Summary
Background
Pemafibrate is a novel, selective peroxisome proliferator‐activated receptor α modulator (SPPARMα). In mice, Pemafibrate improved the histological features of non‐alcoholic steatohepatitis (NASH). In patients with dyslipidaemia, it improved serum alanine aminotransferase (ALT).
Aims
To evaluate the efficacy and safety of Pemafibrate in patients with high‐risk, non‐alcoholic fatty liver disease (NAFLD).
Methods
This double‐blind, placebo‐controlled, randomised multicentre, phase 2 trial randomised 118 patients (1:1) to either 0.2 mg Pemafibrate or placebo, orally, twice daily for 72 weeks. The key inclusion criteria included liver fat content of ≥10% by magnetic resonance imaging‐estimated proton density fat fraction (MRI‐PDFF); liver stiffness of ≥2.5 kPa, by magnetic resonance elastography (MRE); and elevated ALT levels. The primary endpoint was the percentage change in MRI‐PDFF from baseline to week 24. The secondary endpoints included MRE‐based liver stiffness, ALT, serum liver fibrosis markers and lipid parameters.
Results
There was no significant difference between the groups in the primary endpoint (−5.3% vs −4.2%; treatment difference −1.0%, P = 0.85). However, MRE‐based liver stiffness significantly decreased compared to placebo at week 48 (treatment difference −5.7%, P = 0.036), and was maintained at week 72 (treatment difference −6.2%, P = 0.024), with significant reduction in ALT and LDL‐C. Adverse events were comparable between the treatment groups and therapy was well tolerated.
Conclusions
Pemafibrate did not decrease liver fat content but had significant reduction in MRE‐based liver stiffness. Pemafibrate may be a promising therapeutic agent for NAFLD/NASH, and also be a candidate for combination therapy with agents that reduce liver fat content. ClinicalTrials.gov, number: NCT03350165.

1. INTRODUCTION
Non‐alcoholic fatty liver disease (NAFLD) is a disorder characterized by fatty liver, which is identified by either tissue biopsy or diagnostic imaging, with the exclusion of secondary causes including considerable alcohol consumption, hepatitis B/C and drug use. NAFLD can be classified as non‐alcoholic fatty liver where there is no evidence of hepatocellular injury, or fibrosis, or as non‐alcoholic steatohepatitis (NASH), which is characterised by steatosis, inflammation and hepatocellular injury (ballooning). 1 NAFLD mostly develops due to obesity, diabetes mellitus, dyslipidaemia or hypertension, and hepatic involvement is recognised in metabolic syndrome. 12 The number of patients with NAFLD/NASH is increasing worldwide owing to a rise in population with obesity, 1 and the prevalence is estimated to be 20%‐30% and 2%‐6% of the population respectively. 2 , 3
NAFLD/NASH can lead to serious conditions including cirrhosis and liver cancer, 12 and is also associated with an increased risk of cardiovascular events. 4 The guidelines for the management of NAFLD/NASH recommend lifestyle modifications with diet and exercise for weight loss 12 ; however, it is difficult for many patients to maintain long‐term lifestyle modification. Although vitamin E and pioglitazone have been shown to improve some histological features of NASH, neither has been approved for the treatment of NASH and that there is currently no approved treatment for NASH. 5
Peroxisome proliferator‐activated receptors (PPARs) are nuclear hormone receptors that bind to DNA as heterodimers with retinoid X receptors. PPARα is associated with the transcription of genes involved in reducing serum triglycerides (TG) and increasing high‐density lipoprotein‐cholesterol (HDL‐C) and regulates lipid and lipoprotein metabolism. 6 Histological amelioration of NASH has been shown to be associated with an increased expression of PPARα and its target genes, suggesting that PPARα is a potential therapeutic target for NASH. 7
Pemafibrate is a selective PPARα modulator (SPPARMα) that is already approved in Japan for the treatment of hypertriglyceridaemia, 8 , 9 and its efficacy on reducing cardiovascular events is being examined in a large‐scale clinical study, PROMINENT (ClinicalTrials.gov identifier: NCT03071692), conducted in 24 countries worldwide, including Japan, the United States, the United Kingdom and Russia. Pemafibrate regulates the expression of the target genes that are mainly related to lipid and glucose metabolism in the liver. For example, it also increases the expression of genes related to β‐oxidation and lipid transport and enhances energy metabolism via the induction of mitochondrial uncoupling protein 3 gene expression. Non‐clinical studies in animal models of NASH have shown that Pemafibrate improves hepatic lipid content, plasma transaminase level and various pathological findings of NASH (steatosis, ballooning and fibrosis). 10 Previous clinical studies of Pemafibrate in patients with dyslipidaemia demonstrated that it not only reduced the TG level with favourable safety profile but also improved serum liver enzymes (eg alanine aminotransferase [ALT], γ‐glutamyl transpeptidase [GGT] and alkaline phosphatase [ALP]). 6 However, the efficacy of Pemafibrate in the treatment of NAFLD/NASH, with or without dyslipidaemia, compared with that of a placebo has not been examined in a placebo‐controlled clinical study with quantitative imaging biomarkers other than laboratory tests. Therefore, we conducted this PEMA‐FL study (PEMAfibrate randomised placebo‐controlled study in patients with non‐alcoholic Fatty Liver disease) to assess the efficacy and safety of Pemafibrate administered for 72 weeks in high‐risk NAFLD patients with increased liver stiffness and ALT level, with/without dyslipidaemia, by measuring liver fat content and stiffness with magnetic resonance‐based imaging modalities.
2. METHODS
2.1. Study design
This was a placebo‐controlled, randomised, double‐blind, parallel‐group, phase 2 study that examined the efficacy and safety of Pemafibrate in adult patients with NAFLD. The study was conducted from November 2017 to March 2020 at 16 medical centres in Japan. Following a screening period of at least 2 weeks and no more than 8 weeks, eligible patients were randomly assigned to either treatment group and followed up for 72‐week treatment period with no change in dietary and/or exercise guidance throughout the screening and treatment period. The study was conducted in compliance with relevant guidelines, Good Clinical Practice guidance and the Declaration of Helsinki. In addition, the study was approved by the institutional review boards of each institution. This trial is registered with ClinicalTrials.gov, number NCT03350165.
2.2. Patients
Patients with liver fat content, measured by magnetic resonance imaging‐estimated proton density fat fraction (MRI‐PDFF), of ≥10%, liver stiffness, measured by magnetic resonance elastography (MRE), of ≥2.5 kPa, and increased ALT level (>40 U/L for men, >30 U/L for women) were enrolled. Patients were excluded in case of excessive alcohol consumption (≥210 g/week for men and ≥140 g/week for women), body mass index (BMI) <22 kg/m2, uncontrolled diabetes (HbA1c ≥ 8%), impaired renal function (estimated glomerular filtration rate [eGFR] <30 mL min‐1 1.73‐1 m‐2 or on dialysis), cirrhosis, biliary obstruction or chronic liver diseases other than NAFLD. All the inclusion/exclusion criteria are shown in Table S1. Written informed consent was obtained from all patients.
2.3. Randomisation and masking
Randomisation and double‐blind procedures were performed to avoid any bias in patient selection and assessment. The patients were randomised at a 1:1 ratio to receive either Pemafibrate (0.2 mg, twice daily) or placebo, using a dynamic allocation method. Random allocation was performed using an interactive web response system (CAC Croit Corporation, Tokyo, Japan). To minimise bias, the patients, investigators, staff, image analysts, specialists and sponsors remained blinded until the end of the study, even after the primary 24‐week analysis. The primary analysis was carried out by independent unblinded statisticians (University of Tsukuba), and the results were provided to the sponsor; however, any information that could reveal the allocation of patients (individual actual data and individual adverse events etc) was concealed by statisticians. Active drug and placebo tablets and packages were confirmed to be indistinguishable by an external vendor (CAC Croit Corporation) before the study. The original randomisation list was maintained by the external vendor throughout the study. In addition, the randomisation list for emergency use was stored by another external vendor (BELLSYSTEM24, Inc, Tokyo, Japan), and a procedure was prepared to disclose allocation information of a patient if requested by investigators.
