Abstract
Polycyclic aromatic hydrocarbon (PAH)-degrading bacteria were isolated from contaminated estuarine sediment and salt marsh rhizosphere by enrichment using either naphthalene, phenanthrene, or biphenyl as the sole source of carbon and energy. Pasteurization of samples prior to enrichment resulted in isolation of gram-positive, spore-forming bacteria. The isolates were characterized using a variety of phenotypic, morphologic, and molecular properties. Identification of the isolates based on their fatty acid profiles and partial 16S rRNA gene sequences assigned them to three main bacterial groups: gram-negative pseudomonads; gram-positive, non-spore-forming nocardioforms; and the gram-positive, spore-forming group, Paenibacillus. Genomic digest patterns of all isolates were used to determine unique isolates, and representatives from each bacterial group were chosen for further investigation. Southern hybridization was performed using genes for PAH degradation from Pseudomonas putida NCIB 9816-4, Comamonas testosteroni GZ42, Sphingomonas yanoikuyae B1, and Mycobacterium sp. strain PY01. None of the isolates from the three groups showed homology to the B1 genes, only two nocardioform isolates showed homology to the PY01 genes, and only members of the pseudomonad group showed homology to the NCIB 9816-4 or GZ42 probes. The Paenibacillus isolates showed no homology to any of the tested gene probes, indicating the possibility of novel genes for PAH degradation. Pure culture substrate utilization experiments using several selected isolates from each of the three groups showed that the phenanthrene-enriched isolates are able to utilize a greater number of PAHs than are the naphthalene-enriched isolates. Inoculating two of the gram-positive isolates to a marine sediment slurry spiked with a mixture of PAHs (naphthalene, fluorene, phenanthrene, and pyrene) and biphenyl resulted in rapid transformation of pyrene, in addition to the two- and three-ringed PAHs and biphenyl. This study indicates that the rhizosphere of salt marsh plants contains a diverse population of PAH-degrading bacteria, and the use of plant-associated microorganisms has the potential for bioremediation of contaminated sediments.
Contamination of estuarine sediments by highly hydrophobic, low-availability, toxic, organic contaminants has become a significant environmental concern. For example, industrial activities in the New Jersey/New York Harbor estuary have resulted in widespread contamination, and the management of contaminated sediments has become a significant problem. Polycyclic aromatic hydrocarbons (PAHs) are of particular concern because of their toxic, mutagenic, and carcinogenic properties (32). PAHs have also been found to bioaccumulate in aquatic organisms (29). Although PAHs are persistent, mainly due to their high hydrophobicity, a variety of microorganisms (bacteria and fungi) can degrade certain PAHs (for reviews, see references 6 and 44). There is thus a significant interest in studying microorganisms present in contaminated environments as a means for bioremediation.
The fate of PAHs and other organic contaminants in the environment is associated with both abiotic and biotic processes, including volatilization, photooxidation, chemical oxidation, bioaccumulation, and microbial transformation. Microbial activity has been deemed the most influential and significant cause of PAH removal (6). Numerous studies have been conducted on microbial consortia and enrichment, and several diverse genera of bacteria have been isolated (for a review, see reference 6). Recent work has indicated that the stimulation of microbial activity in the rhizosphere of plants can also stimulate biodegradation of various toxic organic compounds (1, 4, 37, 39, 41, 45, 47). This general “rhizosphere effect” is well known in terrestrial systems. The rhizosphere soil has been described as the zone of soil under the direct influence of plant roots and usually extends a few millimeters from the root surface and is a dynamic environment for microorganisms (7). The rhizosphere microbial community is comprised of microorganisms with different types of metabolism and adaptive responses to variation in environmental conditions. The production of mucilaginous material and the exudation of a variety of soluble organic compounds by the plant root play an important part in root colonization and maintenance of microbial growth in the rhizosphere. Microbial activity is thus generally higher in the rhizosphere due to readily biodegradable substrates exuded from the plant (35). Recent studies indicate that degradation of PAHs and polychlorinated biphenyls (PCBs) in soils can be enhanced by plants (37, 47). In the case of PCBs, plant compounds have been shown to induce the cometabolic degradation of PCBs (15, 16).
The activities of microbial communities in sediments and tidal marshes are important in improving sediment quality (pollution degradation), in the biological transformations of major nutrients influencing their availability for plant growth, and in their role in binding sediment particles to improve rooting medium structure. Wetlands have a higher biological activity than many other ecosystems, and they support enhanced biotransformation of toxic chemicals (25). Experiments with Spartina salt marsh mesocosms indicated that biodegradation of oil was influenced by flooding and fertilization conditions (28, 49).
There is extensive information on PAH degradation by both gram-positive and gram-negative bacteria, mostly isolated from soil environments. There is, however, little information on the microbial communities in salt marsh ecosystems and their role in the biodegradation of toxic organic contaminants. In addition, little is known about the ability of these microorganisms to degrade polyaromatic compounds. In the present study, we isolated PAH-degrading bacteria from the salt marsh rhizosphere. We were particularly interested in isolating spore-forming bacteria for two reasons. First, the ability of a bacterium to produce spores and therefore survive adverse conditions would be an attractive feature for remediation of contaminated sites. Secondly, only a few studies have examined the degradation of PAHs by spore-forming bacteria, and none, to our knowledge, involve plant-microbe interactions. Therefore, isolation of spore-forming, PAH-degrading bacteria and subsequent genetic studies of their degradation pathways may lead to the discovery of novel genes involved. In addition, we wanted to evaluate the biodegradation potential of select isolates and determine if reinoculation of the rhizosphere would enhance degradation of contaminated marine sediment.
MATERIALS AND METHODS
Plant and sediment samples.
Salt marsh plant samples were obtained at two sites. The first site, considered to be uncontaminated, was the University of Delaware Marine Station at Lewes, Del. A total of five different plant samples were collected and included Distichlis spicata, Juncus gerardi, Phragmites australis, Spartina alterniflora, and Sporobolus airoides. A minimum of two plants of each species was obtained. The second site was Piles Creek, a contaminated tributary of Arthur Kill, in Linden, N.J. Estuarine sediment and S. alterniflora plant samples were obtained from the intertidal area. A total of three sediment and three plant samples were taken from separate areas within 50 feet of each other. The plant and sediment samples were transported on ice back to the laboratory where they were stored at 4°C until analyzed.
Contaminated sediment for the microbial-sediment slurry biotransformation experiment was obtained from Newtown Creek in the New York Harbor, Brooklyn, N.Y. Material was collected by the Army Corps of Engineers, and a chemical analysis showed the dredged sediment to contain 2 to 7 ppm of the PAHs naphthalene, anthracene, and phenanthrene. The sediment texture consisted of 50% sand, 41% silt, and 9% clay, as determined by the Soil Testing Laboratory at the New Jersey Agricultural Experiment Station. The structure is considered a silt loam, organic matter was 3.7%, and the pH was 7.90.
Bacterial strains, plasmids and media.
