Abstract
Background and aims
Ibogaine is an indole alkaloid used in rituals of the African Bwiti tribe. It is also used in non‐medical settings to treat addiction. However, ibogaine has been linked to several deaths, mainly due to cardiac events called torsades des pointes preceded by QTc prolongation as well as other safety concerns. This study aimed to evaluate the cardiac, cerebellar and psychomimetic safety of ibogaine in patients with opioid use disorder.
Design
A descriptive open‐label observational study.
Setting
Department of psychiatry in a university medical center, the Netherlands.
Participants
Patients with opioid use disorder (n = 14) on opioid maintenance treatment with a lasting wish for abstinence, who failed to reach abstinence with standard care.
Intervention and measurements
After conversion to morphine‐sulphate, a single dose of ibogaine‐HCl 10 mg/kg was administered and patients were monitored at regular intervals for at least 24 hours assessing QTc, blood pressure and heart rate, scale for the assessment and rating of ataxia (SARA) to assess cerebellar side effects and the delirium observation scale (DOS) to assess psychomimetic effects.
Findings
The maximum QTc (Fridericia) prolongation was on average 95ms (range 29‐146ms). Fifty percent of subjects reached a QTc of over 500ms during the observation period. In six out 14 subjects prolongation above 450ms lasted beyond 24 hours after ingestion of ibogaine. No torsades des pointes were observed. Severe transient ataxia with inability to walk without support was seen in all patients. Withdrawal and psychomimetic effects were mostly well‐tolerated and manageable (11/14 did not return to morphine within 24 hours, DOS scores remained below threshold).
Conclusions
This open‐label observational study found that ibogaine treatment of patients with opioid use disorder can induce a clinically relevant but reversible QTc prolongation, bradycardia, and severe ataxia.
Keywords: Addiction, cardiac safety, cerebellar toxicity, detoxification, ibogaine, opioid use disorder
Introduction
Ibogaine is an active alkaloid tryptamine found in the root of Tabernanthe iboga, a shrub found in Central Africa [1]. Ibogaine is the main indole alkaloid of the rootbark extract. It is an entheogen, used in traditional coming‐of‐age rituaIs by the West African Bwiti tribe [2]. It is also used in non‐medical settings by underground providers for the treatment of addiction [3].
Ibogaine has shown some promise in the treatment of addiction, i.e. opioid and cocaine use disorder. Several case–series and small‐scale observational studies have been published, showing a variety of effects: diminished withdrawal, abstinence for varied periods of time, reduction of craving and an increase in overall wellbeing [3, 4, 5, 6]. Moreover, a meta‐analysis of animal studies showed a reduction in self administration of opiates, cocaine and ethanol and a reduction of place preference after ibogaine administration in rat and mouse models of addiction [7].
Concerns about the safety of ibogaine use have also been reported. Studies have shown ibogaine to be associated with torsades des pointes (TdP) after ingestion of ibogaine [8, 9]. In‐vitro studies show that ibogaine prolongs repolarization of cardiomyocytes through human Ether‐a‐go‐go‐related gene (hERG) channel inhibition [10, 11]. This induces lengthening of the QT interval on the electrocardiogram and increases the risk of TdP [9, 11, 12]. Furthermore, ibogaine can reduce heart rate and blood pressure, further increasing the risk for TdP [13].
As well as cardiac risks, ibogaine has been observed to produce reversible ataxia [4]. In rodents this ataxia occurs with cell death of cerebellar purkinje cells [7, 14]. Systematic observations on ataxia in humans are lacking. However, neurological examination in three cases of ibogaine treatment confirms the occurrence of transient ataxia [5]. Anecdotal evidence from case reports for other potential adverse effects of ibogaine include seizures [15], mania [16] and hallucinogen persisting perception disorder [17]. Moreover, one case of suicide during ibogaine use under supervision of an underground treatment provider has been published [18].
Despite concerns about the clinical safety of ibogaine and limited evidence for effectiveness, ibogaine treatments are offered widely across the world, often without medical supervision. The aim of the present study was to evaluate clinical safety of ibogaine in the treatment of patients with opioid use disorder (OUD). Specifically, we assessed the cardiac, cerebellar and behavioral effects of a single administration of ibogaine in patients on opioid substitution treatment during opioid detoxification. We hypothesized that treatment with ibogaine reversibly induces (1) QTc prolongation on the electrocardiogram, (2) ataxia and (3) psychomimetic behavioral changes. We also included some observations on withdrawal, to measure potential benefits and endurability.
Methods
Study design
To investigate the safety of ibogaine in vivo we conducted an open‐label observational study in patients with OUD in opioid substitution treatment (OST). The study was approved by the medical ethical review committee region in Arnhem–Nijmegen (CMO ref.no. 2014/081), and all participants provided written informed consent. The study has been registered under EUDRACT Trial Number 2014‐000354‐11. A data safety monitoring board was installed and consulted after two, seven and 14 patients. Stopping criteria were TdP, death or any other unexpected serious adverse event.
Participants
All recruited patients were diagnosed with OUD according to the DSM‐IV, and were selected from two outpatient addiction clinics (IrisZorg: Arnhem and Nijmegen). Files of 500 outpatients were inspected to approach potential participants. Of these 500 we chose to look into 130 files of patients who were psychosocially stable and on OST. These files were screened for exclusion criteria. Thirty‐six patients deemed eligible were approached to participate; 29 patients were willing and were screened (Fig. 1). Inclusion took place between October 2015 and November 2017. We aimed to recruit 15 patients, and succeeded in including 14. A post‐hoc power analysis showed that with a baseline QTc of 410 (men) and 420 (women) and an increase of 10% QTc (+ 42 ms, giving 462 ms, which is enough for prolongation) with 10 patients 80% power would be reached (α = 0.05, β = 0.2) (Supporting information) [19]
Figure 1.
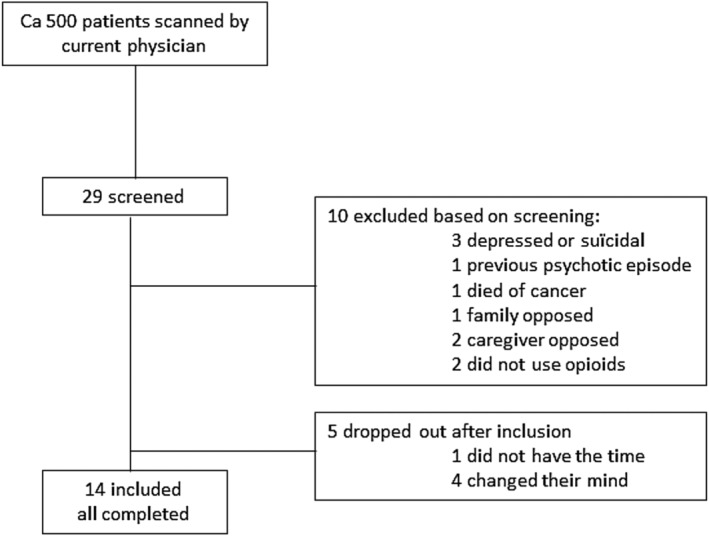
Inclusion flow‐chart.
