Abstract
Background
Patients with bipolar spectrum disorders (BSDs) exhibit an increased risk of Parkinson's disease (PD).
Objective
The aim is to investigate whether a previous diagnosis of BSDs influences the phenotype of PD.
Methods
Of 2660 PD patients followed for at least 6 years (6–27), 250 (BSD‐PD) had BSDs, 6–20 years before PD diagnosis; 48%–43% had a PD or BSD family history, and 34 carried glucocerebrosidase (GBA) and Parkin (PRKN) mutations. The cohort was split into a subset of 213 BSD‐PD patients, compared with 426 matched PD patients without BSDs, and a subset of 34 BSD‐PD and 79 PD patients carrying GBA or PRKN mutations. Carriers of mutations absent in BSD‐PD patients and of synuclein triplication were excluded. Structured clinical interviews and mood disorder questionnaires assessed BSDs. Linear mixed models evaluated the assessment scales over time. Thirteen BSD‐PD patients underwent subthalamic nucleus deep brain stimulation (STN‐DBS) and were compared with 27 matched STN‐DBS‐treated PD patients.
Results
Compared to PD patients, BSD‐PD showed (1) higher frequency of family history of PD (odds ratio [OR] 3.31; 2.32–4.71) and BSDs (OR 6.20; 4.11–9.35) 5); (2) higher incidence of impulse control disorders (hazard ratio [HR] 5.95, 3.89–9.09); (3) higher frequency of functional disorders occurring before PD therapy (HR, 5.67, 3.95–8.15); (4) earlier occurrence of delusions or mild dementia (HR, 7.70, 5.55–10.69; HR, 1.43, 1.16–1.75); and (5) earlier mortality (1.48; 1.11–1.97). Genetic BSD‐PD subjects exhibited clinical features indistinguishable from nongenetic BSD‐PD subjects. STN‐DBS‐treated BSD‐PD patients showed no improvements in quality of life compared to the control group.
Conclusions
BSDs as a prodrome to PD unfavorably shape their course and are associated with detrimental neuropsychiatric features and treatment outcomes. © 2021 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: bipolar spectrum disorders, Parkinson's disease; glucocerebrosidase mutation; parkin mutation, subthalamic nucleus deep brain stimulation
Bipolar spectrum disorders (BSDs), which include bipolar I disorder, bipolar II disorder, and cyclothymic disorder, are rarely discussed in Parkinson's disease (PD), PD with dementia, and dementia with Lewy bodies. 1 , 2 , 3 Recent reviews of neuropsychiatric disorders in PD list only impulse control disorders (ICDs), psychosis with delusions and hallucinations, somatic symptoms and functional disorders (SFDs). 4 , 5 , 6 , 7 Earlier literature, instead, explicitly cited mania or hypomania among the possible complications of PD, addressing caveats. 8 , 9 Several case reports have indicated the prior occurrence or co‐occurrence of BSDs in PD patients. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 The 2006 international guidelines on subthalamic nucleus deep brain stimulation (STN‐DBS) 29 in PD, included hypomania as a preoperative concern, and more recent guidelines repeat the concern. 30 The disappearance of mania/hypomania from the list of psychiatric complications of PD is likely due to the conceptual reframing of nonmotor aspects of PD and identification of ICDs as a specific psychiatric aspect of PD 7 appearing in patients exposed to dopamine agonist (DA) treatments. ICDs include risky behaviors like gambling, hypersexuality, shopping sprees, bulimia, aggressive driving, or hoarding and were first identified as a clinical entity related to dopaminergic treatment exposure by Andew Lees, 31 who named the disorder hedonistic homeostatic behavior.
ICDs of PD became a unique clinical entity and gained a wide space on social media, driven by individual and class‐action lawsuits related to DAs. 32 , 33 However, the behaviors listed among ICDs are quite similar to those listed by the DSM‐5 34 as distinctive aspects of BSDs.
Moreover, a recent large population study and a meta‐analysis study 35 , 36 confirmed earlier epidemiological findings 37 , 38 , 39 and indicated that BSDs are associated with a 3.4 (2–5.6, confidence interval [CI] 95%) increased risk for PD—a result highlighted by editorials. 40 , 41 These studies suggested that the prevalence of BSDs in PD is higher than in control populations. Following the epidemiological reports, new studies addressed BSDs in PD, generically naming the described disorders as hypomania. 42 , 43 , 44 , 45 Guided by earlier literature, 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 and also because, in several legal instances, two senior authors of the present paper were asked to provide an earnest analysis for compensation claims, which were at the time prevalent on social and public media, 46 , 47 , 48 , 49 we started testing all PD patients in our cohort using psychiatric scales and interviews to assess BSDs. When the last epidemiological studies were published, 35 , 36 we thought we could present our data for discussion. A long‐term follow‐up allowed the exploration of genetic predispositions and treatment outcomes in patients who had been diagnosed with BSDs before the onset of PD motor symptoms.
We tested the following hypotheses: (1) a prior diagnosis of BSDs (ie, BSDs assessed before the onset of motor PD symptoms) affects motor symptom progression; (2) BSDs affect nonmotor and psychiatric symptoms of PD; (3) BSDs are related to identifiable genetic predispositions; and (4) PD treatment outcomes are affected in those with a previous diagnosis of BSDs.
Patients and Methods
Cohort Selection
A clinical cohort of PD patients referred to our Movement Disorders Tertiary Center was enrolled between January 1992 and September 2013. The study was approved by the Local Ethics Committee (protocol no. 2098, June 11, 2020, and July 26, 2018, amendment of August 2, 2018), according to the Declaration of Helsinki. Informed consent was obtained from all participants.
A total of 8012 PD patients were enrolled after completing at least two follow‐up visits at 3 and 6 years, ± 6 months, after the first assessment (Fig. 1).
FIG 1.
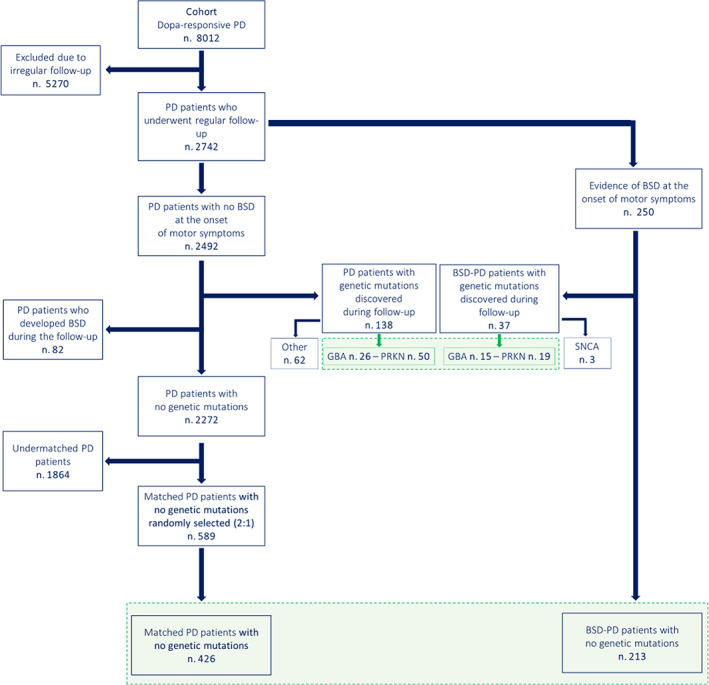
Flowchart and design of the study. The flowchart depicts the cohort's enrollment and the subsequent distinction of the main groups for cross‐sectional (light blue) and longitudinal (green) analyses. The picture also identifies the genetic subsets of Parkinson's disease (PD)‐related genetic mutations. BSD‐PD, bipolar spectrum disorder‐Parkinson's disease; GBA, β‐glucocerebrosidase‐related PD; PRKN, Parkin 2‐related PD; SNCA, α‐synuclein triplication. [Color figure can be viewed at wileyonlinelibrary.com]
A total of 5270 subjects were excluded due to irregular follow‐up or to medical comorbidities and treatments impacting on features and progression of parkinsonian motor symptoms, cognition, and psychiatric symptoms. The remaining 2272 were followed every 6 months for at least 6 years. A 12‐year follow‐up evaluation was completed in 84% of patients. At the first clinical assessment, subjects diagnosed with PD were evaluated for history or presence of psychiatric symptoms. A total of 250 PD subjects with a previous history of BSDs were included in the BSD‐PD group. The subjects who exhibited BSD symptoms only at follow‐up visits were excluded, to avoid potential confounders due to dopaminergic treatments. Genotyping was performed in all subjects with a family history of parkinsonism or of dementia or psychiatric disorders (475 patients) and led to the identification of 175 carriers of PD‐related genetic mutations (online supplemental Appendix e‐1): the only mutations identified in BSD‐PD patients were PRKN (19 patients), GBA (15 patients), and a triplication of the α‐synuclein gene in 3 patients. PRKN and GBA mutations were also identified in 76 PD patients without BSDs. Therefore, carriers of PRKN and GBA mutations were investigated separately (genetic patients). All carriers of other genetic mutations were excluded (Fig. 1); the 3 BSD‐PD patient carriers of α‐synuclein triplication were excluded as they have been described in previous studies (Fig. 1; online supplemental Appendix e‐1).
