Abstract
Aim
To develop a model for predicting renal recovery in cardiac surgery patients with acute kidney injury (AKI) requiring renal replacement therapy (RRT).
Methods
Data from a prospective randomized controlled trial, conducted in a tertiary hospital to compare the survival effect of two dosages of hemofiltration for continuous RRT in cardiac surgery patients between 20 March 2012 and 9 August 2015, were used to develop the model. The outcome was renal recovery defined as alive and dialysis‐free 90 days after RRT initiation. Multivariate logistic regression with a stepwise backward selection of variables based on Akaike Information Criterion was applied to develop the model, which was internally validated using bootstrapping. Model discrimination, calibration and clinical value were assessed using the concordance index (C‐Index), calibration plots and decision curve analysis, respectively.
Results
Totally, 211 patients with AKI requiring RRT (66.8% male) with median age of 57 years were included. The incidence of renal recovery was 33.2% (n = 70). The model included six variables: body mass index stratification, baseline estimated glomerular filtration rate, hypertension, sepsis, mean arterial pressure and mechanical ventilation. The C‐Index for this model was 0.807 (95% CI, 0.744–0.870). After correction by the bootstrap, the C‐Index was 0.780 (95% CI, 0.720–0.845). The calibration plots indicated good consistency between actual observations and model prediction of renal recovery. Decision curve analysis demonstrated the model was clinical usefulness.
Conclusion
We developed and validated a model to predict the chance of renal recovery in cardiac surgery patients with AKI requiring RRT.
Keywords: cardiac surgery, renal replacement therapy, renal recovery, risk assessment
SUMMARY AT A GLANCE
This validated model based on prospective cohort data to predict the chance of renal recovery in cardiac surgery patients, especially after acute dialysis provides values to alert clinical decision and information for post AKI care.
1. INTRODUCTION
Acute kidney injury (AKI) requiring renal replacement therapy (RRT) after cardiac surgery is a serious and common acute medical condition with high mortality and healthcare costs. 1 AKI requiring RRT affects 2%–5% of cardiac surgery patients. 2 , 3 Moreover, the incidence of AKI requiring RRT is increasing. Despite great progress has been made in RRT, recovery of renal function only occurs in 20%–60% of patients with AKI requiring RRT. 4 The remaining patients still need chronic RRT and progress to chronic kidney disease (CKD), which may have an increased long‐term risk of mortality.
Renal function recovery in patients with AKI requiring RRT is considered to be successful when the patient remains alive without the need for RRT. 5 Among those who recovered, only 1%–6% of recovery occurs beyond 90 days of RRT initiation. 5 , 6 Moreover, patients who remain RRT dependent at 90 days after RRT initiation are considered to have end stage renal disease. 4 Therefore, 90 days is the recommended cutoff point for evaluating renal function recovery.
Predicting renal recovery after dialysis upon RRT initiation is of great importance to patients, their families and clinical doctors. Some scholars point out that development of new tools able to predict recovery is a key area for future research. 5 , 7 The ability to predict renal function recovery in these patients is conducive to physician–patient communication, renal care and patient follow‐up. In addition, understanding the likelihood of renal recovery may be beneficial for doctors in making medical decisions. For example, dialysis with a temporary catheter may be more appropriate for patients with a high chance of renal function recovery. Finally, it is useful for clinical studies to recruit suitable subjects and then evaluate the effect of interventions on renal function recovery.
However, to the best of our knowledge, there are currently no models for predicting renal function recovery after AKI requiring RRT in cardiac surgery patients. Therefore, data from our prospective randomized controlled trial study were used to develop a model for predicting renal function recovery 90 days after initiation of RRT in patients with AKI requiring RRT after cardiac surgery.
2. METHODS
2.1. Study population
The present study was a retrospective analysis of the data from the Effect of the Intensity of Continuous Renal Replacement Therapy on patients with cardiac surgery‐associated Acute kidney Injury (CRITERIA) study. The CRITERIA study was a prospective randomized controlled trial conducted in a tertiary hospital to compare the survival effect at 14, 28, 90 and 365 days of two dosages of hemofiltration for continuous RRT in patients underwent cardiac surgery between March 20, 2012, and August 9, 2015. Renal function recovery served as the secondary endpoint in the study (ClinicalTrials.gov Identifier: NCT01560650). Consecutive patients were enrolled if aged >18 years, had AKI after cardiac surgery, and deemed by the treating clinician to require RRT at the Guangdong Provincial People's Hospital between 20 March 2012 and 9 August 2015. The exclusion criteria were previous RRT and existing CKD. The enrolled patients were randomized into two groups: ultrafiltration at a rate of 25 or 35 ml/kg/h. Then, patients underwent continuous RRT at a randomly assigned treatment dose for at least 72 h. As a result, 211 patients were included. More details about the CRITERIA study was provided in the Supplementary Material.
