Abstract
Bladder cancer is the most common malignancy of the urinary tract and arising from the epithelial lining of the urinary bladder. Resistance to cytotoxic therapies is associated with overexpression of oncogenic proteins; including HER2, and Akt in chemotherapy resistance of bladder cancer. Various studies demonstrated that curcuminoids, the most important active phenolic compounds of turmeric (Curcuma longa), have anti‐tumor activities in a wide range of human malignant cell lines. The aim of this study is to evaluate whether curcuminoids (curcumin, demethoxycurcumin (DMC), and bisdemethoxycurcumin) could repress the expression of HER2 in HER2‐overexpressing bladder cancer cells. Among the test compounds, DMC significantly suppressed the expression of HER2, and preferentially inhibited cell proliferation and induced apoptosis in HER2‐overexpressing bladder cancer cells. DMC decreases HER2 level through inhibiting the interaction of HER2 and Hsp90. Our study also indicated that DMC showed additive activity in combination with chemotherapeutic agents, including paclitaxel and cisplatin. These findings show that DMC should be developed further as a new antitumor drug candidate for treatment of HER2‐overexpressing bladder cancer.
Keywords: bladder cancer, demethoxycurcumin, HER2, Hsp90
Abbreviations
- 17‐AAG
17‐(Allylamino)‐17‐demethoxygeldanamycin
- BDMC
bisdemethoxycurcumin
- CDK4
cyclin‐dependent kinase 4
- CHX
cycloheximide
- Cur
curcumin
- DAPI
4′,6‐diamidino‐2‐phenylindole
- DMC
demethoxycurcumin
- DMSO
dimethyl sulfoxide
- EDTA
ethylenediaminetetraacetic acid
- Hsp90
90‐kDa heat shock protein
- MAPK
mitogen‐activated protein kinase
- MG132
Z‐Leu‐Leu‐Leu‐al
- MTT
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide
- PBS
phosphate‐buffered saline
- PI3K
phosphoinositide 3‐kinase
- PI
propidium iodide
- XIAP
X‐linked inhibitor of apoptosis protein
1. INTRODUCTION
Bladder cancer has become the most prevalent cancer of the urinary tract accounting for 76 000 new cases and 16 300 deaths per year in the world. 1 , 2 Currently, a significant proportion of bladder cancer patients show a response to surgical resection of the tumor followed by adjuvant chemotherapy with cisplatin. 3 While, resistance often builds up quickly, cisplatin often loses its efficacy in bladder cancer patients. The mechanisms driving this increased resistance to cisplatin over time are generally unknown.
HER2 is a transmembrane receptor with tyrosine kinase activity that is involved in cell proliferation, survival, metastasis, clinical drug‐resistance, and lower overall survival rates. 4 Its overexpression has been frequently found in various types of human cancers, including bladder cancer. Zhau et al. first found that HER2 overexpression or gene amplification occurred in bladder cancer. 5 Recent studies reported that HER2 overexpression related with high grade and stage and correlated with poor prognosis in bladder cancer. 6 , 7 Moreover, HER2 overexpression was detected frequently in cisplatin and radiation resistant patients. 8 , 9
HER2‐mediated signaling is dependent on HER2 homodimers or heterodimers with other ErbB family members, which could trigger different signaling downstream, such as mitogen‐activated protein kinase (MAPK) pathway and phosphoinositide 3‐kinase (PI3K)/Akt pathway that drivers the tumor development and progression. 10 , 11 Molecular targeting approaches against such specific oncogenic proteins and its signaling pathways have now been investigated to improve therapeutic effects. The previous studies have indicated that reducing the HER2 expression of cancer cells may attenuate its anti‐apoptotic signaling and suppress HER2‐mediated malignant phenotypes. 12 , 13 Therefore, HER2 is not only a potent oncogene, but also an excellent therapeutic target in bladder cancer.
Heat shock protein 90 (Hsp90), a molecular chaperone belonging to the group of heat shock proteins, is known to interact with HER2 for folding, assembly, and stabilization. Moreover, Hsp90 has been reported to protect mutated and overexpressed proteins from misfolding and degradation and play important roles in cell proliferation and survival. 14 , 15 Hsp90 inhibitors destabilize Hsp90 clients by dissociating Hsp90‐client complexes to exert antitumor activity. 16 The Hsp90 inhibitor 17‐(allylamino)‐17‐demethoxygeldanamycin (17‐AAG) binds to an ATP pocket in the amino‐terminal domain of Hsp90, then blocks the binding of HER2, which results in the proteasome‐dependent degradation of HER2. Hsp90 inhibitors can simultaneously inhibit multiple oncogenic pathways on which cancer cells depend to manifest the cancer hallmarks. Thus, Hsp90 is an attractive target for anti‐cancer therapies.
Turmeric (Curcuma longa L.,), a spice component in curry, contains unique phenolic curcuminoids with strong antioxidant and anti‐inflammatory properties. Three curcuminoid constituents found in turmeric are curcumin (CUR), demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC). 17 Research has shown that CUR is highly unstable in alkaline or high‐pH conditions. 18 DMC, a derivative of CUR, lacks one methoxy groups on the aromatic rings of CUR. DMC has similar biological properties to CUR, however, the slightly different chemical structure has led to a more stable chemical properties of DMC. 19 Recent studies have demonstrated that DMC exhibits better biological activities when compared with CUR. 20 , 21 , 22 , 23 Therefore, it is highly interested to investigate the therapeutic potential of DMC in the treatment of various diseases.
It has been reported that DMC induces cytotoxic effects in numerous types of cancer, 18 however, no study exists which shows the effects of DMC on bladder cancer. In this present study, CUR, DMC, and BDMC were chosen for evaluation of their anti‐proliferative effects on human bladder cancer cells. Among them, DMC seemed more sensitive to HER2‐overexpressing bladder cancer cells. Thus, DMC is considered the most promising compound for further study of physiochemical properties and biological mechanisms. Moreover, we examined the combined cytotoxic effects for HER2‐overexpressing bladder cancer cells using the combination of DMC with low dose clinical drugs (Paclitaxel, and Cisplatin). The results suggested that DMC have clinical implications for the treatment of HER2‐overexpressing bladder cancer.
