Abstract
The development of effective (non‐pharmacological) treatment approaches for executive dysfunction in autism spectrum disorder (ASD) requires evidence that factors influencing this domain can be modified by behavioral interventions. The present cross‐sectional study investigated the relative associations of ASD, muscle strength and body mass index with executive function and information processing among the Healthy Brain Network cohort. Patients with ASD (N = 174) and healthy peers (N = 202) aged 5 to 18 years completed cognitive tasks of the NIH toolbox (Pattern Comparison, Flanker, List Sorting, Card Sorting) to assess core components of executive function and information processing. Additionally, anthropometrics and muscle strength were collected from selected items (push‐ups, curl‐ups, trunk lift, and grip strength) of the Fitnessgram battery. Based on structural equation modeling, ASD was related to impaired muscle strength and executive function, when confounders (age, sex, pubertal status, and socioeconomic status) were accounted for. Muscle strength further showed independent contributions to information processing and executive function. This association was moderated by ASD, so that higher muscle strength was related to higher executive function in ASD patients only. The present findings provide a first indication that the promotion of muscle strength may have the potential to generally enhance information processing and to reduce ASD‐related executive dysfunction in children and adolescents.
Lay Summary
In comparison to healthy peers, children with ASD showed impairments in executive function and muscle strength. Moreover, higher muscle strength was independently associated with better executive function, but only in ASD patients. This is a first indication that the promotion of muscle strength, for example, by regular exercise, could contribute to a reduction of ASD‐related executive dysfunction.
Keywords: cognitive performance, development, information processing, mental disorder, physical fitness
INTRODUCTION
A survey of the Autism and Developmental Disabilities Monitoring Network supports that the prevalence of autism spectrum disorder (ASD) increased from 1 in 150 cases in 2006 to 1 in 54 cases in 2016 (Maenner et al., 2020). The global prevalence rate also seems to be rising, such that the management of ASD is a global challenge (Elsabbagh et al., 2012). The leading symptoms of ASD are present in the early developmental stage and encompass repetitive behaviors along with deficits in social communication and interaction. With regard to cognitive abnormalities, meta‐analytical findings suggest that children with ASD have to face pronounced impairments in executive function, which persist across the development (Demetriou et al., 2018). Executive function refers to top‐down control of behavior by employing its core components, such as inhibitory control, working memory, and task‐switching (Miyake & Friedman, 2012). For example, cognitive skills summarized under executive function are necessary for deliberate reasoning, intentional action, emotion regulation, complex social functioning, and adaptation to changing circumstances (Zelazo, 2015). Given the relevance of these cognitive skills for many real‐life domains, it becomes clear that executive function plays a key role for school readiness and concurrent academic achievement in children with ASD (Kim et al., 2020; Pellicano et al., 2017). Executive function has also been suggested to underlie core deficits in adaptive behavior and social communication (Leung et al., 2016; Pugliese et al., 2016). In this respect, some evidence suggests that early executive function predicts later autistic features and adaptive behavior even above and beyond age, intelligence quotient, and theory of mind (i.e., false‐belief understanding) (Kenny et al., 2019). In contrast to the view that ASD core deficits may originate from specific cognitive impairments, an alternative explanation assumes that children with high executive function are able to compensate for atypicalities in brain systems (Johnson, 2012). Despite some differences regarding the nature of such associations, both views highlight the need to target executive function in children with ASD.
The current treatment guidelines do not recommend the routine use of any pharmacological treatment for core symptoms of ASD, although dopamine receptor blockers and psychostimulants have the potential to reduce some deficits related to co‐occurring conditions (Howes et al., 2018). As the limited benefits of these pharmacotherapies do not outweigh their risks (e.g., weight gain, sleep problems, irritability, and emotional outbursts) (Cortese et al., 2012; Kloosterboer et al., 2021), behavioral approaches are preferred for managing ASD (Masi et al., 2017). Executive dysfunction partly underlies core symptoms of ASD, such that factors exerting an influence on this cognitive deficit can inform the development of such behavioral approaches. In this respect, evidence from neurotypical cohorts suggests high performance on executive function tasks in children and adolescents to be linked with high cardiorespiratory fitness and/or muscular strength (de Bruijn et al., 2018; Mora‐Gonzalez et al., 2019; Oberer et al., 2018). This is further supported by a quantitative synthesis of 80 randomized controlled trials showing long‐term cognitive benefits of endurance and resistance exercise across age, although it should be noted that coordinative exercise had even superior effects (Ludyga et al., 2020). A review of the experimental evidence suggests that such exercise‐induced benefits for executive function generalize to neurodevelopmental disorders (Ludyga et al., 2021). However, only a single randomized controlled trial was available for ASD patients (Pan et al., 2017). This limits conclusions on the exercise type that might be most effective. However, the few studies that investigated physical fitness in ASD patients consistently found lower muscular strength (Kern et al., 2013; Pan et al., 2016; Tyler et al., 2014), indicating a greater potential for enhancements of executive function by targeting this aspect of fitness. Moreover, the association between executive function and muscular strength might be moderated by ASD as physical activity programs targeting physical fitness are more efficient in individuals with low cognitive performance (Ishihara et al., 2020). Insights into the relative contribution of muscular strength to ASD‐related cognitive impairments require the consideration of body mass index. This is due to the close relation of both fitness‐related aspects of physical health (García‐Hermoso et al., 2019) and the predictive value of body mass index for children and adolescents' performance on tasks assessing executive function (Blair et al., 2020; Laurent et al., 2020).