The study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Guangdong Provincial People's Hospital (No. GDREC2010118H). Informed consent forms were obtained from all participants.
2.2. Outcomes
The predictive outcome was renal function recovery, defined as remaining alive and no longer requiring RRT at 90 days after RRT initiation. Patients on dialysis at 90 days or who died within 90 days were assigned to the non‐recovered group. 4 , 8
2.3. Potential predictive variables and definitions
Potential predictive variables were collected within 24 h before RRT initiation. If the patients had repeated blood test or multiple laboratory data, the value of the last measurement was included in analysis. Potential variables used to develop the model included the following patient characteristics: demographic characteristics (gender, age, and body mass index [BMI]), baseline estimated glomerular filtration rate (eGFR), left ventricular ejection fraction, comorbidities (such as diabetes, peripheral vascular disease, cerebrovascular disease, hypertension, recent myocardial infarction [occurred within 1 month before surgery], and sepsis), previous heart surgery, surgery type, reoperation, medications within 1 week before RRT initiation (angiotensin enzyme inhibitors or angiotensin receptor inhibitor (ACEI/ARB), nonsteroidal anti‐inflammatory drugs, antibiotics (vancomycin or aminoglycoside), and contrast media exposure), mean arterial pressure (MAP), mechanical ventilation, central venous pressure, AKI severity (defined according to the Kidney Disease Improving Global Outcomes [KDIGO] criteria 9 ), and laboratory findings (haemoglobin, platelet count, serum uric acid and proteinuria). Baseline eGFR was calculated with the Chronic Kidney Disease‐Epidemiology Collaboration formula 10 using the baseline serum creatinine level, which was established as the lowest creatinine level up to 3 months before hospital admission. If pre‐admission creatinine level was unavailable, the minimum serum creatinine level during hospitalization before RRT initiation was used. 9 Sepsis was defined according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). 11 Proteinuria was defined based on the urine dipstick analysis during hospitalization before RRT initiation. Since there are fewer people with urine protein‐heavy (≥2+) proteinuria, proteinuria was categorized into presence or absence. Trace or greater (≥1+) urine dipstick protein levels were defined as the presence.
2.4. Sample size
According to the rule of thumb that a minimum of five events are required for every predictor variable in a logistic model, 12 we estimated that at least 150 patients were required in the development set for nine candidate predictor variables, with an assumed event rate of 30%.
2.5. Statistical analysis
Normally and non‐normally distributed continuous variables were presented as mean ± SD and median (interquartile range), respectively. Differences between groups were assessed by Student's t test for normally distributed variables, Mann–Whitney U for non‐normally distributed variables. Categorical variables were described using frequency (percentage). The comparisons between groups were used as chi‐squared test, and Fisher exact test were applied when more than 20% of cells have expected frequencies of less than five. Multiple imputation with chain equations and an iteration of 20 times was used to estimate the missing data and were merged according to Rubin's rules. 13
In univariate analysis, all variables with a P‐value less than .1 were considered for inclusion in multivariate analysis. If the Spearman's correlation coefficient between variables was ≥0.40, only the variable judged to be more important on a clinical basis was included into the multivariate model. 14 Restricted cubic splines (RCS) were applied to test for possible nonlinear dependency in the relationship between continuous variables and the possibility of renal function recovery. 15 If the spline function presented a non‐linear relation, variables were converted into categorical variables based on previously published literature or clinical expertise. 16 The final model was built using multivariate logistic regression analysis with a stepwise backward selection of variables based on the Akaike Information Criterion (AIC) as a stopping rule. 17 A nomogram was created to facilitate the model's clinical use.
The new model's performance focused on discrimination and calibration. The discrimination was assessed using the Harrel's concordance index (C‐index). C‐index interpretation was similar to that of the area under the receiver operating characteristic curve. The calibration curve was used to assess the calibration. To reduce the overfitting bias and present a more accurate assessment of model performance, Efron's enhanced bootstrap method with 1000 resamples was used to validate the final model. 15 Compared with other ways of internal validation, such as split‐sample modelling and cross‐validation, bootstrap resampling method produced nearly unbiased and stable estimates of predictive accuracy with better efficiency. 18 We used the decision curve analysis (DCA) to evaluate clinical value of the model by calculating net benefits at different threshold probabilities. 19
All analyses and reports for model development and validation complied with the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) guidelines. All statistical analyses were carried out with IBM SPSS v.25.0 (SPSS IBM) and R software (version 4.0.0; https://www.r-project.org).
