Abstract
Background
Skin aging is one of the most concerning issues during the post‐menopausal period. Despite the promising effects of hormonal therapy, there is still concerned about the long‐term outcomes from the treatment. Therefore, nutraceuticals that contain estrogenic and antioxidative effects have gained a lot of attention as an alternative therapy for slowing down skin age‐related changes in women after menopause.
Objective
This study was aimed at evaluating the effects of a combination of nutraceuticals on skin health and antioxidant status in women after menopause.
Methods
Post‐menopausal women aged 45–60 years old were enrolled and randomly allocated (n = 110) equally to either treatment or placebo group (n = 55 per group). The test product, a nutraceutical containing a blend of Glycine max, Cimicifuga racemosa, Vitex agnus‐castus, and Oenothera biennis extracts, was administered over a 12‐week period, with dermatological parameters evaluated at baseline, week 6, and week 12 of the study. Additionally, glutathione (GSH) and malondialdehyde (MDA) levels were detected at baseline and week 12 to evaluate the antioxidant status.
Results
At week 6, skin roughness was significantly improved in the treatment group (n = 50 completed), while at week 12, a significant improvement and large effect sizes observed in skin elasticity (Cohen's d = 1.56, [SDpooled = 0.10]), roughness (d = 1.53, [0.67]), smoothness (d = −1.33, [34.65]), scaliness (d = −0.80 [0.095]), and wrinkles (d = −1.02 [13.68]) compared to placebo (n = 51 completed). Moreover, GSH was significantly increased (d = 1.54 [32.52]) whereas MDA was significantly decreased (d = −1.66, [0.66]) in the test group, compared to placebo. Blood biochemistry, along with vital signs, did not differ between groups, and no subjects reported any adverse throughout the trial.
Conclusion
These data indicate the supplementation with the formulated blend of four herbal extracts is supportive of skin health and antioxidant status in women of menopausal age.
Keywords: antioxidant, menopause, phyto‐estrogen, post‐menopause, skin aging
1. INTRODUCTION
Menopause is defined as a period of 1 year without menstruation as a result of the progressive failure of the ovaries to produce estrogens. It regularly initiates in the late 30 s, and most women experience near‐complete loss of estrogens production by their mid‐50 s. 1 It is estimated that the at‐risk population of peri‐ and post‐menopausal women will reach globally 1.2 billion by 2030. 2
The skin is altered by during the natural aging process in menopausal women. Since estrodiol receptors are expressed in the dermal cellular compartment, changes in dermal cell metabolism are thought to be affected by the reduction in estrogen levels during menopause, which leads to alterations in collagen and glycosaminoglycan turnover. Lower collagen production is related to loss of skin elasticity, while decreased glycosaminoglycans result in loss of hydration and turgor. Consequently, these changes are some of the basic signs of skin aging. Evidence suggests that these changes may be reversed with estrogen administration. 3
Hormone replacement therapies (HRTs) represent the standard‐of‐care treatment for management of menopausal symptoms and delaying skin aging processes. However, a considerable amount of evidence suggests that HRT may increase the risk of cancer in areas where estradiol receptor α is expressed, for example, uterine, breast, and ovarian tissues. 4 Nutraceuticals containing phytoestrogens are a promising alternative therapy, which have been used to alleviate menopausal symptoms and problems associated with skin aging. Phytoestrogens are heterocyclic phenolic compounds occurring naturally in a variety of plant sources that exert estrogenic actions. Due to their structural similarities to estrogens, they can bind to estradiol receptors (ERs), with preference for ERβ, to modulate their downstream activity. 5
There are several notable plant sources of phytoestrogens. Glycine max (soy) germ is the most abundant source of isoflavones with selective estrogen receptor modulating (SERM) and antioxidant polyphenols. It has a higher affinity for ERβ, which can be found in bone, skin, and the cardiovascular tissues, as opposed to the α subtype, which is more prevalent in reproductive and breast tissue. Soy isoflavones have been reported to prevent lipid peroxidation of the skin tissue, stimulate fibroblast proliferation, and reduce collagen degradation. 6 Cimicifuga racemosa (black cohosh) is a medicinal herb containing potent phytochemicals and has been widely used to treat cycle‐related problems, such as premenstrual syndrome (PMS), dysmenorrhea, and menopausal symptoms, while also displaying antioxidant activity. 7 Vitex agnus‐castus (chaste‐tree) berry contains many phytochemicals which are found to be effective in alleviating cycle irregularity and PMS symptoms. Moreover, the antioxidant properties of chasteberry are believed to be suitable for the protection against skin damage in post‐menopausal women. 8 The oil derived from the seeds of Oenothera biennis (evening primrose) seed is a rich source of essential fatty acids, including gamma linolenic acid (GLA), and several types of phytosterols. Evening primrose oil has been shown to improve epidermal barrier function and normalize trans‐epidermal water loss (TEWL), with GLA being the main fatty acid that contributes to skin membrane structure and function. 9
This research is a part of a larger study, which evaluated the effects of nutraceutical containing all four of these medicinal herbs on menopause symptoms. We assessed the effects of this product on a variety skin health parameters (wrinkles, smoothness, roughness, gloss, elasticity, moisture, trans‐epidermal water loss, and melanin index), along with blood testing for safety and oxidative stress status, to determine its potential to improve skin health in post‐menopausal women as an alternative therapy. Clinical studies of the effects of this supplementation on skin health in post‐menopausal women have not, to our knowledge, previously been performed in a prospective, randomized, controlled design.