The present study aimed to examine the relation of ASD, muscular strength, and body mass index with executive function, while accounting for interdependencies. Selective associations with executive function were examined by controlling for basic information processing. Based on the current literature, we expected independent relations of body mass index (Blair et al., 2020; Laurent et al., 2020) and muscular strength with executive function (de Bruijn et al., 2018; Mora‐Gonzalez et al., 2019; Oberer et al., 2018) as well as higher associations in children and adolescents with ASD than in healthy peers (Ishihara et al., 2020).
METHODS
Participants and recruitment
The data used for the present investigation were provided by the Healthy Brain Network (HBN), which used a community‐referred recruitment model (Alexander et al., 2017). Advertisements and announcements were distributed to community members, educators, local care providers, and parents via e‐mail lists and events. Parents, who were concerned about psychiatric symptoms in their child, were encouraged to participate. The data set available at the time of the present investigation included 2480 participants aged 5 to 21 years, who were fluent in English. The main exclusion criteria of HBN concentrated on acute safety concerns (e.g., danger to self or others), cognitive or behavioral impairments that could interfere with participation (e.g., being nonverbal, IQ less than 66) or medical concerns that could confound brain‐related findings (Alexander et al., 2017). The present study was restricted to patients with ASD (according to DSM‐5 diagnosis) and healthy peers (no presence of a mental disorder according to DSM‐5 following semi‐structured psychiatric interviews) aged 5 to 18 years. All participants had to have the capacity to provide assent, with the parent or legal guardian providing written informed consent. The HBN protocol was approved by the Chesapeake Institutional Review Board and followed the guidelines of the Declaration of Helsinki.
Procedures
The extensive HBN study protocol included four laboratory visits to assess a variety of health‐related and other outcomes. The present study used data obtained from diagnostic interviews, psychopathology (Strengths and Difficulties Questionnaire, SDQ), cognitive tasks tapping information processing and executive function (National Institutes of Health Toolbox for the Assessment of Neurological and Behavioral Function, NIH), physical fitness (Fitnessgram), and potential confounders, including socioeconomic (Barratt Simplified Measure of Social Status, BSMSS) and pubertal status (Peterson Puberty Scale, PPS).
ASD diagnosis
Preliminary ASD diagnoses were derived from the child‐ and parent‐based versions of the computerized Kiddie Schedule for Affective Disorders and Schizophrenia. The semi‐structured DSM‐5‐based psychiatric interviews were performed by a licensed clinician and resulted in automated diagnoses. These preliminary diagnoses were verified by the clinical team after considering other data (Autism Spectrum Screening Questionnaire, ASSQ; Autism Diagnostic Interview‐Revised; Autism Diagnostic Observation Schedule) and interactions in the course of the study participation.
Cognitive tasks
Selected tasks from the NIH toolbox were used to assess information processing (i.e., the ability to recognize and respond to simple visual or auditory stimuli), inhibitory control, working memory, and set‐shifting. These cognitive skills were chosen to be able to verify that ASD is related to more pronounced deficits in executive function rather than impairments in information processing (Demetriou et al., 2018). Moreover, this approach is used to determine whether possible associations between muscle strength and cognitive outcomes are selective for ASD‐related impairments or generalize across different cognitive skills. For all employed tasks, age‐corrected standard scores were calculated and used for statistical analyses.
The Pattern Comparison task required participants to indicate whether two visual patterns were the same or different. Type, complexity, and number of stimuli were adjusted to participants' age to ensure adequate variability of performance. Performance was assessed from the number of correct items completed in 90 seconds.
The Flanker task comprised 40 trials, in which a central directional target (fish for children younger than 8 years, arrows for ages 8 and older) was flanked by similar stimuli on the left and right. Depending on the trial type, the flanking stimuli faced in the same (congruent) or different direction (incongruent) compared with the target. Participants were instructed to indicate the direction of the central stimulus, and average response accuracy and reaction time were extracted.
During the List Sorting task, a series of stimuli were presented visually (object) and orally (spoken name). While in one condition, all stimuli belonged to only one of the two categories, stimuli from both categories were shown in the second condition. Participants were instructed to recall all stimuli from each category based on their size. The number of items in each series increased and the test was terminated, when participants failed two trials of the same length. Test scores included the number of correct items across trials.
During the Dimensional Change Card Sorting task, participants were instructed to match a visual target stimulus to one of two stimuli according to the shape or color. Target category (“shape” or “color”) was shown on the screen and delivered orally for children aged less than 8 years. Participants of this age group completed two blocks that either contained shape or color trials. When they succeeded to switch the tasks, a mixed block with 40 trials was administered. In this block, color was crucial on the majority of trials with occasional, unpredictable shifts to shape. Participants aged 8 years or older only completed the mixed block.
Fitness
Participants completed selected items of the Fitnessgram battery, including measurements of body composition (BMI) and muscule strength (push‐ups, curl‐ups, trunk lift, grip strength). For the curl‐up and push‐up tests, participants had to perform as many repetitions as possible (max. 50), with a constant cadence of 1 per 3 seconds. For the trunk lift test, participants lied on their stomach, lifted their upper body off the floor, and held the position for measurement. When tests were performed with the left and right side separately, the average of both sides was used in statistical analysis.
