Abstract
Objectives
Resonance frequency analysis (RFA) is used to monitor implant stability. Its output, the Implant Stability Quotient (ISQ), supposedly correlates with insertion torque, a common measurement of primary stability. However, the reliability of RFA in condensed bone remains unclear.
Material and methods
In this human cadaver study in edentulous jaws and fresh extraction sockets, implants were inserted using a split‐mouth approach into condensed or untreated bone. Mean ISQ, peak insertion torque, and pre‐ and postoperative bone volume fractions (BV/TV) were assessed.
Results
In edentulous jaws, insertion torque and ISQ correlated both in untreated (r = 0.63, p = 0.02) and in condensed (r = 0.82, p < 0.01) bone. In extraction sockets, insertion torque and ISQ only correlated in untreated (r = 0.78, p < 0.01), but not in condensed bone (r = 0.15, p = 0.58). In all edentulous jaws, preoperative BV/TV correlated with insertion torque (r = 0.90, p < 0.0001), ISQ (r = 0.64, p < 0.001), and changes in BV/TV (r = –0.71, p < 0.01). In all extraction sockets, preoperative BV/TV did not correlate with either insertion torque (r = 0.33, p = 0.15), ISQ (r = 0.38, p = 0.09), or changes in BV/TV (r = –0.41, p = 0.09). Joint analysis identified preoperative BV/TV as a predictor of postoperative BV/TV (p < 0.001), insertion torque (p < 0.001), and ISQ (p < 0.001).
Conclusions
RFA is feasible for monitoring stability after late implant placement into condensed bone, but not after immediate placement into condensed fresh extraction sites.
Keywords: bone condensation, edentulous jaw, immediate implant placement, implant stability, resonance frequency analysis
1. INTRODUCTION
Primary mechanical stability is considered a highly important parameter for osseointegration (Lioubavina‐Hack et al., 2006) and secondary biological stability (Monje et al., 2019). Primary stability is the absence of mobility as a function of the mechanical engagement between the implant and the bone; a high insertion torque during surgery is one indicator of this engagement. In addition to insertion torque, several surrogate parameters of implant stability exist, resonance frequency analysis (RFA) being among the most commonly used methods (Lindh et al., 2014). RFA uses electromagnetic pulses to trigger microvibrations in a magnetized transducer temporarily attached to the implant (Meredith et al., 1997; Sennerby & Meredith, 1998). The raw frequency readings are only used in research (Nkenke et al., 2003); for clinical use, they are translated to the arbitrary implant stability quotient (ISQ). The correlation between insertion torque and ISQ remains a matter of controversy (Lages et al., 2018; Levin, 2016; Schliephake et al., 2006). Further, the reliability of RFA remains to be assessed under multiple clinically relevant conditions. Notably, RFA data from implants inserted into surgically condensed bone and implants immediately inserted into fresh extraction sockets are lacking.
Bone condensation is a surgical technique routinely employed where initial bone density is considered insufficient for dental implant placement (Summers, 1994). Traditionally, osteotomes with increasing diameters are used in a stepwise manner to gradually enlarge a relatively narrow pilot hole (Hahn, 1999). This method leads to a lateral compression of the trabecular bone, presumably resulting in increased local density of the fractured bone (Koutouzis et al., 2011; Nkenke et al., 2002). More recently, electromagnetic impact bone condensation was introduced to increase precision through standardized, constant force application and simplified handling, as well as to reduce common side effects of the osteotome technique (e.g., benign paroxysmal positional vertigo) (Crespi, Capparè, & Gherlone, 2013, 2014). Despite its long history, there is currently no consensus on whether or not bone condensing leads to higher primary stability. Using RFA, some previous work attributed higher primary stability to implants placed into condensed bone (Marković et al., 2013), whereas others contradicted that claim (Cehreli et al., 2009; Tabassum et al., 2014). Importantly, RFA was used in these studies without first validating its reliability in condensed bone. A similar lack of profound evidence exists with regard to immediate implant placement. The immediate insertion of implants into fresh extraction sockets (Hämmerle et al., 2004) has become a common surgical protocol with considerable research interest. RFA is widely used to measure stability after immediate placement (Qabbani et al., 2017) and even immediate loading (Juboori et al., 2018). However, bone structure in fresh extraction sockets differs considerably from healed sockets or edentulous bone regions (Araújo & Lindhe, 2005; Cardaropoli et al., 2003). It is thus necessary to investigate whether RFA is reliable in fresh extraction sockets as well.
The validation of RFA in condensed bone as well as fresh extraction sockets is of high relevance for dental implant research and clinical practice alike. To this aim, we performed a cadaver study to investigate the reliability of RFA in implants placed into surgically condensed bone using a split‐mouth design (i.e., one side condensed and one side uncondensed) during either immediate or late implant placement. Previous work has used a comparable cadaver model to study the primary stability of implants (Nkenke et al., 2003). In comparison, here we aimed to assess RFA itself instead of relying on it to measure or predict other parameters, including implant stability. In a more recent cadaver study focusing on the effects of bone condensation, no correlation between insertion torque, RFA, and bone density was reported (Cehreli et al., 2009). In contrast to its femoral head model, we placed implants in edentulous maxillae and maxillary extraction sockets for better clinical applicability of the results. In addition to the clinically measured stability of the implants, we also performed a radiographic assessment of the bone at the implant sites.
