Abstract
Individuals with sex chromosome trisomies ([SCT], XXX, XXY, and XYY)) are at increased risk for neurodevelopmental problems, given that a significant portion of the sex chromosome genes impact brain functioning. An elevated risk for psychopathology has also been described, including attention deficit‐hyperactivity disorder (ADHD). The present study aimed at identifying early markers of ADHD, providing the first investigation of ADHD symptomology in very young children with SCT. The variety, type, and severity of ADHD symptomology in 1–6‐year‐old children with SCT (n = 104) were compared with population‐based controls (n = 101) using the strengths and weaknesses of ADHD symptoms and normal‐behavior (SWAN) parent‐report questionnaire. ADHD symptomology was significantly more prevalent in SCT and already present from toddlerhood on, compared to controls. ADHD inattention symptoms were significantly increased in all karyotypes (XXX, XXY, and XYY), boys with XYY also showed significantly more hyperactivity/impulsivity symptoms than controls. Inattentiveness was more pronounced with increasing age for SCT, in contrast to controls. Within the SCT group, 24% of the children had significantly elevated ADHD symptoms at a clinical level. Already from an early age on, SCT is associated with a risk for ADHD, suggesting that its neurodevelopmental risk lies anchored in early brain maturation. Studying this genetically vulnerable population allows for the prospective study of risk markers to facilitate early and preventive interventions.
Keywords: ADHD, developmental psychopathology, Klinefelter syndrome, sex chromosomes, trisomy X syndrome
1. INTRODUCTION
Sex chromosome trisomies (SCT) are among the most common chromosomal aneuploidies in humans (Hong & Reiss, 2014), with an estimated prevalence around 1 in 650 to 1000 live births (Berglund et al., 2019; Bojesen et al., 2003; Groth et al., 2013; Morris et al., 2008). Karyotypes that result from SCT are 47,XXY (Klinefelter's syndrome) and 47,XYY (XYY syndrome) in males, and 47,XXX (Trisomy X syndrome) in females. Many individuals with SCT experience a significant delay in diagnosis or even nondiagnosis throughout life: estimates of nondiagnosis for all three trisomies range from 12% to 25% (Berglund et al., 2019; Bojesen et al., 2003). Clinically, SCT is characterized by mild, variable, and mostly nonspecific physical features, including minimal facial dysmorphisms, tall stature, and abnormal muscle tone (hypotonia)(Tartaglia et al., 2020). The clinical presentation of SCT is considered to be diverse and heterogeneity in behavioral and cognitive outcomes is rather rule than exception (Giltay & Maiburg, 2010; Groth et al., 2013; Tartaglia et al., 2010). While knowledge of the somatic phenotype of SCT is amply available, more research on specific domains of the neurocognitive and neurobehavioral profile is needed (Pieters et al., 2011). This is especially important considering that a significant fraction of genes on the X chromosome have been linked to brain functioning and X‐chromosome genes are nearly six times more likely to be involved in cognitive performance than genes on the autosomes (Zechner et al., 2001).
Interestingly, neuroimaging studies in individuals with SCT have shown that the X and Y chromosomes impact brain circuits involved in self‐regulation (Hong & Reiss, 2014), which refers to regulation of thoughts, emotions, attention, behavior, and impulses in order to meet goals and adequately respond to the environment (Blair & Diamond, 2008). Such self‐regulatory skills are of great importance with regards to day to day functioning and quality of life, given that optimal self‐regulation promotes positive adjustment and adaptation, as reflected in positive relationships, productivity, achievement, and a positive sense of self (Blair & Diamond, 2008). From a developmental perspective, studies have shown that self‐regulation is associated with important long‐term outcomes such as mental health (Moffitt et al., 2011), social competence (Bradley & Corwyn, 2007; Bradley & Corwyn, 2013), and academic achievement (Eisenberg et al., 2010; Vazsonyi & Huang, 2010), showing that self‐regulation is a vital skill to be acquired in child development. Difficulty with self‐regulation is in line with the types of symptoms of psychopathology that have been described in SCT (van Rijn, 2019), such as autism spectrum disorder (ASD), mood disorders, but especially attention‐deficit/hyperactivity disorder (ADHD).
ADHD is a neurodevelopmental disorder, which is currently defined by a collection of persistent and impaired cognitive and behavioral symptoms, notably inattention, hyperactivity, and impulsivity (DSM‐5: Association, A.P., 2013). Self‐regulation is critical for individuals with ADHD who are challenged in modulating their feelings, thoughts, and responses. With an overall prevalence of 7.2% of ADHD in the general population (Elsabbagh et al., 2012), significantly elevated clinical levels of ADHD symptoms are reported in SCT in several studies across all three karyotypes (van Rijn, 2019). Using dimensional measures across several studies, average estimates of clinical levels of ADHD symptoms are 35% for 47,XXY (with a range of 27%–42%); 49% for 47,XXX (with a range of 27 52%); and 69% for 47,XYY (with a range of 62%–76%). When considering the DSM classification, on average 43% of 47,XXY (with a range of 24%–63%), 49% of 47,XXX (with a range of 25%–49%), and 36% of 47,XYY (with a range of 11%–52%) meet full diagnostic criteria for ADHD. Although the presentation of ADHD‐related symptoms is similarly variable between individuals with SCT, inattentive symptoms are typically the most common in 47,XXY and 47,XXX, whereas 47,XYY boys are likely to present hyperactive/impulsive symptoms as well (Tartaglia et al., 2012).
Previous studies on ADHD symptomology in SCT focused on populations with broad age‐ranges, including participants from middle childhood to adulthood. However, information on early development before the age of 6 years is extremely limited (Urbanus, Swaab, et al., 2020a). This is unfortunate, given that this period in child development is marked by significant advances in brain maturation in typically developing children (Hensch, 2004), making it worthwhile to examine the developmental impact of brain maturation on behavior in a genetically‐at‐risk population. Furthermore, because genetic conditions such as SCT can be identified very early in development (already prenatally), before any clinical behavioral presentation, studying young children with SCT may help to understand the early developmental factors that co‐determine neurobehavioral pathways. This will provide further insights in addition to what we have learned from studying children with psychopathology according to diagnostic criteria based on behavioral presentation. The present study was designed to investigate the developmental impact of SCT on regulation of thoughts, emotions, attention, behavior, and impulses, as expressed in symptoms of ADHD. Rather than considering ADHD as an all‐or‐none phenomenon, the present study was targeted at examining the variation in ADHD symptoms, which may provide a more sensitive measure of early regulation deficiencies in young children.
