Abstract
The formation of a vast number of different multielement active sites in compositionally complex solid solution materials, often more generally termed high‐entropy alloys, offers new and unique concepts in catalyst design, which mitigate existing limitations and change the view on structure–activity relations. We discuss these concepts by summarising the currently existing fundamental knowledge and critically assess the chances and limitations of this material class, also highlighting design strategies. A roadmap is proposed, illustrating which of the characteristic concepts could be exploited using which strategy, and which breakthroughs might be possible to guide future research in this highly promising material class for (electro)catalysis.
Keywords: complex solid solutions, electrocatalysis, energy conversion, high-entropy alloys, materials synthesis
High‐entropy alloys offer a huge variety of multielement active sites on a single catalytic surface. This special polyelemental arrangement implies several different fundamental concepts in structure–activity correlations compared to traditional electrocatalysts, which are summarised and their implications for the possibility to adjust the catalytic properties with different limitations are discussed in detail.
1. Introduction
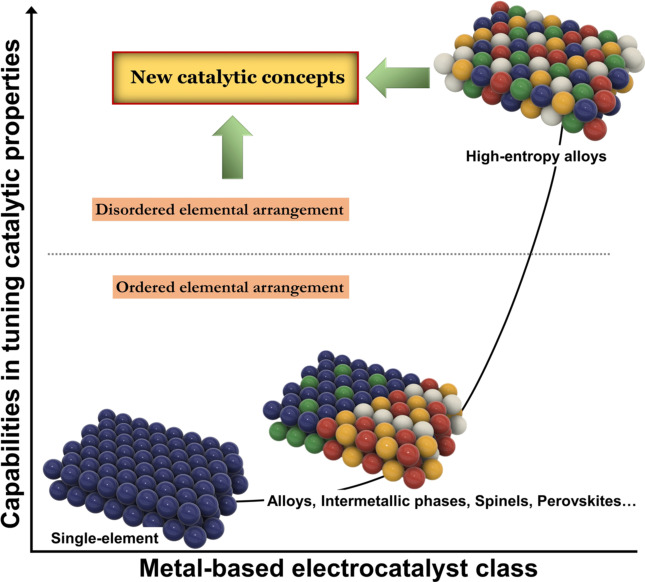
A significant achievement in the design of electrocatalysts is the identification of the correlation between binding energies of reactants/intermediates and activity [1] as a basis to rationalise experimental findings. Tailoring the binding energy by alloying, either in simple alloys [2] or through 3D architectures, [3] allows optimising activity due to the induced electronic interactions. One recent endeavour in developing advanced electrocatalysts is to extend this strategy by exploring the vast multidimensional composition space provided by combining the elements of the periodic table to form multinary alloys. Only negligible parts of this multidimensional composition space have been investigated so far, both theoretically and experimentally. This strategy led to the rise of high‐entropy alloys (HEAs) with five or more elements. [4] There is a rapidly increasing number of publications in this field following up the many successful and auspicious results which were achieved and which are summarised in perspectives and reviews.[ 5 , 6 ]
The focus of these reviews is generally on synthesis routes and related results of activity and stability measurements. Yet, the success is not only based on finding new synergies based on the existing concepts but, more importantly, by enabling a new concept of how the binding energies of reactants, intermediates, and products can be tailored. Many HEAs form a single‐phase compositionally complex solid solution (CCSS) provided suitable synthesis conditions. This can be either accomplished by solely making use of entropic stabilisation. In the case that this is not sufficient, mixing of elements which are not thermodynamically mixable can be achieved by applying non‐equilibrium methods. [7] When a CCSS is successfully formed, all elements are statistically distributed due to entropic stabilisation and multielement active sites are naturally formed on the surface of these materials. Since the binding energy of an active site with the reactants is also governed by the interaction with neighbouring atoms, every active site is different because of different elemental environments. This fundamental difference to any other catalyst class is the basis to explain many distinctive properties and for consideration as a paradigm‐changing concept (“from applying the materials you have to engineering the materials you need”).[ 6 , 8 ]
In this Minireview, we summarise and focus on the conceptual implications of this unique CCSS structure. Chemical intuition approaches for HEAs already yielded catalysts that can compete with state‐of‐the‐art electrocatalysts. We like to emphasise the promising prospect regarding the possibilities when catalyst design can be performed more systematically, which is an essential next step owing to the almost unlimited number of catalyst designs within this class.
2. Results and Discussion
2.1. Terminology—Distinction between “HEA” and “CCSS”
The frequently used term for this catalyst class is “high‐entropy alloy” (HEA) based on the term used in material science. We like to emphasise that additional consideration should be taken into account since there is no general agreement on how HEAs are defined. Initially, the motivation was to increase entropy upon alloying more and more elements. Hence, an initial definition is composition based and requires at least five principal elements with molar fractions of 5–35 at. %. [9] However, these limits were arbitrarily chosen, and a single solid solution phase is not ensured. Therefore, an alternative definition purely refers to entropy, by defining HEAs as alloys that show configurational entropies of above 1.61 R (R=gas constant), with a later refinement lowering the limit to a still arbitrary value of 1.5 R. [10] However, this definition still does not guarantee the presence of a single solid‐solution phase, especially since no temperature effect is included. These HEA definitions do not guarantee the existence of a CCSS phase with a variety of binding sites, which is the decisive feature for the exciting features in the context of electrocatalysis. There are HEAs that form intermetallic or multiple phases, and there are materials with a single CCSS phase, which are not covered by either of the definitions of HEAs. The same holds true for other terms such as “complex concentrated alloys”, “compositionally complex alloys” or “multi‐principal element alloys”, “polyelemental” or “multinary alloys”. For this reason, we like to emphasise that alloying five or more elements is no guarantee to yield a single solid‐solution phase. Calling the alloy a HEA is correct, yet it is not sufficient to specify which catalytic concepts apply. Therefore, to be more precise, we use the term CCSS in this work. This definition covers all alloys that form a single‐phase solid solution and thus, also includes some binary or ternary systems that are not HEAs. Its name arises from the complexity of the structure, where there is no repetitive pattern of elemental arrangement. CCSSs become increasingly stabilised with a higher number of alloying elements, [11] and CCSSs often refer to five or more elements, where the CCSS phase becomes more likely and where the variety in the nature of the active sites becomes significantly increased. Conclusively, the special properties arise upon formation of a CCSS phase, which can be accomplished by increasing entropy, which is the main idea behind HEAs. HEAs refer to the element combination and composition, CCSSs to the phase structure. Utilisation of these terms in electrocatalysis usually assumes both features apply simultaneously, hence just using one term is fine and HEA has been established more widely. Yet the implementation of the CCSS phase should be given in the definition of the respective work and its structure verified when referring to the special properties discussed in the following context.
