Summary
Research has shown that a home‐based educational intervention for patients with chronic kidney disease results in better knowledge and communication, and more living donor kidney transplantations (LDKT). Implementation research in the field of renal care is almost nonexistent. The aims of this study were (1) to demonstrate generalizability, (2) evaluate the implementation process, and (3) to assess the relationship of intervention effects on LDKT‐activity. Eight hospitals participated in the project. Patients eligible for all kidney replacement therapies (KRT) were invited to participate. Effect outcomes were KRT‐knowledge and KRT‐communication, and treatment choice. Feasibility, fidelity, and intervention costs were assessed as part of the process evaluation. Three hundred and thirty‐two patients completed the intervention. There was a significant increase in KRT‐knowledge and KRT‐communication among participants. One hundred and twenty‐nine out of 332 patients (39%) had LDKT‐activity, which was in line with the results of the clinical trials. Protocol adherence, knowledge, and age were correlated with LDKT‐activity. This unique implementation study shows that the results in practice are comparable to the previous trials, and show that the intervention can be implemented, while maintaining quality. Results from the project resulted in the uptake of the intervention in standard care. We urge other countries to investigate the uptake of the intervention.
Keywords: family communication, home‐based educational program, implementation, living kidney donation, patient education
The results of this extensive implementation study showed that home‐based educational interventions for patients with end‐stage kidney disease led to implementation in standard‐care in the Netherlands, and gives great benefits to patients.

Highlights.
This research uniquely evaluates an implementation in the field of renal care: the Kidney Team at Home project.
The implementation process was positively evaluated.
Adhering to the standardized protocol leads to better outcomes.
Results from the implementation process resulted in the uptake and national reimbursement of the intervention in standard care as of 2021.
Introduction
While living donor kidney transplantation (LDKT) is the best treatment option with chronic kidney disease (CKD) in terms of survival and quality of life [1, 2], research has shown that there is inequality in access to LDKT [3, 4]. A number of modifiable factors, such as patients’ knowledge on kidney replacement therapy (KRT), communication with family and friends about KRT, and cultural sensitivity of health care professionals are independently related to the access to LDKT [5, 6]. A home‐based educational intervention has been developed to address these factors. This intervention was tested in the United States in a randomized controlled trial (RCT) by Rodrigue et al. [7, 8]. The intervention took place in the home of the patient and was highly interactive. The patients invited members of their social network to attend a group educational session on KRT at the patient’s home, delivered by health educators. This home‐based educational intervention resulted in better knowledge about LDKT, increased the willingness to talk with the social network about LDKT, and reduced fears and concerns about LDKT compared with patients who received education in the hospital [7]. Based on these findings, two RCTs testing effectiveness of a home‐based educational intervention were conducted in the Netherlands [9, 10].
The first study was aimed at patients who were either newly referred for transplant preparation or already listed for a deceased donor kidney transplantation (DDKT) and unable to find a living donor. The control group received standard care, including standard education by the nephrologist. The experimental group received a home‐based educational intervention in addition to standard care. The patient invited his or her social network for the intervention and two health educators provided information about dialysis and transplantation. Following the work of Rodrigue and colleagues, the intervention was based on the principles and communication techniques drawn from multisystem therapy (MST) [11] to improve family communication and reduce fears and concerns regarding the different KRT. Patients and their social network showed a significant increase in KRT‐knowledge and KRT‐communication. The intervention also resulted in a significant increase of the LDKT‐rate in the experimental group compared with the control group [9].
The second RCT was aimed at patients with CKD who had not yet started KRT and was conducted at three regional hospitals and at the predialysis department of a university hospital. The primary goal of the study was to educate patients and to stimulate the communication between patients and the social network about the different KRT options in order to make a well‐informed decision. Another goal was to monitor the choice of primary KRT‐option after they received this home‐based educational intervention. Both patients and the members of their social network showed a significant increase in KRT‐knowledge and KRT‐communication. Of the 49 patients who initiated with a form of KRT during the two‐year follow‐up, 34 patients underwent a LDKT, 22 of them pre‐emptively [10].
The positive results of these studies led to an implementation project, involving eight hospitals in the Netherlands [12]. The first goal of the project was to assess the generalizability of the results of the previous studies to other regions in the Netherlands. If the project is deemed to be successful, the results could support nationwide deployment of the program as standard care. This study is unique as implementation studies are almost nonexistent in renal care. This is remarkable as large variation exists in how centers treat patients with CKD. For instance, some hospitals refer none of their patients for pre‐emptive transplantation, while other centers have a referral rate of 80% [13]. In the United States, similar variation in referral rates among centers exists [14]. To prevent such variation, and because of the growing interest in research to understand barriers and facilitators of successful implementation [15], the second goal was to evaluate the implementation process of the protocolled intervention in terms of feasibility, fidelity, and intervention costs. Additionally, we were interested in the influence of intervention effects on LDKT‐activity.
To summarize, three research questions emerge. First, are the results of the previous RCTs conducted in the Netherlands replicable to other Dutch regions, thus demonstrating generalizability? Second, is a nationwide implementation viable in terms of feasibility, fidelity, and intervention costs? Third, do patients with higher knowledge and communication skills on KRT have a higher probability of LDKT‐activity?
