Abstract
The Cancer Genome Atlas (TCGA) of a pancreatic cancer cohort identified high MST1R (RON tyrosine kinase receptor) expression correlated with poor prognosis in human pancreatic cancer. RON expression is null/minimal in normal pancreas but elevates from pan‐in lesions through invasive carcinomas. We report using multiple approaches RON directly regulates HIF‐1α, a critical driver of genes involved in cancer cell invasion and metastasis. RON and HIF‐1α are highly co‐expressed in the 101 human PDAC tumors analyzed and RON expression correlated with HIF‐1α expression in a subset of PDAC cell lines. knockdown of RON expression in RON positive cells blocked HIF‐1α expression, whereas ectopic RON expression in RON null cells induced HIF‐1α expression suggesting the direct regulation of HIF‐1α by RON kinase receptor. RON regulates HIF‐1α through an unreported transcriptional mechanism involving PI3 kinase‐mediated AKT phosphorylation and Sp1‐dependent HIF‐1α promoter activity leading to increased HIF‐1α mRNA expression. RON/HIF‐1α modulation altered the invasive behavior of PDAC cells. A small‐molecule RON kinase inhibitor decreased RON ligand, MSP‐induced HIF‐1α expression, and invasion of PDAC cells. Immunohistochemical analysis on RON knockdown orthotopic PDAC tumor xenograft confirmed that RON inhibition significantly blocked HIF‐1α expression. RON/HIF‐1α co‐expression also exists in triple‐negative breast cancer cells, a tumor type that also lacks molecular therapeutic targets. This is the first report describing RON/HIF‐1α axis in any tumor type and is a potential novel therapeutic target.
Keywords: gene expression, invasion, kinases, receptor, signaling, transcription
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) has a very poor prognosis with a 5‐year survival rate of around 6%. 1 The disease is generally diagnosed at an advanced stage with limited opportunities for surgical intervention, leaving chemotherapy as a current standard of care with significant adverse side effects and poor efficacy. 2 Immunotherapy studies with checkpoint inhibitors in PDAC have failed to elicit a similar therapeutic response as seen in some other tumor types. 3 The molecular markers that are involved in the invasive and metastatic process of PDAC are not yet clearly defined and hence lack targeted therapies. 4
A tyrosine kinase receptor, RON (recepteur d’ origine nantais) which belongs to the c‐MET family is reported to be widely expressed in the PDAC tissues. 5 , 6 , 7 , 8 , 9 RON and c‐MET receptors are activated by two distinct ligands, macrophage stimulating protein (MSP) and hepatocyte growth factor (HGF), respectively. 10 RON homodimers are functionally active but also form heterodimers with c‐MET, EGFR, IGFR, and PDGFR. 10 A recent report analyzing The Cancer Genome Atlas (TCGA) of a pancreatic cancer cohort revealed that high RON expression was associated with poor prognostic outcomes. 11 Although RON expression was reported to be null/minimal in the normal pancreas, it is elevated through the progression from PanIN lesions to invasive PDAC with highly active MSP‐RON signaling. 12 All published reports indicate that RON mediates cancer cell invasion and metastasis rather than cancer initiation. RON was reported to be overexpressed in various cancers of epithelial origin. 13 Aberrant RON expression in human PDAC is associated with an aggressive cancer phenotype and decreased disease‐free survival time in patients and an increase in metastasis. knockdown of RON expression in PDAC cells was shown to suppress the primary tumor growth and decreased liver metastasis in an orthotopic animal model system. 14 Moreover, there are small molecule inhibitors and anti‐RON antibody–drug conjugates in preclinical studies to target RON expression/activity. 5 , 15 , 16 , 17 These studies provide the molecular basis and rationale to target RON for therapeutic benefit in PDAC.
In addition to RON, another protein that is established to promote PDAC metastasis is hypoxia‐inducible factor‐1 alpha (HIF‐1α). 18 HIF‐1α is a helix‐loop‐helix transcription factor that drives the expression of many genes vital in all aspects of the cancer cell invasion and metastasis. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 Multiple studies of HIF‐1α and PDAC have shown a significant association between HIF‐1α overexpression and poor prognosis. 32 , 33 , 34 , 35 High HIF‐1α expression in PDAC is a predictor for metastatic disease. 35 , 36 , 37 , 38 , 39 HIF‐1α expression has also been linked to chemotherapy resistance. 40 Hence, RON and HIF‐1α are both associated with PDAC metastasis. However, there are no studies to date exploring if oncogenic RON kinase receptor is one of the contributing factors for HIF‐1α overexpression in PDAC and the relevance of RON/HIF‐1α axis in PDAC or other tumor types.
We now report that these two mediators of PDAC metastasis, RON and HIF‐1α are highly co‐expressed in the 101 human PDAC tumors analyzed and a correlation between RON and HIF‐1α expression exists in a subset of PDAC cell lines. We have provided data through manipulation of RON kinase receptor expression/activity using molecular and pharmacological approaches demonstrating RON kinase receptor tightly regulates steady‐state HIF‐1α expression in PDAC cells. We have also identified that RON regulates HIF‐1α through an unreported transcriptional mechanism in PDAC cells. Significantly, blockade or rescue of RON expression through a molecular approach or inhibition of RON kinase receptor activation through a small molecule RON kinase inhibitor, LCRF altered the invasive phenotype of PDAC cells. The importance of clinical and in vitro data demonstrating the RON/HIF‐1α association in PDAC was corroborated by immunohistochemical (IHC) analysis on the in vivo orthotopic tumor tissue from RON knockdown PDAC cells which revealed RON inhibition blocked HIF‐1α expression. To our knowledge, this is the first report describing the direct regulation of HIF‐1α by RON kinase receptor in any tumor type with potential clinical relevance. Further, these results may have broader implications to other invasive tumor types as we report the existence of RON/HIF‐1α axis in the invasive triple‐negative breast cancer (TNBC) cells, a tumor type that also lacks molecular therapeutic targets.
2. MATERIALS AND METHODS
2.1. Cell culture and reagents
PDAC and TNBC cells were obtained from American Type Culture Collection (ATCC) and BXPC3 vector control, RON knockdown clones were provided by Dr. Freeman. 14 Cell line identities were verified at TGEN by short tandem repeat (STR) profiling using the AmpFISTR Identifiler PCR Amplification Kit (Applied Biosystems). Results were compared with published STR sequences from the ATCC. The STR profiling is repeated once a cell line has been passaged more than 6 months after previous STR profiling. Transient knockdown of RON in CFPAC1 cells was done using siRNA RON (SC‐36434) and HIF‐1α knockdown in BXPC3 cells using siHIF‐1α (SC‐44225) and Lipofectamine 3000 transfection reagent (Invitrogen). Panc1 vector and Panc1 FL‐RON cells were generated through a stable expression of CMV‐Neo and CMV‐FL‐RON (full‐length RON cDNA) plasmids, respectively. Human recombinant MSP was purchased from R&D Systems and RON TKI, LCRF‐0004 (will be referred to as LCRF) was provided by Dr. Raeppel. 41
2.2. The human PDAC tissue microarrays (TMAs)
PDAC tissues analyzed were collected by Dr. Han's research group at TGen, Phoenix as part of an NIH PO1 program pilot grant. Patient samples were collected under protocols approved by the Western Institutional Review Board (WIRB) and patients informed consent was obtained. These are archived tissue samples that are devoid of Protected Health Information (PHI).
