Summary
Fungal infections are increasingly dangerous because of environmentally dispersed resistance to antifungal drugs. Azoles are commonly used antifungal drugs, but they are also used as fungicides in agriculture, which may enable enrichment of azole‐resistant strains of the human pathogen Aspergillus fumigatus in the environment. Understanding of environmental dissemination and enrichment of genetic variation associated with azole resistance in A. fumigatus is required to suppress resistant strains. Here, we focused on eight strains of azole‐resistant A. fumigatus isolated from a single tulip bulb for sale in Japan. This set includes strains with TR34/L98H/T289A/I364V/G448S and TR46/Y121F/T289A/S363P/I364V/G448S mutations in the cyp51A gene, which showed higher tolerance to several azoles than strains harbouring TR46/Y121F/T289A mutation. The strains were typed by microsatellite typing, single nucleotide polymorphism profiles, and mitochondrial and nuclear genome analyses. The strains grouped differently using each typing method, suggesting historical genetic recombination among the strains. Our data also revealed that some strains isolated from the tulip bulb showed tolerance to other classes of fungicides, such as QoI and carbendazim, followed by related amino acid alterations in the target proteins. Considering spatial–temporal factors, plant bulbs are an excellent environmental niche for fungal strains to encounter partners, and to obtain and spread resistance‐associated mutations.
Introduction
Azoles are versatile compounds that show outstanding activity against a wide range of fungi, including plant and human pathogens. These compounds play an essential role in agricultural and clinical settings as fungicides and antifungal drugs (Price et al., 2015; Fisher et al., 2018). Their main mode of action is inhibition of the ergosterol biosynthesis pathway by inhibiting Cyp51, which functions as a 14‐alpha‐demethylase critical for the biosynthesis of ergosterol. Azole fungicides, known as demethylase inhibitors (DMIs), include triazole and imidazole compounds such as tebuconazole, propiconazole, triflumizole and prochloraz. They are widely used to protect crops and fruits against pathogens by application during cultivation and postharvest preservation, as well as for seed disinfection. In medicine, azole drugs are essential options to combat dermatophytes and deep‐seated fungal pathogens, such as Trichophyton rubrum and Aspergillus fumigatus respectively. Azoles are the only class of compound used to control fungi in both agriculture and medicine.
Aspergillus fumigatus is a major causative agent of aspergillosis and is ubiquitously present in the environment as a saprobe. A limited number of antifungals are approved for therapy of A. fumigatus infection; voriconazole (VRCZ) and itraconazole (ITCZ) are the first‐line drugs for the treatment of pulmonary infection (Jenks and Hoenigl, 2018). However, this antifungal therapy is threatened by azole‐resistant A. fumigatus, strains of which have been increasingly isolated since the beginning of this century (Howard et al., 2009). The resistance mechanisms to azole drugs that have been identified in A. fumigatus from clinical settings are mutations in Cyp51A, HMG‐CoA reductase HMG1, and a subunit of CCAAT‐binding complex HapE, and overexpression of cdr1B, which encodes an ABC transporter (Camps et al., 2012; Fraczek et al., 2013; Hagiwara et al., 2016a; Hagiwara et al., 2018; Rybak et al., 2019; Hortschansky et al., 2020; Nywening et al., 2020). These azole resistance mutations are thought to have emerged during therapy with prolonged azole treatment.
However, in addition to treatment‐based resistance, environmentally derived resistance has been considered as a non‐negligible source of azole drug resistance of A. fumigatus during the last decade (Berger et al., 2017; Lestrade et al., 2019). Typical resistant strains from the environment carry a tandem repeat (TR) and single‐nucleotide polymorphisms (SNPs) in the promoter and coding regions of the cyp51A gene respectively. The most prevalent variants are TR34/L98H and TR46/Y121F/T289A, which were isolated for the first time from patients in Europe in 1998 and North America in 2008 respectively (Jeanvoine et al., 2020). The mutants with TR34 typically show high resistance to ITCZ, whereas the strains with TR46 show VRCZ resistance, but some are pan‐azole‐resistant strains. These genotypes were later recovered from different environments worldwide (Hagiwara et al., 2018; Resendiz et al., 2018; Schoustra et al., 2019). Diverse resistant mutants with TRs in the Cyp51A‐encoding gene have been reported (Table 1).
Table 1.
Reported Cyp51A variants with tandem repeats in azole‐resistant A. fumigatus.
| Cyp51A allele | Country | References |
|---|---|---|
| TR34/L98H | Many places | – |
| TR46/Y121F/T289A | Many places | – |
| TR53 | Colombia | Alvarez‐Moreno et al. (2017) |
| TR34/L98H/S302N | The Netherlands | Schoustra et al. (2019) |
| TR34/L98H/F495I | The Netherlands | Schoustra et al. (2019) |
| TR34/L98H/L343H | The Netherlands | Schoustra et al. (2019) |
| TR34/L98H/E356V | The Netherlands | Schoustra et al. (2019) |
| TR34/L98H/S297T/F495I | The Netherlands | Schoustra et al. (2019) and Cao et al. (2020) |
| TR34/L98H/T289A/I364V/G448S | Japan | Nakano et al. (2020), this study |
| TR46/Y121F/T289A/I364V | The Netherlands | Schoustra et al. (2019) |
| TR46/Y121F/M172I/T289A/G448S | The Netherlands, Japan, Iran | Zhang et al. (2017), Nakano et al. (2020), Ahangarkani et al. (2020a), Ahangarkani et al. (2020b), Fraaije et al. (2020) |
| TR46/Y121F/T289A/S363P/I364V/G448S | The Netherlands, Japan | Nakano et al. (2020), Fraaije et al. (2020), Zhang et al. (2021), this study |
| TR3 46/Y121F/M172I/T289A/G448S | The Netherlands, Japan | Zhang et al. (2017), Nakano et al. (2020) |
| TR4 46/Y121F/M172I/T289A/G448S | The Netherlands | Zhang et al. (2021) |
| TR92/Y121F/M172I/T289A/G448S | The Netherlands | Zhang et al. (2021) |
Recently, a possible environmental hot spot for azole‐resistant A. fumigatus was proposed (Zhang et al., 2017). The TR‐type mutants were prevalently isolated from agricultural compost containing azole fungicide residues, whereas azole‐free compost was dominated by azole susceptible A. fumigatus strains. This view was also supported in other studies (Schoustra et al., 2019; Zhang et al., 2021), indicating that azole‐resistant strains are enriched under the selective pressure of environmental azoles. The work by Zhang et al. also suggested that sexual reproduction plays an important role in developing and evolving new cyp51A alleles for drug resistance in compost (Zhang et al., 2017). Taking into consideration that TR‐type drug‐resistant A. fumigatus mutants show cross‐resistance to DMIs (Snelders et al., 2012), azole‐containing environmental niches may serve as evolutionary incubators through genetic recombination.
The propagation of azole‐resistant A. fumigatus has been studied in an epidemiological manner using microsatellite analysis by short tandem repeats for A. fumigatus (STRAf), which is a widely accepted intraspecies typing method with high‐resolution discriminatory power (de Valk et al., 2005). TR‐type mutant strains were spread worldwide. Some isolates from multiple countries were genetically closely related to each other and some had identical microsatellite patterns (Hagiwara et al., 2016b; Wang et al., 2018; Cao et al., 2020; Pontes et al., 2020). Besides such international propagation, intranational clonal expansion was also reported in several countries (Chowdhary et al., 2012; Ahangarkani et al., 2020a; Ahangarkani et al., 2020b). Recent population genomic studies revealed that the azole‐resistant strains are globally distributed. The isolates were divided into two broad clades, and TR mutants belong to the populations in an uneven manner (Sewell et al., 2019). These data suggest that azole resistance primarily expanded by asexual and sexual propagation from a limited number of ancestors with TR‐type mutation, rather than locally and independently emerging in each environment.
It was recently proposed that resistant A. fumigatus strains are transferred internationally via imported plant bulbs (Dunne et al., 2017). Plant bulbs produced in the Netherlands and sold in Ireland were contaminated with TR‐type A. fumigatus mutants. Similar cases were also reported by two independent Japanese groups (Hagiwara, 2020; Nakano et al., 2020); azole‐resistant A. fumigatus with diverse Cyp51A variants were isolated from plant bulbs that were imported from the Netherlands and sold in Japanese gardening shops. These studies suggest that the widespread of azole‐resistant A. fumigatus mutants is attributable in part to trade in agricultural products including plant bulbs.
In the present study, to further understand genetic variations in plant bulb–associated isolates, we focused on eight A. fumigatus strains that were co‐isolated from a single tulip bulb in a previous screening study (Hagiwara, 2020). Sensitivity to medical and agricultural azoles, as well as other classes of fungicides, was compared between the strains. Whole‐genome comparison of the eight strains showed several fragmental overlaps of their genomes, suggesting genetic recombination had occurred between strains in the single bulb. Our work indicates that plant bulbs are not only a vehicle for the pathogen but also a place where the pathogen can evolve its drug resistance.
Results
Variation of Cyp51A mutation in strains from a single bulb
In a previous study, eight strains of A. fumigatus were isolated from a single tulip bulb as different colonies (hereafter referred to as strains 3‐1‐A to 3‐1‐H) (Hagiwara, 2020). Strain 3‐1‐H has no TR or SNPs in cyp51A, whereas TR34 or TR46 occur in combination with various SNPs in the other seven strains (Table 2). Strains 3‐1‐A, 3‐1‐E, 3‐1‐F and 3‐1‐G have a typical variant, TR46/Y121F/T289A. Strain 3‐1‐D has mutations S363P, I364V and G448S as well as TR46/Y121F/T289A. Strains 3‐1‐B and 3‐1‐C have TR34/L98H and mutations T289A, I364V and G448S. Notably, TR34/L98H and G448S are known to play a role in azole resistance, and T289A is typically accompanied by TR46 (Hagiwara et al., 2016a). Thus, the Cyp51A of strains 3‐1‐B and 3‐1‐C showed complicated sequence variation, including three mutations related to azole resistance.
Table 2.