2.2. CCSS Materials as a New Electrocatalyst Class with Unique Features
Several unique features arise upon the formation of CCSS phases:
Fine‐tailoring of binding energies and reaction‐independent applicability: Since the binding energy is a result of many short‐range and long‐range interactions of the binding atom(s) with all neighbouring atoms, an active site can consist of any possible arrangement of the constituent elements. Hence, there is immense flexibility in adjusting the binding energy by selecting constituent CCSS elements and modifying their composition, which gives rise to an almost continuous distribution of binding energies. Since the composition and the selection of the elements can be optimised to adapt to any possible predefined binding energy, different optimisation pathways can be followed for any envisioned electrochemical reaction. Once the atomic arrangements corresponding to specific binding energies are known theoretically, an ideal CCSS catalyst can be designed and synthesised.
Multifunctionality: The active sites are grouped in several “binding peaks” and each peak acts as an almost independent catalytic unit. This feature allows for unique opportunities, for instance, in catalysing multiple reactions at the same surface, which is essential for cascade reactions such as the CO2 reduction reaction. Furthermore, this feature affects the scaling relation. Therefore, this catalyst class might provide otherwise inaccessible characteristics for many key energy conversion reactions, and the most important limitations of conventional catalysts do not necessarily apply for CCSS catalysts.
Element flexibility: Even though poorly understood, interelemental interactions in CCSSs are complex. The first results suggest that the limitations of the single elements do not directly transfer to the CCSS phase. Therefore, it might be possible to replace noble metals with more abundant elements without decreasing performance. Additionally, since there might be multiple element combinations or compositions with similar properties, there may be many suitable catalysts per reaction. These features make it possible to adjust the catalyst design to address the ever‐increasing economic, ecological, and ethical concerns and to react to changes in the market.
A different selectivity concept: Due to the multifunctionality, it becomes more challenging to optimise reactions, where multiple reaction products are possible for a single reactant. This is because one needs to catalyse the reaction of interest while simultaneously ensuring that there is no prominent second functionality catalysing undesired reaction pathways. This is a challenge for reactions where parasitic side reactions are efficiently catalysed as well. This is the case for reduction reactions at low potentials, which generally compete with the parasitic hydrogen evolution reaction. However, when selectivity depends on at which stage of a series of subsequent reactions the conversion is stopped (cascade reactions), the multifunctionality can ensure that the reaction will continue on another site and can be stopped at the product of choice.
Different stability concept: Since multiple elements and their arrangement in the CCSS phase are required to maintain activity, additional effects can affect phase stability, such as segregation, phase transformation and dealloying. However, these effects on phase stability are counterbalanced at least in part by the entropic stabilisation induced by alloying a high number of elements. This thermodynamically benefits the CCSS phase and causes an additional exceptionally high kinetic solid diffusion barrier. [12] Overall, this leads to the complementary effects of thermodynamic stability at high temperatures and kinetic stabilisation at low temperatures.
However, there are also negative aspects such as:
A lower abundance of relevant active sites on the surface. Although multifunctionality has advantages, it also implies that each functionality is present in a lower amount, i.e., only the sites within the optimised binding peak will significantly contribute to the catalytic current. Their lower probability of existence has to be counteracted by their higher activity.
CCSS materials can be thermodynamically stable or metastable at operating conditions. In the latter case, one needs to synthesise them at conditions such as high temperature or high chemical potential, which favour the CCSS phase, and then apply very fast cooling rates to quench and maintain the CCSS phase by kinetic trapping. The synthesis of CCSS particles while adjusting the composition, size, shape, and morphology is challenging; [13] however, many innovative techniques have been reported recently.[ 14 , 15 , 16 ]
2.3. Fundamental Concepts and Catalyst Design Strategies
2.3.1. Modulation of Binding Energies upon Alloying
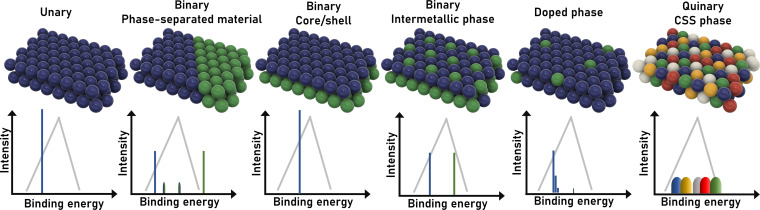
To understand the unique features of CCSS catalysts, the binding energy distribution pattern (BEDP) is the most crucial concept. If one focuses only on on‐top binding of the reactant and assumes an infinite planar crystal surface, for unary catalysts, every binding site is the same, resulting in just one distinct binding energy determined by the intrinsic properties of the element (Figure 1). This assumption is the basis for standard volcano plots in which, for each element, one binding energy is assigned. The addition of a second element and the formation of separated phases yield two peaks in the BEDP of lower intensity corresponding to the individual metal phases. Furthermore, some atoms are positioned at the interphase between both element phases and have different elemental neighbours, resulting in a modulated binding energy. For core/shell particles, every binding site is the same, but the interaction with the different core atoms induces a slight shift in binding energy. For intermetallic phases, the well‐defined arrangement of elements in the crystal structure implies that every atom of a given element has the same neighbour motif, and there will be one binding energy per element. This simple pattern can be extrapolated to higher numbers of elements, that is, a quinary intermetallic phase will provide five distinct binding energies.
