Abstract
Objectives
To evaluate the clinical and biochemical efficacy of laser therapy as an adjunct to non‐surgical treatment in chronic periodontitis.
Methods
A systematic search was performed through the PubMed, EMBASE, and Cochrane Library for eligible articles published as of May 2, 2020, supplemented by information search in the System for Information on Programme Literature in Europe and a manual literature search. Only randomized controlled trials (RCTs) used to compare the adjunctive use of laser and non‐surgical treatment alone with an observation period of at least 6 months were included.
Results
Sixteen RCTs with a total of 525 subjects were included. Meta‐analysis suggested that the additional use of laser to scaling and root planing (SRP) showed significant superiority over SRP alone among most of clinical parameters involved. Regarding the GCF, although volume in the laser group was lower at week 4 and 12, no significant difference was found regarding the cytokines level. Subgroup analysis revealed that the combined therapy produced no significant difference in PD, CAL and PI at most time points for studies in respect to smokers. No treatment‐related adverse events had been reported in the included studies.
Conclusions
Pooled analysis suggested that laser‐assisted non‐surgical treatment improved clinical outcome to SRP alone in the management of non‐smoking chronic periodontitis patients.
Keywords: chronic periodontitis, laser, meta‐analysis, non‐surgical periodontal treatment, systematic review
1. INTRODUCTION
As one of the most prevalent oral diseases worldwide, chronic periodontitis (CP) is a disease characterized by bacterial‐induced inflammation and accompanied by the formation of periodontal pockets, progressive breakdown of periodontal supporting structures, and even tooth loss in susceptible individuals (Meyle & Chapple, 2015).
Due to the close link between bacteria and plaque and CP, eliminating bacterial deposits is undoubtedly the primary goals in periodontal therapy (Koyanagi et al., 2013). Conventional non‐surgical periodontal treatment, primarily consisting of scaling and root planing (SRP), is suggested as the gold standard in clinical practice guidelines for patients with untreated periodontitis (John et al., 2017; Mombelli, 2018). It aims to debride contaminated root surface as well as eliminate the etiologic factors from supra‐ and subgingival regions of the tooth and inflamed tissues (Mombelli, 2018). Its advantage lies in its safety and universal applicability in the vast majority of cases (Sherman et al., 1990). However, there are still some limitations in terms of non‐surgical mechanical treatment.
In areas with difficult access such as deep or winding periodontal pockets, furcation sites, root curvature, and poorly contoured restorations, SRP alone is prone to incomplete elimination of periodontal pathogens (Umeda et al., 2004). The residual subgingival calculus and bacterial deposits remaining on the root surface are likely to result in unsatisfactory treatment effect. Considering the deficiency, various techniques have been recommended as adjunctive approaches, such as laser radiation (Caffesse & Echeverría, 2019; Schwarz et al., 2008).
The most common laser applications for periodontal therapy include erbium‐doped: yttrium aluminum garnet (Er:YAG) laser, erbium, chromium‐doped: yttrium, scandium, gallium, garnet (Er, Cr:YSGG) laser, neodymium‐doped: yttrium aluminum garnet (Nd:YAG) laser, carbon dioxide laser, and diode laser, with wide wavelength range and different physical or biological properties (Cobb, 2006; Cobb 2017). Laser application shows satisfactory bacteriostasis and decontamination ability, because it can remove inactivate bacteria and subgingival calculus, especially those in the sites where traditional periodontal tools cannot reach (Sgolastra et al., 2014; Sjöström & Friskopp, 2002). In addition, lasers also have distinct advantages in hemostasis, better visualization, improved healing, and possible avoidance of the need for surgical intervention (Aoki et al., 2015; Dias et al., 2015; Sella et al., 2015).
Although a number of studies have reported the clinical effectiveness of different kinds of lasers in the treatment of periodontitis, controversy still persists. The effectiveness of lasers in suppressing periodontal pathogens has been demonstrated in animal studies (Jiao et al., 2019; de Oliveira et al., 2011), which suggests a beneficial effect that lasers may produce on the management of chronic periodontitis. However, the clinical trials concerning evaluation of laser therapy as an adjunct to SRP in periodontal treatment failed to draw consistent conclusions. No adequate evidence had been found to support the clinical efficacy of lasers applied in non‐surgical therapy (Salvi et al., 2020; Sanz et al., 2020).
In general, studies have shown that smokers present an unfavorable clinical response to non‐surgical periodontal therapies, such as unobvious reductions in probing depth and lower gains in clinical attachment (Paes Batista da Silva et al., 2016; Petrovic et al., 2013). Many professionals have proposed the use of adjunctive therapeutic approaches for improving the effects of basic periodontal therapy in smokers.
The aim of present research is to investigate whether laser therapy as an adjunct to non‐surgical periodontal therapy can improve the clinical and biochemical efficacy among patients with untreated chronic periodontitis, and whether laser application can yield predictable outcomes in smokers.
2. MATERIAL AND METHODS
2.1. Protocol development
This systematic review and meta‐analysis was conducted and reported according to PRISMA guidelines (Moher et al., 2009). The research focus of the present study is to explore whether adjunctive use of laser for non‐surgical periodontal treatment has better performance in clinical and biochemical outcomes based on 6‐month follow‐up.
2.2. Data sources and search strategy
An electronic search was performed through the following databases (as of May 2, 2020): MEDLINE (PubMed), EMBASE, clinicaltrials.gov, and Cochrane library databases. The search strategies used in scientific databases and number of hits are summarized in Table S1. In addition, System for Information on Grey Literature in Europe and reference lists of identified full‐text articles and relevant reviews were also checked in an attempt to find any additional studies.
2.3. Study selection
Initially, duplicate records were removed using reference management software (Endnote X8) and manual retrieval. Subsequently, titles and abstracts were screened, and for unclear or insufficient information, the full text was obtained. Finally, the selection of articles based on the eligibility criteria was conducted in duplicate by two independent reviewers. Disagreements were resolved through discussion or by a third reviewer.
2.4. Inclusion and exclusion criteria
For the first step, the studies were selected according to the following inclusion criteria:
RCTs on patients diagnosed with periodontitis;
Subjects were allocated to experimental or control group based on having non‐surgical mechanical treatment with one adjunctive type of laser therapy or not;
Follow‐up at least 6 months;
Clinical outcomes included probing depth in baseline and 6 months after treatment as mean ± standard deviation (SD);
Studies were reported in English.
For the second step, preselected studies satisfying all the above‐mentioned standards were excluded if they belonged to any one of the following categories:
Studies including patients with aggressive periodontitis or systemic diseases (e.g., diabetes mellitus or cardiovascular diseases) or receiving any medical treatment expected to affect the progression or therapeutic effect of chronic periodontitis;
Studies including patients who received periodontal treatment within the past 6 months;
Studies including patients in the course of supportive periodontal therapy (SPT);
Studies including antimicrobial photodynamic therapy (aPDT);
Duplicate studies, pilot studies, incomplete data articles, or no full‐text studies;
Insufficient/unclear information not allowing data extraction.
2.5. Data extraction and quality assessment
Data extraction from each eligible study was performed by two independent researchers (Jiang and Feng) using a standard form, and conflicts were resolved by consensus between the two reviewers or approval of the third investigator (Liu). The following data were extracted from each included article: study design, year of publication, country, sample size of study, the number of teeth/sites involved, demographic characteristics, smoking status of participants, parameters of various lasers, details of intervention, length of follow‐up, dropout, and treatment‐related adverse events. Major outcomes included clinical and biochemical parameters after periodontal therapy. If there was any missing information, corresponding authors of relevant studies would be reached.
The methodological quality evaluation and risk of bias assessment were based on the Cochrane Collaboration's tool for assessing risk of bias. All of the discrepancies were solved by discussion or with the help of corresponding authors.
2.6. Statistical analysis
The indicators for primary outcomes are probing depth (PD, mm) and clinical attachment level (CAL, mm); the indicators for secondary outcomes are plaque index (PI) (Silness & Loe, 1964), gingival index (GI) (Loe & Silness, 1963), volume of gingival crevicular fluid (GCF, μl), and cytokine level (pg). As all outcomes provided only continuous data, mean difference (MD) with 95% confidence interval (CI) was used in most of periodontal parameters. In consideration of large difference in measurement methods and details, standardized mean difference (SMD) with 95% CI was counted for cytokine level in GCF. A forest plot was used to visualize the MD or SMD and 95% CI for each study.
