Abstract
Objective
To determine the associations of delirium with urinary tract infection (UTI) and asymptomatic bacteriuria (AB) in individuals aged 65 and older.
Methods
The protocol for this systematic review and meta‐analysis was published on PROSPERO (CRD42020164341). Electronic databases were searched for relevant studies, professional associations and experts in the field were additionally contacted. Studies with control groups reporting associations between delirium and UTI as well as delirium and AB in older adults were included. The random effects model meta‐analysis was conducted using odds ratios (ORs) with 95% confidence intervals (CIs) as effect size measures. The Newcastle‐Ottawa scale was used to rate the studies' quality. Heterogeneity was assessed using the Q and I 2 tests. The effects of potential moderators were investigated by both subgroup and meta‐regression analyses. The risk of publication bias was evaluated using the funnel plot and Egger's test.
Results
Twenty nine relevant studies (16,618 participants) examining the association between delirium and UTI in older adults were identified. The association between delirium and UTI was found to be significant (OR 2.67; 95% CI 2.12–3.36; p < 0.001) and persisted regardless of potential confounders. The association between delirium and AB in older adults in the only eligible study found (192 participants) was insignificant (OR 1.62; 95% CI 0.57–4.65; p = 0.37). All included studies were of moderate quality.
Conclusion
The results of this study support the association between delirium and UTI in older adults. Insufficient evidence was found to conclude on an association between delirium and AB in this age group. These findings are limited due to the moderate quality of the included studies and a lack of available research on the association between delirium and AB. Future studies should use the highest quality approaches for defining both delirium and UTI and consider AB in their investigations.
Keywords: asymptomatic bacteriuria, delirium, urinary tract infection
Key Points
There is a significant association between delirium and UTI in older adults.
The association between delirium and AB in older adults in the only study we could find was statistically insignificant.
Why Does this Paper Matter?
Clinicians should be aware of the importance of assessing UTI symptoms in older adults with delirium and of the possible presence of delirium in older adults referred for treatment of UTI.
INTRODUCTION
The prevalence of delirium in persons aged 65 and older (older adults) varies, depending on the method of assessment and the population studied, from 18%–35% in the general medical ward, to 20%–22% in nursing homes, 25% in geriatric units, and 7%–50% in intensive care units. 1 Delirium in older adults is associated with prolonged hospital stays, complexity of care, institutionalization and high mortality rates as well as considerable difficulties for caregivers and increased healthcare costs. 2 , 3 , 4
Urinary tract infection (UTI) among older adults is also associated with a high number of cases annually and high healthcare costs. 5 , 6 The diagnosis of UTI requires not only confirmed bacteriuria but also the presence of genitourinary symptoms, which often cannot be reliably confirmed in the many delirious individuals who are unable to adequately express themselves, thus posing a challenge to the clinical assessment of such patients. 7 When bacteriuria is detected in older adult patients, many clinicians consider behavioral or mental changes, including delirium, as non‐urinary (also called “non‐specific”) manifestations of UTI, 8 , 9 especially in patients with cognitive impairment, from whom local urinary tract symptoms are often difficult to obtain. 10 Сlinicians often prescribe antibiotics for delirium in patients with confirmed bacteriuria, based on the assumption that anti‐bacterial treatment will manage non‐specific psychiatric symptoms and improve patient outcomes. 11 , 12
The available evidence suggests that there is no clinical benefit (both in terms of preventing further urological problems, in terms of mental disturbances, and in terms of mortality) from the treatment of asymptomatic bacteriuria (AB), which usually has a benign natural course and may resolve on its own or persist despite treatment. 13 , 14 Moreover, such treatment may lead to serious side effects and the possibility of antibiotic‐resistant bacteria. 15 , 16 , 17 There is also evidence that the administration of antibiotics itself is associated with an increased risk of delirium. 18 , 19
However, the studies addressing the association between delirium and UTI in older adults are contradictory. 20 , 21 , 22 , 23 Furthermore, there are limited data on the association between delirium and AB in older adults. 13
There have been three systematic reviews published to date 24 , 25 , 26 that have addressed the relationship between delirium and UTI. Balogun and Philbrick 24 in their systematic review concluded that there may be an association between UTI and delirium in older adults, but the studies reviewed had methodological flaws that may have led to biased results. Chae and Miller 25 did not limit their study to older adults and suggested a potential relationship between UTI and delirium. Mayne and colleagues 26 used the less specific diagnostic category of confusion rather than delirium in their study. The authors concluded that there were insufficient data to determine the relationship between confusion and UTI in older adults, explaining this by poor confusion or UTI definitions as well as inadequate control of confounding factors in individual studies. Previous reviews used less comprehensive search approaches and did not provide a meta‐analytical synthesis. The association between delirium and AB has not been separately systematically studied in any age group.
By answering the main study question, what are the associations of delirium with UTI and AB in older adults, this review should improve the available evidence on these associations.
METHODS
Methods for this systematic review and meta‐analysis have been developed based on recommendations from the PRISMA statement. 27 The protocol for this study, including the definition of the study questions, description of the search strategy, definition of the inclusion and exclusion criteria, description of the approach to assessing risk of bias, and a plan for meta‐analytic synthesis, has previously been published on PROSPERO under number CRD42020164341. 28
Search methods
An electronic search was conducted on January 5, 2021 in the following databases: PubMed, Ovid databases (Ovid Medline, PsycINFO, Embase), Cochrane Library, Scopus, CINAHL, Web of Science (Web of Science Core Collection, KCI‐Korean Journal Database, Medline, Russian Science Citation Index, SciELO Citation Index), Networked Digital Library of Theses and Dissertations, and ProQuest Dissertations & Theses Global. When necessary, authors of potentially relevant publications were contacted directly to request complete usable data. A manual search was also conducted both in journals and in the proceedings of conferences and congresses with the highest impact in the field. We also contacted professional associations and experts in the field to request any further relevant data. Further details can be found in Text S1.
Selection criteria
Study type
Original studies with a control group regardless of their publication type and design (with the exception of case series and case studies) were considered. We included studies reporting the degree of associations, expressed as odds ratios (ORs), between delirium and UTI as well as between delirium and AB, or data to calculate these ORs. We considered studies of individuals (a) with delirium compared with non‐delirium controls, (b) with UTI compared with non‐UTI controls, and (c) with AB compared with non‐bacteriuric controls.