2. MATERIALS AND METHODS
2.1. Chemicals and reagents
CUR, DMC, BDMC, 4′,6‐diamidino‐2‐phenylindole (DAPI), dimethyl sulfoxide (DMSO), and propidium iodide (PI), Trypsin‐Ethylenediaminetetraacetic acid (EDTA), 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT), cycloheximide (CHX), 17‐(allylamino)‐17‐demethoxygeldanamycin (17‐AAG), Z‐Leu‐Leu‐Leu‐al (MG132), G418, wortmannin, Paclitaxel, Cisplatin, anti‐mouse and anti‐rabbit antibodies conjugated to horseradish peroxidase, and β‐Actin antibody were obtained from Sigma‐Aldrich Co. (St. Louis, MO, USA). Cell culture materials were obtained from Invitrogen Life Technologies (Burlington, ON, Canada). Protein A/G‐agarose, X‐linked inhibitor of apoptosis protein (XIAP), cyclin‐dependent kinase 4 (CDK4), HER2 (9G6) and Bax antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Bcl‐2, Bcl‐xL, Survivin, p‐Akt (Ser 473), Akt, caspase‐3, caspase‐9, poly (ADP‐ribose) polymerase (PARP), α‐tubulin, and Raf‐1 antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Hsp90 antibody was from BD Transduction Laboratories (San Jose, CA, USA). HER2 (Ab3) antibody was from Calbiochem (San Diego, CA, USA). Sea Plaque Agarose (low melting temperature agarose) was purchased from Lonza Bioscience (Rockland, ME, USA).
2.2. Cell lines and cell cultures
The human bladder cancer cell lines RT4 (overexpress HER2), and TCCSUP (express the basal level of HER2) were obtained from ATCC (Rockville, MD, USA). In addition, TCCSUP/HER2 was stably transfected with pSV2‐HER2. RPMI 1640 was used for the maintenance of TCCSUP cells and Dulbecco's modified eagle medium was used for RT4 cells and supplemented with 10% fetal bovine serum. Cells were grown in a humidified incubator at 37°C under 5% CO2 in air.
2.3. Western blotting and immunoprecipitation
Whole cell lysate was prepared as described previously. 8 , 9 Approximately 50 μg of total protein lysate was separated in sodium dodecyl sulfate‐polyacrylamide gels for 1 h and subsequently electro‐transferred overnight onto nitrocellulose membranes (Schleicher and Schuell GmbH, Dassel, Germany). Membrane was blocked with 5% nonfat dry milk in 1× Tris‐buffered saline‐T (20 mM Tris–HCl, pH 7.6, 137 mM NaCl and 0.1% Tween‐20) for 1 h at room temperature and immunoblotted with the appropriate antibodies at 4°C overnight, followed by incubation with horseradish peroxidase ‐conjugated secondary antibodies. Protein visualization was performed using an Enhanced chemiluminescence Western blotting kit according to the manufacturer's instructions. For immuneprecipitation assay, 500 μg of total cell lysate was incubated with 1 μg antibody and 50 μl Protein A/G‐agarose at 4°C for overnight. Nonspecifically bound proteins were removed by repeated washings with RIPA‐B buffer without a protease inhibitor. To obtain the Triton X‐100 soluble and insoluble fractions, cells were harvested in 0.3 ml of Triton X‐100 lysis buffer with protease inhibitors (Roche Diagnostics, Mannheim, Germany). After removal of Triton X‐100 soluble supernatants by centrifugation at 600×g for 3 min, and the pellet as the Triton X‐100 insoluble fraction.
2.4. Cell viability assay
Cells were treated with various drug concentrations for the indicated times, the cell viability was determined by MTT dye. After 4 h incubation, cells were solubilized in 200 μl DMSO/well, and the absorbance was measured with a plate reader (Titertek Multiskan MCC/340, Flow Laboratories, McLean, VA, USA) at 570 nm. Each assay was repeated at least three times; cell viability was expressed as a percentage of the control viability.
2.5. Flow cytometry analysis
Cells were treated with various agents for the indicated times. After treatment, cells were harvested, washed twice with phosphate‐buffered saline (PBS) and fixed in 70% ethanol at −20°C overnight. Prior to analysis, washed cell pellet was then resuspended in staining buffer (Triton X‐100 (0.1%, v/v), RNase (100 mg/ml), and PI (80 mg/ml), and incubated at room temperature for 30 min in the dark. The samples were then analyzed by FACScan (Becton‐Dickinson, Mountain View, CA, USA) and cell cycle progression was estimated using the CellQuest Software (BD Technology, Mountain View, CA, USA). The experiment was performed in triplicate.
2.6. Soft agar colony formation assay
TCCSUP/neo, or TCCSUP/HER2 cells (1 × 104) were seeded in 6 cm culture dish containing 0.35% low‐melting agarose over a 0.7% agarose layer in the presence of 20 μM of DMC at 37°C in a humidified 5% CO2 atmosphere for 21 days. Colonies were stained with p‐iodonitrotetrazolium violet (1 mg/ml), and then photographed under a light microscope using the Nikon ACT‐1 program (Version 2.20; LEAD Technologies) and counted using ImageJ (Version 1.48 g; NIH).
2.7. Immunofluorescence analysis
RT4 cells were cyto‐spinned on glass slides, and washed once with PBS, fixed in PBS containing 4% paraformaldehyde for 20 min at room temperature. Cells were permeabilized with 0.2% Triton X‐100 for 10 min and incubated with PBS containing 4% BSA (Sigma; St. Louis, MO, USA) for 30 min. Incubations were performed with HER2 primary antibody diluted in PBS containing 1% BSA for 1 h at room temperature. Afterwards, cover slides were washed and incubated for 30 min with the fluorescein isothiocyanate (FITC)‐conjugated secondary antibody (CHEMICON, Billerica, MA, USA). Fluorescence images were collected on a Zeiss Axioplan.
2.8. Statistical analysis
The data are presented as the mean ± SD from at least three independent experiments. The statistical analyses were performed using One‐way ANOVA. Statistically significant differences among the means were set at *, p < .05; **, p < .01; ***, p < .001.