2.4. Procedures
Patients took their assigned medication (Pemafibrate 0.2 mg tablet or placebo) orally, one tablet at a time, twice daily, for 72 weeks. The adherence to medication was checked by pill counting. Study visits were set every 4 weeks from the start of treatment to week 24 and every 8 weeks from week 24 onwards. Liver fat content and liver stiffness were measured at screening that was performed before randomisation and subsequently at week 0, 24, 48 and 72. Blood samples were collected and analysed at a central measurement institute (LSI Medience Inc, Tokyo, Japan). At week 0 and 12, lipid content of lipoproteins by subclasses was measured by high‐performance liquid chromatography (HPLC) (Skylight Biotech Inc, Akita, Japan).
MRI and MRE imaging and assessment were carried out with reference to previous reports. 11 All imaging assessments were performed blinded. The equipment used in all study sites was standardised to a 3.0T MR Imaging System (GE Healthcare, Little Chalfont, UK). As an application, we used IDEAL‐IQ (Iterative Decomposition of water and fat with Echo Asymmetry and the Least squares estimation quantification sequence) for MRI‐PDFF and MR‐touch for MRE. Detailed imaging conditions and procedures were described in a prespecified manual and standardised for all tests. Imaging was performed at ≥4 hours after meals, and the timing of tests (pre‐breakfast/post‐breakfast to pre‐lunch/post‐lunch) was consistent for each patient throughout the study. Imaging data obtained at the study sites were sent to the imaging data centre (Micron, Inc, Tokyo, Japan.). The image analysts there set the region of interest (ROI) and assessed the images in accordance with the instruction in the prespecified manual. All images obtained from the same patient were assessed by one image analyst throughout the study. The validity of the analysis results was determined by two masked specialists (KI and NT).
2.5. Outcomes
The primary efficacy endpoint was the percentage change in liver fat content measured by MRI‐PDFF from baseline to week 24. Liver stiffness measured by MRE was a secondary parameter, and it was similarly assessed as percentage change from baseline. The MRE‐based liver stiffness measurement (LSM) was categorised into four stages based on the MRE thresholds of 2.61, 2.97, 3.62 and 4.69 kPa for stage 1, 2, 3 and 4, respectively, which well corresponded to biopsy‐proven fibrosis stages as previously described. 12 The proportion of treatment responders according to these imaging data was also assessed based on the following definitions of responders and patients whose conditions worsened: the percentage change from baseline in liver fat content of ≤−30% 13 and ≥+30%; the percentage change from baseline in MRE‐based liver stiffness of ≤−15% 14 and ≥+15% 15 ; and the change from baseline in MRE‐based LSM stage of ≤−1 and ≥+1 respectively. Other efficacy parameters included liver enzymes (ALT, AST and GGT), fibrosis and inflammatory markers (M2BPGi, hyaluronic acid, 7S domain of type IV collagen, ELF test, NAFLD fibrosis score, FIB4 index, NAFIC score and CK‐18 M30) and lipid parameters (total cholesterol, LDL cholesterol, HDL cholesterol, non‐HDL cholesterol and triglycerides). Although ALP, total bilirubin and platelets were specified as safety parameters in the study protocol, they were also analysed as efficacy parameters in the post hoc analysis.
Safety endpoints included the incidence of adverse events and adverse drug reactions during the study. Adverse events were any unfavourable events that did not necessarily have causal relationships with the allocated treatment, and adverse drug reactions were the adverse events whose causal relationships with the treatment could not be ruled out. The changes in laboratory parameters including renal function tests and blood glucose‐related tests were also monitored.
2.6. Statistical analyses
All patients who were randomised and received at least one dose of the study treatment were included in the safety analysis set. Of those, patients who had both baseline and post‐baseline measurements of the efficacy parameters were included in the full analysis set. The efficacy and safety analyses were performed on efficacy analysis set (FAS) and the safety analysis set respectively.
The baseline levels were defined as the mean of levels at screening and week 0 if both were available and as the level at week 0 if screening measurement was unavailable. The efficacy parameters assessed at every 24 weeks and those assessed only at one time point as post‐treatment values were evaluated based on analysis of covariance, with the treatment group (placebo or Pemafibrate) and stratification factor (with or without taking SGLT2 inhibitors) as fixed effects and the baseline value as a covariate. The remaining efficacy parameters, measured at all visits, were evaluated using a mixed‐effects model for repeated measures. This model included the treatment group, stratification factor, week and treatment‐by‐week interaction as fixed effects and baseline as a covariate. An unstructured covariance structure was used to model within‐patient errors.
Response rates were compared using Fisher's exact test. A patient without on‐treatment value was treated as a non‐responder in the responder analysis. Subgroup analyses were performed for efficacy parameters.
For statistical hypothesis testing, a two‐sided test with a significance level of 0.05 was used. Analyses were performed using SAS 9.4 (SAS Institute). Assuming a 20% point of difference of the Pemafibrate group from the placebo group with a standard deviation of 33% for the primary endpoint of percentage change from baseline in liver fat content at week 24, a two‐sided 5% significance level and a 1:1 allocation ratio per group, 44 patients per group were required to ensure 80% power. Considering the possibility of discontinuation and dropout, 50 patients in each group were set as the target sample size.
This study is registered with ClinicalTrials.gov, NCT03350165.
3. RESULTS
Between December 27, 2017 and October 24, 2018, 180 patients were screened, and 118 eligible patients were randomly assigned to the placebo group (n = 60) or the Pemafibrate group (n = 58) (Figure 1). A total of 118 patients were included in FAS. However, one patient in the Pemafibrate group did not undergo post‐treatment imaging assessments due to study withdrawal and was not included in the analysis of imaging parameters. A total of 118 patients were included in the safety analysis.
FIGURE 1.
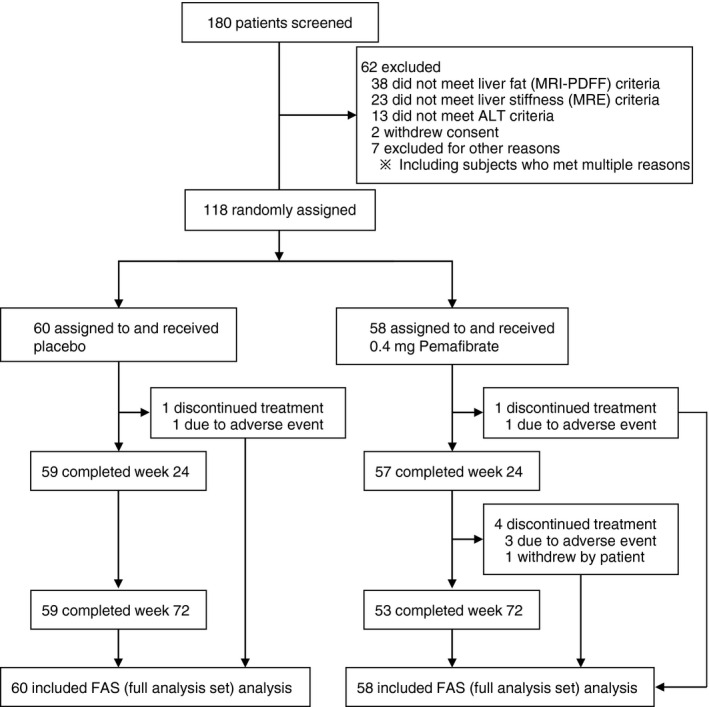
Flow diagram of patient disposition
The baseline patient characteristics are shown in Table 1. The mean age of patients was 53 years, and male patients accounted for more than half of the total study population. 36.4% (43/118) and 57.6% (68/118) of the patients were complicated with type 2 diabetes and dyslipidaemia, respectively. The BMI was approximately 30 kg/m2, with insulin resistance suggested by fasting glucose, insulin and HOMA‐R. Liver fat content and stiffness were 18.4% and 3.1 kPa, respectively, and more than half of the patients were with LSM stage of 2 or more. Serum ALT levels were 88.8 U/L. The treatment groups had similar characteristics with no significant difference in any parameter except for concomitant use of polyunsaturated fatty acids.
