Abstract
Minute amounts of oxygen were supplied to a continuous cultivation of Lactococcus lactis subsp. cremoris MG1363 grown on a defined glucose-limited medium at a dilution rate of 0.1 h−1. More than 80% of the carbon supplied with glucose ended up in fermentation products other than lactate. Addition of even minute amounts of oxygen increased the yield of biomass on glucose by more than 10% compared to that obtained under anaerobic conditions and had a dramatic impact on catabolic enzyme activities and hence on the distribution of carbon at the pyruvate branch point. Increasing aeration caused carbon dioxide and acetate to replace formate and ethanol as catabolic end products while hardly affecting the production of either acetoin or lactate. The negative impact of oxygen on the synthesis of pyruvate formate lyase was confirmed. Moreover, oxygen was shown to down regulate the protein level of alcohol dehydrogenase while increasing the enzyme activity levels of the pyruvate dehydrogenase complex, α-acetolactate synthase, and the NADH oxidases. Lactate dehydrogenase and glyceraldehyde dehydrogenase enzyme activity levels were unaffected by aeration.
Homofermentative lactic acid bacteria (LAB) are used primarily in the dairy industry but may also prove to be advantageous hosts for production of lactate as a bulk chemical or of food additives such as bacteriocins or amino acids using recombinant DNA technology (10, 15). The metabolism of these bacteria is constrained by the requirement for a balance between NADH-producing and -consuming reactions. In the absence of external electron acceptors (e.g., oxygen), the carbon fluxes of catabolism are tightly coupled. In anaerobic culture, glucose may be redox-neutrally converted to lactate or, alternatively, to the mixed acid products formate, acetate, and ethanol in a molar ratio of 2:1:1 (12, 26). However, when oxygen is present in the growth medium, the catabolic carbon fluxes are uncoupled from the redox metabolism due to the NAD+-regenerating activity of NADH oxidases (NOX). This oxygen-related potential for redirecting fluxes is used industrially in connection with the formation of other metabolites such as diacetyl in fermented milk products. Even submillimolar concentrations of diacetyl have a strong impact on the taste of buttermilk and cheese (22). Hence, minute amounts of oxygen may influence an industrial process greatly. In this light, it is surprising that, to our knowledge, no investigations have been carried out on the microaerobic physiology of starter cultures. A number of studies on the effect of oxygen in fully aerated cultures have been published, but little light has been shed on the window ranging from anaerobic to fully aerobic conditions of growth (4, 7, 8, 20).
Interest in the microaerobic physiology of LAB is furthermore spurred by reports on the extreme sensitivity of pyruvate formate-lyase (PFL) to oxygen (1, 25) and the negative effect of oxygen on pfl gene expression (2, 19). Similarly, it has recently been reported that vigorous aeration reduces the transcription of the adhE gene, which encodes alcohol dehydrogenase (ADH) in Lactococcus lactis (3). However, to our knowledge, no data have been published on the effect of controlled aeration on the ADH enzyme protein level. While oxygen inactivates the PFL enzyme, the activity levels of other enzymes are stimulated under aerobic conditions. This is the case for the α-acetolactate synthase (ALS), the initial step in the conversion of pyruvate to acetoin and diacetyl, and the pyruvate dehydrogenase complex (PDH) (8).
Thus, the presence of oxygen in the growth medium influences the metabolism of LAB not only by loosening the tight coupling between catabolic carbon fluxes and the redox metabolism but also by affecting the cellular content of key enzymes. The present investigation was initiated to determine the effect of different levels of controlled mild aeration on product formation and hence on in vivo activities of the enzymes downstream of the pyruvate branch point: PDH, ALS, PFL, and lactate dehydrogenase (LDH). The effect of oxygen on the synthesis of PFL and ADH proteins and on the activity of the enzymes NOX, PDH, ALS, LDH, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was quantified under different growth conditions. As such, the microaerobic physiology of L. lactis MG1363 was determined and the link to levels of enzyme protein or activity under the growth conditions studied was found. To the best of our knowledge, no study of microaerobic conditions of growth has previously been carried out for a homofermentative LAB strain.
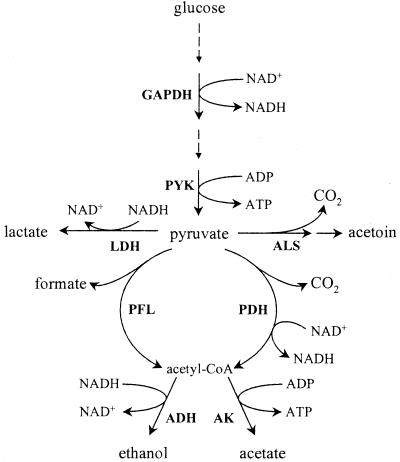
The relevance of the present study is both academic and industrial. Diffusion of air into the growth medium brings about a microaerobic environment at the interface, which may have important implications for production of flavor-associated secondary metabolites. Academically, the influence of oxygen on the distribution of pyruvate among four competing enzymes is a challenge since it involves regulatory control at several levels (Fig. 1). Continuous cultivation was the tool of choice for investigating the effects of varying the dissolved oxygen tension (DOT), since a constant growth rate can be fixed and the in vivo activities of the product forming pathways can be precisely determined. In this way, we showed that minute amounts of oxygen have a dramatic effect on the resulting end product profile.