Materials and methods
Participants & procedure
The home‐based educational intervention was implemented in eight hospitals in the Netherlands; four university hospitals and four regional hospitals. In the Netherlands, kidney transplantations are carried out by university hospitals. Regional hospitals screen their patients for transplantation and refer them to the university hospital for approval for kidney transplantation. Regional hospitals were included to reach those patients who were unable to find a living donor and who have yet to start KRT. For these hospitals, the inclusion criteria for patients were: ≥18 years of age and eligible for all KRT options. In the regional hospitals, medical social workers and dialysis nurses carried out the intervention. The four university hospitals targeted both patients who had yet to start KRT and patients who were already on dialysis, and who were unable to find a living donor. For these hospitals, the inclusion criteria were: ≥18 years of age, currently undergoing dialysis, or expected to start KRT within the coming 12 months and eligible for all KRT options. The patient’s nephrologist determined whether the inclusion criteria were met. In the university hospitals, transplant coordinators were accompanied by psychologists or medical social workers to conduct the intervention. The university hospitals were selected on the following criteria: hospital capacity, to reach as many patients as possible as it would result in a sufficient sample size to evaluate the intervention outcomes, sufficient geographical spread to assess the generalizability to other parts of the Netherlands, and willingness to participate in the implementation project. The four regional hospitals were chosen by the university hospitals as their partner in the transplantation region. The study was approved by the institutional review board of the participating hospitals (Erasmus MC: MEC‐2016‐496).
The educators first approached patients in‐person or by telephone to offer the intervention/recruit them into the study, followed by two home visits. The aim of the first home visit for the educators is to get familiarize themselves with the social network of the patient and to prepare the group educational session. In the second session, the group educational intervention took place. After the second session, patients and invitees were asked by an independent party to evaluate the protocol adherence of the educators. All participants were required to sign an informed consent form, either during recruitment or the first home visit.
Prior to the start of the implementation project, educators received a one‐day training from supervisors educated in family communication and social network resilience. After the training, regular supervision meetings were conducted (one hour, every six weeks) per participating hospital by one clinical psychologist (SI). Furthermore, 4 consortium meetings were organized per year, with the educators (see Consortium), the project group (SR, SI, JB, WW & EM), supervisors (SI & CB), and with the supervising nephrologists (see Consortium). Goals of these meetings were to discuss protocol implementation, inclusion of patients and motivating them to participate and complete the intervention, data entry, protocol adherence, and case studies. A quality assurance system, namely the consortium meetings, supervision, and the independent evaluation with patients and invitees, was put in place to measure and maintain a high degree of protocol adherence.
Questionnaires were completed at two time points: prior to the home‐based educational intervention and after the intervention. Both the patient and at least one invitee completed these questionnaires. A website was used to co‐ordinate data entry over the various study sites. A researcher (SR) monitored every hospital three times during the study period to check accuracy of data entry (comparing the hard‐copy of the questionnaires with the data entered on the website).
This implementation study was conducted between September 2016 and December 2018. All patients approached within this time period were included in the analyses, although some patients received the intervention after this period. Since regional hospitals and university hospitals approached different patient populations, results were reported either per hospital group (university and regional hospital group) or, when of interest, per hospital separately. More details on participants and procedures are described elsewhere [12].
Effect evaluation measures
We measured three effects: KRT‐knowledge, KRT‐communication with individuals from the social network, and the KRT during the 24 months follow‐up after the intervention. KRT‐knowledge was measured using a validated questionnaire: the R3KT‐questionnaire [16]. Answer categories are multiple choice and the number of correct answers is summed to get to a KRT knowledge score (1 to 21). Communication was measured using three items, which could be answered on a Likert scale from 1 (completely disagree) to 5 (completely agree). The questions addressed the attitudes toward communication of patients and invitees with their social network on all forms of KRT. The total communication score is the mean of the three questions. The patients’ premeasurement of these questionnaires was administered at the intake. All invitees were asked to fill in the premeasurement before the start of the group education on a voluntary basis. The postmeasurement was administered on paper directly after the education ended.
The KRT of patients was recorded 6, 12, and 24 months after the intervention date. Treatment modalities were: no KRT necessary yet, peritoneal dialysis, hemodialysis, DDKT, and LDKT. Any LDKT‐activity (either LDKT‐inquiry, work‐up, or actual LDKT) at these time points was also recorded.
Process evaluation measures
To evaluate the implementation, a framework for implementation research (IR) was used. IR examines processes of installation of interventions in standard care to evaluate successful implementation. Proctor et al. [17] introduced a conceptual framework of eight outcomes to evaluate an implementation; acceptability, adoption, appropriateness, feasibility, fidelity, intervention costs, penetration, and sustainability. In this study, we assessed the three outcomes that were most relevant to the project goals: feasibility, fidelity, and intervention costs. Other outcomes, such as acceptability, adoption, and appropriateness were either already researched [18] or were deemed less relevant. Penetration and sustainability should be investigated after the adoption of the intervention in care‐as‐usual.
Feasibility
Feasibility is the extent to which a new treatment can be successfully carried out in a new setting [17]. The concept of feasibility is often operationalized in recruitment and/or participation rates [19, 20]. In this study, the participation rate is defined as the percentage of those who completed an intervention out of those approached. In the implementation project, educators registered every patient who had been approached; whether the patient wanted to receive the home‐based educational intervention or not.
Fidelity
Fidelity is defined as the degree to which an intervention was implemented as it was prescribed in the original protocol [21]. In this implementation project, protocol adherence measure was conducted by a third party by means of a telephone interview with every patient and one invitee who attended the group educational session. The patient and one invitee were asked for their opinion about the extent to which certain topics were discussed during the intervention. Patients and invitees were asked to answer 15 questions. Items were rated on a Likert scale (1‐not at all to 5‐very much). The protocol adherence score was the average score of these 15 items. The questionnaire was based on the protocol adherence measure questionnaire used in MST [22].
