Abstract
Aims
To evaluate the efficacy, sustainability and safety of combined botulinum toxin and polyacrylamide hydrogel (PAHG) therapy to treat urgency and stress components of therapy‐refractory mixed urinary incontinence (MUI) in an elderly study population.
Methods
Fifty‐five women with therapy‐refractory MUI were treated with botulinum toxin and PAHG in one surgical procedure. Urgency urinary incontinence (UUI) and stress urinary incontinence (SUI) outcomes were separately assessed after 4 and 12 months by objective UUI episodes/24 h and cough test, subjective impact of UUI and SUI on quality of life, and subjective International Consultation on Incontinence Questionnaire‐Urinary Incontinence Short Form (ICIQ‐UI SF). MUI outcome was calculated by combining UUI and SUI outcomes. Complications were monitored throughout the study.
Results
At 4 months, objective cure rates were 73%, 53%, and 42%, and subjective cure rates were 71%, 52%, and 50% for SUI, UUI, and MUI. At 12 months, objective cure rates were 73%, 56%, 50% and subjective cure rates were 78%, 42%, and 40% for SUI, UUI, and MUI. The ICIQ‐UI SF score decreased by 9.0 and 8.7 points after 4 and 12 months. All complications were transient and included 22% clean intermittent catheterization immediately after surgery, 33% postvoid residual volumes >100 ml at 14 days, and 13% symptomatic urinary tract infection within the first postoperative month.
Conclusions
The combination of botulinum toxin and PAHG is effective, sustainable and safe to treat therapy‐refractory MUI, even in an elderly and frail study population. Patients benefit from the short surgical procedure without the need for general anaesthesia or discontinuation of anticoagulation.
Keywords: 1‐year follow‐up, Botox, Bulkamid, polyacrylamide hydrogel, stress urinary incontinence, therapy‐refractory urinary incontinence, urgency urinary incontinence
1. INTRODUCTION
Mixed urinary incontinence (MUI) is the combination of both stress urinary incontinence (SUI) and urgency urinary incontinence (UUI), and is defined by the complaint of involuntary leakage associated with effort, sneezing or coughing and also with urgency. 1 MUI is the dominant subtype among ≥65 year‐old women, 2 and is estimated to account for 33% of all incontinence. 3 Whereas the stress component can easily be identified and treated, the urgency component is more difficult to address. 4
MUI is initially treated with behavioral therapy, bladder training, and pelvic floor muscle exercises. 3 Currently, no medication or surgical treatment is specifically indicated for this “mixed” condition. 5 Clinicians commonly turn to a single therapy targeting the predominant symptom, for example, in case of predominant SUI to a sling 4 , 6 or to polyacrylamide hydrogel (PAHG), 6 , 7 , 8 , 9 or in case of predominant UUI to anticholinergics or β‐3 adrenergic agonists. 3 , 5 , 10
The risk of potentiating adverse events such as postvoid residual urine (PVR) and urinary tract infections (UTI), or of a poor outcome with de novo urgency possibly discourages surgeons from a routine combinatory application, especially in a frail patient population. Conversely, these patients would strongly benefit from a short combined intervention without general anesthesia.
To offer this option in the future, this study aims to test a novel combinatory therapy for MUI, by injecting both botulinum toxin to treat UUI and PAHG (Bulkamid®) to treat SUI. Intradetrusor injections of botulinum toxin has been approved for overactive bladder syndrome (OAB) with symptoms of UUI, urgency and frequency in patients who have an inadequate response to or are intolerant of an anticholinergic medicaton. 11 Injection of bulking agents into the urethra to treat SUI has become an alternative to suburethral sling insertions. 12
The present study investigates the efficacy, sustainability and safety of a novel surgical management of MUI by combining UUI and SUI specific treatments in a single operation session. Subjective and objective outcome of both, the UUI and SUI components are evaluated after 4 and 12 months, and a close monitoring of complications brings insight into the safety of this therapy.
2. MATERIALS AND METHODS
Women ≥18 years with a clinical and urodynamic diagnosis of stress dominant MUI, 1 a positive cough stress provocation test, ≥8 micturitions per day, previous unsuccessful conservative, medical or operative treatments for SUI and UUI, and who were willing to use clean intermittent catheterization (CIC) were eligible for study. Exclusion criteria were botulinum toxin or PAHG therapies within the last 12 weeks, pregnancy, lactation, acute UTI and PVR volumes >100 ml.
This single center, prospective observational study (ClinicalTrials.gov NCT02815046) received ethical approval (KEKTGOV2016/12, PB_2018‐00068) and took place from August 2016 to December 2019 in accordance with ICH GCP guidelines and the declaration of Helsinki. All patients gave written informed consent.
