Abstract
Aims
To compare the risks of all‐cause mortality, hepatic outcomes, major adverse cardiovascular events between metformin users and nonusers for patients with diabetes and cirrhosis.
Methods
From the Taiwan's National Health Insurance Research Database, we selected propensity‐score matched metformin users and nonusers from the cohorts of type 2 diabetes mellitus with compensated (n = 26 164) or decompensated liver cirrhosis (n = 15 056) between 1 January 2000 and 31 December 2009, and followed them until 31 December 2010. Cox proportional hazards models with robust sandwich standard error estimates were used to assess risk of investigated outcomes for metformin users.
Results
The incidence rates of mortality during follow‐up were 3.8 and 3.3 per 100 patient‐years (adjusted hazard ratio [aHR] 1.13, 95% confidence interval 1.01–1.25) for metformin users and nonusers, respectively. The incidence rates of cirrhotic decompensation during follow‐up were 5.9 and 4.9 per 100 patient‐years (aHR 1.15, 95% confidence interval 1.04–1.27) for metformin users and nonusers. The risk of death (P for trend <.01) and cirrhotic decompensation (P for trend <.0001) associated with metformin use was significant for those taking metformin for >40 defined daily doses in 90 days or >1000 mg/d. The outcomes of metformin use vs nonuse for type 2 diabetes mellitus with decompensated liver cirrhosis were not statistically different, except that metformin users had higher risk of mortality (aHR 1.15).
Conclusion
Metformin use was associated with higher risks of mortality and cirrhotic decompensation in patients with compensated liver cirrhosis. Prospective studies are required to confirm our results.
Keywords: all‐cause mortality, cardiovascular disease, hepatic failure, hepatocellular carcinoma, metabolic acidosis
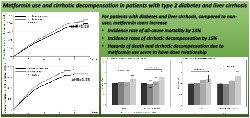
For patients with diabetes and liver cirrhosis, compared to nonusers, metformin users increase incidence rate of all‐cause mortality by 13%, incidence rates of cirrhotic decompensation by 15%. Hazards of death and cirrhotic decompensation due to metformin use seem to have dose relationship.
1. What is already known about this subject
The glucose‐lowering medication use in patients with liver cirrhosis is complicated.
Few studies have investigated appropriate glucose‐lowering medications for persons with diabetes and liver cirrhosis.
What this study adds
Metformin use was associated with 13% higher risk of mortality as compared to nonuse.
Metformin use, as compared to no use, was associated with 15% higher risk of cirrhotic decompensation in patients with compensated cirrhosis.
1. INTRODUCTION
About 29 million people in Europe 1 and 5.5 million Americans live with chronic liver disease or cirrhosis. 2 Compensated liver cirrhosis is associated with 4.7 times higher risk of death than the general population; and the mortality risk of decompensated liver cirrhosis is 9.7‐fold higher. 3 Diabetes mellitus (DM) also increases the likelihood of steatohepatitis and cirrhosis by accumulative dyslipidaemia, free radicals and cytokines. 4
Patients with diabetes and advanced cirrhosis are prone to hypoglycaemia and malnutrition, conditions that make glucose‐lowering therapy difficult. Cirrhosis associated hyperinsulinaemia can further lower the biological efficiency of insulins and oral glucose‐lowering medications; by contrast, many medications are metabolized or excreted by the liver, so the cirrhosis would weaken hepatic mediated function and lead patients to develop hepatotoxicity. 5
Metformin has many physiological advantages. It can activate adenosine monophosphate‐activated protein kinase, upregulate glucose transporter 4 genes, increase glucose uptake and decrease oxidative stress, 6 resulting in reduced hepatic gluconeogenesis and aiding lipid metabolism. It can also induce adiponectin and be helpful with the prevention of hepatic inflammation, steatosis and fibrosis. 4 , 6 In animal models on cirrhotic status, metformin was shown to improve lipid peroxidation, reduce liver injury, alleviate glucose intolerance and insulin resistance. 7 By inducing tumour suppressor liver kinase B1, metformin was also reported to have anticancer and antiaging effects. 8 However, clinical evidence is lacking to clarify if metformin could optimize treatment outcomes for patients with diabetes and cirrhosis.
No clinical trial has specifically targeted the effectiveness of metformin use for patients with coexistent diabetes and cirrhosis, despite few observational studies with moderate numbers of patients being reported. 9 , 10 The glucose‐lowering medication use in patients with liver cirrhosis was complicated, not allowing conclusive findings about the optimal treatment of these patients. Therefore, we conducted this cohort study to investigate the outcomes of metformin use in compensated and decompensated liver cirrhosis with diabetes.
2. METHODS
2.1. Data source
We conducted this cohort study by using Taiwan's National Health Insurance Research Database (NHIRD), which includes healthcare data gathered from at least 99% of the 23 million people of Taiwan. 11 The encrypted information recorded in the NHIRD includes demographics, diagnostic codes according to the International Classification of Diseases 9th revision–Clinical Modification (ICD‐9‐CM) and drug prescriptions. Our study was approved by the institutional review board of the National Health Research Institutes (EC1060704‐E) and was granted a waiver of informed consent.
2.2. Study design
We selected patients in the NHIRD who had both type 2 diabetes and liver cirrhosis between 1 January 2000 and 31 December 2009, and followed them until 31 December 2010 (Figure 1). Type 2 diabetes was ascertained by the ICD‐9‐CM code 250.xx for at least 2 outpatient diagnoses in 1 year or 1 hospitalization and under glucose‐lowering medication treatment. People with a primary diagnosis of ICD‐9‐CM codes 571.5, 571.2 or 571.6 for at least 2 outpatient diagnoses in 1 year or 1 hospitalization were defined as having liver cirrhosis. The algorithm for case definitions of diabetes and cirrhosis based on ICD‐9 coding has been validated by medical record review in previous studies, 12 , 13 showing the accuracy of diabetes and cirrhosis diagnosis was 74.6 and 82.6%, respectively. Patients with cirrhosis and ascites (789.59, 789.5), hepatic encephalopathy (572.2), or variceal bleeding (456.0, 456.2) were defined as having decompensated liver cirrhosis 14 ; patients without these complications were defined as compensated liver cirrhosis. We excluded individuals younger than 20 or older than 100 years, not using glucose‐lowering medications during the study period, who died within 365 days after index date and those who had incomplete data.
FIGURE 1.