Pseudomonas putida NCIB 9816-4 (9), Comamonas testosteroni GZ42 (18), Sphingomonas yanoikuyae B1 (14), and Mycobacterium sp. strain PY01 (Y.-S. Oh and G. J. Zylstra, unpublished data) were chosen as representatives of different genotypes of PAH-degrading organisms. The genes have previously been cloned from these strains into Escherichia coli, and the cloned fragments were used as gene probes for Southern hybridization experiments. Plasmid pDTG112 contains the cloned nah genes from P. putida NCIB 9816-4 (40). A 3.6-kb SalI fragment contains three of the four genes for naphthalene dioxygenase: nahAa (reductase), nahAb (ferredoxin), and nahAc (dioxygenase large subunit) (42). Plasmid pGJZ1822, cloned from C. testosteroni GZ42, has a 6.7-kb SstI-XhoI fragment that contains the genes necessary for the first few steps in PAH degradation (19). Plasmid pGJZ1512, cloned from S. yanoikuyae B1, has a 567-bp SphI fragment that contains bphC encoding a meta-ring cleavage enzyme involved in PAH degradation (26). A 600-bp fragment containing a gene encoding a dioxygenase large subunit involved in PAH degradation by Mycobacterium sp. strain PY01 (J. F. Cigolini and G. J. Zylstra, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. Q-180, 1999) was PCR amplified with the primers 5′-GTTGACCCGTGACGC-3′ and 5′-CTCACTCAAGGCCGG-3′. The PAH-degrading strain C. testosteroni GZ39 was used as a control in hybridization experiments (18).
Mineral salts basal (MSB) medium (43) was used for enrichment cultures and isolation. Solid minimal medium contained 2% noble agar (Difco Laboratories, Detroit, Mich.). Stock concentrations (100 mg ml−1) of naphthalene, biphenyl, and phenanthrene were dissolved in dimethyl formamide and were added to liquid medium at a final concentration of 1 mg ml−1. Naphthalene and biphenyl were added in the vapor phase as crystals in the petri dish lid for solid medium. Phenanthrene was added as a 2% noble agar overlayer onto MSB agar plates at a final concentration of 1 mg ml−1. All PAHs were obtained from Aldrich Chemical Co. (Milwaukee, Wis.) and were a minimum of 98% pure.
Bacterial strains were grown on Trypticase soy (Beckton Dickinson & Co., Cockeysville, Md.) agar (TSA) plates for various phenotypic and fatty acid methyl ester (FAME) analyses. For plasmid preparations, bacterial strains were grown in Luria-Bertani medium (38) containing either kanamycin (50 μg ml−1; Sigma Chemical Co., St. Louis, Mo.) or ampicillin (100 μg ml−1; Sigma Chemical Co.) added from filter-sterilized stock solutions. All bacterial strains and isolates were routinely grown aerobically at 30°C, except for E. coli strains, which were grown at 37°C. Strains were maintained on minimal medium at 4°C, and long-term storage was in 50% glycerol at −80°C.
Enrichment and isolation of PAH-degrading bacteria.
Bacterial enrichment cultures were set up in cotton-plugged Erlenmeyer flasks containing 50 ml of MSB medium using naphthalene, phenanthrene, or biphenyl as the sole source of carbon. Plant rhizosphere and contaminated estuarine sediment were used as sources of the inoculum for the enrichment cultures. Rhizosphere samples were obtained by splitting open the plant root mass, so that the inner roots that had not come in contact with sampling or storage devices could be aseptically cut and collected. One gram of root material was gently shaken to remove loose sediment material and was added to each enrichment culture. Sediment samples were added directly to each enrichment culture using a 1-g subsample. A total of two enrichment cultures for each substrate were set up with each rhizosphere and sediment sample. One set of enrichment cultures was placed directly in a 28°C environmental chamber and shaken at 150 rpm. The second set of enrichments was heat treated for 10 min at 80°C before incubation to enhance isolation of spore-forming bacteria.
The cultures were monitored for the presence of microorganisms by microscopy and gram staining and were subcultured (1:50) into fresh MSB medium containing the selected PAH when growth was detected. After three to four subcultures, the cultures were plated onto MSB agar plates containing the same substrate as the enrichment. Piles Creek sediment and S. alterniflora rhizosphere isolates are denoted as PS and PR, respectively. Delaware isolates obtained from the rhizosphere of S. airoides, S. alterniflora, D. spicata, J. gerardi, or P. australis are denoted as SA, Salt, DS, JG, or PA, respectively. In addition, strains isolated using naphthalene are indicated by an N, while phenanthrene- and biphenyl-enriched isolates are denoted by P and B, respectively.
Phenotypic characterizations.
Phase-contrast microscopy (×1,000 magnification) was used for the morphologic examination of bacterial cells. The strains to be tested were inoculated into 2 ml of TS broth and incubated on a rotary wheel at 37°C. The cells were examined microscopically for motility and morphology at 24, 48, and 72 h and were examined again microscopically after Gram staining.
FAME analysis.
Whole-cell fatty acid analyses were performed on all of the PAH-degrading isolates by growing the cells at 28°C for 24 h on TSA plates. Cellular fatty acids were saponified, methylated, extracted, and analyzed by gas chromatography following the procedures given for the Sherlock Microbial Identification System (MIDI, Inc., Newark, Del.). Identification and comparison were made to the Aerobe (TSBA version 3.9) database of the Sherlock Microbial Identification System. The Dendrogram program of the MIDI software package was used to produce unweighted-pair matchings based on fatty acid compositions.
DNA preparation.
Bacterial strains were grown on either TSA or MSB agar plates supplemented with naphthalene, phenanthrene, or biphenyl. Total genomic DNA was extracted as described by Wilson (48), with the exception of pretreatment with 1.5 mg of lysozyme for 2 h. With the exception of plasmid pDTG112, plasmid DNA was obtained using the QIAprep Spin Miniprep Kit (Qiagen Inc., Santa Clarita, Calif.) according to the manufacturer's directions. For plasmid pDTG112, the plasmid DNA was purified from the total DNA using equilibrium centrifugation in a CsCl-ethidium bromide gradient as described by Sambrook et al. (38). Restriction enzyme digests were performed on the isolated plasmids, and the desired fragments were purified using the Qiaquick gel extraction kit (Qiagen).
Restriction fragment length polymorphism (RFLP).
Total genomic restriction enzyme digests were performed on all of the PAH-degrading isolates using either the BamHI, EcoRI, HindIII, or NotI enzyme as recommended by the supplier (Gibco-BRL, Madison, Wis.). Approximately 2 to 3 μg of each isolate was digested. Electrophoretic analysis of the digested DNA was done on a 0.8% agarose gel at 25 V for 16 to 20 h. The restriction enzyme digest patterns were visualized by UV light following ethidium bromide staining.
16S rDNA analysis.
Sequence analyses of 16S ribosomal DNA (rDNA) were performed on several of the isolates by amplifying the 16S rRNA genes by PCR using the eubacterial primers 27f and 1522r (24). The PCR mixtures (100 μl) contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, 50 pmol of each primer, 2.5 U of Taq DNA polymerase (Gibco-BRL, Grand Island, N.Y.), and ∼10 ng of DNA template. PCR amplification was performed using a Perkin-Elmer GeneAmp PCR System 2400 thermocycler (Applied Biosystems, Foster City, Calif.) programmed as follows: 1 min of denaturation at 94°C; followed by 25 cycles of 96°C for 1 min, 55°C for 1 min, and 72°C for 1 min; with a final extension at 72°C for 10 min. The amplified 1.5-kb PCR products were excised from a 1% agarose gel and purified using the QIAquick gel extraction kit (Qiagen) according to the manufacturer's instructions.
Partial DNA sequences using the primers 27f and 685r (24) were determined directly from the purified PCR products with automated fluorescent Taq cycle sequencing using the ABI 373A Sequencer (Applied Biosystems).
Partial 16S rRNA sequences (ranging from 301 to 1,500 bp) of each isolate were analyzed using the Ribosomal Database Project Sequence Match and Similarity Matrix programs (31) to obtain the most closely matched species.