2.3. Mouse orthotopic pancreatic tumor tissue
Flash‐frozen paraffin‐embedded (FFPE) primary tumor tissue from BXPC3 Vector cells and BXPC3 RON knockdown cells was proved by Dr. Freeman. 14
2.4. Immunohistochemical analysis
Human PDAC tissues and mouse orthotopic pancreatic tumor tissues were stained with H&E and immunohistochemistry using RON (SC 74588‐HRP, 1:100 for 60 min) and HIF‐1α (NB100479, 1:100 for 60 min) primary antibodies were performed as described. 42 RON and HIF‐1α staining intensity in the PDAC tissues was assessed on a scale of 0–3, where 0 is considered negative (0%–5% staining), 1 is weak (5%–10% staining), 2 is moderate (10%–50% staining), and 3 is strong (>50% staining). A board‐certified pathologist, Dr. Zhou performed the blind scoring of the PDAC tissues.
2.5. Western blot analysis
Protein analysis was done on the total cell lysates using RON (SC‐74588), pRON (R&D AF1947), HIF‐1α (CST‐14179), GAPDH (SC‐47724), Actin (SC‐4778), Caspase 3 (CST‐9662), Cleaved Caspase 3 (CST‐9661), pAKT (CST‐4060), and AKT (CST‐9272) antibodies. To analyze the effect of LCRF in blocking the stimulation of RON signaling pathway by MSP, cells were grown in the serum‐free medium for the indicated time in the absence or presence of MSP following 1 h pretreatment with LCRF. Serum starved cells were treated for 24 with a 1‐μM AKT inhibitor, MK‐2206 to determine the involvement of AKT in the RON‐mediated HIF‐1α expression.
2.6. Quantitative real‐time RT‐PCR
RNA was reverse‐transcribed into cDNA and real‐time PCR was performed with SYBR Green PCR mix (Applied Biosystems). RON and HIF‐1α primers were previously described. 43 GAPDH was used as a control.
2.7. Luciferase assay
HIF‐α promoter activity was analyzed by transfection of a 0.69‐kb HIF‐1α promoter‐luciferase reporter plasmid 44 and a control CMV‐Renilla plasmid using Lipofectamine 3000 reagent (Invitrogen). 72 h after transfection, HIF‐1α promoter activity was measured using a Dual‐Luciferase Assay Kit (Promega), and firefly luciferase activity was normalized to renilla‐luciferase activity. To determine the effect of Sp1 on HIF‐1α promoter, CMV‐Sp1 plasmid was cotransfected into cells. Sp1 specificity on HIF‐1α promoter was determined by treating cells with 1 μM Mithramycin for 24 h before luciferase assay.
2.8. Matrigel invasion assay
The invasive behavior of PDAC cells was analyzed. 43 We have used a serum‐free medium to identify the exclusive contribution of RON when cells were stimulated by RON ligand, MSP in the absence or presence of RON TKI LCRF or targeted knockdown of RON, HIF‐1α, or ectopic RON expression in PDAC cells. Cells were stimulated with 200 ng MSP following 1 h pretreatment of 200 or 400 nM LCRF. Following 24 h incubation, cells on the top surface of the chamber were gently removed with cotton swabs. Invaded cells on the undersurface of the membrane were fixed with 70% ethanol and stained with 0.1% crystal violet and captured using ECHO Revolve photomicroscope. Staining intensity was quantified using ImageJ software and represented as relative staining intensity. Quantification data represent the mean ± SD of three separate invasion assays.
2.9. Cell proliferation assay
Cell proliferation analysis was performed using CellTiter nonradioactive protocol as described by the manufacturer (Promega). Briefly, 8000 cells/well were seeded in a 96‐well plate. After overnight incubation, cells were grown in the serum‐free medium for an additional 24 h either in the absence or presence of MSP following 1 h pretreatment with LCRF. The dye solution was then added and incubated for 1 h. Using a plate reader absorbance of the colored formazan product was read at 570 nm.
2.10. Immunofluorescence
Cells were grown in chamber slides, fixed with paraformaldehyde, and incubated with RON or HIF‐1α primary antibodies overnight. Following washing, cells were incubated with secondary antibodies for 2 h. RON and HIF‐1α expression was analyzed using a Carl Zeiss inverted fluorescence microscope using Axio Vision Version 4.8 software. DAPI was used as a nuclear counterstain.
2.11. Statistical analysis
Statistical analysis was performed using GraphPad InStat software (GraphPad Software, Inc.). The significance of differences among groups was determined by one‐way ANOVA, t‐test. Statistical significance was considered as p < 0.05.
3. RESULTS
3.1. RON and HIF‐1α co‐expression in pancreatic ductal adenocarcinomas and pancreatic cancer cells
RON and MSP expression is null/minimal in the normal pancreas but is elevated from preneoplastic PanIN lesions through PDAC and liver metastasis. 12 Similarly, RON expression is null in normal pancreatic ductal epithelial cells but exhibits differential expression in various pancreatic cancer cells. 14 HIF‐1α, a master regulator of genes involved in tumor cell invasion and metastasis is stable under hypoxia (1%–2% O2) but undergoes ubiquitin/proteasome pathway mediated degradation in normoxia (20% O2). HIF‐1α expression is minimal under normoxia in normal human pancreatic ductal epithelial cells but constitutively expressed in several pancreatic cancer cells as well as PDAC tumors and is associated with poor prognosis. 45 , 46 , 47 We analyzed by immunohistochemical analysis the co‐expression of RON and HIF‐1α in 101 PDAC tumors (Figure 1A). RON and HIF‐1α are co‐expressed in all the tumors analyzed. Significantly, 95 out of the 101 tumors analyzed showed high‐intensity staining (>50% i.e., score 3 on a scale of 0–3). We performed Western blot analysis to detect RON and HIF‐1α expression in PDAC cells with GAPDH as an internal control. RON antibody recognizes intracellular 150‐kDa β‐chain of mature RON containing tyrosine kinase domain and 170‐kDa pro‐RON. The HIF‐1α antibody recognizes 93‐kDa unmodified protein as well as 120‐kDa posttranslational modified species. GAPDH antibody detects 37‐kDa species. PDAC cell lines showed differential RON and HIF‐1α expression (Figure 1B). Interestingly, PDAC cells that expressed RON also displayed HIF‐1α which was strikingly absent in RON null cells. The unmodified 93‐kDa steady‐state HIF‐1α protein was the major species observed under normoxia (20% O2). When the cells were grown under hypoxia (1% O2), 93‐kDa unmodified HIF‐1α protein was significantly elevated, and posttranslational modified species was also observed (Supporting Information Figure).
Figure 1.