Statistics
SPSS 25.0 and the AMOS plugin were used for data analyses. In advance, variables with z‐values of kurtosis and/ or skewness exceeding 3.29 were log‐transformed (Kim, 2013). Due to the expectation of missings, analyses were restricted to participants and patients that completed items of the Fitnessgram, physical assessment and the NIH toolbox. This approach was chosen to improve the precision of full maximum likelihood estimation of missing data. One‐way ANOVAs were used to compare anthropometrics (age, height, and body mass index), psychopathology (SDQ, ASSQ), socioeconomic status (BSMSS), muscular fitness (Fitnessgram) and cognitive performance (NIH) between ASD patients and healthy peers. Structural equation modeling (SEM) was employed to investigate the prediction of information processing and executive function by group, body mass index, and muscular fitness. Both executive function and muscular fitness were entered as latent variables, estimated from the NIH toolbox and the Fitnessgram items, respectively. Only indicators that explained a significant proportion of variance, showed factor loadings of β = 0.30 or higher and had less than 50% missing data, were tolerated. To assess the relative contributions of body mass index, muscular fitness, and group to information processing and executive function, interrelations among predictors and outcomes were accounted for by estimating their covariance. Additionally, covariances with sex, age, socioeconomic status, and pubertal status were estimated, if variables showed a statistically significant association with one or more predictors and/or outcomes based on zero‐order correlations (Table S2). Both ASD patients and healthy peers were included in Model 1. To test a moderation of the association between predictors and outcomes, groups were separated in Model 2 (ASD patients) and 3 (healthy peers). The hypothesis that coefficients equal zero was examined with t tests and rejected at p < 0.050. The fits of the models to the data were investigated and considered good at CFI≥0.09 and RMSEA≤0.08.
RESULTS
The final sample comprised 376 participants, because 100 individuals did not complete items of the physical fitness assessments and/or the cognitive test battery (Table S1). Among the ASD patients, frequent co‐occurring conditions were ADHD (N = 121), learning disorder (N = 8), generalized anxiety disorder (N = 6), and other disorders (N = 41; individual disorders occurring in five patients or fewer). In comparison to healthy peers, ASD patients had higher body mass index, higher scores on ASSQ and SDQ, but lower performance on all Fitnessgram items and cognitive tests (Table 1). Whereas age and height did not differ between groups, a higher proportion of boys was found among ASD patients, chi2 = 36.16, p < 0.001.
TABLE 1.
Comparison of psychopathology, anthropometrics, muscle strength and cognitive performance between ASD patients and healthy peers
| Healthy peers (N = 101 f/101 m) | ASD patients (N = 139 m/35 f) | F | η2 | |||
|---|---|---|---|---|---|---|
| M | SE | M | SE | |||
| ASSQ score | 2.6 | 0.3 | 19.1 | 0.8 | 403.02* | 0.52 |
| SDQ score | 6.8 | 0.4 | 15.8 | 0.4 | 258.48* | 0.41 |
| BSMSS score | 51.1 | 0.9 | 47.5 | 1.1 | 6.23* | 0.02 |
| PPS score | 3.4 | 0.2 | 4.7 | 0.2 | 19.14* | 0.07 |
| Age in y | 10.3 | 0.2 | 10.5 | 0.2 | 0.55 | <0.01 |
| Height in inches | 55.5 | 0.5 | 56.8 | 0.6 | 3.02 | <0.01 |
| Body mass index in kg−m−2 | 19.0 | 0.3 | 20.0 | 0.4 | 3.96* | 0.01 |
| Curl‐up | 13.3 | 0.7 | 6.8 | 0.7 | 40.40* | 0.10 |
| Push‐up | 6.8 | 0.5 | 2.9 | 0.4 | 30.13* | 0.08 |
| Trunk lift | 10.0 | 0.2 | 8.3 | 0.2 | 28.43* | 0.07 |
| Grip strength in kg | 24.0 | 1.0 | 20.5 | 1.0 | 5.70* | 0.03 |
| Card Sorting task | 99.4 | 1.3 | 88.6 | 1.2 | 36.92* | 0.09 |
| Flanker task | 92.4 | 0.9 | 85.5 | 1.1 | 24.69* | 0.06 |
| List Sorting task | 101.4 | 1.0 | 91.4 | 1.3 | 39.81* | 0.10 |
| Pattern Comparison task | 93.8 | 1.6 | 88.9 | 1.7 | 4.62* | 0.01 |
Abbreviations: ASSQ, autism spectrum screening questionnaire; BSMSS, Barratt simplified measure of social status; PPS, Peterson puberty scale; SDQ, strengths and difficulties questionnaire.
indicates p < 0.05.
With regard to SEM, preliminary examination of the latent constructs showed acceptable factor loadings for all variables (Model 1 with all latent variables, Figure S1), β ≥ 0.36, p < 0.001. Only for grip strength, missing data exceeded the pre‐specified threshold (Table S2), so that this item was removed from the latent construct. SEM including ASD patients and healthy peers (Model 1, Figure 1) showed that being autistic was moderately related to lower muscle strength, β = −0.38, p < 0.001. In contrast, body mass was not related to muscle strength and/or group. With regard to cognitive performance, group individually predicted executive function, β = −0.37, p < 0.001, so that lower standard scores were moderately related to being autistic. Accounting for this association, high muscle strength predicted both high standard scores on pattern comparison, β = 0.24, p < 0.001, and executive function, β = 0.21, p = 0.004. Independent of the cognitive domain, performance could not be predicted from body mass index. The fits of the data to the model were appropriate, CFI = 0.95, RMSEA = 0.06.
FIGURE 1.
