Summary
Plants are able to detect insect eggs deposited on leaves. In Arabidopsis, eggs of the butterfly species Pieris brassicae (common name large white) induce plant defenses and activate the salicylic acid (SA) pathway. We previously discovered that oviposition triggers a systemic acquired resistance (SAR) against the bacterial hemibiotroph pathogen Pseudomonas syringae.
Here, we show that insect eggs or treatment with egg extract (EE) induce SAR against the fungal necrotroph Botrytis cinerea BMM and the oomycete pathogen Hyaloperonospora arabidopsidis Noco2. This response is abolished in ics1, ald1 and fmo1, indicating that the SA pathway and the N‐hydroxypipecolic acid (NHP) pathway are involved.
Establishment of EE‐induced SAR in distal leaves potentially involves tryptophan‐derived metabolites, including camalexin. Indeed, SAR is abolished in the biosynthesis mutants cyp79B2 cyp79B3, cyp71a12 cyp71a13 and pad3‐1, and camalexin is toxic to B. cinerea in vitro.
This study reveals an interesting mechanism by which lepidopteran eggs interfere with plant–pathogen interactions.
Keywords: Botrytis cinerea, herbivore interactions, indolic metabolism, insect eggs, Pieris brassicae, plant, systemic acquired resistance (SAR)
Introduction
Plants resist insect herbivory through toxic secondary metabolites, anti‐digestive proteins, and emission of volatiles that attract parasitoids. These defenses are generally induced after recognition of herbivore attack, and the resulting transcriptional reprogramming is mainly controlled by the jasmonic acid (JA) pathway (Howe & Jander, 2008; Wu & Baldwin, 2010; Erb & Reymond, 2019). In addition, plants have the ability to detect insect oviposition, even though eggs do not represent a direct threat. Several plant species react to oviposition by activating various direct and indirect defenses (Reymond, 2013; Hilker & Fatouros, 2015). For example, hypersensitive response (HR)‐like necrosis, production of reactive oxygen species (ROS), and emission of volatiles that specifically attract egg parasitoids, are efficient ways to inhibit egg survival or development (Hilker et al., 2002; Fatouros et al., 2014; Geuss et al., 2017; Griese et al., 2017, 2021).
In Arabidopsis, oviposition induces immune responses that are observed during infection with biotroph pathogens, including localized cell death, ROS and callose production, and expression of hundreds of defense‐related genes (Little et al., 2007). Strikingly, the egg‐induced transcriptome contains genes regulated by the salicylic acid (SA) signaling pathway (Little et al., 2007; Lortzing, 2020) and oviposition by the butterfly species Pieris brassicae (common name large white) triggers SA accumulation in local and systemic leaves (Bruessow et al., 2010). Also, responses to oviposition or application of crude egg extract (EE) are similar to the recognition of pathogen‐associated molecular patterns (PAMPs) during PAMP‐triggered immunity (PTI) (Gouhier‐Darimont et al., 2013). In support of this finding, phosphatidylcholines (PCs) were recently identified as insect egg‐derived immunogenic patterns that trigger PTI (Stahl et al., 2020). In addition, early signaling responses to eggs depend on the receptor‐like kinase LecRK‐I.8 (Gouhier‐Darimont et al., 2013, 2019).
When challenged with a primary infection, plants induce a systemic acquired resistance (SAR) that protects distal parts against a secondary infection by a broad range of pathogens (Sticher et al., 1997; Vlot et al., 2009). We previously discovered that oviposition by P. brassicae on Arabidopsis induces a SAR against different strains of the bacterial pathogen Pseudomonas syringae (Hilfiker et al., 2014). Strikingly, this response was also observed in neighboring plants (Orlovskis & Reymond, 2020). Systemic acquired resistance requires the SA pathway and primes distal leaves for faster and enhanced activation of defenses (Jung et al., 2009; Conrath, 2011; Návarová et al., 2012; Shah & Zeier, 2013). In addition, the lysine catabolite N‐hydroxypipecolic acid (NHP) is a critical regulator of SAR. N‐hydroxypipecolic acid originates from the α‐transamination of L‐Lys by the aminotransferase AGD2‐LIKE DEFENCE RESPONSE PROTEIN1 (ALD1) that generates 2,3‐dehydropipecolic acid, which is then reduced to pipecolic acid (Pip) (Ding et al., 2016; Hartmann et al., 2017). Pipecolic acid is further converted to NHP by the N‐hydroxylase FLAVIN‐DEPENDENT MONOOXYGENASE1 (FMO1) (Chen et al., 2018; Hartmann et al., 2018). It was found that Pip and NHP accumulate in local and systemic leaves after leaf inoculation with P. syringae pv maculicola (Psm), and exogenous treatment with Pip or NHP enhanced immunity to Psm. Accordingly, ald1 and fmo1 mutants were unable to mount SAR (Návarová et al., 2012; Hartmann et al., 2018). Thus, evidence is accumulating that both the SA and NHP pathways are required for SAR establishment (Hartmann & Zeier, 2019; Zeier, 2021). Interestingly, treatment of Arabidopsis with P. brassicae EE triggers SA and Pip accumulation in local and distal leaves, and EE‐induced SAR was also shown to depend on the SA and NHP pathways, suggesting a common mechanism between pathogen‐ and egg‐induced SAR (Bruessow et al., 2010; Hilfiker et al., 2014).
Tryptophan‐derived, indolic metabolites are important defense molecules in Arabidopsis, and their biosynthesis is activated by a broad spectrum of herbivores and pathogens (Bednarek et al., 2011; Bednarek, 2012; Kettles et al., 2013; Rajniak et al., 2015; Maier et al., 2021). Conversion of tryptophan to indole‐3‐acetaldoxime is catalyzed by two redundant P450 monooxygenases, CYP79B2 and CYP79B3, from which several branches diverge to generate indole glucosinolates (indole GSs), camalexin, indole‐3‐carboxylic acid (ICA), and other small indolic molecules (Zhao et al., 2002; Bednarek, 2012). Besides their known role as anti‐herbivore compounds (Kim et al., 2008), indole GSs are crucial for resistance against microbial, fungal and oomycete pathogens, primarily as toxic molecules but also as signaling molecules (Bednarek et al., 2009; Clay et al., 2009; Katz et al., 2015). The phytoalexin camalexin accumulates in response to infection by P. syringae pv tomato DC3000 (Pst) and the necrotrophic fungus Botrytis cinerea (Tsuji et al., 1992; Kliebenstein et al., 2005), but also in response to oviposition by P. brassicae (Valsamakis et al., 2020). Camalexin is synthesized by the cytochrome P450 enzyme PHYTOALEXIN DEFICIENT 3 (PAD3), and the pad3‐1 mutant is highly susceptible to B. cinerea (Schuhegger et al., 2006; Ferrari et al., 2007). Indole‐3‐carboxylic acid accumulates in Arabidopsis leaves infected with P. syringae, and its presence in cell walls was correlated with enhanced resistance (Forcat et al., 2010; Stahl et al., 2016). Mutants impaired in ICA conjugates are more susceptible to filamentous pathogens (Pastorczyk et al., 2020). In addition, activation of indolic metabolism is not only restricted to the site of infection. In P. syringae‐infected Arabidopsis, the accumulation of ICA, indole‐3‐carbaldehyde and indole‐3‐ylmethylamine could also be observed in uninfected systemic tissue (Stahl et al., 2016).
Following our discovery that oviposition reduces growth of Pst in distal leaves and knowing that SAR is generally effective against a variety of pathogens, we reasoned that egg recognition might trigger a more general defense response. Here, we tested whether egg‐induced SAR is efficient against the necrotrophic fungus B. cinerea (strain BMM). This plant pathogen has a broad host range and causes grey mold disease, one of the most detrimental fungal diseases in crops (Dean et al., 2012). We show here that P. brassicae oviposition and EE treatment inhibit B. cinerea infection in Arabidopsis. Activation of this response is dependent on the SA and NHP pathways and is absent in plants with impaired indolic metabolism. In addition, we found that EE treatment is also efficient against the oomycete Hyaloperonospora arabidopsidis, suggesting that oviposition protects plants against a broad range of pathogens.
Materials and Methods
Plant and insect growth conditions
Arabidopsis thaliana (Col‐0) plants were grown in potting compost for 4 wk in growth chambers in short day (10 h : 14 h , light : dark) conditions, under 100 µmol m−2 s−1 of light, at 20–22°C and 65% relative humidity. For Hyaloperonospora arabidopsidis disease assays, Col‐0 and ald1‐1 plants were grown on potting soil (mix z2254; Primasta BV, Asten, the Netherlands) at 21°C and 75% relative humidity, under short day conditions. Lines used in this study are described in Supporting Information Methods S1. Pieris brassicae was maintained on Brassica oleracea var. gemmifera in a glasshouse (24°C, 65% relative humidity) (Reymond et al., 2000). Spodoptera littoralis eggs were obtained from Syngenta (Stein AG, Switzerland).
Oviposition and treatment with egg extract
For oviposition, 10–15 pots each containing two plants were placed in a 60 × 60 × 60 cm tent containing around 30 P. brassicae butterflies. After 24 h, eight plants containing one egg batch on each of two leaves were placed in a growth chamber for 4 d. Before hatching, eggs were gently removed with forceps, and two distal leaves were infected with Botrytis cinerea. Control plants were kept in the same conditions without butterflies.
For EE preparation, P. brassicae or S. littoralis eggs were crushed with a pestle in Eppendorf tubes. After centrifugation (14 000 g for 3 min), the supernatant (EE) was collected and stored at −20°C. For at least 4–6 plants per experiment, 2 × 2 µl of EE were spotted under the surface of each of two leaves. Plants were treated 5 d before B. cinerea infection. Untreated plants were used as controls.
For PC application, a PC‐mix (purified from chicken egg, 840051; Avanti Polar Lipids, Alabaster, AL, USA) was solved in 1% dimethylsulfoxide (DMSO), 0.5% Glycerol and 0.1% Tween 20 by sonication. On 4–6 plants, 2 × 2 µl of PC (5 µg µl–1) were spotted under the surface of each of two leaves. This concentration is found in P. brassicae eggs (Stahl et al., 2020). Control plants were treated with 1% DMSO, 0.5% Glycerol and 0.1% Tween 20.
