Abstract
Background
The TCGA molecular groups of endometrial carcinoma are “POLE‐mutated” (POLEmut), “microsatellite‐instable/mismatch repair‐deficient” (MSI/MMRd), “TP53‐mutated/p53‐abnormal” (TP53mut/p53abn), and “no specific molecular profile” (NSMP).
Objective
Prognostic assessment of the TCGA groups in uterine carcinosarcoma (UCS).
Search strategy
Systematic review from January 2000 to January 2021.
Selection criteria
Studies assessing the TCGA groups in UCS.
Data collection and analysis
Progression‐free survival (PFS) and overall survival (OS) were assessed by Kaplan–Meier and Cox analyses (reference: TP53mut/p53abn group) and compared with endometrioid and serous carcinomas (original TCGA cohort), with a significant P < 0.050.
Main results
Five studies with 263 UCS were included. Compared with TP53mut/p53abn UCS, MSI/MMRd UCS showed significantly better PFS (P < 0.001) but similar OS (P = 0.788), whereas NSMP UCS showed similar PFS (P = 0.936) and OS (P = 0.240). Compared with their endometrioid/serous counterparts, NSMP and TP53mut/p53abn UCS showed significantly worse PFS (P < 0.001 and P = 0.004) and OS (P < 0.001 and P < 0.001), while MSI/MMRd UCS showed similar PFS (P = 0.595) but significantly worse OS (P < 0.001). The POLEmut group showed neither recurrences nor deaths in both the UCS and the endometrioid/serous carcinoma cohorts.
Conclusion
POLEmut UCS show excellent prognosis, whereas TP53mut/p53abn and NSMP UCS show a prognosis even worse than that of TP53mut/p53abn endometrioid/serous carcinomas. The prognosis of MSI/MMRd UCS remains to be defined.
Keywords: cancer, endoscopic surgery, gyne‐oncology, laparoscopy, mortality
Short abstract
POLEmut UCS show the same excellent prognosis as POLEmut endometrioid carcinomas, while TP53mut/p53abn and NSMP UCS show a prognosis even worse than TP53mut/p53abn endometrioid/serous carcinomas.
1. INTRODUCTION
Uterine carcinosarcoma (UCS) is a biphasic neoplasm constituted by an epithelial component and a stromal component, both of which are malignant and typically high‐grade. The epithelial component reflects the endometrial carcinoma histotypes, such as serous (most common), endometrioid, clear cells, mixed, and undifferentiated. The stromal component may be “non‐otherwise specified” or reflect the typical uterine sarcomas; in these cases, the stromal component is defined as “homologous”. If a differentiation towards extrauterine mesenchymal tissues is present, the stromal component is defined as “heterologous”. 1 , 2
The classification of UCS has changed over time. Indeed, UCS had previously been listed among the “mixed Müllerian tumors” and was lumped together with uterine sarcomas in terms of staging and treatment. 3 , 4 In recent decades, it has emerged that UCS represents a carcinoma with secondary sarcomatous transformation. 2 Therefore, UCS is now classified as a subtype of endometrial carcinoma and is staged and managed accordingly. 1 , 5 , 6 The rise of The Cancer Genome Atlas (TCGA) molecular classification has brought about a revolution in the prognostic stratification of endometrial carcinoma. The TCGA study and subsequent studies have identified four molecular prognostic groups: the “POLE‐mutated” (POLEmut) group, characterized by excellent prognosis; the “microsatellite‐instable/mismatch repair‐deficient” (MSI/MMRd) group and the “no specific molecular profile” (NSMP) group, characterized by intermediate prognosis; and the “TP53‐mutant/p53‐abnormal” (TP53mut/p53abn) group, characterized by poor prognosis. 7 , 8 , 9 , 10 , 11 , 12 However, the original 2013 TCGA study only included endometrioid and serous carcinomas, 7 and the prevalence and prognostic value of the TCGA groups have been shown to vary across the different histotypes. 13 , 14 , 15 , 16 , 17
Among UCS, the vast majority (>70%) fall into the TP53mut/p53abn group, which is consistent with its aggressive behavior. 16 However, the prognostic value of the other TCGA groups in UCS is not well defined. Moreover, it is unclear if TP53mut/p53abn UCS have a prognosis similar to their endometrioid and serous counterparts. The aim of this quantitative systematic review was to assess the prognostic value of the TCGA groups in UCS and to compare the prognosis of the TCGA groups between UCS and the original 2013 TCGA cohort.
2. MATERIALS AND METHODS
Methods of this study were defined a priori, based on previous studies. 16 , 18 Each review stage was carried out by two independent authors; disagreements, if any, were resolved by consensus. The PRISMA statement 19 was followed to report this study.