Baseline characteristics were assessed at hospital entry. Under peri‐interventional antibiotic prophylaxis with local anesthesia with or without sedation, 20 single doses of botulinum toxin (50–200 units) (Botox®, OnabotulinumtoxinA; Allergan AG) were injected in the detrusor, avoiding the trigone. 13 , 14 Subsequently, about 2 ml (4x ± 0.5 ml) PAHG (Bulkamid®; Contura International A/S) were injected clockwise into four sites of the urethral submucosa. 12 For coaptation, the four depots needed to be localized in the same plane of the proximal third of the urethra. Patients were released from the clinic after successful voiding. Follow‐up visits were planned 14 ± 2 days, 4 ± 2 months and 12 ± 2 months after the intervention.
Co‐primary endpoints were cure and improvement rates of objective and subjective SUI and UUI at months 4 and 12. A 3‐day bladder diary was used to collect UUI characteristics (UUI and urgency episodes/day, micturitions/day, volume per void). 1 , 14 Objective cure was defined by a negative cough test for SUI 15 and no UUI episode/24 h for UUI. 10 , 13 , 14 Subjective cure from general incontinence was defined by an International Consultation on Incontinence Questionnaire‐Urinary Incontinence Short Form (ICIQ‐UI SF) score ≤5. 16 In addition, two questions specifically assessed the self‐evaluated impact of SUI and UUI on quality of life: “Overall, how much does leaking urine while coughing, sneezing or during physical activity interfere with your everyday life?,” and “Overall, how much does leaking urine related to urgency interfere with your everyday life?” The impact was rated on a visual analogue scale (VAS 0–10; 0—no impact, 10—severe impact). Subjective cure of SUI or UUI was defined by a SUI‐VAS or UUI‐VAS score reduction by ≥90%, respectively. Objective cure of MUI was defined by a negative cough test and no UUI episode/24 h. Subjective cure of MUI was defined by both a SUI‐VAS and an UUI‐VAS score reduction by ≥90%.
Objective improvement compared to baseline was defined as a reduced urine leakage in the cough test or a >50% reduction in UUI episodes/day. 14 , 17 Subjective improvement was defined by a SUI‐VAS or UUI‐VAS score reduction ≥75% compared to baseline. 15 MUI was defined as improved if at least one of the respective SUI or UUI components improved, and MUI was defined as failed if at least one of the respective SUI or UUI components failed.
Secondary endpoints at months 4 and 12 included the changes from baseline in number of micturition, urgency and UUI episodes/day, 10 , 14 volume voided/micturition 14 and ICIQ‐UI SF score. 9 , 18 Intra‐ and postoperative safety parameters were assessed during hospitalization, and at the 14‐day, 4‐ and 12‐month follow‐up visits. Urinary retention that required CIC, 13 , 19 PVR volumes, 13 , 19 UTIs 13 , 20 , 21 and pain were recorded. Urethral length, PVR volumes and localization of PAHG depots were assessed by pelvic floor sonography. 15
Sample size calculation was based on an expected proportion of 0.5, a width of confidence interval of 0.3 and a 95% confidence level, and resulted in 43 patients. An inclusion of 55 patients was sufficient to account for a drop‐out rate of 20%. Descriptive statistics were used to examine demographics, medical history, complications and cure/improvement rates. Continuous data were analyzed by paired Wilcoxon signed‐rank test. The significance level was set at α = 0.05. All analyses were performed using R for RStudio Version 3.6.3 for Windows.
3. RESULTS
3.1. Study population and study characteristics
Fifty‐five patients with stress dominant MUI were treated with botulinum toxin (median: 50 units, range: 50–200 units) and PAHG (median 2.0 ml, range: 1.3–3.4 ml) in one single surgical procedure. Baseline characteristics were collected 1 day before surgery (Table 1). Patients' median age was 75 years, the median body mass index was 28.5 kg/m2 22% (12/55) had one, 64% (35/55) had two or more severe comorbidities. Median hospital stay was 2 nights (range: 2–4), and all 55 patients completed the 14‐day and 4‐month visits. Three patients (5.5%) were lost to follow‐up at 12 months. Two patients (3.6%) received a second botulinum toxin injection between the 4‐ and 12‐month visits, and consequently, their urgency‐related parameters were not evaluated at 12 months. Incomplete questionnaires at the 4‐ and 12‐month follow‐ups, missing bladder diaries at 12 months, and one missing cough test and PVR value at 12 months further reduced the available data for investigated parameters. No patient had a second PAHG injection.
Table 1.
