ABSTRACT
Global changes are severely affecting pollinator insect communities worldwide, resulting in repeated patterns of species extirpations and extinctions. Whilst negative population trends within this functional group have understandably received much attention in recent decades, another facet of global changes has been overshadowed: species undergoing expansion. Here, we review the factors and traits that have allowed a fraction of the pollinating entomofauna to take advantage of global environmental change. Sufficient mobility, high resistance to acute heat stress, and inherent adaptation to warmer climates appear to be key traits that allow pollinators to persist and even expand in the face of climate change. An overall flexibility in dietary and nesting requirements is common in expanding species, although niche specialization can also drive expansion under specific contexts. The numerous consequences of wild and domesticated pollinator expansions, including competition for resources, pathogen spread, and hybridization with native wildlife, are also discussed. Overall, we show that the traits and factors involved in the success stories of expanding pollinators are mostly species specific and context dependent, rendering generalizations of ‘winning traits’ complicated. This work illustrates the increasing need to consider expansion and its numerous consequences as significant facets of global changes and encourages efforts to monitor the impacts of expanding insect pollinators, particularly exotic species, on natural ecosystems.
Keywords: invasive species, exotic species, expansion, global change, conservation, butterflies, bees, bumblebees
I. INTRODUCTION
The development of human societies has profoundly impacted global biogeochemical systems to such a degree that the period of time in which we now live has been dubbed the Anthropocene (Lewis & Maslin, 2018; Sage, 2020). From the point of view of biodiversity, this epoch has been marked by a global wave of species extinctions, population extirpations, and negative population trends leading to a significant shift in ecosystem composition (Dirzo et al., 2014). Although species declines have understandably received much attention, this has overshadowed another facet of global change: species undergoing expansion. This latter phenomenon comprises either an increase in the total area of a species’ distribution or a shift from its original distribution, implying expansion into new territories along with the loss of part of their historical range. In both cases, the resulting distribution encompasses new locations with potential consequences for native wildlife (Tomiolo & Ward, 2018).
Three main factors predominantly account for how a subset of species undergo expansion (McGeoch & Latombe, 2016). First, species can respond positively to successful interventions in their habitats that were planned to avoid their extinction (Willis et al., 2009; Neel et al., 2012). Second, steadily increasing evidence shows that populations and species can expand along at least one margin of their distribution range following global changes in temperature and habitats (e.g. Hiddink, Burrows & Molinos, 2015). Third, species can reach new localities and establish inside or outside their native range through human transport (Aizen et al., 2020). While increases in relative abundance, range shifts, and expansions of species as a result of global change are often well documented, the traits of species that allow them to expand and establish new and/or bigger populations are less well understood. Key combinations of life‐history traits are known at least partly to explain such expansions under global changes (Estrada et al., 2016; MacLean & Beissinger, 2017). For example, taxa with a higher dispersal ability are more likely to reach suitable habitats (Travis et al., 2013) and undergo larger range shifts (Buckley & Kingsolver, 2012). Consequently, larger body size, often positively correlated with dispersal ability, shows a high potential to be associated with species demonstrating a geographical range expansion (Lyons, Wagner & Dzikiewicz, 2010; but see Angert et al., 2011). Niche generalism, specifically dietary breadth and range of habitats used, can also affect the ability of an organism to establish in new locations (Braschler & Hill, 2007; Angert et al., 2011), and indeed these two traits are often intercorrelated (Lurgi, López & Montoya, 2012). Other life‐history traits such as a high fecundity, or early and frequent reproduction can also favour the ability of a species to colonize, establish viable populations, and persist in areas beyond its historic distribution (Angert et al., 2011). Although such traits may be important individually, they can present contradictory effects and thus may be counterbalanced. For instance, organisms with larger body size tend to have a lower reproduction rate, leading to antagonistic effects on range expansion (Lurgi et al., 2012).
Given their high species diversity (>5.5 million species; Stork, 2018) and considerable economic importance (almost $60 billion a year in the USA; Losey & Vaughan, 2006), insects represent excellent models to investigate the context‐dependent importance of such traits following ecological disruptions (Halsch et al., 2021). The ongoing shifts of global entomofauna are variable in space, time and intensity at the community, species and population levels, with impacts on natural (e.g. emerging patterns of introgressive hybridization, speciation and extinction) and anthropogenic (e.g. spread of pest species and disease vectors, loss of ecosystem services) biomes. In particular, the response of insect pollinators, mainly bees, butterflies, hoverflies, moths and pollinating wasps, to global changes has garnered much attention due to their role in facilitating the sexual reproduction of both wild plants and crops (Potts et al., 2010; Ollerton, 2017). More than 80% of the world's flowering plants are dependent on insects for pollination (Ollerton, Winfree & Tarrant, 2011), including ~75% of all crops (Vanbergen & The Insect Pollinators Initiative, 2013; Kremen, 2018). Although pollinating insects are widely reported to have experienced drastic population declines (e.g. Settele et al., 2008; Goulson et al., 2015; Kerr et al., 2015; Agrawal & Inamine, 2018; Wagner, 2020; Zattara & Aizen, 2021), a non‐negligible number of species are increasing their abundance, shifting their original distribution area towards new territories, and consequently expanding from their historical distribution ranges (e.g. Duchenne et al., 2020).
In the current context of a global biodiversity crisis, highlighting the factors and the traits that jointly allow a subset of species to benefit from global changes is a fundamental step towards better understanding and predicting the numerous consequences of ongoing global changes. In this context, we aim to review the current understanding of the various factors (Fig. 1) and traits (Table 1) associated with expansions of pollinators. Finally, we discuss the impacts of such expansions on native wildlife.
Fig 1.
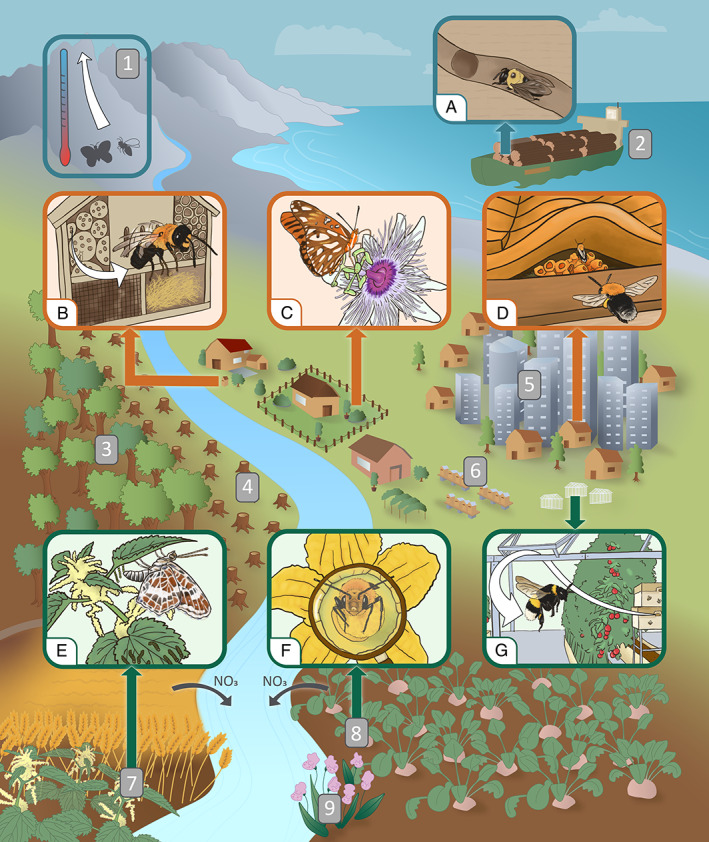
Visual representation of the factors involved in range expansion in pollinators in the Anthropocene: 1, climate change; 2, anthropogenic transport; 3, reforestation; 4, deforestation; 5, urbanization; 6, pollinator domestication; 7, eutrophication; 8, agricultural intensification; 9, plant invasion. A, carpenter bee moved through wood transport; B, invasive megachilid bee benefitting from bee hotels; C, butterfly co‐expanding with its ornamental host; D, bumblebee nesting in a roof; E, butterfly co‐expanding with nitrophilous host; F, squash bee co‐expanding with crop host; G, bumblebee benefitting from domestication. Blue outlines correspond to climate change and anthropogenic transport, orange outlines to novel anthropogenic habitats and green outlines to agricultural intensification and its consequences. Illustrator: Morgane Goyens.
Table 1.
Traits associated with range expansion in pollinators and context in which the trait is beneficial
| Candidate winning trait | Context in which the trait is efficient | References |
|---|---|---|
| Global warming | ||
| Sufficient mobility to: (i) track colder areas (ii) follow warming areas |
Gradual, localized warming Green network to reach higher latitudes or altitudes (i.e. no biotic or abiotic insurmountable obstacles) |
Biella et al. (2020); Martinet et al. (2015); Rasmont et al. (2015); Kerr et al. (2015); Au & Bonebrake (2019); Parmesan et al. (1999); Breed, Stichter & Crone (2012); Pateman et al. (2012); Banaszak, Cibicka & Twerd (2019); Crozier (2003) |
| Plasticity to extreme temperatures | Sudden climatic events, heat waves but with a moderate duration | Zambra et al. (2020); Martinet et al. (2021a , b ); see also Oyen, Giri & Dillon (2016) |
| Inherent association with warmer climates (e.g. tropical, Mediterranean) |
Gradual global temperature rise Warmer winters |
Biella et al. (2020); Martinet et al. (2015); Rasmont et al. (2015); Au & Bonebrake (2019); Banaszak et al. (2019); Noor et al. (2017) |
| Global habitat change | ||
|
Flexibility in nesting material Ability to use human structures for nesting Above‐ground nesting |
Increased anthropic material in ecosystems Increased frequency of impervious surfaces |
Prŷs‐Jones, Kristjánsson & Ólafsson (2016); Prŷs‐Jones (2019); Ballare & Jha (2020); Geslin et al. (2020) |
|
Generalized diet and resource use Flexible diet and opportunism (e.g. nectar robbing) High metabolic performance |
Landscape homogenization Increased spatio‐temporal plant–pollinator mismatches |
Moerman et al. (2016); Vanderplanck et al. (2014); Braby et al. (2014) |
| Specialized diet on expanding host plants |
Host is ornamental Host is a crop Host is itself expanding |
Halsch et al. (2021); López‐Uribe et al. (2016); Ryan et al. (2019); Betzholtz et al. (2013); Vane‐Wright (1993) |
| Association with open habitats | Deforestation | Noor et al. (2017) |
| Association with woody habitats | Increased forested areas | Betzholtz et al. (2013) |
| Larger body size (increased dispersal ability) | Increase in habitat fragmentation | Gérard et al. (2020a ); Pöyry et al. (2009); Freedman et al. (2020) |
| Human facilitation | ||
|
Expected high efficiency as a crop pollinator Easy to manage (e.g. eusociality) Flexibility in phenology |
Increasing need for pollination services (e.g. growing human population, lack of natural pollination services) Social species more likely to be chosen for domestication and exportation |
Geslin et al. (2017) and references therein; Aizen et al. (2020) and references therein; Schweiger et al. (2010) |
| Wood nesting, cavity nesting |
Global trade of wood and other goods by air/sea Palletized system of global trade |
Cane (2003); Fortel et al. (2016); Okabe et al. (2010) and references therein; Sheffield, Heron & Musetti (2020) and references therein |
|
Other facilitating ‘universal’ traits (can apply when the species has already expanded) | ||
|
Territoriality and aggression Competitive advantage for resources |
Poorly competitive natives Regions lacking these behaviours with a ‘naive’ local fauna |
Aizen et al. (2020) and references therein; Geslin et al. (2017) and references therein |
| Parthenogenic, polyandrous reproduction system | Could apply without restriction | Magnacca & King (2013) |
II. POLLINATORS BENEFITING FROM CLIMATE CHANGE
(1). Global impact of climate change on pollinators
Both acute as well as gradual changes in temperature can impact species distributions and population dynamics, and a possible response to these pressures is a shift in distribution across elevation and latitude towards regions that were previously too cool prior to climate warming (Pecl et al., 2017). Climatic conditions directly impact pollinator population dynamics through the modulation of survival (Crozier, 2004; Martinet et al., 2021a ), development (WallisDeVries & van Swaay, 2006; Vanderplanck et al., 2019), fertility (Martinet et al., 2021b ), and dispersal (Parmesan et al., 1999; Hill, Hastings & Botsford, 2002). Moreover, climate partly constrains pollinator distribution based on their thermal requirements (e.g. thermal minima and maxima) (Oyen et al., 2016; Ghisbain et al., 2020). Considering climatic variables independently from other confounding variables is however understandably complex, as climate also impacts pollinators indirectly through environmental changes.