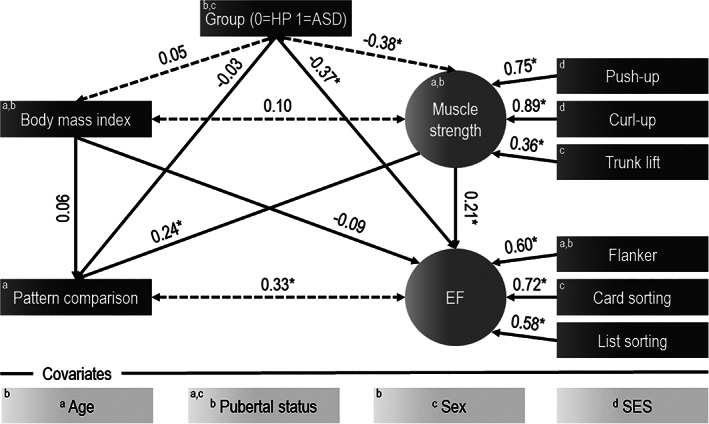
Prediction of information processing and executive function from group, body mass index and muscle strength (Model 1). Standardized regression coefficients are shown above the line. Superscript letters show covariances that were estimated with confounders. *indicates p < 0.05; HP=healthy peers; ASD=patients with autism Spectrum disorder; SES=socioeconomic status
When SEM was performed with ASD patients only (Model 2, Figure 2), the prediction of standard scores on pattern comparison, β = 0.23, p = 0.005, and executive function, β = 0.23, p = 0.024, by muscular strength remained significant. However, when SEM was restricted to healthy peers (Model 3, Figure 2), the predictive value of this aspect of fitness was limited to standard scores on pattern comparison, β = 0.23, p = 0.003. No other differences between models were observed as body mass index showed no relations with muscular strength and cognitive outcomes in both ASD patients and healthy peers. Both Model 2, CFI = 0.98, RMSEA = 0.04, and Model 3, CFI = 0.91, RMSEA = 0.08, showed good model fit.
FIGURE 2.
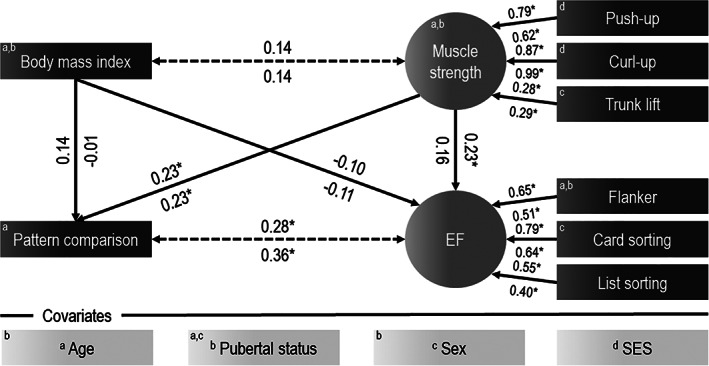
Prediction of information processing and executive function from body mass index and muscle strength in ASD patients (Model 2) and healthy peers (Model 3). Standardized regression coefficients are shown above the line (ASD patients) and below the line (healthy controls). Superscript letters show covariances that were estimated with confounders. *indicates p < 0.05; EF = executive function
DISCUSSION
The results of SEM revealed that ASD was related to both deficits in muscle strength and impaired cognitive performance, when potential confounders (age, sex, pubertal status, and socioeconomic status) were controlled for. Differences in standard scores on cognitive tasks between ASD patients and healthy peers were specific to those demanding executive function. The key finding of the present study was the independent association between muscle strength and this cognitive domain as well as its moderation by ASD.
The association of ASD with executive dysfunction is in line with previous meta‐analytical findings (Demetriou et al., 2018). The lack of differences in information processing between ASD patients and healthy peers argues against a domain‐general cognitive impairment. Although previous findings suggest that the executive function profile might vary based on co‐occurring conditions (Craig et al., 2016), the present study showed that ASD affected working memory, set‐shifting, and inhibitory control in a similar way.
Knowledge of the mechanisms that underlie these impairments is crucial for understanding how they can be reduced. A review of neuroimaging studies supports ASD to be linked with multiple alterations in brain function and structure, but they are not necessarily related to its core deficits (Ecker et al., 2015). However, long‐range functional underconnectivity (O'Reilly et al., 2017) and altered coherence of the fronto‐parietal network in particular seem to contribute to poor executive function in ASD patients (Han & Chan, 2017). As this network subserves cognitive control, an atypical development of related processes may further account for executive dysfunction. In this respect, a meta‐analysis has reported reduced amplitude of P300 component of event‐related potential, indicating abnormalities in the allocation of attentional resources and working memory updating (Cui et al., 2017).
In addition to impaired executive function, patients with ASD showed lower muscle strength than their healthy peers. The uncorrected baseline comparison indicated differences between groups on all items that were used to measure muscle strength. The association of this aspect of fitness and ASD remained even after correction for body mass index and other confounders in SEM. Consequently, the present results further support the deficits in muscle strength in children and adolescents with ASD that were previously observed in very small cohorts (Kern et al., 2013; Pan et al., 2016; Tyler et al., 2014). This might be explained by lower physical activity levels of ASD patients in comparison to healthy peers (McCoy et al., 2016; Tyler et al., 2014), although it should be noted that some findings indicate an engagement in fewer types of physical activity rather than a general difference in the time spent physically active (Bandini et al., 2013). This could be due to the well‐documented deficits in motor competence (Kaur et al., 2018; Liu et al., 2019), which affect the ability to acquire and refine complex motor skills that are required in various physical and sports activities (Holfelder & Schott, 2014). Thus, impaired muscle strength might be a consequence of a low participation in strengthening and toning activities, although interventions promoting such activities have been found to be feasible and lead to benefits in physical fitness and other health‐relevant domains in children and adolescents with ASD (Sowa & Meulenbroek, 2012).
