incidence of OSCC is expected to level off for men, while a further increase is expected for women. The increase in the incidence of oesophageal cancer for men will be caused mainly by the expected increase in the incidence of OAC (10 637 in 2037‐2041). For women, the incidence of OAC is expected to increase from 1627 in 2017 to 2021 to 2622 in 2037 to 2041.
Future predictions for the age‐standardised incidence of OSCC and OAC are presented in Figure 4 for the total population (A) and stratified by sex (B). For OSCC, the incidence is expected to stay more or less stable, with an ASR of 4.8 per 100 000 person‐years in 2014 to 2016 and 4.7 predicted for 2037 to 2041. For OAC, the ASR is expected to increase from 10.7 per 100 000 person‐years in 2014 to 2016 to 13.1 in 2037 to 2041 (Figure 4A). The highest incidence rates are expected for OAC in men, with an expected increase from 18.8 per 100 000 in 2014 to 2016 to 24.4 in 2037 to 2041. For women, the ASR for OSCC and OAC are expected to increase only slightly in the coming decades, with OAC rates ranging from 3.7 in 2014 to 2016 to 4.6 in 2037 to 2041 (Figure 4B).
FIGURE 4.

Calculated (1989‐2016) and predicted (2016‐2041) and predicted age‐standardised incidence rates for OAC and OSCC in the Netherlands (log scale). (A) Age‐standardised incidence rates for OAC and OSCC for men and women combined. (B) Age‐standardised incidence rates for OAC and OSCC for men and women separately. ESP, European Standard Population; OAC, oesophageal adenocarcinoma; OSCC, oesophageal squamous cell carcinoma [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Drawing on 30 years of cancer registration in the Netherlands, the results of the present study indicate that the incidence of oesophageal cancer increased during the period 1989 to 2016 for the two main histological subtypes of oesophageal cancer, OSCC and OAC, with the highest proportion of all oesophageal cancer having been diagnosed between 2010 and 2016. The ASRs of OSCC decreased slightly for men but increased slightly for women. In addition, a steep increase in the incidence of OAC was observed, ranging from 2.8 per 100 000 persons in 1989 to 10.1 in 2016. This increase was more prominent in men than it was in women, and it resulted in an increased risk of OAC for successive generations. Future predictions estimate that the incidence of OAC will increase further to 13.1 per 100 000 persons in 2041. In other words, 13 259 cases of OAC are predicted for the period 2037 to 2041, as compared to the 9386 diagnoses predicted for the period 2017 to 2021.
4.1. Comparisons with other studies
The gradually declining and stabilising incidence rates for OSCC found for men in the present study have previously been reported for the Netherlands, as well as for other Western countries, including Sweden and the United States. Moreover, declining or stabilising incidence rates for OSCC have also been frequently reported in women. 12 , 27 , 28 , 29 , 30 These results are in contrast to the slightly increasing OSCC incidence rates for women in the Netherlands, as found in the present study and as also reported previously by Wang et al. 31 The steep increase in OAC incidence rates found in the present study have also been reported in other Western studies, with more prominently higher increases in the incidence of OAC in men than in women. Furthermore, some studies have reported that these increasing incidence rates have seemed to level off in more recent years, especially for men. 12 , 27 , 28 , 29 , 32 , 33 These findings are also in line with those of the present study, which indicate a slowing of the rate of increase in the incidence of OAC in men after 2005. According to the future predictions in the present study, however, a further increase can be expected in the number of OAC diagnoses in the Netherlands in the coming decades.
Consistent with the present study, birth‐cohort effects have previously been identified as major determinants of trends in the incidence of OAC. 33 In contrast, Edgren et al identify calendar‐period effects rather than birth‐cohort effects as the strongest drivers of OAC incidence rates. 32 The differences in reported APC results may reflect regional differences in exposure to risk factors and/or differences in the methodological designs used in the various studies.
4.2. Explanation of the observed trends
As indicated by the results of the present study, the changing incidence rates of OSCC and OAC are a complex function of age, calendar period and birth cohort, with several factors operating dynamically on the population. The observed predominance of birth‐cohort effects over calendar‐period effects identified in our study contradict the notion that the changes observed in OSCC and OAC incidence rates are the result of changes in population exposure, diagnostic practices and/or disease classifications, as these factors would have affected all age groups equally. In addition, the predominance of birth‐cohort effects and the differing changes in incidence according to histological subtype point to changes in the prevalence of individual risk factors for OSCC and OAC, which differ across generations.