3. RESULTS
3.1. The antitumor effect of curcuminoids on bladder cancer cells
It has recently been demonstrated that curcuminoids exert anticancer effects against various types of cancer. 20 , 21 , 22 , 23 To evaluate the effects of curcuminoids against bladder cancer cells, RT4 (overexpress HER2), and TCCSUP (express the basal level of HER2) cells were treated with various concentrations of curcuminoids (Cur, BDMC, and DMC) for 48 h and cell viability was assessed by MTT assay. Cur, BDMC, and DMC significantly reduced the viability in HER2‐overexpressing RT4 cells. However, the inhibition was much less effective in TCCSUP cells expressing a basal level of HER2 under the same condition (Figure 1A–C). Interestingly, among these curcuminoids, DMC exhibited the most efficient cytotoxic effects in RT4 cells. Collectively, these results show that curcuminoids significantly potentiate the cytotoxic effects of HER2‐overexpressing RT4 cells in a dose‐dependent manner, and DMC exhibit the most efficient cytotoxic effects among these curcuminoids (IC50: Cur, 17.4 μM; BDMC, 15.7 μM; and DMC, 10.3 μM, respectively).
FIGURE 1.
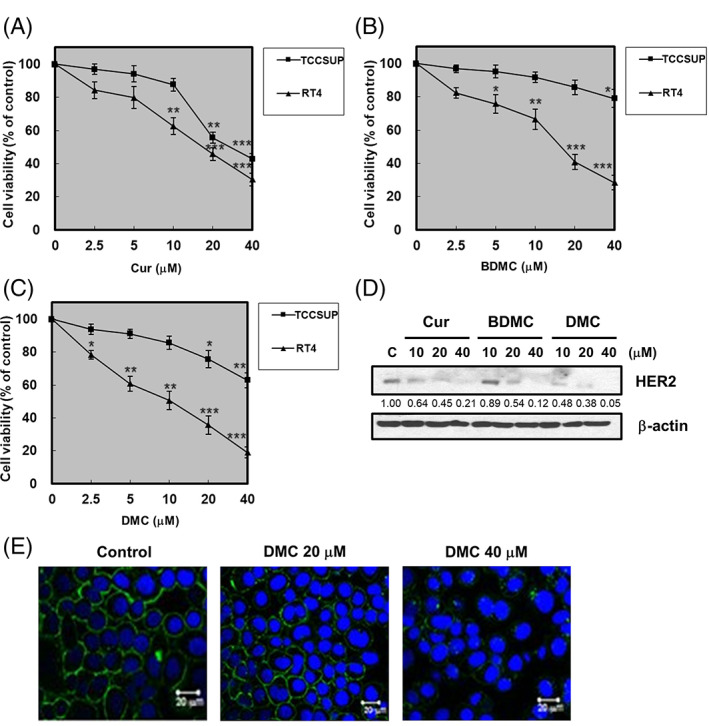
Curcuminoids downregulated HER2 protein expression in HER2‐overexpressing bladder cancer cells. RT4 and TCCSUP cells were treated with various concentrations of (A) curcumin (Cur), (B) bisdemethoxycurcumin (BDMC), and (C) demethoxycurcumin (DMC), as indicated. Cell viability was determined by MTT assays. (D) RT4 cells were treated with vehicle (DMSO), indicated concentrations of Cur, BDMC, and DMC for 48 h followed by Western blot for HER2, and β‐actin. Representative of two separate experiments. Western blot data presented are representative of those obtained in at least three separate experiments. The values below the figures represent change in protein expression of the bands normalized to β‐actin. (E) RT4 cells were grown on coverslips and treated with vehicle (DMSO), indicated concentrations of DMC for 48 h, then subjected to immunofluorescent staining with HER2 and DAPI as described in materials and methods. Fluorescence images were performed by fluorescent microscopy
3.2. Curcuminoids‐induced degradation of HER2
Curcuminoids significantly reduced the viability in HER2‐overexpressing RT4 cells, we next investigated the effects of curcuminoids on reducing the expression of HER2 protein. The Western blotting was performed to examine the HER2 protein level, our result showed that curcuminoids inhibited the expression of HER2 in RT4 cells in a dose‐dependent manner (Figure 1D). Moreover, we found that DMC was more active than Cur and BDMC in inhibiting the expression of HER2 protein levels. Immunofluorescence staining also revealed that DMC inhibited the expression of HER2 in RT4 cells (Figure 1E). Thus, DMC is considered the most promising compound for HER2‐overexpressing bladder cancer.
3.3. DMC induced apoptosis in RT4 cells
In order to investigate whether DMC induced apoptosis, RT4 cells were treated with 20 μM of DMC and stained with PI for analyzing by flow cytometric assays. The results indicated that DMC induced apoptosis in 24 h, 48 h treatment (Figure 2A). For further confirming that DMC induced apoptosis, the changes in the expression of apoptosis‐associated proteins were detected by Western blotting. After treatment with DMC for different concentrations, the RT4 cells exhibited increased PARP activity. The caspase‐3 and ‐9 activities in the RT4 cells were also increased following treatment of DMC (Figure 2B). We next explored the levels of apoptotic proteins that might be involved in DMC‐induced apoptosis. As shown in Figure 2C,D, DMC significantly inhibited the expression of anti‐apoptotic Bcl‐2, Bcl‐xL, Survivin, XIAP proteins in a dose‐dependent manner. However, the levels of the pro‐apoptotic Bax protein were increased by DMC.
FIGURE 2.