FIG. 1.
Metabolic network around the pyruvate node. AK, acetate kinase; PYK, pyruvate kinase. Other abbreviations are defined in the text.
MATERIALS AND METHODS
Strain and culture conditions.
The homofermentative laboratory strain L. lactis ssp cremoris MG1363 (13) was the only organism used in this study. The organism was grown at 30°C in continuous mode in a baffled bioreactor (Applikon, Schiedam, The Netherlands) with a 1,000-ml working volume and fitted with a four-blade Rushton turbine rotating at 350 rpm. The dilution rate (D) was set at 0.1 h−1. The pH was kept constant at 6.6 by automatic addition of 5 M KOH.
The defined growth medium MS10 (6) was supplemented with the following components to sustain growth under aerobic conditions: MnCl2, 1.25 × 10−5 g · liter−1; thiamine, 1 mg · liter; thioctic acid, 2.5 mg · liter; and Sigma antifoam 289, 50 μl · liter−1. The concentration of glucose was 3.00 g · liter−1. The medium was added to the previously autoclaved bioreactor by sterile filtration (pore size, 0.20 μm). A preculture was prepared by transferring a single colony from a petri dish and inoculating a tube containing 10 ml of the same medium as above but where the concentrations of KH2PO4, K2HPO4, and glucose were 9, 7.5, and 10 g · liter−1, respectively. The pre-culture was grown at 30°C. The bioreactor was inoculated to an initial biomass concentration of 1 mg · liter−1. The feed pump was turned on at the end of the exponential growth phase.
An anaerobic steady state was obtained by introducing 50 ml of N2 (99.998% pure) · min−1 into the headspace of the bioreactor. Three different anoxic steady states were obtained by sparging the reactor with 250 N ml of gas composed of N2 (99.998% pure) and atmospheric air (at N2/air ratios of 230:20, 200:50, and 125:125) controlled through the use of two mass flow controllers (Bronkhorst, Holland) per min. At these steady states, DOT was undetectable with a polarographic oxygen sensor (Mettler Toledo). One microaerobic steady state was obtained when a set point DOT value of 5% of the value in equilibrium with air (see below) was maintained by feedback regulation of the ratio of air to N2 fed to the reactor (total flow rate, 250 N ml · min−1). The oxygen electrode was calibrated by sparging the bioreactor with air (100% DOT) and with N2 (0% DOT). In pure water at 30°C, a 100% DOT value corresponds to a saturation oxygen concentration of 2.4 × 10−4 mol · liter−1.
For all conditions, the gas was sterile filtered (pore size, 0.20 μm [Whatman, Ann Arbor, Mich.]) before being introduced into the bioreactor. The off gas was led through a condenser cooled to lower than −8°C and analyzed for its volumetric content of CO2 and O2 by means of an acoustic gas analyser (Brüel and Kjær).
A steady state had been reached when at least five retention times (one retention time being 10 h) had passed since the growth conditions were changed and the concentrations of biomass and carbonated fermentation end products remained unchanged (less than 5% relative deviation) over the last two retention times.
Analysis of fermentation end products and glucose.
Ethanol, acetoin, acetate, formate, lactate, and pyruvate were separated by high-pressure liquid chromatography (AminoHPX-87H column; Bio-Rad, Richmond, Calif.) at 65°C using 5 mM H2SO4 flowing at 0.6 ml · min−1 as the eluent. Ethanol and acetoin were quantified with a Waters 410 refractive index detector (Millipore, Badford, Mass.), while acetate, formate, lactate, and pyruvate were quantified with an Waters 486 absorbance detector set at 210 nm.
Glucose was quantified using a glucose dehydrogenase assay (Roche, Basel, Switzerland) with a Cobas Mira automatic analyzer (Roche).
Determination of bacterial cell dry weight.
Nitrocellulose filters (pore size, 0.45 μm) were used to determine the cell dry weight. The filters were tared after having been dried in a microwave oven at 150 W for 10 min. The biomass from 10 ml of culture was collected on the filter and washed with 10 ml of distilled water before being dried and tared as indicated above.
Enzyme assays in vitro.