Moreover, muscle strength appears to be linked with cognitive performance, given that this aspect of fitness explained a unique proportion of variance on standard scores of the cognitive tasks. Removing grip strength from the latent constructs (due to a high proportion of missing data) did not affect the pattern of results. When the examination of this association was restricted to individual groups, a moderating effect of ASD was revealed. Whereas higher muscle strength was generally related to better information processing, its association with executive function only reached statistical significance in patients with ASD. This lends further evidence to the previously documented influence of baseline cognitive performance on the association of physical activity and executive function (Ishihara et al., 2020). Additionally, low muscle strength in patients with ASD also indicates a greater reserve for adaptations, so that improvements in this fitness‐related aspect may transfer to the domain of the executive function.
The present findings provide insights into the link between muscle strength and executive function in ASD, but the underlying mechanisms remain unclear. However, physical activity and fitness have generally been associated with a favorable alteration of the P300 amplitude of event‐related potentials elicited from executive function tasks in healthy individuals (Kao et al., 2020). Consequently, this is a first indication that the attainment of a high muscle strength could improve executive function in ASD by altering cognitive control processes positively (Cui et al., 2017). In addition, a review (primarily including older populations) has suggested that resistance training in particular benefits cognition via an increased expression of growth factors (i.e. BDNF, IGF‐1) as well as changes in functional and structural properties of the brain (Herold et al., 2019). However, changes in BDNF may not cause improved executive function in ASD, because in contrast to many other mental disorders, ASD is associated with elevated rather than diminished circulating BDNF (Armeanu et al., 2017). Given ASD‐related impairments in functional connectivity, a possible influence of strengthening activities on this property of the brain appears to be a more likely cause of the link between muscle strength and executive function. The first indication in support of this assumption is provided by the observation that increased muscle fatigue leads to a more pronounced connectivity between structures subserving cognitive and motor control (Jiang et al., 2012). The repetition of this mechanism by regular engagement in strengthening activities might therefore trigger a favorable adaption of functional connectivity that partly counteracts some of the atypical brain activation patterns in children and adolescents with ASD.
Despite the relatively large cohort, the cross‐sectional design limits possible implications on the role of muscle strength for partly managing executive dysfunction in ASD. Longitudinal and experimental evidence from healthy cohorts suggests that increases in physical fitness (by exercise) translate into improved executive function (Ludyga et al., 2020; Oberer et al., 2018), but the direction of the effects could also be reversed. In this case, high executive function could contribute to the engagement in activities that promote muscle strength and to the ability to perform best in physical fitness tests due to association of this cognitive domain and self‐regulatory resources (Audiffren & André, 2019). The interpretation of the current findings is further limited by the focus on muscle strength only. Consequently, a similar or different contribution of other fitness‐related aspects, such as cardiorespiratory fitness and motor coordination, to executive function in ASD remains unclear. Furthermore, the results might have been affected by confounders other than age, body mass index, sex, pubertal status, and socioeconomic status. While these variables were accounted for in SEM, the role of co‐occurring conditions for the association of muscle fitness and executive function was not examined as the heterogeneity in these conditions complicated the formation of subgroups. However, the majority of ASD patients in the present sample were also affected by ADHD. Executive dysfunction is a well‐known characteristic of ADHD (Pineda‐Alhucema et al., 2018) and questions whether the associations found are due to this condition rather than ASD. While this question remained unaddressed in the current study, a review comparing patients with ASD or ADHD and patients with ASD with co‐occurring ADHD showed common executive function deficits (Craig et al., 2016). ASD patients with and without co‐occurring ADHD only differed by response inhibition, which was not included in the latent executive function construct of the present analyses. Thus, it seems unlikely that the results were mainly due to co‐occurring ADHD rather than ASD. In addition, the exclusion criteria specified in this study prevent conclusions on whether the present findings can be generalized to non‐verbal individuals with ASD and patients with co‐occurring Intellectual Disability.
In conclusion, children and adolescents with ASD are facing pronounced deficits in muscle strength and executive function. These health‐relevant domains are interrelated in ASD patients, whereas there is no association of muscle strength and executive function in healthy peers. In contrast, this aspect of fitness shows an independent contribution to the domain of information processing irrespective of ASD. Consequently, behavioral interventions targeting muscle strength may have the potential to generally enhance information processing and to reduce ASD‐related executive dysfunction. However, future experimental studies need to verify whether (exercise‐induced) gains in muscular strength and/ or other aspects of fitness (e.g., motor coordination, cardiorespiratory fitness) translate into improved executive function in children with ASD. Given the heterogeneity among ASD patients, these studies also need to address the generalizability of the findings to different subtypes by performing subgroup analyses based on frequent co‐occurring conditions, such as ADHD and intellectual disabilities.
Supporting information
Table S1 Missing data in the full cohort and the analyzed cohort.
Table S2. Zero‐order correlations investigating the association between confounders, predictors and outcomes.
Figure S1. Initial model predicting information processing and executive function from group, body mass index and muscle strength. Notes: Standardized regression coefficients are shown above the line. Superscript letters show covariances that were estimated with confounders. * indicates p < 0.05; HP=Healthy peers; ASD=Patients with Autism Spectrum Disorder; SES=Socioeconomic status
ACKNOWLEDGMENTS
The Healthy Brain Network that provided the underlying data for this study are funded by individual donors and private foundations. No funding was received for the present investigation. Open Access Funding provided by Universitat Basel.