2. MATERIALS AND METHODS
This study was approved by the College of Integrative Medicine's Ethical Review Committee for Human Research (Approval number; 006/62EX) and was performed according to the Declaration of Helsinki guidelines for research involving humans. The clinical protocol was registered with the Thai Clinical Trials Registry (TCTR20190417001) and the WHO‐ICTRP database.
2.1. Sample size calculation
A sample size of 110 was considered as adequately powered, based on discriminating a 10% difference in skin elasticity as the primary endpoint indicator of treatment versus control, with a 10% standard deviation (SD) of effect (α = .05 and β‐1 = .8) and an estimated attrition rate of 10%.
2.2. Subjects
Menopausal women aged 45–60 years were enrolled at the Department of Nutrition, Faculty of Public Health, Mahidol University, Thailand, and included those who had ceased consecutive monthly periods for at least 12 months, and with facial skin showing type II‐III fine lines and wrinkles (Glogau classification). 10 Women were not included if they had botulinum toxin or fillers injected into the facial area in less than 6 months; if they had laser treatment, IPL, dermabrasion, iontophoresis, PRP injection, chemical peels, or other procedures that can alter skin wrinkling and skin aging (up to 1 month prior to treatment); if they had liver or kidney diseases; food allergies; were smokers; were pregnant, or used nutraceuticals or drugs that had estrogenic, or antioxidative activity.
2.3. Study design
Participants were randomly allocated the nutraceutical product or an identically labeled control (placebo) based on a random number generation protocol from www.randomization.com, with allocation blinded to participants and investigators. The treatment group (n = 55) was given the commercial herbal blend formulation, Estosalus® (also marketed as Belle Dame®, Max Biocare Pty Ltd, Victoria, Australia, composition shown in Table 1). The placebo group (n = 55) was given capsules containing soybean oil, matched for physical appearance, odor, and excipient content. Both groups were instructed to take one capsule before breakfast daily for 12 weeks, while maintaining a habitual diet and lifestyle. Clinical examinations were conducted by a medical doctor and a research assistant. Dietary assessments were collected by a food record and analyzed by using the INMUCAL program. Primary dermatological endpoint measures were assessed as baseline (week 0), week 6, and week 12. Secondary biochemical outcomes were evaluated at week 0 and week 12, with compliance evaluated at the last appointment, and adverse effects recorded by the physicians throughout the intervention.
TABLE 1.
Composition of nutraceutical
| Composition (per softgel capsule) | Content |
|---|---|
|
Glycine max (soya bean) seed germ ext. dry conc. equiv. to fresh. Standardized for isoflavones |
7.5 g 100 mg |
| Actaea racemosa (Black cohosh) root & rhizome ext. dry conc. equiv. to dry. Standardized for triterpene glycosides (2 mg) | 520 mg |
| Vitex agnus‐castus (Chasteberry) fruit ext. dry conc. equiv. to dry | 400 mg |
| Evening primrose oil equiv. gamma‐Linolenic acid (50 mg) & Linoleic acid (325 mg) | 500 mg |
2.4. Skin parameter assessments
Physical skin parameters were measured independently by a dermatologist. To prepare for these sessions, subjects had cleaned their face for at least 15 min. Skin elasticity was measured using a cutometer (Cutometer® MPA 580 Courage plus, Khazaka Electronic, GmbH) based on the R2 ratio parameter. Skin tone was assessed for melanin index (Mexameter MX18®) and skin radiance, based on the gloss DSC parameter (Glossymeter GL200®). Skin hydration was based on moisture content, as assessed using a Corneometor CM825®, combined with TEWL using a Tewameter TM300® on left and right upper cheeks at defined locations. Skin texture was assessed using a Visioscan® VC98 microtopography device (Khazaka Electronic GmbH) based on surface assessment of the living skin (SALS) variables of smoothness, roughness, scaliness, and wrinkles density, from readings taken on left and right outer corners of the eyes. Additionally, subjects were asked to complete a questionnaire to record self‐evaluation of skin condition at the end of study.
2.5. Biochemical assessments
Blood samples were taken by clinic staff after overnight fasting at Week 0, 6, and 12. Glutathione (GSH) was analyzed using the reduction of 5,50‐dithiobis‐(2‐nitrobenzoic acid) method. 11 Malonyldialdehyde (MDA) was analyzed by assay of thiobarbituric acid reactive substances (TBARS) activity. 12 Kidney and liver health status were evaluated by whole blood analysis of urea nitrogen (BUN), creatinine (Cr), aspartate aminotransferase (AST), and alanine aminotransferase (ALT). All blood biochemistry tests were performed by N Health Asia medical labs (Bangkok, Thailand).