Inclusion criteria were: 20–60 years of age, a wish for detoxification and abstinence of opioids and prior treatment failure. Exclusion criteria were a history of clinically significant cardiac disease (including ventricular fibrillation, long QT syndrome (LQTS), history of syncope, and ECG abnormalities, including QTc > 450 ms for men and > 470 ms for women), serum potassium > 5.0 mmol/l or < 3.5 mmol/l, severe liver or renal dysfunction (MDRD < 30 ml/min/1,73 m2), or pregnancy. Participants were not allowed to use QT prolonging [20] or CYP2D6 [21] affecting medication, except for methadone (see below). Patients with a history of psychotic symptoms, severe major depressive disorder or suicidality were excluded, based on the Mini‐International Neuropsychiatric Interview (MINI version 5.0.0 R) [22].
Intervention
Participants received ibogaine‐HCl 10 mg/kg orally, administered in a yoghurt mixture, at 8.30 a.m. This dosage is in the lower bound of the range of doses administered in previous studies [3, 4, 6, 13, 23, 24, 25, 26]. Ibogaine hydrochloride for human use was purchased from Phytostan enterprises (Montreal, Quebec, Canada), brand name Remogen [27]. Purity was assessed using a validated liquid chromatography assay with ultraviolet detection [high‐performance liquid chromatography (HPLC)‐UV] by the manufacturer. The purity was confirmed locally by our pharmaceutical laboratory with a validated HPLC‐UV assay and an independent reference substance at purity of 102.3%, with an expected value of 98%–102%. Our batch of ibogaine went past its shelf life in December 2017, after the treatment of the last participant. Before ibogaine administration subjects were given 20 mg of metoclopramide to prevent nausea for comfort and to ensure full ingestion [28].
Outcome measures
Sample characteristics
Age, sex and current medication use were recorded. Substance use and addiction severity were assessed using the Addiction Severity Index (ASI) [30, 31, 32, 33, 34]. The ASI covers seven domains of addiction (medical, employment/support, drug and alcohol use, legal, family/social, psychiatric) documenting life‐time substance use problems and substance use in the past 30 days. It scores the severity of problems experienced as well as a wish/request of the patient for help in these domains, on a scale from 0 to 4 [31, 32, 33, 34, 35]. Higher scores indicate more severe problems. Validation in patients with drug and alcohol use disorders show good internal consistency and reliability [30, 31]. Scoring takes approximately 30 minutes by a trained clinician.
ECG measures
QTc prolongation was assessed using twelve lead ECG measurements performed with a Philips Healthcare, multichannel TC50. QT was corrected using Fridericia's formula (QTcFr = (QT/(RR/1s)^1/3)). QT durations were measured manually by two independent researchers (T.K. and A.I.) in order to obtain a reliable estimation of the QT interval by taking the average of the V5 and II leads [36]. The correlation between the QT intervals calculated by the two researchers was moderate (r = 0.64). Furthermore, Bland–Altman analysis of the manually corrected QTc measurements showed a mean difference between measurements of researchers T.K. and A.I. of 0.89 ms. This means that the measurements were, on average, the same, and neither researcher systematically over‐ or underestimated measurements, indicating no measurement bias. The average absolute difference per measurement was 34 ms, or less than 1 mm on the electrocardiogram (ECG) paper. The average QTc‐value of the two assessors was used in the analyses.
The Philips Healthcare TC50 also provides an automated calculation of the QTc time. These values were used for medical monitoring during treatment. The correlation between the average QTc interval of the assessors and the TC50 QTc interval was strong (r = 0.71, p < 0.01 sig 2‐ tailed). The Bland–Altman plot showed a non‐significant mean difference of + 7 ms for the automatic measurement, with a standard deviation of 26 ms.
Heart rate
The heart rate measured on the ECG was used. Blood pressure was measured after each ECG assessment. A heart rate below 60 beats per minute (bpm) and systolic pressure below 90 mmHg were used as cut‐off for bradycardia and hypotension [37].
Ataxia
Cerebellar ataxia was assessed using the scale for the assessment and rating of ataxia (SARA), a structured clinical assessment applied by a trained physician [38, 39, 40]. The SARA indexes severity of ataxia, often related to cerebellar pathologies. It has eight items (maximum score): gait [8], stance [6], sitting [4], speech [6], finger‐chase test [4], nose–finger test [4], fast alternating movements [4] and a heel–shin test [4], with a total maximum score of 40. The heel–shin test was performed while standing. Higher scores indicate worse performance [38, 39, 40, 41]. The SARA has been found reliable and consistent in several large studies among a range of cerebellar diseases causing ataxia [38, 39, 40, 41].
Delirium
Psychomimetic effects were monitored using the delirium observation screening (DOS) scale, a 13‐item observational scale of verbal and non‐verbal signs of delirium [42]. A score of 3 or higher is indicative of delirium. Scoring was conducted by a trained clinician. The DOS is a reliable, commonly used instrument in many inpatient settings to check for delirium [42]. Any adverse events were noted.
Withdrawal
Withdrawal was measured using the clinical opioid withdrawal scale (COWS), a standardized test for measuring opioid withdrawal used world‐wide [24, 43]. It scores withdrawal on 11 signs and symptoms of withdrawal on a 0–4 or 5 scale. The total scores are translated to a non‐severe scale, which we translated as 0–4 for further analyses.
Study procedures
Before ibogaine treatment, all subjects were admitted to an inpatient clinic and converted from OST to oral morphine sulphate for 8 days, in order to eliminate any QT prolonging effects of methadone and homogenize baseline pharmacotherapy for all participants. Doses of morphine were administered at 4‐hourly intervals. Subjects received the last dose of morphine 4 hours prior to ibogaine administration. Withdrawal was expected to commence between 4–6 hours after the last morphine administration. Subjects were detoxified of any other drugs for at least 8 days prior to participating in the study, with the exception of tobacco. Tobacco smoking was allowed up to half an hour pre‐ingestion and 4–6 hours after ibogaine ingestion, depending on the ability to walk to a smoking area.
