Abstract
Objective
To assess the incidence of severe maternal outcome (SMO), comprising maternal mortality (MM) and maternal near miss (MNM), in Metro East health district, Western Cape Province, South Africa between November 2014 and November 2015 and to identify associated determinants leading to SMO with the aim to improve maternity care.
Methods
Region‐wide population‐based case‐control study. Women were included in the study, if they were maternal deaths or met MNM criteria, both as defined by WHO. Characteristics of women with SMO were compared with those of a sample of women without SMO, matched for age and parity, taken from midwifery‐led obstetrical units from two residential areas in Metro East, using multivariate regression analysis.
Results
Incidence of SMO was 9.1 per 1000 live births, and incidence of MNM was 8.6 per 1000 live births. Main causes of SMO were obstetrical hemorrhage and hypertensive disorders. Factors associated with SMO were HIV (adjusted odds ratio [aOR] 24.8; 95% confidence interval [CI] 10.0–61.6), pre‐eclampsia (aOR 17.5; 95% CI 7.9–38.7), birth by cesarean section (aOR 8.4; 95% CI 5.8–12.3), and chronic hypertension (aOR 2.4; 95% CI 1.1–5.1).
Conclusion
Evaluation of SMO incidence and associated determinants supports optimizing tailored guidelines in Metro‐East health district to improve maternal health.
Keywords: case control, maternal morbidity, maternal mortality, maternal near miss, population‐based, severe maternal outcome, South Africa
1. INTRODUCTION
Improving women's health during and after pregnancy is an international health priority. The maternal mortality ratio (MMR) has traditionally been used as an indicator of the quality of maternity care. Wide variations in MMR across different countries reflect inequalities in access to quality care and highlight huge gaps between the global rich and poor. MMR in 2017 was 462 per 100 000 live births in low‐income countries versus 11 per 100 000 live births in high‐income countries. 1 For South Africa, a middle‐income country with a National Committee for Confidential Enquiries into Maternal Deaths since 1997, MMR in 2016 was 135 per 100 000 live births, with the Western Cape Province having the lowest regional MMR (68.3 per 100 000 live births). 2 , 3
Attention has increasingly shifted toward severe maternal morbidity as an additional quality indicator. 4 , 5 , 6 In this light, WHO has introduced the maternal near miss (MNM) approach (Figure 1), which includes women who survived life‐threatening complications during pregnancy, childbirth or within 42 days of delivery or termination of pregnancy. 3
FIGURE 1.
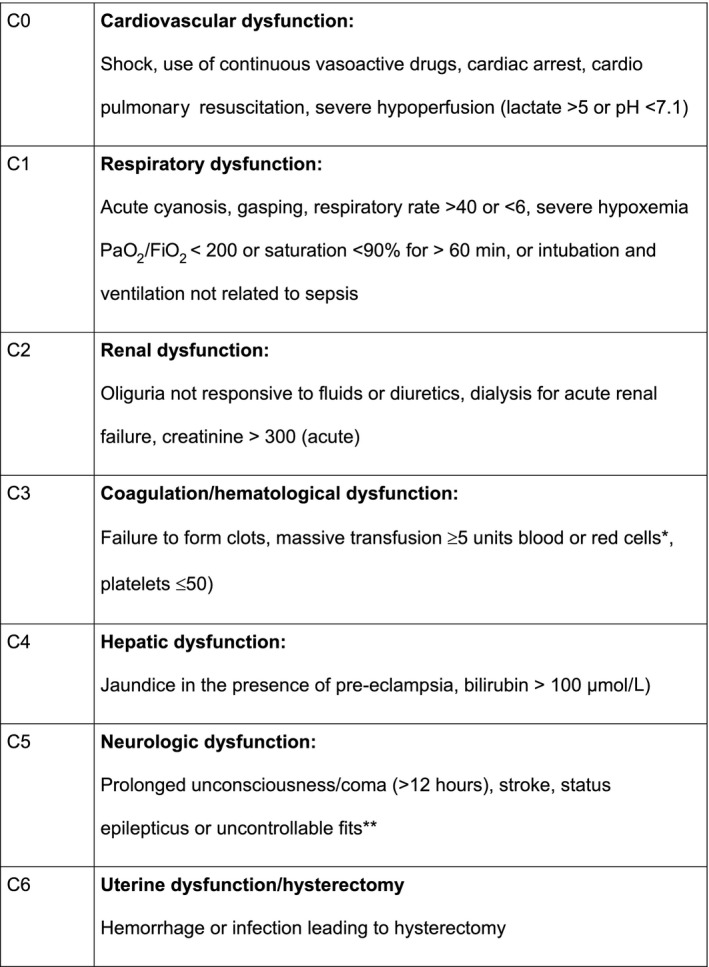
WHO life‐threatening conditions (Maternal near miss criteria). *Used was ≥5 units blood products (including units of red blood cells, fresh frozen plasma, platelets or cryoprecipitate). **Defined in this study uncontrollable fits with more than two fits or not responding to MgSO4
Maternal death and MNM are collectively defined as severe maternal outcome (SMO). 3 , 4 This approach allows the identification of factors associated with SMO that could potentially provide relevant and robust information to prevent SMO. A detailed explanation of the various definitions is given in Figure 2.
FIGURE 2.

Definitions of outcome measures
In Metro East health district, Western Cape Province, South Africa, public maternity care is organized according to a clear referral pathway. Care is divided into three levels (1–3) according to pregnant women's needs. Women with low risk pregnancies will access care in Midwifery Obstetrical Units, which is level 1, district health care. In case of complications, women can be referred to three level 1 district hospitals, mainly run by midwives and medical officers supervised by one or two obstetricians. Severely complicated pregnancies are managed in Tygerberg Hospital (TBH), the academic hospital of Stellenbosch University, providing level 2 and 3 care. TBH is the only level 2 hospital in this district.
Following WHO’s recommendation to analyze SMO as a means to improve maternity care, the objective of this study was to assess the incidence of SMO for the public health sector of Metro East health district and to identify possible determinants associated with SMO.
2. MATERIALS AND METHODS
A region‐wide population‐based case‐control study was performed in Metro East health district, Western Cape Province, South Africa. Ethical approval was obtained from the Health Research Ethics Committee (HREC), Faculty of Health Sciences, Stellenbosch University, on October 3, 2018 (Project ID: 1427, HREC Reference #: S18/02/023). Approval was also obtained from the Provincial Health Authority, the CEO of TBH and the heads of respective departments. A waiver of consent from participants was obtained from HREC, as the study was performed by auditing the hospital records.
All women who were referred to TBH between November 1, 2014 and November 1, 2015 were included in the study if they met the WHO life‐threatening conditions (MNM criteria) or were identified as a maternal death. Identification of women was done by healthcare workers from the Department of Obstetrics and Gynecology in TBH and was overseen by the principle investigator (AH).
Awareness among health workers to identify women was enhanced continuously by having clearly visible posters with instructions in all relevant wards, as well as by sending out monthly phone messages and alerts to all involved healthcare professionals. Data pertaining to women identified as having sustained SMO were collected from their case records. These data were subsequently de‐identified into anonymized case record forms and entered into a separate, password‐protected Microsoft excel database in a secure location in the hospital.
The WHO MNM criteria served as core outcome set. WHO MNM criteria are comprised of seven main “life‐threatening” conditions. 3 In the database, we further subdivided these main categories into more detailed and specific diagnoses (which could be qualified as “potentially life‐threatening” conditions). These specific diagnoses were assessed by the primary investigator and were deemed necessary upon consultation with the study team, to be the first conditions initiating the chain of events that led to SMO as per WHO guidance (Figure 1). Other data entered into the database included general and obstetrical characteristics, maternal and perinatal outcomes and interventions up to 42 days postpartum.
Tygerberg Hospital serves as the only secondary and tertiary level hospital for the population of the Metro East health district. In addition, women from outside the region are also referred for various reasons. All women admitted to TBH from outside the Metro East region were included in the study, but not in the calculations of incidence, mortality index (MI) and MMR (Figure 2).
Data were only collected for women who were admitted to TBH, so the three level 1 referral district hospitals were requested to identify possible MNM cases that had not been referred to TBH from January 2015 to March 2015. This enabled the verification of our assumption that within the existing referral system all women with MNM would eventually be admitted in TBH.
Maternal deaths are routinely recorded and reported throughout South Africa at provincial level, as part of the National Confidential Enquiry into Maternal Death. This allowed easy identification of deaths within the region for the time period under study.
The control group consisted of pregnant women without SMO, included in the ongoing registration of the Safe Passage study in Metro East health district. 7 , 8 This large, prospective multidisciplinary study was initiated by the PASS Network (Prenatal Alcohol in SIDS and Stillbirth), which is designed to investigate the association between prenatal alcohol exposure, sudden infant death syndrome (SIDS) and stillbirth. It also aims determine the biologic basis of the spectrum of phenotypic outcomes of fetal alcohol spectrum disorders from exposure, as modified by environmental and genetic factors that increase risks of stillbirth, SIDS and, in surviving children, fetal alcohol spectrum disorders.