Figure 1.
Schematic comparison of various kinds of catalyst surfaces with the corresponding pattern of binding energies for on‐top binding of a reactant on an idealised infinite single‐crystal lattice, without any step sites, edge sites, defects etc. The sum of the intensity for all peaks is the same for each scenario, yet adjusted here for better legibility.
The concept becomes more complex when two elements are randomly mixed across the surface as displayed for the “doped” phase. Since the sites differ whether none, one, or two atoms of the second element are nearest or second nearest neighbours, all these different sites vary slightly in their binding energy. However, since the binding energy is still primarily determined by the binding atom and most of the neighbouring atoms for all sites are the same, there will be only a small number of distinct peaks in the BEDP. The same concept applies to the CCSS phase, but in a more complex way. Now even for the same element representing the binding atom the various neighbour configurations vary much more, resulting in continuous coverage of binding energies via BEDP peak broadening. Since the interaction with the binding atom is the strongest, all sites with the same binding element can be grouped and form one binding peak, that is, for on‐top binding, there will be as many binding peaks within the BEDP as there are elements within the CCSS. [17] This combined almost continuous distribution of binding energies enables the formation of active sites with the ideal binding energy, that is, the ideal activity, which is the fundamental concept for the unique electrocatalytic properties of CCSS‐based electrocatalysts.
2.3.2. Synthesis and Stability of CCSS Phases
The driving force to achieve the formation of a CCSS must be of entropic origin, which favours alloying of a higher number of elements since the mixing entropy increases with the addition of more elements in a logarithmic‐like progression. [18] The choice of elements also plays a significant role in counteracting enthalpic destabilisation, initially summarised in the Hume‐Rothery rules for binary systems, which were extended to HEA solid solutions. [19] Similarities in atom size, electronegativity, preferred crystal structure etc. enhance CCSS formation. Additionally, entropy decreases when departing from equiatomic compositions. This should be considered for composition optimisation, which can only be pursued until the stability limit of the CCSS phase is reached. The thermodynamic limit is determined by the Gibbs free energy ΔG=ΔH−TΔS, which indicates that the entropic stabilisation increases with temperature. This general correlation is exploited in many proposed “entropy‐driven” synthesis routes. Common wet‐synthesis approaches are challenging due to the different decomposition rates of the metal precursors. Hence, a suitable synthesis method will provide all elements decoupled from any precursors at high temperatures or, more generally, at high energies, where the Gibbs free energy is negative for the given element combination and its molar ratio. When the required energy/temperature is low, synthesis can occur at room temperature, or slower cooling rates are possible. Otherwise, very fast cooling rates are required, maintaining the CCSS phase at room temperature by kinetic trapping. [20] Such an approach can also yield metastable CCSS catalysts of a low number of elements, with high deviation from equiatomic composition, or with higher enthalpic destabilisation. This provides access to a substantially larger number of possible catalyst designs, described as a “combinatorial explosion”. However, it also offers more possibilities for a decrease in stability due to a possible thermodynamically driven degradation, [21] which may limit long‐term stability. Fortunately, a CCSS‐specific core effect is hypothesised to impede such a process. Sluggish diffusion is related to different atom sizes, which induces lattice strain with uneven lattice positions and the trapping of each atom at its given position.[ 10 , 22 ] Hence, there is an additional energy barrier for phase degradation processes, and there are many examples demonstrating the high electrocatalytic stability of CCSS catalysts.[ 7 , 23 ]
Furthermore, this catalyst class allows the incorporation of more abundant transition metals. However, these often tend to oxidise easier, as observed for CCSS materials with partial oxidation of just one or two elements upon annealing. [24] This should also be considered for reactions where this effect impedes activity. [25]
2.3.3. Structure–Activity Correlation
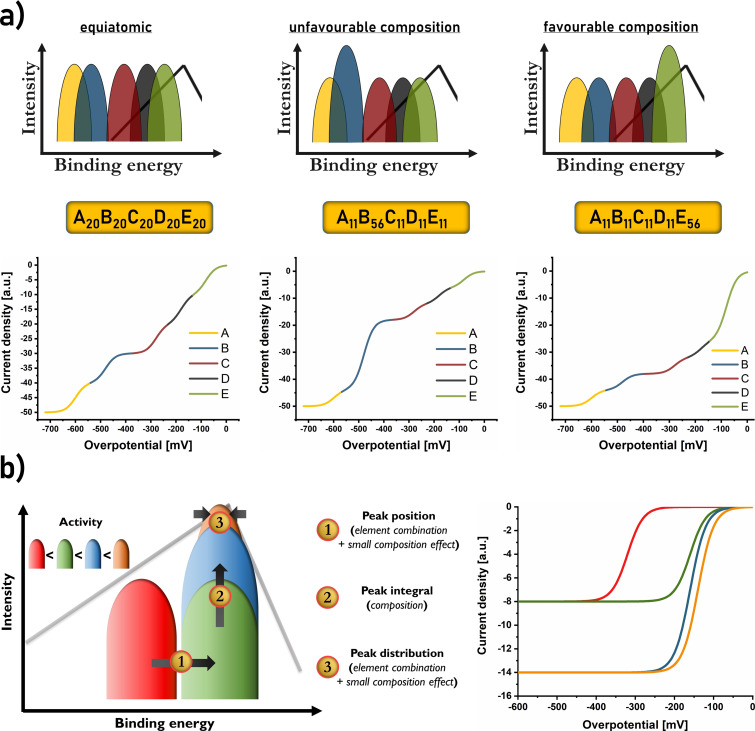