Enzyme extracts were prepared essentially as described previously (12). Approximately 100 ml of culture volume was collected from the bioreactor and centrifuged (6,000 × g for 10 min at 4°C). The supernatant was discarded, and the biomass washed twice in 200 ml of 0.2% (wt/vol) KCl and subsequently resuspended in 5 ml of a pH 7.2 buffer of the following composition: 45 mM Tris, 15 mM tricarballylic acid, 20% (vol/vol) glycerol, 1 mM dithiothreitol, and 4.5 mM MgCl2. The samples were stored at −25°C until analysis. Enzymes were extracted by sonication of cells (five cycles of 30 s alternating with 60 s of cooling on ice). The resulting extract was clarified by centrifugation (10,000 × g for 10 min at 4°C) and immediately analyzed for enzyme activity and enzyme protein levels as specified below. The protein concentration of the enzyme extract was determined by the assay of Lowry et al. (18) using bovine serum albumin as standard.
Enzyme activities of LDH, GAPDH, PDH and NOX were determined at 30°C and pH 7.2. The concentration of NADH was monitored spectrophotometrically at 340 nm (ɛ = 6.22 × 103 M−1 · cm−1). For PDH activity determination, however, the concentration of 2-(p-iodophenyl)-3-p-nitrophenyltetrazolium chloride (INT) was monitored spectrophotometrically at 500 nm (ɛ = 12.4 × 103 M−1 · cm−1). One unit of enzyme was defined as the amount of enzyme required to produce 1 μmol of product per min.
Enzyme activity assays for LDH, GAPDH, and PDH were performed with reaction mixtures exactly as described previously (12).
NOX activity was assayed in 1 ml of reaction mixture which contained 100 mM Tris-HCl buffer (pH 7.2) 5 mM MnSO4, and 0.3 mM NADH. Addition of cellular extract initiated the reaction.
ALS was assayed essentially as described previously (5), in 1 ml of reaction mixture containing 100 mM phosphate buffer (pH 6.5), 0.20 mM cocarboxylase, and 80 mM sodium pyruvate, which was used to initiate the reaction. After incubation at 30°C for 15 min, 200 μl of 0.5 M HCl was added to stop the reaction and convert α-acetolactate to acetoin. The resulting solution was kept at 45°C for 30 min, and then acetoin was quantified by the colorimetric method of Westerfeld (27). The abundance of PFL and ADH in the total protein pool of the organism was quantified using a sandwich enzyme-linked immunosorbent assay (ELISA) as described previously (19). The ELISA signal was corrected for nonspecific binding by running a series of five doublet measurements where the amount of total protein applied was varied. The slope linking the ELISA signal to the amount of total protein was determined. The slopes obtained were normalized with respect to the slope for the anaerobic sample, whereby relative PFL (or ADH) protein levels were determined.
RESULTS
A supply of oxygen leads to an increased yield of biomass on glucose.
In preliminary experiments with a glucose feed concentration of 10 g · liter−1, it was observed that mild aeration of the bioreactor changed the situation of glucose limitation prevalent under anaerobic conditions to one of glucose excess at 5% DOT (data not shown). Since our primary interest lay in studying glucose-limited cultures, the reason for the accumulation of glucose in the bioreactor was not investigated (previous work [14] suggests an inhibitory effect of H2O2 under aerobic conditions and with a high sugar concentration in the feed). By reducing the concentration of glucose in the feed to 3 g · liter−1, we were able to determine five distinct steady states which were all glucose limited (Table 1). One steady state (steady state 1) was anaerobic, while three (steady states 2 to 4) were mildly aerated with increasing ratios of air to N2 in the gas feed but with unmeasurable values of DOT. The final steady state (steady state 5) was obtained for a measured and controlled DOT value of 5%.
TABLE 1.
Biomass concentrations and specific rates of sugar and oxygen uptake as a function of the growth conditionsa
| Steady state | N2/air ratio | Biomass concn (g [cell dry wt] · liter−1) | Yield of biomass on glucose (g [cell dry wt] · g of glucose−1) | Specific uptake rate of glucose (C-mmol · g [cell dry wt]−1 · h−1) | Specific uptake rate of oxygen (mmol of O2 · g [cell dry wt]−1 · h−1) |
|---|---|---|---|---|---|
| 1 | 250:0 | 0.54 ± 0.02 | 0.18 | 18.4 | |
| 2 | 230:20 | 0.60 ± 0.02 | 0.20 | 16.7 | 0.52 |
| 3 | 200:50 | 0.55 ± 0.02 | 0.18 | 18.2 | 1.39 |
| 4 | 125:125 | 0.69 ± 0.04 | 0.22 | 14.4 | 2.83 |
| 5 | 80:170b | 0.68 ± 0.01 | 0.22 | 14.7 | 3.61 |
Total gas flow was 250 N ml · min−1. In steady state 5, DOT was controlled at 5% (13 μmol of O2 · liter−1).
Approximate ratio.
The biomass concentration in the bioreactor, and hence the yield of biomass on glucose, increased with aeration (Table 1). The biomass concentration for the anaerobic steady state was 0.54 g (cell dry weight) · liter−1 and increased with aeration to 0.68 g · liter−1 for a culture aerated to a DOT level of 5%.