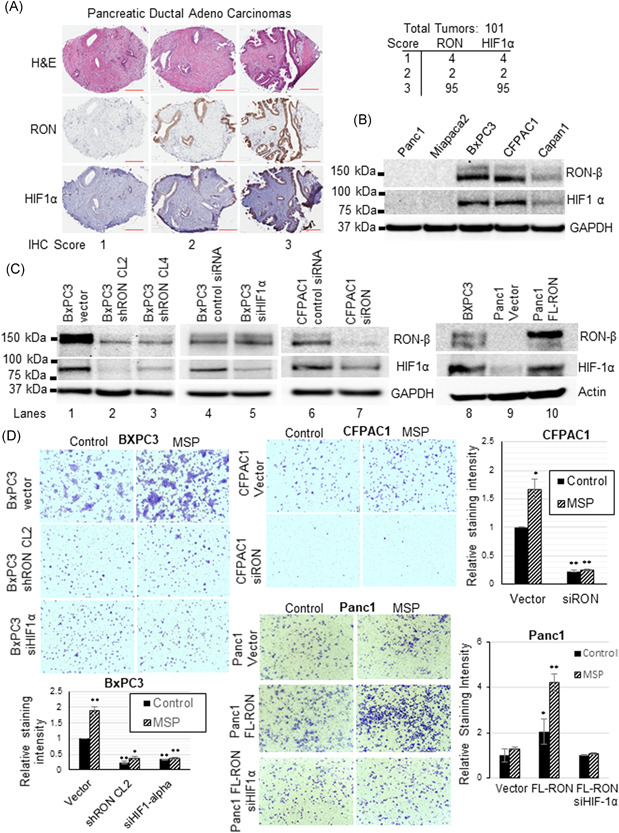
RON and HIF‐1α co‐expression in human PDAC tumors, pancreatic cancer cells, and invasive behavior: (A) Immunohistochemical analysis using RON and HIF‐1α antibodies was performed on 101 PDAC tumors. RON and HIF‐1α proteins are highly co‐expressed in all the tumors analyzed. H&E staining is shown to view the tissue histology. Scale bar 200 µm. (B) RON and HIF‐1α protein expression was analyzed in a panel of pancreatic cancer cells with GAPDH as an internal control. HIF‐1α protein expression under normoxia was observed in RON positive cells but not in RON negative cells. (C) RON and HIF‐1α protein expression was analyzed in control and RON, HIF‐1α knockdown or ectopic RON expressing pancreatic cancer cells. RON or HIF‐1α knockdown significantly inhibited HIF‐1α expression in RON positive cells while ectopic RON induced HIF‐1α expression in RON null PDAC cells. GAPDH or actin is used as a loading control. (D) In vitro matrigel invasion assay either in the absence or presence of RON ligand, MSP (200 ng/ml) was performed in the control and RON, HIF‐1α expression knockdown or RON expression rescued PDAC cells. MSP promoted the invasion of RON/HIF‐1α positive control cells but significantly inhibited in the RON, HIF‐1α expression knockdown cells, while RON expression rescued PDAC cells showed augmented invasion. (E) ImageJ software was used to measure the staining intensity of the invaded cells and quantification data representing the mean ± SD of three separate invasion assays was presented. *p < 0.05 and **p < 0.01 [Color figure can be viewed at wileyonlinelibrary.com]
3.2. RON controls HIF‐1α expression and invasive behavior of PDAC cells
Molecular approaches were used to determine the direct involvement of RON in the regulation of HIF‐1α expression in PDAC cells. RON expression was knockdown in RON positive BXPC3 and CFPAC1 cells, whereas RON expression was rescued in RON null Panc1 cells through ectopic stable expression of full‐length RON cDNA plasmid. We have used control and stable RON expression knockdown BXPC3 clones as well as RON knockdown CFPAC1 cells generated after 48 h following transient transfection with siRNA RON plus CMV vector or CMV FL‐RON expressing Panc1 cells. Transient transfection was also used to generate BXPC3 and Panc1 FL‐RON HIF‐1α knockdown cells. Western blot analysis was done to determine RON and HIF‐1α expression in these cells (Figure 1C). While the control cells (lanes 1, 4, 6, and 8) expressed RON and HIF‐1α, the targeted knockdown of RON reduced HIF‐1α expression in both BXPC3 and CFPAC1 cells (lanes 2, 3, and 7). Keeping RON expression intact and specific knockdown of HIF‐1α in BXPC3 cells (lane 5) confirmed that the protein which was modulated in RON knockdown BXPC3 and CFPAC1 cells (lanes 2, 3, and 7) was indeed HIF‐1α and that a RON/HIF‐1α axis exists in a subset of PDAC. In the reverse, ectopic RON expression in RON null Panc1 cells exhibited a robust induction of HIF‐1α (lane 10) confirming the role of RON in the regulation of HIF‐1α in a subset of PDAC cells. We next ascertained if RON/HIF‐1α axis is critical for MSP mediated invasive phenotype of PDAC cells by carrying out matrigel assay (Figure 1D). The invasion assay was performed in the serum‐free media to determine the exclusive contribution of RON/HIF‐1α signaling pathway when cells are stimulated with MSP. While MSP promoted the invasion of RON/HIF‐1α expressing BXPC3 and CFPAC1 control cells, targeted knockdown of RON or HIF‐1α inhibited MSP‐induced invasive behavior of PDAC cells suggesting a central role of the RON/HIF‐1α axis. The importance of RON/HIF‐1α axis in PDAC cell invasion is further confirmed in Panc1 cells where ectopic RON expression augmented invasive phenotype which was blunted in the HIF‐1α knockdown cells. Staining intensity was quantified using ImageJ software and represented as relative staining intensity in control versus RON or HIF‐1α knockdown and RON expression rescued cells (Figure 1D).