A total of 213 BSD‐PD patients with no PD genetic mutations were matched for gender, age, age at PD onset, and l‐dopa equivalent daily dose (LEDD) with 589 PD patients. As per study design (2:1), of the 589 PD pool, 426 subjects were randomly and blindly selected to constitute the control PD group to be compared with BSD‐PD subjects.
Study Design
The study consisted of cross‐sectional and longitudinal evaluations of three distinct groups, all derived from the original cohort of PD patients:
The core study compared 213 BSD‐PD patients to 426 matched PD patients without BSD, investigated motor, nonmotor, and psychiatric variables.
A substudy of 34 BSD‐PD patients who over the years were found to be carriers of GBA or PRKN mutations was compared to 76 PD carriers of the same mutations, addressing the same variables.
An interventional substudy of 13 BSD‐PD patients who underwent STN‐DBS was compared to 27 PD patients without BSDs who underwent the same procedure during the same period.
PD and BSD Diagnoses
PD was diagnosed following the UK Parkinson's Disease Society Brain Bank clinical diagnostic criteria and l‐dopa responsiveness. 50 Dopamine transporter (DaT) 123I–ioflupane single‐photon emission computed tomography (DAT‐SCAN SPECT) was performed in all BSD‐PD patients and 87% of PD patients 1 to 3 years after the clinical manifestation of parkinsonism and the withdrawal of drugs to avoid possible interferences (online supplemental Appendix e‐2). 51 , 52 BSD‐PD patients were admitted to the study only if DAT‐SCAN SPECT confirmed PD diagnosis. Iatrogenic, vascular, functional, and atypical parkinsonisms were ruled out and excluded from the original cohort.
The BSD diagnosis was determined at the first evaluation, according to current diagnostic criteria (DSM‐IV, 53 IV‐TR, 54 and DSM‐5 34 ). We either confirmed a previous diagnosis performed by psychiatrists (144 of 250 patients) or verified the history of manic/hypomanic symptoms and exposure to treatments with psychiatric interviews, interviews of relatives and general practitioners, and scores of mood disorder scales (106 of 250). All BSD‐PD patients presented with symptoms 6 to 24 years before motor PD symptoms had appeared. A second confirmation for all BSD diagnoses was obtained by independent psychiatrists, who, also, ruled out the presence of schizoaffective, narcissistic, and borderline personality disorders (online supplement Appendix e‐3). BSD‐related symptoms, including depression with/without psychotic traits, were documented by both categorical and qualitative rating scales. Current or historical exposure to antidepressants and/or neuroleptics was recorded in all patients.
Baseline Assessments of BSD‐Related Symptoms
In all patients, Structured Clinical Interview for DSM‐IV (SCID), 55 Young Mania Rating Scale (YMRS), 56 and (from the year 2000) Mood Disorder Questionnaire (MDQ) 57 were administered. All the results were interpreted by independent psychiatrists. In particular, YMRS scores above 20, and MDQ scores indicative of mania, were considered an indication of BSD.
Semistructured interviews conducted by expert psychiatrists further assessed the presence of BSD features. According to DSM‐5, 34 of 213 BSD‐PD patients, 111 were diagnosed as bipolar I, 90 as bipolar II, and 12 as cyclothymic. Of 34 genetic PD patients, 12 were bipolar I and 22 bipolar II.
Longitudinal Assessments
The longitudinal evaluations of BSD symptoms included the estimation of depression severity by Becks Depression Inventory (BDI). 58 , 59 Structured interviews evaluated the presence and severity of ICD and SFD symptoms. Symptom Checklist‐90 (SCL‐90) items, 60 , 61 rated SFDs and also ICDs before 2012 and was, after, converted into ICD scales. 62 These scales list hypersexuality, gambling, shopping, hoarding, and similar behaviors as ICD items. 62 The presence of simple and complex hallucinations or delusions was assessed using the Neuropsychiatric Inventory (NPI)–related items (b) 63 and semistructured interviews. 64
The Mini‐Mental State Examination (MMSE) 65 and the Dementia Rating Scale‐2 (DRS‐2) 66 were used to evaluate cognitive deficits. Mild dementia was diagnosed in subjects with MMSE scores ranging from 23 to 18. MMSE scores lower than 18 supported a dementia diagnosis.
Clinical charts reported acute psychiatric conditions, such as delirium, related or unrelated to treatment manipulations, and catatonia.
Motor symptoms were assessed using the modified Hoehn and Yahr (H&Y) 67 scale and the Unified Parkinson's Disease Rating Scale (UPDRS). 68 REM sleep behavior disorders (RBD), hyposmia, and constipation were rated as categorical variables; 69 , 70 , 71 pain was included in SCL‐90 ratings. 70
Data from 3‐, 6‐, and 12‐year follow‐up visits were analyzed.
Pharmacological Management
No exclusion criteria were applied to drugs used to manage motor symptoms. None of the investigated patients used anticholinergic therapy; 78 BSD patients were treated with lithium 3 to 8 years after the onset of PD motor symptoms; none of the patients had been treated with lithium before the onset of motor symptoms. Valproate, tricyclics, and typical or atypical neuroleptics were not used or withdrawn. Clozapine (6.25–300 mg/d) and quetiapine (25–300 mg/d) were the only antipsychotic drugs prescribed. Depression was treated with venlafaxine (75 patients), duloxetine (62 patients), escitalopram (56 patients), paroxetine (82 patients), or mirtazapine (48 patients).
LEDD was adjusted according to patients' needs. DAs were administered to 1214 PD (of 2492) and 131 PD‐BSD patients, with regimens ranging from 25 to 100 LEDD. After 2004, DAs were progressively withdrawn according to guidelines. 72 Dopamine agonist withdrawal syndrome 73 (DAWS) was diagnosed if severe dysphoria and dysautonomia occurred after withdrawal, thereby prompting the reintroduction of treatment and tapering with add‐on antidepressants.
STN‐DBS
STN‐DBS treatment was performed in 13 BSD‐PD and 27 PD patients between 2003 and 2008. At that time, international 29 , 74 and national criteria and guidelines 30 , 75 did not consider BSDs as exclusion criteria for the procedure, leaving the decision to clinical judgment. Clinical outcomes were evaluated using the UPDRS, 68 Clinical Global Impression, 76 and Parkinson's Disease Quality of life, eight‐item scale. 77
Statistics
Data are reported as mean ± standard deviation (SD) and absolute number and percentage for continuous and categorical and dichotomous variables, respectively. The differences between groups were evaluated by χ2 test for categorical and dichotomous variables (presence/absence of symptoms). Logistic regression models were used to produce odds ratio (OR), with a 95% CI to assess BSD association with other variables. Linear mixed models (LMM) 78 were used to investigate changes (evaluated by test scores) in mood, cognitive, psychotic, and motor symptoms, considering time (baseline, 3‐, 6‐, and 12‐year follow‐up) and groups (BSD‐PD and PD). LMM provides multiplicative models highlighting interactive effects among predictors and additive models for each predictor's individual effects on a given outcome and avoids family‐wise error types.
To evaluate the BSD risk according to time‐dependent PD neuropsychiatric and cognitive symptoms (delusion, depression, ICDs, SFDs, mild dementia, and dementia), Cox proportional hazards models were applied. 79 To avoid truncation, age at onset of PD motor symptoms was used as the time scale. 79 Hazard ratio (HR) and 95% CI were calculated from Cox‐model estimates.
Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). All statistical tests were two sided, and statistical significance was defined at P‐value <0.05.
Results
Cross‐Sectional Analysis
Demographic variables and baseline clinical features of the 213 BSD‐PD and 426 matched PD patients are presented in Tables 1 and 2. Family history of PD and/or BSD patients was threefold (OR 3.31; 95% CI, 2.32–4.71) and sixfold (OR 6.20; 4.11–9.35) more frequent in BSD‐PD.
TABLE 1.