Intervention costs
Intervention costs were assessed using the micro‐costing approach. The eight teams were asked to register how many hours they spent per patient per home‐based intervention including travel, how many patients they have approached, how many patients received the intake session, how many patients received the group educational session (considered a completed intervention), and how many hours the educators spent per year attending training, supervision, and consortium meetings. Furthermore, we registered costs of training, traveling, and consortium meetings. All these cost components were converted into a cost price per completed intervention. For the salary of the personnel, the 2020 collective labor agreement for Dutch personnel of university hospitals was used [23].
Statistical analysis
Analyses were conducted using IBM‐SPSS Statistics version 25 (SPSS Inc., Chicago). Paired T‐tests were used to explore differences between the pre‐ and postmeasurement of the effect outcomes. Cohen’s definition was used for the interpretations of the effect sizes: an effect size of 0.20 is considered a small effect, 0.50 medium, and 0.80 a large effect [24].
To determine the relationship between effect and process evaluation outcomes on LKDT, a Cox‐regression model was used to determine the hazard ratio for patients who received an intervention and had LDKT‐activity as a result of the intervention. Patients who dropped‐out before the second session because of a transplantation were not included in the model, as they did not complete the intervention trajectory. Covariates used in the model were postinterventional knowledge and communication, protocol adherence, and socio‐demographic characteristics that have been shown to influence the access to LDKT: age, gender, ethnicity, and religion [25, 26]. An organization level was also introduced to take into account dependence between patients from the same hospital. Patients who were approached between September 2016 and December 2018 were included in the analysis. The follow‐up period was up to 1st July 2020.
Results
Between 2016 and 2018, 812 patients were approached for the home‐based educational intervention. Of these patients, 332 completed the intervention. Figure 1 shows the flow of these patients and Table 1 shows the characteristics.
Figure 1.
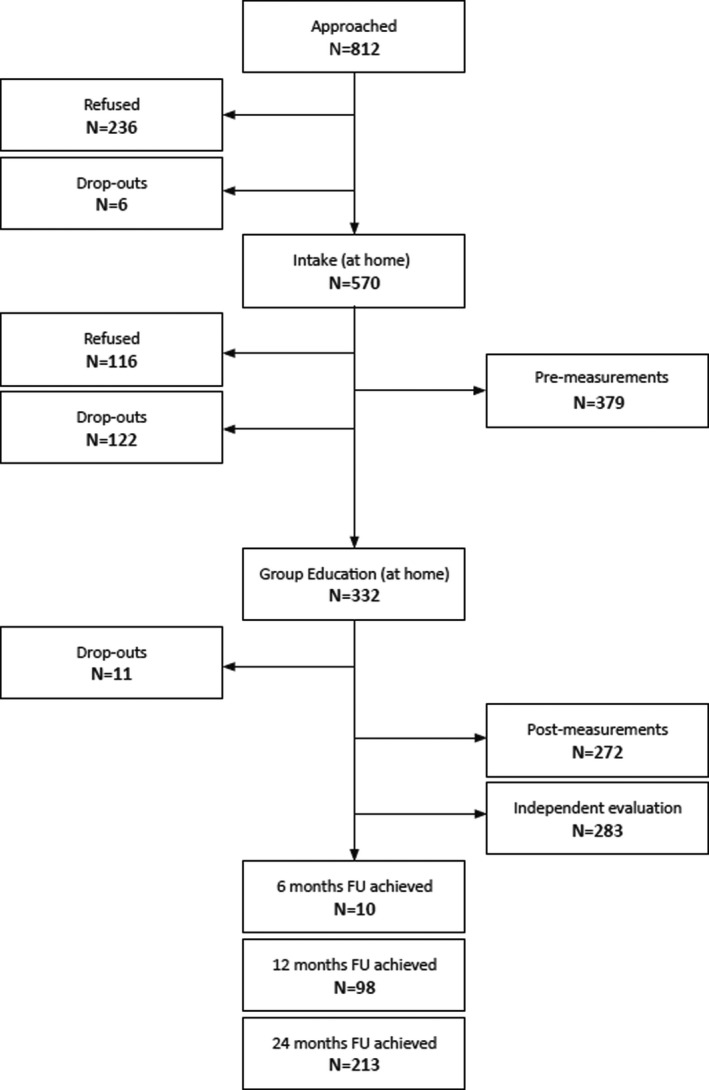
Flow‐chart of the Kidney Team at Home implementation project.
Table 1.
Patient characteristics.
| Characteristics |
Total N = 332 |
University hospitals N = 210 |
Regional hospitals N = 122 |
|---|---|---|---|
| Gender (M/F) | 205/127 | 120/90 | 85/37 |
| Mean age (SD) | 55.2 (13.5) | 52.90 (13.7) | 59.05 (12.2) |
| Married or living together % | 66.9% | 61.4% | 76.2% |
| Religious (Yes/No) % | 40.1%/59.9% | 42.4%/57.6% | 36.1%/63.9% |
| Ethnicity (Western/Non‐Western) % | 69.6%/30.4% | 70.5%/29.5% | 68.0%/32.0% |
| Education level %* | |||
| Low | 12.6% | 9.7% | 17.1% |
| Average | 68.9% | 70.9% | 65.8% |
| High | 18.5% | 19.4% | 17.1% |
| Employment (full or part‐time %) | 125 (37.7%) | 83 (39.5%) | 42 (34.4%) |
| Treatment modality at baseline (%) | |||
| No KRT | 228 (68.4%) | 113 (53.8%) | 115 (94.3%) |
| Hemodialysis | 65 (19.7%) | 61 (29.0%) | 4 (3.3%) |
| Peritoneal dialysis | 32 (9.9%) | 32 (15.2%) | 0 (0.0%) |
| Living with transplant | 7 (2.1%) | 4 (1.9%) | 3 (2.4%) |
| History of transplantation N (%) | 36 (10.8%) | 30 (5.0%) | 6 (4.9%) |
Education level was valued at three levels; Low = elementary school, Average= high school (+some college) and high= at least college degree.
