Abbreviations
- AIT
allergen‐specific immunotherapy
- GPAs
gras pollen allergic patients
- ILCs
innate lymphoid cells
- KLRG1
killer cell lectin‐like receptor subfamily G member 1
- NACs
non‐allergic controls
- RA
retinoic acid
Innate lymphoid cells (ILCs) represent relatively newly described cells of the innate immune system. 1 They are categorized into two main distinct subsets, namely cytotoxic and non‐cytotoxic (helper) ILCs. Cytotoxic ILCs are composed of NK cells (reflecting functions of CD8+ cytotoxic T cells) and lymphoid tissue inducer (LTi) cells (responsible for secondary lymphoid structures development). In contrast, helper ILCs have been divided into three phenotypically and functionally distinct subsets, namely group 1 ILCs (ILC1s), group 2 ILCs (ILC2s), and group 3 ILCs (ILC3s), mirroring T helper (Th)1, Th2, and Th17 cells, respectively. 1 , 2 , 3 , 4 Most recently, the discovery of IL‐10–producing ILCs, followed by identification of retinoic acid (RA)‐mediated pathway of IL‐10–producing ILC2s induction by Morita et al., filled up a missing piece in the puzzle. 4 , 5 The discovery of helper ILCs with regulatory potential raised further questions to fully understand the biology of IL‐10+ ILC2s. This challenge was undertaken by Golebski et al. They characterized the fraction of ILC2s expressing killer cell lectin‐like (KLRG1) receptors. In contrast to KLRG1− cells, KLRG1+ ILC2s released IL‐10 upon activation with IL‐33 and RA. Moreover, IL‐10+KLRG1+ ILC2s overlapped Treg phenotype and attenuated T‐cell responses in IL‐10–mediated mechanism. 6 Those groundbreaking findings open countless possibilities for further research and discussion. We would like to start by disputing how stable is IL‐10+ ILC2s phenotype? Understanding of Treg biology allowed us to comprehend the concepts of cell lineage (distinct, stable cell phenotype, and subsequently specific functions) and cellular plasticity (the capability of mature cells to change their functions and fate upon environmental exposures). 2 , 7 Is ILC2‐mediated IL‐10 production a lasting phenomenon, and can we identify those cells as distinct regulatory ILCs (ILCreg) subpopulation? Or are ILC2s respond to environmental stimulation and, upon RA availability, temporarily produce IL‐10, representing functional adaptation induced by the local microenvironment? The discussion is especially relevant, as flexible changes in lineage stability and cell plasticity are well described in both adaptive and innate immune repertoires. 2 , 7 Additionally, the balanced distribution of ILCs subpopulations functionally affects tissue homeostasis and, therefore, is essential in maintaining health status.
Dysregulated balance in ILCs immunity orchestrates the detrimental innate and adaptive immune responses, thus contribute to the pathology of allergic diseases, such as asthma or rhinosinusitis. 8 In response to allergen exposure, epithelial cells secrete alarmin cytokines, IL‐33, IL‐25, and thymic stromal lymphopoietin (TSLP), which triggers ILC2s responses and subsequently drives type 2 inflammation. 1 , 9 Therefore, functional characterization of circulating ILCs in allergic inflammation is warranted. Increased frequencies of ILC2s were detected in bronchoalveolar lavage fluid from asthmatics, and nasal tissues from patients with seasonal allergic rhinitis and chronic rhinosinusitis with nasal polyps, especially after allergen challenge. 10 Simultaneously, Boonpiyathad et al 11 identified elevated ILC2s numbers in peripheral blood from allergic individuals. In line with this, Golebski et al 6 demonstrated the increased abundance of ILC2s in peripheral blood in grass pollen allergic patients (GPAs) compared to non‐allergic controls (NACs). Single‐cell sequencing analysis showed differential ILC transcriptomic profiles among the investigated groups, with the predominance of dysregulation of ILC2s‐mediated immune response in GPAs during the grass pollen season. 6 The fluctuations in ILCs composition in the course of allergic diseases and their treatment open a window of opportunity to study the concept of innate immune memory referred to as trained immunity/tolerance. 10