2. MATERIAL AND METHODS
2.1. Study design
This split‐mouth human cadaver study was designed and conducted in accordance with the Declaration of Helsinki and its subsequent revisions (World Medical Association, 2013). The ethics committee of the Medical University of Vienna approved the study protocol (No. 1674/2018). Ten freshly frozen, unembalmed head and neck specimens (median age at death: 82 years, 30% female) were split into two groups of five specimens each: late implant placement in edentulous maxillae or maxillary tooth extraction followed by immediate implant placement into the fresh extraction socket. In both groups, the split‐mouth design was applied to test the effect of bone condensing on the outcome parameters. The deceased had bequeathed their bodies to the Division of Anatomy of the Medical University of Vienna for medical research and training purposes prior to their death. We used only complete data in this study and report our results in accordance with STROBE criteria (von Elm et al., 2008).
2.2. Surgical protocol
Preoperatively, the specimens were stored at room temperature for 12 h. In the edentulous jaw group, three implants 4.3 mm in diameter and 10 mm in length (Replace Select Tapered, Nobel Biocare) were placed on one side of the maxilla between the canine and first molar regions following bone condensation using an electrical mallet (Magnetic Mallet, Sweden & Martina) on its lowest setting. First, pilot holes 2.2 mm in diameter and 8 mm in depth were drilled. Subsequently, the electrical mallet was used to expand the pilot holes to the final implant dimensions. In the corresponding sites on the other side of the maxilla, three implants were placed without condensation and in accordance with the manufacturer's drilling protocol. In the fresh extraction socket group, three teeth were extracted on one side of the maxilla between the canine and second molar regions and immediate implants were placed in their extraction sockets following bone condensation using an electrical mallet as described above. Subsequently, the contralateral teeth on the other side of the maxilla were extracted, and immediate implants were placed in their extraction sockets without condensation. After extraction of multirooted teeth, the implants were placed into the palatal socket. The measurements of the pilot holes and the implants were identical to those in the edentulous jaw group. This surgical protocol resulted in four subgroups in total: late implant placement with condensation, late implant placement without condensation, immediate implant placement with condensation, and immediate implant placement without condensation.
2.3. Clinical and radiographic data acquisition and measurements
Preoperative computed tomography (CT) scans of all specimens were performed (Somatom Sensation 4, Siemens) at 120 kV and 80 mAs with a slice thickness of 0.5 mm to serve as baseline and ensure that the bone dimensions allow for implant placement. During the surgeries, we used the surgical motor (Implantmed, W&H) to measure and register the peak implant insertion torque. A handheld RFA device (Beacon, Osstell) was used according to the manufacturer's instructions to measure the ISQ in buccolingual and mesiodistal directions. Following the surgeries and clinical measurements, implants were removed using the surgical motor in a counterclockwise direction. Following the removal of the implants, postoperative CT scans of all specimens were performed. Finally, all jawbones were segmented, and micro‐CT (µCT) scans of the surgical sites were performed (μCT 50, Scanco Medical) at 90 kV and 200 μA with an isotropic resolution of 17.2 μm and an integration time of 500 ms.
Prior to analysis, all radiographic data were pseudonymized by consecutive numbering of the specimens. The pseudonymized CT and µCT scans were exported as DICOM files and transferred to a custom research workstation where we manually checked them for possible misalignments or other errors. For the radiographic analysis of the CT and µCT scans, the implant sites were first localized and marked up as circular shapes in the postoperative scans (axial plane). After that, the accuracy of the generated outlines of the implant sites was controlled in the respective middle slices (coronal plane). If the implant site was in connection to the maxillary sinus, that portion of the site was not marked up so as to not skew the results. To define the region of interest (ROI), the outlines of the implant sites were horizontally expanded by δ = 0.5 mm. The ROI was then defined as a hollow circular cylinder‐like volume where r equals the implant radius in the respective slice and R = r + δ (Figure 1). We then transposed the ROIs to the preoperative scans by precisely aligning the pre‐ and postoperative scans (3D Slicer 4.10.2, available open source under slicer.org) (Figure S1). Finally, we calculated the bone volume fraction (bone volume per total ROI volume, BV/TV) using thresholding, with the threshold set to 300 HU.
FIGURE 1.