Baseline characteristics
| n = 55 | |
|---|---|
| Age (years), median (IQR; min‐max) | 75 (67–81; 47–90) |
| Height (cm), median (IQR; min‐max) | 162 (157–168; 144–176) |
| Weight (kg), median (IQR; min‐max) | 76 (64–88; 44–113) |
| Body mass index (kg/m2), median (IQR; min‐max) | 28.5 (24–33; 18–41) |
| Comorbidities (n), median (IQR; min‐max) | 2 (1–3; 0–10) |
| Parity (n), median (IQR; min‐max) | 2.0 (1.8–3.0; 0–6) |
| Birth mode | |
| Spontaneous delivery, yes (%) | 41 (75) |
| Other (Caesaren, vacuum, forceps), yes (%) | 5 (9) |
| No birth, yes (%) | 9 (16) |
| Previous incontinence surgery, yes (%) | 31 (56) |
| Sling only, yes (%) | 24 (77) |
| Colposuspension only, yes (%) | 4 (13) |
| Sling and colposuspension, yes (%) | 2 (7) |
| Sling and PAHG, yes (%) | 1 (3) |
| Previous prolapse surgery, yes % | 24 (44) |
| Previous colporrhaphy (no mesh), yes % | 12 (50) |
| Previous prolapse surgery with mesh, yes % | 2 (8) |
| Previous prolapse surgery combined (without mesh, with mesh), yes % | 10 (42) |
| Previous hysterectomy, yes (%) | 33 (60) |
Note: Comorbidities were cardiac disease, kidney disease, chronic pulmonary disease, diabetes, neurologic disease, other disease
Abbreviations: IQR, interquartile range; PAHG, polyacrylamide hydrogel.
3.2. Cure/improvement rates for SUI and UUI components at 4 months
At the 4‐month follow‐up, objective cure rates were 73% (40/55) for SUI (cough test) and 53% (29/55) for UUI (UUI episodes), and objective cure/improvement rates were 86% (47/55) for SUI and 78% (43/55) for UUI (Figure 1A). Subjective cure rates were 71% (37/52) for SUI‐VAS and 52% (27/52) for UUI‐VAS, and subjective cure/improvement rates were 79% (41/52) for SUI‐VAS and 69% (36/52) for UUI‐VAS (Figure 1B).
Figure 1.
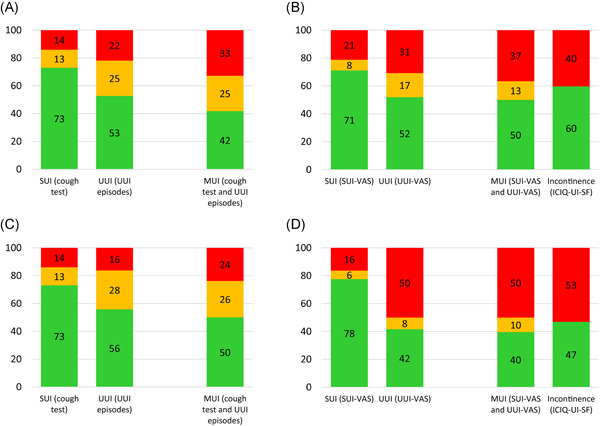
Cure and improved rates of objective and subjective outcomes at 4 and 12 months after treatment. (A) Objective cure/improvement at 4 months, (B) subjective cure/improvement at 4 months, (C) objective cure/improvement at 12 months, (D) subjective cure/improvement at 12 months. The individual parameters measuring stress (SUI) or urgency (UUI) urinary incontinence were separately calculated. Rates for mixed urinary incontinence (MUI) were calculated by combining the respective SUI and UUI parameters. The ICIQ‐UI SF measures subjective overall incontinence. Rates are shown for cure (green), improvement (yellow) and failure (red). Definition of cure, improvement, failure: see Materials and Methods. ICIQ‐UI‐SF, International Consultation on Incontinence Questionnaire‐Urinary Incontinence Short Form; SUI, stress urinary incontinence; UUI, urgency urinary incontinence; VAS, visual analogue scale
3.3. Cure/improvement rates for SUI and UUI components at 12 months
At the 12‐month follow‐up, objective cure rates were 73% (37/51) for SUI (cough test) and 56% (24/43) for UUI (UUI episodes), and objective cure/improvement rates were 86% (44/51) for SUI and 84% (36/43) for UUI (Figure 1C). Subjective cure rates were 78% (38/49) for SUI‐VAS and 42% (20/48) for UUI‐VAS, and subjective cure/improvement rates were 84% (41/49) for SUI‐VAS and 50% (24/48) for UUI‐VAS (Figure 1D).