Southern hybridization using PAH gene probes.
Southern blots were performed on all unique isolates, as determined by RFLP, using gene probes for four different types of PAH dioxygenases. This was accomplished by digesting 2 to 3 μg of genomic DNA from each of the tested isolates with EcoRI according to the manufacturers' directions. In addition, genomic DNA obtained from P. putida NCIB 9816-4, C. testosteroni GZ42, C. testosteroni GZ39, S. yanoikuyae B1, and Mycobacterium sp. strain PY01 was digested and used as positive and negative controls for each hybridization. The digests were run on 0.8% agarose gels in 40 mM Tris–20 mM acetate–2 mM EDTA buffer at 25 V for 16 to 20 h, stained with ethidium bromide, and visualized under UV light prior to transferring onto positively charged nylon membranes (Boehringer Mannheim, Indianapolis, Ind.) using a VacuGene XL Vacuum Blotting System (Pharmacia Biotech, Piscataway, N.J.) The transferred DNA was fixed to the nylon membranes by exposure to a UV cross-linker (Spectronics Corp., Westbury, N.Y.) using a 2× optimal cross-link (120 mJ cm−2). The Southern hybridization protocol was performed using nonradioactive digoxigenin (Boehringer Mannheim) as recommended by the supplier. Gene probes were prepared from the PAH dioxygenase gene fragments by a nonradioactive random primed DNA-labeling method with alkali-labile digoxigenin 11–dUTP. Prehybridization and hybridization were performed at 37°C. Following hybridization, the nylon membrane was washed under low-stringency conditions (0.1× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate] and 0.1% sodium dodecyl sulfate) at room temperature.
Pure culture transformation of PAHs in liquid medium.
Several of the isolates were analyzed for their ability to utilize a variety of aromatic compounds, including naphthalene, phenanthrene, fluorene, pyrene, and biphenyl, individually and as a mixture, in MSB medium. The isolates tested included the gram-negative pseudomonads PR-N7, PR-N9, PR-N10, and PS-P2; the gram-positive non-spore formers PR-N15 and PR-P3; and the spore former PR-P1. Each isolate was tested in duplicate for each substrate or substrates over a time course of 11 days. Cells were grown in 100 ml of MSB containing 5 mM succinate, pelleted by centrifugation (4,000 × g, 10 min), washed with MSB, pelleted again, and suspended in fresh MSB to give a cell density of approximately 107 cells ml−1 for use in transformation experiments. Sterile 7-ml serum vials containing the appropriate carbon source at a final concentration of 100 μM were added from hexane stock solutions, and the material was allowed to evaporate prior to the addition of 2 ml of washed cell suspension. In addition, two controls were added to the experimental design and included a no-cell control (MSB medium only) and a dead-cell control (MSB + 4% formaldehyde + cells). The samples were incubated at 28°C on a rotary shaker at 90 rpm, and the amount of substrate remaining was analyzed over time. At each time point two vials were taken, were extracted with 1 ml of hexane, and were analyzed for the appropriate aromatic substrate using a Hewlett-Packard 5890 Series II Gas Chromatograph using an HP-5MS column and equipped with a 5971 series mass selective detector. Prior to extraction, 20 μM 2-methylnaphthalene was added from a hexane stock solution as an internal standard. Results are reported as the percentage remaining relative to uninoculated controls after 11 days of incubation.
Microbial-sediment slurry biotransformation experiment.
An aerobic slurry system was designed to determine the rate and extent of microbial transformation of contaminated sediment. The rhizosphere-associated phenanthrene-degrading strains PR-P1 and PR-P3 were grown to mid-log phase in TS broth and were harvested by centrifugation at 4,000 × g for 10 min. The cells were washed and pelleted by centrifugation twice using 1/10 salts (8) as the diluent, diluted into 1/10 salts, and inoculated at three different cell concentrations into a PAH-spiked sediment slurry.
Approximately equal volumes (500 ml) of estuarine sediment material, obtained from Newtown Creek, and water were mixed under forced aeration for 1 week prior to bacterial inoculation of slurry microcosms. The microcosms consisted of 50-ml glass serum bottles containing 5 ml of aerated slurry spiked with a mixture of PAHs (naphthalene, fluorene, phenanthrene, and pyrene) and biphenyl from an acetone stock at a concentration of approximately 50 ppm of each. Each bacterial isolate was added to the spiked slurry at 3 inoculum densities, 105, 107, or 109 cells ml−1. Two controls were used consisting of an uninoculated nonsterile slurry control and a sterile (autoclaved three times over 3 days) slurry control. All the bottles were sealed with Teflon stoppers pierced with an 18-gauge cotton-plugged needle, and the cultures were incubated in a 30°C environmental chamber at 90 rpm. The loss of PAHs and biphenyl was analyzed over time.
Entire microcosms were sacrificed in triplicate and extracted for PAH analyses at nine time points over a 56-day period (7-day sampling interval). Twenty parts per million of 2-methylnaphthalene was added as an internal standard, and the content of the entire vial was extracted overnight with 4 ml of hexane and 2 ml of acetone by shaking on a wrist-action shaker at 200 rpm. The samples were centrifuged for 5 min at 300 × g, and the organic phase was removed. The slurry was further extracted using 2 ml of hexane for 4 h by shaking at 200 rpm. The organic phases were combined and analyzed for PAH and biphenyl content by gas chromatography, as described above. Statistical analyses were performed using the Minitab Software Package Release 11.21.
Nucleotide sequence accession numbers.
The 16S rRNA sequences for the PAH-degrading isolates have been deposited in GenBank under accession numbers AF353676 to AF353705.
RESULTS
Isolation of PAH-degrading bacterial strains.
The PAH-degrading strains isolated in this study are listed in Table 1. A total of 42 isolates were obtained from S. alterniflora plant and sediment samples collected from contaminated Piles Creek in New Jersey, using either naphthalene, phenanthrene, or biphenyl as the sole source of carbon. A total of nine isolates were obtained from the four different plant rhizospheres collected from Lewes, Del. However, no PAH-degrading isolates were obtained from the rhizosphere of P. australis from this site. The lower number and diversity of bacteria isolated from this site may be expected due to its uncontaminated nature. Heat treatment prior to enrichment and isolation was included for each of the substrates tested, which resulted in isolation of several spore-forming bacteria. The isolates were characterized on the basis of their morphologic and phenotypic properties and by using a combination of molecular techniques.
TABLE 1.