Demographic and clinical features of patients without genetic mutations
| BSD‐PD (n = 213) | PD (n = 426) | P‐value * | Odds ratio; CI ** | |
|---|---|---|---|---|
| Demographics | ||||
| Sex, n males (%) | 139 (65.3) | 270 (63.4) | 0.64 | 1.01; 0.99–1.03 |
| Age at onset PD, mean age ± SD | 59.86 ± 9.35 | 58.90 ± 9.58 | 0.23 | 1.09; 0.77–1.53 |
| Age at onset BSD, mean age ± SD | 47.26 ± 8.02 | – | ||
| Family history of PD, n (%) | 103 (48.36) | 94 (22.07) | <0.001 | 3.31; 2.32–4.71 |
| Family history of BSD, n (%) | 90 (42.25) | 45 (10.56) | <0.001 | 6.20; 4.11–9.35 |
| Tremor onset, n (%) | 143 (67.14) | 275 (64.55) | 0.52 | 1.12; 0.79–1.59 |
| Bradykinesia onset, n (%) | 169 (79.34) | 346 (81.22) | 0.57 | 0.89; 0.59–1.34 |
| Death before 75 years, n (%) | 30 (14.08) | 26 (6.10) | <0.001 | 1.48; 1.11–1.97 |
| Follow‐up (6–26 years), mean age ± SD | 72.37 ± 8.09 | 72.16 ± 7.59 | 0.76 | 1.00; 0.98–1.03 |
| Follow‐up (0–12 years), mean age ± SD | 70.18 ± 8.99 | 69.11 ± 8.91 | 0.15 | 1.01; 0.99–1.03 |
| Matching at baseline | ||||
| UPDRS total score a | 26.49 ± 7.24 | 27.44 ± 7.33 | 0.53 | |
| Hoehn and Yahr | 1.9 ± 0.5 | 2.1 ± 0.4 | 0.06 | |
| MMSE score | 27.61 ± 1.15 | 27.65 ± 1.35 | 0.66 | |
| LEDD | 551.56 ± 156.41 | 553.52 ± 152.79 | 0.88 | |
| Drug exposure | ||||
| Dopamine agonists, n (%) | 131 (61.50) | 256 (60.09) | 0.73 | 1.06; 0.76–1.49 |
| Dopamine agonist withdrawal syndrome, n (%) | 42 (19.7) | 18 (4.2) | <0.001 | 5.57; 3.12–9.95 |
| Antidepressants, n (%) | 152 (71.36) | 108 (25.35) | <0.001 | 2.02; 1.74–2.35 |
| Neuroleptics, n (%) | 76 (35.68) | 33 (7.75) | <0.001 | 2.45; 1.83–3.28 |
P‐values from the statistical comparisons for demographics, drug exposure, and motor symptoms.
Odds ratio and 95% CI for demographics and drug exposure.
UPDRS subscores are presented in online supplemental Table e‐3.
Abbreviations: BSD‐PD, bipolar spectrum disorder‐Parkinson's disease; CI, confidence interval; SD, standard deviation; UPDRS, Unified Parkinson's Disease Rating Scale; MMSE, Mini‐Mental State Examination; LEDD, l‐dopa equivalent daily doses.
TABLE 2.
Comparison of motor, nonmotor, neuropsychiatric and, cognitive symptoms
| BSD‐PD (n = 213) | PD (n = 426) | P‐value * | |
|---|---|---|---|
| Motor symptoms | |||
| Wearing off, mean age ± SD | 66.70 ± 8.93 | 68.23 ± 9.01 | 0.04 |
| Dyskinesia, mean age ± SD | 63.87 ± 9.09 | 64.87 ± 9.96 | 0.22 |
| Nonmotor symptoms a | |||
| Presence of RBD, hyposmia, constipation, n (%) | 67 (31.46) | 154 (36.15) | 0.24 |
| Neuropsychiatric symptoms b | |||
| Exposure to DAs, n (%) | 131 (61.50) | 256 (60.09) | 0.73 |
| Presence of ICD before DAs, n (%) | 82 (38.50) | 29 (6.81) | <0.001 |
| Onset of ICD before DAs, mean age ± SD (82 vs. 29) | 50.69 ± 8.09 | 54.24 ± 10.09 | 0.06 |
| Presence of ICD with DAs, n (%) | 119 (55.87) | 71 (16.67) | <0.001 |
| Onset of ICD after DAs, mean age ± SD (119 vs. 71) | 56.97 ± 9.92 | 56.99 ± 10.31 | 0.99 |
| Dopamine agonist withdrawal syndrome, n (%) | 42 (19.7) | 18 (4.2) | <0.001 |
| SFD before PD diagnosis, n (%) | 98 (46.01) | 42 (9.86) | <0.001 |
| SFD after PD diagnosis, n (%) | 35 (16.43) | 69 (16.20) | <0.001 |
| Onset of SFD, mean age ± SD | 55.74 ± 6.76 | 62.17 ± 4.03 | <0.001 |
| Presence of hallucinations, n (%) | 145 (68.08) | 296 (69.48) | 0.71 |
| Onset of hallucinations, mean age ± SD | 65.39 ± 8.81 | 64.67 ± 9.17 | 0.44 |
| Presence of delusions, n (%) | 124 (58.22) | 51 (11.97) | <0.001 |
| Onset of delusions, mean age ± SD | 49.35 ± 8.13 | 68.75 ± 7.96 | <0.001 |
| Delusions or hallucinations due to drug, n (%) | 39 (18.31) | 85 (19.95) | 0.62 |
| Presence of depression, n (%) | 170 (79.81) | 176 (41.31) | <0.001 |
| Onset of depression, mean age ± SD | 52.11 ± 8.05 | 59.07 ± 9.73 | <0.001 |
| Presence of delirium, n (%) | 21 (9.86) | 33 (7.75) | 0.37 |
| Delirium in mild dementia, n (%) | 21 (9.86) | 27 (6.34) | 0.11 |
| Catatonia, n (%) | 14 (6.57) | 6 (1.14) | <0.001 |
| Cognitive impairment a | |||
| Presence of mild dementia, n (%) | 142 (66.67) | 255 (59.86) | 0.09 |
| Onset of mild dementia, mean age ± SD | 65.63 ± 5.90 | 67.63 ± 6.38 | 0.002 |
| Presence of dementia, n (%) | 73 (34.27) | 118 (27.70) | 0.09 |
| Onset of dementia, mean age ± SD | 70.78 ± 6.09 | 72.25 ± 4.90 | 0.07 |
Hazard ratios and 95% confidence intervals are reported in online supplemental Table e‐1. Longitudinal comparison of motor, nonmotor, and neuropsychiatric symptoms between groups is shown in online supplemental Figure e‐1, e‐2, and e‐3.
P values from the statistical comparisons for motor, nonmotor, and neuropsychiatric symptoms, including cognitive impairment.
Interviews of the patient and the caregiver evaluated the presence of nonmotor symptoms. The prevalence of each nonmotor symptom is presented in online supplemental Table e‐3.
Survival analyses were performed on neuropsychiatric symptoms and cognitive impairment.
Abbreviations: BSD‐PD, bipolar spectrum disorder‐Parkinson's disease; RBD, REM sleep behavior disorder; DA, dopamine agonist; ICD, impulse control disorder; SFD, somatic symptoms and functional disorders.
PD phenotypes at onset (tremor dominant or akinetic); age of onset for wearing off; and dyskinesia, RBD, hyposmia, and constipation were similar in both groups. The response to pharmacological therapy was not different between the two groups. Despite a similar exposure to DAs (60% of subjects in both groups), the risk of DAWS was higher in the BSD‐PD group (OR 5.57; CI, 3.12–9.95). Higher exposure to antidepressants and neuroleptics was observed in the BSD‐PD group (OR 2.02; CI, 1.74–2.35; and 2.45; 1.83–3.28, respectively).
BSD‐PD patients exhibited significantly increased prevalence of neuropsychiatric symptoms, including ICDs, SFDs, delusions, and depression. There was also a higher prevalence of catatonia in BSD‐PD patients (P < 0.001) (Table 2). Conversely, there was no difference in the frequency of delirium, hallucinations, and delusions associated with LEDD.
Mild dementia appeared at a younger age in BSD‐PD patients. The overall prevalence of dementia was similar in the two groups.
The mortality rate occurring before age 75 years was higher in BSD‐PD patients (OR 1.48; CI, 1.11–1.97).
Longitudinal Study
As shown by Tables 1 and 2 and online supplemental Table e‐1 and Figure e‐1, BSD‐PD patients exhibited an increased risk for neuropsychiatric symptoms. In BSD‐PD patients, ICDs were observed before the onset of PD and PD therapy (HR: 5.95; 95% CI, 3.89–9.09). Increased incidence of ICDs was observed after DA initiation in BSD‐PD compared to PD (HR: 4.16; 95% CI: 3.09–5.60).