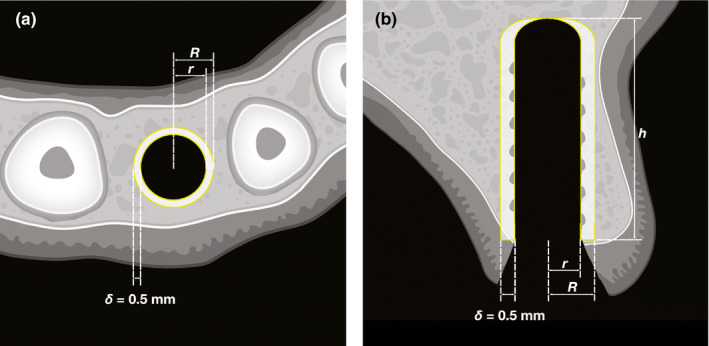
Region of interest definition. A Top‐down view. The inner yellow circle with radius r is the region of interest is an expansion of the implant site by 𝛿 = 0.5 mm. The outer yellow circle with radius R is the outer bounds of the region of interest. The white area between the two yellow circles represents a slice of the volumetric region of interest. B Lateral reconstruction view. The implant length is h = 10 mm. The inner yellow line is the of the sites with radius r in the coronal plane. The outer yellow line represents the reconstruction of the outer bounds of the region of interest after the expansion by 𝛿 = 0.5 mm. The white area between the two yellow lines is the reconstruction of a slice of the volumetric region of interest
2.4. Statistical analysis
The sample size in this study resulted from the availability of freshly frozen, unembalmed head and neck specimens at the Institute of Anatomy of our University. For the analysis, we first collected data in a spreadsheet (Excel 16.29.1 for Mac, Microsoft Corporation), checked the dataset for possible errors, and consequently analyzed it using Prism (Version 9.0.0, GraphPad Software) as well as the R statistical computing environment (Version 3.6.1, R Core Team). Here, we present all parameters as medians and interquartile ranges, unless stated otherwise. We based our statistical analysis on the clinical and quantitative CT measurements. Our primary outcomes were peak insertion torque, ISQ, and BV/TV. For the separate analysis of edentulous jaws and fresh extraction sockets, we used nonparametric tests for all parameters: the Wilcoxon matched‐pairs signed‐rank test for comparisons between split‐mouth pairs and Spearman's rank correlation coefficient to measure the association between different parameters. We set the level of significance at ɑ = 0.05. For the joint analysis of edentulous jaws and extraction sockets, we fitted linear models for postoperative BV/TV, peak insertion torque, and mean ISQ. Treatment group (i.e., condensed or uncondensed) and timing/type of site (i.e., late placement into edentulous jaws or immediate placement into fresh extraction sockets) were considered categorical factors and preoperative BV/TV a metric covariate. Statistical analysis was performed by two researchers (BF and FF).
3. RESULTS
3.1. Clinical parameters in edentulous jaws
First, the implants were inserted into the prepared sites in the edentulous maxillae. For each split‐mouth pair, one site was condensed using an electrical mallet and one site was not. Following full insertion with automatic torque measurement, we manually measured ISQ. We placed 26 implants in five specimens in total that we included in the analyses. In all implant sites, we found highly significant correlations between peak insertion torque and mean ISQ (r = .78, p < .0001), preoperative BV/TV and peak insertion torque (r = .90, p < .0001), as well as between preoperative BV/TV and mean ISQ (r = .64, p < .001) (Table 1 , Figure 2). With regard to split‐mouth sites with or without condensing, peak insertion torque and mean ISQ correlated significantly in sites with condensing (r = .82, p < .01) and in sites without (r = .63, p = .02). In split‐mouth pairs, peak insertion torque did not vary significantly between sites with and without electrical mallet, 26 Ncm (18–46) versus 28 Ncm (22–37, p = .67). Similarly, we found no significant differences in mean ISQ between sites with and without electrical mallet, 74 (63–79) versus 74 (71–77, p = .67) (Table 2). Taken together, RFA and mechanical stability measurements correlated significantly. Further, implant parameters correlated significantly with preoperative BV/TV and were not influenced by electrical mallet use.
TABLE 1.
Correlation analysis
| Edentulous jaws | Fresh extraction sockets | |||||
|---|---|---|---|---|---|---|
| n | r | p | n | r | p | |
| Peak insertion torque—Implant Stability Quotient | ||||||
| Uncondensed bone | 13 | .63 | .02 | 13 | .78 | < .01 |
| Condensed bone | 13 | .82 | < .01 | 16 | .15 | .58 |
| Combined | 26 | .78 | < .0001 | 29 | .61 | < .001 |
| Preoperative BV/TV—peak insertion torque | ||||||
| Uncondensed bone | 13 | .82 | < .01 | 7 | .61 | .17 |
| Condensed bone | 13 | .93 | < .0001 | 13 | –.33 | .27 |
| Combined | 26 | .90 | < .0001 | 20 | .33 | .15 |
| Preoperative BV/TV—Implant Stability Quotient | ||||||
| Uncondensed bone | 13 | .51 | .08 | 7 | .71 | .09 |
| Condensed bone | 13 | .65 | .02 | 13 | –.26 | .38 |
| Combined | 26 | .64 | < .001 | 20 | .38 | .09 |
All r and p values using Spearman's rank correlation coefficient.
Abbreviations: BV, bone volume and TV, total volume of the region of interest.
FIGURE 2.