2.6. Statistical analysis
Data were collected at weeks 0, 6, and 12 for both groups. Dermatology parameters and dietary intake measures were tested for normality using the Shapiro‐Wilk test. The ROUT test was used to detect outliers that were subsequently omitted at Q = 0.1%. Repeated measures ANOVA with Bonferroni correction was used for comparing groups with normal distributions, while multiple comparisons of non‐normal datasets were made with the Kruskal–Wallis rank sum test. Matched data within groups, with no missing data points, were compared using Friedman's test. Proportions of subject satisfaction scores were analyzed by the chi‐squared test. Analyses were performed using Graphpad Prism version 8.3.0, with biological significance assigned for p‐values less than .05. All timepoint values for a specific individual with at least one missing timepoint value (post‐randomization and post‐treatment) were systematically omitted in order to determine treatment efficacy as per protocol (values at baseline, week 6, and week 12). Effect sizes were determined by calculation of Cohen's d statistic with pooled SD for skin parameters and antioxidant status.
3. RESULTS
3.1. Characteristics of subjects at baseline
The CONSORT diagram for subject handling is represented in Figure 1 and shows that out of 180 subjects screened, 110 were randomized. No significant differences in age, weight, BMI, body fat, blood pressure, kidney, or liver function markers between both groups (p > .999) were observed (Table 2), nor were there any differences in energy value or nutrient consumption per day between the two groups (p > .999) (Table 3). There were no adverse effects reported throughout the intervention in either test or control groups. Five subjects in the supplement group and four subjects in the placebo group dropped out; therefore, a total of 101 subjects completed the study period. Rates of compliance were high, with capsule consumption of 98% and 96% in the treatment and placebo group, respectively.
FIGURE 1.
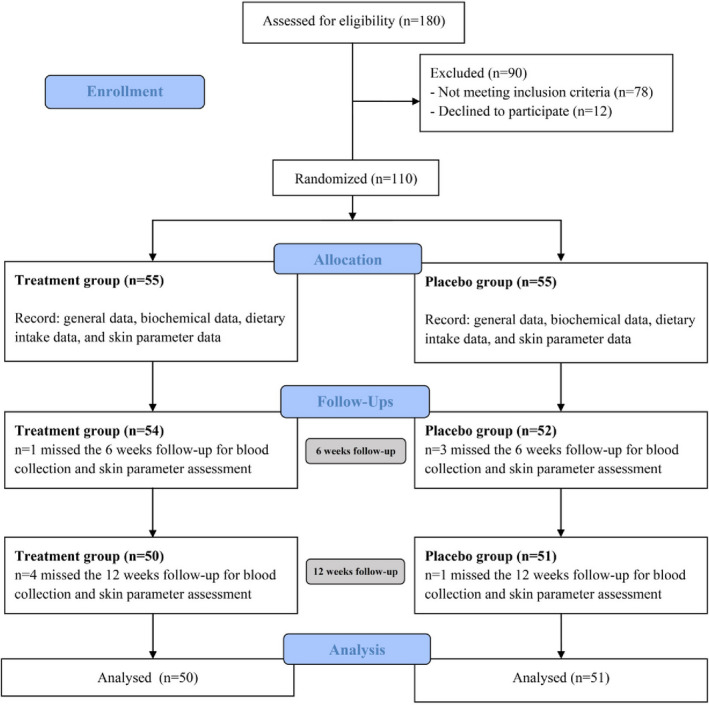
Flow chart for the study sample according to Consolidated Standards of Reporting Trials (CONSORT) guidelines
TABLE 2.
