Abstract
Monoalkylated derivatives of the unusually polar all‐cis 2,3,4,5,6‐ pentafluorocyclohexyl (Janus face) motif are prepared starting from an aryl hydrogenation of 2,3,4,5,6‐ pentafluorophenylacetate methyl ester 15. The method used Zeng's Rh(CAAC) carbene catalyst 4 in the hydrogenation following the protocol developed by Glorius. The resultant Janus pentafluorocyclohexylacetate methyl ester 16 was converted to the corresponding alcohol 18, aldehyde 13, bromide 29 and azide 14 through functional group manipulations, and some of these building blocks were used in Ugi‐multicomponent and Cu‐catalysed click reactions. NBoc protected pentafluoroarylphenylalanine methyl ester 35 was also subject to an aryl hydrogenation, and then deprotection to generate the Janus face β‐pentafluorocyclohexyl‐alanine amino acid 15, which was incorporated into representative members of an emerging class of candidate antiviral compounds. Log P measurements demonstrate that the all‐cis 2,3,4,5,6‐pentafluorocyclohexyl ring system is more polar than a phenyl ring. In overview the paper introduces new building blocks containing this Janus ring and demonstrates their progression to molecules typically used in bioactives discovery programmes.
Keywords: aryl hydrogenation, fluorocyclohexanes, Janus motif, organic chemistry, organofluorine chemistry
Janus all‐cis 2,3,4,5,6‐pentafluorocyclohexyl building blocks have been incorporated into medicinal chemistry type products. These cyclohexanes have polarized faces and represent a unique motif for medicinal chemistry. The paper introduces a range of new accessible building blocks which can be elaborated into a diversity of drug type structures demonstrating their wider utility for exploration in bioactives discovery chemistry.
Introduction
Over the last two decades or so organofluorine chemistry has experienced a ‘Renaissance’, driven by an interest in addressing the synthesis and properties of products carrying isolated −F or −CF3 groups, particularly when attached to sp3 carbons. [1] This is distinct from an immediately previous era which was defined by the perfluorocarbons and fluoropolymers industries or within fine chemicals, with a focus on aryl −F and −CF3 compounds. [2] The prospects of novel bioactives (med‐chem and agrochemicals) resulting from stereogenic fluorine has driven this more recent agenda, and innovation has been stoked by the intense international effort and extraordinary growth in new methods development and catalysis, which defines modern organic chemistry. [3] Access to this next generation of selectively fluorinated alkyls has led to a re‐evaluation of dogmas such as ‘fluorine increases lipophilicity’. In many cases −F or −CF3 substituted alkyls become more polar (less lipophilic) than the hydrocarbon and such fluorine effects are being explored in detail. [4]
We have had a long interest in selective fluorination of alkyl chains, and particularly in a class of molecules which we have termed ‘multi vicinal fluoroalkanes’ (Figure 1a) that are defined by placing a fluorine on each carbon along a chain and with a defined stereochemistry, to limit isomer mixtures. [5] The programme progressed from linear to cyclic alkanes and most recently we have been exploring the synthesis and properties of cyclohexanes with multiple vicinal fluorines attached around the ring with the all‐cis stereochemistry.[ 6 , 7 , 8 , 9 , 10 ] The parent structure, all‐cis 1,2,3,4,5,6‐hexafluorocyclohexane 1 adopts a chair conformation resulting in triaxial C−F bonds, and this arrangement imparts a very high polarity to the ring system as demonstrated by the extraordinary polar properties of 1 (Figure 1b). Cyclohexane 1 has a melting point of 208 °C and a dipole moment of 6.5 D which are particularly high values for an aliphatic. [6] This has been termed a ‘Janus’ ring system as the fluorine and hydrogen faces, face in opposite directions and are responsible for imparting this polarity. [11]
Figure 1.