3.4. Cure/improvement rates for MUI at 4 and 12 months
Objective cure rates for MUI (negative cough test and no UUI episodes) were 42% (23/55) and 50% (21/42) at 4 and 12 months, and objective cure/improvement rates were 67% (37/55) and 76% (32/42) at 4 and 12 months. Subjective cure rates for MUI (SUI‐VAS and UUI‐VAS reduction ≥90%) were 50% (26/52) and 40% (19/48) at 4 and 12 months, and subjective cure/improvement rates were 63% (33/52) and 50% (24/48) at 4 and 12 months.
Subjective cure determined by ICIQ‐UI SF was 60% (31/52) at 4 months and 47% (23/49) at 12 months (Figure 1).
3.5. Objective and subjective outcomes of individual study parameters
Objective and subjective values of investigated study parameters at baseline and follow‐up visits and changes from baseline are shown in Tables 2 and 3. All follow‐up values were significantly different from baseline (p < 0.001) (Table 2).
Table 2.
Objective and subjective values at baseline and at follow‐up visits
| Baseline | 4‐Month visit | 12‐Month visit | |
|---|---|---|---|
| No. UUI episodes/day, median (IQR) (min‐max) | 3.6 (2.2–5.0) | 0.0 (0.0–1.7) a | 0.0 (0.0–1.7) a |
| (0.3–9.7) | (0.0–7.5) | (0.0–6.3) | |
| No. urgency episodes/day, median (IQR) (min‐max) | 8.0 (5.2–8.9) | 2.7 (0.0–5.0) a | 3.3 (1.7–6.0) a |
| (0.0–12.0) | (0–9.3) | (0.0–9.0) | |
| No. micturition episodes/day, median (IQR) (min‐max) | 9.7 (8.6–11.0) | 7.7 (6.3–9.3) a | 7.7 (6.4–9.9) a |
| (8.0–15.7) | (4.0–13.0) | (4.0–12.5) | |
| Volume voided/micturition (ml), median (IQR) (min‐max) | 189 (145–238) | 250 (199–326) a | 225 (194–297) a |
| (69–395) | (94–458) | (102–529) | |
| ICIQ‐UI SF score, median (IQR) (min‐max) | 15.4 (13.0–16.9) | 4.8 (0.08–11.1) a | 5.5 (0.1–8.9) a |
| (8.3–21.0) | (0.0–17.4) | (0.0–20.0) | |
| SUI‐VAS score, median (IQR) (min‐max) | 6.0 (5.0–8.2) | 0.1 (0.0–0.8) a | 0.0 (0.0–0.4) a |
| (0.3–10.0) | (0.0–8.7) | (0.0–9.7) | |
| UUI‐VAS score, median (IQR) (min‐max) | 7.5 (5.0–9.1) | 0.5 (0.0–4.0) a | 1.6 (0.0–4.7) a |
| (0.1–10.0) | (0.0–9.4) | (0.0–10.0) |
Abbreviations: ICIQ‐UI SF, International Consultation on Incontinence Questionnaire‐Urinary Incontinence Short Form; IQR, interquartile range; SUI, stress urinary incontinence; UUI, urgency urinary incontinence; VAS, visual analogue scale.
All follow‐up values were significantly different from baseline (p < 0.001) as determined by the paired Wilcoxon signed‐rank test with significance level 0.05.
Table 3.
Objective and subjective outcome changes from baseline
| Mean change from baseline | 95% CI | Mean % change from baseline | |
|---|---|---|---|
| No. UUI episodes/day | |||
| 4‐Month visit | −2.6 | −3.3, −2.0 | −72 |
| 12‐Month visit | −2.9 | −3.6, −2.2 | −75 |
| No. urgency episodes/day | |||
| 4‐Month visit | −3.9 | −4.8, −3.0 | −55 |
| 12‐Month visit | −3.2 | −4.1, −2.3 | −43 |
| No. micturition episodes/day | |||
| 4‐Month visit | −2.1 | −2.6, −1.6 | −21 |
| 12‐Month visit | −2.0 | −2.5, −1.4 | −19 |
| Volume voided/micturition (ml) | |||
| 4‐Month visit | +64 | +47, +81 | +42 |
| 12‐Month visit | +55 | +29, +81 | +32 |
| ICIQ‐UI SF score | |||
| 4‐Month visit | −9.0 | −11, −7.4 | −61 |
| 12‐Month visit | −8.7 | −10, −7.2 | −60 |
Abbreviations: CI, confidence interval; ICIQ‐UI SF, International Consultation on Incontinence Questionnaire‐Urinary Incontinence Short Form; UUI, urgency urinary incontinence.