3. RESULTS
3.1. Demographic and baseline clinical data for participants
In total, 211 patients were used to develop the predictive model. Patients had a median age of 57 years and a substantial majority (66.8%) of them were men. Ninety days after RRT initiation, the incidence of renal recovery after AKI requiring RRT was 33.2% (n = 70). All patients were classified into two subgroups according to the main endpoint. The baseline characteristics of the study population are detailed in Table 1.
TABLE 1.
Baseline characteristics of patients with acute kidney injury requiring dialysis, stratified according to renal recovery status
| Variable | Total patients (N = 211) | Not recovered (n = 141) | Recovered (n = 70) | p value |
|---|---|---|---|---|
| Age (years) | 57.0 (45.0, 66.0) | 59.0 (46.0, 68.0) | 53.0 (42.0, 61.0) | .005 |
| Male | 141 (66.8%) | 92 (65.2%) | 49 (70.0%) | .490 |
| BMI a | 22.0 ± 3.3 | 21.3 ± 2.9 | 23.3 ± 3.7 | <.001 |
| BMI stratification | <.001 | |||
| <18.5 kg/m2 | 32 (15.2%) | 26 (18.4%) | 6 (8.6%) | |
| 18.5–23.9 kg/m2 | 127 (60.2%) | 92 (65.2%) | 35 (50.0%) | |
| ≧24 kg/m2 | 52 (24.6%) | 23 (16.3%) | 29 (41.4%) | |
| Smoker | 53 (25.1%) | 34 (24.1%) | 19 (27.1%) | .633 |
| LVEF (%) | 59.0 (48.0, 65.0) | 55.0 (46.0, 65.0) | 61.0 (54.0, 66.0) | .152 |
| Baseline serum creatinine (μmol/L) | 86.7 ± 15.0 | 89.2 ± 13.6 | 81.7 ± 16.4 | <.001 |
| Baseline eGFR (ml/min/1.73m2) | 77.0 (63.4, 93.0) | 72.6 (61.7, 86.0) | 86.8 (71.8, 103.5) | <.001 |
| Comorbid disease | ||||
| Hypertension | 71 (33.6%) | 55 (39.0%) | 16 (22.9%) | .019 |
| Diabetes mellitus | 38 (18.0%) | 31 (22.0%) | 7 (10.0%) | .033 |
| Cerebral vascular disease | 14 (6.6%) | 12 (8.5%) | 2 (2.9%) | .120 |
| Peripheral vascular disease | 6 (2.8%) | 4 (2.8%) | 2 (2.9%) | .993 |
| Acute myocardial infarction | 11 (5.2%) | 8 (5.7%) | 3 (4.3%) | .669 |
| Gastrointestinal haemorrhage | 14 (6.6%) | 10 (7.1%) | 4 (5.7%) | .705 |
| Coronary heart disease | 8 (3.8%) | 6 (4.3%) | 2 (2.9%) | .617 |
| Atrial fibrillation | 18 (8.5%) | 13 (9.2%) | 5 (7.1%) | .611 |
| Sepsis | 28 (13.3%) | 23 (16.3%) | 5 (7.1%) | .065 |
| Previous cardiac surgery | 14 (6.6%) | 12 (8.5%) | 2 (2.9%) | .120 |
| Procedure | .673 | |||
| CABG | 22 (10.4%) | 14 (9.9%) | 8 (11.4%) | |
| Valve | 96 (45.5%) | 67 (47.5%) | 29 (41.4%) | |
| Aortic surgery | 47 (22.3%) | 30 (21.3%) | 17 (24.3%) | |
| combined surgery | 32 (15.2%) | 19 (13.5%) | 13 (18.6%) | |
| others | 14 (6.6%) | 11 (7.8%) | 3 (4.3%) | |
| Resurgery | 31 (14.7%) | 22 (15.6%) | 9 (12.9%) | .596 |