Population
Older adults (aged 65 and older) with a diagnosis of delirium, UTI or AB. Comparison subjects were participants without delirium, UTI, or AB respectively. The diagnosis of delirium could have been made either (1) according to DSM or ICD diagnostic criteria (any version); or (2) using a symptom threshold on validated delirium rating scales; or (3) based on records in medical files/registries. The diagnosis of UTI could have been made either (1) on the basis of presence of 1 or more species of bacteria growing in the urine specimen at specified quantitative counts (≥105 colony‐forming units [CFU]/ml or ≥108 CFU/L) in the presence of classic signs or symptoms of (a) cystitis (dysuria, urinary frequency and/or urgency, suprapubic pain or tenderness, hematuria) or (b) pyelonephritis (flank pain, costovertebral angle pain or tenderness) or (c) systemic illness (fever, chills, rigors, marked fatigue, malaise beyond baseline) or (2) based on a listing of the diagnosis of UTI in medical records without additional data on tests performed and presence of UTI symptoms. The diagnosis of AB could have been made either (1) on the basis of presence of 1 or more species of bacteria growing in the urine specimen at specified quantitative counts (≥105 CFU/ml or ≥108 CFU/L) in the absence of signs or symptoms attributable to UTI or (2) on the basis of study‐defined criteria or (3) based on a listing of the diagnosis of AB in medical records.
Outcomes
The outcome measures were ORs expressing the association between delirium and UTI, and between delirium and AB in older adults.
Identification and selection of studies, data extraction, assessment of study quality
These steps were performed blindly by D.K. and R.K. and are described in detail in Text S1. The quality of the studies was assessed using the Newcastle‐Ottawa scale 29 (see Text S2 with the scale content and Table S4 with the detailed results of each study assessment).
Statistical analysis
ORs were extracted when they were available or calculated from the available data. We used random‐effects model in our meta‐analysis. First, we performed a meta‐analysis of ORs across all studies addressing the association between delirium and UTI in older adults. Next, a sensitivity analysis was conducted by (1) removing the study with the highest relative weight and (2) removing the one clear outlier with the highest effect size. Then we conducted a subgroup meta‐analyses of the studies (1) in which participants with delirium were compared with non‐delirium controls, (2) in which participants with UTI were compared with non‐UTI controls, (3) with a diagnosis of delirium based on DSM or ICD criteria, (4) with a diagnosis of UTI based on microbiological urine tests, (5) conducted in the clinical setting, (6) conducted in nursing homes, (7) of population‐based samples.
Additionally, we performed meta‐regression analyses including year of study publication, study country, type, temporality and setting, delirium and UTI assessment method, rating on the Newcastle‐Ottawa scale, age of study participants, their gender distribution and residence status, psychiatric and medical comorbidities, and received medications as regressors (for a complete list of all regressors, see Table S5).
We used Cochran's Q test and I 2 values to assess the heterogeneity of effect sizes. We used funnel plot as well as Egger's regression asymmetry test 30 to assess the presence of the publication bias. We used Duval and Tweedie's “trim and fill” method to examine the effect of the missing results caused by publication bias, if it was detected. 31 With this method, a pooled effect size is recalculated by including the hypothetical missing studies as if they actually existed to augment the observed data so that the funnel plot is more symmetrical.
In the only study found addressing the association between delirium and AB in older adults, the OR was calculated separately.
Analyses were performed using Comprehensive Meta‐Analysis Version 3 (Biostat, Englewood, NJ 2013).
RESULTS
Figure 1 illustrates the study selection process, with 29 studies 20 , 21 , 22 , 23 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 being retained for a meta‐analysis of the associations between delirium and UTI, comprising 16,618 participants, with 26 studies 20 , 21 , 23 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 comparing participants with delirium (4842 individuals) to those without delirium (10,849 individuals), and 3 studies 22 , 39 , 56 comparing participants with UTI (266 individuals) to those without UTI (661 individuals) (for details on each study, see Table 1 and Table S1). A study by Gau and colleagues 22 presented data on the association between delirium and UTI as well as on the association between delirium and AB (192 participants in total: 50 individuals with AB and 142 non‐bacteriuric subjects) and this was the only study we could find that addressed this association: the OR with a 95% confidence interval (CI) for this association was calculated separately (for details see Table 1 and Table S2). Studies not included in the meta‐analysis after evaluation of the full text are listed in Table S3, with reasons for exclusion.
FIGURE 1.
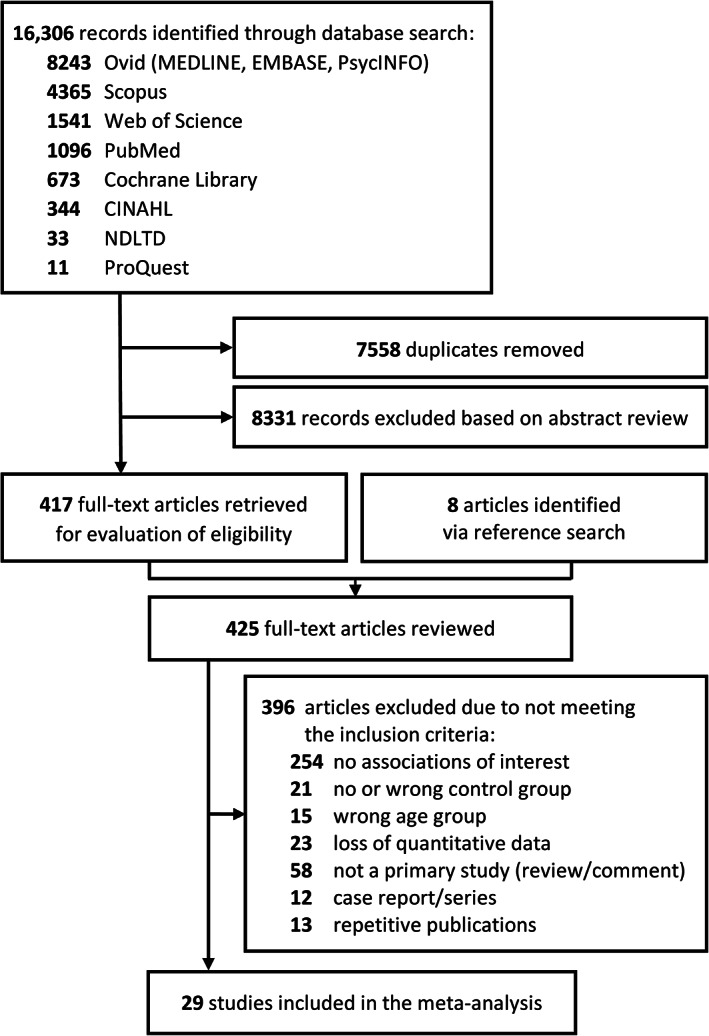
PRISMA diagram of included and excluded studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
TABLE 1.