TABLE 1.
Baseline characteristics of the study population
| Placebo (n = 60) | Pemafibrate (n = 58) | |
|---|---|---|
| Demographics | ||
| Age, years | 53.3 (16.6) | 53.2 (12.5) |
| Male | 37 (61.7) | 31 (53.4) |
| Comorbidities | ||
| Type 2 diabetes a | 25 (41.7) | 18 (31.0) |
| Hyperlipidaemia a | 37 (61.7) | 31 (53.4) |
| Hypertension a | 28 (46.7) | 26 (44.8) |
| Metabolic syndrome b | 41 (68.3) | 38 (65.5) |
| Concomitant drug uses | ||
| Antidiabetics | 17 (28.3) | 14 (24.1) |
| Sulphonylurea | 4 (6.7) | 3 (5.2) |
| SGLT2 inhibitor | 5 (8.3) | 4 (6.9) |
| DPP‐4 inhibitor | 9 (6.7) | 3 (5.2) |
| Metformin | 12 (20.0) | 8 (13.8) |
| Antilipidemics | 36 (60.0) | 29 (50.0) |
| Statin | 26 (43.3) | 18 (31.0) |
| Ezetimibe | 6 (10.0) | 6 (10.3) |
| ω3 polyunsaturated fatty acids c | 6 (10.0) | 0 (0.0) |
| Antihypertensives | 26 (43.3) | 25 (43.1) |
| ARB | 15 (25.0) | 15 (25.9) |
| Vitamin E | 12 (20.0) | 12 (20.7) |
| Liver image | ||
| Liver fat content by MRI‐PDFF, % | 18.1 (5.5) | 18.7 (6.9) |
| MRE‐based liver stiffness, kPa | 3.02 (0.44) | 3.24 (0.81) |
| MRE‐based LSM stage | ||
| ≤1 | 30 (50.0) | 26 (44.8) |
| 2 | 25 (41.7) | 21 (36.2) |
| 3≥ | 5 (8.3) | 11 (19.0) |
| Metabolic factors | ||
| Body weight, kg | 82.0 (24.8) | 80.0 (16.8) |
| Body mass index, kg/m2 | 29.8 (6.5) | 29.5 (4.9) |
| Waist circumference, cm | 99.9 (15.1) | 100.2 (10.4) |
| Fasting glucose, mg/dL | 111 (18) | 110 (17) |
| Fasting insulin, mU/L | 13.0 (7.0) | 12.4 (4.9) |
| HOMA‐R | 3.72 (2.85) | 3.39 (1.45) |
| Haemoglobin A1c, % | 6.13 (0.68) | 6.09 (0.62) |
| Glycated albumin, % | 14.2 (2.56) | 14.1 (2.14) |
| Liver function tests | ||
| ALT, U/L | 94.6 (49.4) | 82.8 (36.6) |
| AST, U/L | 57.3 (26.5) | 54.2 (20.7) |
| GGT, U/L | 78.0 (54.1) | 85.3 (73.4) |
| ALP, U/L | 254 (74) | 260 (76) |
| Total bilirubin, mg/dL | 0.98 (0.41) | 0.94 (0.40) |
| Platelets, 1010/L | 23.1 (5.85) | 23.0 (5.81) |
| Lipids | ||
| Total cholesterol, mg/dL | 202 (37) | 209 (34) |
| LDL cholesterol, mg/dL | 122 (29) | 131 (29) |
| HDL cholesterol, mg/dL | 48.4 (11.3) | 49.0 (8.9) |
| Non‐HDL cholesterol, mg/dL | 154 (36) | 160 (31) |
| Triglycerides, mg/dL | 190 (148) | 166 (63) |
| Inflammatory marker | ||
| CK‐18 M30, U/L | 575 (316) | 480 (281) |
| Fibrosis markers | ||
| Hyaluronic acid, μg/L | 53.5 (56.0) | 68.7 (97.1) |
| 7S domain of type IV collagen, μg/L | 4.78 (1.17) | 5.02 (1.87) |
| M2BPGi | 0.89 (0.39) | 1.03 (0.67) |
| NAFLD fibrosis score | −1.66 (1.69) | −1.68 (1.47) |
| FIB4 index | 1.62 (1.15) | 1.61 (1.00) |
| NAFIC score | 1.9 (1.3) | 1.8 (1.2) |
| ELF test | 9.72 (0.94) | 9.87 (1.01) |
| Renal function tests | ||
| Creatinine, mg/dL | 0.73 (0.16) | 0.71 (0.16) |
| Estimated glomerular filtration rate, mL min‐1 1.73‐1 m‐2 | 83.4 (19.8) | 82.8 (17.0) |
| Single nucleotide polymorphisms | ||
| PNPLA3 (rs738409) | ||
| C/C | 12 (20.0) | 5 (8.6) |
| C/G | 25 (41.7) | 23 (39.7) |
| G/G | 23 (38.3) | 30 (51.7) |
| TM6SF2 (rs58542926) | ||
| C/T + T/T | 48(80.0) | 41(70.7) |
| C/C | 12(20.0) | 17(29.3) |
Data are expressed as mean (SD) or n (%).
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; ARB, angiotensin II receptor blocker; AST, aspartate aminotransferase; CK‐18, cytokeratin 18; DPP‐4, dipeptidyl peptidase‐4; ELF test, enhanced liver fibrosis test; GGT, γ‐glutamyl transferase; HDL, high‐density lipoprotein; HOMA‐R, homoeostasis model assessment‐estimated insulin resistance; LDL, low‐density lipoprotein; LSM, liver stiffness measurement; M2BPGi, mac‐2‐binding protein glycosylation isomer; MRE, magnetic resonance elastography; MRI‐PDFF, magnetic resonance imaging‐proton density fat fraction; PNPLA3, patatin‐like phospholipase domain‐containing protein 3; SGLT2, sodium glucose cotransporter 2; TM6SF2, transmembrane 6 superfamily member 2.
Physician‐reported diagnosis.
Defined as patients with increased waist circumference and two or more risk factors as of abnormalities in serum lipids, blood pressure and fasting plasma glucose in accordance with the diagnostic criteria for metabolic syndrome in Japan.
P < 0.05, in comparison between the treatment groups by Fisher's exact test.
Liver fat content measured by MRI‐PDFF did not significantly change from baseline over 72 weeks including the primary endpoint as of week 24 (Pemafibrate −5.3% vs placebo −4.2%: least square mean difference of the percent change vs placebo, −1.0%, 95% CI −11.5 to 9.4 [P = 0.85]) (Figure 2A; Table 2). However, MRE‐based liver stiffness significantly decreased in the Pemafibrate group at week 24, 48 and 72 by −5.0%, 95% CI −8.5 to −1.6 (P = 0.0049), −9.0%, 95% CI −12.8 to −5.2 (P < 0.0001) and −7.3%, 95% CI −11.1 to −3.5 (P = 0.0002) respectively. Liver stiffness at week 48 and 72 was significantly improved in the treatment group with the least square mean differences of −5.7%, 95% CI −11.0 to −0.4 (P = 0.036) and −6.2%, 95% CI −11.5 to −0.8 (P = 0.024) vs placebo respectively (Figure 2B; Table 2).
FIGURE 2.