General Characteristics and Blood Chemistry of Subjects
| Characteristics and blood chemistry | Treatment | Placebo | P1 | P2 | P3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 6 | Week 12 | Baseline | Week 6 | Week 12 | ||||
| Age (year) | 53.3 (47–57) | ‐ | ‐ | 52.6 (45–58) | ‐ | ‐ | .055 | ‐ | ‐ |
| Weight (kg) | 59.79 ± 10.18 | 59.41 ± 10.16 | 59.20 ± 10.17 | 62.13 ± 11.19 | 61.79 ± 11.17 | 60.45 ± 13.67 | >.999 | >.999 | >.999 |
| BMI (kg/m2) | 23.94 ± 4.03 | 23.82 ± 4.01 | 23.08 ± 5.14 | 24.72 ± 4.28 | 24.58 ± 4.21 | 24.47 ± 4.33 | >.999 | >.999 | >.999 |
| Body fat (%) | 34.42 ± 6.27 | 34.18 ± 6.41 | 33.86 ± 6.52 | 36.21 ± 6.49 | 36.08 ± 6.54 | 36.00 ± 6.71 | >.999 | >.999 | >.999 |
| Blood pressure (mmHg) | |||||||||
| Systolic | 123.1 ± 19.32 | 122.0 ± 18.45 | 120.8 ± 16.30 | 126.5 ± 13.82 | 124.1 ± 11.63 | 124.7 ± 15.54 | >.999 | >.999 | >.999 |
| Diastolic | 78.92 ± 11.27 | 78.78 ± 9.360 | 77.08 ± 12.15 | 82.36 ± 9.209 | 80.36 ± 10.07 | 78.62 ± 10.55 | .662 | >.999 | >.999 |
| Pulse rate (bpm) | 72.60 ± 9.52 | 72.92 ± 10.57 | 72.58 ± 9.47 | 73.96 ± 11.00 | 72.73 ± 10.18 | 75.94 ± 10.75 | >.999 | >.999 | >.999 |
| BUN (mg/dl) | 12.58 ± 2.41 | 12.54 ± 2.44 | 12.94 ± 2.46 | 12.58 ± 1.73 | 13.79 ± 2.56 | 13.73 ± 2.59 | >.999 | .358 | >.999 |
| Cr (mg/dL) | 0.71 ± 0.16 | 0.87 ± 0.17 | 0.66 ± 0.17 | 0.72 ± 0.22 | 0.82 ± 0.22 | 0.67 ± 0.17 | >.999 | .980 | 0.999 |
| AST (U/L) | 21.89 ± 4.80 | 23.17 ± 4.71 | 21.76 ± 4.55 | 23.29 ± 5.44 | 23.84 ± 8.11 | 23.55 ± 6.37 | >.999 | >.999 | >.999 |
| ALT (U/L) | 20.59 ± 8.61 | 20.14 ± 6.98 | 21.80 ± 7.64 | 18.33 ± 5.37 | 18.96 ± 7.04 | 21.92 ± 9.59 | >.999 | >.999 | >.999 |
Values are means ± SD. Means in a row with superscript letters without a common letter differ within group; Significant differences at p < .05. P1 = Comparison of mean between the two groups at baseline; P2 = Comparison of mean between the two groups at week 6; P3 = Comparison of mean between the two groups at week 12; Significant differences at p < .05.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; Cr, creatinine.
TABLE 3.
Total energy and nutrients intake of the subjects
| Dietary Assessment | Treatment | Placebo | P1 | P2 | ||
|---|---|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |||
| Energy (kcal/d) | 1583 ± 448.10 | 1569 ± 477.40 | 1508 ± 455.90 | 1536 ± 409.10 | >.999 | >.999 |
| Carbohydrate (% of energy) | 57.17 ± 9.89 | 53.89 ± 8.14 | 57.90 ± 10.36 | 55.94 ± 12.47 | >.999 | .452 |
| Protein (% of energy) | 15.09 ± 4.31 | 17.79 ± 6.63 | 15.10 ± 4.76 | 15.67 ± 4.00 | >.999 | >.999 |
| Fat (% of energy) | 27.74 ± 8.56 | 28.32 ± 7.27 | 27.39 ± 8.83 | 28.39 ± 10.31 | >.999 | >.999 |
| Cholesterol (mg/d) | 295.30 ± 95.92 | 289.70 ± 83.81 | 292.4 ± 90.29 | 282.5 ± 62.09 | >.999 | >.999 |
| Fiber (g/d) | 11.21 ± 4.87 | 11.56 ± 5.85 | 12.26 ± 7.03 | 12.29 ± 6.26 | >.999 | >.999 |
Values are means ± SD. P1 = Comparison of mean between the two groups at baseline; P2 = Comparison of mean between the two groups at 12 week; Significant differences at p < .05.
3.2. Effects of the nutraceutical on skin condition
Baseline skin parameters were not significantly different between both groups (Table 4). After 6 weeks, test subjects showed a significantly improved skin roughness (p = .018) compared to placebo subjects. After 12 weeks of intervention, nutraceutical supplementation resulted in significant improvements in skin elasticity (p < .0001), roughness (p = .0001), smoothness (p < .0001), scaliness (p = .0052), and wrinkle density (p = .0098) compared with the placebo group (effect sizes are displayed in Table 4), but there were no significant differences in melanin index, gloss, hydration, and TEWL between the two groups (Figures 2, 3, 4, 5, 6, 7).
TABLE 4.
