Abstract
Objective
Cationic guar is an important polysaccharide used as a hair conditioning agent in personal care products. In this article, we report streaming potential data demonstrating its behaviour as it interacts electrostatically with hair. Several cationic guar variants with different molecular weights (MWs) and charge densities (CDs) were examined.
Methods
All experiments were carried out with a custom‐designed streaming potential instrument so that in situ, real‐time data were monitored during the treatment of a hair plug with aqueous solutions of cationic guar and subsequent treatment with anionic surfactants—sodium laureth sulfate (SLES) and cocamidopropyl betaine (CAPB)—commonly found in contemporary shampoo formulations.
Results
The MW of the cationic guar variants plays an integral role in determining the thickness of the adsorbed polymer layer on the hair surface while CD influences the zeta potential. Data were also generated for the treatment of hair with a cationic flexible polymer (polyquaternium‐28) and cationic conditioning surfactant (behentrimonium chloride) to provide a frame of reference. The deposition behaviour on hair of high MW cationic guar variants is distinct from these conventional molecules in terms of its electrokinetic properties. We also examined the electrokinetic behaviour of cationic guar on hair types from different racial backgrounds. While the cationic guar treatment yielded similar results for the different hair types, anionic surfactant treatment resulted in quicker sorption and desorption from African, European 65% grey, and Mulatto hair as compared to Chinese, European dark brown, and Indian hair.
Conclusion
We introduce an in situ technique for measuring the dynamic sorption/desorption of charged molecules on the surface of human hair. Evaluation of a series of cationic guar species revealed varying behaviour depending on the MW and CD of the polysaccharide. Our data also demonstrate differences in the desorption properties of typical shampoo surfactants for hair from diverse racial backgrounds.
Keywords: cationic guar, cocamidopropyl betaine, human hair, sodium laureth sulphate, streaming potential, zeta potential
Cationic guar is an important polysaccharide used as a hair conditioning agent in personal care products. In this article, we report streaming potential data demonstrating its behavior as it interacts electrostatically with hair. Several cationic guar variants with different molecular weights (MWs) and charge densities (CDs) were examined.

Abstrait
Objectif
Le guar cationique est un polysaccharide important utilisé comme conditionneur capillaire dans les produits cosmétiques. Dans ce rapport, nous démontrons l'utilisation de la technique du potentiel de streaming pour étudier comment le guar cationique interagit électrostatiquement avec les cheveux. Plusieurs variantes del guar cationique avec différents poids moléculaires et densités de charge ont été examinés.
Méthodes
Nous avons utilisé un instrument de potentiel de streaming pour les expériences. Les études ont été réalisées en temps réel pour surveiller le traitement de cheveu avec des solutions aqueuses de guar cationique suivi d'un traitement ultérieur avec tensioactifs anioniques comment le sulfate de laureth de sodium et le cocamidopropyle bétaïne, des ingrédients généralement trouvés dans les formulations de shampooing.
Résultats
Le poids moléculaire des variants du guar cationique joue un rôle intégral dans la détermination l'épaisseur de la couche de polymère adsorbée sur la surface des cheveux tandis que le densité de charge influence le potentiel zêta. Des données ont également été générées pour le traitement des cheveux avec un polymère flexible (polyquaternium‐28) et tensioactif de conditionnement cationique (behentrimonium chlorure) pour fournir un cadre de référence. Le comportement de dépôt sur les cheveux des variants de guar cationiques à poids moléculaire élevé est distinct de ces molécules conventionnelles en termes de ses propriétés électrocinétiques. Nous avons également examiné le comportement électrocinétique de guar cationique sur des types de cheveux de différents milieux raciaux. Le traitement avec le guar cationique a donné des résultats similaires pour les différents types de cheveux. En contraste avec ceci, le traitement avec le tensioactif anionique a entraîné une sorption et une désorption plus rapides de cheveux africains, de cheveux européens (65% gris) et de cheveux mulâtres en comparaison à les cheveux chinois, européens et indiens.
Conclusion
Nous introduisons une technique in situ pour mesurer la sorption et la désorption dynamique de molécules chargées à la surface des cheveux humains. L’évaluation d'une série des espèces de guar cationiques ont révélé un comportement variable en fonction du poids moléculaires et densités de charge de le polysaccharide. Nos données démontrent également des différences dans les propriétés de désorption de tensioactifs de shampooing typiques pour les cheveux de diverses origines raciales.
MOTS CLÉS: guar cationique, cocamidopropyle bétaïne, cheveux humains, le sulfate de laureth de sodium, potentiel de streaming, potentiel zêta
INTRODUCTION
Guar gum is a galactomannan polysaccharide from the endosperm of the seeds of the guar plant (Cyamopsis tetragonolobus). It is cultivated mostly in India and Pakistan and is used extensively in food products as a texture and rheology modifier. It is characterized by a wide range of high molecular weights and contains a straight chain polysaccharide of D‐mannose units connected by β (1→4) glycosidic linkages with a pendant galactose unit on alternating mannose units attached by an α (1→6) glycosidic bond. Derivatives of guar gum have become increasingly important in various industries, such as oil field (fracking agents), textiles and paper. Cationic guar gum includes a pendant hydroxypropyl group on the galactose unit with quaternary amine functionality (see molecular structure in Figure 1). Two common synthetic routes for producing cationic guar gum consist of reacting guar gum with 3‐chloro‐2‐hydroxypropyl trimethylammonium chloride or 2,3‐epoxypropyl trimethylammonium chloride, although there are a number of different synthetic approaches that have been explored [1, 2, 3].
FIGURE 1.
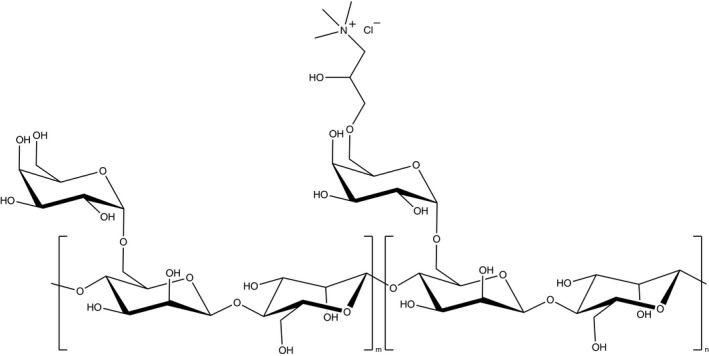
Molecular structure of cationic guar (IUPAC: guar gum, 2‐hydroxy‐3‐(trimethylammonio)propyl ether, chloride; INCI: guar hydroxypropyltrimonium chloride). The m subunit represents the number of native guar gum residues while n refers to the cationic‐modified portion of the molecule. The polymer charge density depends on the number of n units in the molecule
An important application of cationic guar gum in the personal care industry is in shampoos and body washes as a coacervation and conditioning agent [4, 5, 6, 7, 8]. Its quaternary pendant amine group bears a positive charge, making the polymer substantive to human hair, which has a negatively charged surface at pH levels above its isoelectric point—values ranging from 2.45 to 4.5 have been reported in the literature and may be attributed to the chemistry of the surface, which can change as a result of environmental exposure (eg UV radiation, thermal exposure) or chemical cosmetic treatments (eg bleaching, dyeing, etc.) [9]. In general, cationic polymer adsorption on hair occurs due to electrostatic interactions between the polymer and the hair surface. A particular challenge in the scientific community has been to elucidate the process by which cationic polymers deposit onto hair from solutions containing anionic surfactants [10, 11]. This is especially true for cationic guar, which forms coacervates during its dilution from solutions of anionic surfactants, and effectively deposits on the hair surface [12, 13, 14].