Intervention effects
Knowledge & communication
In total, 272 patients and 630 invitees completed the questionnaires both before and after the intervention. There was a significant increase in KRT‐knowledge for both the patients and the invitees in both hospital groups. Patients’ and invitees’ KRT‐communication increased significantly in the university hospitals. In the regional hospitals, the KRT‐communication was high but did not change significantly. Table 2 shows these scores of the patients and invitees per hospital group.
Table 2.
KRT‐knowledge and KRT‐communication – Patients & Invitees.
| Measure (scale range) | N = | Pre‐interventional score (Mean ± SD) | Post‐interventional score (Mean ± SD) | Effect size | p |
|---|---|---|---|---|---|
| Patients – Knowledge (1–21) | |||||
| University Hospitals | 180 | 14.45 ± 4.50 | 18.62 ± 2.18 | 1.18 | <0.001 |
| Regional Hospitals | 92 | 11.94 ± 5.43 | 17.08 ± 3.81 | 1.09 | <0.001 |
| Patients – Communication (1–5) | |||||
| University Hospitals | 179 | 4.07 ± 0.92 | 4.22 ± 0.86 | 0.17 | 0.028 |
| Regional Hospitals | 91 | 4.21 ± 0.92 | 4.27 ± 0.96 | 0.06 | 0.527 |
| Invitees – Knowledge (1–21) | |||||
| University Hospitals | 517 | 11.55 ± 4.30 | 18.42 ± 2.20 | 2.01 | <0.001 |
| Regional Hospitals | 113 | 11.08 ± 5.15 | 17.84 ± 3.16 | 1.58 | <0.001 |
| Invitees – Communication (1–5) | |||||
| University Hospitals | 509 | 3.64 ± 0.90 | 3.95 ± 0.87 | 0.35 | <0.001 |
| Regional Hospitals | 112 | 4.10 ± 0.82 | 4.18 ± 0.84 | 0.10 | 0.373 |
Treatment modality
The follow‐up time was a maximum of 24 months after the intervention date. Two hundred and thirteen patients (64.2%) completed the follow‐up time. Ninety‐eight (29.5%) had their last follow‐up moment at 12 months and 10 (3.0%) patients at 6 months, 11 (3.4%) patients dropped‐out after the group education and before the first follow‐up. Table 3 shows an overview of the treatment modality after the intervention.
Table 3.
Follow‐up (up to 24 months after the intervention).
| Treatment Modality | University hospitals (N = 210) | Regional hospitals (N = 122) | Total (N = 332) |
|---|---|---|---|
| No KRT | 39 (18.6%) | 59 (48.4%) | 98 (29.5%) |
| Hemodialysis | 38 (18.1%) | 19 (15.6%) | 57 (17.2%) |
| Peritoneal dialysis | 27 (12.9%) | 13 (10.7%) | 40 (12.0%) |
| Living Donor Kidney Transplantation after dialysis | 23 (11.0%) | 4 (3.3%) | 27 (8.1%) |
| Pre‐emptive Living Donor Kidney Transplantation | 34 (16.2%) | 15 (12.3%) | 49 (14.8%) |
| Deceased donor transplantation | 40 (19.0%) | 4 (3.3%) | 44 (13.3%) |
| Conservative treatment | 0 (0.0%) | 1 (0.8%) | 1 (0.3%) |
| Died | 4 (1.9%) | 1 (0.8%) | 5 (1.5%) |
| Drop‐out | 5 (2.4%) | 6 (4.9%) | 11 (3.3%) |
Another 46 patients, who were either undergoing HD, PD, or no treatment yet, were in preparation for a living donor transplantation at their final follow‐up moment. In total, 129 (39%) of the 332 patients that completed the intervention had LDKT‐activity (either in preparation or actual LDKT) at some point during their follow‐up.
Process evaluation
Feasibility
The participation rate was 40.9%: 332 of the 812 patients approached completed the intervention. Participation rates between the university hospitals and the regional hospitals differed substantially. The average participation rate of the university hospitals was 34.9% (210 out of 602), while in the regional hospitals the participation rate was 58.1% (122 out of 210). Socio‐demographic variables such as employment, age, and ethnic background could not explain the differences in participation rate. Table 4 shows the participation rate per hospital.
Table 4.
Participation rate per hospital.
| Patients approached | Completed interventions | Participation rate | |
|---|---|---|---|
| University hospital A | 140 | 30 | 21.4% |
| University hospital B | 143 | 60 | 42.0% |
| University hospital C | 156 | 64 | 41.0% |
| University hospital D | 163 | 56 | 34.3% |
| Total university hospitals | 602 | 210 | 34.9% |
| Regional hospital A | 52 | 28 | 53.8% |
| Regional hospital B | 82 | 30 | 36.6% |
| Regional hospital C | 33 | 23 | 70.0% |
| Regional hospital D | 43 | 41 | 95.3% |
| Total regional hospitals | 210 | 122 | 58.1% |
Initially, 460 (57%) patients indicated that they wanted to receive an intervention. Six patients dropped out after the first contact moment, and 105 patients dropped‐out after the first home visit. Most frequently reported reasons for drop out were “Do not want to receive an education (23%),” “received an DDKT in the meantime (11%),” and “not necessary anymore (8.6%).” For nonparticipators, the most reported reasons were: “do not want to receive education (30%),” and “do not find it necessary (20%).”
Fidelity
In total, 118 supervision meetings and 8 consortium meetings took place. The independent third party conducted 283 protocol adherence evaluations with patients and 250 with invitees. The overall average protocol adherence scores given by patients and invitees were 4.71 and 4.65, respectively (on a scale of 1–5).
