Abstract
Background
B‐precursor cell acute lymphoblastic leukemia (B‐ALL) in adults is an aggressive and challenging condition, and patients with relapsed/refractory (R/R) disease after allogeneic stem cell transplantation (SCT), or noncandidates for SCT, have a particularly poor prognosis. The authors investigated the activity of the Fc‐modified anti‐CD19 antibody tafasitamab in adults with R/R B‐ALL (NCT01685021).
Methods
Adults with R/R B‐ALL received single‐agent tafasitamab 12 mg/kg weekly for up to four 28‐day cycles. Patients with complete remission (with or without neutrophil/platelet recovery; complete remission [CR] or complete remission with incomplete count recovery [CRi]) after cycles 2, 3, or 4 could continue tafasitamab every 2 weeks for up to 3 further months. The primary end point was overall response rate (ORR).
Results
Twenty‐two patients were treated (median, 2 prior lines of therapy; range, 1‐8). Six patients completed 2 cycles, and 2 of these patients responded for an ORR of 9%; 16 patients (73%) progressed before their first response assessment. Responses lasted 8 and 4 weeks in the 2 patients with CR and minimal residual disease (MRD)–negative CRi, respectively. Tafasitamab produced rapid B‐cell/blast depletion in 21 of 22 patients within 1 to 2 weeks of first administration. Tafasitamab was well tolerated, with the most frequent adverse events being infusion‐related reactions (59.1%) and fatigue (40.9%). Grade 3 to 4 febrile neutropenia (22.7%) was the most common hematologic adverse event.
Conclusions
Tafasitamab monotherapy was associated with clinical activity in a subset of patients with R/R B‐ALL, including short‐lasting CR and MRD‐negative CRi. Given its favorable tolerability profile, further development of tafasitamab in chemoimmunotherapy combinations and MRD settings should be explored.
Keywords: acute lymphoblastic leukemia, antineoplastic monoclonal antibody, B‐lymphocytes, CD19, MOR208, Xmab5574
Short abstract
In patients with relapsed/refractory B‐precursor acute lymphoblastic leukemia, monotherapy treatment with the Fc‐modified anti‐CD19 antibody tafasitamab was associated with clinical activity, including short‐lasting complete remission and minimal residual disease (MRD)–negative complete remission with incomplete count recovery. In conjuntion with its favorable tolerability profile, further exploration of tafasitamab in chemoimmunotherapy combinations and MRD settings is warranted.
Introduction
Despite the availability of several new therapeutic approaches in recent years, B‐precursor cell acute lymphoblastic leukemia (B‐ALL) in adults remains a challenging malignancy, with more than 60% of patients experiencing relapse and a median overall survival of only 6 to 7 months in relapsing patients. 1 , 2 , 3 , 4 Allogeneic stem cell transplantation (allo‐SCT) is the only available curative therapy, but with complete remission (CR) rates to chemotherapy of 20% to 40% in adults with relapsed/refractory (R/R) ALL, only 10% to 30% of patients go on to receive allo‐SCT. 5
Antibody‐based approaches have focused on the CD19 and CD22 cell surface molecules that are highly expressed on B cells and that, therefore, provide compelling therapeutic targets in R/R B‐ALL. 6 The anti‐CD22 monoclonal antibody‐drug conjugate inotuzumab ozogamicin, 2 the bispecific CD19‐directed CD3 T‐cell engager blinatumomab, 4 and CD19‐targeted chimeric antigen receptor T‐cell (CAR‐T) therapy tisagenlecleucel (in patients up to 25 years of age) 3 have all been explored as salvage therapy in R/R B‐ALL, albeit with modest effects on survival. 6
In the phase 3 (N = 307) inotuzumab ozogamicin study and phase 2 (N = 45) and phase 3 (N = 376) studies of blinatumomab, CR rates (with/without complete hematologic response; CR or complete remission with incomplete count recovery [CRi]) to single‐agent inotuzumab ozogamicin and blinatumomab vary from 34% to 74% in R/R disease, with high rates of minimal residual disease (MRD) negativity among responders (71% to 88%); however, median overall survival (OS) has remained consistent at 7 to 8 months, with 16% to 40% of patients going on to receive allo‐SCT. 7 , 8 , 9 The combination of inotuzumab ozogamicin plus mini‐hyper‐CVD, with or without blinatumomab, as first salvage in 48 patients with Philadelphia chromosome (Ph)‐negative R/R ALL was also associated with high rates of CR and MRD negativity in responders (73% and 93%, respectively) but with 50% of patients proceeding to allo‐SCT and an encouraging median OS of 25 months. 10 CAR‐T therapy with tisagenlecleucel has also achieved high CR/CRi rates in children and adults with R/R B‐ALL, along with an OS probability of 70% at 18 months. 11 , 12