Importantly, climatic variation impacts plant–pollinator interactions by altering the quality and the quantity of plant resources (i.e. pollen and nectar; Carnell, Hulse & Hughes, 2020) or mismatches in the spatio‐temporal distributions of mutualistic partners (Thomson, 2016; Gérard et al., 2020b ). Water stresses (e.g. droughts, floods) are examples of extreme events that are likely to increase in frequency, duration and severity (Dai, 2013; EEA, 2017), and which have numerous effects on plant–pollinator interactions, including modifications in plant and flower size, inflorescence number, floral longevity, and scent (Thomson, 2016; Lambrecht, Morrow & Hussey, 2017; Campbell, Sosenski & Raguso, 2019; Kahl, Lenhard & Joshi, 2019; Descamps, Quinet & Jacquemart, 2020). The food supply for pollinator populations is expected to be altered as these extreme phenomena increase (reviewed in Descamps et al., 2020).
(2). Dealing with the heat or tracking the cold
Several empirical studies have shown strong correlations between global warming (i.e. change in mean temperature) and spatial shift in butterflies (e.g. Parmesan et al., 1999; Breed et al., 2012; Au & Bonebrake, 2019), with more than 60% of studied butterfly species extending their distributions northwards by 35–240 km during the 20th century (Parmesan et al., 1999). Although ~80% of butterfly species included in a more recent study underwent southern range declines, many species with southerly centred ranges showed population growth and shifted their distributions northwards (Breed et al., 2012). This phenomenon is well established in countries like Great Britain, where several studies have demonstrated a temperature‐related shift towards northern regions (e.g. Asher et al., 2001; Pateman et al., 2012). Climate warming has also been proposed as a driving factor behind the colonization of subtropical areas by a diverse array of butterfly and moth species (Chan et al., 2011; Cheng et al., 2019). A key trait involved in these range shifts is that a high proportion of these newcomers are of tropical origin that follow the increasing suitability of subtropical areas for tropical species (Au & Bonebrake, 2019). Such range expansion that is at least partly due to climate change can occur particularly rapidly, as shown for the Indian native butterfly Acraea terpsicore that has colonized new territories of Southeast Asia at an estimated rate of 200 km/year over the last 30 years (Noor et al., 2017).
Amongst the variables involved in expansion, seasonal warming has increasingly been shown to trigger significant effects on range shifts in certain insect pollinators through the expansion of their potential climatic niche. Milder winters, subsequent warmer overwintering conditions, and warmer months during the emergence of queens, have been correlated with the rapid westward colonization of the bumblebee Bombus haematurus by 20% in about 40 years without evidence of dietary niche shift (Biella et al., 2020). Similarly, some heat‐adapted carpenter bees (Apidae, Xylocopa) are expanding their distributions, this expansion being correlated with higher temperature in both winter and summer (Tripodi & Szalanski, 2011; Vickruck & Richards, 2017; Banaszak et al., 2019). Possible mechanistic explanations for these trends might be decreased mortality in a critical phase of an insect's life cycle, as warmer winters could act as a prerequisite for range expansion by increasing larval survivorship (in butterflies, see Crozier, 2003, 2004) or queen success in overwintering (in bumblebees, see Biella et al., 2020). Other life‐history variables can also explain expansion trends, with some butterfly species that do not overwinter as eggs or unfed neonate larvae, as well as multivoltine species, being more prone to show range expansion (Breed et al., 2012). As a result of milder winters, some bumblebee populations have been observed throughout the cold season feeding on under‐exploited flowering plants from urban parks and gardens (Stelzer et al., 2010). Although winter‐foraging could be seen as a favourable adaptive strategy to avoid interspecific competition with other pollinators, such changes in phenology might only be physiologically feasible for pollinators that are able to forage at lower temperatures (e.g. close to 0°C), such as bumblebees (Heinrich, 1979). In species that are already multivoltine however, warmer temperatures during larval development are expected to lead to an additional generation(s) each year (Kiritani, 2006; Gomi et al., 2007). Since 1980 in Europe, a significant proportion of multivoltine butterfly species showed increased frequency of second and subsequent generations, and others even augmented the overall number of generations (Altermatt, 2010).
In Europe and North America, both range shifts and range expansions have only been shown in a minority of the predominantly cold‐adapted bumblebees (Apidae, Bombus) (Kerr et al., 2015; Martinet et al., 2015; Rasmont et al., 2015), while many other members of the genus are regressing (Cameron & Sadd, 2020). A recent study supports the hypothesis that climate (in conjunction with land‐use change and floral availability) has driven an elevation shift in both bumblebees and plants in the mountains of southwestern Europe (Marshall et al., 2020). The observed concurrent changes were however asymmetrical, with plants having shifted on average 100 m higher than bumblebees. High‐elevation habitats are expected to become critically important for cold‐adapted pollinators, as they can act as cold‐temperature refugia from habitats that may no longer exist at lower elevations (Rasmont et al., 2015; Penado, Rebelo & Goulson, 2016). In other species of bumblebees, expansion with retention of the original range (e.g. Bombus haematurus, B. schrencki) could partly be explained by a greater inherent adaptation to acute heat stress during extreme climatic events (i.e. higher critical thermal limit and/or longer time before heat stupor), as suggested by thermal stresses applied to bumblebees under laboratory conditions (Zambra et al., 2020; Martinet et al., 2021a ). Higher resistance to heat stress can ultimately be associated with a higher survival rate that may allow persistence in areas experiencing an increased incidence of heat waves (Martinet et al., 2021a ). Alternatively, the observed retention of their original range could be due to (i) these localities acting as temporary climatic refugia, with the possibility that these relictual populations will die out following further climatic changes, and (ii) a lack of overall sampling in some parts of their range preventing an accurate quantification of their actual regression (Rasmont et al., 2015).
Overall, both gradually increasing average temperatures and the greater frequency of extreme climatic events constitute key factors that can benefit species exhibiting key physiological or ecological traits, such as an inherent adaptation to hotter areas or resilience to heat waves, involved in reducing mortality during critical phases of the pollinator life cycle (Table 1). Although we are not aware of studies explicitly demonstrating positive effects of change in precipitation on the expansion of pollinators, further research is encouraged to investigate whether such events could be responsible for shifting success as a result of physiological adaptation to precipitation regimes.
III. POLLINATORS SURVIVING IN ANTHROPOGENICALLY MODIFIED HABITATS
(1). Thriving inside and outside cities: impact of habitat modification on pollinators
Urbanization leads to the creation of novel ecosystems, including a higher proportion of impervious surfaces, habitat fragmentation and isolation, and a high percentage of exotic plants and novel nesting material (Buchholz & Egerer, 2020). Outside cities, other anthropogenically modified habitats include the use of pesticides, which can impact mortality and behaviour (Henry et al., 2012; Woodcock et al., 2016), fragmentation impacting habitat quality (Senapathi et al., 2015) or loss of floral and nesting resources for insect pollinators (Kleijn & Raemakers, 2008). Globally, habitat destruction and fragmentation, mostly associated with the intensification of agriculture and the consequent eutrophication (Carvalheiro et al., 2020), is a leading factor driving the decline of pollinators (Powney et al., 2019; Duchenne et al., 2020) and insects in general (Wagner, 2020).
(2). New habitats, new opportunities
While anthropogenically induced land‐use and land‐cover change is well known to accelerate rates of extinction in many taxa (Ellis et al., 2010), it can also create new opportunities for a subset of species. Depending on the nature of habitat modification, the identity and composition of the pollinator communities that will be favoured can vary strongly. The creation of novel open habitats helped, for instance, the butterfly Acraea terpsicore expand its range by 7000 km2 across large‐scale palm‐oil and Acacia plantations across Southeast Asia over a 30‐year period (Noor et al., 2017). By contrast, where forest cover has remained stable, such as in Northern Europe, forest species show greater range expansions than species associated with open habitats (Betzholtz et al., 2013). Similarly in southern and eastern Europe, where reforestation occurred following the abandonment of agricultural lands due to socio‐economic changes (Lasanta et al., 2017), species strongly associated with woodlands are favoured (Jenič, Gogala & Grad, 2010).
(3). Dealing with fragmentation
Regardless of habitat modification, fragmentation impacts the number of suitable patches, their shape complexity, their isolation, as well as the proportion of edges (Didham, 2010). The increasing distance between patches of suitable habitats has been suggested to select for larger body size in pollinators, because larger body size can imply higher dispersal capability. In Finland, larger butterflies have been able to shift further distances than smaller ones (Pöyry et al., 2009). In a context of large‐scale migrations, a recent analysis revealed that initial monarch (Danaus plexippus) founders presented large and elongated forewings and were therefore well suited for long‐distance movements, in contrast with populations that ceased migration after establishment (Freedman et al., 2020). In several European countries, bumblebee queen body size has increased over the last century, possibly in response to increased habitat fragmentation (Gérard et al., 2020a ). This pattern has also been observed spatially, with some species of solitary bees displaying a larger body size in more fragmented agricultural landscapes (Warzecha et al., 2016). Alternatively, highly agricultural landscapes can also be associated with lower resource quality and quantity, two factors that negatively impact body size (Roulston & Cane, 2002; Gérard et al., 2018). Moreover, smaller body size can lead to higher overall fitness in these landscapes as smaller offspring require fewer resources in absolute terms to reproduce (Renauld et al., 2016).
(4). Nest opportunists
Landscape changes, particularly urbanization, have made available a completely new array of potential nesting locations for some insect pollinators. Given the mostly impervious nature of soils in cities, above‐ground nesting pollinators are more likely to be selected in urban areas. In Europe, the bumblebee Bombus hypnorum nests in above‐ground cavities, mainly in trees, but frequently takes advantage of human structures such as bird nest boxes, holes in walls, and roofing tiles (Prŷs‐Jones, 2019). This ability, combined with increased forest coverage after 1945 and the urbanization of Europe after 1900, has probably allowed it drastically to increase its abundance, range, and density, including in new countries where it can be the most common species in urban areas (Prŷs‐Jones et al., 2016; BWARS, 2017; Prŷs‐Jones, 2019). In North America, the carpenter bee Xylocopa virginica also benefits from anthropogenic modifications of habitats, this large bee nesting almost exclusively in structures built from milled lumber such as spruce and pine (Ballare & Jha, 2020). In cities, the increasing popularity of bee hotels in public parks also provides new nesting opportunities for bees. These structures have, however, been shown largely to favour the expansion of exotic species, as demonstrated in the South of France where the most common species that emerged from bee hotels was the invasive Megachile sculpturalis (Geslin et al., 2020). A similar pattern has been observed from a survey of almost 600 bee hotels in Canada, in which introduced bees represented nearly half of all bees recorded (MacIvor & Packer, 2015).
(5). Host‐plant opportunists
Some pollinator expansions can be related to the expansion of their respective host plants, either because these hosts are associated with horticultural practices (e.g. domestication of the host plants as ornamental plants), agricultural practices (e.g. domestication of the host plants as crops; selection/support of the wild host plants) and invasion, or because the hosts are themselves expanding following climate changes. For example, the Gulf fritillary butterfly (Agraulis vanillae) has expanded its range in the Western USA in the past 100 years (Shapiro & Manolis, 2007) partly following the use of its host Passiflora as a popular ornamental garden plant (Halsch et al., 2021). Specialization on one specific agricultural crop explains range expansion in the North American squash bee Peponapis pruinosa, a strict pollen specialist of the plant genus Cucurbita. The bee is currently found far beyond the distribution of its ancestral wild host (Cucurbita foetidissima), in these areas relying entirely on domesticated Cucurbita pepo used in commercial and domestic agriculture. Thanks to this anthropogenic facilitation, P. pruinosa has attained one of the largest geographical ranges of North American native bees (López‐Uribe et al., 2016). Similarly, although at a much broader scale, the cabbage white butterfly Pieris rapae, an agricultural pest, has undergone a dramatic expansion following the global use of brassicaceous crops on which its larvae feed (e.g. cabbage, canola, bok choy, turnips). The species is believed to have originated in Europe and to have started expanding ~1200 years ago following the diversification of brassicaceous crops and the development of human trade routes such as the Silk Road (Hiura, 1968; Fukano et al., 2012). Over the last 160 years, P. rapae successfully continued its global invasion through multiple independent introductions and is now present on all continents except South America and Antarctica (Ryan et al., 2019). Another consequence of agricultural expansion has been increased fertilizer use that has favoured plant species associated with nitrogen‐rich habitats, a potentially important driver of range expansion in butterfly species with a nitrogen‐favoured larval diet (Betzholtz et al., 2013). An index assessing the level at which nitrogen‐rich habitats can impact butterflies has been developed and could be used to forecast which species could benefit from anthropogenic habitats (WallisDeVries & van Swaay, 2017). Away from agriculture, climate‐driven expansion of key host plants partly explains the dramatic northern expansion of the specialist giant swallowtail butterfly (Papilio cresphontes) by 180 km per decade in North America, an expansion that is also associated with warmer, wetter climate conditions in both overwintering and active flight stages of this pollinator (Wilson et al., 2021).