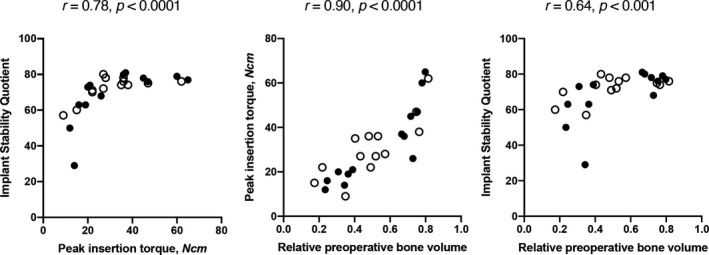
Correlations in edentulous jaws. Blank circles show sites without, filled circles show sites with bone condensing. All r and p values using Spearman's rank correlation coefficient
TABLE 2.
Split‐mouth comparisons in edentulous jaws
| Uncondensed bone | Condensed bone | p | |
|---|---|---|---|
| Clinical parameters | |||
| Peak insertion torque, Ncm | 28 (22–37) | 26 (18–46) | .67 |
| Implant Stability Quotient | 74 (71–77) | 74 (63–79) | .67 |
| Radiographic parameters | |||
| BV/TV, preoperative CT | 0.49 (0.38–0.66) | 0.67 (0.33–0.74) | .74 |
| BV/TV, postoperative CT | 0.41 (0.35–0.50) | 0.39 (0.27–0.49) | .11 |
| BV/TV, postoperative µCT | 0.88 (0.77–0.93) | 0.87 (0.76–0.95) | .95 |
Medians and interquartile ranges. P‐values using Wilcoxon matched‐pairs signed‐rank test.
Abbreviations: BV, bone volume; CT, computed tomography; TV, total volume of the region of interest; and µCT, micro‐computed tomography.
3.2. Radiographic parameters in edentulous jaws
Next, the implants were removed, and the bone samples were scanned again. Postoperative BV/TV was measured using both CT and µCT. Between the two methods, there was a highly significant correlation in postoperative BV/TV (r = .81, p < .0001). Therefore, we used CT data in our further analyses for their comparability with the preoperative scans. Postoperative BV/TV did not vary significantly between sites with and without electrical mallet, 0.39 (0.27–0.49) versus 0.41 (0.35–0.50, p = .11) (Table 2). In all implant sites, changes in BV/TV correlated significantly with preoperative BV/TV (r = –.71, p < .01). This correlation was independent of electrical mallet use. Further, a discrete cluster of implant sites that showed the largest decrease in BV/TV exclusively contained sites that showed a preoperative BV/TV of > 0.60. Sites with a preoperative BV/TV of ≤ 0.60 showed either a smaller decrease or even an increase in BV/TV between pre‐ and postoperative scans (Figure 3). In sum, a significant correlation showed an inverse relationship between preoperative BV/TV and BV/TV change, independently of electrical mallet use.
FIGURE 3.
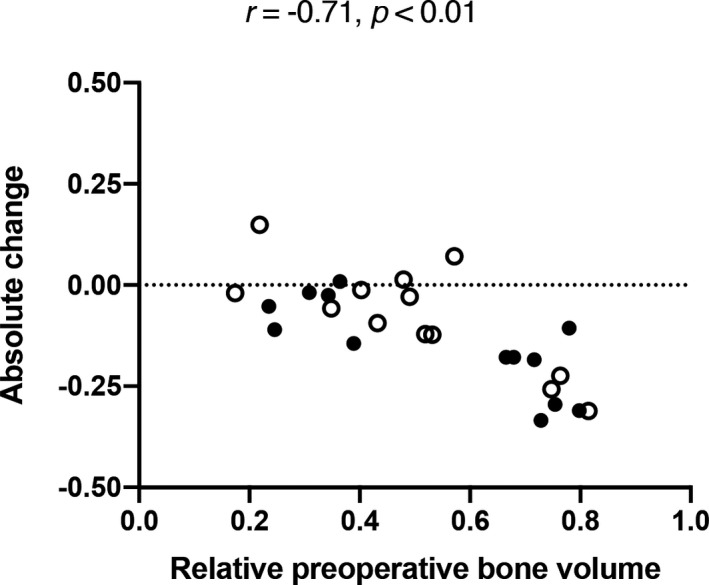
Preoperative relative bone volume and its absolute change in edentulous jaws. Blank circles show sites without, filled circles show sites with bone condensing. All r and p values using Spearman's rank correlation coefficient
3.3. Clinical and radiographic parameters after implant placement in fresh extraction sockets
Analogously to our methods in edentulous jaws, we inserted implants into fresh extraction sockets. Again, one site per split‐mouth pair was condensed using an electrical mallet, and all implants were removed after torque and ISQ measurement. We placed 29 implants in five specimens in total that we included in the analyses. Similarly to edentulous jaws, peak insertion torque and mean ISQ correlated significantly (r = .61, p < .001). However, we found no significant correlations between preoperative BV/TV and peak insertion torque (r = .33, p = 0.15), as well as between BV/TV and mean ISQ (r = .38, p = .09) (Table 1 , Figure 4). With regard to split‐mouth sites with or without condensing, peak insertion torque and mean ISQ only correlated significantly in sites without condensing (r = .78, p < .01), but not in sites with condensing (r = .15, p = .58). In all implant sites, changes in BV/TV showed a tendency toward negative correlation with preoperative BV/TV, although this result is not significant (r = –.41, p = .09) (Figure 5). Due to the specific nature of immediate implant placement, some specimens ended up with very little bone in the preoperative ROIs. These sites were excluded from our further radiographic analyses to minimize the risk of skewed data. The resulting low sample size did not allow for radiological intragroup comparisons between contralateral implant sites. These data show that RFA and mechanical stability measurements correlated significantly, albeit the correlation was not quite as strong as in edentulous jaws.