4.2.1. Risk factors in relation to the declining and stabilising risk of OSCC
Tobacco smoking and moderate‐to‐heavy alcohol consumption are the two strongest risk factors for OSCC, particularly in combination, as they act synergistically. 6 , 34 Both may cause chronic irritation and inflammation of the oesophageal mucosa. 35 In an Australian case‐control study, the total fraction of OSCC attributable to smoking and alcohol consumption combined was 58%, and it was substantially higher for men than it was for women (78% vs 38%). 36 Given the increased risk of OSCC associated with smoking and alcohol consumption, changes in the prevalence of these factors may explain the changes observed in the trends for OSCC incidence. The percentage of men and women smokers in the Netherlands has decreased from over 42% for men and 29% for women in 1990 to 27% and 19%, respectively, in 2017. 37 Alcohol consumption decreased as well, although less pronounced compared to smoking. 38 Taken together, these trends thus suggest that the decreased incidence of OSCC in men is largely due to a reduction in smoking. No overall decline in the risk of OSCC was observed for women, although this risk was slightly less for women in the most recent birth cohorts. Differences in the smoking patterns of men and women suggest that women may lag somewhat behind men with regard to OSCC trends.
4.2.2. Risk factors relating to the increasing risk of OAC
GORD and BO are the main factors associated with increased risk of OAC. 7 , 8 , 9 Over the past several decades, the prevalence of GORD has risen in Western countries. 39 , 40 Accordingly, the incidence of BO has increased in the Netherlands as well. 41 , 42 Given that both GORD and BO are on the rise, both of these conditions may have contributed to the high increase in the incidence of OAC, as revealed in the present study. Given that the prevalence of BO has levelled off in the Netherlands in more recent years, and considering a time lag of approximately 5 to 10 years between the onset of BO and progression to OAC, the increasing incidence of OAC may be expected to reach a plateau in a few years as well. 43 , 44 The increasing trend in the prevalence of OAC may have also been influenced by decreases in Helicobacter pylori infections, which reduce the secretion of gastric acid, and therefore gastro‐oesophageal reflux. The decline in the prevalence of H. pylori infections in Western countries could thus have contributed to the increasing incidence of OAC. 33 , 45
A considerable body of evidence also links a high incidence of OAC to a high prevalence of obesity, especially with regard to abdominal (visceral) fat, which is more common among males. 9 , 46 , 47 Identified as contributing to the development of GORD, 48 obesity also constitutes an independent risk factor for OAC. 47 , 49 In the most highly developed countries, 43% of all OAC cases are estimated to be attributable to obesity. 2 The increasing trend in the incidence of OAC may thus be explained in part by the prevalence of obesity. The steady increase in the prevalence of overweight and obesity among both men and women in the Netherlands runs parallel to the increasing rates of OAC reported, thus logically suggesting that overweight and obesity contribute to the incidence of OAC. 50 Abdominal (visceral) obesity is particularly likely to have been a factor in this increase, and it may explain why OAC is more common in men than it is in women. 51
4.3. Strengths and limitations of the study
The most important strength of the present study is that it examines long‐term trends in the incidence of OSCC and OAC in the Netherlands by age, sex, calendar period and birth cohort based on data from 1989 to 2016, as collected by the NCR. The NCR has been reported to be more than 95% complete for all cancer diagnoses in the Netherlands. 14 Moreover, the data were collected prospectively and independently of the present study, thereby limiting the influence of systematic recall or information bias and reducing the effect of selection bias, as the entire Dutch population was included. 52 We were able to use these data to make predictions for the incidence of OSCC and OAC incidence for each decade through 2041.
The main limitation of the present study is the lack of information on individual risk factors, which impeded direct analyses of potential reasons for the changes observed in the incidence of OSCC and OAC. For this reason, we can only speculate about aetiological clues to these trends. Another limitation of our study is that the incidence rates for the most recent birth cohorts were low and should thus be interpreted with caution. In addition, APC analyses require several assumptions.
Information on differentiation grade and TNM stages were missing for between 14% and 36% of the reported diagnoses of oesophageal carcinoma. Although cases from later periods were less likely to be missing information on TNM stage, they were more likely to lack information on differentiation grade. Because the proportion of missing data does not differ across the histological subtypes and sex, we do not expect that the lack of this information had any influence on the incidence trends reported for these groups.