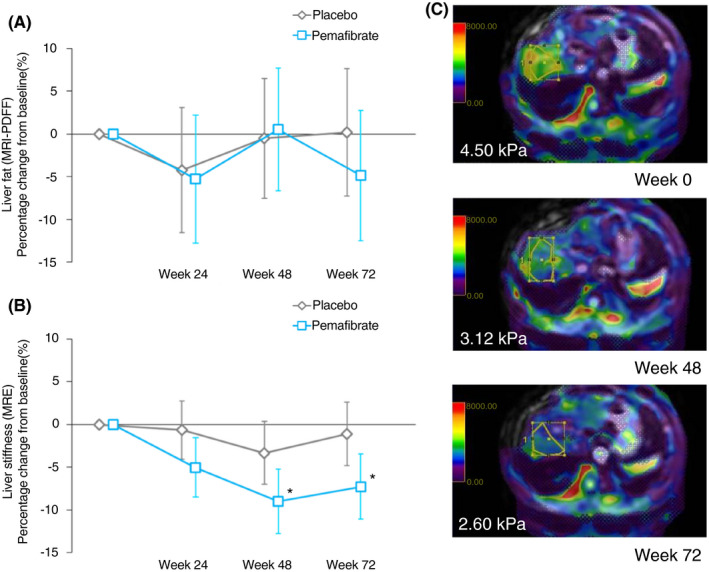
Percentage change from baseline to 72 weeks in liver fat content by MRI‐PDFF (A) and liver stiffness by MRE (B). Data are expressed as least square mean. Error bars show 95% CI. *P < 0.05 vs placebo. Representative images of MRE of a patient (C). ROIs, the areas surrounded by yellow line, were set in the right lobe of the liver in accordance with the instruction in the prespecified manual [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Results of liver imaging and serum markers of liver function, lipids, inflammation and fibrosis
| Placebo (n = 60) | Pemafibrate (n = 58) | Difference of Pemafibrate vs Placebo | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 24 | Week 48 | Week 72 | Week 24 | Week 48 | Week 72 | Week 24 | Week 48 | Week 72 | |
| Liver imaging | |||||||||
| Liver fat content by MRI‐PDFF | −4.2 [−11.6, 3.1] | −0.5 [−7.5, 6.5] | 0.2 [−7.2, 7.7] | −5.3 [−12.8, 2.2] | 0.5 [−6.7, 7.7] | −4.9 [−12.5, 2.8] | −1.0 [−11.5, 9.4] | 1.0 [−9.0, 11.1] | −5.1 [−15.8, 5.6] |
| P valuea | 0.85 | 0.84 | 0.35 | ||||||
| Responder, % (n) | 18.3 (11) | 11.7 (7) | 11.7 (7) | 20.7 (12) | 10.3 (6) | 22.4 (13) | |||
| [9.5, 30.4] | [4.8, 22.6] | [4.8, 22.6] | [11.2, 33.4] | [3.9, 21.2] | [12.5, 35.3] | ||||
| P valueb | 0.82 | 1.00 | 0.14 | ||||||
| Liver stiffness by MRE | −0.7 [−4.0, 2.7] | −3.3 [−7.0, 0.3] | −1.1 [−4.8, 2.6] | −5.0 [−8.5, −1.6] | −9.0 [−12.8, −5.2] | −7.3 [−11.1, −3.5] | −4.4 [−9.3, 0.5] | −5.7 [−11.0, −0.4] | −6.2 [−11.5, −0.8] |
| P valuea | 0.078 | 0.036* | 0.024* | ||||||
| Responder, % (n) | 15.0 (9) [7.1, 26.6] | 18.3 (11) [9.5, 30.4] | 15.0 (9) [7.1, 26.6] | 19.0 (11) [9.9, 31.4] | 37.9 (22) [25.5, 51.6] | 25.9 (15) [15.3, 39.0] | |||
| P valueb | 0.63 | 0.024* | 0.17 | ||||||
| Liver function tests | |||||||||
| ALT | −12.5 [−20.8, −4.1] | −15.8 [−24.3, −7.3] | −10.2 [−19.2, −1.3] | −39.5 [−48.1, −31.0] | −42.5 [−51.3, −33.8] | −43.8 [−53.2, −34.5] | −27.1 [−39.1, −15.1] | −26.8 [−39.0, −14.5] | −33.6 [−46.5, −20.7] |
| P valuec | <0.0001*** | <0.0001*** | <0.0001*** | ||||||
| AST | −6.5 [−17.7, 4.6] | −9.6 [−20.1, 1.0] | −5.1 [−16.5, 6.3] | −9.2 [−20.5, 2.1] | −9.7 [−20.6, 1.1] | −9.6 [−21.4, 2.2] | −2.7 [−18.6, 13.2] | −0.2 [−15.3, 15.0] | −4.5 [−20.9, 12.0] |
| P valuec | 0.74 | 0.98 | 0.59 | ||||||
| GGT | −8.4 [−14.0, −2.8] | −8.9 [−15.8, −2.1] | −7.3 [−14.1, −0.4] | −51.3 [−57.0, −45.6] | −43.8 [−50.8, −36.8] | −47.2 [−54.3, −40.0] | −43.0 [−51.97, −35.0] | −34.9 [−44.7, −25.0] | −39.9 [−49.8, −30.0] |
| P valuec | <0.0001*** | <0.0001*** | <0.0001*** | ||||||
| ALP | 6.1 [2.8, 9.4] | 4.3 [0.8, 7.9] | 5.6 [1.9, 9.2] | −33.6 [−36.9, −30.2] | −35.3 [−38.9, −31.7] | −34.0 [−37.8, −30.3] | −39.7 [−44.4, −35.0] | −39.6 [−44.7, −34.5] | −39.6 [−44.8, −34.4] |
| P valuec | <0.0001*** | <0.0001*** | <0.0001*** | ||||||
| Total bilirubin | −4.5 [−10.1, 1.1] | 3.7 [−1.6, 9.0] | −1.1 [−6.8, 4.6] | −21.1 [−26.7, −15.4] | −20.9 [−26.4, −15.4] | −19.8 [−25.8, −13.9] | −16.6 [−24.5, −8.6] | −24.6 [−32.3, −17.0] | −18.7 [−27.0, −10.5] |
| P valuec | 0.0001** | <0.0001*** | <0.0001*** | ||||||
| Platelets | 0.9 [−1.4, 3.2] | −1.0 [−3.7, 1.6] | 3.0 [−0.3, 6.2] | 13.4 [11.1, 15.7] | 15.1 [12.4, 17.8] | 15.6 [12.3, 19.0] | 12.5 [9.2, 15.8] | 16.1 [12.3, 19.9] | 12.7 [8.0, 17.4] |
| P valuec | <0.0001*** | <0.0001*** | <0.0001*** | ||||||
| Lipids | |||||||||
| Total cholesterol | 0.4 [−2.6, 3.4] | 1.6 [−1.1, 4.4] | 3.7 [0.8, 6.7] | −11.2 [−14.2, −8.1] | −11.6 [−14.4, −8.7] | −11.1 [−14.2, −8.1] | −11.5 [−15.8, −7.3] | −13.2 [−17.1, −9.2] | −14.9 [−19.1, −10.6] |
| P valuec | <0.0001*** | <0.0001*** | <0.0001*** | ||||||
| LDL cholesterol | −1.1 [−5.6, 3.5] | 2.1 [−1.9, 6.2] | 2.7 [−1.6, 6.9] | −14.7 [−19.3, −10.1] | −14.2 [−18.4, −10.1] | −12.5 [−16.8, −8.1] | −13.7 [−20.1, −7.2] | −16.4 [−22.2, −10.5] | −15.1 [−21.2, −9.0] |
| P valuec | 0.0001** | <0.0001*** | <0.0001*** | ||||||
| HDL cholesterol | 5.9 [2.3, 9.5] | 3.8 [0.7, 7.0] | 4.5 [1.0, 7.9] | −1.7 [−5.3, 2.0] | −6.4 [−9.6, −3.2] | −7.4 [−10.9, −3.8] | −7.6 [−12.7, −2.5] | −10.2 [−14.7, −5.7] | −11.9 [−16.8, −6.9] |
| P valuec | 0.0040** | <0.0001*** | <0.0001*** | ||||||
| Non−HDL cholesterol | −1.0 [−4.6, 2.6] | 1.3 [−2.1, 4.6] | 3.9 [0.2, 7.5] | −13.8 [−17.5, −10.2] | −13.0 [−16.4, −9.5] | −11.9 [−15.7, −8.2] | −12.8 [−18.0, −7.7] | −14.2 [−19.1, −9.4] | −15.8 [−21.0, −10.6] |
| P valuec | <0.0001*** | <0.0001*** | <0.0001*** | ||||||
| Triglycerides | −3.5 [−10.0, 3.0] | −1.3 [−8.6, 5.9] | 1.1 [−5.0, 7.3] | −34.6 [−41.2, −28.0] | −27.1 [−34.6, −19.7] | −31.5 [−38.0, −25.1] | −31.1 [−40.4, −21.9] | −25.8 [−36.2, −15.4] | −32.7 [−41.6, −23.8] |
| P valuec | <0.0001*** | <0.0001*** | <0.0001*** | ||||||
| Inflammatory marker | |||||||||
| CK‐18 M30 | −7.3 [−21.7, 7.2] | −10.4 [−28.6, 7.7] | 2.2 [−15.6, 20.0] | −16.9 [−31.6, −2.2] | −8.3 [−26.8, 10.2] | 2.3 [−15.9, 20.4] | −9.6 [−30.3, 11.1] | 2.2 [−23.9, 28.3] | 0.0 [−25.5, 25.6] |