A supply of oxygen strongly affects pyruvate metabolism.
Based on the known dilution rate (D = 0.1 h−1) and the measured steady-state concentrations of glucose, biomass, and catabolic products (Tables 1 and 2), we estimated the specific in vivo activities through the enzymes metabolizing pyruvate and involved in NAD+ regeneration (Fig. 2 and 3). For all five steady states, 94% or more of the glucose carbon was recovered in biomass and end products. These data furthermore showed that in our experimental plan, several patterns of behavior ranging from anaerobic metabolism (ethanol, formate, and acetate besides lactate) to aerobic metabolism (more acetate, some CO2, but no ethanol or formate) were covered (Table 2).
TABLE 2.
Distribution of glucose to different products
| Steady state | Amt (C-mmol · liter−1) of:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Biomassb | Lactate | Formate | Ethanol | Acetate | Acetoin | Pyruvate | CO2c | Sum | |
| 1 | 0.00 | 8.37 | 17.56 | 25.39 | 21.79 | 24.18 | 1.16 | 0.89 | NA | 99.85 |
| 2 | 0.00 | 9.25 | 10.99 | 27.27 | 15.92 | 32.30 | 1.52 | 0.00 | NA | 97.25 |
| 3 | 0.00 | 8.48 | 10.88 | 26.25 | 9.15 | 38.95 | 0.23 | 0.00 | NA | 93.93 |
| 4 | 0.00 | 10.69 | 9.78 | 14.81 | 0.34 | 49.23 | 0.97 | 0.99 | 10.46d | 97.27 |
| 5 | 0.00 | 10.48 | 18.22 | 0.00 | 0.00 | 44.56 | 0.00 | 0.00 | 22.28d | 95.54 |
It was assumed that 43% of the carbon atoms found in the ash-free biomass (Mw = 24.8 g · C-mol−1) stemmed from glucose (P. Loubière, personal communication) and that biomass including ash had a molecular mass of 27.8 g · C-mol−1 (17).
The CO2 production converted to C-mmol · liter−1 was calculated as CO2 = [(ethanol + acetate + acetoin)/2] − formate. The rationale behind this formula was that the synthesis of 1 C-mol of ethanol or acetate from pyruvate inferred the synthesis of 1 C-mol of either formate or CO2. Similarly 1 C-mole of CO2 is synthesized concomitantly with 2 C-mol of acetoin. For steady states 1, 2, and 3, no CO2 production could be calculated (NA).
CO2 was only included in the carbon balance for steady states 4 and 5.
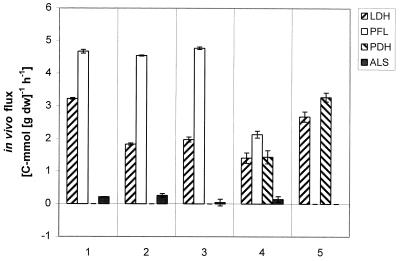
FIG. 2.
Distribution of carbon at the pyruvate branch point as a function of aeration. As an example, the in vivo specific activity of PFL at steady state 3 was computed as follows: rPFL = concentration of formate × dilution rate/biomass concentration = 26.25 (C-mmol · liter−1) × 0.1 (h−1)/0.58 (g [dry weight] · liter−1 = 4.77 C-mmol · g (dry weight)−1 · h−1. The in vivo specific activity of PDH was computed as rPDH = [(racetate kinase + rADH)/2] − rPFL in millimoles of carbon per gram (dry weight) per hour.
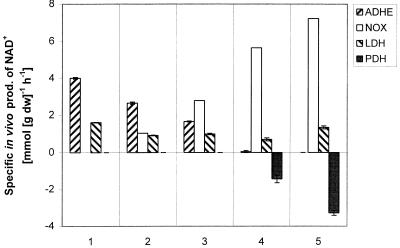
FIG. 3.
Synthesis of NAD+ by ADH, LDH, NOX, and PDH as function of growth conditions. As an example, the in vivo specific activity through ADH for steady state 2 was calculated as follows: rADH = (concentration of ethanol) × (moles of NAD+ generated per C-mole of ethanol generated) × (dilution rate)/(biomass concentration) = 15.92 (C-mmol · liter−1) × 1 (mmol of NAD+ · C-mmol−1) × 0.1 (h−1)/0.60 (g [dry weight] · liter−1) = 2.65 mmol of NAD+ · g (dry weight)−1 · h−1. The in vivo flux through NOX was calculated as rNOX = (number of moles of NAD+ generated per mole of O2 consumed) × (specific uptake rate of oxygen).
By measuring the oxygen concentration in the gas inlet and outlet, it was possible to estimate the actual rate of oxygen consumption (Table 1). This was assumed to be equal to the specific in vivo activity of the NADH oxidases (Fig. 3), since these presumably are the only enzymes involved in oxygen consumption in L. lactis.