2.1. Search strategy and study selection
Four electronic databases (Scopus, ISI Web of Science, PubMed, Google Scholar) were searched from January 2000 to January 2021 for all studies assessing the TCGA molecular signatures in UCS. We adopted the following word combination: (uterine OR endometrial) AND (carcinosarcoma OR mixed malignant Müllerian) AND (TCGA OR ProMisE OR ultramutated OR POLE OR hypermutated OR mismatch OR microsatellite OR copy number OR TP53 OR p53). We also assessed the reference lists of eligible studies. We included all studies that assessed the TCGA groups and survival outcomes in UCS. Given the rarity of POLEmut UCS, 16 we also included studies that did not assess POLE status. Exclusion criteria, defined a priori, were: sample size less than 10, data not extractable, reviews.
2.2. Data extraction
PICO 18 of our study were: P (population) = women with UCS; I (intervention or risk factor) = POLEmut, MSI/MMRd and NSMP groups; C (comparator) = TP53mut/p53abn group; O (outcome) = progression‐free survival (PFS) and overall survival (OS). The TP53mut/p53abn group was used as reference (comparator) because it is the most represented group in UCS. Data were extracted without modifications.
2.3. Risk of bias assessment
The QUADAS‐2 20 were used to assess the risk of bias within studies, as previously described. 16 , 18 Four domains were assessed: (1) “patient selection” domain (were selection criteria and period of enrollment reported and adequate?); (2) “index test” domain (were immunohistochemical and/or molecular methods reported and adequate); (3) “reference standard” domain (were follow‐up data reported and adequate?); and (4) “flow” (were all patients assessed for the TCGA classification and survival outcomes?). The risk of bias was categorized as “low”, “unclear”, or “high” as previously described. 16 , 18
2.4. Data analysis
PFS and OS for each TCGA group in UCS were represented graphically by using Kaplan–Meier curves. Cox regression survival analysis was used to calculate hazard ratio (HR) for recurrence and death in the TCGA group of UCS. Two analyses were performed: in the first analysis, HR was calculated in each TCGA group of UCA by using the TP53mut/p53abn group as reference; in the second analysis, each TCGA group of UCS was compared with the TCGA groups in the original TCGA series published in 2013, which included 232 patients with endometrial carcinoma (endometrioid, serous, and mixed). A P value less than 0.05 was considered significant. Statistical Package for Social Science (SPSS) 18.0 package (IBM Corp., Armonk, NY, USA) was used for the analyses.
3. RESULTS
3.1. Study selection and characteristics
Five studies 21 , 22 , 23 , 24 , 25 with 263 UCS patients were included (Figure S1). The POLEmut group was assessed in three studies by using POLE sequencing. The MSI/MMRd group was assessed by MMR immunohistochemistry in three studies and by MSI testing in two studies. The TP53mut/p53abn group was assessed by p53 immunohistochemistry in two studies, by TP53 sequencing in two studies and by both methods in the remaining study. Characteristics of the included studies are reported in Table 1.
TABLE 1.
Characteristics of the included studies
| Study | Country | Period of enrollment | Sample size | POLEmut | MSI/MMRd | TP53mut/p53abn | NSMP | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Test | Positive (%) | Test | Positive (%) | test | positive (%) | positive (%) | ||||
| McConechy 2015 | Canada | Unclear | 30 | Mol | 1 (3.3) | IHC | 1 (3.3) | mol, IHC | 23 (76.7) | 5 (16.7) |
| Cherniak 2017 | USA | Unclear | 57 | Mol | 1 (1.8) | Mol | 2 (3.5) | mol | 50 (87.7) | 4 (7) |
| Jones 2019 | USA | 2007–2012 | 27 | Not assessed | Not assessed | IHC | 12 (44.4) | IHC | 11 (40.7) | 4 (14.8) |
| Gotoh 2019 | Japan | 1998–2015 | 92 | Mol | 10 (10.9) | Mol | 24 (26.1) | mol | 49 (53.3) | 9 (9.8) |
| Saijo 2019 | Japan | 2007–2017 | 57 | Not assessed | Not assessed | IHC | 6 (10.5) | IHC | 34 (59.6) | 17 (29.8) |
Abbreviations: IHC, immunohistochemistry; Mol, molecular analysis.
3.2. Risk of bias assessment
For the patient selection domain, three studies were considered at low risk of bias and two studies at unclear risk (period of enrollment not reported). For the index test domain, three studies were considered at low risk of bias and two studies at unclear risk (POLE mutations not assessed). For the “reference standard” domain, all studies were considered at low risk of bias. For the “flow” domain, the risk of bias was considered low for four studies and unclear for one study (only part of the cases was assessed by MMR immunohistochemistry). Authors’ judgments are reported in Figure S2.