3.6. Safety outcomes
No serious adverse events were observed. There was no intraoperative complication, and postoperative complications were transient. One patient experienced not clearly localized pain, and two had minimal hematuria. CIC was necessary for 12/55 (22%) patients during the first postoperative day(s), and 5 of them had a PVR >100 ml at hospital release, which spontaneously resolved without further catheterization. Eighteen patients (18/55, 33%) had PVR volumes >100 ml at 14 days, 4/55 (7.3%) at 4 months, and 2/51 (3.9%) at 12 months (Table 4). Median PVR was 20 ml (range: 0–100 ml) at baseline, 70 ml (range: 0–480 ml) at 14 days, 30 ml (range: 0–300 ml) at 4 months, and 15 ml (range: 0–220 ml) at 12 months. Mean PVR increases from baseline are shown (Table 4). Four patients (4/55, 7.3%) had a PVR increase ≥200 ml at one of the postoperative visits, while 40/55 (73%) patients did not have a PVR increase >100 ml at any postoperative visit.
Table 4.
Safety parameters
| n = 55 | |
|---|---|
| UTI, symptomatic 0–1 month, yes (%) | 7 (13) |
| UTI, recurrent 0–6 months, yes (%) | 4 (7.3) |
| UTI, recurrent 0–12 months, yes (%) | 4 (7.7) |
| CIC during hospitalization, yes (%) | 12 (22) |
| PVR >100 ml at 14 days, yes (%) | 18 (33) |
| PVR >100 ml at 4 months, yes (%) | 4 (7.3) |
| PVR >100 ml at 12 months, yes (%) | 2 (3.9) |
| PVR ≥ 200 ml increase from baseline at one of the postoperative visits, yes (%) | 4 (7.3) |
| mean PVR increase from baseline | |
| At 14 days (ml), mean (SD) | +71 (93) |
| At 4 months (ml), mean (SD) | +20 (41) |
| At 12 months (ml), mean (SD) | +4.3 (43) |
Note: 12‐Month UTI data available for 52 patients; 12‐month PVR data available for 51 patients.
Abbreviations: CIC, clean intermittent catheterization; PVR, postvoid residual urine; UTI, urinary tract infection.
The rate of symptomatic UTIs was 13% (7/55) within the first postoperative month. Four (4/7, 57%) of these patients also had a PVR volume >100 ml at the 14‐day visit. The rate of recurrent UTIs was 7.3% (4/55) after 6 months and 7.7% (4/52) after 1 year (Table 4). Overall, six patients (6/55, 11%) had recurrent UTIs in the first postoperative year, three of them already had recurrent UTIs before the operation. Thirty‐seven patients (37/55, 71%) had no UTI within the first postoperative year. All UTIs were uncomplicated with no upper urinary tract involvement.
4. DISCUSSION
4.1. Summary of study results
This is the first study simultaneously treating both the urgency and stress components of MUI in a single combined operation with botulinum toxin and PAHG. The combination therapy was potent, long lasting, and with low risk, even for our elderly and frail study population. All subjective and objective UUI and SUI parameters significantly improved 4 and 12 months after the intervention.
Cure rates were better for SUI than for UUI, while cure rates were similar for objective and subjective SUI, or objective and subjective UUI, respectively (Figure 1). Subjective cure determined by ICIQ‐UI SF was similar to subjective cure/improvement of MUI. Only subjective UUI decreased from 4 to 12 months, while subjective SUI and objective SUI and UUI stayed equally successful at the 4‐ and 12‐month visits.
4.2. Treatment of MUI, other studies
Treatment and evaluation of MUI usually concentrated on the predominant symptom and not on the mixed condition. Therapy with a sling was assessed by cough/pad tests and satisfaction and resulted in 72% cure at the 8‐month follow‐up, 6 or 85% cure at the 4‐year follow‐up. 4 A therapy with solifenacin reported 49% cure defined by no UUI episode/3 days after 12 weeks. 5 A therapy with tolterodine ER produced a statistically significant decrease in the weekly UUI episodes compared with placebo after 8 weeks, 3 and a therapy with mirabegron of a mixed UUI/MUI population resulted in 46.3% cure defined by zero UUI episode/day after 18 weeks. 10
To relate our data to published outcomes after PAHG or botulinum toxin treatment we compared similar outcome parameters. However, these comparisons are only approximate and limited by different patient inclusion criteria, dosage, injection technique and follow‐up periods.
4.3. Outcome after PAHG therapy, comparison to other studies
One other study also investigated PAHG treatment of sole MUI patients. 7 A significant improvement of subjective MUI severity from VAS 10 to 6 was found at the 3‐month follow‐up. In our study, SUI‐VAS decreased from 6 to 0.1, and UUI‐VAS from 7.5 to 0.5 at the 4‐month follow‐up.