There are a number of methods that can be used to determine the deposition of a cationic polymer onto hair. Some of these methods include fluorescent labelling in combination with optical microscopy, X‐ray photoelectron spectroscopy, radiotracer techniques and atomic force microscopy. While these techniques have utility, they do not provide quantitative dynamic data describing the sorption process. Streaming potential is a very effective technique to monitor the deposition of charged molecules onto human hair and wool [15, 16, 17, 18, 19, 20, 21, 22]. The earliest studies on hair examined the influence of salts on the adsorption behaviour of cationic cellulose ether [15]. While simple anions were shown to have little effect on the sorption of cationic cellulose ether, cations inhibited their sorption on hair in the following order: La3+ > Al3+ > Fe3+ > Ca2+ > Fe2+ > Cs+ > Na+ > Li+. These data suggest that cations interact with surface charges on the fibre and the degree of the charge and size of the ion play a key role in regulating this interaction. However, it should be noted these differences are not observed at all salt concentrations.
Streaming potential studies by Jachowicz and coworkers were carried out with a custom‐designed instrument capable of monitoring electrokinetic data (streaming potential and conductivity) and flow rate of the streaming potential solution [16, 17, 18, 19]. The latter parameter provides the permeability of the hair fibre assembly (porous plug) and allows for the calculation of the thickness of the deposited polymer layer. The work by Jachowicz et al. enabled the in situ analysis of a wide variety of molecules, such as those found in shampoos and conditioners (cationic surfactants, anionic surfactants, cationic polymers, silicone oils, etc.), providing information about their substantivity and removability from hair.
In this article, we present novel data on the deposition of cationic guar derivatives onto the surface of human hair. Using a custom‐designed streaming potential instrument, we monitor streaming potential (zeta potential at the hair fibre surface), thickness of the deposited polymeric layer, and pH and conductivity of the streaming potential solution. We report the influence of MW and CD of cationic guar on its hair deposition properties. In addition, we examine binding behaviour of cationic guar on various ethnic types of hair. Additional experiments were carried out using a miniature tensile tester to determine the force required to comb through an assembly of hair fibres in the wet state. Typically, cationic polymers decrease the wet combing forces of hair. The motivation for conducting combing measurements stemmed from the desire to elucidate any possible correlation between zeta potential and practical use parameters related to the everyday application of cationic guar to hair.
MATERIALS AND METHODS
The majority of the work reported in this article entails streaming potential and permeability measurements leading to the calculation of zeta potential and thickness of the adsorbed polymer layer. In addition, mechanical measurements of the force required to comb through a wet hair fibre assembly were carried out with a miniature tensile tester. These data permitted a correlation between the two techniques providing information related to the relevance of zeta potential in consumer‐perceivable hair care applications.
Hair samples
All hair was purchased from International Hair Importers and Products, Inc. (Glendale, New York, USA). The majority of the tests comparing different variants of cationic guar were carried out with European dark brown hair. Studies were also carried out to compare the differences between various hair types including Indian, Chinese, European dark brown, European light brown, European 65% grey, Mulatto and African. All hair was shampooed twice with 3% (w/w) SLES:CAPB (12:2) prior to conducting experiments. SLES (Steol CS‐130) was obtained from Jeen International (Fairfield, New Jersey, USA), while the source of CAPB (Amphosol CA) was Stepan (Northfield, Illinois, USA).
Cationic guar variants
Variants of cationic guar were obtained from Ashland, Inc. (Wilmington, Delaware, USA) and are characterized by their solution viscosity (MW) and CD. Table 1 contains a list of the various cationic guar species examined in this work.
TABLE 1.
Viscosity, CD, trade name and designation of the cationic guar variants investigated in this study. Information obtained from Reference [23]. Unless otherwise indicated, viscosity measurements were conducted on 1% (w/w) solutions of the polymers
| Guar variant | Trade name | CD | Viscosity (cps) |
|---|---|---|---|
| High CD‐high viscosity | N‐Hance 3215 | High | 4200 b |
| High CD‐very low viscosity | AquaCat PF 618 | High | <100a |
| Medium CD‐high viscosity | N‐Hance 3196 | Medium | 4500 b |
| Medium CD‐low viscosity | N‐Hance CCG 45 | Medium | 40 b |
| Low CD‐high viscosity | N‐Hance 3000 | Low | 2700 c |
A 10% (w/w) solution was tested.
Brookfield RV (20 rpm).
Brookfield LV (6 rpm).
For comparison, we also conducted streaming potential measurements of hair treated with a typical conditioning surfactant and polymer containing cationic functionality. Behentrimonium chloride (tradename: Jeequat BTMC‐85%) was obtained from Jeen International. The cationic polymer, polyquaternium‐28, contains vinyl pyrrolidone (VP) and methacrylamidopropyl trimethylammoniumchloride (MAPTAC) monomers, and was obtained from Ashland, Inc. (tradename: Conditioneze NT‐20).
Electrokinetic permeability analysis
Electrokinetic and permeability data were collected using a custom‐designed streaming potential instrument. The streaming potential instrument employed in this study is based on the same operating principles as that reported in Jachowicz et al. [18]. As shown in Figure 2, it consists of a central control unit connected to the streaming potential cell, Consort C861 multiparameter analyser (Consort nv, Turnhout, Belgium), Pennsylvania model 7300 bench scale (Pennsylvania Scale Company, Lancaster, Pennsylvania, USA), 10 L low density poly(ethylene) carboy (VWR Scientific, Radnor, Pennsylvania, USA), and two 1 gal low density poly(ethylene) bottles (VWR Scientific). Tygon tubing R‐3603 (Norton Performance Plastic Corporation, Akron, Ohio, USA) connects the various components and allows streaming potential solution to flow from one part of the instrument to another. All water used in the streaming potential experiments was purified with a Milli‐Q Integral 5 (MilliporeSigma, Merck KGaA, Darmstadt, Germany) water purification system at a resistivity of 18.0 MΩ⋅cm at 25 °C.
FIGURE 2.
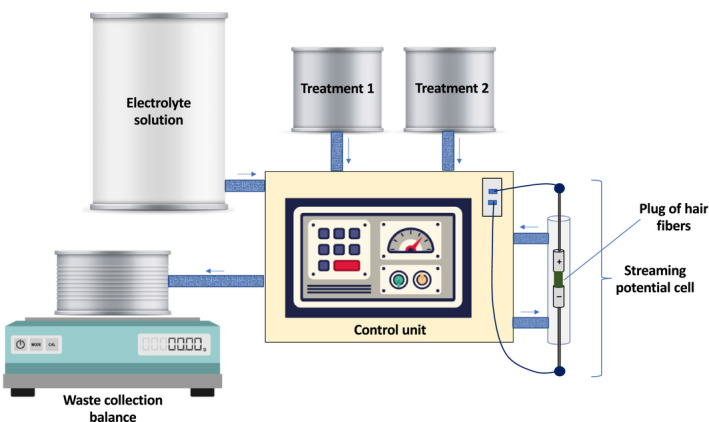
Illustration of the customed‐designed streaming potential instrument used in this study
An electrolyte solution (5 × 10−5 M KCl) flows from the electrolyte solution deposit tank (10 L carboy) through the streaming potential cell to the waste collection chamber where the quantity of solution is monitored as a function of time to determine the flow rate. The direction of solution of flow in the streaming potential instrument is illustrated by the small arrows. ACS reagent grade (99.0‐100.5%) KCl was obtained from Sigma‐Aldrich (St. Louis, Missouri, USA). Two treatment containers are connected to the same flow loop as the electrolyte solution. In all of the examples provided in this report, Treatment 1 (1 gal bottle) consist of a 0.01% (w/w) solution of a cationic guar variant while Treatment 2 (1 gal bottle) was a 0.3% (w/w) solution of SLES:CAPB (12:2) intended to represent a typical surfactant composition found in most commercial shampoos. Please note that much lower concentrations of polymers and surfactants were employed in this work than normal application concentrations due to the tightly packed nature of the hair fibre plug in the streaming potential cell. The streaming potential instrument was pressurized to 6.8 ‐ 7.0 bar using compressed air, allowing the streaming potential solution to flow properly during the course of an experiment. The streaming potential cell was constructed with poly (methylmethacrylate) and two perforated Ag/AgCl electrodes that contain six holes that are 1 mm in diameter, which evenly distributes the flow of solution through the hair plug between the two electrodes. Each electrode is 0.5 mm thick and has a diameter of 11 mm. For each experiment, 2 g hair swatches are shampooed with 3% (w/w) SLES. The hair is extensively rinsed to remove shampoo followed by drying. Then, 0.5 g of hair was cut into 4‐6 mm pieces after wetting with the KCl electrolyte solution. The hair snippets were then soaked in KCl solution for 1 hour. The wet snippets were then placed between the streaming potential electrodes to form a hair plug. The gap between the electrodes was 7.5 mm.