(a) Acyclic multivicinal fluoroalkane chain, adopts a helical conformation. (b) Janus face aspect imparts high polarity to cyclohexane 1 and derivatives 2 with all‐cis fluorines. (c) Monoalkylated derivatives 2 favour the more polar triaxial C−F conformation with an equatorial alkyl group. [10]
Most recently [10] we have explored monoalkylated structures of all‐cis pentafluorocyclohexane represented by 2 a and 2 b, where R is either Me or Et respectively. These systems adopt a conformation, both in gas phase calculations and in solid state structures, where the R substituent lies equatorial and there are three triaxial C−F bonds, rather than the R group axial and with two diaxial C−F bonds. Therefore against expectation, the preferred conformation is the more polar for mono alkylated ring systems. There is a recent and developing interest in applying this motif to solution state coordination of anions [12] and for controlled supramolecular assembly of polymers [13] due to the self association of the polar rings, however these derivatives have yet to be explored in a medicinal chemistry context. In that context the Janus all‐cis pentafluorocyclohexyl ring system is anticipated to have unique properties not represented by any other substituent in organic chemistry as it has an electronegative fluorine face and an electropositive hydrogen face, and both faces have the potential to interact,[8][12] with polar amino acid side chains of opposite electrostatic polarity in protein receptors or enzymes.
Previously [14] we have reported on the preparation of building blocks for the incorporation of all‐cis 2,3,5,6 ‐ tetrafluorocyclohexane motifs into molecular architectures appropriate to bioactives discovery with illustrative examples shown in Figure 2. For example amino acid 4 could be prepared [14d] in various protected forms and the peptoid product 6 [14c] represents a range of products which were generated through Ugi and Passerini multicomponent reactions. [15] With such compounds in hand it became apparent that the fluorine of the 2,3,5,6 ‐ tetrafluorocyclohexane ring system rendered these compounds significantly more hydrophilic relative to their aromatic and non‐fluorinated cyclohexyl analogues. [16] This is a striking feature of the ring system as fluorine is generally considered to impart lipophilicity, however in these ring systems the fluorine imparts hydrophilicity, as determined by comparative Log P measurements, illustrated for the anilines 7–10 in Figure 2. This is due to the polar nature of the fluorocyclohexane ring with water associating to the protic face of the Janus rings through hydrogen bonding. [16] In this paper we now explore the all‐cis 1,2,3,4,5‐pentafluorocyclohexane ring system in a similar context. Until recently this arrangement of a substituted cyclohexane and with five fluorine around the ring was not synthetically accessible, however the Glorius lab have developed [17] a catalytic fluoroaryl hydrogenation which we have used here to access this class of compounds. The prototype example shown in Figure 3 demonstrated the efficient hydrogenation of hexafluorobenzene 11 using Zeng's CAAC catalyst 12[ 18] to generate hexafluorocyclohexane 1, and the method was extended to prepare a range of all‐cis fluorocyclohexanes and also with different functionalities. [17]
Figure 2.
Previous disclosures on all‐cis 2,3,5,6 ‐ tetrafluorocyclohexane motifs. (a) An amino acid derivative 4; [14d] (b) aldehyde 5 as an Ugi component. (c). Log P trends across anilines 7–10 indicate increased hydrophilicity with increased fluorination. [16]
Figure 3.
Catalyst and cis‐selective hydrogenation of hexafluorobenzene 11 to give 1 as reported by Glorius.[ 17 , 18 ]
In this paper we report the preparation of a range of building blocks incorporating the all‐cis 2,3,4,5,6‐pentafluoro‐ cyclohexypentafluoro cyclohexyl motif but with a particular focus on elaborations of aldehyde 13, azide 14 and amino acid 15 as illustrated in Figure 4.
Figure 4.
Key building blocks 13–15 carrying the all‐cis 2,3,4,5,6‐pentafluorocyclohexyl motif used in this study.