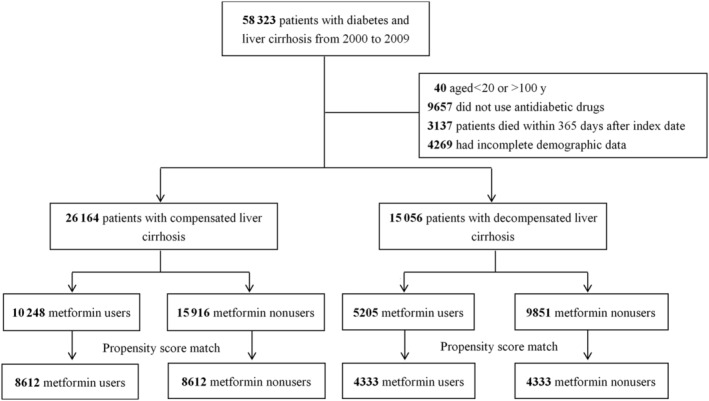
Flowchart of selection of study subjects
2.3. Procedures
We defined the second date of concurrent diagnosis of type 2 DM (T2DM) and liver cirrhosis as the comorbid date (Figure 2). Patients who had ever taken metformin within 90 days after the comorbid date were defined as metformin users, and those who were not prescribed metformin in this period as nonusers. We defined the 91st day after the comorbid date as the index date. We identified variables that were thought to be the potential confounders of our study, including demographics, comorbidities diagnosed within 1 year before the index date, and medications (e.g., glucose‐lowering medications, antihypertensive drugs, statin and aspirin). The comorbid disorders selected in this study included hepatitis B virus (HBV) infection (070.2, 070.3, V02.61) and hepatitis C virus (HCV) infection (070.41, 070.44, 070.51, 070.54, 070.70, 070.71, V02.62). To truly reflect characteristics of our study subjects who were patients with diabetes and liver cirrhosis, we used the chronic illness with complexity index to quantify patients' comorbidity profiles, 15 and the diabetes complications severity index score 16 was used to define the diabetes severity.
FIGURE 2.
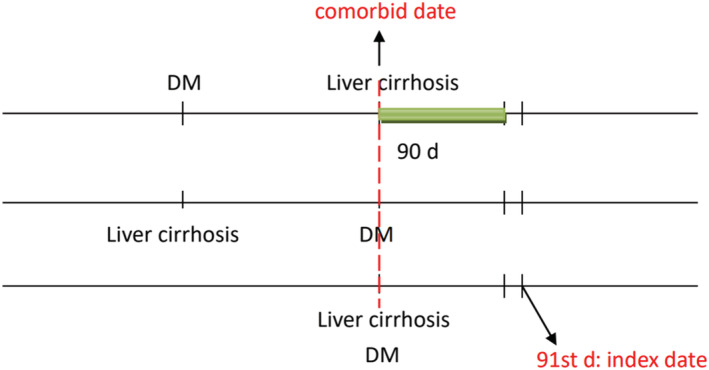
Patients who had ever taken metformin within 90 days after the comorbid date were defined as metformin users; the 91st day after the comorbid date was defined as the index date. DM, diabetes mellitus
2.4. Main outcomes
All‐cause mortality was the main outcome of this study. Death was defined by discharge from hospital with certified death (the discharge date was defined as the death date) or termination of the NHI coverage after discharge from hospital due to a critical illness and no further healthcare utilization in the NHI records for more than 1 year (the end of NHI coverage was defined as the death date). We assessed the incidence rates of cirrhotic decompensation (the composite of variceal bleeding, ascites and hepatic encephalopathy), 14 oesophageal varices with bleeding, abdominal ascites, hepatic encephalopathy, jaundice (782.4) and hepatic failure (characterized by jaundice, hepatic encephalopathy and an elevated prothrombin time, as coded by ICD‐9‐CM 570, 572.2, 572.4, 572.8) to evaluate progression of liver cirrhosis. We also assessed incidence rate of hepatocellular carcinoma (HCC, 155.x) and major adverse cardiovascular events (MACE), including stroke (430–437), ischaemic heart disease (410–414) and heart failure (428). Incidence of hospitalization due to hypoglycaemia (251.0x, 251.1x, or 251.2x) and metabolic acidosis (276.2) were calculated to evaluate possible complications of treatments.
2.5. Statistical analyses
We used propensity score matching to optimize comparability between metformin users and nonusers. 17 The propensity score was estimated for every patient by a nonparsimonious multivariable logistic regression, with receipt of metformin as the dependent variable. We included clinically relevant covariates in the analysis as independent variables (Table 1). The nearest‐neighbour algorithm was applied to construct matched pairs, assuming that the proportion of 0.995–1.0 was perfect. 18
TABLE 1.
Baseline characteristics of metformin users vs. matched nonusers in type 2 diabetes patients with compensated liver cirrhosis
| Medication | Before propensity score match | After propensity score match | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metformin users | Metformin nonusers | Metformin users | Metformin nonusers | |||||||
| n (10 248) | % | n (15 916) | % | P value | n (8612) | % | n (8612) | % | P value | |
| Age (y) | ||||||||||
| Mean (SD) | 55.4 (12.1) | 57.4 (13.0) | <.001 | 56.1 (12.1) | 56.2 (12.4) | .37 | ||||
| Diabetes duration (y) | ||||||||||
| Mean (SD) | 2.0 (2.3) | 1.7 (2.0) | <.001 | 1.8 (2.2) | 1.8 (2.1) | .02 | ||||
| Sex | ||||||||||
| Male | 7209 | 70.4% | 11 051 | 69.4% | .12 | 6046 | 70.2% | 6022 | 69.9% | .69 |
| Female | 3039 | 29.7% | 4865 | 30.6% | 2566 | 29.8% | 2590 | 30.1% | ||
| Comorbid disorders | ||||||||||
| HBV | 1973 | 19.3% | 2733 | 17.2% | <.001 | 1587 | 18.4% | 1567 | 18.2% | .69 |
| HCV | 2102 | 20.5% | 2967 | 18.6% | <.001 | 1694 | 19.7% | 1699 | 19.7% | .92 |
| CIC index | ||||||||||
| 0 | 370 | 3.6% | 434 | 2.7% | <.001 | 293 | 3.4% | 315 | 3.7% | .36 |
| 1 | 3168 | 30.9% | 4463 | 28.0% | 2580 | 30.0% | 2639 | 30.6% | ||
| ≥2 | 6710 | 65.5% | 11 019 | 69.2% | 5739 | 66.6% | 5658 | 65.7% | ||
| DCSI score | ||||||||||
| 0 | 6240 | 60.9% | 9922 | 62.3% | <.001 | 5344 | 62.1% | 5494 | 63.8% | .05 |
| 1 | 1785 | 17.4% | 2337 | 14.7% | 1418 | 16.5% | 1371 | 15.9% | ||
| ≥2 | 2223 | 21.7% | 3657 | 23.0% | 1850 | 21.5% | 1747 | 20.3% | ||
| Antihypertensive drugs | ||||||||||
| ACEI/ARB | 3243 | 31.7% | 3663 | 23.0% | <.001 | 2398 | 27.8% | 2404 | 27.9% | .92 |
| β‐blockers | 2768 | 27.0% | 3841 | 24.1% | <.001 | 2233 | 25.9% | 2209 | 25.7% | .68 |
| Calcium‐channel blockers | 2673 | 26.1% | 3573 | 22.5% | <.001 | 2117 | 24.6% | 2086 | 24.2% | .58 |