Intervention costs
On average, educators spent 22.1 hours on each completed intervention. Incorporated in the total adjusted hours are the number of patients (1.43) that have to be approached in order to include one patient, and the proportion of completed interventions per patient included (0.59). In the Dutch context, the cost per intervention amounts to €2500–€3000. Table 5 shows a breakdown of the estimated number of hours per intervention. Sessions 1 and 2 are home visits and include traveling hours.
Table 5.
Breakdown of hours spent per intervention.
| Activity | Hours |
|---|---|
| Approaching patients | 1.3 |
| Home visit 1 | 4.4 |
| Home visit 2 (both educators) | 15.6 |
| Training (both educators) | 0.8 |
| Total hours per intervention | 22.1 |
| Supervision (supervisor, consultant and both educators) | 2.9 |
| Multidisciplinary consultation | 0.3 |
| Total hours | 25.3 |
Intervention effects & LDKT‐activity
Figure 2 shows the cumulative hazard ratio of LDKT‐activity. Postinterventional knowledge (HR = 1.267; CI = 1.134–1.416; P < 0.001) and protocol adherence (HR = 2.328; CI = 1.102–4.917; P = 0.027) were positively related to the rate of LDKT, indicating that a higher protocol adherence increases the probability of LDKT‐activity. A younger age (HR: 0.976; CI = 0.951–0.983; P < 0.001) was also significantly related to the rate of LDKT‐activity. Postinterventional communication was not related to the rate of LDKT‐activity. Eventually, 222 patients completed both the postmeasurement questionnaires and the independent evaluation.
Figure 2.
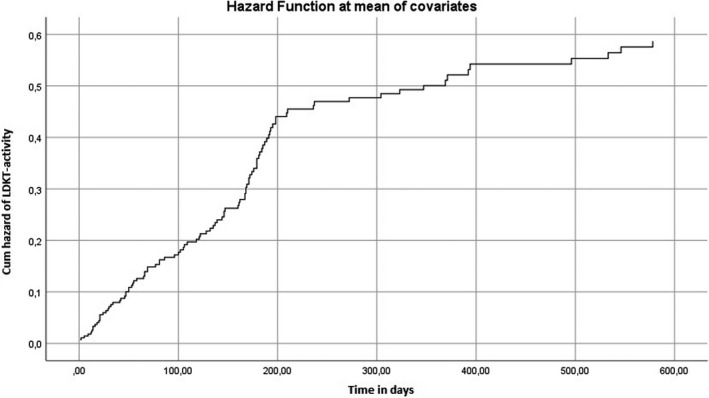
This graph depicts the cumulative hazard time‐to‐event data for the rate of LDKT‐activity. Covariates used were: age, postinterventional knowledge, postinterventional communication, gender, religion and ethnicity (Western/non‐Western).
Discussion
Conclusions
The favorable findings of this implementation project resulted in national uptake of the intervention in the Netherlands as of 2021. To our knowledge, this is the first time a psychosocial intervention has gone from bench to bedside and been implemented as part of standard care and reimbursement at a national level in a kidney replacement therapy program worldwide. Results demonstrate that the results of the previous studies are generalizable and replicable. Protocol adherence was high throughout the implementation project and the intervention comes with relatively low costs. The relationship between intervention effects and LDKT‐activity shows support for the importance of the protocol and quality assessment.
Intervention effect evaluation
Results of the previous RCTs [9, 10] were replicated in terms of increase in KRT‐knowledge for all participating hospitals and KRT‐communication in the university hospitals. Communication skills did not significantly improve for patients and invitees in the regional hospitals. This might be because of a ceiling effect, as both patients and invitees scored relatively high on the premeasurement of the questionnaires. Patient’s treatment choice after the intervention is also in line with the results of the previous studies. Among participants with follow‐up data, 39% had LDKT‐activity, which is comparable to the previous RCT in which 29 of 71 (40%) had LDKT‐activity [9]. It is reassuring that the implementation project yields comparable results to those from the previous conducted studies. Results of a RCT are not always generalizable to a naturalistic setting, since a RCT often involves a homogenous population and is often conducted in a somewhat artificial environment, which differs from how the majority of patients react in practice [27]. However, in this study, we found evidence supporting generalizability of findings that home‐based education can successfully be implemented throughout the Netherlands.
Process evaluation
The overall participation rate was relatively low (40.9%), which had several reasons. First, the project involved questionnaires and written informed consent, which is often a barrier to participate in a study [28]. Second, 122 patients dropped‐out after the first home‐visit, because some patients underwent a transplantation, were not eligible for transplantation or found a living donor candidate in the meantime. Initially, 57% of the patients had signed the informed consent form. Thus, the participation rate might be significantly higher when the intervention is implemented in standard care without research components.
There was variability among the hospitals in terms of the participation rate of patients. Among the university hospitals, participation rates ranged between 21.4% and 42.0%. In the regional hospital group, this rate ranged from 36.6% to 95.3%. The difference between the hospital groups could be explained by the different target population. Contrary to university hospitals, regional hospitals only targeted pre‐KRT patients who were more recently been diagnosed with CKD and are less familiar with the information on treatments and are probably more interested in an educational intervention. This is also reflected by the preinterventional knowledge, which was lower in the pre‐KRT group.
Another contributing factor for the variability of the participation rate might be a form of selection bias. The hospitals with the highest participation rate had the lowest number of patients approached. It is possible that the educators of these hospitals offered the intervention to patients who were most likely to participate. Unfortunately, data on the case load of each hospital were not available. Other important factors may be the timing, the method, and the team member who approached the patients for participation.