Skin parameters of subjects
| Skin Parameters | Treatment | Placebo | P1 | P2 | P3 | Effect size (Test vs Placebo) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 6 | Week 12 | Baseline | Week 6 | Week 12 | Cohen's d [SD pooled ] | ||||
| R2 ratio (Cutometer®) | 0.57 ± 0.10 | 0.66 ± 0.09 | 0.72 ±0.10 | 0.61 ± 0.09 | 0.61 ± 0.12 | 0.61 ± 0.09 | .5770 | .2592 | <.0001 | 1.56 [0.10] |
| Melanin index (Mexameter MX18®) | 237.10 ± 62.60 | 236.30 ± 62.99 | 233.10 ± 57.13 | 231.50 ± 57.66 | 232.00 ± 56.02 | 230.00 ± 58.74 | >.999 | >.999 | >.999 | −0.11 [22.8] |
| Gloss DSC Value (Glossymeter GL200®) | 5.29 ± 1.23 | 5.43 ± 1.59 | 5.28 ± 1.19 | 5.49 ± 1.77 | 5.49 ± 1.57 | 5.41 ± 1.73 | >.999 | >.999 | >.999 | 0.055 [1.11] |
| Skin hydration (Corneometer CM825®) | 72.35 ± 7.06 | 76.02 ± 8.38 | 73.21 ± 7.64 | 73.62 ± 8.85 | 78.92 ± 7.15 | 77.77 ± 8.42 | >.999 | >.999 | .0412 | −0.45 [7.27] |
| TEWL (g/h/ m2) (Tewameter TM300®) | 11.70 ± 3.66 | 8.87 ± 3.24 | 10.52 ± 1.73 | 12.45 ± 3.50 | 9.74 ± 3.32 | 10.86 ± 2.02 | >.999 | >.999 | >.999 | 0.11 [3.51] |
| SELS parameters | ||||||||||
| SEsm | 212.70 ± 43.23 | 183.40 ± 33.27 | 170.70 ± 30.70 | 202.20 ± 42.02 | 199.60 ± 36.99 | 206.10 ± 42.73 | >.999 | .3386 | <.0001 | −1.33 [34.65] |
| SEr | 2.62 ± 1.01 | 3.09 ± 1.01 | 3.48 ± 1.10 | 2.71 ± 0.77 | 2.49 ± 0.56 | 2.53 ± 0.69 | .206 | .018 | .0001 | 1.53 [0.67] |
| SEsc | 0.62 ± 0.19 | 0.60 ± 0.11 | 0.57 ± 0.11 | 0.63 ± 0.10 | 0.64 ± 0.09 | 0.65 ± 0.10 | >.999 | >.999 | .0052 | −0.80 [0.095] |
| SEw | 76.21 ± 17.19 | 70.12 ± 13.90 | 65.12 ± 10.59 | 72.35 ± 15.42 | 72.78 ± 14.36 | 74.48 ± 14.50 | >.999 | >.999 | .0098 | −1.02 [13.68] |
Values are means ± SD. P1 = Comparison of mean between the two groups at baseline; P2 = Comparison of mean between the two groups at week 6; P3 = Comparison of mean between the two groups at week 12; Significant differences at p < .05. R2 ratio = skin elasticity; Cohen's d = baseline‐corrected difference between treatment and placebo means at completion. Bold indicates a large treatment effect size, with direction indication by sign.
Abbreviations: SEr, skin roughness; SEsc, skin scaliness; SEsm, skin smoothness; Sew, wrinkles; TEWL, trans‐epidermal water loss (g/h/m2).
FIGURE 2.
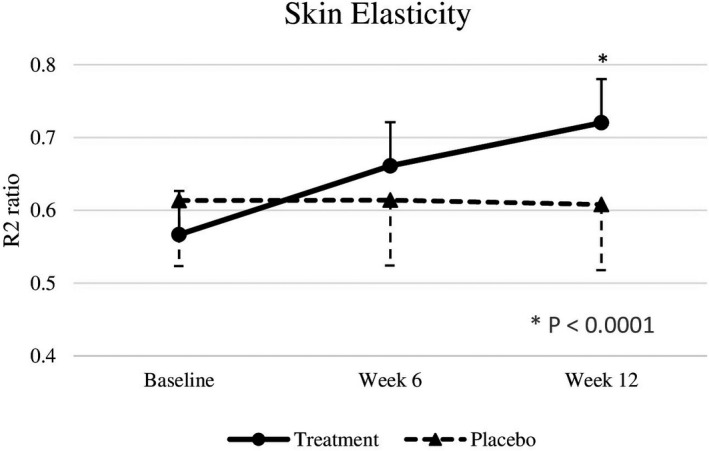
R2 ratio at baseline, week 6, and week 12. Significant differences at p < .05
FIGURE 3.
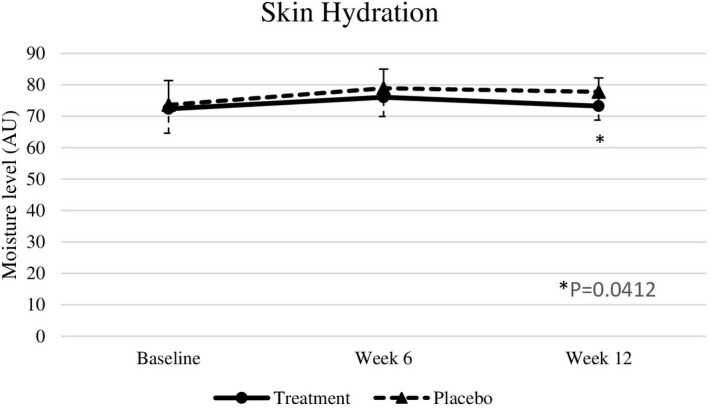
Moisture level at baseline, week 6, and week 12. Significant differences at p < .05
FIGURE 4.