Safety and tolerability are of particular importance in older patients or those with comorbid conditions, especially with respect to suitability for intensive chemotherapy, and may contribute considerably to regimen choice. 5 , 6 Nonchemotherapeutic approaches are also associated with characteristic adverse events (AEs), such as veno‐occlusive liver disease/sinusoidal obstruction syndrome with inotuzumab ozogamicin 7 (of particular concern for patients who progress to allo‐SCT 13 ) and cytokine release syndrome and neurologic events with blinatumomab. 8 , 14 Blinatumomab also requires a 4‐week continuous intravenous (iv) infusion via a portable pump, 8 , 14 which may be challenging for patients. CAR‐T therapy generally requires pre‐infusion lymphodepleting chemotherapy and is frequently associated with cytokine release syndrome (median onset 3 days) and neurologic events within 8 weeks after infusion. 12
Therefore, adult patients with R/R B‐ALL need additional, more effective and better‐tolerated treatment options. To this end, several combination studies involving these novel approaches are ongoing. 1
Tafasitamab is an Fc‐modified, humanized, anti‐CD19 antibody with enhanced antibody‐dependent cell‐mediated cytotoxicity, antibody‐dependent cell‐mediated phagocytosis, and direct cytotoxicity. 15 It has been investigated as a single agent and in combination with immunomodulatory imide drugs and targeted agents in R/R CD19+ B‐cell malignancies, such as chronic lymphocytic leukemia 16 and R/R non‐Hodgkin lymphoma, 17 , 18 , 19 and has been shown to induce natural killer (NK) cell–mediated lysis of ALL cells from pediatric and adult patients. 20
Tafasitamab was recently approved by the Food and Drug Administration (FDA) in combination with lenalidomide for the treatment of R/R diffuse large B‐cell lymphoma (DLBCL) in transplant‐ineligible patients. 21 The aim of this study was to evaluate the efficacy and safety of tafasitamab monotherapy in adult patients with R/R B‐ALL.
Materials and Methods
MOR208C202 was a multicenter, single‐arm, open‐label, phase 2a study evaluating the efficacy and safety of tafasitamab in adolescent and adult patients with R/R B‐ALL, performed at 3 sites in the United States. Eligible patients were ≥16 years old with previously treated, Ph‐negative B‐ALL and had experienced progression after at least 1 prior therapy. Patients with Ph‐positive B‐ALL who were refractory or intolerant to at least 1 tyrosine kinase inhibitor were also eligible, as were patients with mixed phenotype acute leukemia with a B‐cell immunophenotype. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 to 2 and adequate hepatic and renal function.
Patients were not eligible if they had received any previous treatment with an anti‐CD19 antibody or fragments, an anti‐CD20 therapy within 4 weeks before study treatment, or prior allo‐SCT within 3 months of study treatment, or if they had acute graft‐versus‐host disease.
The primary end point was overall response rate (ORR), including morphologic CR, CRi, or partial remission (PR), based on best response achieved during the treatment period. Secondary end points included duration of response, time to hematologic relapse, incidence and severity of AEs, patients with MRD by flow cytometry with a minimal sensitivity of 10‐4, number and proportion of patients subsequently receiving allo‐SCT, number and proportion of patients with immunogenic responses to treatment, as well as Cmax, Tmax, and trough concentration of tafasitamab. Exploratory biomarkers included the longitudinal assessment of B‐cells and NK cells according to local standards and CD19/CD20 assessment of B‐cell blasts (CD45low/CD16/CD14/CD3‐negative cells) via a centralized assay (ICON Laboratory Services, Whitesboro, New York) of antibody binding capacity using BD Quantibrite™ beads.
Patients received tafasitamab (12 mg/kg iv) weekly in up to four 28‐day cycles, with an additional loading dose on day 4 of cycle 1. Patients responding after cycle 1 could receive up to 3 additional 4‐week cycles followed by tafasitamab every 2 weeks for 3 months or until progressive disease (PD) (Fig. 1). Responses and disease status were evaluated after every cycle. Response assessment was based on complete blood counts, bone marrow aspirates or biopsy (until CR or CRi), and CT scans (if applicable).
Figure 1.