3.3. RON regulates HIF‐1α expression through PI3 kinase signaling pathway mediated transcriptional mechanism
We previously reported the involvement of PI3K and ERK signaling pathways in cancer cell invasion. 43 knockdown of HIF‐1α expression reduced MSP‐induced invasion of PDAC cells (Figure 1D). We tested the hypothesis that RON through PI3K mediated AKT activation (phosphorylation of Ser 473) regulates Sp1‐dependent HIF‐1α expression in PDAC cells (Figure 2A). RON positive cells (BXPC3 control) and RON knockdown cells (BXPC3 shRON cl2) were grown to 70% confluence in complete medium and switched to serum‐free medium and grown for another 24 h to analyze the exclusive contribution of RON signaling pathway in the induction of HIF‐1α expression. Western blot analysis was performed using RON, pAKT, AKT, Sp1, HIF‐1α, and actin antibodies. RON antibody recognizes 150 kDa, HIF‐1α antibody 93 kDa, AKT and pAKT antibodies 62 kDa, Sp1 antibody 95 and 105 kDa species, respectively. Decreased phosphorylation of PI3K substrate AKT along with reduced HIF‐1α expression was observed in the RON knockdown cells with no apparent change in total AKT and a minor change, if any in the sp1 expression was observed (Figure 2A). The specificity of AKT in the RON‐mediated HIF‐1α protein expression was determined by treating the serum‐starved RON/HIF‐1α positive BXPC3 cells with 1 μM AKT inhibitor, MK‐2206 which was recently shown to inhibit AKT phosphorylation in these cells. 48 Inhibition of AKT phosphorylation blocked HIF‐1α expression in RON positive BXPC3 cells (Figure 2A). To determine if the decrease in HIF‐1α protein expression in BXPC3 RON knockdown PDAC cells was due to a decrease in HIF‐1α mRNA expression, we carried out real‐time RT‐PCR analysis on BXPC3 control and RON knockdown clones using RON, HIF‐1α, and GAPDH primers (Figure 2B). While RON and HIF‐1α mRNA were detected in the BXPC3 control cells, RON knockdown clones exhibited a dramatic decrease in the HIF‐1α mRNA expression suggesting the involvement of a transcriptional mechanism in the regulation of HIF‐1α by RON in the PDAC cells. GAPDH mRNA was amplified simultaneously for an endogenous control and represented as RON/GAPDH mRNA and HIF‐1α/GAPDH mRNA. Decrease in HIF‐1α transcription can be evaluated by analyzing the HIF‐1α promoter activity in the RON positive and RON knockdown cells. HIF‐1α promoter region is well‐characterized with no distinct TATA box in the transcription initiation region and multiple Sp1 transcription factor binding sites in the core promoter (Figure 2C). We obtained the 0.69 kb HIF‐1α promoter‐luciferase reporter plasmid from Dr. Yu's lab 44 and initially confirmed the activation of HIF‐1α promoter by Sp1 thorough analysis of the activities of this promoter‐luciferase reporter in MDA MB 231 breast cancer cells in the absence or presence of CMV‐Sp1. An Sp1 dose‐dependent increase in HIF‐1α promoter activity was observed (Figure 2C) confirming the involvement of Sp1 in the regulation of HIF‐1α expression. To determine if the loss of Sp1‐dependent HIF‐1α promoter activity was a contributing factor to decreased HIF‐α mRNA expression observed in the RON knockdown PDAC cells, we have analyzed the activities of this HIF‐1α promoter construct in RON positive BXPC3 vector cells in the absence or presence of Sp1‐DNA binding inhibitor, Mithramycin A which was previously shown to inhibit HIF‐1α promoter‐luciferase activity 49 and RON knockdown (BXPC3 shRON) PDAC cells. While high HIF‐1α promoter activity was noticed in the RON/HIF‐1α expression positive BXPC3 vector cells, Sp1‐dependent HIF‐1α promoter activity was almost abrogated in the Sp1 inhibitor, Mithramycin A treated BXPC3 cells as well as RON knockdown HIF‐1α expression blocked BXPC3 shRON cells (Figure 2D).
Figure 2.
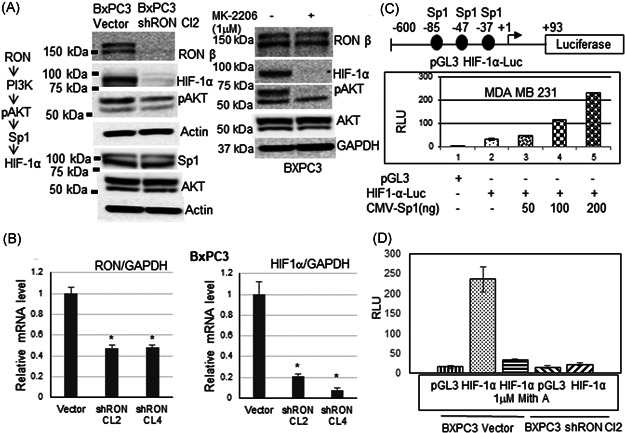
Transcriptional regulation of HIF‐1α through PI3K mediated RON Kinase pathway: (A) Western blot analysis on the total cell lysates from serum‐starved RON positive BXPC vector control, RON knockdown BXPC3 shRON cl2 cells and AKT inhibitor, MK‐2206 treated BXPC3 cells was performed with various antibodies. RON knockdown or AKT inhibitor, MK‐2206 inhibited PI3K mediated AKT phosphorylation and HIF‐1α expression with no apparent changes in other proteins tested. (B) Quantitative real‐time RT‐PCR using RON, HIF‐1α, and GAPDH internal control primers was performed on the total RNA from BXPC3 vector control, RON knockdown clones 2 and 4. RON knockdown significantly decreased HIF‐1α mRNA expression. *p < 0.05. (C) Sp1 transcription factor effect on HIF‐1α promoter activity was analyzed in breast cancer cells. An Sp dose‐dependent increase in HIF‐1α promoter activity was observed. (D) HIF‐1α promoter activity was analyzed in RON positive BXPC3 vector control in the absence or presence of Sp1 inhibitor, Mithramycin A, and RON knockdown BXPC3 sh RON cl2 cells. HIF‐1α promoter activity was blunted in the Mithramycin A treated and RON knockdown cells
3.4. Blockade of RON receptor activation inhibited HIF‐1α expression and invasion of PDAC cells
A pharmacological approach using a small molecule RON inhibitor was employed to determine if blocking RON ligand, MSP‐induced RON receptor activation (tyrosine phosphorylation in the kinase domain) inhibits HIF‐1α expression and invasive behavior of PDAC cells. LCRF, a small molecule RON TKI was recently reported to block MSP/RON signaling and inhibit the growth of malignant pleural mesothelioma. 41 To determine if LCRF is effective in blocking RON phosphorylation and activation in PDAC cells, we initially carried out an LCRF dose–response analysis and determined 400 nM dosage was effective in blocking RON ligand, MSP‐induced phosphorylation of RON tyrosine kinase receptor. We then grew BXPC3 cells in the serum‐free medium overnight and stimulated with 200 ng MSP following 1 h pretreatment with 400 nM LCRF (Figure 3A). Total RON and phospho‐RON along with internal control GAPDH protein expression were analyzed over a time course (0–4 h). While MSP stimulated RON receptor phosphorylation in the control cells, LCRF pretreatment inhibited RON ligand, MSP‐induced phosphorylation. Total RON or GAPDH expression was unaffected. We next analyzed if blockade of RON receptor activation by LCRF affects HIF‐1α expression in PDAC cells (Figure 3A). BXPC3 and CFPAC1 cells were seeded and incubated overnight in complete medium and switched to serum‐free medium and grown for an additional 24 h in the absence or presence of 200 ng MSP following 1 h pretreatment with 200 or 400 nM LCRF. Western blot analysis using HIF‐1α and GAPDH antibodies was performed. MSP stimulated HIF‐1α expression in the control cells while small‐molecule RON TKI was effective in blocking the MSP‐induced HIF‐1α expression. A matrigel invasion assay was carried out to determine if blockade of RON kinase receptor activation by LCRF inhibits RON ligand, MSP‐induced invasion of PDAC cells (Figure 3B). BXPC3 and CFPAC1 cells were plated in the serum‐free medium and stimulated with RON ligand, MSP (200 ng) either in the presence or absence of 200 or 400 nM LCRF. 24 h later cells that were invaded through matrigel were stained with crystal violet and photographed. MSP stimulated the invasion of RON/HIF‐1α positive BXPC3 and CFPAC1 cells through matrigel while RON TKI was effective in reducing the MSP influence. Staining intensity was quantified using ImageJ software and represented as relative cell staining intensity in control versus LCRF treated cells (Figure 3B).
Figure 3.
