ABSTRACT
Food withdrawal as a health‐enhancing measure has beneficial effects on aging, disease prevention, and treatment. However, the cellular and molecular mechanisms involving gut microbial changes and metabolic consequences resulting from food withdrawal have yet to be elucidated. In this study, we subjected lean and obese mice to a dietary intervention that consisted of a 4‐d complete food withdrawal and an 8‐d 50% food withdrawal, and we studied changes in cecal microbiome and host serum metabolome. The abundance of potentially pathogenic Proteo‐bacteria was decreased and Akkermansia muciniphila was elevated by food withdrawal in mice fed a high‐fat diet (HFD). Meanwhile, food withdrawal decreased the abundance of metabolites in branched chain amino acid, lipid, and free fatty acid metabolisms in host serum, more so in HFD mice than in normal mice. Microbial predicted function also showed that food withdrawal decreased the abundance of microbes associated with predicted diseases in the HFD group but not in the normal chow group. Correlation between the microbiome data and metabolomics data revealed a strong association between gut microbial and host metabolic changes in response to food withdrawal. In summary, our results showed that food withdrawal was safer and more metabolically beneficial to HFD‐induced obese mice than to normal lean mice, and the beneficial effects were primarily derived from the changes in gut microbiota, which were closely associated with the host metabolome.—Zheng, X., Zhou, K., Zhang, Y., Han, X., Zhao, A., Liu, J., Qu, C., Ge, K., Huang, F., Hernandez, B., Yu, H., Panee, J., Chen, T., Jia, W., Jia, W. Food withdrawal alters the gut microbiota and metabolome in mice. FASEB J. 32, 4878–4888 (2018). www.fasebj.org
Keywords: fasting, microbiome, metabolomics
Abbreviations
- BCAA
branched chain amino acid
- FC
fold change
- FFA
free fatty acid
- H-postF
HFD-post-food withdrawal
- H-preF
HFD-pre-food withdrawal
- HFD
high-fat diet
- MUFA
monounsaturated fatty acid
- N-postF
normal diet-post-food withdrawal
- N-preF
normal diet-pre-food withdrawal
- OGTT
oral glucose tolerance test
- OTU
operation taxonomic unit
- PCOA
principal coordinate analysis
- PICRUSt
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States
- PLS-DA
partial least squares discrimination analysis
- PUFA
polyunsaturated fatty acid
- SFA
saturated fatty acid
Food withdrawal, also known as bigu in Chinese, is a lifestyle intervention that involves abstinence from food consumption. It has been practiced in various regions of the world for millennia, and many religious groups incorporate periods of food withdrawal into their rituals (1). In traditional Chinese culture, the concept of food withdrawal is interpreted as maintaining well‐being without eating particular foodstuffs or any food for a period of time (2, 3). The number of individuals who have adopted food withdrawal as a lifestyle change has been steadily increasing. Study findings have pointed to various beneficial effects of food withdrawal such as reduced body weight, delayed aging, and improved health. In humans, food withdrawal helps reduce obesity, hypertension, asthma, and rheumatoid arthritis; in rodents, intermittent or periodic food withdrawal protects against diabetes, cancer, heart disease, and neurodegeneration (1, 4–6). Recent mechanistic studies have highlighted the beneficial roles of food withdrawal in adaptive cellular responses that reduce oxidative damage and inflammation, optimize energy metabolism, and boost cellular protection (1, 7–10).
Despite its increasing popularity, food withdrawal is still a subject of intense medical debate. Adverse outcomes have also been observed in individuals practicing food withdrawal, including development or exacerbation of eating disorders, overreliance on caffeine‐rich beverages such as coffee, or development of food intolerances and inflammation (11–14). Thus, there is a pressing need to improve the safety of food withdrawal practices by identifying individuals suitable for this intervention and by understanding the mechanistic pathways underlying the beneficial or detrimental effects of food withdrawal.
In addition, the impact of food withdrawal on gut microbial composition and metabolic function, as well as its association with host metabolic health, has yet to be determined. Previous studies using 16S rRNA gene sequencing documented changes in colonic and cecal microbiomes of 5 species of vertebrates after food withdrawal: tilapia, toads, geckos, quail, and mice. The results revealed differences in the food withdrawal‐induced changes in the microbiome across the host species (15). Reports have also shown that caloric restriction switched metabolism from lipid biosynthesis to fatty acid catabolism and to downstream β‐oxidation (16, 17). Another recent study on mice has shown that intermittent food withdrawal stimulated beige fat development within white adipose tissue and dramatically ameliorated obesity, insulin resistance, and hepatic steatosis (18). The gut microbiota and microbial metabolites orchestrate the effects of intermittent food withdrawal on white adipose beiging and metabolic improvement. However, no study has comprehensively investigated food withdrawal‐induced changes in gut microbial composition and function or their association with host metabolism.
The aim of the present study was to test the hypothesis that food withdrawal is safer and more beneficial to obese mice than to lean mice and that food withdrawal–induced changes in host metabolism are associated with changes in gut microbiota composition and function. C57BL/6J mice were used to mimic stepwise initiation and conclusion of food withdrawal by lean or obese individuals. Lean and obese mice were fed a normal chow or a high‐fat diet (HFD), respectively, for 6 wk at baseline. All mice were subjected to 50% food withdrawal on the normal chow for 4 d, followed by a complete food withdrawal for 4 d, and another 50% food withdrawal on the normal chow for 4 d, and recovered on the normal chow ad libitum for 2 wk. Blood and cecal content samples were collected before the first 50% food withdrawal and after the 2‐wk recovery for metabolomic and 16S metagenomic analyses.
MATERIALS AND METHODS
Animal experiment
The experimental design complied with the ethical principles of animal experimentation [including the 3Rs (reduction, refinement, and replacement)] provided in the Supplemental Data. The study protocol was approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital. A total of 40 C57BL/6J mice (male, 4 wk old) were purchased from Shanghai Laboratory Animal Center (SLAC; Shanghai, China). The mice were acclimated for 1 wk before experimentation. All mice were maintained in a controlled environment (20–22°C, 12‐h light/dark cycle)andwerehousedinindividualventilatedcagesunder specific pathogen‐free conditions with free access to ultrapure water. During the experiment, body weight and food intake of all animals were recorded once a week except for during the food withdrawal period when body weight was measured daily.