Taken together, the aim of the present study was to evaluate the developmental impact of SCT on ADHD symptoms, with regards to variety, type, and severity, in an international, sample of young children (1–6 years old), compared to population‐based control sample. To our knowledge, the current study is the first to investigate the neurodevelopmental risks in terms of ADHD symptoms of young children with SCT during toddlerhood and the preschool period. Comparing age‐related differences in a large sample of predominantly prenatally diagnosed SCT children with control peers could provide prospective insight into the early impact of SCT on self‐regulation in the developing young brain. Furthermore, by identifying the recruitment strategy, our study also allowed for an empirical investigation of phenotypic differences within the SCT group. Due to advances in noninvasive prenatal testing technology, it is expected that the number of prenatal diagnoses of SCT will substantially increase over the coming years (Tartaglia et al., 2020). Knowledge on the early development is therefore also greatly needed to guide genetic counseling and improve clinical care.
2. METHODS
2.1. Participants
The current study is part of a larger international longitudinal study (the TRIXY Early Childhood Study, at Leiden University in the Netherlands, including research sites in the Netherlands and the United States of America [USA]). The TRIXY Early Childhood Study investigates the social, emotional, and behavioral development of young children with a trisomy of the X/Y chromosomes (TRIXY, https://www.universiteitleiden.nl/en/social‐behavioural‐sciences/education‐and‐child‐studies/trixycenterofexpertise). For the current study, children aged 1 up to and including 6 years (at baseline) were included.
In total, 104 children with SCT with a mean age of 43.85 months (SD = 22.57, range 11–86 months) and 101 population‐based controls (control group = CG) with a mean age of 43.30 months (SD = 19.50, range 12–77 months) participated with their primary caregiver. Parental education of the primary caregiver, assessed using the Hollingshead ratings of educational attainment, showed that most of the primary caregivers had at least a post high school degree or training (SCT: MD = 5.95, SD = 1.16, CG: MD = 5.61, SD = 1.39). The SCT and CGs did not differ significantly with regards to age (t[203] = 0.186, p = 0.852) nor parental education (t[203] = 1.891, p = 0.060). However, group differences existed with regards to gender distribution (i.e., as expected the CG included significantly more girls than the SCT group: X 2 [1, N = 205] = 12.698, p < 0.001). As for the timing of SCT diagnosis, 71 children (68.3%) had a prenatal diagnosis (i.e., because of [routine] prenatal screening, abnormal ultrasound findings, or advanced maternal age) versus 33 children (31.7%) with a postnatal diagnosis (i.e., because of developmental delay, physical and/or growth problems, or medical concerns). More than half of children with XXY did not receive testosterone replacement therapy (51.0%, n = 25) at any given time in their development. With regards to ADHD‐diagnosis in the family, parental reports showed that in the SCT group 13 parents (12.5%) and seven siblings (6.7%) had a diagnosis of ADHD.
Children with SCT were recruited from two sites: first, the Trisomy of the X and Y chromosomes (TRIXY) Center of Expertise at Leiden University in the Netherlands (n = 46) that recruited children from all Dutch‐speaking countries in Western Europe, and second, the eXtraordinarY Kids Clinic in Developmental Pediatrics at Children's Hospital Colorado in Denver, USA (n = 58) that recruited children from across the USA. Recruitment of children with SCT took place with the help of clinical genetics departments, pediatricians, and national advocacy or support groups for (parents of) individuals with SCT with recruitment flyers and postings on the internet (e.g., TRIXY website and the eXtraordinarY Kids Facebook page). For the SCT group, ascertainment bias was recorded and three subgroups were identified: (a) “active prospective follow‐up” (51.0% of the SCT group), (b) “information seeking parents” (29.8% of the SCT group), and (c) “clinically referred cases” (19.2% of the SCT group). Control participants were recruited from elementary schools and daycare centers from the western part of the Netherlands.
All participants were Dutch‐ or English‐speaking (child and parent) and without history of traumatic brain injury, severely impaired hearing or sight, or colorblindness. For children in the SCT group, trisomy in at least 80% of the cells was confirmed by standard karyotyping. Researchers requested parents to present a copy of the karyotyping report of the child that was provided by their clinician at time of diagnosis. Karyotyping of the child was done by clinical genetic departments, based on the appropriate guidelines for chromosomal karyotyping. The controls were not subjected to genetic screening, due to ethical reasons. These children were considered a representation of the general population and given the prevalence of SCT is ~1 in 1000, the risk of having one or more children with undiagnosed SCT in the control group was considered minimal and acceptable.
2.2. Ethics and procedure
This study was approved by the Medical Research and Ethical Committee of Leiden University Medical Center in the Netherlands and the Colorado Multiple Institutional Review Board (COMIRB) in the USA. Researchers from Leiden University were responsible for project and data management (i.e., training and supervision of researchers, processing, and scoring of data). Written informed consent was obtained from all parents/guardians. The primary caregiving parent (92% mother) of the child completed the questionnaires, either in Dutch or in English, using the online survey software Qualtrics (http://www.qualtrics.com/).
2.3. Instruments
2.3.1. ADHD symptoms
The strengths and weaknesses of ADHD symptoms and normal‐behavior (SWAN) were selected as a screening tool for ADHD symptomology. The SWAN is a parent‐report questionnaire designed to reflect the entire range of attention skills in both nonclinical as well as clinical populations (Swanson et al., 2012). The SWAN rating scale provides a continuous distribution of both positive and negative evaluations of attention behaviors (Polderman et al., 2007; Swanson et al., 2012), by using a 7‐point scale anchored to average behavior (i.e., far below average = 3, below average = 2, somewhat below average = 1, average = 0, somewhat above average = −1, above average = −2, and far above average = −3). The questionnaire consists of 18 items that reflect the 18 DSM‐5 ADHD symptoms, divided into two subscales of nine items corresponding to the domains of inattention (items 1–9) and hyperactivity/impulsivity (items 10–18). Positive scores indicate parental report of experienced difficulty in attentional skill above average, whereas negative scores indicate better skills than average. The mean of all 18 item scores results in the combined scale score and there are also mean total subscale scores on the inattention items and the nine hyperactive/impulsive items. The scales reportedly show good internal consistency, validity, and reliability in different international samples and studies (Polderman et al., 2007; Swanson et al., 2012, 2017). In the current study, the internal consistency coefficients were 0.91 (combined), 0.87 (inattention), and 0.87 (hyperactivity/impulsivity) which indicate good to excellent internal validity.