With the micro‐costing approach, it was estimated that in the Netherlands the cost for an intervention lies between €2500 and €3000. These costs include costs of the consortium meetings, supervision, training, interpreter, and protocol adherence measure. The total costs were adjusted for the number of prematurely terminated interventions and first sessions, that is, the cost estimation accounts for the number of patients that have to be approached to achieve a complete intervention. A cost‐effectiveness analysis should reveal whether the intervention is cost‐effective compared with standard care.
Protocol adherence was high throughout the project and the intervention costs were relatively low. Most attention must be paid to the recruitment of patients when implementing the intervention. Offering the intervention in an early disease stage might reduce the number of drop‐outs.
Intervention effects & LDKT‐activity
Multivariable analysis showed a significant relationship between the rate of LDKT‐activity and age of the patients, postinterventional knowledge, and protocol adherence scores. This result indicates the importance of implementing the home‐based educational intervention according to a standardized protocol and the clinical relevance of the quality assurance system. A higher age and lower knowledge are often associated with a reduced likelihood of LDKT [25, 29].
Limitations
There are a few limitations to this study. First, the evaluation of the effect of the intervention on KRT‐communication may have been suboptimal. The questionnaire consisted of three questions regarding communication and the postintervention measurement was performed directly after the intervention. The main reason to administer the questionnaire on the same day as the intervention was to ensure a high response rate. However, it failed to capture the effects of the intervention on communication in the days and weeks after the intervention.
Second, cultural differences, differences in organization of the nephrology department, and difference in standard educational materials and current education practices might all have an influence on the intervention effects. Hence, one needs to be cautious drawing conclusions while comparing the different hospitals with one another.
Third, it is difficult to draw conclusions on the differences in uptake across the hospitals. The variability in the participation rate is interesting from an implementation point of view. However, there is very little data on nonparticipants, nor are data on active hospital files and data on comorbidities of patients available. Moreover, it is difficult to determine the role of individual healthcare professionals during the initial approach, the hospitals and the timing and placement in the care pathway.
Practical implications
Addressing disparities in LDKT has been highlighted as a research priority [30]. Here, we showed that the intervention can also be implemented in multiple regions and different types of hospitals while maintaining impact and quality. This approach should be assessed in other countries to further support generalizability. In the United States, several RCTs have been performed regarding home‐based education for CKD‐patients [8, 31]. Also in the United Kingdom, initiatives to address disparities have been undertaken with home‐based education [32, 33, 34]. The results of these single‐center studies were favorable.
A set of recommendations emerged from this study. We recommend that the quality assurance structure of regular supervision and the independent evaluation should remain an integral part of the intervention. It safeguards the quality of the intervention at small incremental costs, justified by the fact that protocol adherence is associated with a higher probability of LDKT‐activity. In addition, we recommend that the home‐based educational intervention should be integrated in the nephrology guidelines, offering the intervention before patients start with any form of KRT, if possible [35]. In this way, patients receive complete information on all types of KRT, so they are fully informed prior to decision‐making regarding their treatment. Importantly, a timely educational intervention can also facilitate pre‐emptive transplantation, which avoids the harmful effects of dialysis in terms of morbidity, mortality, and deterioration of the quality of life, and is associated with better patient and graft survival than transplantation after a period of dialysis [36, 37, 38, 39].
Authorship
Steef Redeker: Participated in: Research design, writing of the paper, performance of the research, data analysis. Emma K. Massey: Participated in: Research design, writing of the paper, performance of the research, data analysis. Charlotte Boonstra: Participated in: Research design, writing of the paper, performance of the research. Jan J. van Busschbach: Participated in: Research design, writing of the paper, performance of the research. Reinier Timman: Participated in: data analysis, writing of the paper. Harald F.H. Brulez: Participated in: Writing of the paper, performance of the research. Daan A.A.M.J. Hollander Participated in: Writing of the paper, performance of the research. Luuk B. Hilbrands: Participated in: Writing of the paper, performance of the research. Frederike Bemelman: Participated in: Writing of the paper, performance of the research. Stefan P. Berger: Participated in: Writing of the paper, performance of the research. Jacqueline van de Wetering: Participated in: Writing of the paper, performance of the research. René M.A. van den Dorpel: Participated in: Writing of the paper, performance of the research. Margriet Dekker‐Jansen: articipated in: Writing of the paper, performance of the research. Willem Weimar: Participated in: Research design, writing of the paper, performance of the research, data analysis. Sohal Y. Ismail: Participated in: Research design, writing of the paper, performance of the research, data analysis.
Funding
The implementation project was supported by Zorgverzekeraars Nederland.
Conflict of interest
All authors have no competing interests to report.
Ethical approval
The medical ethics review committees of all participating hospitals have approved the study (Erasmus MC: MEC‐2011‐004). The study is in accordance with the Declaration of Istanbul (2008) and the Declaration of Helsinki (2000). All patients who participated in the study, gave written consent by signing the informed consent form.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
Acknowledgments
The authors thank the patients and their family and friends for participating in the project. All authors express their gratitude to the participating hospitals, the educators and their supervisors. Finally, the authors express their gratitude to Marian van Noord for organizational support and to the supervisory committee for advising the project team throughout the project.
Appendix 1.