The mice were randomly divided into 2 groups (n =20/ group). The normal group was fed a normal chow (10% calories from fat), and the HFD group was fed a diet with 59% calories derived from coconut oil. The normal chow and HFD treatments lasted for 6 wk. At the end of the 6 wk, 9 mice in each group underwent an oral glucose tolerance test (OGTT) and were euthanized 2 d later for liver, serum, and cecal content sample collection. The 2 groups of samples were defined as normal‐pre‐food withdrawal (N‐preF) and HFD–pre–food withdrawal (H‐preF). The remaining 22 animals (11/group) were all fed the normal chow that provided 50% calories of their baseline need (i.e., 50% food withdrawal) for 4 d, followed by complete food withdrawal for 4 d, and then another 50% food withdrawal on the normal chow for 4 d. The mice had free access to drinking water during the food withdrawal period. The physical status of each mouse was closely observed 3 times a day, and body weight was measured daily during this period. Mice were euthanized when their weight loss in 1 d was >8% or when the cumulative score reached 6 according to the physical evaluation criteria provided in the Supplemental Data. In the following 2 wk, mice from both groups were fed the normal chow ad libitum. All of the mice underwent an OGTT at the end of the 2 wk and were euthanized after 2 d for liver, serum, and cecal content sample collection. The 2 groups of samples were defined as normal‐post‐food withdrawal (N‐postF) and HFD‐post‐food withdrawal (H‐postF).
For OGTT, a glucose solution (1.5 g/kg) was orally administered to the mice after they were unfed for 6 h. Glucose levels were measured in tail vein blood samples by using a glucose analyzer (OneTouch Ultra; LifeScan, Johnson & Johnson, Milpitas, CA, USA) at 0, 15, 30, 60, and 120 min after the glucose load.
For sample collection, mice were unfed overnight and then euthanized for blood harvesting. The blood was centrifuged at 3000 g for 15 min at 4°C for serum collection. Liver and cecal contents were carefully collected and kept in liquid nitrogen before transfer to a —80°C freezer for storage.
Serum metabolite assessment
Serum metabolites were profiled by using the protocols of “Metabolite profile” and “Bile acid quantitation” established by our laboratory based on gas chromatography‐time‐of‐flight mass spectrometry and ultra‐performance liquid chromatography‐triple‐quadrupole mass spectrometry. Detailed methods are provided in the Supplemental Data.
Cecal microbiota assessment
Seven cecal content samples from each of the 4 groups (N‐preF, N‐postF, H‐preF, and H‐postF) were randomly selected for microbiota 16S rRNA analysis. Microbial genome DNA was extracted from these samples by using a QiaAmp DNA stool Mini Kit (51504; Qiagen, Germantown, MD, USA) and following the manufacturer's protocol. Successful DNA isolation was confirmed by using agarose gel electrophoresis.
The V4–V5 hypervariable regions of 16S rRNA were PCR amplified from the microbial genomic DNA harvested from the cecal samples and were used for the remainder of the study. PCR amplification of the V4–V5 region of bacterial 16S rRNA genes was performed by using the forward primer 515F (5′‐GTG‐CCAGCMGCCGCGGTAA‐3′) and the reverse primer 907R (5 ‘‐CCGTCAATTCMTTTRAGTTT‐3′). Sample‐specific 7‐bp barcodes were incorporated into the primers for multiplex sequencing. The PCR conditions were as follows: 1 predenaturation cycle at 94°C for 4 min, 25 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 45 s, and elongation at 72°C for 30 s, with 1 postelongation cycle at 72°C for 5 min. The PCR amplicon products were separated on 0.8% agarose gels and then extracted. Only PCR products without primer dimers and contaminant bands were used for sequencing by synthesis. Bar‐coded V4‐V5 amplicons were sequenced by using the paired‐end method of the MiSeq System (Illumina, Inc, San Diego, CA) with a 6‐cycle index read. Sequences with an average phred score <25, ambiguousbases, homopolymer runs exceeding 6 bp, primer mismatches, or sequence lengths shorter than 100 bp were removed. Only sequences with an overlap longer than 10 bp and without any mismatch were assembled according to their overlap sequence. Reads that could not be assembled were discarded. Barcode and sequencing primers were trimmed from sequence reads.
Bacterial operation taxonomic units (OTUs) were counted for each sample to express the richness of bacterial species with an identity cutoff of 97%. The OTU abundance of each sample was generated at the species level. The mean length of all effective bacterial sequences without primers was 223 bp. Bacterial OTUs were generated by using the uclust function in the Quantitative Insights Into Microbial Ecology (QIIME; http://qiime.org/scripts/pick_otus.html) database. Taxon‐dependent analysis was conducted by using the Greengene Database (19). Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUST) was used to identify differences in predictive metagenome function (20). Functions were predicted from the normalized OTUs for KEGG orthologs.
Statistical analysis
Taxonomy abundance was normalized to the summation of OTUs in each sample. A phylogenetic tree was constructed based on the 16S sequence alignment using FastTree in Mothur (https://www.mothur.org/). Unweighted UniFrac was run using the resulting tree, and principal coordinate analysis (PCOA) was performed on the resulting matrix. Partial least squares discrimination analysis (PLS‐DA) and orthogonal partial least squares discriminant analysis were conducted in the Simca‐P 13.0 Software package (Umetrics, Umea, Sweden). Mann‐Whitney U tests and Spearman correlation were performed by using SPSS v.13.0 software (IBM SPSS Statistics, IBM Corp., Armonk, NY, USA). Data are expressed as means ± sem. Significance is established at P < 0.05.
RESULTS
Effects of food withdrawal on body weight, liver weight, and glucose tolerance
The experimental procedure is outlined in Fig. 1A . The mice were fednormalchow(normal group) or HFD(HFD group) for 6 wk, followed by a 4‐d 50% food withdrawal on normal chow, a 4‐d complete food withdrawal, and another 4‐d50% food withdrawal on normal chow. Three days into the complete food withdrawal, 2 mice in the normal group had significant body weight loss and physical weakening, and thus were euthanized. The rest of the mice were in good condition during food withdrawal. To observe the long‐lasting effect of food withdrawal, and to eliminate the confounding effect of stress response immediately after food withdrawal, all mice had a 2‐wk recovery with access to normal chow ad libitum before the post‐food withdrawal time point. The body weight (Fig. 1B ) of the animals in both the normal and HFD groups decreased during the first 50% food withdrawal and the complete food withdrawal periods, stabilized during the second 50% food withdrawal period, and increased after being given access to normal diet ad libitum. The body weight of the HFD group was significantly higher than that of the normal group from 4 wk into the HFD treatment until the end of the second 50% food withdrawal. During the first week of the free‐feeding recovery, HFD mice gained an average of 5.2 g in body weight, which was significantly lower than the 7.7‐g body weight gain in normal mice (P < 0.05). At the end of the 2‐wk recovery, the 2 groups had similar body weight.