The percentage of variability across studies attributable to heterogeneity beyond chance was assessed using the chi‐squared‐based Q test (p < .1 was considered indicative of significance) and the I 2 statistic. If the test indicated substantial or considerable heterogeneity (I2 > 50%), a random effects model would be used for summary statistics. Otherwise (I2 ≤ 50%), a fixed effect model would be applied. For the hypothesis test, the critical value of statistical significance was set at p < .05 (two‐tailed Z test).
Subgroup analysis was conducted to identify potential factors related to the efficacy of adjunctive laser therapy. A sensitivity analysis with omission of one study at a time was conducted to identify heterogeneity. Where sufficient studies were available, publication bias was assessed by the Egger test. All analyses were conducted using RevMan software (version 5.3).
3. RESULTS
3.1. Search results
Of 1863 articles that were retrieved through electronic and manual searching, 690 were excluded due to duplication. By screening titles and abstracts, 71 articles were reserved for full‐text reading and the rest was eliminated. After a detailed assessment of full text, 16 qualified articles remained for our meta‐analysis (Aykol et al., 2011; Dereci et al., 2016; Eltas & Orbak, 2012; Euzebio Alves et al., 2013; Everett et al., 2017; Gundogar et al., 2016; Magaz et al., 2016; Makhlouf et al., 2012; Rotundo et al., 2010; Saglam et al., 2014; Sanz‐Sanchez et al., 2015; Sezen et al., 2020; Ustun et al., 2014, 2018; Zengin Celik et al., 2019; Zhou et al., 2019). The flow diagram of the study selection is shown in Figure 1.
FIGURE 1.
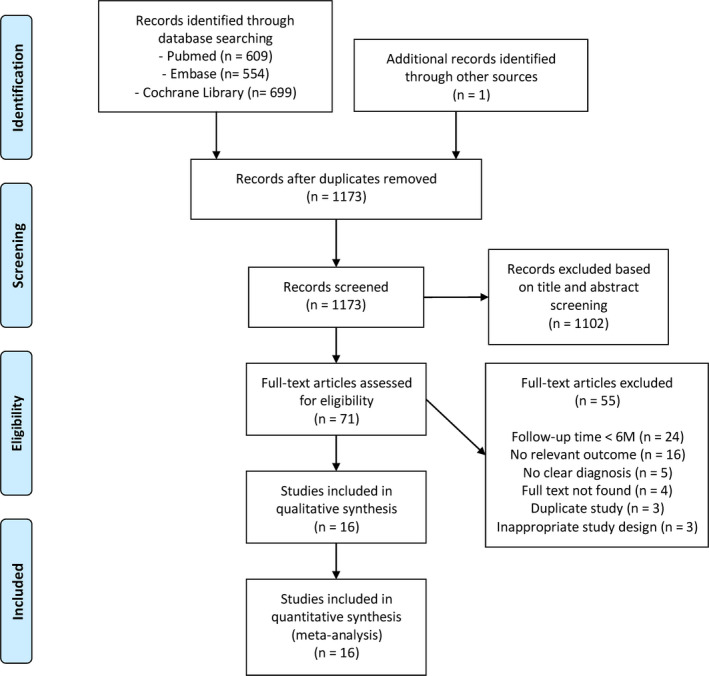
Flow diagram of selection process
3.2. Study characteristics and quality assessment
A total of 525 systemically healthy patients diagnosed with chronic periodontitis were randomized in 16 pooled studies, of which 507 finished the trials covered in present study. The general characteristics of the included studies and parameters, usage of the employed laser appliance in experimental groups, are, respectively, summarized in Tables 1 and 2. The qualities were judged based on Cochrane Handbook for Systematic Reviews of Interventions. The majority of studies displayed a low or unclear risk of bias. The risk of bias profile is summarized and presented in Figure 2.
TABLE 1.
Characteristics of included studies
| Study | Country | Study Design | Patient characteristics | Sample size | Average age (range) | Male | Smoking status | Teeth/sites involved | Treatment | Follow‐up (weeks) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L/C | L/C | L/C | L | C | L | C | L | C | |||||
| Rotundo et al. (2010) | Italy | Split‐mouth, RCT | Moderate to advanced CP | 27 | 50.5 ± 11.7 | 9 | 12 smokers (<10 cigarettes/day) | One quadrant of the mouth (419 sites) | One quadrant of the mouth (422 sites) | Laser + SRP | SRP | 24 | |
| Aykol et al. (2011) | Turkey | parallel, RCT | moderate to advanced CP | 18/18 | 43.56 ± 6.7 (31–58)/42.22 ± 7.53 (31–53) | 11/11 | 9 smokers (≥10 cigarettes/day) | 9 smokers (≥10 cigarettes/day) | Full‐mouth (2,508 sites) | Full‐mouth (2,688 sites) | SRP + laser | SRP | 24 |
| Eltas and Orbak (2012) (1) | Turkey | Split‐mouth, RCT | Generalized moderate CP | 26 | 42.7 ± 5.1 (32–51) | 13 | Smokers (>10 cigarettes/day for >5 years) | One tooth each quadrant, two quadrants | One tooth each quadrant, two quadrants | SRP + laser | SRP + placebo laser | 24 | |
| Eltas and Orbak (2012) (2) | Turkey | Split‐mouth, RCT | Generalized moderate CP | 26 | 44.1 ± 4.9 (34–52) | 13 | Non‐smokers | One tooth each quadrant, two quadrants | One tooth each quadrant, two quadrants | SRP + laser | SRP + placebo laser | 24 | |
| Makhlouf et al. (2012) | Egypt | Split‐mouth, RCT | CP | 16 | 22–50 | 4 | Non‐smokers | 2–3 teeth of one side | 2–3 teeth of the other side | SRP + laser | SRP + sham laser | 48 | |
| Euzebio Alves et al. (2013) | Brazil | Split‐mouth, RCT | SEVERE CP | 37 | 46.8 ± 8.11 (37–64) | 13 | Non‐smokers | A single‐rooted teeth | A contralateral single‐rooted teeth | SRP + laser | SRP + placebo laser | 24 | |
| Saglam et al. (2014) | Turkey | Parallel, RCT | CP | 15/15 | 42.13 ± 9.05/40.83 ± 7.64 | 10/8 | Non‐smokers | Non‐smokers | Full‐mouth | Full‐mouth | SRP + laser | SRP | 24 |
| Ustun et al. (2014) | Turkey | Split‐mouth, RCT | Generalized CP | 21 | 40.23 ± 10.18 | 7 | Non‐smokers | A quadrant of the mouth | The contralateral quadrant of the mouth | Laser + SRP | SRP | 24 | |
| Sanz‐Sanchez et al. (2015) | Spain | Parallel, RCT | Initial‐moderate CP | 19/21 | 48.5 (37–71)/56.8 (39–71) | 7/5 | 10 smokers | 7 smokers | Full‐mouth | Full‐mouth | SRP + laser (one week apart) | Two sessions of SRP within one week (one side per session) | 48 |
| Dereci et al. (2016) | Turkey | Parallel, RCT | CP | 30/30 | 43.7 ± 3.1 | 31 | Smokers and non‐smokers | Smokers and non‐smokers | Full‐mouth | Full‐mouth | SRP 1 time + laser 3 times within 7 days | SRP 3 times within 7 days + placebo laser | 24 |
| Gundogar et al. (2016) | Turkey | Split‐mouth, RCT | Generalized CP | 25 | 40.44 ± 8.69 (28–57) | 9 | Non‐smokers | One side of the mouth | The other side of the mouth | SRP + laser 4 times within 7 days | SRP | 24 | |
| Magaz et al. (2016) | Spain | Split‐mouth, RCT | Moderate CP | 30 | 48.5 ± 9.4 | 10 | 4 Smokers (< 10 cigarettes/day) | Two quadrants (sites ≥4 mm) | Two contralateral quadrants (maxilla + mandible) (sites ≥4 mm) | SRP + laser | SRP | 24 | |
| Everett et al. (2017) | USA | Split‐mouth, RCT | CP | 14 | 34–65 | 5 | Non‐smokers | One side of the mouth | The other side of the mouth | SRP + laser | SRP + sham laser | 24 | |
| Ustun et al. (2018) | Turkey | Parallel, RCT | CP | 20/20 | 44.05 ± 6.16 (36–59)/45.8 ± 6.53 (35–58) | 9/12 | Non‐smokers | Non‐smokers | Full‐mouth | Full‐mouth | SRP + laser | SRP | 24 |
| Zengin Celik et al. (2019) | Turkey | Parallel, RCT | Moderate and advanced CP | 19/19 | 38.4 ± 7.5 (25–58) | 19 | Non‐smokers | Non‐smokers | Full‐mouth | Full‐mouth | SRP + Laser | SRP | 24 |
| Zhou et al. (2019) | China | Split‐mouth, RCT | CP | 27 | 49 ± 9.6 (35–70) | 11 | Non‐smokers | A quadrant of the mouth | A quadrant of the mouth (other side) | Laser + SRP | SRP | 24 | |
| Sezen et al. (2020) | Turkey | Split‐mouth, RCT | Moderate to advanced CP | 32 | 37.25 ± 11.87 (18–65) | 18 | Non‐smokers | One side of the upper jaw | The other side of the upper jaw | SRP + Laser | SRP | 24 | |
Abbreviations: C, control group; CP, chronic periodontitis; L, Laser group; RCT, randomized controlled trial.