Main characteristics a of included studies reporting associations of delirium with urinary tract infections and asymptomatic bacteriuria in older adults
| Study | Study design | Approaches to the diagnosis of delirium, UTI, and AB |
|---|---|---|
| Alvarez‐Perez, 2017 32 | Retrospective сohort study |
Delirium: DSM‐5 criteria UTI: record in medical charts |
| Amado Tineo, 2013 33 | Prospective cross‐sectional study |
Delirium: CAM UTI: record in medical charts |
| Anderson, 2010 20 | Prospective cohort study |
Delirium: CAM UTI: record in medical charts |
| Arshi, 2018 34 | Prospective cohort study |
Delirium: record in medical charts UTI: record in medical charts |
| Brouquet, 2010 21 | Prospective cohort study |
Delirium: CAM UTI: record in medical charts |
| de Bortoli Pereira, 2018 35 | Prospective cross‐sectional study |
Delirium: CAM UTI: record in medical charts |
| Edlund, 2001 36 | Prospective cohort study |
Delirium: DSM‐IV criteria UTI: record in medical charts |
| Edlund, 2006 37 | Prospective cohort study |
Delirium: DSM‐IV criteria UTI: record in medical charts |
| Elsamadicy, 2017 38 | Retrospective cohort study |
Delirium: DSM‐5 criteria UTI: record in medical charts |
| Eriksson, 2010 39 | Prospective cross‐sectional study |
Delirium: DSM‐IV criteria UTI: record in medical charts |
| Eriksson, 2011 40 | Prospective cross‐sectional study |
Delirium: DSM‐IV criteria UTI: record in medical charts |
| Gau, 2009 22 | Retrospective case–control study |
Delirium: record in medical charts UTI: positive microbiological urine tests and presence of UTI symptoms AB: positive microbiological urine tests and absence of UTI symptoms |
| Gual, 2018 41 | Prospective cohort study |
Delirium: CAM UTI: record in medical charts |
| Jitapunkul, 1992 23 | Prospective cohort study |
Delirium: DSM‐III‐R criteria UTI: record in medical charts |
| Khurana. 2002 42 | Prospective cohort study |
Delirium: ICD‐10 research criteria UTI: record in medical charts |
| Kobayashi, 2017 43 | Retrospective cohort study |
Delirium: record in medical charts UTI: record in medical charts |
| Large, 2013 44 | Prospective cohort study |
Delirium: CAM UTI: record in medical charts |
| Lundström, 2004 45 | Control group from an RCT considered as a prospective cohort |
Delirium: DSM‐IV criteria UTI: record in medical charts |
| Lundström, 2005 46 | Control group from an RCT considered as a prospective cohort |
Delirium: DSM‐IV criteria UTI: record in medical charts |
| Marcantonio, 2005 47 | Prospective cohort study |
Delirium: CAM UTI: record in medical charts |
| Morandi, 2019 48 | Retrospective cohort study |
Delirium: CAM UTI: record in medical charts |
| Olofsson, 2005 49 | Prospective cohort study |
Delirium: DSM‐IV criteria UTI: record in medical charts |
| Olofsson, 2018 50 | Prospective cohort study |
Delirium: DSM‐IV‐TR criteria UTI: record in medical charts |
| Perez‐Ros, 2018 51 | Retrospective case–control study |
Delirium: DSM‐IV criteria, CAM UTI: record in medical charts |
| Raats, 2015 52 | Prospective cohort study |
Delirium: DSM‐IV criteria, DOSS UTI: record in medical charts |
| Sandberg, 1999 53 | Prospective cross‐sectional study |
Delirium: DSM‐III‐R criteria UTI: record in medical charts |
| Schuurmans, 2003 54 | Prospective cohort study |
Delirium: DSM‐IV criteria UTI: record in medical charts |
| Stroomer‐van Wijk, 2016 55 | Prospective case–control study |
Delirium: DSM‐IV criteria UTI: record in medical charts |
| Wojszel, 2018 56 | Prospective cohort study |
Delirium: DOSS UTI: positive microbiological urine tests and presence of UTI symptoms |
Abbreviations: AB, asymptomatic bacteriuria; CAM, Confusion Assessment Method; DOSS, Delirium Observation Screening Scale; DSM, Diagnostic and Statistical Manual of Mental Disorders (number refers to the edition); ICD, International Classification of Diseases (number refers to the edition); RCT, randomized controlled trial; UTI, urinary tract infection.
For further details, see Tables S1 and S2.
The results of all analyses relating the associations between delirium and UTI in older adults are summarized in Table 2, which reports the effect sizes (ORs with 95% CI) as well as tests of heterogeneity and publication bias (Egger's test).
TABLE 2.
Summary of results demonstrating pooled odds ratios in the main meta‐analysis, sensitivity analysis and subgroup meta‐analysis of associations between delirium and urinary tract infections in older adults
| Heterogenity | Egger's test | |||||||
|---|---|---|---|---|---|---|---|---|
| Type of analysis | N | OR (95% CI) | p value | Q | p value | I 2 | t | p value |
| All studies 20 , 21 , 22 , 23 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 | 29 | 2.67 (2.12–3.36) | <0.001 | 70.87 | <0.001 | 60.49 | 2.95 | 0.007 |
| After removing the study with the highest weight 34 | 28 | 2.77 (2.24–3.43) | <0.001 | 47.22 | 0.009 | 42.83 | 1.06 | 0.301 |
| After removing one clear outlier 55 | 28 | 2.57 (2.06–3.20) | <0.001 | 63.07 | <0.001 | 57.19 | 2.62 | 0.014 |
| Studies in which participants with delirium were compared with non‐delirium controls 20 , 21 , 23 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 | 26 | 2.62 (2.05–3.34) | <0.001 | 59.16 | <0.001 | 57.74 | 2.81 | 0.010 |
| Studies in which participants with UTI were compared with non‐UTI controls 22 , 39 , 56 | 3 | 3.07 (1.33–7.06) | 0.008 | 10.29 | 0.006 | 80.56 | 1.03 | 0.490 |
| Studies with a diagnosis of delirium based on DSM or ICD criteria 23 , 32 , 36 , 37 , 38 , 39 , 40 , 42 , 45 , 46 , 49 , 50 , 53 , 54 , 55 | 15 | 3.01 (2.25–4.01) | <0.001 | 22.82 | 0.063 | 38.64 | 2.75 | 0.017 |
| Studies with a diagnosis of UTI based on microbiological urine tests 22 , 56 | 2 | 4.15 (1.32–13.05) | 0.015 | 4.88 | 0.027 | 79.50 | NA | NA |
| Studies conducted in the clinical setting 21 , 22 , 23 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 41 , 42 , 43 , 44 , 45 , 46 , 48 , 49 , 50 , 52 , 54 , 55 , 56 | 23 | 3.22 (2.37–4.37) | <0.001 | 63.94 | <0.001 | 65.59 | 3.11 | 0.005 |
| Studies conducted in nursing homes 20 , 47 , 51 | 3 | 1.64 (1.09–2.48) | 0.018 | 2.14 | 0.343 | 6.60 | 0.52 | 0.696 |
| Studies of population‐based samples 39 , 40 | 2 | 2.19 (1.52–3.14) | <0.001 | 1.19 | 0.276 | 15.90 | NA | NA |
Abbreviations: N, number of studies included in the analysis; NA, not applicable; OR, odds ratio; 95% CI, 95% confidence interval.