Figure 1.
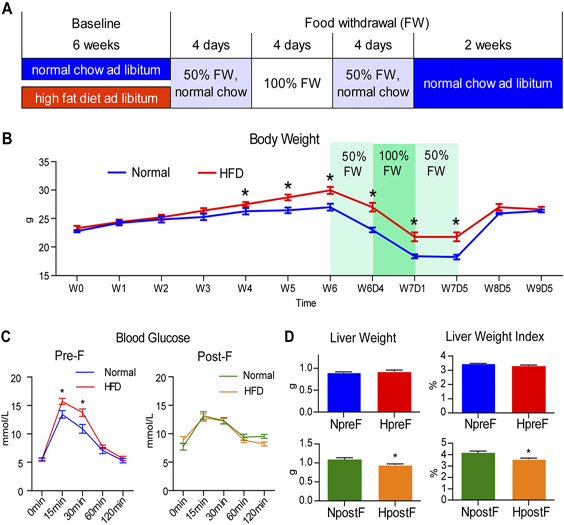
Experimental outline and the effects of food withdrawal on body weight, liver weight, and glucose tolerance. A) Outline of animal treatments, which consisted of a 6‐wk ad libitum normal chow (for normal mice) or HFD (for obese mice) treatment at baseline, a 4‐d 50% food withdrawal (FW) with normal chow, a 4‐d complete FW, another 4‐d 50% FW with normal chow, and a 2‐wk recovery with ad libitum access to normal chow. The end of the 6‐wk baseline treatment was defined as the pre‐food withdrawal time point, and the end of the 2‐wk recovery was defined as the post‐food withdrawal time point. B) Body weight of the normal and HFD groups throughout the experiment. C) The blood glucose levels during the OGTT (0, 15, 30, 60, and 120 min postloading) of the animals in the normal and HFD groups before and after food withdrawal. D) Liver weight and liver weight index (liver weight/body weight × 100) of the animals in the normal and HFD groups before and after food withdrawal. Data are expressed as means ± sem. Group differences were assessed by using the Mann‐Whitney U test. *P < 0.05 when comparing normal and HFD groups.
The OGTT was performed before food withdrawal and after the 2‐wk recovery (Fig. 1C). Before the food withdrawal, the blood glucose level of the HFD mice was significantly higher than that of the normal group at 15 and 30 min postloading, implying impaired glucose tolerance in the HFD group. The 2 groups had similar OGTT results after the 2‐wk recovery. The liver weight and the liver weight index (liver weight/body weight × 100) were similar between the 2 groups before food withdrawal but were lower in the HFD group than in the normal group after the 2‐wk recovery (Fig. 1D ). Thus, the food withdrawal intervention normalized body weight and glucose tolerance and decreased liver weight in HFD mice.
Effects of food withdrawal on cecal microbiota
The impact of food withdrawal on cecal microbiota composition in the normal and HFD groups was evaluated by using 16S rRNA gene sequencing. To visualize the group differences in bacterial taxa composition, the β‐diversity of microbial composition was calculated by using unweighted PCOA. The PCOA plot (Fig. 2A) shows that at baseline, the N‐preF and N‐postF groups were separated along the PC2 axis, and the H‐preF and H‐postF groups were separated along the PC1 axis; however, at the conclusion of the food withdrawal intervention, N‐postF and H‐postF converged to the same position. Additional PCOA plots comparing N‐preF vs. N‐postF and H‐preF vs. H‐postF can be found in Supplemental Figure S1A, B. We further evaluated interindividual similarity at the phylum level using the hierarchical clustering of the samples from the 4 groups (Fig. 2B ). Samples from the N‐preF and H‐preF groups not only completely separated from each other but also separated from the N‐postF and H‐postF groups (although N‐postF and H‐postF overlapped). Figure 2A, B shows that the food withdrawal dramatically shifted the microbial composition in both normal and HFD mice, and all mice acquired similar microbiome composition after food withdrawal, regardless of their dietary and metabolic patterns at baseline.
Figure 2.
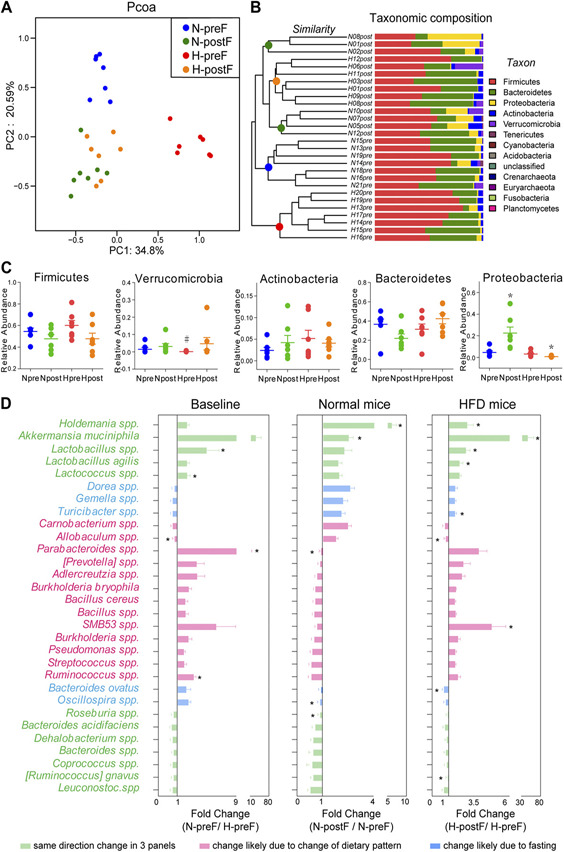
Effects of food withdrawal on cecal microbiota. A) PCOA plot generated by using microbiota OTU metrics based on the Bray‐Curtis similarity for the N‐preF, N‐postF, H‐preF, and H‐postF groups (n = 7/group). B) The hierarchical cluster based on the Bray‐Curtis similarity of the samples from the N‐preF, N‐postF, H‐preF, and H‐postF groups. The bar plot shows the abundance of each phylum in each sample. C) The 5 most abundant phyla in the N‐preF, N‐postF, H‐preF, and H‐postF groups. Data are expressed as means ± sem. Group differences were assessed by using the Mann‐Whitney U test. *P < 0.05 when comparing N‐postF vs. N‐preF, or H‐postF vs. H‐preF, #P < 0.05 when comparing N‐preF vs. H‐preF. D) Fold changes of the annotated microbes at the genus or species level. Data are expressed as means ± sem. Group differences were assessed by using the Mann‐Whitney U test. *P < 0.05.