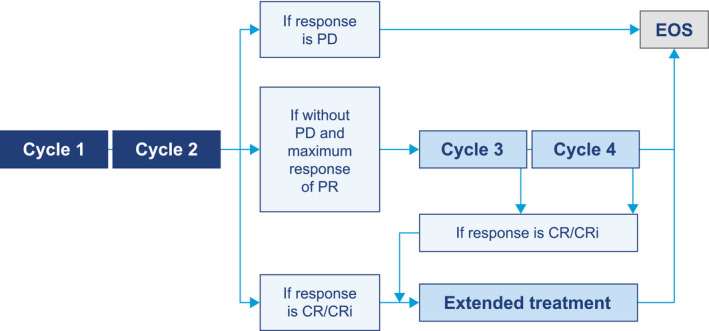
Treatment algorithm. CR indicates complete remission; CRi, complete remission with incomplete count recovery; EOS, end of study; PD, progressive disease; PR, partial remission.
During cycle 1, patients received premedication including antipyretics (acetaminophen, 650 mg orally or iv or equivalent), antihistamines (diphenhydramine, 25‐50 mg iv or equivalent) and glucocorticosteroids (methylprednisolone, 80‐120 mg iv or equivalent). If no infusion‐related reactions (IRRs) were experienced during cycle 1, premedication could be stopped for subsequent cycles at the discretion of the investigator.
CR was defined as a bone marrow blast count of <5% with normal peripheral counts for neutrophils (≥1 × 109/L) and platelets (≥100 × 109/L). 22 CRi was defined as CR with incomplete recovery of neutrophils or platelets. PR was defined as a ≥50% decrease in bone marrow blasts but remaining in the 6% to 25% range (with normal peripheral counts). PD was defined as a ≥100% increase in circulating blasts, or an increase in blasts as judged by the investigator as inappropriate for further study continuation.
Statistical Methods
With a target sample size of 30 patients, it was assumed that 27 patients would be included in the intent‐to‐treat (ITT) population. With an observed ORR of 20%, the expected 95% confidence interval (CI) would be 5% to 35%, indicating that the true ORR was greater than 0. The primary end point (ORR) was estimated in the ITT population according to best response achieved at any time during the study. In addition, an exact 95% CI was generated for the ORR.
The secondary end points were analyzed descriptively using summary statistics for continuous data and frequency tables for categorical data. The 95% CIs are presented for means or rates.
Descriptive statistics were calculated with nonparametric 95% CI for mean change from baseline where appropriate.
Results
Patients
Of 30 patients screened, 22 received treatment between April 2013 and early termination of the study on February 28, 2015, because of a low proportion of responses to single‐agent therapy.
Baseline patient and disease characteristics are shown in Table 1. The median age of patients was 52 years, with a median of 13 months since B‐ALL diagnosis and a median of 2 prior lines of therapy (range, 1‐8). The rate of best response to their last line of therapy was 32% (7 of 22 patients; all CR). All patients had previously received chemotherapy, including 7 patients who had received chemoimmunotherapy (including rituximab in 6 cases), and 6 patients had previously received allo‐SCT, with a range of 15 to 152 weeks between allo‐SCT and cycle 1 day 1 in the present study.
TABLE 1.
Patient Baseline and Disease Characteristics
| Characteristic | Patients (N = 22) |
|---|---|
| Median age, y (range) | 52.0 (16.0‐79.0) |
| Male, n (%) | 12 (54.5) |
| Median time since ALL diagnosis, mo (range) | 13.0 (1.7‐322.5) |
| ECOG performance status, No. (%) | |
| 0 | 7 (31.8) |
| 1 | 10 (45.5) |
| 2 | 5 (22.7) |
| ALL subtype, No. (%) | |
| Acute pre‐B‐lymphoblastic leukemia | 15 (68.2) |
| Acute pro‐B‐lymphoblastic leukemia | 2 (9.1) |
| Mature B‐lymphoblastic leukemia | 1 (4.5) |
| Common B‐lymphoblastic leukemia | 1 (4.5) |
| Philadelphia‐positive B‐ALL | 2 (9.1) |
| Other (Pre‐B‐ALL in CR1) | 1 (4.5) |
| ALL cytogenetics, No. (%) | |
| t(4;11)/11q23 | 3 (13.6) |
| t(9;22) | 2 (9.1) |
| t(1;19) | 4 (18.2) |
| 14q32 | 1 (4.5) |
| Low hypodiploidy/complex karyotype | 1 (4.5) |
| Other | 11 (50) |
| Median prior lines of therapy, No. (range) | 2 (1‐8) |
| Prior allogeneic stem cell transplantation, No. (%) | 6 (27.3) |
| Prior umbilical cord blood transplantation, No. (%) | 1 (4.5) |
| Prior ALL therapies, No. (%) | |
| Chemotherapy/chemoimmunotherapy a | 22 (100) |
| Radiation therapy | 2 (9.1) |
| POMP maintenance | 2 (9.1) |
| Best response to last therapy, No. (%) | |
| Complete remission | 7 (31.8) |
| Stable disease | 4 (18.2) |
| Progressive disease | 6 (27.3) |
| Unknown | 5 (22.7) |
Abbreviations: ALL, acute lymphoblastic leukemia; B‐ALL, B‐precursor acute lymphoblastic leukemia; ECOG, Eastern Cooperative Oncology Group; POMP, mercaptopurine, vincristine, methotrexate and prednisone.