Intra‐ and interplant SAR experiments were performed according to a previously published protocol (Orlovskis & Reymond, 2020).
Culture of Botrytis cinerea, infection and growth assessment
Botrytis cinerea strain BMM (Zimmerli et al., 2001) was grown on 1× potato dextrose agar (PDA, 39 g l−1; Difco, Chemie Brunschwig AG, Basel, Switzerland) for 10–14 d in darkness at 20–25°C. Spores were harvested in water and filtered through wool to remove hyphae. Spores were diluted in half‐strength potato dextrose broth (PDB, 12 g l−1; Difco) to 5 × 105 spores ml−1 for inoculation. One 5 µl droplet of spore suspension was deposited on the adaxial surface of two leaves per plant. To facilitate spore germination, inoculated plants were kept under a water‐sprayed transparent lid to maintain high humidity in a growth chamber under dim light (around 2 µmol m−2 s−1) during the whole period of infection. After 3 d, lesion size measurements were made using ImageJ software v.2.0.0‐rc‐65/1.51u (http://imagej.nih.gov/ij).
To visualize B. cinerea structures, inoculated leaves were stained with lactophenol‐trypan blue for 2 h at 37°C, cleared in boiling 95% EtOH and stored in 70% EtOH. Observations of B. cinerea hyphae were made using a Leica MZ16A stereomicroscope fitted with a DFC310FX camera (Leica Microsystems, Heerbrugg, Switzerland). Images were analyzed with ImageJ.
To quantify B. cinerea growth, relative expression of Bc Tubulin was measured by quantitative polymerase chain reaction (qPCR; for details, see Methods S2).
Determination of antifungal activity
Camalexin (Glixx Laboratories, Hopkinton, MA, USA) and ICA (Sigma‐Aldrich) were dissolved in DMSO. Plugs (diameter 0.5 cm) were taken from a 7‐d‐old B. cinerea culture on 1× PDA and transferred to six‐well plates supplemented with different concentrations of camalexin and ICA in PDA. Control plates contained 0.1% DMSO. For each treatment, radial growth of the fungal colony was measured on two plates (n = 12) after 24 h of incubation at 23°C in darkness. Mycelial growth inhibition (MGI) was calculated using the following formula: MGI % = ((C–T)/C) × 100, where C is the average colony diameter on control plates, and T is the average colony diameter on treated plates.
Infection with Hyaloperonospora arabidopsidis and Pseudomonas syringae
Infection assays were performed with H. arabidopsidis isolate Noco2 (100 spores μl–1). The pathogen was maintained on Arabidopsis Col‐0 and transferred weekly to fresh 10‐d old seedlings. Spores were collected from a Ws‐eds1 mutant to achieve the high level of inoculum used. Two leaves of each tested plant were treated with 2 × 2 µl of EE 1 d before pathogen challenge. Untreated plants were used as controls. The H. arabidopsidis spore suspension was applied with a spray gun. Plants were subsequently left to dry to the air for c. 30 min and incubated at 100% humidity at 16°C. Eight days post inoculation, disease severity was determined visually. For spore counts, four systemic leaves from 4–5 EE‐treated or control plants were weighed and suspended in 2 ml of water after which the number of spores mg–1of plant tissue was determined.
Infection with Pseudomonas syringae pv tomato DC3000 has been described previously (Hilfiker et al., 2014).
Exogenous applications of pipecolic acid
One day before B. cinerea infection, 10 ml of a 1 mM D,L‐Pip (Sigma‐Aldrich) solution was pipetted onto each pot containing one plant. Control plants were supplemented with 10 ml of water.
Salicylic acid quantification and infiltration
Total SA was measured using the bacterial biosensor Acinetobacter sp. ADPWH_lux. (Huang et al., 2005, 2006), as described previously (Stahl et al., 2020). For each sample, six leaf discs of 0.7 cm diameter from three plants were pooled and analyzed. For SA infiltration, plant genotypes were infiltrated with 0.25 or 0.5 mM solutions on the abaxial side of two leaves per plant with a 1 ml needleless syringe. H2O was infiltrated as control. After 4 h, plants were harvested for SA quantification or further infected with B. cinerea for 3 d before lesion measurement. For the reporter line PR1::GUS, half of each leaf was infiltrated with 0.5 mM SA, and beta‐glucuronidase (GUS) analysis was performed after 4 h (Bruessow et al., 2010).
Metabolite analyses
For each sample, between 10 and 12 leaves (two leaves per plant) were harvested per time point and per treatment. Leaves were then pooled, frozen and ground with a pestle and mortar in liquid nitrogen. Next, 100 mg of frozen leaf powder was placed in a 1.5 ml Eppendorf tube, and 500 µl of extraction buffer (80% methanol, 19.5% water and 0.5% formic acid) was added. After centrifugation (3 min at 14 000 g ), 200 µl aliquots were transferred into vials. Camalexin content was measured using ultra‐high‐performance liquid chromatography coupled to tandem mass spectrometry (UHPLC‐MS/MS) (Balmer et al., 2018) and indolic metabolites by quadrupole time‐of‐flight mass spectrometry (UHPLC‐QTOFMS) (Böttcher et al., 2014). Indole‐3‐carboxylic acid conjugates were quantified as ICA equivalents. The protocol for GS analysis has been described previously (Glauser et al., 2012; for details, see Methods S3).
Insect performance assays
Plants were sprayed with either half‐strength PDB or B. cinerea spore suspension at a concentration of 5 × 105 spores ml−1. After 48 h, five freshly hatched P. brassicae larvae were placed on each of 11 pots, each containing two plants, in a transparent plastic box and kept in a growth chamber during the experiment. Plants were replaced every 3 d by a new set of inoculated plants in order to keep a constant amount of material for feeding larvae. After 6 d of feeding, larvae were weighed on a precision balance (Mettler‐Toledo, Greifensee, Switzerland) and placed back on the plants until a final weight measurement after 12 d.
Statistical analyses
Data were analyzed using R software v.3.5.2 (http://www.R‐project.org). Normal distribution and variance homogeneity of data were evaluated with the Shapiro–Wilk test and Levene’s test, respectively. If not normal, data were log‐transformed to enable analyses with parametric tests.
To compare CTL and EE within the same genotype in SAR bioassays, we used a linear mixed model fit by the restricted maximum likelihood (REML) algorithm (package lme4 in R) using plant treatment as a fixed factor and experimental block as a random factor. For multiple comparisons between genotypes, data were analyzed using a linear mixed model with a post‐hoc general linear hypothesis test with Tukey contrasts, using plant treatment as a fixed factor and experimental block as a random factor. In both analyses, each block included data from each independent replicate consisting of 8–30 leaves from different plants and from different pots. For feeding bioassays, we used Welch’s t‐test. For metabolite quantifications, we used ANOVA with the Tukey test for post‐hoc comparison. Information on sample sizes and summary statistics can be found in Table S1.
Results
Oviposition by Pieris brassicae reduces Botrytis cinerea infection
Pieris brassicae butterflies were allowed to oviposit on Arabidopsis plants, and 4 to 5 d later eggs were gently removed, just before the hatching of larvae. Two distal leaves were then infected with B. cinerea BMM by drop inoculation, and the lesion size was measured after 3 d (Fig. 1a). Compared to control plants, oviposited plants showed a significantly reduced infection (Fig. 1b). As a complementary experiment, plants were pretreated with P. brassicae EE. The amount of EE applied onto each plant was equivalent to two egg batches (20–30 eggs per batch). A similar reduction of B. cinerea infection was observed on EE‐treated plants compared to control plants (Fig. 1c). This result confirms previous observations that EE treatment mimics responses triggered by natural oviposition (Little et al., 2007; Bruessow et al., 2010; Gouhier‐Darimont et al., 2019; Orlovskis & Reymond, 2020; Stahl et al., 2020).
Fig. 1.
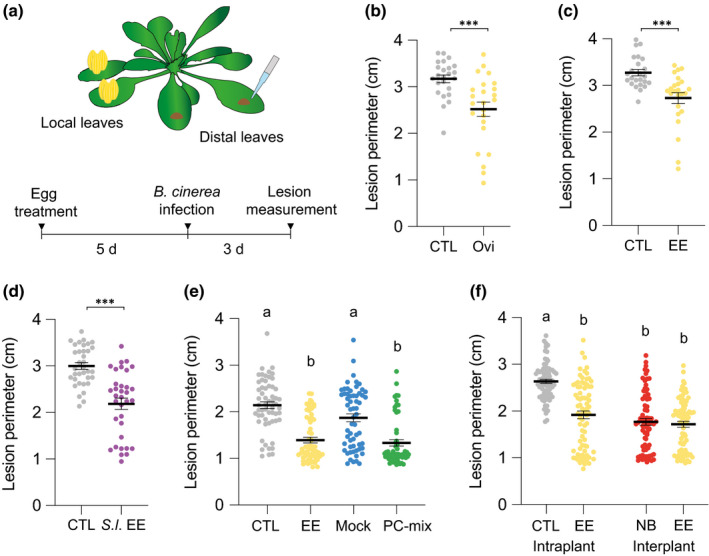
Oviposition and treatment with egg extract (EE) reduce Botrytis cinerea BMM infection. (a) Experimental design. (b–d) Effect of 5 d‐pretreatment with Pieris brassicae oviposition (Ovi) (b), P. brassicae EE (c), or Spodoptera littoralis EE (d) on B. cinerea growth. Lesion perimeter in distal leaves was measured 3 d after inoculation. Inoculated plants without pretreatment were used as controls (CTL). (e) Plants were pretreated with either P. brassicae EE or a 5 µg µl−1 solution of phosphatidylcholine (PC)‐mix from chicken egg. Respective controls consisted of CTL plants or plants treated with a mock solution (Mock). (f) Egg extract pretreatment reduced B. cinerea growth in distal leaves when compared to CTL plants grown separately (intraplant systemic acquired resistance (SAR)). No difference in B. cinerea growth was observed when EE‐treated and untreated neighbor plants (NB) were in the same pot (interplant SAR). Means ± SE of three independent experiments are shown (n = 8–30 per experiment). Significant differences between control and treated plants are indicated (linear mixed model; ***, P < 0.001). Lowercase letters indicate significant difference at P < 0.05 (linear mixed model and post‐hoc general linear hypothesis test with Tukey contrasts). Dots indicate individual values.