Population analyses revealed that the bacterial population in the cecum was dominated by Firmicutes, Bacteroidetes, Proteobacteria, Actinobacterh, and Verrucomicrobia, and the abundance of these 5 phyla was compared between groups (Fig. 2C ). Food withdrawal increased the abundance of Proteobacteria in normal mice but decreased that in HFD mice. We further studied the group differences at the genus and species levels. Thirty microbes annotated at genus and species levels were selected because there was no 0 value in their relative abundance across all of these samples. Figure 2D illustrates the fold change (FC) of N‐preF/ H‐preF (baseline difference), N‐postF/N‐preF (food withdrawal‐induced changes in normal mice), and H‐postF/H‐preF (food withdrawal‐induced changes in HFD mice). The green bars show microbes with same‐direction FC in all 3 comparisons, implicating healthy diet and food withdrawal–induced similar changes in these microbes; Holdemania species, Akkermansia muciniphila, and Lactobacillus species had the highest FC. Strikingly, food withdrawal increased the abundance of A. muciniphila in HFD mice. The pink bars show same‐direction FC between N‐preF/H‐preF and H‐postF/H‐preF, implicating changes possiblymainlyinducedbydietarypattern, withParabacteroides species and SMB53 species having the highest FC. The blue bars show the same‐direction FC between N‐postF/N‐preF and H‐postF/H‐preF, suggesting changes likely due to food withdrawal, with Turicibacter species and Bacteroides ovatus having significant FC, albeit relatively small.
Effects of food withdrawal on serum metabolome
The metabolome profiling was conducted on gas chromatography‐time‐of‐flight mass spectrometry and ultra‐performance liquid chromatography‐triple‐quadrupole mass spectrometry platforms. A total of 116 metabolites were identified, including bile acids, fatty acids, amino acids, and carbohydrates (Supplemental Table S2). The PLS‐DA scores plot (Fig. 3A ) shows obvious separations between N‐preF and N‐postF, and between H‐preF and H‐postF, with the latter seemingly farther apart than the former. Additional orthogonal partial least squares discriminant analysis score plots comparing N‐preF vs. N‐postF and H‐preF vs. H‐postF can be found in Supplemental Fig. S2A, B.
Figure 3.
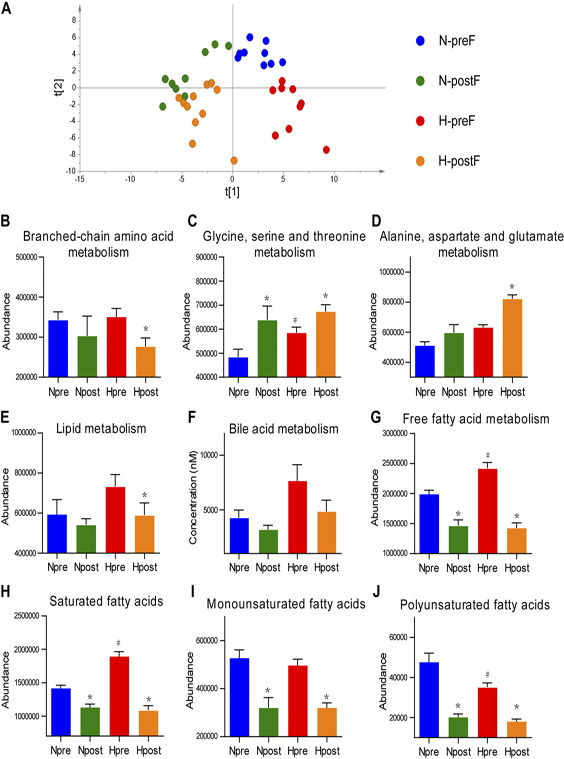
Effects of food withdrawal on the serum metabolome. A) The PLS‐DA score plot of metabolites generated by using the metabolite intensity from the N‐preF (n = 9), N‐postF (n = 9), H‐preF (n = 9), and H‐postF (n = 11) groups. B–J) The abundance of metabolite pathways from the N‐preF, N‐postF, H‐preF, and H‐postF groups. Data are expressed as means ± sem. Group differences were assessed by using the Mann‐Whitney U test. *P < 0.05 when comparing N‐postF vs. N‐preF, or H‐postF vs. H‐preF, #P < 0.05 when comparing N‐preF vs. H‐preF.
We further evaluated the effect of food withdrawal on host metabolic alteration in terms of abundance of metabolic pathways. The abundance of 1 metabolic pathway of 1 sample was the sum of the abundance of detected metabolites typically found in that pathway. The results showed that food withdrawal decreased the abundance of branched chain amino acid (BCAA) metabolism (Fig. 3B ) but increased those of glycine, serine, and threonine metabolism (Fig. 3C) and alanine, aspartate, and glutamate metabolism (Fig. 3D ). Food withdrawal also decreased the abundance of lipid metabolism (Fig. 3E ), bile acid metabolism (Fig. 3F ), and free fatty acid (FFA) metabolism (Fig. 3G ). We further categorized FFAs into saturated fatty acid (SFA) (Fig. 3H ), monounsaturated fatty acid (MUFA) (Fig. 3I ), and polyunsaturated fatty acid (PUFA) (Fig. 3J ) and found that food withdrawal decreased the abundance of all 3 subcategories of FFAs. The food withdrawal– induced decreases in BCAA, lipids, and FFA were more pronounced in the HDF group than in the normal group.