TABLE 2.
Laser parameters and usage of included studies
| Study | Laser type | Product name | Material of tip (diameter) | Wavelength (nm) | Power (W) | Energy level | Energy/power density | Duration of irradiation | Frequency of irradiation | Method | Post‐treatment instructions |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rotundo et al. (2010) | Er:YAG laser | Smart 2,940 Plus, Calenzan, Firenze, Italy | Fiber tip (0.5mm) | 2,940 | n.r. | 150 mJ/pulse | n.r. | n.r. | Once | From coronal to apical direction with an inclination of the fiber tip of about 20° | Supportive periodontal therapy at week 1, 4, 12 and 24 weeks |
| Aykol et al. (2011) | Low‐level laser | Fotona XD‐2, Fotona, Ljubljana, Slovenia | n.r. | 808 | 0.25 | n.r. | 4 J/cm2 | Incisors and premolars: 10s, molars: 20 s | 3 times: on the 1, 2, and 7 days after treatment | Non‐contact technique (The application distance was 0.5 to 1 cm to the gingiva) | n.r. |
| Eltas and Orbak (2012) (1) | Nd:YAG lasers | n.r. | Fiber optic tip | 1,064 | 1 | 100 mJ | n.r. | 30 s/site, 120 s/tooth | Once | Insert the tip at the bottom of the periodontal pocket and slowly moved from apical to coronal in a sweeping motion (mesially, distally, buccally, and lingually) | n.r. |
| Eltas and Orbak (2012) (2) | Nd:YAG lasers | n.r. | Fiber optic tip | 1,064 | 1 | 100 mJ | n.r. | 30 s/site, 120 s/tooth | Once | Insert the tip at the bottom of the periodontal pocket and slowly moved from apical to coronal in a sweeping motion (mesially, distally, buccally, and lingually) | n.r. |
| Makhlouf et al. (2012) | Low‐level laser | Petrolaser apparatus model SL−202 | Fiber tip | 830 | 0.1 | n.r. | 3 J/cm2 | 30 s/site, 60 s/tooth | 10 sessions for every site | The tip was positioned externally at the base of the pocket, lingually and buccally in slight contact, at a 90°angle to the long axis of the tooth, starting apically and moving upward to the top of the pocket | Oral hygiene compliance weekly for 6 months (only superficial debris was removed) |
| Euzebio Alves et al. (2013) | Diode laser | ZAP Softlase, Pleasant Hill, USA | Fiber optic tip (400 µm) | 808 ± 5 | 1.5 | n.r. | 1,193.7 W/cm2 | 20 s/site | Twice: 1 day and 1 week after SRP of experimental sites | The optic was parallel to the long axis of the tooth, 1mm coronal to the base of the pocket, and was moved coronally with sweeping movements | Periodontal maintenance at 3 months |
| Saglam et al. (2014) | Diode laser | Ezlase, Biolase, USA | Fiber optic tip (300 μm) | 940 | 1.5 | n.r. | 15 J/cm2 | 20 s/tooth | Once | The tip was inserted into the periodontal pocket base in parallel alignment with the root surface, slowly moved from apical to coronal in a sweeping motion (mesially to distally at the buccal aspect for 10 s and distally to mesially at the lingual aspect for 10 s) | n.r. |
| Ustun et al. (2014) | Diode laser | Fotona XD‐2, Fotona d.d., Slovenia | Fiber tip (320 μm) | 810 | 1.25 | n.r. | n.r. | 20 s/site, 80 s/tooth | Once | The tip inserted at the bottom of the periodontal pocket, slowly moved from apical to coronal in a sweeping motion (mesially, distally, lingually and buccally) | n.r. |
| Sanz‐Sanchez et al. (2015) | Er:YAG laser | DS‐001A, Electro Medical System, Nyon, Switzerland | Sapphire tip | n.r. | n.r. | 160 mJ | n.r. | n.r. | Once | The tip was inserted along the pocket and swiped along the root surface | Supragingivally polish and a low abrasive polishing paste at each follow‐up visit |
| Dereci et al. (2016) | Er, Cr:YSGG laser | Biolase, Irvine, California, USA | Fiber optic tip RFPT 5‐14 | n.r. | 1.5 | n.r. | n.r. | n.r. | 3 times within 7 days | Bottom‐up technique: with an angulation of 10° to the root surface, in a bottom to upward direction with circulation movements in contact with the pocket, each pocket was irradiated once per session | n.r. |
| Gundogar et al. (2016) | Low‐level laser (GaAlAs diode laser) | CHEESE, GIGAA Laser, Wuhan, China | n.r. (1 cm) | 980 | 0.4 | n.r. | 7.64 J/cm2 | 15 s/tooth | Four times: immediately after SRP and on the 1, 3, and 7 day after treatment | Non‐contact from the buccal surface and tip‐tissue distance was approximately 5 mm | n.r. |
| Magaz et al. (2016) | Er, Cr:YSGG laser | Waterlase, Biolase | Fiber optic tip (600μm) | 2,780 | 1 | 50 mJ/pulse | n.r. | 60 s/tooth | Once | The tip was led in parallel paths with an inclination of 5 to 15° toward the root surface, from the coronal to the apical aspects of the pocket | n.r. |
| Everett et al. (2017) | Carbon dioxide (CO2) laser | Azuryt CTL 1,401, CO2 | Fiber optic tip (762 μm) | 10,600 | Initial pass: 4, second pass: 8 | n.r. | Initial pass: 25–50 J/cm2, second pass: 75–100 J/cm2 | 4 s/tooth | Four times: immediately after SRP and on the 10, 20 and 30 day postscaling | Maintaining a parallel orientation to the long axis of the tooth to the base of the probing depth, the laser was continuously “dragged” in the pocket from distal to the mesial portion in 2 s (once on the buccal and once on the lingual portion of the pockets) | Supragingival prophylaxis, oral hygiene instructions at 10, 20, and 30 days postscaling |
| Ustun et al. (2018) | Er, Cr:YSGG laser | Waterlase iplus, Biolase, Irvine, CA, USA | Fiber optic tip RFPT 5‐14 | n.r. | 1.5 | n.r. | n.r. | n.r. | Once | n.r. | n.r. |
| Zengin Celik et al. (2019) | Er:YAG laser | Light‐Walker AT, Fotona, Ljubljana, Slovenia | Quartz tip (0.6mm) | n.r. | n.r. | 150 mJ/pulse | n.r. | n.r. | Once | From the coronal to the apical direction in slow parallel paths at an inclination of 15–20° to the root surface | Supragingival scaling for the first postoperative month at 2‐week intervals and at 3‐month intervals |
| Zhou et al. (2019) | Er:YAG laser | LITETOUCH, Syneron, Yokneam Elite, Israel | Fiber tip (0.8 mm) | n.r. | n.r. | Hard tissue: 100 mJ/pulse, soft tissue: 50 mJ/pulse | n.r. | n.r. | Once | In a coronal to apical direction in slow parallel paths at an inclination of 15–20° to the root surface | n.r. |
| Sezen et al. (2020) | Er, Cr:YSGG laser | n.r. | Elastic RFPT5−14 tip (580 μm) | n.r. | 1.2 | 40 mJ | n.r. | 30 s/site | Once | Bottom‐up technique: the tip was placed at the bottom of the pocket and moved slowly in a coronal direction, circulating parallel to the surface of the teeth | n.r. |
Abbreviations: n.r., not reported; SRP, scaling and root planing.
FIGURE 2.
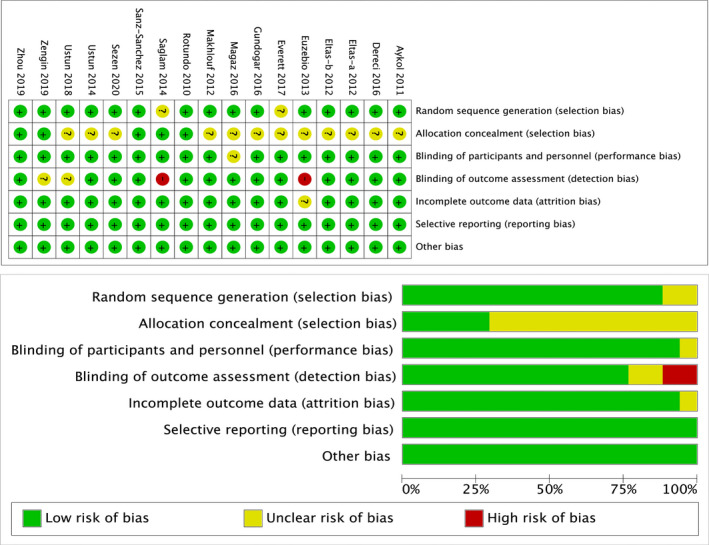
Assessing risk of bias in included studies by Cochrane risk of bias tools
3.3. Clinical efficacy assessment
At baseline, no significant difference was observed between the laser and control groups for all outcomes. All of the incorporated studies reported PD data during the follow‐up period. Pooled analysis suggested that the additional use of laser for SRP showed lower PD at week 4 (MD = −0.47, 95% CI −0.71 to −0.23; I2 = 85%, p = .0001), week 12 (MD = −0.29, 95% CI −0.53 to −0.05; I2 = 94%, p = .02) and week 24 (MD = −0.24, 95% CI −0.41 to −0.06; I2 = 91%, p = .008) after treatment, with statistically remarkable difference. However, heterogeneity was especially high at most time points (Figure 3).
FIGURE 3.
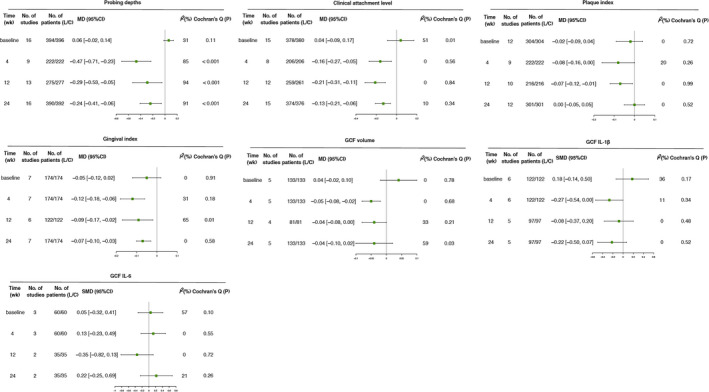
Incorporated forest plot of clinical (PD, CAL, PI and GI) and biochemical (GCF volume, IL‐1β level, and IL‐6 level) parameters during follow‐up
Fifteen studies reported CAL data corresponding to their respective follow‐up periods. After treatment, the CAL was significantly lower in the laser group, and the difference was statistically significant at week 4 (MD = −0.16, 95% CI −0.27 to −0.05; I2 = 0%, p = .004), week 12 (MD = −0.21, 95% CI −0.31 to −0.11; I2 = 0%, p < .0001) and week 24 (MD = −0.13, 95% CI −0.21 to −0.06; I2 = 10%, p = .0005). No heterogeneity was found.
Regarding the PI and GI, the indexes were significantly lower in the laser group at all time points during follow‐up period except for PI at week 24.
3.4. Biochemical efficacy assessment
Synthesis of these five studies revealed that the GCF volume in the laser group was significantly lower at week 4 (MD = −0.05, 95% CI −0.08 to −0.02; I2 = 0%, p = .002) and a marginal lower at week 12 (MD = −0.04, 95% CI −0.08 to 0; I2 = 33%, p = .05), while no significant difference was found after a follow‐up of 24 weeks, with moderate heterogeneity. There was almost no significant difference regarding the IL‐1β and IL‐6 level in GCF. All these results are shown in Figure 3.
3.5. Adverse event assessment
Safety was evaluated upon assessing the adverse effects that occurred during and after treatment. Of the 16 reports, no laser‐related side effects were observed in seven studies. Other nine studies did not mention the relevant side effects in the whole process.
3.6. Subgroup analysis
3.6.1. Study design
The subgroup analysis of six studies with parallel design revealed a lower PD, with significant difference at week 4 (MD = −0.68, 95% CI −1.06 to −0.31; I2 = 77%, p = .0004), but without significant differences at week 12 and week 24 in terms of high heterogeneity, as illustrated in Figure 4a. The CAL and PI were significantly lower in the laser group at week 12, week 24 and week 4, week 12, respectively. In contrast, the pooled result based on split‐mouth models demonstrated a significant lower PD in the laser group at all time points. However, no significant difference was found regarding CAL or PI.
FIGURE 4.