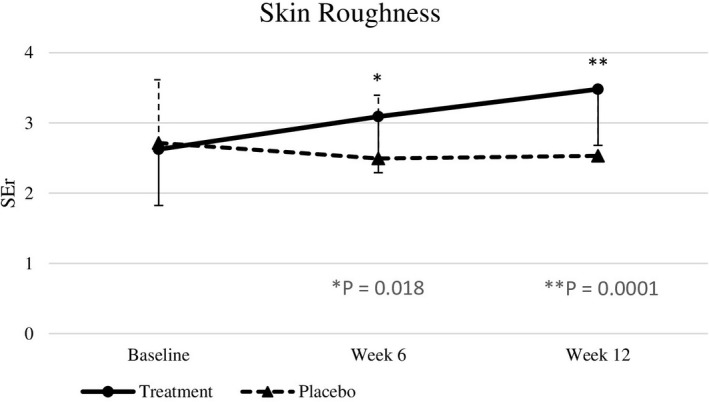
SEr at baseline, week 6, and week 12. Significant differences at p < .05
FIGURE 5.
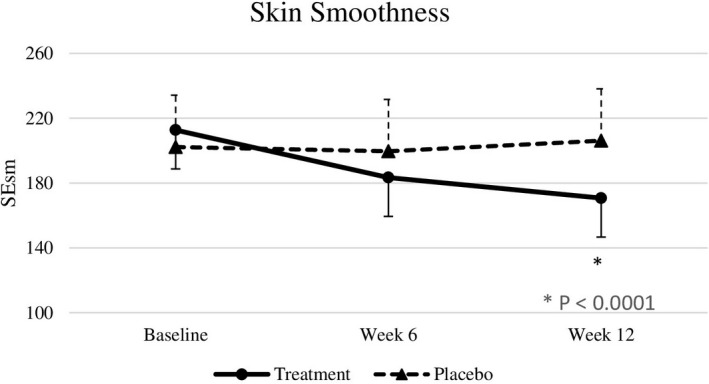
SEsm at baseline, week 6, and week 12. Significant differences at p < .05.
FIGURE 6.
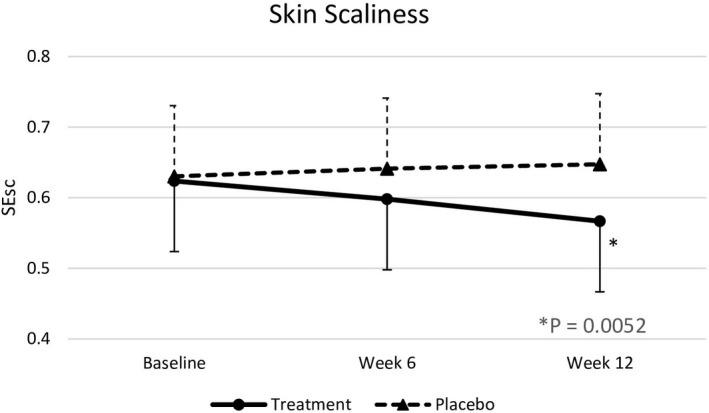
SEsc at baseline, week 6, and week 12. Significant differences at p < .05
FIGURE 7.
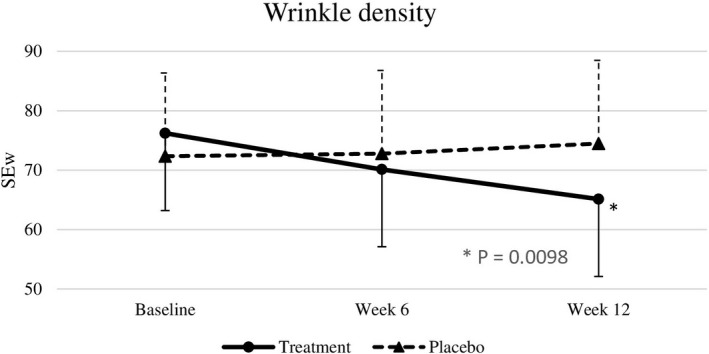
SEw at baseline, week 6, and week 12. Significant differences at p < .05
3.3. Effects of the nutraceutical on antioxidant status
According to Table 5, baseline antioxidant status did not differ significantly between the two groups. The treatment group demonstrated significant increases in GSH levels (p = .0242) and a corresponding decrease in the level of MDA (p < .0001) compared with the placebo group, indicating an overall improvement in oxidative stress status.
TABLE 5.