Cyp51A variation and microsatellite typing of the strains in this study.
| Strain ID | Cyp51A variation | 2A | 2B | 2C | 3A | 3B | 3C | 4A | 4B | 4C |
|---|---|---|---|---|---|---|---|---|---|---|
| 3‐1‐A | TR46/Y121F, T289A | 10 | 20 | 8 | 44 | 9 | 10 | 8 | 10 | 7 |
| 3‐1‐B | TR34/L98H, T289A, I364V, G448S | 23 | 10 | 9 | 35 | 9 | 6 | 8 | 10 | 18 |
| 3‐1‐C | TR34/L98H, T289A, I364V, G448S | 23 | 10 | 9 | 35 | 9 | 6 | 8 | 10 | 18 |
| 3‐1‐D | TR46/Y121F, T289A, S363P, I364V, G448S | 24 | 20 | 12 | 45 | 9 | 11 | 8 | 10 | 18 |
| 3‐1‐E | TR46/Y121F, T289A | 26 | 20 | 12 | 36 | 9 | 22 | 8 | 14 | 31 |
| 3‐1‐F | TR46/Y121F, T289A | 25 | 20 | 12 | 45 | 11 | 6 | 10 | 12 | 18 |
| 3‐1‐G | TR46/Y121F, T289A | 23 | 10 | 9 | 36 | 9 | 6 | 12 | 10 | 7 |
| 3‐1‐H | wt | 23 | 19 | 15 | 33 | 11 | 7 | 13 | 9 | 5 |
Varied sensitivity to azoles in the strains from a single bulb
As previously reported, strains 3‐1‐A to G, which have TRs in cyp51A, showed VRCZ resistance (>32 μg ml−1) in minimum inhibitory concentration tests (Hagiwara, 2020). To further understand the susceptibility to azole drugs, colony growth was evaluated on potato dextrose agar (PDA) containing 10 μg ml−1 of VRCZ (Fig. 1A). Strains 3‐1‐B, 3‐1‐C and 3‐1‐D were more tolerant to VRCZ than the other strains. When grown on medium containing DMIs (triflumizole, imazalil, prochloraz, tebuconazole, epoxiconazole, or difenoconazole), strain 3‐1‐H, which harbours WT Cyp51A, showed the greatest growth inhibition among the strains. Strains 3‐1‐B, 3‐1‐C and 3‐1‐D were less affected by the DMIs (except prochloraz) (Fig. 1B). On the basis of colony diameter measurement, strains 3‐1‐B, 3‐1‐C and 3‐1‐D showed higher tolerance to VRCZ and DMIs than strains 3‐1‐A, 3‐1‐E, 3‐1‐F and 3‐1‐G (Fig. 1C). These results suggest that the combination of TR and G448S mutation increases resistance to azole compounds.
Fig. 1.
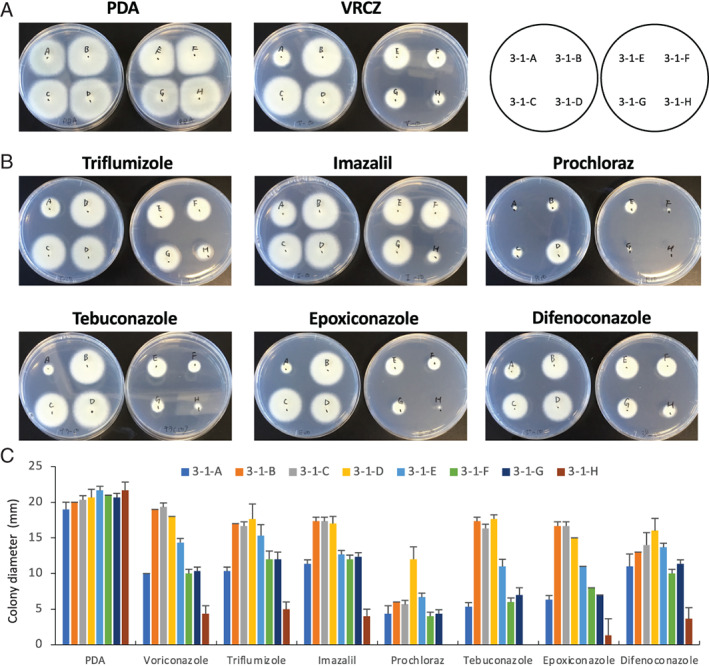
Colony growth of A. fumigatus strains isolated from a single tulip bulb on potato dextrose agar (PDA) containing azoles.
A. Growth on PDA containing voriconazole (VRCZ). Each strain was inoculated on PDA with dimethylsulfoxide (DMSO) as a control or 10 μg ml−1 VRCZ and was incubated for 48 h.
B. Growth on PDA containing demethylase inhibitors (DMIs). Each strain was inoculated on PDA with DMSO as a control or 10 μg ml−1 DMI and was incubated for 48 h.
C. Colony diameter on PDA containing VRCZ or DMIs. Each strain was inoculated on PDA with DMSO as a control or 10 μg ml−1 azole and incubated for 28 h. Error bars represent standard deviations based on three independent replicates.
The expression levels of genes related to azole resistance were examined in the eight strains by quantitative real‐time (qRT)‐PCR. Compared with strain 3‐1‐H, which has the WT cyp51A gene, strains with a TR in the cyp51A gene showed higher expression of cyp51A (Fig. 2A). Overexpression of cdr1B, which encodes an ABC transporter, has been reported to confer azole resistance. Thus, the expression level of cdr1B was also determined in the eight strains by qRT‐PCR. Strains 3‐1‐B and 3‐1‐C showed relatively high expression levels of cdr1B (Fig. 2B).
Fig. 2.
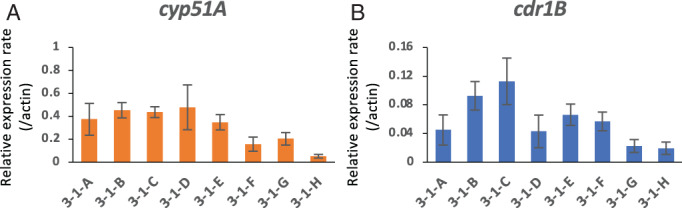
Gene expression analysis by quantitative real‐time (qRT)‐PCR. Expression levels of cyp51A (A) and cdr1B (B) were determined in the eight strains isolated from a single tulip bulb. The strains were cultured in potato dextrose broth for 18 h. The actin gene was used as an internal control. Error bars represent standard deviations based on three independent replicates.
Microsatellite typing analysis of tulip bulb isolates
As described in the Introduction, different Japanese group has isolated TR‐type azole‐resistant A. fumigatus strains from plant bulbs that were imported from the Netherlands (Nakano et al., 2020). To investigate the genetic relationships of plant bulb isolates between the different studies, microsatellite analysis using STRAf was performed (Table 2). Besides the 8 strains from a single tulip bulb, this analysis included 10 (NGS‐ER1, NGS‐ER16, NGS‐ER15, NGS‐ER2, NGS‐ER10, NGS‐ER6, NGS‐ER7, NGS‐ER3, NGS‐ER5 and NGS‐ER4) and four (1‐1‐B, 3‐3‐A, 3‐3‐B and 3‐3‐C) TR‐type strains that were previously reported in Nakano et al. (2020) and Hagiwara (2020) respectively (Fig. 3). In addition, TR‐type A. fumigatus isolated in different countries were also included from the literature (Hagiwara et al., 2016b; Chen et al., 2019). Among the eight strains from a single tulip bulb, the STRAf patterns of 3‐1‐B and 3‐1‐C matched perfectly. Strain 3‐1‐D is closely related to them, as this strain contains the same number of STRs in four of the nine panels. Similarly, strain 3‐1‐D shares the same number of STRs as strain 3‐1‐F in four of the nine panels. Interestingly, some strains that were isolated in Nakano et al. (2020) showed a close relationship with our strains. NGS‐ER15 had an STR pattern similar to that of our strains 3‐1‐B and 3‐1‐C (five of the nine panels), which is consistent with these strains having the same Cyp51A allele (TR34/L98H/T289A/I364V/G448S). Strains NGS‐ER6 and NGS‐ER7 are closely related to strains 3‐3‐A and 3‐3‐B that were isolated from a single another tulip bulb in our previous study (Hagiwara, 2020). Note that these extraordinarily close relatives were isolated from plant bulbs in different laboratories.
Fig. 3.
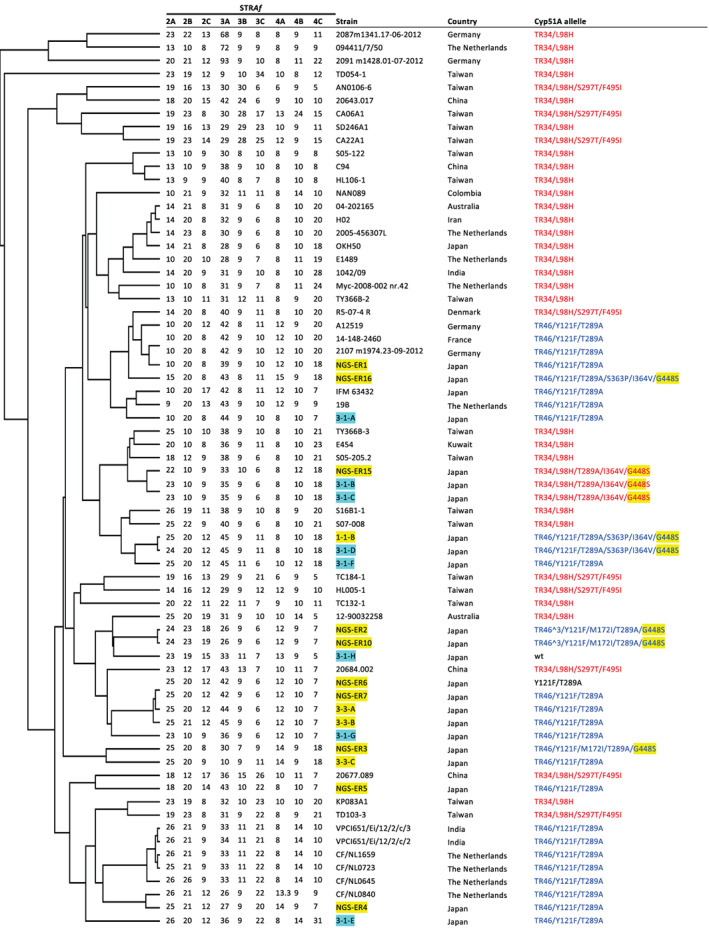
Microsatellite‐typing analysis of A. fumigatus strains with tandem repeat (TR) mutations. The dendrogram was constructed using short tandem repeat for A. fumigatus (STRAf) patterns of the strains. The nine STR panels are shown. The strains listed refer to the literature (Hagiwara et al., 2016b; Chen et al., 2019; Hagiwara, 2020; Nakano et al., 2020). The names of strains isolated from plant bulbs in this study or other study are highlighted in pale blue or yellow respectively. Cyp51A alleles with TR34 are indicated in red, and those with TR46 in blue.