2.4. Statistical analyses
2.4.1. Raw scores
For analyses, raw scores on the SWAN were used to compare SCT and CG children. Furthermore, to assess for clinical risk, a cut‐off score was used. The cut‐off scores for the SWAN subscales were calculated following guidelines of Swanson et al. (2012), the developers of the SWAN, by using the mean + 1.65 SD (in z‐scores) from the control sample as cut‐off. This method has been verified across studies with differential methodologies and proven useful in identifying the abnormal prevalence of ADHD symptoms in 4% of the population (for review: see Brites et al. (2015). In the current study, cut‐off scores were 0.52 (inattention subscale), and 0.79 (hyperactivity/impulsivity subscale) and resulted in either “below” or “at‐risk” category. Also, because the items on the SWAN contain the exact 18 DSM‐5 diagnostic criteria for ADHD, participants could be categorized into one of the DSM ADHD subtypes. This was done by following the steps taken by Tartaglia et al. (2010), who used the predecessor of the SWAN in a similar sample. Participants were determined to meet criteria for ADHD if they were noted to have moderate to severe symptoms in six of the nine inattentive items (ADHD‐Inattentive subtype), or in six of the nine hyperactive/impulsive items (ADHD‐hyperactive impulsive subtype), or in six of the nine items in both inattentive and hyperactive/impulsive domains (ADHD‐combined subtype).
2.4.2. Age groups
Participants were divided into three groups based on their age: (a) 1–2‐year‐old group (ranging 11–35 months), (b) 3–4‐year‐old group (ranging 36–59 months), and (c) 5–6‐year‐old group (ranging 60–86 months).
2.4.3. Analyses
Statistical Package for the Social Sciences (SPSS) version 25 was used for statistical analyses. General group comparisons were performed using independent sample t‐tests. Correlations between age and ADHD symptoms within research groups were investigated using Pearson's correlation analysis. Group differences in ADHD symptoms were examined using univariate analysis of variance (ANOVA), within each of the specific age group, to investigate developmental trajectory. In addressing the relation between age and ADHD symptoms, correlational analyses within the SCT were performed without IQ as a covariate. This approach was based on the work of Dennis et al. (2009) who have argued that correcting for IQ may obscure developmental vulnerabilities that are due to shared processes in terms of overall brain development, resulting in type 2 errors (false negatives). Effect sizes for t‐test analyses were calculated with Cohen's d, with 0.2 being a small, 0.5 being a medium and 0.8 being a large effect (Cohen, 1977). Level of significance was set at p ≤ 0.05, two‐tailed. After analyses were completed, participants with extreme outliers on one of the three SWAN variables (Z > 3) were identified and all analyses were rerun without these participants to assess their influence on the results. When applicable, the influence of the outliers is described in the results section.
3. RESULTS
3.1. ADHD symptomology
Table 1 shows the mean scores of the SCT group and control group on the SWAN rating scale. Independent samples t‐tests were used to test the differences in mean scores on the SWAN (sub)scales between groups. Children with SCT had significantly more ADHD symptoms in general and specifically more inattentive ADHD symptoms than controls, with medium effect sizes (see Table 1). For the hyperactive/impulsive ADHD symptoms, there was no significant difference in mean scores between children with SCT and controls.
TABLE 1.
Means, SD, and t‐test statistics for SCT and CG groups for ADHD symptoms
| SCT (N = 104) | CG (N = 101) | Group differences | |||
|---|---|---|---|---|---|
| M ± SD | M ± SD | t | p | Cohen's d | |
| SWAN‐combined | 0.03 ± 0.68 | −0.30 ± 0.52 | 3.95 | <0.001 | 0.6 |
| SWAN‐inattention | 0.09 ± 0.72 | −0.45 ± 0.59 | 5.78 | <0.001 | 0.8 |
| SWAN‐hyperactive/impulsive | −0.02 ± 0.75 | −0.15 ± 0.57 | 1.46 | 0.146 | 0.2 |
Note: Negative means represent scores above average on the SWAN, as impairment is rated as more positive.
Abbreviations: CG, control group; SCT, sex chromosome trisomies; SWAN, Strengths and Weaknesses of ADHD symptoms and Normal‐behavior.
3.2. Karyotypes
To examine whether these increased ADHD symptoms were present in all SCT karyotypes, separate independent samples t‐tests were performed for each karyotype to test for mean differences on the SWAN (sub)scales. Children with SCT were compared to their control peers matched on gender (XXX vs. XX, XXY vs. XY, and XYY vs. XY). Descriptives and t‐test statistics are given in Table 2.
TABLE 2.
ADHD symptoms between groups: karyotype‐specific comparisons
| XXX (N = 33) | XX (N = 57) | XXY (N = 49) | XY (N = 44) | XYY (N = 22) | XY (N = 44) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M ± SD | M ± SD | p | d | M ± SD | M ± SD | p | d | M ± SD | M ± SD | p | d | |
| SWAN combined | 0.09 ± 0.65 | −0.33 ± 0.56 | <0.01 | 0.7 | −0.18 ± 0.60 | −0.26 ± 0.46 | n.s. | – | 0.41 ± 0.72 | −0.26 ± 0.46 | <0.001 | 1.1 |
| SWAN inattention | 0.21 ± 0.74 | −0.46 ± 0.63 | <0.001 | 1.0 | −0.11 ± 0.63 | −0.43 ± 0.53 | <0.01 | 0.5 | 0.33 ± 0.78 | −0.43 ± 0.53 | <0.001 | 1.1 |
| SWAN hyperactive/impulsivity | −0.03 ± 0.70 | −0.20 ± 0.61 | n.s. | – | −0.24 ± 0.67 | −0.09 ± 0.52 | n.s. | – | 0.50 ± 0.75 | −0.09 ± 0.52 | <0.001 | 0.9 |
Note: Negative means represent scores above average on the SWAN, as impairment is rated as more positive.
Abbreviation: SWAN, Strengths and Weaknesses of ADHD symptoms and Normal behavior.
For girls with XXX, parents reported significantly more ADHD symptoms in general and specifically more inattentive ADHD symptoms compared to control girls, with medium to large effect sizes. There was no significant difference with the control girls for hyperactive/impulsive ADHD symptoms (p = 0.211). For boys with XXY, parents also reported significantly more inattentive ADHD symptoms compared to control boys, with a medium effect size. There were no significant differences with the control boys for hyperactive/impulsive ADHD symptoms (t = −1.248, p = 0.215) and total ADHD symptoms (t = 0.746, p = 0.458). Finally, for boys with XYY, parents reported significantly more ADHD symptoms in general compared to control boys, with difficulties in both the inattention domain as the hyperactive/impulsive domain, with large effect sizes. Thus, increased ADHD symptoms were found in all karyotypes with difficulties primarily in the inattention domain. In the XYY group, the ADHD symptoms were more pronounced and included problems in the hyperactive/impulsive domain, in addition to inattention difficulties.
3.3. Age‐related effects
Given the finding that increased ADHD symptoms were present in all children with SCT with only minor karyotype‐specific differences and the fact that all ages were represented equally across karyotypes, we were able to investigate the impact of age on ADHD symptoms in the total SCT group, above and beyond karyotype. To examine the developmental trajectory of ADHD symptoms in children with SCT and controls, separate correlation analyses were performed within the SCT and control groups between age and the three SWAN (sub)scales. The results showed a significant correlation between age and inattentive ADHD symptoms in the SCT group (r = 0.234, p < 0.02), whereas no such relationship existed in the control group (r = 0.022, p = 0.824). Using the Fisher r‐to‐z transformation, the significance of the difference between the two correlation coefficients was tested and yielded a borderline significant difference in strength of the correlation (z = 1.53, one‐tailed p = 0.063). Furthermore, in both groups there was no significant relationship between age and total ADHD symptoms (SCT: r = 0.158, p = 0.109, CG: r = −0.040, p = 0.681), and age and hyperactive/impulsive ADHD symptoms (SCT: r = 0.060, p = 0.519, CG: r = −0.098, p = 0.331). In other words, inattentive ADHD symptoms increased with age for children with SCT, while for controls ADHD symptoms were not related to age and appeared to present relatively similar across ages. To further identify which specific age groups may drive differences between SCT and controls in terms of cross‐sectional age trajectory, participants were divided into three age‐groups (1–2 years, 3–4 years, 5–6 years) and separate post‐hoc ANOVAs were performed within each age‐group with ADHD symptoms (SWAN combined scale, SWAN inattention subscale, SWAN hyperactive/impulsive subscale) as dependent variables and research group (SCT vs. CG) as independent variable. Table 3 shows the descriptive statistics for all ANOVAs (also see Figure 1).
TABLE 3.
ADHD symptoms across age groups
| 1–2‐year‐olds | 3–4‐year‐olds | 5–6‐year‐olds | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCT (N = 35) | CG (N = 31) | SCT (N = 40) | CG (N = 43) | SCT (N = 29) | CG (N = 27) | |||||||||
| M ± SD | M ± SD | p | d | M ± SD | M ± SD | p | d | M ± SD | M ± SD | p | d | |||
| SWAN combined | −0.04 ± 0.55 | −0.28 ± 0.51 | n.s. | – | −0.05 ± 0.61 | −0.33 ± 0.53 | <0.05* | 0.5 | 0.24 ± 0.86 | −0.27 ± 0.52 | <0.02* | 0.7 | ||
| SWAN inattention | −0.06 ± 0.47 | −0.50 ± 0.64 | <0.01* | 0.8 | −0.01 ± 0.63 | −0.44 ± 0.54 | <0.01* | 0.7 | 0.38 ± 0.98 | −0.40 ± 0.62 | <0.01* | 1.0 | ||
| SWAN hyperactivity/impulsivity | −0.03 ± 0.71 | −0.05 ± 0.50 | n.s. | – | −0.09 ± 0.67 | −0.23 ± 0.65 | n.s. | – | 0.09 ± 0.89 | −0.15 ± 0.53 | n.s. | – | ||
Note: Negative means represent scores above average on the SWAN, as impairment is rated as more positive. Effect sizes displayed in Cohen's d.
Abbreviation: SWAN, Strengths and Weaknesses of ADHD symptoms and Normal behavior.
*Significant at p < 0.05.
FIGURE 1.
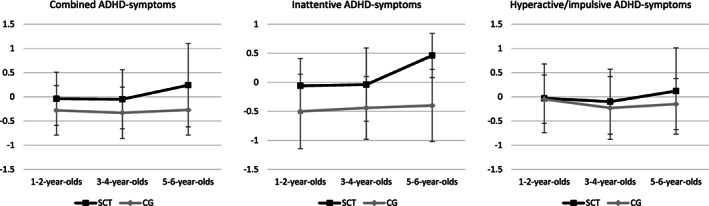
Mean scores for ADHD‐symptomatology at different ages: SCT versus CG. ADHD, attention deficit‐hyperactivity disorder; CG, control group; SCT, sex chromosome trisomies
In the 1–2‐year‐old age group, univariate ANOVAs for the SWAN (sub)scales indicated significant differences between SCT and controls for inattentive ADHD symptoms only, with a large effect size. No significant group differences between SCT and controls were found for total ADHD symptoms or hyperactive/impulsive ADHD symptoms. In other words, 1–2‐year‐olds with SCT did not show increased hyperactivity or impulsivity but showed more inattentiveness as compared to controls. In the 3–4‐year‐old and 5–6‐year‐old groups, univariate ANOVAs for the SWAN (sub)scales indicated significant differences for total ADHD symptoms and inattentive ADHD symptoms, with medium effect sizes in the younger group and large effect sizes in the older group. No significant group differences were found for hyperactive/impulsive ADHD symptoms, indicating that in 3–6‐year‐olds, children with SCT show similar amounts compared to controls. In other words, the 3–6‐year‐old children with SCT did not show increased hyperactivity or impulsivity compared to controls but showed more ADHD symptoms in general and more inattentive behaviors.
3.4. Clinical risk
In addition to average outcomes, we were also interested how many of the children with SCT had scores above clinical cut‐off, indicating the severity of ADHD symptoms and the increased risk for ADHD symptomatology. Based on the cut‐off score (calculated by taking the Z + 1.65 SD in the CG of each subscale), the number of children with SCT above cut‐off were divided by the total number of SCT participants. Results indicated that 24.0% of the children with SCT showed significant inattentive ADHD symptoms, along with 10.6% of the SCT children having hyperactive and impulsive ADHD symptoms above clinical cut‐off.