Seven patients received previous immunotherapies that were classified by the investigator as part of a chemotherapeutic regimen (rituximab in 6 cases [including 1 patient who also received experimental moxetumomab] and experimental inotuzumab ozogamicin in 1 case).
A total of 16 patients discontinued treatment before the first response assessment, including 11 patients who experienced rapid disease progression. Other reasons for withdrawal were (each n = 1): death (sepsis, not related to treatment), abnormal laboratory value (neutropenia; 0.89 × 109 cells/L), withdrawn consent, receipt of new cancer therapy, and withdrawal after missing 2 consecutive visits (cycle 1 day 8 and cycle 1 day 15). Six patients (27.3%) completed the study (ie, completed ≥2 cycles with ≥1 disease assessment), and 2 patients had responses qualifying them for further treatment cycles.
Efficacy
In the ITT population, 2 out of 22 patients responded (one CR and one CRi) with an ORR of 9% (95% CI, 1.1‐29.2) (Fig. 2). Three patients had stable disease (14%; qualifying for neither remission nor disease progression), 16 patients had disease progression (73%), and 1 patient (5%) had no response assessment (Table 2).
Figure 2.
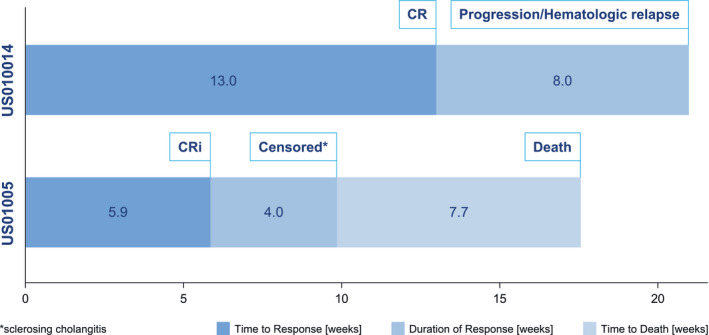
Time on study for responders. CRi indicates complete remission with incomplete count recovery.
TABLE 2.
ORRs
| Response Rates | Patients (N = 22), No. (%) |
|---|---|
| ORR (CR, CRi, or PR) | 2 (9.1) [95% CI, 1.1‐29.2] |
| CR | 1 (4.5) |
| CRi | 1 (4.5) |
| PR | 0 (0) |
| SD | 3 (13.6) |
| PD | 16 (72.7) |
| No response assessment after 2 cycles (PD) | 1 (4.5) |
Abbreviations: CI, confidence interval; CR, complete remission; CRi, complete remission with incomplete count recovery; ORR, overall response rate; PD, progressive disease; PR, partial remission; SD, stable disease.
The patient with a CR was a 47‐year‐old male who experienced this response after 13 weeks on treatment, with hematologic relapse after a further 8 weeks. This patient had low hypodiploid Ph‐negative pro‐B‐ALL, with 35 chromosomes in the leukemic cells, and had a history of childhood ALL (entered study 27 years after first ALL diagnosis) with 2 prior systemic regimens (undefined multi‐agent chemotherapy in childhood [CR1 for 248 months]; hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone [hyper‐CVAD; CR2 for 26 months]; and maintenance with mercaptopurine, vincristine, methotrexate, and prednisone maintenance therapy).
The patient with CRi was a 46‐year‐old female who entered the study, 7 months after her first ALL diagnosis, experienced CRi after 6 weeks on study, and was censored after a further 4 weeks because of an AE (severe sclerosing cholangitis). This patient had Ph‐negative pre‐B ALL with an adverse (near‐haploid) karyotype and had previously received treatment with hyper‐CVAD (with a short CR1 for 4 months) and allo‐SCT (approximately 15 weeks before study entry), then with cyclophosphamide and dexamethasone (with a response of disease progression), indicating that she was refractory to allo‐SCT; her ALL cytogenetics were 35,XX,‐2,‐3,‐4,‐7,‐1,‐12,‐13,‐15,‐16,‐17,‐20,del(22)(q12)[7]/36,SL,+del(22)(q12)[11]/46,XX[2].