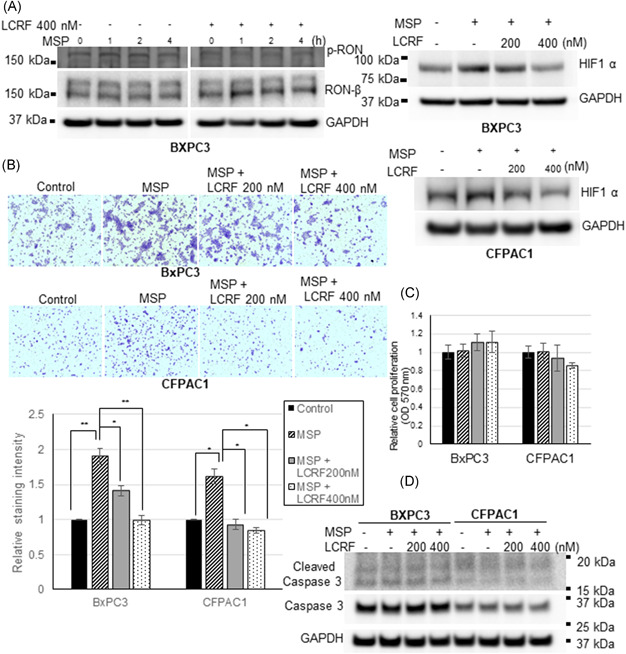
RON TKI, LCRF blocks MSP‐induced RON phosphorylation/activation and inhibits HIF‐1α expression and invasion of pancreatic cancer cells. (A) Serum starved BXPC3 cells were stimulated with MSP 200 ng/ml either in the absence or presence of RON TKI, LCRF for the indicated time and phospho RON, total RON, and GAPDH protein expression was analyzed. MSP‐induced RON phosphorylation in the control cells, but RON TKI inhibited MSP‐induced RON phosphorylation. PDAC cells were grown in a serum‐free medium containing 200 ng/ml MSP for 24 h either in the absence or presence of RON TKI, LCRF, and HIF‐1α, GAPDH protein expression was analyzed. MSP‐induced HIF‐1α expression in the control cells, but RON TKI was effective at 400 nM concentration in significantly blocking the MSP‐induced HIF‐1α expression. (B) Matrigel invasion assay was performed in the serum‐free medium containing 200 ng/ml MSP either in the absence or presence of RON TKI, LCRF. MSP‐induced invasion of both the pancreatic cancer cells. However, RON TKI at 400 nM concentration significantly inhibited MSP‐induced invasion. ImageJ software was used to measure the staining intensity of the invaded cells and quantification data representing three separate invasion assays was presented. *p < 0.05 and **p < 0.01. (C) Pancreatic cancer cells were seeded in a 96‐well plate and incubated overnight. Cells are then changed to serum‐free medium containing 200 ng/ml MSP and grown for 24 h either in the absence or presence of RON TKI, LCRF. Cell proliferation was determined using nonradioactive CellTiter method. Very minimal alteration in the cell proliferation was observed in the control versus LCRF treated cells. (D) Pancreatic cancer cells were grown in the serum‐free medium containing 200 ng/ml MSP either in the absence or presence of RON TKI, LCRF. Total and cleaved Caspase 3 protein expression along with GAPDH control was analyzed. No apparent change in the total or cleaved Caspase 3 was observed between control and RON TKI treated pancreatic cancer cells [Color figure can be viewed at wileyonlinelibrary.com]
3.5. RON TKI LCRF blocks PDAC cell invasion without significantly altering cell proliferation or cell death
We performed cell proliferation and cell survival analysis to determine if reduced cell growth or increased cell death was a contributing factor to the decreased invasion observed in the LCRF treated PDAC cells. BXPC3 and CFPAC1 cells were grown in the serum‐free medium for 24 h with 200 ng MSP following 1 h pretreatment of 200 or 400 nM LCRF, as utilized in the invasion assay. 24 h later cell proliferation was determined by nonradioactive method in the control, RON ligand, MSP stimulated as well as LCRF treated PDAC cells (Figure 3C). There appear to be no statistically significant changes in the proliferation rates among control, MSP‐stimulated, and RON TKI treated PDAC cells. Caspases play an important role in the regulation of apoptosis. Caspase‐3 is considered as one of the key executioners of the cell death pathway and its activation involved proteolytic processing from an inactive zymogen (34 kDa) to active p17 and p12 fragments. To determine if enhanced cell death was involved in the decreased cell invasion noticed in the RON TKI treated PDAC cells, total and cleaved caspase 3 protein expression was analyzed by western blot in the PDAC cells grown for 24 h in the presence of 200 ng MSP following 1 h pretreatment of 200 or 400 nM LCRF (Figure 3D). Total and cleaved caspase fragments were observed in the control, MSP‐stimulated, and RON TKI treated cells. No apparent change either in the total or cleaved caspases was observed.
3.6. RON knockdown reduced HIF‐1α expression in the human PDAC tumor xenograft
Orthotopic implantation of BXPC3 vector control cells and RON knockdown cells into the pancreas of 15 athymic nude mice significantly suppressed primary tumor growth and decreased lymph‐node and liver metastasis, 80% in vector control versus 26% in RON knockdown with a significant p value of 0.0092. 14 We obtained the FFPE tissue from BXPC3 vector control and RON knockdown tumor xenografts to determine if decreased HIF‐1α expression in the RON knockdown tissue was a contributing factor to the reduction in the primary tumor growth and decreased metastasis observed. Following deparaffinization, immunohistochemical analysis using RON, HIF‐1α antibodies was performed on the control and RON knockdown tissues (Figure 4A). While high RON and HIF‐1α expression was detected in the BXPC3 vector control tissue, RON knockdown tissue exhibited a significant reduction in RON and HIF‐1α expression. H&E was used as a counterstain and showed no apparent changes in histology.
Figure 4.