In general, an inherently broader diet is likely best for facilitating species expansion, as the ability to consume a wider variety of available resources will allow dietary shifts depending on resource availability (Roger et al., 2017). In Europe, the large majority of strongly expanding bee species are generalists, while specialized species are mostly stable or decreasing (Rasmont et al., 2015; Scheper et al., 2015). Among Australian butterflies, several species have expanded their ranges since 1970 (Peters, Smithers & Rushworth, 2010) and a common trait was the ability of the larvae to forage on non‐native larval food plants (Braby et al., 2014). Similarly, Nymphalinae butterflies showing a greater dietary breadth (i.e. with a more diverse host‐plant use) are naturally more widespread than butterflies with narrower host‐plant use (Slove & Janz, 2011). Whether a broader dietary breadth is a cause or a consequence of range expansion in such pollinators could be contextual. An increasing dietary breadth could lead to a range expansion if the range of the pollinator was originally restricted by the range of its host plant (Janz & Nylin, 2008). Alternatively, if the original range of a species is constricted by a factor other than diet (e.g. isotherms), a range expansion caused by an external factor (e.g. a temperature rise) can put the pollinator in contact with novel resources that could ultimately be used through ecological fitting (Agosta & Klemens, 2008). However, if the inherent dietary flexibility of the species is too low, host switching is unlikely to occur, as dietary shifts generally occur over evolutionary time (Larkin, Neff & Simpson, 2008; Dellicour et al., 2014).
Regardless of dietary breadth, the introduction of non‐native plants into ecosystems can promote the expansion of non‐native pollinators and vice versa. This phenomenon, reported as invasive mutualism or alien mutualism, can strongly erode native pollination networks by modifying the strength of interactions and distributions of asymmetries to the advantage of ‘super‐generalist’ alien species during invasion (Aizen, Morales & Morales, 2008). The invasive mutualism of non‐native honeybees and the exotic yellow star thistle (Centaurea solstitialis), both introduced to the western USA in the 1800s, is a key factor that allowed the expansion of both aliens (Barthell et al., 2001). Similarly, human‐driven colonization by milkweeds (Asclepias spp.) in non‐native locations has aided the dramatic expansion of the highly mobile monarch butterfly across both the Pacific and Atlantic oceans in the 19th century (Vane‐Wright, 1993; Pierce et al., 2014).
Finally, an inherent physiological ability to perform well on a wide variety of diets or on novel diets is a key factor in range shifts, as is the case for the globally expanding generalist bumblebee species Bombus terrestris (Moerman et al., 2016; see Section IV.2). More studies investigating and comparing the metabolic performance of pollinators under stressful conditions (e.g. heat stress; Tomlinson et al., 2015) could provide key insights into the mechanisms allowing expanding species to arrive and establish in new areas. In contrast to bees, the majority of butterfly species are generally much more tightly bound to specific host plants (Forister et al., 2015), although instances of host‐plant shifts have attracted much recent attention (e.g. Chaturvedi et al., 2018; Singer & Parmesan, 2019). Given that larval diet in butterflies is directly related not only to larval performance (Couture, Serbin & Townsend, 2015) but also to forewing characteristics and flight abilities in adults (Johnson et al., 2014; Reim et al., 2019), subsequent impacts are expected on adult migration, especially in a context of climate warming (Soule, Decker & Hunter, 2020).
IV. POLLINATORS BENEFITING FROM HUMAN INTRODUCTION
(1). Expansion and invasion in human‐introduced pollinators
Human introductions of pollinating insects can be deliberate (e.g. domestication followed by intentional introduction to new areas), although most are accidental and caused by the international transport of goods (Russo, 2016). This introduction of non‐native pollinator species to new geographical areas can subsequently lead to their establishment (Braby et al., 2014) and therefore expansion from their original range. This phenomenon is mostly observed on islands where non‐native pollinators can become the most abundant pollinator species, but invasions can also occur on the mainland (Russo, 2016). At the global scale, introduction of managed pollinators to improve pollination services has predominantly involved bees. The two most abundant and widespread introduced bee species are the buff‐tailed bumblebee (Bombus terrestris) (see Section IV.2) and the Western honey bee, Apis mellifera, both eusocial species (Goulson, 2003; Garibaldi et al., 2013; Kleijn et al., 2015). With the notable exception of Megachile rotundata (Pitts‐Singer & Cane, 2011), solitary bees have been introduced less extensively (e.g. the megachilid Osmia ribifloris for blueberry pollination from the west to the east coast of the USA, or the halictid Nomia melanderi for alfalfa pollination from North America to New Zealand) (Stubbs, Drummond & Osgood, 1994; Howlett & Donovan, 2010). Overall, most human‐introduced pollinators are found within two families, Megachilidae (33 non‐native species) and Apidae (30 non‐native species) (Russo, 2016).
Some species are seen as ‘tramp’ species, meaning they are particularly prone to anthropogenic dispersal due to inherent combinations of traits that appear beneficial in particular contexts. Of the 30 non‐native bee species that have established themselves in North America (Cane, 2003; Gibbs & Sheffield, 2009), most share two key traits: they are cavity‐nesters and non‐parasitic. While the former trait can directly facilitate establishment in exotic species (see example of Megachile sculpturalis in Section III.4), the latter is mainly due to non‐parasitic pollinators having the advantage of not depending on a host for survival. Noticeable examples of species in which cavity‐nesting is an advantage for range expansions include carpenter bees (Xylocopa), a genus of wood‐nesting species that are commonly found outside their original ranges accompanying the global trade of wood (e.g. Okabe et al., 2010). Short travel times of driftwood logs in oceans could also promote short‐distance dispersal in these wood‐nesting bees (Sheffield et al., 2020).
Reproductive strategy can also be an important factor associated with expansion, as proposed for Ceratina dentipes, a bee originating from Southeast Asia and now rapidly spreading across Hawaii and the South Pacific (Groom et al., 2017; Shell & Rehan, 2019). Because to date only females have been caught in Hawaii, the species is thought to be capable of parthenogenic reproduction, a trait that could help facilitate invasion, as a population could be founded by a single mated female (Magnacca & King, 2013). Another way populations can be buffered against the challenge of colonizing new environments is through female multiple mating or polyandry, which can facilitate colonization by protecting against inbreeding, reducing the costs of mating with infertile or incompatible males (Lewis et al., 2020).
Several other behavioural and morphological characteristics are likely to facilitate expansion in human‐introduced pollinators, such as the aggressive territorial behaviour displayed by the males of Anthidium manicatum, a species native to Europe and northern Africa now established in Asia, North America, South America, New Zealand and the Azores (e.g. Strange et al., 2011; Soper & Beggs, 2013). A major consequence of this aggressive behavior (Colla, 2016) is the exclusion of native bumble bees from floral resources in invaded sites (Graham et al., 2019). In the Caribbean, aggressive Africanized honeybees fully replaced the European honeybee, followed by subsequent loss of aggressive behaviour (Rivera‐Marchand, Oskay & Giray, 2012; Ackerman, 2021). Finally, aggressive behaviour could have acted as a facilitating trait for the spread of the Asian‐native bee Megachile sculpturalis in both the USA and Europe, notably through evictions of native species and subsequent occupation of their nests (Roulston & Malfi, 2012; Le Feon et al., 2018; Geslin et al., 2020). Among other factors, this rapid expansion may be due to both its polylectic diet and its ability to nest in a wide range of cavities (see Section III.4), facilitating survival in a wide variety of habitats (Fortel et al., 2016). Wasp pollinators in Vespidae have also frequently been mistakenly introduced into new areas, with some species becoming invasive and threatening native species (Beggs et al., 2011). Their success is likely due to more efficient social and foraging behaviours, a generalist predatory lifestyle, and their aggression (Gamboa, Greig & Thom, 2002; Armstrong & Stamp, 2003).
Records of invasions following human introduction in other pollinators such as butterflies and hoverflies seem less common. For instance, while more than 10 exotic butterfly species have been introduced to Australia, only three have become established (Braby et al., 2014). Finally, although many hoverfly species have also been actively introduced globally for pest management, this family is far less studied and no examples are readily available (Hardy, 1964).
(2). Case study of a winner: the worldwide success story of the recently domesticated buff‐tailed bumblebee
One of the most famous cases of a pollinator expanding its range due to human facilitation is the bumblebee Bombus terrestris. Since the late 1980s, this species, native to the western Palaearctic, has been exported across the globe for pollination of greenhouse crops, particularly tomatoes (Velthuis & Van Doorn, 2006). However, the species rapidly showed invasive characteristics and now raises major conservation concerns where it has been imported (Geslin & Morales, 2015; Aizen et al., 2020; Cameron & Sadd, 2020).
The global success of B. terrestris can be explained by an array of ecological and physiological features that make it highly adaptable, facilitating its survival and reproduction in a wide variety of habitats. First, B. terrestris has a large and highly flexible diet (Rasmont et al., 2008; Boustani et al., 2020), even in areas outside its native range where it is able to use indigenous plants (Hingston et al., 2002; Goulson, 2003). It demonstrates the ability to rob nectar from plants, allowing (i) carbohydrate intake without the need to expend energy and time in handling flowers with complex morphologies (as suggested in A. mellifera; Dedej & Delaplane, 2005), and (ii) consumption of nectar from plants with nectaries otherwise inaccessible to its short tongue. This ability has been observed outside of its native range and is reported to decrease nectar availability for other pollinators (Sáez et al., 2017). Under laboratory conditions, this species produces larger pupae than other bumblebees even on resources of lower quality (Vanderplanck et al., 2014; Moerman et al., 2016). They also have some degree of tolerance to pathogens that may have spilled over from this species detrimentally to infect native bees not previously exposed to these pathogens in areas of introduction (Arbetman et al., 2013).
In addition to these characteristics, B. terrestris copes well with a variable climate. The species shows a high tolerance to a sudden and lasting hyperthermic stress, the major consequence of heat waves (Zambra et al., 2020; Martinet et al., 2021a ) and has also been able to expand its range during recent increases in annual mean temperature (Martinet et al., 2015). In addition to its physiological resilience to climate and diet, B. terrestris is a comparatively early‐emerging bee, enabling it to benefit from spring resources to found a nest and initiate its colony before its competitors. Unlike most declining bumblebee species, its phenology is bivoltine (i.e. with two generations per year) or even winter active in suitable locations, allowing it to benefit from a larger array of floral resources throughout the year (Stelzer et al., 2010). Consequently, social generalist bees with broad diets such as B. terrestris (but also A. mellifera) may suffer less from both temporal and spatial mismatches than solitary, univoltine and specialized bees which are generally restricted to narrower activity periods in which the host plants are available (Schweiger et al., 2010). In addition, the large number of workers in social bee species optimizes egg production and food intake, contrasting with the lifestyle of solitary bee species (Stevens, Hogendoorn & Schwarz, 2007).
With its combination of climatic tolerance, dietary flexibility, and strong dispersal ability, coupled with advantageous life‐history traits such as a long phenology and sociality, Bombus terrestris provides key insights into our understanding of ‘winning traits’ for a pollinator in the context of global change.
V. CONSEQUENCES OF RANGE EXPANSION ON NATIVE ECOSYSTEMS
(1). Competition with native pollinators
There is surprisingly little evidence for competition between native species and expanding non‐managed species for floral and nesting resources, although some cases have been reported (e.g. Portman, Tepedino & Tripodi, 2019). Many cases of expansions concern pollinators using resources with very low levels of competition, an element that could facilitate the expansion of such species. Documented examples of competition for nesting resources between non‐managed exotic and native bees include the wood‐nesting exotic bee Lithurgus chrysurus and the native Megachile spp. in North America (Rust et al., 2004), and M. sculpturalis (see Section III.4).
On the other hand, competition between native species and massively introduced managed species (MIMS) has raised much interest, notably when MIMS integrate into native plant–pollinator networks (Geslin et al., 2017). During recent decades, the use of MIMS has become more prevalent following the increased dependency of agriculture on managed insect pollination in ecosystems (Aizen et al., 2008; Lautenbach et al., 2012). The main consequence of the introduction of MIMS seems to be exploitative competition for floral resources (Stout & Morales, 2009; but see Nishikawa et al., 2019; Iwasaki et al., 2018). Such an overlap can trigger a cascade of effects on wild pollinators including a decrease in fitness (Elbgami et al., 2014), in visitation frequency to specific plants (Shavit, Dafni & Ne'eman, 2009), and in shifts towards alternative plants (Walther‐Hellwig et al., 2006). In addition to potential spatio‐temporal overlaps in floral choices, competition and exclusion of native pollinators from nest sites by introduced MIMS have been increasingly suggested as significant impacts, notably in bumblebees (Matsumura, Yokoyama & Washitani, 2004; Inoue, Yokoyama & Washitani, 2008; Inoue & Yokoyama, 2010).