Allergen‐specific immunotherapy (AIT) is a therapeutic strategy applied in allergic patients to build a tolerance to allergens, allowing for a long‐lasting reduction in symptoms. The efficacy of AIT was mainly underscored through adaptive immune responses involving Th2, Treg, and B regulatory cells. 12 Innate immune cell compartment is also an essential part of tolerance induction to the allergen. Following the concept of trained tolerance, we previously showed that AIT decreased the frequency of circulating ILC2s. 10 Additionally, the capability of ILC2s to the conversion to Treg‐like, IL‐10–producing ILCs raised broad attention as a potential cellular biomarker for monitoring the efficacy of AIT and predicting its long‐term disease‐modifying benefits. In this context, Golebski et al 6 investigated the role of AIT‐induced IL‐10–producing ILC2s in building and maintaining immunotolerance to sensitizing allergens. Subsequently, the authors asked whether IL‐10–producing ILC2s may regulate the epithelial barrier integrity, which disruption implicates the development of most allergic diseases. 9 As expected, IL‐10–producing ILC2s exert a protective effect against nasal epithelial barrier disintegration upon grass pollen allergen (Phleum pratense, Phlp) exposure or restore its integrity through blocking of IL‐6 and IL‐8 release. Given ILCs’ plasticity, the authors aimed to explore whether peripheral blood‐derived ILCs in NACs, GPAs, and house dust mite allergics were able to produce IL‐10. Interestingly, only ILC2s collected from non‐allergic individuals secreted IL‐10 in response to alarmin stimulation, whereas ILC2s from allergic donors lost this capability. Once activated with alarmins and allergens, ILC2s from healthy donors produce IL‐10, in contrast to allergic patients. The mechanism underlying reduction in IL‐10–producing cell frequency in allergic individuals compared to NACs remains elusive. Are they any epigenetic signatures involved in IL‐10 region availability and phenotype switch in those cells? For now, this question remains open. Finally, implicating clinical relevance, the authors confirmed the restoration of the IL‐10+ ILC2s population in allergic patients undergoing grass pollen sublingual AIT compared to placebo‐treated patients. 6 Those impressing results showed the importance and high potential of IL‐10–producing ILC2s in AIT‐mediated immune tolerance (Figure 1).
FIGURE 1.
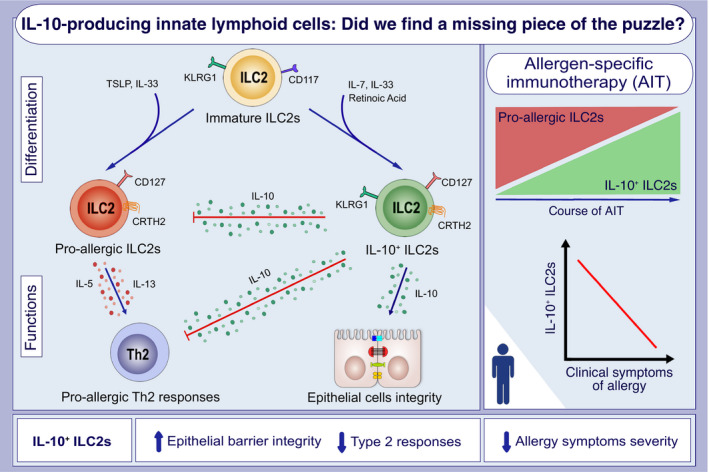
Immature type 2 innate lymphoid cells (ILC2s), depending on the external stimulation, differentiate into i) pro‐allergic ILC2s (upon TSLP and IL‐33), or ii) IL‐10–producing ILC2s (upon IL‐7, IL‐33, and retinoic acid). Pro‐allergic ILC2s secrete IL‐5 and IL‐13, subsequently inducing Th2 responses. On the opposite, IL‐10+ ILC2 inhibit pro‐allergic, type 2 responses mediated by ILC2s and Th2 cells by secreting regulatory cytokine IL‐10 and increasing epithelial barrier integrity. Allergic patients undergoing allergen‐specific immunotherapy (AIT) present increased frequencies of IL‐10–producing ILC2s in the course of treatment. Furthermore, enhanced IL‐10+ ILC2s are related to decreased allergy symptoms severity. KLRG1, killer cell lectin‐like receptor subfamily G member 1; TSLP, thymic stromal lymphopoietin
Undoubtedly, the understanding of the AIT‐induced mechanism of immunotolerance advanced in recent years. Further characterization of microenvironmental regulation of IL‐10 production by ILC2s represents an exciting subject of research. An extraordinary capability of ILCs to reflect changes in cytokines and chemokines profiles in the course of allergic diseases may help to predict clinical response to AIT in allergic patients. Further testing and modifications of ILCs responses will allow us to better understand its applicability as a cellular biomarker for assessment efficacy, safety, and putative long‐term benefits upon AIT. So, did we find a missing piece of the puzzle? We definitely made a picture more complete, and there is still a lot of fun ahead. We are very excited to learn more.