In this multicenter study, pregnant women at high risk for maternal drinking during pregnancy were recruited in the Northern Plains, USA, and in Cape Town, South Africa from August 2007 to January 2015. 7 , 8
A woman was eligible if all of the following criteria were met: (1) able to provide informed consent, (2) pregnant with one or two fetuses, (3) 16 years of age or older, (4) pregnancy duration of at least 6+0 weeks and not at the delivery admission, and (5) able to speak English or Afrikaans.
Inclusion in Cape Town was in two residential areas within Metro East and comprised all women who were pregnant or gave birth in this low‐risk population attending the Midwifery Obstetrical Units (level 1, district health service). Data collected in the Safe Passage study from January 2014 to January 2015 were controls for the SMO group.
Descriptive data are presented as numbers and percentages, as well as means ± standard deviation when distributed normally or medians (interquartile range) in the case of skewed data. SMO was dichotomized as either having SMO or not. MI was calculated by dividing the number of maternal deaths by the number of women with SMO. The assumption behind the MI is that a lower quality of maternity care is reflected by a higher MI.
Women with missing data are reported in the tables. Data were analyzed for single associated determinants through univariate logistic regression using SMO as outcome. To identify associations of combinations of determinants with SMO, multivariable logistic regression was performed. Women with SMO were matched to controls by age (with maximum 2 years difference) and parity (exact matching) in a 1:2 ratio for cases and controls.
Data were analyzed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA). There was no patient involvement in data interpretation or analysis.
3. RESULTS
During the 12‐month study period 32 161 live births occurred in Metro East health district. Twenty women died and 379, of whom 111 from outside Metro East region, were identified as having sustained MNM. This accounted for an MMR of 49.7 per 100 000 live births and an incidence of MNM of 8.6 per 1000 live births. These results gave an incidence of SMO of 9.1 per 1000 live births and an MI of 6.9%.
WHO MNM categories of organ failure are described in Table 1, which also contains interventions.
TABLE 1.
Categories and main interventions among women with SMO according to WHO MNM criteria a
| SMO (n = 399) | |
|---|---|
| Life‐threatening condition (MNM criteria) | n = 395b |
| Coagulation/hematologic | 162 (40.6) |
| Respiratory | 81 (20.3) |
| Uterine | 55 (13.8) |
| Neurologic | 48 (12.0) |
| Cardiovascular | 37 (9.3) |
| Renal | 9 (2.3) |
| Hepatic | 3 (0.8) |
| Interventions | |
| Intensive care unit (1–71 days) | 86/389 (21.6) |
| Obstetrical critical care unit (1–15 days) | 249/381 (62.4) |
| Hysterectomy | 59/399 (14.8) |
| More than five blood products | 137/380 (34.3) |
| Laparotomy | 15/399 (3.8) |
| Inotropic usec | 20/399 (5.0) |
| Intubationc | 106/397 (26.6) |
Abbreviations: MNM, maternal near miss; SMO, severe maternal outcome.
Values are given as number (percentage) or as number/total number (percentage).
Four in the unknown category.
Additions to the interventions of the WHO tool.
The three most common WHO MNM categories were: (1) coagulation/hematologic dysfunction in 162/399 (40.6%) women, (2) respiratory dysfunction in 81/399 (20.3%) women, and (3) uterine dysfunction in 55/399 (13.8%) women. In 295/399 (74.4%) women one dysfunction category was present; 75/399 (18.8%) had two categories and 24/399 (6.1%) had dysfunction in three or more categories.
Hypertensive disorders of pregnancy and obstetrical hemorrhage were the principle underlying conditions ultimately leading to SMO, shown in the detailed list of diagnoses that initiated the chain of events leading to SMO (Table 2).
TABLE 2.