The anode and cathode are connected to the instrument control unit by alligator clips and electrical wiring, allowing the measured potential difference to be converted to a digital signal, which is relayed to a standard desktop computer operating Windows 7 (Microsoft Corporation, Redmond, Washington, USA) and containing the custom software designed for the instrument. The Consort C861 multiparameter analyser is connected to a pH and conductivity electrode positioned along the flow route of the streaming potential solution.
The zeta potential () was calculated using the Smoluchowski equation:
| (1) |
where and are the viscosity and dielectric constant, respectively, of the KCl electrolyte solution, is the conductivity of the streaming potential solution, E is the streaming potential and P is the pressure driving the flow of the solution.
We also estimated the thickness () of the adsorbed layer on the hair surface using Equation 2:
| (2) |
where is the average pore radius (21.4 μm) of the fibre plug, is the flow rate before deposition, and is the flow rate after deposition [18].
Mechanical measurements of combing force
Combing analysis was achieved using a miniature tensile tester (Model 170) manufactured by Dia‐Stron, Ltd. (Hampshire, UK). The combing measurements were carried out on wet hair with the following instrumental parameters: range, 2000 G; gauge, 2 G; size, 50 mm; phase 1 (extension), 350%; phase 2, 0%; phase 3, 0%; and phase 4, 0%. In all experiments, hair tresses were combed several times to remove entanglements before performing combing measurements.
To better differentiate between treatments, studies were conducted on European light brown hair that was bleached in two regions of the tress using an acrylic (Acme Plastics, Woodland Park, New Jersey, USA) frame (two pieces) containing two sheets of silicone rubber (McMaster‐Carr, Elmhurst, Illinois, USA) material sandwiched together by the frame.
A hair tress (2 g with dimensions of 3.175 × 20.32 cm) was placed between the two silicone rubber sheets and bleaching treatment was carried out by placing the bleaching paste in the windows region of the frame and thoroughly saturating the fibres [24]. Hair was subjected to a one‐hour bleaching cycle with 120 g of Clairol Professional BW 2 Powder Lightner (The Wella Corporation, Woodland Hills, California, USA) and 147 mL of Salon Care Professional 20 Volume Clear Developer (Arcadia Beauty Labs LLC, Reno, Nevada, USA). The resulting mixture was applied to damp hair.
A photograph of one piece of the acrylic frame alongside a hair tress bleached with the device is provided in Figure 3. Also included in the figure is a representative figure of a combing curve obtained for a bleached hair tress. After bleaching, the entire hair tress was treated with a 1% (w/w) solution of the cationic guar variant. Wet combing measurements were obtained after bleaching and after treatment with cationic guar. The combing work in the window treatment regions of the combing curve was calculated using the Dia‐Stron, Ltd. Windows application software.
FIGURE 3.
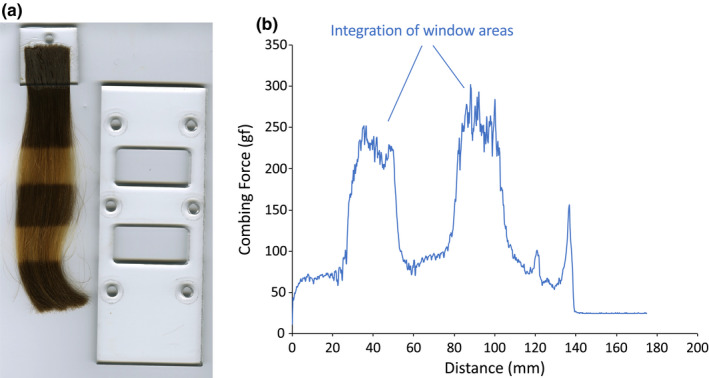
(a) Photograph of a hair tress after bleaching with a window treatment frame. Note that only one part of the window treatment frame (one of the acrylic portions) is shown in the photograph. (b) Wet combing curve of hair after the bleaching treatment
RESULTS AND DISCUSSION
The adsorption of thin films of macromolecules on the surface of hair is an important area of research, especially in the application of shampoos and conditioners, which can modify its surface characteristics and tactile properties. Adsorption is driven by entropy and the interactions between the macromolecules and the surface [25]. In this study, we monitor the interactions of a cationic surfactant (behentrimonium chloride), flexible polymer (polyquaternium‐28) and polysaccharide (cationic guar) with the surface of human hair using streaming potential. We also monitored the effects of an anionic shampoo system (SLES‐CAPB) on the binding properties of the conditioning treatments. The surfactants and polymers can be absorbed or adsorbed to hair. Additional studies of cationic guar variants allowed us to determine the influence of CD and MW on their electrokinetic behaviour. The adsorption behaviour of cationic guar and SLES‐CAPB was examined on hair types from various ethnic origins allowing us to identify two types of behaviour. Finally, mechanical wet combing forces were measured for hair fibres assembled in a tress. Using a specialized technique that consisted of bleaching the hair tress in selected regions, we were able to identify a relationship between cationic guar charge density and combing force reduction.
Zeta potential of hair
The electrical charges on the hair surface determine the zeta potential at the hair fibre liquid interface when hair is immersed in an aqueous solution. These charges arise due to the amino acid functionalities present on the surface of the hair fibre and are affected by the pH of the electrolyte solution. The low isoelectric point of hair suggests that its surface is populated with a considerable quantity of acidic groups. Zeta potential is also influenced by ions and molecules in the solution phase that can be adsorbed on the surface of hair [26]. Figure 4 contains an illustration of a negatively charged hair fibre containing oppositely charged ions in the Stern layer and a mixture of positive and negative ions in the diffuse layer. The slipping plane is located at the boundary between the Stern and diffuse layer, and is defined as the interface separating mobile and immobile fluid. The zeta potential (typically presented in units of mV) is the potential difference in this region. The KCl electrolyte solution used in the streaming potential experiment essentially provides the necessary ions for this phenomenon to take place.
FIGURE 4.
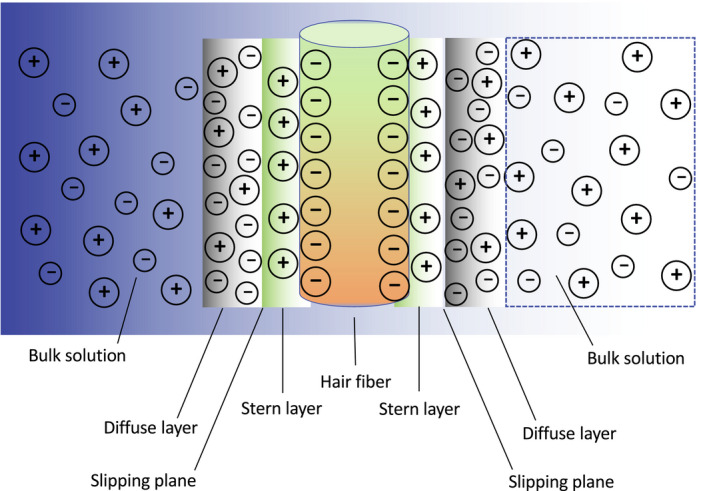
Illustration of the ion concentration surrounding the hair fibre surface when hair is immersed in an aqueous electrolyte solution
Hair treated with a conventional cationic surfactant
Long chain alkyl quaternary surfactants and cationic polymers are typically employed as conditioning agents for hair due to their electrostatic attraction to the negatively charged hair surface. The in situ streaming potential instrument used in this work is ideally suited to dynamically monitor the sorption and desorption of molecules from hair. To establish a baseline, we first present data obtained for one of the most commonly used cationic surfactants in hair conditioner formulations, behentrimonium chloride. Structurally, it has a quaternary amine polar group attached to a twenty‐two carbon aliphatic chain. Unfortunately, there is little information available in the literature about the mechanism of interaction of cationic surfactants with the hair surface [27]. In addition to being cationic, the surfactants must have a substantial aliphatic component (at least C12 and up to C22) in order to adsorb and remain on the hair surface, even after rinsing. This implies that in addition to a charge driven mechanism, there is also a hydrophobic element, which relies on van der Waals interactions between the hydrophobic moieties of the cationic surfactants. Typically, long chain alcohols are formulated with the cationic surfactants in conditioning preparations. It is believed that the surfactants form aggregates with the fatty alcohols, allowing them to also bind to the hair surface [9].