An increased frequency of SFDs, before and after PD diagnosis, was observed in the BSD‐PD group (HR: 5.67; 95% CI: 3.95–8.15; and HR: 1.55; 95% CI:1.18–2.03, respectively). BSD‐PD subjects also exhibited increased frequency and younger age of onset for delusions (HR: 7.70; 95% CI: 5.55–10.69), depression (HR: 3.43; 95% CI: 2.76–4.24), and mild dementia (HR: 1.43; 95% CI 1.16–1.75).
Longitudinal Changes in the LMM Analysis
According to LMM predictive assessments of longitudinal variations, a multiplicative effect was observed for MMSE, DRS‐2, BDI, and SCL‐90 scores, showing an increased risk for earlier worsening of cognition, depression, and psychiatric symptoms in BSD‐PD patients over time (online supplemental Table e‐2 and Figures e‐2, e‐3). Conversely, for the NPI‐total score, only an additive effect was observed for BSD‐PD subjects. As for motor symptom changes, despite the presence of a multiplicative effect for H&Y scores and additive effects for UPDRS scores, a substantial overlap between the two groups was observed (online supplemental Figure e‐4). For LEDD variations, only time effect was found, indicating no differences between the groups.
Bipolar Disorder I and II Comparisons
LMM also compared the 111 patients with bipolar disorder I and 90 patients with bipolar disorder II, assessing MMSE, UPDRS, H&Y, BDI, and NPI scores: time effect was invariably <0.001, assessing the effect of progression, but group and time per group comparisons resulted in values ranging from <0.1984 to <0.479.
YMRS changes through time did not predict progression of motor or psychiatric scores, <0.1349.
Age onset of BSDs was not predictive of PD symptom progression.
Genetic Substudy
PRKN patients in both groups were younger than GBA carriers, as shown in Table 3. Online supplemental Figure e‐2 shows the age of onset of ICDs, SFDs, and delusions in comparison with the onset of motor symptoms.
TABLE 3.
Clinical characteristics of genetic BSD‐PD and PD groups
| GBA BSD‐PD (n = 15) | GBA PD (n = 26) | PRKN BSD‐PD (n = 19) | PRKN PD (n = 50) | |
|---|---|---|---|---|
| Age at onset PD | 58.87 ± 9.66 | 63.80 ± 8.04 | 39.37 ± 5.92 | 45.34 ± 6.10 |
| Family history PD (%) | 5 (33.33) | 26 (100.00) | 11 (57.89) | 50 (100.00) |
| Family history BSD (%) | 6 (40.00) | 9 (34.62) | 9 (47.37) | 23 (46.00) |
| Age at onset BSD | 44.80 ± 5.41 | – | 31.37 ± 3.02 | – |
| Dystonia at onset (%) | – | – | 12 (63.16) | 17 (34.00) |
| Tremor at onset (%) | 8 (53.33) | 16 (61.54) | 10 (52.63) | 23 (46.00) |
| Akinesia at onset % | 6 (40.00) | 11 (42.31) | 7 (36.84) | 21 (42.00) |
| DA exposure (%) | 6 (40.00) | 15 (57.69) | 12 (63.16) | 26 (52.00) |
| ICD with DA (%) | 6 (40.00) | 13 (50.00) | 12 (63.16) | 19 (38.00) |
| ICD before treatment (%) | 9 (60.00) | 5 (19.23) | 13 (68.42) | 17 (34.00) |
| SFD (%) | 10 (66.67) | 7 (26.92) | 13 (68.42) | 7 (14.00) |
| Delusions (%) | 12 (80.00) | 9 (34.62) | 3 (15.79) | 6 (12.00) |
| Hallucinations (%) | 15 (100.00) | 22 (84.62) | 12 (63.16) | 12 (24.00) |
| Catatonia (%) | 2 (13.33) | – | 1 (5.26) | – |
| DAWS (%) | 4 (26.67) | 6 (23.08) | 3 (15.79) | 8 (16) |
| Wearing off (age at onset) | 63.93 ± 8.82 | 67.28 ± 7.92 | 41.28 ± 5.66 | 51.31 ± 6.03 |
| Dyskinesia (age at onset) | 64.71 ± 8.62 | 69.09 ± 7.83 | 42.22 ± 6.81 | 53.04 ± 6.21 |
| Dementia (%) | 5 (33.33) | 12 (46.15) | 6 (31.58) | 21 (42.00) |
| Follow‐up duration (y) | 7.80 ± 1.70 | 6.15 ± 1.71 | 7.95 ± 1.96 | 8.56 ± 2.82 |
| YMRS | 30.27 ± 6.13 | 6.25 ± 3.86 | 29.42 ± 6.97 | 9.83 ± 3.16 |
Hazard ratios and 95% confidence intervals are reported in online supplemental Table e‐1.
The comparison of neuropsychiatric symptoms between PD and BSD‐PD group is presented in online supplemental Figure e‐4.
Abbreviations: BSD, bipolar spectrum disorder; PD, Parkinson's disease; GBA, β‐glucocerebrosidase‐related PD; PRKN, Parkin 2–related PD; DA, dopamine agonist; ICD, impulse control disorder; SFD, somatic symptoms and functional disorders; DAWS, dopamine agonist withdrawal syndrome; YMRS, Young Mania Rating Scale.
ICDs appeared before the manifestation of motor symptoms in 9 GBA BSD‐PD patients and SFDs in 10. The occurrence of delusions, hallucinations, and SFDs was higher in GBA BSD‐PD than GBA PD patients. DA treatment induced ICDs in 19 patients (6 GBA BSD‐PD, 13 GBA PD). Catatonia was observed in 2 BSD‐PD carriers of GBA mutations (Table 3).
For PRKN mutation carriers, ICDs, SFDs, and delusions were higher in BSD‐PD than PD carriers of the same mutation. In one PRKN carrier with BSD‐PD and dementia, akinetic‐negativism type catatonia was observed.
HRs for psychiatric symptoms were similar in carriers of PRKN and GBA mutations and in the groups of patients without known genetic mutations (online supplemental Table e‐1 and Figure e‐4).
Outcomes after STN‐DBS
STN‐DBS reduced dyskinesia in all 13 BSD‐PD patients, decreased motor fluctuations in 7, and led to LEDD reduction in all but one. Conversely, all BSD‐PD patients reported no improvements in their quality of life and persistence of subjectively severe motor fluctuations. One patient exhibited agitation, delirium, and aggressiveness on waking up from surgery. Five patients showed signs of long‐lasting (1–14 months) manic states associated with delusions. The comparison with 27 PD patients revealed differences only in subjective reports of quality of life, which was significantly improved only in the 27 patients without BSDs. Table 4 provides the comparisons.
TABLE 4.
Clinical outcomes of patients treated with STN‐DBS
| BSD‐PD (n = 13) | PD (n = 27) | |
|---|---|---|
| Gender n males (%) | 5 (38.46) | 13 (48.15) |
| Age at onset of PD, mean ± SD | 43.67 ± 7.66 | 44.30 ± 7.99 |
| Disease duration, mean ± SD | 17.50 ± 5.66 | 16.81 ± 5.23 |
| Age at STN‐DBS, mean ± SD | 60.33 ± 7.05 | 61.22 ± 5.20 |
| CGI‐I patient, mean ± SD | 4.42 ± 0.67 | 1.52 ± 0.58 |
| CGI‐I rater, mean ± SD | 1.33 ± 0.49 | 1.26 ± 0.45 |
| Before | After | Before | After | |
|---|---|---|---|---|
| UPDRS total, mean ± SD | 53.08 ± 11.41 | 17.58 ± 4.72 | 52.26 ± 10.56 | 18.93 ± 4.60 |
| UPDRS 32–33, mean ± SD | 6.67 ± 1.37 | 1.33 ± 0.78 | 6.70 ± 1.14 | 1.37 ± 0.74 |
| UPDRS 36–39, mean ± SD | 6.17 ± 0.72 | 1.00 ± 0.85 | 6.04 ± 0.81 | 0.93 ± 0.78 |
| LEDD, mean ± SD | 834.17 ± 90.60 | 354.17 ± 96.43 | 820.74 ± 98.52 | 377.04 ± 62.13 |
| PDQ‐8, mean ± SD | 28.83 ± 2.04 | 24.00 ± 3.20 a | 28.67 ± 1.83 | 13.41 ± 2.08 |
| CGI‐S, mean ± SD | 5.83 ± 0.58 | 2.25 ± 0.45 | 5.81 ± 0.83 | 2.30 ± 0.47 |
Notice that the only item not showing significant improvement after STN‐DBS is PDQ‐8 in the 13 BSD‐PD patients. P < 0.0001 in all other comparisons.