A further examination of this group of children revealed that most of the children were older than 5 years. Because the SWAN questionnaire contains the 18 DSM‐5 diagnostic criteria for ADHD, it was also possible to categorize these children, based on the parental report scores, into one of three subtypes of ADHD (similar to the DSM‐5 subtypes): ADHD‐inattentive subtype, ADHD‐hyperactive/impulsive subtype, and ADHD‐combined subtype. A fourth category was included that represented no ADHD. For this sub‐analysis, we only examined children with SCT of 5 years and older. Almost half (44.8%) of the 5–6‐year‐old children met behavioral criteria of ADHD, with 31.0% predominantly inattentive symptoms and 13.8% presenting both inattentive and hyperactive/impulsive symptoms (combined).
3.5. Additional analyses (ascertainment bias and recruitment site)
3.5.1. Ascertainment bias
To examine whether ascertainment bias was relevant to the increased risk for ADHD symptoms, three separate between‐subjects ANOVAs were performed with ADHD symptoms (SWAN combined, inattention, and hyperactivity/impulsivity (sub)scales) as dependent variables and ascertainment bias within the SCT group (prospective follow‐up, information seeking parents, clinically referred cases) as independent variable. Because age did not significantly differ between the three groups, it was not included as a covariate in the analysis. There were no significant differences in degree of ADHD symptoms (Pillai's trace = 0.052, F[6200] = 0.884, p = 0.508): how children enrolled in the study did not appear to affect the degree of ADHD symptoms (also see Table 4).
TABLE 4.
Differences in ADHD symptoms: ascertainment bias within the SCT group
| Prospective follow‐up (N = 53) | Information seeking parents (N = 31) | Clinically referred cases (N = 20) | ||
|---|---|---|---|---|
| M ± SD | M ± SD | M ± SD | p | |
| SWAN combined | −0.03 ± 0.73 | −0.03 ± 0.57 | 0.30 ± 0.68 | 0.142 |
| SWAN inattention | 0.01 ± 0.78 | 0.04 ± 0.51 | 0.37 ± 0.78 | 0.137 |
| SWAN hyperactivity/impulsivity | −0.06 ± 0.75 | −0.10 ± 0.75 | 0.23 ± 0.74 | 0.248 |
Note: Negative means represent scores above average on the SWAN, as impairment is rated as more positive.
Abbreviations: SCT, sex chromosome trisomies; SWAN, Strengths and Weaknesses of ADHD symptoms and Normal‐behavior.
3.5.2. Recruitment site
To examine whether recruitment site was relevant in the increased risk for ADHD symptoms in children with SCT, three separate between‐subjects ANCOVAs were performed with ADHD symptoms (SWAN Combined, Inattention, and Hyperactivity/Impulsivity (sub)scales) as dependent variables and recruitment site (the Netherlands, USA) as independent variable. Because age differed significantly between the two groups (i.e., SCT children from the USA are significantly younger), it was included as a covariate in the analysis as well. There were no significant group differences in ADHD symptoms (Pillai's trace = 0.005, F[6200] = 0.157, p = 0.925): the country from which children were recruited and assessed did not appear to affect the degree of ADHD symptoms. Parenting rating of ADHD symptoms were similar on all three (sub)scales in the two research sites: total ADHD symptoms (NL: M = 0.07, SD = 0.75, USA: M = 0.01, SD = 0.63), inattention ADHD symptoms (NL: M = 0.15, SD = 0.85, USA: M = 0.04, SD = 0.59), and hyperactive/impulsive ADHD symptoms (NL: M = −0.01, SD = 0.76, USA: M = −0.03, SD = 0.74).
4. DISCUSSION
This is one of the first case‐controlled studies investigating the early impact of SCT on regulation of thought and behavior as expressed in ADHD symptomology in a large international sample of young children (1–6 years old). Type and severity of ADHD symptoms were measured using a sensitive, well‐known, and widely used instrument (the SWAN rating scale), which allows for capturing the full range of attentional behaviors that reflect symptoms of ADHD in daily life, not limited to classification of ADHD as an all‐or‐none phenomenon. The current study showed that, on average, the level of ADHD symptoms in SCT was higher than in the general population sample, in the full 1–6 year age range. More specifically, children with SCT had more behavioral challenges in the domain of inattention reported by their parents, indicating more difficulties with regulating their attention. Furthermore, behaviors associated with ADHD increased with age, more strongly so in the SCT group, although differences with control peers were already evident for the youngest age‐group (1–2–year‐olds). From a clinical perspective, 24% of the children with SCT had scores in the clinical range on parent‐report, indicating significantly elevated levels of ADHD symptoms. Levels of ADHD behaviors were largely similar across karyotypes, although boys with an extra Y chromosome showed more and broader impairments than children with an extra X chromosome. In addition to inattention difficulties, boys with 47,XYY also exhibited difficulties with hyperactivity and impulsivity. Ascertainment bias and country of recruitment were not relevant to the increased risk of ADHD symptoms, underlining the robustness of these findings.
The most notable finding of this study is that the increased risk for ADHD‐symptomology previously reported in older children and adults with SCT, was already found in 1–2‐year‐olds with SCT. Previous studies have shown that attentional difficulties are part of the behavioral profile of children with SCT, with predominantly inattention behaviors and to a lesser extent hyperactive and impulsive behavior (Ross et al., 2012; Tartaglia et al., 2012). The current study suggests that these attentional difficulties already exist in very young children with SCT, pointing to a significant neurodevelopmental risk from toddlerhood onward. Given the fact that a significant fraction of the genes on the sex chromosomes are involved in brain development, this elevated risk for attentional difficulties may be one of the first signs that the child's genetic makeup has impacted the brain's development and, more specifically, the brain areas that are important for self‐regulation. The self‐regulation problems in this young SCT group corresponds to what has been described in older children, adolescents, and adults with SCT, in terms of various and diverse symptoms of psychopathology, such as ASD and ADHD. Considering the importance of self‐regulation for adaptive functioning, participation in society, and mental health, these early signs of ADHD symptomology may mark ‘at risk’ developmental pathways within this genetic population. It is important to point out that although differences between children with SCT and controls on average were significant with medium to large effect sizes, only a subgroup of children had scores in the clinical range. Thus, while some parents may already be recognizing some early attentional concerns in their child compared to peers their age, there are also many parents who do not report any or only mild concerns.