| P valuea | 0.36 | 0.87 | 1.00 | ||||||
| Fibrosis markers | |||||||||
| M2BPGi | −13.5 [−20.1, −6.9] | −13.7 [−18.1, −9.3] | −11.1 [−16.6, −5.6] | −27.3 [−34.0, −20.6] | −29.7 [−34.2, −25.2] | −32.0 [−37.6, −26.3] | −13.8 [−23.3, −4.4] | −16.0 [−22.3, −9.7] | −20.9 [−28.8, −13.0] |
| P valuea | 0.0044** | <0.0001*** | <0.0001*** | ||||||
| Hyaluronic acid | 32.3 [10.6, 53.9] | 21.1 [−7.2, 49.3] | 20.9 [3.1, 38.7] | 6.3 [−15.7, 28.4] | −5.2 [−34.0, 23.5] | 3.7 [−14.4, 21.8] | −25.9 [−56.9, 5.1] | −26.3 [−66.7, 14.1] | −17.1 [−42.6, 8.3] |
| P valuea | 0.10 | 0.20 | 0.19 | ||||||
| 7S domain of type IV collagen | 7.6 [3.0, 12.2] | 5.6 [1.1, 10.1] | 4.9 [−0.7, 10.6] | 4.9 [0.3, 9.6] | 3.3 [−1.3, 7.9] | 1.6 [−4.2, 7.3] | −2.7 [−9.3, 3.9] | −2.3 [−8.8, 4.1] | −3.3 [−11.4, 4.7] |
| P valuea | 0.42 | 0.48 | 0.41 | ||||||
| ELF test | 0.1 [−1.5, 1.6] | −1.1 [−2.5, 0.3] | −0.4 [−2.0, 1.1] | −1.6 [−3.2, −0.0] | −1.7 [−3.1, −0.3] | −1.8 [−3.4, −0.3] | −1.7 [−3.9, 0.6] | −0.6 [−2.6, 1.4] | −1.4 [−3.6, 0.8] |
| P valuea | 0.14 | 0.56 | 0.21 | ||||||
| NAFLD fibrosis score | −38.0 [−84.4, 8.4] | −25.5 [−86.8, 35.7] | −30.4 [−64.5, 3.4] | −19.5 [−66.7, 27.6] | −30.2 [−92.6, 32.3] | −19.2 [−53.8, 15.4] | 18.5 [−47.7, 84.7] | −4.6 [−92.1, 82.8] | 11.3 [−37.2, 59.8] |
| P valuec | 0.59 | 0.90 | 0.67 | ||||||
| FIB4 index | −2.6 [−9.5, 4.3] | −1.3 [−8.2, 5.7] | −3.1 [−11.4, 5.3] | 1.6 [−5.4, 8.6] | 1.6 [−5.5, 8.8] | 2.5 [−6.1, 11.1] | 4.2 [−5.7, 14.1] | 2.9 [−7.1, 12.9] | 5.6 [−6.4, 17.6] |
| P valuec | 0.41 | 0.60 | 0.38 | ||||||
| NAFIC score | 12.1 [−6.6, 30.8] | −2.1 [−21.6, 17.5] | 9.3 [−10.5, 29.2] | −6.1 [−25.7, 13.5] | 1.6 [−19.2, 22.4] | −5.8 [−26.8, 15.3] | −18.2 [−45.3, 8.9] | 3.7 [−24.9, 32.2] | −15.1 [−44.0, 13.8] |
| P valuea | 0.19 | 0.80 | 0.30 | ||||||
Data are expressed as least square mean of the percentage change from baseline [95% CI] unless otherwise indicated as % (n). Missing data were imputed by carrying the last observation forward. The measured values of cytokeratin 18 that exceeded the upper limit of quantitation of 1000 (U/L) were substituted with 1000.1(U/L). MRI‐PDFF responder was defined as patients with the percentage change in MRI‐PDFF of ≤−30%. MRE responder was defined as patients with the percentage change in MRE‐based liver stiffness of ≤−15%. Least square mean and 95% CI were estimated by analysis of covariance or mixed‐effect model for repeated measures.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK‐18, cytokeratin 18; ELF test, enhanced liver fibrosis test; GGT, γ‐glutamyl transferase; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; M2BPGi, mac‐2‐binding protein glycosylation isomer; MRE, magnetic resonance elastography; MRI‐PDFF, magnetic resonance imaging‐proton density fat fraction.
*P value <0.05, **P value <0.01, ***P value <0.001.
P value for comparison between the treatment groups by t‐test with analysis of covariance.
P value for comparison between the treatment groups by Fisher's exact test.
P value for comparison between the treatment groups by t‐test with mixed‐effect model for repeated measures.
The proportion of MRI‐PDFF responders, whose MRI‐PDFF reduced by 30% or more, showed no significant difference between the groups; However, there was a trend toward more MRE responders, whose liver stiffness reduced by 15% or more, in the Pemafibrate group throughout the study period with a significantly greater proportion in the Pemafibrate group at week 48 (Table 2). When the ‘worsened’ category was added in the responder analyses as described in the Methods to better understand how the treatment responses of non‐responders, there were a significantly smaller proportion of “worsened” patients in the Pemafibrate group in terms of MRE‐based LSM stage at weeks 24 and 72 (Figure S1C). The subgroup analyses of the percentage change in MRI‐PDFF and MRE‐based liver stiffness showed no significant interaction with any evaluated factors except for metabolic syndrome status on the treatment effect on MRI‐PDFF (Figure S2).
Significant reductions in ALT, GGT and ALP levels were observed in the Pemafibrate group (Table 2). These changes occurred initially at week 4 and persisted until the end of the study (Figure 3; Table S2). The proportion of patients whose levels of ALT and AST were below the upper limit of normal level was consistently higher in the Pemafibrate group (Figure S3). Pemafibrate significantly reduced the mac‐2‐binding protein glycosylation isomer (M2BPGi), compared with the placebo throughout the study (Table 2). Other serum fibrosis markers such as hyaluronic acid, type IV collagen 7S and ELF test were not significantly different between the two groups (Table 2). In the subpopulation of MRE responders at week 72, each efficacy parameter showed a greater improvement at week 72 (Figure S4).
FIGURE 3.