Consistent with observations of lesion size, hyphal development was also significantly reduced in distal leaves (Fig. S1). In addition, expression of B. cinerea β‐tubulin gene was significantly lower in plants pretreated with EE (Fig. S1), providing independent confirmation that EE pretreatment inhibits B. cinerea growth. Then, a time‐course experiment indicated that inhibition of B. cinerea infection can also be observed in local leaves pretreated with EE from 2 to 3 d after infection, and that this protection reaches distal leaves only after 3 d (Fig. S2).
To explore the generality of egg‐derived inhibition of B. cinerea infection, we treated plants with EE from the generalist herbivore Spodoptera littoralis. Like with P. brassicae EE, pretreatment with S. littoralis EE significantly reduced fungal infection (Fig. 1d). Pretreatment with a solution of phosphatidylcholines (PC‐mix), known to contain bioactive PCs found in P. brassicae eggs (Stahl et al., 2020), inhibited B. cinerea infection to the same extent as EE, indicating that egg‐induced SAR is triggered following perception of an egg‐associated molecular pattern (Fig. 1e).
We previously found that egg‐induced SAR against P. syringae extends to neighboring plants through an as‐yet unknown root‐mediated signal (Orlovskis & Reymond, 2020). Strikingly, inhibition of B. cinerea growth after EE pretreatment was also observed in untreated plants that were grown in the same pot (Fig. 1f). Thus, EE pretreatment of focal plants activates resistance in neighbors against different pathogens. Future investigations might reveal whether the same signal is used for both responses and whether this phenomenon operates between different species.
Egg extract‐induced systemic acquired resistance against Botrytis cinerea requires salicylic acid and N‐hydroxypipecolic acid signaling
Signaling of Arabidopsis responses to P. brassicae oviposition involves early LecRK‐I.8 activity followed by triggering of the SA pathway (Gouhier‐Darimont et al., 2013, 2019; Stahl et al., 2020). In the lecrk‐I.8 T‐DNA knockout mutant, infection by B. cinerea was similar to that observed in Col‐0. However, EE pretreatment did not trigger SAR in the mutant, indicating that LecRK‐I.8 contributes to the triggering of SAR but not to basal resistance against this fungus (Fig. 2a).
Fig. 2.
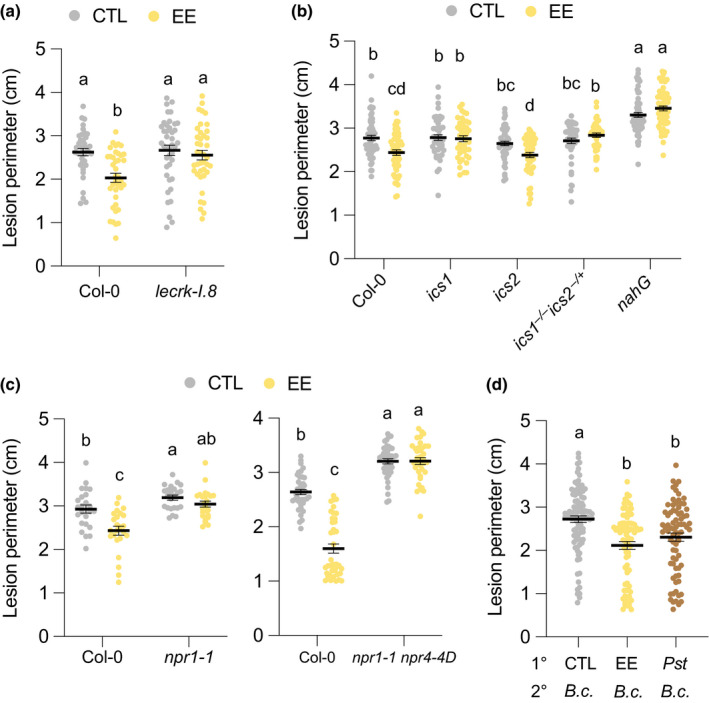
Egg extract‐induced systemic acquired resistance depends on the salicylic acid pathway. (a–c) Plant genotypes were pretreated with Pieris brassicae egg extract (EE) for 5 d and further infected with Botrytis cinerea BMM for 3 d. Lesion perimeter was measured in control (CTL) and distal leaves from EE‐treated plants (EE). The double mutant ics1 ics2 was homozygous for ics1 (−/−) and heterozygous for ics2 (−/+). (d) Local leaves (1°) were untreated (CTL), treated with EE for 5 d (EE) or infiltrated with Pseudomonas syringae pv tomato DC3000 (Pst) for 2 d. Distal leaves (2°) were then inoculated with B. cinerea spore suspension (B.c.) for 3 d before lesion perimeter measurement. Means ± SE of three independent experiments are shown (n = 8–28 per experiment). Lowercase letters indicate significant difference at P < 0.05 (linear mixed model and post‐hoc general linear hypothesis test with Tukey contrasts). Dots indicate individual values.
Salicylic acid biosynthesis requires primarily the activity of ISOCHORISMATE SYNTHASE 1(ICS1), with a limited contribution of its homolog ICS2 (Garcion et al., 2008). Egg extract‐induced SAR was absent in ics1 (sid2‐1 allele (Nawrath & Métraux, 1999)) and in the semi‐homozygous ics1 −/− ics2 −/+, whereas it was conserved in ics2, demonstrating the crucial role of ICS1 in this response (Fig. 2b). Also, SAR was lost in the SA‐degrading transgenic line nahG (Fig. 2b). In line with the SAR phenotype, EE treatment induced strong SA accumulation in Col‐0 and ics2, while levels were undetectable in ics1, ics1 −/− ics2 −/+, and nahG (Fig. S3). Although we could not test a fully homozygous ics1 ics2 double mutant, which shows severely impaired growth (Garcion et al., 2008), our data indicate that ICS1‐dependent SA accumulation is required for the systemic induction of defense by EE treatment, with no apparent contribution of ICS2.
In PTI signaling, NON EXPRESSOR OF PR GENES1 (NPR1) and NPR3/NPR4 are important downstream modulators of defense gene expression (Zhou & Zhang, 2020). They all bind SA, but NPR1 acts as a positive activator of transcription, whereas NPR3 and NPR4 are repressors (Zhou & Zhang, 2020). We previously found that EE‐induced PR1 expression was significantly reduced in npr1‐1 (Gouhier‐Darimont et al., 2013). Here, EE treatment did not not significantly reduce B. cinerea growth in npr1‐1, although there was a trend for a weak response in the mutant (Fig. 2c). A residual signaling activity in npr1‐1 is postulated to be due to the inhibition of NPR3/NPR4 repressor activity by SA (Liu et al., 2020). Indeed, using the npr1‐1 npr4‐4D double mutant, which includes the gain‐of‐function mutant npr 4‐4D and is blocked in SA signaling (Liu et al., 2020), we could not detect any SAR (Fig. 2c). The double mutant and npr1‐1 were also significantly more susceptible to B. cinerea in the absence of EE pretreatment. Thus, these findings demonstrate a contribution of SA signalling to basal resistance and EE‐induced SAR against B. cinerea.
Collectively, our data suggest that activation of PTI signaling by insect eggs generates SAR against B. cinerea BMM. To test whether this is similar to a bacterial‐induced SAR, we infiltrated primary leaves with Pst for 2 d before infecting distal leaves with B. cinerea. Strikingly, EE or Pst pretreatment led to a similar inhibition of fungal growth compared to untreated controls (Fig. 2d).
The NHP pathway is central for bacterial‐induced SAR and is required for the establishment of primed defenses in systemic leaves (Hartmann & Zeier, 2019; Zeier, 2021). We have previously shown that this pathway is also necessary for egg‐induced SAR against bacterial pathogens (Hilfiker et al., 2014). Here, we used the Pip biosynthesis mutant ald1 and the fmo1 mutant impaired in Pip conversion to NHP. Upon secondary B. cinerea infection, both mutants showed a lack of EE‐induced SAR, indicating that the NHP pathway is required for systemic inhibition of fungal growth (Fig. 3a). To further assess the role of Pip in EE‐induced SAR, we exogenously applied 1 mM Pip solution to the soil 1 d before B. cinerea inoculation. In Col‐0, Pip application alone did not inhibit B. cinerea growth and did not enhance EE‐induced SAR (Fig. 3b). This suggests that Pip is not sufficient to activate SAR in the absence of an EE‐derived stimulus. However, Pip application to ald1 was able to restore SAR after EE treatment, indicating that Pip can complement the biosynthesis mutant and acts downstream of an EE stimulus. Finally, Pip application had no effect on fmo1, which did not display EE‐induced SAR in any condition, confirming the need for hydroxylation of Pip by FMO1 to generate the active SAR signal NHP (Fig. 3b).
Fig. 3.