| Valuables at RRT initiation | ||||
| MAP (mmHg) | 78.8 ± 12.6 | 77.2 ± 12.6 | 81.9 ± 12.0 | .011 |
| Mechanical ventilation | 181 (85.8%) | 125 (88.7%) | 56 (80.0%) | .090 |
| Vasoactive drug above 3 kinds | 100 (47.4%) | 69 (48.9%) | 31 (44.3%) | .524 |
| CVP (cmH2O) | 15.0 (11.0, 19.0) | 15.0 (11.0, 19.0) | 15.0 (12.0, 20.0) | .366 |
| AKI stage 3 | 125 (59.2%) | 79 (56.0%) | 46 (65.7%) | .178 |
| GCS score | 3.0 (3.0, 15.0) | 3.0 (3.0, 14.5) | 6.0 (3.0, 15.0) | .144 |
| Drugs use | ||||
| Aminoglycosides or vancomycin antibiotics | 23 (10.9%) | 16 (11.3%) | 7 (10.0%) | .767 |
| ACEI or ARB | 21 (10.0%) | 11 (7.8%) | 10 (14.3%) | .138 |
| NSAID | 25 (11.8%) | 19 (13.5%) | 6 (8.6%) | .299 |
| Contrast media exposure | 21 (10.0%) | 16 (11.3%) | 5 (7.1%) | .337 |
| Laboratory data | ||||
| Haemoglobin (g/L) | 97.0 (86.0, 109.0) | 95.0 (86.0, 109.0) | 101.5 (90.0, 110.0) | .149 |
| Platelet count (×109/L) | 94.0 (58.0, 140.0) | 93.0 (56.0, 137.0) | 97.0 (66.0, 154.0) | .472 |
| Serum uric acid (μmol/L) | 465.0 (348.0, 566.0) | 465.0 (348.0, 576.0) | 465.0 (368.0, 548.0) | .962 |
| Glucose (mmol/L) | 9.9 (7.9, 13.4) | 9.9 (7.8, 13.5) | 10.0 (8.2, 13.1) | .879 |
| HbA1c (%) | 6.0 (5.7, 6.6) | 6.1 (5.7, 6.6) | 5.8 (5.6, 6.7) | .256 |
| ALT (U/L) | 60.0 (28.0, 309.0) | 65.0 (28.0, 410.0) | 52.0 (27.0, 210.0) | .474 |
| Total bilirubin (μmol/L) | 36.0 (20.4, 61.4) | 36.3 (20.8, 61.9) | 34.7 (20.4, 53.4) | .534 |
| Serum albumin (g/L) | 31.8 (27.7, 36.0) | 31.0 (27.2, 34.7) | 34.2 (29.7, 37.5) | .003 |
| CO2CP (mmol/L) | 24.6 (21.9, 27.8) | 24.6 (22.2, 27.9) | 24.8 (21.4, 27.2) | .591 |
| Serum potassium (mmol/L) | 4.2 (3.8, 4.7) | 4.2 (3.7, 4.7) | 4.2 (3.8, 4.7) | .623 |
| Lactic acid (mmol/L) | 3.5 (1.5, 6.4) | 4.6 (1.5, 7.6) | 2.8 (1.4, 5.1) | .035 |
| Lactic acid ≧2.0 mmol/L | 137 (64.9%) | 94 (66.7%) | 43 (61.4%) | .453 |
| Serum Chlorine (mmol/L) | 104.5 (99.8, 106.9) | 104.5 (100.1, 106.9) | 104.4 (99.8, 106.6) | .722 |
| Proteinuria | 36 (17.1%) | 26 (18.4%) | 10 (14.3%) | .450 |
Abbreviations: ACEI/ARB, angiotensin converting enzyme inhibitior/angiotensin receptor blocker; AKI, acute kidney injury; ALT, alanine Aminotransferase; BMI, body mass index; CABG, coronary artery bypass grafting; CO2CP, carbon dioxide‐combining power.; CVP, central venous pressure; eGFR, estimated glomerular filtration rate; GCS, glasgow coma scale; HbA1c, glycated haemoglobin; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; NSAID, non‐steroidal anti‐inflammatory drugs; RRT, renal replacement therapy.
Data were expressed as mean ± SD.
