Abstract
Injury to the A2 pulley is caused by high eccentric forces on the flexor‐tendon–pulley system. Accurate diagnosis is necessary to identify the most appropriate treatment options. This review summarizes the literature with respect to using ultrasound (US) to diagnose A2 pulley injuries, compares ultrasound to magnetic resonance imaging and computed tomography, and identifies current knowledge gaps. The results suggest that US should be used as the primary imaging modality given high accuracy, relatively low cost, ease of access, and dynamic imaging capabilities. Manual resistance is beneficial to accentuate bowstringing, but further research is needed to determine best positioning for evaluation.
Keywords: CT, finger injury, MRI, rock climbing, US
Abbreviations
- CT
computed tomography
- DIP
distal interphalangeal
- MRI
magnetic resonance imaging
- PIP
proximal interphalangeal
- TP
tendon to phalanx
- US
ultrasound
The annular pulleys are fibrous envelopes that enhance flexor tendon functions by holding the tendons against the phalanges. Of the five pulleys, the A2 pulley is the thickest and strongest, and has an average length of 16.3 mm. 1 Anatomically, the A2 pulley stretches from the cranial part of the proximal phalanx to the junction of the proximal two‐thirds and distal‐third of the proximal phalanx (Figure 1). When the A2 pulley is significantly injured, bowstringing of the flexor tendons occurs, which can lead to a range of motion loss and reduced finger function. Bowstringing is a result of the flexor tendon gaining an increased mechanical advantage over the extensors, causing the tendon to separate from the phalanx and restrict digit extension. 2
Figure 1.
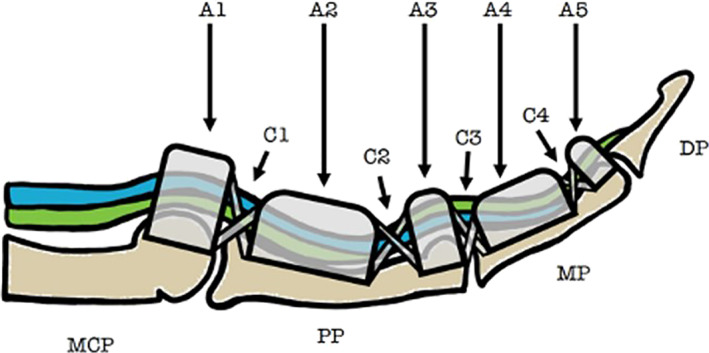
Sagittal depiction of the anatomy of the finger flexor pulley system. The flexor digitorum superficialis (blue) and flexor digitorum profundus (green) are displayed. Annular pulleys are represented using A1–A5 and cruciate pulleys using C1–C4. MCP, metacarpophalangeal joint; PP, proximal phalanx; MP, middle phalanx; DP, distal phalanx.
Biomechanical analysis has shown that injury to the A2 pulley likely occurs when the pulley is eccentrically loaded, resulting in high tensile forces between the pulley and tendon. 3 Consequently, eccentric flexor tendon loading is generally considered to be one of the greatest risk factors for A2 pulley injury. 3 A2 pulley injuries have been reported in rock climbers, baseball players, bowlers, and other persons who engage in activities that involve a sudden extension force applied to an acutely flexed digit. 4 , 5 , 6 , 7 Pulley injuries have also been frequently reported in patients who have received multiple steroid injections near the pulley site. 8 Cadaver studies have further investigated the biomechanics of loading within the pulley system to determine which pulley is most prone to injury, with one study reporting that the A2 pulley is most likely to rupture, followed by the A3, A4, and A1 pulleys. 9
Rock climbing has the highest incidence of A2 pulley injuries of all sports. 10 A survey of competitive rock climbers in the United States found that 26% of climbers were found to have flexor pulley injuries involving A2. 4 This is because climbers will often support their entire body weight using one or two fingers in a flexed position, imparting significant stress on the pulleys. In addition, climbers will sometimes use a crimp grip (Figure 2) to successfully latch on to small surface areas while climbing. The crimp grip positions the proximal interphalangeal (PIP) joint at 90+ degrees of flexion and the distal interphalangeal (DIP) joint in maximal hyperextension, 11 which places 36 times more force on the A2 pulley than a standard slope grip. 12 This underlying tension has been described to exceed the threshold for A2 pulley rupture. 12
Figure 2.

Representation of crimp grip of the fingers in a typical climbing position.
Clinically, A2 pulley tears can present either acutely or chronically and range in severity from small, partial tears to large, complete tears. Acute pulley tears can involve a sudden sharp pain that occurs during activity with an audible “pop” followed by finger swelling. 3 Chronic injuries are caused by repetitive microtrauma to the pulley, which can lead to symptoms of focal tenderness and pain while gripping. Bowstringing is generally considered to be indicative of a major A2 pulley injury as it typically results in cosmetic and functional deficiencies. 13 The clinical diagnosis of bowstringing can be particularly challenging because pain and swelling can obscure bowstringing, and lower severity injuries may present with more subtle bowstringing. 13 Therefore, clinicians must often rely on advanced imaging to make an accurate diagnosis and determine the best available treatment options, which may range from observation to surgical reconstruction. Several modalities have been proposed for this purpose, including ultrasound (US), magnetic resonance imaging (MRI), and computed tomography (CT).
US is a high‐resolution, accessible, cost‐effective musculoskeletal imaging modality that can be used to visualize the soft tissue structures of the fingers as well as the adjacent bony surfaces (Figure 3). 14 It is the only imaging modality that offers the ability to dynamically evaluate the pulley–tendon complex and easily compare the affected versus contralateral side. 13 For example, in addition to static imaging, the examiner can use real‐time motion of the flexor‐tendon–pulley system to differentiate it from surrounding structures and better characterize A2 pulley injuries. Specifically, resisted finger flexion can dynamically accentuate bowstringing, and the extent of bowstringing can be compared to the contralateral side. 14 Similar to US, MRI also provides excellent visualization of the A2 pulley and can identify indirect signs of A2 pulley injury such as edema and an increased tendon to phalanx (TP) distance. However, unlike US, MRI is more costly, less readily available, and does not provide the ability to perform dynamic testing. Static forces may be applied during MRI scan, but real‐time motion assessment is generally precluded by motion artifact. 15 , 16 It can also be difficult to visualize tears on routine sagittal MRI images depending on the size of the cuts, whereas US could more feasibly capture such images. CT shares many of the challenges of MRI including relatively high cost and the absence of dynamic imaging capabilities. Although CT does allow for exceptional visualization of osseous anatomy, it is not as detailed as US and MRI when imaging soft tissue structures.
Figure 3.