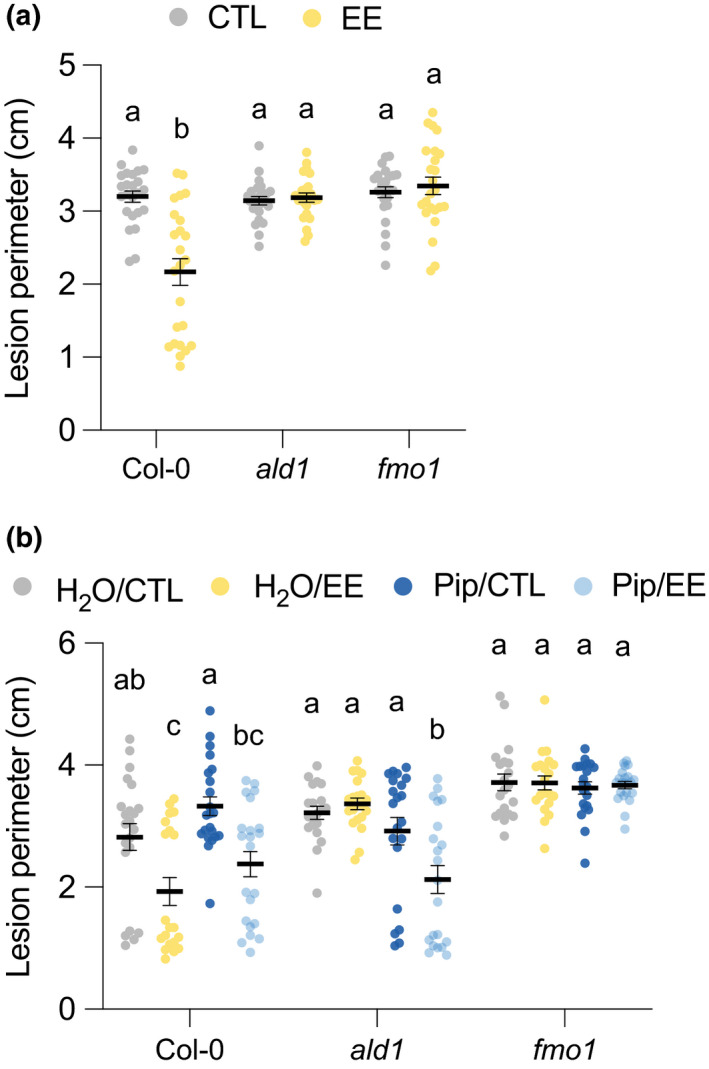
Egg extract (EE)‐induced systemic acquired resistance depends on the N‐hydroxy‐pipecolic acid pathway. (a) Plant genotypes were pretreated with Pieris brassicae EE for 5 d and further infected with Botrytis cinerea BMM for 3 d. Lesion perimeter was measured in control (CTL) and distal leaves from EE‐treated plants (EE). Means ± SE of three independent experiments are shown (n = 8 per experiment). Lowercase letters indicate significant difference at P < 0.05 (linear mixed model and post‐hoc general linear hypothesis test with Tukey contrasts). (b) Plant genotypes were pretreated with P. brassicae EE for 5 d and further infected with B. cinerea. H2O or 1 mM pipecolic acid (Pip) was applied to the soil 1 d before infection, and lesion perimeter measurements were recorded 3 d after infection. Means ± SE of three independent experiments are shown (n = 6–8 per experiment). For each genotype, different lowercase letters indicate significant differences at P < 0.05 (ANOVA followed by Tukey’s Honest Significant Difference test). Dots indicate individual values.
We next reasoned that the combined accumulation of SA and NHP may be sufficient to generate SAR. We first established that leaf SA infiltration was able to induce PR1 expression using a PR1::GUS reporter plant (Fig. S4a) and that infiltration of 0.25 mM SA in Col‐0 yielded the same amounts that accumulate after 5 d of EE treatment (Fig. S4b). However, treatment with SA alone or in combination with Pip was not able to inhibit B. cinerea growth in Col‐0, ald1 or fmo1, suggesting that induction of SAR by EE requires additional components.
Camalexin is involved in egg extract‐induced systemic acquired resistance
Given the reported role of indolic metabolism in plant immunity, we asked whether any indolic biosynthetic branch is responsible for the inhibition of B. cinerea infection. The cyp79b2 cyp79b3 double mutant is blocked in tryptophan conversion to indole‐3‐acetaldoxime (IAOx), a central molecule from which several indolics derive, including indole GSs, 4‐hydroxyindole‐3‐carbonyl nitrile (4‐OH‐ICN), camalexin, and ICA (Böttcher et al., 2014; Rajniak et al., 2015) (Fig. 4a). Strikingly, EE‐induced SAR was abolished in cyp79b2 cyp79b3 (Fig. 4b). In addition, the mutant was significantly more susceptible to B. cinerea infection in the absence of EE pretreatment, indicating that tryptophan‐derived compounds are important for both basal resistance and SAR.
Fig. 4.
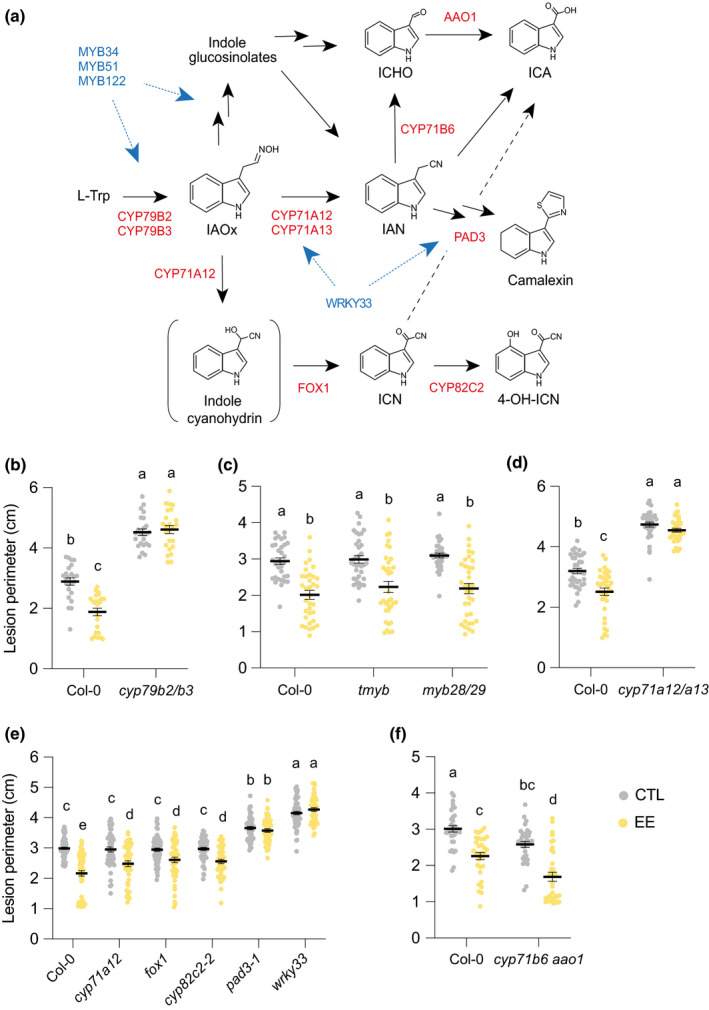
Establishment of egg extract (EE)‐induced systemic acquired resistance requires tryptophan‐derived compounds. (a) Simplified scheme of biosynthesis of tryptophan derivatives and position of biosynthesis (red) and regulatory (blue) genes tested in this study. Brackets indicate an unstable intermediate. Several arrows indicate multiple steps. 4‐OH‐ICN, 4‐hydroxy‐ICN; IAN, indole‐3‐acetonitrile; IAOx, indole‐3‐acetaldoxime; ICA, indole‐3‐carboxylic acid; ICHO, indole‐3‐carbaldehyde; ICN, indole carbonyl nitrile; L‐Trp, tryptophan. (b–f) Plant genotypes were pretreated with Pieris brassicae EE for 5 d and further infected with Botrytis cinerea BMM for 3 d. Lesion perimeter was measured in control (CTL) and distal leaves from EE‐treated plants (EE). Means ± SE of three independent experiments are shown (n = 8–21 per experiment). Lowercase letters indicate significant difference at P < 0.05 (linear mixed model and post‐hoc general linear hypothesis test with Tukey contrasts). Dots indicate individual values. The ‘tmyb’ label on the x‐axis in part (c) represents myb34 myb51 myb122.
Indole GSs contribute to Arabidopsis immunity against bacterial and fungal pathogens, including B. cinerea (Bednarek et al., 2009; Clay et al., 2009; Xu et al., 2016). MYB34, MYB51, and MYB122 transcription factors regulate the biosynthesis of indole GSs, but also of camalexin and other Trp‐derived metabolites (Frerigmann & Gigolashvili, 2014; Frerigmann et al., 2016). The myb34 myb51 myb122 (tmyb) triple mutant lacks indole GSs, but not aliphatic GSs (Frerigmann & Gigolashvili, 2014). However, EE‐induced inhibition of B. cinerea growth was similar in Col‐0 and in the indole GS mutant (Fig. 4c). In addition, a myb28 myb29 double mutant lacking aliphatic GSs (Beekwilder et al., 2008) displayed EE‐induced SAR, suggesting that neither GS type is involved in the inhibition of B. cinerea BMM growth (Fig. 4c). Next, whole‐leaf concentrations of both GS classes were not significantly different between treated and control plants over a time‐course of 12 h to 48 h after inoculation, indicating that neither EE nor B. cinerea induced GS accumulation (Fig. S5a,c; Table S2). In addition, we confirmed that tmyb lacks indole GSs, even after EE or B. cinerea treatment, but has wild‐type levels of aliphatic GSs (Fig. S5b,d; Table S3).
In leaves, oxidation of IAOx by CYP71A12 and CYP71A13 generates IAN, which is the common precursor of 4‐OH‐ICN, ICA, and camalexin (Fig. 4a). Egg extract‐induced SAR was abolished in the cyp71a12 cyp71a13 double mutant, suggesting the involvement of one or several of these metabolites (Fig. 4d). To assess the specific role of 4‐OH‐ICN, which exhibits antimicrobial activity (Rajniak et al., 2015), we used mutants in three consecutive biosynthetic steps. We observed a significant EE‐induced SAR in cyp71a12, fox1, and cyp82c2‐2, thus eliminating 4‐OH‐ICN as a player in EE‐induced SAR against B. cinerea BMM (Fig. 4e).