Other PAHG studies investigated a mixed MUI/SUI population. 8 , 9 , 18 , 22 Subanalysis showed a better outcome for genuine SUI than for MUI. 9 , 18 , 22 Therefore, our treatment might be less successful for our MUI patients than for a mixed MUI/SUI population. However, the ICIQ‐UI SF score in our study improved from 15.4 at baseline to 4.8 and 5.5 at the 4 and 12‐month follow‐ups, which was equal or even better than published outcomes for mixed MUI/SUI populations. In one study with a mixed UI population (56.5% MUI, 31% SUI, 7.1% UUI, 5.3% nonspecified), 18 the ICIQ‐UI SF score dropped from 16.05 to 10.58 at the 3‐month follow‐up. In another study with 50% MUI/50% SUI, 9 , 22 it decreased from 15.0 to 7.0 at the 12‐month follow‐up, and in a further study with 42.2% MUI/57.8% SUI from 15 to 5 at the 12‐month follow‐up. 8
None of our patients required a reinjection of PAHG within the 12‐month follow‐up period. This was a major difference to the other studies that reported 35%, 9 8%, 8 43%, 12 or 77% reinjections and 36% re‐reinjections, 23 or offered a further injection if the procedure was unsuccessful. 7
4.4. Outcome after botulinum toxin therapy, comparison to other studies
Botulinum toxin injection is a standard therapy for UUI/OABwet. Published outcomes with 100 units of botulinum toxin 12 weeks after injection were compared to our outcomes with 50 units (median, range: 50–200 units) 4 months after injection: Change from baseline in UUI episodes/day were −2.65, 14 −3.40, 17 −2.63, 24 or −3.13, 19 and −2.6 in our study. The cure rate (no UUI episode/day) was 22.9%, 14 55%, 17 37%, 24 or 19%, 19 ours was 53%. Mean percentage decreases from baseline in UUI episodes/day, micturition episodes/day, and urgency episodes/day were −53.2%, −19.7%, and −41.1%, 13 our corresponding numbers were −72%, −21%, and −55%. Mean increase from baseline in voided volume/micturition was +41.1 ml, 14 +47.9 ml, 17 or +29.47 ml, 19 our corresponding number was +64 ml. Thus, our combination therapy for MUI with PAHG and low dose botulinum toxin achieved similar or better outcomes than a therapy for OABwet with 100 units botulinum toxin.
Fifty units of botulinum toxin have a higher efficacy (4‐month cure 64%) in our study than 50 units in other studies (3‐month cure 15.8% 17 or 29.8% 24 ). The treatment effect also lasted longer. Only 4% needed a reinjection before the 12‐month visit that was much lower than the 71.5% reported for patients treated with 100 units who requested a repeat injection within 1 year. 11
4.5. Postoperative PVR volumes, comparison to other studies
Botulinum toxin therapy takes effect after approximately 1–2 weeks and lasts for 4–10 months, 11 , 14 , 24 whereas PAHG therapy takes effect immediately after the injection and can last for up to 7 years. 8 , 25 , 26 Effects are not only reflected by the timing of symptom alleviation, but also by the temporal manifestation of complications, such as urinary retention.
Twenty‐two percent of our patients had PAHG‐related postoperative urinary retention which followed complete spontaneous resolution within a few days of CIC. This was higher than in other studies that reported urinary retention rates of 5.7%, 23 1.1%, 9 1.9%, 7 13%, 15 or 8.2%. 25
Mean PVR volume increase from baseline as a consequence of botulinum toxin injection were highest (+71 ml) at the 14‐day visit, and decreased to +20 ml after 4 months. Other studies with 100 units of botulinum toxin reported similar kinetics, with a maximum increase after 2 weeks (+49.5 ml, 14 +50 ml, 24 +48.78 ml 19 ), and a decrease at 12 weeks (+32.6 ml, 14 +35 ml, 24 +15.53 ml 19 ). The fraction of patients with a postoperative PVR increase ≥200 ml was similar to published data (7.3% [our study] vs. 8.8% 13 ). Also, the fraction with postoperative PVR increase ≤100 ml was similar (73% [our study] vs. 75.8% 13 ). Similar to other studies, 17 , 24 PVR increases from baseline were dose dependent, with mean increases of +60 ml after 50 units and +101 ml after 100 units at the 14‐day visit.