3.2. Feature selection and model construction
Univariate analysis showed that age, BMI, baseline serum creatinine, baseline eGFR, hypertension, diabetes, sepsis, MAP, mechanical ventilation, serum albumin and serum lactic acid upon RRT initiation were associated with renal function recovery, P < .1 (Table 1). Age and baseline serum creatinine level were excluded because both variables are multicollinear with baseline eGFR (Figure S2). BMI and serum lactic acid level were transformed into categorical variables due to the nonlinear associations between them and probability of renal recovery, as indicated by the RCS curves (Figure S3). Then, multivariate logistic regression analysis with a stepwise backward selection of variables based on AIC was applied to establish the final model. As a result, BMI stratification, baseline eGFR, hypertension, sepsis, MAP and mechanical ventilation were the best predictors of renal recovery (Table 2). A nomogram was also created according to the logistic regression results to offer clinicians a quantitative tool for predicting individual probability of renal recovery (Figure 1).
TABLE 2.
Multivariate logistic regression analysis of variables for predicting renal recovery after acute kidney injury requiring renal replacement therapy
| Variables | β | p | OR | 95% CI |
|---|---|---|---|---|
| BMI stratification | <.001 | |||
| <18.5 kg/m2 | 1 | |||
| 18.5–23.9 kg/m2 | 0.476 | .394 | 1.609 | (0.539, 4.804) |
| ≧24 kg/m2 | 2.155 | <.001 | 8.629 | (2.581, 28.848) |
| Baseline eGFR (ml/min/1.73m2) | 0.046 | <.001 | 1.047 | (1.026, 1.068) |
| Hypertension | −0.955 | .019 | 0.385 | (0.173, 0.857) |
| Sepsis | −0.946 | .112 | 0.388 | (0.121, 1.248) |
| MAP | 0.030 | .039 | 1.030 | (1.001, 1.060) |
| Mechanical ventilation | −1.130 | .021 | 0.323 | (0.123, 0.846) |
| Constant | −6.376 | <.001 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; MAP, mean arterial pressure.
FIGURE 1.
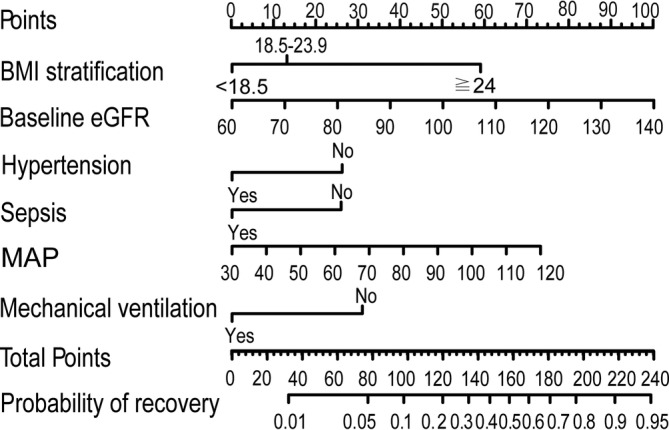
Nomogram for predicting renal function recovery 90 days after renal replacement therapy initiation in patients with acute kidney injury after cardiac surgery. The probability of renal function recovery for each patient after AKI requiring RRT was estimated by drawing on each variable axis. Plotting vertical lines from each variable axis to the top points scale to get the score of each variable. Calculate the sum points of all variables. After locating the sum points of all variables on the total points scale axis, drawing a downward vertical line from the total points scale axis to the probability axis. Then a personalized probability of renal function recovery after AKI requiring RRT was obtained
3.3. Model validation
The model demonstrated good discrimination with a C‐index of 0.807 (95% CI, 0.744–0.870). After correction using the bootstrap method, the C‐index was 0.780 (95% CI, 0.720–0.845). Furthermore, the calibration plot presented that the apparent and bias‐corrected curves have a close fit to the ideal curve, indicating excellent accordance between the model prediction and the actual observations of renal function recovery (Figure 2A).
FIGURE 2.
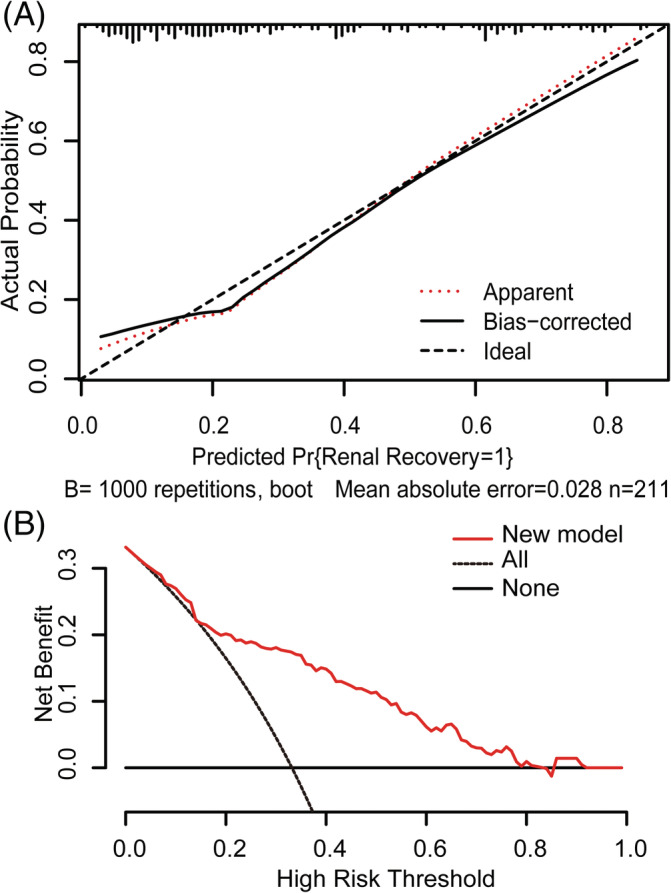
Model calibration and decision curve analyses. (A) Internal model calibration curves (bootstrap = 1000 repetitions). Calibration plot illustrates the relationship between actual occurrence of renal recovery and predicted probability according to the model. The ideal curve along the 45° line represents model calibration in which predicted values are the same as actual outcomes. Apparent and bias‐corrected curves have a close fit to the ideal curve, indicating better predictive accuracy of the model. (B) Decision curve analyses for prediction models. Y‐axis is for net benefit and x‐axis for threshold probability. Dashed and solid black lines represent hypothesis that all patients and no patients had renal recovery after acute kidney injury requiring renal replacement therapy, respectively. The net benefit was computed by subtracting the proportion of false positives from proportion of true positives in all patients and weighting the relative harm driven by false positives. Threshold probability occurs when the expected benefit of treatment avoidance is equal to the expected benefit of treatment. Net benefits for the new model are presented for each decision threshold. The new model was positive across the most range of decision thresholds
3.4. Clinical usefulness
The DCA curve showed that within most range of prediction thresholds, using the new model to predict the chance of renal function recovery generated a net benefit (Figure 2B).
4. DISCUSSION
The present study developed and internally validated a model to predict the probability of renal function recovery 90 days after initiation of RRT in patients with AKI requiring RRT after cardiac surgery. The model included six readily available and objectively measured variables: BMI stratification, baseline eGFR, hypertension, sepsis, MAP and mechanical ventilation. The model demonstrated good performance and was clinically useful across a range of threshold probabilities. A nomogram was created to facilitate the model's use in clinical practice.
To date, few studies have assessed renal function recovery after AKI requiring RRT in patients with cardiac surgery. The incidence of renal function recovery after AKI requiring RRT ranging from 20% to 60% has been reported in critical patients. 4 The present study indicated renal function recovery after AKI requiring RRT at 90 days after RRT initiation was 33.2%, which is comparable to previous reports.
Currently, there are little data predicting renal recovery after initiation of RRT in patients with AKI requiring RRT after cardiac surgery. Prior studies have indicated that several factors, such as baseline eGFR, hypertension, sepsis and MAP, are potential predictors of renal function recovery after AKI requiring RRT in critical patients. 20 , 21 The present study corroborated previous study results. Surprisingly, low BMI was found to be a risk factor for non‐recovery in patients with AKI requiring RRT. A prospective study of 16 264 patients with acute myocardial infarction demonstrated that a low BMI is a predictor of persistent renal dysfunction after percutaneous coronary intervention. 22 Similar to the present results, another retrospective study showed that a low BMI is a risk factor for 90‐day non‐recovery of renal function in elderly patients with AKI. 23 Prior studies have shown that critical patients mostly present with hypermetabolism and high energy consumption. Critically ill patients with a low BMI have less efficient immune systems and are prone to inflammation, which affects recovery after kidney injury and aggravates mortality. 24 In addition, plasma leptin levels are elevated in patients with a high BMI. 25 Experiments on rats showed that leptin may protect against progression of multiple‐organ dysfunction syndrome in response to endotoxemia. 24 High BMI activates the renin angiotensin system, which induces haemodynamic improvement and then alleviates ischaemia of vital organs. 26 However, a high BMI is associated with hypertension, diabetes, aggravated oxidative stress, causing glomerular hyperfiltration and renal injury aggravation. 27 Therefore, the relationship between recovery of renal function and BMI remains unclear.