Genome sequencing and comparison between strains
To gain more insight into genetic differences or relatedness, genomes of the eight strains (3‐1‐A to H) were sequenced using the Illumina platform. Complete mitochondrial genomes were successfully obtained for the strains [31 749–31 770 base pairs (bp) long] (Table 3). A phylogenetic tree was constructed using the mitochondrial genomes and those of other strains (IFM 61407, IFM 59365 and IFM 61578) that had been clinically isolated in Japan (Takahashi‐Nakaguchi et al., 2015) (Fig. 4A). This dendrogram indicated that the eight strains isolated from the tulip bulb can be divided into three groups. Group m1 contains strains 3‐1‐A, 3‐1‐D and 3‐1‐G; strains 3‐1‐B, 3‐1‐C, 3‐1‐E and 3‐1‐F are in Group m2. Strain 3‐1‐H was distantly positioned from both Groups m1 and m2. Differences in the length of the mitochondrial genome well reflect the grouping, suggesting that strains within each group are very close relatives. In the microsatellite typing analysis described above, strains 3‐1‐B, 3‐1‐C, 3‐1‐D and 3‐1‐F were grouped into the same clade, but this was inconsistent with the grouping based on mitochondrial genomes, in which strain 3‐1‐D was not in the same group as strains 3‐1‐B, 3‐1‐C and 3‐1‐F.
Table 3.
Results of genome sequencing for strains isolated from a single tulip bulb.
| Strain ID | Total length of chromosomes (bp) | GC (%) | # of proteins | Mitochondrial genome (bp) | Mito group | CSP type | Mating type | SNP in hmg1 |
|---|---|---|---|---|---|---|---|---|
| 3‐1‐A | 28 889 155 | 49.342 | 9492 | 31 770 | m1 | t02 | mat1‐1 | E105K, S212P, Y564H |
| 3‐1‐B | 28 519 682 | 49.352 | 9359 | 31 763 | m2 | t02 | mat1‐1 | S212P, Y564H |
| 3‐1‐C | 28 533 261 | 49.355 | 9490 | 31 763 | m2 | t02 | mat1‐1 | S212P, Y564H |
| 3‐1‐D | 29 087 830 | 49.324 | 9515 | 31 770 | m1 | t02 | mat1‐1 | E105K, S212P, Y564H |
| 3‐1‐E | 28 703 796 | 49.398 | 9464 | 31 763 | m2 | t02 | mat1‐1 | S212P, Y564H |
| 3‐1‐F | 28 808 510 | 49.492 | 9537 | 31 763 | m2 | t02 | mat1‐2 | S212P, Y564H |
| 3‐1‐G | 29 178 518 | 49.295 | 9543 | 31 770 | m1 | t02 | mat1‐1 | E105K, S212P, Y564H |
| 3‐1‐H | 28 716 638 | 49.611 | 9617 | 31 749 | m3 | t01 | mat1‐1 | S212P, Y564H |
Fig. 4.
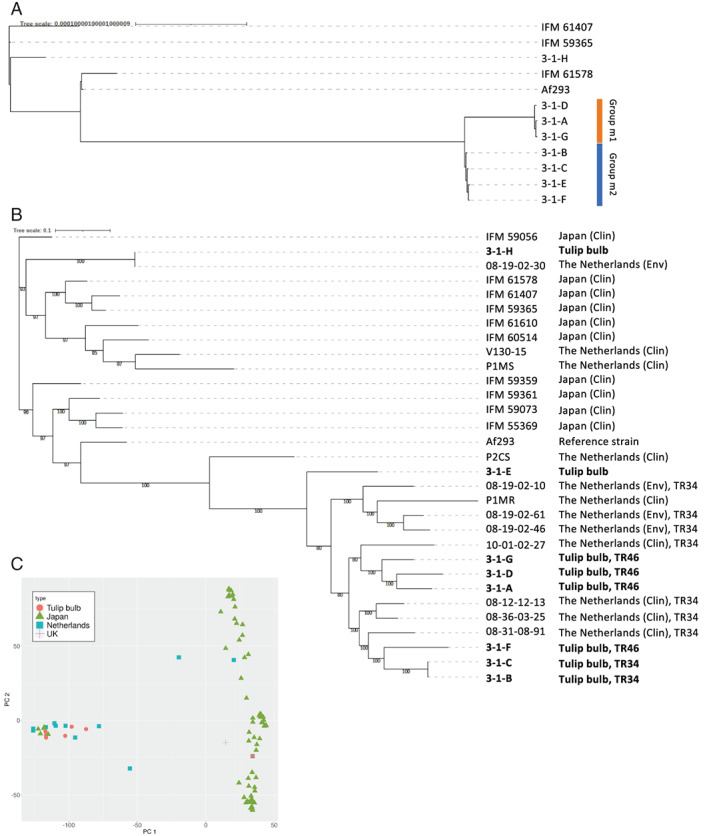
Phylogenetic trees constructed using mitochondrial (A) and nuclear (B) genomes. The trees were constructed using genomes of strains isolated from a single tulip bulb (3‐1‐A to 3‐1‐H) or clinically isolated in a previous study (Takahashi‐Nakaguchi et al., 2015) and shown in the literature (Fan et al., 2021), as well as A. fumigatus reference strain Af293. The clinical and environmental isolates are indicated as Clin and Env respectively. The types of tandem repeat in the cyp51A gene are depicted as TR34 or TR46 if the strain possesses the allele.
C. Principal component analysis of 75 185 polymorphic loci of 96 strains was conducted. The strain and genome data used for the analyses are provided in Table S1.
Nuclear genomes of the eight strains were compared with the reference genome of A. fumigatus strain Af293 (retrieved from AspGD, http://www.aspgd.org/); 92.2% to 93.6% of the Af293 genome was covered in the eight strains, and 69 943–79 384 SNPs were detected the genomes of the eight strains compared with the sequence of Af293 (Table 4). Phylogenetic analysis of the eight strains and previously sequenced strains including the isolates from Japan and the Netherlands was performed by using concatenated sequences of the SNP positions (Takahashi‐Nakaguchi et al., 2015; Fan et al., 2021) (Fig. 4B). Among the eight strains, 3‐1‐H was distantly positioned from the other seven strains (3‐1‐A to ‐G) in the dendrogram, whereas 3‐1‐H was very closely related to the Netherlands' environmental isolate 08‐19‐02‐30 (Abdolrasouli et al., 2015). The other seven strains showed moderately close genetic relatedness to each other and to the strains of the Netherlands, most of which have TR mutation. Strains 3‐1‐B and 3‐1‐C showed the closest relationship, which was supported by the largest number (75 484) of common SNPs against Af293 (Table 4). This is consistent with the results of microsatellite and mitochondrial genome typing. Nevertheless, in the mitochondrial genome typing, strain 3‐1‐E was in Group m2 with strains 3‐1‐B, 3‐1‐C and 3‐1‐F; however, strain 3‐1‐E was relatively distant from these three strains in phylogenetic analysis based on the nuclear genome (Fig. 4B).
Table 4.
Summary of SNPs in strains isolated from a single tulip bulb.
| % of covered positions | # of common SNPs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # of SNPs | 3‐1‐A | 3‐1‐B | 3‐1‐C | 3‐1‐D | 3‐1‐E | 3‐1‐F | 3‐1‐G | 3‐1‐H | ||
| 3‐1‐A | 92.7% | 74,858 | ||||||||
| 3‐1‐B | 92.2% | 77,709 | 56,717 | |||||||
| 3‐1‐C | 92.2% | 77,873 | 56,852 | 75,484 | ||||||
| 3‐1‐D | 93.3% | 78,387 | 60,722 | 58,958 | 59,009 | |||||
| 3‐1‐E | 92.6% | 76,211 | 53,708 | 52,539 | 52,712 | 50,947 | ||||
| 3‐1‐F | 93.0% | 75,546 | 57,638 | 57,673 | 57,825 | 54,169 | 51,842 | |||
| 3‐1‐G | 93.0% | 79,384 | 62,638 | 63,534 | 63,599 | 64,147 | 52,463 | 57,704 | ||
| 3‐1‐H | 93.6% | 69,943 | 29,767 | 33,662 | 33,632 | 34,092 | 32,742 | 31,065 | 33,711 | |
To gain deeper insight into geographic affiliation of the tulip isolates, principal component analysis of polymorphic loci was performed using total of 12 Netherlands and 75 Japanese isolates (Table S1). There were apparently different two populations one of which contains most of Japanese strains (Fig. 4C). In another population, seven of the tulip strains and Netherlands strains were included. These results suggested that the tulip bulbs had not been locally contaminated in Japan, but carried the strains from the Netherlands.
From the genome sequences, CSP typing was performed, which can typify strains by sequence variation at a single locus (csp: Afu3g08990) (Klaassen et al., 2009). The results showed that seven strains (3‐1‐A to 3‐1‐G) carried an identical type (t02), but strain 3‐1‐H had type t01. Sequence analysis for mating type revealed that all but strain 3‐1‐F harboured mat1‐1, whereas 3‐1‐F carried mat1‐2 (Table 3).
The mutation in hmg1 gene encoding a 3‐hydroxy‐3‐methylglutaryl‐coenzyme‐A reductase has been recently reported to be involved in resistance to triazoles (Rybak et al., 2019; Arai et al., 2021). From the genome sequences, SNPs in hmg1 were extracted. There are E105K/S212P/Y564H in 3‐1‐A, ‐D and ‐G strains, whereas S212P/Y564H was found in the rest of strains (3‐1‐B, ‐C, ‐E, ‐F and ‐H) (Table 3). This classification was consistent with that of mitochondrial genome (Fig. 4A).