4.4. Consequences and future implications
Given the increasing risk, changing demographics (population ageing and growth) and increasing trend in obesity, the results of our study suggest that OAC has become the predominant form of oesophageal cancer in the Netherlands, and that the incidence and absolute number of oesophageal cancer diagnoses, and particularly OAC, are likely to continue increasing sharply. These trends are likely to generate a significant increase in the overall demand on resources and costs, primarily in specialised centres. Public health services should prepare now for the expected increase in the number of OAC patients in order to safeguard the quality of care in the years to come. To date, most OAC patients are diagnosed at a locally advanced or even metastatic stage, which has a 5‐year survival rate of less than 5%. 3 Only a minority (10%) of patients are diagnosed at an early stage, at which endoscopic treatment can lead to a 5‐year survival rate exceeding 80%. 53
Improvements in early detection are thus crucial in order to improve survival. To date, population‐level screening for preinvasive stages (eg, BO) is not recommended for oesophageal cancer, due to its overall low prevalence rate, the high cost of endoscopic screening and a substantial risk of complications. Novel, less‐invasive screening methods (eg, nonendoscopic cell‐collection devices and exhaled‐breath analyses) are currently under development, and they may offer a more favourable approach to screening for OAC in the future. 54 , 55 For now, screening followed by surveillance for nonneoplastic BO and endoscopic treatment for dysplastic BO should be considered for individuals with multiple risk factors for oesophageal cancer (eg, GORD, older age, male gender and obesity). 56 Primary prevention measures, including obesity control and smoking cessation, remain key factors in reducing the incidence of OAC.
The combined effects of an ageing population, the increasing incidence and early detection of cancer, and the availability of more effective treatments are likely to produce a substantial increase in the number of OAC patients and BO patients in need of surveillance. Stakeholders will need to expand current facilities and find ways of improving efficiency in the delivery of OAC survivorship care. Furthermore, a more personalised approach to surveillance is needed for patients with newly diagnosed BO, such that those who are at the highest risk are treated and those who are at the lowest risk are discharged from further surveillance. 57
Overall, the results of our study support the need for both primary prevention through lifestyle interventions and secondary prevention through the identification of individuals who are at high risk for OAC, in order to control the incidence of OAC, both now and in the future.
5. CONCLUSION
In summary, our study reveals a decrease in the risk of OSCC and an increase in the risk of OAC for successive birth cohorts in the Netherlands. The increased risk of OAC was more prominent than the decreased risk of OSCC, especially for men. Public health services in the Netherlands should therefore pay more attention to the changing epidemiology of OSCC and OAC, in addition to prioritising resources, diminishing the prevalence of modifiable risk factors and identifying new approaches to the prevention, early detection, surveillance and treatment of oesophageal cancer.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
ETHICS STATEMENT
Because our study was based on the number of newly diagnosed oesophageal cancer cases and publicly available population sizes without personal records, ethical approval and informed consent from participants were legally unnecessary.
Supporting information
Appendix S1: Supporting Information
de Vegt F, Gommers JJJ, Groenewoud H, et al. Trends and projections in the incidence of oesophageal cancer in the Netherlands: An age‐period‐cohort analysis from 1989 to 2041. Int. J. Cancer. 2022;150(3):420-430. doi: 10.1002/ijc.33836
DATA AVAILABILITY STATEMENT
The data underlying the findings of our study are available from the corresponding author upon reasonable request and with permission from the Netherlands Comprehensive Cancer Organisation.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body‐mass index in 2012: a population‐based study. Lancet Oncol. 2015;16:36‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Putten M, de Vos‐Geelen J, Nieuwenhuijzen GAP, et al. Long‐term survival improvement in oesophageal cancer in The Netherlands. Eur J Cancer. 2018;94:138‐147. [DOI] [PubMed] [Google Scholar]
- 4. Abbas G, Krasna M. Overview of esophageal cancer. Ann Cardiothorac Surg. 2017;6:131‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564‐1571. [DOI] [PubMed] [Google Scholar]
- 6. Dong J, Thrift AP. Alcohol, smoking and risk of oesophago‐gastric cancer. Best Pract Res Clin Gastroenterol. 2017;31:509‐517. [DOI] [PubMed] [Google Scholar]
- 7. Hvid‐Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch‐Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375‐1383. [DOI] [PubMed] [Google Scholar]
- 8. Olsen CM, Pandeya N, Green AC, Webb PM, Whiteman DC, Australian CS. Population attributable fractions of adenocarcinoma of the esophagus and gastroesophageal junction. Am J Epidemiol. 2011;174:582‐590. [DOI] [PubMed] [Google Scholar]
- 9. Nimptsch K, Steffen A, Pischon T. Obesity and oesophageal cancer. Recent Results Cancer Res. 2016;208:67‐80. [DOI] [PubMed] [Google Scholar]
- 10. Reither EN, Hauser RM, Yang Y. Do birth cohorts matter? Age‐period‐cohort analyses of the obesity epidemic in the United States. Soc Sci Med. 2009;69:1439‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: age‐period and age‐cohort models. Stat Med. 1987;6:449‐467. [DOI] [PubMed] [Google Scholar]
- 12. Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am J Gastroenterol. 2017;112:1247‐1255. [DOI] [PubMed] [Google Scholar]
- 13. Britton J, Gadeke L, Lovat L, et al. Research priority setting in Barrett's oesophagus and gastro‐oesophageal reflux disease. Lancet Gastroenterol Hepatol. 2017;2:824‐831. [DOI] [PubMed] [Google Scholar]
- 14. IKNL Netherlands Comprehensive Cancer Organisation (IKNL). https://iknl.nl/en/about-iknl Accessed November 15, 2020.