Similar results were observed in a prior study, with a decreased chance of renal recovery in patients who experienced mechanical ventilation upon RRT initiation. 28 However, the mechanism underlying this finding is not fully understood. Mechanical ventilation may be an indicator of disease severity. The relationship between ventilation use and renal recovery should be further studied.
Renal recovery after AKI was shown to be related to AKI severity in several studies, where the possibility of renal function recovery decreased with AKI aggravation. 21 However, AKI severity upon RRT initiation was not included in the present model. Patients included in present study were classified as AKI stage 2 or 3. Some prior studies have also found no significant difference with regard to RRT requirement after 90 days between AKI stage 2 and AKI stage 3 for RRT initiation. 29
Diabetes was a risk factor for the non‐recovery of renal function after AKI. 21 However, some studies could not demonstrate any difference in renal recovery after RRT between patients with and without diabetes. 23 , 30 In the univariate analysis, the rate of patients with diabetes in the non‐recovery group was higher than that in the recovery group, which was consistent with the medical common sense. However, diabetes was not selected in the final model. The possible reason may be that patients with normal renal function were included, reducing the effect of CKD associated with comorbidities, such as diabetic, on the renal recovery. Moreover, diabetes may be partially collinear with other variables such as BMI, sepsis and hypertension. The contribution of diabetes to outcomes had been reflected by other variables.
The present study has the following strengths. A model was developed to predict the possibility of renal recovery in patients upon initiation of RRT rather than at the time of discharge, which provides a uniform time point for evaluation of renal recovery and can thus better guide clinical practice. The time of discharge may be affected by some factors unrelated to patient condition. Furthermore, the currently recommended 90 days were used as the cutoff point for evaluating renal function recovery. Therefore, the present results are generalizable and comparable. Finally, except model discrimination and calibration, its clinical value was also assessed using DCA, which may aid clinical decision‐making.
However, some limitations should be noted in this study. Firstly, despite the fact that data from a prospective randomized controlled trial were used, the sample size was relatively small and limited to a single centre. Thus, the model must be validated at other centres before it is widely used. Secondly, variables needed to calculate Charlson Index were not fully collected at the time when the CRITERIA study was designed. We could not calculated the effect of Charlson Index, thus were unable to fully assessed comorbidities and the combination effect on renal recovery. Lastly, like most previous studies, renal function recovery was defined as survival and freedom from dialysis. Despite the KDIGO guidelines providing recommendations for RRT cessation, this decision is subjective and has low manipulability in practice. Furthermore, RRT weaning is determined based on urine output, renal function markers and patient condition. The appropriate timing for RRT weaning and definition of successful cessation in patients with AKI requiring RRT have not been standardized yet.
5. CONCLUSION
We used data from a prospective randomized controlled trial to develop and validate a model for predicting the probability of renal function recovery 90 days after initiation of RRT in patients with AKI requiring RRT after cardiac surgery. The model consisted of six readily available and objectively measured variables. The resulting model may be beneficial for doctor‐patient communication, treatment decision‐making and rational utilization of medical resources.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Appendix S1. Supporting Information
Figure S1 Flow chart outlining participant selection of the CRITERIA study
Figure S2 Correlation among potential predictors (dark colour indicates stronger correlation).
Figure S3 Restricted cubic spline plots representing relationships between potential continuous predictors and renal function recovery.
ACKNOWLEDGEMENTS
This study was supported by the Guangdong Science and Technology Project (2017A070709008), Guangzhou Science and Technology Project (201604020037), Fundamental Research Funds for the Central Universities (No. 2018MS24), and the Medical Scientific Research Foundation of Guangdong Province (A2019038). The funders had no role in study design, data collection, analysis, or interpretation, report writing or decision to submit the article for publication.
Hu P, Song L, Liang H, et al. Prospective model for predicting renal recovery in cardiac surgery patients with acute kidney injury requiring renal replacement therapy. Nephrology. 2021;26:586–593. 10.1111/nep.13878
Penghua Hu, Li Song, Huaban Liang and Yuanhan Chen contributed equally to this work.
Funding information Fundamental Research Funds for the Central Universities, Grant/Award Number: 2018MS24; Guangdong Science and Technology Project, Grant/Award Number: 2017A070709008; Guangzhou Science and Technology Project, Grant/Award Number: 201604020037; Medical Scientific Research Foundation of Guangdong Province, Grant/Award Number: A2019038
REFERENCES
- 1. Wang Y, Bellomo R. Cardiac surgery‐associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13(11):697‐711. [DOI] [PubMed] [Google Scholar]
- 2. Bagshaw SM, Darmon M, Ostermann M, et al. Current state of the art for renal replacement therapy in critically ill patients with acute kidney injury. Intensive Care Med. 2017;43(6):841‐854. [DOI] [PubMed] [Google Scholar]
- 3. O'Neal JB, Shaw AD, Billings FT. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care. 2016;20(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cerdá J, Liu KD, Cruz DN, et al. Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol. 2015;10(10):1859‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee BJ, Hsu CY, Parikh R, et al. Predicting renal recovery after dialysis‐requiring acute kidney injury. Kidney Int Rep. 2019;4(4):571‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hickson LJ, Chaudhary S, Williams AW, et al. Predictors of outpatient kidney function recovery among patients who initiate hemodialysis in the hospital. Am J Kidney Dis. 2015;65(4):592‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. 2017;13(4):241‐257. [DOI] [PubMed] [Google Scholar]
- 8. Srisawat N, Wen X, Lee M, et al. Urinary biomarkers and renal recovery in critically ill patients with renal support. Clin J Am Soc Nephrol. 2011;6(8):1815‐1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2:1‐138. [Google Scholar]
- 10. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315(8):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. 2007;165(6):710‐718. [DOI] [PubMed] [Google Scholar]
- 13. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377‐399. [DOI] [PubMed] [Google Scholar]
- 14. Sohl E, Heymans MW, de Jongh RT, et al. Prediction of vitamin D deficiency by simple patient characteristics. Am J Clin Nutr. 2014;99(5):1089‐1095. [DOI] [PubMed] [Google Scholar]
- 15. Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361‐387. [DOI] [PubMed] [Google Scholar]
- 16. Kim Y, Margonis GA, Prescott JD, et al. Nomograms to predict recurrence‐free and overall survival after curative resection of adrenocortical carcinoma. JAMA Surg. 2016;151(4):365‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. [DOI] [PubMed] [Google Scholar]
- 18. Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774‐781. [DOI] [PubMed] [Google Scholar]
- 19. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak. 2006;26(6):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Macedo E, Mehta RL. Renal recovery after acute kidney injury. Contrib Nephrol. 2016;187:24‐35. [DOI] [PubMed] [Google Scholar]
- 21. Forni LG, Darmon M, Ostermann M, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43(6):855‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choe JC, Cha KS, Ahn J, Park JS, Lee HW, Oh JH. Korea acute myocardial infarction registry investigators. Persistent renal dysfunction after percutaneous coronary intervention in patients with acute myocardial infarction. Angiology. 2017;68(2):159‐167. [DOI] [PubMed] [Google Scholar]
- 23. Li Q, Zhao M, Du J, Wang X. Outcomes of renal function in elderly patients with acute kidney injury. Clin Interv Aging. 2017;12:153‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schiffl H. Obesity and the survival of critically ill patients with acute kidney injury: a paradox within the paradox? Kidney Dis (Basel). 2020;6(1):13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robinson MK, Mogensen KM, Casey JD, et al. The relationship among obesity, nutritional status, and mortality in the critically ill. Crit Care Med. 2015;43(1):87‐100. [DOI] [PubMed] [Google Scholar]
- 26. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548‐2556. [DOI] [PubMed] [Google Scholar]
- 27. MacLaughlin HL, Blacklock RM, Wright K, et al. Obesity and recovery from acute kidney injury (Ob AKI): a prospective cohort feasibility study. BMJ Open. 2019;9(3):e24033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ihara K, Ishigami J, Inoshita S. Predictors of withdrawal from renal replacement therapy among patients with acute kidney injury requiring renal replacement therapy. Clin Exp Nephrol. 2019;23(6):814‐824. [DOI] [PubMed] [Google Scholar]
- 29. Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315(20):2190‐2199. [DOI] [PubMed] [Google Scholar]
- 30. Rennie TJ, Patton A, Dreischulte T, Bell S. Incidence and outcomes of acute kidney injury requiring renal replacement therapy: a retrospective cohort study. Nephron. 2016;133(4):239‐246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Figure S1 Flow chart outlining participant selection of the CRITERIA study
Figure S2 Correlation among potential predictors (dark colour indicates stronger correlation).
Figure S3 Restricted cubic spline plots representing relationships between potential continuous predictors and renal function recovery.