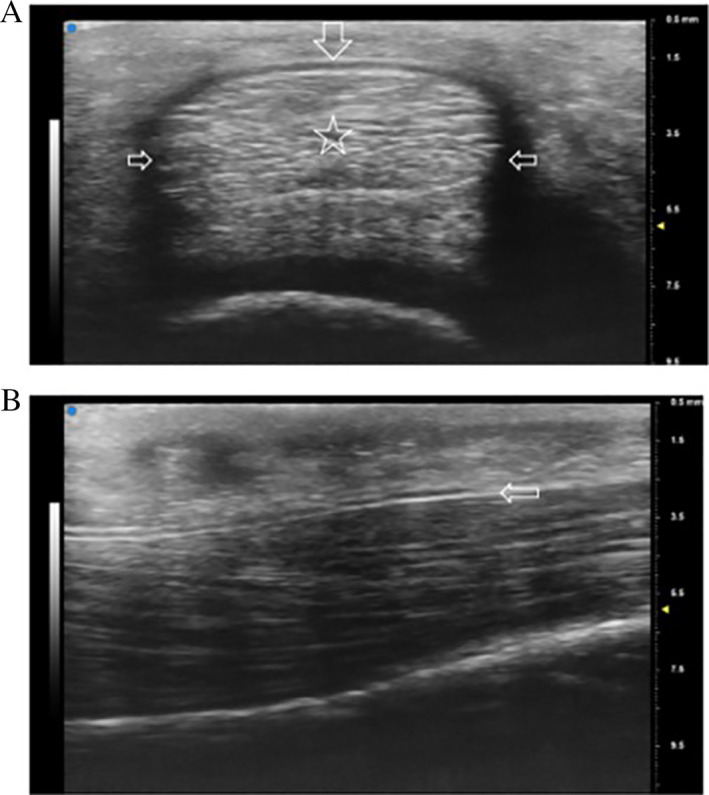
Normal annular A2 pulley depicted with a 20 to 46 MHz transducer (VisualSonics VEVO MD). A, Transverse ultrasound image obtained over the proximal phalanx demonstrating the A2 pulley as a thin band surrounding the flexor tendons. Note its relatively hyperechoic appearance on the volar portion (top arrow pointing down) and relatively hypoechoic appearance on both sides of the tendon as a result of anisotropy (middle arrows pointing inward). B, Longitudinal ultrasound image of the A2 pulley depicting the leading edge (arrow) over the proximal phalanx with the flexor tendons underneath (right = distal).
Given that US provides a unique blend of high resolution and dynamic imaging, it is not surprising that US has been used more commonly than MRI and CT to evaluate A2 pulley injuries. The primary purpose of this review is to summarize the clinical and cadaveric US studies, compare methods and findings against the other primary modalities (MRI, CT), and acknowledge research gaps that may best aid clinicians in pulley injury diagnoses.
Methods and Results
A literature search was conducted in May 2021 in the MEDLINE/PubMed database on studies published up until the search date. Searches were performed using a variation of the terms “A2 pulley,” “bowstringing,” and “pulley rupture.” Only one search limitation was applied—language was restricted to English only. The searches yielded a combined 690 results. Articles were excluded if they did not mention the A2 pulley or did not include US, MRI, or CT. Accordingly, 607 articles were excluded during title and abstract review, leaving 83 articles to move on to full‐text review. An additional 61 articles were excluded during full‐text review, leaving 22 articles to be included in this review for data extraction. Of the 22 articles, 12 included US only, 14 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 6 MRI only, 28 , 29 , 30 , 31 , 32 , 33 and 1 CT only, 34 with 3 including a combination of imaging methods (2 articles including US and MRI 35 , 36 ; 1 article including US, MRI, and CT 15 ). Sample demographics are provided in Table 1 and data extraction results in Table 2, with both tables organized into two sections: cadaver studies and clinical (non‐cadaver) studies.
Table 1.
Demographics Table
| Authors | Year | Experimental Group | Control Group | ||||
|---|---|---|---|---|---|---|---|
| Experimental Group | Climbing Experience (Years Climbing) | N Subjects (Male: Female) | N Fingers | Mean Age (Range) in Years | N Fingers | ||
| Cadaver studies | |||||||
| Hauger et al. 15 | 2000 | Cadavers | N/A | 4 (3:1) | 24 | 73.5 (68–78) | N/A |
| Leeflang & Coert 17 | 2014 | Cadavers | N/A | 8 (NS) | 24 | 76 (73–82) | N/A |
| Bayer et al. 28 | 2015 | Cadavers | N/A | 10 (7:3) | 21 | 75.5 (47–85) | N/A |
| Schoffl et al. 18 | 2017 | Cadavers | N/A | 10 (6:4) | 34 | 75.5 (NS) | N/A |
| Schoffl et al. 19 | 2018 | Cadavers | N/A | 9 (0:9) | 18 | 73.2 (NS) | N/A |
| Clinical studies | |||||||
| Parellada et al. 29 | 1996 | HV | N/A | 3 (3:0) | 3 | NS (23–59) | N/A |
| Le Viet et al. 34 | 1996 | RC; MI | NS | 7 (7:0) | 14* | 40 (NS); 38 (NS) | N/A |
| Gabl et al. 30 | 1998 | RC | 6–10 UIAA (NS) | 13 (12:1) | 13 | 27 (20–51) | N/A |
| Bodner et al. 35 | 1999 | RC; MI | NS | 32 (32:0) | 64* | 25 (18–42) | 40 |
| Klauser et al. 20 | 1999 | RC | 7–11 UIAA (5–25) | 34 (29:5) | 136 | 29.7 (21–54) | 80 |
| Martinoli et al. 36 | 2000 | RC | NS | 16 (16:0) | 19* | 27 (22–37) | 40 |
| Klauser et al. 21 | 2000 | RC | 8–11 UIAA (5–16) | 52 (NS) | 208 | 29.7 (NS) | 80 |
| Klauser et al. 14 | 2002 | RC | 8–11 UIAA (3–12) | 64 (NS) | 256 | 21.7 (18–35) | N/A |
| Schoffl et al. 22 | 2006 | RC | 8.53 ± 1.11 UIAA (NS) | 21 (19:2) | 27 | 34 (22–59) | N/A |
| Guntern et al. 31 | 2007 | RC | 6b–8c + French system (NS) | 8 (7:1) | 28 | 32.5 (19–43) | N/A |
| Bassemir et al. 23 | 2015 | HV | NS | 200 (100:100) | 1600* | 41.1 (NS) | N/A |
| Schneeberger & Schweizer 24 | 2016 | RC | On‐sight 7.87, redpoint 8.44 (1–30) | 45 (NS) | 47 | 33.4 (21.8–56.2) | N/A |
| Reissner et al. 25 | 2018 | HV | N/A | 10 (6:4) | 20 | 33 (18–60) | N/A |
| Hoff & Greenberg 32 | 2018 | HV | N/A | 1 (NS) | 2 | NS | N/A |
| Schellhammer & Vantorre 33 | 2019 | RC; HV | NS | 3 (3:0); 14 (9:5) | 22 | NS | N/A |
| Iruretagoiena‐Urbieta et al. 26 | 2020 | RC | NS | 29 (NS) | 58 | 33 (22–41) | 20* |
| Scheibler, Janig, & Schweizer 27 | 2021 | RC; MI | 6c–8b French system (NS) | 11 (9:2) | 12 | 39 (25–55) | N/A |
HV, healthy volunteers; MI, miscellaneous injury; N/A, not applicable (not included in study); NS, not stated (not specified in study). RC, Rock climbers; UIAA, Union Internationale des Associations d'Alpinisme.