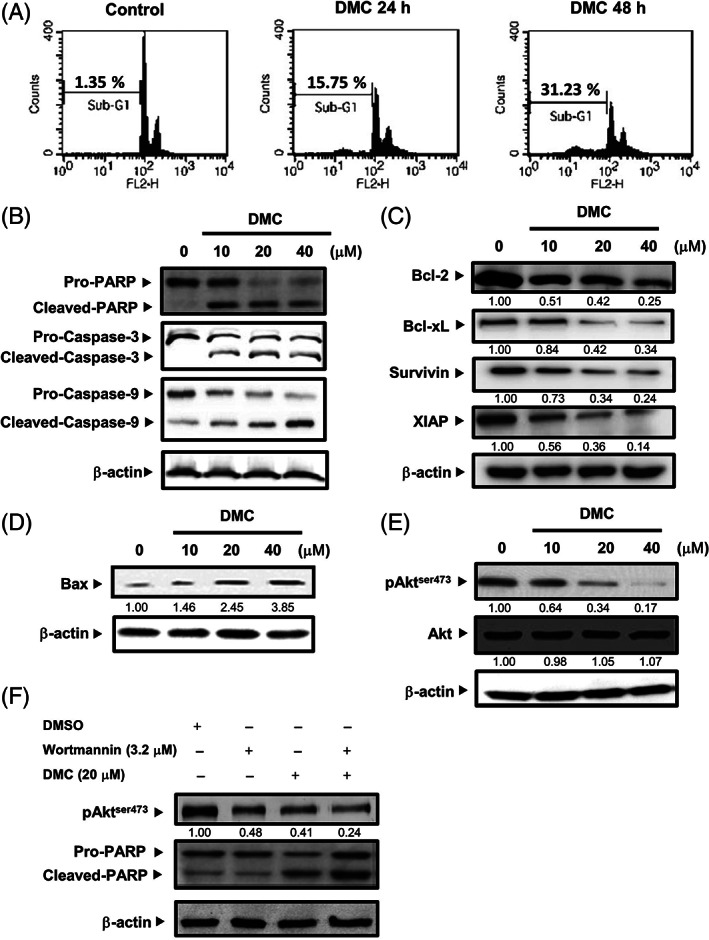
Demethoxycurcumin (DMC) induced apoptosis through the inhibition of PI3k/Akt signaling pathway in RT4 cells. (A) RT4 cells were treated at time 0 with DMSO alone (Control), 20 μM DMC for the indicated periods. Cells were harvested and stained with PI and the apoptosis distribution was analyzed by flow cytometry. (B) RT4 cells were treated with vehicle (DMSO), indicated concentrations of DMC for 48 h followed by Western blot for PARP, Caspase‐3, Caspase‐9, and β‐Actin. (C) RT4 cells were treated with vehicle (DMSO), indicated concentrations of DMC for 48 h followed by Western blot for Bcl‐2, Bcl‐xL, Survivin, XIAP, and β‐actin. (D) RT4 cells were treated with vehicle (DMSO), indicated concentrations of DMC for 48 h followed by Western blot for Bax and β‐actin. (E) RT4 cells were treated with vehicle (DMSO), indicated concentrations of DMC for 48 h followed by Western blot for phospho‐Aktser473, Akt, and β‐actin. (F) Before treatment with 20 μM DMC, RT4 cells were pretreated with 3.2 μM Wortmannin. RT4 cells were treated with vehicle (DMSO), indicated concentrations of DMC for 48 h followed by Western blot for phospho‐Aktser473, PARP, and β‐actin. Western blot data presented are representative of those obtained in at least three separate experiments. The values below the figures represent change in protein expression of the bands normalized to β‐actin
3.4. DMC induced apoptosis by inactivation of the PI3‐kinase (PI3K)/Akt signaling pathway
Given the critical role of PI3K/Akt pathway in controlling cell survival/death in cancer cells, we determined whether the Akt activity is associated with apoptotic effects of DMC. Our results found that DMC markedly reduced the levels of p‐Akt (Ser 473) in RT4 cells without significantly altering the levels of total Akt (Figure 2E). In order to further identify the role of Akt in the DMC‐induced apoptosis in RT4 cells, the effect of PI3K inhibitor wortmannin was examined. We found that pre‐treatment of RT4 cells with wortmannin enhanced DMC‐induced apoptosis (Figure 2F). Taken together, these data imply that the cytotoxicity of DMC may be related to the inhibition of PI3K/Akt pathway.
3.5. DMC had preferential activity against HER2‐overexpressing bladder cancer cells
To evaluate the effects of DMC on HER2‐induced cell proliferation, TCCSUP cells were stably transfected with pSV2‐HER2 and then treated with 20 μM DMC for 24 and 48 h. Our result showed that DMC preferentially inhibited the proliferation of TCCSUP/HER2 cells (Figure 3A). Treatment with 20 μM DMC for 48 h inhibited over 60% of growth in TCCSUP/HER2 cells, however, the growth inhibition by DMC was much less in parental TCCSUP cell lines expressing a basal level of HER2.
FIGURE 3.
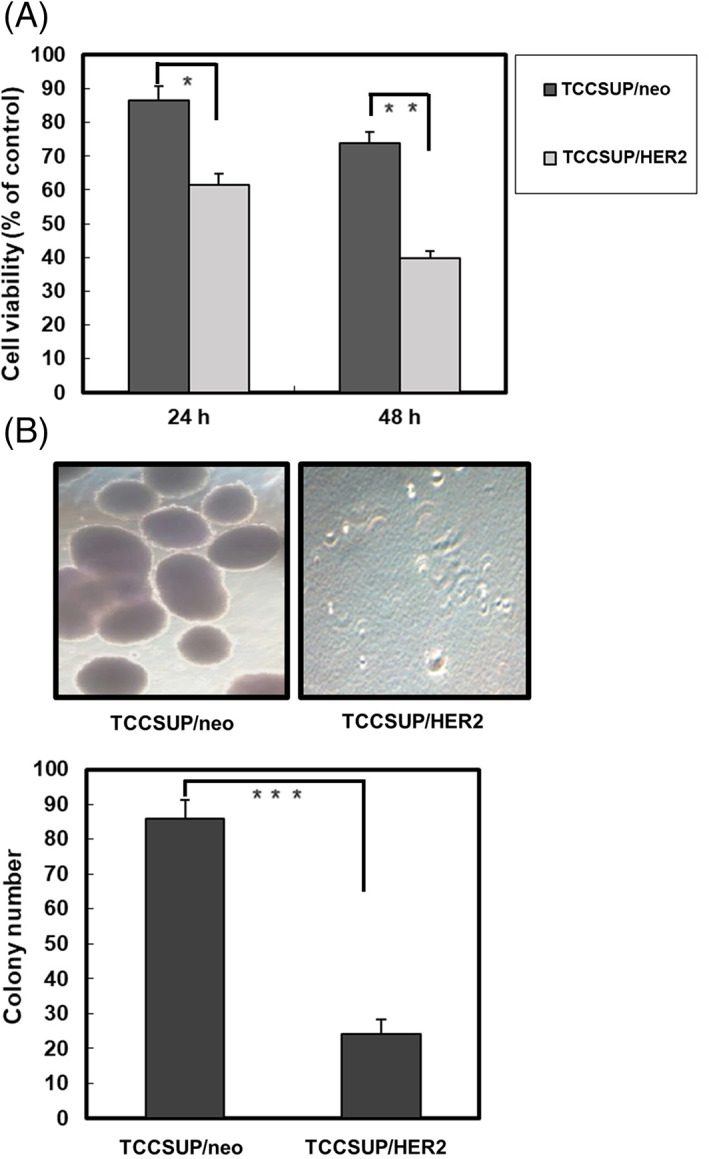
Demethoxycurcumin (DMC) preferentially inhibited the proliferation of HER2‐overexpressing bladder cancer cells. (A) TCCSUP/neo and TCCSUP/HER2 cells were treated with 20 μM DMC for the indicated periods. Cell viability was determined by MTT assays. (B) TCCSUP/neo, or TCCSUP/HER2 cells (1 × 104) were seeded in 6 cm culture dish, containing 0.35% low‐melting agarose over a 0.7% agarose layer. They were treated with 20 μM of DMC for 21 days. Column, mean of three independent experiments. Bars represent the SD. Asterisk, values significantly different from the control. *, p < .05; **, p < .01; ***, p < .001
3.6. DMC preferentially inhibited the colony‐forming activity in HER2‐overexpressing bladder cancer cells
Examining the effects of DMC on HER2‐induced anchorage independent growth, we seeded TCCSUP/neo or TCCSUP/HER2 cells into soft agar in the presence of 20 μM DMC and monitored them for colony formation. DMC preferentially inhibited the colony‐forming activity of TCCSUP/HER2 cells (Figure 3B). Overall, these results suggest that DMC preferentially suppresses HER2‐mediated transformation phenotype of bladder cancer cells.