FIGURE 4.
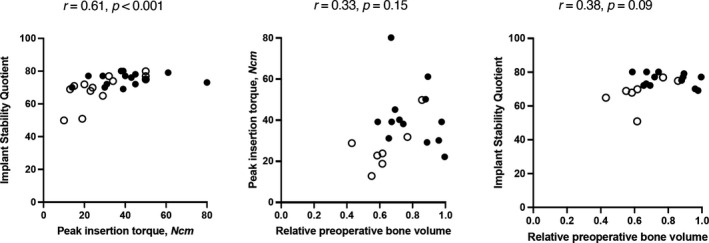
Correlations in fresh extraction sockets. Blank circles show sites without, filled circles show sites with bone condensing. All r and p values using Spearman's rank correlation coefficient
FIGURE 5.
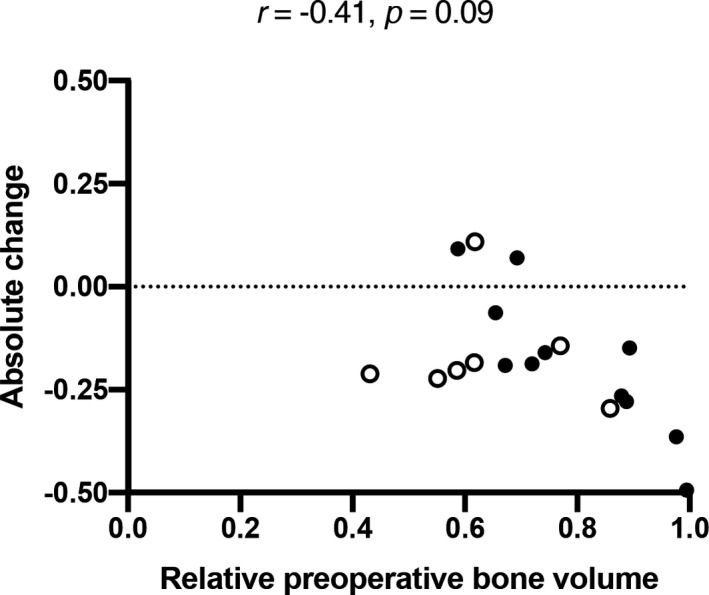
Preoperative relative bone volume and its absolute change in fresh extraction sockets. Blank circles show sites without, filled circles show sites with bone condensing. All r and p values using Spearman's rank correlation coefficient
3.4. Joint analysis of edentulous jaws and fresh extraction sockets
Finally, a joint analysis of all specimens was performed using linear models for postoperative BV/TV, peak insertion torque, and mean ISQ. The empty jaw sites exhibiting very little bone in the preoperative ROIs, as described above, remained excluded for this analysis. Thus, 40 implants in eight specimens in total were included in the analyses (Figure 6). Postoperative BV/TV was affected only by preoperative BV/TV (p < .001), but not by treatment group (p = .92) or timing/type of site (p = .18). Peak insertion torque was affected by preoperative BV/TV (p < .001) as well as timing/type of site (p = .03), but not by treatment group (p = .38). Mean ISQ was affected only by preoperative BV/TV (p < .001), but not by treatment group (p = .67) or timing/type of site (p = .33). These data suggest that preoperative BV/TV was a strong predictor of the peak insertion torque and mean ISQ, whereas electrical mallet use and timing/type of site (immediate placement into fresh extraction sockets versus late placement into healed sites) were not.
FIGURE 6.
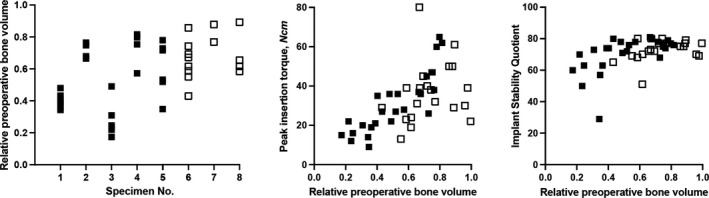
Joint analysis of edentulous jaws and fresh extraction sockets. Blank squares show fresh extraction sockets, filled squares show sites in empty jaws
4. DISCUSSION
In this human cadaver study, we used a split‐mouth design to investigate whether RFA is a reliable tool to measure implant stability in two specific clinical situations: implant placement into surgically condensed and immediate implant placement into fresh extraction sockets. Our main finding is that in edentulous jaws, mean ISQ correlated with peak insertion torque in both uncondensed and condensed bone. However, in fresh extraction sockets, condensation led to a loss of correlation between mean ISQ and peak insertion torque. In fresh extraction sockets, ISQ thus only correlated with peak insertion torque in uncondensed bone. Our secondary findings regarding the effect of bone condensation are somewhat more complex and need cautious interpretation. For one, bone condensation did not improve either the peak insertion torque or the mean ISQ in edentulous jaws. However, correlations were generally stronger in condensed than uncondensed edentulous bone. In fresh extraction sockets, we found nearly exact opposite results. Bone condensation led to higher mean ISQ but not higher peak insertion torque. Correlations were generally stronger in uncondensed than condensed extraction sockets. A joint analysis of all specimens using linear models for postoperative BV/TV, peak insertion torque, and mean ISQ identified preoperative BV/TV as a strong predictor of all three parameters.