Figure 5 contains a zeta potential profile for hair subjected to two treatment cycles with behentrimonium chloride, each subsequently followed by treatment with SLES‐CAPB. At the bottom far left of the plot, the zeta potential of untreated hair is plotted over a timeframe of 15 min demonstrating its negative surface charge characteristics. During this period, the KCl solution flows through the hair plug. This is followed by a 5‐min treatment with behentrimonium chloride (indicated as cationic surfactant in the figure) and then a 15‐min rinsing step (20‐35 min) with the electrolyte solution. During the rinsing stage, there is a downward slope that corresponds to excess behentrimonium chloride being removed from the hair surface.
FIGURE 5.
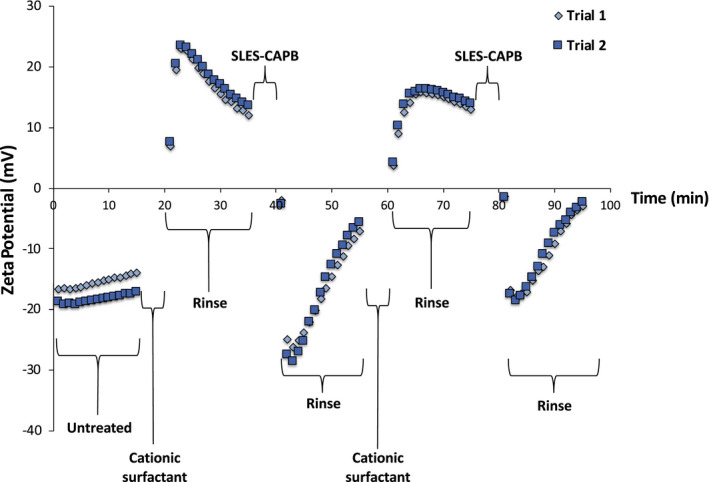
Zeta potential data of hair as a function of flow time before, during and after treatment with behentrimonium chloride and SLES‐CAPB. Trial 1 and Trial 2 refer to two different trials that were carried out on different hair plugs
Between 35 and 40 min, a solution of SLES‐CAPB passes through the hair plug. Immediately, the zeta potential drops significantly due to some removal of behentrimonium chloride as well as the agglomeration of adsorbed SLES bearing a negative charge on the surface of the hair. More than likely, negatively charged SLES probably interacts with the cationic groups of behentrimonium chloride. As the hair plug is rinsed (40‐55 min), SLES is removed from the hair and the surface becomes less negatively charged. We should note that in the case of behentrimonium chloride treatment of hair not all of the SLES is removed from the fibre plug. This is reflected by the slope of the plot, which does not reach a plateau.
A second treatment with behentrimonium chloride is administered between 55 and 60 min followed by a rinse step from 60 to 75 min. Note the difference in the slope of the data after the first treatment (20‐35 min) versus the second treatment (60‐75 min). After the first treatment, excess behentrimonium chloride that is not electrostatically bound to the hair is gradually rinsed off. However, after the second treatment a plateau is reached immediately. At the onset of the first treatment cycle, the hair surface is free of cationic and anionic ingredients. However, during the second treatment cycle, behentrimonium chloride could interact with residual SLES‐CAPB on the surface by electrostatic and van der Waals interactions, which would result in the formation of a complex. The removal of this complex would not result in a significant change in the zeta potential.
A second treatment cycle with SLES‐CAPB is carried out (75‐80 min) followed by a subsequent rinse cycle (80‐95 min). Overall, we find that this technique is extremely reproducible as demonstrated by the data plotted for Trials 1 and 2 in Figure 5. Based on the flow rate data, we calculated the deposited thickness of a layer of behentrimonium chloride on the surface of hair according to Equation 2 and found the following values after each cycle: 0.03 ± 0.06 μm (first treatment); 0.02 ± 0.03 μm (first SLES‐CAPB treatment); 0.01 ± 0.02 μm (second treatment); and 0.04 ± 0.03 μm (second SLES‐CAPB treatment). Essentially, the thickness of the layer is only a fraction of a micron—on the order of tens of nanometers—and is extremely difficult to measure with accuracy using this technique. In the sections below, we discuss polymeric treatments, which yield more reproducible data.
Hair treated with a conventional cationic copolymer
The zeta potential plot for polyquaternium‐28 is plotted alongside behentrimonium chloride in Figure 6. Often times we observe differences in the zeta potential of the untreated hair plug. This could be attributed to the packing of the fibres in the streaming potential cell. Even when there are differences between untreated hair samples in the first rinse cycle, comparable values of zeta potential are found after the first treatment cycle with a cationic surfactant or cationic polymer. In Figure 6, there is a clear distinction after the first treatment cycle with the cationic species. The zeta potential for behentrimonium chloride is greater in magnitude than that measured for polyquaternium‐28. This is not surprising since we would expect the cationic surfactant to have higher charge density over a given region of the hair surface than the polymer, which has an 80:20 monomer ratio (w/w) of VP to MAPTAC. Also notable in the zeta potential plots is the difference between slopes during the rinsing stage after the first treatment with the cationic species. The slope is much greater for hair treated with behentrimonium chloride than with polyquaternium‐28. This seems to suggest that the cationic surfactant molecules form multiple layers on the hair surface—many without forming an electrostatic bond—and are probably removed during the rinsing cycle. In the case of polyquaternium‐28, it appears that a greater percentage of the polymer that initially deposits on the surface of hair remains there throughout the rinse cycle.
FIGURE 6.

Zeta potential profile of hair treated with polyquaternium‐28 as compared to behentrimonium chloride
There is a clear distinction between behentrimonium chloride and polyquaternium‐28 when the hair is subsequently treated with SLES‐CAPB (see the second rinse cycle in Figure 6 between 40 and 55 min). The zeta potential of the polymer‐treated hair decreases, but not nearly as much as hair treated with the cationic surfactant. Possibly, there is greater charge density on the surface of behentrimonium chloride‐treated hair facilitating greater interaction with SLES. However, after 15 min of rinsing, the zeta potential of cationic surfactant‐treated hair almost reaches levels of polyquaternium‐28‐treated hair. A possible explanation could be that the cationic polymer forms a coacervate (in situ) with the anionic surfactant, which could prevent excessive SLES molecules from penetrating into the slipping plan where the zeta potential is recorded.