Abbreviations: BSD‐PD, bipolar spectrum disorder‐Parkinson's disease; STN‐DBS, subthalamic nucleus deep brain stimulation; CGI, Clinical Global Impression; CGI‐S, Clinical Global Impression‐severity of disease; CGI‐I, Clinical Global Impression‐improvement; SD, standard deviation; UPDRS, Unified Parkinson's Disease Rating Scale: items 32–33, dyskinesias; items 36–39, off‐status; LEDD, l‐dopa equivalent daily dose; PDQ‐8, Parkinson's Disease Questionnaire‐8.
Qualitative Clinical Elements
As neuropsychiatric disorders can benefit from qualitative descriptions, we summarized four case presentations and narrative case histories, fictionalized and anonymized by expert psychiatrists in the supplementary materials (online supplemental appendices e‐4 and e‐5).
Inadequate compliance with clinical prescriptions was evidenced by frequent refusal to accept treatment changes, despite side effects: 32 BSD‐PD patients, 4 BSD‐PD carriers of PRKN mutation, and 3 BSD‐PD carriers of GBA mutations restarted DA treatment, after withdrawal, despite the occurrence of severe ICDs, pedal edema, antecollis with myopathy, and kidney failure. Two of the 13 BSD‐PD patients who underwent STN‐DBS had concealed the history of their BSDs to gain access to surgical procedures.
Discussion
Our study confirms epidemiological studies 35 , 36 , 37 , 38 , 39 , 40 , 41 and case reports 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 indicating that BSDs are associated with PD and suggests that BSDs may be a prodrome to PD, which is associated with a greater prevalence of psychiatric, somatoform, and cognitive complications; inadequate improvements after STN‐DBS; and higher mortality. High genetic susceptibility for BSD‐PD is suggested by the high prevalence of a family history of PD and BSD. Although we found only PRKN and GBA (and synuclein triplication) mutations in our BSD‐PD patients, it should be mentioned that previous studies on leucine‐rich repeat kinase 2 (PARK8) and α‐synuclein (PARK4) mutation carriers also showed BSDs in these patients. 80 , 81 , 82
We could not provide a reliable estimate of the prevalence of BSD before PD because of referral selection bias related to tertiary center selection and, possibly, to our attention to favor long‐term compliance in BSD‐PD patients. As previous epidemiological studies reported PD occurrence in BSD populations, 35 , 36 , 37 , 38 , 39 in a reversed epidemiological design, further epidemiological studies are needed to confirm/challenge prevalence estimates in the present study (250/8012; 3%). Comparison of patients with bipolar I and bipolar II disorders did not show differences in progressions of motor symptoms or psychiatric symptoms or dementia. This was unexpected, as current literature suggests that bipolar I patients are subject to worse clinical outcomes than patients with bipolar II or cyclothymia. 62 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 Our interpretation is that the mixed effect of PD symptoms, cognitive decline, and treatments masked and blunted differences. Our sample size of GBA and PRKN carriers is too small to allow a comparison between bipolar disorder I and II patients or between patients with early (adolescent/young adult) and later onset of BSD. Further research in this area is needed.
BSD‐PD and PD patients were similar in severity and progression of motor symptoms, and the presence of known nonmotor symptoms like RBD, hyposmia, and constipation did not separate BSD‐PD from PD. Also, hallucinations were not statistically different between the two groups (online supplemental Figure e‐1). This finding is likely explained by the specific occurrence of visual hallucinations in PD. 2
The incidence and prevalence of all other neuropsychiatric symptoms, including SFDs, ICDs, and delusions, were significantly increased in the BSD‐PD group.
These results call for a discussion regarding psychiatric categorizations.
Various criteria have been suggested to distinguish neuropsychiatric symptoms of PD 2 , 62 , 83 from those appearing in DSM‐5. The DSM‐5 stresses SFD comorbidity with BSDs or with narcissistic and/or borderline personality disorders. 34 Our findings, showing higher SFD prevalence when BSD features are present, are in line with this formulation.
The different disorders listed among PD ICD 3 , 7 , 62 , 83 do not appear in DSM‐5 categorizations of compulsions but for hoarding. Indeed, the similarity of ICDs in PD with the symptom list of bipolar disorders is striking. DSM‐5 34 describes increased goal‐directed activities (sex, social activities) and excessive involvement in high‐risk activities (buying sprees, risky economic decisions), closely matching the behavioral disturbances classified as ICDs in PD. The anecdotal evidence gathered by reviewing social media narratives produced by PD patients with severe ICDs supports this notion by describing clear examples of manic states. 47 , 48 , 49
Also, the finding that some of the BSD‐PD patients were self‐administering DAs despite medical warnings suggests that DAs may act as an inducer of manic–hypomanic states. Consequently, DAWS could be reframed as the equivalent of depressive episodes triggered by antidepressant withdrawal in bipolar patients. In agreement with our suggestion of a BSD‐PD subphenotype, several studies described ICDs appearing before any exposure to dopaminergic drugs in PD 91 , 92 and GBA and PRKN mutation carriers. 93 , 94 The online supplemental appendix e‐6 addresses issues of categorization.
Our findings on delusions and catatonia also support the need to identify BSDs in a subtype of PD patients.
Finally, our data on outcomes of DBS‐treated BSD patients indicate an unfavorable outlook. This is consistent with a previous report 95 and highlights the concerns about hypomania/mania noted as potential pre‐ and postoperative problems in the STN‐DBS guidelines. 29 We provide further support to this statement.
Conclusions
PD associated with BSDs can be a phenotypic subtype of PD. Our study indicates that psychiatric examinations are helpful and needed in PD patients, as shown by an earlier report that tested patients undergoing STN‐DBS with extensive psychiatric interviews and scales 95 , 96 and found a high incidence of hypomania. Ours, and these studies, therefore, support the suggestion that, in PD, disorders other than the ones conventionally listed by reviews on neuropsychiatric aspects 1 , 2 , 3 are found if structured interviews and appropriate scales are used. Further studies should investigate the effects of hypomania emerging after the onset of PD motor symptoms or after exposure to treatments and understand whether this subphenotype represents the end of a continuum encompassing all neuropsychiatric aspects of PD or a separate entity related to complex predispositions.
Recent imaging findings in PD psychosis and BSDs highlight a shared dysfunction of the frontal control systems and disconnection with the default mode network 5 , 84 , 85 , 86 enhancing internal narrative 97 with the appearance of psychotic elements, like hallucinations, delusions, disinhibition, SFDs, 98 , 99 , 100 , 101 with cyclic suppression of reality checking. A recent hypothesis suggests that, due to the phylogenetic evolution of the frontal lobes, a process not aligned with the development of subcortical systems equipped with a matching array of modulatory neurotransmitters, 102 the frontal control system can be the weak point that becomes the preferential target for neurodegeneration, producing converging behavioral phenotypes.
Our hypothesis complies with an approach to bipolar disorders based on multiple prototypes, including primary and secondary forms. 103
Author Roles
M.O.: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data.
A.I.: analysis or interpretation of data.
C.C.: drafting/revision of the manuscript for content, including medical writing for content.
M.R.: drafting/revision of the manuscript for content, including medical writing for content.
R.F.: analysis or interpretation of data.
A.J.E.: drafting/revision of the manuscript for content, including medical writing for content.
L.S.B.: drafting/revision of the manuscript for content, including medical writing for content.
J.‐P.T.: drafting/revision of the manuscript for content, including medical writing for content.
M.G.: drafting/revision of the manuscript for content, including medical writing for content.
G.M.: drafting/revision of the manuscript for content, including medical writing for content.
E.M.V.: drafting/revision of the manuscript for content, including medical writing for content.
A.T.: drafting/revision of the manuscript for content, including medical writing for content.
L.B.: drafting/revision of the manuscript for content, including medical writing for content.
S.D.P.: drafting/revision of the manuscript for content, including medical writing for content.
F.D.: drafting/revision of the manuscript for content, including medical writing for content.
S.L.S.: drafting/revision of the manuscript for content, including medical writing for content; study concept or design.
Supporting information
APPENDIX S1. Supporting Information
Acknowledgments
M.O. and S.L.S. contributed to the conception and design of the study. R.F. and A.D.I. organized the database and performed the statistical analysis. M.O. and S.L.S. wrote the manuscript and supervised all the data. All the other authors contributed to manuscript revisions, read and approved the submitted version, evaluated patients, and performed the different assessments. Open Access Funding provided by Universita degli Studi Gabriele d'Annunzio Chieti Pescara within the CRUI‐CARE Agreement.