PAH-degrading isolates obtained from contaminated estuarine sediment and salt marsh plant rhizosphere and comparison of bacterial identification using FAME and partial 16S rRNA gene sequences of several of the strains
| Isolatea | Source | PAHb | FAME analysisc
|
Partial 16S rRNA gene sequenced
|
||
|---|---|---|---|---|---|---|
| Best match | SI | Best match | % Similarity | |||
| DS-N1 | D. spicata | N | Brevibacillus brevis | 0.45 | P. validus | 99.6 |
| DS-N2 | D. spicata | N | P. validuse | 0.92 | P. validus | 96.7 |
| DS-N3 | D. spicata | N | P. validus | 0.81 | P. validus | 97.9 |
| JG-N1 | J. gerardi | N | P. validus | 0.80 | NDf | |
| JG-N2 | J. gerardi | N | P. validus | 0.81 | P. validus | 96.0 |
| JG-N3 | J. gerardi | N | P. validus | 0.87 | ND | |
| SA-N1 | S. airoides | N | Paenibacillus sp. | Poor | P. validus | 96.0 |
| Salt-N1 | S. alterniflora | N | Pseudomonas fluorescens | 0.59 | ND | |
| Salt-N2 | S. alterniflora | N | P. putida | 0.80 | ND | |
| PR-N1 | S. alterniflora | N | Paenibacillus sp. | Poor | P. validus | 97.3 |
| PR-N2 | S. alterniflora | N | Paenibacillus sp. | Poor | ND | |
| PR-N3 | S. alterniflora | N | P. validus | 0.87 | ND | |
| PR-N4 | S. alterniflora | N | P. validus | 0.86 | P. validus | 98.0 |
| PR-N5 | S. alterniflora | N | Paenibacillus sp. | Poor | P. validus | 96.9 |
| PR-N6 | S. alterniflora | N | Pseudomonas chlororaphis | 0.85 | ND | |
| PR-N7 | S. alterniflora | N | P. stutzeri | 0.88 | P. stutzeri | 99.0 |
| PR-N8 | S. alterniflora | N | P. putida | 0.87 | ND | |
| PR-N9 | S. alterniflora | N | P. putida | 0.87 | P. putida | 99.0 |
| PR-N10 | S. alterniflora | N | P. putida | 0.82 | P. putida | 98.0 |
| PR-N11 | S. alterniflora | N | Paenibacillus sp. | Poor | ND | |
| PR-N12 | S. alterniflora | N | Paenibacillus sp. | Poor | ND | |
| PR-N13 | S. alterniflora | N | P. validus | 0.81 | P. validus | 98.2 |
| PR-N14 | S. alterniflora | N | N. asteroides | 0.77 | Rhodococcus ruber | 97.1 |
| PR-N15 | S. alterniflora | N | N. brasiliensis | 0.21 | Tsukamurella wratislaviensis | 99.8 |
| PR-N16 | S. alterniflora | N | Paenibacillus sp. | Poor | P. validus | 97.3 |
| PR-N17 | S. alterniflora | N | N. asteroides | 0.57 | ND | |
| PR-N18 | S. alterniflora | N | Nocardia sp. | 0.14 | ND | |
| PR-N19 | S. alterniflora | N | P. validus | 0.93 | P. validus | 97.3 |
| PR-N20 | S. alterniflora | N | P. validus | 0.84 | P. validus | 98.0 |
| PR-N21 | S. alterniflora | N | P. validus | 0.75 | P. validus | 98.9 |
| PR-N22 | S. alterniflora | N | P. validus | 0.81 | P. validus | 98.5 |
| PR-P1 | S. alterniflora | P | P. validus | 0.85 | P. validus | 98.5 |
| PR-P2 | S. alterniflora | P | P. validus | 0.90 | ND | |
| PR-P3 | S. alterniflora | P | A. oxydans | 0.46 | A. oxydans | 95.4 |
| PR-P6 | S. alterniflora | P | P. validus | 0.88 | ND | |
| PR-P9 | S. alterniflora | P | P. validus | 0.91 | P. validus | 99.5 |
| PR-P10 | S. alterniflora | P | P. validus | 0.91 | P. validus | 99.8 |
| PR-P11 | S. alterniflora | P | P. validus | 0.70 | P. validus | 99.5 |
| PR-P12 | S. alterniflora | P | F. resinovorum | 0.79 | S. subarctica | 99.3 |
| PR-P13 | S. alterniflora | P | P. validus | 0.91 | P. validus | 97.9 |
| PR-B1 | S. alterniflora | B | P. validus | 0.87 | ND | |
| PR-B2 | S. alterniflora | B | B. brevis | 0.34 | P. validus | 98.4 |
| PS-N1 | Sediment | N | P. pabuli | 0.26 | P. validus | 97.1 |
| PS-N2 | Sediment | N | P. fluorescens | 0.47 | ND | |
| PS-N3 | Sediment | N | N. asteroides | 0.71 | ND | |
| PS-N4 | Sediment | N | P. stutzeri | 0.89 | ND | |
| PS-N5 | Sediment | N | P. stutzeri | 0.86 | ND | |
| PS-N6 | Sediment | N | P. validus | 0.33 | P. validus | 96.4 |
| PS-P1 | Sediment | P | Nocardioform | NDg | ND | |
| PS-P2a | Sediment | P | Alcaligenes xylosoxidans | 0.65 | Alcaligenes faecalis | 98.4 |
| PS-P2b | Sediment | P | A. xylosoxidans | 0.56 | ND | |
DS, JG, SA, and salt isolates obtained from plants collected from Lewes, Del. PR and PS designates isolates obtained at Piles Creek, N.J.
PAH carbon source in enrichment culture. N, naphthalene; P, phenanthrene; and B, biphenyl.
FAME identification was performed using the aerobic (TSBA) library version 3.9 of the Microbial Identification System (MIDI, Inc.). SI values below 0.2 are listed as poor, and the match is given only to the genus.
16S rRNA genes were amplified by PCR using universal primers. The PCR products were partially sequenced (∼500 bp, ranging from 292 to 1,488 bp) and were compared to the Ribosomal Database Project database to obtain the most closely matched species.
P. validus listed as the invalid synonym P. gordonae in MIDI library.
ND, not determined.
Identification based on presence of fatty acid 10-methyl 18:0 (TSBA).
FAME analysis.
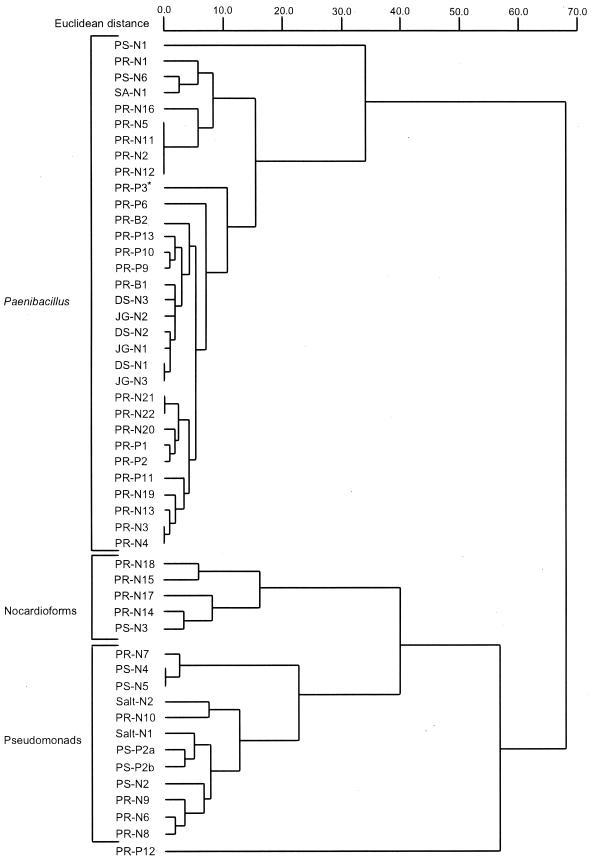
The tentative identification and similarity index (SI) of all of the isolates using the aerobic (TSBA) library version 3.9 of the Microbial Identification System is shown in Table 1. A pairwise-comparison Euclidean-distance dendrogram based on whole-cell fatty acid compositions of the PAH-degrading isolates (except isolate PS-P1) was constructed, revealing that the isolates fall within three main groups (Fig. 1). Group 1 included the endospore-forming Paenibacillus, group 2 included the gram-positive nocardioforms, and group 3 included the gram-negative pseudomonads. One of the isolates, PR-P12, falling outside these groups was tentatively identified as Flavobacterium resinovorum (SI = 0.79) (Table 1). Isolate PR-P3 clustered within the Paenibacillus group but had the closest match to Arthrobacter oxydans (SI = 0.46). In addition, one of the isolates, PS-P1, was not identified on several attempts by the MIDI Microbial Identification System but was considered a member of the nocardioform group based on the presence of 10-methyl 18:0 fatty acid (tuberculostearic acid).