After the second treatment cycle with the cationic species, there is another rinsing step (60‐75 min) where the deposition behaviour of both ingredients changes relative to the first treatment. We typically observe this behaviour with all types of cationic species used to treat hair. It demonstrates that in the case of polyquaternium‐28‐treated hair there is no excess of polymer that is rinsed from the surface. Nearly all of the polymer that electrostatically interacts with the hair surface during the treatment phase remains throughout the rinse cycle. In the case of behentrimonium chloride, there is an excess at the beginning of the rinse cycle that is progressively removed during rinsing, although to a lesser extent than was the case for the first treatment cycle rinsing (20‐35 min). The rinse cycle (80‐95 min) after the second SLES‐CAPB treatment shows results similar to that found after the first SLES‐CAPB treatment, although with slightly higher magnitude of positive charge. Unlike behentrimonium chloride‐treated hair, SLES is more easily rinsed from the surface of polyquaternium‐28‐treated hair. A plateau is not reached during the SLES‐CAPB rinse cycle for behentrimonium chloride‐treated hair as there may be greater quantities of SLES counterions due to the greater charge density on the surface of hair of behentrimonium chloride relative to polyquaternium‐28.
The thickness of a deposited layer of polyquaternium‐28 was determined to be: 0.39 ± 0.01 μm (first treatment); 0.13 ± 0.14 μm (first SLES‐CAPB treatment); 0.81 ± 0.11 μm (second treatment); and 0.42 ± 0.01 μm (second SLES‐CAPB treatment). After the first and second treatment cycle, the thickness of the polymeric film is approximately in the range of the cuticle step height (ca. 0.50 μm). Interestingly, the thickness decreases after shampooing, which suggests that initially after treatment there are multiple layers of polymer on the hair—polymer close to the surface adsorbed to the hair and polymer above adsorbed to underlying polymer. After shampooing and rinsing, much of the polymer adsorbed to polymer is removed with only a thin layer remaining on the surface of hair.
Hair treated with cationic guar
The zeta potential curve for hair treated with a cationic guar variant (low CD‐high viscosity) is provided in Figure 7. For comparison, the corresponding curve for polyquaternium‐28 is plotted alongside it. During the rinse cycle (20‐35 min) after the first treatment with the cationic polymers, it is apparent that there are greater values of zeta potential and a larger decreasing slope for polyquaternium‐28 as compared to cationic guar. The difference in magnitude of zeta potential is more than likely due to the lower charge per unit area on the surface of the hair for this particular cationic guar variant, which is classified as low CD‐high viscosity. The values for zeta potential for cationic guar are nearly flat after treatment and during rinsing. Such behaviour could suggest that the colloidal particles of cationic guar are larger than polyquaternium‐28. More than likely, cationic guar has stronger intermolecular forces resulting in the deposition of a larger colloid on the surface. Very little or no excess guar is removed during rinsing. This phenomenon is similar to that already touched upon in the previous section when comparing behentrimonium chloride and polyquaternium‐28. In this case, there may be some excess polyquaternium‐28, relative to cationic guar, that is on or near the surface but is not electrostatically bound and can be removed through the rinsing step.
FIGURE 7.
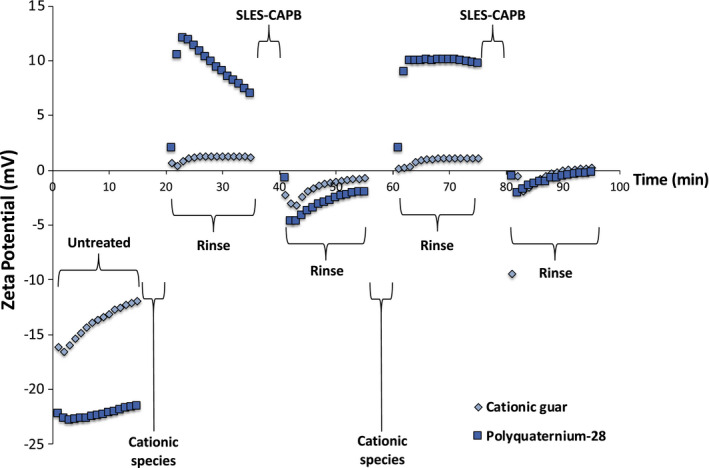
Zeta potential profile of hair treated with low CD‐high viscosity cationic guar as compared to polyquaternium‐28
Similar behaviour is observed for the two polymers during the rinsing cycle (40‐55 min) after treatment with SLES‐CAPB. The zeta potential is lower (more negative) for polyquaternium‐28, which could be due to the excess presence of negatively charged SLES counterions. The second treatment and subsequent rinse cycle (60‐75 s) is very similar to the first one for cationic guar. Curiously, after both treatments there is a slight increase in zeta potential. This behaviour is very common in some of the tested cationic guar variants.
Influence of CD and MW on cationic guar deposition
To better understand the deposition behaviour of cationic guar on hair, we generated streaming potential data for several variants with distinct CD and MW (characterized by viscosity). Figure 8 contains a comparison of low and high charge density variants with comparable molecular weight characteristics. As anticipated, treatment with high CD‐high viscosity cationic guar left the hair surface more positively charged than treatment with the low CD‐high viscosity variant. This behaviour is observed after both rinsing cycles after treatment with cationic guar. In the case of these two cationic guar variants, we do not find a large difference between them after SLES‐CAPB treatment. This may suggest that SLES forms a coacervate with cationic guar, which would minimize the influence of anionic charge during its deposition on the hair surface.
FIGURE 8.
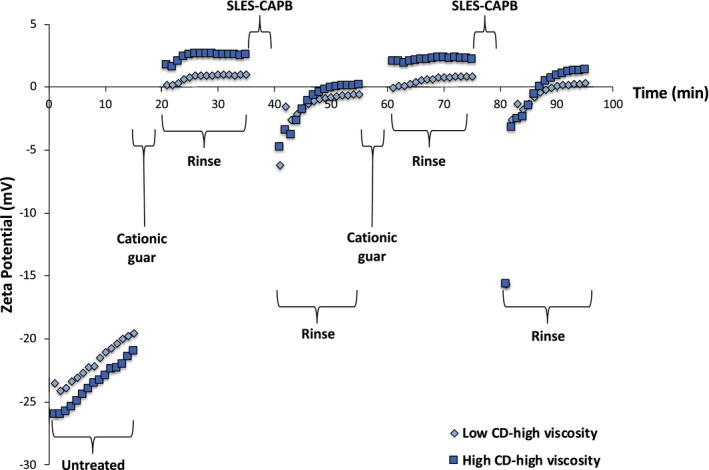
Zeta potential profile of hair treated with low CD‐high viscosity and high CD‐high viscosity cationic guar
The effect of MW, or viscosity, on the deposition characteristics of cationic guar are provided in Figure 9. High CD‐high viscosity cationic guar was compared to high CD‐very low viscosity cationic guar. There is a clear distinction between the two variants. In addition to the difference in zeta potential, the rinse cycle after treatment (16‐20 min and 60‐75 min) for the high CD‐very low viscosity variant seems to behave similar to a linear, flexible chain synthetic polymer. The greatest difference between the two species when comparing the zeta potential plots after SLES‐CAPB treatment is the magnitude of charge on the surface. The zeta potential profile of the high CD‐very low viscosity cationic guar derivative is very similar to behentrimonium chloride after SLES‐CAPB treatment (Figure 6).
FIGURE 9.