Duration of Response
Among the 2 responding patients, the CR lasted 56 days until hematologic relapse, and the CRi lasted 28 days until the patient was censored because of an AE (sclerosing cholangitis). The patient with CR was consistently MRD‐positive and eligible for allo‐SCT, and the patient with CRi was considered MRD‐negative by the investigator, although none of the study patients received an allo‐SCT during or after the study.
AEs and Immunogenicity
Most AEs experienced by patients were of mild to moderate severity (77%) and of grade 1 to 2 toxicity (71%). The most common AEs (any grade) were IRRs (n = 13; 59.1%) and fatigue (n = 9; 40.9%), and the most common hematologic AE was febrile neutropenia (n = 5; 22.7%) (Table 3).
TABLE 3.
Treatment‐Emergent AEs
| AE Category | Patients (N = 22), No. (%) [Events] |
|---|---|
| AEs, ≥20% (any grade) | |
| Infusion‐related reaction | 13 (59.1) [15] |
| Fatigue | 9 (40.9) [10] |
| Hypokalemia | 6 (27.3) [14] |
| Pyrexia | 6 (27.3) [7] |
| Constipation | 6 (27.3) [6] |
| Nausea | 6 (27.3) [6] |
| Hyperglycemia | 6 (27.3) [6] |
| Febrile neutropenia | 5 (22.7) [10] |
| Hyperkalemia | 5 (22.7) [6] |
| Dyspnea | 5 (22.7) [5] |
| Hematologic AEs, ≥5% (any grade) a | |
| Febrile neutropenia | 5 (22.7) [10] |
| Anemia | 4 (18.2) [6] |
| Neutrophil count decreased | 4 (18.2) [6] |
| Platelet count decreased | 3 (13.6) [4] |
| White blood cell count decreased | 2 (9.1) [3] |
| Thrombocytopenia | 2 (9.1) [2] |
| Grade ≥3 AEs, ≥10% | |
| Febrile neutropenia | 5 (22.7) [10] |
| Hyperglycemia | 5 (22.7) [5] |
| Sepsis | 4 (18.2) [4] |
| Neutrophil count decreased | 4 (18.2) [5] |
| Platelet count decreased | 3 (13.6) [4] |
| Lung infection/pneumonia | 3 (13.6) [3] |
| Tumor lysis syndrome | 3 (13.6) [3] |
| Hypertension | 3 (13.6) [3] |
| Treatment‐emergent AEs leading to death | |
| Sepsis | 2 (9.1) [2] |
| Disease progression | 2 (9.1) [2] |
| Sclerosing cholangitis b | 1 (4.5) [1] |
Abbreviations: AE, adverse event; SOC, system organ class; PT, preferred terms.
Six additional patients (27.3%) had disease progression reported as an AE as defined in the study protocol.
Hematologic AEs were MedDRA coded to either SOC blood and lymphatic system disorders (including PTs anemia, neutropenia, and thrombocytopenia) or SOC investigations (including PTs neutrophil count decreased, white blood cell count decreased, and platelet count decreased) depending on the AE term (verbatim) reported by the investigator.
Suspected to be related to treatment.
Seventeen patients experienced serious AEs (SAEs; 77.3%). The most frequent SAEs were pyrexia (22.7%) and febrile neutropenia (18.2%). SAEs were suspected to be tafasitamab‐related by the investigators in 4 patients (18.2%: sclerosing cholangitis; disseminated fungal infection; tumor lysis syndrome; and [in 1 patient] febrile neutropenia, tumor lysis syndrome, and pneumonitis). Six patients (27.3%) experienced an AE leading to death (Table 3); 5 deaths were considered unrelated to treatment (2 cases of sepsis, 2 cases of PD, and 1 nontreatment‐emergent SAE of death). The case of sclerosing cholangitis in the refractory patient after allo‐SCT who experienced transient CRi led to fulminant liver failure and was suspected to be related to treatment but could also have been related to a preexisting graft versus host disease.
All IRRs occurred during day 1 of cycle 1 and were assessed as nonserious (all were grade 1 or 2, with the exception of one grade 3 event), and all patients recovered on the same day, after interruption of study drug infusion and symptomatic treatment if necessary. The most common grade ≥3 AEs were febrile neutropenia, thrombocytopenia (including platelet count decreased), neutropenia (including neutrophil count decreased), sepsis (including enterococcal sepsis), and hyperglycemia (each 22.7%) (Table 3).
None of the 22 patients treated with tafasitamab tested positive for anti‐drug antibodies.