| Diuretics | 3393 | 33.1% | 5399 | 33.9% | .17 | 2867 | 33.3% | 2813 | 32.7% | .38 |
| Other antihypertensives | 560 | 5.5% | 848 | 5.3% | .63 | 474 | 5.5% | 474 | 5.5% | >.99 |
| Glucose‐lowering medications | ||||||||||
| Sulfonylurea | 7133 | 69.6% | 5786 | 36.4% | <.001 | 5505 | 63.9% | 5515 | 64.0% | .87 |
| Meglitinide | 817 | 8.0% | 926 | 5.8% | <.001 | 577 | 6.7% | 623 | 7.2% | .17 |
| α‐glucosidase inhibitor | 896 | 8.7% | 800 | 5.0% | <.001 | 598 | 6.9% | 585 | 6.8% | .70 |
| Thiazolidinedione | 594 | 5.8% | 306 | 1.9% | <.001 | 286 | 3.3% | 274 | 3.2% | .61 |
| Sitagliptin | 58 | 0.6% | 33 | 0.2% | <.001 | 29 | 0.3% | 26 | 0.3% | .69 |
| Insulin | 2161 | 21.1% | 2325 | 14.6% | <.001 | 1566 | 18.2% | 1491 | 17.3% | .13 |
| Other drugs | ||||||||||
| Statin | 1010 | 9.9% | 792 | 5.0% | <.001 | 676 | 7.9% | 641 | 7.4% | .32 |
| Aspirin | 1537 | 15.0% | 1812 | 11.4% | <.001 | 1150 | 13.4% | 1122 | 13.0% | .53 |
SD, standard deviation; HBV, hepatitis B virus; HCV, hepatitis C virus; CIC, chronic illness with complexity; DCSI, diabetes complications severity index; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Crude and multivariable adjusted Cox proportional hazards models with robust sandwich standard error estimates were used to compare the outcomes between metformin users and nonusers. All of the analyses were done on an as‐treated basis. The metformin users were censored (i.e. stop following up) if they stopped metformin use after the index date; by contrast, the metformin nonusers were censored if they started to use metformin after the defined index date. Because the competing risks of death might confound the estimates of risks for other outcomes, we applied the competing risk analysis for adjustment.
The results are shown as adjusted hazard ratios (aHR) with 95% confidence interval of metformin users, as compared with nonusers. We checked the proportional‐hazards assumption using the Schoenfeld residuals test and complementary log–log plots. To calculate risk of mortality, we censored patients at the time of defined death or the end of study, whichever came first. For other investigated outcomes, we censored patients on the date of death, the date of respective outcomes, or end of follow‐up on 31 December 2010, whichever occurred first. We compared the cumulative incidence of mortality and cirrhotic decompensation over time between metformin users and nonusers by the Fine and Gray's sub‐distribution hazard model.
To assess dose‐effect, we analysed the risk of mortality and cirrhotic decompensation by the cumulative defined daily dose (DDD) during the period of 90‐day metformin exposure (≤15 DDD, 16–40 DDD, or >40 DDD) and the prescribed daily dose (≤500 mg, 501–1000 mg, >1000 mg per day), relative to nonuse of metformin. DDD is the assumed average maintenance dose used for its main indication in adults, which is 2000 mg for metformin.
We also conducted several additional sensitivity analyses by: (i) excluding participants who were followed up for <180 days, to see the consistence of the investigated outcomes and to prevent selection bias by unintentionally excluding some cirrhotic patients with shorter lifespan; (ii) adding an inverse probability of censoring weight (IPCW) 19 , 20 as a covariate to the intention‐to‐treat model to account for nonrandom switches from metformin users to nonusers, or vice versa, during the observation period. The IPCW method assigned higher weights to the outcomes for those who have not changed status of metformin use, by which we could minimize the bias caused by the nonrandom censoring; (iii) updating the metformin use status annually and modelling metformin exposure as a time‐varying variable; and (iv) adding the variables of alcoholic disorders, HBV therapy (lamivudine, tenofovir disoproxil, adefovir dipivoxil, entecavir and telbivudine) or HCV therapy (interferons) to confirm our results (the adding variable model). A 2‐tailed P‐value <.05 was considered as significant. SAS version 9.4 and Stata SE version 11.0 were used for the analyses.
3. RESULTS
Between 1 January 2000 and 31 December 2009, a total of 26 164 patients were diagnosed with type 2 diabetes and compensated liver cirrhosis, while 15 056 patients were diagnosed as having T2DM with decompensated liver cirrhosis. The flowchart of the study subjects is depicted in Figure 1.
Before matching, several differences were noted between the 2 groups (Table 1). After propensity score matching, 8612 paired diabetes subjects with compensated liver cirrhosis were selected. The matched pairs were similar with respect to all covariates. The mean age of the cohort was 56.2 years; the mean duration of diabetes was 1.8 years; the prevalence of HBV and HCV infections were 18.3 and 19.7%.
In the matched cohort of T2DM with compensated liver cirrhosis, 733 (8.51%) metformin users and 666 (7.73%) nonusers died during follow‐up (incidence rate 3.8 vs 3.3 per 100 patient‐y). The multivariable models showed metformin users had significantly higher mortality risk (aHR 1.13 and 1.05 for adjusted model 1 and 2, respectively; Table 2).
TABLE 2.
Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with compensated liver cirrhosis
| Metformin users | Metformin nonusers | Crude a | Adjusted model 1 b | Adjusted model 2 c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Events | Person‐y | Incidence rate | Events | Person‐y | Incidence rate | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value |
| All‐cause mortality | 733 | 19 317 | 3.8% | 666 | 19 893 | 3.3% | 1.12 (1.01–1.25) | .03 | 1.13 (1.01–1.25) | .03 | 1.05 (0.96–1.14) | .28 |
| Cirrhotic decompensation | 854 | 14 567 | 5.9% | 726 | 14 921 | 4.9% | 1.16 (1.05–1.28) | .003 | 1.15 (1.04–1.27) | .006 | 1.24 (1.14–1.35) | <.001 |
| Oesophageal varices | 581 | 16 658 | 3.5% | 471 | 17 004 | 2.8% | 1.22 (1.08–1.38) | .002 | 1.18 (1.05–1.34) | .007 | 1.32 (1.19–1.47) | <.001 |
| Hepatic ascites | 472 | 16 747 | 2.8% | 456 | 17 001 | 2.7% | 1.02 (0.89–1.16) | .81 | 0.99 (0.87–1.13) | .91 | 1.12 (1.01–1.24) | .03 |
| Hepatic encephalopathy | 477 | 17 075 | 2.8% | 472 | 17 474 | 2.7% | 1.00 (0.88–1.14) | .95 | 0.99 (0.87–1.12) | .85 | 1.07 (0.97–1.19) | .18 |
| HCC | 471 | 15 022 | 3.1% | 441 | 15 209 | 2.9% | 1.06 (0.93–1.20) | .40 | 1.06 (0.93–1.21) | .40 | 1.08 (0.97–1.20) | .14 |
| Hepatic failure | 533 | 16 281 | 3.3% | 488 | 16 543 | 2.9% | 1.08 (0.95–1.22) | .23 | 1.07 (0.94–1.21) | .30 | 1.11 (1.01–1.23) | .04 |
| Jaundice | 97 | 18 179 | 0.5% | 97 | 18 748 | 0.5% | 0.99 (0.75–1.31) | .94 | 1.00 (0.76–1.33) | .98 | 1.03 (0.82–1.31) | .79 |
| MACE | 417 | 10 945 | 3.8% | 396 | 11 266 | 3.5% | 1.04 (0.91–1.19) | .56 | 1.07 (0.93–1.22) | .36 | 0.95 (0.86–1.06) | .37 |
| Stroke | 304 | 15 535 | 2.0% | 276 | 15 764 | 1.8% | 1.09 (0.92–1.28) | .32 | 1.10 (0.93–1.29) | .28 | 1.06 (0.93–1.20) | .38 |
| Ischaemic heart disease | 302 | 13 627 | 2.2% | 286 | 14 062 | 2.0% | 1.04 (0.89–1.23) | .61 | 1.04 (0.88–1.23) | .63 | 0.97 (0.86–1.10) | .64 |
| Heart failure | 151 | 17 010 | 0.9% | 168 | 17 249 | 1.0% | 0.88 (0.70–1.09) | .24 | 0.90 (0.72–1.12) | .33 | 0.89 (0.75–1.06) | .20 |
| Hypoglycemia | 31 | 19 034 | 0.2% | 36 | 19 462 | 0.2% | 0.84 (0.52–1.36) | .48 | 0.83 (0.51–1.35) | .44 | 0.89 (0.61–1.30) | .53 |
| Metabolic acidosis | 40 | 19 136 | 0.2% | 38 | 19 328 | 0.2% | 1.04 (0.67–1.62) | .86 | 1.05 (0.67–1.64) | .84 | 1.01 (0.72–1.42) | .97 |
CI, confidence interval; HCC, hepatocellular carcinoma; MACE, major adverse cardiac event, including stroke, ischaemic heart disease and heart failure.
Crude model: univariate analysis in the as‐treated model.
Adjusted model 1: as‐treated analysis adjusted for age, sex, diabetes mellitus duration (y), comorbid disorder (hepatitis B or C infection), chronic illness with complexity index (0, 1, ≥2), diabetes complications severity index score (0, 1, ≥2), antihypertensive drugs (angiotensin converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, calcium‐channel blockers, diuretics, other antihypertensives), glucose‐lowering medications (sulfonylureas, meglitinides, α‐glucosidase inhibitor, thiazolidinedione, sitagliptin, insulin), statin and aspirin.
Adjusted model 2: intention‐to‐treat model adjusted for age, sex, diabetes mellitus duration (y), comorbid disorder (hepatitis B or C infection), chronic illness with complexity index (0, 1, ≥2), diabetes complications severity index score (0, 1, ≥2), antihypertensive drugs (angiotensin converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, calcium‐channel blockers, diuretics, other antihypertensives), glucose‐lowering medications (sulfonylureas, meglitinides, α‐glucosidase inhibitor, thiazolidinedione, sitagliptin, insulin), statin, aspirin and inverse probability of censoring weight.
As shown in Table 2, compared to the nonusers, metformin users had higher risk in developing variceal bleeding (aHR 1.18) and cirrhotic decompensation (aHR 1.15); but had no significant difference in the risks of ascites (aHR 0.99), hepatic encephalopathy (aHR 0.99) and hepatic failure (aHR 1.07). If we used IPCW to account for nonrandom censoring, metformin use could significantly increase risk of cirrhotic decompensation (aHR 1.24), oesophageal varices bleeding (aHR 1.24), hepatic ascites (aHR 1.12) and hepatic failure (aHR 1.11). Figure 3 depicts the cumulative incidence of mortality and cirrhotic decompensation of metformin users and nonusers in T2DM with compensated liver cirrhosis. However, metformin users did not have higher risks of developing HCC, jaundice, MACE, hypoglycaemia or metabolic acidosis.
FIGURE 3.
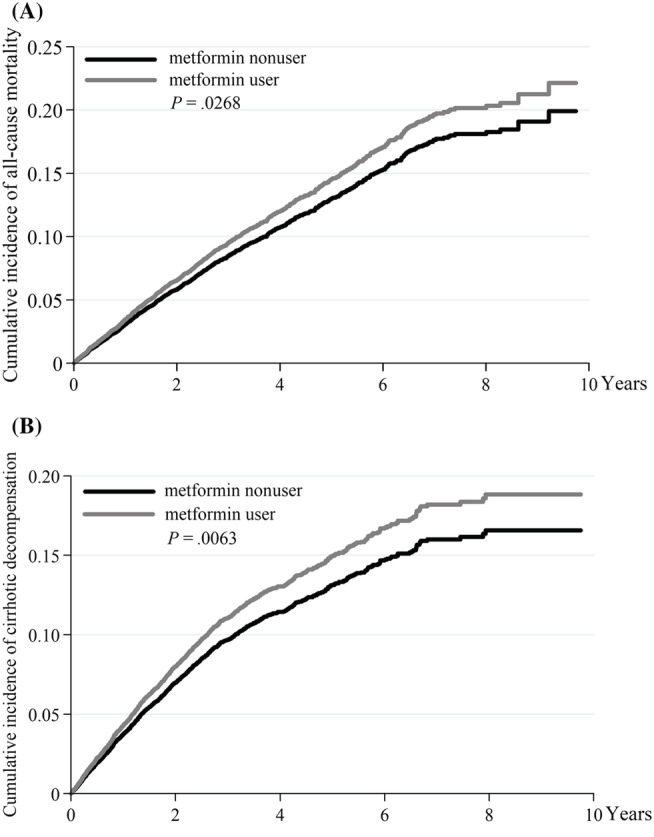
The cumulative incidence of mortality (A) and cirrhotic decompensation (B) between metformin users and nonusers in T2DM with compensated liver cirrhosis, by the Fine and Gray's sub‐distribution hazard method
As compared with patients not using metformin, those who received ≤15 DDD (aHR 1.25) and 16–40 DDD (aHR 1.24) of metformin did not have significantly higher risks of death, but the risk for those who received >40 DDD was significantly higher (aHR 1.38). The P‐value for dose–response trend was .0067. Similarly, compared with nonusers, those who took ≤500 mg (aHR 1.18) or 501–1000 mg (aHR 1.35) metformin per day did not have higher risks of death, but the risk for those who received >1000 mg/d was significantly higher (aHR 1.42; P for trend = .0029; Figure 4).