Several pathways can lead to ICA formation, from degradation of indole GSs, conversion of IAN, and hydrolysis of ICN (Fig. 4a). Some steps involve the activity of CYP71B6 and/or AAO1. Egg extract‐induced SAR was similar in Col‐0 and the cyp71b6 aao1 double mutant (Fig. 4f). However, ICA analysis revealed that this metabolite accumulated strongly after B. cinerea infection, with or without EE pretreatment, and that concentrations were similar in Col‐0 and cyp71b6 aao1 (Fig. S6). Also, ICA conjugates were induced by EE treatment but not by B. cinerea, though the concentrations were similar between Col‐0 and cyp71b6 aao1 (Fig. S7). This finding suggests another route for ICA accumulation during B. cinerea infection or EE treatment, similar to what was recently reported for the necrotroph Plectosphaerella cucumerina (Pastorczyk et al., 2020). However, ICA and ICA conjugate accumulation was also unaffected in cyp71a12 cyp71a13, although this mutant displayed no EE‐induced SAR (Fig. S6 and S7). This finding indicates that IAN is not a precursor for ICAs in these conditions and that the impaired SAR in this mutant cannot be attributed to a lack of ICAs.
Finally, SAR was abolished in pad3‐1, a mutant of CYP71B15 that catalyzes the last step in camalexin biosynthesis (Schuhegger et al., 2006) and forms a core metabolon with CYP71A12/A13 and CYP79B2 (Mucha et al., 2019). Consistently, SAR was also absent in wrky33, a mutant of the transcription factor WRKY33 that regulates the expression of camalexin biosynthesis genes (Birkenbihl et al., 2012; Liu et al., 2015; Zhou et al., 2020) (Fig. 4e). Also, both mutants displayed significantly enhanced basal susceptibility to B. cinerea compared to Col‐0. Thus, these data point to camalexin as a key component of EE‐induced SAR against B. cinerea BMM.
Camalexin accumulation
The finding that EE‐induced SAR was lost in mutants impaired in camalexin biosynthesis led us to quantify this metabolite in response to EE treatment and/or B. cinerea infection. First, EE treatment triggered a strong accumulation of camalexin, but this was only observed in local leaves (Fig. S8). This finding could explain the reduced B. cinerea growth in local leaves after EE treatment (Fig. S2). Then, B. cinerea infection produced a significant increase in camalexin from 12 h to 48 h after inoculation; however, this increase was similar when local leaves were pretreated with EE (Fig. 5a,b). In addition, the camalexin accumulation found in both ald1 and ics1 was similar to that observed in Col‐0, irrespective of EE pretreatment (Fig. 5a,b). These data suggest that the SA and NHP pathways are crucial to generate the SAR signal but not for B. cinerea‐induced camalexin production. Further analysis of camalexin concentrations every 3 h between 12 and 24 h after B. cinerea infection did not reveal an earlier or enhanced accumulation of this metabolite.
Fig. 5.
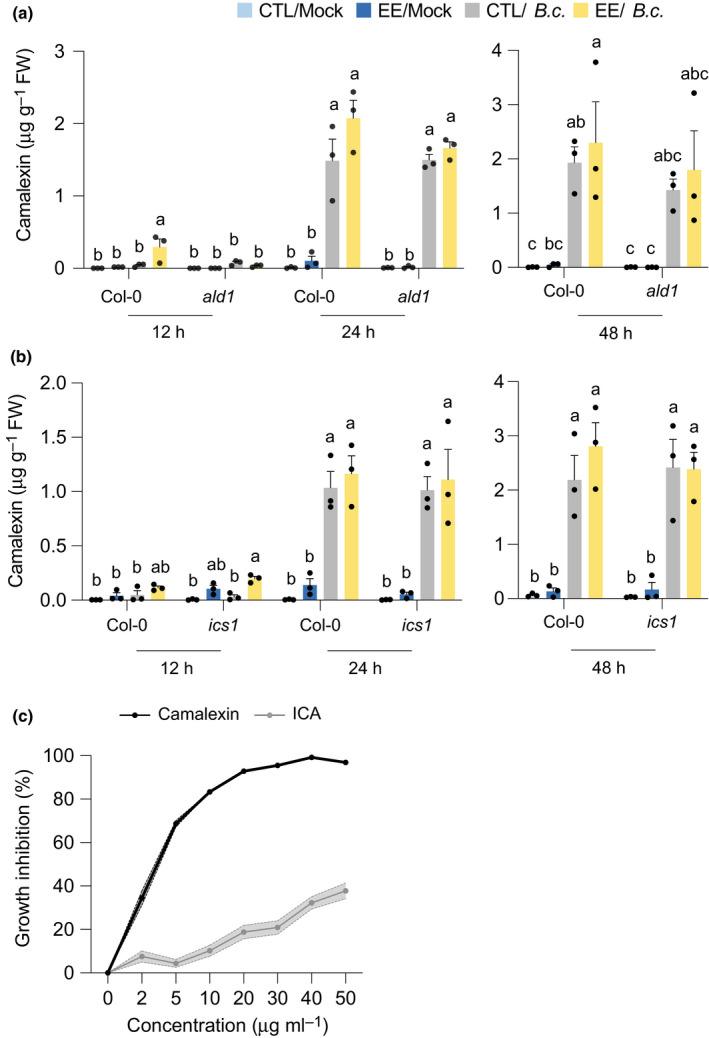
Camalexin accumulation after egg extract and Botrytis cinerea BMM treatment. Plant genotypes were pretreated with Pieris brassicae egg extract (EE) for 5 d, and distal leaves were further treated with a mock solution or infected with B. cinerea. Untreated plants were used as controls (CTL). (a,b) Camalexin concentrations were measured in distal leaves. Means ± SE of three independent experiments are shown (n = 10–12 per experiment). For each time point, different letters indicate significant differences at P < 0.05 (ANOVA followed by Tukey’s Honest Significant Difference test). (c) In vitro growth inhibition assay. Radial growth of a B. cinerea colony growing on potato dextrose agar plates supplemented with different concentrations of camalexin or indole‐3‐carboxylic acid (ICA) was measured after 24 h of incubation. Solid line, mean; shaded band, SE (n = 12). This experiment was repeated twice with similar results.
Camalexin secretion to the leaf surface is crucial for defense against B. cinerea in Arabidopsis (Khare et al., 2017; He et al., 2019). To test if EE pretreatment might prime camalexin secretion after B. cinerea infection, we incubated infected leaves for 30 s in 80% MeOH and measured camalexin concentrations in the solution. However, surface camalexin concentrations were similar in CTL or EE‐treated Col‐0 plants from 12 to 24 h after infection, and the same was true for ald1 plants (Fig. S9).
Camalexin has known antifungal properties against B. cinerea (Ferrari et al., 2003; Kliebenstein et al., 2005). In addition, ICA is toxic to diverse plant fungal pathogens in vitro (Kavitha et al., 2010; Pedras & Hossain, 2011). To test the antifungal role of these indolics against the strain used in this study, we monitored B. cinerea BMM growth in vitro on plates supplemented with increasing concentrations. Camalexin showed a steep dose‐dependent effect on fungal growth, reaching > 90% of inhibition between 20 and 50 µg ml−1. This inhibition was similar to that observed previously for sensitive B. cinerea strains DGUS‐1 and GLUK‐1 (Kliebenstein et al., 2005). Interestingly, ICA also inhibited B. cinerea growth, although with a weaker activity (37% inhibition at 50 µg ml−1) (Fig. 5c).
To ensure that the conserved EE‐induced SAR that was observed in different indolic mutants was not due to a compensatory overaccumulation of camalexin, we quantified this compound in response to EE treatment and/or B. cinerea infection. However, camalexin concentrations were not different from Col‐0 in cyp71a12 or cyp71b6 aao1. On the contrary, camalexin was undetectable in cyp71a12 cyp71a13 and pad3‐1, which is consistent with their impaired EE‐induced SAR (Fig. S10). In addition, B. cinerea infection triggered similar ICA accumulation in Col‐0 and pad3‐1, strongly suggesting that the absence of EE‐induced SAR in this mutant is specifically due to the lack of camalexin (Fig. S6).
Besides their role in regulating indole GS biosynthesis, MYB34, MYB51, and MYB122 have also been shown to differentially regulate the accumulation of camalexin and ICA in response to UV, flagellin or P. cucumerina treatments (Frerigmann et al., 2015, 2016). However, both indolic metabolites accumulated similarly in Col‐0 and tmyb after B. cinerea infection, indicating that the contribution of these MYBs to different branches of the Trp pathway depends on the (a)biotic conditions considered (Figs S6, S10).
Reduced performance of Pieris brassicae larvae on Botrytis cinerea‐infected plants
Whole‐plant reduction of B. cinerea infection by insect egg pretreatment may benefit hatching larvae. To test the effect of B. cinerea on P. brassicae larvae, we measured insect performance on infected plants. After 12 d, larvae were significantly smaller when feeding on infected plants compared to plants sprayed with PDB only (Fig. 6).
Fig. 6.
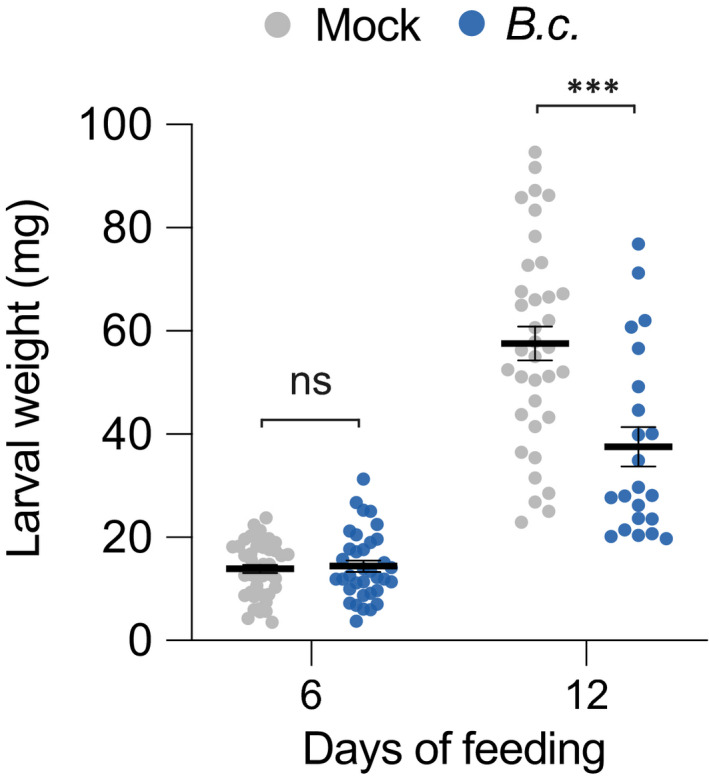
Pieris brassicae larval development is inhibited in Botrytis cinerea BMM‐infected plants. Plants were sprayed with a suspension of B. cinerea spores (B.c.) or mock solution (control; CTL). Freshly hatched P. brassicae were then placed on plants for a total of 12 d. Newly infected plants were placed every 3 d, in order to have sufficient material for the larvae to feed on. Larval weight was recorded after 6 and 12 d. Means ± SE are shown (n = 22–43). Significant differences between control and infected plants are indicated (Welch’s two sample t‐test; ***, P < 0.001; ns, not significant). This experiment was repeated twice with similar results. Dots indicate individual values.