Another main finding of this study is that ADHD symptoms were more pronounced with increasing age; in children with SCT, older age was associated with higher levels of inattentive ADHD symptoms, whereas in controls these symptoms presented stable across ages. Looking at the different age‐groups, the presence of ADHD symptoms was the most pronounced in the oldest age‐group of children (5–6‐year‐olds), with large effect sizes. The result that age was significantly related to attentional self‐regulatory difficulties in children with SCT, but not in controls, could reflect increasing problems that may emerge and present more profoundly with age. This relates to what is called the “growing into deficit” phenomenon (Rourke et al., 1983). As a result of neuroanatomical maturation, the functionality of the brain increases which is reflected in behavioral opportunities and advancing neurocognitive functions in the developing child. The development of neurocognitive functions occurs in a relative stepwise pattern, in which the next step is dependent on the succession of previous steps. Early disturbances of the neuroanatomical growth, for a substantial part driven by genetic make‐up, could therefore impact the succession of the upcoming developmental steps. However, the effects of some of these disturbances may only emerge into behavioral difficulties at a later point in time when a developmental task is presented for which the brain is not yet fully equipped. Also, several neurocognitive functions come “online” at different and later stages of development, due to the maturation process of the brain, making it possible that the effect of early disturbances may only become noticeable many years later in development. Albeit cross‐sectionally, our results indicate that self‐regulation problems as expressed in ADHD symptoms may emerge with increasing age in children with SCT, which stresses the importance of a developmental perspective on neurobehavioral outcome in individuals with SCT. Longitudinal studies are needed to provide further clarity on the developmental trajectories.
The current findings contribute to a clearer understanding of the behavioral profile of young children with SCT and specifically show that self‐regulatory difficulties with regards to attention are part of the variability and heterogeneity of the SCT behavioral profile. Studying children with a genetic disposition that can be diagnosed prenatally provides a unique opportunity to examine developmental genetic‐behavioral‐pathways, implementing a prospective approach that goes beyond describing problematic behavior and instead focuses on identifying early markers of “at risk” development, irrespective of outcomes. From a neuropsychological perspective, it is interesting to examine which information processing deficits related to self‐regulation might underly the behavioral profile of children with SCT. Neuro‐imaging studies consistently show neuroanatomical and functional differences relative to control peers (Hong & Reiss, 2014), addressing the relevant research question if and how underlying neurocognitive functions might relate to the behavioral profile of young children with SCT. Earlier studies (Lee et al., 2011; van Rijn & Swaab, 2015) have already shown that difficulties with executive functions present across the lifespan of individuals with SCT (e.g., school‐aged, adolescents, and adults). Moreover, there is also some evidence that executive dysfunction and self‐regulation could be linked, with studies showing associations between impaired executive functions and increasing externalizing behavior problems symptoms of ADHD and ASD (van Rijn & Swaab, 2015). Investigating the early relations between developing neurocognitive functions and the behavioral profile may help in identifying children with SCT who are prone to developing self‐regulatory difficulties and may provide targets for early intervention. Research is also needed to investigate whether the attentional difficulties in SCT are a consequence of problems in other domains (e.g., cognitive, social, or emotional deficits) or whether these difficulties represent a broader impairment in regulatory skills in general. This would be interesting given that preliminary results from the same sample of SCT children showed that the behavioral profile of these children is diverse and heterogeneous (Urbanus, van Rijn, & Swaab, 2020b), suggesting that regulatory difficulties are present and persistent in multiple developmental domains (e.g., social, emotional, and behavioral).
Even though the current study is the first to date that examines the development of a large, international cohort of young children with SCT to well‐matched control peers, our findings should be interpreted in light of several limitations. Due to the limited distribution of children with different karyotypes over the separate age‐groups, specific questions could not be examined. For example, it might have been interesting to examine whether the development of attentional difficulties across ages is similar for different karyotypes. Furthermore, the current study examined cross‐sectional differences with regards to age and attentional behaviors. A longitudinal design is needed to add validity to the developmental outcomes found in this study. Thirdly, although parents were asked to report on any known diagnosis of ADHD in the family, we did not examine the relation between background genes and the vulnerability for ADHD, due to limited sample size and therefore limited power to test this hypothesis. However, now that we have established the increased risk for ADHD in this population, an interesting follow‐up question would be whether a part of the increased risk is attributed to a genetic familiar vulnerability. However, this calls for a meticulous designed study of background loading, in which affected first‐ and second‐degree family members are identified properly and where genetic factors are related to more developmental domains, other than ADHD alone. Lastly, the current study did not examine the effect of early testosterone hormone treatment on the behavioral profile in the SCT subgroup with XXY. Only a randomized and placebo‐controlled trial could provide adequate and reliable insight into the effects of testosterone in infants with Klinefelter: one of which is currently underway (PI Davis, NCT03325647).
The results of this study also have important implications for clinical care. Although the focus of this study was to describe the broad attentional profile of children with SCT, rather than considering ADHD as an all‐or‐nothing clinical phenomenon, and most children with SCT do not have significant problems in this area, a subgroup of children with SCT are at a substantial risk and might meet full diagnostic criteria of ADHD. These results indicate that all professionals working with individuals with SCT should be aware of the broad behavioral profile and provide routine monitoring and screening of (attentional) regulatory difficulties from an early age on. Following clinical standards with regards to ADHD assessment (e.g., diagnostic interviewing, neuropsychological assessment, and collateral information from school and parents), an early recognition of ADHD (symptoms) in children with SCT calls for early intervention and treatment. Specifically neuropsychological assessment could provide useful information on an individual's strengths and weaknesses and his/her accompanying needs. Early intervention is important because our results show that, as compared to children from the general population, ADHD symptoms are found to be more pronounced with increasing age in SCT. No different from children with ADHD without SCT, treatment for children with SCT and a clinical diagnosis of ADHD ought to be multimodal and focused on limiting the impact of the attentional difficulties on development. Above all, psychoeducation and support for parents and (pre)school with frequent follow‐ups should be included in the treatment plan. Although pharmaceutical treatment is often considered part of the treatment plan for ADHD and has been reported to be effective for symptom improvement in older children with SCT and ADHD (Tartaglia et al., 2012), careful consideration is needed when deciding on implementing medication for a child in the preschool age group. It should include balancing the benefits and risk of medication in the important period in brain maturation of these young children. Furthermore, cultural differences in the use of psychostimulants may also apply. Thus, when considering pharmaceutical treatment, parents should seek out consultation and guidance from a licensed psychiatrist or developmental‐behavioral pediatrician with experience in complex neurodevelopmental disorders.