4.6. Postoperative UTI, comparison to other studies
Symptomatic UTIs within the first month after the intervention can be related to the treatment. 20 Thirteen percent of our patients were affected. This was slightly above published UTI ratios after PAHG alone (3.5%–8.4% 9 , 12 , 15 , 23 ), and similar to UTI ratios after botulinum toxin injection (5%–39% 13 , 14 , 17 , 19 , 20 , 24 ). Risk factors for UTI that also apply to our study population were high PVR volumes, estrogen deficiency, previous urogenital surgery, a previous UTI, urge incontinence, or poor medical health. 21
4.7. Rationale for the successful therapy
What could be a reason for long‐lasting effects of the combined therapy? For PAHG, the relatively high postoperative CIC ratio of 22% and the ongoing effect without requiring re‐injections might be associated, 25 and due to a new injection technique. Crucial for efficient bulking are the preoperative urethral length measurement, the positioning of the depots at same level of the proximal third of the urethra, the injection of 4 depots instead of 3, injection of 2 ml PAHG, and the urethroscopic control of urethral lumen closure while injecting the bulking agent.
For botulinum toxin, the combination with PAHG might account for its durable success. We hypothesize that the PAHG depots prevent urethral funneling and block the entrance of urine into the proximal urethra. Consequently, pressure points in the bladder neck triggering micturition are not stimulated, 27 and the sensation of “urgency” is not elicited.
4.8. Limitations
In this prospective observational study at a single tertiary urogynecological center, a set of variables was simultaneously assessed to show the proof of concept of a new combination therapy for a multifactorial disease. Larger follow‐up multicenter studies with more patients are required to verify these preliminary data. Particularly, randomized controlled trials with the same study population comparing combined botulinum toxin/PAHG with botulinum toxin or PAHG alone are of great interest.
5. CONCLUSION
The combination of PAHG and botulinum toxin is an effective, long lasting and safe method to treat stress dominant MUI. Patients can highly benefit from this combined therapy with only one very short intervention, not requiring general anaesthesia nor discontinuation of anticoagulation. The therapy has low risks and is especially suited for therapy‐refractory, obese, frail and elderly MUI patients. A stationary setting is recommended to closely monitor postoperative PVR. An optimized PAHG injection technique and injection of 50 instead of 100 units of botulinum toxin seem crucial for a good treatment outcome.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Volker Viereck: Project development, patient recruitment/treatment, manuscript editing. Marianne Gamper: Study protocol editing, data analysis, manuscript writing/editing. Claudia Walser: Study protocol writing/ethics, data collection, manuscript editing. Debra Fesslmeier: Data collection/analysis, statistics, manuscript writing/editing. Julia Münst: Patient recruitment, data analysis, manuscript editing. Irena Zivanovic: Patient recruitment/treatment, manuscript editing.
Viereck V, Gamper M, Walser C, Fesslmeier D, Münst J, Zivanovic I. Combination therapy with botulinum toxin and bulking agent—An efficient, sustainable, and safe method to treat elderly women with mixed urinary incontinence. Neurourology and Urodynamics. 2021;40:1820‐1828. 10.1002/nau.24757
Patient recruitment, data collection, statistical analysis and manuscript writing/editing were done at Cantonal Hospital Frauenfeld, Switzerland
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4‐20. [DOI] [PubMed] [Google Scholar]
- 2. Minassian VA, Stewart WF, Hirsch AG. Why do stress and urge incontinence co‐occur much more often than expected? Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1429‐1440. [DOI] [PubMed] [Google Scholar]
- 3. Khullar V, Cardozo L, Dmochowski R. Mixed incontinence: current evidence and future perspectives. Neurourol Urodyn. 2010;29:618‐622. [DOI] [PubMed] [Google Scholar]
- 4. Rezapour M, Ulmsten U. Tension‐Free vaginal tape (TVT) in women with mixed urinary incontinence—a long‐term follow‐up. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(Suppl 2):S15‐S18. [DOI] [PubMed] [Google Scholar]
- 5. Staskin DR, Te AE. Short‐ and long‐term efficacy of solifenacin treatment in patients with symptoms of mixed urinary incontinence. BJU Int. 2006;97:1256‐1261. [DOI] [PubMed] [Google Scholar]
- 6. Kulseng‐Hanssen S, Husby H, Schiotz HA. The tension free vaginal tape operation for women with mixed incontinence: do preoperative variables predict the outcome? Neurourol Urodyn. 2007;26:115‐121. [DOI] [PubMed] [Google Scholar]