The utility of aldehyde 13 is explored in Ugi multicomponent reactions, [15] the azide in Cu‐catalysed ‘click’ reactions [19] and the amino acid is incorporated into known coronavirus inhibitor analogues.[ 20 , 21 ] It emerges as a general phenomenon that the replacement of cyclohexyl or aryl ring by the all‐cis 2,3,4,5,6‐pentafluorocyclohexyl moiety results in a significant reduction in Log P values, which suggests that these motifs would be quite acceptable starting points, for example as fragments for bioactives and drug discovery programmes.
Results and Discussion
The pentafluoroaryl phenylacetate ester 16 is readily available and offered a convenient starting point for building block development. Aryl hydrogenation using the Zeng catalyst 12 [17] and following Glorius protocols [16] resulted in an efficient conversion to the corresponding cyclohexyl ester 17 as the major product with the 5‐defluorinated co‐product 18 as a minor product (17 : 18, 11 : 1). The regiospecific nature of the defluorination was established by X‐ray crystallography of 18 (Scheme 1). This minor co‐product could be removed by chromatography and 17 was progressed to further transformations. Reduction of the ester moiety of 17 to aldehyde 13 was carried out in a two‐step protocol as a one‐step controlled reduction with DIBAL did not offer any yield advantage. Accordingly, ester 17 was reduced to alcohol 19 with DIBAL and this product was conveniently oxidised to aldehyde 13 after exposure to Dess‐Martin periodinane. [22]
Scheme 1.
i. 12 (1 mol%), H2 (50 bar), hexane, 4 Å MS, r.t., 16h; ii. DIBAL, THF, 0 °C to r.t, 16h, 83%; iii. Dess‐Martin periodinane, THF, r.t, 45min, 85%. X‐ray structure of 17.
1H NMR analysis revealed that aldehyde 13 readily forms a hemiacetal with [2H4]‐methanol suggesting a significant electron withdrawing effect of the pentafluorocyclohexane ring system. None‐ the‐less it was a good substrate in a number of 4‐component Ugi reactions, of which the products 20–25 are summarised in Figure 5.
Figure 5.
General Ugi multicomponent procedure with aldehyde 13 showing isolated yields and product structures 20–25.
Two of these products (20 and 25) were amenable to X‐ray structure analysis as shown in Figure 6 and this assisted in confirming their peptoid like structures. In order to elucidate the effect of the 2,3,4,5,6‐all‐cis‐pentafluorocyclohexane ring on lipophilicity of this product class, Log P values were determined for products 20–22 and compared to their corresponding analogues 26–28 with a phenyl ring in place of the 2,3,4,5,6‐all‐cis‐pentafluorocyclohexane rings. The Log P values were determined by HPLC (acetonitrile/water) as described previously[ 14 , 16 ] and the comparative data are presented in Figure 7. It is clear that replacement of a phenyl ring by the all‐cis 2,3,4,5,6‐pentafluorocyclohexyl moiety increases hydrophilicity, with the Log P decreasing by 0.4–0.65 Log P units. This trend is consistent with that previously observed for the phenyltetrafluorocyclohexyl motifs, [14] and also the all cis‐1,2,3‐trifluorocyclopropyl motif,24 and it suggests that this aspect reinforces its utility as a candidate isostere [25] for hit optimisation studies.
Figure 6.

X‐ray crystal structures of Ugi multicomponent products 20 and 25.
Figure 7.
Comparative Log P values of Ugi fluorocyclohexyl products 20–22 with the corresponding benzyl products 26–28.
In other functional group interconversions, alcohol 19 was readily converted to the corresponding bromide 29 by an Appel reaction, [26] and the bromide to azide 14 as shown in Figure 8a. Azide 14 is a crystalline solid and the corresponding X‐ray is also shown in Figure 8a. Azide 14 could be readily reduced under Staudinger conditions [27] to amine 34 to provide another building block. Azide 14 was also a relatively efficient substrate in a series of Cu‐catalysed ‘click’ reactions [19] with phenylacetylenes to give the products 30–33 as illustrated in Figure 8b.
Figure 8.