Underlying primary diagnosis that qualified a woman for the MNM criteria a
| Direct causes (n = 329) | |
|---|---|
| Hypertension (n = 157; 39.3%) | |
| HELLP | 80 (20.0) |
| Eclampsia | 42 (10.5) |
| Pulmonary edema | 33 (8.3) |
| Liver rupture | 1 (0.3) |
| Acute renal failure | 1 (0.3) |
| Chronic hypertension | 0 |
| Pregnancy‐induced hypertension | 0 |
| Pre‐eclampsia | 0 |
| Acute fatty liver | 0 |
| Hemorrhage (n = 106; 26.6%) | |
| Placental abruption ± hypertension | 44 (11.0) |
| Bleeding related to CS during/after | 23 (5.8) |
| Uterine atony | 10 (3.0) |
| Retained placenta | 9 (2.7) |
| Perineal trauma | 6 (1.8) |
| Placenta previa | 5 (1.5) |
| Morbidly adherent placenta | 5 (1.5) |
| Ruptured uterus with(out) scar | 3 (0.9) |
| Uterine inversion | 1 (0.3) |
| Cervical trauma | 0 |
| Sepsis (n = 34; 8.5%) | |
| Puerperal sepsis after CS | 26 (7.9) |
| Puerperal sepsis after vagina birth | 5 (1.5) |
| Chorioamnionitis | 3 (0.9) |
| Other (n = 32; 8.0%) | |
| Ectopic | 13 (3.3) |
| Miscarriage | 2 (0.5) |
| Hyperemesis gravidarum | 0 |
| General anesthetic complication | 1 (0.3) |
| Spinal/epidural anesthetic complication | 1 (0.3) |
| Thrombo‐embolism | 2 (0.5) |
| Pulmonary embolism | 1 (0.3) |
| Amniotic fluid embolism | 1 (0.3) |
| Acute collapse (undefined) | 1 (0.3) |
| Other | 10 (2.5) |
| Indirect causes (n = 68) | |
|---|---|
| Non‐pregnancy‐related infection (n = 35; 8.8%) | |
| Respiratory tract | 18 (4.5) |
| Tuberculosis | 7 (1.7) |
| HIV | 3 (0.75) |
| Urinary tract | 3 (0.75) |
| Other | 4 (1.0) |
| Underlying medical disease (n = 33; 8.3%) | |
| Cardiac | 11 (2.8) |
| Endocrine | 5 (1.3) |
| Respiratory | 4 (1.0) |
| Trauma/suicide | 4 (1.0) |
| Hematologic | 2 (0.5) |
| Genitourinary | 2 (0.5) |
| Autoimmune | 1 (0.3) |
| Psychiatry | 2 (0.5) |
| Neoplasm | 2 (0.5) |
| Gastrointestinal | 0 |
| Central nervous system | 0 |
Abbreviations: CS, cesarean section; HELLP, hemolysis, elevated liver enzymes and low platelet count; MNM, maternal near miss.
Values are given as number (percentage).
In Table 3, general and obstetrical characteristics are depicted for women with SMO (399) compared with women without SMO (1005). Among 399 women with SMO, the principle underlying condition occurred antepartum in 189/399 (47.4%), peripartum in 54/399 (13.5%), and postpartum in 152/399 (38.1%).
TABLE 3.
Maternal and obstetrical characteristics for women with SMO in relation to women without SMO a
| SMO (n = 399) | Non‐SMO (n = 1005) | |
|---|---|---|
| Age, year | 27.0 ± 6.6 | 25.3 5.9b |
| Home language | (n = 389) | (n = 1014) |
| Xhosa | 199 (49.9) | 0 (0) |
| Afrikaans | 162 (40.6) | 795 (79.1) |
| English | 13 (3.2) | 87 (8.7) |
| Other | 15 (3.9) | 132 (12.2) |
| Marital status | (n = 387) | (n = 1005) |
| Unmarried | 310 (77.7)e | 741 (73.8)e |
| Married | 77 (19.3) | 264 (26.3) |
| Income in South African Randf | (n = 387) | (n = 674) |
| No income | 262 (67.7) | 0 (0) |
| 1–500 | 5 (1.3) | 122 (12.1) |
| 500–4000 | 105 (27.1) | 551 (54.8) |
| ≥400 | 15 (3.9) | 1 (0.1) |
| Gestational age, week | (n = 361) | (n = 1005) |
| ≤24 | 26 (7.2) | 15 (1.5) |
| 24–37 | 211 (58.4) | 139 (13.9) |
| ≥37 | 124 (34.3) | 851 (84.7) |
| BMI | 28.6 ± 7.7c | 25.4 ± 5.8d |
| HIV positive | 88/378 (23.3) | 14/1003 (1.4) |
| CD4 count >0 <200 | 20/87 (23.0) | NA |
| On antiretroviral drugs | 65/87 (74.7) | NA |
| Unknown | 10/87 (11.5) | NA |
| Hypertensive disorder | 149/399 | 142/1001 (14.2) |
| Chronic hypertension | 44 (11.0) | 40 (4.0) |
| Pregnancy‐induced hypertension | 21 (5.2) | 86 (8.6) |
| Pre‐eclampsia | 84 (21.1) | 16 (1.6) |
| Mode of birth | (n = 398) | (n = 989) |
| Induction | 147 (36.8) | 126 (13.4) |
| Spontaneous vaginal birth | 131 (32.9) | 780 (78.9) |
| Assisted vaginal birth | 7 (1.8) | 38 (3.8) |
| Cesarean sectiong | 218 (55.0) | 6,3 (16.5) |
| Neonatal outcome, births | (n = 390) | (n = 1005) |
| Live birth | 232/364 (63.7) | 985/1016 (97) |
| Stillbirth | 110/364 (30.2) | 17/1016 (1.7) |
| Neonatal death | 5/364 (1.3) | 6/1016 (0.6) |
| Sets of twins | 17/36 (4.7) | 11/1016 (1.1) |
| Otherh | 443/390 (11.0) | 14/1005 (1.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); SMO, severe maternal outcome.