Ludyga, S. , Pühse, U. , Gerber, M. , & Mücke, M. (2021). Muscle strength and executive function in children and adolescents with autism spectrum disorder. Autism Research, 14(12), 2555–2563. 10.1002/aur.2587
REFERENCES
- Alexander, L. M. , Escalera, J. , Ai, L. , Andreotti, C. , Febre, K. , Mangone, A. , Vega‐Potler, N. , Langer, N. , Alexander, A. , Kovacs, M. , Litke, S. , O'Hagan, B. , Andersen, J. , Bronstein, B. , Bui, A. , Bushey, M. , Butler, H. , Castagna, V. , Camacho, N. , … Milham, M. P. (2017). An open resource for transdiagnostic research in pediatric mental health and learning disorders. Scientific Data, 4, 170181. 10.1038/sdata.2017.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armeanu, R. , Mokkonen, M. , & Crespi, B. (2017). Meta‐analysis of BDNF levels in autism. Cellular and Molecular Neurobiology, 37(5), 949–954. 10.1007/s10571-016-0415-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audiffren, M. , & André, N. (2019). The exercise‐cognition relationship: A virtuous circle. Journal of Sport and Health Science, 8(4), 339–347. 10.1016/j.jshs.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandini, L. G. , Gleason, J. , Curtin, C. , Lividini, K. , Anderson, S. E. , Cermak, S. A. , Maslin, M. , & Must, A. (2013). Comparison of physical activity between children with autism spectrum disorders and typically developing children. Autism: The International Journal of Research and Practice, 17(1), 44–54. 10.1177/1362361312437416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair, C. , Kuzawa, C. W. , & Willoughby, M. T. (2020). The development of executive function in early childhood is inversely related to change in body mass index: Evidence for an energetic tradeoff? Developmental Science, 23(1), e12860. 10.1111/desc.12860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese, S. , Castelnau, P. , Morcillo, C. , Roux, S. , & Bonnet‐Brilhault, F. (2012). Psychostimulants for ADHD‐like symptoms in individuals with autism spectrum disorders. Expert Review of Neurotherapeutics, 12(4), 461–473. 10.1586/ern.12.23 [DOI] [PubMed] [Google Scholar]
- Craig, F. , Margari, F. , Legrottaglie, A. R. , Palumbi, R. , de Giambattista, C. , & Margari, L. (2016). A review of executive function deficits in autism spectrum disorder and attention‐deficit/hyperactivity disorder. Neuropsychiatric Disease and Treatment, 12, 1191–1202. 10.2147/NDT.S104620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, T. , Wang, P. P. , Liu, S. , & Zhang, X. (2017). P300 amplitude and latency in autism spectrum disorder: A meta‐analysis. European Child & Adolescent Psychiatry, 26(2), 177–190. 10.1007/s00787-016-0880-z [DOI] [PubMed] [Google Scholar]
- de Bruijn, A. G. M. , Hartman, E. , Kostons, D. , Visscher, C. , & Bosker, R. J. (2018). Exploring the relations among physical fitness, executive functioning, and low academic achievement. Journal of Experimental Child Psychology, 167, 204–221. 10.1016/j.jecp.2017.10.010 [DOI] [PubMed] [Google Scholar]
- Demetriou, E. A. , Lampit, A. , Quintana, D. S. , Naismith, S. L. , Song, Y. J. C. , Pye, J. E. , Hickie, I. , & Guastella, A. J. (2018). Autism spectrum disorders: A meta‐analysis of executive function. Molecular Psychiatry, 23(5), 1198–1204. 10.1038/mp.2017.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker, C. , Bookheimer, S. Y. , & Murphy, D. G. M. (2015). Neuroimaging in autism spectrum disorder: Brain structure and function across the lifespan. The Lancet Neurology, 14(11), 1121–1134. 10.1016/S1474-4422(15)00050-2 [DOI] [PubMed] [Google Scholar]
- Elsabbagh, M. , Divan, G. , Koh, Y.‐J. , Kim, Y. S. , Kauchali, S. , Marcín, C. , Montiel‐Nava, C. , Patel, V. , Paula, C. S. , Wang, C. , Yasamy, M. T. , & Fombonne, E. (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Research: Official Journal of the International Society for Autism Research, 5(3), 160–179. 10.1002/aur.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Hermoso, A. , Ramírez‐Campillo, R. , & Izquierdo, M. (2019). Is muscular fitness associated with future health benefits in children and adolescents? A systematic review and meta‐analysis of longitudinal studies. Sports Medicine (Auckland, N.Z.), 49(7), 1079–1094. 10.1007/s40279-019-01098-6 [DOI] [PubMed] [Google Scholar]
- Han, Y. M. Y. , & Chan, A. S. (2017). Disordered cortical connectivity underlies the executive function deficits in children with autism spectrum disorders. Research in Developmental Disabilities, 61, 19–31. 10.1016/j.ridd.2016.12.010 [DOI] [PubMed] [Google Scholar]
- Herold, F. , Törpel, A. , Schega, L. , & Müller, N. G. (2019). Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements ‐ a systematic review. European Review of Aging and Physical Activity: Official Journal of the European Group for Research into Elderly and Physical Activity, 16, 10. 10.1186/s11556-019-0217-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holfelder, B. , & Schott, N. (2014). Relationship of fundamental movement skills and physical activity in children and adolescents: A systematic review. Psychology of Sport and Exercise, 15(4), 382–391. 10.1016/j.psychsport.2014.03.005 [DOI] [Google Scholar]