3.7. DMC downregulated HER2 protein expression by modulating the protein stability of HER2
We next examined the effects of DMC on HER2 protein stability, RT4 cells were treated with protein synthesis inhibitor CHX or with a combination of CHX and DMC, and then the relative levels of HER2 protein in these cells were detected. As shown in Figure 4A, a combination of CHX and DMC downregulated HER2 protein was faster than that treated only with CHX. This indicated that DMC downregulated HER2 protein through a post‐translational mechanism in HER2‐overexpressing bladder cancer cells. To further investigate whether DMC downregulated HER2 protein was due to proteolytic degradation, we tested the effect of the proteasome inhibitor MG132 on HER2 expression. Our result found that DMC downregulated HER2 protein in both Triton X‐100‐soluble and Triton X‐100‐insoluble cellular fractions. However, a combination of MG132 and DMC could inhibit DMC‐mediated downregulation of HER2 protein in the Triton X‐100‐insoluble cellular fraction (Figure 4B). Collectively, these results suggest that DMC downregulated HER2 protein by the ubiquitin‐proteasome pathway.
FIGURE 4.
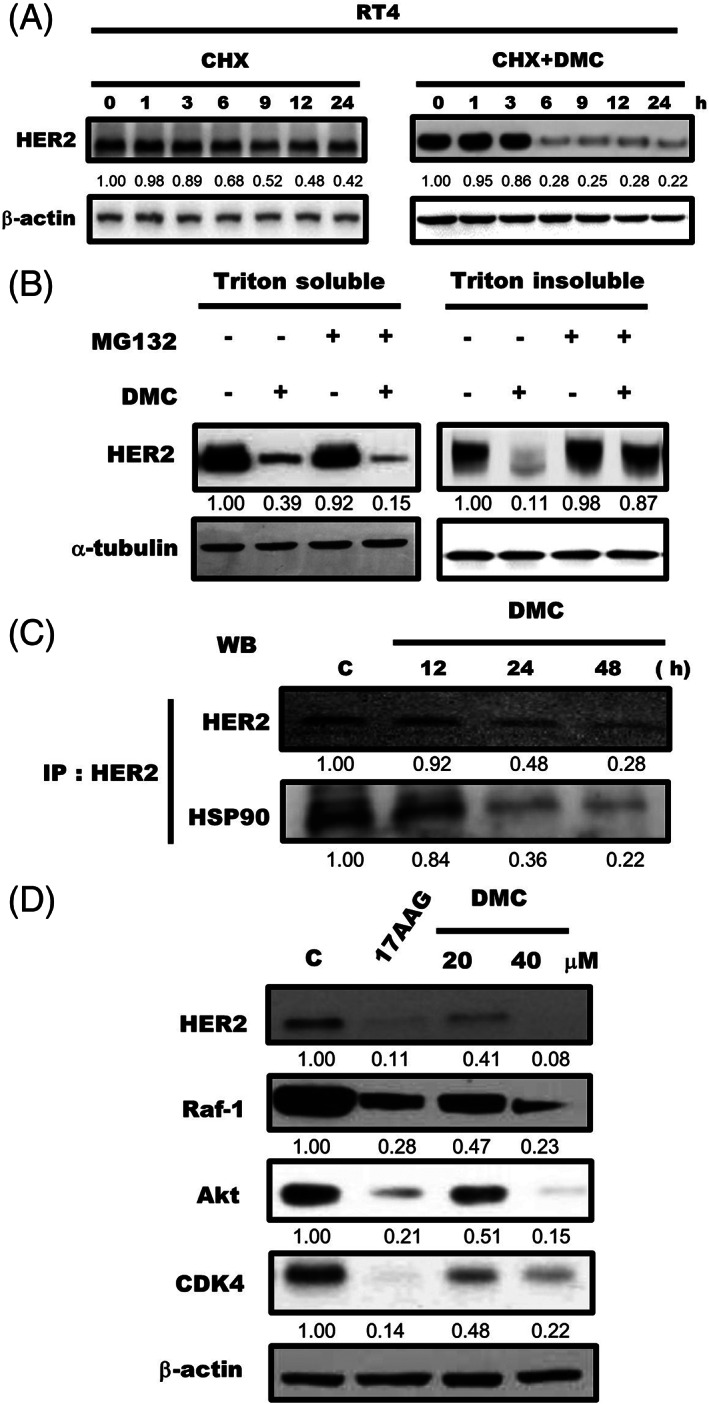
Demethoxycurcumin (DMC) downregulated HER2 expression by dissociating of Hsp90 from HER2. (A) RT4 cells were cultured with 20 μg/ml cycloheximide (CHX) in the presence or absence of 20 μM DMC for the indicated times followed by Western blot for HER2 and β‐Actin. (B) RT4 cells were pretreated with MG132 (20 μM) for 30 min followed by 20 μM DMC for 9 h, and Triton X‐100–soluble and Triton X‐100–insoluble cell lysates were prepared and a followed by Western blotting for HER2 and β‐actin. (C) RT4 cells were treated with 20 μM DMC for the indicated periods. Lysates of RT4 cells were immunoprecipitated with antibodies against HER2 and immunoblotting was performed using anti‐HER2 and anti‐Hsp90 antibodies. (D) RT4 cells were treated with Hsp90 inhibitor 17AAG (10 μM) or DMC (20 and 40 μM) for 48 h followed by Western blot for HER2, Raf‐1, AKT, CDK4, and β‐actin. Western blot data presented are representative of those obtained in at least three separate experiments. The values below the figures represent change in protein expression of the bands normalized to β‐actin
3.8. DMC caused dissociation of Hsp90 from HER2 and promoted downregulation of HER2 protein
Hsp90 is a molecular chaperone that is crucial for the stability and maturation of the HER2 protein. 24 We next examined whether DMC downregulated HER2 protein by disassociating HER2 with Hsp90. As shown in Figure 4C, after treatment with 20 μM DMC, Hsp90 dissociated from HER2 protein complex. Previous study indicated that inhibition of Hsp90 would result in down‐regulation of multiple oncogenic proteins, therefore, we examined whether DMC could decrease the levels of Hsp90 client proteins in RT4 cells. As shown in Figure 4D, Hsp90 inhibitor 17AAG, and DMC caused a progressive decline in client proteins of Hsp90 (HER2, Raf‐1, Akt, CDK4). Our result demonstrated that DMC induced down‐regulation of oncogenic Hsp90 client proteins.