Egg extract‐induced systemic acquired resistance against Hyaloperonospora arabidopsidis
To test whether EE‐induced SAR can target other plant pathogens, we monitored infection of the oomycete Hyaloperonospora arabidopsidis Noco2 (Hpa), which is an obligate biotroph that causes downy mildew on Arabidopsis (Coates & Beynon, 2010). Egg extract pretreatment strongly enhanced resistance against Hpa. Remarkably, < 10% of systemic leaves from EE‐treated plants showed symptoms of infection, whereas > 90% of control plants were infected. By contrast, ald1 plants were fully infected in the presence or absence of EE pretreatment (Fig. 7a). Similarly, the spore number on systemic leaves of EE‐treated plants was drastically reduced in Col‐0, whereas this effect was much less pronounced in ald1 (Fig. 7b). These results illustrate a wide‐ranging protective effect of EE treatment and the important role of the NHP pathway in this response. This finding also supports the observation that exogenous application of Pip or NHP confers resistance to Hpa in Arabidopsis (Hartmann et al., 2018).
Fig. 7.
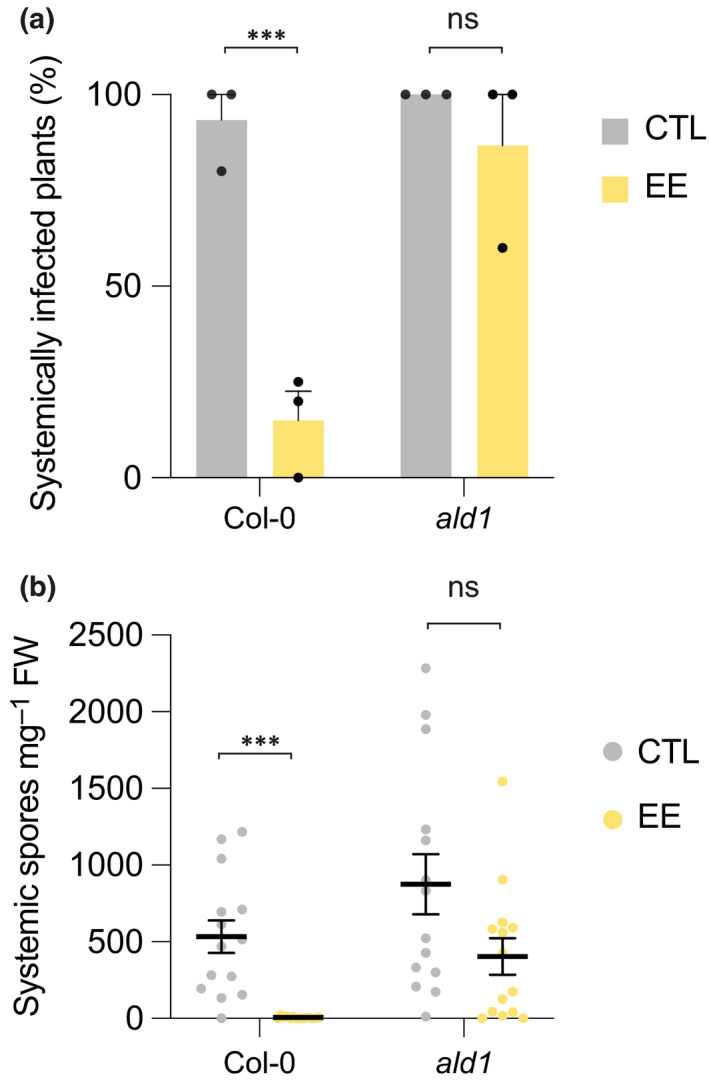
Egg extract‐induced systemic acquired resistance reduces Hyaloperonospora arabidopsidis Noco2 infection. Effect of 1 d‐pretreatment with Pieris brassicae egg extract (EE) on H. arabidopsidis infection in distal leaves was measured 8 d after inoculation. Inoculated plants without pretreatment were used as controls. Percentage of systemically infected plants (a) or number of spores on systemic leaves (b) were quantitated. Mean values ± SE of three independent experiments are shown (n = 4–5 per experiment). Significant differences between control and treated plants are indicated ((a) Pearson's chi‐squared test; (b) Welch’s two sample t‐test; ***, P < 0.001; ns, not significant).
Discussion
Our data show that activation of the SA pathway in response to oviposition leads to a systemic enhanced protection against a necrotrophic fungus. This finding is somewhat surprising given that plant resistance to necrotrophs is generally known to require JA/ethylene (ET) pathways (Pieterse et al., 2012). There are, however, reports pointing to a contribution of SA signaling in defense against B. cinerea. Exogenous SA application decreased B. cinerea lesion size, and the ein2‐1 npr1‐1 double mutant was more susceptible than the single ET mutant ein2‐1 (Ferrari et al., 2003). Enhanced basal susceptibility of ics1 (sid2‐1) and npr1‐1 to B. cinerea was reported (Nie et al., 2017). Also, phenotypic and transcriptomic analyses of Arabidopsis plants infected with B. cinerea isolates support a more intricate role of the JA and SA pathways in resistance (Zhang et al., 2017). There is also growing evidence that the trophic lifestyle of B. cinerea is more plastic than previously thought (van Kan et al., 2014; Veloso & van Kan, 2018), and this may explain why the SA pathway may contribute in part to defense against this fungus. In line with these findings, we show here that nahG, npr1‐1 and npr1‐1 npr4‐4D, which are SA signaling null mutants, have elevated basal susceptibility to B. cinerea BMM.
Interestingly, oviposition was also shown to enhance defenses against chewing larvae, a resistance that normally requires the JA pathway (Lortzing et al., 2019, 2020; Valsamakis et al., 2020). In addition, P. brassicae eggs trigger the accumulation of JA and JA‐Ile in Arabidopsis (Valsamakis et al., 2020). The reported specificity and antagonism of the SA/JA pathways (Pieterse et al., 2012) may thus not be as strict as anticipated and may depend on the plant–biotic interaction considered.
We also demonstrate that EE‐induced SAR against B. cinerea BMM and H. arabidopsidis Noco2 requires the NHP pathway, consistent with our previous finding with EE‐induced SAR against Pst and Psm (Hilfiker et al., 2014). There is thus apparently a shared mechanism for SAR activation in response to insect eggs and bacteria, which both trigger SA and NHP pathways. In line with this hypothesis, we found that Pst infection of local leaves triggers SAR against B. cinerea. It would be interesting to test whether any biotic stress that leads to SA accumulation generates a similar NHP‐dependent SAR or whether additional specific cues from the attacker are necessary. Noticeably, we found that Pip complementation of ald1 was not sufficient to restore SAR against B. cinerea but that EE pretreatment was necessary, implying an additional egg‐derived signal. Also, we could not induce SAR by co‐treatment with Pip and SA, indicating that more components are needed. Alternatively, SA infiltration might have been unable to replace the natural EE‐induced SA accumulation and generation of a SAR signal. By contrast, Pip complementation of ald1 was sufficient to restore SAR against Psm (Návarová et al., 2012). More work will be needed to investigate what distinguishes bacteria‐ from egg‐induced SAR at the molecular level, but results from previous work and this study indicate that both phenomena depend on the SA and NHP pathways (Hartmann & Zeier, 2019; Zeier, 2021).
We identified camalexin as a crucial indolic compound potentially associated with the execution of EE‐induced SAR against a sensitive B. cinerea strain. Indeed, EE‐induced SAR is abolished in Arabidopsis mutants that are blocked in camalexin accumulation but not in mutants impaired in the biosynthesis of other Trp‐derived metabolites, like indole GS or 4‐OH‐ICN. We also show that camalexin is toxic to the B. cinerea BMM strain used in this study. Interestingly, bacteria‐induced SAR against P. syringae developed in hydroponically cultivated cyp79b2 cyp79b3 (Stahl et al., 2016), indicating a dispensable function of tryptophan‐derived compounds for SAR towards bacterial challenge. This finding points to a difference in the establishment of SAR between bacterial and fungal pathogens. In support of this hypothesis, camalexin has been shown not to protect plants against bacterial pathogens (Bednarek, 2012).
While we present genetic evidence for a role of camalexin in EE‐induced SAR against the BMM strain, measurements of camalexin accumulation in the infected leaf, and specifically at the leaf surface, could not reveal a difference between control and EE‐pretreated plants. In addition, although ald1 and ics1 mutants are impaired in the generation of a SAR signal, they displayed similar camalexin concentrations to Col‐0 after B. cinerea infection. For instance, B. cinerea is known to rapidly detoxify camalexin into IAN and ICA (Pedras et al., 2011). As our in vitro toxicity assay showed that ICA is significantly less antifungal than camalexin, one hypothesis could be that EE pretreatment inhibits camalexin detoxification by B. cinerea. Alternatively, camalexin may be metabolized in planta to an as‐yet unknown potent antifungal compound, and this conversion would be enhanced by SAR signal(s). Also, EE‐induced SAR may potentiate the toxicity of camalexin towards B. cinerea. An alternative explanation could be that the higher basal susceptibility of mutants that lack camalexin cannot be overcome by the egg‐induced SA/NHP‐dependent SAR, which may operate via a distinct mechanism. Clearly, further research will be needed to elucidate the molecular steps that connect egg‐triggered SA/NHP pathways to the inhibition of B. cinerea BMM, whether this is through indolic metabolism or through other defensive compounds.