Under anaerobic conditions, pyruvate was distributed between mainly two enzymes: LDH and PFL (steady state 1 in Fig. 2). As aeration was increased (steady states 2 and 3), the flux through the PFL was not affected, showing that this enzyme, although reported to be extremely sensitive to oxygen (1, 19, 25, 29) may function even when L. lactis consumes oxygen. On further increase of aeration (steady state 4), PFL activity was dramatically reduced, and at steady state 5, with 5% DOT, the activity was practically zero. PDH displays a behavior opposite to that of PFL. There was no activity in steady states 1, 2, and 3, but gradually the synthesis of acetyl coenzyme A (acetyl-CoA) was taken over by PDH. There was no obvious trend in LDH activity with increasing aeration. The ALS activity was close to zero in all five steady states, which confirms that lactococci produce only minor amounts of acetoin in the absence of citrate as an alternative source for pyruvate.
Under anaerobic conditions, LDH and ADH were the main enzymes responsible for the reoxidation of NADH produced in glycolysis (steady state 1 in Fig. 3). Even the most modest supply of oxygen (steady states 2 and 3) clearly resulted in NOX taking over the NAD+-regenerating role of ADH, while the LDH flux remained almost constant. The in vivo activity of PFL was virtually unaffected by modest aeration, whereas the in vivo ADH activity was observed to drop as soon as oxygen became available (Fig. 2 and 3). A further increase in aeration resulted in NOX being the major enzyme responsible for the oxidation of NADH, with LDH contributing only modestly. PDH activity results in the formation of NADH; therefore, PDH contributed negatively to the NAD+ balance for steady states 4 and 5.
Aeration affects the enzyme levels.
The in vitro levels of GAPDH and of enzymes downstream of the pyruvate branch point were determined in an attempt to obtain the pattern of regulation triggered by the presence of oxygen in the medium (Table 3). An increase in aeration had no effect on the enzyme activity levels of GAPDH, while the flux through the enzyme, quantified as the specific rate of glucose uptake, decreased with increasing aeration (Table 1). This was surprising since increasing aeration was expected to reduce the ratio of NADH to NAD+ (9, 23), hence relieving the inhibitory effect of an elevated ratio on GAPDH activity (11). The data therefore suggest that the enzyme was insensitive to the putative variation of the NADH/NAD+ ratio. This led us to believe that the ratio, even for the anaerobic steady state, was so low that it hardly had any inhibitory effect on the in vivo activity of the GAPDH enzyme. The level of LDH in vitro activity was not affected by increasing aeration.
TABLE 3.
Specific in vitro activities of GAPDH, NOX, LDH, PDH, and ALS and protein levels of ADH and PFL as function of culture conditionsa
| Steady state | Enzyme activity (μmol · min−1 · mg of protein−1)
|
Amt (% of anaerobic level) of:
|
|||||
|---|---|---|---|---|---|---|---|
| GAPDH | LDH | PDH | ALS | NOX | PFL | ADH | |
| 1 | 0.152 | 7.88 | 0.07 | 0.31 | 0.16 | 100 | 100 |
| 2 | 0.302 | 7.14 | 0.04 | 0.13 | 0.12 | 54 | 18 |
| 3 | 0.303 | 8.58 | 0.03 | 0.25 | 0.16 | 42 | 21 |
| 4 | 0.307 | 8.28 | 0.13 | 0.43 | 0.39 | 14 | 1 |
| 5 | 0.223 | 8.57 | 0.78 | 0.89 | 0.63 | 10 | 0 |
ADH and PFL levels were normalized to the values found for anaerobic growth (steady state 1). Standard deviations on the determination of specific enzyme activities were less than 20% except for the more cumbersome analysis of GAPDH, for which the standard deviation was 40%.
The specific in vitro activities of PDH, ALS, and NOX increased with aeration (Table 3), which correlates with the observed in vivo specific activities of these enzymes (Fig. 2 and 3).
The low but nonzero in vitro activities of PDH and NOX for steady state 1 were, however, surprising, since these enzymes are not normally associated with anaerobiosis. Although no gaseous oxygen was supplied to the bioreactor, the medium feed contained dissolved air, which was the only conceivable source of oxygen under these circumstances since a continuous flushing of the headspace of the bioreactor with N2 (99.996% pure) ensured that no atmospheric oxygen could enter the bioreactor. It was necessary to determine whether the minute supply of oxygen through the liquid feed was inducing the expression of the genes encoding NOX and PDH or whether a low “dormant” level of these enzymes ensured a state of preparedness of the organism in case the growth environment turned aerobic. NOX and PDH activities were therefore also assayed in protein extracts of cells grown in batch cultures on the same growth medium with 10 g of lactose · liter−1. Lactose was preferred to glucose, since a specific growth rate of 0.15 h−1 is obtained on lactose, i.e., a growth rate similar to the dilution rate of 0.1 h−1. Strictly anaerobic conditions were ensured by thoroughly flushing the growth medium with N2 prior to inoculation and maintaining a N2 blanket during the cultivation. NOX was not detectable in these cell extracts, while PDH showed a clear activity of 0.07 μmol/min/mg. Lack of detectable NOX activity for strictly anaerobic conditions implies that the organism is not ready for a radical switch from strictly anaerobic to aerobic conditions. Under the glucose-limited fermentation conditions used in this study, even traces of oxygen were sufficient to induce NOX synthesis, as observed in the cell extracts taken from the anaerobic steady state.