Our findings relate to those of others as the correlations between insertion torque and ISQ in late implant placement are comparable to earlier preclinical (Isoda et al., 2012) and clinical studies (Sarfaraz et al., 2018). It has been previously suggested that the correlation between peak insertion torque and ISQ is conditional on an insertion torque value between 30 and 50 Ncm (Baldi et al., 2018). We did not aim to test this hypothesis. Nevertheless, it should be noted that 63% of implants from the groups showing correlations between peak insertion torque and mean ISQ had a peak insertion torque outside of that suggested range. Even though our findings differ from a recent systematic review of twelve studies with regard to correlation between insertion torque and ISQ (Lages et al., 2018), the quality of evidence in that review was deemed low due to the poor quality and high risk of bias the included work. With regard to immediate implant placement, we were unable to thoroughly compare our results to those of others as the correlation between peak insertion torque and ISQ has not been previously studied in fresh extraction sockets.
It is plausible to assume that the inherent heterogeneity of fresh extraction sockets can explain some of our results. Even with the precision of a split‐mouth design and careful extraction procedures, the anatomy of contralateral extraction sockets has an inevitably higher variability than contralateral edentulous bone regions. While edentulous bone regions can vary in the composition of cortical and spongy bone, extraction sockets can further vary based on tooth anatomy as well as eventual pathological processes. We aimed to limit these factors by carefully selecting contralateral split‐mouth pairs before including them in this study as well as not including teeth with extensive pathological processes. The higher macroanatomical variability of fresh extraction sockets could further explain why we found weaker correlations in fresh extraction sockets than in edentulous bone regions. In fresh extraction sockets, some correlation coefficients were even negative in condensed and positive in uncondensed bone. While our sample size is one possible explanation for this finding, we found no such differences in edentulous bone with a similar sample size.
The strength of this study lies in the novelty and relevance of the research question, data quality from three radiographic measurements per sample, algorithmic radiographic measurements, as well as automated torque measurement and registration. The only manual working steps susceptible to human error in our analyses were the RFA measurement and the localization of the ROI. It should be noted that there is currently no way of automating RFA, and the ROI was manually localized to ensure the highest fidelity of the results. The main limitations to our study are its relatively low sample size, based on the availability of freshly frozen and unembalmed head and neck specimens, as well as the removal of the implants before measuring postoperative BV/TV which we deemed necessary in order to avoid metal artifacts in the ROI. The clinical applicability of our results is limited by the high age of the donors. Our study is further limited by the fact that in some samples, the surgical procedure of immediate implant placement led to compromised preoperative CT measurements (i.e., very little bone in the ROI), forcing us to exclude some samples from our radiographic analyses. This was an unforeseen consequence of the surgeries. The limitations concerning sample size and the exclusion of some sockets from the immediate implant placement group could potentially be addressed in a future study with higher initial sample sizes. We further did not analyze the bone microstructure in this study. Further analyses should thus include volumetric bone density measurement. Another limitation is the potentially considerable macroanatomical variability of contralateral extraction sockets. We believe that our split‐mouth design reduces bias based on macroanatomical variability, for we assessed sites from the same specimen. Nevertheless, it should be noted that this variability can still pose a challenge when comparing contralateral sites.
The clinical relevance and applicability of our results are twofold. First, our results ultimately suggest that RFA is a valid surrogate measurement for implant stability after late implant placement with or without, as well as immediate implant placement without bone condensation. Second, our data point to a loss of correlation between peak insertion torque and mean ISQ following bone condensation of the fresh extraction socket. Consequently, we advise caution when relying on ISQ values after immediate implant placement into condensed extraction sockets. Our results also have indications for future research into this field. The loss of correlation between peak insertion torque and mean ISQ in condensed extraction sockets needs further investigation.
5. CONCLUSION
Within the limitations of our study, we conclude that the highly significant correlations found between peak insertion torque and mean ISQ support RFA as a plausible tool to monitor stability after both immediate and late implant placement without bone condensation. In fresh extraction sockets, bone condensation led to a loss of correlation between peak insertion torque and mean ISQ.
AUTHOR CONTRIBUTIONS
Balazs Feher: Data curation (equal); Project administration (equal); Visualization (lead); Writing‐original draft (lead). Florian Frommlet: Data curation (equal); Investigation (equal); Methodology (equal); Validation (equal); Visualization (equal). Reinhard Gruber: Conceptualization (equal); Data curation (equal); Methodology (equal); Visualization (equal); Writing‐original draft (equal). Lena Hirtler: Formal analysis (equal); Investigation (equal); Supervision (equal). Christian Ulm: Investigation (lead); Supervision (equal); Writing‐review & editing (equal). Ulrike Kuchler: Conceptualization (lead); Investigation (equal); Methodology (equal); Project administration (lead); Supervision (equal); Writing‐review & editing (lead).