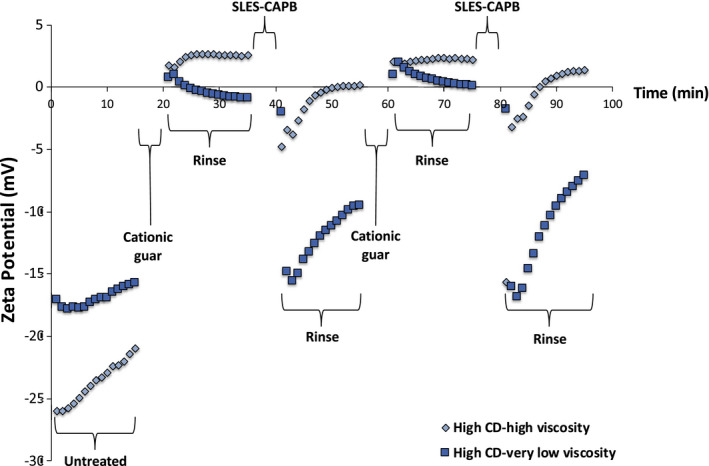
Zeta potential profile of hair treated with high CD‐high viscosity and high CD‐very low viscosity cationic guar
In general, we find that the zeta potential after treatment with anionic surfactants (SLES‐CAPB) of hair pre‐treated with a cationic polymer is greater than hair pre‐treated with a cationic surfactant. Cationic surfactants are removed from the surface of hair after repeated shampooing. On the other hand, once a cationic polymer (polysaccharide or flexible chain) adsorbs to the surface of hair it is not easily removed by anionic surfactant treatment. This phenomenon stems from two principles regarding polymer adsorption. First, there is an increase in entropy when a polyelectrolyte is adsorbed on a surface from solution due to the release of counterions in the solution from the polyelectrolyte and surface [25]. Therefore, one would expect a greater entropic contribution with increasing charge density of the polymer. Second, in order to remove a cationic polymer from the surface, all of the electrostatic bonds between the polymer and hair would have to be broken simultaneously [11]. In addition, if a coacervate is formed with cationic guar during the SLES‐CAPB treatment cycle, this would facilitate polymer deposition to an even greater extent.
Examination of the thickness of the deposited layers on the hair surface for cationic guar derivatives yielded interesting results. Table 2 contains the deposited thin film thickness data after the first and second treatments with cationic guar followed by SLES‐CAPB. Immediately apparent is the dependence of the adsorbed polymer layer on the viscosity (ie MW) of the cationic guar variant. The higher viscosity samples leave considerably thicker films on the hair surface. When comparing the three high viscosity samples, there is no apparent dependence on CD. For comparison, the low and very low viscosity cationic guar derivatives result in much thinner deposition layers, approaching levels anticipated for a flexible polymer. In the case of the high viscosity samples, subsequent treatment with SLES‐CAPB appears to reduce the thickness of adsorbed polymer layer. However, in the case of the low and very low viscosity samples the thickness stays the same after SLES‐CAPB treatment. In the case of most polymers we have investigated by the streaming potential technique, there is normally a slight increase in the thickness of the deposited layer after the second treatment, but this normally reaches a plateau after several treatments.
TABLE 2.
Calculated thickness of the adsorbed polymer layer according to Equation 2. Data are provided in μm
| Cationic guar Treatment 1 | SLES‐CAPB Treatment 1 | Cationic guar Treatment 2 | SLES‐CAPB Treatment 2 | |
|---|---|---|---|---|
| High CD‐high viscosity | 6.01 ± 1.30 | 2.97 ± 0.76 | 8.39 ± 2.17 | 5.35 ± 1.57 |
| Medium CD‐high viscosity | 8.93 ± 0.89 | 4.50 ± 0.59 | 11.24 ± 1.00 | 7.75 ± 1.00 |
| Low CD‐high viscosity | 7.00 ± 0.86 | 4.75 ± 0.79 | 9.24 ± 1.30 | 6.88 ± 0.89 |
| Medium CD‐low viscosity | 1.02 ± 0.05 | 1.28 ± 0.53 | 1.88 ± 0.38 | 1.96 ± 0.78 |
| High CD‐very low viscosity | 0.00 ± 0.00 | 0.18 ± 0.13 | 0.12 ± 0.10 | 0.21 ± 0.16 |
Mechanical wet combing analysis of hair treated with cationic guar derivatives
One of the most common techniques to measure the surface properties of hair is wet combing analysis. The forces encountered during wet combing—as opposed to dry combing—are thought to arise from swelling of the fibres and increased adhesion between the fibres, which both would impede the motion of a comb through a hair fibre assembly [28]. Most conditioning agents reduce wet combing forces. In part, the surface tension properties of a treatment are believed to contribute to the level of wet combing forces [29].
To better understand the effects of cationic guar MW and CD on the conditioning properties of hair, we conducted wet combing analysis on fine hair bleached in two regions of the tress. The use of fine hair allowed us to minimize the amount of error usually associated with wet combing measurements. Measurements of untreated, bleached hair were conducted first, followed by treatment of the entire tress with cationic guar, and then additional combing measurements. In the analysis, the two regions of the combing curves corresponding to the window regions (where bleaching was administered) were integrated before and after treatment. Average values were obtained by measuring three tresses twice. Again, this special technique (fine hair bleached in the windows region) was chosen due to its ability to discern small differences between treatments. Therefore, it should be noted that the streaming potential data presented throughout this report were collected using virgin dark brown hair. We investigated the effects of bleaching and subsequent treatment with cationic guar on the streaming potential profiles (data not shown) and only found differences in the magnitude of the various parameters as compared to virgin hair.
Figure 10 contains a graph of the combing work values reported in gf⋅cm for a number of cationic guar variants. The order of sequence in the bar chart corresponds to the greatest (left side) to least (right side) difference between untreated bleached hair and bleached hair treated with the respective cationic guar variant. The actual difference in combing work is provided on the chart and ranges from 0.0138 to 0.0695 gf⋅cm. Overall, it appears that there is a trend demonstrating that CD plays a greater role than MW at determining the combing reduction properties of cationic guar.
FIGURE 10.
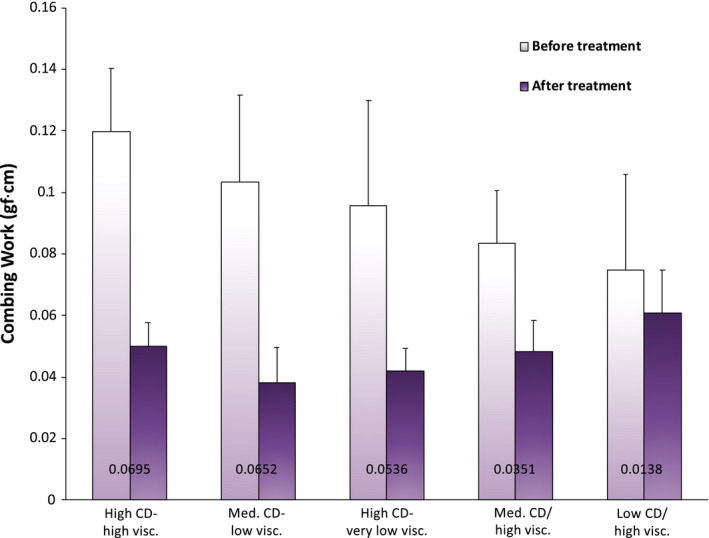
Combing work data (integrated window regions) obtained from wet combing curves for hair treated with various cationic guar variants. The number reported on each bar pair corresponds to the difference between treated and untreated hair for that particular treatment
Behaviour of cationic guar on different types of hair
The interaction of cationic guar with hair was investigated for various hair types including African, Chinese, European dark brown, European 65% grey, Indian and Mulatto hair. In previous work, it was reported that the amino acid composition is the same for all hair types [30]. Further, a recent study carried out with an ethnically diverse pool of individuals from South Africa detailed the proteomic evaluation of hair and did not find racial differences in the quantitative evaluation of keratins, keratin‐associated proteins, histone proteins and desmosomes [31]. Despite these findings, data by several groups, including our own, show that the lipid content of African hair is higher than for other hair types [32]. Asian hair has the most circular cross section while African hair is the most elliptical. In addition, African hair has a lower radial swelling rate than Asian and Caucasian hair, which could be attributed to its higher lipid content. In terms of mechanical (tensile) properties, it is generally found that the break stress and elongation at break is lower in African hair than Asian and Caucasian hair. Most of the investigated differences in hair types from various racial origin focus on the bulk properties of the fibre imparted by the cortex. With the exception of lipid analysis, differences in the outermost layers of the cuticle have yet to be reported. Nevertheless, we investigated the deposition properties of cationic guar on various types of hair.