Pharmacokinetics
After the first infusion, mean Tmax was 4.5 hours and mean Cmax was ~306 μg/mL. Mean serum trough concentrations of tafasitamab at steady state were ~177 and ~198 μg/mL before the seventh and ninth doses, respectively. Mean Cmax at steady state was ~457 and ~443 µg/mL after the seventh and ninth doses, respectively.
B‐Cell and NK Cell Effects
Peripheral B‐cell/lymphoblast depletion was observed within 1 to 2 weeks after the first infusion of tafasitamab for all but 1 patient (Fig. 3); the median relative B‐cell/lymphoblast depletion reached 91.8% at cycle 1 day 8. For the 2 responding patients, B‐cell counts decreased from 715 cells/μL (baseline) to 25 cells/μL (C1D15) for the patient with CR, and reached 4 cells/μL (C1D15) for the patient with CRi (baseline sample not collected). At screening, a high level of expression of CD19 relative to CD20 was confirmed, with all patients having CD19+ B‐lymphoblasts (Figs. 4A,B).
Figure 3.
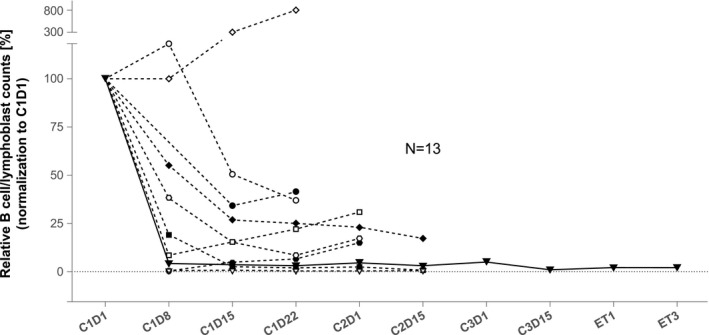
Peripheral B‐cell/lymphoblast counts. C, cycle; D, day.
Figure 4.
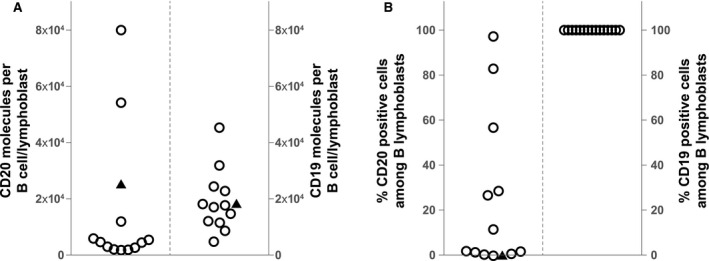
(A) Baseline CD19/CD20 expression levels per B lymphoblas, and (B) frequency of CD19/20‐positive cells among peripheral B lymphoblasts. The median CD20 expression is 4885 molecules per cell, and the median CD19 level is 17,878 molecules (not plotted). The black triangle shows 25,763 CD20 molecules and 18,257 CD19 molecules (n = 13 patients) on the B lymphoblast surface.
Most patients showed a decrease in peripheral NK cell counts during the first 2 weeks of treatment, with a median reduction of 60% at cycle 1 day 8, which recovered slightly at cycle 1 day 22.
Discussion
This study of single‐agent tafasitamab in adult patients with R/R B‐ALL (1‐8 prior lines of therapy) demonstrated modest activity, with 2 responders out of 22 patients (1 CR and 1 CRi; ORR 9% by ITT analysis) receiving extended therapy. Most patients experienced PD before reaching 2 treatment cycles, indicating the aggressive nature of this disease. The study was terminated before the planned recruitment was complete because of a low proportion of responses to single‐agent therapy and a low likelihood of meeting the protocol‐specified sample size.
The indication of clinical activity with tafasitamab monotherapy in R/R B‐ALL was supported by a rapid reduction in B‐cells/lymphoblasts in 12 of 13 evaluable patients. Observed steady‐state Cmax and trough levels of tafasitamab were in line with that expected from previous clinical experience in R/R B‐cell non‐Hodgkin lymphoma, 17 and it is unlikely that higher doses or a more frequent dosing schedule would lead to better efficacy.
The present study does not indicate that single‐agent therapy with tafasitamab is sufficient in R/R B‐ALL, compared with response rates of 36% to 44% observed with blinatumomab and 74% to 79% with inotuzumab ozogamicin. 5 A more promising development approach may be to use tafasitamab to augment or deepen the treatment response in the MRD setting. This approach was demonstrated in a pediatric population after allo‐SCT, where a similar Fc‐modified anti‐CD19 antibody produced significant reductions in MRD levels of ≥1 log (4 of 14 patients), or to below the patient‐individual detection limit (6 of 14 patients) before, as well as after, allo‐SCT. 23 Support for adult patients in an MRD setting following upfront treatment after induction/consolidation could, therefore, also be considered.