FIGURE 4.
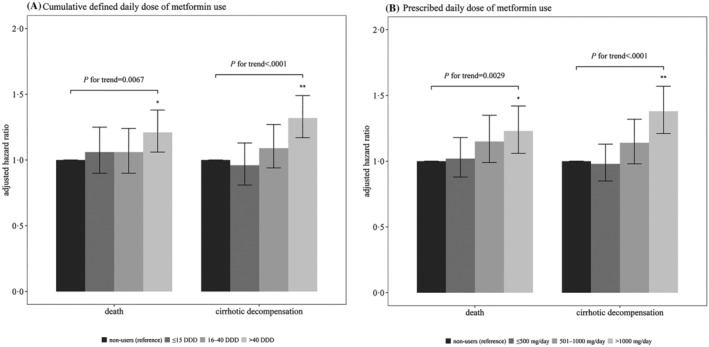
Outcomes of various dosage of metformin use compared to nonusers in patients with type 2 diabetes mellitus and compensated liver cirrhosis. (A) by defined daily dose (DDD); (B) by prescribed daily dose (mg/d). *: P < .05, **: P < .001. The error bars correspond to the 95% confidence intervals
As compared with patients not using metformin, those who received a cumulative DDD ≤15 (aHR 1.13) and > 40 (aHR 1.49) had significantly higher risks of cirrhotic decompensation; but for those who received a cumulative DDD of 16–40, the risk of developing cirrhotic decompensation was not significant (aHR 1.27). The P‐value for trend was <.0001. Similarly, compared with patients not using metformin, those who took metformin ≤500 mg/d (aHR 1.13) did not have higher risk of cirrhotic decompensation; but those who took 501–1000 (aHR 1.32) or >1000 (aHR 1.57) mg/d had significantly higher risks of developing cirrhotic decompensation. The P‐value for trend was <.0001 (Figure 4).
After propensity score matching, the 4333 matched pairs of diabetes patients with decompensated cirrhosis were similar with respect to all covariates, as shown in Supplementary Table S1. The multivariable models showed metformin users had significantly higher risk of mortality (aHR 1.15, P = .03). Metformin users did not have higher risks of developing HCC, hepatic failure, MACE, hypoglycaemia or metabolic acidosis. The IPCW model did not show any significant results for metformin use in patients with decompensated cirrhosis (Table 3).
TABLE 3.
Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with decompensated liver cirrhosis
| Metformin users | Metformin nonusers | Crude a | Adjusted model 1 b | Adjusted model 2 c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Events | Person–year | Incidence rate | Events | Person–year | Incidence rate | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value |
| All‐cause mortality | 522 | 10 084 | 5.2% | 460 | 10 717 | 4.3% | 1.17 (1.03–1.32) | .02 | 1.15 (1.02–1.31) | .03 | 0.98 (0.89–1.09) | .77 |
| HCC | 233 | 6995 | 3.3% | 201 | 7559 | 2.7% | 1.18 (0.98–1.42) | .09 | 1.19 (0.98–1.44) | .08 | 1.06 (0.90–1.24) | .49 |
| Hepatic failure | 310 | 5756 | 5.4% | 271 | 6093 | 4.4% | 1.16 (0.98–1.36) | .08 | 1.17 (1.00–1.37) | .06 | 1.08 (0.95–1.24) | .24 |
| MACE | 149 | 6327 | 2.4% | 163 | 6535 | 2.5% | 0.90 (0.72–1.12) | .36 | 0.92 (0.73–1.14) | .43 | 0.89 (0.74–1.07) | .21 |
| Stroke | 103 | 8333 | 1.2% | 104 | 8798 | 1.2% | 0.99 (0.76–1.29) | .92 | 1.02 (0.78–1.33) | .90 | 0.97 (0.78–1.21) | .79 |
| Ischaemic heart disease | 92 | 7761 | 1.2% | 98 | 8144 | 1.2% | 0.93 (0.71–1.23) | .62 | 0.94 (0.71–1.24) | .65 | 0.91 (0.73–1.14) | .42 |
| Heart failure | 67 | 8780 | 0.8% | 68 | 9187 | 0.7% | 0.98 (0.70–1.37) | .90 | 0.98 (0.70–1.38) | .90 | 0.89 (0.67–1.18) | .41 |
| Hypoglycaemia | 23 | 9826 | 0.2% | 13 | 10 523 | 0.1% | 1.77 (0.89–3.54) | .11 | 1.80 (0.90–3.60) | .10 | 1.21 (0.72–2.02) | .48 |
| Metabolic acidosis | 26 | 9922 | 0.3% | 16 | 10 476 | 0.2% | 1.63 (0.89–3.00) | .12 | 1.64 (0.90–3.01) | .11 | 0.94 (0.60–1.46) | .77 |
CI, confidence interval; HCC, hepatocellular carcinoma, MACE, major adverse cardiac event, including stroke, ischaemic heart disease and heart failure.
Crude model: univariate analysis in the as‐treated model.
Adjusted model 1: as‐treated analysis adjusted for age, sex, diabetes mellitus duration (y), comorbid disorder (hepatitis B or C infection), chronic illness with complexity index (0, 1, ≥2), diabetes complications severity index score (0, 1, ≥2), antihypertensive drugs (angiotensin converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, calcium‐channel blockers, diuretics, other antihypertensives), glucose‐lowering medications (sulfonylureas, meglitinides, α‐glucosidase inhibitor, thiazolidinedione, sitagliptin, insulin), statin and aspirin.