Figure 11 contains a zeta potential plot comparing European dark brown and Mulatto hair treated with low CD‐high viscosity cationic guar. At first glance, both hair types appear to behave similarly when treated with the cationic guar variant. However, there are subtle differences in the zeta potential profiles that are very reproducible from one experiment to another. For example, there is flatter plateau at the beginning of both rinse steps (20‐35 min and 60‐75 min) right after treatment with cationic guar for Mulatto as compared to European dark brown hair. After making this observation on numerous occasions, we came to the conclusion that Mulatto hair has a more open structure allowing cationic guar to more quickly bind to the surface. Likewise, the zeta potential values at the beginning of the rinsing cycle after treatment with SLES‐CAPB demonstrate that there is a greater negative charge present in the case of European dark brown hair. In this case, it probably indicates that SLES‐CAPB rinses from Mulatto with greater ease than from European dark brown hair due to its more open structure. In fact, we made the same observation for African and European 65% grey hair. Overall, our findings suggest that African, European 65% grey, and Mulatto hair might more quickly desorb SLES‐CAPB than Chinese, European dark brown and Indian hair. This might be a structural attribute of the different hair types. For example, African, European 65% grey and Mulatto hair may have a more open structure that allows for quicker sorption and desorption (or diffusion into or out of the fibre) of ingredients.
FIGURE 11.
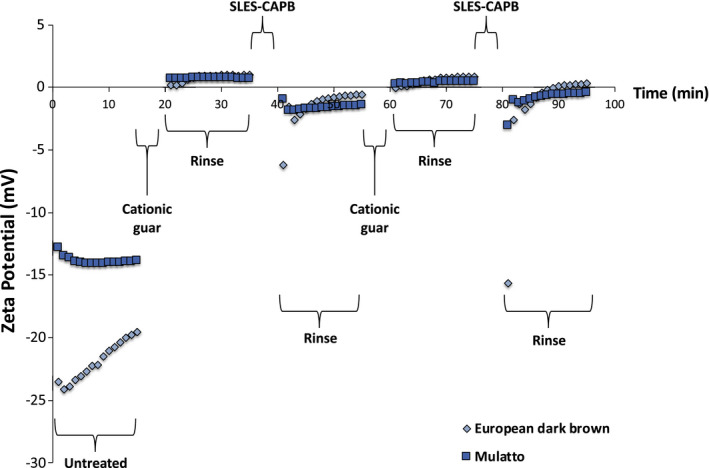
Zeta potential profile for European dark brown and Mulatto hair treated with low CD‐high viscosity cationic guar
Conductivity and pH of the streaming potential solution
Thus far, we have shown data of the zeta potential of hair treated with anionic surfactants, cationic surfactants and cationic polymers with various degrees of MW and CD. The determination of zeta potential of hair is dependent on the streaming potential solution conductivity. The pH also affects zeta potential since changes in pH can alter the surface chemistry of hair—protonating or deprotonating pendant and terminal acid and amide groups of the protein amino acids—thereby influencing the streaming potential values. As noted in the experimental section of this report, we monitor pH, conductivity, streaming potential, temperature and flow rate during each experiment. At very low concentrations, one might expect the KCl electrolyte solution to be very sensitive to small changes in pH or conductivity caused by adding surfactants or polymers to the streaming potential cell.
Figure 12 contains a plot of streaming potential solution conductivity as a function of the duration of the experiment. At the far left of the plot, there is a section labelled as untreated. This is the rinse stage of pre‐washed untreated hair. The conductivity of the streaming potential solution after passing through the various hair plugs of untreated hair (1‐15 min) is reproducible from experiment to experiment. Treatment with cationic guar is administered between 16 and 20 min. After treatment (16‐20 min), the conductivity begins to decrease until it plateaus between 2.7 and 5.5 µS/cm for the different cationic guar variants. When the surfactant solution of SLES‐CAPB is introduced to the system (36‐40 min), there is a spike in the conductivity. However, there is a plateau of the conductivity after thorough rinsing (41‐55 min).
FIGURE 12.

Conductivity profiles of hair treated with various cationic guar derivatives
As a result of the second cycle of cationic guar treatment (56‐60 min), there is another small peak in the conductivity curves, which again seems to level off during the rinse cycle (61‐75 min). Finally, a second SLES‐CAPB treatment is administered (76‐80 min), which causes an increase in the conductivity for all cationic guar variants, which is slightly higher than that observed in the first cycle. During the final rinse stage (81‐95 min), the conductivity appears to plateau for nearly all of the samples, except one of the high CD variants.
Our results suggest that the choice of cationic guar species used to treat the hair influence the measured conductivity. The conductivity of an electrolyte solution is a measure of its ability to conduct electricity due to the ion concentration. To some extent, the number of ions present in solution is proportional to the amount of charge transferred between the electrodes of a conductivity meter. It should be noted that not all ions conduct electricity to the same degree. For example, ions that move slower through solution do not conduct electricity as well as ions that have greater speed and mobility.
Comparing the different cationic guar variants in Figure 12, the MW (viscosity) and CD appear to affect the conductivity. Treatment of hair with the higher molecular weight species results in conductivity values greater than lower molecular variants. Within the higher MW samples (low CD‐high viscosity, medium CD‐high viscosity and high CD‐high viscosity), treatment of hair with lower CD cationic guar results in higher conductivity readings. It could be that the lower CD of the macroion results in less binding of the ions from the electrolyte solution with the polymer‐coated hair fibre. Therefore, more of the ions from the streaming potential solution would be free and detectable during the conductivity measurements. The same trend is observed when comparing the lower MW species (medium CD‐low viscosity and medium CD‐very low viscosity). As for the differences between high and low MW cationic guar treatment, ions from the streaming potential solution may be able to move more freely and access the charge sites of the lower molecular weight analogs. Again, this might leave less free ions in the streaming potential solution, and therefore, decrease the conductivity. Likewise, conductivity measurements of behentrimonium chloride and polyquaternium‐28‐treated hair resulted in similar fluctuations, although the recovery time is slightly greater probably due to their higher CDs than the cationic guar variants.
The pH profile for the streaming potential solution after treatment of hair with high CD‐very low viscosity cationic guar is provided in Figure 13. Essentially, treatment with all of the cationic guar variants resulted in very similar pH profiles. The pH of the streaming potential solution after passing through the streaming potential cell for untreated hair is generally pH 5.5. There is a small change in pH after treatment with guar. In some cases, there is a slight increase while in others a small decrease is observed. The pH change due to cationic guar treatment only fluctuates during the online treatment cycle (ie at 21‐35 min and 56‐60 min) and returns to normal during the rinsing stage. Similarly, treatment with SLES‐CAPB (ie 36‐40 and 76‐80 min) causes the pH to rise to just below pH 6.5, but only during the treatment stage. As in the case with the cationic guar treatment, the pH returns to a normal steady value during the rinsing step. Similar trends in pH were observed for treatments with behentrimonium chloride and polyquaternium‐28.
FIGURE 13.

pH profile of hair treated with high CD‐very low viscosity cationic guar
CONCLUSIONS
In this study, we present data on the electrokinetics of the deposition of cationic guar variants on human hair. The deposition behaviour is controlled by CD and MW of the sample. CD has a direct effect on the measured zeta potential of the treated fibres. On the other hand, MW has greater influence on the thickness of the deposited thin film. Mechanical wet combing experiments of hair tresses demonstrated that CD actually plays the dominant role in determining the amount of combing force reduction of hair fibre assemblies in the form of a tress. We also examined the behaviour of cationic guar and SLES‐CAPB on various types of hair. We found that curly (African and mulatto) and European 65% grey hair behaved differently as compared to Chinese, European dark brown and Indian hair. For example, SLES‐CAPB desorbs more quickly from curly and grey hair than the other types we tested, which may suggest they have a more open structure and morphology, at least at the cuticle level.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the technical contributions of Drs. Janusz Jachowicz and Marek Zielinski in the design and construction of the streaming potential instrument. Also, much gratitude goes to Dr. Michael Franzke and Ms. Lidia Kulcsar from Ashland for critically reading the text and offering their insight. The authors would like to acknowledge the financial support provided by Ashland Specialty Ingredients, G.P. This article is dedicated to our coauthor Donna Laura, one of the most meticulous scientists with whom we have had the pleasure to collaborate.