Participants of the Nierteam aan Huis consortium
Bemelman F.J. van der Pant K.A.M.I.; Zwiers N.; Fleur W.; Espineira‐Ramirez R.Y.; Van Milgen‐Adriaens M. (Amsterdam UMC); van de Wetering J.; Weimar W.; Massey E.K.; Ismail S.Y.; van Noord‐Haubrich M.A.A.; Busschbach J.J.; Redeker S.; Jansen D.L.; Wageveld‐Sanderson J.C.; van Berkel I.; (Erasmus MC); Clarisse I.; Hollander D.; van de Linde P.; van Dijk C; de Rooij A. (Jeroen Bosch Ziekenhuis); van den Dorpel M.A.; Versluijs‐Rovers E.E.; van Dongen‐Segeren G.C.A.; Schelfhout R.; van Kooij A.C.; van Krieken A.S.; Koevermans M.M.; van der Marel T. (Maasstad Ziekenhuis); Brulez H.; Zwiers‐Visser L.; Wisse E.M.; Brouns N.C.J.; Dubbelman P.S.; Holling B.; Smulders R.M.; (OLVG); Dooper I.; Hilbrands L.B.; Lobeek M.; Hopman S.P.F. (Radboudumc); Sanders J.S.F.; Berger S.P.; Crop M.J.; Brugman C.A.; Scholten‐Greben H.G.K.; Waijer J.; Kisteman M.J.; Van der Laan J. (UMCG); Mahangoe M.; Poorterman‐Stokvis I.; Dekker‐Janssen M.A.; Hutten I.A.M.; Potgieter J.H.; Brok S.J.; Kettelerij H. (ZGT); Boonstra A.C. (De Viersprong).
Kidney Team at Home consortium members are presented in Appendix 1.
Contributor Information
Steef Redeker, Email: s.redeker@erasmusmc.nl.
the Kidney Team at Home consortium:
J Bemelman, K.A.M.I. van der Pant, N. Zwiers, W. Fleur, R.Y. Espineira‐Ramirez, M. Van Milgen‐Adriaens, J. van de Wetering, W. Weimar, E.K. Massey, S.Y. Ismail, M.A.A. van Noord‐Haubrich, J.J. Busschbach, S. Redeker, D.L. Jansen, J.C. Wageveld‐Sanderson, I. van Berkel, I. Clarisse, D. Hollander, P. van de Linde, C van Dijk, A. de Rooij, M.A. van den Dorpel, E.E. Versluijs‐Rovers, G.C.A. van Dongen‐Segeren, R. Schelfhout, A.C. van Kooij, A.S. van Krieken, M.M. Koevermans, T. van der Marel, H. Brulez, L. Zwiers‐Visser, E.M. Wisse, N.C.J. Brouns, P.S. Dubbelman, B. Holling, R.M. Smulders, I. Dooper, L.B. Hilbrands, M. Lobeek, S.P.F. Hopman, J.S.F. Sanders, S.P. Berger, M.J. Crop, C.A. Brugman, H.G.K. Scholten‐Greben, J. Waijer, M.J. Kisteman, J. Van der Laan, M. Mahangoe, I. Poorterman‐Stokvis, M.A. Dekker‐Janssen, I.A.M. Hutten, J.H. Potgieter, S.J. Brok, H. Kettelerij, and A.C. Boonstra
References
- 1. Mahillo B, Carmona M, Alvarez M, White S, Noel L, Matesanz R. 2009 global data in organ donation and transplantation: activities, laws, and organization. Transplantation 2011; 92: 1069. [DOI] [PubMed] [Google Scholar]
- 2. Kramer A, Pippias M, Noordzij M, et al. The European Renal Association ‐ European Dialysis and Transplant Association (ERA‐EDTA) Registry Annual Report 2015: a summary. Clin Kidney J. 2018; 11: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roodnat JI, van de Wetering J, Zuidema W, et al. Ethnically diverse populations and their participation in living kidney donation programs. Transplantation 2010; 89: 1263. [DOI] [PubMed] [Google Scholar]
- 4. Taylor DM, Bradley JA, Bradley C, et al. Limited health literacy is associated with reduced access to kidney transplantation. Kidney Int 2019; 95: 1244. [DOI] [PubMed] [Google Scholar]
- 5. Gillis KA, Lees JS, Ralston MR, et al. Interaction between socioeconomic deprivation and likelihood of pre‐emptive transplantation: influence of competing risks and referral characteristics – a retrospective study. Transpl Int 2019; 32: 153. [DOI] [PubMed] [Google Scholar]
- 6. Ismail SY, Claassens L, Luchtenburg AE, et al. Living donor kidney transplantation among ethnic minorities in the Netherlands: a model for breaking the hurdles. Patient Educ Couns 2013; 90: 118. [DOI] [PubMed] [Google Scholar]
- 7. Rodrigue JR, Cornell DL, Lin JK, Kaplan B, Howard RJ. Increasing live donor kidney transplantation: a randomized controlled trial of a home‐based educational intervention. Am J Transplant 2007; 7: 394. [DOI] [PubMed] [Google Scholar]
- 8. Rodrigue JR, Paek MJ, Egbuna O, et al. Making house calls increases living donor inquiries and evaluations for blacks on the kidney transplant waiting list. Transplantation 2014; 98: 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ismail SY, Luchtenburg AE, Timman R, et al. Home‐based family intervention increases knowledge, communication and living donation rates: a randomized controlled trial. Am J Transplant 2014; 14: 1862. [DOI] [PubMed] [Google Scholar]
- 10. Massey EK, Gregoor PJ, Nette RW, et al. Early home‐based group education to support informed decision‐making among patients with end‐stage renal disease: a multi‐centre randomized controlled trial. Nephrol Dial Transplant 2016; 31: 823. [DOI] [PubMed] [Google Scholar]
- 11. Henggeler SW, Schaeffer CM. Multisystemic therapy((R)): Clinical overview, outcomes, and implementation research. Fam Process 2016; 55: 514. [DOI] [PubMed] [Google Scholar]
- 12. Redeker S, Oppe M, Visser M, et al. Cost‐effectiveness of a home‐based group educational programme on renal replacement therapies: a study protocol. BMJ Open 2019; 9: e025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Renine . Nierfunctie vervanging Nederland. Utrecht: Nefrovisie, 2020. [Google Scholar]