Baseline of all outcomes were measured 30 minutes before administration of ibogaine. K+Ca2+ and Mg2+ were checked to be within normal ranges prior to ibogaine administration. ECGs were then performed every half hour for the first 12 hours. Thereafter, ECG measurements were performed every hour in case of persistent QTc prolongation (> 450 ms for men; > 470 ms for women) or every 4 hours if automatic QTc time was shortening and below 500 ms. ECG measurements continued for 24 hours after administration. After 24 hours a cardiologist assessed if monitoring needed to continue. If QTc exceeded 500 ms, participants received a magnesium bolus infusion of 2 g in 10 minutes, followed by 2 g of magnesium over the next 10 hours for myocardial stabilization. If necessary, subjects could be transferred to the coronary care unit (CCU) for continuous cardiac monitoring.
The SARA and COWS were assessed at 2, 6, 10 and 24 hours after administration of ibogaine. The DOS scale was assessed every hour for the first 12 hours after administration of ibogaine.
Statistical analyses
Demographics, type of substitution therapy, current substance use and addiction severity were summarized using descriptive statistics.
Based on the Food and Drug Administration (FDA) guidelines (Guidance for Industry, Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non‐Antiarrhythmic Drugs), we used the following outcome measures: the difference between the QTc before administration and the maximum QTc during the observation period (ΔQTcMax) per subject, the proportion of subjects with a QTc > 500 ms at any given time and the proportion of subjects with a QTc > 500 ms and > 450 ms at each measurement [44]. During the evening and night subjects were left sleeping, if deemed safe, therefore fewer measurements were taken during the night. The measurements t27–t30 (evening), t31–39 (night) and t40–t48 (morning) were pooled and renamed ‘evening’, ‘night’ and ‘morning’.
The number of subjects developing bradycardia or hypotension and the mean maximum drop in heart rate and blood pressure during the first 12 hours were calculated [37]. The number of magnesium supplementations and adverse events such as TdP, seizures and vomiting were counted. Average total SARA scores and per‐item SARA scores were calculated at each time‐point. Our intention was to calculate the time to onset of withdrawal. COWS scores remained low, however, so the number of measurements with a non‐zero score was summarized.
As 10 participants scored zero and four scored one to two points on the DOS during the observation period, no statistical analyses were performed and psychomimetic effects were reported qualitatively.
All statistical analyses were performed using IBM SPSS statistics version 25 and Microsoft Excel.
Results
Patient characteristics, medical history, drug use and vitals are summarized in Table 1. Patients scored on average between 0 and 1 on all ASI domains except drug use, reflecting a stable psychosocial situation and a wish for abstinence. All subjects had a history of polysubstance (ab)use. Heart rate, blood pressure and QTc were within the normal range.
Table 1.
Subject characteristics and baseline measurements.
| Characteristics | n = 14 |
| Sex M/F | 12/2 |
| Methadone/buprenorphine | 12/2 |
| Age (median; 25th and 75th percentile) | 48 (44–51) |
| ASI a | Average (SD) |
| Physical | 0,85 (1,14) |
| Work | 0,46 (0,78) |
| Alcohol | 0,77 (1,48) |
| Drugs | 3,23 (1,83) |
| Judicial | 0,38 (1,12) |
| Family and social | 0,46 (0,52) |
| Psychological and emotional | 1,00 (1,22) |
| Total | 1,02 (1,16) |
| Drug use b | |
| Alcohol | 2/14 |
| Amphetamine | 0/14 |
| Benzodiazepines | 3/14 |
| Cannabis | 4/14 |
| Cocaine | 7/14 |
| Heroin | 8/14 |
| Tobacco | 13/14 |
| Baseline clinical measurements (25th and 75th percentiles) | |
| Baseline median QTc | 411 ms (387–434 ms) |
| Baseline median HR | 70 bpm (63–80 bpm) |
| Baseline mean diastolic blood pressure | 78 mmHg (75–88 mmHg) |
| Baseline mean systolic blood pressure | 129 mmHg (119–146 mmHg) |
Addiction severity index scores range from 0 to 4. A score of 0 means that the subject does not consider this domain a problem or needs no help, and a score of 4 means that the subject experiences the domain to be a big problem and would like help;
frequency of any drug use 1 month prior to detoxification. SD = standard deviation; bpm = beats per minute; HR = heart rate; M/F = male/female.
Primary outcomes
ECG changes
The main findings are presented in Table 2. The ΔQTcMax varied greatly, with a median of a 95 ms (Fig. 2). Half the participants reached a QTc of >500 ms; the proportion of subjects with a QTc > 500 ms at any given time varied between 7–21% (Fig. 3). After 24 hours, QTc was still > 450 ms in 29% of subjects (Fig. 3). No TdP were observed on ECG and no clinical signs of such were seen. QTc prolongation was highly variable over time, showing spikes up and down (Supporting information, Fig. 5). This variation also occurred in the automatic measurements (data not shown).
Table 2.
Primary and secondary outcomes.
| Outcomes | |
|---|---|
| ΔQTcMax a (median, SD, range) | 102 ms (36 ms; 40–168 ms) |
| No. of subjects with QTc > 500 ms event | 7/14 |
| No. of subjects with QTc > 450 ms after 24 hours | 6/14 |
| Magnesium infusions | 8/14 |
| No of subjects with bradycardia (< 60 bpm) | 7/14 |
| Median maximum decrease in heart rate | 9 bpm |
| No. of subjects with hypotension (systolic < 90 mmHg) | 0/14 |
| Median maximum decrease in blood pressure | 22 mmHg |
| Median and average COWS b scores | 0; 0.08 |
| No. of subjects not on morphine after 24 hours | 11/14 |
| Average maximum SARA c score | 13.7 points |
| No. of subjects with DOS d score > 2 | 0/14 |
| Subjective duration of ibogaine experience | 3–7 hours |
The maximum prolongation of QT interval from baseline, corrected for heart rate using Fridericia's formula;
clinical opioid withdrawal scale (COWS). The median and average COWS scores were used only if no reversal to morphine;
scale for the assessment and rating of ataxia;
delerium observation scale (DOS). SD = standard deviation; SARA = assessment and rating of ataxia; bpm = beats per minute.
Figure 2.
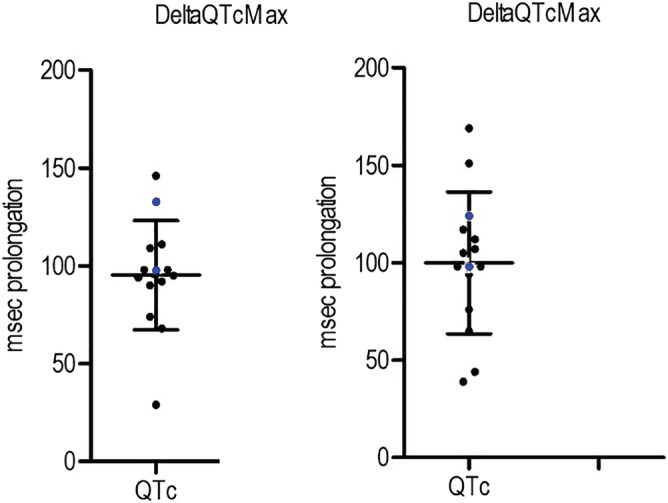
ΔQTcMax per subject with mean and standard deviation bars (102 ms; 36 ms, females in blue) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
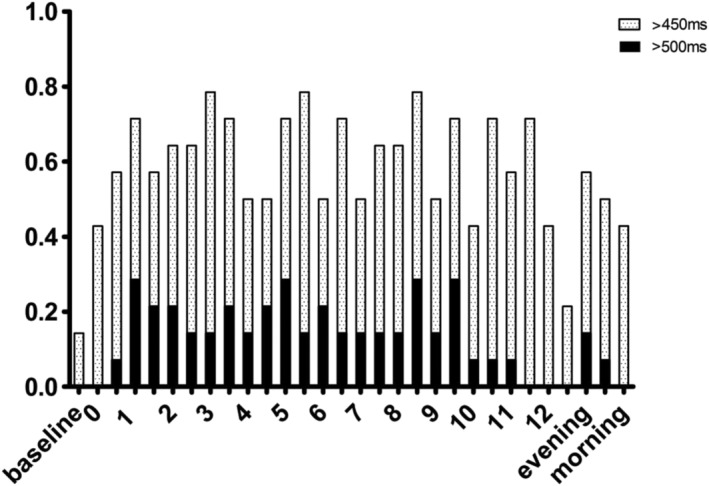
QTc prolongation proportions of subjects with a QTc time exceeding 450 and 500 ms during the first 24 hours. Evening, night‐time and morning (24 hours) measurements are grouped into their respective categories
QTc is known to be slightly longer in women, and measurements up to 470 ms are deemed normal [19]. Both women included in our study had measurements above 500 ms, with baseline measurements of 438 ms and 441 ms. Eight subjects received magnesium infusions, due to QT prolongation over 500 ms on automatic ECG measurements. No seizures occurred.
Secondary outcomes
Heart rate, blood pressure and adverse events
During the first 12 hours after administration mild bradycardia (c. 50 bpm) and a decrease in blood pressure occurred (Table 2). The only observed adverse event was vomiting, observed in two patients more than 2 hours after ibogaine ingestion.
Ataxia
The SARA scores increased from baseline to maximum in 2–6 hours after ingestion (Fig. 4). All subjects developed clinical signs of cerebellar ataxia, with full remission within 24 hours after ibogaine administration. Five patients scored above zero [1, 2] after 24 hours; they were tested again 24 hours later, with full remission of ataxia. Signs of ataxia were mainly observed in gait, standing and the heel–shin tests, with subjects needing support by a nurse to go to the bathroom (for scores per item; see Supporting information, Fig. 6).
Figure 4.
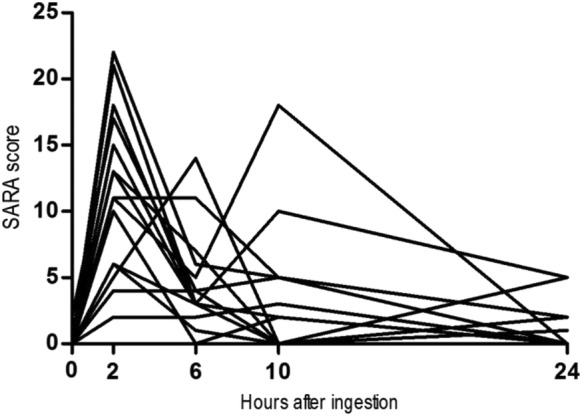
Scale for the assessment and rating of ataxia (SARA) scores. This shows the progression of SARA scores of all individuals. The highest scores are mainly in the 2–6‐hour range
Psychomimetic effects
The DOS scores were zero at baseline. In 10 subjects no delirious signs were observed; the other four participants scored one to two points during treatment. Our clinical observation was that all subjects were mostly lying quietly on their beds for c. 4–8 hours. They reported wakeful dreaming and reliving memories. One subject seemingly grabbed at items that were not there and three were not adequately spatially orientated. This experience lasted approximately 3–7 hours.
Withdrawal
Withdrawal severity remained low during the observation period of 24 hours after ibogaine ingestion for most subjects. Only five measurements gave a score of 1. Three subjects requested a return to morphine substitution based on a subjective feeling of treatment failure. No measurements of COWS or SOWS were done immediately prior to restarting morphine, as this was not part of the procedure and subjects were allowed to return to morphine unconditionally. Resumption of these 3 individuals was at 3.5, 10 and 19.5 hours after ibogaine ingestion.
Discussion
This study aimed to investigate safety of ibogaine‐HCl (10 mg/kg) in patients with OUD in OST undergoing acute opioid withdrawal. All but one patient developed a degree of QTc prolongation, with half of the patients developing a QTc exceeding 500 ms. Although reversible, this QTc prolongation is a clinically relevant cardiac safety risk, including risk of TdP, even after a relatively small dose of ibogaine [26]. Furthermore, bradycardia and decreased blood pressure were observed, as well as transient ataxia. During the first 24 hours, subjects experienced mostly mild withdrawal and transient psychomimetic effects, which were well‐tolerated. Three out of fourteen subjects returned to morphine treatment within this timeframe.
The observed QTc prolongation is in agreement with case reports of subjects admitted to emergency departments after ibogaine ingestion. A QTc of > 500 ms is associated with a large increase [odds ratio (OR) = 11.2, 95% confidence interval (CI) = 4.6–27] in the risk of an adverse cardiovascular disorder in patients presenting with a drug overdose to the emergency room (ER) [45]. The patients in these case reports showed a prolonged QTc, together with ventricular fibrillation, TdP or both [9]. Other illicit substances or pre‐existing cardiac pathology appeared relevant in some of these patients. Our study shows that in a well‐controlled experimental setting, ibogaine produces a clinically relevant QTc‐prolongation in patients without pre‐existing cardiac abnormalities. This indicates that administration of ibogaine should be restricted to well‐controlled settings with strict cardiac monitoring.
In‐vitro studies strongly suggest that the QTc prolonging effect of ibogaine and its metabolite noribogaine results from inhibition of cardiac hERG‐potassium channels [9, 10, 11]. As hERG channels are crucial for cardiac repolarization, inhibition of these channels results in prolongation of the action potential with subsequent prolonged depolarization of cardiomyocytes. Our observations show QTc prolongation in a clinical setting, reproducing the in‐vitro results [9, 10, 11, 12]. The observed bradycardia further adds to the risk of TdP [46].