Values are given as mean ± standard deviation, as number (percentage) or as number/total number (percentage).
Age known from 1003 women.
n =315.
n =992.
This group contains single and having a partner, but not married.
Per month in South African Rand (1 GBP is 21.0 SAR in November 2020).
Elective and emergency cesarean section and hysterotomy.
This group contains missing data and early pregnancies (<28 weeks or <700 g in case of unsure gestation).
Of the 399 women with SMO, 16/399 (4%) were referrals from home, 144/399 (36.1%) from Midwifery Obstetrical Units (level 1), 149/399 (37.3%) from level 1, and 39/399 (9.8%) from rural level 2 hospitals outside Metro East. Forty women (10%) had only attended pregnancy care in TBH. The majority of women who sustained SMO gave birth by cesarean section (CS) (218/398; 57.4%), of which 207/218 (95.0%) were emergency CS and 11/218 (5.05%) were elective CS. Reasons for emergency CS were fetal distress in 99/207 (47.8%) women and poor progress of labor in 25/207 (12.1%) women. In 95/218 (43.5%) women who gave birth by CS, SMO happened before CS. In 123/218 (56.4%) women, SMO happened during or after CS.
Pre‐existing conditions such as diabetes mellitus and autoimmune disease as well as substance use were not presented in the tables because numbers were too small to be significant.
Babies of women with SMO had poorer perinatal outcomes than babies of women without SMO, as described previously. 9 The SMO group had 232/364 (63.7%) live births and 110/364 (30.2%) stillbirths, compared with neonates of women without SMO, who comprised 985/1004 (97%) live births and 17/1004 (1.7%) stillbirths.
Univariable regression analyses revealed that factors associated with SMO were positive HIV status, hypertensive disorders of pregnancy and chronic hypertension, CS, and obesity with a two‐fold increase from obesity to severe obesity (Table 4). Applying 1:2 matching, 342 of 399 women with SMO could be matched with 784 women in the non‐SMO group. After matching for age and parity, multivariable regression analysis showed how multiple factors together were associated with SMO: positive HIV status (adjusted odds ratio [aOR] 24.8; 95% confidence interval [CI] 10.0–61.6), presence of pre‐eclampsia (aOR 17.5; 95% CI 7.9–38.7), and presence of chronic hypertension (aOR 2.45; 95% CI 1.1–5.1) were strong influencers. Birth by CS was also associated with SMO (aOR 8.4; 95% CI 5.8–12.3). In this model, obesity and pregnancy‐induced hypertension were not statistically significantly associated with SMO.
TABLE 4.
Associations with SMO (univariable and multivariable regression analysis)
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR | 95% CI | aOR | 95% CI | |
| Age | 1.05 | 1.03–1.07 | na | na |
| Parity | 1.01 | 0.87–1.12 | na | na |
| BMI | ||||
| Normal (BMI 18.5–24.9) | Ref. | Ref. | ||
| Underweight (BMI <18.5) | 0.91 | 0.45–1.73 | 0.59 | 0.24–1.43 |
| Overweight (BMI 25.0–29.9) | 1.89 | 1.39–2.74 | 1.51 | 0.80–2.41 |
| Obese (BMI 30.0–40.0) | 2.23 | 1.61–3.11 | 1.37 | 0.88–2.21 |
| Severe obesity (BMI >40.0) | 8.11 | 4.12–16.21 | 1.32 | 0.41–3.82 |
| Positive HIV | 20.12 | 11.51–35.42 | 24.82 | 10.01–61.62 |
| Cesarean section | 7.13 | 5.51–9.32 | 8.43 | 5.83–12.31 |
| Hypertensive disorder | ||||
| No hypertension | Ref. | Ref. | ||
| Chronic hypertension | 2.57 | 1.72–4.11 | 2.43 | 1.11–5.11 |
| Pregnancy‐induced hypertension | 0.04 | 0.25–0.65 | 0.05 | 0.02–0.13 |
| Pre‐eclampsia | 11.72 | 8.83–15.52 | 17.52 | 7.91–38.72 |
Abbreviations: aOR, adjusted odds ratio; BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); CI, confidence interval; na, not applicable; OR, odds ratio; Ref., reference; SMO, severe maternal outcome.