(2). Co‐expansion with exotic host plants
Introduction of non‐native pollinator species may promote the spread or co‐expansion of non‐native plant species as the introduced bees could mainly forage on exotic flowers, potentially increasing their seed set and therefore fitness (Stout, Kells & Goulson, 2002; Morales & Aizen, 2006). For example, more than 80% of plants visited by the invasive Anthidium manicatum in New Zealand are exotic species (Lamiaceae and Plantaginaceae), although it also visits native plants (Soper & Beggs, 2013). Such non‐native mutualisms can lead to the formation of ‘invasion complexes’, in which non‐native pollinators disproportionally visit non‐native plants for their resources, thereby promoting their reproduction (Morales & Aizen, 2002, 2006). However, range expansion into anthropogenic habitats could also potentially rescue native plant species when wild native pollinators are scarce (Sanguinetti & Singer, 2014).
(3). Transmission of diseases and pathogens
Invader species can displace the resident fauna if they are tolerant to diseases that they vector, and that have detrimental effects on native species (Holt & Bonsall, 2017). Transmission of pathogens is increasingly discussed in the context of global expansion of managed insect pollinators, with growing evidence showing that they can be transmitted to wild species (Gisder & Genersch, 2017). Again, the most documented examples come from the intensely managed bumblebees. Data also show that non‐native solitary bee species can transmit parasites to closely related indigenous species (Bosch, 1992; McKinney & Park, 2013; Hedtke et al., 2015) and such pathogen transmissions may result in distribution shifts in native species. In Sweden, the northward expansion of the butterfly Araschnia levana has induced shifts in resident species distributions, with a possible explanation being parasitoid‐driven competition generating significant mortality in the native species (Audusseau et al., 2017, 2020).
Honeybees and more recently bumblebees have been intensely domesticated for the purposes of crop pollination, to such an extent that hundreds of thousands of colonies are now moved across countries on an annual basis. In particular, the recent globalization in the commercial use of bumblebees has caused a huge expansion of their range, raising concerns due to suspected impacts on the pathogenosphere of indigenous species. Growing evidence shows that parasite transmission by these anthropogenically expanding species, i.e. managed species, could be correlated with the decline of native species (Cameron et al., 2011). Evidence supports the hypothesis that pathogen spillover to native communities occurs in the proximity of greenhouses in which commercial colonies are installed (Graystock, Goulson & Hughes, 2014). In practice, a large number of foragers escape from greenhouses and share floral resources with wild indigenous bees, with the potential for transmission of pathogens to wild populations via contact with flowers (Colla et al., 2006). At the continental scale, there is growing evidence that European bumblebee colonies have carried at least two pathogens (Apicystis bombi and Crithidia bombi) to the Americas and parts of Asia (Cameron & Sadd, 2020). European strains of parasites have been found in Eastern Asia, highlighting the massive parasite migration that has accompanied the expansion of commercialized bumblebees globally (Goka, Okabe & Yoneda, 2006). The case of South America is particularly alarming, since the timing of the geographic expansion of domesticated B. terrestris appears to be congruent with the decline of the endemic Patagonian Bombus dahlbomii, which is now known to host at least one of the invader's pathogens (Arbetman et al., 2013).
Domesticated pollinators can therefore facilitate the spread of non‐native diseases to wild populations, triggering epidemics that could eventually affect both community structure and pollination services. Much work is still required to understand fully the consequences of the global pollinator trade on pathogen prevalence and its consequences on wild pollinator faunas (Aizen et al., 2020).
(4). Impacting crop yield
Non‐native pollinators such as honey bees that outcompete native species, have been found to be less‐efficient pollinators that can result in poorer fruit set and quality than pollination by native bees for some crop plants (see Winfree et al., 2007; Garibaldi et al., 2013). In extreme cases of invasion (e.g. by MIMS), a high abundance of non‐native pollinators can translate into excessively frequent pollinator visits per flower, exacerbating costs to the plant (e.g. nectar robbing or flower damage; Sáez et al., 2018). An oversaturation of pollination services, although not strictly limited to contexts in which exotic pollinators are present, is increasingly recognized as detrimental for fruit production and may even diminish yields of pollinator‐dependent crops (reviewed in Aizen et al., 2020). Apart from harmful effects of pollinators on plants during visits or during the pollination process itself, the global expansion of crop pests also negatively impacts crop yields, such as the butterfly Pieris rapae (McKinlay, 1992; Ryan et al., 2019).
(5). Mating with native taxa
In addition to range expansions perturbing community interactions, introduced species can also impact local populations by creating novel points of contact among closely related species that were previously geographically isolated. When close relatives interact, introgression may occur (Bartomeus et al., 2020; Cejas et al., 2020). This can challenge the integrity of species, potentially threatening rare species (Rhymer & Simberloff, 1996), subspecies distinctions (Lecocq et al., 2016), and species’ fitness (Barton & Hewitt, 1989). Commercial introduction of the European bumble bee B. terrestris to Japan has led to reports of high levels of mating with the closely related endemic species B. hypocrita, resulting in reduced fertility (Kanbe et al., 2008; Tsuchida et al., 2019), and introduction of the eastern North American species B. impatiens into the range of its sister species in Mexico (B. ephippiatus) has raised similar concerns (Duennes et al., 2017). Similarly, increased overlap of range limits of swallowtail butterflies Papilio glaucus and P. canadensis as a result of range expansions with climate change are thought to have generated hybrids with differing phenology and potentially host use (Mercader, Aardema & Scriber, 2009). Introgression also can lead to selective transfer of advantageous alleles between resident and introduced lineages that can enhance the ability of an introduced species to survive in its environment, enabling it to expand its range further. Such adaptive introgression has been demonstrated in mimetic neotropical Heliconius butterflies. Study of loci driving wing patterns has revealed that some of these butterfly species have acquired their mimetic phenotypes through introgression with other mimetic species (Dasmahapatra et al., 2012; Kronforst, 2012). Once alleles for these color patterns were transferred to the new species, they became immediately adaptive, enabling the species to expand into new mimicry zones. Through similar processes, as alleles are shared among related pollinators as a consequence of range expansion, adaptive alleles may be transferred both within and across species that facilitate improved fitness in their newly occupied ranges, thus leading to further range expansion (Pfennig, Kelly & Pierce, 2016).
Overall, a substantial range of effects can be expected on native plant–pollinator systems following the expansion of insect pollinators. Two major issues must, however, be clearly separated: MIMS and non‐managed pollinators. This separation is crucial because regulatory authorities have much more control on the former, and are responsible for the uncontrolled invasions that have occurred where regulations have been lacking (Aizen et al., 2020; Bartomeus et al., 2020). Although a few facilitative interactive effects of MIMS have been suggested (namely a spillover of shared plant resources and transfer of social information to optimize foraging, see Geslin et al., 2017), these cases are not likely to compensate for the ecological disruptions caused by MIMS. The second case of expansion (non‐managed pollinators) is much more difficult to control as it results from a combination of biotic (e.g. species traits) and abiotic (e.g. climate change) conditions that are much harder to predict (Fig. 1, Table 1). In this particular case, the role of governments predominantly lies in controlling and mitigating the anthropogenic factors that mostly cause the expansions, namely global warming and habitat destruction.
VI. CONCLUSIONS
While much has been written in recent decades about the threats and ecological traits associated with global biodiversity declines, the scientific literature contains significantly fewer syntheses focused on the characteristics of expanding species. Here, we reviewed the traits (Table 1) and factors (Fig. 1) that have facilitated the expansions of a fraction of the pollinating entomofauna, allowing this group to take advantage of what are generally deleterious global environmental changes.
Although the characteristics allowing species to expand are mostly taxon specific and context dependent, various traits including high mobility, high resistance to acute heat stress, and inherent adaptations to warmer climates appear to be important in allowing pollinators to persist and even expand in the face of climate change. Similarly, an overall level of flexibility regarding resource requirements is common in expanding species, although dietary niche specialization can also be involved in range expansion under specific contexts.
Importantly, expansion of wild and domesticated species can show diverse impacts on indigenous wildlife, including resource competition, co‐expansion with exotic plants, pathogen spread, detrimental impacts on crop yield, and hybridization with indigenous wildlife.
The context‐dependent nature of each of these success stories renders generalizations towards ultimate candidate ‘winning traits’ complicated. Similarly, the consequences of these expansions appear hard to predict and to disentangle from confounding biotic or abiotic disruptions.
There has been no global evaluation of how the combined patterns of decline and expansion of pollinators will affect our ecosystems and food security in the near future. What is clear however is that our current reliance on massively introduced pollinators is far from sustainable and must be considered a serious issue for the conservation of global ecosystems.
Further standardized work gathering quantitative data to reveal the rates and magnitude of range shifts is strongly encouraged to investigate the spatial and temporal aspects of expansions. In addition, further work investigating specific traits such as species fecundity and individual mobility are needed to understand population shifts better across space and time.
Continuously monitoring populations and identifying the relative contributions of both traits and factors involved with increases in range size and abundance remain crucial aspects of our understanding of how species react to environmental changes globally. Most importantly, these efforts need to be made at a much larger geographic scale than is presently the case, given that our global knowledge on insect conservation is derived predominantly from Europe and North America.
Considering patterns of both decline and expansion is fundamental to assess accurately current biodiversity changes at a global level. The present review underlines the importance for ecologists to understand how wildlife reacts to global changes, and we hope it will encourage future work on our understanding of patterns of species expansions worldwide.
VII. ACKNOWLEDGEMENTS
We thank two anonymous reviewers and the editorial board of Biological Reviews for their constructive comments. The authors express their gratitude to Morgane Goyens for creating the summary figure. This work was partly supported by the Fonds National de la Recherche Scientifique (F.R.S.‐FNRS) and the Fonds Wetenschappelijk Onderzoek (FWO) under EOS project named CLIPS (n°3094785). G.G. is supported by a F.R.S.‐FNRS PhD grant “Aspirant” and T.J.W. by a F.R.S‐FNRS postdoctoral grant Chargé de Recherches.