- 15. WHO . International Classification of Diseases for Oncology (ICD‐O). 3rd ed. World Health Organization; 2013. https://apps.who.int/iris/bitstream/handle/10665/96612/9789241548496_eng.pdf?sequence=1&isAllowed=y [Google Scholar]
- 16. Hermanek P, Sobin LH. TNM Classification of Malignant Tumours. 4th ed. Heidelberg: Springer Science & Business Media; 1987. [Google Scholar]
- 17. Sobin LH, Fleming ID. TNM Classification of Malignant Tumors. 5th ed. New York: John Wiley & Sons; 1997. [Google Scholar]
- 18. Wittekind C, Sobin L. TNM Classification of Malignant Tumours; 6th ed. New York: John Wiley & Sons; 2002. [Google Scholar]
- 19. Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th ed. John Wiley & Sons; 2009. [Google Scholar]
- 20. Statistics Netherlands (CBS) . https://www.cbs.nl/en-gb Accessed November 15, 2020.
- 21. Pace M, Lanzieri G, Glickman M, et al. Revision of the European Standard Population. Eurostat Methodologies and Working Papers. Luxembourg: Publications Office of the European Union; 2013. [Google Scholar]
- 22. Joinpoint Regression Program . Statistical Methodology and Applications Branch , Surveillance Research Program. Version 4.7.0.0 ed. USA: National Cancer Institute.
- 23. Belikov A. The Poisson process is the universal law of cancer development: driver mutations accumulate randomly, silently, at constant rate and for many decades, likely in stem cells. bioRxiv. 2018. 10.1101/231027 [DOI] [Google Scholar]
- 24. Murphy CC, Yang YC. Use of age‐period‐cohort analysis in cancer epidemiology research. Curr Epidemiol Rep. 2018;5:418‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moller B, Fekjaer H, Hakulinen T, et al. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med. 2003;22:2751‐2766. [DOI] [PubMed] [Google Scholar]
- 26. Moller B, Fekjaer H, Hakulinen T, et al. Prediction of cancer incidence in the Nordic countries up to the year 2020. Eur J Cancer Prev. 2002;11(suppl 1):S1‐S96. [PubMed] [Google Scholar]
- 27. Offman J, Pesola F, Sasieni P. Trends and projections in adenocarcinoma and squamous cell carcinoma of the oesophagus in England from 1971 to 2037. Br J Cancer. 2018;118:1391‐1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Otterstatter MC, Brierley JD, De P, et al. Esophageal cancer in Canada: trends according to morphology and anatomical location. Can J Gastroenterol. 2012;26:723‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xie S‐H, Mattsson F, Lagergren J. Incidence trends in oesophageal cancer by histological type: an updated analysis in Sweden. Int J Cancer Epidemiol Detect Prev. 2017;47:114‐117. [DOI] [PubMed] [Google Scholar]
- 30. Castro C, Bosetti C, Malvezzi M, et al. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980‐2011) and predictions to 2015. Ann Oncol. 2014;25:283‐290. [DOI] [PubMed] [Google Scholar]
- 31. Wang Q‐L, Xie S‐H, Wahlin K, Lagergren J. Global time trends in the incidence of esophageal squamous cell carcinoma. Clin Epidemiol. 2018;10:717‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edgren G, Adami HO, Weiderpass E, Nyren O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013;62:1406‐1414. [DOI] [PubMed] [Google Scholar]
- 33. Kong CY, Kroep S, Curtius K, et al. Exploring the recent trend in esophageal adenocarcinoma incidence and mortality using comparative simulation modeling. Cancer Epidemiol Biomarkers Prev. 2014;23:997‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prabhu A, Obi KO, Rubenstein JH. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta‐analysis. Am J Gastroenterol. 2014;109:822‐827. [DOI] [PubMed] [Google Scholar]
- 35. Jain S, Dhingra S. Pathology of esophageal cancer and Barrett's esophagus. Ann Cardiothorac Surg. 2017;6:99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pandeya N, Olsen CM, Whiteman DC. Sex differences in the proportion of esophageal squamous cell carcinoma cases attributable to tobacco smoking and alcohol consumption. Cancer Epidemiol. 2013;37:579‐584. [DOI] [PubMed] [Google Scholar]
- 37. Zantinge E, Plasmans M, van der Wilk E. Trend in roken volwassenen 1990‐2017; 2019. https://www.volksgezondheidenzorg.info/onderwerp/roken/cijfers‐context/trends#node‐trend‐roken‐volwassenen. Accessed December 2020. [Google Scholar]
- 38. Trimbos Institute . https://www.trimbos.nl/english/ Accessed December 6, 2020.
- 39. Boeckxstaens G, El‐Serag HB, Smout AJPM, Kahrilas PJ. Symptomatic reflux disease: the present, the past and the future. Gut. 2014;63:1185‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. El‐Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut. 2014;63:871‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Soest EM, Dieleman JP, Siersema PD, Sturkenboom MC, Kuipers EJ. Increasing incidence of Barrett's oesophagus in the general population. Gut. 2005;54:1062‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peters Y, Al‐Kaabi A, Shaheen NJ, et al. Barrett oesophagus. Nat Rev Dis Primers. 2019;5:35. [DOI] [PubMed] [Google Scholar]
- 43. Masclee GM, Coloma PM, de Wilde M, Kuipers EJ, Sturkenboom MC. The incidence of Barrett's oesophagus and oesophageal adenocarcinoma in the United Kingdom and The Netherlands is levelling off. Aliment Pharmacol Ther. 2014;39:1321‐1330. [DOI] [PubMed] [Google Scholar]
- 44. Peters Y, Honing J, Kievit W, et al. Incidence of progression of persistent nondysplastic Barrett's esophagus to malignancy. Clin Gastroenterol Hepatol. 2019;17:869‐877. e5. [DOI] [PubMed] [Google Scholar]
- 45. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta‐analysis. Gastroenterology. 2017;153:420‐429. [DOI] [PubMed] [Google Scholar]
- 46. O'Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH‐AARP Diet and Health Study. Gut. 2012;61:1261‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol. 2011;8:340‐347. [DOI] [PubMed] [Google Scholar]
- 48. Long E, Beales IL. The role of obesity in oesophageal cancer development. Therap Adv Gastroenterol. 2014;7:247‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hoyo C, Cook MB, Kamangar F, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol. 2012;41:1706‐1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Plasmans M, Zantinge E. Trend overgewicht volwassenen; 2019. https://www.volksgezondheidenzorg.info/onderwerp/overgewicht/cijfers‐context/trends#node‐trend‐overgewicht‐volwassenen. Accessed December 2020. [Google Scholar]
- 51. Ryan AM, Rowley SP, Fitzgerald AP, Ravi N, Reynolds JV. Adenocarcinoma of the oesophagus and gastric cardia: male preponderance in association with obesity. Eur J Cancer. 2006;42:1151‐1158. [DOI] [PubMed] [Google Scholar]
- 52. Thygesen LC, Ersbøll AK. When the entire population is the sample: strengths and limitations in register‐based epidemiology. Eur J Epidemiol. 2014;29:551‐558. [DOI] [PubMed] [Google Scholar]
- 53. Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett's esophagus and mortality from esophageal adenocarcinoma: a population‐based cohort study. Am J Gastroenterol. 2014;109:1215‐1222. [DOI] [PubMed] [Google Scholar]
- 54. Fitzgerald RC, di Pietro M, O'Donovan M, et al. Cytosponge‐trefoil factor 3 versus usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet. 2020;396:333‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peters Y, Schrauwen RWM, Tan AC, Bogers SK, de Jong B, Siersema PD. Detection of Barrett's oesophagus through exhaled breath using an electronic nose device. Gut. 2020;69:1169‐1172. [DOI] [PubMed] [Google Scholar]
- 56. Qumseya BJ, Bukannan A, Gendy S, et al. Systematic review and meta‐analysis of prevalence and risk factors for Barrett's esophagus. Gastrointest Endosc. 2019;90:707‐717.e1. [DOI] [PubMed] [Google Scholar]
- 57. Parasa S, Vennalaganti S, Gaddam S, et al. Development and validation of a model to determine risk of progression of Barrett's esophagus to neoplasia. Gastroenterology. 2018;154:1282‐1289. e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
The data underlying the findings of our study are available from the corresponding author upon reasonable request and with permission from the Netherlands Comprehensive Cancer Organisation.