Causes of maternal death (20/399; 0.05%) were tuberculosis (five deaths), assault (three deaths), septic shock (three deaths), eclampsia (two deaths), placental abruption in the presence of pre‐eclampsia/disseminated intravascular coagulation (two deaths), and one each of suicide, traditional medicine, cervical cancer, acute respiratory distress syndrome, and postpartum hemorrhage.
In 3 months of data collection in surrounding level 1 hospitals, three women with MNM presenting in any of these three hospitals were not referred to TBH.
4. DISCUSSION
The incidence of women with SMO in Metro East region of 9.1 per 1000 live births corresponds with previously published data for middle‐income countries. 10 , 11 , 12 , 13 , 14 The WHO MNM tool allowed systematic analysis and indicated that hypertensive disorders of pregnancy and obstetrical hemorrhage were the principle underlying conditions associated with SMO. A positive HIV status, CS, pre‐eclampsia, and chronic hypertension were factors also associated with SMO.
The number of maternal deaths was relatively low in this setting, as was the MI of 6.9% compared with similar level‐of‐income settings, indicating relatively high quality of care. The incidence of SMO (9.1 per 1000 live births) in Metro East health district was slightly higher than in previous reports for Pretoria in 2013–2014 (SMO 5.1 per 1000 live births) 15 and in Metro West in 2014 (SMO 6.5 per 1000 live births). 16 This might be due to differences in data collection methods or in the application of WHO MNM criteria and warrants closer investigation.
The WHO MNM organ‐based criteria were helpful to systematically select cases, even though this involved extracting data from patient files and entering these into a separate database, which was relatively labor intensive and will probably not be possible in most resource‐poor settings. As only women with life‐threatening conditions were included, the figures do not allow for estimations of potentially life‐threatening conditions. Some women were found to be clinically very ill, but did not fit into any specific category of the WHO MNM criteria. Applying a criterion ‘other life‐threatening conditions as determined by the clinician’ may be considered.
The WHO life‐threatening condition categories are generic, so the provision of more specific diagnoses leading to SMO provided much more insight into the actual clinical problems. The authors suggest including a more comprehensive list of underlying diagnoses/“potentially life‐threatening” conditions into the tool.
A positive HIV status is strongly associated with SMO. Presence of HIV in pregnancy in South Africa of about 20% is described in the literature and confirmed in this study. 17 , 18 , 19 The majority of women with HIV in this study (65/87; 75%) were treated with antiretroviral therapy according to national guidelines and 20/87 (23%) had CD4 counts <200 cells per cubic millimeter of blood.
The high number of CS worldwide is an alarming trend 20 , 21 , 22 , 23 , 24 and in the SMO population, the mode of birth was CS in the majority of women (218/398; 55.0%). Whether CS was the actual start of the chain of events leading to SMO or part of the management of an underlying condition already present before CS and contributing to SMO is unclear, but in 123/218 (56.4%) women SMO only happened during or after CS, suggesting a possible association with mode of birth in some of these women. The results also indicate that adverse perinatal outcomes are clearly associated with SMO.
With specific reference to low‐ and middle‐income countries, collecting data for research remains a challenge, given the fact, that digitalization of a clearly defined referral pathway is often not present. 25 This should in theory limit the number of missed cases as all women with SMO are treated in well designated high‐level‐of‐care hospitals. Audit of SMO from which to learn lessons is extremely important to educate, explain, put in place safeguards, and effect changes to ensure similar outcomes are avoided. In TBH, women with SMO were discussed and evaluated in weekly MM and morbidity meetings and feedback was provided to the referral clinics in order to improve the quality of care. In this regard the joint effort for evaluation of reliable SMO incidence and associated determinants paves the way for optimizing tailored guidelines in the Metro‐East health district to improve maternal health.
A strength of this study is its region‐wide representation of more than 30 000 births. In addition, data collection was performed by a single dedicated investigator, allowing for uniform interpretation of the findings. Another strength was the use of a control group from the same region during the same time period, enabling identification of associated factors. This study showed that including only women who were admitted to the obstetrical critical care unit or the general intensive care unit, a method often used in other MNM studies, would have led to underreporting of SMO because almost 40% of women with SMO in this sample were never admitted to such units and stayed in the obstetrical or gynecology wards, in many cases due to bed shortages.
Although data collection of the large population was accurate, a limitation of the study was that only the public health sector was included. Data from the private health sector, which comprises about 15% of births in the region, were not available. Additionally, although most women were referred to TBH, some cases may inevitably have been missed as shown in our additional analysis in three level 1 hospitals.