3.3. Prognostic analysis
In the PFS analysis, none of the POLEmut carcinomas progressed among the UCS and in the 2013 TCGA cohort. In UCS, MSI/MMRd cases showed significantly better PFS than TP53mut/p53abn cases, with an HR of 0.19 (95% confidence interval [CI] 0.08–0.46; P < 0.001), but no significant difference was found between NSMP and TP53mut/p53abn cases (HR 1.02, 95% CI 0.59–1.78; P = 0.936). Compared with the correspondent groups in the 2013 TCGA cohorts, MSI/MMRd UCS showed similar PFS (HR 0.75, 95% CI 0.26–2.19; P = 0.595), while NSMP and TP53mut/p53abn UCS showed significantly worse PFS, with HR of 5.31 (95% CI 2.44–11.59; P < 0.001) and 2.18 (95% CI 1.29–3.69; P = 0.004), respectively (Figure 1).
FIGURE 1.
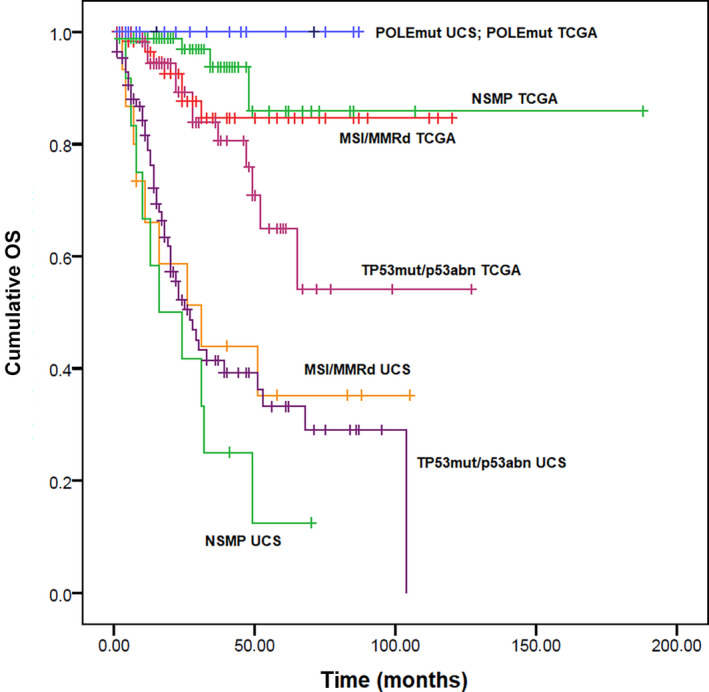
Kaplan–Meier curves for progression‐free survival in uterine carcinosarcoma and in the 2013 TCGA cohort, stratified based on the four TCGA molecular prognostic groups [Colour figure can be viewed at wileyonlinelibrary.com]
In the OS analysis, none of the POLEmut patients died among the UCS and in the 2013 TCGA cohort. In UCS, MSI/MMRd and NSMP cases showed similar OS to the TP53mut/p53abn cases, with HR of 0.91 (95% CI 0.44–1.87; P = 0.788) and 1.51 (95% CI 0.76–2.99; P = 0.240), respectively. Compared with the correspondent groups in the 2013 TCGA cohorts, MSI/MMRd, NSMP, and TP53mut/p53abn UCS showed significantly worse OS, with HR of 5.90 (95% CI 2.19–15.86; P < 0.001), 22.02 (95% CI 6.90–70.27; P < 0.001), and 3.51 (95% CI 1.86–6.64; P < 0.001), respectively (Figure 2). Survival analysis results are reported in Tables 2 and 3.
FIGURE 2.