In vitro protein levels of PFL and ADH were quantified by an ELISA. This assay does not allow the active and inactive forms of PFL to be distinguished. Hence, the total amount of PFL protein, relative to the anaerobic steady state, was measured. The assay nevertheless made possible an estimation of the impact of oxygen on the levels of ADH and PFL, which has not been possible before because of the extreme difficulty in measuring this activity (Table 3). While the oxygen-related trend observed for PFL was expected (2, 19), no actual correlation between controlled microaerobic conditions and intracellular PFL protein levels had been established. Furthermore, to our knowledge, no reports are available on the influence of small amounts of oxygen on in vitro protein levels of ADH.
Except for LDH, it appears that oxygen in minute amounts triggers a response affecting the in vitro levels of enzymes downstream of the pyruvate branch point. This response created the potential for redirecting the carbon flux from the combination of PFL and ADH toward PDH and ALS. Furthermore, the increased activity of NOX under aerobic conditions helped to regenerate NAD+ consumed in the glycolysis.
Regulation of in vivo activities. (i) PFL.
For steady states 2 and 3, the specific in vivo activity of PFL was unaltered compared to the anaerobic level. This was the case despite the approximately two fold-lower PFL protein level observed for steady states 2 and 3 (Table 3). This shows that the PFL protein level was not limiting for formate synthesis under the anaerobic conditions studied here. It is doubtful that the limitation of PFL activity under anaerobic conditions could be attributed to the intracellular levels of glyceraldehyde-3-phosphate and dihydroxyacetone phosphate, both allosteric inhibitors of the enzyme (25), since it has been shown that at a comparable dilution rate (D = 0.12 h−1), the intracellular levels of the two metabolites are far below the values which would lead to inhibition (28).
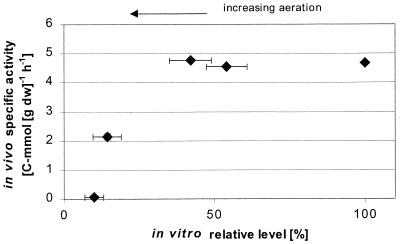
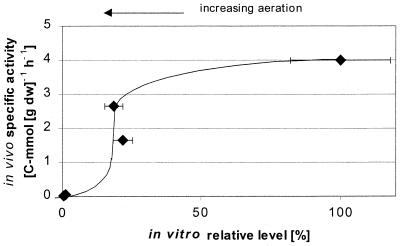
From a certain aeration level (steady states 3, 4, and 5), the presence of oxygen seems to have a dominating control over the in vivo activity of PFL and hence on the flux to formate. This may be due to the observed down regulation of PFL synthesis or, alternatively, to irreversible inactivation of PFL (19). The ratio of inactive to active PFL protein may have increased with aeration since absolutely no formate synthesis was observed for steady state 5 in spite of a significant in vitro protein level, which should have sustained a detectable production of formate if the enzyme had been fully active (Fig. 4).
FIG. 4.
In vivo specific activity of PFL versus the intracellular level of the enzyme (sum of active and inactive forms), expressed as a percentage of the amount found under anaerobic conditions of growth. Horizontal error bars represent the standard deviations of five duplicate determinations of the protein in the sample. Vertical error bars representing the standard deviation of three determinations of the in vivo specific activity of the enzyme are not visible since the standard deviations were low on the scale of the figure.
(ii) ADH.
Ethanol synthesis decreased drastically on slight aeration (Table 2), which is ascribed partly to a reduced protein level of ADH in the cell (Table 3). Furthermore, the sigmoidal shape of the relationship linking flux through ADH to the in vitro ADH level suggests that increased aeration lowered the NADH/NAD+ ratio, leading to a higher degree of inhibition of ADH (Fig. 5). It appears from our data that the ADH protein level present under anaerobic conditions does not limit the rate of ethanol synthesis. The same conclusion was reached for the PFL protein level and formate synthesis.
FIG. 5.
In vivo specific activity of ADH versus the intracellular level of the enzyme, expressed as a percentage of the amount found under anaerobic conditions of growth. Error bars are defined in the legend to Fig. 4. Steady states 4 and 5 are both close to the origin.