Relevant conflicts of interest/financial disclosures: M.O. has served on the scientific advisory boards of GlaxoSmithKline, Novartis, Lundbeck, Eisai, Valeant, Medtronic, and Newron; has received speaker honoraria from Zambon, the World Parkinson Congress, the Movement Disorder Society, and the Atypical Dementias Congress; has received publishing royalties from Springer; was an invited guest and lecturer for the Mental Disorders in Parkinson Disease Congress; serves on the editorial board of Medicine (Baltimore); has been employed as a speaker for Boehringer Ingelheim, GlaxoSmithKline, UCB, and Zambon; and has received research support from the Italian Ministry of Health and the Italian Ministry of Education. A.J.E. has received grant support from the NIH and The Michael J. Fox Foundation; personal compensation as a consultant/scientific advisory board member for AbbVie, Neuroderm, Neurocrine, Amneal, Adamas, Acadia, Acorda, InTrance, Sunovion, Lundbeck, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from USWorldMeds, Acadia, and Sunovion. S.L.S. has no conflicts of interest. M.D.G. is the president of the Italian Society of Psychiatry (SIP). J.‐P.T. is supported by the NIHR Newcastle Biomedical Research Centre and has received honoraria from GE Healthcare for delivering educational presentations on Lewy body disease. Outside of this work, J.‐P.T. has acted as a consultant for Kyowa Kirin and Heptares Sosei and received grant funding from Heptares Sosei.
Funding agencies: This work has been supported by nonprofit agencies (the Italian Department of Health [RF‐2013‐02358785 and NET‐2011‐02346784‐1], the AIRAlzh Onlus [ANCC‐COOP], European Union's Horizon 2020 research and innovation program under the Marie Skłodowska‐Curie grant agreement iMIND—no. 84166, the Alzheimer's Association—Part the Cloud: Translational Research Funding for Alzheimer' Disease [18PTC‐19‐602325], and the Alzheimer's Association—GAAIN Exploration to Evaluate Novel Alzheimer's Queries [GEENA‐Q‐19‐596282]). J.‐P.T. is supported by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre (BRC) based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University.
[The copyright line for this article was changed on 06 September 2021, after original online publication.]
Data availability statement
Dataset available upon request.
References
- 1. Galvin JE, Pollack J, Morris JC. Clinical phenotype of Parkinson disease dementia. Neurology 2006;67:1605–1611. 10.1212/01.wnl.0000242630.52203.8f [DOI] [PubMed] [Google Scholar]
- 2. Ffytche DH, Creese B, Politis M, et al. The psychosis spectrum in Parkinson disease. Nat Rev Neurol 2017;13:81–95. 10.1038/nrneurol.2016.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weintraub D, Mamikonyan E. The neuropsychiatry of Parkinson disease: a perfect storm. Am J Geriatr Psychiatry 2019;27:998–1018. 10.1016/j.jagp.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Onofrj M, Bonanni L, Manzoli L, et al. Cohort study on somatoform disorders in Parkinson disease and dementia with Lewy bodies. Neurology 2010;74:1598–1606. 10.1212/WNL.0b013e3181df09dd [DOI] [PubMed] [Google Scholar]
- 5. Onofrj M, Espay AJ, Bonanni L, et al. Hallucinations, somatic‐functional disorders of PD‐DLB as expressions of thalamic dysfunction. Mov Disord 2019;34:1100–1111. 10.1002/mds.27781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wissel BD, Dwivedi AK, Merola A, et al. Functional neurological disorders in Parkinson disease. J Neurol Neurosurg Psychiatry 2018;89:566–571. 10.1136/jnnp-2017-317378 [DOI] [PubMed] [Google Scholar]
- 7. Kelly MJ, Baig F, Hu MTM, et al. Spectrum of impulse control behaviours in Parkinson's disease: pathophysiology and management. J Neurol Neurosurg Psychiatry 2020;91:703–711. 10.1136/jnnp-2019-322453 [DOI] [PubMed] [Google Scholar]
- 8. Brown RG, Marsden CD. How common is dementia in Parkinson's disease? Lancet 1984;324:1262–1265. 10.1016/S0140-6736(84)92807-1 [DOI] [PubMed] [Google Scholar]
- 9. Klawans HL. Behavioral alterations and the therapy of parkinsonism. Clin Neuropharmacol 1982;5(suppl 1):S29–S37. 10.1097/00002826-198200051-00006 [DOI] [PubMed] [Google Scholar]
- 10. Engmann B. Bipolar affective disorder and Parkinson's disease. Case Rep Med 2011;2011:3–5. 10.1155/2011/154165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monreal JA, Staner L. Mania, parkinson disease and risperidone. Case report. Actas Esp Psiquiatr 2001;29(5):349–350. [PubMed] [Google Scholar]
- 12. Cannas A, Spissu A, Floris GL, et al. Bipolar affective disorder and Parkinson's disease: a rare, insidious and often unrecognized association. Neurol Sci 2002;23(suppl 2):67–68. 10.1007/s100720200073 [DOI] [PubMed] [Google Scholar]
- 13. Oliveira CA, Agostinho CS, Avelino MJ, et al. 2133 – Late‐onset bipolar disorder: literature review and a case report of inaugural acute mania on an elderly woman with parkinson disease. Eur Psychiatry 2013;28:1. 10.1016/S0924-9338(13)77019-6 21920709 [DOI] [Google Scholar]
- 14. Kummer A, Dias FMV, Cardoso F, et al. Low frequency of bipolar disorder, dopamine dysregulation syndrome, and punding in Brazilian patients with Parkinson's disease. Braz J Psychiatry 2010;32(1):62–65. 10.1590/s1516-44462010000100012 [DOI] [PubMed] [Google Scholar]
- 15. Wang J‐K, Lee H‐C. Pharmacotherapy in bipolar depression comorbid with Parkinson's disease: a case report. J Neuropsychiatry Clin Neurosci 2015;27:e213–e217. 10.1176/appi.neuropsych.14090227 [DOI] [PubMed] [Google Scholar]
- 16. Pettorruso M, Fasano A, De Risio L, et al. Punding in non‐demented Parkinson's disease patients: relationship with psychiatric and addiction spectrum comorbidity. J Neurol Sci 2016;362:344–347. 10.1016/j.jns.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 17. Steinlechner S, Hagenah J, Rumpf H‐J, et al. Associations of specific psychiatric disorders with isolated focal dystonia, and monogenic and idiopathic Parkinson's disease. J Neurol 2017;264(6):1076–1084. 10.1007/s00415-017-8488-x [DOI] [PubMed] [Google Scholar]
- 18. Sablaban IM, Sivananthan M. A man made manic: levodopa‐carbidopa‐induced mania in traumatic brain injury. Prim Care Companion CNS Disord 2019;21(1):18–22. 10.4088/PCC.18br02379 [DOI] [PubMed] [Google Scholar]
- 19. Kurlan R, Dimitsopulos T. Selegiline and manic behavior in Parkinson's disease. Arch Neurol 1992;49:1231. 10.1001/archneur.1992.00530360029012 [DOI] [PubMed] [Google Scholar]
- 20. Przedborski S, Liard A, Hildebrand J. Induction of mania by apomorphine in a depressed parkinsonian patient. Mov Disord 1992;7:285–287. 10.1002/mds.870070318 [DOI] [PubMed] [Google Scholar]
- 21. Harsch HH, Miller M, Young LD. Induction of mania by L‐dopa in a nonbipolar patient. J Clin Psychopharmacol 1985;5(6):338–339. [PubMed] [Google Scholar]
- 22. Pearlman CAJ. Manic behavior and levodopa. N Engl J Med 1971;285(23):1326–1327. 10.1056/NEJM197112022852325 [DOI] [PubMed] [Google Scholar]
- 23. Ryback RS, Schwab RS. Manic response to levodopa therapy. Report of a case. N Engl J Med 1971;285(14):788–789. 10.1056/NEJM197109302851409 [DOI] [PubMed] [Google Scholar]
- 24. Tune LE, Folstein M, Rabins P, Jayaram G, McHugh P. Familial manic‐depressive illness and familial Parkinson's disease: a case report. Johns Hopkins Med J 1982;151(2):65–70. [PubMed] [Google Scholar]
- 25. Larmande P, Palisson E, Saikali I, et al. Disappearance of akinesia in Parkinson disease during a manic attack. Rev Neurol (Paris) 1993;149(10):557–558. [PubMed] [Google Scholar]
- 26. Kim E, Zwil AS, McAllister TW, et al. Treatment of organic bipolar mood disorders in Parkinson's disease. J Neuropsychiatry Clin Neurosci 1994;6(2):181–184. 10.1176/jnp.6.2.181 [DOI] [PubMed] [Google Scholar]
- 27. Kulisevsky J, Avila A, Berthier ML. Bipolar affective disorder and unilateral parkinsonism after a brainstem infarction. Mov Disord 1995;10(6):799–802. 10.1002/mds.870100618 [DOI] [PubMed] [Google Scholar]
- 28. Maier F, Merkl J, Ellereit AL, et al. Hypomania and mania related to dopamine replacement therapy in Parkinsonʼs disease. Parkinsonism Relat Disord 2014;20:421–427. 10.1016/j.parkreldis.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 29. Lang AE, Houeto JL, Krack P, et al. Deep brain stimulation: preoperative issues. Mov Disord 2006;21(suppl 14):S171–S196. 10.1002/mds.20955 [DOI] [PubMed] [Google Scholar]
- 30. Ravina B, Marder K, Fernandez HH, et al. Diagnostic criteria for psychosis in Parkinson's disease: report of an NINDS, NIMH work group. Mov Disord 2007;22:1061–1068. 10.1002/mds.21382 [DOI] [PubMed] [Google Scholar]
- 31. Giovannoni G, O'Sullivan JD, Turner K, et al. Hedonistic homeostatic dysregulation in patients with Parkinson's disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry 2000;68:423–428. 10.1136/jnnp.68.4.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Terry M, McDermid R. Pfizer agrees to pay off Parkinson patients who developed gambling and sex addiction while on cabaser. 2015. https://www.biospace.com/article/pfizer-agrees-to-pay-off-parkinson-patients-who-developed-gambling-and-sex-addiction-while-on-cabaser-/
- 33. Whitehead L. Patients to launch class action over Parkinson's drug. 2008. https://www.abc.net.au/news/2008-01-22/patients-to-launch-class-action-over-parkinsons/1019298.