There are number of B. cinerea strains that are resistant or less sensitive to camalexin, for instance through transporter‐mediated efflux (Kliebenstein et al., 2005; Stefanato et al., 2009). Whether EE‐induced SAR is efficient against these strains, and through which mechanism/metabolite, are important questions that deserve future investigation.
We showed that concentrations of indole GSs are not affected by EE treatment nor by B. cinerea BMM infection. Given that we sampled whole leaves, however, we cannot exclude the possibility that more specific changes occurred at a finer scale. Indeed, previous work has shown higher indole and aliphatic GS concentrations at increasing distance from the B. cinerea lesion, and this could be modulated by EE pretreatment (Kliebenstein et al., 2005). However, the finding that tmyb and myb28 myb29 are still able to develop a normal EE‐induced SAR against the BMM strain strongly suggests that GSs do not play a role in this phenomenon. Although this tmyb lacks indole GSs (Frerigmann & Gigolashvili, 2014), it is also impaired in the UV‐induced conversion of IAOx to several indolic metabolites, including camalexin and ICA, albeit not in response to infection with P. cucumerina (Frerigmann et al., 2016). We also found that tmyb has wild‐type camalexin and ICA levels after B. cinerea infection, further supporting the role of camalexin in EE‐induced SAR. Interestingly, PEN2‐dependent metabolism of indole GS has been reported to be important for innate immunity against bacterial and fungal pathogens, and is connected to the SA pathway (Bednarek et al., 2009; Clay et al., 2009; Bednarek, 2012). However, conservation of EE‐induced SAR in tmyb makes it unlikely that indole GS derivatives play a signaling role in the response against the BMM strain.
Our observation that GS mutants and Col‐0 have a similar basal resistance to the BMM strain differs from the findings of other studies indicating that aliphatic and/or indole GSs are involved in basal resistance to certain B. cinerea strains (Buxdorf et al., 2013). Similarly, the cyp82c2 mutant displayed increased susceptibility to B. cinerea in other studies, suggesting an important role for 4‐OH‐ICN (Rajniak et al., 2015; Liu et al., 2020). Thus, the role of GS and 4‐OH‐ICN in EE‐induced SAR may also depend on the strain considered and should be explored further.
CYP71A12 and CYP71A13 are required for the conversion of IAOx to IAN, which can be metabolized to camalexin or ICA. Our finding that EE‐induced SAR is absent in cyp71a12/a13, although this mutant still accumulates wild‐type levels of ICA after B. cinerea infection, suggests that this indolic metabolite is not the main contributor here. A way of obtaining definitive proof would be to test a mutant that lacks ICA exclusively and observe no defect in EE‐induced SAR. However, given that ICA concentrations were also unaffected in tmyb and in cyp71b6 aao1, illustrating several redundant routes to ICA biosynthesis, such a mutant may be difficult to obtain. Interestingly, a role for indolic metabolites in Arabidopsis resistance against isolates of the necrotrophic fungus P. cucumerina has been reported (Sanchez‐Vallet et al., 2010). The cyp79b2 cyp79b3 mutant was fully susceptible to P. cucumerina infection but camalexin and indole GSs played a minor role in resistance, implying the existence of other antifungal tryptophan derivatives. Strikingly, metabolite profiling after P. cucumerina infection revealed a significant accumulation of ICA, and mutants affected in biosynthesis of ICA conjugates were more susceptible to this pathogen (Sanchez‐Vallet et al., 2010; Gamir et al., 2012; Pastorczyk et al., 2020). There is thus evidence that ICA may contribute to defense against some fungal pathogens. The relatively weak yet significant toxicity of ICA towards B. cinerea BMM in vitro supports this hypothesis and requires further evaluation.
Egg extract‐induced SAR also targets the oomycete H. arabidopsidis Noco2 in an NHP‐dependent way and raises the question of the nature of plant defense compounds involved in this response. In Arabidopsis, basal immunity to H. arabidopsidis is activated by the detection of microbe‐associated molecular patterns (Oome et al., 2014) and involves the concerted action of Enhanced Disease Susceptibility1 (EDS1) and Phytoalexin Deficient4 (PAD4), followed by mobilization of the SA pathway (Rietz et al., 2011). Interestingly, local pre‐treatment with the molecular pattern nlp24 led to systemic resistance against H. arabidopsidis (Albert et al., 2015). Although camalexin accumulates after H. arabidopsidis infection (Mert‐Türk et al., 2003), previous studies have shown that pad3‐1 and cyp79b2 cyp79b3 do not show enhanced susceptibility, suggesting that camalexin and other tryptophan‐derived metabolites are not crucial for resistance (Glazebrook et al., 1997; Stuttmann et al., 2011). Again, further work will be necessary to identify metabolites or defense proteins involved in EE‐induced SAR against this oomycete.
Studies with Pieris brassicae have revealed that prior egg deposition primes plants for a better defense against hatching larvae and, interestingly, that this phenomenon depends on the SA pathway and requires ALD1 (Geiselhardt et al., 2013; Pashalidou et al., 2015; Bonnet et al., 2017; Lortzing et al., 2019; Valsamakis et al., 2020). Our observation that P. brassicae larvae perform poorly on B. cinerea‐infected plants may suggest that egg‐induced SAR would be beneficial for the insect. However, in light of the findings described in this article, the situation might be different in the context of natural oviposition on plants that become infested. Egg‐induced priming of defenses might overcome the potential benefit of feeding on healthier plants. Further experiments will be needed to answer this question. Also, given that larval feeding creates open wounds that are potential entries for opportunistic pathogens, plants may have evolved egg‐induced SAR to anticipate such threat.
That insect eggs protect Arabidopsis against Pseudomonas syringae (Hilfiker et al., 2014), B. cinerea BMM and H. arabidopsidis Noco2 is remarkable given the different lifestyles of these pathogens. Intriguingly, treatment of Arabidopsis leaves with rhamnolipids was shown to induce resistance against the same three pathogens in an SA‐dependent manner (Sanchez et al., 2012). Rhamnolipids are produced by several bacterial species and are potent activators of immunity (Vatsa et al., 2010). Similarly, we recently found that insect egg‐derived PCs are responsible for the activation of immune responses, including SA accumulation, and that this response depends on LecRK‐I.8 (Stahl et al., 2020). We show here that EE‐induced SAR against B. cinerea is also induced by PCs and requires LecRK‐I.8. It would be interesting to test whether rhamnolipids and egg PCs inhibit pathogen growth through a similar mechanism. In addition, assays with different plant species, including crops, and leaf pathogens will be needed to explore the generality of egg‐ and PC‐induced SAR. If validated, this may lead to the development of an efficient strategy to control a broad range of diseases.
In conclusion, this study shows that insect eggs activate a SAR targeting a necrotrophic fungus and an oomycete pathogen. This phenomenon extends to neighboring plants, requires the SA and NHP pathways, and may involve indolic metabolism. Whether insects, plants, or both benefit from such a SAR will require further studies, but this finding illustrates a fascinating aspect of plant–herbivore interactions.
Author contributions
EA, ES, TMR, GVdA and PR conceived the research plans. EA, GG, ES, EB, JZ and TMR performed the experiments and analyzed the data. PR wrote the article with contributions from all the authors.
Supporting information
Table S1 Replicate numbers and summary statistics.
Fig. S1 Egg extract (EE) treatment reduces Botrytis cinerea growth in distal leaves.
Fig. S2 Time course of EE‐induced reduction of B. cinerea infection.
Fig. S3 Salicylic acid (SA) quantification in SA biosynthesis mutants.
Fig. S4 Exogenous SA infiltration does not trigger EE‐induced systemic acquired resistance (SAR).
Fig. S5 Glucosinolates concentrations in EE‐ and B. cinerea‐treated plants.
Fig. S6 Indole‐3‐carboxylic acid (ICA) accumulates in response to B. cinerea infection.
Fig. S7 ICA conjugates accumulate in response to EE treatment.
Fig. S8 EE induces camalexin accumulation in local leaves.
Fig. S9 Early time course of camalexin accumulation.
Fig. S10 Camalexin concentrations in various indolic mutants.
Methods S1 Lines used in this study.
Methods S2 Measurement of B. cinerea growth by quantitative polymerase chain reaction (qPCR).
Methods S3 Metabolite analyses.
Table S2 Time‐course of single glucosinolate species accumulation.