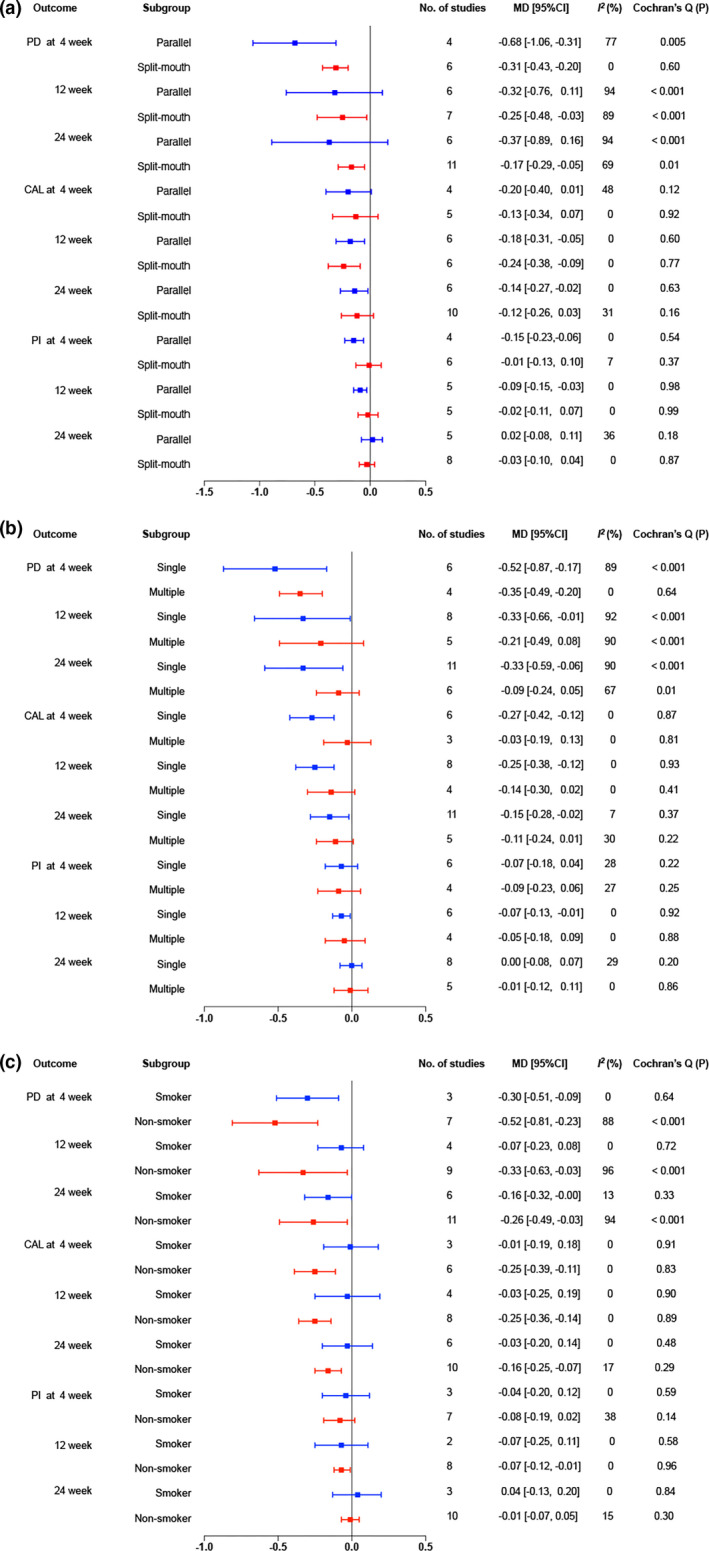
Further subgroup analysis for clinical performance according to smoking status. (a) Forest plot of the PD during follow‐up. (b) Forest plot of the CAL during follow‐up. (c) Forest plot of the PI during follow‐up
3.6.2. Laser irradiation times
For the subgroup of single‐time irradiation, the additional use of laser for SRP showed significant superiority over SRP alone in terms of PD and CAL at week 4, week 12, and week 24 after treatment. For the subgroup of multiple‐time irradiation, pooled analysis suggested that no significant difference was observed in the three parameters between the laser and control groups, except for PD at week 4 (MD = −0.35, 95% CI −0.49 to −0.2; I2 = 0%, p < .00001). However, heterogeneity was substantially high at most time points in PD (Figure 4b).
3.6.3. Smoking status
For the subgroup composed of smokers, the combined therapy resulted in a lower PD with significant difference at week 4 (MD = −0.3, 95% CI −0.51 to −0.09; I2 = 0%, p = .005) and it indicated a borderline significant lower at 24 weeks after treatment. For the subgroup of non‐smokers, the additional use of laser to SRP showed significant lower PD at all time points (Figure 4c). Heterogeneity was especially high in subgroup of non‐smokers. Sensitivity analysis showed that one study affected the heterogeneity the most (Table S2).
For the subgroup which enrolled smokers, the combined therapy did not bring significant difference in CAL at week 4 and 24. In contrast, the subgroup of non‐smokers, the CAL, was significantly lower in the laser group, and the difference was statistically significant at week 4 (MD = −0.25, 95% CI −0.39 to −0.11; I2 = 0%, p = .0004), week 12 (MD = −0.25, 95% CI −0.36 to −0.14; I2 = 0%, p < .00001) and week 24 (MD = −0.16, 95% CI −0.25 to −0.07; I2 = 17%, p = .0007).
For the subgroup which enrolled smokers, there was no significant difference between the experimental and control groups at all time points for PI. For non‐smokers, the PI was significantly lower in the laser group at week 12 (MD = −0.07, 95% CI −0.12 to −0.01; I2 = 0%, p = .02), while no significant difference was found at week 4 and week 24. No heterogeneity was found regarding the CAL and PI at all time points.
3.7. Sensitivity analysis and publication bias
In our systematic review and meta‐analysis, a sensitivity analysis of variability was conducted only when considerable heterogeneity (I2 > 50%) was indicated. See more details in Table S2. No significant publication bias was detected (Figure 5).
FIGURE 5.
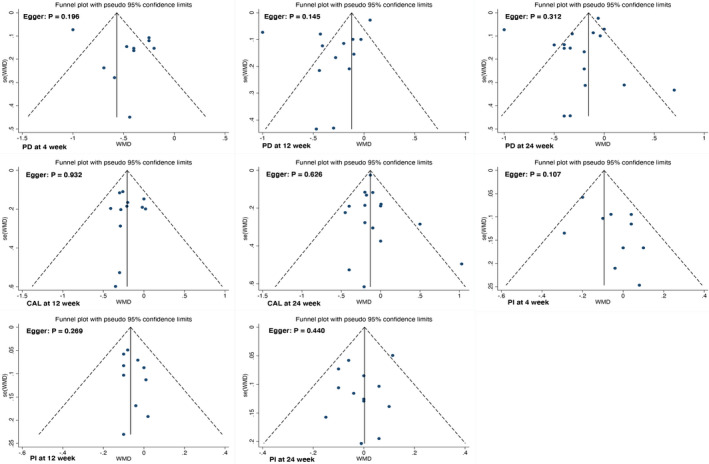
Funnel plot for PD, CAL, and PI. Notes: Only parameters discussed in over 10 papers were conducted bias analyses
4. DISCUSSION
This systematic review and meta‐analysis indicated that major classic clinical indicators (PD, CAL, GI, PI) of periodontitis, within the follow‐up of 6 months, had a significant improvement when comparing SRP + laser to SPR alone. The encouraging results we obtained may be partly attributed to the advantages of lasers over conventional methods. In areas that are inaccessible for mechanical instruments, such as deep pockets, furcations, or tight root proximity, lasers could reduce subgingival bacterial loads and exert bio‐stimulatory, bactericidal effects. That is conductive to a more thorough treatment (Ishikawa et al., 2009).
In this meta‐analysis, the effects of laser therapy on reducing periodontal inflammation were also evaluated through the analysis of GCF. However, except for the GCF volume and IL‐1β level in the laser group at week 4, no significant difference was found in a short‐term or medium‐term observation. Considering the inconsistency of measurement and the paucity of studies regarding the GCF volume and cytokine level, it is quite difficult to draw a definitive conclusion with regard to the biochemical effect of laser therapy at present.
Smoking is a generally acknowledged risk factor for chronic periodontitis, and smokers usually present poor clinical response to periodontal therapy (Kibayashi et al., 2007). In order to eliminate this potential risk factor and reduce the heterogeneity caused by the enrollment of smokers, we conducted a subgroup meta‐analysis to assess how the effectiveness of laser + SRP was influenced by smoking, thereby elucidates the synergistic effect of lasers and SRP more accurately.
Overall, for the subgroup in which smokers were enrolled, the additional use of laser for SRP did not show any significant difference over SRP alone in terms of CAL and PI. In contrast, non‐smokers undergoing combined therapy showed significant clinical improvements both for short‐term and medium‐term period. The results of subgroup analysis clearly revealed a negative impact of smoking habit on clinical efficacy of the combined therapy. Given the results, for long‐time smokers, especially refractory cases, other adjunctive therapeutic approaches need to be explored for a more predictable and effective treatment of periodontitis.
In our meta‐analysis, subgroup analysis was performed only for three clinical outcomes due to limited studies. Besides, considering the situation that the number of cigarettes smoked per day and number of years of smoking were given in rough introduction in some studies, detailed smoking status is encouraged to be included in future clinical trials to further explore whether there is a dose‐based relationship between the frequency of smoking and the therapeutic effect brought by adjunctive use of lasers.
Study design is very important when devising study protocols. The split‐mouth model is a popular in vivo experimental design in oral research, in which different intervention is randomly performed for each side of the mouth. Split‐mouth design has some potential advantages. For instance, it can reduce the variability among patients and enhance statistical power of the study when sample size is relatively small (Hujoel & DeRouen, 1992). However, attention should be given to a biased estimate of treatment efficacy as a result of the potential “carry‐across” effect between treated quadrants within one patient (Lesaffre et al., 2007). Another aspect to be noticed is that the oral microenvironment is integral and indivisible. Split‐mouth trials showed deficiencies in assessing microbiological or biochemical changes, with the risk of translocation of pathogenic bacteria and cytokines within the oral cavity (Lesaffre et al., 2007). Our subgroup analysis showed a relatively ideal result in primary outcomes in split‐mouth design and most of which revealed consistent trends with parallel‐arm design. It seems that split‐mouth design is feasible for clinical assessment. The original concerns on cross‐effects and unpredictable “leakage” between quadrants of lasers might not exist or the influence might not be strong enough to change the result. However, more RCTs need to be carried out in the future to evaluate the impact of different study designs on the microbiological or biochemical performance of the adjunctive use of lasers with SRP.