The interindividual variation in the extent, timing and duration of QTc prolongation might result from several interindividual pharmacokinetic and pharmacodynamic differences. For instance, ibogaine is metabolized to noribogaine by CYP2D6, an enzyme of the cytochromeP450, that has strong interindividual variation in activity in humans. Genetic variation in the CYP2D6 genotype results in poor, intermediate, extensive and ultrarapid metabolizers [47, 48, 49]. This variation can cause variation in the first‐pass effect, bioavailability, as well as elimination of ibogaine and availability of noribogaine. Although our subjects did not take any medication affecting CYP2D6 activity, such medication is commonly used, particularly in psychiatry. This might add further risk of cardiotoxicity of ibogaine in clinical practice, with presumably highest risks in poor metabolizers and those taking CYP2D6 inhibiting medication.
Even though none of our participants had QTc times exceeding 500 ms after 24 hours, QTc was still > 450 ms after 24 hours in 29% of subjects. It is important to note that the metabolite noribogaine has also been associated with QTc prolongation, although some suggest noribogaine to be less potent compared to ibogaine itself [29, 50]. The half‐life of serum ibogaine is 1–6 hours and that of noribogaine is 28–49 hours [29]. This might further add to persistent QTc prolongation, as observed in some individuals [9].
In line with previous observations in animals and humans, transient ataxia occurred after administration of ibogaine [3, 7]. Based on the known half‐life and Tmax of ibogaine it seems likely that ibogaine itself, and not is metabolites, is responsible for this observation. The transient ataxia resolved long before noribogaine levels peak. It has been suggested that impaired motor control might be due to the oneirogeinic experience that occur during ibogaine treatment [3]. However, we hypothesize that an effect of ibogaine on the cerebellum can however not be ruled out. Indeed, cerebellar toxicity of ibogaine is observed in animal models much higher doses are given [51, 52, 53, 54]. Translation of these animal data to humans is however complex. The pronounced effects of ibogaine on motor coordination of distal limbs and balance is in accordance with a higher susceptibility of the vermis to toxicity effects as opposed to the cerebellar cortex [55]. This may be because the vermis is closer to the CSF, in which toxins may enter [55]. A human autopsy report on a woman receiving multiple doses of ibogaine did not show cerebellar damage [56]. There are no reports of long lasting neurologic effects of ibogaine treatment. Effects on the human cerebellum need further study, for instance using neuro‐imaging techniques. Interestingly, the cerebellum has also been implicated to modulate dopaminergic neurons in the ventral tegmental area (VTA) in the limbic system, and as such might play a role in addictive behaviors [57]. Future studies should investigate whether cerebellar effects of ibogaine play a role in its alleged anti‐addictive properties [57].
Despite clear effects, no subjects had substantial increases on the DOS scale. Although no severe behavioral changes occurred in our subjects, they reported having psychomimetic experiences of closed eye visuals and vivid memories. One subject reported visual hallucinations. In the current study, these so‐called ‘oneirogenic experiences’ were generally well‐tolerated. However, literature and internet fora suggest that longer‐lasting disturbing behavioral changes can occur after ibogaine ingestion [58, 59]. As such, ibogaine administration in settings with expertise in handling people experiencing oneirogenic and/or psychotic symptoms is advisable.
Although the mechanism of action of ibogaine is poorly understood, it is known that ibogaine has a high affinity for several receptor sites, including N‐methyl‐D‐aspartate receptor (NMDA), κ‐ and μ‐opioid receptors as well as sigma‐2 receptor sites [51]. The observed mild withdrawal during the first 24 hours after ingestion in the current study might be related to an effect on opioid receptors, although any mechanistic conclusions cannot be based on the current clinical observations.
Taken together, our findings of serious (cardiac) side effects of ibogaine hamper the clinical utility of ibogaine in the treatment of substance use disorders. Given the limited evidence for effectiveness, the presumed clinical benefits may not outweigh the observed cardiac risks. If the side effects are dose related, an alternative to the single high dose ibogaine treatment regimen might be a repeated low dosing approach, which may produce a more favorable safety profile although accumulation of noribogaine (with a much longer t1/2) and subsequent QTc prolongation is a risk. Multiple dosing should only be performed under strict monitoring of QTc and based on CYP2D6 genotyping.
The current findings should be interpreted in the light of several limitations. First, we selected a specific group of subjects with OUD on OST with a wish for abstinence and a stable psychosocial situation, as reflected by their ASI scores. Furthermore, we excluded people with known liver or cardiac disease, which are both common among chronic illicit opioid users. The selection was made to limit the risk of increased exposure to ibogaine, cardiac events and psychosocial destabilization. The safety concerns presented here might thus be even more pertinent for the overall sample of patients with OUD or other addictions, especially those with cardiac pathology.
Secondly, only 14 patients were included in the current study. Although our study shows systematic effects on QTc time (primary outcome measure), the study is underpowered to detect rare, severe adverse events such as TdP, seizures or severe psychosis. As for TdP, our ECG monitoring was not continuous and short episodes may even have been missed. Furthermore, the provided dose of 10 mg/kg is in the lower bound of dosages in previous research settings, potentially contributing to an underestimation of ibogaine's toxic effects. Further studies using higher doses therefore seem superfluous based on the current findings.
Thirdly, all patients received metoclopramide to prevent nausea and vomiting. Both ibogaine and metoclopramide are metabolized by CYP2D6 and in vitro findings suggest limited competitive inhibition of metabolism. Yet, it cannot be fully ruled out that this may have had an effect on bioavailabilty of ibogaine (by limiting the first pass effect) and prolonging the half‐life (by limiting metabolism) [60]. However, this interaction has been suggested to only occur at very high ibogaine plasma levels of above 100uM [60]. Moreover, Metoclopramide is not listed in the Micromedex or uptodate‐interaction checker as having a significant interaction with other, well known CYP2D6 substrates, such as tamoxifen and metoprolol [61].
Next, CredibleMeds lists metoclopramide as a QT‐prolonging drug [62]. However, little quantative studies are available. One study found 2 mg of metoclopramide i.v. to have a mild QTc‐prolonging effect of about 1%, or 3‐4 ms [65]. Another study showed metoclopramide to affect beat‐to‐beat fluctuations, which may explain some of the interval variation seen in our results [65, 66]. As the QT‐prolonging effects of metoclopramide are small, we do not deem this to be of major concern regarding the observed impressive QTc‐prolongation. Yet, given the lack of a placebo control group, which we consider unethical in this population, any confounding effects of metoclopramide or opioid withdrawal itself cannot be fully ruled out.
Lastly, our washout period for opioid substitution treatment was eight days. No urine samples on drug testing were available during the inpatient study period. It can thus not be fully ruled out that any (residual) methadone was present at the start of ibogaine treatment. However, it is highly unlikely that (residual) methadone had a major contribution to the observed QT‐prolongation after ibogaine ingestion. QTc was within normal range during methadone treatment (at screening) and in the IBM Micromedex no interactions of methadone or buprenorphine with ibogaine leading to higher plasma levels are reported, eg based on replacement of protein‐binding by these drugs [61]. Taken together, possible drug‐drug interactions with metoclopramide, methadone or buprenorphine on metabolism or QT‐prolongation will not have a major effect on the massive QT‐prolongation observed. If choosing an antiemetic or other concomitant medication with ibogaine treatment both QT‐prolonging and CYP2D6 inhibiting effects should be ruled out, and an individual correction formula (QTcI) made in accordance with current FDA (or EMA) guidelines [67].
In our study we focused upon safety outcomes after ibogaine ingestion; however, we observed some promise in alleviating withdrawal [26]. Patients with OUD have limited treatment options, the mainstay being detoxification combined with psychosocial treatments or substitution programs [68]. Any potential beneficial effect of new treatment options are thus worth exploring in a research context, but the risks of ibogaine treatment observed here may not outweigh the potential benefits [51, 68]. For future work with ibogaine we strongly advise treatments to take place under careful clinical monitoring to ensure patient safety. Research reports on efficacy of ibogaine should also report on these safety issues.
In conclusion, this open‐label observational study in patients with OUD in opioid substitution treatment in acute withdrawal showed that ibogaine induces a clinically relevant reversible QTc prolongation. Other observed transient adverse effects of ibogaine were bradycardia and severe ataxia. Based on the current findings, the use of ibogaine outside a well‐controlled medical context (i.e. by underground providers) should be avoided due to its high cardiac risk profile.
Clinical trial registration
This study has been registered under EUDRACT trial number 2014‐000354‐11.
Declaration of interests
None.
Author contributions
Thomas Knijver: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; visualization. Arnt Schellekens: Conceptualization; data curation; investigation; methodology; resources; supervision. Maarten Belgers: Conceptualization; funding acquisition; investigation; methodology; resources. Rogier Donders: Data curation; formal analysis; visualization. Toon Van Oosteren: Conceptualization; funding acquisition. Kees Kramers: Conceptualization; investigation; methodology; resources; supervision. Robbert Verkes: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision.
Supporting information
Table S3 Suggested Bazett‐Corrected QTc Values for Diagnosing QT Prolongation.
Table S4 Ranges of normal serum levels of potassium, calcium and magnesium.
Figure S5 Manual QTc measurements per subject. The individual measurements of the QTc using Bazett's formula on manual QT measurements during the first 24 hours after ibogaine ingestion.
Figure S6 Average ataxia scores with standard error bars per item of the SARA. Measurements taken within 24 hours after ibogaine ingestion.
Acknowledgements
For aid in conducting our research we would like to thank Sanders Dekkers, Jan Leijtens, Saskia Delis, Georghe Pop, Sjoerd Westra and Anne Loes In der Maur, NISPA and the nursing staff of IrisZorg and Radboud UMC. We thank Stichting Het Hoogeland, a non‐profit organization supporting addiction research, for funding.
Knuijver, T. , Schellekens, A. , Belgers, M. , Donders, R. , van Oosteren, T. , Kramers, K. , and Verkes, R. (2022) Safety of ibogaine administration in detoxification of opioid‐dependent individuals: a descriptive open‐label observational study. Addiction, 117: 118–128. 10.1111/add.15448
References
- 1. National Center for Biotechnology Information . PubChem Compound Database; CID=197060. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/197060 (accessed 16 October 16, 2018).
- 2. Fernandez J. W. Bwiti: An Ethnography of the Religious Imagination in Africa. Princeton, NJ: Princeton University Press; 1982. [Google Scholar]
- 3. Mash D. C., Duque L., Page B., Allen‐Ferdinand K. Ibogaine detoxification transitions opioid and cocaine abusers between dependence and abstinence: clinical observations and treatment outcomes. Front Pharmacol 2018; 9: 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheppard S. G. A preliminary investigation of ibogaine: case reports and recommendations for further study. J Subst Abuse Treat 1994; 11: 379–385. [DOI] [PubMed] [Google Scholar]
- 5. Luciano D. Observations on treatment with ibogaine. Am J Addict 1998; 7: 89–90. [DOI] [PubMed] [Google Scholar]
- 6. Alper K. R., Lotsof H. S., Frenken G. M., Luciano D. J., Bastiaans J. Ibogaine in acute opioid withdrawal. an open label case series. Ann NY Acad Sci 2000; 909: 257–259. [DOI] [PubMed] [Google Scholar]
- 7. Belgers M., Leenaars M., Homberg J. R., Ritskes‐Hoitinga M., Schellekens A. F., Hooijmans C. R. Ibogaine and addiction in the animal model, a systematic review and meta‐analysis. Transl Psychiatry 2016; 6: e826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alper K. R., Stajić M., Gill J. R. Fatalities temporally associated with the ingestion of ibogaine. J Forensic Sci 2012; 57: 398–412. [DOI] [PubMed] [Google Scholar]
- 9. Koenig X., Hilber K. The anti‐addiction drug ibogaine and the heart: a delicate relation. Molecules 2015; 20: 2208–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koenig X., Kovar M., Boehm S., Sandtner W., Hilber K. Anti‐addiction drug ibogaine inhibits hERG channels: a cardiac arrhythmia risk. Addict Biol 2014; 19: 237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thurner P., Stary‐Weinzinger A., Gafar H., Gawali V. S., Kudlacek O., Zezula J., et al. Mechanism of hERG channel block by the psychoactive indole alkaloid ibogaine. J Pharmacol Exp Ther 2014; 348: 346–358. [DOI] [PubMed] [Google Scholar]
- 12. Koenig X., Kovar M., Rubi L., Mike A. K., Lukacs P., Gawali V. S., et al. Anti‐addiction drug ibogaine inhibits voltage‐gated ionic currents: a study to assess the drug's cardiac ion channel profile. Toxicol Appl Pharmacol 2013; 273: 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mash D. C., Kovera C. A., Pablo J., Tyndale R., Ervin F. R., Kamlet J. D., et al. Ibogaine in the treatment of heroin withdrawal. Alkaloids Chem Biol 2001; 56: 155–171. [DOI] [PubMed] [Google Scholar]
- 14. Schep L. J., Slaughter R. J., Galea S., Newcombe D. Ibogaine for treating drug dependence. What is a safe dose? Drug Alcohol Depend 2016; 166: 1–5. [DOI] [PubMed] [Google Scholar]
- 15. Breuer L., Kasper B. S., Schwarze B., Gschossmann J. M., Kornhuber J., Müller H. H. ‘Herbal seizures’—atypical symptoms after ibogaine intoxication: a case report. J Med Case Rep 2015; 9: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marta C. J., Ryan W. C., Kopelowicz A., Koek R. J. Mania following use of ibogaine: a case series. Am J Addict 2015; 24: 203–205. [DOI] [PubMed] [Google Scholar]
- 17. Knuijver T., Belgers M., Markus W., Verkes R. J., van Oosteren T., Schellekens A. Hallucinogen persisting perception disorder after Ibogaine treatment for opioid dependence. J Clin Psychopharmacol 2018; 38: 646–648. [DOI] [PubMed] [Google Scholar]
- 18. Kagie R. De Heks van Kockengen [Witch of Kockengen]. Available at: https://www.vn.nl/de‐heks‐van‐kockengen/2012 (accessed July 2019).