For a quinary alloy, at least five binding peaks in the BEDP are present, and their intensity integral is determined by the probability of the binding atom at the surface. Increasing the molar ration of a given element will likewise increase the number of sites within this peak (Figure 2 a). Moreover, not only on‐top binding, but also twofold or threefold hollow site adsorption is possible, increasing the number of adsorption peaks to 15 or 35, respectively. Recent models suggest that for the oxygen reduction reaction (ORR), the binding geometry will change between on‐top (*OH and *OOH) and threefold hollow site adsorption (*O) for the different intermediates at CCSS surfaces, and hence also the number and nature of binding peaks will vary between the reaction steps.[ 26 , 27 ] This unique concept lowers the probability of the presence of the best binding peak, but also enables unique opportunities to optimise different intermediate steps individually, hence breaking the traditional scaling relation. The combined current response of all sites within one binding peak of the BEDP yields one “current wave” in a voltammogram (Figure 2 a, bottom) with the overpotential related to the binding energy position of the peak maximum and the plateau intensity related to the integral, i.e., the number of sites within the peak.[ 25 , 28 ] Since the plateau current is not due to mass transport limitation but due to the maximum attainable turnover at the respective sites, it is possible to observe more than one current wave in the corresponding voltammograms. In such cases, composition optimisation should be used to increase the number of sites within the first and most active current wave (by increasing the molar ratio of the binding element(s) they all have in common) in order to not rely on contributions of more than the best binding peak. This strategy was shown recently, simultaneously demonstrating the almost independent nature of each binding peak. [25] However, even if just one current wave is visible, composition optimisation is highly valuable since the current linearly scales with the number of sites within the related binding peak.
Figure 2.
a) Schematic illustration of how each CCSS binding peak of the BEDP yields one current wave in voltammetric measurements, where activity and plateau current depend on binding energy shift and peak integral, respectively. Hence, adjusting the composition affects the current wave proportions. In this representation, the absence of any mass‐transport effects allows visibility of all current waves. b) Since the most active current wave is of the highest interest, effects of element combination and composition on the binding peak are presented, and the effect on the corresponding current wave is shown.
This fundamental concept makes it possible to derive a general strategy to optimise the catalytic activity of CCSS materials. Focusing on the best binding peak of each CCSS where it is essential to 1) find an element combination for which this peak is as narrow as possible with a position as close as possible to the optimal binding energy for the reaction of choice, and to 2) optimise the composition to maximise the peak integral while still maintaining the CCSS phase and not violating the first requirement (Figure 2 b). Increasing the peak integral is performed by increasing the molar ratio of the centre atom(s) of the sites forming this binding peak. The limits of this increase vary for each element combination (enthalpy of mixing and number of elements) and cannot be predicted yet. However, with the opportunity to kinetically freeze the CCSS phase by using non‐equilibrium processes such as fast quenching, molar fractions of about 60–70 % of one element are realistic, where the decrease in relevant surface sites compared to a unary catalyst becomes marginal, and the gain in catalytic turnover by fine‐tailoring of the binding energy can significantly surpass this counteracting effect since improvements by orders of magnitudes are possible. Consequently, the catalytic activity of an optimised CCSS can become substantially higher while simultaneously enabling access to more abundant element combinations.
However, the multielement interaction is complex, and predicting a suitable element combination to match these design strategies is not straightforward. The binding atom is still the most relevant. This suggests implementing elements that show promising activity for the target reaction alone, as the so‐far best guess for chemical intuition. Indeed, this approach was applied in many of the successful studies to propose active HEA‐type catalysts for the ORR,[ 29 , 30 ] the oxygen evolution reaction (OER), [31] the hydrogen evolution reaction (HER), [32] CO oxidation, [30] CO reduction, [33] CO2 reduction,[ 33 , 34 ] NH3 synthesis, [14] NH3 decomposition, [35] MeOH oxidation, [36] EtOH oxidation, [37] and Li‐O2 batteries. [16] The unique applicability of CCSS materials to any electrochemical reaction due to the flexibility in which the binding energy can be addressed was successfully demonstrated. However, there is evidence that the position of the individual elements in volcano plots alone is not decisive. In the first model where the BEDP was derived, [17] the order of binding peaks is similar to the order of single elements in ORR volcano plots, but still two positions are reversed, and the Pd‐based binding peak is closest to the volcano top, even though Pt is also present. Furthermore, Cr‐Mn‐Fe‐Co‐Ni showed good activity with respect to the ORR,[ 25 , 28 , 29 ] even though all individual elements are far from a good volcano position. Recent work introduced the importance of Bader charges and thus electron donor–acceptor interactions between the elements to predict binding energy shifts in alloys. [38] The overlap of orbitals, which is affected by atom size differences, will also play a role.
DFT calculations may contain most of this information, and indeed, such a model predicted experimental composition trends accurately. [27] However, it requires a lot of computation power and time to model a single element combination. Given the millions of possible element combinations (2 118 760 possible ways to combine 50 elements to form quinary alloys and 15 890 700 possibilities to combine 50 elements to form senary alloys), having general guidelines at hand would be advantageous. From an experimental perspective, an element combination screening could focus on equiatomic compositions and consider only the overpotential position of the first current wave to identify promising candidates, [28] followed by composition optimisation to increase their current intensity, [25] according to the concept illustrated in Figure 2 a. For this purpose, the inflection point of the first current wave is suggested to be used as the activity descriptor, which can also be applied for cases where only one current wave is visible before any activity‐independent limitation induces a current decline. [25] In this way, the catalyst design can be optimised in terms of element combination and composition. However, properties like particle size, shape, crystallinity, morphology etc. also play a role, which has been largely unexplored to date for CCSS catalysts. Particles in one of the earliest studies [29] were amorphous [39] but still highly active. This is one possible outcome based on the introduced fundamental concepts, as the multielement sites are still present, but its theoretical investigation is challenging since it requires a distinct crystal structure with atoms at fixed positions. In another study, it was suggested that the composition is more important to achieve higher activity than size and crystal structure. [40] For the particle size effect, the increasing contribution of edge and corner sites with decreasing NP diameters implies the enhanced contribution of different geometrical arrangements of sites where the electronic interaction between the different elements is altered and the combined analysis of geometry, electronic interaction, and stability should be considered. Further, it was demonstrated that the huge variety in different sites implies that not all theoretically possible sites can be present at a single NP. [41] Since the immense number of NPs in a catalyst powder averages out this effect, this does not affect the overall performance, yet one should be aware that analysis of a single NP is not necessarily representative for the whole NP population.
2.3.4. Selectivity and Multifunctionality
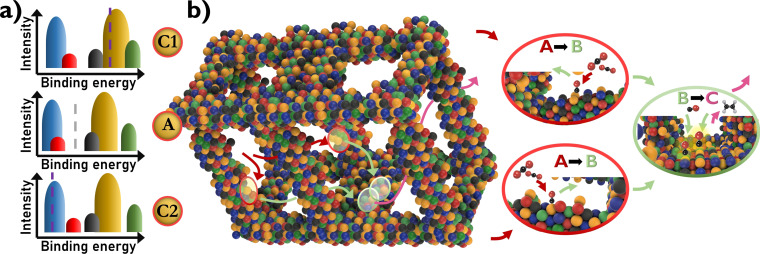
As discussed previously, a quinary CCSS has at least five binding peaks in the BEDP (on‐top binding), implying that binding the reactant at each of the five possible elements will result in a different binding energy group. When multiple reaction products are possible, not only optimisation of the most relevant binding peak becomes essential, but simultaneously, a negligible coverage of undesired binding energies by the other peaks should occur (Figure 3 a – scenario “A”). For different reactants, binding energies of other intermediates might be relevant, and different BEDPs have to be considered. The peak integrals are consistent (for unaltered binding geometries), yet the position and peak distribution might be different. This makes applications for many reactions quite challenging, and guidance by theory becomes valuable. For cascade reactions, this unique feature might provide the solution to obtain products of a higher number of required cascade steps, even with good selectivity. Since the intermediate products desorb from the surface between each step, they can bind at a different site for the subsequent reaction. Hence, the number of functionalities required corresponds to the number of cascade steps involved, which is easily possible with CCSS catalysts. Realisation of such a catalyst design benefits from theoretical guidance to predict which element combinations yield suitable BEDPs. A step in this direction was performed with respect to the CO2 reduction reaction with the general goal to suppress the HER as much as possible while having good CO turnover numbers. Multiple CCSS combinations were tested. Their composition was optimised to reduce binding sites favourable for the HER and simultaneously to increase the number of binding sites for the reduction of CO2 to CO. [33] Ultimately, the formed CO is further reduced to valuable higher‐carbon products in the following reaction step, and favourable binding sites for this step should be considered. An additional requirement is to force the formed CO to bind again at the catalytic surface before its diffusional loss to the bulk of the electrolyte, which would interrupt the reaction sequence. For this goal, the combination of the CCSS concept with a 3D architecture, which traps the formed intermediate products within the catalyst network for the duration of the envisaged reaction sequence to the final product, is proposed (Figure 3 b). For the CO2 reduction reaction, gas diffusion electrodes providing confined internal reaction volumes already assist this strategy.
Figure 3.
a) Schematic BEDPs for C1: first cascade reaction step, A: alternative, undesired reaction, and C2: second cascade reaction step. The vertical lines represent the ideal binding energies for each reaction. The binding energy refers to the respective reactants, which can be different for each step. Thus, the peak pattern is altered. b) Combining the CCSS concept with a 3D architecture to trap formed intermediate products within the catalyst network and force their subsequent cascade reaction until the final product is obtained.
One major issue concerning the limiting reactions for the utilisation of hydrogen as a sustainable energy carrier, namely the ORR and OER, is the scaling relation of reaction intermediates. The scaling relation inherently lowers the catalytic activity by about six orders of magnitude compared to an ideal catalyst, even though catalysts were found with the best compromising binding energies located precisely at the top of the volcano. [42] Since the reaction intermediates do not desorb from the surface, with the exception of H2O2 in the case of the 2+2 electron transfer pathway during the ORR, the cascade concept cannot be applied. However, it was shown recently that the scaling between *O and *OH as well as *OOH is not valid anymore at CCSS surfaces. *O binds at threefold hollow sites, whereas *OH and *OOH bind at on‐top sites. Since the active sites consist of different elements for a CCSS surface, the transition states do not correlate anymore. [26] Still, the scaling between *OH and *OOH persists in this consideration. One central question is whether the scaling between *OH and *OOH can be overcome, for example, by a change in the reaction mechanism or 3D architectures. Making use of the unique multifunctionality of the CCSS class, for example, by utilising any of the earlier proposed strategies,[ 42 , 43 ] can serve as a guideline. For these reactions, the scaling exists due to the binding via the oxygen for all intermediates. For different reactions with an inherent scaling relation but differently bound elements, the multifunctionality of CCSS surfaces may provide a unique way to mitigate this limitation in such a way that different reactants, that is, different binding elements, are correlated to different BEDPs, which provides an interesting avenue for methanol fuel cell catalysts, for instance.