Comparison of genome‐wide SNP frequency pattern
Inconsistency in strain typing among the typing methods using the mitochondrial and chromosomal genome sequences caused us to speculate that genetic recombination had occurred between the strains isolated from the single tulip bulb. To help test this hypothesis, the SNP frequency and distribution were investigated and compared among the strains in a genome‐wide manner (Fig. S1). There were several regions where the patterns of SNP frequency markedly differed among the strains (Fig. S1). For example, regions 5‐A, 5‐B and 5‐C on chromosome 5 were particularly characteristic (Fig. 5A). In region 5‐A, strains 3‐1‐A, 3‐1‐E and 3‐1‐H showed similar patterns of SNP frequency. In region 5‐B, the pattern of strain 3‐1‐A was similar to that of strains 3‐1‐F and 3‐1‐H. In region 5‐C, the pattern of strain 3‐1‐A was similar to that of strains 3‐1‐E, 3‐1‐G and 3‐1‐H. These results indicate that strain 3‐1‐A shares parts of the sequence of chromosome 5 with strains 3‐1‐E, 3‐1‐F, 3‐1‐G and 3‐1‐H. Such intergenomic variations were also found on other chromosomes (Figs 5B and S1). These results showed a genome‐wide mosaic pattern of SNP frequency, which is indicative of genetic recombination events in the strains.
Fig. 5.
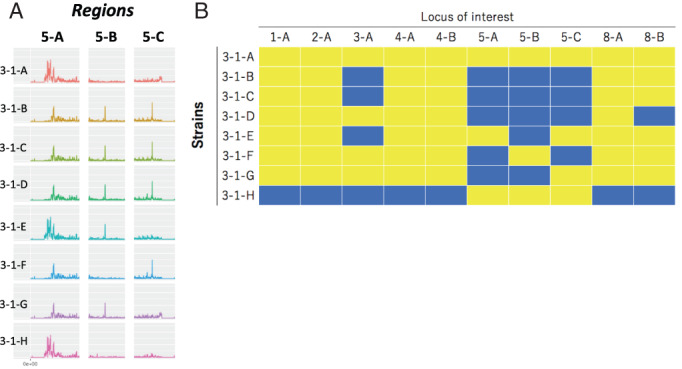
Differences in patterns of single nucleotide polymorphism (SNP) frequency among the strains isolated from a single tulip bulb.
A. The SNP presence patterns are compared in certain regions on chromosome 5 (5‐A, 5‐B and 5‐C).
B. The patterns in each region could typically be divided into two groups, which are indicated by yellow or blue panels. Ten genomic loci are shown and compared among the eight strains.
Comparing genome‐wide distribution of orthologous genes
To further investigate genome shuffling in the strains, we compared the patterns of orthologous among the strains isolated from the tulip bulb. First, the genes shared with the reference genome of A. fumigatus strain Af293 were investigated based on reciprocal blast hits (RBHs), which resulted in the isolates containing 8196 to 8322 orthologues of genes in strain Af293 (Table 5). The positions of the orthologues were generally evenly distributed in the strains, although fewer orthologues were found on chromosome 7. Notably, different patterns of orthologue content were displayed in some regions of the genomes of the various strains (Fig. 6). That is, some sets of strains have lost particular sets of genes, and other sets of genes have been lost in other sets of strains. Hence, the set of strains that shares an orthologue pattern is different at each locus (Fig. 6). This suggests repeated genome shuffling among the strains.
Table 5.
The number of orthologous genes in the strains isolated from a single tulip bulb compared with reference strain A. fumigatus Af293.
| # of orthologous genes compared with strain Af293 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chromosome | Af293 | 3‐1‐A | 3‐1‐B | 3‐1‐C | 3‐1‐D | 3‐1‐E | 3‐1‐F | 3‐1‐G | 3‐1‐H |
| chr1 | 1642 | 1369 | 1355 | 1377 | 1371 | 1369 | 1365 | 1370 | 1370 |
| chr2 | 1640 | 1433 | 1406 | 1425 | 1425 | 1411 | 1436 | 1427 | 1433 |
| chr3 | 1395 | 1163 | 1163 | 1169 | 1179 | 1152 | 1173 | 1179 | 1189 |
| chr4 | 1253 | 1089 | 1075 | 1093 | 1090 | 1098 | 1096 | 1085 | 1095 |
| chr5 | 1367 | 1151 | 1156 | 1160 | 1153 | 1153 | 1162 | 1156 | 1153 |
| chr6 | 1249 | 1068 | 1067 | 1074 | 1058 | 1069 | 1073 | 1070 | 1080 |
| chr7 | 651 | 490 | 478 | 476 | 483 | 489 | 493 | 494 | 489 |
| chr8 | 628 | 502 | 496 | 507 | 497 | 505 | 506 | 491 | 513 |
| Total | 9825 | 8265 | 8196 | 8281 | 8256 | 8246 | 8304 | 8272 | 8322 |
Fig. 6.
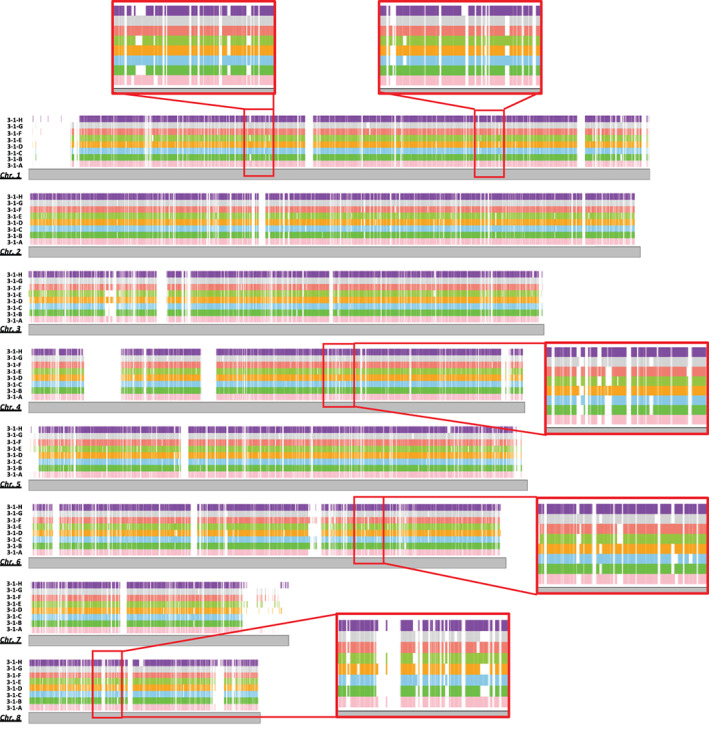
Visualization of genome‐wide orthologous gene content in the strains isolated from a single tulip bulb. Orthologous genes were searched against the reference genome of A. fumigatus strain Af293. The presence of an orthologue is indicated by coloured ribbons for each strain (3‐1‐A to 3‐1‐H). Some regions are enlarged to enable easier comparison of the patterns of gene content.
Varied tolerance to agricultural fungicides
As these strains were derived from a horticultural product, they may have been exposed to agricultural fungicides besides DMIs. Hence, the susceptibility of the eight strains to QoI (pyraclostrobin), SDHI (boscalid), methyl benzimidazole carbamate (carbendazim) and phenylpyrrole (fludioxonil) was evaluated on PDA plates. There was no significant difference among the strains in susceptibility to fludioxonil and boscalid (Fig. 7A and B). However, the colony of strain 3‐1‐H was smaller than those of the other seven strains on the medium containing pyraclostrobin or carbendazim. These results suggest that there is varied tolerance to pyraclostrobin and carbendazim among the strains.
Fig. 7.
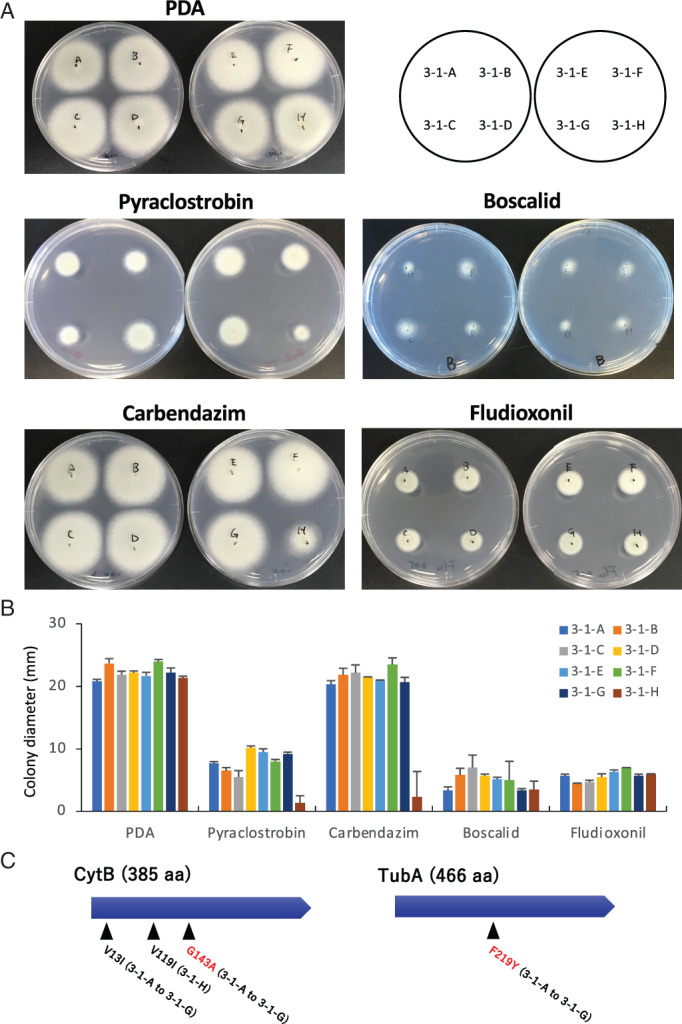
Colony growth of A. fumigatus isolated from a single tulip bulb on PDA containing fungicide.