- 19. Goldenberg I., Moss A. J., Zareba W. QT interval: how to measure it and what is ‘normal’. J Cardiovasc Electrophysiol 2006; 17: 333–336. [DOI] [PubMed] [Google Scholar]
- 20. The Arizona QT drugslist. Available at: https://crediblemeds.org/ (accessed October 2017).
- 21. Flockhart DA. Drug Interactions: Cytochrome P450 Drug Interaction Table. Indiana University. Clinical Pharmacology Research Institute, Indiana University School of Medicine; 2007. Available at: /clinpharm/ddis/clinical‐table/ (accessed October 2017).
- 22. Sheehan D. V., Lecrubier Y., Sheehan K. H., Amorim P., Janavs J., Weiller E., et al. The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 1998; 59: 22–33 quiz 4–57. [PubMed] [Google Scholar]
- 23. Brown T. K., Alper K. Treatment of opioid use disorder with ibogaine: detoxification and drug use outcomes. Am J Drug Alcohol Abuse 2018; 44: 24–36. [DOI] [PubMed] [Google Scholar]
- 24. Malcolm B. J., Polanco M., Barsuglia J. P. Changes in withdrawal and craving scores in participants undergoing opioid detoxification utilizing ibogaine. J Psychoactive Drugs 2018; 50: 256–265. [DOI] [PubMed] [Google Scholar]
- 25. Schenberg E. E., de Castro Comis M. A., Chaves B. R., da Silveira D. X. Treating drug dependence with the aid of ibogaine: a retrospective study. J Psychopharmacol 2014; 28: 993–1000. [DOI] [PubMed] [Google Scholar]
- 26. Noller G. E., Frampton C. M., Yazar‐Klosinski B. Ibogaine treatment outcomes for opioid dependence from a twelve‐month follow‐up observational study. Am J Drug Alcohol Abuse 2017; 44: 37–46. [DOI] [PubMed] [Google Scholar]
- 27. inc. M. e. f. L. E. P. Ibogaïne Alliance website with information Remogen Ibogaine: https://www.ibogainealliance.org/conferences/vancouver-2012/archive/bob-sisko/; 2014. (accessed July 2021)
- 28. Preparaat tekst metoclopramide , https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/m/metoclopramideconsulted (accessed July 2021).
- 29. Glue P., Lockhart M., Lam F., Hung N., Hung C. T., Friedhoff L. Ascending‐dose study of noribogaine in healthy volunteers: pharmacokinetics, pharmacodynamics, safety, and tolerability. J Clin Pharmacol 2015; 55: 189–194. [DOI] [PubMed] [Google Scholar]
- 30. DeJong C. A., Willems J. C., Schippers G. M., Hendriks V. M. The Addiction Severity Index: reliability and validity in a Dutch alcoholic population. Int J Addict 1995; 30: 605–616. [DOI] [PubMed] [Google Scholar]
- 31. Hendriks V. M., Kaplan C. D., van Limbeek J., Geerlings P. The Addiction Severity Index: reliability and validity in a Dutch addict population. J Subst Abuse Treat 1989; 6: 133–141. [DOI] [PubMed] [Google Scholar]
- 32. McLellan A. T., Luborsky L., Cacciola J., Griffith J., Evans F., Barr H. L., et al. New data from the Addiction Severity Index. reliability and validity in three centers. J Nerv Ment Dis 1985; 173: 412–423. [DOI] [PubMed] [Google Scholar]
- 33. McLellan A. T., Cacciola J. C., Alterman A. I., Rikoon S. H., Carise D. The Addiction Severity Index at 25: origins, contributions and transitions. Am J Addict 2006; 15: 113–124. [DOI] [PubMed] [Google Scholar]
- 34. Stöffelmayr B. E., Mavis B. E., Kasim R. M. The longitudinal stability of the addiction severity index. J Subst Abuse Treat 1994; 11: 373–378. [DOI] [PubMed] [Google Scholar]
- 35. Kosten T. R., Rounsaville B. J., Kleber H. D. Concurrent validity of the addiction severity index. J Nerv Ment Dis 1983; 171: 606–610. [DOI] [PubMed] [Google Scholar]
- 36. Postema P. G., Wilde A. A. The measurement of the QT interval. Curr Cardiol Rev 2014; 10: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Available at: https://www.mayoclinic.org/diseases‐conditions/high‐blood‐pressure/diagnosis‐treatment/drc‐20373417 (accessed July 2020).
- 38. Schmitz‐Hübsch T., du Montcel S. T., Baliko L., Berciano J., Boesch S., Depondt C., et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 2006; 66: 1717–1720. [DOI] [PubMed] [Google Scholar]
- 39. Weyer A., Abele M., Schmitz‐Hübsch T., Schoch B., Frings M., Timmann D., et al. Reliability and validity of the scale for the assessment and rating of ataxia: a study in 64 ataxia patients. Mov Disord 2007; 22: 1633–1637. [DOI] [PubMed] [Google Scholar]
- 40. Yabe I., Matsushima M., Soma H., Basri R., Sasaki H. Usefulness of the scale for assessment and rating of ataxia (SARA). J Neurol Sci 2008; 266: 164–166. [DOI] [PubMed] [Google Scholar]
- 41. Schmitz‐Hübsch T., Fimmers R., Rakowicz M., Rola R., Zdzienicka E., Fancellu R., et al. Responsiveness of different rating instruments in spinocerebellar ataxia patients. Neurology 2010; 74: 678–684. [DOI] [PubMed] [Google Scholar]
- 42. Jones R. N., Cizginer S., Pavlech L., Albuquerque A., Daiello L. A., Dharmarajan K., et al. Assessment of instruments for measurement of delirium severity: a systematic review. JAMA Intern Med 2019; 179: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Handelsman L., Cochrane K. J., Aronson M. J., Ness R., Rubinstein K. J., Kanof P. D. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse 1987; 13: 293–308. [DOI] [PubMed] [Google Scholar]
- 44. US Food and Drug Administration (FDA) . Report E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non‐Antiarrhythmic Drugs. International Council for Harmonisation of Technical Requirements (ICH). Rockville, MD: FDA; 2005.
- 45. Manini A. F., Nair A. P., Vedanthan R., Vlahov D., Hoffman R. S. Validation of the prognostic utility of the electrocardiogram for acute drug overdose. J Am Heart Assoc 2017; 6: e004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Namboodiri N. Bradycardia‐induced torsade de pointes—an arrhythmia less understood. Ind Pacing Electrophysiol J 2010; 10: 435–438. [PMC free article] [PubMed] [Google Scholar]
- 47. Baumann M. H., Pablo J., Ali S. F., Rothman R. B., Mash D. C. Comparative neuropharmacology of ibogaine and its O‐desmethyl metabolite, noribogaine. Alkaloids Chem Biol 2001; 56: 79–113. [DOI] [PubMed] [Google Scholar]
- 48. Glue P., Winter H., Garbe K., Jakobi H., Lyudin A., Lenagh‐Glue Z., et al. Influence of CYP2D6 activity on the pharmacokinetics and pharmacodynamics of a single 20 mg dose of ibogaine in healthy volunteers. J Clin Pharmacol 2015; 55: 680–687. [DOI] [PubMed] [Google Scholar]
- 49. Maciulaitis R., Kontrimaviciute V., Bressolle F. M., Briedis V. Ibogaine, an anti‐addictive drug: pharmacology and time to go further in development. A narrative review. Hum Exp Toxicol 2008; 27: 181–194. [DOI] [PubMed] [Google Scholar]
- 50. Baumann M. H., Pablo J. P., Ali S. F., Rothman R. B., Mash D. C. Noribogaine (12‐hydroxyibogamine): a biologically active metabolite of the antiaddictive drug ibogaine. Ann NY Acad Sci 2000; 914: 354–368. [DOI] [PubMed] [Google Scholar]
- 51. Litjens R. P., Brunt T. M. How toxic is ibogaine? Clin Toxicol 2016; 54: 297–302. [DOI] [PubMed] [Google Scholar]
- 52. O'Hearn E., Molliver M. E. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience 1993; 55: 303–310. [DOI] [PubMed] [Google Scholar]
- 53. Molinari H. H., Maisonneuve I. M., Glick S. D. Ibogaine neurotoxicity: a re-evaluation. Brain Res 1996: 737: 255–262. [DOI] [PubMed] [Google Scholar]
- 54. Scallet A. C., Ye X., Rountree R., Nony P., Ali S. F. Ibogaine produces neurodegeneration in rat, but not mouse, cerebellum. Neurohistological biomarkers of Purkinje cell loss. Ann N Y Acad Sci 1996: 801: 217–226. [DOI] [PubMed] [Google Scholar]
- 55. Cavanagh J. B., Holton J. L., Nolan C. C. Selective damage to the cerebellar vermis in chronic alcoholism: a contribution from neurotoxicology to an old problem of selective vulnerability. Neuropathol Appl Neurobiol 1997: 23: 355–363. [PubMed] [Google Scholar]
- 56. Mash D. C., Kovera C. A., Buck B. E., Norenberg M. D., Shapshak P., Hearn W. L. et al. Medication development of ibogaine as a pharmacotherapy for drug dependence. Ann N Y Acad Sci 1998: 844: 274‐292. [PubMed] [Google Scholar]
- 57. Carta I., Chen C. H., Schott A. L., Dorizan S., Khodakhah K. Cerebellar modulation of the reward circuitry and social behavior. Science 2019; 363: eaav0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schenberg E. E., de Castro Comis M. A., Alexandre J. F. M., Tófoli L. F., Rasmussen Chaves B. D., da Silveira D. X. A phenomenological analysis of the subjective experience elicited by ibogaïne in the context of a drug dependence treatment. J Psychedel Stud 2017; 1: 1–10. [Google Scholar]
- 59. Heink A., Katsikas S., Lange‐Altman T. Examination of the phenomenology of the ibogaine treatment experience: role of altered states of consciousness and psychedelic experiences. J Psychoactive Drugs 2017; 49: 201–208. [DOI] [PubMed] [Google Scholar]
- 60. Livezey M. R., Briggs E. D., Bolles A. K., Nagy L. D., Fujiwara R., Furge L. L. Metoclopramide is metabolized by CYP2D6 and is a reversible inhibitor, but not inactivator, of CYP2D6. Xenobiotica 2014: 44: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. IBM . Micromedex® (electronic version). IBM Watson Health, Greenwood Village, Colorado, USA. Available at: https://www.micromedexsolutions.com/ (Accessed July 23, 2021).
- 62. Woosley R. L., Heise C. W., Romero K. A. https://www.Crediblemeds.org, QTdrugs List, Accessed July 23, 2021). AZCERT, Inc. 1822 Innovation Park Dr. Oro Valley, AZ 85755.
- 63. Chou C. C., Wu D. Torsade de pointes induced by metoclopramide in an elderly woman with preexisting complete left bundle branch block. Chang Gung Med J 2001: 24: 805–809. [PubMed] [Google Scholar]
- 64. Siddique S. M., Shariff N., Vesuwala N., Hafiz T. Metoclopramide as a possible cause of prolonged QT syndrome and torsade de pointes in a patient with heart failure and renal insufficiency. Ann Intern Med 2009: 150: 502–504. [DOI] [PubMed] [Google Scholar]
- 65. Fleming G. F., Vokes E. E., McEvilly J. M., Janisch L., Francher D., Smaldone L. Double‐blind, randomized crossover study of metoclopramide and batanopride for prevention of cisplatin‐induced emesis. Cancer Chemother Pharmacol 1991; 28: 226–227. [DOI] [PubMed] [Google Scholar]
- 66. Ellidokuz E., Kaya D. The effect of metoclopramide on QT dynamicity: double‐blind, placebo‐controlled, cross‐over study in healthy male volunteers. Aliment Pharmacol Ther 2003: 18: 151–155. [DOI] [PubMed] [Google Scholar]
- 67. FDA . E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non‐Antiarrhythmic Drugs Questions and Answers (R3) Guidance for Industry JUNE 2017; 2017.
- 68. Toce M. S., Chai P. R., Burns M. M., Boyer E. W. Pharmacologic treatment of opioid use disorder: a review of pharmacotherapy, adjuncts, and toxicity. J Med Toxicol 2018; 14: 306–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S3 Suggested Bazett‐Corrected QTc Values for Diagnosing QT Prolongation.
Table S4 Ranges of normal serum levels of potassium, calcium and magnesium.
Figure S5 Manual QTc measurements per subject. The individual measurements of the QTc using Bazett's formula on manual QT measurements during the first 24 hours after ibogaine ingestion.
Figure S6 Average ataxia scores with standard error bars per item of the SARA. Measurements taken within 24 hours after ibogaine ingestion.