- 14. Patzer RE, Plantinga LC, Paul S, et al. Variation in dialysis facility referral for kidney transplantation among patients with end‐stage renal disease in georgia. JAMA 2015; 314: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davies P, Walker AE, Grimshaw JM. A systematic review of the use of theory in the design of guideline dissemination and implementation strategies and interpretation of the results of rigorous evaluations. Implement Sci 2010; 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ismail SY, Timmerman L, Timman R, et al. A psychometric analysis of the Rotterdam Renal Replacement Knowledge‐Test (R3K‐T) using item response theory. Transpl Int 2013; 26: 1164. [DOI] [PubMed] [Google Scholar]
- 17. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011; 38: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Massey EK, Hilhorst MT, Nette RW, et al. Justification for a home‐based education programme for kidney patients and their social network prior to initiation of renal replacement therapy. J Med Ethics 2011; 37: 677. [DOI] [PubMed] [Google Scholar]
- 19. Beidas RS, Becker‐Haimes EM, Adams DR, et al. Feasibility and acceptability of two incentive‐based implementation strategies for mental health therapists implementing cognitive‐behavioral therapy: a pilot study to inform a randomized controlled trial. Implement Sci 2017; 12: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Apter AJ, Wan F, Reisine S, et al. Feasibility, acceptability and preliminary effectiveness of patient advocates for improving asthma outcomes in adults. J Asthma 2013; 50: 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stains M, Vickrey T. Fidelity of implementation: an overlooked yet critical construct to establish effectiveness of evidence‐based instructional practices. CBE Life Sci Educ. 2017; 16: rm1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chapman JE, Scheidow AJ, Henggeler SW, Halliday‐Boykins C, Cunningham PB. Developing a measure of therapist Adherence to Contingency Management: An Application of the Many‐Facet Rasch Model. J Childe Adolesc Subst Abuse. 2008; 17: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centra NFvUM . Cao universitair medische centra 2018‐2020: nfu.nl: 2019. [Available from: https://www.nfu.nl/img/pdf/19.2084_Umcs_Uitgave_2019_NL_Cao_umc_2018‐2020_v3‐4‐2019.pdf.
- 24. Cohen J. A power primer. Psychol Bull 1992; 112: 155. [DOI] [PubMed] [Google Scholar]
- 25. Roodnat JI, Laging M, Massey EK, et al. Accumulation of unfavorable clinical and socioeconomic factors precludes living donor kidney transplantation. Transplantation 2012; 93: 518. [DOI] [PubMed] [Google Scholar]
- 26. Ismail SY, Luchtenburg AE, Kal‐V Gestel JA, et al. Modifiable factors in access to living‐donor Kidney transplantation among diverse populations. Transplantation 2013; 96: 586. [DOI] [PubMed] [Google Scholar]
- 27. Roche N, Reddel HK, Agusti A, et al. Integrating real‐life studies in the global therapeutic research framework. Lancet Respir Med 2013; 1: e29. [DOI] [PubMed] [Google Scholar]
- 28. Knottnerus JA, Tugwell P. Prevention of premature trial discontinuation: how to counter Lasagna's law. J Clin Epidemiol 2016; 80: 1–2. [DOI] [PubMed] [Google Scholar]
- 29. Wu DA, Robb ML, Watson CJE, et al. Barriers to living donor kidney transplantation in the United Kingdom: a national observational study. Nephrol Dial Transplant 2017; 32: 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodrigue JR, Kazley AS, Mandelbrot DA, Hays R, LaPointe RD, Baliga P. Living donor kidney transplantation: overcoming disparities in live kidney donation in the US—Recommendations from a consensus conference. Clin J Am Soc Nephrol 2015; 10: 1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodrigue JR, Cornell DL, Kaplan B, Howard RJ. A randomized trial of a home‐based educational approach to increase live donor kidney transplantation: Effects in blacks and whites. Am J Kidney Dis. 2008; 51: 663‐670. [DOI] [PubMed] [Google Scholar]
- 32. Buffin J, Little R, Jain N, Warrens AN. A peer outreach initiative to increase the registration of minorities as organ donors. Clin Kidney J. 2015; 8: 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Papalois VE, Theodosopoulou M. Optimizing Health Literacy for Improved Clinical Practices. Hershey, PA: IGI Global, 2018. [Google Scholar]
- 34. Scotland OD. Renal Education and Choices @ Home (REACH) Project: Organ Donation Scotland; 2018. [Available from: https://www.organdonationscotland.org/news‐events/renal‐education‐and‐choices‐home‐reach‐project.
- 35. Knight RJ, Teeter LD, Graviss EA, et al. Barriers to preemptive renal transplantation: a single center questionnaire study. Transplantation 2015; 99: 576. [DOI] [PubMed] [Google Scholar]
- 36. Jay CL, Dean PG, Helmick RA, Stegall MD. Reassessing preemptive kidney transplantation in the united states: are we making progress? Transplantation 2016; 100: 1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT. Preemptive kidney transplantation: the advantage and the advantaged. J Am Soc Nephrol 2002; 13: 1358. [DOI] [PubMed] [Google Scholar]
- 38. Abramowicz D, Hazzan M, Maggiore U, et al. Does pre‐emptive transplantation versus post start of dialysis transplantation with a kidney from a living donor improve outcomes after transplantation? A systematic literature review and position statement by the Descartes Working Group and ERBP. Nephrol Dial Transplant 2016; 31: 691. [DOI] [PubMed] [Google Scholar]
- 39. Helmick RA, Jay CL, Price BA, Dean PG, Stegall MD. Identifying barriers to preemptive kidney transplantation in a living donor transplant cohort. Transplant Direct. 2018; 4: e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.