REFERENCES
- Ackerman, J. D. (2021). Island invasions by introduced honey bees: what can be expected for Puerto Rico and the Caribbean? Frontiers in Ecology and Evolution 8, 556744. [Google Scholar]
- Agosta, S. J. & Klemens, J. A. (2008). Ecological fitting by phenotypically flexible genotypes: implications for species associations, community assembly and evolution. Ecology Letters 11, 1123–1134. [DOI] [PubMed] [Google Scholar]
- Agrawal, A. A. & Inamine, H. (2018). Mechanisms behind the monarch's decline. Science 360, 1294–1296. [DOI] [PubMed] [Google Scholar]
- Aizen, M. A. , Arbetman, M. P. , Chacoff, N. P. , Chalcoff, V. R. , Feinsinger, P. , Garibaldi, L. A. , Harder, L. D. , Morales, C. L. , Sáez, A. & Vanbergen, A. J. (2020). Invasive bees and their impact on agriculture. In The Future of Agricultural Landscapes, Part I. Advances in Ecological Research (eds Bohan D. A. and Vanbergen A. J.), pp. 49–92. Elsevier, London. [Google Scholar]
- Aizen, M. A. , Morales, C. L. & Morales, J. M. (2008). Invasive mutualists erode native pollination webs. PLoS Biology 6, 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altermatt, F. (2010). Climatic warming increases voltinism in European butterflies and moths. Proceedings of the Royal Society B: Biological Sciences 277(1685), 1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angert, A. L. , Crozier, L. G. , Rissler, L. J. , Gilman, S. E. , Tewksbury, J. J. & Chunco, A. J. (2011). Do species’ traits predict recent shifts at expanding range edges? Ecology Letters 14, 677–689. [DOI] [PubMed] [Google Scholar]
- Arbetman, M. P. , Meeus, I. , Morales, C. L. , Aizen, M. A. & Smagghe, G. (2013). Alien parasite hitchhikes to Patagonia on invasive bumblebee. Biological Invasions 15, 489–494. [Google Scholar]
- Armstrong, T. R. & Stamp, N. E. (2003). Colony productivity and foundress behaviour of a native wasp versus an invasive social wasp. Ecological Entomology 28, 635–644. [Google Scholar]
- Asher, J. , Warren, M. , Fox, R. , Harding, P. , Jeffcoate, G. & Jeffcoate, S. (2001). The Millennium Atlas of Butterflies in Britain and Ireland. Oxford University Press, Oxford. [Google Scholar]
- Au, T. F. & Bonebrake, T. C. (2019). Increased suitability of poleward climate for a tropical butterfly (Euripus nyctelius) (Lepidoptera: Nymphalidae) accompanies its successful range expansion. Journal of Insect Science 19(6), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audusseau, H. , Le Vaillant, M. , Janz, N. , Nylin, S. , Karlsson, B. & Schmucki, R. (2017). Species range expansion constrains the ecological niches of resident butterflies. Journal of Biogeography 44, 28–38. [Google Scholar]
- Audusseau, H. , Ryrholm, N. , Stefanescu, C. , Tharel, S. , Jansson, C. , Champeaux, L. , Shaw, M. R. , Raper, C. , Lewis, O. T. , Janz, N. & Schmucki, R. (2020). Altered parasitism of a butterfly assemblage associated with a range‐expanding species. Biorxiv. 10.1101/2020.02.13.947440. [DOI] [Google Scholar]
- Ballare, K. M. & Jha, S. (2020). Genetic structure across urban and agricultural landscapes reveals evidence of resource specialization and philopatry in the Eastern carpenter bee, Xylocopa virginica L. Evolutionary Applications 14(1), 136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszak, J. , Cibicka, W. B. & Twerd, L. (2019). Possible expansion of the range of Xylocopa violacea L. (Hymenoptera, Apiformes, Apidae) in Europe. Turkish Journal of Zoology 43, 650–656. [Google Scholar]
- Barthell, J. F. , Randall, J. M. , Thorp, R. W. & Wenner, A. M. (2001). Promotion of seed set in yellow star‐thistle by honey bees: evidence of an invasive mutualism. Ecological Applications 11, 1870–1883. [Google Scholar]
- Bartomeus, I. , Molina, F. P. , Hidalgo‐Galiana, A. & Ortego, J. (2020). Safeguarding the genetic integrity of native pollinators requires stronger regulations on commercial lines. Ecological Solutions and Evidence 1, e12012. [Google Scholar]
- Barton, N. H. & Hewitt, G. M. (1989). Adaptation, speciation and hybrid zones. Nature 341, 497–503. [DOI] [PubMed] [Google Scholar]
- Beggs, J. R. , Brockerhoff, E. G. , Corley, J. C. , Kenis, M. , Masciocchi, M. , Muller, F. , Rome, Q. & Villemant, C. (2011). Ecological effects and management of invasive alien Vespidae. BioControl 56, 505–526. [Google Scholar]
- Betzholtz, P.‐E. , Pettersson, L. B. , Ryrholm, N. & Franzén, M. (2013). With that diet, you will go far: trait‐based analysis reveals a link between rapid range expansion and a nitrogen‐favoured diet. Proceedings of the Royal Society B: Biological Sciences 280, 20122305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biella, P. , Ćetković, A. , Gogala, A. , Neumayer, J. , Sárospataki, M. , Šima, P. & Smetana, V. (2020). Northwestward range expansion of the bumblebee Bombus haematurus into Central Europe is associated with warmer winters and niche conservatism. Insect Science 28, 861–872. 10.1111/1744-7917.12800. [DOI] [PubMed] [Google Scholar]
- Bosch, J. (1992). Parasitism in wild and managed populations of the almond pollinator Osmia cornuta Latr. (Hymenoptera: Megachilidae). Journal of Apicultural Research 31, 77–82. [Google Scholar]
- Boustani, M. , Yammine, W. , Nemer, N. , Abou Fakhr Hammad, E. , Michez, D. & Rasmont, P. (2020). Distribution and flower visitation records of bumblebees in Lebanon (Hymenoptera: Apidae). Annales de la Société entomologique de France (N.S.) 56, 115–124. [Google Scholar]
- Braby, M. F. , Bertelsmeier, C. , Sanderson, C. & Thistleton, B. M. (2014). Spatial distribution and range expansion of the tawny Coster butterfly, Acraea terpsicore (Linnaeus, 1758) (Lepidoptera: Nymphalidae), in South‐East Asia and Australia. Insect Conservation and Diversity 7(2), 132–143. [Google Scholar]
- Braschler, B. & Hill, J. K. (2007). Role of larval host plants in the climate‐driven range expansion of the butterfly Polygonia c‐album . Journal of Animal Ecology 76, 415–423. [DOI] [PubMed] [Google Scholar]
- Breed, G. A. , Stichter, S. & Crone, E. E. (2012). Climate‐driven changes in northeastern US butterfly communities. Nature Climate Change 3, 142–145. [Google Scholar]
- Buchholz, S. & Egerer, M. (2020). Functional ecology of wild bees in cities: towards a better understanding of trait‐urbanization relationships. Biodiversity and Conservation 29, 2779–2801. [Google Scholar]
- Buckley, L. B. & Kingsolver, J. G. (2012). Functional and phylogenetic approaches to forecasting species’ responses to climate change. Annual Review of Ecology, Evolution, and Systematics 43, 205–226. [Google Scholar]
- BWARS (Bees, Wasps & Ants Recording Society) (2017). Bombus hypnorum map. http://www.bwars.com/index.php?q=content/bombus-hypnorum-2014. Accessed 24.03.2019.
- Cameron, S. A. , Lozier, J. D. , Strange, J. P. , Koch, J. B. , Cordes, N. , Solter, L. F. & Griswold, T. L. (2011). Patterns of widespread decline in North American bumble bees. Proceedings of the National Academy of Sciences of the United States of America 108, 662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, S. A. & Sadd, B. M. (2020). Global trends in bumble bee health. Annual Review of Entomology 65, 209–232. [DOI] [PubMed] [Google Scholar]
- Campbell, D. R. , Sosenski, P. & Raguso, R. A. (2019). Phenotypic plasticity of floral volatiles in response to increasing drought stress. Annals of Botany 123, 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane, J. H. (2003). Exotic nonsocial bees (Hymenoptera: Apiformes) in North America: ecological implications. In For Non‐Native Crops, Whence Pollinators of the Future? (eds Stricler K. and Cane J. H.), pp. 113–126. Thomas Say Publications in Entomology, Proceedings, Entomological Society of America, Lanham. [Google Scholar]
- Carnell, J. D. , Hulse, R. A. & Hughes, W. O. H. (2020). A review of nutrition in bumblebees: the effect of caste, life‐stage and life history traits. In Advances in Insect Physiology (Volume 59, ed. Jurenka R.), pp. 71–129. Academic Press, London. [Google Scholar]
- Carvalheiro, L. G. , Biesmeijer, J. C. , Franzén, M. , Aguirre‐Gutiérrez, J. , Garibaldi, L. A. , Helm, A. , Michez, D. , Pöyry, J. , Reemer, M. , Schweiger, O. , van den Leon, B. , WallisDeVries, M. F. & Kunin, W. E. (2020). Soil eutrophication shaped the composition of pollinator assemblages during the past century. Ecography 43, 209–221. [Google Scholar]
- Cejas, D. , López‐López, A. , Muñoz, I. , Ornosa, C. & De la Rúa, P. (2020). Unveiling introgression in bumblebee (Bombus terrestris) populations through mitogenome‐based markers. Animal Genetics 51, 70–77. [DOI] [PubMed] [Google Scholar]
- Chan, A. , Cheung, J. , Sze, P. , Wong, A. , Wong, E. & Yau, E. (2011). A review of the local restrictedness of Hong Kong butterflies. Hong Kong Biodiversity Newsletter 21, 1–2. [Google Scholar]
- Chaturvedi, S. , Lucas, L. K. , Nice, C. C. , Fordyce, J. A. , Forister, M. L. & Gompert, Z. (2018). The predictability of genomic changes underlying a recent host shift in Melissa blue butterflies. Molecular Ecology 27, 2651–2666. [DOI] [PubMed] [Google Scholar]
- Cheng, W. , Kendrick, R. C. , Guo, F. , Xing, S. , Tingley, M. W. & Bonebrake, T. C. (2019). Complex elevational shifts in a tropical lowland moth community following a decade of climate change. Diversity and Distributions 25, 514–523. [Google Scholar]
- Colla, S. R. (2016). Status, threats and conservation recommendations for wild bumble bees (Bombus spp.) in Ontario, Canada: a review for policymakers and practitioners. Natural Areas Journal 36, 412–426. [Google Scholar]
- Colla, S. R. , Otterstatter, M. C. , Gegear, R. J. & Thomson, J. D. (2006). Plight of the bumble bee: pathogen spillover from commercial to wild populations. Biological Conservation 129, 461–467. [Google Scholar]
- Couture, J. J. , Serbin, S. P. & Townsend, P. A. (2015). Elevated temperature and periodic water stress alter growth and quality of common milkweed (Asclepias syriaca) and monarch (Danaus plexippus) larval performance. Arthropod‐Plant Interactions 9, 149–161. [Google Scholar]
- Crozier, L. (2003). Winter warming facilitates range expansion: cold tolerance of the butterfly Atalopedes campestris . Oecologia 135, 648–656. [DOI] [PubMed] [Google Scholar]
- Crozier, L. (2004). Warmer winters drive butterfly range expansion by increasing survivorship. Ecology 85, 231–241. [Google Scholar]
- Dai, A. (2013). Increasing drought under global warming in observations and models. Nature Climate Change 3, 52–58. [Google Scholar]
- Dedej, S. & Delaplane, K. (2005). Net energetic advantage drives honey bees (Apis mellifera L.) to nectar larceny in Vaccinium ashei Reade. Behavavioural Ecology and Sociobiology 57, 398–403. [Google Scholar]
- Dasmahapatra, K. K. , Walters, J. R. , Briscoe, A. D. , Davey, J. W. , Whibley, A. , Nadeau, N. J. , Zimin, A. V. , Hughes, D. S. T. , Ferguson, L. C. , Martin, S. H. , Salazar, C. , Lewis, J. J. , Adler, S. , Ahn, S.‐J. , Baker, D. A. , et al. (2012). Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellicour, S. , Lecocq, T. , Kuhlmann, M. , Mardulyn, P. & Michez, D. (2014). Molecular phylogeny, biogeography, and host plant shifts in the bee genus Melitta (Hymenoptera: Anthophila). Molecular Phylogenetics and Evolution 70, 412–419. [DOI] [PubMed] [Google Scholar]
- Descamps, C. , Quinet, M. & Jacquemart, A.‐L. (2020). The effects of drought on plant–pollinator interactions: what to expect? Environmental and Experimental Botany 182, 104297. [Google Scholar]
- Didham, R. (2010). Ecological Consequences of Habitat Fragmentation. Encyclopedia of Life Sciences, John Wiley & Sons, Chichester. [Google Scholar]
- Dirzo, R. , Young, H. S. , Galetti, M. , Ceballos, G. , Isaac, N. J. B. & Collen, B. (2014). Defaunation in the Anthropocene. Science 345, 401–406. [DOI] [PubMed] [Google Scholar]
- Duchenne, F. , Thébault, E. , Michez, D. , Gérard, M. , Devaux, C. , Rasmont, P. , Vereecken, N. J. & Fontaine, C. (2020). Long‐term effects of global change on occupancy and flight period of wild bees in Belgium. Global Change Biology 26(12), 6753–6766. [DOI] [PubMed] [Google Scholar]
- Duennes, M. A. , Petranek, C. , de Bonilla, E. P. D. , Mérida‐Rivas, J. , Martinez‐López, O. , Sagot, P. , Vandame, R. & Cameron, S. A. (2017). Population genetics and geometric morphometrics of the Bombus ephippiatus species complex with implications for its use as a commercial pollinator. Conservation Genetics 18, 553–572. [Google Scholar]