- 7. Mohr S, Marthaler C, Imboden S, Monga A, Mueller MD, Kuhn A. Bulkamid (PAHG) in mixed urinary incontinence: what is the outcome? Int Urogynecol J. 2017;28:1657‐1661. [DOI] [PubMed] [Google Scholar]
- 8. Pai A, Al‐Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid((R))) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent European J Urol. 2015;68:428‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lose G, Sørensen HC, Axelsen SM, Falconer C, Lobodasch K, Safwat T. An open multicenter study of polyacrylamide hydrogel (Bulkamid(R)) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herschorn S, Chapple CR, Abrams P, et al. Efficacy and safety of combinations of mirabegron and solifenacin compared with monotherapy and placebo in patients with overactive bladder (SYNERGY study). BJU Int. 2017;120:562‐575. [DOI] [PubMed] [Google Scholar]
- 11. Nitti VW, Ginsberg D, Sievert KD, et al. Durable efficacy and safety of long‐term onabotulinumtoxinA treatment in patients with overactive bladder syndrome: final results of a 3.5‐year study. J Urol. 2016;196:791‐800. [DOI] [PubMed] [Google Scholar]
- 12. Itkonen Freitas AM, Mentula M, Rahkola‐Soisalo P, Tulokas S, Mikkola TS. Tension‐free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372‐378. [DOI] [PubMed] [Google Scholar]
- 13. Chapple C, Sievert KD, MacDiarmid S, et al. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomised, double‐blind, placebo‐controlled trial. Eur Urol. 2013;64:249‐256. [DOI] [PubMed] [Google Scholar]
- 14. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189:2186‐2193. [DOI] [PubMed] [Google Scholar]
- 15. Zivanovic I, Rautenberg O, Lobodasch K, von Bünau G, Walser C, Viereck V. Urethral bulking for recurrent stress urinary incontinence after midurethral sling failure. Neurourol Urodyn. 2017;36:722‐726. [DOI] [PubMed] [Google Scholar]
- 16. Kuszka A, Gamper M, Walser C, Kociszewski J, Viereck V. Erbium:YAG laser treatment of female stress urinary incontinence: midterm data. Int Urogynecol J. 2020;31:1859‐1866. [DOI] [PubMed] [Google Scholar]
- 17. Denys P, Le Normand L, Ghout I, et al. Efficacy and safety of low doses of onabotulinumtoxinA for the treatment of refractory idiopathic overactive bladder: a multicentre, double‐blind, randomised, placebo‐controlled dose‐ranging study. Eur Urol. 2012;61:520‐529. [DOI] [PubMed] [Google Scholar]
- 18. Hansen MF, Lose G, Kesmodel US, Gradel KO. A national population‐based cohort study of urethral injection therapy for female stress and mixed urinary incontinence: the Danish Urogynaecological Database, 2007‐2011. Int Urogynecol J. 2017;28:1309‐1317. [DOI] [PubMed] [Google Scholar]
- 19. Yokoyama O, Honda M, Yamanishi T, et al. OnabotulinumtoxinA (botulinum toxin type A) for the treatment of Japanese patients with overactive bladder and urinary incontinence: results of single‐dose treatment from a phase III, randomized, double‐blind, placebo‐controlled trial (interim analysis). Int J Urol. 2020;27:227‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christiansen FE, Pedersen TB, Juel J, Kirkeby HJ. Single‐centre experience with intradetrusor injection of onabotulinumtoxinA: a retrospective study of the years 2003‐2012 in a Danish population. Scand J Urol. 2017;51:392‐396. [DOI] [PubMed] [Google Scholar]
- 21. Aydin A, Ahmed K, Zaman I, Khan MS, Dasgupta P. Recurrent urinary tract infections in women. Int Urogynecol J. 2015;26:795‐804. [DOI] [PubMed] [Google Scholar]
- 22. Toozs‐Hobson P, Al‐Singary W, Fynes M, Tegerstedt G, Lose G. Two‐year follow‐up of an open‐label multicenter study of polyacrylamide hydrogel (Bulkamid(R)) for female stress and stress‐predominant mixed incontinence. Int Urogynecol J. 2012;23:1373‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sokol ER, Karram MM, Dmochowski R. Efficacy and safety of polyacrylamide hydrogel for the treatment of female stress incontinence: a randomized, prospective, multicenter North American study. J Urol. 2014;192:843‐849. [DOI] [PubMed] [Google Scholar]
- 24. Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double‐blind, placebo controlled, randomized, dose ranging trial. J Urol. 2010;184:2416‐2422. [DOI] [PubMed] [Google Scholar]
- 25. Giammò A, Geretto P, Ammirati E, et al. Urethral bulking with Bulkamid: an analysis of efficacy, safety profile, and predictors of functional outcomes in a single‐center cohort. Neurourol Urodyn. 2020;39:1523‐1528. [DOI] [PubMed] [Google Scholar]
- 26. Brosche T, Kuhn A, Lobodasch K, Sokol ER. Seven‐year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harms L, Emons G, Bader W, Lange R, Hilgers R, Viereck V. Funneling before and after anti‐incontinence surgery—a prognostic indicator? Part 2: tension‐free vaginal tape. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:289‐294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