FIG. 1.
Euclidean-distance dendrogram based on the whole-cell fatty acid compositions of the sediment and rhizosphere PAH-degrading isolates. Sediment and rhizosphere isolates are denoted as PS and PR, respectively. In addition, strains isolated using naphthalene are indicated by an N, while phenanthrene- and biphenyl-enriched isolates are denoted by a P and B, respectively. ∗, isolate PR-P3 is an Arthrobacter sp.
The dendrogram (Fig. 1) also shows subclusters within each of these groups. In the spore-forming Paenibacillus group there were two subgroups. The members of the smaller subgroup, consisting of isolates PS-N1, PR-N16, PR-N5, PR-N11, PR-N2, PR-N12, PS-N6, PR-N1, and SA-N1, all exhibited a mucoid colony morphology when grown on solid media and had a low SI to either Paenibacillus alvei, Paenibacillus pabuli, or Paenibacillus validus (Table 1). In contrast, the larger subgroup within the Paenibacillus cluster exhibited a nonmucoid, mottled colony morphology when grown on solid media, and with the exception of PR-B2 and DS-N1, had a high SI to P. validus (Table 1). The five isolates found within the nocardioform group also formed two subgroups. Isolates PR-N18 and PR-N15 both had a cream-colored, waxy colony morphology and were identified, with low SIs, as Nocardia asteroides and Nocardia brasiliensis, respectively (Table 1). The remaining three nocardioforms had an orange, waxy colony morphology and were identified with a high SI as N. asteroides (Table 1). In the pseudomonad group, the three isolates PR-N7, PS-N4, and PS-N5 were identified with a high match to Pseudomonas stutzeri (Table 1). These three isolates cluster closely together in relation to the remaining pseudomonad isolates. Of the P. stutzeri strains, PS-N5 and PR-N7 exhibit a small, crinkled, tan colony morphology, while PS-N4 exhibits a small, tan colony morphology. The crinkled, tan morphology can give rise to noncrinkled colonies resembling PS-N4. This dual colony morphology is commonly seen in P. stutzeri (Norberto Palleroni, personal communication).
Genomic restriction enzyme digests.
RFLP analysis of total genomic DNA digested with BamHI, EcoRI, HindIII, and NotI enzymes was used as a screening tool to determine unique isolates. A large number of gels were performed encompassing all of the cultured isolates. The gels were examined visually, and an isolate was considered unique based on the presence of a unique banding pattern. Isolates which shared a banding pattern and had similar FAME profiles were grouped together, and a single isolate from the group was chosen as a representative strain for sequencing and Southern hybridization experiments. The RFLP data are summarized below. All of the six nocardioform isolates were found to be unique. Of the 12 original pseudomonad isolates, 10 were found to have different restriction enzyme banding patterns. Shared RFLP patterns were seen between the pseudomonad isolates PS-N4 and PS-N5 and between PS-P2a and PS-P2b. The large Paenibacillus group, containing 31 isolates, was found to have 19 isolates exhibiting unique RFLP patterns. Shared banding patterns were seen between the mucoid colony morphology Paenibacillus isolates PR-N5, PR-N11, PR-N2, and PR-N12. In addition, shared banding patterns were seen between the nonmucoid colony morphology Paenibacillus isolates PR-B2 and PR-B1; JG-N1, DS-N1, and JG-N3; PR-N19 and PR-N20; PR-N21 and PR-N22; PR-P1, PR-P2, and PR-P6; and PR-N13, PR-N3, and PR-N4.
16S rRNA gene analyses.
Several of the unique isolates from each of the three major groups were further identified by partial sequencing of their 16S rRNA gene. In general, the 16S rDNA sequence analysis confirmed the identification based on fatty acid profiles. Within the Paenibacillus group the isolates identified as P. validus by FAME also had a high sequence similarity to P. validus. The smaller subgroup of isolates having a low SI to P. alvei, P. pabuli, or P. validus by FAME had a low similarity to P. validus based on their partial 16S rRNA gene sequence (Table 1). Strain PR-P3, which was an outlier within the Paenibacillus group, was identified with the closest match by both FAME and 16S rDNA analysis to A. oxydans. The norcardioform isolates PR-N14 and PR-N15 were identified as belonging to the genera Rhodococcus and Tsukamurella, respectively. The identification of the Pseudomonas isolates as P. putida and P. stutzeri by FAME was confirmed by 16S rDNA phylogenetic analysis. The outlying isolate PR-P12 was identified with a high 16S rDNA sequence similarity to Sphingomonas subarctica.
Southern hybridization with PAH gene probes.
All of the unique isolates, as determined by RFLP, were tested for the presence of previously described genes for PAH dioxygenases. A large number of Southern hybridizations were performed, with much of the data revealing no hybridization results. Therefore, we present the data in text form with one figure exemplifying the nah gene hybridization results for isolates representing the three major bacterial groups (pseudomonads, nocardiofoms, and Paenibacillus).
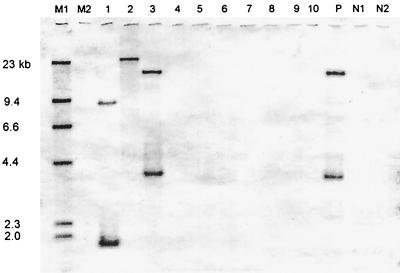
All of the unique pseudomonad isolates with the exception of PS-P2 hybridized to the classical nah genes from P. putida NCIB 9816-4. The unique pseudomonad isolates PR-N10, PS-N2, PR-N6, Salt-N1, and Salt-N2 gave the same pattern of hybridization as the control strain P. putida NCIB 9816-4 (Fig. 2). This double-banding pattern was a result of an EcoRI digestion of the genomic DNA. The positive bands were at 18.5 and 3.7 kb. Pseudomonad isolate PR-N7 also gave a double-banding pattern to the nah gene probe; however, the bands were at 9.0 and 1.9 kb. In addition, all of the pseudomonad isolates, except for PS-P2 and PS-N5, hybridized to the nag genes cloned from C. testosteroni GZ42. Of the pseudomonad isolates hybridizing to the nag gene, six isolates (Salt-N1, Salt-N2, PR-N6, PS-N2, PR-N10, and PR-N7) gave the same single-band pattern, while the remaining two isolates (PR-N8 and PR-N9) gave a different single-band pattern distinguishable from the other six isolates. However, the nocardioforms, Paenibacillus isolates, Arthrobacter isolate PR-P3, or the sphingomonad isolate PR-P12 did not hybridize to either of these PAH dioxygenase genes (Fig. 2). None of the tested PAH-degrading isolates hybridized to the gene probe from S. yanoikuyae B1, and only norcardioform isolates PR-N18 and PS-P1 hybridized to the PAH oxygenase gene cloned from Mycobacterium sp. strain PY01.
FIG. 2.