- Howes, O. D. , Rogdaki, M. , Findon, J. L. , Wichers, R. H. , Charman, T. , King, B. H. , Loth, E. , McAlonan, G. M. , McCracken, J. T. , Parr, J. R. , Povey, C. , Santosh, P. , Wallace, S. , Simonoff, E. , & Murphy, D. G. (2018). Autism spectrum disorder: Consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. Journal of Psychopharmacology (Oxford, England), 32(1), 3–29. 10.1177/0269881117741766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara, T. , Drollette, E. S. , Ludyga, S. , Hillman, C. H. , & Kamijo, K. (2020). Baseline cognitive performance moderates the effects of physical activity on executive functions in children. Journal of Clinical Medicine, 9(7), 2071. 10.3390/jcm9072071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Z. , Wang, X.‐F. , Kisiel‐Sajewicz, K. , Yan, J. H. , & Yue, G. H. (2012). Strengthened functional connectivity in the brain during muscle fatigue. NeuroImage, 60(1), 728–737. 10.1016/j.neuroimage.2011.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M. H. (2012). Executive function and developmental disorders: The flip side of the coin. Trends in Cognitive Sciences, 16(9), 454–457. 10.1016/j.tics.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Kao, S.‐C. , Cadenas‐Sanchez, C. , Shigeta, T. T. , Walk, A. M. , Chang, Y.‐K. , Pontifex, M. B. , & Hillman, C. H. (2020). A systematic review of physical activity and cardiorespiratory fitness on P3b. Psychophysiology, 57(7), e13425. 10.1111/psyp.13425 [DOI] [PubMed] [Google Scholar]
- Kaur, M. , Srinivasan, S. M. , & Bhat, A. N. (2018). Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without autism Spectrum disorder (ASD). Research in Developmental Disabilities, 72, 79–95. 10.1016/j.ridd.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny, L. , Cribb, S. J. , & Pellicano, E. (2019). Childhood executive function predicts later autistic features and adaptive behavior in young autistic people: A 12‐year prospective study. Journal of Abnormal Child Psychology, 47(6), 1089–1099. 10.1007/s10802-018-0493-8 [DOI] [PubMed] [Google Scholar]
- Kern, J. K. , Geier, D. A. , Adams, J. B. , Troutman, M. R. , Davis, G. A. , King, P. G. , & Geier, M. R. (2013). Handgrip strength in autism spectrum disorder compared with controls. Journal of Strength and Conditioning Research, 27(8), 2277–2281. 10.1519/JSC.0b013e31827de068 [DOI] [PubMed] [Google Scholar]
- Kim, H.‐Y. (2013). Statistical notes for clinical researchers: Assessing normal distribution (2) using skewness and kurtosis. Restorative Dentistry & Endodontics, 38(1), 52–54. 10.5395/rde.2013.38.1.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. H. , Buzzell, G. , Faja, S. , Choi, Y. B. , Thomas, H. R. , Brito, N. H. , Shuffrey, L. C. , Fifer, W. P. , Morrison, F. D. , Lord, C. , & Fox, N. (2020). Neural dynamics of executive function in cognitively able kindergarteners with autism spectrum disorders as predictors of concurrent academic achievement. Autism: The International Journal of Research and Practice, 24(3), 780–794. 10.1177/1362361319874920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloosterboer, S. M. , de Winter, B. C. M. , Reichart, C. G. , Kouijzer, M. E. J. , de Kroon, M. M. J. , van Daalen, E. , Ester, W. A. , Rieken, R. , Dieleman, G. C. , Altena, D. , Bartelds, B. , Schaik, R. H. N. , Nasserinejad, K. , Hillegers, M. H. J. , Gelder, T. , Dierckx, B. , & Koch, B. C. P. (2021). Risperidone plasma concentrations are associated with side effects and effectiveness in children and adolescents with autism spectrum disorder. British Journal of Clinical Pharmacology, 87(3), 1069–1081. 10.1111/bcp.14465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent, J. S. , Watts, R. , Adise, S. , Allgaier, N. , Chaarani, B. , Garavan, H. , Potter, A. , & Mackey, S. (2020). Associations among body mass index, cortical thickness, and executive function in children. JAMA Pediatrics, 174(2), 170–177. 10.1001/jamapediatrics.2019.4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, R. C. , Vogan, V. M. , Powell, T. L. , Anagnostou, E. , & Taylor, M. J. (2016). The role of executive functions in social impairment in autism Spectrum disorder. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence, 22(3), 336–344. 10.1080/09297049.2015.1005066 [DOI] [PubMed] [Google Scholar]
- Liu, T. , Kelly, J. , Davis, L. , & Zamora, K. (2019). Nutrition, BMI and motor competence in children with autism Spectrum disorder. Medicina (Kaunas, Lithuania), 55(5), 135. 10.3390/medicina55050135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludyga, S. , Gerber, M. , Pühse, U. , Looser, V. N. , & Kamijo, K. (2020). Systematic review and meta‐analysis investigating moderators of long‐term effects of exercise on cognition in healthy individuals. Nature Human Behaviour, 4(6), 603–612. 10.1038/s41562-020-0851-8 [DOI] [PubMed] [Google Scholar]
- Ludyga, S. , Pühse, U. , Gerber, M. , & Kamijo, K. (2021). How children with neurodevelopmental disorders can benefit from the neurocognitive effects of exercise. Neuroscience and Biobehavioral Reviews, 127, 514–519. 10.1016/j.neubiorev.2021.04.039 [DOI] [PubMed] [Google Scholar]