In the control group, some women had eclampsia or HELLP (hemolysis, elevated liver enzymes and low platelet count) syndrome, even though these women did not meet the WHO MNM criteria applied in the study. If there was access to a control group without these conditions, a larger effect of the associated factors may have been seen.
This systematic analysis of women with SMO with the help of the WHO MNM tool gave insight into pregnancy‐related hypertension and obstetrical hemorrhage as the principle causes of SMO. A positive HIV status, birth by CS, and presence of pre‐eclampsia or chronic hypertension were identified as factors associated with SMO.
CONFLICTS OF INTEREST
The authors have no conflict of interest.
AUTHOR CONTRIBUTIONS
AH and LV designed the concept of the study and identified women with SMO. AH did the literature search, collected data and prepared the first draft with ES and TvdA and finalized the draft on the basis of comments from other authors and reviewer feedback. SG provided access to clinical data in the district. AH and MtW performed data analysis. TvdA, ES, LV, SG, MtW, JvR, JdV, GT contributed to interpretation of the data analysis, reviewed study protocol, and critically commented on all drafts of the manuscript. JdV and GT provided overall guidance and supervision. All authors approved the final manuscript for publication.
ACKNOWLEDGMENTS
We thank Prof Hein Odendaal and Lucy T Brink, MSc, for sharing the useful data of the Safe Passage study that was used as the non‐SMO group. We thank staff members of all participating facilities who contributed to data collection and the Western Cape Department of Health and the CEOs of the hospital for allowing access to the folders and permission to publish the data.
Heitkamp A, Vollmer (Murray) L, van den Akker T, et al. Great saves or near misses? Severe maternal outcome in Metro East, South Africa: A region‐wide population‐based case‐control study. Int J Gynecol Obstet. 2022;157:173–180. 10.1002/ijgo.13739
REFERENCES
- 1. World Health Organization . Trends in Maternal Mortality: 2000 to 2017: Estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: WHO; 2019. [Google Scholar]
- 2. Damian DJ, Njau B, Lisasi E, Msuya SE, Boulle A. Trends in maternal and neonatal mortality in South Africa: a systematic review. Syst Rev. 2019;8(1):76. 10.1186/s13643-019-0991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization Library . Evaluating the Qualitative Care of Severe Pregnancy Complications. The WHO Near Miss Approach for Maternal Health. Geneva: WHO; 2011. [Google Scholar]
- 4. Say L, Souza JP, Pattinson RC. WHO working group on Maternal Mortality and Morbidity classifications. Maternal near miss—towards a standard tool for monitoring quality of maternal health care. Best Pract Res Clin Obstet Gynaecol. 2009;23(3):287‐296. 10.1016/j.bpobgyn.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 5. Pattinson RC, Buchmann E, Mantel G, Schoon M, Rees H. Can enquiries into severe acute maternal morbidity act as a surrogate for maternal death enquiries? BJOG. 2003;110(10):889‐893. [PubMed] [Google Scholar]
- 6. Mantel GD, Buchmann E, Rees H, Pattinson RC. Severe acute maternal morbidity: a pilot study of a definition for a near‐miss. Br J Obstet Gynaecol. 1998;105(9):985‐990. 10.1111/j.1471-0528.1998.tb10262.x. [DOI] [PubMed] [Google Scholar]
- 7. Dukes KA, Burd L, Elliott AJ, et al. PASS Research Network. The safe passage study: design, methods, recruitment, and follow‐up approach. Paediatr Perinat Epidemiol. 2014;28(5):455‐465. 10.1111/ppe.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elliott AJ, Kinney HC, Haynes RL, et al. Concurrent prenatal drinking and smoking increases risk for SIDS: Safe Passage Study report. EClinicalMedicine. 2020;20(19):100247. 10.1016/j.eclinm.2019.100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aminu M, Unkels R, Mdegela M, Utz B, Adaji S, van den Broek N. Causes of and factors associated with stillbirth in low‐ and middle‐income countries: a systematic literature review. BJOG. 2014;121(Suppl 4):141‐153. 10.1111/1471-0528.12995. [DOI] [PubMed] [Google Scholar]