Estimated based on descriptions in the paper.
Table 2.
Summary of Studies Using US, MRI, and CT in Diagnostics of A2 Pulley Injuries
| Authors | Year | Imaging Modality | Clinical System | Finger Positioning | Resistance Applied | Diagnostic Criteria | TP Location/Measurement | Sensitivity/Specificity |
|---|---|---|---|---|---|---|---|---|
| Cadaver studies | ||||||||
| Hauger et al. 15 | 2000 | CT | 2 mm slices, 1 mm increments |
MCP: full extension PIP: 60° flexion DIP: 10° flexion |
500 g weight with traction |
Complete tear: 2–5 mm Incomplete tear: 0–3 mm Combined tear: 5–8 mm |
Junction of proximal two‐thirds and distal one‐third of the proximal phalanx | NT |
| MRI | 1.5‐T with a phased array wrist coil |
MCP: full extension PIP: 60° flexion DIP: 10° flexion |
500 g weight with traction |
Complete tear: 2–5 mm Incomplete tear: 0–3 mm Combined tear: 5–8 mm |
Junction of proximal two‐thirds and distal one‐third of the proximal phalanx | NT | ||
| US | 12 MHz transducer |
MCP: full extension PIP: 60° flexion DIP: 10° flexion |
500 g weight with traction and simultaneous counter‐pressure at the fingertip |
Complete tear: 2–5 mm Incomplete tear: 0–3 mm Combined tear: 5–8 mm |
Junction of proximal two‐thirds and distal one‐third of the proximal phalanx | NT | ||
| Leeflang & Coert 17 | 2014 | US | Frequency band 20–60 MHz, center frequency 40 MHz transducer |
MCP: 15° flexion PIP: 15° flexion DIP: 15° flexion |
FDP loaded to 100 N, FDS preloaded to 5 N | Area between flexor tendon and palmar side of proximal phalanx | 10 mm distal to MCP; Size of area (mm2) between proximal phalanx and flexor tendon >5 mm | NT |
| Bayer et al. 28 | 2015 | MRI | 1.5 T with a dedicated eight channel orthopedic foot coil | Crimp Grip and Neutral | 10 N–1.0 kg weight attached to a pulley system to the FDS and FDP |
*Complete tears: 2.29 ± 1.82 mm (crimp grip) 1.10 ± 0.67 mm (neutral) |
50% length of the proximal phalanx | .93/1.0 in crimp and .79/.86 in neutral dissection |
| Schoffl et al. 18 | 2017 | US | 14 MHz transducer |
MCP: NS PIP: full available flexion DIP: up to 30° flexion |
10 N flexor tendons | Complete tear: >1.9 mm | Over A2 pulley | 1.0/.94 Dissection |
| Schoffl et al. 19 | 2018 | US | 18 MHz transducer |
MCP: NS PIP: 30° flexion DIP: full available flexion |
10 N flexor tendons | Complete tear: >2 mm | Middle of proximal phalanx | .94/1.0 Dissection |
| Clinical studies | ||||||||
| Parellada et al. 29 | 1996 | MRI | 1.5 T | Finger at full extension and 30–45° flexion a | None | Bowstringing b | NT | NT |
| Le Viet et al. 34 | 1996 | CT | 1.5 mm slices |
MCP: NS PIP: flexion DIP: extension |
Manual NS c | Bowstringing | NT | NT |
| Gabl et al. 30 | 1998 | MRI | 1.5 T with surface coil |
MCP: full extension PIP: 60° flexion DIP: 10° flexion |
Manual Subject |
Complete tear: Bowstringing from PIP joint to base of proximal phalanx Incomplete tear: Bowstringing less than to base of proximal phalanx |
NT | NT |
| Bodner et al. 35 | 1999 | MRI | 1.5 T with surface coil |
MCP: NS PIP: 40° flexion DIP: 10° flexion |
Manual subject | Complete tear: ≥3 mm extended, ≥5 mm flexed | Midpart of proximal phalanx, at bony attachment of the check rein ligament |
1.0/1.0 Surgical visualization d |
| US | 10 MHz transducer |
MCP: NS PIP: 40° flexion DIP: 10° flexion |
Manual examiner | Complete tear: ≥3 mm extended, ≥5 mm flexed | Midpart of proximal phalanx, at bony attachment of the check rein ligament |
1.0/1.0 Surgical Visualization |
||
| Klauser et al. 20 | 1999 | US | 10 MHz transducer |
MCP: extension PIP: 40° flexion DIP: 10° flexion |
Manual NS c |
Complete tear: >.3 cm at rest, >.5 cm forced flexion Bowstringing Tenosynovitis Tendon and pulley thickening Tendon gliding Cysts/fibrous tissue Fluid collection |
NS | NT |
| Martinoli et al. 36 | 2000 | MRI | MRI: 2 T with surface coil or a dedicated extremity coil | 45° flexion | Manual Examiner |
Bowstringing Synovial sheath effusion |
At the level of the proximal phalanx/pulley | NT |
| US | US: 12–5 MHz and 10–13 MHz transducers | 45° flexion | Manual Examiner |
Bowstringing Tenosynovitis Thickened hypoechoic pulley |
At the level of the proximal phalanx/pulley | NT | ||
| Klauser et al. 21 | 2000 | US | 13–5 MHz transducer |
MCP: extension PIP: 40° flexion DIP: 10° flexion |
Manual NS c |
Bowstringing Tendon thickening Tendon sheath cyst Fibrous tissue Fluid collection |
At the level of the base of the proximal phalanx in the area of the A2 pulley | NT |
| Klauser et al. 14 | 2002 | US | 12 MHz transducer |
MCP: extension PIP: 40° flexion DIP: 10° flexion |
Manual Examiner |
Complete tear: ≥3 mm Incomplete tear: <3 mm Combined tear (A2 + A3): ≥5 mm |
15–20 mm from the base of the proximal phalanx where the flexor tendon lies close to the phalanx | .98/1.0 MRI |
| Schoffl et al. 22 | 2006 | US | 10 MHz transducer | Forced flexion and extension | Manual examiner | Complete tear: >2 mm | Middle of the proximal phalanx | NT |
| Guntern et al. 31 | 2007 | MRI | 3 T with a dedicated wrist coil | Flexion and extension a | None | Complete tear: ≥2 mm | Mid‐diaphysis level in sagittal plane |
.88/1.0 Clinical exam |
| Bassemir et al. 23 | 2015 | US | 18 MHz transducer |
MCP: extension PIP: extension DIP: 30° flexion |
500 g weight with custom sling | N/A | Level of proximal‐ to middle‐third of the proximal phalanx | NT |
| Schneeberger & Schweizer 24 | 2016 | US | 17.5 MHz transducer |
MCP: extension PIP: 40° flexion DIP: 10° flexion |
Manual NS c | Bowstringing | Middle of the proximal phalanx | NT |
| Reissner et al. 25 | 2018 | US | 17 MHz transducer | Resting position | None | Bowstringing | 15 mm from the base of the proximal phalanx | NT |
| Hoff & Greenberg 32 | 2018 | MRI | 1.5 T | Crimp grip | Subject grasped glass container | Complete tear: ≥2 mm | Middle of the proximal phalanx | NT |
| Schellhammer & Vantorre 33 | 2019 | MRI | 1.5 T with a dedicated knee coil | 6 consecutive finger positions from extension to full flexion | None | Bowstringing | Middle of the proximal phalanx | NT |
| Iruretagoiena‐Urbieta et al. 26 | 2020 | US | 24 MHz transducer |
MCP: neutral PIP: 40° flexion DIP: 10° flexion |
Manual Examiner | Complete tear: >2 mm | Middle of the proximal phalanx | NT |
| Scheibler, Janig, & Schweizer 27 | 2021 | US | NS |
MCP: NS PIP: NS DIP: NS |
Loading of the flexor tendon | NS | Middle of the proximal phalanx | NT |
DIP, distal interphalangeal joint; FDP, flexor digitorum profundus; FDS, flexor digitorum superficialis; MCP, metacarpophalangeal joint; N/A, not applicable (not included in study); NS, not stated (not specified in study); NT, not taken; PIP, proximal interphalangeal joint; T, tesla; TP, tendon to phalanx distance.
When specific values for finger joints are not included, the information was not provided in the manuscript.
Bowstringing = qualitative.
Person applying resistance.
Gold Standard used *reported as averages.
Ultrasound (US)
US was the most common form of imaging used to evaluate A2 pulley injuries. Out of 15 US studies, 4 (27%) evaluated A2 injuries in cadaver models, 9 (60%) in rock climbers, and 2 (13%) in healthy volunteers. Each study evaluated the A2 pulley both statically and dynamically. Sensitivity and specificity for ultrasound were reported in 4 studies (27%, 2 clinical and 2 cadaveric), with sensitivities ranging from 0.94 to 1.00 and specificities ranging from 0.94 to 1.00, when compared with surgical visualization of the tear, MRI, and/or dissection (see Table 2).
Multiple qualitative and quantitative measures for diagnosis were used. Qualitatively, in both clinical and cadaveric studies, A2 pulley injuries were generally identified by an absence of the pulley within the sagittal plane and/or a tendinous gap during resisted flexion (bowstringing). In clinical studies, injuries were also identified by one or more of the following factors: (1) fluid along the pulley or within the flexor tendon sheath, (2) a flexor tendon sheath cyst, (3) presence of fibrous tissue, (4) PIP or DIP joint fluid, or (5) irregular thickening of the A2 pulley (Figure 4). In an effort to more precisely characterize the degree of pulley disruption, quantitative measures were performed in 7 studies (47%, 4 clinical and 3 cadaveric) by specifically measuring TP distance with defined cutoff values (see example in Figure 5). Methods varied in terms of how bowstringing and TP distance were evaluated, with 6 studies (40%, 5 clinical and 1 cadaveric) that measured from the midpoint of the proximal phalanx without specifically stating how this was determined, and 3 studies (20%, 2 clinical and 1 cadaveric) that defined particular points between 10 and 20 mm from the base of the proximal phalanx (which included the midpoint). All 15 studies (100%) evaluated bowstringing in the long axis to the tendon with only 7 studies (47%) also investigating bowstringing in the short axis.
Figure 4.
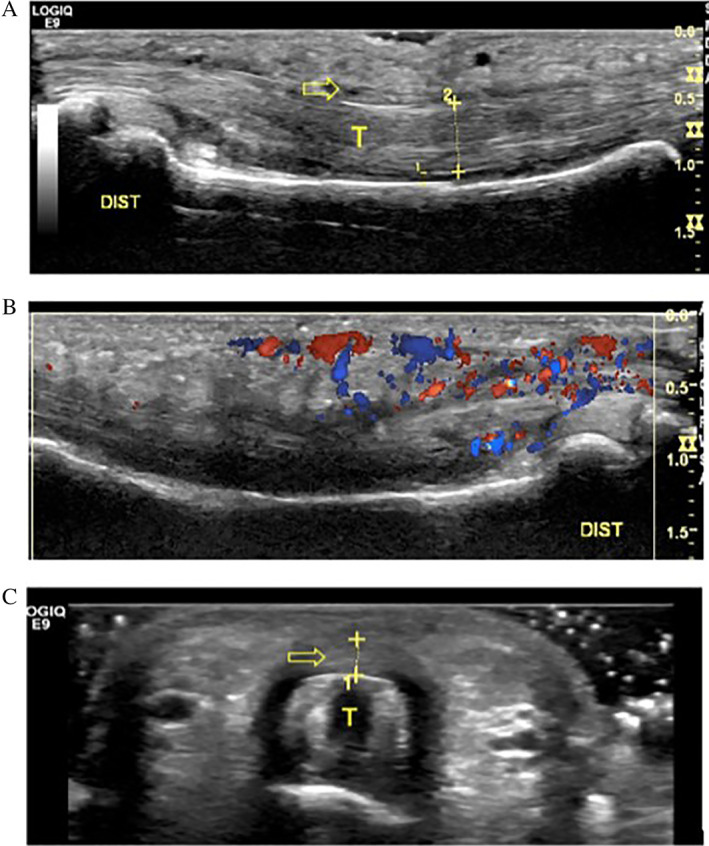
Ultrasound findings of a 21‐year‐old male rock climber with partial tear of the A2 pulley and tendinosis. A, Long axis image of the flexor digitorum tendons (T) and A2 pulley (arrow) demonstrating tendon thickening. B, Long axis image of tendons with notable hyperemia displayed on Doppler. C, SAX image exhibiting thickening of a relatively poorly defined tear of the A2 pulley (arrow) overlying the flexor tendons (T). Dist, distal.