Antioxidant Status of Subjects
| Antioxidant biomarkers | Treatment | Placebo | P1 | P2 | Effect size (Test vs. Placebo) | ||
|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | Cohen's d [SD pooled ] | |||
| GSH (µmol/L) | 481.50 ± 127.40 | 528.80 ± 117.80 | 460.70 ± 123.20 | 458.00 ± 123.10 | >.999 | .0242 | 1.54 [32.52] |
| MDA (µmol/L) | 4.31 ± 0.88 | 3.50 ± 0.93 | 4.45 ± 0.87 | 4.75 ± 0.88 | >.999 | <.0001 | −1.66 [0.66] |
Values are means ± SD. P1 = Comparison of mean between the two groups at baseline; P2 = Comparison of mean between the two groups at week 12; Significant differences at p <.05. Cohen's d = baseline‐corrected difference between treatment and placebo means at completion. Bold indicates a large treatment effect, with direction indication by sign.
Abbreviations: GSH, reduced glutathione; MDA, Malondialdehyde.
3.4. Satisfaction assessments
Subjects in the nutraceutical group were more satisfied than placebo subjects with almost all aspects of their perceived skin health at week 6 and even more satisfied at week 12 (smoothness; p < .0001, moisture; p = .0012, elasticity; p < .0001, and wrinkles; p < .0001). The level of satisfaction with dark spot appearance was comparable between treatment and test subjects presented in Table 6.
TABLE 6.
Satisfaction of subjects
| Topic | Number of subjects satisfied (percentage) | p | |||
|---|---|---|---|---|---|
| Treatment | Placebo | ||||
| Week 6 | Week 12 | Week 6 | Week 12 | ||
| Smoothness | 34* (65.4%) | 42* (80.8%) | 12 (23%) | 15 (28.8%) | <.0001 |
| Moisture | 28* (53.8%) | 34* (65.4%) | 16 (30.8%) | 19 (36.5%) | .0012 |
| Elasticity | 41* (78.8%) | 46* (88.5%) | 10 (19.2%) | 8 (15.4%) | <.0001 |
| Dark spots | 11 (21.1%) | 15 (28.8%) | 8 (15.4%) | 10 (19.2%) | .3920 |
| Wrinkles | 32* (61.5%) | 40* (76.9%) | 17 (32.7%) | 21 (40.4%) | <.0001 |
Significant differences between treatment and placebo.
Values are numbers (percentages). P = Comparison of the value between the two groups at week 12; Significant differences at p <.05.
4. DISCUSSION
In this clinical trial, the skin at the lateral aspect of both eyes was assessed to evaluate the potential anti‐aging effects of the test nutraceuticals. At the end of the study, the results demonstrated intake of the test product was effective in improving skin elasticity, skin smoothness, skin scaliness, and skin roughness compared with placebo. Significant intragroup differences in these parameters were also observed in the treatment group. Presumably, the increased skin elasticity was mediated to large extent by the activation of estrogen receptor‐β by soy isoflavones, which may have stimulated collagen and elastin content, and therefore mechanical integrity. A previous study also reported that 40 mg/day of soy isoflavones for 12 weeks significantly improved in fine wrinkles and elasticity of malar skin. 6 This result complemented the improvements observed in skin smoothness, roughness, and scaliness in our study, in line with previous reports in relation to the effects of evening primrose oil in restoring epidermal barrier structure and function.9
Meanwhile, no significant effects of the nutraceutical were observed on the skin melanin index, skin gloss, skin hydration, and TEWL. In one previous study, the skin hydration and TEWL significantly improved after administration of evening primrose oil 3 g per day for 12 weeks.9 This indicated that the concentration of evening primrose oil used in this study, which is 500 mg/day, might not be enough to significantly regenerate the whole epidermal barrier function. Apart from skin barrier function, hydration also relates to the vasculature of the skin. Although the activation of ER tends to increase the epidermal and vascular‐endothelial growth factors, it has been reported that 6 months of estrogen therapy was not able to restore cutaneous microvasculature. 13 With regard to the skin melanin index, evidence related to the effects of isoflavones and other ingredients on melanin pigment is limited.
In addition, a significant improvement was observed in GSH and MDA levels in the treatment group. This indicates not only improved endogenous antioxidant activity, but also lowered plasma markers of lipid peroxidation, suggesting a dual benefit on direct oxidative radical reduction and support of protective mechanisms within the body. Accordingly, a previous study revealed that chasteberry extract could increase reduced GSH concentration and increase catalase, glutathione reductase, glutathione peroxidase, and glutathione‐S‐transferase activities in animal models. 14 Moreover, in mice fed a basal diet with or without 1.08 g of an isoflavone‐rich soy isolate, the level of liver MDA after 60 days was found to be significantly lower in the treatment compared with the control. 15 Soy isoflavone administration in women has also been shown to increase GSH levels by fourfold, compared to placebo, in another study by Jamilian et al. 16
One limitation of this research was that phenolic metabolites originating from the nutraceutical were not measured in blood samples following oral supplementation. This would complement future studies to determine the pharmacokinetics, mechanism of action, together with longer term safety and efficacy of this complementary medicine alongside standard estrogen replacement therapy in post‐menopausal women.