Adjusted model 2: intention‐to‐treat model adjusted for age, sex, diabetes mellitus duration (y), comorbid disorder (hepatitis B or C infection), chronic illness with complexity index (0, 1, ≥2), diabetes complications severity index score (0, 1, ≥2), antihypertensive drugs (angiotensin converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, calcium‐channel blockers, diuretics, other antihypertensives), glucose‐lowering medications (sulfonylureas, meglitinides, α‐glucosidase inhibitor, thiazolidinedione, sitagliptin, insulin), statin, aspirin and inverse probability of censoring weight.
Instead of excluding participants who were followed up for <365 days, we repeated the assessment by excluding those followed up for <180 days (Tables S2 and S3). The metformin users with compensated liver cirrhosis were associated with significantly higher risk of cirrhotic decompensation (Tables S2). Metformin use as compared with no use in decompensated liver cirrhosis was not associated with risk of all‐cause mortality, HCC, hepatic failure, MACE, hypoglycaemia or metabolic acidosis (Table S3). The time‐varying analysis disclosed that metformin use in compensated cirrhosis was significantly associated with higher risks of cirrhotic decompensation, variceal bleeding, hepatic ascites and hepatic failure (Table S4). Metformin use as compared with no use in decompensated cirrhosis was not associated with any investigated outcomes (Table S5). Our additional analysis for adding types of cirrhosis as covariates (the adding variable analysis) disclosed that approximately 18.2, 19.9 and 22.0% of patients with compensated cirrhosis have HBV infection, HCV infection and alcoholic disorders, respectively (Table S6); approximately 20.3, 19.2 and 31.9% of patients with decompensated cirrhosis have HBV infection, HCV infection and alcoholic disorders, respectively (Table S7). When including HBV and HCV therapy as covariates, the new model exhibited that metformin use in compensated cirrhosis was significantly associated with higher risks of mortality, cirrhotic decompensation and variceal bleeding (Table S8); metformin use in decompensated cirrhosis was significantly associated with higher risk of all‐cause mortality (Table S9).
4. DISCUSSION
Our results showed that metformin use was associated with higher risks of mortality and cirrhotic decompensation in patients with diabetes and compensated liver cirrhosis. These higher risks were dose dependent and persistent in the sensitivity tests. It also significantly associated with higher risk of mortality in patients with decompensated liver cirrhosis.
Zhang et al. used a hospitalized cohort to compare 172 patients who continued metformin use for at least 3 months with 78 patients who discontinued metformin use within 3 months after cirrhosis, concluding that continuous use of metformin was associated with longer survival. 9 Zhang's study may not be able to reflect a true benefit of metformin use in subjects with liver cirrhosis due to: (i) the discontinuation group had higher model for end‐stage liver disease and Child–Pugh scores, indicating higher severity in liver dysfunction; and (ii) immortal time bias (the continuation group was guaranteed to live for > 3 months because the definition of continuation and the index date).
Nkontchou et al. conducted a hospital‐based cohort study for patients with HCV induced liver cirrhosis, recruiting 26 subjects with metformin use and 74 patients receiving other glucose‐lowering medications. 10 The Nkontchou study indicated that aHR for metformin users compared to nonusers was 0.19 for HCC development and 0.22 for liver‐related death or transplantation; metformin use could prevent cirrhotic patients from liver‐related death or cancer by about 80%. However, results derived from studies with small sample size might be interpreted cautiously. Vilar‐Gomez et al. investigated 191 patients with diabetes and biopsy‐proven nonalcoholic steatohepatitis and fibrosis or cirrhosis, revealing that long‐term metformin uses might reduce the risks of all‐cause mortality, liver transplant and HCC. 21 There is some difference between Vilar‐Gomez's and our studies. First, all our patients have cirrhosis and some of the cirrhosis come from viral hepatitis. Second, Vilar‐Gomez's patients are from biopsy‐proven case series, our patients are from population based administrative database. Third, the case numbers are different.
Metformin has some adverse effects, including gastrointestinal discomforts and reduction of serum vitamin B12 with long‐term use. Its use in advanced chronic kidney disease significantly increases the risk of all‐cause mortality. 22 Metformin use could increase the risk of lactic acidosis, but only few cirrhotic patients with lactic acidosis have been reported. 23 Our study demonstrated that the use of metformin in compensated and decompensated cirrhotic patients was not associated with higher risk of metabolic acidosis.
Diabetes could aggravate the serious complications of liver cirrhosis. 4 Ampuero et al. reported that metformin could inhibit glutaminase activity and protect patients from hepatic encephalopathy, 24 but our study found that using metformin in compensated liver cirrhosis could significantly increase the risks of variceal bleeding and cirrhotic decompensation, except hepatic encephalopathy. The difference might be due to different study methods, patient numbers and some of our participants having chronic viral infection, different from Ampuero's patients, most of whom had nonalcoholic fatty liver disease.
Metformin does not undergo hepatic metabolism and is excreted unchanged by glomerular filtration and tubular secretion into urine. 25 It is not expected to cause or exacerbate liver injury. The case report disclosed that metformin use had the chance of drug‐induced hepatitis. 26 Animal studies demonstrated that metformin can accumulate in rat liver 4 times more than the plasma concentration, and can accumulate at 100 times higher concentration in the mitochondria. Through the organic cation transporter 1, metformin can enter cells and inhibit complex I of the mitochondrial electron transport chain, which could decrease ATP production and lead to energy stress. 27 Supratherapeutic concentrations of metformin can cause mitochondrial dysfunction through impairing the main site of energy production; they could dose‐dependently induce mitochondrial dysfunction and cytotoxicity of hepatocytes. 28 Mitochondrial injury has the potential to cause significant liver damage; persistent mitochondrial dysfunction could progress to portal hypertension and chronic liver failure. 29 Pre‐existing hepatic disorders also can augment the hepatic toxic effect. Many hepatic failure syndromes are linked by the common pathway of mitochondrial injury. 30 The above‐mentioned clues might be able to explain the results of our study. One animal study disclosed that metformin can reduce hepatic resistance and portal pressure in cirrhotic rats, 31 but our results suggested that metformin may increase portal pressure in patients with coexisting diabetes and cirrhosis. Because the physiology, pathology and pharmacological effects may be different between animal models and human subjects, we need to do further studies to check the change of portal pressure after metformin use in patients with coexisted diabetes and liver cirrhosis.
The UK Prospective Diabetes Study disclosed that metformin could decrease the risk of cardiovascular complication, 32 but the use of metformin in our cohorts did not have a beneficial effect on MACE. One study has reported that cardiovascular disease was rare in cirrhotic patients 33 ; this might be related to a shorter duration of diabetes in cirrhosis, reduced life expectancy of these patients, or some protective factors from cirrhosis (e.g., low platelet count and coagulation factors). Because cirrhotic patients have a lower incidence of cardiovascular events, metformin might be difficult to provide cardiovascular protection in these patients.