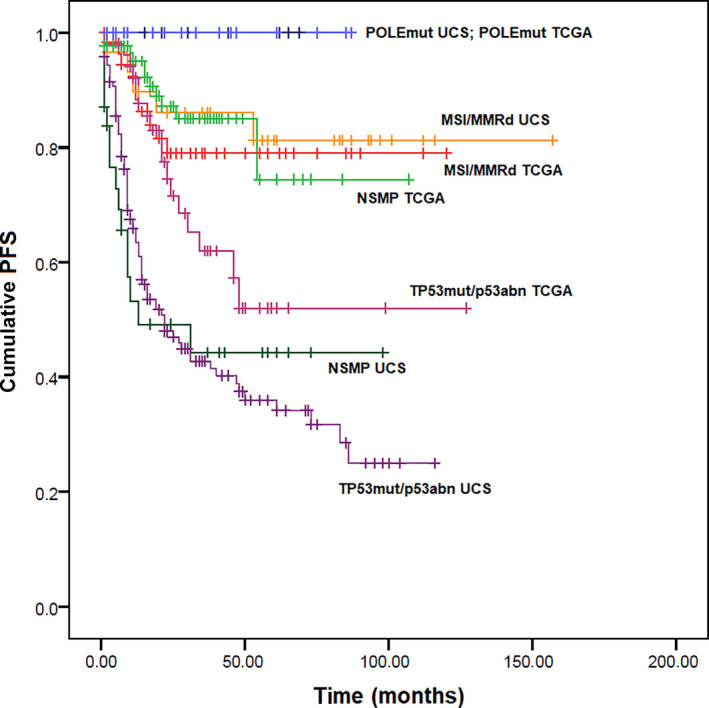
Kaplan–Meier curves for overall survival in uterine carcinosarcoma and in the 2013 TCGA cohort, stratified based on the four TCGA molecular prognostic groups [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Progression‐free survival and overall survival in the TCGA groups of uterine carcinosarcoma, using the p53abn group as reference
| TCGA group (UCS) | Progression‐free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| POLEmut | NV | NV | NV | NV | NV | NV |
| MMRd | 0.19 | 0.08–0.46 | <0.001 | 0.91 | 0.44–1.87 | 0.788 |
| NSMP | 1.02 | 0.59–1.78 | 0.936 | 1.51 | 0.76–2.99 | 0.240 |
| p53abn (reference) | 1.00 | — | — | 1.00 | — | — |
Abbreviations: CI, confidence interval; HR, hazard ratio; UCS, uterine carcinosarcoma.
NV, not evaluable, because no event occurred in POLEmut cases.
TABLE 3.
Progression‐free survival and overall survival in the TCGA groups of uterine carcinosarcoma, using the correspondent groups in the 2013 TCGA cohort as reference
| TCGA group (UCS vs 2013 TCGA cohort) | Progression‐free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| POLEmut | NV a | NV | NV | NV | NV | NV |
| MMRd | 0.75 | 0.26–2.19 | 0.595 | 5.90 | 2.19–15.86 | <0.001 |
| NSMP | 5.31 | 2.44–11.59 | <0.001 | 22.02 | 6.90–70.27 | <0.001 |
| p53abn | 2.18 | 1.29–3.69 | 0.004 | 3.51 | 1.86–6.64 | <0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; UCS, uterine carcinosarcoma.
NV, not evaluable, because no event occurred in POLEmut cases.
4. DISCUSSION
This study showed that POLEmut UCS had the same excellent prognosis as POLEmut endometrioid carcinomas, whereas TP53mut/p53abn and NSMP UCS had a prognosis even worse than TP53mut/p53abn endometrioid/serous carcinomas. MSI/MMRd UCS showed similar PFS but worse OS than their endometrioid counterpart.
In the last decades, the risk stratification of endometrial carcinoma has been based on poorly reproducible histologic features and on simplistic clinical classifications. 26 , 27 , 28 , 29 Since their publication in 2013, the TCGA findings have progressively changed the approach to the study of endometrial carcinoma. 6 , 7 , 30 The “Proactive Molecular Risk Classifier for Endometrial Cancer” (ProMisE) is a surrogate of the TCGA classification, which has allowed a wider applicability of the TCGA groups. 8 , 9 , 10 The ProMisE has indeed introduced the use of mismatch repair protein immunohistochemistry as a surrogate of microsatellite instability molecular testing, and of p53 immunohistochemistry as a surrogate of somatic copy number alteration testing. 7 , 8 , 9 , 31 , 32 In 2020, the TCGA prognostic groups have been integrated in the European Society of Gynaecological Oncology/ European Society for Therapeutic Radiotherapy and Oncology/ European Society of Pathology (ESGO/ESTRO/ESP) guidelines for management of endometrial carcinoma. According to such guidelines, all POLEmut carcinomas up to FIGO (the International Federation of Gynecology & Obstetrics) Stage II should be considered at low risk, while all TP53mut/p53abn carcinomas with myometrial invasion should be considered at high risk. In contrast, the risk stratification of the MSI/MMRd group and NSMP group is affected by pathologic prognostic factors such as FIGO grade, histotype, lymphovascular space invasion, and deep myometrial invasion. 6 Although such integrated classification represents an important step forward in the management of endometrial carcinomas, there are still some points that should be clarified. Despite being lumped together in the guidelines, the NSMP and MSI/MMRd groups may diverge in selected subsets of endometrial carcinomas. In particular, the NSMP group seems to be much more prognostically heterogeneous than the MSI/MMRd group. 7 , 8 , 9 , 10 , 11 , 12 , 21 , 22 , 33 , 34 , 35 Furthermore, the ESGO/ESTRO/ESP guidelines place all non‐endometrioid carcinomas in the same risk group regardless of the TCGA signature (except for the POLEmut group). 6 Possible differences between different histotypes within the TP53mut/p53abn group are also disregarded.