5. CONCLUSION
To conclude, in this study it was found that young children with SCT (47,XXX, 47,XXY, and 47,XYY) are at an increased risk for ADHD symptoms, specifically inattentiveness, and that this risk is already present from toddlerhood onward. The elevated risk is roughly similar across all three karyotypes, with boys with an extra Y chromosome also showing more hyperactive/impulsive symptoms compared to controls. Moreover, the results showed that ADHD symptoms are higher with increasing age in children with SCT, in line with relevant self‐regulation skills coming “on‐line” over the course of neurodevelopment, depending on brain maturation. The current findings suggest that self‐regulatory skills, as expressed in symptoms of ADHD, are already impaired in young children with SCT, leading to the proposition that neurodevelopmental problems are likely anchored in early brain development of individuals with SCT. Furthermore, these insights give rise to the hypothesis that the differential behavioral problems of this population in later development might be associated to early self‐regulatory difficulties. Self‐regulation might be a key factor in explaining behavioral difficulties, also because of its importance in typical development. Future studies are necessary to examine neurocognitive measures of self‐regulation, given that different information processing deficits could relate to the behavioral problems associated with SCT. Moreover, studies with a longitudinal approach could provide insight into the developmental trajectories of young children with SCT and investigate how self‐regulatory skills develop in this population as well as its predictive value over time. Nevertheless, these early signs of self‐regulatory deficits might serve as an at‐risk marker in SCT, allowing the identification of children with at‐risk development and guide preventive and early interventions optimizing outcomes of these children. From a clinical perspective, clinicians should be aware of the neurodevelopmental risk with regards to self‐regulation in children with SCT and monitor the neurodevelopment of these children, given that a significant portion of these children at this young age are already at clinical risk for elevated ADHD symptoms.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This study was funded by a grant from the Dutch Organization for Scientific Research (NWO funding # 016.165.397 to Sophie van Rijn). Work in Colorado was partially supported by infrastructure of NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. Contents are the authors' sole responsibility and do not necessarily represent official NIH views. The authors thank the families that participated in our study, and the research assistants and students for their help with data collection and processing.
Kuiper, K. , Swaab, H. , Tartaglia, N. , & van Rijn, S. (2021). Early developmental impact of sex chromosome trisomies on attention deficit‐hyperactivity disorder symptomology in young children. American Journal of Medical Genetics Part A, 185A:3664–3674. 10.1002/ajmg.a.62418
Funding information National Institutes of Health, Grant/Award Number: UL1 TR002535; Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Grant/Award Number: 016.165.397
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Association, A. P . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association. [Google Scholar]
- Berglund, A. , Viuff, M. H. , Skakkebæk, A. , Chang, S. , Stochholm, K. , & Gravholt, C. H. (2019). Changes in the cohort composition of turner syndrome and severe non‐diagnosis of Klinefelter, 47, XXX and 47, XYY syndrome: A nationwide cohort study. Orphanet Journal of Rare Diseases, 14(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair, C. , & Diamond, A. (2008). Biological processes in prevention and intervention: The promotion of self‐regulation as a means of preventing school failure. Development and Psychopathology, 20(3), 899–911. 10.1017/S0954579408000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen, A. , Juul, S. , & Gravholt, C. H. (2003). Prenatal and postnatal prevalence of Klinefelter syndrome: A national registry study. The Journal of Clinical Endocrinology & Metabolism, 88(2), 622–626. [DOI] [PubMed] [Google Scholar]
- Bradley, R. H. , & Corwyn, R. (2013). From parent to child to parent…: Paths in and out of problem behavior. Journal of Abnormal Child Psychology, 41(4), 515–529. 10.1007/s10802-012-9692-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, R. H. , & Corwyn, R. F. (2007). Externalizing problems in fifth grade: Relations with productive activity, maternal sensitivity, and harsh parenting from infancy through middle childhood. Developmental Psychology, 43(6), 1390–1401. 10.1037/0012-1649.43.6.1390 [DOI] [PubMed] [Google Scholar]
- Brites, C. , Salgado Azoni, C. , Ferreira, T. , Lima, R. , & Ciasca, S. M. (2015). Development and applications of the SWAN rating scale for assessment of attention deficit hyperactivity disorder: A literature review. Brazilian Journal of Medical and Biological Research = Revista Brasileira de Pesquisas Medicas e Biologicas, 48, 965–972. 10.1590/1414-431X20154528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. D. (1977). Statistical power analysis for the behavioural sciences. Academic. [Google Scholar]
- Dennis, M. , Francis, D. J. , Cirino, P. T. , Schachar, R. , Barnes, M. A. , & Fletcher, J. M. (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15(3), 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg, N. , Valiente, C. , & Eggum, N. D. (2010). Self‐regulation and school readiness. Early Education and Development, 21(5), 681–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh, M. , Divan, G. , Koh, Y.‐J. , Kim, Y. S. , Kauchali, S. , Marcín, C. , Montiel‐Nava, C. , Patel, V. , Paula, C. S. , Wang, C. , Yasamy, M. T. , & Fombonne, E. (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Research: Official Journal of the International Society for Autism Research, 5(3), 160–179. 10.1002/aur.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltay, J. C. , & Maiburg, M. C. (2010). Klinefelter syndrome: Clinical and molecular aspects. Expert Review of Molecular Diagnostics, 10(6), 765–776. [DOI] [PubMed] [Google Scholar]