Our study had some strengths. First, this was a population‐based cohort study in the real‐world practice setting with a long‐term follow‐up. This study has the largest sample size regarding studying on metformin use in T2DM with liver cirrhosis. Second, we detailed the analysis of mortality, cirrhotic decompensation, metabolic acidosis and cardiovascular complications, tried to give a comprehensive description of outcomes for metformin use in T2DM with liver cirrhosis. Third, we derived our results from several study designs including propensity score matching, competing risk adjustment, precise error estimate for Cox proportional hazards models, IPCW adjustment to account for bias caused by nonrandom censoring, and the effect of time‐varying exposure of metformin. Our study design could also avoid immortal time bias by using a fixed exposure period (within 90 days after the comorbid date) to categorize metformin use and nonuse, and setting the 91st date as the index date for both groups.
Nevertheless, this study is subject to some limitations. First, the NHIRD did not provide complete information about patients' body weight, alcohol consumption, cigarette smoking, physical activity or family history—all of which might influence the measured outcomes. Second, this dataset was short of biochemical results; therefore, we could not calculate the Child–Pugh class and the model for end‐stage liver disease scores to classify the severity of liver cirrhosis. Instead, we used clinical diagnosis to separate patients into compensated and decompensated liver cirrhosis. Some patients with mild–moderate ascites or minimal hepatic encephalopathy may not be fully captured in outpatient clinics, which may lead to underdiagnosis of decompensated cirrhosis and affect study results. However, we tried to put in as many important variables as possible to balance the study and control groups to increase their comparability. Third, the cohort study was generally subject to some inevitable unknown confounding factors; a prospective randomized study would be needed to verify our results.
Finally, in this nationwide cohort study, metformin use in diabetes with liver cirrhosis was associated with higher risks of mortality and cirrhotic decompensation. Prospective studies are required to confirm our results.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
F.S.Y., Y.H.H., C.C.H.: participated in the study concept and design; Y.R.L., M.C.H., S.J.S., C.C.H.: participated in the acquisition of data, F.S.Y., Y.H.H., Y.R.L.: participated in the statistical analysis and interpretation of data; F.S.Y., S.J.S., C.C.H.: participated in the drafting of the manuscript; F.S.Y., M.C.H., C.M.H., Y.R.L., Y.H.H., C.C.H.: participated in the critical revision of the manuscript for important intellectual content, C.C.H., C.M.H.: participated in obtaining funding; Y.R.L., S.J.S., M.C.H., Y.H.H., C.M.H.: participated in the administrative, technical or material support; C.C.H., S.J.S.: participated in the study supervision.
ETHICS APPROVAL
Our study was approved by the institutional review board of the National Health Research Institutes (EC1060704‐E).
PATIENT CONSENT
The information recorded in the NHIRD was encrypted before release, such that patients or care providers could not be identified. Therefore, we were granted a waiver of the informed consent.
Supporting information
TABLE S1 Baseline characteristics of metformin users vs. matched nonusers in type 2 diabetes patients with decompensated liver cirrhosis
TABLE S2 Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with compensated liver cirrhosis (excluding those who had follow up for <180 days)
TABLE S3 Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with decompensated liver cirrhosis (excluding those who had follow up for <180 days)
TABLE S4 Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with compensated liver cirrhosis (time‐varying exposure)
TABLE S5 Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with decompensated liver cirrhosis (time‐varying exposure)min users vs. matched nonusers in type 2 diabetes patients with compensated liver cirrhosis (adding variables analysis)
TABLE S7 Baseline characteristics of metformin users vs. matched nonusers in type 2 diabetes patients with decompensated liver cirrhosis (adding variables analysis)
TABLE S8 Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with compensated liver cirrhosis (adding variables analysis)
TABLE S9 Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with decompensated liver cirrhosis (adding variables analysis)
ACKNOWLEDGEMENTS
This study was based on data from the NHIRD provided by the NHI Administration, Taiwan. The interpretation of data and conclusion reported herein do not represent those of the NHI Administration. This is a PI initiated research project. There is no external funding source supporting this analysis.
Yen F‐S, Huang Y‐H, Hou M‐C, et al. Metformin use and cirrhotic decompensation in patients with type 2 diabetes and liver cirrhosis. Br J Clin Pharmacol. 2022;88(1):311-322. 10.1111/bcp.14970
The authors confirm that the Principal Investigator for this paper is Professor Chih‐Cheng Hsu, who had direct clinical responsibility for the study results, database and statements of this manuscript.
DATA AVAILABILITY STATEMENT
Data are available from the National Health Insurance Research Database managed by Taiwan National Health Insurance Administration. Requests for data should be sent as a formal proposal to the NHIRD (https://dep.mohw.gov.tw/DOS/np-2500-113.html).
REFERENCES
- 1. HEPAMAP . A Roadmap for Hepatology Research in Europe: An Overview for Policy Makers 2019. http://www.easl.eu/medias/EASLimg/News/3f9dd90221ef292_file.pdf. Accessed January 15, 2019.
- 2. American Liver Foundation . Your Liver 2019. https://liverfoundation.org/for-patients/about-the-liver/. Accessed January 28, 2019.