A. Growth on PDA containing fungicide. Each strain was inoculated onto PDA with DMSO as a control, or QoI (pyraclostrobin; 10 μg ml−1), SDHI (boscalid; 2.5 μg ml−1), methyl benzimidazole carbamate (carbendazim; 5 μg ml−1), or phenylpyrrole (fludioxonil; 0.2 μg ml−1), and incubated for 48 h.
B. Colony diameter on PDA containing fungicide. Each strain was incubated for 30 h. Error bars represent standard deviations based on three independent replicates.
C. Amino acid substitutions detected in CytB and TubA of A. fumigatus strains isolated from a tulip bulb.
From the genome sequences, mutations that are possibly responsible for tolerance to carbendazim and pyraclostrobin were searched in the target molecules tubulin and cytochrome b that are encoded by tubA (Afu1g10910) and cytB (AfuMt00001) respectively (Fig. 6C). The amino acid substitution F219Y was found in TubA of strains 3‐1‐A to 3‐1‐G. This substitution has been reported in several carbendazim‐resistant strains of plant pathogenic fungi (Yarden and Katan, 1993; Zhou et al., 2020). To investigate how the mutation is distributed in human pathogenic A. fumigatus genomes, the SNP database in FungiDB was explored. According to the dataset, 18% (14 of 77 strains) of A. fumigatus contain F219Y in TubA. Notably, eight of the 77 strains were isolated from the environment, and four of these possess the amino acid substitution.
In CytB, mutations V13I and G143A were found in strains 3‐1‐A to 3‐1‐G, and V119I was found in 3‐1‐H. G143A in CytB has been reported to confer the resistance to QoI in many plant pathogenic fungi (Samuel et al., 2011; Bolton et al., 2013), suggesting that this mutation in A. fumigatus is related to low sensitivity to QoI fungicide. As mitochondrial genome sequences are scarce in public databases, we investigated the sequence of the cytb gene in nine strains that were clinically isolated in a previous study and whose mitochondrial genome sequence is at least partly available (Takahashi‐Nakaguchi et al., 2015). Among these nine strains, no G143A mutation was observed, whereas seven of the strains contain V119I (as also observed in our strain 3‐1‐H).
Discussion
The distribution of azole‐resistant A. fumigatus in natural environments has drawn increasing attention in recent years, with special interest in where the resistant strains have emerged, inhabit, and have been translocated to. However, deep understanding is still lacking. In this work, to fill in gaps in knowledge, we focused on strains that were isolated from a single tulip bulb.
Sexual reproduction of A. fumigatus was demonstrated in laboratory conditions in 2009 (O'Gorman et al., 2009). After this discovery, researchers paid more attention to the pan‐genome of clinical isolates of this pathogenic fungus. Population genetics study using linkage disequilibrium analysis for genetic markers supported the view that A. fumigatus reproduces asexually and sexually in natural habitats (Klaassen et al., 2012). This might accelerate gene flow among geographic regions, potentially blurring the geographic structure for some populations. Although there is accumulating evidence for genetic recombination in nature, proving the occurrence of sexual reproduction is difficult unless one can directly collect cleistothecia and ascospores formed in the environment. Genetic recombination in sexual development is suggested to cause the emergence of TR mutation in cyp51A gene through unequal crossover (Zhang et al., 2017). Thus, sexual reproduction is considered both to spread mutations by fusion with other strains and production of progeny and to locally produce de novo TR mutations, which could affect the prevalence of resistance to drugs and fungicides. Our data show that seven of the eight isolates from a single tulip bulb contain TR mutations, and the genetic variation between the TR‐containing strains is low compared with that between apparently independent strains. In addition, on the basis of genome‐wide distributions of SNPs and orthologous genes, genetic recombination is likely to have occurred between the seven strains.
Co‐isolation of the strains from a single bulb indicates that they have had much opportunities to physically interact with each other inside or on the bulb. In addition to the close spatial relationship of the fungal strains, they may interact for a long time. In the conventional process of plant bulb production, bulbs are multiplied from a parental bulb. This bulb multiplication is continued every year, which presumably causes the sustained presence of the fungi on/inside the bulbs. As described here, several strains were attached to a single bulb. These strains might have encountered others and genetically mixed many times. Once mutations giving rise to resistance to azoles emerged, the mutations could be preferentially and stably retained in the microbial community inside the bulb.
Sequencing analysis of the eight strains produced complete mitochondrial genomes and chromosomal genomes. The mitochondrial genome was of great help in interpreting whether there had been sexual reproduction among the strains. On the basis of mitochondrial genome sequences, the strains with TR mutation can be classed into Groups m1 and m2 (Fig. 4A). The length and sequences of the mitochondrial genomes are highly conserved in each group, indicating that they are genetically close progenies. However, the chromosomal genomes were diverse among the strains with TR mutation, excepting strains 3‐1‐B and 3‐1‐C which had approximately 97% of SNPs against Af293 in common (Table 4). The differences in grouping based on mitochondrial and chromosomal genomes strongly suggested a genetic recombination event. We therefore propose that the complete mitochondrial genome is valuable for gaining deeper insight into genetic relatedness among and between environmental and clinical isolates.
Strains with complicated cyp51A alleles have been reported in the literature and this article (Table 1). For instance, we have isolated A. fumigatus strains with TR34/L98H/T289A/I364V/G448S (3‐1‐B and 3‐1‐C) and TR46/Y121F/T289A/S363P/I364V/G448S (3‐1‐D) mutations in the cyp51A gene. TR34/L98H is a typical TR‐type mutation conferring resistance to ITCZ and in some cases to VRCZ, whereas TR46/Y121F/T289A confers resistance to VRCZ and in most cases to ITCZ (van Ingen et al., 2015; Buil et al., 2018). Amino acid substitution G448S contributes to resistance to VRCZ and occasionally to ITCZ (Bellete et al., 2010; Toyotome et al., 2016; Cao et al., 2020). Our finding that three strains (3‐1‐B, 3‐1‐C and 3‐1‐D) showed a higher tolerance to VRCZ and some DMIs than strains with only TR46/Y121F/T289A mutation is suggestive of elevation of tolerance to azole drugs by combining mutations. Importantly, strains with G448S mutation have been isolated not only from clinical samples but also from soil (Cao et al., 2020). We cannot rule out the possibility that the G448S mutation originally emerged and was retained in strains with TR46/Y121F/T289A under the selective pressure of fungicides.
The A. fumigatus strains used in the present work were isolated from a tulip bulb by culturing at 45°C on plates containing medium supplemented with fluconazole to select fungi that were resistant to fluconazole (Hagiwara, 2020). In total in that study, A. fumigatus was isolated from 50.8% of tulip bulbs (96/189), and strains isolated from 20.6% of the bulbs (39/189) had TR mutation. Because A. fumigatus is a saprophytic fungus that widely inhabits soil, compost, plant debris, wood chips, the air and aquatic environments, it was not surprising that half of the tulip bulbs were contaminated with A. fumigatus. However, we have no idea how the fungus resides on or inside the bulbs from a biological viewpoint. Because of the high frequency of A. fumigatus isolation from tulip bulbs, there might be certain mechanisms by which A. fumigatus colonizes and infects the plant tissue, enabling persistence across bulb progenies. Notably, some A. fumigatus strains were isolated from Citrus macrocarpa, Myricaria laxiflora, Ligusticum wallichii and Moringa oleifera (Arora and Kaur, 2019; Qin et al., 2019; Francisco et al., 2020; Li et al., 2020) as an endophyte. In general, however, the view that A. fumigatus has an endophytic mode in its life cycle remains to be established. In consideration of the dynamic mobilization of A. fumigatus in the environment, its association with plants may be overlooked, and we should pay more attention to it.
Recently, several field studies were published in which the prevalence of azole‐resistant A. fumigatus was investigated in association with fungicide use. Work by Zhou et al. (2021) demonstrated that the concentration of triazoles in the soil of greenhouses was not significantly correlated with azole susceptibility of isolates. In another study from Germany, a low frequency of azole‐resistant isolates from crop fields was reported regardless of azole fungicide use (Barber et al., 2020). A study by Fraaije et al. (2020) also reported a low number of azole‐resistant isolates in the soils of wheat‐cropping fields subjected to fungicide treatment. The authors considered that arable crop production is low risk for development of azole resistance. Conversely, a large‐scale survey across China was conducted, which showed that the residual level of azole fungicides in paddy soils positively correlated with the prevalence of azole‐resistant A. fumigatus (Cao et al., 2021). Field research on azole‐resistant A. fumigatus has started in many countries. More studies are required on the effects of fungicide use on the occurrence and spread of azole‐resistant A. fumigatus in the environment, including agricultural and horticultural settings.
In plant bulbs, there may be other pathogenic and nonpathogenic fungi beside A. fumigatus. They are also exposed to fungicides when the bulbs are treated with fungicide. Repeated use of fungicides would facilitate the occurrence of resistance mutations in non‐targeted fungi as well as in the target fungi of the pesticide. In the present study, we found that mutations in CytB and TubA that are related to resistance to QoI and carbendazim fungicides respectively, were detected in A. fumigatus strains as an example of non‐target fungi. These mutations might have been resulted from fungicide exposure during bulb production. Importantly, identical mutations of A. fumigatus were reported by Fraaije et al. (2020) and are found in database. These findings suggest that mutations related to resistance to antifungal agents are already present in the genomes of environmental fungi regardless of their pathogenicity. The boundary between acquired and natural resistance to antifungal compounds may become unclear in the near future.
Materials and methods
Strains and culture conditions
Strains 3‐1‐A to 3‐1‐H used in this study were obtained in previous study and were isolated from a single tulip bulb (Hagiwara, 2020). For plate and liquid cultures, PDA and PDB were used respectively. For colony growth tests, 105 conidia of each strain were inoculated and incubated for 48 h at 37°C before taking pictures. In susceptibility tests, 10 μg ml−1 VRCZ, imazalil, prochloraz, triflumizole, tebuconazole, epoxiconazole and difenoconazole were respectively added to PDA. The control plate contained the equivalent volume of dimethylsulfoxide (DMSO). For measuring colony diameter, the culture time was 28 or 30 h. The data were obtained in triplicate, and the mean and standard deviation are presented. The fungicides fludioxonil, carbendazim, boscalid and pyraclostrobin were used at 0.2, 5, 2.5 and 10 μg ml−1 respectively.