Effects of food withdrawal on potential microbial function
We analyzed the potential function of gut microbiota based on combined KEGG pathways and PICRUSt analysis. Group differences in microbial function related to metabolism of amino acids, lipids, bile acids, and FFA are shown in Fig. 4A. Food withdrawal significantly decreased the abundance of alanine, aspartate, and glutamate metabolism in the microbiome of HFD mice, which was opposite the change in metabolite abundance in the host serum metabolome. Food withdrawal also decreased gene abundance in BCAA biosynthesis and increased that in BCAA degradation in the microbiome of HFD mice (although these changes were not statistically significant). These changes coincided with the decreased metabolite abundance of BCAA metabolism in host serum. Furthermore, food withdrawal decreased (albeit nonsignificantly) gene abundance in lipid and bile acid metabolism in the microbiome, regardless of baseline dietary pattern of the mice, which seemed to be in line with the changes in host metabolite abundance in these pathways. Food withdrawal in HFD mice consistently decreased the abundance of potential functions related to disease, particularly those related to neurodegenerative diseases, metabolic diseases, and cancers. Conversely, food withdrawal in normal mice seemed to increase the abundance of the disease‐related genes, but the changes were not statistically significant.
Figure 4.

Cecal microbiota functional changes and correlations between the host metabolome and cecal microbiome. A) Microbial PICRUSt‐predicted KEGG functions relevant to metabolism and diseases. Data are expressed as means ± sem. Group differences were assessed by using the Mann‐Whitney U test. *P < 0.05 when comparing N‐postF vs. N‐preF, or H‐postF vs. H‐preF. B) Spearman correlations of the relative abundance of cecal microbial genus or species and the abundance of metabolite pathways in host serum (n = 28). The r values are represented by gradient colors, with red cells indicating positive correlations and blue cells indicating negative correlations. *P < 0.05.
Correlations between microbiota composition and host metabolism
We further correlated the relative abundance of cecal microbes with the abundance of metabolic pathways in host serum (Fig. 4B ). Several microbes, such as Holdemania species, Streptococcus species, SMB53, and Ruminococcus gnavus, significantly correlated with multiple metabolic pathways in the host. Several host metabolic pathways, such as those of BCAA, bile acid, and FFA, also significantly correlated with the abundance of multiple microbes. These correlations suggest that the structure of cecal microbiota is closely related to host metabolism.
DISCUSSION
Short‐term food withdrawal or intermittent food withdrawal (reduced meal frequency) has been shown to extend lifespan and increase resistance to aging‐related diseases in rodents and monkeys, as well as improve the health of obese or overweight people (1, 5, 21, 22). We designed the animal experiments to simulate 2 conditions under which people may use food withdrawal as a lifestyle intervention. The first condition is that lean and healthy people try to improve fitness through prolonged food withdrawal. The second condition is that people who are used to an HFD, and have obesity and other metabolic diseases, try to improve their health through prolonged food withdrawal. Under the latter condition, people most likely will also adapt to a healthy diet before and after food withdrawal. We hypothesized that food withdrawal would profoundly change microbiota composition and function, and host metabolism thus would have a long‐lasting effect. We therefore allowed a 2‐wk free‐feeding recovery before evaluating the effect of food withdrawal in mice, which not only avoided potential confounding effects of food withdrawal‐related stress responses but also revealed relatively long‐term effects of food withdrawal. The duration of food withdrawal was determined based on veterinarian consultation and a thorough review of previously published mouse studies (Supplemental Table S1). Fifty percent food withdrawal was used to gradually start and finish the food withdrawal intervention to allow time for metabolic adaptation. All mice were individually housed in clean cages to eliminate cannibalism, as well as to reduce residual chow contamination and coprophagy, thus ensuring the accuracy of food withdrawal.
Our results suggested potential negative effects of relatively long‐term food withdrawal on normal lean mice. Three days of complete food withdrawal after 4 d of 50% food withdrawal resulted in an 18% mortality (eutha‐nization) in normal mice, indicating that severe food withdrawal may impose a considerable level of risk in lean people. Figure 2C shows that relatively long‐term food withdrawal significantly increased the abundance of Proteobacteria in cecal microbiota in normal mice. Many pathogenic genera, including Escherichia, Salmonella, Vibrio, Helicobacter, and Yersinia, are found under the Pro‐teobacteria phylum (23). Figure 4A also illustrates a trend that relatively long‐term food withdrawal seemed to increase the abundance of disease‐related genes in the microbiota of normal mice. Therefore, the risk of severe food withdrawal for lean people may primarily be in the changes of microbiota. All HFD mice successfully endured the severe food deprivation and were morbidity‐free during the food withdrawal, possibly due to their ample energy reserve in fat tissue. Food withdrawal normalized body weight and glucose tolerance in the HFD mice, demonstrating this intervention had significant metabolic benefit in these mice.
Food withdrawal drastically increased the abundance of A. muciniphila in HFD mice, compared with that in normalmice(Fig. 2D ). A. muciniphila is a mucin‐degrading bacterium that resides in the mucus layer and regulates the cross‐talk between the host and gut microbiota (24). A. muciniphila has been reported to prevent fat mass gain (24, 25). It also mitigates metabolic endotoxemia‐induced inflammation (26), mediates negative effects of IFN‐γ on glucose metabolism (27), and protects against adipose tissue inflammation and insulin resistance (24, 25). A recent study showed that A. muciniphila had the capability to predict personal effects of caloric restriction treatment (28), and individuals with higher baseline abundance of A. muciniphila before caloric restriction were associated with better clinical outcomes after caloric restriction. Food withdrawal also significantly increased the abundance of Lactobacillus species in HFD mice. Lactobacillus species dominate murine microbiota under a chow diet but are suppressed by HFD, which explains the fact that at baseline, the abundance of Lactobacillus species in normal mice was higher than that in HFD mice. The abundance of Lactobacillus species was found to be negatively correlated with fat mass gain (29). Lactobacillus strains, such as Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, are used as probiotics and found to reduce HFD‐induced obesity, insulin resistance, and inflammation (30, 31). Food withdrawal significantly increased the abundance of Holdemania species in both normal and HFD mice. Holdemania species was found to be associated with being lean in Japanese men (32) but also correlated with clinical indicators of impaired lipid and glucose metabolism (33).
Notably, food withdrawal significantly decreased the abundance of metabolites in the BCAA metabolic pathway in HFD mice (Fig. 3B ), which may contribute to the improvement in glucose tolerance. A number of studies have reported that circulating levels of BCAA are increased in obese individuals and are associated with worse metabolic health and future insulin resistance or type 2 diabetes mellitus (34, 35). BCAAs directly promote insulin resistance, possibly via disruption of insulin signaling in skeletal muscle. The underlying cellular mechanisms may include activation of the mammalian target of rapamycin complex 1, JUN, and insulin receptor substrate‐1 signaling pathways in skeletal muscle (36–38). Decreased abundance of BCAA metabolism in the circulation of HFD mice after food withdrawal intervention might therefore have contributed to restoring insulin sensitivity and improving glucose tolerance in these mice.