- 3. Fleming KM, Aithal GP, Card TR, West J. All‐cause mortality in people with cirrhosis compared with the general population: a population‐based cohort study. Liver Int. 2012;32(1):79‐84. 10.1111/j.1478-3231.2011.02517.x [DOI] [PubMed] [Google Scholar]
- 4. Elkrief L, Rautou PE, Sarin S, Valla D, Paradis V, Moreau R. Diabetes mellitus in patients with cirrhosis: clinical implications and management. Liver Int. 2016;36(7):936‐948. 10.1111/liv.13115 [DOI] [PubMed] [Google Scholar]
- 5. Garcia‐Compean D, Jaquez‐Quintana JO, Gonzalez‐Gonzalez JA, Maldonado‐Garza H. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15(3):280‐288. 10.3748/wjg.15.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uygun A, Kadayifci A, Isik AT, et al. Metformin in the treatment of patients with non‐alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19(5):537‐544. 10.1111/j.1365-2036.2004.01888.x [DOI] [PubMed] [Google Scholar]
- 7. Xu H, Zhou Y, Liu Y, et al. Metformin improves hepatic IRS2/PI3K/Akt signaling in insulin‐resistant rats of NASH and cirrhosis. J Endocrinol. 2016;229(2):133‐144. 10.1530/JOE-15-0409 [DOI] [PubMed] [Google Scholar]
- 8. Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin‐dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804‐10812. 10.1158/0008-5472.CAN-07-2310 [DOI] [PubMed] [Google Scholar]
- 9. Zhang X, Harmsen WS, Mettler TA, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014;60(6):2008‐2016. 10.1002/hep.27199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nkontchou G, Cosson E, Aout M, et al. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. 2011;96(8):2601‐2608. 10.1210/jc.2010-2415 [DOI] [PubMed] [Google Scholar]
- 11. Cheng TM. Taiwan's new national health insurance program: genesis and experience so far. Health Aff. 2003;22(3):61‐76. 10.1377/hlthaff.22.3.61 [DOI] [PubMed] [Google Scholar]
- 12. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 2005;104(3):157‐163. [PubMed] [Google Scholar]
- 13. Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47(5):e50‐e54. 10.1097/MCG.0b013e3182688d2f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mukerji AN, Patel V, Jain A. Improving survival in decompensated cirrhosis. Int J Hepatol. 2012;2012:318627. 10.1155/2012/318627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meduru P, Helmer D, Rajan M, Tseng CL, Pogach L, Sambamoorthi U. Chronic illness with complexity: implications for performance measurement of optimal glycemic control. J Gen Intern Med. 2007;22(Suppl 3):408‐418. 10.1007/s11606-007-0310-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and health care utilization. Am J Manag Care. 2008;14(1):15‐23. [PMC free article] [PubMed] [Google Scholar]
- 17. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17(19):2265‐2281. [DOI] [PubMed] [Google Scholar]
- 18. Iezzoni LI. Risk adjustment for measuring healthcare outcomes. Chicago, IL: Health Administration Press; 1997. [Google Scholar]
- 19. Breskin A, Cole SR, Westreich D. Exploring the Subtleties of Inverse Probability Weighting and Marginal Structural Models. Epidemiology. 2018;29(3):352‐355. 10.1097/EDE.0000000000000813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cole SR, Hernán MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158(7):687‐694. 10.1093/aje/kwg206 [DOI] [PubMed] [Google Scholar]
- 21. Vilar‐Gomez E, Vuppalanchi R, Desai A, et al. Long‐term metformin use may improve clinical outcomes in diabetic patients with non‐alcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis. Aliment Pharmacol Ther. 2019;50(3):317‐328. 10.1111/apt.15331 [DOI] [PubMed] [Google Scholar]
- 22. Hung SC, Chang YK, Liu JS, et al. Metformin use and mortality in patients with advanced chronic kidney disease: national, retrospective, observational, cohort study. Lancet Diabetes Endocrinol. 2015;3(8):605‐614. 10.1016/S2213-8587(15)00123-0 [DOI] [PubMed] [Google Scholar]
- 23. Edwards CM, Barton MA, Snook J, David M, Mak VH, Chowdhury TA. Metformin‐associated lactic acidosis in a patient with liver disease. QJM. 2003;96(4):315‐316. 10.1093/qjmed/hcg049 [DOI] [PubMed] [Google Scholar]
- 24. Ampuero J, Ranchal I, Nuñez D, et al. Metformin inhibits glutaminase activity and protects against hepatic encephalopathy. PLoS ONE. 2012;7(11):e49279. 10.1371/journal.pone.0049279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamed AE, Abas B, Shaltout I, et al. Managing diabetes and liver disease association, guidelines (consensus) development. J Endocrinol Diabetes Obes. 2015;3(3):1‐19. [Google Scholar]
- 26. Babich MM, Pike I, Shiffman ML. Metformin‐induced acute hepatitis. Am J Med. 1998;104(5):490‐492. 10.1016/s0002-9343(98)00088-6 [DOI] [PubMed] [Google Scholar]
- 27. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577‐1585. 10.1007/s00125-017-4342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Protti A, Lecchi A, Fortunato F, et al. Metformin overdose causes platelet mitochondrial dysfunction in humans. Crit Care. 2012;16(5):R180. 10.1186/cc11663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carr A, Morey A, Mallon P, Williams D, Thorburn DR. Fatal portal hypertension, liver failure, and mitochondrial dysfunction after HIV‐1 nucleoside analogue‐induced hepatitis and lactic acidaemia. Lancet. 2001;357(9266):1412‐1414. 10.1016/S0140-6736(00)04579-7 [DOI] [PubMed] [Google Scholar]
- 30. Fromenty B, Pessayre D. Inhibition of mitochondrial beta‐oxidation as a mechanism of hepatotoxicity. Pharmacol Ther. 1995;67(1):101‐154. 10.1016/0163-7258(95)00012-6 [DOI] [PubMed] [Google Scholar]
- 31. Tripathi DM, Erice E, Lafoz E, et al. Metformin reduces hepatic resistance and portal pressure in cirrhotic rats. Am J Physiol Gastrointest Liver Physiol. 2015;309(5):G301‐G309. 10.1152/ajpgi.00010.2015 Epub 2015 Jul 2 [DOI] [PubMed] [Google Scholar]
- 32. UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854‐865. 10.1016/S0140-6736(98)07037-8 [DOI] [PubMed] [Google Scholar]
- 33. Marchesini G, Ronchi M, Forlani G, et al. Cardiovascular disease in cirrhosis—a point‐prevalence study in relation to glucose tolerance. Am J Gastroenterol. 1999;94(3):655‐662. 10.1111/j.1572-0241.1999.00931.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Baseline characteristics of metformin users vs. matched nonusers in type 2 diabetes patients with decompensated liver cirrhosis
TABLE S2 Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with compensated liver cirrhosis (excluding those who had follow up for <180 days)
TABLE S3 Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with decompensated liver cirrhosis (excluding those who had follow up for <180 days)
TABLE S4 Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with compensated liver cirrhosis (time‐varying exposure)
TABLE S5 Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with decompensated liver cirrhosis (time‐varying exposure)min users vs. matched nonusers in type 2 diabetes patients with compensated liver cirrhosis (adding variables analysis)
TABLE S7 Baseline characteristics of metformin users vs. matched nonusers in type 2 diabetes patients with decompensated liver cirrhosis (adding variables analysis)
TABLE S8 Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with compensated liver cirrhosis (adding variables analysis)
TABLE S9 Outcomes of metformin users vs. matched nonusers in type 2 diabetes patients with decompensated liver cirrhosis (adding variables analysis)
Data Availability Statement
Data are available from the National Health Insurance Research Database managed by Taiwan National Health Insurance Administration. Requests for data should be sent as a formal proposal to the NHIRD (https://dep.mohw.gov.tw/DOS/np-2500-113.html).