3.9. DMC enhanced the chemotherapeutic efficacy of paclitaxel and cisplatin by downregulating HER2
To investigate the biological effect of downregulation of HER2 protein mediated by DMC, Paclitaxel and Cisplatin were applied to RT4 or TCCSUP cells in combination with 20 μM of DMC. The combination of DMC and Paclitaxel (Figure 5A), or Cisplatin (Figure 5C) enhanced the chemotherapeutic efficacy in HER2‐overexpressing RT4 cells. However, the combination of DMC and Paclitaxel (Figure 5B), or Cisplatin (Figure 5D) has no significantly enhanced effect in TCCSUP (express the basal level of HER2) cells. Our results clearly demonstrated that DMC enhanced the chemotherapeutic efficacy of Paclitaxel and Cisplatin on HER2‐overexpressing bladder cancer cells and inhibited the HER2‐induced drug resistance of tumor cells.
FIGURE 5.
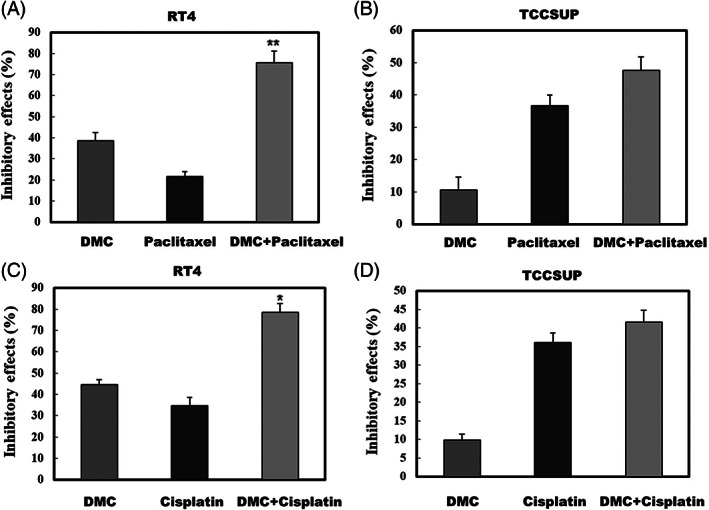
Effect of the addition of demethoxycurcumin (DMC) to clinical drugs (Paclitaxel and Cisplatin) on the proliferation of bladder cancer cells. RT4 and TCCSUP cells were treated with 20 μM DMC, (A, B) 400 nM Paclitaxel, (C, D) Cisplatin either alone or in combination for 48 h. Cell viability was determined by MTT assays
4. DISCUSSION
Previous studies suggested that HER2 expression is associated with poor prognosis for bladder cancer patients. Therefore, it is crucial to develop novel drugs targeting HER2‐overexpressing bladder cancer. The major observation reported here is that the curcuminoids effectively downregulated HER2 protein in HER2‐overexpressing bladder cancer cells. Interestingly, we found that one of the curcuminoids, DMC, was more effective than others in downregulating the HER2 protein level. DMC preferentially suppressed the proliferation and transformation phenotype of HER2‐overexpressing bladder cancer cells, but not the cell lines expressing basal levels of HER2. DMC provided a basis for the development of potent agents that can pharmacologically target HER2, the molecular mechanisms will be the subject of future investigation.
Recently, several other natural products are known to have the effect of downregulating HER2 protein. Our previous studies found that flavonoid apigenin induced apoptosis of the HER2‐overexpressing breast cancer cells by downregulating HER2 protein. Apigenin downregulated HER2 protein by disassociating HER2 with GRP94 (endoplasmic reticulum hsp90 chaperone). 25 We also found that various flavonoids downregulated HER2 protein depended on the position of B ring and the existence of the 3′ or 4′‐hydroxyl group on the 2‐phenyl group. 26 We have shown that aloe‐emodin downregulated HER‐2 through the downregulation of YB‐1. 27 Gang et al., found that fisetin induced apoptosis of HER2‐overexpressing breast cancer by induction of HER2 proteasomal degradation. 28 Mary et al., indicated that genistein, the main soy isoflavone, downregulated HER2 protein through an ER‐independent mechanism, 29
Recent studies have demonstrated that PI3K/Akt signaling in HER2‐overexpressing cancer cells may be directly linked to the proliferation and anti‐apoptosis, here, we found that DMC mainly exerted its anti‐tumor in inhibiting the HER2/PI3K/Akt pathway. Recently, Lin et al. indicated that DMC significantly inhibited on‐target cisplatin (CDDP) resistance protein, excision repair cross‐complementary 1 (ERCC1), via PI3K/Akt in nonsmall cell lung carcinoma cells (NSCLC). 30 Rakesh et al., reported that DMC inhibited proliferation and induced apoptosis through Akt/NF‐κB signaling pathway inhibition. 31 Huang et al., found that DMC induced the apoptosis of osteosarcoma cancer cells through repression of Akt signaling pathway. 32 Our results were in agreement with those of previous studies, DMC might exert their cytotoxic anti‐tumor activity through the inhibition of PI3K/Akt pathway.