Southern hybridization of selected isolates with the nah genes for naphthalene degradation from P. putida NCIB 9816-4. EcoRI-digested genomic DNA was probed with a 3.5-kb SalI fragment from P. putida NCIB 9816-4 containing the genes for naphthalene dioxygenase. M1 and M2 are the digoxigenin-labeled and high-molecular-weight markers, respectively. Lanes 1 to 4 are the pseudomonad isolates PR-N7, PR-N9, PR-N10, and PS-P2; lane 5 is the sphingomonad isolate PR-P12; lanes 6 and 7 are the nocardioform isolates PR-N14 and PR-N15; lane 8 is Arthrobacter sp. strain PR-P3; and lanes 9 and 10 represent the mucoid and nonmucoid Paenibacillus isolates PR-N5 and PR-P1, respectively. P is the positive control P. putida NCIB 9816-4, and N1 and N2 are the negative controls C. testosteroni GZ39 and S. yanoikuyae B1, respectively.
Transformation studies using pure cultures.
Isolates representing each group (pseudomonad, nocardioform, and Paenibacillus) and the Arthrobacter isolate PR-P3 were tested for the ability to degrade PAHs in cell suspensions. The isolates were tested for the ability to utilize PAHs individually or as a mixture containing naphthalene, fluorene, phenanthrene, pyrene, and biphenyl. Table 2 lists the amount (percent) of PAH remaining after 11 days of incubation. Results from cultures containing a mixture of PAHs were similar to those of cultures containing a single PAH (data not shown). None of the tested isolates was able to utilize pyrene in liquid suspension within the 11-day incubation time; however, all of the isolates were able to utilize naphthalene completely or partially (PS-P2 and PR-N10). In general, the strains isolated on phenanthrene (PS-P2, PR-P3, and PR-P1) were able to utilize a greater number of PAHs than the strains isolated on naphthalene (Table 2).
TABLE 2.
Abilities of several isolates to utilize a variety of PAHs
| Isolatea | % PAH remainingb
|
||||
|---|---|---|---|---|---|
| Naphthalene | Biphenyl | Fluorene | Phenanthrene | Pyrene | |
| Pseudomonads | |||||
| PR-N7 | 0 | 85 | 100 | 85 | 90 |
| PR-N9 | 0 | 69 | 78 | 71 | 90 |
| PR-N10 | 40 | 90 | 97 | 89 | 100 |
| PS-P2 | 44 | 51 | 79 | 28 | 100 |
| Gram-positive non- spore formers | |||||
| PR-N15 | 0 | 84 | 80 | 68 | 100 |
| PR-P3 | 0 | 84 | 42 | 1 | 100 |
| Spore former | |||||
| PR-P1 | 0 | 0 | 29 | 8 | 100 |
Substrate utilization experiments were performed using dense-cell suspensions in MSB medium containing the appropriate PAH at a final concentration of 100 μM. Samples were incubated at 28°C at 90 rpm, were extracted with hexane, and were analyzed by gas chromatography.
Percentage remaining was calculated relative to uninoculated controls after 11 days of incubation.
Sediment slurry transformation studies.
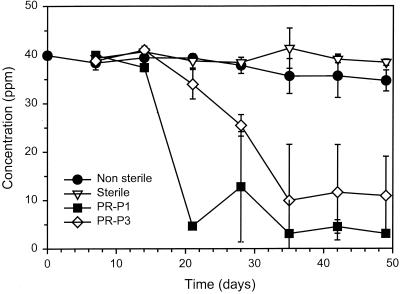
Two gram-positive PAH-degrading isolates, PR-P1 (P. validus) and PR-P3 (A. oxydans), were inoculated at cell densities ranging from 105 to 109 cells ml−1 into an aerobic marine sediment slurry containing a mixture of PAHs (naphthalene, fluorene, phenanthrene, and pyrene) and biphenyl at a concentration of 50 ppm for each substrate. The transformation experiment was designed to follow the disappearance of a mixture of PAHs over time, and the results would determine whether specifically inoculated rhizosphere bacteria could enhance transformation over the indigenous microbial community. Uninoculated and inoculated sediment slurries both showed loss of the two- and three-ringed PAHs within 2 weeks of incubation (data not shown). However, pyrene transformation was only observed in treatments inoculated with strain PR-P1 or PR-P3 at 109 cells ml−1 (Fig. 3). The sterile slurry microcosms showed no loss of pyrene, indicating that pyrene transformation was microbially mediated. In addition, the indigenous bacteria present in the nonsterile slurry microcosms were not responsible for the loss of pyrene seen in the inoculated microcosms. Pyrene transformation was not observed in the microcosms inoculated to a cell density of 105 or 107 cells ml−1. Microcosms inoculated with the spore-forming P. validus strain PR-P1 showed a more rapid loss of pyrene than did microcosms inoculated with the non-spore-forming A. oxydans strain PR-P3 (Fig. 3). A large variation was seen between the triplicate samples analyzed for the inoculated microcosms. A two-sample t test was performed on the pyrene transformation data. The t test indicated that the pyrene concentrations in the PR-P1- and PR-P3-inoculated microcosms were, by day 35, significantly different (95% confidence, P < 0.05) from the sterile and nonsterile control microcosms. By day 50 the PR-P1 and PR-P3 high inoculum density microcosms were found to contain mean concentrations of 3 and 11 ppm of pyrene, respectively (not significantly different from each other).
FIG. 3.
Loss of pyrene in contaminated sediment slurry inoculated with either the Paenibacillus sp. strain PR-P1 or the Arthrobacter sp. strain PR-P3. Data points represent the means of triplicate samples. Error bars indicate the standard deviation. Where no error bar is present, the value is less than the height of the symbol.
DISCUSSION
We present a study of the culturable PAH-degrading bacteria associated with the rhizosphere of several salt marsh plant species in contaminated and uncontaminated estuarine sediments. In addition, a pasteurization method was successful in isolating spore-forming bacteria. Numerous studies have demonstrated the importance of the rhizosphere effect on degradation of organic contaminants. Most of these studies have examined terrestrial plants and agricultural chemicals (1, 2, 27); few have looked at the influence of plant-associated microorganisms on the fate of PCBs (15, 16) and PAHs (34, 39). There have been a limited number of studies on PAH degradation involving wetland or salt marsh ecosystems, but none have studied the diversity of PAH-degrading microorganisms present (28, 30, 49).
Recently, more studies have focused on PAH degradation in marine and estuarine ecosystems (3, 11, 12, 13, 17, 20, 21, 46). No studies have been conducted on the PAH-degrading microorganisms associated with salt marsh plants. In the present study, we isolated a variety of PAH-degrading microorganisms from the rhizosphere of the salt marsh grasses S. alterniflora, J. gerardi, D. spicata, and S. airoides. We found both gram-positive (predominantly nocardioform) and gram-negative (predominantly pseudomonad) bacteria, and a pasteurization technique prior to enrichment resulted in the isolation of spore-forming (exclusively Paenibacillus sp.) bacteria.
The rhizosphere has been reported to be predominantly associated with gram-negative bacteria (7); however, this view has been changing with more studies using molecular tools (5). Indeed, the culturable diversity of PAH-degrading microorganisms in this study would lend evidence to this trend. Many of the bacterial species and genera that we isolated have been reported to degrade PAHs previously. Few reports have been made regarding Paenibacillus and its ability to degrade PAHs. Pichinoty et al. (36) described Bacillus gordonae sp. nov., later to be emended as P. validus (22), which was able to utilize p-hydroxybenzoate, phthalate, isophthalate, protocatechuate, trimellitate, quinate, phenol, p-cresol, and naphthalene. Recently, Meyer et al. (33) reported the isolation of a PAH-degrading tentative Paenibacillus sp. from tar oil-contaminated soil.