REFERENCES
- 1. Labeau M., Guar and guar derivatives. In Polymers for a Sustainable Environment and Green Energy, Eds. Matyjaszewski K., Möller M., (Elsevier, Amsterdam, Netherlands, 2012), pp. 195–203. [Google Scholar]
- 2. Pal S., Mal D., Singh R., Synthesis and characterization of cationic guar gum: a high performance flocculating agent. J. Appl. Polym. Sci. 105, 3240–3245 (2007). [Google Scholar]
- 3. Wan X., Guo C., Feng J., Yu T., Chai X., Chen G., Xie W., Determination of the degree of substitution of cationic guar gum by headspace‐based gas chromatography during its synthesis. J. Agric. Food Chem. 65, 7012–7016 (2017). [DOI] [PubMed] [Google Scholar]
- 4. Bujak T., Nizioł‐Łukaszewska Z., Ziemlewska A., Amphiphilic cationic polymers as effective substances improving the safety of use of body wash gels. Int. J. Biol. Macromol. 147, 973–979 (2020). [DOI] [PubMed] [Google Scholar]
- 5. Chiron S., Performance and sensorial benefits of cationic guar in hair care applications. Cosmet Toil. 119(2), 47–50, 52 (2004). [Google Scholar]
- 6. Freeland M., Holder I., Tucker J., Cationic guar gum. Cosmet Toil. 99(6), 83–87 (1984). [Google Scholar]
- 7. Biasotti B., Colombo R., Fumagalli C., Riccaboni M., Langella V., Botto E., Gatti K., Rolling ball viscometer: a new method for performances prediction of cationic guar derivatives. H&PC Today. 12(3), 71–73 (2017). [Google Scholar]
- 8. Fevola M.. Guar hydroxypropyltrimonium chloride. Cosmet Toil, 127(1), 16, 18‐21 (2012). [Google Scholar]
- 9. Robbins C., Chemical and Physical Behavior of Hair. 5th ed., (Springer, Heidelberg, Germany, 2012). [Google Scholar]
- 10. Banerjee S., Cazeneuve C., Baghdadli N., Ringeissen S., Léonforte F., Leermakers F., Luengo G., Modeling of polyelectrolyte adsorption from micellar solutions onto biomimetic substrates. J. Phys. Chem. B. 121(37), 8638–8651 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Llamas S., Guzmán E., Ortega F., Baghdadli N., Cazeneuve C., Rubio R., Luengo G., Adsorption of polyelectrolytes and polectrolytes‐surfactant mixtures at surfaces: a physico‐chemical approach to a cosmetic challenge. Adv. Coll. Inter. Sci. 222, 461–487 (2015). [DOI] [PubMed] [Google Scholar]
- 12. Anthony O., Marques C., Richetti P., Bulk and surface behavior of cationic guars in solutions of oppositely charged surfactants. Langmuir. 14, 6086–6095 (1998). [Google Scholar]
- 13. Svensson A., Huang L., Johnson E., Nylander T., Piculell L., Surface deposition and phase behavior of oppositely charged polyion/surfactant ion complexes. 1. Cationic guar versus cationic hydroxyethylcellulose in mixtures with anionic surfactants. ACS Appl. Mater. Interfaces. 1(11), 2431–2442 (2009). [DOI] [PubMed] [Google Scholar]
- 14. Yuan Z., Wang J., Niu X., Ma J., Qin X., Li L., Shi L., Wu Y., Guo X., A study of the surface adhesion and rheology properties of cationic conditioning polymers. Ind. Eng. Chem. Res. 58, 9390–9396 (2019). [Google Scholar]
- 15. Faucher J., Goddard E., Hannan R., Sorption and desorption of a cationic polymer by human hair: effects of salt solutions. Text Res. J. 47(9), 616–620 (1977). [Google Scholar]
- 16. Jachowicz J., Williams C., Fingerprinting of cosmetic formulations by dynamic electrokinetic and permeability analysis. I. Shampoos. J. Soc. Cosmet. Chem. 45(6), 309–336 (1994). [Google Scholar]
- 17. Jachowicz J., Fingerprinting of cosmetic formulations by dynamic electrokinetic and permeability analysis. II. Hair conditioners. J. Soc. Cosmet. Chem. 46(2), 100–116 (1995). [Google Scholar]
- 18. Jachowicz J., Maxey S., Williams C., Sorption/desorption of ions by dynamic electrokinetic and permeability analysis of fiber plugs. Langmuir. 9(11), 3085–3092 (1993). [Google Scholar]
- 19. Jachowicz J., Berthiaume M., Garcia M., The effect of the amphiprotic nature of human hair keratin on the adsorption of high charge density cationic polyelectrolytes. Colloid Polym. Sci. 263(10), 847–858 (1985). [Google Scholar]
- 20. Dussaud A., Breen P., Koczo K., Characterization of the deposition of silicone copolymers on keratin fibers by streaming potential measurements. Colloids Surf. A. 434, 102–109 (2013). [Google Scholar]
- 21. Algie J., Baird K., Foulds R., Robinson V., The felting of wool fabrics washed at various values of pH, and the isoelectric point determined by streaming potential measurements. Text. Res. J. 44(10), 767–771 (1974). [Google Scholar]
- 22. Capablanca J., Watt I., Factors affecting the zeta potential at wool fiber surfaces. Text. Res. J. 56(1), 49–55 (1986). [Google Scholar]
- 23. N‐Hance Cationic Guar and AquaCat Cationic Guar Solutions: Products for Personal Care. (Ashland, Inc., Wilmington, DE; 2010). [Google Scholar]
- 24. Jachowicz J., Helioff M., Spatially resolved combing analysis. J. Soc. Cosmet. Chem. 48, 93–105 (1997). [Google Scholar]
- 25. Guzmán E., Ortega F., Baghdadli N., Luengo G., Rubio R., Effect of the molecular structure on the adsorption of conditioning polyelectrolytes on solid substrates. Colloids Surf. A. 375, 209–218 (2011). [Google Scholar]
- 26. Luxbacher T., Electrokinetic properties of natural fibres. In Handbook of Natural Fibres, Volume 2: Processing and Applications, Ed. Kozłowski R., (Woodhead Publishing, Cambridge, 2012). [Google Scholar]
- 27. Arai M., Suzuki T., Kaneko Y., Miyake M., Nishikawa N., Properties of aggregates of amide guanidine type cationic surfactant with 1‐hexadecanol adsorbed on hair. In Studies in Surface Science and Catalysis 132, Eds. Iwasawa Y., Oyama N., Kunieda H., (Elsevier Science, Amsterdam, Netherlands, 2001), pp. 1005–1008. [Google Scholar]
- 28. Newman W., Cohen G., Hayes C., A quantitative characterization of combing force. J. Soc. Cosmet. Chem. 24, 773–782 (1973). [Google Scholar]
- 29. Kamath Y., Weigmann H., Measurement of combing forces. J. Soc. Cosmet. Chem. 37, 111–124 (1986). [Google Scholar]
- 30. Wolfram L., Human hair: a unique physicochemical composite. J. Am. Acad. Dermatol. 48, S106–S114 (2003). [DOI] [PubMed] [Google Scholar]
- 31. Adeola H., Khumalo N., Arowolo A., Mehlala N., No difference in the proteome of racially and geometrically classified scalp hair sample from a South African cohort: Preliminary findings. J. Proteom. 226, 103892 (2020). [DOI] [PubMed] [Google Scholar]
- 32. Martí M., Barba C., Manich A., Rubio L., Alonso C., Coderch L., The influence of hair lipids in ethnic hair properties. Int. J. Cosmet. Sci. 38, 77–84 (2016). [DOI] [PubMed] [Google Scholar]