2.3.5. Future Challenges
The heterogeneity in the catalytic surface, namely different elements, their various electronic interactions, strain effects, possible partial oxidation etc. makes the detailed description of the active CCSS surface very complex. The exact structure can be affected by many factors, most notably by element selection and composition, but also by the crystal and microstructure, 3D architecture, other morphological effects, reaction conditions, and more. This makes the analysis of the activity of CCSS catalysts a multivariate challenge which, together with the combinatorial explosion, enables tailoring of the properties in many ways. To systematically control these influencing parameters, the underlying concepts must be considered, and essential key correlations have already been found and are discussed in this Minireview. Furthermore, the mechanical properties of HEAs have been explored for many years.[ 10 , 22 , 44 ] However, research concerning electrochemical properties has just started. Indeed, many open challenges are still unresolved, such as:
Understanding the elemental interactions in order to predict suitable element combinations for each application;
Understanding the composition effect extending the BEDP peak integral aspect, but also understanding the shift in binding energy;
Understanding the composition limits of a single CCSS phase with respect to element choices and exposed conditions while distinguishing between volume and surface composition;
Understanding the stability effect and how to tune it while distinguishing between chemical and mechanical stability;
Understanding the effect of crystal structure, size, morphology etc. on activity, selectivity, and stability;
Developing a database that summarises all information, including calculated and experimental data;
Implementing high‐throughput methods to generate a large amount of empirical data; this implies the availability of theoretical models as well as experimental setups;
Designing suitable synthesis routes, which allow controlling the parameters that tune the electrochemical properties and furthermore allow upscaling to industrial scale
Identifying additional concepts regarding how to overcome the scaling relation, how to master cascade reactions or discovering possible not yet identified capabilities; and
Ultimately finding the optimal electrocatalyst including the next best options for each reaction under application conditions.
For achieving these goals, a strong collaboration between theory (models), (high‐throughput) computation, and experimental verification or empirical data generation and specifically machine learning tools for identification of trends in this very complex and multivariate interaction‐rich catalyst class is very important and rewarding since the huge composition space enabled by the mixing capabilities cannot be explored with standard approaches alone in a reasonable time.
3. Conclusion
The electrocatalyst class of complex solid solutions provides more than extending the present catalyst principles based on more combination options. The accumulation of entropy as described with the HEA concept enables the presence of a single‐phase solid solution where all elements are mixed under the formation of millions of different multielement active sites, which opens up unique concepts not bound to many of the limitations of common electrocatalysts. Owing to the various factors that affect the electrocatalytic properties with almost unlimited options to combine them, it is possible to tailor the properties of the materials in an unprecedented manner. However, control over this process is very complex. Yet, the combinatorial explosion is not a curse but a blessing since, when mastered, the different boundary conditions combined with the immense flexibility will provide access to an explosion of catalyst properties as well. The capabilities in theory include optimisation of properties (activity, stability, selectivity) in an unprecedented manner, simultaneous utilisation of more abundant elements (cost, sustainability), and optimisation of multiple properties almost individually for a given material (enabling new applications). We have highlighted which steps of this process have already been achieved, specifically how the most important structural changes can be exploited to manipulate the electrochemical current response. We have further suggested key goals to be accomplished to push this catalyst class to its limits, which possibly outperform conventional catalysts in many aspects, especially for more complex reactions.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Tobias Löffler studied chemistry at Ruhr University Bochum with one semester abroad at University Helsinki. He completed his PhD in Bochum at the Center for Electrochemical Sciences with Prof. Schuhmann. For his research he was awarded the GDCh “Förderpreis Elektrochemie” and the Jury Award of the “Energieforschungspreis Wasserstoff” of the state of North Rhine Westphalia both in 2020. He is currently a postdoctoral researcher at the Center for Interface‐Dominated High Performance Materials (ZGH) in Bochum.

Biographical Information
Alfred Ludwig is Professor for Materials Discovery and Interfaces at the Institute for Materials of Ruhr University Bochum (RUB). In 1999 he received his Ph.D. in mechanical engineering from University of Karlsruhe. He then worked at the caesar research center in Bonn and became head of the research group “Combinatorial Materials Science” in 2002. At the same time, he started at RUB as junior professor and became full professor in 2012. He initiated and organised the research center ZGH (Center for Interface‐Dominated High Performance Materials) of which he is the scientific director.

Biographical Information
Jan Rossmeisl is Professor of theoretical chemistry and he heads the Center for High Entropy Alloy Catalysis at the Department of Chemistry at Copenhagen University. Before joining the University of Copenhagen in April 2015, he was an Associate Professor and group leader for the theoretical catalysis group at the Department of Physics at the Technical University of Denmark. He holds master's (2000) and Ph.D. (2004) degrees in physics from the Technical University of Denmark. His research interests include electrocatalysis, energy conversion, atomic scale simulations, and rational interface design for catalysis.

Biographical Information
Wolfgang Schuhmann studied chemistry at the University of Karlsruhe, and completed his Ph.D. with F. Korte at the Technical University of Munich in 1986. After finishing his habilitation at Technical University of Munich in 1993, he was appointed professor for Analytical Chemistry at the Ruhr University Bochum in 1996. His research interests cover a broad spectrum of different fields of electrochemistry, including micro‐ and nanoelectrochemistry, scanning electrochemical microscopy and related techniques, biosensors, biofuel cells, and the development of electrocatalysts for energy conversion reactions.