Tafasitamab could be administered safely to patients with B‐ALL and was well tolerated; treatment‐emergent AEs were most frequently mild to moderate, nonserious IRRs and fatigue, with febrile neutropenia being the most common hematologic AE. Tafasitamab‐related SAEs occurred in 4 of the 17 patients who experienced SAEs, indicating that most SAEs were related to the underlying disease. IRRs were manageable with best supportive care and did not lead to treatment discontinuation. Cytopenias and febrile neutropenia were mostly related to the underlying disease and consistent with prior experience in R/R chronic lymphocytic leukemia (CLL) 16 and R/R non‐Hodgkin lymphoma. 17
Combination therapy with tafasitamab has proven to be an effective option in R/R DLBCL in the L‐MIND study (tafasitamab plus lenalidomide [NCT02399085] 24 ), which led to accelerated approval by the FDA. In patients with newly diagnosed, previously untreated DLBCL, tafasitamab with or without lenalidomide in addition to standard R‐CHOP is being evaluated in the First‐MIND study (NCT04134936). Tafasitamab plus lenalidomide is also being studied in R/R CLL (NCT02005289), as is tafasitamab plus idelalisib or venetoclax (COSMOS; NCT02639910).
In conclusion, tafasitamab monotherapy was associated with clinical activity in a subset of patients with R/R B‐ALL, including short‐lasting CR/CRi. Given its favorable tolerability profile, in future studies tafasitamab can be readily combined with or included in other anti‐leukemia regimens, or to treat patients with MRD.
About Tafasitamab
Tafasitamab is a humanized Fc‐modified cytolytic CD19 targeting monoclonal antibody. In 2010, MorphoSys licensed exclusive worldwide rights to develop and commercialize tafasitamab from Xencor, Inc.
Tafasitamab incorporates an XmAb® engineered Fc domain, which mediates B‐cell lysis through apoptosis and immune effector mechanism including Antibody‐Dependent Cell‐Mediated Cytotoxicity (ADCC) and Antibody‐Dependent Cellular Phagocytosis (ADCP).
In January 2020, MorphoSys and Incyte entered into a collaboration and licensing agreement to further develop and commercialize tafasitamab globally. Following accelerated approval by the U.S. Food and Drug Administration in July 2020, tafasitamab is being co‐commercialized by MorphoSys and Incyte in the United States. Incyte has exclusive commercialization rights outside the United States.
Funding Support
This work was sponsored by MorphoSys AG.
Conflict of Interest Disclosures
Rebecca B. Klisovic reports support from Incyte and MorphoSys AG during the conduct of the study and support for attending meetings from Novartis and participation on a data safety monitoring board or advisory board for Incyte outside the submitted work. Wolfram Brugger is an employee of MorphoSys AG and has stock or stock options in AstraZeneca. Maren Dirnberger‐Hertweck is an employee of MorphoSys AG. Mark Winderlich is an employee of MorphoSys AG. Sumeet V. Ambarkhane is an employee of MorphoSys AG. Elias J. Jabbour reports research grants and consulting fees from AbbVie, Amgen, Adaptive Biotechnology, BMS, Pfizer, Takeda, Genentech, Incyte, and MorphoSys AG. Wing H. Leung has nothing to disclose.
Author Contributions
All authors contributed to the study design, data collection, data analysis and data interpretation, and writing or review of this article.
Klisovic RB, Leung WH, Brugger W, Dirnberger‐Hertweck M, Winderlich M, Ambarkhane SV, Jabbour EJ. A phase 2a, single‐arm, open‐label study of tafasitamab, a humanized, Fc‐modified, anti‐CD19 antibody, in patients with relapsed/refractory B‐precursor cell acute lymphoblastic leukemia. Cancer. 2021. 10.1002/cncr.33796
We thank the patients and their families, clinical researchers, and their teams and hospitals that have participated in this study.
This study is registered at ClinicalTrials.gov (NCT01685021).
Medical writing assistance was provided by Martin Quinn, PhD (Syneos Health), and funded by MorphoSys AG.
References
- 1. Samra B, Jabbour E, Ravandi F, et al. Evolving therapy of adult acute lymphoblastic leukemia: state‐of‐the‐art treatment and future directions. J Hematol Oncol. 2020;13:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. BESPONSA prescribing information . US Food & Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761040s000lbl.pdf. Accessed February 26, 2021.
- 3. KYMRIAH prescribing information . US Food & Drug Administration. https://www.fda.gov/files/vaccines%2Cblood%26biologics/published/Package‐Insert‐‐‐KYMRIAH.pdf. Accessed February 26, 2021.