5. CONCLUSION
We established that, compared to a placebo, daily supplementation with a commercial nutraceutical containing four medicinal herbs improved indices of facial skin health, including elasticity, roughness, smoothness, scaliness, and wrinkle density after 12 weeks in menopausal women. This corresponded with increased antioxidant (GSH) and lowered lipid peroxidation (MDA), indicating a more optimal oxidative stress status. Taken together, these findings point to a measurable anti‐aging effect of the formula in women with age‐related declines in skin structure and integrity.
CONFLICT OF INTERESTS
No conflict of interests has been declared.
AUTHORS’ CONTRIBUTIONS
PT, MM, PS, and AB conceived and designed the study. PT, PS, RW, and AB were responsible for recruitment of the subjects and data collection. PT, MM, PS, and AB participated in data analysis and interpretation. PT and AB drafted the manuscript. All authors read and approved the final manuscript.
ETHICAL STATEMENT
This study was approved by the College of Integrative Medicine's Ethical Review Committee for Human Research (Approval number; 006/62EX).
ACKNOWLEDGMENTS
The authors thank all research assistants of the Department of Nutrition, Faculty of Public Health, Mahidol University, Thailand, for their invaluable assistance and site for study.
Tumsutti P, Maiprasert M, Sugkraroek P, Wanitphakdeedecha R, Bumrungpert A. Effects of a combination of botanical actives on skin health and antioxidant status in post‐menopausal women: A randomized, double‐blind, placebo‐controlled clinical trial. J Cosmet Dermatol. 2022;21:2064–2072. 10.1111/jocd.14345
Funding information
This study was funded by Max Biocare Pty Ltd, Australia.
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Goodman NF, Cobin RH, Ginzburg SB, Katz IA, Woode DE. American association of clinical endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of menopause. Endocr Pract. 2011;17:1‐25. [DOI] [PubMed] [Google Scholar]
- 2. Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006;96(7):1226‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raine‐Fenning NJ, Brincat MP. Skin aging and menopause: implications for treatment. Am J Clin Dermatol. 2003;4(6):371‐378. [DOI] [PubMed] [Google Scholar]
- 4. Zhou B, Sun Q, Cong R, et al. Hormone replacement therapy and ovarian cancer risk: a meta‐analysis. Gynecol Oncol. 2008;108(3):641‐651. [DOI] [PubMed] [Google Scholar]
- 5. Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERα) and beta (ERβ): subtype‐selective ligands and clinical potential. Steroids. 2014;90:13‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Izumi T, Saito M, Obata A, Arii M, Yamaguchi H, Matsuyama A. Oral intake of soy isoflavone aglycone improves the aged skin of adult women. J Nutr Sci Vitaminol. 2007;53(1):57‐62. [DOI] [PubMed] [Google Scholar]
- 7. Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev. 2012;2012(9):CD007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roemheld‐Hamm B. Chasteberry. Am Fam Physician. 2005;72(5):821‐824. [PubMed] [Google Scholar]
- 9. Muggli R. Systemic evening primrose oil improves the biophysical skin parameters of healthy adults. Int J Cosmet Sci. 2005;27(4):243‐249. [DOI] [PubMed] [Google Scholar]
- 10. Zhang J, Hou W, Feng S, Chen X, Wang H. Classification of facial wrinkles among Chinese women. J Biomed Res. 2017;31(2):108‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bumrungpert A, Pavadhgul P, Kalpravidh RW. Camellia oil‐enriched diet attenuates oxidative stress and inflammatory markers in hypercholesterolemic subjects. J Med Food. 2016;19(9):895‐898. [DOI] [PubMed] [Google Scholar]
- 12. Bumrungpert A, Lilitchan S, Tuntipopipat S, Tirawanchai N, Komindr S. Ferulic acid supplementation improves lipid profiles, oxidative stress, and inflammatory status in hyperlipidemic subjects: a randomized, double‐blind, placebo‐controlled clinical trial. Nutrients. 2018;10(6):713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Accorsi‐Neto A, Haidar M, Simões R, Simões M, Soares‐Jr J, Baracat E. Effects of isoflavones on the skin of postmenopausal women: a pilot study. Clinics. 2009;64(6):505‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ibrahim AY, El‐Newary SA, Youness ER, Ibrahim AM, El‐Kashak WA. Protective and therapeutic effect of Vitex agnus‐castus against prostate cancer in rat. J Appl Pharm Sci. 2017;7(12):133‐143. [Google Scholar]
- 15. Ibrahim WH, Habib HM, Chow CK, Bruckner GG. Isoflavone‐rich soy isolate reduces lipid peroxidation in mouse liver. Int J Vitam Nutr Res. 2008;78(4–5):217‐222. [DOI] [PubMed] [Google Scholar]
- 16. Jamilian M, Asemi Z. The effects of soy isoflavones on metabolic status of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2016;101(9):3386‐3394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