In this study, we assessed the prognosis of the TCGA groups in UCS compared with their counterparts in the original 2013 TCGA cohort, which comprised endometrioid and serous carcinomas. 7 We found that POLEmut UCS showed neither recurrences nor deaths, a result that was superimposable on the POLEmut endometrioid carcinomas of the 2013 TCGA cohort. The excellent prognosis of the POLEmut groups was also demonstrated in clear‐cell carcinomas, mixed carcinomas, and even undifferentiated/dedifferentiated carcinomas. 34 , 35 , 36 , 37 These findings agree with the ESGO/ESTRO/ESP guidance, supporting that all POLEmut endometrial carcinomas should be considered at low risk regardless of histotype. 6
Regarding the TP53mut/p53abn group, we found that UCS of this group showed poor PFS and poor OS, consistently with what observed in other histotypes. 7 , 33 , 34 , 35 , 37 , 38 , 39 In addition, we found that TP53mut/p53abn UCS showed significantly poorer PFS and OS than their 2013 TCGA counterparts (mostly serous carcinomas). Given that the TP53mut/p53abn group is the most represented in UCS, this finding supports that UCS is overall more aggressive than the prototypical type II endometrial cancer. This is in agreement with previous studies that suggested a worse prognosis for UCS compared to serous and clear cell carcinoma. 40 , 41 , 42 Remarkably, the NCCN guidelines recommend adjuvant therapy for UCS even in the case of disease limited to the endometrium with no residual on the final hysterectomy specimens; such recommendation is not made for serous and clear cell carcinoma. 5
Regarding the NSMP group, we found that UCS of this group showed a poor prognosis, similar to that of TP53mut/p53abn UCS and significantly poorer than that of TP53mut/p53abn endometrioid/serous carcinomas. This confirms the extreme variability in the prognosis of the NSMP group, which appears good‐to‐intermediate in low‐grade endometrioid carcinomas, intermediate in high‐grade endometrioid carcinomas, and poor in non‐endometrioid carcinomas. 7 , 33 , 37 , 39 This also supports a worse prognosis for UCS compared with the classical type II endometrial carcinomas, as discussed above.
The prognostic analysis of the MSI/MMRd group showed discordance between PFS and OS in UCS. In the PFS analysis, MSI/MMRd UCS showed an intermediate prognosis similar to that of endometrial carcinomas (all endometrioid) of the same group in the 2013 TCGA cohort. In the OS analysis, MSI/MMRd UCS showed a very poor prognosis similar to that of TP53mut/p53abn and NSMP UCS and worse than any 2013 TCGA group. By assessing the possible causes for such discrepancy, we noted that most MSI/MMRd UCS in the OS analysis derived from a study with an unusually high prevalence of the MSI/MMRd signature. 23 Indeed, almost half (44.4%) of UCS in that study were MSI/MMRd, against an average percentage less than 10%. 16 Such a high percentage of MSI/MMRd cases is analogous to that observed in undifferentiated/dedifferentiated carcinoma. 14 Interestingly, dedifferentiated carcinoma may show morphologic and immunohistochemical overlap with UCS. 43 Indeed, both are biphasic tumors with an overt carcinomatous component and a dyshesive malignant component. 1 , 14 , 16 The dyshesive component of dedifferentiated carcinoma may show cell spindling, rhabdoid morphology, and myxoid stroma, which mimic a sarcomatous component. 44 Furthermore, UCS may sometimes show a low‐grade carcinoma component, as is typically observed in dedifferentiated carcinoma, whereas the latter may show a high‐grade carcinoma component, which is more typical of UCS. 25 , 45 In dedifferentiated carcinoma, the MSI/MMRd group seems to have a poor prognosis similar to that of the TP53mut/p53abn and NSMP groups 18 ; by contrast, in other non‐endometrioid carcinomas, the MSI/MMRd signature is associated with improved prognosis. 33 , 34 , 35 , 37 , 46 On this account, we hypothesize that the poor prognosis of MSI/MMRd UCS in our analysis might be a result of the inclusion of dedifferentiated carcinomas. Recently, it has been suggested that the highly aggressive behavior of undifferentiated/dedifferentiated carcinomas may be due to mutations in proteins of the SWI/SNF complex (ARID1B, SMARCA4/BRG1, SMARCB1/INI1), which occur in about two‐thirds of cases 18 , 45 , 47 ; the loss of these proteins on immunohistochemistry seems to be a specific diagnostic marker of undifferentiated/dedifferentiated carcinoma. 48 We believe that further studies are necessary to define the biologic behavior of MSI/MMRd. For this purpose, it would be advisable to perform SWI/SNF protein immunohistochemistry in all cases that may raise the possibility of a dedifferentiated carcinoma. Such a procedure would allow the exclusion of highly aggressive dedifferentiated carcinoma and achieve a more precise prognostic definition of MSI/MMRd UCS.
A limitation of our results is the low number of patients in the groups other than TP53mut/p53abn, especially in the POLEmut group and in the OS analysis. Furthermore, we had no sufficient data to perform a multivariate survival analysis, because not all studies reported individual clinical data. Finally, we could not review histologic and immunohistochemical findings to confirm the diagnosis of UCS.
In conclusion, the TCGA classification significantly stratifies OS and PFS in UCS. POLEmut UCS show an excellent prognosis similar to that of POLEmut endometrioid carcinomas, supporting their inclusion in the same low‐risk category for management purposes. TP53mut/p53abn and NSMP UCS show a very poor prognosis, seemingly even worse than that of serous carcinomas. Whether MSI/MMRd UCS should be considered intermediate‐risk like MSI/MMRd carcinomas remains to be defined; for this purpose, a screening with SWI/SNF protein immunohistochemistry would be useful to exclude highly aggressive dedifferentiated carcinomas, which may mimic UCS and confound the results.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
The study was conceived by AT, AR, DR, DA, AS, RS, and GFZ and the protocol was developed by AT, AR, DR, DA, GA, GS, AM, AS, RS, GFZ. Electronic search was oerfirned by GS and NDA; study selection by MV and GA; data extraction by AM and DR; risk of bias assessment by PC and FI; and data analysis by AT and AR. Disagreements were resolved by AT, AR, DR, DA, GA, MV, GS, NDA, PC, FI, AM, AS, RS, and GFZ. Interpretation was by AT, AR, DR, DA, PC, FI, AM, AS, RS, and GFZ. The first draft was written by DR, DA, GA, MV, GS, NDA, and FI and was revised by AT, AR, AS, RS, PC, AM, and GFZ. The study was supervised by AT, AR, AM, AS, RS, and GFZ.
Supporting information
Fig S1
Fig S2
Supplementary Material
ACKNOWLEDGMENTS
Open Access Funding provided by Universita degli Studi di Napoli Federico II within the CRUI‐CARE Agreement. [Correction added on 09‐May‐2022, after first online publication: CRUI‐CARE funding statement has been added.]
Travaglino A, Raffone A, Raimondo D, et al. Prognostic value of the TCGA molecular classification in uterine carcinosarcoma. Int J Gynecol Obstet. 2022;158:13–20. doi: 10.1002/ijgo.13937
Renato Seracchioli and Gian Franco Zannoni contributed equally to the study.
REFERENCES
- 1. WHO Classification of Tumours Editorial Board . Female Genital Tumours. (WHO classification of tumours series, 5th edn, Vol. 4. International Agency for Research on Cancer; 2020. [Google Scholar]
- 2. Matsuzaki S, Klar M, Matsuzaki S, Roman LD, Sood AK, Matsuo K. Uterine carcinosarcoma: contemporary clinical summary, molecular updates, and future research opportunity. Gynecol Oncol. 2021;160(2):586‐601. [DOI] [PubMed] [Google Scholar]
- 3. Kurman R, Carcangiu M, Herrington C, Young R. World Health Organization Classification of Tumors of Female Reproductive Organs, 4th edn. International Agency for Research on Cancer (IARC) Press; 2014. [Google Scholar]
- 4. Nam JH, Park JY. Update on treatment of uterine sarcoma. Curr Opin Obstet Gynecol. 2010;22(1):36‐42. [DOI] [PubMed] [Google Scholar]
- 5. Abu‐Rustum NR, Yashar CM, Bradley K, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) – Uterine neoplasms. Version 1.2021 – October 20, 2020.
- 6. Concin N, Matias‐Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31(1):12‐39. [DOI] [PubMed] [Google Scholar]
- 7. Network CGAR, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Talhouk A, McConechy MK, Leung S, et al. A clinically applicable molecular‐based classification for endometrial cancers. Br J Cancer. 2015;113(2):299‐310. 10.1038/bjc.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: a simple, genomics‐based clinical classifier for endometrial cancer. Cancer. 2017;123(5):802‐813. [DOI] [PubMed] [Google Scholar]
- 10. Kommoss S, McConechy MK, Kommoss F, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population‐based case series. Ann Oncol. 2018;29(5):1180‐1188. [DOI] [PubMed] [Google Scholar]
- 11. Raffone A, Travaglino A, Mascolo M, et al. TCGA molecular groups of endometrial cancer: pooled data about prognosis. Gynecol Oncol. 2019;155(2):374‐383. [DOI] [PubMed] [Google Scholar]
- 12. Britton H, Huang L, Lum A, et al. Molecular classification defines outcomes and opportunities in young women with endometrial carcinoma. Gynecol Oncol. 2019;153(3):487‐495. [DOI] [PubMed] [Google Scholar]
- 13. Travaglino A, Raffone A, Mascolo M, et al. Clear cell endometrial carcinoma and the TCGA classification. Histopathology. 2020;76(2):336‐338. [DOI] [PubMed] [Google Scholar]
- 14. Travaglino A, Raffone A, Mascolo M, et al. TCGA molecular subgroups in endometrial undifferentiated/dedifferentiated carcinoma. Pathol Oncol Res. 2020;26(3):1411‐1416. [DOI] [PubMed] [Google Scholar]
- 15. Travaglino A, Raffone A, Mollo A, et al. TCGA molecular subgroups and FIGO grade in endometrial endometrioid carcinoma. Arch Gynecol Obstet. 2020;301(5):1117‐1125. [DOI] [PubMed] [Google Scholar]
- 16. Travaglino A, Raffone A, Gencarelli A, et al. TCGA classification of endometrial cancer: the place of carcinosarcoma. Pathol Oncol Res. 2020;26(4):2067‐2073. [DOI] [PubMed] [Google Scholar]
- 17. Travaglino A, Raffone A, Stradella C, et al. Impact of endometrial carcinoma histotype on the prognostic value of the TCGA molecular subgroups. Arch Gynecol Obstet. 2020;301(6):1355‐1363. [DOI] [PubMed] [Google Scholar]
- 18. Santoro A, Angelico G, Travaglino A, et al. Clinico‐pathological significance of TCGA classification and SWI/SNF proteins expression in undifferentiated/dedifferentiated endometrial carcinoma: a possible prognostic risk stratification. Gynecol Oncol. 2021;161(2):629‐635. 10.1016/j.ygyno.2021.02.029. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529‐536. [DOI] [PubMed] [Google Scholar]
- 21. McConechy MK, Hoang LN, Chui MH, et al. In‐depth molecular profiling of the biphasic components of uterine carcinosarcomas. J Pathol Clin Res. 2015;1(3):173‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cherniack AD, Shen H, Walter V, et al. Integrated molecular characterization of uterine carcinosarcoma. Cancer Cell. 2017;31(3):411‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones TE, Pradhan D, Dabbs DJ, Bhargava R, Onisko A, Jones MW. Immunohistochemical markers with potential diagnostic, prognostic, and therapeutic significance in uterine carcinosarcoma: a clinicopathologic study of 43 cases. Int J Gynecol Pathol. 2021;40(1):84‐93. [DOI] [PubMed] [Google Scholar]
- 24. Gotoh O, Sugiyama Y, Takazawa Y, et al. Clinically relevant molecular subtypes and genomic alteration‐independent differentiation in gynecologic carcinosarcoma. Nat Commun. 2019;10(1):4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saijo M, Nakamura K, Ida N, et al. Histologic appearance and immunohistochemistry of DNA mismatch repair protein and p53 in endometrial carcinosarcoma: impact on prognosis and insights into tumorigenesis. Am J Surg Pathol. 2019;43(11):1493‐1500. [DOI] [PubMed] [Google Scholar]
- 26. Suarez AA, Felix AS, Cohn DE. Bokhman Redux: endometrial cancer "types" in the 21st century. Gynecol Oncol. 2017;144(2):243‐249. [DOI] [PubMed] [Google Scholar]
- 27. Zannoni GF, Vellone VG, Arena V, et al. Does high‐grade endometrioid carcinoma (grade 3 FIGO) belong to type I or type II endometrial cancer? A clinical‐pathological and immunohistochemical study. Virchows Arch. 2010;457(1):27‐34. [DOI] [PubMed] [Google Scholar]
- 28. Bae HS, Kim H, Young Kwon S, Kim KR, Song JY, Kim I. Should endometrial clear cell carcinoma be classified as Type II endometrial carcinoma? Int J Gynecol Pathol. 2015;34(1):74‐84. [DOI] [PubMed] [Google Scholar]
- 29. Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular‐based classification. Virchows Arch. 2004;444(3):213‐223. [DOI] [PubMed] [Google Scholar]
- 30. McAlpine J, Leon‐Castillo A, Bosse T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J Pathol. 2018;244(5):538‐549. [DOI] [PubMed] [Google Scholar]