- Groth, K. A. , Skakkebæk, A. , Høst, C. , Gravholt, C. H. , & Bojesen, A. (2013). Klinefelter syndrome—a clinical update. The Journal of Clinical Endocrinology & Metabolism, 98(1), 20–30. [DOI] [PubMed] [Google Scholar]
- Hensch, T. K. (2004). Critical period regulation. Annual Review of Neuroscience, 27, 549–579. 10.1146/annurev.neuro.27.070203.144327 [DOI] [PubMed] [Google Scholar]
- Hong, D. S. , & Reiss, A. L. (2014). Cognitive and neurological aspects of sex chromosome aneuploidies. The Lancet. Neurology, 13(3), 306–318. 10.1016/S1474-4422(13)703028 [DOI] [PubMed] [Google Scholar]
- Lee, N. R. , Wallace, G. L. , Clasen, L. S. , Lenroot, R. K. , Blumenthal, J. D. , White, S. L. , Celano, M. J. , & Giedd, J. N. (2011). Executive function in young males with Klinefelter (XXY) syndrome with and without comorbid attention‐deficit/hyperactivity disorder. Journal of the International Neuropsychological Society: JINS, 17(3), 522–530. 10.1017/S1355617711000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt, T. E. , Arseneault, L. , Belsky, D. , Dickson, N. , Hancox, R. J. , Harrington, H. , Houts, R. , Poulton, R. , Roberts, B. W. , Ross, S. , Sears, M. R. , Thomson, W. M. , & Caspi, A. (2011). A gradient of childhood self‐control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences, 108(7), 2693–2698. 10.1073/pnas.1010076108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J. K. , Alberman, E. , Scott, C. , & Jacobs, P. (2008). Is the prevalence of Klinefelter syndrome increasing? European Journal of Human Genetics, 16(2), 163–170. [DOI] [PubMed] [Google Scholar]
- Pieters, J. , Kooper, A. J. A. , van Kessel, A. G. , Braat, D. D. M. , & Smits, A. P. T. (2011). Incidental prenatal diagnosis of sex chromosome aneuploidies: Health, behavior, and fertility. ISRN Obstetrics and Gynecology, 2011, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polderman, T. J. C. , Derks, E. M. , Hudziak, J. J. , Verhulst, F. C. , Posthuma, D. , & Boomsma, D. I. (2007). Across the continuum of attention skills: A twin study of the SWAN ADHD rating scale. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 48(11), 1080–1087. 10.1111/j.1469-7610.2007.01783.x [DOI] [PubMed] [Google Scholar]
- Ross, J. L. , Roeltgen, D. P. , Kushner, H. , Zinn, A. R. , Reiss, A. , Bardsley, M. Z. , McCauley, E. , & Tartaglia, N. (2012). Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Pediatrics, 129(4), 769–778. 10.1542/peds.2011-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke, B. P. , Bakker, D. J. , Fisk, J. L. , & Strang, J. D. (1983). Child neuropsychology. Guilford Press. [Google Scholar]
- Swanson, J. M. , Arnold, L. E. , Molina, B. S. G. , Sibley, M. H. , Hechtman, L. T. , Hinshaw, S. P. , Abikoff, H. B. , Stehli, A. , Owens, E. B. , Mitchell, J. T. , Nichols, Q. , Howard, A. , Greenhill, L. L. , Hoza, B. , Newcorn, J. H. , Jensen, P. S. , Vitiello, B. , Wigal, T. , Epstein, J. N. , … Kraemer, H. C. (2017). Young adult outcomes in the follow‐up of the multimodal treatment study of attention‐deficit/hyperactivity disorder: Symptom persistence, source discrepancy, and height suppression. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 58(6), 663–678. 10.1111/jcpp.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, J. M. , Schuck, S. , Porter, M. M. , Carlson, C. , Hartman, C. A. , Sergeant, J. A. , Clevenger, W. , Wasdell, M. , McCleary, R. , & Lakes, K. (2012). Categorical and dimensional definitions and evaluations of symptoms of ADHD: History of the SNAP and the SWAN rating scales. The International Journal of Educational and Psychological Assessment, 10(1), 51–70. [PMC free article] [PubMed] [Google Scholar]
- Tartaglia, N. , Cordeiro, L. , Howell, S. , Wilson, R. , & Janusz, J. (2010). The spectrum of the behavioral phenotype in boys and adolescents 47, XXY (Klinefelter syndrome). Pediatric Endocrinology Reviews: PER, 8(1), 151. [PMC free article] [PubMed] [Google Scholar]
- Tartaglia, N. , Howell, S. , Davis, S. , Kowal, K. , Tanda, T. , Brown, M. , Boada, C. , Alston, A. , Crawford, L. , Thompson, T. , van Rijn, S. , Wilson, R. , Janusz, J. , & Ross, J. (2020). Early neurodevelopmental and medical profile in children with sex chromosome trisomies: Background for the prospective eXtraordinarY babies study to identify early risk factors and targets for intervention. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 184(2), 428–443. 10.1002/ajmg.c.31807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia, N. R. , Ayari, N. , Hutaff‐Lee, C. , & Boada, R. (2012). Attention‐deficit hyperactivity disorder symptoms in children and adolescents with sex chromosome aneuploidy: XXY, XXX, XYY, and XXYY. Journal of Developmental and Behavioral Pediatrics: JDBP, 33(4), 309–318. 10.1097/DBP.0b013e31824501c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus, E. , Swaab, H. , Tartaglia, N. , Cordeiro, L. , & van Rijn, S. (2020a). The behavioral profile of children aged 1–5 years with sex chromosome trisomy (47,XXX, 47,XXY, 47,XYY). American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 184(2), 444–455. 10.1002/ajmg.c.31788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus, E. , van Rijn, S. , & Swaab, H. (2020b). A review of neurocognitive functioning of children with sex chromosome trisomies: Identifying targets for early intervention. Clinical Genetics, 97(1), 156–167. 10.1111/cge.13586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn, S. , & Swaab, H. (2015). Executive dysfunction and the relation with behavioral problems in children with 47,XXY and 47,XXX. Genes, Brain and Behavior, 14(2), 200–208. 10.1111/gbb.12203 [DOI] [PubMed] [Google Scholar]
- van Rijn, S. (2019). A review of neurocognitive functioning and risk for psychopathology in sex chromosome trisomy (47,XXY, 47,XXX, 47, XYY). Current Opinion in Psychiatry, 32(2), 79–84. 10.1097/YCO.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazsonyi, A. T. , & Huang, L. (2010). Where self‐control comes from: On the development of self‐control and its relationship to deviance over time. Developmental Psychology, 46(1), 245–257. 10.1037/a0016538 [DOI] [PubMed] [Google Scholar]
- Zechner, U. , Wilda, M. , Kehrer‐Sawatzki, H. , Vogel, W. , Fundele, R. , & Hameister, H. (2001). A high density of X‐linked genes for general cognitive ability: A run‐away process shaping human evolution? Trends in Genetics: TIG, 17(12), 697–701. 10.1016/s0168-9525(01)02446-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