(a) Reactions forming azide 14, amine 34 and ‘click’ products 30–33. X‐ray structures of triazole 31, azide 14, and amine hydrochloride of 34. (b) ‘Click’ triazole products 30–33 derived from 14 and an appropriate phenyl acetylene.
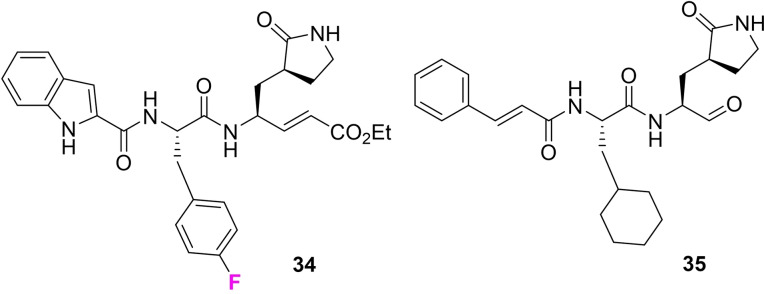
A potentially interesting building block of wide utility is amino acid 15. We report its synthesis here and then its incorporation into the structural skeleton of a class of antiviral analogues typified by 34 and 35 in Figure 9. This structural series of peptidomimetic aldehydes and esters has emerged as potent inhibitors of the human enterovirus 71(EV‐71) and coronavirus (SARS) protease, [20] and interest in this class of compounds has received a particular and recent focus as a starting point to find inhibitors of the COVID‐19 coronavirus. [21] In particular those compounds 34 and 35 containing fluoroaryl‐ phenylalanine and cyclohexyl‐amino acid show good antiviral activities. [20] In the context of exploring the all‐cis 2,3,4,5,6‐pentafluorocyclohexyl moiety in drug discovery, the two aldehydes 45 and 48 (Scheme 2) and the two esters 52 and 53 (Scheme 3) of this class of antivirals were prepared carrying the Janus ring in place of phenyl or cyclohexyl. These syntheses required amino acid 15 which could be prepared by aryl hydrogenation of the pentafluoroaryl analogue of fully protected phenylalanine 36, itself prepared after methylation of the commercially available NBoc protected L‐amino acid. [28] Hydrogenation using the Glorius/Zeng protocol[ 16 , 17 ] generated the cyclohexylamino acid 37 in good yield. The pentafluorocyclohexyl ring system is susceptible to base decomposition, and selective ester hydrolysis under acidic conditions proved difficult, therefore the free amino acid 15 was generated and its structure confirmed by X‐ray analysis (inset in Scheme 2). This amino acid was then re‐protected with the NBoc group to give 38. [29] The synthesis to the target aldehydes 45 and 48 with either a cinnamate or indole‐carboxylate amide could then be completed following the published [20c] protocols as illustrated in Scheme 2. Similarly, the corresponding conjugated ethyl ester analogues 52 and 53, carrying the pendant cinnamate or indole‐carboxylate amides respectively were prepared also from NBoc protected amino acid 38 following the previous protocols, [20d] with only minor modifications as illustrated in Scheme 3. During the Boc deprotection of 50 to get 51 in methanol, trans esterification of ethyl ester to methyl ester was observed (1 : 1 mixture) and the reaction of 51 upon reaction with 42 gave a 1 : 1 mixture of 52. Knowing this, the de protection of Boc for the synthesis of 53 was performed in ethanol and achieved pure 53.
Figure 9.
Representatives 34 and 35 of lead Enterovirus‐71 and SARS‐2 proteases inhibitors. [20]
Scheme 2.
Synthesis of antiviral candidates 45 and 48 from amino acid 15.
Scheme 3.
Syntheses of antiviral candidates 52 and 53.