Acknowledgements
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) in the framework of the research unit “UNODE – unusual anode reactions” (FOR 2982 [433304666]) as well as under Germany's Excellence Strategy‐EXC 2033‐390677874‐RESOLV. J.R. thanks the Danish National Research Foundation for funding CHEAC, DNRF‐149. The authors acknowledge helpful discussions with René Bansner and Patrick Wilde. Open Access funding enabled and organized by Projekt DEAL.
T. Löffler, A. Ludwig, J. Rossmeisl, W. Schuhmann, Angew. Chem. Int. Ed. 2021, 60, 26894.
References
- 1. Jiao Y., Zheng Y., Jaroniec M., Qiao S. Z., Chem. Soc. Rev. 2015, 44, 2060. [DOI] [PubMed] [Google Scholar]
- 2. Stephens I. E. L., Bondarenko A. S., Grønbjerg U., Rossmeisl J., Chorkendorff I., Energy Environ. Sci. 2012, 5, 6744. [Google Scholar]
- 3. Mavrikakis M., Hammer B., Nørskov J. K., Phys. Rev. Lett. 1998, 81, 2819. [Google Scholar]
- 4. Tsai C.-F., Wu P.-W., Lin P., Chao C.-G., Yeh K.-Y., Jpn. J. Appl. Phys. 2008, 47, 5755. [Google Scholar]
- 5.
- 5a. Ma Y., Ma Y., Wang Q., Schweidler S., Botros M., Fu T., Hahn H., Brezesinski T., Breitung B., Energy Environ. Sci. 2021, 14, 2883; [Google Scholar]
- 5b. Tomboc G. M., Kwon T., Joo J., Lee K., J. Mater. Chem. A 2020, 8, 14844; [Google Scholar]
- 5c. Li H., Zhu H., Zhang S., Zhang N., Du M., Chai Y., Small Struct. 2020, 1, 2000033; [Google Scholar]
- 5d. Xin Y., Li S., Qian Y., Zhu W., Yuan H., Jiang P., Guo R., Wang L., ACS Catal. 2020, 10, 11280; [Google Scholar]
- 5e. Amiri A., Shahbazian-Yassar R., J. Mater. Chem. A 2021, 9, 782. [Google Scholar]
- 6. Löffler T., Savan A., Garzón-Manjón A., Meischein M., Scheu C., Ludwig A., Schuhmann W., ACS Energy Lett. 2019, 4, 1206. [Google Scholar]
- 7. Li T., Yao Y., Huang Z., Xie P., Liu Z., Yang M., Gao J., Zeng K., Brozena A. H., Pastel G., et al., Nat Catal 2021, 4, 62. [Google Scholar]
- 8.J. Bishop-Moser, D. Miracle, Manufacturing high entropy alloys: Pathway to industrial competitiveness, 2018.
- 9. Yeh J.-W., Chen S.-K., Lin S.-J., Gan J.-Y., Chin T.-S., Shun T.-T., Tsau C.-H., Chang S.-Y., Adv. Eng. Mater. 2004, 6, 299. [Google Scholar]
- 10. Pickering E. J., Jones N. G., Int. Mater. Rev. 2016, 61, 183. [Google Scholar]
- 11. Yao Y., Huang Z., Hughes L. A., Gao J., Li T., Morris D., Zeltmann S. E., Savitzky B. H., Ophus C., Finfrock Y. Z., et al., Matter 2021, 4, 2340. [Google Scholar]
- 12. Tsai K.-Y., Tsai M.-H., Yeh J.-W., Acta Mater. 2013, 61, 4887. [Google Scholar]
- 13. Buonsanti R., Loiudice A., Mantella V., Acc. Chem. Res. 2021, 54, 754. [DOI] [PubMed] [Google Scholar]
- 14. Yao Y., Huang Z., Xie P., Lacey S. D., Jacob R. J., Xie H., Chen F., Nie A., Pu T., Rehwoldt M., et al., Science 2018, 359, 1489. [DOI] [PubMed] [Google Scholar]
- 15. Bondesgaard M., Broge N. L. N., Mamakhel A., Bremholm M., Iversen B. B., Adv. Funct. Mater. 2019, 29, 1905933. [Google Scholar]
- 16. Wang X., Dong Q., Qiao H., Huang Z., Saray M. T., Zhong G., Lin Z., Cui M., Brozena A., Hong M., et al., Adv. Mater. 2020, 32, 2002853. [DOI] [PubMed] [Google Scholar]
- 17. Batchelor T. A. A., Pedersen J. K., Winther S. H., Castelli I. E., Jacobsen K. W., Rossmeisl J., Joule 2019, 3, 834–835. [Google Scholar]
- 18. Yeh J. W., Chen Y. L., Lin S. J., Chen S. K., Mater. Sci. Forum 2007, 560, 1. [Google Scholar]
- 19. Pei Z., Yin J., Hawk J. A., Alman D. E., Gao M. C., npj Comput Mater 2020, 6, 50. [Google Scholar]
- 20. Ludwig A., Matter 2021, 4, 2100. [Google Scholar]
- 21.
- 21a. Ferrari A., Körmann F., Appl. Surf. Sci. 2020, 533, 147471; [Google Scholar]
- 21b. Bracq G., Laurent-Brocq M., Perrière L., Pirès R., Joubert J.-M., Guillot I., Acta Mater. 2017, 128, 327. [Google Scholar]
- 22. Miracle D. B., Senkov O. N., Acta Mater. 2017, 122, 448. [Google Scholar]
- 23.
- 23a. Zhang D., Zhao H., Wu X., Deng Y., Wang Z., Han Y., Li H., Shi Y., Chen X., Li S., et al., Adv. Funct. Mater. 2021, 31, 2006939; [Google Scholar]
- 23b. Jin Z., Lyu J., Zhao Y.-L., Li H., Lin X., Xie G., Liu X., Kai J.-J., Qiu H.-J., ACS Mater. Lett. 2020, 2, 1698. [Google Scholar]
- 24.