Food withdrawal also decreased the abundance of FFA metabolism, including those of SFAs, MUFAs, and PUFAs in serum, regardless of baseline diet, possibly because these metabolites were used by host tissues for energy production during food withdrawal (39). Reportedly, SFAs were much less mobilizable from adipose tissue to blood than PUFAs (40), and therefore PUFAs might have been depleted faster than SFAs from fat tissue during food withdrawal; how this differential mobilization rate of FFAs might have (possibly transiently) affected mouse metabolism is unknown. Although MUFAs and PUFAs are commonly known for their beneficial effects on metabolic health, unsaturated fatty acids also induce syntheses of proinflammatory prostaglandins and leukotrienes, and elevated levels of PUFAs may contribute to obesity‐associated inflammation (41, 42). A recent study from our group found that ~2 dozen of mono‐ or poly‐unsaturated FFA (represented by C20:3 n6) increased in obese subjects with metabolic disorders compared with metabolically healthy lean or obese control subjects, and these increases were normalized after caloric restriction or metabolic surgery (43). More in‐depth studies on MUFA and PUFA during food withdrawal are required to understand the metabolic consequences associated with their changes.
SFAs are generally associated with detrimental effects on health, including promoting inflammation, insulin resistance, and hyperglycemia (44–46). Food withdrawal decreased the abundance of SFA metabolism more in HFD mice than in normal mice, and this change may have contributed to the metabolic improvement in these mice after food withdrawal.
The results also showed that the level of lactate (Supplemental Fig. S3) was increased after food withdrawal, although without statistical significance. This observation was consistent with a recent study reporting that intermittent food withdrawal treatment resulted in a shift in the gut microbiota composition, leading to elevation of the fermentation product lactate and to the selective up‐regulation of monocarboxylate transporter 1 expression in beige cells (47).
These food withdrawal‐induced changes in host metabolism were at least partially due to changes in micro‐biota. For example, abundance of host metabolites in FFA metabolism had strong inverse correlations with the abundance of A. muciniphila and species of Holdemania, Lactococcus, Lactobacillus, and SMB53 in microbiota (Fig. 4B ), and food withdrawal significantly increased the abundance of these 5 bacterial species in HFD mice (Fig. 2D ). Similar situations can also be found for host metabolites in BCAA metabolism.
There were several limitations to the present study (1). The experiment was designed to mimic the real‐world situation when food withdrawal and dietary change most likely co‐occur. The purpose of the experiment was to evaluate the collective effects of these lifestyle changes. We are cognizant that such experimental design did not allow us to tease apart the effects of caloric restriction from those of dietary change. In future studies, we will consider treating HFD mice with normal chow, with and without concurrent caloric restriction, to separate the effects of dietary change and caloric restriction (2). Food withdrawal significantly decreased liver weight in HFD mice. We did not perform further analysis in the liver, and therefore what had changed in the liver and whether these changes contributed to metabolic improvement in the HFD mice remain unknown (3). To further delineate the relationship between microbiome and metabolome, and their separate, additive, or synergistic effects on metabolic health, we will also consider use of germ‐free or commensal depleted animal models to study food withdrawal (4). The human metabolic system is far more complex than that of mice, and results generated from this mouse study therefore only reflect food withdrawal‐induced microbiota and metabolism alterations in mice, not in humans. Carefully designed clinical studies are required to reliably evaluate the effects of prolonged food withdrawal in people with different BMIs, dietary habits, and metabolic health status.
Taken together, the data indicate that the food withdrawal intervention had dramatic effects on cecal micro‐biota composition and host metabolism in mice, especially in HFD mice. Mice treated with HFD at baseline had normalized body weight and glucose tolerance and decreased liver weight after food withdrawal. Microbial‐predicted function showed that food withdrawal significantly decreased the abundance of microbial genes associated with diseases in HFD mice but indicated a trend to increase that in normal mice. All HFD mice survived food withdrawal, but 2 normal mice failed to endure the relatively long‐term food deprivation. Therefore, this study supports our hypothesis that food withdrawal is safer and more beneficial for obese mice than for lean mice, and the beneficial effects are associated with changes in gut microbiota composition and function, which correlate with host metabolism.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31500954, 31501079, and 81772530) and the International Science and Technology Cooperation Program of China (2014DFA31870). The authors declare no conflicts of interest.
Biographies
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
X. Zheng, K. Zhou, Y. Zhang, and Wei Jia designed the study; X. Zheng, Y. Zhang, X. Han, and F. Huang performed the animal study and collected samples; X. Zheng, A. Zhao, J. Liu, C. Qu, and K. Ge acquired the data for analyses; X. Zheng and T. Chen analyzed the data; X. Zheng and K. Zhou drafted the manuscript; and B. Hernandez, H. Yu, J. Panee, Weiping Jia, and Wei Jia critically revised the manuscript.
Zheng, X. , Zhou, K. , Zhang, Y. , Han, X. , Zhao, A. , Liu, J. , Qu, C. , Ge, K. , Huang, F. , Hernandez, B. , Yu, H. , Panee, J. , Chen, T. , Jia, W. , Jia, W. Food withdrawal alters the gut microbiota and metabolome in mice. FASEB J. 32, 4878–4888 (2018). www.fasebj.org
These authors contributed equally to this work.
Contributor Information
Xiaojiao Zheng, Email: joyzheng99@sjtu.edu.cn.
Wei Jia, Email: wjia@cc.hawaii.edu.