There are many parameters of lasers that may have an impact on the effect, among which dose applied during laser application is an important one. Due to the limitation of the number of studies, we only conducted a subgroup meta‐analysis on laser irradiation times. For primary outcomes, the additional use of laser showed significant superiority at all time points in single exposure group. However, multiple‐exposure subgroup only showed a significant lower PD at week 4. The results revealed that single‐time laser irradiation had a better treatment effect. Moreover, it is worth noting that low‐level laser was applied for at least three times. This is probably because low‐level lasers are thought to function through the interaction of light with the cell and tissue instead of breakdown of tissue from high‐power laser. This interaction might also be affected by other parameters, such as wavelength, power, energy density, treatment duration, intervention time, method of application, and condition of tissue (Kellesarian et al., 2017; Varma et al., 2020). That is why a definitive conclusion with regard to the irradiation dose cannot be drawn at present.
The results of the present analysis are in partial agreement with those of a previous review by Jia et al. (2020), which revealed a better clinical effectiveness in CAL gain within 6 months when laser‐assisted periodontal treatment was performed. But CAL was chosen as the only observed indicator in Jia's research. The meta‐analysis by Cheng et al. (2016) suggested that the adjunctive laser therapy might be effective in reducing PD in a relatively short term (3 months), which was consistent with our findings. But no more follow‐up data are available. Moreover, the above‐mentioned literature has limitations in that smokers and non‐smokers with chronic periodontitis were analyzed together or smoking status was ignored in analysis.
Compared with other meta‐analyses, the present research has several strengths. First, we set half a year as the limit of follow‐up period in our inclusion criteria, aiming at tracking not only the short‐term but also the longer effects of laser‐assisted treatment. Second, we investigated the role of study design, laser irradiation times, and smoking in the combined therapy based on the analysis by subgroup in turn. No previous meta‐analysis has ever been performed for such types of subgroup analyses so far. Third, the outcomes in this meta‐analysis include both clinical and biochemical parameters so as to comprehensively explore the changes brought about by laser and non‐surgical mechanical treatment.
However, there are several limitations in our study. First, only the literature in English is included, which may result in omission of information from the literature in other languages. Second, the types of outcomes collected and analyzed in present study were not comprehensive. For example, if microbes had been observed at the same time, the results might be more convincing. Although many types of lasers have functions in eliminating bacterial endotoxins from the root surface and showing antibacterial effect in removing periodontal pathogen in vitro (Dodani et al., 2019; Javali et al., 2019), the microbiological efficacy indicates conflicting results in insufficient clinical trials (Arcuri et al., 2020). Other important factors that should not be ignored are the additional cost and working time. It is necessary to assess the validity as well as the cost‐effectiveness of this new approach, which might lead to more extensive and comprehensive guide in adjunctive use of laser in non‐surgical periodontal treatment.
The results of this meta‐analysis must be interpreted with caution due to the heterogeneity among studies. Considerable heterogeneity exists at most time points in PD, and the sensitivity analyses suggest that the heterogeneity could be decreased to a certain degree by excluding the studies of Saglam et al (Table S2). This might be caused by individual difference in patients diagnosed with chronic periodontitis, as different degrees and different rates of progress are all involved. Besides, unlike other protocols, full‐mouth subgingival SRP was performed in a single appointment in this clinical procedure and the whole‐mouth clinical indexes were all measured. The marked variation in involved teeth/sites could probably be another factor that contributes to increased heterogeneity. Within the highly heterogeneous set of confounding variables, such as different types of lasers and corresponding parameters, including wavelength, energy, power density, material, and size of tip, duration of irradiation and frequency of application were extremely variable in the selected studies, showing that there are no established protocols on the optimal use of laser in dental practice.
In order to yield more convincing and consistent results, potential confounding factors such as patient characteristics, development stage of the disease, selection criteria for sampling sites, irradiation frequency and method, post‐treatment instruction, and supportive periodontal therapy need to be properly standardized in the future. Meanwhile, further high‐quality studies are needed for the assessment of the costs and duration of treatment, as well as microbial and biochemical outcomes, to optimize and accurately evaluate the impact of adjunctive laser therapy.
5. CONCLUSION
Laser therapy has the potential to improve clinical outcome variables in both short‐ and medium‐term treatments. However, smoking may diminish the adjunct effect of laser therapy.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
AUTHOR CONTRIBUTIONS
Yiyang Jiang: Formal analysis; Methodology; Writing‐original draft; Writing‐review & editing. Jie Feng: Methodology; Writing‐review & editing. Juan Du: Data curation; Methodology. Jingfei Fu: Data curation. Yitong Liu: Data curation. Lijia Guo: Supervision. Yi Liu: Supervision.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/odi.13847.
Supporting information
Table S1
Table S2
ACKNOWLEDGEMENTS
This work was supported by grants from the National Nature Science Foundation of China (81974149 and 81991504 to Y.L, 81600891 to L.G), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201703 to Y.B), the Beijing Baiqianwan Talents Project (2017A17 to Y.L), Beijing Municipal Administration of Hospitals' Ascent Plan (DFL20181501 to Y.L), Beijing Municipal Science & Technology Commission (Z16100000516203 to L.G), and Beijing Municipal Administration of Hospitals' Youth Programme (QML20181501).
Jiang Y, Feng J, Du J, et al. Clinical and biochemical effect of laser as an adjunct to non‐surgical treatment of chronic periodontitis. Oral Dis. 2022;28:1042–1057. 10.1111/odi.13847
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- Aoki, A. , Mizutani, K. , Schwarz, F. , Sculean, A. , Yukna, R. A. , Takasaki, A. A. , Romanos, G. E. , Taniguchi, Y. , Sasaki, K. M. , Zeredo, J. L. , Koshy, G. , Coluzzi, D. J. , White, J. M. , Abiko, Y. , Ishikawa, I. , & Izumi, Y. (2015). Periodontal and peri‐implant wound healing following laser therapy. Periodontology 2000, 68(1), 217‐269. 10.1111/prd.12080 [DOI] [PubMed] [Google Scholar]
- Arcuri, C. , Petro, E. , Sollecchia, G. , Mummolo, S. , & Marzo, G. (2020). Laser in periodontal pockets: In vivo and in vitro study. Journal of Biological Regulators and Homeostatic Agents, 34(3 Suppl. 1), 139–146. [PubMed] [Google Scholar]
- Aykol, G. , Baser, U. , Maden, I. , Kazak, Z. , Onan, U. , Tanrikulu‐Kucuk, S. , Ademoglu, E. , Issever, H. , & Yalcin, F. (2011). The effect of low‐level laser therapy as an adjunct to non‐surgical periodontal treatment. Journal of Periodontology, 82(3), 481–488. 10.1902/jop.2010.100195 [DOI] [PubMed] [Google Scholar]
- Caffesse, R. G. , & Echeverría, J. J. (2019). Treatment trends in periodontics. Periodontology 2000, 79(1), 7–14. 10.1111/prd.12245 [DOI] [PubMed] [Google Scholar]
- Cheng, Y. , Chen, J. W. , Ge, M. K. , Zhou, Z. Y. , Yin, X. , & Zou, S. J. (2016). Efficacy of adjunctive laser in non‐surgical periodontal treatment: A systematic review and meta‐analysis. Lasers in Medical Science, 31(1), 151–163. 10.1007/s10103-015-1795-5 [DOI] [PubMed] [Google Scholar]
- Cobb, C. M. (2006). Lasers in periodontics: A review of the literature. Journal of Periodontology, 77(4), 545–564. 10.1902/jop.2006.050417 [DOI] [PubMed] [Google Scholar]
- Cobb, C. M. (2017) . Lasers and the treatment of periodontitis: the essence and the noise. Periodontol 2000, 75(1), 205–295. 10.1111/prd.12137 [DOI] [PubMed] [Google Scholar]
- de Oliveira, R. R. , Novaes, A. B. , Garlet, G. P. , de Souza, R. F. , Taba, M. , Sato, S. , de Souza, S. L. S. , Palioto, D. B. , Grisi, M. F. M. , & Feres, M. (2011). The effect of a single episode of antimicrobial photodynamic therapy in the treatment of experimental periodontitis. Microbiological profile and cytokine pattern in the dog mandible. Lasers in Medical Science, 26(3), 359–367. 10.1007/s10103-010-0864-z [DOI] [PubMed] [Google Scholar]
- Dereci, O. , Hatipoglu, M. , Sindel, A. , Tozoglu, S. , & Ustun, K. (2016). The efficacy of Er, Cr:YSGG laser supported periodontal therapy on the reduction of peridodontal disease related oral malodor: A randomized clinical study. Head & Face Medicine, 12(1), 20. 10.1186/s13005-016-0116-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, S. B. F. , Fonseca, M. V. A. , dos Santos, N. C. C. , Mathias, I. F. , Martinho, F. C. , Junior, M. S. , Jardini, M. A. N. , & Santamaria, M. P. (2015). Effect of GaAIAs low‐level laser therapy on the healing of human palate mucosa after connective tissue graft harvesting: Randomized clinical trial. Lasers in Medical Science, 30(6), 1695–1702. 10.1007/s10103-014-1685-2 [DOI] [PubMed] [Google Scholar]
- Dodani, K. , Khare, N. , Bathini, C. , Mishra, S. , Inamdar, M. N. , & Nasha, A. (2019). An in vitro study of bactericidal effect of gallium aluminium arsenide laser on anaerobic photosensitized periodontopathics. The Journal of Contemporary Dental Practice, 20(3), 385–389. 10.5005/jp-journals-10024-2526 [DOI] [PubMed] [Google Scholar]
- Eltas, A. , & Orbak, R. (2012). Clinical effects of Nd:YAG laser applications during nonsurgical periodontal treatment in smoking and nonsmoking patients with chronic periodontitis. Photomedicine and Laser Surgery, 30(7), 360–366. 10.1089/pho.2011.3184 [DOI] [PubMed] [Google Scholar]
- Euzebio Alves, V. T. , de Andrade, A. K. P. , Toaliar, J. M. , Conde, M. C. , Zezell, D. M. , Cai, S. , Pannuti, C. M. , & De Micheli, G. (2013). Clinical and microbiological evaluation of high intensity diode laser adjutant to non‐surgical periodontal treatment: A 6‐month clinical trial. Clinical Oral Investigations, 17(1), 87–95. 10.1007/s00784-012-0703-7 [DOI] [PubMed] [Google Scholar]
- Everett, J. D. , Rossmann, J. A. , Kerns, D. G. , & Al‐Hashimi, I. (2017). Laser assisted non‐surgical periodontal therapy: A double blind, randomized clinical trial. The Open Dentistry Journal, 11, 79–90. 10.2174/1874210601711010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundogar, H. , Senyurt, S. Z. , Erciyas, K. , Yalim, M. , & Ustun, K. (2016). The effect of low‐level laser therapy on non‐surgical periodontal treatment: A randomized controlled, single‐blind, split‐mouth clinical trial. Lasers in Medical Science, 31(9), 1767–1773. 10.1007/s10103-016-2047-z [DOI] [PubMed] [Google Scholar]
- Hujoel, P. P. , & DeRouen, T. A. (1992). Validity issues in split‐mouth trials. Journal of Clinical Periodontology, 19(9 Pt 1), 625–627. 10.1111/j.1600-051x.1992.tb01709.x [DOI] [PubMed] [Google Scholar]
- Ishikawa, I. , Aoki, A. , Takasaki, A. A. , Mizutani, K. , Sasaki, K. M. , & Izumi, Y. (2009). Application of lasers in periodontics: True innovation or myth? Periodontology 2000, 50, 90–126. 10.1111/j.1600-0757.2008.00283.x [DOI] [PubMed] [Google Scholar]
- Javali, M. A. , AlQahtani, N. A. , Ahmad, I. , & Ahmad, I. (2019). Antimicrobial photodynamic therapy (light source; methylene blue; titanium dioxide): Bactericidal effects analysis on oral plaque bacteria: An in vitro study. Nigerian Journal of Clinical Practice, 22(12), 1654–1661. 10.4103/njcp.njcp_189_19 [DOI] [PubMed] [Google Scholar]
- Jia, L. , Jia, J. , Xie, M. , Zhang, X. , Li, T. , Shi, L. , Shi, H. , & Zhang, X. (2020). Clinical attachment level gain of lasers in scaling and root planing of chronic periodontitis: A network meta‐analysis of randomized controlled clinical trials. Lasers in Medical Science, 35(2), 473–485. 10.1007/s10103-019-02875-5 [DOI] [PubMed] [Google Scholar]
- Jiao, Y. , Tay, F. R. , Niu, L. N. , & Chen, J. H. (2019). Advancing antimicrobial strategies for managing oral biofilm infections. International Journal of Oral Science, 11(3), 28. 10.1038/s41368-019-0062-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, M. T. , Michalowicz, B. S. , Kotsakis, G. A. , & Chu, H. (2017). Network meta‐analysis of studies included in the Clinical Practice Guideline on the nonsurgical treatment of chronic periodontitis. Journal of Clinical Periodontology, 44(6), 603–611. 10.1111/jcpe.12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellesarian, S. V. , Malignaggi, V. R. , Majoka, H. A. , Al‐Kheraif, A. A. , Kellesarian, T. V. , Romanos, G. E. , & Javed, F. (2017). Effect of laser‐assisted scaling and root planing on the expression of pro‐inflammatory cytokines in the gingival crevicular fluid of patients with chronic periodontitis: A systematic review. Photodiagnosis and Photodynamic Therapy, 18, 63–77. 10.1016/j.pdpdt.2017.02.010 [DOI] [PubMed] [Google Scholar]
- Kibayashi, M. , Tanaka, M. , Nishida, N. , Kuboniwa, M. , Kataoka, K. , Nagata, H. , Nakayama, K. , Morimoto, K. , & Shizukuishi, S. (2007). Longitudinal study of the association between smoking as a periodontitis risk and salivary biomarkers related to periodontitis. Journal of Periodontology, 78(5), 859–867. 10.1902/jop.2007.060292 [DOI] [PubMed] [Google Scholar]
- Koyanagi, T. , Sakamoto, M. , Takeuchi, Y. , Maruyama, N. , Ohkuma, M. , & Izumi, Y. (2013). Comprehensive microbiological findings in peri‐implantitis and periodontitis. Journal of Clinical Periodontology, 40(3), 218–226. 10.1111/jcpe.12047 [DOI] [PubMed] [Google Scholar]
- Lesaffre, E. , Garcia Zattera, M. J. , Redmond, C. , Huber, H. , & Needleman, I. (2007). Reported methodological quality of split‐mouth studies. Journal of Clinical Periodontology, 34(9), 756–761. 10.1111/j.1600-051X.2007.01118.x [DOI] [PubMed] [Google Scholar]
- Loe, H. , & Silness, J. (1963). Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontologica Scandinavica, 21, 533–551. 10.3109/00016356309011240 [DOI] [PubMed] [Google Scholar]
- Magaz, V. R. , Alemany, A. S. , Alfaro, F. H. , & Molina, J. N. (2016). Efficacy of adjunctive Er, Cr:YSGG laser application following scaling and root planing in periodontally diseased patients. The International Journal of Periodontics & Restorative Dentistry, 36(5), 715–721. 10.11607/prd.2660 [DOI] [PubMed] [Google Scholar]
- Makhlouf, M. , Dahaba, M. M. , Tuner, J. , Eissa, S. A. , & Harhash, T. A. (2012). Effect of adjunctive low level laser therapy (LLLT) on nonsurgical treatment of chronic periodontitis. Photomedicine and Laser Surgery, 30(3), 160–166. 10.1089/pho.2011.3069 [DOI] [PubMed] [Google Scholar]
- Meyle, J. , & Chapple, I. (2015). Molecular aspects of the pathogenesis of periodontitis. Periodontology 2000, 69(1), 7–17. [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. BMJ, 339, b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombelli, A. (2018). Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000, 76(1), 85–96. 10.1111/prd.12147 [DOI] [PubMed] [Google Scholar]
- Paes Batista da Silva, A. , Barros, S. P. , Moss, K. , Preisser, J. , Marchesan, J. T. , Ward, M. , & Offenbacher, S. (2016). Microbial profiling in experimentally induced biofilm overgrowth among patients with various periodontal states. Journal of Periodontology, 87(1), 27–35. 10.1902/jop.2015.150328 [DOI] [PubMed] [Google Scholar]
- Petrovic, M. , Kesic, L. , Obradovic, R. , Savic, Z. , Mihailovic, D. , Obradovic, I. , AvdicSaracevic, M. , JanjicTrickovic, O. , & Janjic, M. (2013). Comparative analysis of smoking influence on periodontal tissue in subjects with periodontal disease. Materia Socio Medica, 25(3), 196–198. 10.5455/msm.2013.25.196-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotundo, R. , Nieri, M. , Cairo, F. , Franceschi, D. , Mervelt, J. , Bonaccini, D. , Esposito, M. , & Pini‐Prato, G. (2010). Lack of adjunctive benefit of Er:YAG laser in non‐surgical periodontal treatment: A randomized split‐mouth clinical trial. Journal of Clinical Periodontology, 37(6), 526–533. 10.1111/j.1600-051X.2010.01560.x [DOI] [PubMed] [Google Scholar]
- Saglam, M. , Kantarci, A. , Dundar, N. , & Hakki, S. S. (2014). Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: A randomized, controlled clinical trial. Lasers in Medical Science, 29(1), 37–46. 10.1007/s10103-012-1230-0 [DOI] [PubMed] [Google Scholar]
- Salvi, G. E. , Stähli, A. , Schmidt, J. C. , Ramseier, C. A. , Sculean, A. , & Walter, C. (2020). Adjunctive laser or antimicrobial photodynamic therapy to non‐surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47(Suppl 22), 176–198. 10.1111/jcpe.13236 [DOI] [PubMed] [Google Scholar]
- Sanz, M. , Herrera, D. , Kebschull, M. , Chapple, I. , Jepsen, S. , Berglundh, T. , Sculean, A. , Tonetti, M. S. , Merete Aass, A. , Aimetti, M. , Kuru, B. E. , Belibasakis, G. , Blanco, J. , Bol‐van den Hil, E. , Bostanci, N. , Bozic, D. , Bouchard, P. , Buduneli, N. , Cairo, F. , … Wennström, J. (2020). Treatment of stage I‐III periodontitis‐The EFP S3 level clinical practice guideline. Journal of Clinical Periodontology, 47(Suppl 22), 4–60. 10.1111/jcpe.13290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz‐Sanchez, I. , Ortiz‐Vigon, A. , Matos, R. , Herrera, D. , & Sanz, M. (2015). Clinical efficacy of subgingival debridement with adjunctive erbium:Yttrium‐aluminum‐garnet laser treatment in patients with chronic periodontitis: A randomized clinical trial. Journal of Periodontology, 86(4), 527–535. 10.1902/jop.2014.140258 [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Aoki, A. , Becker, J. , & Sculean, A. (2008). Laser application in non‐surgical periodontal therapy: A systematic review. Journal of Clinical Periodontology, 35(8 Suppl), 29–44. 10.1111/j.1600-051X.2008.01259.x [DOI] [PubMed] [Google Scholar]
- Sella, V. R. G. , do Bomfim, F. R. C. , Machado, P. C. D. , da Silva Morsoleto, M. J. M. , Chohfi, M. , & Plapler, H. (2015). Effect of low‐level laser therapy on bone repair: A randomized controlled experimental study. Lasers in Medical Science, 30(3), 1061–1068. 10.1007/s10103-015-1710-0 [DOI] [PubMed] [Google Scholar]
- Sezen, D. , Hatipoglu, M. , & Ustun, K. (2020). Evaluation of the clinical and biochemical efficacy of erbium, chromium:Ytrium‐scandium‐gallium‐garnet (ER, CR:YSGG) laser treatment in periodontitis. Lasers in Medical Science, 35, 1567–1575. 10.1007/s10103-020-02990-8 [DOI] [PubMed] [Google Scholar]
- Sgolastra, F. , Severino, M. , Petrucci, A. , Gatto, R. , & Monaco, A. (2014). Nd:YAG laser as an adjunctive treatment to nonsurgical periodontal therapy: A meta‐analysis. Lasers in Medical Science, 29(3), 887–895. 10.1007/s10103-013-1293-6 [DOI] [PubMed] [Google Scholar]
- Sherman, P. R. , Hutchens, L. H. Jr , Jewson, L. G. , Moriarty, J. M. , Greco, G. W. , & McFall, W. T. Jr (1990). The effectiveness of subgingival scaling and root planning. I. Clinical detection of residual calculus. Journal of Periodontology, 61(1), 3–8. 10.1902/jop.1990.61.1.3 [DOI] [PubMed] [Google Scholar]
- Silness, J. , & Loe, H. (1964). Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontologica Scandinavica, 22, 121–135. 10.3109/00016356408993968 [DOI] [PubMed] [Google Scholar]
- Sjöström, L. , & Friskopp, J. (2002) .Laser treatment as an adjunct to debridement of periodontal pockets. Swedish Dental Journal, 26(2), 51–57. [PubMed] [Google Scholar]
- Umeda, M. , Takeuchi, Y. , Noguchi, K. , Huang, Y. , Koshy, G. , & Ishikawa, I. (2004). Effects of nonsurgical periodontal therapy on the microbiota. Periodontology 2000, 36, 98–120. 10.1111/j.1600-0757.2004.03675.x [DOI] [PubMed] [Google Scholar]
- Ustun, K. , Erciyas, K. , Sezer, U. , Senyurt, S. Z. , Gundogar, H. , Ustun, O. , & Oztuzcu, S. (2014). Clinical and biochemical effects of 810 nm diode laser as an adjunct to periodontal therapy: A randomized split‐mouth clinical trial. Photomedicine and Laser Surgery, 32(2), 61–66. 10.1089/pho.2013.3506 [DOI] [PubMed] [Google Scholar]
- Ustun, K. , Hatipoglu, M. , Daltaban, O. , Felek, R. , & Firat, M. Z. (2018). Clinical and biochemical effects of erbium, chromium: Yttrium, scandium, gallium, garnet laser treatment as a complement to periodontal treatment. Nigerian Journal of Clinical Practice, 21(9), 1150–1157. 10.4103/njcp.njcp_51_18 [DOI] [PubMed] [Google Scholar]
- Varma, S. R. , AlShayeb, M. , Narayanan, J. , Abuhijleh, E. , Hadi, A. , Jaber, M. , & Abu Fanas, S. (2020). Applications of lasers in refractory periodontitis: A narrative review. Journal of International Society of Preventive and Community Dentistry, 10(4), 384–393. 10.4103/jispcd.JISPCD_241_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin Celik, T. , Saglam, E. , Ercan, C. , Akbas, F. , Nazaroglu, K. , & Tunali, M. (2019). Clinical and microbiological effects of the use of erbium: Yttrium‐aluminum‐garnet laser on chronic periodontitis in addition to nonsurgical periodontal treatment: A randomized clinical trial‐6 months follow‐up. Photobiomodulation, Photomedicine, and Laser Surgery, 37(3), 182–190. 10.1089/photob.2018.4510 [DOI] [PubMed] [Google Scholar]
- Zhou, X. , Lin, M. , Zhang, D. , Song, Y. , & Wang, Z. (2019). Efficacy of Er:YAG laser on periodontitis as an adjunctive non‐surgical treatment: A split‐mouth randomized controlled study. Journal of Clinical Periodontology, 46(5), 539–547. 10.1111/jcpe.13107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