Our first analysis of all 29 available studies of associations between delirium and UTI found a significant pooled effect size (OR 2.67; 95% CI 2.12–3.36; p‐value <0.001) (Figure 2). Heterogeneity was high and significant, Egger's test was also significant, indicating publication bias. Correction of publication bias using Duval and Tweedie's method yielded the following result: OR 2.17; 95% CI 1.72–2.74 (see Figure S1).
FIGURE 2.
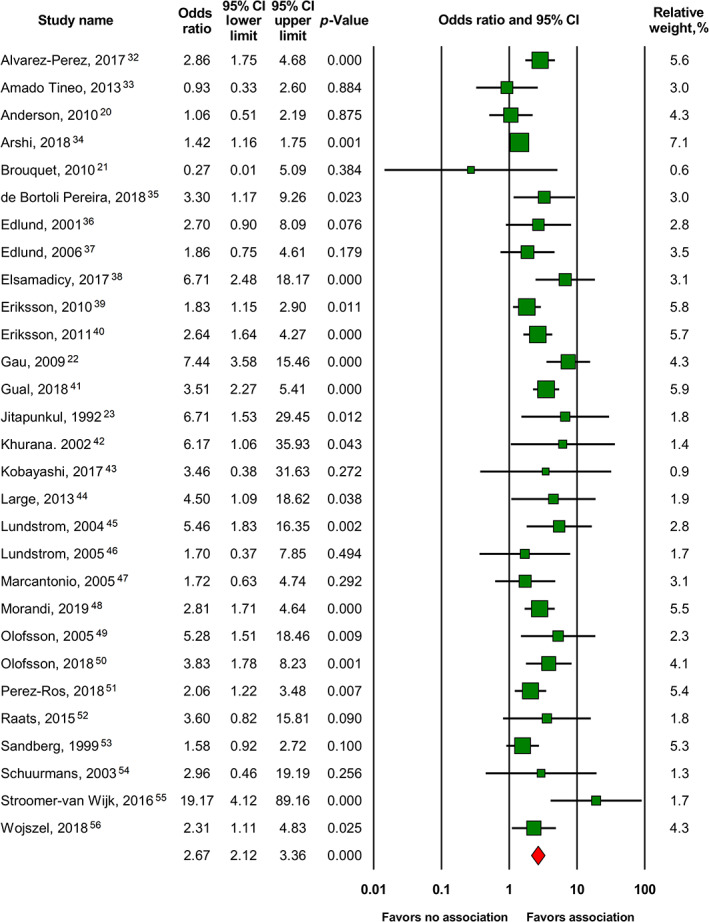
Forest plot of the main meta‐analysis of 29 studies 20 , 21 , 22 , 23 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 expressing associations between delirium and UTI in older adults. 95% CI, 95% confidence interval
After removing the study with the highest weight 34 as well as after removing one clear outlier with the highest effect size, 55 results remained significant. Subgroup meta‐analyses of studies (1) in which participants with delirium were compared with non‐delirium controls, 20 , 21 , 23 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 (2) in which participants with UTI were compared with non‐UTI controls, 22 , 39 , 56 (3) with a diagnosis of delirium based on DSM or ICD criteria, 23 , 32 , 36 , 37 , 38 , 39 , 40 , 42 , 45 , 46 , 49 , 50 , 53 , 54 , 55 (4) with a diagnosis of UTI based on microbiological urine tests, 22 , 56 (5) conducted in the clinical setting, 21 , 22 , 23 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 41 , 42 , 43 , 44 , 45 , 46 , 48 , 49 , 50 , 52 , 54 , 55 , 56 (6) conducted in nursing homes, 20 , 47 , 51 and (7) of population‐based samples 39 , 40 each found a statistically significant pooled OR (see Figures S2–S17). Thus, the results of all sensitivity and subgroup meta‐analyses (see Table 2 and Figures S2–S17) were also statistically significant.
Meta‐regression analysis showed that year of study publication, study country, type, temporality and setting, delirium and UTI assessment method, rating on the Newcastle‐Ottawa scale, age of study participants, their gender distribution, and residence status did not significantly influence the pooled ORs. Among the comorbid psychiatric and medical conditions we examined as regressors only the presence of dehydration, urinary catheter, and urinary retention had a statistically significant effect on the pooled OR. In a meta‐regression analysis, we also found a statistically significant effect of receiving benzodiazepines and drugs with anticholinergic properties on the pooled ORs. For details on meta‐regression analysis, see Tables S5–S13.
The association between delirium and AB in older adults in the only study reporting this association that we could find 22 was statistically insignificant: OR 1.62; 95% CI 0.57–4.65; p‐value 0.37.
DISCUSSION
To our knowledge, this is the first meta‐analysis of the associations of delirium with UTI and AB. Only delirium and UTI were found to be significantly associated.
Through subgroup meta‐analysis, the association between delirium and UTI persisted regardless of whether individuals with delirium were compared with non‐delirium individuals, or individuals with UTI were compared with non‐UTI individuals. This association remains significant when considering (1) only those studies in which delirium was diagnosed according to formal diagnostic criteria and (2) only those studies in which the diagnosis of UTI was based on positive results of microbiological urine tests combined with the presence of UTI symptoms, making it unlikely that our results were biased by non‐optimal approaches to detecting delirium and UTI.
According to both subgroup meta‐analysis and meta‐regression analysis, the association between delirium and UTI in older adults was similar in clinical, nursing home, and population‐based samples; thus, clinical referral bias cannot account for this association.
Meta‐regression analysis showed that gender, age, and differences defining delirium and UTI also had no statistically significant effect on the association between delirium and UTI, which is consistent with the results of our subgroup meta‐analysis. There was also no statistically significant effect of study design (cohort, case–control, cross‐sectional) and temporality (prospective and retrospective) on the association we studied. The effect of the study country did not reach statistical significance. This observation should be interpreted with caution as only one or two studies were available for most countries.
It is noteworthy that preexisting dementia did not show a statistically significant impact on the association between delirium and UTI in older adults in the meta‐regression analysis. It is conceivable that recognition of both delirium and UTI is more difficult in individuals with dementia, making such an association less detectable. This issue requires further study.
Among the moderators related to somatic conditions we found only dehydration, urinary retention, and the presence of a urinary catheter were found to have significant effects on the association between delirium and UTI in older adults. However, the use of a urinary catheter has previously been associated with both delirium and UTI. 57 , 58 The associations of delirium with dehydration and urinary retention have also been reported, 59 , 60 , 61 and there are some suggestions of a possible association between UTI and urinary retention. 62 Although data on the association between UTI and dehydration are contradictory, an adequate hydration is thought to improve the results of antimicrobial therapy for UTI. 63 , 64 In the meta‐regression analysis focusing on medication status we found a statistically significant effect of benzodiazepines and drugs with anticholinergic properties on the association between delirium and UTI. However, the association between delirium and drugs from both of these groups has already been described previously. 65 , 66 , 67
Study quality was not a significant predictor in the meta‐regression analysis, which is consistent with our meta‐analysis of subgroups demonstrating that significant results persisted when only the most rigorous studies were included, that is, studies in which the diagnosis of delirium was based on formal diagnostic criteria, and studies in which UTI was diagnosed based on results of microbiological urine tests.
There was statistically significant heterogeneity in both the main meta‐analysis and most subgroup meta‐analyses. However, in the sensitivity analysis with the removal of the study with the highest relative weight, the heterogeneity expressed by the I 2 value decreased from 60.49 (substantial) to 42.83 (moderate). The degree of heterogeneity was not significant in the subgroup meta‐analysis that included studies with a diagnosis of delirium based on formal criteria, as well as in two smaller subgroup meta‐analyses that included three studies conducted in nursing homes and two studies of population‐based samples.
There was also statistically significant publication bias in both the main meta‐analysis and most subgroup meta‐analyses. However, the publication bias was insignificant in the sensitivity analysis with the removal of the study with the highest relative weight as well as in two small subgroup meta‐analyses involving three studies in which participants with UTI were compared with non‐UTI controls and three studies conducted in nursing homes. Where publication bias was observed, we attempted to correct the results obtained using Duval and Tweedie's “trim and fill” method, which identified potentially missing studies to add predominantly to the left side of the funnel plots to ensure symmetry. Adjusted ORs were slightly attenuated but remained significant (for details see Figures S1–S17).
Cross‐sectional data are insufficient to identify causality in the association between delirium and UTI. The vast majority of the included longitudinal studies lack data on urine examination findings at baseline. However, in two studies 22 , 56 where such data are available, the number of individuals with and without delirium is pooled for the entire follow‐up period. Thus, we cannot show whether one condition temporarily precedes another, thus contributing to its emergence.
In the absence of a clear signal from longitudinal studies, several possibilities arise: (1) UTI or related factors may increase the risk of developing delirium; (2) delirium may increase the risk of developing UTI; (3) delirium and UTI share some biological risk factors. Regarding the first possibility, there is convincing evidence that inflammation, which is part of the infectious process, itself increases the risk of delirium. 68 , 69 As for the second hypothesis, we were unable to assess comprehensively the effect of delirium subtypes, its symptoms severity, or frequency on the association between delirium and UTI, because of the paucity of data. However, the administration of drugs with anticholinergic side effects, as well as benzodiazepines, which are known to increase the risk of delirium, 65 , 66 , 67 had a statistically significant effect on the association between delirium and UTI. It also seems reasonable that the inability of patients with delirium to maintain proper personal hygiene may increase the risk of UTI and complicate its treatment. This point represents an area for future research. The third hypothesis of shared biological dysfunction in delirium and UTI reflects the idea that many conditions traditionally thought to be nervous system disorders also involve changes in other physiological systems 70 , 71 , 72 , 73 including the immune system.
Despite extensive searching, we were unable to assess meta‐analytically the association between delirium and AB in older adults: our results were limited to only one suitable study, which showed no statistically significant association between the two conditions.
Our results should be seen within the context of some limitations. First, we tried to include unpublished research data by contacting experts in the field, but were ultimately unable to obtain any unpublished data. Second, there were a group of studies whose results could potentially be used in our meta‐analysis if the necessary quantitative data were reported in publications, but we were only able to obtain these data from a few authors. Third, we were unable to find any study in which both conditions (UTI or AB and delirium) were defined according to the highest diagnostic standards: in all publications one of the conditions was identified based on a record in the medical files. Fourth, the limited data available on the association between delirium and AB in the older adults should also be taken into account. All of the above suggests that our evidence is of moderate quality and further research is needed to investigate both the associations and the factors affecting them.
Some limitations are also related to the results of the meta‐regression analysis. First, these results are mostly based on subsets of studies. Second, the influence of a number of somatic conditions on the association between delirium and UTI could not be properly assessed, as the individual studies did not report subtypes of the respective conditions (e.g., diabetes mellitus, type I or II) that were important for understanding such an effect. Third, we were only able to assess the effect of a small group of medications on the association we examined because many of the included studies did not report data on the medications taken by participants.
Our findings have important clinical and medical implications. Clinicians should be aware, of the importance of assessing UTI symptoms in older adults with delirium, and of the possible presence of delirium in older adults referred for treatment of UTI. Due to the lack of relevant data, no reliable conclusions can be drawn about the association between delirium and AB in older adults from our study, which points to the need for high‐quality prospective research addressing this association.
The effects of treatment of UTI and AB on their associations with delirium are of particular interest for further research, as clinicians are ultimately interested in whether treatment of UTI and AB can improve delirium. In this context, the impact of treatment of usually benign AB in those subgroups of older adults who due to varying factors are at higher risk of developing UTI and urosepsis also merits further closer study. The influence of individual types of organisms (including those associated with a higher incidence of drug resistance) remains open to further research as well.
CONFLICT OF INTEREST
None of the authors is affiliated with any organization or institution that has any financial interest in this publication. No author has any conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
D.K. led the project management. D.K. and R.K. implemented the search strategy, and performed all stages of study screening, quality assessment, and data extraction in a double‐blind setting. D.K. performed the statistical processing of the data. D.K. and R.K. prepared the draft article. S.K. and E.L. accompanied the project as supervisors and contributed substantially to the interpretation of the results and the preparation of the final manuscript. All authors read and approved the final version of the manuscript.
SPONSOR'S ROLE
The study was not sponsored.
FINANCIAL DISCLOSURE
This study was supported by in‐house funding from the University Hospital of Old Age Psychiatry and Psychotherapy at the University of Bern. No commercial, public or non‐profit sectors provided any specific grants for this study.
Supporting information
Text S1. Details on the methods of the study
Text S2. Items of the Newcastle‐Ottawa Quality Assessment Scale
Table S1. Characteristics of included studies reporting an association between delirium and urinary tract infection in adults aged 65 and older
Table S2. Characteristics of the only included study reporting an association between delirium and asymptomatic bacteriuria in adults aged 65 and older.