DATA AVAILABILITY STATEMENT
The source data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting information
Fig S1
Supplementary Material
ACKNOWLEDGEMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Medical University of Vienna. The authors declare no conflicts of interest with respect to the authorship and/or publication of this article. The authors thank Drs. Paul Inkofer and Sophie Filipitsch as well as Mr. Patrick Heimel for their help with the analyses. The present study did not receive any funding. The source data that support the findings of this study are available from the corresponding author upon reasonable request.
Feher, B. , Frommlet, F. , Gruber, R. , Hirtler, L. , Ulm, C. , & Kuchler, U. (2021). Resonance frequency analysis of implants placed in condensed bone. Clinical Oral Implants Research, 32, 1200–1208. 10.1111/clr.13817
REFERENCES
- Araújo, M. G. , & Lindhe, J. (2005). Dimensional ridge alterations following tooth extraction. An experimental study in the dog. Journal of Clinical Periodontology, 32(2), 212–218. 10.1111/j.1600-051X.2005.00642.x [DOI] [PubMed] [Google Scholar]
- Baldi, D. , Lombardi, T. , Colombo, J. , Cervino, G. , Perinetti, G. , Di Lenarda, R. , & Stacchi, C. (2018). Correlation between insertion torque and implant stability quotient in tapered implants with knife‐edge thread design. BioMed Research International, 2018, 7201093. 10.1155/2018/7201093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardaropoli, G. , Araújo, M. , & Lindhe, J. (2003). Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. Journal of Clinical Periodontology, 30(9), 809–818. 10.1034/j.1600-051X.2003.00366.x. [DOI] [PubMed] [Google Scholar]
- Cehreli, M. C. , Kökat, A. M. , Comert, A. , Akkocaoğlu, M. , Tekdemir, I. , & Akça, K. (2009). Implant stability and bone density: assessment of correlation in fresh cadavers using conventional and osteotome implant sockets. Clinical Oral Implants Research, 20(10), 1163–1169. 10.1111/j.1600-0501.2009.01758.x [DOI] [PubMed] [Google Scholar]
- Crespi, R. , Capparè, P. , & Gherlone, E. (2013). Electrical mallet provides essential advantages in maxillary bone condensing. A prospective clinical study. Clinical Implant Dentistry and Related Research, 15(6), 874–882. 10.1111/j.1708-8208.2011.00432.x [DOI] [PubMed] [Google Scholar]
- Crespi, R. , Capparè, P. , & Gherlone, E. (2014). A comparison of manual and electrical mallet in maxillary bone condensing for immediately loaded implants: a randomized study. Clinical Implant Dentistry and Related Research, 16(3), 374–382. 10.1111/j.1708-8208.2012.00485.x [DOI] [PubMed] [Google Scholar]
- Hahn, J. (1999). Clinical uses of osteotomes. Journal of Oral Implantology, 25(1), 23–29. 10.1563/1548‐1336(1999)025<0023:Cuoo>2.3.Co;2 [DOI] [PubMed] [Google Scholar]
- Hämmerle, C. H. , Chen, S. T. , & Wilson, T. G. (2004). Consensus statements and recommended clinical procedures regarding the placement of implants in extraction sockets. International Journal of Oral & Maxillofacial Implants, 19(Suppl), 26–28. [PubMed] [Google Scholar]
- Isoda, K. , Ayukawa, Y. , Tsukiyama, Y. , Sogo, M. , Matsushita, Y. , & Koyano, K. (2012). Relationship between the bone density estimated by cone‐beam computed tomography and the primary stability of dental implants. Clinical Oral Implants Research, 23(7), 832–836. 10.1111/j.1600-0501.2011.02203.x [DOI] [PubMed] [Google Scholar]
- Juboori, M. J. A. , Attas, M. A. A. , Gomes, R. Z. , & Alanbari, B. F. (2018). Using resonance frequency analysis to compare delayed and immediate progressive loading for implants placed in the posterior maxilla: A pilot study. The Open Dentistry Journal, 12, 801–810. 10.2174/1745017901814010801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutouzis, T. , Koutouzis, G. , Tomasi, C. , & Lundgren, T. (2011). Immediate loading of implants placed with the osteotome technique: one‐year prospective case series. Journal of Periodontology, 82(11), 1556–1562. 10.1902/jop.2011.100751 [DOI] [PubMed] [Google Scholar]
- Lages, F. S. , Douglas‐de Oliveira, D. W. , & Costa, F. O. (2018). Relationship between implant stability measurements obtained by insertion torque and resonance frequency analysis: A systematic review. Clinical Implant Dentistry and Related Research, 20(1), 26–33. 10.1111/cid.12565 [DOI] [PubMed] [Google Scholar]
- Levin, B. P. (2016). The correlation between immediate implant insertion torque and implant stability quotient. The International Journal of Periodontics & Restorative Dentistry, 36(6), 833–840. 10.11607/prd.2865 [DOI] [PubMed] [Google Scholar]
- Lindh, C. , Oliveira, G. H. C. , Leles, C. R. , do Carmo Matias Freire, M. , & Ribeiro‐Rotta, R. F. (2014). Bone quality assessment in routine dental implant treatment among Brazilian and Swedish specialists. Clinical Oral Implants Research, 25(9), 1004–1009. 10.1111/clr.12221 [DOI] [PubMed] [Google Scholar]
- Lioubavina‐Hack, N. , Lang, N. P. , & Karring, T. (2006). Significance of primary stability for osseointegration of dental implants. Clinical Oral Implants Research, 17(3), 244–250. 10.1111/j.1600-0501.2005.01201.x [DOI] [PubMed] [Google Scholar]
- Marković, A. , Mišić, T. , Miličić, B. , Calvo‐Guirado, J. L. , Aleksić, Z. , & Ðinić, A. (2013). Heat generation during implant placement in low‐density bone: effect of surgical technique, insertion torque and implant macro design. Clinical Oral Implants Research, 24(7), 798–805. 10.1111/j.1600-0501.2012.02460.x [DOI] [PubMed] [Google Scholar]
- Meredith, N. , Book, K. , Friberg, B. , Jemt, T. , & Sennerby, L. (1997). Resonance frequency measurements of implant stability in vivo. A cross‐sectional and longitudinal study of resonance frequency measurements on implants in the edentulous and partially dentate maxilla. Clinical Oral Implants Research, 8(3), 226–233. 10.1034/j.1600-0501.1997.080309.x [DOI] [PubMed] [Google Scholar]
- Monje, A. , Ravidà, A. , Wang, H. L. , Helms, J. A. , & Brunski, J. B. (2019). Relationship between primary/mechanical and secondary/biological implant stability. The International Journal of Oral & Maxillofacial Implants, 34, s7–s23. 10.11607/jomi.19suppl.g1 [DOI] [PubMed] [Google Scholar]
- Nkenke, E. , Hahn, M. , Weinzierl, K. , Radespiel‐Tröger, M. , Neukam, F. W. , & Engelke, K. (2003). Implant stability and histomorphometry: A correlation study in human cadavers using stepped cylinder implants. Clinical Oral Implants Research, 14(5), 601–609. 10.1034/j.1600-0501.2003.00937.x [DOI] [PubMed] [Google Scholar]
- Nkenke, E. , Kloss, F. , Wiltfang, J. , Schultze‐Mosgau, S. , Radespiel‐Tröger, M. , Loos, K. , & Neukam, F. W. (2002). Histomorphometric and fluorescence microscopic analysis of bone remodelling after installation of implants using an osteotome technique. Clinical Oral Implants Research, 13(6), 595–602. 10.1034/j.1600-0501.2002.130604.x [DOI] [PubMed] [Google Scholar]
- Qabbani, A. A. , Razak, N. H. A. , Kawas, S. A. , Sheikh Abdul Hamid, S. , Wahbi, S. , & Samsudin, A. R. (2017). The efficacy of immediate implant placement in extraction sockets for alveolar bone preservation: A clinical evaluation using three‐dimensional cone beam computerized tomography and resonance frequency analysis value. Journal of Craniofacial Surgery, 28(4), e318–e325. 10.1097/scs.0000000000003569 [DOI] [PubMed] [Google Scholar]
- Sarfaraz, H. , Johri, S. , Sucheta, P. , & Rao, S. (2018). Study to assess the relationship between insertion torque value and implant stability quotient and its influence on timing of functional implant loading. The Journal of Indian Prosthodontic Society, 18(2), 139–146. 10.4103/jips.jips_203_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliephake, H. , Sewing, A. , & Aref, A. (2006). Resonance frequency measurements of implant stability in the dog mandible: experimental comparison with histomorphometric data. International Journal of Oral and Maxillofacial Surgery, 35(10), 941–946. 10.1016/j.ijom.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Sennerby, L. , & Meredith, N. (1998). Resonance frequency analysis: Measuring implant stability and osseointegration. Compendium of Continuing Education in Dentistry, 19(5), 493–498, 500, 502, quiz 504. [PubMed] [Google Scholar]
- Summers, R. B. (1994). A new concept in maxillary implant surgery: the osteotome technique. Compendium, 15(2), 152, 154–156. 158 passim; quiz 162. [PubMed] [Google Scholar]
- Tabassum, A. , Meijer, G. J. , Walboomers, X. F. , & Jansen, J. A. (2014). Evaluation of primary and secondary stability of titanium implants using different surgical techniques. Clinical Oral Implants Research, 25(4), 487–492. 10.1111/clr.12180 [DOI] [PubMed] [Google Scholar]
- von Elm, E. , Altman, D. G. , Egger, M. , Pocock, S. J. , Gøtzsche, P. C. , & Vandenbroucke, J. P. (2008). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Journal of Clinical Epidemiology, 61(4), 344–349. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- JAMA, (2013). World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. 310(20), 2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Supplementary Material
Data Availability Statement
The source data that support the findings of this study are available from the corresponding author upon reasonable request.