- EEA (European Environment Agency) (2017). Climate Change, Impacts and Vulnerability in Europe 2016: An Indicator‐Based Report. Publications Office of the European Union, Luxembourg. [Google Scholar]
- Elbgami, T. , Kunin, W. E. , Hughes, W. O. H. & Biesmeijer, J. C. (2014). The effect of proximity to a honeybee apiary on bumblebee colony fitness, development, and performance. Apidologie 45, 504–513. [Google Scholar]
- Ellis, E. C. , Klein Goldewijk, K. , Siebert, S. , Lightman, D. & Ramankutty, N. (2010). Anthropogenic transformation of the biomes, 1700 to 2000. Global Ecology and Biogeography 19, 589–606. [Google Scholar]
- Estrada, A. , Morales‐Castilla, I. , Caplat, P. & Early, R. (2016). Usefulness of species traits in predicting range shifts. Trends in Ecology & Evolution 31, 190–203. [DOI] [PubMed] [Google Scholar]
- Forister, M. L. , Novotny, V. , Panorska, A. K. , Baje, L. , Basset, Y. , Butterill, P. T. , Cizek, L. , Coley, P. D. , Dem, F. , Diniz, I. R. , Drozd, P. , Fox, M. , Glassmire, A. E. , Hazen, R. , Hrcek, J. , et al. (2015). The global distribution of diet breadth in insect herbivores. Proceedings of the National Academy of Sciences of the United States of America 112, 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortel, L. , Henry, M. , Guilbaud, L. , Mouret, H. & Vaissière, B. E. (2016). Use of human‐made nesting structures by wild bees in an urban environment. Journal of Insect Conservation 20, 239–253. [Google Scholar]
- Freedman, M. G. , Jason, C. , Ramırez, S. R. & Strauss, S. Y. (2020). Host plant adaptation during contemporary range expansion in the monarch butterfly. Evolution 74(2), 377–391. [DOI] [PubMed] [Google Scholar]
- Fukano, Y. , Satoh, T. , Hirota, T. , Nishide, Y. & Obara, Y. (2012). Geographic expansion of the cabbage butterfly (Pieris rapae) and the evolution of highly UV‐reflecting females. Insect Science 19, 239–246. [Google Scholar]
- Gamboa, G. J. , Greig, E. I. & Thom, M. C. (2002). The comparative biology of two sympatric paper wasps, the native Polistes fuscatus and the invasive Polistes dominulus (Hymenoptera, Vespidae). Insectes Sociaux 49, 45–49. [Google Scholar]
- Garibaldi, L. A. , Steffan‐Dewenter, I. , Winfree, R. , Aizen, M. A. , Bommarco, R. , Cunningham, S. A. , Kremen, C. , Carvalheiro, L. G. , Harder, L. D. , Afik, O. , Bartomeus, I. , Benjamin, F. , Boreux, V. , Cariveau, D. , Chacoff, N. P. , et al. (2013). Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339, 1608–1611. [DOI] [PubMed] [Google Scholar]
- Gérard, M. , Martinet, B. , Maebe, K. , Marshall, L. , Smagghe, G. , Vereecken, N. J. , Vray, S. , Rasmont, P. & Michez, D. (2020a). Shift in size of bumblebee queens over the last century. Global Change Biology 26, 1185–1195. [DOI] [PubMed] [Google Scholar]
- Gérard, M. , Michez, M. , Debat, V. , Meeus, I. , Piot, N. , Sculfort, O. , Vastrade, M. , Smaaghe, G. & Vanderplanck, M. (2018). Stressful conditions reveal decrease in size, modification of shape but relatively stable asymmetry in bumblebee wings. Scientific Reports 8, 15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard, M. , Vanderplanck, M. , Wood, T. & Michez, D. (2020b). Global warming and plant–pollinator mismatches. Emerging Topics in Life Sciences 4(1), 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geslin, B. , Gachet, S. , Deschamps‐Cottin, M. , Flacher, F. , Ignace, B. , Knoploch, C. , Meineri, É. , Robles, C. , Ropars, L. , Schurr, L. & Le Féon, V. (2020). Bee hotels host a high abundance of exotic bees in an urban context. Acta Oecologica 105, 103556. [Google Scholar]
- Geslin, B. , Gauzens, B. , Baude, M. , Dajoz, I. , Fontaine, C. , Henry, M. , Ropars, L. , Rollin, O. , Thebault, E. & Vereecken, N. J. (2017). Massively introduced managed species and their consequences for plant–pollinator interactions. Advances in Ecological Research 57, 147–199. [Google Scholar]
- Geslin, B. & Morales, C. L. (2015). New records reveal rapid geographic expansion of Bombus terrestris Linnaeus, 1758 (Hymenoptera: Apidae), an invasive species in Argentina. Check List 11, 3–5. [Google Scholar]
- Ghisbain, G. , Michez, D. , Marshall, L. , Rasmont, P. & Dellicour, S. (2020). Wildlife conservation strategies should incorporate both taxon identity and geographical context ‐ further evidence with bumblebees. Diversity and Distributions 26, 1741–1751. [Google Scholar]
- Gibbs, J. & Sheffield, C. S. (2009). Rapid range expansion of the wool‐carder bee, Anthidium manicatum (Linnaeus) (Hymenoptera: Megachilidae), in North America. Journal of the Kansas Entomological Society 82, 21–29. [Google Scholar]
- Gisder, S. & Genersch, E. (2017). Viruses of commercialized insect pollinators. Journal of Invertebrate Pathology 147, 51–59. [DOI] [PubMed] [Google Scholar]
- Goka, K. , Okabe, K. & Yoneda, M. (2006). Worldwide migration of parasitic mites as a result of bumblebee commercialization. Population Ecology 48, 285–291. [DOI] [PubMed] [Google Scholar]
- Gomi, T. , Nagasaka, M. , Fukuda, T. & Hagihara, H. (2007). Shifting of the life cycle and life‐traits of the fall webworm in relation to climate change. Entomologia Experimentalis et Applicata 125, 179–184. [Google Scholar]
- Goulson, D. (2003). Effects of introduced bees on native ecosystems. Annual Review of Ecology, Evolution, and Systematics 34, 1–26. [Google Scholar]
- Goulson, D. , Nicholls, E. , Botias, C. & Rotheray, E. L. (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957. [DOI] [PubMed] [Google Scholar]
- Graham, K. K. , Eaton, K. , Obrien, I. & Starks, P. T. (2019). Anthidium manicatum, an invasive bee, excludes a native bumble bee, Bombus impatiens, from floral resources. Biological Invasions 21, 1089–1099. [Google Scholar]
- Graystock, P. , Goulson, D. & Hughes, W. O. H. (2014). The relationship between managed bees and the prevalence of parasites in bumblebees. PeerJ 2, e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom, S. V. C. , Stevens, M. I. , Ramage, T. & Schwarz, M. P. (2017). Origins and implications of apid bees (Hymentopera: Apidae) in French Polynesia. Entomological Science 20, 65–75. [Google Scholar]
- Halsch, C. A. , Shapiro, A. M. , Thorne, J. H. , Waetjen, D. P. & Forister, M. L. (2021). A winner in the Anthropocene: changing host plant distribution explains geographical range expansion in the gulf fritillary butterfly. Ecological Entomology 45, 652–662. [Google Scholar]
- Hardy, D. E. (1964). Insects of Hawaii. Volume 11, Diptera: Brachycera, Family Dolichopodidae, Cyclorrhapha, Series Aschiza, Families Lonchopteridae, Phoridae, Pipunculidae, and Syrphidae. University of Hawaii Press, Honolulu. [Google Scholar]
- Hedtke, S. M. , Blitzer, E. J. , Montgomery, G. A. & Danforth, B. N. (2015). Introduction of non‐native pollinators can lead to trans‐continental movement of bee‐associated fungi. PLoS One 10(6), e0130560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich, B. (1979). Bumblebee Economics. Harvard University Press, Cambridge. [Google Scholar]
- Henry, M. , Beguin, M. , Requier, F. , Rollin, O. , Odoux, J.‐F. , Aupinel, P. , Aptel, J. , Tchamitchian, S. & Decourtye, A. (2012). A common pesticide decreases foraging success and survival in honey bees. Science 336, 348–350. [DOI] [PubMed] [Google Scholar]
- Hiddink, J. G. , Burrows, M. T. & Molinos, J. G. (2015). Temperature tracking by North Sea benthic invertebrates in response to climate change. Global Change Biology 21, 117–129. [DOI] [PubMed] [Google Scholar]
- Hill, M. F. , Hastings, A. & Botsford, L. W. (2002). The effects of small dispersal rates on extinction times in structured metapopulation models. The American Naturalist 160, 389–402. [DOI] [PubMed] [Google Scholar]
- Hingston, A. B. , Marsden‐Smedley, J. , Driscoll, D. A. , Corbett, S. , Fenton, J. , Anderson, R. , Plowman, C. , Mowling, F. , Jenkin, M. , Matsui, K. , Bonham, K. J. , Ilowski, M. , Mcquillan, P. B. , Yaxley, B. , Reid, T. , et al. (2002). Extent of invasion of Tasmanian native vegetation by the exotic bumblebee Bombus terrestris (Apoidea: Apidae). Austral Ecology 27(2), 162–172. [Google Scholar]
- Hiura, I. (1968). Monshirochou‐zoku no Rekishi. Konchu to Shizen 3, 9–15 (in Japanese). [Google Scholar]
- Holt, R. D. & Bonsall, M. B. (2017). Apparent Competition. Annual Reviews 48, 447–471. [Google Scholar]
- Howlett, B. G. & Donovan, B. J. (2010). A review of New Zealand's deliberately introduced bee fauna: current status and potential impacts. New Zealand Entomologist 33, 92–101. [Google Scholar]
- Inoue, M. N. & Yokoyama, J. (2010). Competition for flower resources and nest sites between Bombus terrestris (L.) and Japanese native bumblebees. Applied Entomology and Zoology 45, 29–35. [Google Scholar]
- Inoue, M. N. , Yokoyama, J. & Washitani, I. (2008). Displacement of Japanese native bumblebees by the recently introduced Bombus terrestris (L.) (Hymenoptera: Apidae). Journal of Insect Conservation 12, 135–146. [Google Scholar]
- Iwasaki, J. M. , Dickinson, K. J. M. , Barratt, B. I. P. , Mercer, A. R. , Jowett, T. W. D. & Lord, J. M. (2018). Floral usage partitioning and competition between social (Apis mellifera, Bombus terrestris) and solitary bees in New Zealand: niche partitioning via floral preferences? Austral Ecology 43, 937–948. [Google Scholar]
- Janz, N. & Nylin, S. (2008). The oscillation hypothesis of host‐plant range and speciation. In Specialization, Speciation and Radiation: The Evolutionary Biology of Herbivorous Insects (ed. Tilmon K. J.), pp. 203–215. University of California Press, Berkeley. [Google Scholar]
- Jenič, A. , Gogala, A. & Grad, J. (2010). Bombus haematurus (Hymenoptera: Apidae), new species in the Slovenian bumblebee fauna. Acta Entomologica Slovenica 18, 168–170. [Google Scholar]
- Johnson, H. , Solensky, M. J. , Satterfield, D. A. & Davis, A. K. (2014). Does skipping a meal matter to a butterfly's appearance? Effects of larval food stress on wing morphology and color in monarch butterflies. PLoS One 9(4), e93492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl, S. M. , Lenhard, M. & Joshi, J. (2019). Compensatory mechanisms to climate change in the widely distributed species Silene vulgaris . Journal of Ecology 107, 1918–1930. [Google Scholar]
- Kanbe, Y. , Okada, I. , Yoneda, M. , Goka, K. & Tsuchida, K. (2008). Interspecific mating of the introduced bumblebee Bombus terrestris and the native Japanese bumblebee Bombus hypocrita sapporoensis results in inviable hybrids. Naturwissenschaften 95, 1003–1008. [DOI] [PubMed] [Google Scholar]
- Kerr, J. T. , Pindar, A. , Galpern, P. , Packer, L. , Potts, S. G. , Roberts, S. M. , Rasmont, P. , Schweiger, O. , Colla, S. R. , Richardson, L. L. , Wagner, D. L. , Gall, L. F. , Sikes, D. S. & Pantoja, A. (2015). Climate change impacts on bumblebees converge across continents. Science 349(6244), 177–180. [DOI] [PubMed] [Google Scholar]
- Kiritani, K. (2006). Predicting impacts of global warming on population dynamics and distribution of arthropods in Japan. Population Ecology 48, 5–12. [Google Scholar]
- Kleijn, D. & Raemakers, I. (2008). A retrospective analysis of pollen host plant use by stable and declining bumble bee species. Ecology 89, 1811–1823. [DOI] [PubMed] [Google Scholar]
- Kleijn, D. , Winfree, R. , Bartomeus, I. , Carvalheiro, L. G. , Henry, M. , Isaacs, R. , Klein, A.‐M. , Kremen, C. , M'Gonigle, L. K. , Rader, R. , Ricketts, T. H. , Williams, N. M. , Lee Adamson, N. , Ascher, J. S. , Báldi, A. , et al. (2015). Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nature Communications 6, 7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen, C. (2018). The value of pollinator species diversity. Science 359, 741–742. [DOI] [PubMed] [Google Scholar]
- Kronforst, M. R. (2012). Mimetic butterflies introgress to impress. PLoS Genetics 8, e1002802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht, S. C. , Morrow, A. & Hussey, R. (2017). Variation in and adaptive plasticity of flower size and drought‐coping traits. Plant Ecology 218, 647–660. [Google Scholar]
- Larkin, L. L. , Neff, J. L. & Simpson, B. B. (2008). The evolution of a pollen diet: host choice and diet breadth of Andrena bees (Hymenoptera: Andrenidae). Apidologie 39, 133–145. [Google Scholar]