Log P values were determined[ 10 , 16 ] for products 45, 48, 52 and 53. The values were in the range Log P ∼1.5–2.0. These are low values and in a good range for candidate drug development, and they indicate again that the all‐cis 2,3,4,5,6‐pentafluoro‐cyclohexyl ring system possesses good properties for bioactive molecule discovery. In the event the candidate antivirals 45, 48, 52 and 53 were assessed for their antiviral activity against Zika virus and the SARS‐CoV‐2 virus, however they did not show any activity in these assays. [30]
Conclusion
Several building blocks have been prepared by direct aryl hydrogenation of all‐ 2,3,4,5,6‐pentafluorophenyl acetaldehyde 16 and the NBoc‐protected methyl ester of pentafluoroaryl phenylalanine. In particular a series of candidate bioactives were prepared from cyclohexylacetaldehyde 13 and amino acid 15. Aldehyde 13, was used to prepare a range of Ugi multicomponent products and the amino acid to access direct analogues of known peptidomimetic aldehydes and esters. Comparative Log P determinations between phenyl and all‐cis 2,3,4,5,6‐pentafluorocyclohexyl analogues illustrates an increase in hydrophilicity for the fluorocyclohexanes and suggests a utility for this motif in bioactives discovery programmes. These results should encourage the further exploration of this motif in medicinal chemistry.
X‐ray crystallography
Deposition Number(s) 2098747 (14), 2098748 (15), 2098749 (17), 2098750 (18), 2098751 (19), 2098752 (20), 2098753 (25), 2098754 (31), 2098755 (34HCl), 2098756 (37), 2098757 (53) contain(s) the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
We thank EPSRC for a grant (EP/R013799/1) and Studentships (JLC) through the EPSRC CRITICAT CDT programme and also the Chinese Scholarship Council (CY). We also thank Professor Arvind H Patel at the MRC Centre for Virus Research at the University of Glasgow for antiviral assays.
J. L. Clark, R. M. Neyyappadath, C. Yu, A. M. Z. Slawin, D. B. Cordes, D. O'Hagan, Chem. Eur. J. 2021, 27, 16000.
References
- 1.
- 1a.S. Meyer, J. Hafliger, R. Gilmour, Chem. Sci. 2021, 12, 10686–10695; [DOI] [PMC free article] [PubMed]
- 1b.R. Hevey, Chem. Eur. J. 2021, 27, 2240–2253; [DOI] [PubMed]
- 1c.I. Ojima (Ed.), ‘Frontiers of Organic Fluorine Chemistry’, World Scientific Europe, 2020;
- 1d.Q. H. Liu, C. F. Ni, J. B. Hu Hu, Nat. Sci. Rev. 2017, 4, 303–325;
- 1e.A. Harsanyi, G. Sandford, Green Chem. 2015, 17, 2081–2086;
- 1f.P. Kirsch, ‘Modern Fluoroorganic Chemistry. Synthesis, Reactivity, Applications.’ 2nd Edn., Wiley-VCH, Weinheim, 2013.
- 2.
- 2a.R. D. Chambers, ‘Fluorine in Organic Chemistry’ 2nd Edn., Blackwell Publishing Ltd., Oxford, 2004;
- 2b.M. Hudlicky, A. E. Pavlath, ‘Chemistry of Organofluorine Compounds II,’ ACS Monograph 187, American Chemical Society, Washington 1995.
- 3.
- 3a. Zhu W., Zhen X., Wu J., Cheng Y., An J., Ma X., Liu J., Qin Y., Zhu H., Xue J., Jiang X., Nat. Commun. 2021, 12, 3957; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b. Scheidt F., Schäfer M., Sarie J. C., Daniliuc C. G., Molloy J. J., Gilmour R., Angew. Chem. Int. Ed. 2018, 57, 16431–16435; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 16669–16673; [Google Scholar]
- 3c. Banik S. M., Medley J. W., Jacobsen E. N., J. Am. Chem. Soc. 2016, 138, 5000–5003; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3d. Molnar I. G., Gilmour R., J. Am. Chem. Soc. 2016, 138, 5004–5007; [DOI] [PubMed] [Google Scholar]
- 3e. Beeson T. D., MacMillan D. W. C., J. Am. Chem. Soc. 2005, 127, 8826–8828; [DOI] [PubMed] [Google Scholar]
- 3f. Hintermann L., Togni A., Angew. Chem. Int. Ed. 2000, 39, 4359–4362; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2000, 112, 4530–4533. [Google Scholar]
- 4.
- 4a. Jeffries B. F. J., Wang Z., Felstead H. R., Le Questel J. Y., Scott J., Chiarparin E., Graton J., Linclau B., J. Med. Chem. 2020, 63, 1002–1031; [DOI] [PubMed] [Google Scholar]
- 4b. Huchet Q. A., Trapp N., Kuhn B., Wagner B., Fischer H., Kratochwil N. A., Carreira E. M., Müller K., J. Fluorine Chem. 2017, 198, 34–46; [Google Scholar]
- 4c. Vorberg R., Trapp N., Zimmerli D., Wagner B., Fischer H., Kratochwil N. A., Kansy M., Carreira E. M., Müller K. M., ChemMedChem 2016, 11, 2216–2239; [DOI] [PubMed] [Google Scholar]
- 4d. Bôhm H.-J, Banner D., Bendels S., Kansy M., Kuhn B., Müller K., Obst-Sander U., Stahl M., ChemBioChem. 2004, 5, 637–643. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. O'Hagan D., Chem. Eur. J. 2020, 26, 7981–7997; [DOI] [PubMed] [Google Scholar]
- 5b. Al-Maharik N., Cordes D. B., Slawin A. M. Z., Bühl M., O'Hagan D., Org. Biomol. Chem. 2020, 18, 878–887; [DOI] [PubMed] [Google Scholar]
- 5c. O′Hagan D., J. Org. Chem. 2012, 77, 3689–3699; [DOI] [PubMed] [Google Scholar]
- 5d. Hunter L., Kirsch P., Slawin A. M. Z., O'Hagan D., Angew. Chem. Int. Ed. 2009, 48, 5457–5460; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 5565–5568. [Google Scholar]
- 6. Keddie N. S., Slawin A. M. Z., Lebl T., Philp D., O'Hagan D., Nat. Chem. 2015, 7, 483–488. [DOI] [PubMed] [Google Scholar]
- 7. Cormanich R. A., Keddie N., Rittner R., O'Hagan D., Bühl M., Phys. Chem. Chem. Phys. 2015, 44, 29475–29478. [DOI] [PubMed] [Google Scholar]
- 8. Ziegler B. E., Lecours M., Marta R. A., Featherstone J., Fillion E., Hopkins W. S., Steinmetz V., Keddie N. S., O'Hagan D., McMahon T. B., J. Am. Chem. Soc. 2016, 138, 7460–7463. [DOI] [PubMed] [Google Scholar]
- 9. Lecours M. J., Marta R. A., Steinmetz V., Keddie N., Fillion E., O'Hagan D., McMahon T. B., Hopkins W. S., J. Phys. Chem. Lett. 2017, 8, 109–113. [DOI] [PubMed] [Google Scholar]
- 10. Clark J. L., Taylor A., Geddis A., Neyyappadath R. M., Piscelli B. A., Yu C., Cordes D. B., Slawin A. M. Z., Cormanich R. A., Guldin S., O'Hagan D., Chem. Sci. 2021, 12, 9712–9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santschi N., Gilmour R., Nat. Chem. 2015, 7, 467–468. [DOI] [PubMed] [Google Scholar]
- 12. Shyshov O., Siewerth K. A., von Delius M., Chem. Commun. 2018, 54, 4353–4355. [DOI] [PubMed] [Google Scholar]
- 13. Shyshov O., Haridas S. V., Pesce L., Qi H., Gardin A., Bochicchio D., Kaiser U., Pavan G. M., von Delius M., Nat. Commun. 2021, 12, 3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.
- 14a.T. Bykova, N. Al-Maharik, A. M. Z. Slawin, M. Bühl, T. Lebl, D. O’ Hagan, Chem. Eur. J. 2018, 24, 13290–13296; [DOI] [PubMed]
- 14b.T. Bykova, N. Al-Maharik, A. M. Z. Slawin, D. O'Hagan, Beilstein J. Org. Chem. 2017, 13, 728–733; [DOI] [PMC free article] [PubMed]
- 14c. Bykova T., Al-Maharik N., Slawin A. M. Z., O'Hagan D., Org. Biomol. Chem. 2016, 14, 1117–1123; [DOI] [PubMed] [Google Scholar]
- 14d. Ayoup M. S., Cordes D. B., Slawin A. M. Z., O'Hagan D., Org. Biomol. Chem. 2015, 13, 5621–5624; [DOI] [PubMed] [Google Scholar]
- 14e. Salah Ayoup M., Cordes D. B., Slawin A. M. Z., O'Hagan D., Beilstein J. Org. Chem. 2015, 11, 2671–2676; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14f. Durie A. J., Fujiwara T., Cormanich R., Bűhl M., Slawin A. M. Z., O'Hagan D., Chem. Eur. J. 2014, 20, 6259–6263. [DOI] [PubMed] [Google Scholar]
- 15.
- 15a. Wang Q., Wang D.-X., Wang M.-X., Zhu J., Acc. Chem. Res. 2018, 51, 1290–1300; [DOI] [PubMed] [Google Scholar]
- 15b. Ugi I., Dömling A., Hörl W., Endeavour 1994, 18, 115–122. [Google Scholar]
- 16. Rodil A., Bosisio S., Ayoup M. S., Quinn L., Cordes D. B., Slawin A. M. Z., Murphy C. D., Michel J., O'Hagan D., Chem. Sci. 2018, 9, 3023–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.
- 17a. Nairoukh Z., Wollenburg M., Schlepphorst C., Bergander K., Glorius F., Nat. Chem. 2019, 11, 264–270; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17b. Wiesenfeldt M. P., Knecht T., Schlepphorst C., Glorius F., Angew. Chem. Int. Ed. 2018, 57, 8297–8300; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 8429–8432; [Google Scholar]
- 17c. Wiesenfeldt M. P., Nairoukh Z., Li W., Glorius F., Science 2017, 357, 908–912. [DOI] [PubMed] [Google Scholar]
- 18.
- 18a. Zhang X., Ling L., Luo M., Zeng X., Angew. Chem. Int. Ed. 2019, 58, 16785–16789; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 16941–16945; [Google Scholar]
- 18b. Wei Y., Rao B., Cong X., Zeng X., J. Am. Chem. Soc. 2015, 137, 9250–9253. [DOI] [PubMed] [Google Scholar]
- 19.
- 19a. Tornøe C. W., Christensen C., Meldal M., J. Org. Chem. 2002, 67, 3057–3064; [DOI] [PubMed] [Google Scholar]
- 19b. Rostovtsev V. V., Green L. G., Fokin V. V., Sharpless K. B., Angew. Chem. Int. Ed. 2002, 41, 2596–2599; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2002, 114, 2708–2711. [Google Scholar]
- 20.
- 20a. Dai W., Jochmans D., Xie H., Yang H., Li J., Su H., Chang D., Wang J., Peng J., Zhu L., Nian Y., Hilgenfeld R., Jiang H., Chen K., Zhang L., Xu Y., Neyts J., Liu H., J. Med. Chem. 2021, 64, 10.1021/acs.jmedchem.0c02258; [DOI] [PubMed] [Google Scholar]
- 20b. Dai W., Zhang B., Jiang X.-M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F., Li C., Li Y., Bai F., Wang H., Cheng X., Cen X., Hu S., Yang X., Wang J., Liu X., Xiao G., Jiang H., Rao Z., Zhang L.-K., Xu Y., Yang H., Liu H., Science 2020, 363, 1331–1335; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20c. Zhai Y., Zhao X., Cui Z., Wang M., Wang Y., Li L., Sun Q., Yang X., Zeng D., Liu Y., Sun Y., Lou Z., Shang L., Yin Z., J. Med. Chem. 2015, 58, 9414–9420; [DOI] [PubMed] [Google Scholar]
- 20d. Kuo C.-J., Shie J.-J., Fang J.-M., Yen G.-R., Hsu J. T. A., Liu H.-G., Tseng S.-N., Chang S.-C., Lee C.-Y., Shih S.-R., Liang P.-H., Bioorg. Med. Chem. 2008, 16, 7388–7398; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20e. Anand K., Ziebuhr J., Wadhwani P., Mesters J. R., Hilgenfeld R., Science 2003, 300, 1763–1767. [DOI] [PubMed] [Google Scholar]
- 21.
- 21a. Hoffman R. L., Kania R. S., Brothers M. A., Davies J. F., Ferre R. A., Gajiwala K. S., He M., Hogan R. J., Kozminski K., Li L. Y., Lockner J. W., Lou J., Marra M. T., Mitchell L. J., Murray B. W., Nieman J. A., Noell S., Planken S. P., Rowe T., Ryan K., Smith G. J., Solowiej J. E., Steppan C. M., Taggart B., J. Med. Chem. 2020, 63, 12725–12747; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21b. Zhang L., Lin D., Kusov Y., Nian Y., Ma Q., Wang J., von Brunn A., Leyssen P., Lanko K., Neyts J., de Wilde A., Snijder E. J., Liu H., Hilgenfeld R., J. Med. Chem. 2020, 63, 4562–4578. [DOI] [PubMed] [Google Scholar]
- 22. Dess D. B., Martin J. C., J. Org. Chem. 1983, 48, 4155–4156. [Google Scholar]
- 23.
- 23a. Giaginis C., Tsantili-Kakoulidou A., J. Liq. Chromatogr. Relat. Technol. 2008, 31, 79–96; [Google Scholar]
- 23b. Du C. M., Valko K., Bevan C., Reynolds D., Abraham M. H., Anal. Chem. 1998, 70, 4228–4234. [Google Scholar]
- 24. Fang Z., Cordes D. B., Slawin A. M. Z., O'Hagan D., Chem. Commun. 2019, 55, 10539–10542. [DOI] [PubMed] [Google Scholar]
- 25.
- 25a. Johnson B. M., Shu Y. Z., Zhuo X. L., Meanwell N. A., J. Med. Chem. 2020, 63, 6315–6386; [DOI] [PubMed] [Google Scholar]
- 25b. Mei H., Han J., White S., Graham D. J., Izawa K., Sato T., Fustero S., Meanwell N. A., Soloshonok V. A., Chem. Eur. J. 2020, 26, 11349–11390; [DOI] [PubMed] [Google Scholar]
- 25c. Gillis E. P., Eastman K. J., Hill M. D., Donnelly D. J., Meanwell N. A., J. Med. Chem. 2015, 58, 8315–8359. [DOI] [PubMed] [Google Scholar]
- 26. Appel R., Angew. Chem. Int. Ed. 1975, 14, 801–811; [Google Scholar]; Angew. Chem. 1975, 87, 863–874. [Google Scholar]
- 27. Gololobov Y. G., Kasukhin L. F., Tetrahedron 1992, 48, 1353–1406. [Google Scholar]
- 28. Geurink P. P., Liu N., Spaans M. P., Downey S. L., van den Nieuwendijk A. M. C. H., van der Marel G. A., Kisselev A. F., Florea B. I., Overkleeft H. S., J. Med. Chem. 2010, 53, 2319–2323; N-Boc-L-(pentafluoroaryl)phenylalanine - Fluorochem. Ltd., Catalogue No 008086; CAS Number 34702–60-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vaz E., Fernandez-Saurez M., Muñoz L., Tetrahedron: Asymmetry 2003, 14, 1935–1942. [Google Scholar]
- 30.Antiviral assays were conducted the MRC-Centre for Virus Research at the University of Glasgow, UK.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information