REFERENCES
- 1. Longo, V. D. , and Mattson, M. P. (2014) Fasting: molecular mechanisms and clinical applications. CellMetab. 19, 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pregadio, F. (2008) Bigu abstention from cereals. In The Encyclopedia of Taoism, pp. 233–234, Routledge, London: [Google Scholar]
- 3. Eisen, M. Chinese Bigu for Yang Sheng. Accessed October 15, 2011, at http://yang-sheng.com/?tag=bigu
- 4. Choi, I. Y. , Piccio, L. , Childress, P. , Bollman, B. , Ghosh, A. , Brandhorst, S. , Suarez, J. , Michalsen, A. , Cross, A.H. , Morgan, T.E. , Wei, M. , Paul, F. , Bock, M. , and Longo, V. D. (2016) Adiet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 15, 2136–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brandhorst, S. , Choi, I. Y. , Wei, M. , Cheng, C. W. , Sedrakyan, S. , Navarrete, G. , Dubeau, L. , Yap, L. P. , Park, R. , Vinciguerra, M. , Di Biase, S. , Mirzaei, H. , Mirisola, M. G. , Childress, P. , Ji, L. , Groshen, S. , Penna, F. , Odetti, P. , Perin, L. , Conti, P.S. , Ikeno, Y. , Kennedy, B. K. , Cohen, P. , Morgan, T. E. , Dorff, T. B. , and Longo, V. D. (2015) A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 22, 86–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Longo, V. D. , and Panda, S. (2016) Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 23, 1048–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee, C. , Safdie, F.M. , Raffaghello, L. , Wei, M. , Madia, F. , Parrella, E. , Hwang, D. , Cohen, P. , Bianchi, G. , and Longo, V. D. (2010) Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 70, 1564–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee, C. , Raffaghello, L. , Brandhorst, S. , Safdie, F. M. , Bianchi, G. , Martin-Montalvo, A. , Pistoia, V. , Wei, M. , Hwang, S. , Merlino, A. , Emionite, L. , de Cabo, R. , and Longo, V. D. (2012) Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 4, 124ra27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raffaghello, L. , Lee, C. , Safdie, F.M. , Wei, M. , Madia, F. , Bianchi, G. , and Longo, V. D. (2008) Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. USA 105, 8215–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Safdie, F. M. , Dorff, T. , Quinn, D. , Fontana, L. , Wei, M. , Lee, C. , Cohen, P. , and Longo, V. D. (2009) Fasting and cancer treatment in humans: a case series report. Aging (Albany N.Y.) 1, 988–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stice, E. , Davis, K. , Miller, N. P. , and Marti, C. N. (2008) Fasting increases risk for onset of binge eating and bulimic pathology: a 5-year prospective study. J. Abnorm. Psychol. 117, 941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng, M. F. , Rozin, P. , and Teitelbaum, P. (1971) Starvation retards development of food and water regulations. J. Comp. Physiol. Psychol. 76, 206–218 [DOI] [PubMed] [Google Scholar]
- 13. Placanica, J.L. , Faunce, G.J. , and Soames Job, R.F. (2002) The effect of fasting on attentional biases for food and body shape/weight words in high and low eating disorder inventory scorers. Int. J. Eat. Disord. 32, 79–90 [DOI] [PubMed] [Google Scholar]
- 14. Laessle, R. G. , Schweiger, U. , and Pirke, K. M. (1988) Depression as a correlate of starvation in patients with eating disorders. Biol. Psychiatry 23, 719–725 [DOI] [PubMed] [Google Scholar]
- 15. Kohl, K. D. , Amaya, J. , Passement, C. A. , Dearing, M. D. , and McCue, M. D. (2014) Unique and shared responses of the gut microbiota to prolonged fasting: a comparative study across five classes of vertebrate hosts. FEMS Microbiol. Ecol. 90, 883–894 [DOI] [PubMed] [Google Scholar]
- 16. Selman, C. , Kerrison, N. D. , Cooray, A. , Piper, M. D. , Lingard, S. J. , Barton, R.H. , Schuster, E.F. , Blanc, E. , Gems, D. , Nicholson, J.K. , Thornton, J. M. , Partridge, L. , and Withers, D. J. (2006) Coordinated multitissue transcriptional and plasma metabonomic profiles following acute caloric restriction in mice. Physiol. Genomics 27, 187–200 [DOI] [PubMed] [Google Scholar]
- 17. Richards, S. E. , Wang, Y. , Lawler, D. , Kochhar, S. , Holmes, E. , Lindon, J. C. , and Nicholson, J. K. (2008) Self-modeling curve resolution recovery of temporal metabolite signal modulation in NMR spectroscopic data sets: application to a life-long caloric restriction study in dogs. Anal. Chem. 80, 4876–4885 [DOI] [PubMed] [Google Scholar]
- 18. Li, G. , Xie, C. , Lu, S. , Nichols, R. G. , Tian, Y. , Li, L. , Patel, D. , Ma, Y. , Brocker, C.N. , Yan, T. , Krausz, K.W. , Xiang, R. , Gavrilova, O. , Patterson, A. D. , and Gonzalez, F. J. (2017) Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 26, 672–685.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeSantis, T. Z. , Hugenholtz, P. , Larsen, N. , Rojas, M. , Brodie, E. L. , Keller, K. , Huber, T. , Dalevi, D. , Hu, P. , and Andersen, G.L. (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Langille, M. G. , Zaneveld, J. , Caporaso, J. G. , McDonald, D. , Knights, D. , Reyes, J. A. , Clemente, J. C. , Burkepile, D. E. , Vega Thurber, R. L. , Knight, R. , Beiko, R. G. , and Huttenhower, C. (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31, 814–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei, M. , Brandhorst, S. , Shelehchi, M. , Mirzaei, H. , Cheng, C. W. , Budniak, J. , Groshen, S. , Mack, W.J. , Guen, E. , Di Biase, S. , Cohen, P. , Morgan, T.E. , Dorff, T. , Hong, K. , Michalsen, A. , Laviano, A. , and Longo, V. D. (2017) Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mattison, J. A. , Colman, R. J. , Beasley, T. M. , Allison, D. B. , Kemnitz, J. W. , Roth, G. S. , Ingram, D. K. , Weindruch, R. , de Cabo, R. , and Anderson, R.M. (2017) Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 8, 14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Madigan, M. , and Martinko, J. (2005) Brock Biology of Microorganisms, Prentice Hall, Upper Saddle River, NJ, USA: [Google Scholar]