Quantitative real‐time RT‐PCR
Strains were cultured in PDB at 37°C for 18 h and harvested. The mycelia were frozen in liquid nitrogen, and total RNA was isolated using Sepasol Super G (Nacalai Tesque, Kyoto, Japan). cDNA was obtained by reverse transcription reaction using the total RNA sample and ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan).
Real‐time RT‐PCR was performed using Brilliant III Ultra‐Fast SYBR Green QPCR Master Mix (Agilent Technologies, Santa Clara, CA, USA) as described previously (Ninomiya et al., 2020). Relative expression ratios were calculated using the comparative cycle threshold (Ct) method. The actin‐encoding gene was used as a normalization reference. Each sample was tested in triplicate, and the standard deviation is presented. The primer sets used were described in Hagiwara et al. (2017).
Microsatellite typing
Microsatellite typing was performed as described previously (Hagiwara et al., 2014). Briefly, nine microsatellite regions of approximately 400 bp were PCR amplified using purified genome DNA as a template and sequenced by the Sanger method. The repeat numbers of each locus were counted from the sequences. A dendrogram was constructed using Cluster 3.0 by hierarchical clustering with City‐block distance for average linkage and drawn using Treeview ver. 1.1.6r2 (de Hoon et al., 2004; Saldanha, 2004).
Genome sequencing
Whole‐genome sequencing using next‐generation methods was performed as described previously (Hagiwara et al., 2018). In brief, we extracted genomic DNA from overnight‐cultured mycelia with NucleoSpin Plant II (Takara Bio, Shiga, Japan). For paired‐end library preparation, an NEBNext Ultra DNA Library Prep Kit (New England BioLabs, MA, USA) and NEBNext Multiplex Oligos (New England BioLabs) were used in accordance with the manufacturer's instructions. A total of 11 strains including 3‐1‐A to 3‐1‐H, IFM 59365, IFM 61407 and IFM 61578 were sequenced. Paired‐end sequencing (150‐bp) on a HiSeq 4000 system (Illumina, San Diego, CA, USA) was carried out by GENEWIZ (South Plainfield, NJ, USA).
SNP detection
In addition to the abovementioned 11 strains, we used raw data for seven strains for comparison, which have been taken in a study by Takahashi‐Nakaguchi et al. (2015). Adapters and low‐quality bases from Illumina reads were trimmed by fastp (ver. 0.20.1) (Chen et al., 2018). Filtered reads were aligned against the A. fumigatus strain Af293 reference genome using BWA (ver. 0.7.17‐r1188) (Li and Durbin, 2009). SNP detection was performed as described previously (Hagiwara et al., 2018). Briefly, SNPs were identified by using SAMtools (ver. 1.9) (Li et al., 2009) and filtered with >20‐fold coverage, >30 mapping quality and 75% consensus using in‐house scripts (Tenaillon et al., 2012; Suzuki et al., 2014).
Phylogenetic tree construction
Among the strains that were sequenced, mitochondrial genomes of 12 strains were available and aligned by MAFFT (ver. 7.475) (Katoh and Standley, 2013). A phylogenetic tree was constructed using multithreaded RAxML (ver. 8.2.12) (Stamatakis, 2014), the GTRCAT model, and 1000 bootstrap replicates, and visualized by iTOL (Letunic and Bork, 2019). For the genome‐wide phylogenetic analysis of 31 and 96 strains, Genome Analysis ToolKit (GATK) (ver. 4.1.2.0) (McKenna et al., 2010) was applied to detect the polymorphic loci on the chromosomal genomes according to Zhao et al. (2021). Then, 82 014 polymorphic loci of 31 strains were used for construction of a phylogenetic tree by the methods described above. Principal component analysis of 75 185 polymorphic loci of 96 strains was conducted by TASSEL (ver. 5.2.73) (Bradbury et al., 2007). A list of the strains and genomes used for the analyses is provided in Table S1.
Genome assembly and gene prediction
Mitochondrial genomes were assembled and annotated using GetOrganelle (ver. 1.6.4) (Jin et al., 2020) and MITOS2 (Bernt et al., 2013) respectively. To filter the mitochondrial reads, trimmed reads were aligned against mitochondrial genomes by BWA (ver. 0.7.17‐r1188) (Li and Durbin, 2009), and the mapped reads were filtered by SAMtools (ver. 1.9) (Li et al., 2009) and SeqKit (Shen et al., 2016). Contigs were assembled by VelvetOptimiser (ver. 2.2.6) (Zerbino and Birney, 2008), followed by generation of a simulated mate‐paired library using wgsim (ver. 0.3.1‐r13) (https://github.com/lh3/wgsim). The assembly of nuclear genomes was carried out by ALLPATHS‐LG (ver. R52488) (Gnerre et al., 2011). The annotation of assembled nuclear genomes was performed by the Funannotate pipeline (ver. 1.7.4) (https://funannotate.readthedocs.io/en/latest/) as described previously (Takahashi et al., 2021). Following identification of repeat sequences by RepeatModeler (ver. 1.0.11) (http://www.repeatmasker.org/RepeatModeler.html) and RepeatMasker (ver. 4.0.7) (https://www.repeatmasker.org), Funannotate ab initio prediction was performed with the option ‘‐‐busco_seed_species = aspergillus_fumigatus’ by Augustus (ver. 3.3.3) (Stanke et al., 2006), GeneMark‐ES (ver. 4.38) (Ter‐Hovhannisyan et al., 2008), GlimmerHMM (ver. 3.0.4) (Majoros et al., 2004) and SNAP (ver. 2006‐07‐28) (Ian, 2004) using exon hints from the proteins of A. fumigatus Af293 and N. fischeri NRRL 181 downloaded from the Aspergillus Genome Database (http://www.aspgd.org/) (Cerqueira et al., 2014). The completeness of draft genomes and predicted proteins was evaluated by BUSCO (ver. 4.0.6) (Seppey et al., 2019) with the database eurotiales_odb10. Most tools were obtained through Bioconda (Grüning et al., 2018).
Detection of orthologous genes
Orthologous relationships with A. fumigatus Af293 were determined by RBH with criteria BLASTp (ver. 2.9.0+) coverage >80% and identity >80% (Camacho et al., 2009).
Visualization of genome‐wide distribution of SNPs and orthologous genes
SNP frequency in each 1‐kb window was calculated and plotted in 250‐bp steps using Python (Van Rossum and Drake, 2009) and R (R Core Team, 2019) scripts. The orthologous genes of A. fumigatus Af293 in each strain were visualized by R script.
Author Contributions
H.T. and D.H. designed the research; H.T., S.O., Y.K., S.‐i.U. and D.H. performed experiments; H.T. contributed new materials/tools; H.T. and D.H. analysed data; and H.T. and D.H. wrote the manuscript.
Supporting information
Fig. S1. Genome‐wide SNP frequency compared among the eight strains (3‐1‐A to 3‐1‐H). The 10 regions where the pattern is characteristically distinct among the strains are marked by red boxes.
Table S1. List of strains used for SNP typing analyses
Acknowledgements
This study was supported by a grant from the Institute for Fermentation, Osaka (to D.H.). H.T. was partly supported by the National Bioscience Database Center (NBDC) of the Japan Science and Technology Agency (JST), and JSPS KAKENHI Grant Numbers 21K07001 and 16H06279. D.H. and H.T. were partly supported by AMED, Grant Number JP19fm0208024. We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Data Availability Statement
The genome sequencing data are deposited to DDBJ as DRA011961. BioSample accession(s): SAMD00322244–SAMD00322251.