- Maenner, M. J. , Shaw, K. A. , Baio, J. , Washington, A. , Patrick, M. , Di Rienzo, M. , Christensen, D. L. , Wiggins, L. D. , Pettygrove, S. , Andrews, J. G. , Lopez, M. , Hudson, A. , Baroud, T. , Schwenk, Y. , White, T. , Rosenberg, C. R. , Lee, L.‐C. , Harrington, R. A. , Huston, M. , … Dietz, P. M. (2020). Prevalence of autism Spectrum disorder among children aged 8 years ‐ autism and developmental disabilities monitoring network, 11 sites, United States, 2016. Morbidity and Mortality Weekly Report. Surveillance Summaries (Washington, D.C. 2002), 69(4), 1–12. 10.15585/mmwr.ss6904a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi, A. , DeMayo, M. M. , Glozier, N. , & Guastella, A. J. (2017). An overview of autism Spectrum disorder, heterogeneity and treatment options. Neuroscience Bulletin, 33(2), 183–193. 10.1007/s12264-017-0100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy, S. M. , Jakicic, J. M. , & Gibbs, B. B. (2016). Comparison of obesity, physical activity, and sedentary Behaviors between adolescents with autism Spectrum disorders and without. Journal of Autism and Developmental Disorders, 46(7), 2317–2326. 10.1007/s10803-016-2762-0 [DOI] [PubMed] [Google Scholar]
- Miyake, A. , & Friedman, N. P. (2012). The nature and Organization of Individual Differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21(1), 8–14. 10.1177/0963721411429458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora‐Gonzalez, J. , Esteban‐Cornejo, I. , Cadenas‐Sanchez, C. , Migueles, J. H. , Molina‐Garcia, P. , Rodriguez‐Ayllon, M. , Henriksson, P. , Pontifex, M. B. , Catena, A. , & Ortega, F. B. (2019). Physical fitness, physical activity, and the executive function in children with overweight and obesity. The Journal of Pediatrics, 208, 50–56.e1. 10.1016/j.jpeds.2018.12.028 [DOI] [PubMed] [Google Scholar]
- Oberer, N. , Gashaj, V. , & Roebers, C. M. (2018). Executive functions, visual‐motor coordination, physical fitness and academic achievement: Longitudinal relations in typically developing children. Human Movement Science, 58, 69–79. 10.1016/j.humov.2018.01.003 [DOI] [PubMed] [Google Scholar]
- O'Reilly, C. , Lewis, J. D. , & Elsabbagh, M. (2017). Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLoS One, 12(5), e0175870. 10.1371/journal.pone.0175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, C.‐Y. , Chu, C.‐H. , Tsai, C.‐L. , Sung, M.‐C. , Huang, C.‐Y. , & Ma, W.‐Y. (2017). The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism: The International Journal of Research and Practice, 21(2), 190–202. 10.1177/1362361316633562 [DOI] [PubMed] [Google Scholar]
- Pan, C.‐Y. , Tsai, C.‐L. , Chu, C.‐H. , Sung, M.‐C. , Ma, W.‐Y. , & Huang, C.‐Y. (2016). Objectively measured physical activity and health‐related physical fitness in secondary school‐aged male students with autism Spectrum disorders. Physical Therapy, 96(4), 511–520. 10.2522/ptj.20140353 [DOI] [PubMed] [Google Scholar]
- Pellicano, E. , Kenny, L. , Brede, J. , Klaric, E. , Lichwa, H. , & McMillin, R. (2017). Executive function predicts school readiness in autistic and typical preschool children. Cognitive Development, 43, 1–13. 10.1016/j.cogdev.2017.02.003 [DOI] [Google Scholar]
- Pineda‐Alhucema, W. , Aristizabal, E. , Escudero‐Cabarcas, J. , Acosta‐López, J. E. , & Vélez, J. I. (2018). Executive function and theory of mind in children with ADHD: A systematic review. Neuropsychology Review, 28(3), 341–358. 10.1007/s11065-018-9381-9 [DOI] [PubMed] [Google Scholar]
- Pugliese, C. E. , Anthony, L. G. , Strang, J. F. , Dudley, K. , Wallace, G. L. , Naiman, D. Q. , & Kenworthy, L. (2016). Longitudinal examination of adaptive behavior in autism Spectrum disorders: Influence of executive function. Journal of Autism and Developmental Disorders, 46(2), 467–477. 10.1007/s10803-015-2584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa, M. , & Meulenbroek, R. (2012). Effects of physical exercise on autism Spectrum disorders: A meta‐analysis. Research in Autism Spectrum Disorders, 6(1), 46–57. 10.1016/j.rasd.2011.09.001 [DOI] [Google Scholar]
- Tyler, K. , MacDonald, M. , & Menear, K. (2014). Physical activity and physical fitness of school‐aged children and youth with autism spectrum disorders. Autism Research and Treatment, 2014, 312163–312166. 10.1155/2014/312163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo, P. D. (2015). Executive function: Reflection, iterative reprocessing, complexity, and the developing brain. Developmental Review, 38, 55–68. 10.1016/j.dr.2015.07.001 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Missing data in the full cohort and the analyzed cohort.
Table S2. Zero‐order correlations investigating the association between confounders, predictors and outcomes.
Figure S1. Initial model predicting information processing and executive function from group, body mass index and muscle strength. Notes: Standardized regression coefficients are shown above the line. Superscript letters show covariances that were estimated with confounders. * indicates p < 0.05; HP=Healthy peers; ASD=Patients with Autism Spectrum Disorder; SES=Socioeconomic status