DISCUSSION
Under anaerobic conditions, pyruvate is converted to lactate by LDH and to acetyl-CoA and formate by PFL. Acetyl-CoA fuels the synthesis of lipids, of acetate through acetate kinase, and of ethanol through ADH. ATP production accompanies the synthesis of acetate, whereas ethanol production regenerates NAD+ consumed in the glycolysis and in the biomass synthesis. By supplying oxygen to the growth medium, NAD+ regeneration was taken over by NADH oxidases, whereby the organism can redirect the flux from ethanol to increased acetate synthesis, thus producing more ATP. This led to a higher yield of biomass on glucose and clearly demonstrates that ATP synthesis limits biomass formation when the organism is grown at a dilution rate of 0.1 h−1 under anaerobic conditions. NOX most probably displayed a higher affinity for NADH than did ADH, judging from the decrease in specific ethanol production as oxygen was introduced.
Oxygen, even at undetectable levels, triggered a response down regulating the capacity of the PFL-ADH pathway and increasing the in vitro activity levels of ALS, NOX, and PDH. In contrast, GAPDH and LDH in vitro activity was not affected by increasing aeration. A significant redirection of carbon at the pyruvate branch point ensued from the altered enzyme levels. Our results show that PFL and PDH can function simultaneously, although this requires fine-tuned conditions of growth.
The anaerobic product spectrum consisting of formate, acetate, ethanol, and lactate was replaced with the aerobic product pattern (acetate, CO2, and lactate) for low values of DOT (5% DOT, corresponding to 13 μmol of O2 · liter−1). This underlines the strong influence of low oxygen levels on a continuous cultivation operated at low dilution rate.
We ascribe the lack of acetoin synthesis to the rather low level of the glycolytic flux obtained for D = 0.1 h−1 used in our study. This prevents a large pool of intracellular pyruvate from building up; because of the low affinity of ALS for pyruvate (16, 24), a high intracellular concentration of pyruvate (e.g., by decomposition of citrate) is needed if carbon is to be directed through ALS.
The molecular mechanism of oxygen action is unknown, but it is reasonable to assume that oxygen plays a significant role on the level of gene expression and hence on enzyme levels. Increased aeration diminished the synthesis of PFL and ADH proteins while raising the activity levels of ALS, NOX, and PDH. Furthermore, oxygen is known to irreversibly inhibit PFL by inducing peptide bond cleavage. The presence of oxygen will probably also change the NADH/NAD+ ratio and thus the in vivo activity of enzymes involving this pair of cofactors (GAPDH, PDH, LDH, ADH, and NOX). We have tried hard to measure NADH in cellular extracts on a fluorometer by using an enzyme-linked assay described previously (12), but the presence of interferences in the extract, particularly under aerobic conditions, impeded the analysis (16). This has prevented us from confirming a correlation between the NADH/NAD+ ratio and the in vivo specific activities of the enzymes mentioned above.
Preliminary results from our laboratory show that the oxygen-induced regulatory cascade leading to higher ALS, NOX, and PDH activity is not only controlled by aeration but also subjected to glucose repression (data not shown).
This work demonstrates the strong impact of a minute oxygen supply on pyruvate metabolism and suggests that both altered gene expression and varied redox levels play a predominant role in regulation of this metabolism.
REFERENCES
- 1.Abbe K, Takahashi S, Yamada T. Involvement of oxygen-sensitive pyruvate formate lyase in mixed acid fermentation by Streptococcus mutans under strictly anaerobic conditions. J Bacteriol. 1982;152:175–182. doi: 10.1128/jb.152.1.175-182.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnau J, Jørgensen F, Madsen S, Vrang A, Israelsen H. Cloning, expression and characterization of the Lactococcus lactis pfl gene, encoding pyruvate formate lyase. J Bacteriol. 1997;179:5884–5891. doi: 10.1128/jb.179.18.5884-5891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnau J, Jørgensen F, Madsen S, Vrang A, Israelsen H. Cloning of the Lactococcus lactis adhE gene, encoding a multifunctional alcohol dehydrogenase, by complementation of a fermentative mutant of Escherichia coli. J Bacteriol. 1998;180:3049–3055. doi: 10.1128/jb.180.12.3049-3055.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borch E, Molin G. The aerobic growth and product formation of Lactobacillus, Leuconostoc, Brochothrix and Carnobacterium in batch cultures. Appl Microbiol Biotechnol. 1989;30:81–88. [Google Scholar]
- 5.Boumerdassi H, Monnet C, Desmazeaud M, Corrieu G. Isolation and properties of Lactococcus lactis subsp. biovar diacetylactis CNRZ 483 mutants producing diacetyl and acetoin from glucose. Appl Environ Microbiol. 1997;63:2293–2299. doi: 10.1128/aem.63.6.2293-2299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocaign-Bousquet M, Garrigues C, Novak L, Lindley N D, Loubière P. Rational development of a simple synthetic medium for the sustained growth of Lactococcus lactis. J Appl Bacteriol. 1995;79:108–116. [Google Scholar]
- 7.Cogan J F, Walsh D, Condon S. Impact of aeration on the metabolic end-products formed from glucose and galactose by Streptococcus lactis. J Appl Bacteriol. 1989;66:77–84. [Google Scholar]
- 8.Condon S. Responses of lactic acid bacteria to oxygen. FEMS Microbiol Rev. 1987;46:269–280. [Google Scholar]
- 9.de Graef M, Alexeeva S, Snoep J, Teixera de Mattos M J. The steady-state internal redox state (NADH/NAD) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli. J Bacteriol. 1999;181:2351–2357. doi: 10.1128/jb.181.8.2351-2357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vos W M. Metabolic engineering of sugar catabolism in lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:223–242. doi: 10.1007/BF00395934. [DOI] [PubMed] [Google Scholar]
- 11.Even S, Garrigues C, Loubière P, Lindley N D, Cocaign-Bousquet M. Pyruvate metabolism in Lactococcus lactis is dependent upon on glyceraldehyde-3-phosphate dehydrogenase activity. Metab Eng. 1999;1:198–205. doi: 10.1006/mben.1999.0120. [DOI] [PubMed] [Google Scholar]
- 12.Garrigues C, Loubière P, Lindley N D, Cocaign-Bousquet M. Control of the shift from homolactic to mixed acid fermentation in Lactococcus lactis: predominant role of the NADH/NAD+ ratio. J Bacteriol. 1997;179:5282–5287. doi: 10.1128/jb.179.17.5282-5287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grufferty R, Condon S. Effects of fermentation sugar on hydrogen peroxide accumulation by Streptococcus lactis C10. J Dairy Res. 1983;50:481–489. [Google Scholar]
- 15.Hols P, Kleerebezem M, Schanck A N, Andre N, Ferain T, Hugenholtz J, Delcour J, de Vos W M. Conversion of Lactococcus lactis from homolactic to homoalanine fermentation through metabolic engineering. Nat Biotechnol. 1999;17:588–592. doi: 10.1038/9902. [DOI] [PubMed] [Google Scholar]
- 16.Jensen N B S. Ph.D. thesis. Technical University of DenmarkLyngby, Denmark: Department of Biotechnology; 1999. [Google Scholar]
- 17.Loubière P, Cocaign-Bousquet M, Matos J, Goma G, Lindley N D. Influence of end-products inhibition and nutrients limitations on the growth of Lactococcus lactis subsp. lactis. J Appl Bacteriol. 1997;82:95–100. [Google Scholar]
- 18.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Melchiorsen C R, Jokumsen K V, Villadsen J, Johnsen M G, Israelsen H, Arnau J. Synthesis and posttranslational regulation of pyruvate formate lyase in Lactococcus lactis. J Bacteriol. 2000;182:4783–4788. doi: 10.1128/jb.182.17.4783-4788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mickelson M N. Aerobic metabolism of Streptococcus agalactiae. J Bacteriol. 1967;94:184–191. doi: 10.1128/jb.94.1.184-191.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novak L. Ph.D. thesis. Toulouse, France: Institut National Sciences Appliquées; 1998. [Google Scholar]
- 22.Parker R B, Eliker P R. Effect of spoilage bacteria on diacetyl content and flavour of cottage cheese. J Dairy Sci. 1953;36:843–847. [Google Scholar]
- 23.Snoep J. Regulation of pyruvate catabolism in Enterococcus faecalis. Ph.D. thesis. Amsterdam, The Netherlands: Department of Microbiology, University of Amsterdam; 1992. [Google Scholar]
- 24.Snoep J, Teixera de Mattos M, Starrenburg J C, Hugenholtz J. Isolation, characterization, and physiological role of the pyruvate dehydrogenase complex and α-acetolactate synthase of Lactococcus lactis subsp. lactis bv. diacetylactis. J Bacteriol. 1992;174:4838–4841. doi: 10.1128/jb.174.14.4838-4841.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi S, Abbe K, Yamada T. Purification of pyruvate formate lyase from Streptococcus mutans and its regulatory properties. J Bacteriol. 1982;149:1034–1040. doi: 10.1128/jb.149.3.1034-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas T, Ellwood D, Longyear M. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979;138:109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westerfeld W W. A colorimetric determination of blood acetoin. J Biol Chem. 1945;161:495–502. [PubMed] [Google Scholar]
- 28.Yamada T, Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975;124:55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada T, Takahashi-Abbe S, Abbe T. Effects of oxygen on pyruvate formate lyase in situ and sugar metabolism of Streptococcus mutans and Streptococcus sanguis. Infect Immun. 1985;47:129–134. doi: 10.1128/iai.47.1.129-134.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]