References
- Abdolrasouli, A. , Rhodes, J. , Beale, M.A. , Hagen, F. , Rogers, T.R. , Chowdhary, A. , et al. (2015) Genomic context of azole resistance mutations in Aspergillus fumigatus determined using whole‐genome sequencing. mBio 6: e00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahangarkani, F. , Badali, H. , Abbasi, K. , Nabili, M. , Khodavaisy, S. , de Groot, T. , and Meis, J.F. (2020a) Clonal expansion of environmental triazole resistant Aspergillus fumigatus in Iran. J Fungi (Basel) 6: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahangarkani, F. , Puts, Y. , Nabili, M. , Khodavaisy, S. , Moazeni, M. , Salehi, Z. , et al. (2020b) First azole‐resistant Aspergillus fumigatus isolates with the environmental TR46 /Y121F/T289A mutation in Iran. Mycoses 63: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez‐Moreno, C. , Lavergne, R.A. , Hagen, F. , Morio, F. , Meis, J.F. , and Le Pape, P. (2017) Azole‐resistant Aspergillus fumigatus harboring TR34/L98H, TR46/Y121F/T289A and TR53 mutations related to flower fields in Colombia. Sci Rep 7: 45631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai, T. , Umeyama, T. , Majima, H. , Inukai, T. , Watanabe, A. , Miyazaki, Y. , and Kamei, K. (2021) Hmg1 mutations in Aspergillus fumigatus and their contribution to triazole susceptibility. Med Mycol: myab026. [DOI] [PubMed] [Google Scholar]
- Arora, D.S. , and Kaur, N. (2019) Antimicrobial potential of fungal endophytes from Moringa oleifera . Appl Biochem Biotechnol 187: 628–648. [DOI] [PubMed] [Google Scholar]
- Barber, A.E. , Riedel, J. , Sae‐Ong, T. , Kang, K. , Brabetz, W. , Panagiotou, G. , Deising, H.B. , and Kurzai, O. (2020). Effects of agricultural fungicide use on Aspergillus fumigatus abundance, antifungal susceptibility, and population structure. mBio, 11, e02213–20. 10.1128/mBio02213-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellete, B. , Raberin, H. , Morel, J. , Flori, P. , Hafid, J. , and Manhsung, R.T. (2010) Acquired resistance to voriconazole and itraconazole in a patient with pulmonary aspergilloma. Med Mycol 48: 197–200. [DOI] [PubMed] [Google Scholar]
- Berger, S. , El Chazli, Y. , Babu, A.F. , and Coste, A.T. (2017) Azole resistance in Aspergillus fumigatus: a consequence of antifungal use in agriculture? Front Microbiol 8: 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt, M. , Donath, A. , Jühling, F. , Externbrink, F. , Florentz, C. , Fritzsch, G. , et al. (2013) MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol 69: 313–319. [DOI] [PubMed] [Google Scholar]
- Bolton, M.D. , Rivera, V. , and Secor, G. (2013) Identification of the G143A mutation associated with QoI resistance in Cercospora beticola field isolates from Michigan, United States. Pest Manag Sci 69: 35–39. [DOI] [PubMed] [Google Scholar]
- Bradbury, P.J. , Zhang, Z. , Kroon, D.E. , Casstevens, T.M. , Ramdoss, Y. , and Buckler, E.S. (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23: 2633–2635. [DOI] [PubMed] [Google Scholar]
- Buil, J.B. , Hagen, F. , Chowdhary, A. , Verweij, P.E. , and Meis, J.F. (2018) Itraconazole, voriconazole, and posaconazole CLSI MIC distributions for wild‐type and azole‐resistant Aspergillus fumigatus isolates. J Fungi (Basel) 4: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho, C. , Coulouris, G. , Avagyan, V. , Ma, N. , Papadopoulos, J. , Bealer, K. , and Madden, T.L. (2009) BLAST+: architecture and applications. BMC Bioinformatics 10: 421. 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps, S.M. , Dutilh, B.E. , Arendrup, M.C. , Rijs, A.J. , Snelders, E. , Huynen, M.A. , et al. (2012) Discovery of a HapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS One 7: e50034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, D. , Wang, F. , Yu, S. , Dong, S. , Wu, R. , Cui, N. , et al. (2021) Prevalence of azole‐resistant Aspergillus fumigatus is highly associated with azole fungicide residues in the fields. Environ Sci Technol 55: 3041–3049. [DOI] [PubMed] [Google Scholar]
- Cao, D. , Wu, R. , Dong, S. , Wang, F. , Ju, C. , Yu, S. , et al. (2020) Five‐year survey (2014 to 2018) of azole resistance in environmental Aspergillus fumigatus isolates from China. Antimicrob Agents Chemother 64: e00904‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira, G.C. , Arnaud, M.B. , Inglis, D.O. , Skrzypek, M.S. , Binkley, G. , Simison, M. , et al. (2014) The Aspergillus genome database: multispecies curation and incorporation of RNA‐Seq data to improve structural gene annotations. Nucl Acids Res 42: D705–D710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Zhou, Y. , Chen, Y. , and Gu, J. (2018) fastp: an ultra‐fast all‐in‐one FASTQ preprocessor. Bioinformatics 34: i884–i890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.C. , Kuo, S.F. , Wang, H.C. , Wu, C.J. , Lin, Y.S. , Li, W.S. , and Lee, C.H. (2019) Azole resistance in Aspergillus species in southern Taiwan: an epidemiological surveillance study. Mycoses 62: 1174–1181. [DOI] [PubMed] [Google Scholar]
- Chowdhary, A. , Kathuria, S. , Xu, J. , Sharma, C. , Sundar, G. , Singh, P.K. , et al. (2012) Clonal expansion and emergence of environmental multiple‐triazole‐resistant Aspergillus fumigatus strains carrying the TR₃₄/L98H mutations in the cyp51A gene in India. PLoS One 7: e52871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoon, M.J. , Imoto, S. , Nolan, J. , and Miyano, S. (2004) Open source clustering software. Bioinformatics 20: 1453–1454. [DOI] [PubMed] [Google Scholar]
- de Valk, H.A. , Meis, J.F. , Curfs, I.M. , Muehlethaler, K. , Mouton, J.W. , and Klaassen, C.H. (2005) Use of a novel panel of nine short tandem repeats for exact and high‐resolution fingerprinting of Aspergillus fumigatus isolates. J Clin Microbiol 43: 4112–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne, K. , Hagen, F. , Pomeroy, N. , Meis, J.F. , and Rogers, T.R. (2017) Intercountry transfer of Triazole‐resistant Aspergillus fumigatus on plant bulbs. Clin Infect Dis 65: 147–149. [DOI] [PubMed] [Google Scholar]
- Fan, Y. , Wang, Y. , Korfanty, G.A. , Archer, M. , and Xu, J. (2021) Genome‐wide association analysis for triazole resistance in Aspergillus fumigatus . Pathogens 10: 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, M.C. , Hawkins, N.J. , Sanglard, D. , and Gurr, S.J. (2018) Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360: 739–742. [DOI] [PubMed] [Google Scholar]
- Fraaije, B. , Atkins, S. , Hanley, S. , Macdonald, A. , and Lucas, J. (2020) The multi‐fungicide resistance status of Aspergillus fumigatus populations in arable soils and the wider European environment. Front Microbiol 11: 599233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraczek, M.G. , Bromley, M. , Buied, A. , Moore, C.B. , Rajendran, R. , Rautemaa, R. , et al. (2013) The cdr1B efflux transporter is associated with non‐cyp51a‐mediated itraconazole resistance in Aspergillus fumigatus . J Antimicrob Chemother 68: 1486–1496. [DOI] [PubMed] [Google Scholar]
- Francisco, J.C.E. , Rivera, W.L. , and Vital, P.G. (2020) Influences of carbohydrate, nitrogen, and phosphorus sources on the citric acid production by fungal endophyte Aspergillus fumigatus P3I6. Prep Biochem Biotechnol 50: 292–301. [DOI] [PubMed] [Google Scholar]
- Gnerre, S. , Maccallum, I. , Przybylski, D. , Ribeiro, F.J. , Burton, J.N. , Walker, B.J. , et al. (2011) High‐quality draft assemblies of mammalian genomes from massively parallel sequence data. Proc Natl Acad Sci U S A 108: 1513–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüning, B. , Dale, R. , Sjödin, A. , Chapman, B.A. , Rowe, J. , Tomkins‐Tinch, C.H. , et al. (2018) Bioconda: sustainable and comprehensive software distribution for the life sciences. Nat Methods 15: 475–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara, D. (2020) Isolation of azole‐resistant Aspergillus fumigatus from imported plant bulbs in Japan and the effect of fungicide treatment. J Pestic Sci 45: 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara, D. , Arai, T. , Takahashi, H. , Kusuya, Y. , Watanabe, A. , and Kamei, K. (2018) Non‐cyp51A azole‐resistant Aspergillus fumigatus isolates with mutation in HMG‐CoA reductase. Emerg Infect Dis 24: 1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara, D. , Miura, D. , Shimizu, K. , Paul, S. , Ohba, A. , Gonoi, T. , et al. (2017) A novel Zn2‐Cys6 transcription factor AtrR plays a key role in an azole resistance mechanism of Aspergillus fumigatus by co‐regulating cyp51A and cdr1B expressions. PLoS Pathog 13: e1006096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara, D. , Takahashi, H. , Fujimoto, M. , Sugahara, M. , Misawa, Y. , Gonoi, T. , et al. (2016b) Multi‐azole resistant Aspergillus fumigatus harboring Cyp51A TR46/Y121F/T289A isolated in Japan. J Infect Chemother 22: 577–579. [DOI] [PubMed] [Google Scholar]
- Hagiwara, D. , Takahashi, H. , Watanabe, A. , Takahashi‐Nakaguchi, A. , Kawamoto, S. , Kamei, K. , and Gonoi, T. (2014) Whole‐genome comparison of Aspergillus fumigatus strains serially isolated from patients with aspergillosis. J Clin Microbiol 52: 4202–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara, D. , Watanabe, A. , Kamei, K. , and Goldman, G.H. (2016a) Epidemiological and genomic landscape of azole resistance mechanisms in Aspergillus fungi. Front Microbiol 21: 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortschansky, P. , Misslinger, M. , Mörl, J. , Gsaller, F. , Bromley, M.J. , Brakhage, A.A. , et al. (2020) Structural basis of HapEP88L‐linked antifungal triazole resistance in Aspergillus fumigatus . Life Sci Alliance 3: e202000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, S.J. , Cerar, D. , Anderson, M.J. , Albarrag, A. , Fisher, M.C. , Pasqualotto, A.C. , et al. (2009) Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 15: 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ian, K. (2004) Gene finding in novel genomes. BMC Bioinformatics 5: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanvoine, A. , Rocchi, S. , Bellanger, A.P. , Reboux, G. , and Millon, L. (2020) Azole‐resistant Aspergillus fumigatus: a global phenomenon originating in the environment? Med Mal Infect 50: 389–395. [DOI] [PubMed] [Google Scholar]
- Jenks, J.D. , and Hoenigl, M. (2018) Treatment of Aspergillosis. J Fungi (Basel) 4: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, J.J. , Yu, W.B. , Yang, J.B. , Song, Y. , de Pamphilis, C.W. , Yi, T.S. , et al. (2020) GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol 21: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, K. , and Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen, C.H.W. , de Valk, H.A. , Balajee, S.A. , and Meis, J.F.G.M. (2009) Utility of CSP typing to sub‐type clinical Aspergillus fumigatus isolates and proposal for a new CSP type nomenclature. J Microbiol Methods 77: 292–296. [DOI] [PubMed] [Google Scholar]