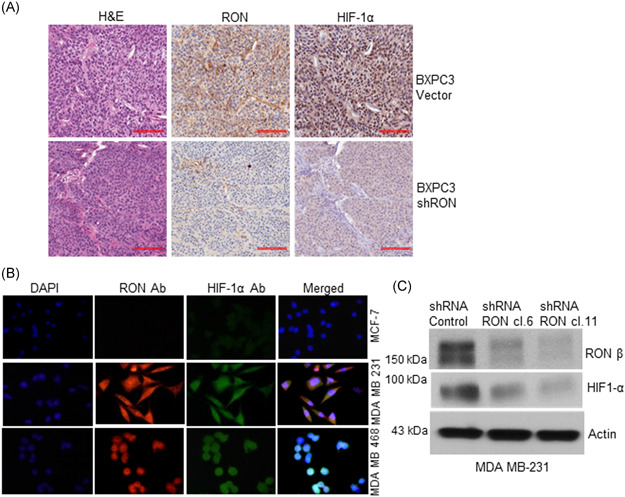
RON knockdown inhibited HIF‐1α expression in the human pancreatic tumor xenograft and RON, HIF‐1α co‐expression in TNBC cells. (A) RON and HIF‐1α expression was analyzed in the BXPC3 control and RON knockdown xenograft FFPE tissue. High RON and HIF‐1α expression was detected in the BXPC3 control xenograft tissue. However, RON knockdown xenograft tissue exhibited a significantly decreased HIF‐1α expression. Scale bar: 100 µm. (B) Immunofluorescence analysis was done on the invasive MDA MB 231, MDA MB 468 TNBC cells, and noninvasive MCF‐7 non‐TNBC cells. RON and HIF‐1α co‐expression was detected in the TNBC cells but not in the non‐TNBC cells. (C) RON and HIF‐1α protein expression was analyzed by Western blot analysis in the MDA MB 231 vector control and RON knockdown clones. RON and HIF‐1α are expressed in the vector control cells, but RON knockdown significantly reduced HIF‐1α expression [Color figure can be viewed at wileyonlinelibrary.com]
3.7. RON/HIF‐1α axis exists in triple‐negative breast cancer cells (TNBC)
To determine if the relevance of the RON/HIF‐1α axis is limited to PDAC or may be present in other tumor types we analyzed RON and HIF‐1α expression in TNBC cells, a tumor type which also has a poor prognosis due to lack of targeted therapies. Immunofluorescence analysis using RON and HIF‐1α antibodies was performed on the invasive MDA MB 231, MDA MB 468 TNBC cells along with non‐TNBC MCF‐7 cells (Figure 4B). DAPI was used as a nuclear counterstain. While RON and HIF‐1α co‐expression was observed in the TNBC cells it was remarkably absent in the non‐TNBC MCF‐7 cells. RON expression was observed both in the cytoplasm and nucleus, a finding that is not surprising since nuclear RON has been found to bind the c‐Jun promoter and regulate its expression. 50 We analyzed by Western blots RON and HIF‐1α expression in the MDA MB 231 vector control and RON knockdown clones to determine if RON regulates HIF‐1α expression in TNBC cells as observed in the PDAC cells (Figure 4C). While RON and HIF‐1α expression was detected in the MDA MB 231 vector control cells, RON knockdown significantly reduced HIF‐1α expression.
4. DISCUSSION
Prognosis for PDAC remains poor due to late diagnosis and metastasis. Standard treatment options include chemo and radiotherapy, but these therapies have many adverse side effects and limited efficacy. Immunotherapies have not yet had a significant impact on PDAC outcomes, necessitating the exploration of novel molecular targets for therapeutic development. Independent studies of RON and HIF‐1α expression support that both are involved in the development of chemo and radiotherapy resistance in PDAC. However, no studies have explored the potential relevance of a RON/HIF‐1α axis in PDAC. Significantly, levels of RON and its ligand, MSP as well as enzyme matriptase which activates MSP were found to be low in normal human pancreatic tissue but elevated from preneoplastic PanIN lesions through PDAC with liver metastasis suggesting a potential role for MSP/RON signaling in PDAC development and metastasis to adjacent and distant tissues, thus providing molecular rationale to explore RON signaling pathway for future therapeutic development in PDAC.
Our analysis of the 101 human PDAC tumors revealed that RON and HIF‐1α are co‐expressed in all the tumors analyzed. Remarkably, an overwhelming majority (95 out of 101 tumors) displayed high expression of both RON and HIF‐1α (Figure 1A). This is the first reported observation in any tumor type. Targeted knockdown of RON in PDAC cells through shRNA‐based molecular approach inhibited HIF‐1α expression, while rescue of RON expression in RON null PDAC cells exhibited robust induction of HIF‐1α demonstrating a direct role for RON signaling in the regulation of HIF‐1α in a subset of PDAC cells (Figure 1C). We have shown the potential involvement of an unreported transcriptional mechanism in the regulation of HIF‐1α by RON since real‐time RT‐PCR analysis showed a significant decrease in the HIF‐1α mRNA expression in RON knockdown PDAC cells (Figure 2B). Additional studies involving HIF‐1α promoter analysis confirmed the transcriptional regulation of HIF‐1α by RON in PDAC cells. We previously reported the involvement of PI3 kinase/AKT role in the RON‐mediated invasion of TNBC cells. 43 PI3k/AKT mediated phosphorylation of Sp1 transcription factor and its binding to VEGF promoter was shown to be involved in the regulation of VEGF gene expression and tumor cell angiogenesis. 51 However, we have observed only minimal changes, if any in Sp1 protein levels/phosphorylation status between RON positive and RON knocked‐down PDAC cells. HIF‐1α gene promoter region is characterized. 52 It lacks a distinct TATA box and is GC‐rich with multiple Sp1 binding sites in the promoter region with additional binding sites for transcriptional regulators, such as NFkB, CREB, Ap1, Ap2, Stat3, and hypoxia‐responsive element, and so on. The −200 bp core promoter contains 3 Sp1 transcription factor binding sites and is essential for HIF‐1α gene expression. 49 However, Stat3 was also reported to be required for HIF‐1α mRNA expression in tumor cells. 44 Consequently, it is plausible that PI3k/AKT induced Sp1 transcription factor binding to HIF‐1α core promoter region may trigger binding of additional co‐regulators of HIF‐1α gene transcription in the RON signaling pathway. However, additional regulation at the translational/posttranslational level cannot be ruled out and will be a focus of our future studies.
As RON has been documented to be involved in the development and progression of various tumor types, 13 preclinical/clinical efforts are being aggressively pursued to develop small molecule or antibody‐drug conjugate therapies. 15 , 16 , 17 Recently, a small molecule RON inhibitor, LCRF was reported to block MSP/RON signaling and inhibited the growth of malignant pleural mesothelioma. 41 Notably, we found that this RON TKI was effective in blocking the MSP/RON signaling pathway induced HIF‐1α expression in the PDAC cells (Figure 3A). We also show that both RON and HIF‐1α are involved in the MSP/RON signaling‐induced PDAC cell invasion since the targeted blocking of either RON or HIF‐1α decreased the invasive potential of PDAC cells (Figure 1C). Interestingly, the RON TKI mediated decrease in PDAC cell invasion (Figure 3B) was not due to a decrease in cell proliferation or increased cell death as there is no appreciable difference between control and RON TKI treated PDAC cells (Figures 3C and D). This finding is consistent with a previous report indicating that RON ligand, MSP promoted metastasis in a mouse model of breast cancer without altering cell proliferation or cell survival. 53 RON through HIF‐1α may regulate invasion and metastasis by influencing the capability of tumor cells to migrate and invade extracellular matrix and blood vessels without affecting cell proliferation or cell survival.
The potential involvement of RON/HIF‐1α axis in the human PDAC tumor growth and metastasis to adjacent lymph nodes and liver was supported by the immunohistochemical analysis on the tumor xenograft where RON knockdown tumor showed reduced HIF‐1α expression (Figure 4A). It was reported that although RON knockdown suppressed tumor growth up to 7 weeks, tumors began to grow a few weeks later due to compensatory hyperactivation of c‐MET, a comember of the RON family. 14 It is plausible that c‐MET regulated tumorigenicity may also involve HIF‐1α and HIF‐1α expression in cancer stem cells was cited as a contributing factor for tumor recurrence following successful chemotherapy in some tumor types. HIF‐1α inhibitors are currently in clinical use for cardiovascular disease and a range of HIF‐1α inhibitors are in preclinical/clinical development for oncology. 54 Perhaps a combinatory approach with RON and HIF‐1α inhibitors may offer therapeutic benefit in RON/HIF‐1α positive PDAC tumors.