Figure 5.
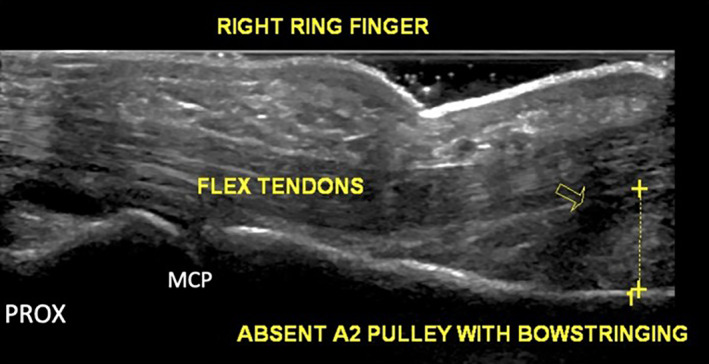
Example of bowstringing (arrow) in a patient with pulley injury. Long axis image of the flexor digitorum tendons demonstrates absence of the A2 pulley and a TP distance of 6.4 mm.
Regarding positioning of the fingers, 14 of the 15 studies (93%) used provocative positioning to stress the A2 pulley and accentuate bowstringing. The PIP and DIP were most often placed in flexion against resistance. Across all four US cadaver studies, the PIP was placed in at least 15° flexion and the DIP in at least 10° flexion. In the 11 clinical studies, the most common position was with the PIP in at least 40° (55%) and DIP in at least 10° (64%) of flexion.
Magnetic Resonance Imaging (MRI)
MRI was the second most common imaging method used to evaluate A2 pulley injuries. Out of 9 total MRI studies, A2 injuries were evaluated in 2 studies (22%) in cadaver models, 5 studies (56%) in rock climbers, and 3 studies (33%) in healthy volunteers (note: one study examined rock climbers and healthy volunteers). Sensitivity and specificity for MRI were reported in 3 studies (33%, 2 clinical and 1 cadaveric), with sensitivities ranging from 0.79 to 1.00 and specificities ranging from 0.86 to 1.00, when compared with surgical visualization of the tear, clinical diagnosis by physical examination, and/or dissection (see Table 2).
The majority of the 9 MRI studies varied in their methodology, with no standard position used to evaluate the pulleys. Five studies (56%) measured TP distance at the midpoint of the proximal phalanx without specifically describing how this was determined, 1 study (11%) measured at the level of the pulley, and 1 study (11%) measured the TP distance at the junction of the proximal two‐thirds and at the distal one‐third of the proximal phalanx. Four studies (44%) reported that qualitative bowstringing was seen on MRI, which was indicative of A2 pulley tears. The remaining 5 studies (56%) used TP distance of ≥2 mm as diagnostic criteria (note: one of these studies gave an average of 2.29 rather than a threshold of ≥2 mm).
Six studies (67%) performed provocative positioning or testing of the finger to stress the A2 pulley and accentuate bowstringing, whereas 3 studies (33%) did not apply any resistance. Of the 3 studies (33%) that reported degrees in flexion of the PIP and DIP, PIP ranged from 40 to 60° and DIP was at 10°. The remaining 6 studies (67%) provided only qualitative descriptions of finger positioning (e.g., neutral, flexion, crimp grip).
Computed Tomography (CT)
Only 2 studies were identified that utilized CT to evaluate the pulleys. One study (50%) was performed in cadavers and compared CT findings to that of US and MRI, 15 and the other study (50%) was done in rock climbers and persons with miscellaneous injuries using CT only. 34 In the first study, Hauger and colleagues 15 used cadavers and placed the PIP in 60° flexion and the DIP in 10° flexion, applied resistance using a 500 g weight with traction, and measured the TP distance at the junction of the proximal two‐thirds and distal one‐third of the proximal phalanx. In the second study, Le Viet and colleagues 34 studied rock climbers and persons with miscellaneous injuries with their PIPs flexed and DIPs extended. Manual resistance was applied and only qualitative bowstringing was reported. Neither study reported sensitivity and specificity against a gold standard. Specific diagnostic criteria are referenced in Table 2.
Discussion
The primary purpose of this review was to summarize the literature investigating the role of US to evaluate A2 pulley injuries, compare the accuracy of US versus MRI and CT when possible, and identify knowledge gaps to facilitate future research. Our review identified 22 studies investigating A2 pulley injuries with US (15 studies), MRI (9 studies), or CT (2 studies), with US being the most common used modality. Our review suggests that US is a superior method to visualize and diagnose A2 pulley injuries. Compared with US and MRI, CT was unable to directly visualize the pulleys and could only depict the gross morphology of the flexor tendons. 15 Compared with CT, both US and MRI provide superior soft tissue imaging without ionizing radiation. Although US and MRI appear to be similarly accurate based on reported sensitivities and specificities, 14 , 18 , 19 , 28 , 31 , 35 US is more accessible, less costly, allows easy side‐to‐side comparison, and facilitates dynamic assessment of the flexor‐tendon–pulley system. 13 Regardless of the type of imaging modality used (US, MRI, CT), we identified significant variability in the methods used to assess the A2 pulley with respect to hand/finger positioning, use of resistance, and diagnostic criteria. Nonetheless, some general statements can be made to summarize the findings in the currently available literature.
For positioning, the patient (or cadaveric specimen) was most often placed with the PIP in 40+ degrees of flexion and the DIP in 10° of flexion. Some amount of resistance to flexion was typically applied to place the pulley–tendon complex on tension and accentuate bowstringing. This resistance was applied using various methods but most frequently to the fingertip via pressure from the investigator. Resistance to flexion was most commonly performed in US studies, in which US provided the opportunity to dynamically assess and stress the flexor‐tendon–pulley complex. It has been shown that the amount of force on the A2 pulley is directly proportional to the external force on the fingertip; therefore, resistance against the fingertip most closely resembles the mechanism of injury and increases sensitivity for the detection of bowstringing. 11 A form of resistance or stress testing was done in 14 of 15 US studies, 6 of 9 MRI studies, and 2 of 2 CT studies.