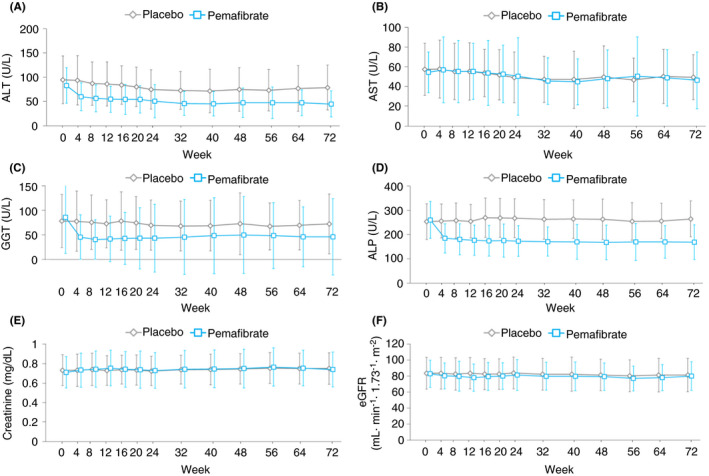
Measured levels of liver (A‐D) and renal function (E, F) markers over 72 weeks. Data are expressed as mean. Error bars show SD. ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ‐glutamyl transpeptidase; ALP, alkaline phosphatase; eGFR, estimated glomerular filtration rate [Colour figure can be viewed at wileyonlinelibrary.com]
Pemafibrate treatment significantly reduced the total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), HDL‐C, non‐HDL‐C and TG levels. These reductions were maintained over 72 weeks (Figure 4; Table 2; Table S2). The subclass analysis of HDL‐C showed that the cholesterol content in the smaller HDL particles increased whereas that in the larger HDL particles decreased in the Pemafibrate group (Figure S5).
FIGURE 4.
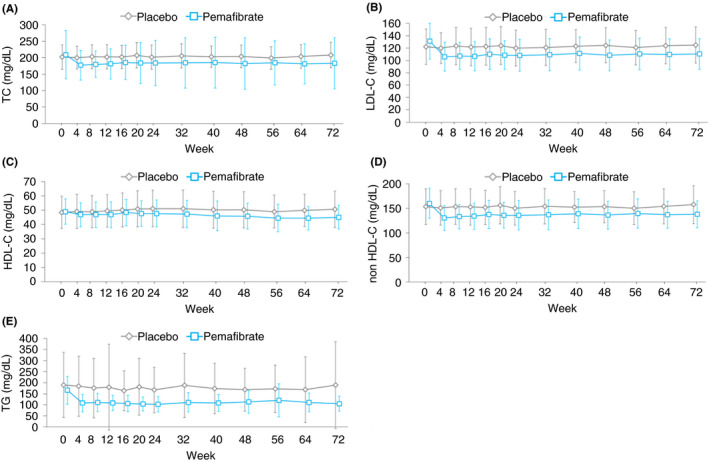
Measured levels of lipid markers over 72 weeks. Data are expressed as mean. Error bars show SD. TC, total cholesterol; LDL‐C, low‐density lipoprotein‐cholesterol; HDL‐C, high‐density lipoprotein‐cholesterol; TG, triglyceride [Colour figure can be viewed at wileyonlinelibrary.com]
During the 72‐week study period, adverse events were mostly mild and moderate in severity, with only one severe adverse event in each group. The proportion of patients with at least one or more adverse events was 86.2% (50/58) in the Pemafibrate and 85.0% (51/60) in the placebo group, and adverse drug reactions were 17.2% (10/58) and 11.7% (7/60) respectively (Table 3). Six serious adverse events (obstructive pancreatitis, erysipelas, facial bone fracture, altered state of consciousness, cerebral infarction and nephrolithiasis) occurred in five patients in the Pemafibrate group. Four serious adverse events (retinal haemorrhage, duodenal ulcer perforation, contusion and uterine polyp) occurred in three patients in the placebo group. These adverse events were not study drug related. There were no deaths throughout the study. Four patients in the Pemafibrate group were withdrawn owing to adverse events (diabetes mellitus, altered state of consciousness, cerebral infarction and generalised rash) and one patient in the placebo group owing to a liver disorder. Regarding the incidence of total adverse events, serious adverse events and adverse events leading to drug discontinuation, no significant differences were observed between the groups. The same was applicable for individual adverse events. No safety concerns were observed regarding renal function such as serum creatinine and eGFR (Figure 3). In addition, there were no clinically relevant changes in glycaemic markers and body weight (Figure S6; Table S2).
TABLE 3.
Adverse events
| Placebo | Pemafibrate | |
|---|---|---|
| (n = 60) | (n = 58) | |
| Death | 0 | 0 |
| Serious adverse events | 3 (5.0) | 5 (8.6) |
| Treatment‐related serious adverse events | 0 | 0 |
| Adverse events leading to discontinuation | 1 (1.7) | 4 (6.9) |
| Treatment‐related adverse events leading to discontinuation | 1 (1.7) | 0 |
| Adverse events | 51 (85.0) | 50 (86.2) |
| Relatively common adverse events (≥5%) | ||
| Gastrointestinal disorders | ||
| Constipation | 3 (5.0) | — |
| Dental caries | 1 (1.7) | 3 (5.2) |
| Diarrhoea | 3 (5.0) | 7 (12.1) |
| Vomiting | 3 (5.0) | 1 (1.7) |
| General and general disorders and administration site conditions | ||
| Pyrexia | 3 (5.0) | 2 (3.4) |
| Infectious and parasitic diseases | ||
| Bronchitis | 2 (3.3) | 4 (6.9) |
| Conjunctivitis | 3 (5.0) | — |
| Cystitis | 3 (5.0) | 2 (3.4) |
| Gastroenteritis | 3 (5.0) | 2 (3.4) |
| Influenza | 2 (3.3) | 3 (5.2) |
| Nasopharyngitis | 24 (40.0) | 24 (41.4) |
| Injury, poisoning and procedural complications | ||
| Ligament sprain | — | 3 (5.2) |
| Contusion | 3 (5.0) | 2 (3.4) |
| Laboratory tests | ||
| Blood creatinine phosphokinase increased | 2 (3.3) | 4 (6.9) |
| Glycosylated haemoglobin increased | — | 3 (5.2) |
| Metabolism and nutrition disorders | ||
| Dehydration | 1 (1.7) | 3 (5.2) |
| Diabetes mellitus | 3 (5.0) | 4 (6.9) |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia | 3 (5.0) | 4 (6.9) |
| Back pain | 3 (5.0) | 9 (15.5) |
| Respiratory, thoracic and mediastinal disorders | ||
| Cough | — | 3 (5.2) |
| Upper respiratory tract inflammation | 3 (5.0) | 3 (5.2) |
| Skin and subcutaneous tissue disorders | ||
| Rash | — | 4 (6.9) |
| Vascular disorders | ||
| Hypertension | 5 (8.3) | 2 (3.4) |
Data are n (%).
4. DISCUSSION
To our knowledge, this phase 2 study is the first to evaluate the efficacy and safety of Pemafibrate in patients with NAFLD in a 72‐week placebo‐controlled design. The patients with NAFLD were regarded as high risk due to both increased liver stiffness and ALT, and its baseline levels of MRE‐based liver stiffness were similar to levels in other clinical trial which enrolled patients with biopsy‐proven NASH and stage 1‐3 fibrosis. 16 In this population, Pemafibrate did not reduce the liver fat content, but significantly decreased MRE‐based liver stiffness. The change in liver stiffness was accompanied with a significant improvement in serum liver enzymes such as ALT, GGT and ALP. Furthermore, serum lipids such as LDL‐C and TG also significantly decreased. Meanwhile, the incidence of adverse events was similar between the groups, and the long‐term treatment with Pemafibrate was well tolerated. Similar efficacy and safety were observed in subgroup patients with baseline TG levels of 150 mg/dL or more; it was explored taking into account that Pemafibrate is approved in Japan for the treatment of hyperlipidaemia (Tables S3‐S6).
In AMLN‐diet‐induced NASH model mice, Pemafibrate improved the histological findings of the liver such as steatosis, ballooning and fibrosis. 10 On the contrary, in STAM NASH model mice, Pemafibrate did not reduce the total liver fat content but reduced ballooning and hepatic macrovesicular steatosis. 17 These animal studies suggested that PPARα activation by Pemafibrate promotes lipid turnover and simultaneously induces TG hydrolysis, fatty acid β‐oxidation, TG synthesis from dihydroxyacetone phosphate and TG re‐esterification in the liver. 9 The finding that Pemafibrate increased hepatic glucose uptake in a clinical pharmacology study 18 suggests that fatty acid turnover was promoted in the liver. Because hepatic macrovesicular steatosis has been suggested to be associated with the development of NASH, 19 further investigation including liver biopsy is warranted to understand why the liver fat content did not decrease but liver stiffness suggested the potential amelioration of NASH conditions.
The decrease in the MRE‐based liver stiffness was the most interesting finding in the present study because it may be collectively supported by the percentage changes in some of the other serum markers. MRE‐based liver stiffness well predicted the fibrosis stage diagnosed with liver biopsy. 20 , 21 As for the treatment response, a decrease of ≥15% in liver stiffness was associated with a significant improvement in fibrosis markers. 14 Taking it as the cut‐off value to define a responder, the rate of responders was higher in the Pemafibrate group throughout the study period although a statistical significance was found only at week 48 (Table 2). In addition, there was a significant decrease in M2BPGi, which is a novel marker reflecting the progression of liver fibrosis and is associated with MRE‐based liver stiffness independent of age, 22 together with greater reductions in liver enzymes and other fibrosis markers such as ELF test, hyaluronic acid and type 4 collagen 7S in MRE responders than non‐responders in the Pemafibrate group, although there were significant interaction only in AST (Figure S4). It is well known that advanced liver fibrosis is associated with an increased risk of developing liver‐related events and predicts the prognosis of patients with NAFLD. 23 The significant reductions in MRE‐based liver stiffness and serum liver markers suggest the improvement in liver fibrosis. However, considering that MRE‐based liver stiffness is also correlated with liver inflammation, these findings may be suggestive of the improvement in liver inflammation as a driver. 20 It needs further research to confirm the histological improvement, and its consequence for the prognosis of patients will be of the next interests.
In a study with NASH model mice, Pemafibrate improved liver fibrosis with the reduction in collagen 1α1 mRNA expression in the liver where the ALT level and the expression of pro‐inflammatory genes also decreased. 10 Therefore, this finding suggests that it might improve liver fibrosis via the alleviation of inflammation and/or direct suppression of liver fibrosis. As previously shown in clinical trials in patients with dyslipidaemia, 6 Pemafibrate significantly reduced the serum levels of ALT, GGT and ALP in the present study. Especially, the ALT reduction with Pemafibrate by approximately 40% from baseline throughout the treatment period was greater than that with elafibranor (GFT505), which is a dual PPARα/δ agonist developed for NAFLD/NASH treatment. 24 Therefore, it is reasonable to expect that Pemafibrate will have a stronger treatment effect on inflammation in NAFLD/NASH.
We found that Pemafibrate reduces the LDL‐C, non‐HDL‐C and TG levels unlike several drugs that have been developed for the management of NASH and shows negative effects on blood lipids (eg FXR agonists, FGF‐19 and ACCi). 5 , 25 , 26 Because NAFLD/NASH is a significant risk factor of cardiovascular events, 4 these effects of Pemafibrate on blood lipids may provide an additional benefit for cardiovascular disease prevention in patients with NAFLD. While previous clinical studies of Pemafibrate in patients with hyperlipidaemia have consistently shown an increase in HDL‐C, in the present study, we observed a decrease in HDL‐C. This may be an overall effect of the reduction and increase in cholesterol in the larger and smaller HDL particles respectively. This may also be considered beneficial as smaller HDL particles play a critical role in the reverse cholesterol transport system. 27 The beneficial effects of Pemafibrate on the reverse cholesterol transport have been examined in basic and clinical research. 28 , 29
In terms of safety, Pemafibrate treatment for 72 weeks was well tolerated in the present study population. The incidence of adverse events was similar in both treatment groups, and there were few concerns about liver, renal or skeletal muscle‐related adverse events. For renal function, the present study reproduced that Pemafibrate did not affect serum creatinine and eGFR, as observed in previous studies in patients with dyslipidaemia. 6 , 18 This should be positively recognised as NAFLD/NASH is associated with increased prevalence of CKD. 30
There were several limitations to this study. First, liver biopsy was not performed. Although non‐invasive and quantitative imaging assessments, such as those used in the present study, have been confirmed to be well associated with liver biopsy results, 20 , 21 , 31 the response of MRE‐based liver stiffness to Pemafibrate treatment was not confirmed with liver histology. However, although liver biopsy remains a gold standard for the diagnosis of NASH 12 and the only approved surrogate endpoint for the development of drugs for NASH, there is no established surrogate endpoint to predict the true clinical outcome as a result of treatment. Additionally, the number of patients was small, and the study was conducted only at Japanese institutions. A larger scale, multinational confirmatory trial with liver biopsy will be needed to verify the efficacy of Pemafibrate in NAFLD/NASH and liver fibrosis and to generalise the findings to a broader spectrum of population.
In summary, in this double‐blind, placebo‐controlled, randomised, multicentre, phase 2 study in patients with high‐risk NAFLD, Pemafibrate treatment for 72 weeks did not reduce liver fat content, but it significantly reduced MRE‐based liver stiffness. Thus, the reduction in MRE‐based liver stiffness may reflect the amelioration of hepatic fibrosis and lobular inflammation by the significant changes in serum liver markers. The significant reductions in serum lipid parameters may also provide additional benefit in terms of cardiovascular disease risk. Moreover, the favourable safety profile of Pemafibrate will be a further additional value when considering long‐term and concomitant medication for the management of NAFLD/NASH. Therefore, Pemafibrate, a novel SPPARMα, may be a promising therapeutic agent for NAFLD/NASH, and also be a candidate for combination therapy with agents that reduce liver fat.
AUTHORSHIP
Guarantor of the article: Atsushi Nakajima.
Author contributions: A Nakajima, Y Eguchi, H Suganami, T Nojima, R Tanigawa, M Iizuka and R Loomba designed the study; M Yoneda, K Imajo, N Tamaki, R Tanigawa, M Iizuka and Y Iida were responsible for data collection; H Suganami and T Nojima analysed the data; A Nakajima, Y Eguchi, M Yoneda, K Imajo, N Tamaki, R Tanigawa, M Iizuka, Y Iida and R Loomba interpreted the data; all authors participated in manuscript review and writing; R Tanigawa, M Iizuka and Y Iida were responsible for preparing the tables and figures. A statement indicating that all authors approved the final version of the manuscript. All authors had access to the study data and reviewed and approved the final manuscript.
Supporting information
Fig S1‐6
Table S1‐6
ACKNOWLEDGEMENTS
We acknowledge the investigators and patients who participated in this study. This study was conducted at Hokkaido University Hospital, Hokkaido (Naoya Sakamoto); Asahikawa Medical University, Hokkaido (Kazunobu Aso, Koji Sawada); Aomori Prefectural Central Hospital, Aomori (Hiroshi Numao); Yamagata University Hospital, Yamagata (Yoshiyuki Ueno); Fukuwa Clinic, Tokyo (Yasushi Fukushima); Yokohama City University Hospital, Kanagawa (Masato Yoneda); Saiseikai Yokohamashi Tobu Hospital, Kanagawa (Shigeru Nakano), Graduate School of Medical and Dental Sciences, Niigata University, Niigata (Shuji Terai, Kenya Kamimura); Ogaki Municipal Hospital, Gifu (Toshifumi Tada, Satoshi Yasuda), Seirei Hamamatsu General Hospital, Shizuoka (Masamichi Nagasawa); Hamamatsu University Hospital, Shizuoka (Yoshimasa Kobayashi, Kazuhito Kawata); Iwata City Hospital, Shizuoka (Yuzo Sasada, Kazumi Iino); Chutoen General Medical Center, Shizuoka (Masahiro Takayanagi); Shiga University of Medical Science Hospital, Shiga (Katsutaro Morino, Rie Osaki, Takehide Fujimoto); Kurume University School of Medicine, Fukuoka (Takumi Kawaguchi); and Fukuoka University Faculty of Medicine, Fukuoka (Makoto Irie, Kazuhide Takata).