Overall, our studies involving PDAC clinical samples, in vitro data in pancreatic cancer cells along immunohistochemical analysis on in vivo human PDAC tumor xenograft establish that a novel RON/HIF‐1α signaling axis exists in pancreatic cancer and potentially other invasive tumors such as TNBCs.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Akihisa Kato contributed in vitro data on PDAC cells; Serina Ng and Haiyong Han contributed IHC data; Wendi Zhou scored IHC data; Amalraj Thangasamy contributed in vitro data on TNBC cells; Stephane Raeppel contributed RON TKI; Michael Fallon and Sushovan Guha provided clinical expertise; Sudhakar Ammanamanchi designed the study and wrote the manuscript.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
The authors thank our long‐time collaborators Dr. James W. Freeman and Dr. Shuzie Zhao for providing the RON expression manipulated PDAC cells and FFPE tumor tissue. This study was funded by a grant from the Banner Health Foundation (S.A.) and The Uehara Memorial Foundation (A.K. research fellowship).
Kato A, Ng S, Thangasamy A, et al. A potential signaling axis between RON kinase receptor and hypoxia‐inducible factor‐1 alpha in pancreatic cancer. Molecular Carcinogenesis. 2021;60:734‐745. 10.1002/mc.23339
REFERENCES
- 1. Knudsen ES, Balaji U, Mannakee B, et al. Pancreatic cancer cell lines as patient‐derived avatars: genetic characterization and functional utility. Gut. 2018;67:508‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bishi S, Brossart P, Feldmann G. Current therapeutic options for pancreatic ductal adenocarcinoma. Oncol Res Treat. 2018;41(10):590‐594. [DOI] [PubMed] [Google Scholar]
- 3. Tan E, El‐Rayes B. Pancreatic cancer and immunotherapy: resistance mechanisms and proposed solutions. J Gastrointest Cancer. 2019;50:1‐8. [DOI] [PubMed] [Google Scholar]
- 4. Aslan M, Shahbazi R, Ulubayaram K, Ozpolat B. Targeted therapies for Pancreatic cancer and hurdles ahead. Anticancer Res. 2018;38:6591‐6606. [DOI] [PubMed] [Google Scholar]
- 5. Yao H‐P, Hudson R, Wang M‐H. RON receptor tyrosine kinase in pancreatic ductal adenocarcinoma: pathogenic mechanism in malignancy and pharmaceutical target for therapy. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188360. [DOI] [PubMed] [Google Scholar]
- 6. Chakedis J, French R, Babicky M, et al. Characterization of RON protein isoforms in pancreatic cancer: implications for biology and therapeutics. Oncotarget. 2016;7:45959‐45975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chakedis J, French R, Babicky M, et al. A novel protein osoform of the RON tyrosine kinase receptor transforms human pancreatic duct epithelial cells. Oncogene. 2016;35:3249‐3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang CM, Babicky ML, Lowy AM. The RON receptor tyrosine kinase in pancreatic cancer pathogenesis and its implications for future targeted therapies. Pancreas. 2014;43:183‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomas RM, Toney K, Fenoglio‐Preiser C, et al. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res. 2007;67:6075‐6082. [DOI] [PubMed] [Google Scholar]
- 10. Yao H‐P, Zhou Y‐Q, Zhang R, Wang M‐H. MSP‐RON signaling in cancer: pathogenesis and therapeutic potential. Nat Rev Cancer. 2013;13:466‐481. [DOI] [PubMed] [Google Scholar]
- 11. Babicky ML, Harper MM, Chakedis J, et al. MST1R kinase accelerates pancreatic cancer progression via effects on both epithelial cells and macrophages. Oncogene. 2019;38:5599‐5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li C, Morvaridi S, Lam G, et al. MSP‐RON signaling is activated in the transition from pancreatic intraepithelial neoplasia (PanIN) to pancreatic ductal adenocarcinoma (PDAC). Front Physiol. 2019;10:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fahem N, Welm AL. RON signaling is a key mediator of tumor progression in many human cancers. Cold Spring Harb Symp Quant Biol. 2016;81:177‐188. [DOI] [PubMed] [Google Scholar]
- 14. Zhao S, Cao L, Freeman JW. Knockdown of RON receptor kinase delays but does not prevent tumor progression while enhancing HGF/MET signaling in pancreatic cancer cell lines. Oncogenesis. 2013;2:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng JY, Sharma S, Zhou YQ, et al. Synergistic activities of MET/RON inhibitor BMS‐777607 and mTOR inhibitor AZD8055 to polyploid cells derived from pancreatic cancer and cancer stem cells. Mol Cancer Ther. 2014;13:37‐48. [DOI] [PubMed] [Google Scholar]
- 16. Yao HP, Feng L, Weng TH, et al. Preclinical efficacy of anti‐RON antibody‐drug conjugate Zt/g4‐MMAE for therapy of pancreatic cancer overexpressing RON receptor tyrosine kinase. Mol Pharm. 2018;15:3260‐3271. [DOI] [PubMed] [Google Scholar]
- 17. Zarei O, Benvenuti S, Ustun‐Alkan F, Hamzeh‐Mivehroud M, Dastmalchi S. Strategies of targeting the extracellular domain of RON tyrosine kinase receptor for cancer therapy and drug delivery. J Cancer Res Clin Oncol. 2016;142:2429‐2446. [DOI] [PubMed] [Google Scholar]
- 18. Yuen A, Diaz B. The impact of hypoxia in pancreatic cancer invasion and metastasis. Hypoxia. 2014;14:91‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun X, Zhang Y, Li B, Yang H. MTA1 promotes the invasion and migration of pancreatic cancer cells potentially through the HIF‐1α/VEGF pathway. J Recept Signal Transduct Res. 2018;38:352‐358. [DOI] [PubMed] [Google Scholar]
- 20. Kong F, Kong X, Du Y, et al. STK33 promotes growth and progression of pancreatic cancer as a critical downstream mediator of HIF‐1α . Cancer Res. 2017;77:6851‐6862. [DOI] [PubMed] [Google Scholar]
- 21. Wei H, Xu Z, Liu F, et al. Hypoxia induces oncogene Yes‐associated protein 1 nuclear translocation to promote pancreatic ductal adenocarcinoma invasion via epithelial‐mesenchymal transition. Tumour Biol. 2017;39:39. [DOI] [PubMed] [Google Scholar]
- 22. Cui XG, Han ZT, He SH, et al. HIF1/2α mediates hypoxia‐induces LDHA expression in human pancreatic cancer cells. Oncotarget. 2017;8:24840‐24852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Logsdon DP, Grimard M, Luo M, et al. Regulation of HIF‐1α under hypoxia by APE1/Ref‐1 impacts CA9 expression: dual targeting in patient‐derived 3D pancreatic cancer models. Mol Cancer Ther. 2016;15:2722‐2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen S, Chen JZ, Zhang JQ, et al. Hypoxia induces TWIST‐activated epithelial‐mesenchymal transition and proliferation of pancreatic cancer cells in vitro and in nude mice. Cancer Lett. 2016;383:73‐84. [DOI] [PubMed] [Google Scholar]
- 25. Zhu S, He C, Deng S, et al. MiR‐548an, transcriptionally downregulated by HIF‐1α/HDAC1 suppresses tumorigenesis of pancreatic cancer by targeting vimentin expression. Mol Cancer Ther. 2016;15:2209‐2219. [DOI] [PubMed] [Google Scholar]