- 24. Everard, A. , Belzer, C. , Geurts, L. , Ouwerkerk, J. P. , Druart, C. , Bindels, L. B. , Guiot, Y. , Derrien, M. , Muccioli, G. G. , Delzenne, N. M. , de Vos, W.M. , and Cani, P.D. (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 110, 9066–9071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schneeberger, M. , Everard, A. , Gómez-Valadés, A. G. , Matamoros, S. , Ramírez, S. , Delzenne, N. M. , Gomis, R. , Claret, M. , and Cani, P. D. (2015) Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 5, 16643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li, J. , Lin, S. , Vanhoutte, P. M. , Woo, C. W. , and Xu, A. (2016) Akkermansia muciniphila protectsagainst atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe-/- mice. Circulation 133, 2434–2446 [DOI] [PubMed] [Google Scholar]
- 27. Greer, R. L. , Dong, X. , Moraes, A. C. , Zielke, R. A. , Fernandes, G. R. , Peremyslova, E. , Vasquez-Perez, S. , Schoenborn, A. A. , Gomes, E. P. , Pereira, A. C. , Ferreira, S. R. , Yao, M. , Fuss, I. J. , Strober, W. , Sikora, A.E. , Taylor, G.A. , Gulati, A.S. , Morgun, A. , and Shulzhenko, N. (2016) Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism. Nat. Commun. 7, 13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dao, M. C. , Everard, A. , Aron-Wisnewsky, J. , Sokolovska, N. , Prifti, E. , Verger, E. O. , Kayser, B. D. , Levenez, F. , Chilloux, J. , Hoyles, L. , Dumas, M. E. , Rizkalla, S. W. , Dore, J. , Cani, P. D. , and Clement, K. ; MICRO-Obes Consortium . (2016) Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65, 426–436 [DOI] [PubMed] [Google Scholar]
- 29. Lecomte, V. , Kaakoush, N.O. , Maloney, C. A. , Raipuria, M. , Huinao, K D. , Mitchell, H. M. , and Morris, M. J. (2015) Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One 10, e0126931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park, D. Y. , Ahn, Y. T. , Park, S. H. , Huh, C. S. , Yoo, S. R. , Yu, R. , Sung, M. K. , McGregor, R. A. , and Choi, M.S. (2013) Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One 8, e59470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Esposito, E. , Iacono, A. , Bianco, G. , Autore, G. , Cuzzocrea, S. , Vajro, P. , Canani, R. B. , Calignano, A. , Raso, G. M. , and Meli, R. (2009) Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J. Nutr. 139, 905–911 [DOI] [PubMed] [Google Scholar]
- 32. Kobayashi, T. , Osaki, T. , and Oikawa, S. (2015) Use of T-RFLP and seven restriction enzymes to compare the faecal microbiota of obese and lean Japanese healthy men. Benef. Microbes 6, 735–745 [DOI] [PubMed] [Google Scholar]
- 33. Lippert, K. , Kedenko, L. , Antonielli, L. , Kedenko, I. , Gemeier, C. , Leitner, M. , Kautzky-Willer, A. , Paulweber, B. , and Hackl, E. (2017) Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabol c syndrome n older adults. Benef. Microbes 8, 545–556 [DOI] [PubMed] [Google Scholar]
- 34. Chen, T. , Ni, Y. , Ma, X. , Bao, Y. , Liu, J. , Huang, F. , Hu, C. , Xie, G. , Zhao, A. , Jia, W. , and Jia, W. (2016) Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep. 6, 20594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang, T. J. , Larson, M. G. , Vasan, R. S. , Cheng, S. , Rhee, E. P. , McCabe, E. , Lewis, G. D. , Fox, C.S. , Jacques, P. F. , Fernandez, C. , O'Donnell, C. J. , Carr, S. A. , Mootha, V.K. , Florez, J.C. , Souza, A. , Melander, O. , Clish, C. B. , and Gerszten, R. E. (2011) Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Newgard, C.B. , An, J. , Bain, J.R. , Muehlbauer, M.J. , Stevens, R.D. , Lien, L. F. , Haqq, A. M. , Shah, S. H. , Arlotto, M. , Slentz, C. A. , Rochon, J. , Gallup, D. , Ilkayeva, O. , Wenner, B. R. , Yancy, W. S. Jr. , Eisenson, H. , Musante, G. , Surwit, R. S. , Millington, D. S. , Butler, M. D. , and Svetkey, L. P. (2009) Abranched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patti, M. E. , Brambilla, E. , Luzi, L. , Landaker, E. J. , and Kahn, C. R. (1998) Bidirectional modulation of insulin action by amino acids. J. Clin. Invest. 101, 1519–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lynch, C.J. , and Adams, S.H. (2014) Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 10, 723–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cahill, G. F. Jr. , (2006) Fuel metabolism in starvation. Annu. Rev. Nutr. 26, 1–22 [DOI] [PubMed] [Google Scholar]
- 40. Conner, W. E. , Lin, D.S. , and Colvis, C. (1996) Differential mobilization of fatty acids from adipose tissue. J. Lipid Res. 37, 290–298 [PubMed] [Google Scholar]
- 41. Perreault, M. , Zulyniak, M. A. , Badoud, F. , Stephenson, S. , Badawi, A. , Buchholz, A. , and Mutch, D. M. (2014) A distinct fatty acid profile underlies the reduced inflammatory state of metabolically healthy obese individuals. PLoS One 9, e88539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Steffen, B. T. , Steffen, L. M. , Tracy, R. , Siscovick, D. , Hanson, N. Q. , Nettleton, J. , and Tsai, M.Y. (2012) Obesity modifies the association between plasma phospholipid polyunsaturated fatty acids and markers of inflammation: the Multi-Ethnic Study of Atherosclerosis. Int. J. Obes. 36, 797–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ni, Y. , Zhao, L. , Yu, H. , Ma, X. , Bao, Y. , Rajani, C. , Loo, L. W. , Shvetsov, Y. B. , Yu, H. , Chen, T. , Zhang, Y. , Wang, C. , Hu, C. , Su, M. , Xie, G. , Zhao, A. , Jia, W. , and Jia, W. (2015) Circulating unsaturated fatty acids delineate the metabolic status of obese individuals. EBioMedicine 2, 1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El-Assaad, W. , Buteau, J. , Peyot, M. L. , Nolan, C. , Roduit, R. , Hardy, S. , Joly, E. , Dbaibo, G. , Rosenberg, L. , and Prentki, M. (2003) Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology 144, 4154–4163 [DOI] [PubMed] [Google Scholar]
- 45. Kennedy, A. , Martinez, K. , Chuang, C. C. , LaPoint, K. , and Mcintosh, M. (2009) Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J. Nutr. 139, 1–4 [DOI] [PubMed] [Google Scholar]
- 46. Funaki, M. (2009) Saturated fatty acids and insulin resistance. J. Med. Invest. 56, 88–92 [DOI] [PubMed] [Google Scholar]
- 47. Li, G. , Xie, C. , Lu, S. , Nichols, R.G. , Tian, Y. , Li, L. , Patel, D. , Ma, Y. , Brocker, C.N. , Yan, T. , Krausz, K.W. , Xiang, R. , Gavrilova, O. , Patterson, A. D. , and Gonzalez, F. J. (2017) Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 26, 801 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material