- 34. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM‐5™. Arlington, VA: American Psychiatric Association, 2013; 2013. [Google Scholar]
- 35. Huang MH, Cheng CM, Huang KL, et al. Bipolar disorder and risk of Parkinson disease: a nationwide longitudinal study. Neurology 2019;92:E2735–E2742. 10.1212/WNL.0000000000007649 [DOI] [PubMed] [Google Scholar]
- 36. Faustino PR, Duarte GS, Chendo I, et al. Risk of developing Parkinson disease in bipolar disorder: a systematic review and meta‐analysis. JAMA Neurol 2020;77:192–198. 10.1001/jamaneurol.2019.3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li X, Sundquist J, Hwang H, et al. Impact of psychiatric disorders on Parkinson's disease: a nationwide follow‐up study from Sweden. J Neurol 2008;255:31–36. 10.1007/s00415-007-0655-z [DOI] [PubMed] [Google Scholar]
- 38. Nilsson FMFM, Kessing LVV, Bolwig TGG. Increased risk of developing Parkinson's disease for patients with major affective disorder: a register study. Acta Psychiatr Scand 2008;104:380–386. 10.1111/j.1600-0447.2001.00372.x [DOI] [PubMed] [Google Scholar]
- 39. Lin HCHL, Lin HCHL, Chen YH. Psychiatric diseases predated the occurrence of Parkinson disease: a retrospective cohort study. Ann Epidemiol 2014;24:206–213. 10.1016/j.annepidem.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 40. Pontone GM, Koch G. An association between bipolar disorder and Parkinson disease: when mood makes you move. Neurology 2019;92:1125–1126. 10.1212/WNL.0000000000007641 [DOI] [PubMed] [Google Scholar]
- 41. Dols A, Lemstra AW. Parkinsonism and bipolar disorder. Bipolar Disord 2020;22:413–415. 10.1111/bdi.12888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schwartz F, Tahmasian M, Maier F, et al. Overlapping and distinct neural metabolic patterns related to impulsivity and hypomania in Parkinson's disease. Brain Imaging Behav 2019;13:241–254. 10.1007/s11682-017-9812-x [DOI] [PubMed] [Google Scholar]
- 43. Bacciardi S, Elefante C, Brancati GE, et al. Bipolar Spectrum disorders in Parkinson's disease: a systematic evaluation. CNS Spectr 2020;1–7. 10.1017/S1092852920002126 [DOI] [PubMed] [Google Scholar]
- 44. Pelzer EA, Dillenburger B, Grundmann S, et al. Hypomania and saccadic changes in Parkinson's disease: influence of D2 and D3 dopaminergic signalling. NPJ Parkinsons Dis 2020;6:5–12. 10.1038/s41531-019-0107-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Canesi M, Lavolpe S, Cereda V, et al. Hypomania, depression, Euthymia: new evidence in Parkinson's disease. Behav Neurol 2020;2020:5139237. 10.1155/2020/5139237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le iene show/Il morbo di Parkinson e il gioco dʼazzardo, Lega Nord: intervista a Riccardo Bossi. 2012. https://www.ilsussidiario.net/news/cinema-televisione-e-media/2012/4/12/le-iene-show-il-morbo-di-parkinson-e-il-gioco-d-azzardo-lega-nord-intervista-a-riccardo-bossi-video/267210/
- 47. Grow K. Ozzy Osbourne reveals Parkinsonʼs disease diagnosis. 2020. https://www.rollingstone.com/music/music-news/ozzy-osbourne-parkinsons-disease-940329
- 48. Roberts G. Parkinsonʼs disease drugʼs cause of high sex drive and gambling addiction side effect revealed in study. 2019. https://www.abc.net.au/news/2019-10-28/parkinson-disease-side-effect-gambling-addiction-high-sex-drive/11645290
- 49.Not "Depression": Manic‐Depression and Robin Williams. A deadly disease, whether we accept it or not. 2014. https://www.psychologytoday.com/intl/blog/mood-swings/201408/not-depression-manic-depression-and-robin-williams
- 50. Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinsonʼs disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. 10.1136/jnnp.55.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sossi V, Dinelle K, Schulzer M, et al. Levodopa and pramipexole effects on presynaptic dopamine PET markers and estimated dopamine release. Eur J Nucl Med Mol Imaging 2010;37:2364–2370. 10.1007/s00259-010-1581-3 [DOI] [PubMed] [Google Scholar]
- 52. Schillaci O, Pierantozzi M, Filippi L, et al. The effect of levodopa therapy on dopamine transporter SPECT imaging with123I‐FP‐CIT in patients with Parkinsonʼs disease. Eur J Nucl Med Mol Imaging 2005;32:1452–1456. 10.1007/s00259-005-1922-9 [DOI] [PubMed] [Google Scholar]
- 53. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 54. American Psychiatric Association , Arlington (VA). Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 55. First MB, Gibbon M, Spitzer R, et al. Structured Clinical Interview for DSM‐IV Axis II Personality Disorders, (SCID‐II). Washington, DC; American Psychiatric Press, Inc.; 1997. [Google Scholar]
- 56. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978;133:429–435. 10.1192/bjp.133.5.429 [DOI] [PubMed] [Google Scholar]
- 57. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 2000;157:1873–1875. 10.1176/appi.ajp.157.11.1873 [DOI] [PubMed] [Google Scholar]
- 58. Beck AT, Guth D, Steer RA, et al. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther 1997;35:785–791. 10.1016/s0005-7967(97)00025-9 [DOI] [PubMed] [Google Scholar]
- 59. Beck A, Steer R, Brown G. BDI‐Fast Screen for Medical Patients: Manual. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 60. Derogatis LR. The SCL‐90 Manual I: Scoring, administration and procedures for the SCL‐90. Giunti Psychometrics, Florence (Italy): Clinical Psychometrics Unit; 1977. [Google Scholar]
- 61. Kim SW, Dysken MW, Kuskowski M. The symptom checklist‐90: obsessive‐compulsive subscale: a reliability and validity study. Psychiatry Res 1992;41:37–44. 10.1016/0165-1781(92)90016-v [DOI] [PubMed] [Google Scholar]
- 62. Weintraub D, Mamikonyan E, Papay K, et al. Questionnaire for impulsive‐compulsive disorders in Parkinsonʼs disease‐rating scale. Mov Disord 2012;27:242–247. 10.1002/mds.24023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Levin HS, High WM, Goethe KE, et al. The neurobehavioural rating scale: assessment of the behavioural sequelae of head injury by the clinician. J Neurol Neurosurg Psychiatry 1987;50:183–193. 10.1136/jnnp.50.2.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Onofrj M, Taylor JP, Monaco D, et al. Visual hallucinations in PD and Lewy body dementias: old and new hypotheses. Behav Neurol 2013;27:479–493. 10.3233/BEN-129022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Folstein M, Folstein S, McHugh P. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 66. Jurica P, Leitten C, Mattis S. Dementia Rating Scale‐2 (DRS‐2): Professional Manual. Lutz, FL; Psychological Assessment Resources, 2001; 2001. [Google Scholar]
- 67. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology 1998;50:318–334. 10.1212/wnl.50.2.318 [DOI] [PubMed] [Google Scholar]
- 68. Fahn S, Elton R, Members of the UPDRS Development Committee . Unified Parkinsonʼs Disease Rating Scale. In: Fahn S, Marsden C, Calne D, Goldstein ME, eds. Recent Developments in Parkinsonʼs Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163, 293–304. [Google Scholar]
- 69. Picillo M, Pellecchia MT, Erro R, et al. The use of University of Pennsylvania smell identification test in the diagnosis of Parkinsonʼs disease in Italy. Neurol Sci 2014;35:379–383. 10.1007/s10072-013-1522-6 [DOI] [PubMed] [Google Scholar]
- 70. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinsonʼs Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 71. Trenkwalder C, Kohnen R, Högl B, et al. Parkinsonʼs disease sleep scale‐validation of the revised version PDSS‐2. Mov Disord 2011;26:644–652. 10.1002/mds.23476 [DOI] [PubMed] [Google Scholar]