We wanted to determine the ability of the rhizosphere and estuarine sediment isolates to degrade a variety of PAHs, individually and in combination, in order to determine their biodegradation and bioremediation potentials. The pure culture transformation experiments showed that phenanthrene-enriched isolates were able to utilize a greater variety of other PAHs than the naphthalene-enriched isolates, although none degraded pyrene in minimal media. The microbial slurries demonstrated a greater transformation activity than did the minimal-medium dense-cell experiments. The greater activity in the sediment slurries indicates that the organic or inorganic constituents or the extant microbial community of the sediment stimulated the activity of the inoculated strains. Siciliano and Germida (41) reported that inoculants required an unknown soil factor to degrade 2-chlorobenzoic acid in the rhizosphere of Dahurian wild rye. It should be noted that both the pure culture and microbial slurry experiments were performed under highly oxygenated conditions and that the limited diffusion of oxygen into organic-rich sediments has been found to restrict PAH biodegradation in the natural environment (10). The anoxic conditions found in most sediments, however, may not be the case in tidal and salt marsh ecosystems where plants provide oxygen to their deeper roots. For example, Spartina is known to pump oxygen into the rhizosphere (23). Indeed, when the plants were collected at low tide, highly oxidized areas were observed around the root system in comparison to the bulk nonrhizosphere sediment. Of specific interest is evaluating the role of oxygen cycling by roots of salt marsh plants in the enhancement of PAH biodegradation by rhizosphere-associated microorganisms.
Many different types of PAH oxygenase genes have been described (for a review, see reference 50). Recent studies using known PAH degradation genes as probes indicate that previously undescribed genes are present (3). In our study, 75% of the pseudomonad isolates hybridized to the classical nah gene from P. putida NCIB 9816-4, and approximately the same number hybridized to the nag genes cloned from C. testosteroni GZ42. The absence of homology of the Paenibacillus isolates to all of the tested gene probes indicates the possibility of novel PAH degradation genes.
Based on our results (morphologic and phenotypic characterization), there is a wide variation between the PAH-degrading isolates, indicating that the rhizosphere of S. alterniflora contains a diverse population of PAH-degrading bacteria. In addition, vegetated salt marsh ecosystems may hold promise as a means of remediation of contaminated coastal environments.
ACKNOWLEDGMENTS
This work was supported by a grant from The State of New Jersey Commission on Science and Technology. G.J.Z. acknowledges the support of the NSF under grant MCB-9723921.
We thank John Gallagher of the University of Delaware Halophyte Biology Laboratory for supplying marsh plants; John Cigolini, Jon Dennis, Lisa Newman, and Michael Murillo for helpful discussions and assistance; and Gregory Flanagan and Quinn Im for technical assistance.
REFERENCES
- 1.Anderson T A, Kruger E L, Coats J R. Enhanced degradation of a mixture of three herbicides in the rhizosphere of a herbicide-tolerant plant. Chemosphere. 1994;28:1551–1557. [Google Scholar]
- 2.Arthur E L, Perkovich B S, Anderson T A, Coats J R. Degradation of an atrazine and metolachlor herbicide mixture in pesticide-contaminated soils from two agrochemical dealerships in Iowa. Water Air Soil Pollut. 2000;119:75–90. [Google Scholar]
- 3.Berardesco G, Dyhrman S, Gallagher E, Shiaris M P. Spatial and temporal variation of phenanthrene-degrading bacteria in intertidal sediments. Appl Environ Microbiol. 1998;64:2560–2565. doi: 10.1128/aem.64.7.2560-2565.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle J J, Shann J R. Biodegradation of phenol, 2,4-DCP, 2,4-D, and 2,4,5-T in field-collected rhizosphere and nonrhizosphere soils. J Environ Qual. 1995;24:782–785. [Google Scholar]
- 5.Cattelan A J, Hartel P G, Fuhrmann J J. Bacterial composition in the rhizosphere of nodulating and non-nodulating soybean. Soil Sci Soc Am J. 1998;62:1549–1555. [Google Scholar]
- 6.Cerniglia C E. Biodegradation of polycyclic aromatic hydrocarbons. Curr Opin Biotechnol. 1993;4:331–338. [Google Scholar]
- 7.Curl E A, Truelove B. The rhizosphere. Berlin, Germany: Springer-Verlag; 1986. [Google Scholar]
- 8.Daane L L, Molina J A E, Berry E C, Sadowsky M J. Influence of earthworm activity on gene transfer from Pseudomonas fluorescens to indigenous soil bacteria. Appl Environ Microbiol. 1996;62:515–521. doi: 10.1128/aem.62.2.515-521.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies J I, Evans W C. Oxidative metabolism of naphthalene by soil pseudomonads. Biochem J. 1964;91:251–261. doi: 10.1042/bj0910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLaune R D, Patrick W H, Casselman M E. Effect of sediment pH and redox conditions on degradation of benzo(a)pyrene. Mar Pollut Bull. 1981;12:251–253. [Google Scholar]
- 11.Dyksterhouse S E, Gray J P, Herwig R P, Lara J C, Staley J T. Cycloclasticus pugetii gen. nov., sp. nov., an aromatic hydrocarbon-degrading bacterium from marine sediments. Int J Syst Bacteriol. 1995;45:116–123. doi: 10.1099/00207713-45-1-116. [DOI] [PubMed] [Google Scholar]
- 12.Geiselbrecht A D, Hedlund B P, Tichi M A, Staley J T. Isolation of marine polycylic aromatic hydrocarbon (PAH)-degrading Cycloclasticus strains from the Gulf of Mexico and composition of their PAH degradation ability with that of Puget Sound Cycloclasticus strains. Appl Environ Microbiol. 1998;64:4703–4710. doi: 10.1128/aem.64.12.4703-4710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geiselbrecht A D, Herwig R P, Deming J W, Staley J T. Enumeration and phylogenetic analysis of polycyclic aromatic hydrocarbon-degrading marine bacteria from Puget Sound sediments. Appl Environ Microbiol. 1996;62:3344–3349. doi: 10.1128/aem.62.9.3344-3349.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson D T, Roberts R L, Wells M C, Kobal V M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973;50:211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert E S, Crowley D E. Plant compounds that induce polychlorinated biphenyl biodegradation by Arthrobacter sp. strain B1B. Appl Environ Microbiol. 1997;63:1933–1938. doi: 10.1128/aem.63.5.1933-1938.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert E S, Crowley D E. Repeated application of carvone-induced bacteria to enhance biodegradation of polychlorinated biphenyls in soil. Appl Microbiol Biotechnol. 1998;50:489–494. doi: 10.1007/s002530051325. [DOI] [PubMed] [Google Scholar]
- 17.Gilewicz M, Ni'matuzahroh, Nadalig T, Budzinski H, Doumenq P, Michotey V, Bertrand J C. Isolation and characterization of a marine bacterium capable of utilizing 2-methylphenanthrene. Appl Microbiol Biotechnol. 1997;48:528–533. doi: 10.1007/s002530051091. [DOI] [PubMed] [Google Scholar]