- 10. Cecatti JG, Souza JP, Parpinelli MA, et al. Brazilian Network for Surveillance of Severe Maternal Morbidity. Brazilian network for the surveillance of maternal potentially life threatening morbidity and maternal near‐miss and a multidimensional evaluation of their long term consequences. Reprod Health. 2009;24(6):15. 10.1186/1742-4755-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heemelaar S, Josef M, Diener Z, et al. Maternal near‐miss surveillance, Namibia. Bull World Health Organ. 2020;98(8):548‐557. 10.2471/BLT.20.251371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shen FR, Liu M, Zhang X, Yang W, Chen YG. Factors associated with maternal near‐miss morbidity and mortality in Kowloon Hospital, Suzhou, China. Int J Gynaecol Obstet. 2013;123(1):64‐67. [DOI] [PubMed] [Google Scholar]
- 13. Adamu AN, Okusanya BO, Tukur J, et al. Maternal near‐miss and death among women with hypertensive disorders in pregnancy: a secondary analysis of the Nigeria Near‐miss and Maternal Death Survey. BJOG. 2019;126(Suppl 3):12‐18. 10.1111/1471-0528.15427. [DOI] [PubMed] [Google Scholar]
- 14. Sotunsa JO, Adeniyi AA, Imaralu JO, et al. Maternal near‐miss and death among women with postpartum haemorrhage: a secondary analysis of the Nigeria Near‐miss and Maternal Death Survey. BJOG. 2019;126(Suppl 3):19‐25. 10.1111/1471-0528.15624. [DOI] [PubMed] [Google Scholar]
- 15. Soma‐Pillay P, Pattinson RC, Langa‐Mlambo L, Nkosi BS, Macdonald AP. Maternal near miss and maternal death in the Pretoria Academic Complex, South Africa: a population‐based study. S Afr Med J. 2015;105(7):578‐663. 10.7196/SAMJnew.8038. [DOI] [PubMed] [Google Scholar]
- 16. Iwuh IA, Fawcus S, Schoeman L. Maternal near‐miss audit in the Metro West maternity service, Cape Town, South Africa: a retrospective observational study. S Afr Med J. 2018;108(3):171‐175. 10.7196/SAMJ.2018.v108i3.12876. [DOI] [PubMed] [Google Scholar]
- 17. van der Spuy ZM. HIV and reproductive health: a South African experience. Reprod Biomed Online. 2009;18(Suppl 2):3‐10. 10.1016/s1472-6483(10)60441-5. [DOI] [PubMed] [Google Scholar]
- 18. Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384(9939):258‐271. 10.1016/S0140-6736(14)60164-1. Erratum in: Lancet. 2014;384(9948):1098. [DOI] [PubMed] [Google Scholar]
- 19. South Africa Every Death Counts Writing Group , Bradshaw D, Chopra M, et al. Every death counts: use of mortality audit data for decision making to save the lives of mothers, babies, and children in South Africa. Lancet. 2008;371(9620):1294‐1304. 10.1016/S0140-6736(08)60564-4. Erratum in: Lancet. 2008 Sep 27;372(9644):1150. [DOI] [PubMed] [Google Scholar]
- 20. Visser GHA, Ayres‐de‐Campos D, Barnea ER, et al. FIGO position paper: how to stop the caesarean section epidemic. Lancet. 2018;392(10155):1286‐1287. 10.1016/S0140-6736(18)32113-5. [DOI] [PubMed] [Google Scholar]
- 21. Bishop D, Dyer RA, Maswime S, et al. ASOS investigators. Maternal and neonatal outcomes after caesarean delivery in the African Surgical Outcomes Study: a 7‐day prospective observational cohort study. Lancet Glob Health. 2019;7(4):e513‐e522. 10.1016/S2214-109X(19)30036-1. Erratum in: Lancet Glob Health. 2019 Aug;7(8):e1019. [DOI] [PubMed] [Google Scholar]
- 22. Sobhy S, Arroyo‐Manzano D, Murugesu N, et al. Maternal and perinatal mortality and complications associated with caesarean section in low‐income and middle‐income countries: a systematic review and meta‐analysis. Lancet. 2019;393(10184):1973‐1982. 10.1016/S0140-6736(18)32386-9. [DOI] [PubMed] [Google Scholar]
- 23. Moodley J, Pattinson RC, Fawcus S, Schoon MG, Moran N, Shweni PM. National Committee on Confidential Enquiries into Maternal Deaths in South Africa. The confidential enquiry into maternal deaths in South Africa: a case study. BJOG. 2014;121(Suppl 4):53‐60. 10.1111/1471-0528.12869. [DOI] [PubMed] [Google Scholar]
- 24. Maswime S, Buchmann E. Causes and avoidable factors in maternal death due to cesarean‐related hemorrhage in South Africa. Int J Gynaecol Obstet. 2016;134(3):320‐323. 10.1016/j.ijgo.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 25. Sewankambo NK, Katamba A. Health systems in Africa: learning from South Africa. Lancet. 2009;374(9694):957‐959. 10.1016/S0140-6736(09)61244-7. [DOI] [PubMed] [Google Scholar]