Declaration of personal interests: A Nakajima has received personal fees from Astellas Pharma, Bioferrumin Pharma, EA Pharma, Kowa Company, Ltd., Mylan EPD and Mochida Pharma; grants from Astellas Pharma, Biofermin Pharma, EA Pharma, Kowa Company, Ltd. and Mylan EPD. Y Eguchi has received personal fees from Dainippon Sumitomo Pharmacy, Gilead Sciences, Kowa Company, Ltd. and Novo Nordisk. M Yoneda has received personal fees from Kowa Company, Ltd. H Suganami, T Nojima, R Tanigawa, M Iizuka and Y Iida are employees of Kowa Company, Ltd. R Loomba has served as a consultant for Alnylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol‐Myers Squibb, CohBar, Eli Lilly, Galmed Pharmaceuticals, Gilead Sciences, Glympse Bio, Inipharm, Intercept, Ionis, Janssen, Kowa Research Institute, Inc, Madrigal Pharmaceuticals, Metacrine, Inc, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Sagimet, 89 bio and Viking Therapeutics. In addition, R Loomba's institution has received grant supports from Allergan, AstraZeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Genfit, Gilead Sciences, Intercept, Inventiva, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Pfizer and Siemens. R Loomba is also a co‐founder of Liponexus, Inc. R Loomba has received funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), DOD PRCRP (W81XWH‐18‐2‐0026), NIDDK (U01DK061734, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835) and NIAAA (U01AA029019). K Imajo and N Tamaki have nothing to disclose.
Nakajima A, Eguchi Y, Yoneda M, et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator‐activated receptor α modulator (SPPARMα), versus placebo in patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54:1263–1277. 10.1111/apt.16596
The Handling Editor for this article was Professor Geoffrey Dusheiko, and it was accepted for publication after full peer‐review.
Funding information
This study was funded by Kowa Company, Ltd. The funder of the study contributed to the study design, data collection, data analysis, data interpretation and writing of the report. All authors had access to the study data and reviewed and approved the final manuscript.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Chalasani N, Younossi Z, Lavine Joel E, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005‐2023. [DOI] [PubMed] [Google Scholar]
- 2. National Guideline Centre . NICE Guideline. 2016. [Google Scholar]
- 3. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease‐Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73‐84. [DOI] [PubMed] [Google Scholar]
- 4. Lonardo A, Nascimbeni F, Mantovani A, et al. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68(2):335‐352. [DOI] [PubMed] [Google Scholar]
- 5. Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53(3):362‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamashita S, Masuda D, Matsuzaka Y. Pemafibrate, a new selective PPARα modulator: drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr Atheroscler Rep. 2020;22(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Francque S, Verrijken A, Caron S, et al. PPARα gene expression correlates with severity and histological treatment response in patients with non‐alcoholic steatohepatitis. J Hepatol. 2015;63(1):164‐173. [DOI] [PubMed] [Google Scholar]
- 8. Fruchart J‐C. Pemafbrate (K‐877), a novel selective peroxisome proliferator‐activated receptor alpha modulator for management of atherogenic dyslipidaemia. Cardiovasc Diabetol. 2017;16:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non‐alcoholic fatty liver disease. J Hepatol. 2015;62(3):720‐733. [DOI] [PubMed] [Google Scholar]
- 10. Honda Y, Kessoku T, Ogawa Y, et al. Pemafibrate, a novel selective peroxisome proliferator‐activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci Rep. 2017;14(7):42477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non‐invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2016;65(5):1006‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2019;17(4):630‐637.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel J, Bettencourt R, Cui J, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol. 2016;9(5):692‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loomba R, Lawitz E, Ghalib R, et al. SAT‐489—longitudinal changes in liver stiffness by magnetic resonance elastography (MRE), liver fibrosis, and serum markers of fibrosis in a multi‐center clinical trial in nonalcoholic steatohepatitis (NASH). J Hepatol. 2017;66(1):S671. [Google Scholar]
- 15. Ajmera VH, Liu A, Singh S, et al. Clinical utility of an increase in magnetic resonance elastography in predicting fibrosis progression in nonalcoholic fatty liver disease. Hepatology. 2020;71(3):849‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanyal A, Charles ED, Neuschwander‐Tetri BA, et al. Pegbelfermin (BMS‐986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non‐alcoholic steatohepatitis: a randomized, double‐blind, placebo‐controlled, phase 2a trial. Lancet. 2019;392(10165):2705‐2717. [DOI] [PubMed] [Google Scholar]
- 17. Sasaki Y, Asahiyama M, Tanaka T, et al. Pemafibrate, a selective PPARα modulator, prevents non‐alcoholic steatohepatitis development without reducing the hepatic triglyceride content. Sci Rep. 2020;10(1):7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuba I, Matsuba R, Yamashita S, et al. Effects of a novel selective peroxisome proliferator‐activated receptor‐a modulator, Pemafibrate, on hepatic and peripheral glucose uptake in patients with hypertriglyceridemia and insulin resistance. J Diabetes Investig. 2018;9(6):1323‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saponaro C, Gaggini M, Carli F, et al. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients. 2015;7(11):9453‐9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150(3):626.e7‐637.e7. [DOI] [PubMed] [Google Scholar]
- 21. Jayakumar S, Middleton MS, Lawitz EJ, et al. Longitudinal correlations between MRE, MRI‐PDFF, and liver histology in patients with non‐alcoholic steatohepatitis: analysis of data from a phase II trial of selonsertib. J Hepatol. 2019;70(1):133‐141. [DOI] [PubMed] [Google Scholar]
- 22. Tamaki N, Higuchi M, Kurosaki M, et al. Wisteria floribunda agglutinin‐positive mac‐2 binding protein as an age‐independent fibrosis marker in nonalcoholic fatty liver disease. Sci Rep. 2019;9(1):10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology. 2015;61(5):1547‐1554. [DOI] [PubMed] [Google Scholar]
- 24. Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an agonist of the peroxisome proliferator‐activated receptor‐α and ‐δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150(5):1147.e5‐1159.e5. [DOI] [PubMed] [Google Scholar]
- 25. Stephen AH, Rinella ME, Abdelmalek MF, et al. NGM282 for treatment of non‐alcoholic steatohepatitis: a multicentre, randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet. 2018;391(10126):1174‐1185. [DOI] [PubMed] [Google Scholar]
- 26. Loomba R, Kayali Z, Noureddin M, et al. GS‐0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155(5):1463.e6‐1473.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Camont L, Lhomme M, Rached F, et al. Small, dense high‐density lipoprotein‐3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti‐inflammatory, and antiapoptotic functionalities. Arterioscler Thromb Vasc Biol. 2013;33(12):2715‐2723. [DOI] [PubMed] [Google Scholar]
- 28. Hennuyer N, Duplan I, Paquet C, et al. The novel selective PPARα modulator (SPPARMα) Pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis. 2016;249:200‐208. [DOI] [PubMed] [Google Scholar]
- 29. Yamashita S, Arai H, Yokote K, et al. Effects of Pemafibrate (K‐877) on cholesterol efflux capacity and postprandial hyperlipidemia in patients with atherogenic dyslipidemia. J Clin Lipidol. 2018;12(5):1267.e4‐1279.e4. [DOI] [PubMed] [Google Scholar]
- 30. Mantovani A, Petracca G, Beatrice G, et al. Non‐alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta‐analysis. Gut. 2020;gutjnl‐2020‐323082. [DOI] [PubMed] [Google Scholar]
- 31. Stine JG, Munaganuru N, Barnard A, et al. Change in MRI‐PDFF and histologic response in patients with nonalcoholic steatohepatitis: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2020;S1542–3565(20):31220‐31229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐6
Table S1‐6
Data Availability Statement
Research data are not shared.