- 18.Goyal A K, Zylstra G J. Molecular cloning of novel genes for polycyclic aromatic hydrocarbon degradation from Comamonas testosteroni GZ39. Appl Environ Microbiol. 1996;62:230–236. doi: 10.1128/aem.62.1.230-236.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal A K, Zylstra G J. Genetics of naphthalene and phenanthrene degradation by Comamonas testosteroni. J Ind Microbiol Biotechnol. 1997;19:401–407. doi: 10.1038/sj.jim.2900476. [DOI] [PubMed] [Google Scholar]
- 20.Guerin W F, Jones G E. Mineralization of phenanthrene by a Mycobacterium sp. Appl Environ Microbiol. 1988;54:937–944. doi: 10.1128/aem.54.4.937-944.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedlund B P, Geiselbrecht A D, Bair T J, Staley J T. Polycyclic aromatic hydrocarbon degradation by a new marine bacterium, Neptunomonas naphthovorans gen. nov., sp. nov. Appl Environ Microbiol. 1999;65:251–259. doi: 10.1128/aem.65.1.251-259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heyndrickx M, Vandemeulebroecke K, Scheldeman P, Hoste B, Kersters K, De Vos P, Logan N A, Aziz A M, Ali N, Berkeley R C W. Paenibacillus (formerly Bacillus) gordonae (Pichinoty et al. 1986) Ash et al. 1994 is a later subjective synonym of Paenibacillus (formerly Bacillus) validus (Nakamura 1984) Ash et al. 1994: emended description of P. validus. Int J Syst Bacteriol. 1995;45:661–669. doi: 10.1099/00207713-45-4-661. [DOI] [PubMed] [Google Scholar]
- 23.Howes B L, Teal J M. Oxygen loss from and its relationship to salt marsh oxygen balance. Oecologia. 1994;97:431–438. doi: 10.1007/BF00325879. [DOI] [PubMed] [Google Scholar]
- 24.Johnson J L. Similarity analysis of rRNAs. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. pp. 683–700. [Google Scholar]
- 25.Kadlec R, Knight R L. Treatment wetlands. Boca Raton, Fla: Lewis Publishers; 1995. [Google Scholar]
- 26.Kim E, Zylstra G J. Molecular and biochemical characterization of two meta-cleavage dioxygenases involved in biphenyl and m-xylene degradation by Beijerinckia sp. strain B1. J Bacteriol. 1995;177:3095–3103. doi: 10.1128/jb.177.11.3095-3103.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lappin H M, Greaves M P, Slater J H. Degradation of the herbicide mecoprop [2-(2-methyl-4-chlorphenoxy)propionic acid] by a synergistic microbial community. Appl Environ Microbiol. 1985;49:429–433. doi: 10.1128/aem.49.2.429-433.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Q, Mendelssohn I A, Henry C B, Roberts P O, Walsh M M, Overton E B, Portier R J. Effects of bioremediation agents on oil degradation in mineral and sandy salt marsh sediments. Environ Technol. 1999;20:825–837. [Google Scholar]
- 29.Lotufo G R. Bioaccumulation of sediment-associated fluoranthene in benthic copepods: uptake, elimination and biotransformation. Aquat Toxicol. 1998;44:1–15. [Google Scholar]
- 30.Machate T, Noll H, Behrens H, Kettrup A. Degradation of phenanthrene and hydraulic characteristics in a constructed wetland. Water Res. 1997;31:554–560. [Google Scholar]
- 31.Maidak B L, Cole J R, Lilburn T G, Parker C T, Jr, Saxman P R, Stredwick J M, Garrity G M, Li B, Olsen G J, Pramanik S, Schmidt T M, Tiedje J M. The RDP (Ribosomal Database Project) continues. Nucleic Acids Res. 2000;28:173–174. doi: 10.1093/nar/28.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menzie C A, Potocki B B, Santodonato J. Exposure to carcinogenic PAHs in the environment. Environ Sci Technol. 1992;26:1278–1284. [Google Scholar]
- 33.Meyer S, Mose R, Neef A, Stahl U, Kämpfer P. Differential detection of key enzymes of polyaromatic-hydrocarbon-degrading bacteria using PCR and gene probes. Microbiology. 1999;145:1731–1741. doi: 10.1099/13500872-145-7-1731. [DOI] [PubMed] [Google Scholar]
- 34.Nichols T D, Wolf D C, Rogers H B, Beyrouty C A, Reynolds C M. Rhizosphere microbial populations in contaminated soils. Water Air Soil Pollut. 1997;95:165–178. [Google Scholar]
- 35.Paul E A, Clark F E. Soil microbiology and biochemistry. New York, N.Y: Academic Press; 1989. [Google Scholar]
- 36.Pichinoty F, Waterbury J B, Mandel M, Asselineau J. Bacillus gordonae sp. nov., une nouvelle espace appartenant au second groupe morphologique, dégrandant divers composés aromatiques. Ann Inst Pasteur/Microbiol (Paris) 1986;137A:65–78. doi: 10.1016/s0769-2609(86)80006-0. [DOI] [PubMed] [Google Scholar]
- 37.Reilley K A, Banks M K, Schwab A P. Dissipation of polycyclic aromatic hydrocarbons in the rhizosphere. J Environ Qual. 1996;25:212–219. [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Schwab A P, Banks M K. Biologically mediated dissipation of polyaromatic hydrocarbons in the root zone. In: Anderson T A, Coats J R, editors. Bioremediation through rhizosphere technology. Washington, D.C.: American Chemical Society; 1994. pp. 132–141. [Google Scholar]
- 40.Serdar C M, Gibson D T. Studies of nucleotide sequence homology between naphthalene-utilizing strains of bacteria. Biochem Biophys Res Commun. 1989;164:772–779. doi: 10.1016/0006-291x(89)91526-x. [DOI] [PubMed] [Google Scholar]
- 41.Siciliano S D, Germida J J. Enhanced phytoremediation of chlorobenzoates in rhizosphere soil. Soil Biol Biochem. 1999;31:299–305. [Google Scholar]
- 42.Simon M J, Osslund T D, Saunders R, Ensley B D, Suggs S, Harcourt A, Suen W-C, Cruden D L, Gibson D T, Zylstra G J. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB 9816-4. Gene. 1993;127:31–37. doi: 10.1016/0378-1119(93)90613-8. [DOI] [PubMed] [Google Scholar]
- 43.Stanier R Y, Palleroni N J, Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966;43:159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland J B, Rafii F, Khan A A, Cerniglia C E. Mechanisms of polycyclic aromatic dydrocarbon degradation. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: John Wiley and Sons, Inc.; 1995. pp. 269–306. [Google Scholar]
- 45.Walton B T, Anderson T A. Microbial degradation of trichloroethylene in the rhizosphere: potential application to biological remediation of waste sites. Appl Environ Microbiol. 1990;56:1012–1016. doi: 10.1128/aem.56.4.1012-1016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West P A, Okpokwasili G C, Brayton P R, Grimes D J, Colwell R R. Numerical taxonomy of phenanthrene-degrading bacteria isolated from the Chesapeake Bay. Appl Environ Microbiol. 1984;48:988–993. doi: 10.1128/aem.48.5.988-993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wetzel S C, Banks M K, Schwab A P. Rhizosphere effects on the degradation of pyrene and anthracene in soil. In: Kruger E L, Anderson T A, Coats J R, editors. Phytoremediation of soil and water contaminants. Washington, D.C.: American Chemical Society; 1996. pp. 254–262. [Google Scholar]
- 48.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons; 1994. pp. 2.4.1–2.4.5. [DOI] [PubMed] [Google Scholar]
- 49.Wright A L, Weaver R W, Webb J W. Oil bioremediation in salt marsh mesocosms as influenced by N and P fertilization, flooding, and season. Water Air Soil Pollut. 1997;95:179–191. [Google Scholar]
- 50.Zylstra G J, Kim E, Goyal A. Comparative molecular analysis of genes for polycyclic aromatic hydrocarbon degradation. Genet Eng. 1997;19:257–269. doi: 10.1007/978-1-4615-5925-2_14. [DOI] [PubMed] [Google Scholar]