- 24a. Li Y. J., Kostka A., Savan A., Ludwig A., J. Alloys Compd. 2018, 766, 1080; [Google Scholar]
- 24b. Li Y. J., Savan A., Kostka A., Stein H. S., Ludwig A., Mater. Horiz. 2018, 5, 86. [Google Scholar]
- 25. Löffler T., Waag F., Gökce B., Ludwig A., Barcikowski S., Schuhmann W., ACS Catal. 2021, 11, 1014. [Google Scholar]
- 26. Pedersen J. K., Batchelor T. A. A., Yan D., Skjegstad L. E. J., Rossmeisl J., Curr. Opin. Electrochem. 2021, 26, 100651. [Google Scholar]
- 27. Batchelor T. A. A., Löffler T., Xiao B., Krysiak O. A., Strotkötter V., Pedersen J. K., Clausen C. M., Savan A., Li Y., Schuhmann W., et al., Angew. Chem. Int. Ed. 2021, 60, 6932; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 7008. [Google Scholar]
- 28. Löffler T., Savan A., Meyer H., Meischein M., Strotkötter V., Ludwig A., Schuhmann W., Angew. Chem. Int. Ed. 2020, 59, 5844–5850; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 5893–5900. [Google Scholar]
- 29. Löffler T., Meyer H., Savan A., Wilde P., Garzón Manjón A., Chen Y.-T., Ventosa E., Scheu C., Ludwig A., Schuhmann W., Adv. Energy Mater. 2018, 8, 1802269. [Google Scholar]
- 30. Qiu H.-J., Fang G., Wen Y., Liu P., Xie G., Liu X., Sun S., J. Mater. Chem. A 2019, 7, 6499. [Google Scholar]
- 31.
- 31a. Glasscott M. W., Pendergast A. D., Goines S., Bishop A. R., Hoang A. T., Renault C., Dick J. E., Nat. Commun. 2019, 10, 2650; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31b. Qiu H.-J., Fang G., Gao J., Wen Y., Lv J., Li H., Xie G., Liu X., Sun S., ACS Mater. Lett. 2019, 1, 526. [Google Scholar]
- 32.
- 32a. Wu D., Kusada K., Yamamoto T., Toriyama T., Matsumura S., Gueye I., Seo O., Kim J., Hiroi S., Sakata O., et al., Chem. Sci. 2020, 11, 12731–12736; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32b. Gao S., Hao S., Huang Z., Yuan Y., Han S., Lei L., Zhang X., Shahbazian-Yassar R., Lu J., Nat. Commun. 2020, 11, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pedersen J. K., Batchelor T. A. A., Bagger A., Rossmeisl J., ACS Catal. 2020, 10, 2169. [Google Scholar]
- 34. Nellaiappan S., Katiyar N. K., Kumar R., Parui A., Malviya K. D., Pradeep K. G., Singh A. K., Sharma S., Tiwary C. S., Biswas K., ACS Catal. 2020, 10, 3658–3663. [Google Scholar]
- 35. Xie P., Yao Y., Huang Z., Liu Z., Zhang J., Li T., Wang G., Shahbazian-Yassar R., Hu L., Wang C., Nat. Commun. 2019, 10, 4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.
- 36a. Tsai C.-F., Yeh K.-Y., Wu P.-W., Hsieh Y.-F., Lin P., J. Alloys Compd. 2009, 478, 868; [Google Scholar]
- 36b. Yusenko K. V., Riva S., Carvalho P. A., Yusenko M. V., Arnaboldi S., Sukhikh A. S., Hanfland M., Gromilov S. A., Scripta Mater. 2017, 138, 22. [Google Scholar]
- 37. Wu D., Kusada K., Yamamoto T., Toriyama T., Matsumura S., Kawaguchi S., Kubota Y., Kitagawa H., J. Am. Chem. Soc. 2020, 142, 13833. [DOI] [PubMed] [Google Scholar]
- 38. Xu X., Guo Y., Bloom B. P., Wei J., Li H., Li H., Du Y., Zeng Z., Li L., Waldeck D. H., ACS Nano 2020, 14, 17704. [DOI] [PubMed] [Google Scholar]
- 39. Garzón-Manjón A., Meyer H., Grochla D., Löffler T., Schuhmann W., Ludwig A., Scheu C., Nanomaterials 2018, 8, 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manjón A. G., Löffler T., Meischein M., Meyer H., Lim J., Strotkötter V., Schuhmann W., Ludwig A., Scheu C., Nanoscale 2020, 12, 23570. [DOI] [PubMed] [Google Scholar]
- 41.E. B. Tetteh, L. Banko, O. A. Krysiak, T. Löffler, B. Xiao, S. Varhade, S. Schumacher, A. Savan, C. Andronescu, A. Ludwig, et al., Electrochem. Sci. Adv. 2021, https://doi.org/10.1002/elsa.202100105.
- 42. Kulkarni A., Siahrostami S., Patel A., Nørskov J. K., Chem. Rev. 2018, 118, 2302. [DOI] [PubMed] [Google Scholar]
- 43.
- 43a. Busch M., Halck N. B., Kramm U. I., Siahrostami S., Krtil P., Rossmeisl J., Nano Energy 2016, 29, 126; [Google Scholar]
- 43b. Montoya J. H., Seitz L. C., Chakthranont P., Vojvodic A., Jaramillo T. F., Nørskov J. K., Nat. Mater. 2017, 16, 70. [DOI] [PubMed] [Google Scholar]
- 44. Li Z., Zhao S., Ritchie R. O., Meyers M. A., Prog. Mater. Sci. 2019, 102, 296. [Google Scholar]