- 26. Gao C, Li S, Zhao T, et al. SCF, regulated by HIF‐1α promotes pancreatic ductal adenocarcinoma progression. PLOS One. 2015;10:0121338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao T, Ren H, Li J, et al. LASP1 is a HIF‐1α target gene critical for metastasis of pancreatic cancer. Cancer Res. 2015;75:111‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao X, Gao S, Ren H, et al. Hypoxia‐inducible factor‐1 promotes pancreatic ductal adenocarcinoma invasion and metastasis by activating transcription of the actin‐binding protein fascin. Cancer Res. 2014;74:2455‐2464. [DOI] [PubMed] [Google Scholar]
- 29. Zhu GH, Huang C, Feng ZZ, Lv XH, Qiu ZJ. Hypoxia‐induced snail expression through transcriptional regulation by HIF‐1α in pancreatic cancer cells. Dig Dis Sci. 2013;58:3503‐3515. [DOI] [PubMed] [Google Scholar]
- 30. Buchler P, Reber HA, Buchler M, et al. Hypoxia inducible factor 1 regulates VEGF expression in human pancreatic cancer. Pancreas. 2003;26:56‐64. [DOI] [PubMed] [Google Scholar]
- 31. Yoon DY, Buchler P, Saarikoski ST, Hines OJ, Reber HA, Hankinson O. Identification of genes differentially induced by hypoxia in pancreatic cancer cells. Biochem Biophys Res Commun. 2001;288:882‐886. [DOI] [PubMed] [Google Scholar]
- 32. Song Z, Ren H, Gao S, Zhao X, Zhang H, Hao J. The clinical significance and regulation mechanism of hypoxia inducible factor 1 and miR‐191 expression in pancreatic cancer. Tumour Biol. 2014;35:11319‐11328. [DOI] [PubMed] [Google Scholar]
- 33. Miyake K, Yoshizumi T, Imura S, et al. Expression of HIF‐1α, histone deacetylase and metastasis‐associated protein 1 in pancreatic carcinoma: correlation with poor prognosis with possible regulation. Pancreas. 2008;36:1‐9. [DOI] [PubMed] [Google Scholar]
- 34. Sun HC, Qiu ZJ, Liu J, et al. Expression of HIF‐1α and associated proteins in pancreatic ductal adenocarcinoma and their impact on prognosis. Int J Oncol. 2007;30:1359‐1367. [PubMed] [Google Scholar]
- 35. Matsuo Y, Ding Q, Desaki R, et al. HIF‐1α plays a pivotal role in hepatic metastasis of pancreatic cancer: an immunohistochemical study. J Hepatobiliary Pancreat Sci. 2014;21:105‐112. [DOI] [PubMed] [Google Scholar]
- 36. Colbert LE, Fisher SB, Balci S, et al. High nuclear HIF‐1α expression is a predictor of distant recurrence in patients with resected pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2015;91:631‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoffman AC, Mori R, Vallbohmer D, et al. High expression of HIF‐1α is a predictor of clinical outcome in patients with pancreatic ductal adenocarcinomas and correlated to PDGFA, VEGF and bFGF. Neoplasia. 2008;10:674‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akakura N, Kobayashi M, Horiuchi I, et al. Constitutive expression of HIF‐1α renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res. 2001;61:6548‐6554. [PubMed] [Google Scholar]
- 39. Couvelard A, O'Toole D, Leek R, et al. Expression of hypoxia‐inducible factors is correlated with the presence of a fibrotic focus and angiogenesis in pancreatic ductal adenocarcinomas. Histopathology. 2005;46:668‐676. [DOI] [PubMed] [Google Scholar]
- 40. Wang R, Cheng L, Xia J, Wang Z, Wu Q, Wang Z. Gemcitabine resistance is associated with epithelial‐mesenchymal transition and induction of HIF‐1α in pancreatic cancer cells. Curr Cancer Drug Targets. 2014;14:407‐417. [DOI] [PubMed] [Google Scholar]
- 41. Baird A‐M, Easty D, Jarzabek M, et al. When RON MET TAM in mesothelioma: all druggable for one, and one drug for all? Front Endocrinol. 2019;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vaseva A, Blake DR, Gilbert TSK, et al. KRAS suppression‐induced degradation of Myc is antagonized by a MEK5‐ERK5 compensatory mechanism. Cancer Cell. 2018;34:807‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thangasamy A, Rogge J, Ammanamanchi S. Recepteur d’ Origine Nantais tyrosine kinase is a direct target of hypoxia‐inducible factor‐1α‐mediated invasion of breast cancer cells. J Biol Chem. 2009;283:14001‐14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niu G, Briggs J, Deng J, et al. Signal transducer and activator of transcription3 is required for hypoxia‐inducible factor‐1α RNA expression in both tumor cells and tumor associated myeloid cells. Mol Cancer Res. 2008;7:1099‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McGinn O, Gupta VK, Dauer P, et al. Inhibition of hypoxic response decreases stemness and reduces tumorigenic signaling due to impaired assembly of HIF1 transcription complex in pancreatic cancer. Sci Rep. 2017;7:7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Akakura N, Kobayashi M, Horiuchi I, et al. Constitutive expression of hypoxia inducible factor‐1a renders pancreatic cancer cell resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res. 2001;61:6548‐6554. [PubMed] [Google Scholar]
- 47. Ye L‐Y, Zhang Q, Bai X‐L, Pankaj P, Hu Q‐D, Liang T‐B. Hypoxia‐inducible factor‐1α expression and its clinical significance in pancreatic cancer. Pancreatology. 2014;14:391‐397. [DOI] [PubMed] [Google Scholar]
- 48. Wang Z, Luo G, Qiu Z. Akt inhibitor MK‐2206 reduces pancreatic cancer cell viability and increases the efficacy of gemcitabine. Oncol Lett. 2020;19:1999‐2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Minet E, Ernest I, Michel G, et al. HIF1A gene transcription is dependent on a core promoter sequence encompassing activating and initiation sequences located upstream from the transcription initiation site and cis elements located within the 5’ UTR. Biochem Biophys Res Commun. 1999;261:534‐539. [DOI] [PubMed] [Google Scholar]
- 50. Chang H‐Y, Liu H‐S, Lai M‐D, et al. Hypoxia promotes nuclear translocation and transcriptional function in the oncogenic tyrosine kinase RON. Cancer Res. 2014;74:4549‐4562. [DOI] [PubMed] [Google Scholar]
- 51. Pore N, Liu S, Shu HK, et al. Sp1 is involved in Akt‐mediated induction of VEGF expression through a HIF‐1α‐independent mechanism. Mol Biol Cell. 2004;15:4841‐4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Iyer NV, Leung SW, Semenza GL. The human HIF‐1α gene: HIF‐1α structure and evolutionary conservation. Genomics. 1998;52:159‐165. [DOI] [PubMed] [Google Scholar]
- 53. Welm AL, Sneddon JB, Taylor C, et al. The macrophage‐stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci USA. 2007;104:7570‐7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fallah J, Rini B. HIF‐1 inhibitors: status of current clinical development. Curr Oncol Rep. 2019;21:6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.