- 72. Fox SH, Katzenschlager R, Lim S‐Y, et al. International Parkinson and movement disorder society evidence‐based medicine review: update on treatments for the motor symptoms of Parkinson's disease. Mov Disord 2018;33:1248–1266. 10.1002/mds.27372 [DOI] [PubMed] [Google Scholar]
- 73. Solla P, Fasano A, Cannas A, et al. Dopamine agonist withdrawal syndrome in Parkinson's disease. J Neurol Sci 2017;382:47–48. 10.1016/j.jns.2017.08.3263 [DOI] [PubMed] [Google Scholar]
- 74. Defer GL, Widner H, Marié RM, et al. Core assessment program for surgical interventional therapies in Parkinsonʼs disease (CAPSIT‐PD). Mov Disord 1999;14:572–584. [DOI] [PubMed] [Google Scholar]
- 75. Abbruzzese G, Albanese A, Antonini A, et al. Linee Guida per il Trattamento della Malattia di Parkinson 2002. Neurol Sci 2002;23:S1–S63.12032582 [Google Scholar]
- 76. Guy W, National Institute of Mental Health (U.S.). Psychopharmacology Research Branch.; Early Clinical Drug Evaluation Program . ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 77. Jenkinson C, Fitzpatrick R, Peto V, et al. The PDQ‐8: development and validation of a short‐form parkinsonʼs disease questionnaire. Psychol Health 1997;12:805–814. 10.1080/08870449708406741 [DOI] [Google Scholar]
- 78. Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- 79. Kim M, Paik MC, Jang J, et al. Cox proportional hazards models with left truncation and time‐varying coefficient: application of age at event as outcome in cohort studies. Biom J 2017;59:405–419. 10.1002/bimj.201600003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Goldwurm S, Zini M, Di Fonzo A, et al. LRRK2 G2019S mutation and Parkinson's disease: a clinical, neuropsychological and neuropsychiatric study in a large Italian sample. Parkinsonism Relat Disord 2006;12:410–419. [DOI] [PubMed] [Google Scholar]
- 81. Singleton AB, Farrer M, Johnson J, et al. [alpha]‐synuclein locus triplication causes Parkinson's disease. Science 2003;302:841. [DOI] [PubMed] [Google Scholar]
- 82. Olgiati S, Thomas A, Quadri M, et al. Early‐onset parkinsonism caused by alpha‐synuclein gene triplication: clinical and genetic findings in a novel family. Parkinsonism Relat Disord 2015;21:981–986. 10.1016/j.parkreldis.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 83. Okai D, Askey‐Jones S, Mack J, et al. Parkinsonʼs impulse‐control scale for the severity rating of impulse‐control behaviors in parkinsonʼs disease: a semistructured clinical assessment tool. Mov Disord Clin Pract 2016;3:494–499. 10.1002/mdc3.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee SE, Khazenzon AM, Trujillo AJ, et al. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain 2014;137:3047–3060. 10.1093/brain/awu248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Franciotti R, Falasca NW, Bonanni L, et al. Default network is not hypoactive in dementia with fluctuating cognition: an Alzheimer disease/dementia with Lewy bodies comparison. Neurobiol Aging 2013;34:1148–1158. 10.1016/j.neurobiolaging.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 86. Franciotti R, Delli Pizzi S, Perfetti B, et al. Default mode network links to visual hallucinations: a comparison between Parkinson's disease and multiple system atrophy. Mov Disord 2015;30:1237–1247. 10.1002/mds.26285 [DOI] [PubMed] [Google Scholar]
- 87. Abé C, Ekman CJ, Sellgren C, et al. Cortical thickness, volume and surface area in patients with bipolar disorder types I and II. J Psychiatry Neurosci 2016;41(4):240–250. 10.1503/jpn.150093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Caseras X, Lawrence NS, Murphy K, et al. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry 2013;170(5):533–541. 10.1176/appi.ajp.2012.12020169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sanchez‐Moreno J, Bonnin CM, Gonzales‐Pinto A, et al. Factors associated with poor functional outcome in bipolar disorder: sociodemographic, clinical, and neurocognitive variables. Acta Psychiatr Scand 2018;138(2):145–154. 10.1111/acps.12894 [DOI] [PubMed] [Google Scholar]
- 90. Vrabie M, Marinescu V, Talaşman A, et al. Cognitive impairment in manic bipolar patients: important, understated, significant aspects. Ann Gen Psychiatry 2015;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Baig F, Kelly MJ, Lawton MA, et al. Impulse control disorders in Parkinson disease and RBD: a longitudinal study of severity. Neurology 2019;93:E675–E687. 10.1212/WNL.0000000000007942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Voon V, Mehta AR, Hallett M, et al. Impulse control disorders in Parkinson's disease: recent advances. Curr Opin Neurol 2011;24:324–330. 10.1097/WCO.0b013e3283489687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Morgante F, Fasano A, Ginevrino M, et al. Impulsive‐compulsive behaviors in parkin ‐associated Parkinson disease. Neurology 2016;87:1436–1441. 10.1212/WNL.0000000000003177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Petrucci S, Ginevrino M, Trezzi I, et al. GBA‐related Parkinson's disease: dissection of genotype‐phenotype correlates in a large Italian cohort. Mov Disord 2020;35:2106–2111. 10.1002/mds.28195 [DOI] [PubMed] [Google Scholar]
- 95. Houeto JL, Mesnage V, Mallet L, et al. Behavioural disorders, Parkinson's disease and subthalamic stimulation. J Neurol Neurosurg Psychiatry 2002;72:701–707. 10.1136/jnnp.72.6.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rieu I, Martinez‐Martin P, Pereira B, et al. International validation of a behavioral scale in Parkinson's disease without dementia. Mov Disord 2015;30:705–713. 10.1002/mds.26223 [DOI] [PubMed] [Google Scholar]
- 97. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 2003;100(1):253–258. 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shine JM, Muller AJ, O'Callaghan C, Hornberger M, et al. Abnormal connectivity between the default mode and the visual system underlies the manifestation of visual hallucinations in Parkinson's disease: a task‐based fMRI study. NPJ Parkinsons Dis 2015;1:15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yao N, Shek‐Kwan Chang R, Cheung C, et al. The default mode network is disrupted in Parkinson's disease with visual hallucinations. Hum Brain Mapp 2014;35(11):5658–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fernández‐Corcuera P, Salvador R, Monté GC, et al. Bipolar depressed patients show both failure to activate and failure to de‐activate during performance of a working memory task. J Affect Disord 2013;148:170–178. 10.1016/j.jad.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 101. Meda SA, Ruaño G, Windemuth A, et al. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci U S A 2014;111:2066–2075. 10.1073/pnas.1313093111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Diederich NJ, Uchihara T, Grillner S, et al. The evolution‐driven signature of Parkinson's disease. Trends Neurosci 2020;43:475–492. 10.1016/j.tins.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 103. Akiskal HS, Pinto O. The evolving bipolar spectrum. Prototypes I, II, III, and IV. Psychiatr Clin North Am 1999;22:517–534, vii. 10.1016/s0193-953x(05)70093-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1. Supporting Information
Data Availability Statement
Dataset available upon request.