In this study, we presented evidence that the degradation of HER2 after CHX plus DMC treatment was faster than treatment with CHX alone. This result implicated that a possible post‐transcriptional mechanism for HER2 degradation. HSP90 has been recognized as an important chaperone protein for a vast array of client proteins including HER2. We attempted to identify whether DMC disrupted the association of Hsp90 with HER2. DMC exerted its destabilizing effects by dissociating HER2 from Hsp90, and such dissociation preceded the proteolytic degradation of HER2 at the plasma membrane. Yanyan et al., suggest that the binding of (−)‐EGCG to Hsp90 impairs the association of Hsp90 with HER2, thereby inducing degradation of HER2, resulting antiproliferating effects in pancreatic cancer cells. 33
Chemotherapy remains the standard of care for patients with muscle‐invasive bladder cancer. However, recent studies have shown that HER2 overexpression is associated with higher rates of disease recurrent and lower complete response rates after chemotherapy. Therefore, combined treatment with HER2 inhibitor and chemotherapy might be an option to efficiently overcome chemoresistance of HER2‐overexpressing bladder cancer cells. In the present study, we demonstrated that the combination of DMC and paclitaxel (Figure 5A), or cisplatin (Figure 5C) enhanced the chemotherapeutic efficacy in HER2‐overexpressing RT4 cells, but did not have the same effect in the TCCSUP cell lines, which express basal levels of HER2. Teng et al., showed DMC as a chemosensitizing agent through the inhibition of P‐glycoprotein (P‐gp). 34 Shi et al., implicated that low‐dose DMC significantly enhanced the effect of temozolomide on glioma cells by increasing intracellular level of reactive oxygen species production, activating caspase‐3‐dependent apoptotic pathway, and inactivating of JAK/STAT3 signaling pathway in GBMs. 35 Chen et al., demonstrated that the combination of DMC with cisplatin might restore DDP sensitivity in cisplatin‐resistant nonsmall lung cancer cells through the inhibition of ERCC1. 36 Our and previous results suggest that DMC is potential to be developed further as specific target of co‐chemotherapeutic agents for cancer treatment.
5. CONCLUSIONS
Taken together, the novel findings of DMC in the present study may help improve the efficacy of preventive or therapeutic compounds against HER2‐overexpressing bladder cancer.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Sheng‐Tang Wu and Tzong‐Der Way: Conception and design, interpretation of data; Chien‐Chang Kao, Yi‐Ching Cheng and Ming‐Hsin Yang: Acquisition and analysis of data, drafting the manuscript; Tai‐Lung Cha, Guang‐Huan Sun, Ying‐Chao Lin and Hao‐Kuang Wan: Conception and design, given final approval of the version to be published; Chi‐Tang Ho: drafting and revising the manuscript. All authors have read and approved the manuscript.
ACKNOWLEDGMENT
The investigation was supported by a research grants from Tri‐Service General Hospital Research Foundation (TSGH‐C105‐054 and TSGH‐C106‐043). Thanks are also due to support (in part) by the grant from China Medical University (CMU109‐S‐53).
Kao C‐C, Cheng Y‐C, Yang M‐H, et al. Demethoxycurcumin induces apoptosis in HER2 overexpressing bladder cancer cells through degradation of HER2 and inhibiting the PI3K/Akt pathway. Environmental Toxicology. 2021;36(11):2186-2195. 10.1002/tox.23332
Funding information China Medical University, Grant/Award Number: CMU109‐S‐53; Research Foundation; Tri‐Service General Hospital
Contributor Information
Sheng‐Tang Wu, Email: doc20283@gmail.com.
Tzong‐Der Way, Email: tdway@mail.cmu.edu.tw.
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Nielsen ME, Smith AB, Meyer AM, et al. Trends in stage‐specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006. Cancer. 2014;120(1):86‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inazu M. Choline transporter‐like proteins CTLs/SLC44 family as a novel molecular target for cancer therapy. Biopharm Drug Dispos. 2014;35(8):431‐449. [DOI] [PubMed] [Google Scholar]
- 4. Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19(13):3159‐3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhau HE, Zhang X, von Eschenbach AC, et al. Amplification and expression of the c‐erb B‐2/neu proto‐oncogene in human bladder cancer. Mol Carcinog. 1990;3(5):254‐257. [DOI] [PubMed] [Google Scholar]
- 6. Simonetti S, Russo R, Ciancia G, Altieri V, De Rosa G, Insabato L. Role of polysomy 17 in transitional cell carcinoma of the bladder: immunohistochemical study of HER2/neu expression and fish analysis of c‐erbB‐2 gene and chromosome 17. Int J Surg Pathol. 2009;17(3):198‐205. [DOI] [PubMed] [Google Scholar]
- 7. Fleischmann A, Rotzer D, Seiler R, Studer UE, Thalmann GN. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol. 2011;60(2):350‐357. [DOI] [PubMed] [Google Scholar]
- 8. Koga F, Yoshida S, Tatokoro M, et al. ErbB2 and NFkappaB overexpression as predictors of chemoradiation resistance and putative targets to overcome resistance in muscle‐invasive bladder cancer. PLoS One. 2011;6(11):e27616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chakravarti A, Winter K, Wu CL, et al. Expression of the epidermal growth factor receptor and her‐2 are predictors of favorable outcome and reduced complete response rates, respectively, in patients with muscle‐invading bladder cancers treated by concurrent radiation and cisplatin‐based chemotherapy: a report from the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 2005;62(2):309‐317. [DOI] [PubMed] [Google Scholar]
- 10. Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9(7):463‐475. [DOI] [PubMed] [Google Scholar]