- 4. BLINCYTO prescribing information . US Food & Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125557s017lbl.pdf. Accessed February 26, 2021.
- 5. DeAngelo DJ, Jabbour E, Advani A. Recent advances in managing acute lymphoblastic leukemia. Am Soc Clin Oncol Educ B. 2020;40:330‐342. [DOI] [PubMed] [Google Scholar]
- 6. Jabbour E, Pui CH, Kantarjian H. Progress and innovations in the management of adult acute lymphoblastic leukemia. JAMA Oncol. 2018;4:1413‐1420. [DOI] [PubMed] [Google Scholar]
- 7. Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long‐term survival follow‐up from the randomized, phase 3 INO‐VATE study. Cancer. 2019;125:2474‐2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martinelli G, Dombret H, Chevallier P, et al. Complete molecular and hematologic response in adult patients with relapsed/refractory (R/R) Philadelphia chromosome‐positive B‐precursor acute lymphoblastic leukemia (ALL) following treatment with blinatumomab: results from a phase 2 single‐arm, multicen. Blood. 2015;126:679. [Google Scholar]
- 10. Jabbour E, Sasaki K, Ravandi F, et al. Chemoimmunotherapy with inotuzumab ozogamicin combined with mini‐hyper‐CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome–negative acute lymphoblastic leukemia in first salvage. Cancer. 2018;124:4044‐4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grupp SA, Maude SL, Rives S, et al. Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory (r/r) acute lymphoblastic leukemia. Blood. 2018;132(suppl 1):895. [Google Scholar]
- 12. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B‐cell lymphoblastic leukemia. N Engl J Med. 2018;378:439‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kebriaei P, Cutler C, Lima MD, et al. Management of important adverse events associated with inotuzumab ozogamicin: expert panel review. Bone Marrow Transplant. 2018;53:449‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B‐precursor acute lymphoblastic leukaemia: a multicentre, single‐arm, phase 2 study. Lancet Oncol. 2015;16:57‐66. [DOI] [PubMed] [Google Scholar]
- 15. Horton HM, Bernett MJ, Pong E, et al. Potent in vitro and in vivo activity of an Fc‐engineered anti‐CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68:8049‐8057. [DOI] [PubMed] [Google Scholar]
- 16. Woyach JA, Awan F, Flinn IW, et al. A phase 1 trial of the Fc‐engineered CD19 antibody XmAb5574 (MOR00208) demonstrates safety and preliminary efficacy in relapsed CLL. Blood. 2014;124:3553‐3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jurczak W, Zinzani PL, Gaidano G, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B‐cell non‐Hodgkin's lymphoma. Ann Oncol. 2018;29:1266‐1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B‐cell lymphoma (L‐MIND): a multicentre, prospective, single‐arm, phase 2 study. Lancet Oncol. 2020;21:978‐988. [DOI] [PubMed] [Google Scholar]
- 19. Staber PB, Jurczak W, Brugger W, et al. Primary analysis of anti‐CD19 tafasitamab (MOR208) treatment in combination with idelalisib or venetoclax in R/R CLL patients who failed prior BTK inhibitor therapy (COSMOS trial). Blood. 2019;134(suppl 1):1754. [Google Scholar]
- 20. Kellner C, Zhukovsky EA, Pötzke A, et al. The Fc‐engineered CD19 antibody MOR208 (XmAb5574) induces natural killer cell‐mediated lysis of acute lymphoblastic leukemia cells from pediatric and adult patients. Leukemia. 2013;27:1595‐1598. [DOI] [PubMed] [Google Scholar]
- 21. US Food & Drug Administration FDA grants accelerated approval to tafasitamab‐cxix for diffuse large B‐cell lymphoma. https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐grants‐accelerated‐approval‐tafasitamab‐cxix‐diffuse‐large‐b‐cell‐lymphoma. Accessed February 26, 2021.
- 22. NCCN Clinical Practice Guidelines in Oncology: Acute Lymphoblastic Leukemia. v1.2020 . National Comprehensive Cancer Network. https://www.nccn.org/. Accessed February 26, 2021.
- 23. Seidel UJE, Schlegel P, Grosse‐Hovest L, et al. Reduction of minimal residual disease in pediatric B‐lineage acute lymphoblastic leukemia by an Fc‐optimized CD19 antibody. Mol Ther. 2016;24:1634‐1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salles G, Duell J, Gonzalez‐Barca E, et al. Long‐term outcomes from the phase II L‐MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B‐cell lymphoma. EHA Library. 2020;293691:EP1201. [DOI] [PubMed] [Google Scholar]