Table S3. The list of articles excluded at the full text screening phase with indication of reasons for exclusion
Table S4. Quality assessment of included studies according to the Newcastle‐Ottawa Scale
Table S5. Complete list of all moderators of the meta‐regression analysis
Table S6. List of data from each included study used for meta‐regression
Table S7. Results of a meta‐regression with the country of study as a categorical moderator
Table S8. Results of a meta‐regression with the type of the study as a categorical moderator
Table S9. Results of a meta‐regression with the study temporality as a categorical moderator
Table S10. Results of a meta‐regression with the study setting as a categorical moderator
Table S11. Results of a meta‐regression with the method to establish the diagnosis of delirium as a categorical moderator
Table S12. Results of a meta‐regression with the method to establish the diagnosis of UTI as a categorical moderator
Table S13. Results of a meta‐regression with numerical moderators
Figure S1. Funnel plot. All studies
Figure S2. Forest plot. After removing the study with the highest weight
Figure S3. Funnel plot. After removing the study with the highest weight
Figure S4. Forest plot. After removing one clear outlier
Figure S5. Funnel plot. After removing one clear outlier
Figure S6. Forest plot. Studies comparing participants with delirium to controls without delirium
Figure S7. Funnel plot. Studies comparing participants with delirium to controls without delirium
Figure S8. Forest plot. Studies comparing participants with UTI to non‐UTI controls
Figure S9. Funnel plot. Studies comparing participants with UTI to non‐UTI controls
Figure S10. Forest plot. Studies with a diagnosis of delirium based on DSM or ICD criteria
Figure S11. Funnel plot. Studies with a diagnosis of delirium based on DSM or ICD criteria
Figure S12. Forest plot. Studies with a diagnosis of UTI based on microbiological urine tests
Figure S13. Forest plot. Studies conducted in the clinical setting
Figure S14. Funnel plot. Studies conducted in the clinical setting
Figure S15. Forest plot. Studies conducted in nursing homes
Figure S16. Funnel plot. Studies conducted in nursing homes
Figure S17. Forest plot. Studies of population‐based samples
ACKNOWLEDGMENTS
The authors thank Dr. Francisco Jose Alvarez‐Perez, Dr. José Percy Amado‐Tineo, and Dr. Jen‐Tzer Gau for sharing additional information on the included studies. The authors are also grateful to Alexander Rubanovich PhD, DSc and Sven Trelle PD, MD for collegial advice on methodological issues. Open Access Funding provided by Universitat Bern.
Krinitski D, Kasina R, Klöppel S, Lenouvel E. Associations of delirium with urinary tract infections and asymptomatic bacteriuria in adults aged 65 and older: A systematic review and meta‐analysis. J Am Geriatr Soc. 2021;69(11):3312-3323. doi: 10.1111/jgs.17418
REFERENCES
- 1. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kennedy M, Enander RA, Tadiri SP, Wolfe RE, Shapiro NI, Marcantonio ER. Delirium risk prediction, healthcare use and mortality of elderly adults in the emergency department. J Am Geriatr Soc. 2014;62(3):462‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leslie DL, Marcantonio ER, Zhang Y, Leo‐Summers L, Inouye SK. One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shankar KN, Hirschman KB, Hanlon AL, Naylor MD. Burden in caregivers of cognitively impaired elderly adults at time of hospitalization: a cross‐sectional analysis. J Am Geriatr Soc. 2014;62(2):276‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curns AT, Holman RC, Sejvar JJ, Owings MF, Schonberger LB. Infectious disease hospitalizations among older adults in the United States from 1990 through 2002. Arch Intern Med. 2005;165(21):2514‐2520. [DOI] [PubMed] [Google Scholar]
- 6. Simmering JE, Tang F, Cavanaugh JE, Polgreen LA, Polgreen PM. The increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998–2011. Open Forum Infect Dis. 2017;4(1):ofw281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rowe TA, Juthani‐Mehta M. Diagnosis and management of urinary tract infection in older adults. Infect Dis Clin North Am. 2014;28(1):75‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ducharme J, Neilson S, Ginn JL. Can urine cultures and reagent test strips be used to diagnose urinary tract infection in elderly emergency department patients without focal urinary symptoms? CJEM. 2007;9(2):87‐92. [DOI] [PubMed] [Google Scholar]
- 9. Walker S, McGeer A, Simor AE, Armstrong‐Evans M, Loeb M. Why are antibiotics prescribed for asymptomatic bacteriuria in institutionalized elderly people? A qualitative study of physicians' and nurses' perceptions. CMAJ. 2000;163(3):273‐277. [PMC free article] [PubMed] [Google Scholar]
- 10. D'Agata E, Loeb MB, Mitchell SL. Challenges in assessing nursing home residents with advanced dementia for suspected urinary tract infections. J Am Geriatr Soc. 2013;61(1):62‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eyer MM, Lang M, Aujesky D, Marschall J. Overtreatment of asymptomatic bacteriuria: a qualitative study. J Hosp Infect. 2016;93(3):297‐303. [DOI] [PubMed] [Google Scholar]
- 12. Rousham E, Cooper M, Petherick E, Saukko P, Oppenheim B. Overprescribing antibiotics for asymptomatic bacteriuria in older adults: a case series review of admissions in two UKhospitals. Antimicrob Resist Infect Control. 2019;8(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dasgupta M, Brymer C, Elsayed S. Treatment of asymptomatic UTI in older delirious medical in‐patients: a prospective cohort study. Arch Gerontol Geriatr. 2017;72:127‐134. [DOI] [PubMed] [Google Scholar]
- 14. Zalmanovici Trestioreanu A, Lador A, Sauerbrun‐Cutler MT, Leibovici L. Antibiotics for asymptomatic bacteriuria. Cochrane Database Syst Rev. 2015;4:CD009534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lob SH, Nicolle LE, Hoban DJ, Kazmierczak KM, Badal RE, Sahm DF. Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010–2014. Diagn Microbiol Infect Dis. 2016;85(4):459‐465. [DOI] [PubMed] [Google Scholar]
- 16. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308‐1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thaden JT, Lewis SS, Hazen KC, et al. Rising rates of carbapenem‐resistant enterobacteriaceae in community hospitals: a mixed‐methods review of epidemiology and microbiology practices in a network of community hospitals in the southeastern United States. Infect Control Hosp Epidemiol. 2014;35(8):978‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhattacharyya S, Darby RR, Raibagkar P, Gonzalez Castro LN, Berkowitz AL. Antibiotic‐associated encephalopathy. Neurology. 2016;86(10):963‐971. [DOI] [PubMed] [Google Scholar]
- 19. Warstler A, Bean J. Antimicrobial‐induced cognitive side effects. Ment Health Clin. 2016;6(4):207‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson CP, Ngo LH, Marcantonio ER. Complications in postacute care are associated with persistent delirium. J Am Geriatr Soc. 2012;60(6):1122‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brouquet A, Cudennec T, Benoist S, et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg. 2010;251(4):759‐765. [DOI] [PubMed] [Google Scholar]
- 22. Gau JT, Shibeshi MR, Lu IJ, et al. Interexpert agreement on diagnosis of bacteriuria and urinary tract infection in hospitalized older adults. J Am Osteopath Assoc. 2009;109(4):220‐226. [PubMed] [Google Scholar]
- 23. Jitapunkul S, Pillay I, Ebrahim S. Delirium in newly admitted elderly patients: a prospective study. QJM. 1992;83(1):307‐314. [PubMed] [Google Scholar]
- 24. Balogun SA, Philbrick JT. Delirium, a symptom of UTI in the elderly: fact or fable? A systematic review. Can Geriatr J. 2014;17(1):22‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chae JH, Miller BJ. Beyond urinary tract infections (UTIs) and delirium: a systematic review of UTIs and neuropsychiatric disorders. J Psychiatr Pract. 2015;21(6):402‐411. [DOI] [PubMed] [Google Scholar]
- 26. Mayne S, Bowden A, Sundvall PD, Gunnarsson R. The scientific evidence for a potential link between confusion and urinary tract infection in the elderly is still confusing – a systematic literature review. BMC Geriatr. 2019;19(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krinitski D, Kasina R, Kloeppel S, Lenouvel E. Urinary tract infection, asymptomatic bacteriuria and delirium in people aged 65 and over: protocol for a systematic review and meta‐analysis of associations between common medical and mental conditions. PROSPERO: International Prospective Register of Systematic Reviews . 2020;CRD42020164341. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020164341.