- Lasanta, T. , Arnáez, J. , Pascual, N. , Ruiz‐Flaño, P. , Errea, M. P. & Lana‐Renault, N. (2017). Space–time process and drivers of land abandonment in Europe. Catena 149, 810–823. [Google Scholar]
- Lautenbach, S. , Seppelt, R. , Liebscher, J. & Dormann, C. F. (2012). Spatial and temporal trends of global pollination benefit. PLoS One 7, e35954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Feon, V. , Aubert, M. , Genoud, D. , Andrieu‐Ponel, V. , Westrich, P. & Geslin, B. (2018). Range expansion of the Asian native giant resin bee Megachile sculpturalis (Hymenoptera, Apoidea, Megachilidae) in France. Ecology & Evolution 8, 1534–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecocq, T. , Coppée, A. , Michez, D. , Brasero, N. , Rasplus, J.‐Y. , Valterová, I. & Rasmont, P. (2016). The alien's identity: consequences of taxonomic status for the international bumblebee trade regulations. Biological Conservation 195, 169–176. [Google Scholar]
- Lewis, S. L. & Maslin, M. A. (2018). The Human Planet. How We Created the Anthropocene. Pelican Books, London. [Google Scholar]
- Lewis, R. C. , Pointer, M. D. , Friend, L. A. , Vasudeva, R. , Bemrose, J. , Sutter, A. , Gage, M. J. G. & Spurgin, L. G. (2020). Polyandry provides reproductive and genetic benefits in colonising populations. Ecology and Evolution 10, 10851–10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Uribe, M. M. , Cane, J. H. , Minckley, R. L. & Danforth, B. N. (2016). Crop domestication facilitated rapid geographical expansion of a specialist pollinator, the squash bee Peponapis pruinosa . Proceedings of the Royal Society B: Biological Sciences 283, 20160443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losey, J. E. & Vaughan, M. (2006). The economic value of ecological services provided by insects. BioScience 56, 311–323. [Google Scholar]
- Lurgi, M. , López, B. C. & Montoya, J. M. (2012). Novel communities from climate change. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 2913–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons, S. K. , Wagner, P. J. & Dzikiewicz, K. (2010). Ecological correlates of range shifts of Late Pleistocene mammals. Philosophical Transactions of the Royal Society B: Biological Sciences 365, 3681–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIvor, J. S. & Packer, L. (2015). ‘Bee hotels’ as tools for native pollinator conservation: a premature verdict? PLoS One 10, e0122126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean, S. A. & Beissinger, S. R. (2017). Species’ traits as predictors of range shifts under contemporary climate change: a review and meta‐analysis. Global Change Biology 23, 4094–4105. [DOI] [PubMed] [Google Scholar]
- Magnacca, K. N. & King, C. B. A. (2013). Assessing the Presence and Distribution of 23 Hawaiian Yellow‐Faced Bee Species on Lands Adjacent to Military Installations on O'ahu and Hawai'i Island. Report, Pacific Cooperative Studies Unit, University of Hawaii at Manoa, Honolulu. [Google Scholar]
- Marshall, L. , Perdijk, F. , Dendoncker, N. , Kunin, W. , Roberts, S. & Biesmeijer, J. C. (2020). Bumblebees moving up: shifts in elevation ranges in the Pyrenees over 115 years. Proceedings of the Royal Society B: Biological Sciences 287, 20202201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet, B. , Dellicour, S. , Ghisbain, G. , Przybyla, K. , Zambra, E. , Lecocq, T. , Boustani, M. , Baghirov, R. , Michez, D. & Rasmont, P. (2021a). Global effects of extreme temperatures on wild bumblebees. Conservation Biology. 10.1111/cobi.13685. [DOI] [PubMed] [Google Scholar]
- Martinet, B. , Rasmont, P. , Cederberg, B. , Evrard, D. , Ødegaard, F. , Paukkunen, J. & Lecocq, T. (2015). Forward to the north: two Euro‐Mediterranean bumblebee species now cross the Arctic Circle. Annales de la Société Entomologique de France 51, 303–309. [Google Scholar]
- Martinet, B. , Zambra, E. , Przybyla, K. , Lecocq, T. , Nonclercq, D. , Rasmont, P. , Michez, D. & Hennerbert, E. (2021b). Mating under climate change: impact of simulated heatwaves on reproduction of model pollinators. Functional Ecology 35(3), 739–752. [Google Scholar]
- Matsumura, C. , Yokoyama, J. & Washitani, I. (2004). Invasion status and potential ecological impacts of an invasive alien bumblebee, Bombus terrestris L. (Hymenoptera: Apidae) naturalized in southern Hokkaido, Japan. Global Environmental Research 8, 51–66. [Google Scholar]
- McGeoch, M. A. & Latombe, G. (2016). Characterizing common and range expanding species. Journal of Biogeography 43, 217–228. [Google Scholar]
- McKinlay, R. G. (ed.) (1992). Vegetable Crop Pests. Palgrave Macmillan UK, London. [Google Scholar]
- McKinney, M. I. & Park, Y.‐L. (2013). Distribution of Chaetodactylus krombeini (Acari: Chaetodactylidae) within Osmia cornifrons (Hymenoptera: Megachilidae) nests: implications for population management. Experimental and Applied Acarology 60, 153–161. [DOI] [PubMed] [Google Scholar]
- Mercader, R. J. , Aardema, M. L. & Scriber, J. M. (2009). Hybridization leads to host‐use divergence in a polyphagous butterfly sibling species pair. Oecologia 158, 651–662. [DOI] [PubMed] [Google Scholar]
- Moerman, R. , Vanderplanck, M. , Roger, N. , Declèves, S. , Wathelet, B. , Rasmont, P. , Fournier, D. & Michez, D. (2016). Growth rate of bumblebee larvae is related to pollen amino acids. Journal of Economic Entomology 109, 25–30. [DOI] [PubMed] [Google Scholar]
- Morales, C. L. & Aizen, M. A. (2002). Does invasion of exotic plants promote invasion of exotic flower visitors? A case study from the temperate forests of the southern Andes. Biological Invasions 4, 87–100. [Google Scholar]
- Morales, C. L. & Aizen, M. A. (2006). Invasive mutualisms and the structure of plant‐pollinator interactions in the temperate forests of north‐west Patagonia, Argentina. Journal of Ecology 94, 171–180. [Google Scholar]
- Neel, M. C. , Leidner, A. K. , Haines, A. , Goble, D. D. & Scott, J. M. (2012). By the numbers: how is recovery defined by the US endangered species act? BioScience 62, 646–657. [Google Scholar]
- Nishikawa, Y. , Shimamura, T. , Kudo, G. & Yabe, K. (2019). Habitat use and floral resource partitioning of native and alien bumblebees in the coastal grassland—rural landscape. Journal of Insect Conservation 23, 677–687. [Google Scholar]
- Noor, S. , Othman, N. L. , Abang, F. & Dieng, H. (2017). Spatial distribution and range expansion of an exotic butterfly Acraea terpsicore (Linnaeus, 1758) (Nymphalidae: Heliconiinae) in Borneo. American Scientific Research Journal for Engineering, Technology, and Sciences 38(2), 168–193. [Google Scholar]
- Okabe, K. , Masuya, H. , Kawazoe, K. & Makino, S. (2010). Invasion pathway and potential risks of a bamboo‐nesting carpenter bee, Xylocopa tranquebarorum (Hymenoptera: Apidae), and its micro‐associated mite introduced into Japan. Applied Entomology and Zoology 45, 329–337. [Google Scholar]
- Ollerton, J. (2017). Pollinator diversity: distribution, ecological function, and conservation. Annual Review of Ecology, Evolution, and Systematics 48, 353–376. [Google Scholar]
- Ollerton, J. , Winfree, R. & Tarrant, S. (2011). How many flowering plants are pollinated by animals? Oikos 120, 321–326. [Google Scholar]
- Oyen, K. J. , Giri, S. & Dillon, M. E. (2016). Altitudinal variation in bumble bee (Bombus) critical thermal limits. Journal of Thermal Biology 59, 52–57. [DOI] [PubMed] [Google Scholar]
- Parmesan, C. , Ryrholm, N. , Stefanescu, C. , Hill, J. K. , Thomas, C. D. , Descimon, H. , Huntley, B. , Kaila, L. , Kullberg, J. , Tammaru, T. , Tennent, W. J. , Thomas, J. A. & Warren, M. (1999). Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399, 579–583. [Google Scholar]
- Pateman, R. M. , Hill, J. K. , Roy, D. B. , Fox, R. & Thomas, C. D. (2012). Temperature‐dependent alterations in host use drive rapid range expansion in a butterfly. Science 336(6084), 1028–1030. [DOI] [PubMed] [Google Scholar]
- Pecl, G. T. , Araújo, M. B. , Bell, J. D. , Blanchard, J. , Bonebrake, T. C. , Chen, I.‐C. , Clark, T. D. , Colwell, R. K. , Danielsen, F. , Evengård, B. , Falconi, L. , Ferrier, S. , Frusher, S. , Garcia, R. A. , Griffis, R. B. , et al. (2017). Biodiversity redistribution under climate change: impacts on ecosystems and human well‐being. Science 355, eaai9214. [DOI] [PubMed] [Google Scholar]
- Penado, A. , Rebelo, H. & Goulson, D. (2016). Spatial distribution modelling reveals climatically suitable areas for bumblebees in undersampled parts of the Iberian Peninsula. Insect Conservation and Diversity 9, 391–401. [Google Scholar]
- Peters, J. V. , Smithers, C. N. & Rushworth, G. D. (2010). Changes in the range of Hasora khodahaslia (Swinhoe) (Insecta: Lepidoptera: Hesperiidae) in eastern Australia ‐ a response to climate change? In The Natural History of Sydney (eds Lunney D., Hutchings P. and Hochuli D.), pp. 219–226. Royal Zoological Society of New South Wales, Mosman. [Google Scholar]
- Pfennig, K. S. , Kelly, A. L. & Pierce, A. A. (2016). Hybridization as a facilitator of species range expansion. Proceedings of the Royal Society B: Biological Sciences 283, 20161329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, A. A. , Zalucki, M. P. , Bangura, M. , Udawatta, M. , Kronforst, M. R. , Altizer, S. , Haeger, J. F. & de Roode, J. C. (2014). Serial founder effects and genetic differentiation during worldwide range expansion of monarch butterflies. Proceedings of the Royal Society B: Biological Sciences 281, 20142230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts‐Singer, T. L. & Cane, J. H. (2011). The alfalfa leafcutting Bee, Megachile rotundata: the world's most intensively managed solitary bee. Annual Review of Entomology 56, 221–237. [DOI] [PubMed] [Google Scholar]
- Portman, Z. M. , Tepedino, V. J. & Tripodi, A. D. (2019). Persistence of an imperiled specialist bee and its rare host plant in a protected area. Insect Conservation and Diversity 12, 183–192. [Google Scholar]
- Potts, S. G. , Biesmeijer, J. C. , Kremen, C. , Neumann, P. , Schweiger, O. & Kunin, W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends in Ecology & Evolution 25, 345–353. [DOI] [PubMed] [Google Scholar]
- Powney, G. D. , Carvell, C. , Edwards, M. , Morris, R. K. A. , Roy, H. E. , Woodcock, B. A. & Isaac, N. J. B. (2019). Widespread losses of pollinating insects in Britain. Nature Communications 10, 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöyry, J. , Luoto, M. , Heikkinen, R. K. , Kuussaari, M. & Saarinen, K. (2009). Species traits explain recent range shifts of Finnish butterflies. Global Change Biology 15, 732–743. [Google Scholar]
- Prŷs‐Jones, O. (2019). Preadaptation to the vertical: an extra dimension to the natural history and nesting habits of the Tree Bumble Bee, Bombus (Pyrobombus) hypnorum . Journal of Apicultural Research 58, 643–659. [Google Scholar]
- Prŷs‐Jones, O. E. , Kristjánsson, K. & Ólafsson, E. (2016). Hitchhiking with the Vikings? The anthropogenic bumblebee fauna of Iceland – past and present. Journal of Natural History 50, 2895–2916. [Google Scholar]
- Rasmont, P. , Coppee, A. , Michez, D. & De Meulemeester, T. (2008). An overview of the Bombus terrestris (L. 1758) subspecies (Hymenoptera: Apidae). Annales de la Société entomologique de France (N.S.) 44, 243–250. [Google Scholar]
- Rasmont, P. , Franzén, M. , Lecocq, T. , Harpke, A. , Roberts, S. P. M. , Biesmeijer, K. , Castro, L. , Cederberg, B. , Dvořák, L. , Fitzpatrick, Ú. , Gonseth, Y. , Haubruge, E. , Mahé, G. , Manino, A. , Michez, D. , et al. (2015). Climatic risk and distribution atlas of European bumblebees. Biorisk 10, 1–246. [Google Scholar]
- Reim, E. , Eichhorn, D. , Roy, J. D. , Steinhoff, P. O. M. & Fischer, K. (2019). Nutritional stress reduces flight performance and exploratory behavior in a butterfly. Insect Science 26, 897–910. [DOI] [PubMed] [Google Scholar]
- Renauld, M. , Hutchinson, A. , Loeb, G. , Poveda, K. & Connelly, H. (2016). Landscape simplification constrains adult size in a native ground‐nesting bee. PLoS One 11, e0150946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhymer, J. M. & Simberloff, D. (1996). Extinction by hybridization and introgression. Annual Review of Ecology and Systematics 27, 83–109. [Google Scholar]