CONFLICT OF INTEREST
All authors have nothing to disclose.
Tynecka and Radzikowska contributed equally as first‐authors.
REFERENCES
- 1. Vivier E, Artis D, Colonna M, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174(5):1054‐1066. [DOI] [PubMed] [Google Scholar]
- 2. Bal SM, Golebski K, Spits H. Plasticity of innate lymphoid cell subsets. Nat Rev Immunol. 2020;20(9):552‐565. [DOI] [PubMed] [Google Scholar]
- 3. Golebski K, Ros XR, Nagasawa M, et al. IL‐1β, IL‐23, and TGF‐β drive plasticity of human ILC2s towards IL‐17‐producing ILCs in nasal inflammation. Nat Commun. 2019;10(1):2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morita H, Kubo T, Rückert B, et al. Induction of human regulatory innate lymphoid cells from group 2 innate lymphoid cells by retinoic acid. J Allergy Clin Immunol. 2019;143(6):2190. [DOI] [PubMed] [Google Scholar]
- 5. Wang S, Xia P, Chen Y, et al. Regulatory innate lymphoid cells control innate intestinal inflammation. Cell. 2017;171(1):201. [DOI] [PubMed] [Google Scholar]
- 6. Golebski K, Layhadi JA, Sahiner U, et al. Induction of IL‐10‐producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity. 2021;54(2):291. [DOI] [PubMed] [Google Scholar]
- 7. DuPage M, Bluestone JA. Harnessing the plasticity of CD4+ T cells to treat immune‐mediated disease. Nat Rev Immunol. 2016;16(3):149‐163. [DOI] [PubMed] [Google Scholar]
- 8. Ebbo M, Crinier A, Vély F, Vivier E. Innate lymphoid cells: major players in inflammatory diseases. Nat Rev Immunol. 2017;17(11):665‐678. [DOI] [PubMed] [Google Scholar]
- 9. Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021. 10.1038/s41577-021-00538-7 [DOI] [PubMed] [Google Scholar]
- 10. Eljaszewicz A, Ruchti F, Radzikowska U, et al. Trained immunity and tolerance in innate lymphoid cells, monocytes, and dendritic cells during allergen‐specific immunotherapy. J Allergy Clin Immunol. 2021;147(5):1865‐1877. [DOI] [PubMed] [Google Scholar]
- 11. Boonpiyathad T, Tantilipikorn P, Ruxrungtham K, et al. IL‐10‐producing innate lymphoid cells increased in patients with house dust mite allergic rhinitis following immunotherapy. J Allergy Clin Immunol. 2021;147(4):1507. [DOI] [PubMed] [Google Scholar]
- 12. Shamji MH, Layhadi JA, Sharif H, Penagos M, Durham SR. Immunological responses and biomarkers for allergen‐specific immunotherapy against inhaled allergens. J Allergy Clin Immunol Pract. 2021;9(5):1769‐1778. [DOI] [PubMed] [Google Scholar]