- 29. Ottawa Hospital Research Institute . The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses . Accessed January 4, 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 30. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56(2):455‐463. [DOI] [PubMed] [Google Scholar]
- 32. Alvarez‐Perez FJ, Paiva F. Prevalence and risk factors for delirium in acute stroke patients. A retrospective 5‐years clinical series. J Stroke Cerebrovasc Dis. 2017;26(3):567‐573. [DOI] [PubMed] [Google Scholar]
- 33. Amado Tineo J, Chucas Ascencio L, Rojas Moya C, Pintado Caballero S, Cerrón Aguilar C, Vásquez AR. Factores asociados a síndrome confusional agudo en adultos mayores internados en emergencia de un hospital terciario. Anal Facul Med. 2013;74(3):193‐197. [Google Scholar]
- 34. Arshi A, Lai WC, Chen JB, Bukata SV, Stavrakis AI, Zeegen EN. Predictors and sequelae of postoperative delirium in geriatric hip fracture patients. Geriatr Orthop Surg Rehabil. 2018;9:2151459318814823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Bortoli PF, Lopes MA. Delirium in elderly inpatients admitted to clinical wards prevalence and investigation of clinical conditions in a Brazilian sample. Dement Neuropsychol. 2018;12(2):152‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Edlund A, Lundstrom M, Brannstrom B, Bucht G, Gustafson Y. Delirium before and after operation for femoral neck fracture. J Am Geriatr Soc. 2001;49(10):1335‐1340. [DOI] [PubMed] [Google Scholar]
- 37. Edlund A, Lundstrom M, Karlsson S, Brannstrom B, Bucht G, Gustafson Y. Delirium in older patients admitted to general internal medicine. J Geriatr Psychiatry Neurol. 2006;19(2):83‐90. [DOI] [PubMed] [Google Scholar]
- 38. Elsamadicy AA, Wang TY, Back AG, et al. Post‐operative delirium is an independent predictor of 30‐day hospital readmission after spine surgery in the elderly (≥65years old): a study of 453 consecutive elderly spine surgery patients. J Clin Neurosci. 2017;41:128‐131. [DOI] [PubMed] [Google Scholar]
- 39. Eriksson I, Gustafson Y, Fagerstrom L, Olofsson B. Prevalence and factors associated with urinary tract infections (UTIs) in very old women. Arch Gerontol Geriatr. 2010;50(2):132‐135. [DOI] [PubMed] [Google Scholar]
- 40. Eriksson I, Gustafson Y, Fagerstrom L, Olofsson B. Urinary tract infection in very old women is associated with delirium. Int Psychogeriatr. 2011;23(3):496‐502. [DOI] [PubMed] [Google Scholar]
- 41. Gual N, Morandi A, Perez LM, et al. Risk factors and outcomes of delirium in older patients admitted to postacute care with and without dementia. Dement Geriatr Cogn Disord. 2018;45(1–2):121‐129. [DOI] [PubMed] [Google Scholar]
- 42. Khurana PS, Sharma PS, Avasthi A. Risk factors in delirious geriatric general medical inpatients. Indian J Psychiatry. 2002;44(3):266‐272. [PMC free article] [PubMed] [Google Scholar]
- 43. Kobayashi K, Imagama S, Ando K, et al. Risk factors for delirium after spine surgery in extremely elderly patients aged 80 years or older and review of the literature: Japan Association of spine surgeons with ambition multicenter study. Global Spine J. 2017;7(6):560‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Large MC, Reichard C, Williams JT, et al. Incidence, risk factors, and complications of postoperative delirium in elderly patients undergoing radical cystectomy. Urology. 2013;81(1):123‐128. [DOI] [PubMed] [Google Scholar]
- 45. Lundström M. Delirium in Old Patients with Femoral Neck Fracture: Risk Factors, Outcome, Prevention and Treatment. Umeå: Faculty of Medicine, Department of Community Medicine and Rehabilitation, Umeå universitet; 2004. [Google Scholar]
- 46. Lundström M, Edlund A, Karlsson S, Brannstrom B, Bucht G, Gustafson Y. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622‐628. [DOI] [PubMed] [Google Scholar]
- 47. Marcantonio ER, Kiely DK, Simon SE, et al. Outcomes of older people admitted to postacute facilities with delirium. J Am Geriatr Soc. 2005;53(6):963‐969. [DOI] [PubMed] [Google Scholar]
- 48. Morandi A, Mazzone A, Bernardini B, et al. Association between delirium, adverse clinical events and functional outcomes in older patients admitted to rehabilitation settings after a hip fracture: a multicenter retrospective cohort study. Geriatr Gerontol Int. 2019;19(5):404‐408. [DOI] [PubMed] [Google Scholar]
- 49. Olofsson B, Lundstrom M, Borssen B, Nyberg L, Gustafson Y. Delirium is associated with poor rehabilitation outcome in elderly patients treated for femoral neck fractures. Scand J Caring Sci. 2005;19(2):119‐127. [DOI] [PubMed] [Google Scholar]
- 50. Olofsson B, Persson M, Bellelli G, Morandi A, Gustafson Y, Stenvall M. Development of dementia in patients with femoral neck fracture who experience postoperative delirium – A three‐year follow‐up study. Int J Geriatr Psychiatry. 2018;33(4):623‐632. [DOI] [PubMed] [Google Scholar]
- 51. Perez‐Ros P, Martinez‐Arnau FM, Baixauli‐Alacreu S, Garcia‐Gollarte JF, Tarazona‐Santabalbina F. A predictive model of the prevalence of delirium in elderly subjects admitted to nursing homes. Endocr Metab Immune Disord Drug Targets. 2018;18(4):355‐361. [DOI] [PubMed] [Google Scholar]
- 52. Raats JW, van Eijsden WA, Crolla RM, Steyerberg EW, van der Laan L. Risk factors and outcomes for postoperative delirium after major surgery in elderly patients. PLoS One. 2015;10(8):e0136071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sandberg O, Gustafson Y, Brannstrom B, Bucht G. Clinical profile of delirium in older patients. J Am Geriatr Soc. 1999;47(11):1300‐1306. [DOI] [PubMed] [Google Scholar]