- Klaassen, C.H.W. , Gibbons, J.G. , Fedorova, N.D. , Meis, J.F. , and Rokas, A. (2012) Evidence for genetic differentiation and variable recombination rates among Dutch populations of the opportunistic human pathogen Aspergillus fumigatus . Mol Ecol 21: 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestrade, P.P.A. , Meis, J.F. , Melchers, W.J.G. , and Verweij, P.E. (2019) Triazole resistance in Aspergillus fumigatus: recent insights and challenges for patient management. Clin Microbiol Infect 25: 799–806. [DOI] [PubMed] [Google Scholar]
- Letunic, I. , and Bork, P. (2019) Interactive tree of life (iTOL) v4: recent updates and new developments. Nucl Acids Res 47: W256–W259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , and Durbin, R. (2009) Fast and accurate short read alignment with Burrows‐Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Handsaker, B. , Wysoker, A. , Fennell, T. , Ruan, J. , Homer, N. , et al. (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Chen, J.F. , Qin, L.L. , Li, X.H. , Cao, Z.X. , Gu, Y.C. , et al. (2020) Two new sesquiterpenes produced by the endophytic fungus Aspergillus fumigatus from Ligusticum wallichii . J Asian Nat Prod Res 22: 138–143. [DOI] [PubMed] [Google Scholar]
- Majoros, W.H. , Pertea, M. , and Salzberg, S.L. (2004) TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene‐finders. Bioinformatics 20: 2878–2879. [DOI] [PubMed] [Google Scholar]
- McKenna, A. , Hanna, M. , Banks, E. , Sivachenko, A. , Cibulskis, K. , Kernytsky, A. , et al. (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next‐generation DNA sequencing data. Genome Res 20: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano, Y. , Tashiro, M. , Urano, R. , Kikuchi, M. , Ito, N. , Moriya, E. , et al. (2020) Characteristics of azole‐resistant Aspergillus fumigatus attached to agricultural products imported to Japan. J Infect Chemother 26: 1021–1025. [DOI] [PubMed] [Google Scholar]
- Ninomiya, A. , Urayama, S.I. , Suo, R. , Itoi, S. , Fuji, S.I. , Moriyama, H. , and Hagiwara, D. (2020) Mycovirus‐induced tenuazonic acid production in a rice blast fungus Magnaporthe oryzae . Front Microbiol 11: 1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nywening, A.V. , Rybak, J.M. , Rogers, P.D. , and Fortwendel, J.R. (2020) Mechanisms of triazole resistance in Aspergillus fumigatus . Environ Microbiol 22: 4934–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gorman, C.M. , Fuller, H.T. , and Dyer, P.S. (2009) Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus . Nature 457: 471–474. [DOI] [PubMed] [Google Scholar]
- Pontes, L. , Beraquet, C.A.G. , Arai, T. , Pigolli, G.L. , Lyra, L. , Watanabe, A. , et al. (2020) Aspergillus fumigatus clinical isolates carrying CYP51A with TR34/L98H/S297T/F495I substitutions detected after four‐year retrospective azole resistance screening in Brazil. Antimicrob Agents Chemother 64: e02059‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C.L. , Parker, J.E. , Warrilow, A.G. , Kelly, D.E. , and Kelly, S.L. (2015) Azole fungicides ‐ understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag Sci 71: 1054–1058. [DOI] [PubMed] [Google Scholar]
- Qin, W. , Liu, C. , Jiang, W. , Xue, Y. , Wang, G. , and Liu, S. (2019) A coumarin analogue NFA from endophytic Aspergillus fumigatus improves drought resistance in rice as an antioxidant. BMC Microbiol 19: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2019) R: a language and environment for statistical computing. In R Foundation for Statistical Computing. Vienna: Austria. https://www.R-project.org/. [Google Scholar]
- Resendiz Sharpe, A. , Lagrou, K. , Meis, J.F. , Chowdhary, A. , Lockhart, S.R. , Verweij, P.E. , and ISHAM/ECMM Aspergillus Resistance Surveillance Working Group . (2018) Triazole resistance surveillance in Aspergillus fumigatus . Med Mycol 56: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak, J.M. , Ge, W. , Wiederhold, N.P. , Parker, J.E. , Kelly, S.L. , Rogers, P.D. , and Fortwendel, J.R. (2019) Mutations in hmg1, challenging the paradigm of clinical triazole resistance in Aspergillus fumigatus . mBio 10: e00437‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha, A.J. (2004) Java Treeview—extensible visualization of microarray data. Bioinformatics 20: 3246–3248. [DOI] [PubMed] [Google Scholar]
- Samuel, S. , Papayiannis, L.C. , Leroch, M. , Veloukas, T. , Hahn, M. , and Karaoglanidis, G.S. (2011) Evaluation of the incidence of the G143A mutation and cytb intron presence in the cytochrome bc‐1 gene conferring QoI resistance in Botrytis cinerea populations from several hosts. Pest Manag Sci 67: 1029–1036. [DOI] [PubMed] [Google Scholar]
- Schoustra, S.E. , Debets, A.J.M. , Rijs, A.J.M.M. , Zhang, J. , Snelders, E. , Leendertse, P.C. , et al. (2019) Environmental hotspots for azole resistance selection of Aspergillus fumigatus, The Netherlands. Emerg Infect Dis 25: 1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppey, M. , Manni, M. , and Zdobnov, E.M. (2019) BUSCO: assessing genome assembly and annotation completeness. Methods Mol Biol 1962: 227–245. [DOI] [PubMed] [Google Scholar]
- Sewell, T.R. , Zhu, J. , Rhodes, J. , Hagen, F. , Meis, J.F. , Fisher, M.C. , and Jombart, T. (2019) Nonrandom distribution of azole resistance across the global population of Aspergillus fumigatus . mBio 10: e00392‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, W. , Le, S. , Li, Y. , and Hu, F. (2016) SeqKit: a cross‐platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS One 11: e0163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelders, E. , Camps, S.M. , Karawajczyk, A. , Schaftenaar, G. , Kema, G.H. , van der Lee, H.A. , et al. (2012) Triazole fungicides can induce cross‐resistance to medical triazoles in Aspergillus fumigatus . PLoS One 7: e31801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis, A. (2014) RAxML version 8: a tool for phylogenetic analysis and post‐analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke, M. , Schöffmann, O. , Morgenstern, B. , and Waack, S. (2006) Gene prediction in eukaryotes with a generalized hidden Markov model that uses hints from external sources. BMC Bioinformatics 7: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, S. , Horinouchi, T. , and Furusawa, C. (2014) Prediction of antibiotic resistance by gene expression profiles. Nat Commun 5: 5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, H. , Umemura, M. , Ninomiya, A. , Kusuya, Y. , Shimizu, M. , Urayama, S. , et al. (2021) Interspecies genomic variation and transcriptional activeness of secondary metabolism‐related genes in Aspergillus section Fumigati. Front Fungal Biol 2: 656751. 10.3389/ffunb.2021.656751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi‐Nakaguchi, A. , Muraosa, Y. , Hagiwara, D. , Sakai, K. , Toyotome, T. , Watanabe, A. , et al. (2015) Genome sequence comparison of Aspergillus fumigatus strains isolated from patients with pulmonary aspergilloma and chronic necrotizing pulmonary aspergillosis. Med Mycol 53: 353–360. [DOI] [PubMed] [Google Scholar]
- Tenaillon, O. , Rodríguez‐Verdugo, A. , Gaut, R.L. , McDonald, P. , Bennett, A.F. , Long, A.D. , et al. (2012) The molecular diversity of adaptive convergence. Science 335: 457–461. [DOI] [PubMed] [Google Scholar]
- Ter‐Hovhannisyan, V. , Lomsadze, A. , Chernoff, Y.O. , and Borodovsky, M. (2008) Gene prediction in novel fungal genomes using an ab initio algorithm with unsupervised training. Genome Res 18: 1979–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyotome, T. , Fujiwara, T. , Kida, H. , Matsumoto, M. , Wada, T. , and Komatsu, R. (2016) Azole susceptibility in clinical and environmental isolates of Aspergillus fumigatus from eastern Hokkaido, Japan. J Infect Chemother 22: 648–650. [DOI] [PubMed] [Google Scholar]
- van Ingen, J. , van der Lee, H.A. , Rijs, T.A. , Zoll, J. , Leenstra, T. , Melchers, W.J. , and Verweij, P.E. (2015) Azole, polyene and echinocandin MIC distributions for wild‐type, TR34/L98H and TR46/Y121F/T289A Aspergillus fumigatus isolates in The Netherlands. J Antimicrob Chemother 70: 178–181. [DOI] [PubMed] [Google Scholar]
- Van Rossum, G. , and Drake, F.L. (2009) Python 3 Reference Manual. Scotts Valley, CA: CreateSpace. [Google Scholar]
- Wang, H.C. , Huang, J.C. , Lin, Y.H. , Chen, Y.H. , Hsieh, M.I. , Choi, P.C. , et al. (2018) Prevalence, mechanisms and genetic relatedness of the human pathogenic fungus Aspergillus fumigatus exhibiting resistance to medical azoles in the environment of Taiwan. Environ Microbiol 20: 270–280. [DOI] [PubMed] [Google Scholar]
- Yarden, O. , and Katan, T. (1993) Mutations leading to substitutions at amino acid 198 and 200 of beta–tubulin that correlate with benomyl‐resistant phenotypes of field strains of Botrytis cinerea . Phytopathology 83: 1478–1483. [Google Scholar]
- Zerbino, D.R. , and Birney, E. (2008) Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 18: 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Lopez Jimenez, L. , Snelders, E. , Debets, A.J.M. , Rietveld, A.G. , Zwaan, B.J. , et al. (2021) Dynamics of Aspergillus fumigatus in azole fungicide‐containing plant waste in The Netherlands (2016‐2017). Appl Environ Microbiol 87: e02295‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Snelders, E. , Zwaan, B.J. , Schoustra, S.E. , Meis, J.F. , van Dijk, K. , et al. (2017) A novel environmental azole resistance mutation in Aspergillus fumigatus and a possible role of sexual reproduction in its emergence. mBio 8: e00791‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, S. , Ge, W. , Watanabe, A. , Fortwendel, J.R. , and Gibbons, J.G. (2021) Genome‐wide association for itraconazole sensitivity in non‐resistant clinical isolates of Aspergillus fumigatus . Front Fungal Biol 1: 617338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, D. , Korfanty, G.A. , Mo, M. , Wang, R. , Li, X. , Li, H. , et al. (2021) Extensive genetic diversity and widespread azole resistance in greenhouse populations of Aspergillus fumigatus in Yunnan, China. mSphere 6: e00066‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z. , Duan, Y. , and Zhou, M. (2020) Carbendazim‐resistance associated β2 ‐tubulin substitutions increase deoxynivalenol biosynthesis by reducing the interaction between β2 ‐tubulin and IDH3 in Fusarium graminearum . Environ Microbiol 22: 598–614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Genome‐wide SNP frequency compared among the eight strains (3‐1‐A to 3‐1‐H). The 10 regions where the pattern is characteristically distinct among the strains are marked by red boxes.
Table S1. List of strains used for SNP typing analyses
Data Availability Statement
The genome sequencing data are deposited to DDBJ as DRA011961. BioSample accession(s): SAMD00322244–SAMD00322251.