- Rivera‐Marchand, B. , Oskay, D. & Giray, T. (2012). Gentle africanized bees on an oceanic Island. Evolutionary Applications 5, 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger, N. , Moerman, R. , Carvalheiro, L. G. , Aguirre‐Guitiérrez, J. , Jacquemart, A.‐L. , Kleijn, D. , Lognay, G. , Moquet, L. , Quinet, M. , Rasmont, P. , Richel, A. , Vanderplanck, M. & Michez, D. (2017). Impact of pollen resources drift on common bumblebees in NW Europe. Global Change Biology 23, 68–76. [DOI] [PubMed] [Google Scholar]
- Roulston, T. H. & Cane, J. H. (2002). The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evolutionary Ecology 16, 49–65. [Google Scholar]
- Roulston, T. & Malfi, R. (2012). Aggressive eviction of the eastern carpenter bee (Xylocopa virginica Linnaeus) from its nest by the giant resin bee (Megachile sculpturalis Smith). Journal of the Kansas Entomological Society 85, 387–388. [Google Scholar]
- Russo, L. (2016). Positive and negative impacts of non‐native bee species around the world. Insects 7(4), 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust, R. W. , Cambon, G. , Grossa, J.‐P. T. & Vaissière, B. E. (2004). Nesting biology and foraging ecology of the wood‐boring bee Lithurgus chrysurus (Hymenoptera: Megachilidae). Journal of the Kansas Entomological Society 77, 269–279. [Google Scholar]
- Ryan, S. F. , Lombaert, E. , Espeset, A. , Vila, R. , Talavera, G. , Dinca, V. , Doellman, M. M. , Renshaw, M. A. , Eng, M. W. , Hornett, E. A. , Li, Y. , Pfrender, M. E. & Shoemaker, D. (2019). Global invasion history of the agricultural pest butterfly Pieris rapae revealed with genomics and citizen science. Proceedings of the National Academy of Sciences of the United States of America 116(40), 20015–20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez, A. , Morales, C. L. , Garibaldi, L. A. & Aizen, M. A. (2017). Invasive bumble bees reduce nectar availability for honey bees by robbing raspberry flower buds. Basic and Applied Ecology 19, 26–35. [Google Scholar]
- Sáez, A. , Morales, J. M. , Morales, C. L. , Harder, L. D. & Aizen, M. A. (2018). The costs and benefits of pollinator dependence: empirically based simulations predict raspberry fruit quality. Ecological Applications 28, 1215–1222. [DOI] [PubMed] [Google Scholar]
- Sage, R. F. (2020). Global change biology: a primer. Global Change Biology 26, 3–30. [DOI] [PubMed] [Google Scholar]
- Sanguinetti, A. & Singer, R. B. (2014). Invasive bees promote high reproductive success in Andean orchids. Biological Conservation 175, 10–20. [Google Scholar]
- Scheper, J. , Bommarco, R. , Holzschuh, A. , Potts, S. G. , Riedinger, V. , Roberts, S. P. M. , Rundlöf, M. , Smith, H. G. , Steffan‐Dewenter, I. , Wickens, J. B. , Wickens, V. J. & Kleijn, D. (2015). Local and landscape‐level floral resources explain effects of wildflower strips on wild bees across four European countries. Journal of Applied Ecology 52, 1165–1175. [Google Scholar]
- Schweiger, O. , Biesmeijer, J. C. , Bommarco, R. , Hickler, T. , Hulme, P. E. , Klotz, S. , Kühn, I. , Moora, M. , Nielsen, A. , Ohlemüller, R. , Petanidou, T. , Potts, S. G. , Pyšek, P. , Stout, J. C. , Sykes, M. T. , et al. (2010). Multiple stressors on biotic interactions: how climate change and alien species interact to affect pollination. Biological Reviews 85(4), 777–795. [DOI] [PubMed] [Google Scholar]
- Senapathi, D. , Carvalheiro, L. G. , Biesmeijer, J. C. , Dodson, C.‐A. , Evans, R. L. , McKerchar, M. , Morton, R. D. , Moss, E. D. , Roberts, S. P. M. , Kunin, W. E. & Potts, S. G. (2015). The impact of over 80 years of land cover changes on bee and wasp pollinator communities in England. Proceedings of the Royal Society B: Biological Sciences 282, 20150294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settele, J. , Kudrna, O. , Harpke, A. , Kuhn, I. , van Swaay, C. , Verovnik, R. , Warren, M. , Wiemers, M. , Hanspach, J. , Hickler, T. , Kuhn, E. , van Halder, I. , Veling, K. , Vliegenthart, A. , Wynhoff, I. & Schweiger, O. (2008). Climatic Risk Atlas of European Butterflies. Pensoft, Sofia‐Moscow. [Google Scholar]
- Shapiro, A. M. & Manolis, T. D. (2007). Field Guide to Butterflies of the San Francisco Bay and Sacramento Valley Regions. California Natural History Guides, Berkeley. [Google Scholar]
- Shavit, O. , Dafni, A. & Ne'eman, G. (2009). Competition between honeybees (Apis mellifera) and native solitary bees in the Mediterranean region of Israel—implications for conservation. Israel Journal of Plant Sciences 57, 171–183. [Google Scholar]
- Sheffield, C. , Heron, J. & Musetti, L. (2020). Xylocopa sonorina Smith, 1874 from Vancouver, British Columbia, Canada (Hymenoptera: Apidae, Xylocopinae) with comments on its taxonomy. Biodiversity Data Journal 8, e49918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shell, W. A. & Rehan, S. M. (2019). Invasive range expansion of the small carpenter bee, Ceratina dentipes (Hymenoptera: Apidae) into Hawaii with implications for native endangered species displacement. Biological Invasions 21, 1155–1166. [Google Scholar]
- Singer, M. C. & Parmesan, C. (2019). Butterflies embrace maladaptation and raise fitness in colonizing novel host. Evolutionary Applications 12, 1417–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slove, J. & Janz, N. (2011). The relationship between diet breadth and geographic range size in the butterfly subfamily Nymphalinae – a study of global scale. PLoS One 6, e16057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper, J. & Beggs, J. (2013). Assessing the impact of an introduced bee, Anthidium manicatum, on pollinator communities in New Zealand. New Zealand Journal of Botany 51, 213–228. [Google Scholar]
- Soule, A. J. , Decker, L. E. & Hunter, M. D. (2020). Effects of diet and temperature on monarch butterfly wing morphology and flight ability. Journal of Insect Conservation 24, 961–975. [Google Scholar]
- Stelzer, R. J. , Chittka, L. , Carlton, M. & Ings, T. C. (2010). Winter active bumblebees (Bombus terrestris) achieve high foraging rates in urban Britain. PLoS One 5, e9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, M. I. , Hogendoorn, K. & Schwarz, M. P. (2007). Evolution of sociality by natural selection on variances in reproductive fitness: evidence from a social bee. BMC Evolutionary Biology 7, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork, N. E. (2018). How many species of insects and other terrestrial arthropods are there on earth? Annual Review of Entomology 63, 31–45. [DOI] [PubMed] [Google Scholar]
- Stout, J. C. , Kells, A. R. & Goulson, D. (2002). Pollination of the invasive exotic shrub Lupinus arboreus (Fabaceae) by introduced bees in Tasmania. Biological Conservation 106, 425–434. [Google Scholar]
- Stout, J. C. & Morales, C. L. (2009). Ecological impacts of invasive alien species on bees. Apidologie 40, 388–409. [Google Scholar]
- Strange, J. P. , Koch, J. B. , Gonzalez, V. H. , Nemelka, L. & Griswold, T. (2011). Global invasion by Anthidium manicatum (Linnaeus) (Hymenoptera: Megachilidae): assessing potential distribution in North America and beyond. Biological Invasions 13, 2115–2133. [Google Scholar]
- Stubbs, C. S. , Drummond, F. A. & Osgood, E. A. (1994). Osmia ribifloris biedermannii and Megachile rotundata (Hymenoptera: Megachilidae) introduced into the lowbush blueberry agroecosystem in Maine. Journal of the Kansas Entomological Society 67, 173–185. [Google Scholar]
- Thomson, D. M. (2016). Local bumble bee decline linked to recovery of honey bees, drought effects on floral resources. Ecology Letters 19, 1247–1255. [DOI] [PubMed] [Google Scholar]
- Tomiolo, S. & Ward, D. (2018). Species migrations and range shifts: a synthesis of causes and consequences. Perspectives in Plant Ecology, Evolution and Systematics 33, 62–77. [Google Scholar]
- Tomlinson, S. , Dixon, K. W. , Didham, R. K. & Bradshaw, S. D. (2015). Physiological plasticity of metabolic rates in the invasive honey bee and an endemic Australian bee species. Journal of Comparative Physiology B 185, 835–844. [DOI] [PubMed] [Google Scholar]
- Travis, J. M. J. , Delgado, M. , Bocedi, G. , Baguette, M. , Bartoń, K. , Bonte, D. , Boulangeat, I. , Hodgson, J. A. , Kubisch, A. , Penteriani, V. , Saastamoinen, M. , Stevens, V. M. & Bullock, J. M. (2013). Dispersal and species’ responses to climate change. Oikos 122, 1532–1540. [Google Scholar]
- Tripodi, A. D. & Szalanski, A. L. (2011). Further range extension of Xylocopa micans Lepeletier (Hymenoptera: Apidae). Journal of the Kansas Entomological Society 84, 163–164. [Google Scholar]
- Tsuchida, K. , Yamaguchi, A. , Kanbe, Y. & Goka, K. (2019). Reproductive interference in an introduced bumblebee: polyandry may mitigate negative reproductive impact. Insects 10(2), 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbergen, A. J. & The Insect Pollinators Initiative (2013). Threats to an ecosystem service: pressures on pollinators. Frontiers in Ecology and the Environment 11, 251–259. [Google Scholar]
- Vanderplanck, M. , Martinet, B. , Carvalheiro, L. G. , Rasmont, P. , Barraud, A. , Renaudeau, C. & Michez, D. (2019). Ensuring access to high‐quality resources reduces the impacts of heat stress on bees. Scientific Reports 9, 12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderplanck, M. , Moerman, R. , Rasmont, P. , Lognay, G. , Wathelet, B. , Wattiez, R. & Michez, D. (2014). How does pollen chemistry impact development and feeding behaviour of polylectic bees? PLoS One 9, e86209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane‐Wright, R. I. (1993). The Columbus hypothesis: an explanation for the dramatic 19th century range expansion of the monarch butterfly. In Biology and Conservation of the Monarch Butterfly (eds Malcolm S. B. and Zalucki M. P.), pp. 179–187. Natural History Museum of Los Angeles County, Los Angeles. [Google Scholar]
- Velthuis, H. H. W. & van Doorn, A. (2006). A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 37, 421–451. [Google Scholar]
- Vickruck, J. L. & Richards, M. H. (2017). Nesting habits influence population genetic structure of a bee living in anthropogenic disturbance. Molecular Ecology 26, 2674–2686. [DOI] [PubMed] [Google Scholar]
- Wagner, D. L. (2020). Insect declines in the Anthropocene. Annual Review of Entomology 65, 457–480. [DOI] [PubMed] [Google Scholar]
- WallisDeVries, M. F. & van Swaay, C. A. M. (2006). Global warming and excess nitrogen may induce butterfly decline by microclimatic cooling. Global Change Biology 12, 1620–1626. [Google Scholar]
- WallisDeVries, M. F. & van Swaay, C. A. M. (2017). A nitrogen index to track changes in butterfly species assemblages under nitrogen deposition. Biological Conservation 212, 448–453. [Google Scholar]
- Walther‐Hellwig, K. , Fokul, G. , Frankl, R. , Büchler, R. , Ekschmitt, K. & Wolters, V. (2006). Increased density of honeybee colonies affects foraging bumblebees. Apidologie 37, 517–532. [Google Scholar]
- Warzecha, D. , Diekötter, T. , Wolters, V. & Jauker, F. (2016). Intraspecific body size increases with habitat fragmentation in wild bee pollinators. Landscape Ecology 31, 1449–1455. [Google Scholar]
- Willis, S. G. , Hill, J. K. , Thomas, C. D. , Roy, D. B. , Fox, R. , Blakeley, D. S. & Huntley, B. (2009). Assisted colonization in a changing climate: a test‐study using two U.K. butterflies. Conservation Letters 2, 46–52. [Google Scholar]
- Wilson, J. K. , Casajus, N. , Hutchinson, R. A. , Mcfarland, K. P. , Kerr, J. T. , Berteaux, D. , Larrivee, M. & Prudic, K. L. (2021). Climate change and local host availability drive the northern range boundary in the rapid expansion of a specialist insect herbivore, Papilio cresphontes . Frontiers in Ecology and Evolution 9, 579230. [Google Scholar]
- Winfree, R. , Williams, N. M. , Dushoff, J. & Kremen, C. (2007). Native bees provide insurance against ongoing honey bee losses. Ecology Letters 10, 1105–1113. [DOI] [PubMed] [Google Scholar]
- Woodcock, B. A. , Isaac, N. J. B. , Bullock, J. M. , Roy, D. B. , Garthwaite, D. G. , Crowe, A. & Pywell, R. F. (2016). Impacts of neonicotinoid use on long‐term population changes in wild bees in England. Nature Communications 7, 12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambra, E. , Martinet, B. , Brasero, N. , Michez, D. & Rasmont, P. (2020). Hyperthermic stress resistance of bumblebee males: test case of Belgian species. Apidologie 51, 911–920. [Google Scholar]
- Zattara, E. E. & Aizen, M. A. (2021). Worldwide occurrence records reflect a global decline in bee species richness. One Earth 4, 114–123. [Google Scholar]