- 54. Schuurmans MJ, Duursma SA, Shortridge‐Baggett LM, Clevers G‐J, Pel‐Littel R. Elderly patients with a hip fracture: the risk for delirium. Appl Nurs Res. 2003;16(2):75‐84. [DOI] [PubMed] [Google Scholar]
- 55. Stroomer‐van Wijk AJ, Jonker BW, Kok RM, van der Mast RC, Luijendijk HJ. Detecting delirium in elderly outpatients with cognitive impairment. Int Psychogeriatr. 2016;28(8):1303‐1311. [DOI] [PubMed] [Google Scholar]
- 56. Wojszel ZB, Toczynska‐Silkiewicz M. Urinary tract infections in a geriatric sub‐acute ward‐health correlates and atypical presentations. Eur Geriatr Med. 2018;9(5):659‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852‐857. [PubMed] [Google Scholar]
- 58. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Blackburn T, Dunn M. Cystocerebral syndrome. Acute urinary retention presenting as confusion in elderly patients. Arch Intern Med. 1990;150(12):2577‐2578. [DOI] [PubMed] [Google Scholar]
- 60. Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119(6):474‐481. [DOI] [PubMed] [Google Scholar]
- 61. Waardenburg IE. Delirium caused by urinary retention in elderly people: a case report and literature review on the “cystocerebral syndrome”. J Am Geriatr Soc. 2008;56(12):2371‐2372. [DOI] [PubMed] [Google Scholar]
- 62. Kaplan SA, Wein AJ, Staskin DR, Roehrborn CG, Steers WD. Urinary retention and post‐void residual urine in men: separating truth from tradition. J Urol. 2008;180(1):47‐54. [DOI] [PubMed] [Google Scholar]
- 63. Beetz R. Mild dehydration: a risk factor of urinary tract infection? Eur J Clin Nutr. 2003;57(suppl 2):S52‐S558. [DOI] [PubMed] [Google Scholar]
- 64. Lean K, Nawaz RF, Jawad S, Vincent C. Reducing urinary tract infections in care homes by improving hydration. BMJ Open Qual. 2019;8(3):e000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011;40(1):23‐29. [DOI] [PubMed] [Google Scholar]
- 66. Flacker JM, Cummings V, Mach JR Jr, Bettin K, Kiely DK, Wei J. The association of serum anticholinergic activity with delirium in elderly medical patients. Am J Geriatr Psychiatry. 1998;6(1):31‐41. [PubMed] [Google Scholar]
- 67. Han L, McCusker J, Cole M, Abrahamowicz M, Primeau F, Elie M. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med. 2001;161(8):1099‐1105. [DOI] [PubMed] [Google Scholar]
- 68. Cunningham C. Systemic inflammation and delirium: important co‐factors in the progression of dementia. Biochem Soc Trans. 2011;39(4):945‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Simone MJ, Tan ZS. The role of inflammation in the pathogenesis of delirium and dementia in older adults: a review. CNS Neurosci Ther. 2011;17(5):506‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev. 2018;98(1):477‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65(3):229‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. 2018;33(11):1428‐1457. [DOI] [PubMed] [Google Scholar]
- 73. Qureshi IA, Mehler MF. Towards a ‘systems’‐level understanding of the nervous system and its disorders. Trends Neurosci. 2013;36(11):674‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text S1. Details on the methods of the study
Text S2. Items of the Newcastle‐Ottawa Quality Assessment Scale
Table S1. Characteristics of included studies reporting an association between delirium and urinary tract infection in adults aged 65 and older
Table S2. Characteristics of the only included study reporting an association between delirium and asymptomatic bacteriuria in adults aged 65 and older.
Table S3. The list of articles excluded at the full text screening phase with indication of reasons for exclusion
Table S4. Quality assessment of included studies according to the Newcastle‐Ottawa Scale
Table S5. Complete list of all moderators of the meta‐regression analysis
Table S6. List of data from each included study used for meta‐regression
Table S7. Results of a meta‐regression with the country of study as a categorical moderator
Table S8. Results of a meta‐regression with the type of the study as a categorical moderator
Table S9. Results of a meta‐regression with the study temporality as a categorical moderator
Table S10. Results of a meta‐regression with the study setting as a categorical moderator
Table S11. Results of a meta‐regression with the method to establish the diagnosis of delirium as a categorical moderator
Table S12. Results of a meta‐regression with the method to establish the diagnosis of UTI as a categorical moderator
Table S13. Results of a meta‐regression with numerical moderators
Figure S1. Funnel plot. All studies
Figure S2. Forest plot. After removing the study with the highest weight
Figure S3. Funnel plot. After removing the study with the highest weight
Figure S4. Forest plot. After removing one clear outlier
Figure S5. Funnel plot. After removing one clear outlier
Figure S6. Forest plot. Studies comparing participants with delirium to controls without delirium
Figure S7. Funnel plot. Studies comparing participants with delirium to controls without delirium
Figure S8. Forest plot. Studies comparing participants with UTI to non‐UTI controls
Figure S9. Funnel plot. Studies comparing participants with UTI to non‐UTI controls
Figure S10. Forest plot. Studies with a diagnosis of delirium based on DSM or ICD criteria
Figure S11. Funnel plot. Studies with a diagnosis of delirium based on DSM or ICD criteria
Figure S12. Forest plot. Studies with a diagnosis of UTI based on microbiological urine tests
Figure S13. Forest plot. Studies conducted in the clinical setting
Figure S14. Funnel plot. Studies conducted in the clinical setting
Figure S15. Forest plot. Studies conducted in nursing homes
Figure S16. Funnel plot. Studies conducted in nursing homes
Figure S17. Forest plot. Studies of population‐based samples