- 31. Raffone A, Travaglino A, Cerbone M, et al. Diagnostic accuracy of immunohistochemistry for mismatch repair proteins as surrogate of microsatellite instability molecular testing in endometrial cancer. Pathol Oncol Res. 2020;26(3):1417‐1427. [DOI] [PubMed] [Google Scholar]
- 32. Raffone A, Travaglino A, Cerbone M, et al. Diagnostic accuracy of p53 immunohistochemistry as surrogate of TP53 sequencing in endometrial cancer. Pathol Res Pract. 2020;216(8):153025. [DOI] [PubMed] [Google Scholar]
- 33. Stelloo E, Bosse T, Nout RA, et al. Refining prognosis and identifying targetable pathways for high‐risk endometrial cancer; a TransPORTEC initiative. Mod Pathol. 2015;28(6):836‐844. [DOI] [PubMed] [Google Scholar]
- 34. Kim SR, Cloutier BT, Leung S, et al. Molecular subtypes of clear cell carcinoma of the endometrium: opportunities for prognostic and predictive stratification. Gynecol Oncol. 2020;158(1):3‐11. [DOI] [PubMed] [Google Scholar]
- 35. DeLair DF, Burke KA, Selenica P, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol. 2017;243(2):230‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Espinosa I, Lee CH, D'Angelo E, Palacios J, Prat J. Undifferentiated and dedifferentiated endometrial carcinomas with POLE exonuclease domain mutations have a favorable prognosis. Am J Surg Pathol. 2017;41(8):1121‐1128. [DOI] [PubMed] [Google Scholar]
- 37. Conlon N, Da Cruz Paula A, Ashley CW, et al. Endometrial carcinomas with a "Serous" component in young women are enriched for DNA mismatch repair deficiency, Lynch syndrome, and POLE exonuclease domain mutations. Am J Surg Pathol. 2020;44(5):641‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bosse T, Nout RA, McAlpine JN, et al. Molecular classification of Grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am J Surg Pathol. 2018;42(5):561‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Joehlin‐Price A, Van Ziffle J, Hills NK, Ladwig N, Rabban JT, Garg K. Molecularly classified uterine FIGO Grade 3 endometrioid carcinomas show distinctive clinical outcomes but overlapping morphologic features. Am J Surg Pathol. 2021;45(3):421‐429. [DOI] [PubMed] [Google Scholar]
- 40. Taskin OÇ, Onder S, Topuz S, et al. A selected immunohistochemical panel aids in differential diagnosis and prognostic stratification of subtypes of high‐grade endometrial carcinoma: a clinicopathologic and immunohistochemical study at a single institution. Appl Immunohistochem Mol Morphol. 2017;25(10):696‐702. [DOI] [PubMed] [Google Scholar]
- 41. Zhang C, Hu W, Jia N, et al. Uterine carcinosarcoma and high‐risk endometrial carcinomas: a clinicopathological comparison. Int J Gynecol Cancer. 2015;25(4):629‐636. [DOI] [PubMed] [Google Scholar]
- 42. Prueksaritanond N, Chantape W. Comparative survival outcomes of uterine papillary serous carcinoma, clear cell carcinoma, grade 3 endometrioid adenocarcinoma, and carcinosarcoma of endometrial cancer in Rajavithi Hospital. J Med Assoc Thail. 2016;99(Suppl 2):S75‐S83. [PubMed] [Google Scholar]
- 43. Murali R, Davidson B, Fadare O, et al. High‐grade endometrial carcinomas. Int J Gynecol Pathol. 2019;38:S40‐S63. 10.1097/PGP.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kihara A, Amano Y, Matsubara D, Fukushima N, Fujiwara H, Niki T. BRG1, INI1, and ARID1B deficiency in endometrial carcinoma: a clinicopathologic and immunohistochemical analysis of a large series from a single institution. Am J Surg Pathol. 2020;44(12):1712‐1724. [DOI] [PubMed] [Google Scholar]
- 45. Busca A, Parra‐Herran C, Nofech‐Mozes S, et al. Undifferentiated endometrial carcinoma arising in the background of high‐grade endometrial carcinoma – expanding the definition of dedifferentiated endometrial carcinoma. Histopathology. 2020;77(5):769‐780. [DOI] [PubMed] [Google Scholar]
- 46. Köbel M, Tessier‐Cloutier B, Leo J, et al. Frequent mismatch repair protein deficiency in mixed endometrioid and clear cell carcinoma of the endometrium. Int J Gynecol Pathol. 2017;36(6):555‐561. [DOI] [PubMed] [Google Scholar]
- 47. Tessier‐Cloutier B, Coatham M, Carey M, et al. SWI/SNF‐deficiency defines highly aggressive undifferentiated endometrial carcinoma. J Pathol Clin Res. 2021;7(2):144‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kang EY, Tessier‐Cloutier B, Duggan MA, Stewart CJR, Lee CH, Köbel M. Loss of ARID1B and SMARCB1 expression are specific for the diagnosis of dedifferentiated/undifferentiated carcinoma in tumours of the upper gynaecologic tract and cervix. Histopathology. 2021; 10.1111/his.14333. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Supplementary Material