- 11. Agus DB, Akita RW, Fox WD, et al. Targeting ligand‐activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127‐137. [DOI] [PubMed] [Google Scholar]
- 12. Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19(53):6102‐6114. [DOI] [PubMed] [Google Scholar]
- 13. Menard S, Tagliabue E, Campiglio M, Pupa SM. Role of HER2 gene overexpression in breast carcinoma. J Cell Physiol. 2000;182(2):150‐162. [DOI] [PubMed] [Google Scholar]
- 14. Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761‐772. [DOI] [PubMed] [Google Scholar]
- 15. Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70‐based chaperone machinery. Exp Biol Med (Maywood). 2003;228(2):111‐133. [DOI] [PubMed] [Google Scholar]
- 16. Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90‐pp60v‐src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91(18):8324‐8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jayaprakasha GK. Jagan Mohan Rao L, Sakariah KK. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J Agric Food Chem. 2002;50(13):3668‐3672. [DOI] [PubMed] [Google Scholar]
- 18. Hatamipour M, Ramezani M, Tabassi SAS, Johnston TP, Ramezani M, Sahebkar A. Demethoxycurcumin: a naturally occurring curcumin analogue with antitumor properties. J Cell Physiol. 2018;233(12):9247‐9260. [DOI] [PubMed] [Google Scholar]
- 19. Han G, Bi R, Le Q, Zhao LL, Dong Y, Lin QH. Study on effect of demethoxycurcumin in curcuma long on stability of curcumin. Zhong Yao Cai. 2008;31(4):592‐594. [PubMed] [Google Scholar]
- 20. Shieh JM, Chen YC, Lin YC, et al. Demethoxycurcumin inhibits energy metabolic and oncogenic signaling pathways through AMPK activation in triple‐negative breast cancer cells. J Agric Food Chem. 2013;61(26):6366‐6375. [DOI] [PubMed] [Google Scholar]
- 21. Hung CM, Su YH, Lin HY, et al. Demethoxycurcumin modulates prostate cancer cell proliferation via AMPK‐induced down‐regulation of HSP70 and EGFR. J Agric Food Chem. 2012;60(34):8427‐8434. [DOI] [PubMed] [Google Scholar]
- 22. Yodkeeree S, Chaiwangyen W, Garbisa S, Limtrakul P. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down‐regulation of MMPs and uPA. J Nutr Biochem. 2009;20(2):87‐95. [DOI] [PubMed] [Google Scholar]
- 23. Luthra PM, Kumar R, Prakash A. Demethoxycurcumin induces Bcl‐2 mediated G2/M arrest and apoptosis in human glioma U87 cells. Biochem Biophys Res Commun. 2009;384(4):420‐425. [DOI] [PubMed] [Google Scholar]
- 24. Kim LS, Kim JH. Heat shock protein as molecular targets for breast cancer therapeutics. J Breast Cancer. 2011;14(3):167‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Way TD, Kao MC, Lin JK. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu‐overexpressing breast cancer cells via the phosphatidylinositol 3‐kinase/Akt‐dependent pathway. J Biol Chem. 2004;279(6):4479‐4489. [DOI] [PubMed] [Google Scholar]
- 26. Way TD, Kao MC, Lin JK. Degradation of HER2/neu by apigenin induces apoptosis through cytochrome c release and caspase‐3 activation in HER2/neu‐overexpressing breast cancer cells. FEBS Lett. 2005;579(1):145‐152. [DOI] [PubMed] [Google Scholar]
- 27. Ma JW, Hung CM, Lin YC, Ho CT, Kao JY, Way TD. Aloe‐emodin inhibits HER‐2 expression through the downregulation of Y‐box binding protein‐1 in HER‐2‐overexpressing human breast cancer cells. Oncotarget. 2016;7(37):58915‐58930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo G, Zhang W, Dang M, Yan M, Chen Z. Fisetin induces apoptosis in breast cancer MDA‐MB‐453 cells through degradation of HER2/neu and via the PI3K/Akt pathway. J Biochem Mol Toxicol. 2019;33(4):e22268. [DOI] [PubMed] [Google Scholar]
- 29. Sakla MS, Shenouda NS, Ansell PJ, Macdonald RS, Lubahn DB. Genistein affects HER2 protein concentration, activation, and promoter regulation in BT‐474 human breast cancer cells. Endocrine. 2007;32(1):69‐78. [DOI] [PubMed] [Google Scholar]
- 30. Lin CY, Hung CC, Wang CCN, Lin HY, Huang SH, Sheu MJ. Demethoxycurcumin sensitizes the response of non‐small cell lung cancer to cisplatin through downregulation of TP and ERCC1‐related pathways. Phytomedicine. 2019;53:28‐36. [DOI] [PubMed] [Google Scholar]
- 31. Kumar R, Lal N, Nemaysh V, Luthra PM. Demethoxycurcumin mediated targeting of MnSOD leading to activation of apoptotic pathway and inhibition of Akt/NF‐kappaB survival signalling in human glioma U87 MG cells. Toxicol Appl Pharmacol. 2018;345:75‐93. [DOI] [PubMed] [Google Scholar]
- 32. Huang C, Lu HF, Chen YH, Chen JC, Chou WH, Huang HC. Curcumin, demethoxycurcumin, and bisdemethoxycurcumin induced caspase‐dependent and ‐independent apoptosis via Smad or Akt signaling pathways in HOS cells. BMC Complement Med Ther. 2020;20(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Y, Zhang T, Jiang Y, Lee HF, Schwartz SJ, Sun D. (−)‐Epigallocatechin‐3‐gallate inhibits Hsp90 function by impairing Hsp90 association with cochaperones in pancreatic cancer cell line Mia Paca‐2. Mol Pharm. 2009;6(4):1152‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Teng YN, Hsieh YW, Hung CC, Lin HY. Demethoxycurcumin modulates human P‐glycoprotein function via uncompetitive inhibition of ATPase hydrolysis activity. J Agric Food Chem. 2015;63(3):847‐855. [DOI] [PubMed] [Google Scholar]
- 35. Shi L, Sun G. Low‐dose DMC significantly enhances the effect of TMZ on glioma cells by targeting multiple signaling pathways both in vivo and in vitro. Neuromolecular Med. 2015;17(4):431‐442. [DOI] [PubMed] [Google Scholar]
- 36. Chen Y, Hong C, Chen X, Qin Z. Demethoxycurcumin increases the sensitivity of cisplatin‐resistant non‐small lung cancer cells to cisplatin and induces apoptosis by activating the caspase signaling pathway. Oncol Lett. 2020;20(5):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.