For diagnostics, some studies used more qualitative methods such as visualization of bowstringing (typically assessed in the longitudinal plane of the digit) or the presence of tenosynovitis, fluid surrounding the pulley (in particular when located posterior to the tendon), pulley thickening (assessed in the transverse plane), or the presence of cysts. Others directly measured the TP distance to quantify bowstringing. When TP distance was measured, the site of measurement varied significantly across studies but was most commonly located at the midpoint of the proximal phalanx. Determination of how the midpoint was identified, however, was never well defined (e.g., tip of the distal phalanx to tip of proximal phalanx). Sensitivity and specificity values were only reported for a portion of the US (27%) and MRI (33%) studies, but generally reflected high accuracy for diagnosing A2 pulley tears, based on MRI, surgical, clinical, and cadaveric gold standards. Of potential clinical relevance, lowest sensitivity and specificity values were reported by studies in which the hand and fingers were placed in a neutral position, while the higher values occurred in studies that placed the hand and fingers in crimp grip position (which included resistance to flexion). 28 Interestingly, although the full crimp grip produces the highest stress on the A2 pulley, 37 none of the US studies to date have used this position to evaluate the A2 pulley (Figure 6). Future US investigations should consider utilizing the full crimp position to evaluate A2 pulley injuries.
Figure 6.
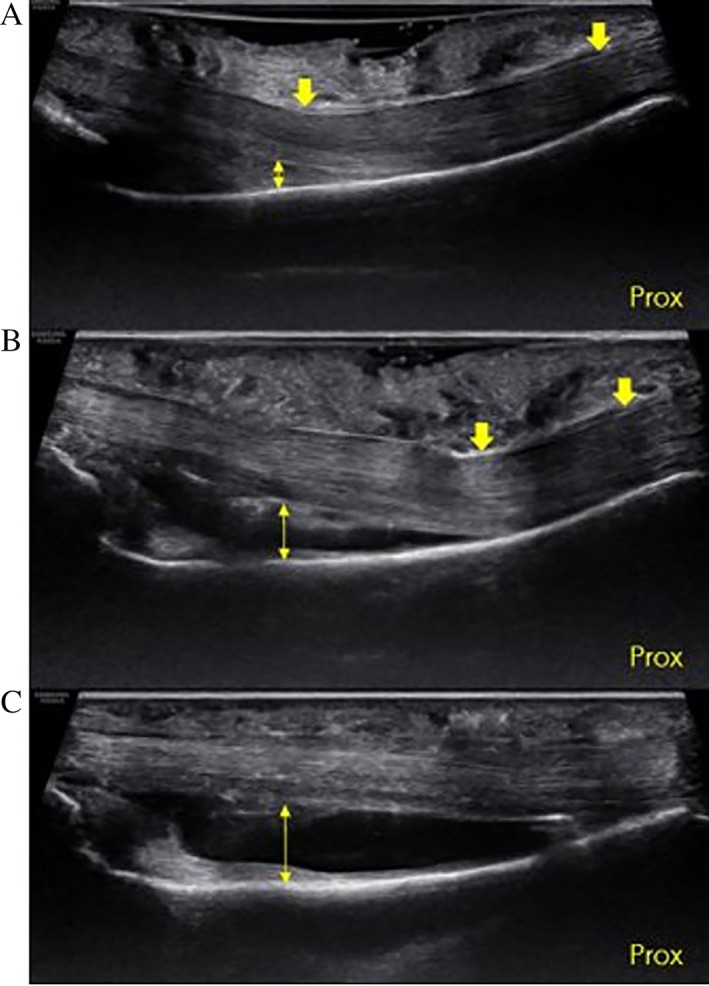
Ultrasound image of a cadaveric specimen. Long axis view of long finger showing the proximal–distal extent of the proximal phalanx (Prox, proximal). A, Intact A2 pulley (down arrows) in the crimp grip position using a gel standoff. Note how the superficial part of the tendons deflect at the distal end of A2, and the TP distance (double‐headed arrow) is greater than 0 at distal A2 under normal conditions. B, Same specimen following US guided release of the distal 50% of A2 (down arrows). The point of tendon deflection has moved proximally, and the TP distance (double‐headed arrow) has increased. C, Same specimen following US guided release 100% of A2. The bowstringing has increased and the tendon is completely separated from the palmar aspect of the phalanx (double‐headed arrow).
Based on our literature review, there is a need for standardizing positioning and measurement prior to further defining the role of diagnostic imaging in A2 pulley injuries. This is particularly the case with respect to US because US provides the opportunity to place the hand and fingers in multiple different positions. However, choosing the best site at which to measure the pulley remains challenging. The proximal edge of the A2 pulley is difficult to evaluate due to its proximity to (and sometimes confluence with) the A1 pulley and its relative thinness compared to the distal edge (Figure 7). Directly evaluating the distal edge of the A2 pulley can be problematic given its high anatomic variability, as there is a difference in the location of the A2 pulley in relation to where the ulnar and radial slips of the flexor digitorum superficialis diverge. In addition, we hypothesize that an intact vinculum (Figure 8) may contribute to a false‐negative evaluation of bowstringing or TP distance, although this has not yet been described. It is possible that with advancements in technology and the use of higher‐frequency transducers and higher Tesla MRI machines that clinicians may be able to better visualize these given structures. With enhanced imaging, diagnostics may not rely on indirect signs of rupture with measurement discrepancies, but rather direct visualization. Future research is warranted to determine how finger position and point of measurement impact outcomes in defining the ideal method for assessment.
Figure 7.
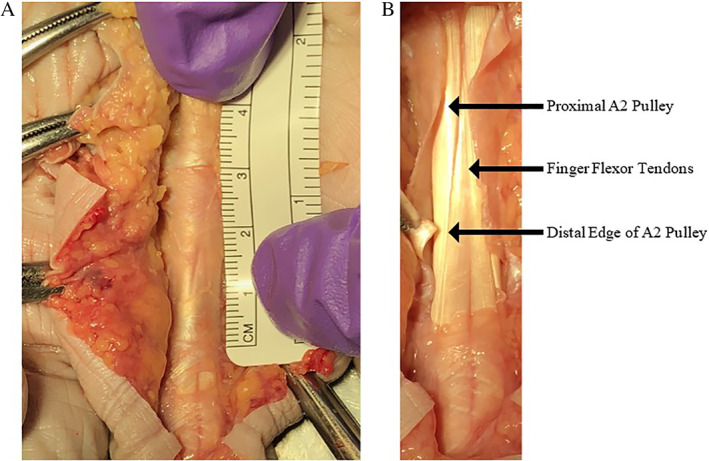
Cadaveric image of the A2 pulley. A, The proximal fibers of A2 blend with the A1 pulley, creating a challenge in directly distinguishing the transition zone between the two pulleys. B, The arrows point to the proximal A2 pulley, flexor tendon, and the distal edge of the A2 pulley. Note that the leading edge is thickened in comparison to the proximal portion. The thinning of the proximal portion of the A2 pulley creates difficulty in identifying it with ultrasound imaging, a situation that can be improved with a high‐frequency transducer.
Figure 8.
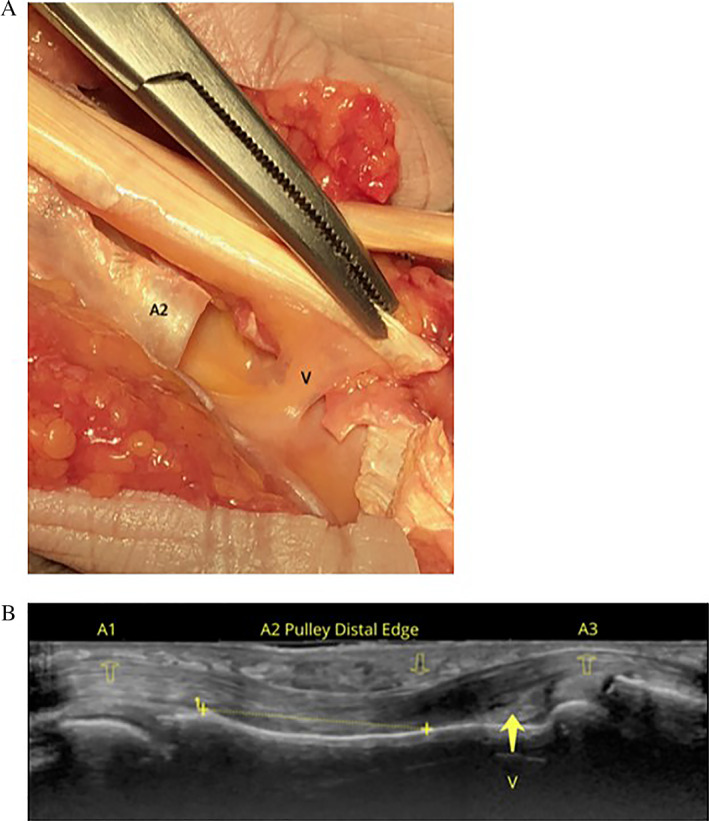
Intact vinculum. A, Cadaveric image depicting the vinculum (V). Note its relationship to the A2 pulley. B, Image B is a longitudinal ultrasound image with an 18 MHz transducer depicting the A2 pulley over the proximal phalanx with the flexor tendons underneath. The A1 and A3 pulleys are also depicted in the image demonstrating the anatomical relationship to the region of the vinculum.
It is important to refine the diagnosis of A2 pulley injuries because detection and characterization of these injuries determine treatment. In general, prognosis and treatment options are based on the number (A2 versus combined) and severity of injured pulleys. Consequently, accurate diagnosis is a prerequisite for triage. Our review has revealed that relatively few studies provide defined cutoff values for TP distance to assist clinicians with this decision‐making. For example, Hauger and colleagues described a partial A2 pulley tear as a TP distance of 0–3 mm, complete tear 2–5 mm, and combination tear of multiple pulleys 5–8 mm for both US and MRI based on simulated lesions in a cadaver model. 15 A similar definition was used in an US study by Klauser et al. in which a partial A2 tear was defined as <3 mm and a complete tear as ≥3 mm, using MRI as a gold standard. 14 However, in an US study by Schoffl and colleagues, >2 mm identified a complete tear based on a cadaver model, 19 and an MRI study by Bodner et al. allowed up to 5 mm of TP distance before diagnosing a complete tear. 35 As previously discussed, it is highly likely that methodological differences account for the majority of the differences in diagnostic criteria published in the literature. This situation presents both a challenge and an opportunity for future research to systematically determine the standards of evaluation.
Conclusion
The results of this review suggest that US should be used as the primary imaging modality to assess A2 pulley injuries due to its high accuracy, relatively low cost, ease of access, and dynamic imaging capabilities. Furthermore, manually resisted flexion is likely beneficial to stress the A2 pulley and accentuate bowstringing. Future research should determine the best positions in which to qualitatively and quantitatively evaluate the A2 pulley to assist clinicians in appropriate triage of isolated and combined A2 pulley injuries.
The authors wish to acknowledge Michael Farrell, MD and Blynn L. Shideler III, BS for assistance with formation of figures. This work was supported in part by the Uniformed Services University, Department of Physical Medicine & Rehabilitation, Musculoskeletal Injury Rehabilitation Research for Operational Readiness (MIRROR) (HU00011920011).
References
- 1. Smith J, Rizzo M, Lai JK. Sonographically guided percutaneous first annular pulley release: cadaveric safety study of needle and knife techniques. J Ultrasound Med 2010; 29:1531–1542. [DOI] [PubMed] [Google Scholar]
- 2. Heithoff SJ, Millender LH, Helman J. Bowstringing as a complication of trigger finger release. J Hand Surg Am 1988; 13:567–570. [DOI] [PubMed] [Google Scholar]
- 3. Crowley TP. The flexor tendon pulley system and rock climbing. J Hand Microsurg 2012; 4:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rohrbough JT, Mudge MK, Schilling RC. Overuse injuries in the elite rock climber. Med Sci Sports Exerc 2000; 32:1369–1372. [DOI] [PubMed] [Google Scholar]
- 5. Lourie GM, Hamby Z, Raasch WG, Chandler JB, Porter JL. Annular flexor pulley injuries in professional baseball pitchers: a case series. Am J Sports Med 2011; 39:421–424. [DOI] [PubMed] [Google Scholar]
- 6. Patel P, Schucany WG, Toye L, Ortinau E. Flexor tendon pulley injury in a bowler. Proc (Bayl Univ Med Cent) 2012; 25:282–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dowd MB, Fuentes EO. Closed traumatic A2 pulley rupture: rare mechanism of injury. J Hand Surg Eur Vol 2009; 34:548–549. [DOI] [PubMed] [Google Scholar]
- 8. Gyuricza C, Umoh E, Wolfe SW. Multiple pulley rupture following corticosteroid injection for trigger digit: case report. J Hand Surg Am 2009; 34:1444–1448. [DOI] [PubMed] [Google Scholar]
- 9. Manske PR, Lesker PA. Strength of human pulleys. Hand 1977; 9:147–152. [DOI] [PubMed] [Google Scholar]
- 10. El‐Sheikh Y, Wong I, Farrokhyar F, Thoma A. Diagnosis of finger flexor pulley injury in rock climbers: a systematic review. Can J Plast Surg 2006; 14:227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schweizer A. Biomechanical properties of the crimp grip position in rock climbers. J Biomech 2001; 34:217–223. [DOI] [PubMed] [Google Scholar]
- 12. Vigouroux L, Quaine F, Labarre‐Vila A, Moutet F. Estimation of finger muscle tendon tensions and pulley forces during specific sport‐climbing grip techniques. J Biomech 2006; 39:2583–2592. [DOI] [PubMed] [Google Scholar]
- 13. Bianchi S, Martinoli C, de Gautard R, Gaignot C. Ultrasound of the digital flexor system: Normal and pathological findings. J Ultrasound 2007; 10:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klauser A, Frauscher F, Bodner G, et al. Finger pulley injuries in extreme rock climbers: depiction with dynamic US. Radiology 2002; 222:755–761. [DOI] [PubMed] [Google Scholar]
- 15. Hauger O, Chung CB, Lektrakul N, et al. Pulley system in the fingers: normal anatomy and simulated lesions in cadavers at MR imaging, CT, and US with and without contrast material distention of the tendon sheath. Radiology 2000; 217:201–212. [DOI] [PubMed] [Google Scholar]
- 16. Jacobson JA. Musculoskeletal ultrasound and MRI: which do I choose? Semin Musculoskelet Radiol 2005; 9:135–149. [DOI] [PubMed] [Google Scholar]
- 17. Leeflang S, Coert JH. The role of proximal pulleys in preventing tendon bowstringing: pulley rupture and tendon bowstringing. J Plast Reconstr Aesthet Surg 2014; 67:822–827. [DOI] [PubMed] [Google Scholar]
- 18. Schöffl I, Hugel A, Schöffl V, Rascher W, Jüngert J. Diagnosis of complex pulley ruptures using ultrasound in cadaver models. Ultrasound Med Biol 2017; 43:662–669. [DOI] [PubMed] [Google Scholar]
- 19. Schöffl I, Deeg J, Lutter C, Bayer T, Schöffl V. Diagnosis of A3 pulley injuries using ultrasound. Sportverletz Sportschaden 2018; 32:251–259. [DOI] [PubMed] [Google Scholar]
- 20. Klauser A, Bodner G, Frauscher F, Gabl M, Zur ND. Finger injuries in extreme rock climbers. Assessment of high‐resolution ultrasonography. Am J Sports Med 1999; 27:733–737. [DOI] [PubMed] [Google Scholar]
- 21. Klauser A, Frauscher F, Bodner G, et al. Value of high‐resolution ultrasound in the evaluation of finger injuries in extreme sport climbers. Ultraschall Med 2000; 21:73–78. [DOI] [PubMed] [Google Scholar]
- 22. Schöffl VR, Einwag F, Strecker W, Schöffl I. Strength measurement and clinical outcome after pulley ruptures in climbers. Med Sci Sports Exerc 2006; 38:637–643. [DOI] [PubMed] [Google Scholar]
- 23. Bassemir D, Unglaub F, Hahn P, Müller LP, Bruckner T, Spies CK. Sonographical parameters of the finger pulley system in healthy adults. Arch Orthop Trauma Surg 2015; 135:1615–1622. [DOI] [PubMed] [Google Scholar]
- 24. Schneeberger M, Schweizer A. Pulley ruptures in rock climbers: outcome of conservative treatment with the pulley‐protection splint‐a series of 47 cases. Wilderness Environ Med 2016; 27:211–218. [DOI] [PubMed] [Google Scholar]
- 25. Reissner L, Zechmann‐Mueller N, Klein HJ, Calcagni M, Giesen T. Sonographic study of repair, gapping and tendon bowstringing after primary flexor digitorum profundus repair in zone 2. J Hand Surg Eur Vol 2018; 43:480–486. [DOI] [PubMed] [Google Scholar]
- 26. Iruretagoiena‐Urbieta X, De la Fuente‐Ortiz de Zarate J, Blasi M, Obradó‐Carriedo F, Ormazabal‐Aristegi A, Rodríguez‐López ES. Grip force measurement as a complement to high‐resolution ultrasound in the diagnosis and follow‐up of A2 and A4 finger pulley injuries. Diagnostics (Basel) 2020; 10:206. 10.3390/diagnostics10040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scheibler AG, Janig C, Schweizer A. Primarily conservative treatment for triple (A2‐A3‐A4) finger flexor tendon pulley disruption. Hand Surg Rehabil 2021; 40:314–318. [DOI] [PubMed] [Google Scholar]
- 28. Bayer T, Fries S, Schweizer A, Schöffl I, Janka R, Bongartz G. Stress examination of flexor tendon pulley rupture in the crimp grip position: a 1.5‐tesla MRI cadaver study. Skeletal Radiol 2015; 44:77–84. [DOI] [PubMed] [Google Scholar]
- 29. Parellada JA, Balkissoon AR, Hayes CW, Conway WF. Bowstring injury of the flexor tendon pulley system: MR imaging. AJR Am J Roentgenol 1996; 167:347–349. [DOI] [PubMed] [Google Scholar]
- 30. Gabl M, Rangger C, Lutz M, Fink C, Rudisch A, Pechlaner S. Disruption of the finger flexor pulley system in elite rock climbers. Am J Sports Med 1998; 26:651–655. [DOI] [PubMed] [Google Scholar]
- 31. Guntern D, Goncalves‐Matoso V, Gray A, Picht C, Schnyder P, Theumann N. Finger A2 pulley lesions in rock climbers: detection and characterization with magnetic resonance imaging at 3 tesla—initial results. Invest Radiol 2007; 42:435–441. [DOI] [PubMed] [Google Scholar]
- 32. Hoff MN, Greenberg TD. MRI sport‐specific pulley imaging. Skeletal Radiol 2018; 47:989–992. [DOI] [PubMed] [Google Scholar]
- 33. Schellhammer F, Vantorre A. Semi‐dynamic MRI of climbing‐associated injuries of the finger. Skeletal Radiol 2019; 48:1435–1437. [DOI] [PubMed] [Google Scholar]
- 34. Le Viet D, Rousselin B, Roulot E, Lantieri L, Godefroy D. Diagnosis of digital pulley rupture by computed tomography. J Hand Surg Am 1996; 21:245–248. [DOI] [PubMed] [Google Scholar]
- 35. Bodner G, Rudisch A, Gabl M, Judmaier W, Springer P, Klauser A. Diagnosis of digital flexor tendon annular pulley disruption: comparison of high frequency ultrasound and MRI. Ultraschall Med 1999; 20:131–136. [DOI] [PubMed] [Google Scholar]
- 36. Martinoli C, Bianchi S, Nebiolo M, Derchi LE, Garcia JF. Sonographic evaluation of digital annular pulley tears. Skeletal Radiol 2000; 29:387–391. [DOI] [PubMed] [Google Scholar]
- 37. Schöffl I, Oppelt K, Jüngert J, Schweizer A, Neuhuber W, Schöffl V. The influence of the crimp and slope grip position on the finger pulley system. J Biomech 2009; 42:2183–2187. [DOI] [PubMed] [Google Scholar]