Table S3 Single glucosinolate species in Col‐0 and the tmyb mutant.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
This research was supported by a grant (310030_200372) from the Swiss National Science Foundation to PR, by the Less is More grant (847.13.006) of the Netherlands Organization for Scientific Research to GVdA and TMR, and by a grant (ZE467/6–2) of the German Research Foundation (DFG) to JZ. We thank Blaise Tissot and Caroline Gouhier‐Darimont for maintenance of plants and insects, and Steve Lassueur for help with metabolite analyses. We thank N. Clay (Yale University, USA), E. Glawischnig (TUM Münich, Germany), I. Somssich (MPI Koeln, Germany) and Y. Zhang (University of British Columbia, CA) for sharing mutants. We thank the anonymous reviewers for useful comments on a previous version of the manuscript. Open Access Funding provided by Universite de Lausanne.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
- Albert I, Böhm H, Albert M, Feiler CE, Imkampe J, Wallmeroth N, Brancato C, Raaymakers TM, Oome S, Zhang H et al. 2015. An RLP23–SOBIR1–BAK1 complex mediates NLP‐triggered immunity. Nature Plants 1: 15140. [DOI] [PubMed] [Google Scholar]
- Balmer A, Pastor V, Glauser G, Mauch‐Mani B. 2018. Tricarboxylates induce defense priming against bacteria in Arabidopsis thaliana . Frontiers in Plant Science 9: 1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek P. 2012. Chemical warfare or modulators of defence responses – the function of secondary metabolites in plant immunity. Current Opinion in Plant Biology 15: 407–414. [DOI] [PubMed] [Google Scholar]
- Bednarek P, Piślewska‐Bednarek M, Loren V, van Themaat E, Maddula RK, Svatos A, Schulze‐Lefert P. 2011. Conservation and clade‐specific diversification of pathogen‐inducible tryptophan and indole glucosinolate metabolism in Arabidopsis thaliana relatives. New Phytologist 192: 713–726. [DOI] [PubMed] [Google Scholar]
- Bednarek P, Pislewska‐Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez‐Vallet A et al. 2009. A glucosinolate metabolism pathway in living plant cells mediates broad‐spectrum antifungal defense. Science 323: 101–106. [DOI] [PubMed] [Google Scholar]
- Beekwilder J, van Leeuwen W, van Dam NM, Bertossi M, Grandi V, Mizzi L, Soloviev M, Szabados L, Molthoff JW, Schipper B et al. 2008. The impact of the absence of aliphatic glucosinolates on insect herbivory in Arabidopsis. PLoS ONE 3: e2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenbihl RP, Diezel C, Somssich IE. 2012. Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiology 159: 266–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet C, Lassueur S, Ponzio C, Gols R, Dicke M, Reymond P. 2017. Combined biotic stresses trigger similar transcriptomic responses but contrasting resistance against a chewing herbivore in Brassica nigra . BMC Plant Biology 17: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher C, Chapman A, Fellermeier F, Choudhary M, Scheel D, Glawischnig E. 2014. The Biosynthetic pathway of indole‐3‐carbaldehyde and indole‐3‐carboxylic acid derivatives in Arabidopsis. Plant Physiology 165: 841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruessow F, Gouhier‐Darimont C, Buchala A, Metraux J‐P, Reymond P. 2010. Insect eggs suppress plant defence against chewing herbivores. The Plant Journal 62: 876–885. [DOI] [PubMed] [Google Scholar]
- Buxdorf K, Yaffe H, Barda O, Levy M. 2013. The effects of glucosinolates and their breakdown products on necrotrophic fungi. PLoS ONE 8: e70771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y‐C, Holmes EC, Rajniak J, Kim J‐G, Tang S, Fischer CR, Mudgett MB, Sattely ES. 2018. N‐hydroxy‐pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proceedings of the National Academy of Sciences, USA 115: E4920–E4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. 2009. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates ME, Beynon JL. 2010. Hyaloperonospora arabidopsidis as a pathogen model. Annual Review of Phytopathology 48: 329–345. [DOI] [PubMed] [Google Scholar]
- Conrath U. 2011. Molecular aspects of defence priming. Trends in Plant Science 16: 524–531. [DOI] [PubMed] [Google Scholar]
- Dean R, van Kan JAL, Pretorius ZA, Hammond‐Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J et al. 2012. The top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology 13: 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding P, Rekhter D, Ding Y, Feussner K, Busta L, Haroth S, Xu S, Li X, Jetter R, Feussner I et al. 2016. Characterization of a pipecolic acid biosynthesis pathway required for systemic acquired resistance. Plant Cell 28: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Reymond P. 2019. Molecular interactions between plants and insect herbivores. Annual Review of Plant Biology 70: 527–557. [DOI] [PubMed] [Google Scholar]
- Fatouros NE, Pineda A, Huigens ME, Broekgaarden C, Shimwela MM, Figueroa Candia IA, Verbaarschot P, Bukovinszky T. 2014. Synergistic effects of direct and indirect defences on herbivore egg survival in a wild crucifer. Proceedings of the Royal Society of London, Series B: Biological Sciences 281: 20141254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Denoux C, De Lorenzo G, Ausubel FM, Dewdney J. 2007. Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiology 144: 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. 2003. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. The Plant Journal 35: 193–205. [DOI] [PubMed] [Google Scholar]
- Forcat S, Bennett M, Grant M, Mansfield JW. 2010. Rapid linkage of indole carboxylic acid to the plant cell wall identified as a component of basal defence in Arabidopsis against hrp mutant bacteria. Phytochemistry 71: 870–876. [DOI] [PubMed] [Google Scholar]
- Frerigmann H, Gigolashvili T. 2014. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana . Molecular Plant 7: 814–828. [DOI] [PubMed] [Google Scholar]
- Frerigmann H, Glawischnig E, Gigolashvili T. 2015. The role of MYB34, MYB51 and MYB122 in the regulation of camalexin biosynthesis in Arabidopsis thaliana . Frontiers in Plant Science 6: 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerigmann H, Piślewska‐Bednarek M, Sanchez‐Vallet A, Molina A, Glawischnig E, Gigolashvili T, Bednarek P. 2016. Regulation of pathogen‐triggered tryptophan metabolism in Arabidopsis thaliana by MYB transcription factors and indole glucosinolate conversion products. Molecular Plant 9: 682–695. [DOI] [PubMed] [Google Scholar]
- Gamir J, Pastor V, Cerezo M, Flors V. 2012. Identification of indole‐3‐carboxylic acid as mediator of priming against Plectosphaerella cucumerina . Plant Physiology and Biochemistry 61: 169–179. [DOI] [PubMed] [Google Scholar]
- Garcion C, Lohmann A, Lamodière E, Catinot J, Buchala A, Doermann P, Metraux J‐P. 2008. Characterization and biological function of the ISOCHORISMATE SYNTHASE2 gene of Arabidopsis. Plant Physiology 147: 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiselhardt S, Yoneya K, Blenn B, Drechsler N, Gershenzon J, Kunze R, Hilker M. 2013. Egg laying of Cabbage White butterfly (Pieris brassicae) on Arabidopsis thaliana affects subsequent performance of the larvae. PLoS ONE 8: e59661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuss D, Stelzer S, Lortzing T, Steppuhn A. 2017. Solanum dulcamara’s response to eggs of an insect herbivore comprises ovicidal hydrogen peroxide production. Plant, Cell & Environment 40: 2663–2677. [DOI] [PubMed] [Google Scholar]
- Glauser G, Schweizer F, Turlings TCJ, Reymond P. 2012. Rapid profiling of intact glucosinolates in Arabidopsis leaves by UHPLC‐QTOFMS using a charged surface hybrid column. Phytochemical Analysis 23: 520–528. [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Zook M, Mert F, Kagan I, Rogers EE, Crute IR, Holub EB, Hammerschmidt R, Ausubel FM. 1997. Phytoalexin‐deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics 146: 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouhier‐Darimont C, Schmiesing A, Bonnet C, Lassueur S, Reymond P. 2013. Signalling of Arabidopsis thaliana response to Pieris brassicae eggs shares similarities with PAMP‐triggered immunity. Journal of Experimental Botany 64: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouhier‐Darimont C, Stahl E, Glauser G, Reymond P. 2019. The Arabidopsis lectin receptor kinase LecRK‐I.8 is involved in insect egg perception. Frontiers Plant Science 10: 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griese E, Caarls L, Bassetti N, Mohammadin S, Verbaarschot P, Bukovinszkine’Kiss G, Poelman EH, Gols R, Schranz ME, Fatouros NE. 2021. Insect egg‐killing: a new front on the evolutionary arms‐race between brassicaceous plants and pierid butterflies. New Phytologist 230: 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griese E, Dicke M, Hilker M, Fatouros NE. 2017. Plant response to butterfly eggs: inducibility, severity and success of egg‐killing leaf necrosis depends on plant genotype and egg clustering. Scientific Reports 7: 7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M, Kim D, Bernsdorff F, Ajami‐Rashidi Z, Scholten N, Schreiber S, Zeier T, Schuck S, Reichel‐Deland V, Zeier J. 2017. Biochemical principles and functional aspects of pipecolic acid biosynthesis in plant immunity. Plant Physiology 174: 124–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M, Zeier J. 2019. N‐hydroxypipecolic acid and salicylic acid: a metabolic duo for systemic acquired resistance. Current Opinion in Plant Biology 50: 44–57. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Zeier T, Bernsdorff F, Reichel‐Deland V, Kim D, Hohmann M, Scholten N, Schuck S, Bräutigam A, Hölzel T et al. 2018. Flavin monooxygenase‐generated N‐hydroxypipecolic acid is a critical element of plant systemic immunity. Cell 173: 456–469. [DOI] [PubMed] [Google Scholar]
- He Y, Xu J, Wang X, He X, Wang Y, Zhou J, Zhang S, Meng X. 2019. The Arabidopsis pleiotropic drug resistance transporters PEN3 and PDR12 mediate camalexin secretion for resistance to Botrytis cinerea . Plant Cell 31: 2206–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker O, Groux R, Bruessow F, Kiefer K, Zeier J, Reymond P. 2014. Insect eggs induce a systemic acquired resistance in Arabidopsis. The Plant Journal 80: 1085–1094. [DOI] [PubMed] [Google Scholar]
- Hilker M, Fatouros NE. 2015. Plant responses to insect egg deposition. Annual Review of Entomology 60: 493–515. [DOI] [PubMed] [Google Scholar]
- Hilker M, Kobs C, Varama M, Schrank K. 2002. Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. Journal of Experimental Biology 205: 455–461. [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G. 2008. Plant immunity to insect herbivores. Annual Review of Plant Biology 59: 41–66. [DOI] [PubMed] [Google Scholar]
- Huang WE, Huang L, Preston GM, Naylor M, Carr JP, Li Y, Singer AC, Whiteley AS, Wang H. 2006. Quantitative in situ assay of salicylic acid in tobacco leaves using a genetically modified biosensor strain of Acinetobacter sp. ADP1. The Plant Journal 46: 1073–1083. [DOI] [PubMed] [Google Scholar]
- Huang WE, Wang H, Zheng H, Huang L, Singer AC, Thompson I, Whiteley AS. 2005. Chromosomally located gene fusions constructed in Acinetobacter sp. ADP1 for the detection of salicylate. Environmental Microbiology 7: 1339–1348. [DOI] [PubMed] [Google Scholar]
- Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT. 2009. Priming in systemic plant immunity. Science 324: 89–91. [DOI] [PubMed] [Google Scholar]
- Katz E, Nisani S, Yadav BS, Woldemariam MG, Shai B, Obolski U, