Abstract
As people with cystic fibrosis (CF) live longer and healthier lives, increasing numbers are considering the full range of reproductive options for their futures, including parenthood, pregnancy, or pregnancy prevention. As the face of CF changes, the CF care model must adapt to meet the reproductive health needs of both parents and nonparents with CF. This article summarizes the reproductive goals and family‐building concerns faced by people with CF, including fertility, pregnancy, and alternative paths to parenthood, the impact of parenthood on mental and physical health, and important future research.
Keywords: cystic fibrosis, family‐building, fertility, parenthood
1. INTRODUCTION
The quantity and quality of the lives of people with cystic fibrosis (CF) are increasing. 1 Consequently, more people with CF are expressing the desire to become parents. A 2005 Australian survey found that 84% of men with CF wanted children, 2 whereas a 2018 survey found that 78% of adolescent and young adult women with CF in the United States intended to become parents. 3 The number of people with CF having pregnancies 1 and anecdotally, the number of men who are becoming fathers is increasing. Indeed, the impact of highly effective modulator therapies (HEMT) will allow many people with CF to consider all reproductive options, including parenthood, pregnancy, or pregnancy prevention. To address the changing priorities of people with CF afforded by increased longevity, CF care providers must consider optimal incorporation of all aspects of reproductive health into the CF care model. This manuscript provides an overview of the reproductive goals and family‐building concerns faced by people with CF, including fertility, pregnancy, and alternative paths to parenthood, the known mental and physical health impacts of parenthood, and important directions for future research.
2. REPRODUCTIVE DECISION‐MAKING AND COUNSELING
The decision of whether to become a parent is complex for people with CF, and CF is a major factor in reproductive decision‐making. Both parents and nonparents with CF express concerns around balancing their roles as both parent and patient, communicating with children about CF, and the impact of anticipated health decline and early mortality on children. 4 Parents with CF report “being a parent on compressed time,” reflecting parenting with both a limited life expectancy and complex daily treatments, and the necessity to prioritize these often‐conflicting needs. 5 Specifically related to the decision to become pregnant, women with CF report not being forthcoming about their reproductive desires for fear of being judged by their CF team. 6 A recent systematic review found that many people with CF report a positive outlook on parenthood, despite potential negative impacts on health, treatment adherence, the need for coping strategies for parental stresses, and the pressure of time related to mortality. 7
A recent qualitative study exploring the reproductive decision support needs and preferences of women with CF demonstrated the unique reproductive health care needs of this population and the uncertainty and disjointed care they often face when making family planning decisions. 8 Women with CF desire to have tailored, disease‐specific reproductive health discussions with their CF team, beginning in early adolescence. 9 The majority of adolescent and young adult women with CF desire such conversations to be initiated by their CF team, whereas 50% of surveyed older adult women with CF would prefer to initiate such conversations themselves. 10 , 11 Men with CF also desire clear reproductive health discussions with their CF team and families during adolescence. 2 Recent work among women with CF highlights the utility of a disease‐specific reproductive goals decision aid to encourage relevant parenting, pregnancy, and contraceptive discussions with care providers. 8
As people with CF commonly experience suboptimal and fragmented reproductive health care provision, it is crucial to promote effective collaborations between the CF team, primary care providers, and reproductive health specialists. Given the heritability of CF, the role of genetic counselors is especially important as people with CF consider their reproductive options. 12 To meet the range of reproductive needs for people with CF, many reproductive health specialists may have a role in the comprehensive care of those with CF including those in obstetrics and gynecology, maternal‐fetal medicine, reproductive endocrinology and fertility services, and urology. It is critical that such providers understand the complexities of modern CF care, including the impact of HEMT on quality of life and life expectancy. Additionally, it is important to consider the psychological burden of such reproductive decisions and partnership with a mental health provider familiar with these complex concerns would be beneficial.
It is also important to consider and respect the family‐building and parenting desires of transgender and gender‐diverse individuals. 13 , 14 Provision of gender‐affirming care is a crucial first step and universal, nonjudgmental communication about family planning is advised. Furthermore, consideration of fertility preservation may be warranted as some gender‐affirming treatments impact fertility potential. 15 , 16
As many people with CF desire pregnancy prevention, contraceptive counseling and provision are imperative as part of routine comprehensive care in CF. Key clinical considerations and research related to contraception are detailed in the companion paper in this issue. 17
3. FERTILITY
Over 97% of all males with CF are infertile based on CF transmembrane conductance regulator (CFTR) protein‐related congenital bilateral absence of the vas deferens (CBAVD), and atresia or absence of the epididymis 18 (Figure 1A,B). Males with mutations in CFTR without other CF clinical manifestations still have infertility associated with atrophy of seminal vesicles and epididymis with or without an abnormal vas deferens suggesting that this aspect of embryonic development is incredibly sensitive to CFTR function. Semen analysis for the presence or absence of sperm is the mainstay of diagnosing infertility in men with CF. Physical exam by an experienced care provider usually confirms the presence of a vas deferens, but ultrasound, cross‐sectional contrast computed tomography (CT), and magnetic resonance imaging (MRI) will identify the pathogenesis of azoospermia in most men. 19
Figure 1.
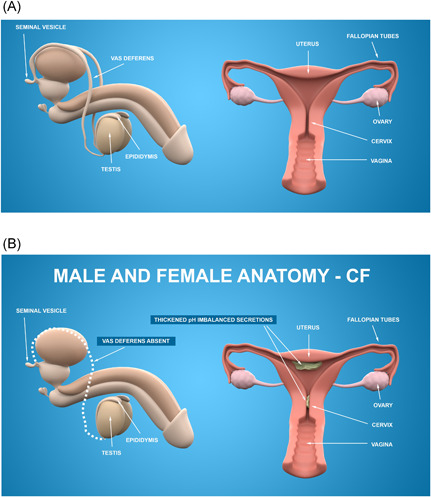
Male and female reproductive anatomy. (A) Reproductive anatomy in people without cystic fibrosis (CF). (B) The majority of men with CF are born with congenital bilateral absence of the vas deferens (CBAVD). Although the majority of women with CF are fertile, fertility can be impaired by thickened, acidic mucus resulting from malfunctioning CF‐transmembrane conductance regulator (CFTR) in the reproductive tract [Color figure can be viewed at wileyonlinelibrary.com]
It is estimated that approximately 35% of females with CF attempting to conceive are infertile (compared to 5%–15% in the general population). 20 The etiology of female infertility in CF is believed to be multifactorial and can be related to nutritional deficiencies, defective CFTR in the reproductive tract, and lower ovarian reserve. Delayed puberty in some females with CF suggests an impaired hypothalamic–pituitary–gonadal axis defect as a potential cause of anovulation and associated subfertility. 21 CFTR dysfunction throughout the female reproductive tract results in thick, acidic mucus that may act as a physical barrier to sperm entrance into the uterus and likely also impairs sperm capacitation (the ability of the sperm to fertilize the ovum) 20 (Figure 1). Imaging in the evaluation of female infertility includes use of saline ultrasound, a transvaginal ultrasound performed while sterile saline is infused into the uterus to detect uterine abnormalities, or hysterosalpingogram (HSG), which uses radiographs to assess patency of the fallopian tubes and to determine if the uterine cavity is normal. 22 , 23 More recently, sono‐HSG has been developed, which is similar to HSG but uses ultrasound instead of radiation. 24 Demonstration of reproductive tract abnormalities or obstruction will guide infertility management. Impact of HEMT on improved female fertility has been widely reported with the etiology likely due to their beneficial effect on cervical mucus and normalization of bicarbonate secretion in the uterus and fallopian tubes. 20 , 25 , 26 , 27
Although female fertility in CF may be improved by HEMT treatment, it is unlikely that HEMT given to males with CF will impact infertility based on their CBAVD. For example, Sun and colleagues administered ivacaftor to pregnant ferrets homo‐ or heterozygous for the Gly551Asp (G551D) mutation. While rescue of pancreatic function was demonstrated in kits heterozygous for G551D and a knock‐out mutation or homozygous for G551D, only those ferret kits homozygous for G551D who were exposed in utero throughout pregnancy experienced rescue of the vas deferens and epididymis. 28 Furthermore, there are no cases reported in the literature of rescue of fertility in males with CF treated with ivacaftor since the time of its approval in 2012. The impact of HEMT on fertility rescue and prevention of CBAVD via administration to mothers of male fetuses and neonates with CF has yet to be evaluated.
Assisted reproductive technology (ART) is the overarching term used for all fertility‐related therapy techniques for males and females (Figures 2 and 3). Genetic counseling should be considered before initiating ART, and preimplantation genetic testing (PGT) may be performed on the embryo in certain methods. Success rates depend on patient characteristics and treatment, and are not always CF related. Figure 2 highlights commonly used ART.
Figure 2.
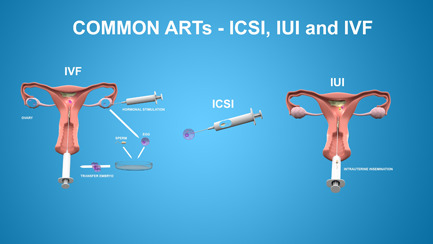
Common assisted reproductive technology (ART) used to achieve pregnancy includes IVF, ICSI, and IUI. Hormonal stimulation of ovarian follicle production is often combined with IVF, IUI, or artificial donor insemination. Egg retrieval is performed by using an ultrasound‐guided needle into each ovarian follicle. Traditional IVF requires a large number of sperm to be combined in vitro to fertilize an oocyte. ICSI allows one sperm alone to achieve fertilization by directly injecting it into the cytoplasm of an oocyte. Embryos or sperm are placed directly into the uterus via IUI. ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; IUI, intrauterine insemination [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3.

Commonly used assisted reproductive techniques for sperm retrieval include testicular sperm extraction (TESE), testicular sperm aspiration (TESA), percutaneous epididymal extraction (PESA), and microsurgical epididymal sperm extraction (MESA). Following sperm retrieval, intracytoplasmic sperm injection (ICSI), intrauterine insemination (IUI), and in vitro fertilization (IVF) are required to achieve pregnancy [Color figure can be viewed at wileyonlinelibrary.com]
For females with CF, stimulation of ovulation often involves treatments such as clomiphene citrate, a selective estrogen receptor modulator, or parenteral gonadotropins. Ovarian hyperstimulation syndrome, a potentially life‐threatening complication, may occur to varying degrees with symptoms potentially mimicking pulmonary exacerbation with dyspnea, pleural effusions, and gastrointestinal symptoms. 29 Stimulation of ovarian follicle production is often combined with IVF, IUI, or artificial donor insemination. Among ARTs, IVF and intrauterine insemination (IUI) are the most widely used methods. In IUI, semen is obtained from the partner or donor and placed directly into the uterus via a catheter. IVF requires that egg and sperm be combined ex vivo and subsequently implanted (Figure 2). Egg retrieval is performed by using an ultrasound‐guided needle into each ovarian follicle. Traditional IVF requires a large number of sperm (50,000–100,000) to be combined in vitro to fertilize an oocyte. A technique called intracytoplasmic sperm injection (ICSI) allows one sperm alone to achieve fertilization by directly injecting it into the cytoplasm of an oocyte. 30 IUI is less effective but generally less expensive than IVF, but cost varies within countries as well as US states.
IVF may be utilized by partners of males with CF or females with CF who have impaired fertility. In females, failure to conceive following IUI is also a rationale for IVF. Success rates of such procedures often depend on maternal age and the underlying cause of infertility; however, no CF‐specific outcome data exist. For females under 35 years of age, there is an overall 46%–47% success rate of a live birth; this decreases to a range of 2.8%–3.5% after age 42 years. 31 , 32 People with CF may potentially experience a reduced success rate of live births with IVF based on CF‐related complications including abnormal lung function, CF‐related diabetes, pancreatic insufficiency and protein‐calorie malnutrition, thickened cervical mucus and altered motility of fallopian tube cilia, as well as presumed abnormal vaginal and cervical pH leading to decreased sperm motility and survival in an abnormally acidic environment. 20 , 21
For males with CF, several ART options exist. As noted above, IVF allows males with CF to use their own sperm for fertilization. The main options for collecting sperm for diagnostic and fertilization purposes include testicular sperm aspiration (TESA), percutaneous epididymal sperm aspiration (PESA), and testicular sperm extraction (TESE) (Figure 3), followed by ICSI (Figure 2). With TESA, sperm for fertilization are identified in 1/3–1/2 of males and the numbers increase approximately 10% when followed by TESE. 33 , 34 The rates of clinical pregnancy with ART are similar to those in the non‐CF population at close to 50% but there is also an increase in the risk of miscarriages. 35
In animal reproductive models, no impaired fertility or genotoxicity was observed based on exposure to the individual components of HEMT. 36 However, it should be noted that males who wish to undergo ART and completely avoid exposure of sperm in the testicle to CFTR modulators must discontinue modulators for the life of an average sperm, approximately 80 days. Furthermore, because levels of CFTR modulators in seminal fluid are expected to approximate those in plasma, and 100% will be absorbed through the vaginal wall, 37 men wishing to avoid even minimal exposure of the conceptus to CFTR modulators should use condoms throughout the partner's pregnancy.
4. PREGNANCY
Historically, many care providers discouraged people with CF from becoming pregnant due to fears of maternal and fetal morbidity and mortality. To support these concerns, an early study of pregnancy in CF showed women with severe lung disease tended to have lower weight infants. 38 However, as the median age of survival improved, increasing numbers of people with CF became pregnant. A US registry study examining pregnancies in females with CF showed that those who became pregnant were initially healthier and had better 10‐year survival rates than females with CF who did not become pregnant; even with adjustment for initial severity of CF disease, pregnancy did not decrease survival. 39
In the pre‐HEMT era, people with CF had higher risks of several serious complications during pregnancy than occur in the general population, including preterm infants (adjusted OR [aOR]: 2.3; 95% CI: 1.2–4.4) with higher rates of infants with congenital anomalies (aOR: 2.6; 95% CI: 1.4–5.0), cesarean delivery (aOR 2.4; 95% CI: 1.2–4.6), severe preeclampsia (aOR 1.7; 95% CI: 0.2–12), pneumonia (aOR: 56.5; 95% CI: 43.0−74.1), requirement for mechanical ventilation (aOR: 18.3; 95% CI: 10.8–31.2), and (although rare) death (aOR: 76.0; 95% CI: 31.6–183). 40 , 41 The increased rate of complications was thought due to underlying disease and failure to gain adequate weight. In addition to underlying pulmonary disease, other comorbidities can complicate pregnancies including poorly controlled CF‐related diabetes and pulmonary hypertension. 40 Importantly, pregnancy does not seem to alter the subsequent clinical course for people with CF with mild to moderate pulmonary disease. 42 , 43 , 44 However, the frequency of treatment for pulmonary exacerbations may increase during pregnancy. 45
In the post‐HEMT era, the general health of people with CF treated with HEMT is improving; pulmonary function has improved and stabilized and the ability to achieve weight gain may no longer be an issue in the majority of pregnancies. However, people of advanced maternal age (>35 years) and those with lower lung function and body mass index (BMI) have achieved pregnancy on HEMT. 46 The impact on outcomes of such pregnancies in the setting of HEMT is relatively unknown. Furthermore, CFRD remains an active issue for many with CF, and careful assessment and management of glycemic control should be provided.
To further understand pregnancy in the HEMT era, the Maternal and Fetal Outcomes in the Era of Modulators (MAYFLOWERS), a prospective, multicenter observational project that will enroll approximately 285 women with CF will begin in the United States in summer 2021. 47 The primary goal of the study is to understand the impact of pregnancy on health outcomes in people with CF and their infants. One of the MAYFLOWERS substudies will assess the role of continuous glucose monitoring in pregnancy in CF.
4.1. Care during pregnancy
CF care providers should address reproductive health concerns with people with CF on a regular basis. Such discussions should include addressing reproductive goals, contraception needs, and pregnancy planning to guide recommendations for partner genetic testing for CF and for achieving optimal and stable pulmonary function before conception. Group prenatal care has been shown to be helpful for pregnancy outcomes in the general population. 48 However, in‐person group care would be challenging in people with CF due to infectious disease control. Use of telehealth may be an exciting avenue to support people with CF in prenatal care. 49
The European Respiratory Society/Thoracic Society of Australia and New Zealand Task Force recently wrote a comprehensive review on reproductive management of women with chronic airway disease, including CF. 40 Their review, published before the widespread use of HEMT, highlights the importance of pregnancy planning, including recommendations related to considerations of baseline lung function, CF‐related diabetes control, close monitoring of nutrition and weight gain, treatment of increased gestational reflux, and emphasis that expectant mothers should remain active. During pregnancy, it is critically important that people with CF attend multidisciplinary CF clinics on a regular basis, including input by the pulmonologist, nutritionist, social worker, and pharmacist (if available). If prepregnancy obstetrics consultation did not occur, early referral to an obstetric team specializing in high‐risk pregnancies including those in people with CF, and regular communication between the CF and obstetrics teams is essential.
4.2. CF medications during pregnancy
People with CF must take numerous medications to maintain their health status. 50 , 51 However, many therapeutics cross the placenta and/or can be transferred in breast milk. Therefore, when a person becomes pregnant, they and their providers must devise a medication management plan that will prevent deterioration of health while simultaneously minimizing risk to the fetus. Historically, to delineate potential risks to the developing fetus or lactating infant from medications consumed by the mother, the US Federal Drug Administration (FDA) designated medications in categories A–D or X. 52 Category A medications were those that had adequate data in pregnancy that showed no risk of harming the fetus versus Category X medications that were ones for which animal and/or human fetal risk had been demonstrated. After 2015, the Pregnancy and Lactation Labeling Rule (PLLR) replaced the lettering system. Sponsors must describe the results of testing in animal reproductive models (including the dose evaluated in relationship to the maximum recommended human dose) and report whether there are adequate and well‐controlled studies in pregnancy to characterize the drug‐associated risks of birth defects or miscarriage. 52
Two recent reviews describe in detail the risks associated with commonly used medications in the care of people with CF. 40 , 53 The majority of medications used in CF are considered safe in pregnancy and lactation, including the use of inhaled mucolytics such as hypertonic saline and dornase alpha, pancreatic enzymes, fat‐soluble vitamins (vitamin A should be given at <10,000 IU/day), and insulin. Inhaled antibiotics are considered probably safe as there is minimal systemic absorption. Although azithromycin is frequently used in the acute treatment of infection by obstetricians, chronic use has not been studied in pregnancy. Large population studies of acute use of this drug showed no risk to small risks, and it is considered probably safe for use during pregnancy. 54 Notable exceptions for the safe use of CF medications in pregnancy include the azoles, intravenous aminoglycosides (particularly in the first trimester), rifamycins, trimethoprim/sulfamethoxazole, and most of the immunosuppressants used in lung transplant recipients (described in detail in the lung transplant section below).
Reproductive animal data for CFTR modulators demonstrates that each of the modulators is transferred across the placenta (and is present in breast milk), but causes no genotoxicity or congenital birth defects at normal human doses. 36 , 55 , 56 , 57 Furthermore, while data suggest that this class of drugs crosses the blood–brain barrier, no neurodevelopmental toxicity has been reported. 58 Though animal data was not concerning, pregnant people were excluded from the Phase III trials, and, thus, our current knowledge is based on case reports and case series. 26 , 59 , 60 Two recent surveys of CF care providers regarding maternal and fetal outcomes of people who continued on CFTR modulators for some or all of their pregnancies provide some reassurance about the use of CFTR modulators during pregnancy. 61 , 62 These studies reported data from a total of 110 pregnancies during which the mother took a CFTR modulator during all or part of pregnancy. Miscarriage rates for people on CFTR modulators were below those reported for the general population. 63 The overwhelming majority of maternal and infant complications that occurred were consistent with the known higher incidence in people with CF, and rated by care providers as unrelated to CFTR modulator use. 42 , 64 Two cases of severe congenital anomalies were deemed unrelated to modulator use based on maternal risk factors. Additionally, in a limited retrospective data set focused on fertility, O'Connor and colleagues reported no known pregnancy complications in 14 people with CF who became pregnant within a mean of 8 weeks following HEMT initiation. 27 Finally, a female infant born with CF (F508del homozygous but with a negative newborn screen and pancreatic sufficiency) to a mother with CF who took HEMT throughout pregnancy was born healthy. 65 Although the summary of these data suggests no alarming signals for use of CFTR modulator therapy during pregnancy, data from the MAYFLOWERS study will enable care providers to offer evidence‐based guidance to people regarding use of CFTR modulators in pregnancy. The substudy assessing maternal and infant pharmacokinetics will contribute to our understanding of the comparative concentrations of CFTR modulators in mother and infant, and thus the infant's potential exposure in utero and during lactation.
While the available retrospective data for use of CFTR modulators in pregnancy is reassuring, ivacaftor did cause congenital cataracts when directly administered to neonatal rats. 55 This finding led to a label recommendation for baseline and follow‐up cataract evaluation in children receiving ivacaftor alone or ivacaftor‐containing products. 56 , 57 Although none of the infants in the case series or surveys developed cataracts following exposure in utero or during lactation, formal ophthalmological testing was performed infrequently. 60 , 61 , 62 Thus, it remains unknown if CFTR modulators taken by mothers can lead to cataracts in their offspring. Trimble and colleagues also reported transient transaminitis of unclear relationship to the mother's lumacaftor‐ivacaftor use in a breastfeeding infant. 66 Although the concentration of these drugs in the infant's plasma during lactation was quite low (2.7% and 0.5% compared to mother's levels, respectively), dosing of CFTR modulators and incidence of transaminitis have not been established in infants <4 months of age. Therefore, care providers of infants exposed to CFTR modulators through lactation should consider a plan for reflexive or routine monitoring of liver function tests.
4.3. Considerations for pregnancy in lung transplant recipients
People with CF are capable of becoming pregnant after lung transplantation, but there are many risks to consider. As noted above, pregnancy risks in people with CF before a transplant correlate with disease severity. 67 , 68 This same correlation applies to those who are transplant recipients but additional challenges, such as maternal rejection of the transplanted lung and infant complications secondary to maternal health status or exposure to immunosuppressive medications are important to consider.
Based on data from a small multicenter case series and registry analysis, maternal and fetal complications associated with pregnancy after a lung transplantation, specifically preterm delivery and low infant birth weight, are high. 69 , 70 , 71 A study using data from the National Transplantation Pregnancy Registry from 1991 to 2010 reported 30 pregnancies in 21 lung transplant recipients, of which 10 had CF. 72 More than half had live births with few associated life‐threatening complications and no reports of permanent disability in the infants. However, the incidence of preterm birth was 60% and there were 11 infant complications and 2 neonatal deaths. When comparing the pregnancies in transplanted recipients with CF and without CF, there was a higher rate of rejection during pregnancy in CF, but there was a lower rate of spontaneous abortion. For live births, mean gestational age was similar but mean birth weight was lower in CF. Most recently, in an abstract from 2020, investigators used the Transplant Pregnancy Registry International to search pregnant lung transplant recipients from 1992 to 2019. 71 They reported 51 pregnancy outcomes (including multiple births) in 36 recipients resulting in 31 live births (61%), 14 miscarriages (before 20 weeks' gestation) (28%), 5 terminations (10%), no stillbirths (after 20 weeks' gestation), and 1 ectopic pregnancy. They also reported 62% of infants born with a birth weight of <2500 g.
An additional consideration is increased risk of rejection of the transplanted lung. In general, lung transplant recipients experience a higher rate of rejection than other organ recipients. 72 In the studies referenced above, 7%–24% of lung transplant recipients with CF who became pregnant experienced graft rejection. 70 , 71 The cause of these increased rates of rejection are unknown, but may be due to providers and recipients decreasing immunosuppressive medications to protect the fetus or rarely due to antibody‐mediated rejection from human leukocyte antigen sensitization. 73 For this reason, some lung transplant centers request the recipients avoid pregnancy all together, whereas others recommend waiting at least 2 years after transplantation to have the opportunity to safely achieve the lowest possible doses of immunosuppressive medications before pregnancy.
Caution is advised with immunosuppressive medication use during pregnancy. Therapies such as cyclosporine and tacrolimus require frequent monitoring as frequent dose adjustments may be needed due to maternal weight changes. Certain immunosuppressive agents such as rapamycin inhibitors (i.e., mycophenolate mofetil) should be avoided during conception or pregnancy due to teratogenic effects. 74 Other immunosuppressant medications, such as prednisone, azathioprine, and calcineurin inhibitors, have limited information regarding use and management during pregnancy. Breastfeeding is generally not advised for a lung transplant recipient as many immunosuppressant medications pass into breast milk and may harm the infant. Although prior studies have included infants that were breastfed, data on outcomes were limited and further research is needed. 71 Long‐term implications of health and parenting experiences of lung transplant recipients must also be considered and investigated further.
Overall, successful pregnancies are possible after lung transplantation, but this option should be considered with extreme caution as these are high‐risk pregnancies with associated risks for maternal graft rejection, prematurity, and low birthweight infants. An honest conversation between care providers and transplant recipients, ideally before transplant, is needed to discuss reproductive goals and family‐building options, including key information on contraception and the known risks of pregnancy. Importantly, before lung transplantation, discussion of fertility preservation is warranted due to the teratogenic effects of common immunosuppressants. 75
5. ALTERNATIVE PATHS TO PARENTHOOD
Other options for family‐building include fostering, adoption, and surrogacy. There is limited CF‐specific data available on the health impact and the prevalence of these paths to parenthood. 7 However, people with CF have pursued and do qualify for each of these options. Figure 4 highlights the variety of paths to parenthood for people with CF.
Figure 4.

Paths to parenthood. While some people with cystic fibrosis will choose pregnancy to build their families, other options for family‐building include adoption (assuming the legal parenting responsibilities for a child whose parents or legal guardians are unable to care for them), step‐parenthood (assuming full or partial responsibility for a partner's children), foster care (providing a home for one or more children while their own family is temporarily unable to care for them), and surrogacy (utilizing someone else who becomes pregnant and carries a child specifically for another person) [Color figure can be viewed at wileyonlinelibrary.com]
Fostering is when a person provides a home for one or more children while their own family is temporarily unable to care for them. Adoption is when a person assumes the legal parenting responsibilities for a child whose parents or legal guardians are unable to care for them. Options for adoption vary based on geographic location, but can include domestic adoption (adopting a child from one's own country) or international adoption (adopting a child from another country). Each has its advantages and disadvantages, and different adoption agencies may have their own guidelines and requirements for placing children with families. Importantly, each state and country has its own regulations, processes, and costs for fostering or adopting children. As a CF team, it may be helpful to be familiar with local agencies or guidelines related to these options.
Surrogacy is when someone else becomes pregnant and carries a child specifically for another person. There is gestational and traditional surrogacy. In gestational surrogacy, IVF is used (Figure 2), and one or more eggs from the female partner (or an egg donor) is fertilized with sperm from the male partner (or a sperm donor), and the developing embryo is implanted in the surrogate. In traditional surrogacy, the surrogate is inseminated with semen from the male partner or a donor. Traditional surrogacy, which relies on the surrogate's egg, can have additional legal complications because the surrogate is the biological mother of the child. Surrogacy may be a good option for people with CF, depending on their baseline health, fertility, or partner's carrier status. Similar to adoption and foster care, regulations around surrogacy vary by US state and country. Again, people with CF may find it reassuring to receive some guidance or information related to local surrogacy laws from their CF team as they navigate these options and build their family. The ability of the CF team to provide such guidance will likely require local outreach to governing bodies.
6. MENTAL HEALTH ASPECTS OF FAMILY‐BUILDING AND PARENTING
CF teams can provide support and respond to changes in mental health throughout the process of decision‐making, becoming a parent, and raising a family. Infertility or pregnancy loss may result in stress, anxiety, and depression for both men and women. 76 , 77 , 78 , 79 , 80 Considering parenthood through ART, adoption, or fostering can also raise complex issues, including grief and loss, impact on relationships, and disclosure to children and others. 81 , 82 , 83 The American Society for Reproductive Medicine/Society for ART recommends psychoeducational consultation for people undergoing ART procedures, with referral for mental health evaluation and treatment as needed. 84 Limited evidence exists regarding effective psychological interventions to address psychological distress, coping, relationship functioning, and parenting in those facing subfertility or infertility and considering alternative options for family‐building. 85 , 86 , 87 , 88 , 89
For people of any gender, a range of positive and negative expectations, feelings and experiences can accompany the role shifts of becoming a parent. 90 , 91 , 92 Mental health symptoms often emerge in the peripartum period, and women with a prior history of psychiatric disorder are at elevated risk. 93 Most women notice transient shifts in anxiety, mood, and sleep in the first 2 weeks postpartum. 94 About one out of five meet the criteria for major depression during or in the first year after pregnancy, and half of the postpartum depressive episodes begin during pregnancy. 94 Women with bipolar disorder often suffer postpartum exacerbations, with 17% experiencing depression severe enough to require hospitalization, mania, or psychosis. 95 While rare in the general population, perinatal psychosis is a psychiatric emergency, can be triggered by peripartum endocrine and immunological changes in the absence of prior history, and carries a high risk of relapse following subsequent pregnancies. 95 Perinatal anxiety, obstetric traumatic stress, and obsessions/compulsions are also common. 93 , 96 , 97 , 98
Multiple professional organizations recommend screening for depression and anxiety during pregnancy and postpartum. 99 , 100 , 101 The Edinburgh Depression Scale and Patient Health Questionnaire‐9 (PHQ‐9) are often used to detect depression in obstetric populations. 93 The PHQ‐9 provides useful information about somatic symptoms (e.g., fatigue, sleep, appetite), which may be attributable to mood/anxiety, perinatal physiologic changes, parenting an infant, and/or CF, and have bidirectional interactions with physical and mental health. Like the PHQ‐9, the Generalized Anxiety Disorder 7‐Item Scale is routinely used by CF teams, 102 , 103 , 104 but it does not include items about obsessions/compulsions or trauma. Given the adverse impact of substance use on maternal and child health, its routine screening is also recommended for pregnant people. 105 , 106
Psychotherapy and social support are typically first‐line interventions for mild to moderate peripartum psychiatric symptoms, with a strong evidence base for cognitive behavioral therapy. 94 However, access, expense, and time burden may limit feasibility. Low social support and parenting self‐efficacy increase the risk of depression in new parents, and interventions may target infant attachment in at‐risk families. 93 , 94 , 107
During preconception planning, pregnancy, and breastfeeding, deciding whether to continue, initiate, or change psychotropic medication is a collaborative process taking into account individual risk tolerance and preferences, analogous to discussions around using CFTR modulators or other CF therapies peripartum. Women and their care providers should consider personal treatment history and likelihood of relapse of symptoms, 95 , 108 potential consequences of untreated psychiatric symptoms for both mother and child, 94 , 109 , 110 and the benefits and known and unknown risks of pharmacotherapies. 111 , 112 Some psychotropic medications (e.g., fluoxetine) are well‐studied and frequently continued during pregnancy and lactation, whereas others have limited available safety data. 111 , 112 Exposure to polypharmacy should be limited when possible. 111 However, antidepressant doses may need to be increased in the second to third trimester due to physiologic changes of pregnancy. 112 Medications with high teratogenicity (e.g., valproate) are best avoided in women of childbearing age. 111 , 112
People with chronic physical illness or poor physical health postpartum may be at higher risk for perinatal psychiatric symptoms. 93 , 113 In a multinational study during the COVID‐19 pandemic, breastfeeding people (n = 5134) more often reported elevated symptoms of depression and anxiety if they had a chronic physical illness. 114 In a study of nearly 29,000 pregnancies, people with chronic physical illness, and specifically those with pulmonary disease, were no more likely to plan pregnancy than those without a medical condition, and those with mental illness or Type 2 diabetes engaged in less planning. 115 In a population surveillance survey, attending a doctor's visit for concomitant chronic physical illness substantially reduced the risk of avoiding care assistance for postpartum depression among White, Hispanic, and African American women. 116
Specific investigations related to the mental health implications of family‐building and pregnancy in CF and optimal intervention strategies are lacking. A qualitative study of men and women with CF indicated the emotional and practical impact of infertility, pregnancy, and parenting with CF, and the desire for conversation, education, and support around these concerns. 4 Most recently, a qualitative study of French parents with CF and their spouses highlighted that, while most express joy in parenthood and feel that it motivates them to self‐care, they desire additional support during the ART process and in discussing CF with their children. 117 The CF Foundation Mental Health Advisory Committee created educational materials for adults with CF who are parenting; other online resources providing psychoeducation and referral to perinatal support groups and mental healthcare are also available. Table 1 summarizes selected mental health resources related to parenthood and family‐building for people with CF. With depression and anxiety screening programs already in place, and the advantage of longitudinal relationships, CF teams are well‐positioned to implement prevention and intervention strategies for people with CF who seek to become parents.
Table 1.
Selected resources on mental health aspects of family‐building and parenting
| Resource | Infertility and ART | Adoption, fostering | Pregnancy loss | Pregnancy, postpartum, and breastfeeding | Parenting/children's mental health |
|---|---|---|---|---|---|
| Massachusetts General Hospital Center for Women's Mental Health (https://womensmentalhealth.org) | X | X | |||
| American Society for Reproductive Medicine (https://www.reproductivefacts.org/) | X | ||||
| Resolve: The National Infertility Association (https://resolve.org) | X | X | X | ||
| HHS Child Welfare Information Gateway (https://www.childwelfare.gov/) | X | ||||
| Postpartum Support International (PSI) (https://postpartum.net/) | X | X | |||
| Cystic Fibrosis Foundation (https://www.cff.org) | X | ||||
| Parenting at a Challenging Time (PACT) (https://www.mghpact.org/) | X | ||||
| American Academy of Child and Adolescent Psychiatry (AACAP) (https://www.aacap.org/AACAP/Families_and_Youth/Family_Resources/Home.aspx) | X | ||||
| American Psychological Association (https://www.apa.org/helpcenter/family) | X | ||||
| Consortium for Science‐Based Information on Children, Youth and Families (https://infoaboutkids.org/) | X |
7. PHYSICAL HEALTH IMPACTS OF PARENTHOOD
The different stages of childhood present varying physical challenges to every parent, but may do so particularly for those with CF. In a recent survey to characterize treatment burden in CF, people with CF reported spending 2–3 h per day on the many tasks required to maintain their health, including taking oral and inhaled therapies, cleaning of equipment, performing airway clearance, and managing diabetes. 118 Parents caring for newborns experience sleep deprivation, 119 even in the absence of these additional tasks required to maintain their own health. The attempts to split time between caring for children and managing self‐care could lead to decreased adherence to therapy, which can adversely impact health. 120 Additionally, approximately one third of pulmonary exacerbations are thought to be initiated following contraction of an upper respiratory tract infection 121 , 122 ; thus, when children start in daycare or school settings, parents with CF often have an increased risk of repeated exposure to viral pathogens.
Few studies have formally evaluated the short‐ and long‐term impacts of parenthood on the physical health of people with CF, and there is a particular paucity of studies in fathers with CF. In one study conducted using the French CF registry among 48 men with moderate disease, fathers had a nonsignificant trend toward increased outpatient care center visits, but they did not experience clinical deterioration in lung function or BMI in the 3 years following paternity compared to matched men with CF who did not become fathers. 123 In contrast, Bianco and colleagues recently evaluated health outcomes in fathers in the United Kingdom with moderate disease and found a statistically significant decline in weight and a trend toward a decline in lung function in the first 12 months of fatherhood. Half of men with severe disease at the time of fatherhood died or underwent transplant in the 12–15 months after they became fathers. 124 Two relatively large studies of long‐term follow‐up of mothers with CF have been conducted. As noted above (Section 4, pregnancy), data from the US CF registry (1985–1997) demonstrated that women with CF who became pregnant had higher baseline lung function and better 10‐year survival than women who did not become pregnant. 39 Data collected from 1994 to 2005 in the Epidemiologic Study of Cystic Fibrosis showed that baseline lung function was higher in women who became pregnant than in those who did not become pregnant. 45 Although mothers did not experience accelerated disease progression in the 18 months following pregnancy, mothers did require more courses of IV antibiotics, had more illness‐related outpatient visits, and lower quality of life scores for physical functional, vitality, health perceptions, and respiratory symptoms compared to women who did not become mothers. Because all of these studies were conducted in the premodulator era and/or excluded those on CFTR modulators, the ability of HEMT to mitigate the potential adverse impacts of parenthood is currently unknown.
8. FUTURE DIRECTIONS
It is clear that as people with CF continue to live longer, healthier lives, more and more adults will choose to become parents. It is anticipated that those who start HEMT very early in life might not have the many associated complications of CF disease and current burden and cost of therapy. However, those who start HEMT as adults must consider the balance they will need to achieve between self‐care and care of their children. 4 While the US CF registry contains a wealth of information regarding multiple aspects of CF health, 1 there is only one question related to pregnancy in women, and there are no questions related to becoming a parent by routes other than pregnancy for people with CF. Other international registries collect limited data on parenthood timing and route. Thus, registry collection modification and additional data sources are needed to comprehensively evaluate the physical or mental health impacts of parenthood on people with CF.
Importantly, investigators in France are prospectively evaluating the perceptions, experiences, expectations, and needs of both people with CF who become parents (Parenting Concerns in Patients With CF [MucoPar]) as well as those of children whose parents have CF (Concerns of Children Whose Parents Have CF [MUCOKIDS]). 125 , 126 This study will provide valuable data regarding psychosocial aspects of parenthood. Investigators in the United States are developing patient‐centered decision aids to assist women with CF recognize and realize their reproductive goals in the context of their disease. 127 To better characterize and explore the lived experience of parents with CF, investigators are also conducting individual interviews and PhotoVoice‐based qualitative studies among mothers and fathers with CF. 128 , 129 Given the paucity of data available about the physical health impacts of parenthood for people with CF in the current era, a prospective, multicenter study is greatly needed. Such a study would extensively aid clinicians in their desire to provide evidence‐based guidance to prospective parents with CF.
CONFLICT OF INTERESTS
All authors declare that there are no conflict of interests directly related to this work.
AUTHOR CONTRIBUTIONS
Traci Kazmerski: Conceptualization (lead); writing original draft (lead); writing review and editing (lead). Natalie West: Conceptualization (supporting); writing original draft (equal); writing review and editing (supporting). Raksha Jain: Conceptualization (supporting); writing original draft (equal); writing review and editing (supporting). Ahmet Uluer: Conceptualization (supporting); writing original draft (equal); writing review and editing (supporting). Anna M. Georgiopoulos: Conceptualization (supporting); writing original draft (equal); writing review and editing (supporting). Moira L. Aitken: Conceptualization (supporting); writing original draft (equal); writing review and editing (supporting). Jennifer Taylor‐Cousar: Conceptualization (equal); resources (lead); supervision (lead); writing original draft (equal); writing review and editing (equal).
ACKNOWLEDGMENTS
The authors would like to acknowledge Miriam Bernard, BA, for her assistance with formatting this manuscript, and the Asher Family Fund. TMK, NW, MA, RJ, and JLTC receive grant support from the Cystic Fibrosis Foundation (WEST19Y3, KAZMER19Y3, TAYLOR19Y3, AITKEN19Y3, JAIN19Y3) to support the Women's Health Research Working Group in Cystic Fibrosis and directly support this study.
Kazmerski TM, West NE, Jain R, et al. Family‐building and parenting considerations for people with cystic fibrosis. Pediatric Pulmonology. 2022;57:S75‐S88. 10.1002/ppul.25620
REFERENCES
- 1.2019 Patient Registry Annual Data Report. Bethesda (MD): Cystic Fibrosis Foundation; 2020. https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2019-Patient-Registry-Annual-Data-Report.pdf
- 2. Sawyer SM, Farrant B, Cerritelli B, Wilson J. A survey of sexual and reproductive health in men with cystic fibrosis: new challenges for adolescent and adult services. Thorax. 2005;60(4):326‐330. 10.1136/thx.2004.027599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kazmerski TM, Sawicki GS, Miller E, et al. Sexual and reproductive health behaviors and experiences reported by young women with cystic fibrosis. J Cyst Fibros. 2018;17(1):57‐63. 10.1016/j.jcf.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 4. Hailey CE, Tan JW, Dellon EP, Park EM. Pursuing parenthood with cystic fibrosis: reproductive health and parenting concerns in individuals with cystic fibrosis. Pediatr Pulmonol. 2019;54(8):1225‐1233. 10.1002/ppul.24344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barker H, Moses J, O′Leary C. ‘I've got to prioritise': being a parent with cystic fibrosis. Psychol Health Med. 2017;22(6):744‐752. 10.1080/13548506.2016.1233345 [DOI] [PubMed] [Google Scholar]
- 6. Kazmerski TM, Gmelin T, Slocum B, Borrero S, Miller E. Attitudes and decision making related to pregnancy among young women with cystic fibrosis. Matern Child Health J. 2017;21(4):818‐824. 10.1007/s10995-016-2181-z [DOI] [PubMed] [Google Scholar]
- 7. Jacob A, Journiac J, Fischer L, Astrologo L, Flahault C. How do cystic fibrosis patients experience parenthood? A systematic review. J Health Psychol. 2021;26(1):60‐81. 10.1177/1359105320916539 [DOI] [PubMed] [Google Scholar]
- 8. Leech MM, Roe AH, Stransky OM, Talabi MB, Borrero S, Kazmerski TM. Exploring the reproductive decision support needs and preferences of women with cystic fibrosis. Contraception. 2020;103(1):32‐37. 10.1016/j.contraception.2020.10.004 [DOI] [PubMed] [Google Scholar]
- 9. Kazmerski TM, Borrero S, Tuchman LK, et al. Provider and patient attitudes regarding sexual health in young women with cystic fibrosis. Pediatrics. 2016;137(6). 10.1542/peds.2015-4452 [DOI] [PubMed] [Google Scholar]
- 10. Kazmerski TM, Sawicki GS, Miller E, et al. Sexual and reproductive health care utilization and preferences reported by young women with cystic fibrosis. J Cyst Fibros. 2018;17(1):64‐70. 10.1016/j.jcf.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 11. Kazmerski TM, Stransky OM, Taylor‐Cousar JL, et al. Sexual and reproductive health behaviors and experiences of adult women with cystic fibrosis. Pediatr Pulmonol. 2020;55(S138):91. 10.1002/ppul.25089 [DOI] [Google Scholar]
- 12. Foil KE, Powers A, Raraigh KS, Wallis K, Southern KW, Salinas D. The increasing challenge of genetic counseling for cystic fibrosis. J Cyst Fibros. 2019;18(2):167‐174. 10.1016/j.jcf.2018.11.014 [DOI] [PubMed] [Google Scholar]
- 13. Kidd KM, Sequeira GM, Voss RV, et al. Caring for gender diverse youth with cystic fibrosis. J Cyst Fibros. 2020;19(6):1018‐1020. 10.1016/j.jcf.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonnington A, Dianat S, Kerns J, et al. Society of family planning clinical recommendations: contraceptive counseling for transgender and gender diverse people who were female sex assigned at birth. Contraception. 2020;102(2):70‐82. 10.1016/j.contraception.2020.04.001 [DOI] [PubMed] [Google Scholar]
- 15. Chiniara LN, Viner C, Palmert M, Bonifacio H. Perspectives on fertility preservation and parenthood among transgender youth and their parents. Arch Dis Child 2019;104(8):739‐744. 10.1136/archdischild-2018-316080 [DOI] [PubMed] [Google Scholar]
- 16. Ainsworth AJ, Allyse M, Khan Z. Fertility preservation for transgender individuals: a review. Mayo Clin Proc. 2020;95(4):784‐792. 10.1016/j.mayocp.2019.10.040 [DOI] [PubMed] [Google Scholar]
- 17. West NE, Kazmerski TM, Taylor‐Cousar JL, et al. Optimizing sexual and reproductive health across the lifespan in people with cystic fibrosis. Pediatr Pulmon. 2021.. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaplan E, Shwachman H, Perlmutter AD, Rule A, Khaw KT, Holsclaw DS. Reproductive failure in males with cystic fibrosis. N Engl J Med. 1968;279(2):65‐69. 10.1056/NEJM196807112790203 [DOI] [PubMed] [Google Scholar]
- 19. Kim B, Kawashima A, Ryu J‐A, Takahashi N, Hartman RP, King BF. Imaging of the seminal vesicle and vas deferens. Radiographics. 2009;29(4):1105‐1121. 10.1148/rg.294085235 [DOI] [PubMed] [Google Scholar]
- 20. Hughan KS, Daley T, Rayas MS, Kelly A, Roe A. Female reproductive health in cystic fibrosis. J Cyst Fibros. 2019;18(Suppl 2):S95‐S104. 10.1016/j.jcf.2019.08.024 [DOI] [PubMed] [Google Scholar]
- 21. Hodges CA, Palmert MR, Drumm ML. Infertility in females with cystic fibrosis is multifactorial: evidence from mouse models. Endocrinology. 2008;149(6):2790‐2797. 10.1210/en.2007-1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luciano DE, Exacoustos C, Luciano AA. Contrast ultrasonography for tubal patency. J Minim Invasive Gynecol. 2014;21(6):994‐998. 10.1016/j.jmig.2014.05.017 [DOI] [PubMed] [Google Scholar]
- 23. Papaioannou S, Bourdrez P, Varma R, Afnan M, Mol BW, Coomarasamy A. Tubal evaluation in the investigation of subfertility: a structured comparison of tests. BJOG. 2004;111(12):1313‐1321. 10.1111/j.1471-0528.2004.00403.x [DOI] [PubMed] [Google Scholar]
- 24. Case AM, Pierson RA. Clinical use of sonohysterography in the evaluation of infertility. J Obstet Gynaecol Can. 2003;25(8):641‐648. 10.1016/s1701-2163(16)30122-0 [DOI] [PubMed] [Google Scholar]
- 25. Taylor‐Cousar JL. CFTR modulators: impact on fertility, pregnancy, and lactation in women with cystic fibrosis. J Clin Med. 2020;9(9):2706. 10.3390/jcm9092706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jain R, Taylor‐Cousar JL. Fertility, pregnancy and lactation considerations for women with CF in the CFTR modulator era. J Pers Med. 2021;11(5). 10.3390/jpm11050418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O′Connor KE, Goodwin DL, Nesmith A, et al. Elexacafator/tezacaftor/ivacaftor resolves subfertility in females with CF: a two center case series. J Cyst Fibros. 2021;20(3):399‐401. 10.1016/j.jcf.2020.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun X, Yi Y, Yan Z, et al. In utero and postnatal VX‐770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci Transl Med. 2019;11:eaau7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fiedler K, Ezcurra D. Predicting and preventing ovarian hyperstimulation syndrome (OHSS): the need for individualized not standardized treatment. Reprod Biol Endocrinol. 2012;10:32. 10.1186/1477-7827-10-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Persily JB, Vijay V, Najari BB. How do we counsel men with obstructive azoospermia due to CF mutations? A review of treatment options and outcomes. Transl Androl Urol. 2021;10(3):1467‐1478. 10.21037/tau-19-681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention. Assisted reproductive technology fertility clinic success rates report. 2020. US Deptartment of Health and Human Services; 2018. https://www.cdc.gov/art/reports/2018/fertility-clinic.html [Google Scholar]
- 32. Society for Assisted Reproductive Technology . 2019. National Summary Report. 2020. Accessed June 28, 2021. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2019
- 33. Houwen J, Lundin K, Söderlund B, Bergh C, Kremer JAM, Ekerhovd E. Efficacy of percutaneous needle aspiration and open biopsy for sperm retrieval in men with non‐obstructive azoospermia. Acta Obstet Gynecol Scand. 2008;87(10):1033‐1038. 10.1080/00016340802356891 [DOI] [PubMed] [Google Scholar]
- 34. Lu S, Cui Y, Li X, et al. Association of cystic fibrosis transmembrane‐conductance regulator gene mutation with negative outcome of intracytoplasmic sperm injection pregnancy in cases of congenital bilateral absence of vas deferens. Fertil Steril. 2014;101(5):1255‐1260. 10.1016/j.fertnstert.2014.01.033 [DOI] [PubMed] [Google Scholar]
- 35. de Souza DaS, Faucz FR, Pereira‐Ferrari L, Sotomaior VS, Raskin S. Congenital bilateral absence of the vas deferens as an atypical form of cystic fibrosis: reproductive implications and genetic counseling. Andrology. 2018;6(1):127‐135. 10.1111/andr.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.TRIKAFTA™ (elexacaftor/tezacaftor/ivacaftor; ivacaftor) Tablets Package Insert. Vertex Pharmaceuticals Inc. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212273s000lbl.pdf
- 37. Scialli AR, Bailey G, Beyer BK, et al. Potential seminal transport of pharmaceuticals to the conceptus. Reprod Toxicol. 2015;58:213‐221. 10.1016/j.reprotox.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 38. Cheng EY, Goss CH, McKone EF, et al. Aggressive prenatal care results in successful fetal outcomes in CF women. J Cyst Fibros. 2006;5(2):85‐91. 10.1016/j.jcf.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 39. Goss CH, Rubenfeld GD, Otto K, Aitken ML. The effect of pregnancy on survival in women with cystic fibrosis. Chest. 2003;124(4):1460‐1468. 10.1378/chest.124.4.1460 [DOI] [PubMed] [Google Scholar]
- 40. Middleton PG, Gade EJ, Aguilera C, et al. ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases. Eur Respir J. 2020;55(2):1901208. 10.1183/13993003.01208-2019 [DOI] [PubMed] [Google Scholar]
- 41. Ashcroft A, Chapman SJ, Mackillop L. The outcome of pregnancy in women with cystic fibrosis: a UK population‐based descriptive study. BJOG. 2020;127:1696‐1703. 10.1111/1471-0528.16423 [DOI] [PubMed] [Google Scholar]
- 42. Patel EM, Swamy GK, Heine RP, Kuller JA, James AH, Grotegut CA. Medical and obstetric complications among pregnant women with cystic fibrosis. Am J Obstet Gynecol. 2015;212:98e1. 10.1016/j.ajog.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 43. Thorpe‐Beeston JG, Madge S, Gyi K, Hodson M, Bilton D. The outcome of pregnancies in women with cystic fibrosis‐‐single centre experience 1998‐2011. BJOG. 2013;120:354‐361. 10.1111/1471-0528.12040 [DOI] [PubMed] [Google Scholar]
- 44. Canny GJ. Pregnancy in patients with cystic fibrosis. CMAJ. 1993;149:805‐806. [PMC free article] [PubMed] [Google Scholar]
- 45. Schechter MS, Quittner AL, Konstan MW, Millar SJ, Pasta DJ, McMullen A. Long‐term effects of pregnancy and motherhood on disease outcomes of women with cystic fibrosis. Ann Am Thorac Soc. 2013;10(3):213‐219. 10.1513/AnnalsATS.201211-108OC [DOI] [PubMed] [Google Scholar]
- 46. Taylor‐Cousar JL, Jain R. Maternal and fetal outcomes following elexacaftor‐tezacaftor‐ivacaftor use during pregnancy and lactation. J Cyst Fibros. 2021;S1569‐1993(21):00055‐2. 10.1016/j.jcf.2021.03.006 [DOI] [PubMed] [Google Scholar]
- 47.Prospective Study of Pregnancy in Women With Cystic Fibrosis (MAYFLOWERS). ClinicalTrials.gov Identifier: NCT04828382. Posted April 2, 2021.
- 48. Group Prenatal Care. ACOG Committee Opinion No. 731 . American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;131:e104‐e108. 10.1097/AOG.0000000000002526 [DOI] [PubMed] [Google Scholar]
- 49. Peahl AF, Powell A, Berlin H, et al. Patient and provider perspectives of a new prenatal care model introduced in response to the coronavirus disease 2019 pandemic. Am J Obstet Gynecol. 2021;224(4):384.e1‐384.e11. 10.1016/j.ajog.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Castellani C, Duff AJA, Bell SC, et al. ECFS best practice guidelines: the 2018 revision. J Cyst Fibros. 2018;17(2):153‐178. 10.1016/j.jcf.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 51. Flume PA, Mogayzel PJ Jr., Clinical Practice Guidelines for Pulmonary Therapies Committee , et al. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180(9):802‐808. 10.1164/rccm.200812-1845PP [DOI] [PubMed] [Google Scholar]
- 52.Content and Format of Labeling for Human Prescription Drug and Biological Products; Requirements for Pregnancy and Lactation Labeling. U.S. Food and Drug Administration. 2014 December 3; Accessed May 9, 2021. https://www.fda.gov/drugs/labeling-information-drug-products/pregnancy-and-lactation-labeling-drugs-final-rule [PubMed]
- 53. Magm Kroon, Akkerman‐Nijland AM, Rottier BL, Koppelman GH, Akkerman OW, Touw DJ. Drugs during pregnancy and breast feeding in women diagnosed with cystic fibrosis ‐ an update. J Cyst Fibros. 2018;17(1):17‐25. 10.1016/j.jcf.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 54. Taylor‐Cousar JL, Jain R, Kazmerski TM, et al. Concerns regarding the safety of azithromycin in pregnancy‐relevance for women with cystic fibrosis. J Cyst Fibros. 2021;20(3):395‐396. 10.1016/j.jcf.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 55.KALYDECO® (ivacaftor) tablets Package Insert. Vertex Pharmaceuticals Inc. 2012, Accessed December 2020. https://pi.vrtx.com/files/uspi_ivacaftor.pdf
- 56.ORKAMBI® (lumacaftor/ivacaftor) tablets Package Insert. Vertex Pharmaceuticals Inc. 2015, Accessed August 2018. https://pi.vrtx.com/files/uspi_lumacaftor_ivacaftor.pdf
- 57.SYMDEKO™ (tezacaftor/ivacaftor) tablets Package Insert. Vertex Pharmaceuticals. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210491lbl.pdf
- 58. Qiu F, Habgood M, Schneider‐Futschik EK. The balance between the safety of mother, fetus, and newborn undergoing cystic fibrosis transmembrane conductance regulator treatments during pregnancy. ACS Pharmacol Transl Sci. 2020;3(5):835‐843. 10.1021/acsptsci.0c00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor‐tezacaftor‐ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809‐1819. 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Heijerman HGM, Mckone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double‐blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940‐1948. 10.1016/S0140-6736(19)32597-8. Erratum in: Lancet. 2020 May 30;395(10238):1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nash EF, Middleton PG, Taylor‐Cousar JL. Outcomes of pregnancy in women with cystic fibrosis (CF) taking CFTR modulators ‐ an international survey. J Cyst Fibros 2020;19(4):521‐526. 10.1016/j.jcf.2020.02.018 [DOI] [PubMed] [Google Scholar]
- 62. Taylor‐Cousar JL, Jain R. Maternal and fetal outcomes following elexacaftor‐tezacaftor‐ivacaftor use during pregnancy and lactation. J Cyst Fibros. 2021;20(3):402‐406. 10.1016/j.jcf.2021.03.006 [DOI] [PubMed] [Google Scholar]
- 63. US Department of Health and Human Services . Office on Women's Health: Pregnancy Loss. https://www.womenshealth.gov/pregnancy/youre-pregnant-now-what/pregnancy-loss
- 64. Jelin AC, Sharshiner R, Caughey AB. Maternal co‐morbidities and neonatal outcomes associated with cystic fibrosis. J Matern Fetal Neonatal Med. 2017;30(1):4‐7. 10.3109/14767058.2016.1161747 [DOI] [PubMed] [Google Scholar]
- 65. Fortner CN, Seguin JM, Kay DM. Normal pancreatic function and false‐negative CF newborn screen in a child born to a mother taking CFTR modulator therapy during pregnancy. J Cyst Fibros. 2021;S1569‐1993(21):00100‐00104. 10.1016/j.jcf.2021.03.018 [DOI] [PubMed] [Google Scholar]
- 66. Trimble A, McKinzie C, Terrell M, Stringer E, Esther CR, Jr. Measured fetal and neonatal exposure to Lumacaftor and Ivacaftor during pregnancy and while breastfeeding. J Cyst Fibros. 2018;17(6):779‐782. 10.1016/j.jcf.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reynaud Q, Rousset Jablonski C, Poupon‐Bourdy S, et al. Participating Centers of the French Cystic Fibrosis Registry. Pregnancy outcome in women with cystic fibrosis and poor pulmonary function. J Cyst Fibros. 2020;19(1):80‐83. 10.1016/j.jcf.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 68. Edenborough FP, Mackenzie WE, Stableforth DE. The outcome of 72 pregnancies in 55 women with cystic fibrosis in the United Kingdom 1977‐1996. BJOG. 2000;107(2):254‐261. 10.1111/j.1471-0528.2000.tb11697.x [DOI] [PubMed] [Google Scholar]
- 69. Gyi KM, Hodson ME, Yacoub MY. Pregnancy in cystic fibrosis lung transplant recipients: case series and review. J Cyst Fibros. 2006;5(3):171‐175. 10.1016/j.jcf.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 70. Shaner J, Coscia LA, Constantinescu S, et al. Pregnancy after lung transplant. Prog Transplant. 2012;22(2):134‐140. 10.7182/pit2012285 [DOI] [PubMed] [Google Scholar]
- 71. Daly AT, Coscia L, Nathan HM, Hasz RD, Constantinescu S, Moritz MJ. Pregnancy outcomes in 36 lung transplant recipients. Transplantation. 2020;104(S3):16‐26. 10.1097/01.tp.0000701776.03628.64 [DOI] [Google Scholar]
- 72. Parulekar AD, Kao CC. Detection, classification, and management of rejection after lung transplantation. J Thorac Dis. 2019;11(Suppl 14):S1732‐S1739. 10.21037/jtd.2019.03.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. O'Boyle PJ, Smith JD, Danskine AJ, Lyster HS, Burke MM, Banner NR. De novo HLA sensitization and antibody mediated rejection following pregnancy in a heart transplant recipient. Am J Transplant. 2010;10(1):180‐183. 10.1111/j.1600-6143.2009.02875.x [DOI] [PubMed] [Google Scholar]
- 74. Davis‐Kankanamge C, Higgins J, Allsworth JE, Strickland J. Menstruation and contraception patterns of female adolescent transplant recipients. Pediatr Transplant. 2020;24(7):e13817. 10.1111/petr.13817 [DOI] [PubMed] [Google Scholar]
- 75. Ladores S, Bray LA, Brown J. "If we would have known": a couple's regret over a missed opportunity to have a biological child after lung transplantation. J Patient Exp. 2018;5(4):320‐322. 10.1177/2374373518778861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kazemi A, Torabi M, Abdishahshahani M. Adjustment toward infertility mediates the relationship between coping, depression and anxiety in men: a confirmatory analysis. Eur J Obstet Gynecol Reprod Biol. 2021;258:48‐52. 10.1016/j.ejogrb.2020.12.049 [DOI] [PubMed] [Google Scholar]
- 77. Warchol‐Biedermann K. The etiology of infertility affects fertility quality of life of males undergoing fertility workup and treatment. Am J Mens Health. 2021;15(2):1557988320982167. 10.1177/1557988320982167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hedegaard S, Landersoe SK, Olsen LR, Krog MC, Kolte AM, Nielsen HS. Stress and depression among women and men who have experienced recurrent pregnancy loss: focusing on both sexes. Reprod Biomed Online. 2021;(21):1172‐1180. 10.1016/j.rbmo.2021.03.012 [DOI] [PubMed] [Google Scholar]
- 79. Farren J, Jalmbrant M, Falconieri N, et al. Posttraumatic stress, anxiety and depression following miscarriage and ectopic pregnancy: a multicenter, prospective, cohort study. Am J Obstet Gynecol. 2020;222(4):367.e1‐367.e22. 10.1016/j.ajog.2019.10.102 [DOI] [PubMed] [Google Scholar]
- 80. Davoudian T, Gibbins K, Cirino NH. Perinatal loss: the impact on maternal mental health. Obstet Gynecol Surv. 2021;76(4):223. 10.1097/OGX.0000000000000874 [DOI] [PubMed] [Google Scholar]
- 81. Barnett ER, Cleary SE, Butcher RL, Jankowski MK. Children's behavioral health needs and satisfaction and commitment of foster and adoptive parents: do trauma‐informed services make a difference? Psychol Trauma. 2019;11(1):73‐81. 10.1037/tra0000357 [DOI] [PubMed] [Google Scholar]
- 82. Grotevant HD, Lo AY. Adoptive parenting. Curr Opin Psychol. 2017;15:71‐75. 10.1016/j.copsyc.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 83. Greenfeld DA. Effects and outcomes of third‐party reproduction: parents. Fertil Steril. 2015 Sep;104(3):520‐524. 10.1016/j.fertnstert.2015.07.1128 [DOI] [PubMed] [Google Scholar]
- 84. Practice Committee of the American Society for Reproductive Medicine and the Practice Committee for the Society for Assisted Reproductive Technology . Electronic address: ASRM@asrm.org. Guidance regarding gamete and embryo donation. Fertil Steril. 2021;S0015‐0282(21):00078‐00079. 10.1016/j.fertnstert.2021.01.045 [DOI] [Google Scholar]
- 85. Verkuijlen J, Verhaak C, Nelen WL, Wilkinson J, Farquhar C. Psychological and educational interventions for subfertile men and women. Cochrane Database Syst Rev. 2016;3(3):CD011034. 10.1002/14651858.CD011034.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gaitzsch H, Benard J, Hugon‐Rodin J, Benzakour L, Streuli I. The effect of mind‐body interventions on psychological and pregnancy outcomes in infertile women: a systematic review. Arch Womens Ment Health. 2020;23(4):479‐491. 10.1007/s00737-019-01009-8 [DOI] [PubMed] [Google Scholar]
- 87. Ying L, Wu LH, Loke AY. The effects of psychosocial interventions on the mental health, pregnancy rates, and marital function of infertile couples undergoing in vitro fertilization: a systematic review. J Assist Reprod Genet. 2016;33(6):689‐701. 10.1007/s10815-016-0690-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Raad G, Tanios J, Azoury J, Daher A, Fakih C, Bakos HW. Neurophysiology of cognitive behavioural therapy, deep breathing and progressive muscle relaxation used in conjunction with ART treatments: a narrative review. Hum Reprod Update. 2021;27(2):324‐338. 10.1093/humupd/dmaa048 [DOI] [PubMed] [Google Scholar]
- 89. Schoemaker NK, Wentholt WGM, Goemans A, Vermeer HJ, Juffer F, Alink LRA. A meta‐analytic review of parenting interventions in foster care and adoption. Dev Psychopathol. 2020;32(3):1149‐1172. 10.1017/S0954579419000798 [DOI] [PubMed] [Google Scholar]
- 90. Law S, Ormel I, Babinski S, et al. Dread and solace: talking about perinatal mental health. Int J Ment Health Nurs. 2021. 10.1111/inm.12884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Márquez F, Lucchini C, Bertolozzi MR, et al. Being a first‐time father. Their experiences and meanings: a qualitative systematic review. Rev Chil Pediatr. 2019;90(1):78‐88. 10.32641/rchped.v90i1.821 [DOI] [PubMed] [Google Scholar]
- 92. Tasker F, Gato J. Gender identity and future thinking about parenthood: a qualitative analysis of focus group data with transgender and non‐binary people in the United Kingdom. Front Psychol. 2020;11:865. 10.3389/fpsyg.2020.00865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. van der Zee‐van den Berg AI, Boere‐Boonekamp MM, Groothuis‐Oudshoorn CGM, Reijneveld SA. Postpartum depression and anxiety: a community‐based study on risk factors before, during and after pregnancy. J Affect Disord. 2021;286:158‐165. 10.1016/j.jad.2021.02.062 [DOI] [PubMed] [Google Scholar]
- 94. Earls MF, Yogman MW, Mattson G, Rafferty J, Committee on Psychosocial Aspects of Child and Family Health . Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143(1):e20183260. 10.1542/peds.2018-3259 [DOI] [PubMed] [Google Scholar]
- 95. Wesseloo R, Kamperman AM, Munk‐Olsen T, Pop VJ, Kushner SA, Bergink V. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta‐analysis. Am J Psychiatry. 2016;173(2):117‐127. 10.1176/appi.ajp.2015.15010124 [DOI] [PubMed] [Google Scholar]
- 96. Cirino NH, Knapp JM. Perinatal posttraumatic stress disorder: a review of risk factors, diagnosis, and treatment. Obstet Gynecol Surv. 2019;74(6):369‐376. 10.1097/OGX.0000000000000680 [DOI] [PubMed] [Google Scholar]
- 97. Schobinger E, Stuijfzand S, Horsch A. Acute and post‐traumatic stress disorder symptoms in mothers and fathers following childbirth: a prospective cohort study. Front Psychiatry. 2020;11:562054. 10.3389/fpsyt.2020.562054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Miller ES, Hoxha D, Wisner KL, Gossett DR. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health (Larchmt). 2015;24(10):825‐830. 10.1089/jwh.2014.5063 [DOI] [PubMed] [Google Scholar]
- 99. American Psychiatric Association . Position statement on screening and treatment of mood and anxiety disorders during pregnancy and postpartum. December 2020. https://www.psychiatry.org/home/policy-finder
- 100. Screening for Perinatal Depression. ACOG Committee Opinion No. 757 . American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e208‐e212. 10.1097/AOG.0000000000002927 [DOI] [PubMed] [Google Scholar]
- 101. Rafferty J, Mattson G, Earls MF, Yogman MW, Committee on Psychosocial Aspects of Child and Family Health . Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143(1):e20183259. 10.1542/peds.2018-3260 [DOI] [PubMed] [Google Scholar]
- 102. Quittner AL, Abbott J, Hussain S, et al. Integration of mental health screening and treatment into cystic fibrosis clinics: evaluation of initial implementation in 84 programs across the United States. Pediatr Pulmonol. 2020;55(11):2995‐3004. 10.1002/ppul.24949 [DOI] [PubMed] [Google Scholar]
- 103. Quittner AL, International Committee on Mental Health, EPOS Trial Study Group , et al. International Committee on Mental Health in Cystic Fibrosis: Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus statements for screening and treating depression and anxiety. Thorax. 2016;71(1):26‐34. 10.1136/thoraxjnl-2015-207488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Abbott J, Havermans T, ECFS Mental Health Working Group , et al. Mental health screening in cystic fibrosis centres across Europe. J Cyst Fibros. 2019;18(2):299‐303. 10.1016/j.jcf.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 105. Hostage JC, Brock J, Craig W, Sepulveda D. Integrating screening, brief intervention and referral to treatment (SBIRT) for substance use into prenatal care. Matern Child Health J. 2020;24(4):412‐418. 10.1007/s10995-020-02892-9 [DOI] [PubMed] [Google Scholar]
- 106. Boden SL, Jones CW, Cabacungan ET. Improved maternal and infant outcomes with serial, self‐reported early prenatal substance use screening. Matern Child Health J. 2021;25:1118‐1125. 10.1007/s10995-021-03127-1 [DOI] [PubMed] [Google Scholar]
- 107. Ansari NS, Shah J, Dennis CL, Shah PS. Risk factors for postpartum depressive symptoms among fathers: a systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2021;100:1186‐1199. 10.1111/aogs.14109 [DOI] [PubMed] [Google Scholar]
- 108. Bayrampour H, Kapoor A, Bunka M, Ryan D. The risk of relapse of depression during pregnancy after discontinuation of antidepressants: a systematic review and meta‐analysis. J Clin Psychiatry. 2020;81(4):19r13134. 10.4088/JCP.19r13134 [DOI] [PubMed] [Google Scholar]
- 109. Faleschini S, Rifas‐Shiman SL, Tiemeier H, Oken E, Hivert MF. Associations of prenatal and postnatal maternal depressive symptoms with offspring cognition and behavior in mid‐childhood: a prospective cohort study. Int J Environ Res Public Health. 2019;16(6):1007. 10.3390/ijerph16061007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Garman EC, Cois A, Tomlinson M, Rotheram‐Borus MJ, Lund C. Course of perinatal depressive symptoms among South African women: associations with child outcomes at 18 and 36 months. Soc Psychiatry Psychiatr Epidemiol. 2019;54(9):1111‐1123. 10.1007/s00127-019-01665-2 [DOI] [PubMed] [Google Scholar]
- 111. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215‐234. 10.1016/j.clp.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 112. Betcher HK, Wisner KL. Psychotropic treatment during pregnancy: research synthesis and clinical care principles. J Womens Health (Larchmt). 2020;29(3):310‐318. 10.1089/jwh.2019.7781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Brown HK, Qazilbash A, Rahim N, Dennis CL, Vigod SN. Chronic medical conditions and peripartum mental illness: a systematic review and meta‐analysis. Am J Epidemiol. 2018;187(9):2060‐2068. 10.1093/aje/kwy080 [DOI] [PubMed] [Google Scholar]
- 114. Ceulemans M, Foulon V, Ngo E, et al. Mental health status of pregnant and breastfeeding women during the COVID‐19 pandemic – a multinational cross‐sectional study. Acta Obstet Gynecol Scand. 2021;100:1219‐1229. 10.1111/aogs.14092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. De Wolff MG, Johansen M, Rom AL, Midtgaard J, Tabor A, Hegaard HK. Degree of pregnancy planning and recommended pregnancy planning behavior among women with and without chronic medical conditions – a large hospital‐based cross‐sectional study. Acta Obstet Gynecol Scand. 2020;100:1051‐1060. 10.1111/aogs.14069 [DOI] [PubMed] [Google Scholar]
- 116. Manso‐Córdoba S, Pickering S, Ortega MA, Asúnsolo Á, Romero D. Factors related to seeking help for postpartum depression: a secondary analysis of New York City PRAMS Data. Int J Environ Res Public Health. 2020;17(24):9328. 10.3390/ijerph17249328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jacob A, Hubert D, Grenet D, Brain C, Flahault C. Parenthood experience of cystic fibrosis patients and their spouses. Abstracts of the 44th European Cystic Fibrosis Conference. J Cyst Fibros. 2021;20(S1):P255. 10.1016/S1569-1993(21)01280-7 [DOI] [Google Scholar]
- 118. Davies G, Rowbotham NJ, Smith S, et al. Characterising burden of treatment in cystic fibrosis to identify priority areas for clinical trials. J Cyst Fibros. 2020;19(3):499‐502. 10.1016/j.jcf.2019.10.025 [DOI] [PubMed] [Google Scholar]
- 119. Haddad S, Dennis CL, Shah PS, Stremler R. Sleep in parents of preterm infants: a systematic review. Midwifery. 2019;73:35‐48. 10.1016/j.midw.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 120. Narayanan S, Mainz JG, Gala S, Tabori H, Grossoehme D. Adherence to therapies in cystic fibrosis: a targeted literature review. Expert Rev Respir Med. 2017;11(2):129‐145. 10.1080/17476348.2017.1280399 [DOI] [PubMed] [Google Scholar]
- 121. Flight WG, Bright‐Thomas RJ, Tilston P, et al. Incidence and clinical impact of respiratory viruses in adults with cystic fibrosis. Thorax. 2014;69(3):247‐253. 10.1136/thoraxjnl-2013-204000 [DOI] [PubMed] [Google Scholar]
- 122. Hoek RA, Paats MS, Pas SD, et al. Incidence of viral respiratory pathogens causing exacerbations in adult cystic fibrosis patients. Scand J Infect Dis. 2013;45(1):65‐69. 10.3109/00365548.2012.708942 [DOI] [PubMed] [Google Scholar]
- 123. Duguépéroux I, Hubert D, Dominique S, Bellis G, De Braekeleer M, Durieu I. Paternity in men with cystic fibrosis: a retrospective survey in France. J Cyst Fibros. 2006;5(4):215‐221. 10.1016/j.jcf.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 124. Bianco B, Horsley A, Brennan A. Implications of fatherhood in cystic fibrosis. Paediatr Respir Rev. 2019;31:18‐20. 10.1016/j.prrv.2019.02008 [DOI] [PubMed] [Google Scholar]
- 125.Parenting Concerns in Patients With Cystic Fibrosis (MucoPar). ClinicalTrials.gov Identifier: NCT04133246. Posted October 21, 2019.
- 126.Concerns of Children Whose Parents Have Cystic Fibrosis (MUCOKIDS). ClinicalTrials.gov Identifier: NCT04702386. Posted January 8, 2021..
- 127. Stransky OM, Pam M, Ladores SL, et al. Engaging stakeholders in the development of a reproductive goals decision aid for women with cystic fibrosis. Abstract for the 2021 North American CF Conference, in press. [DOI] [PMC free article] [PubMed]
- 128.Fibrosis Cystic Foundation Clinical Pilot and Feasibility Award, KAZMER20A0; PI: Traci M Kazmerski.
- 129. Wang C, Burris MA. Photovoice: concept, methodology, and use for participatory needs assessment. Health Educ Behav. 1997;24(3):369‐387. 10.1177/109019819702400309 [DOI] [PubMed] [Google Scholar]


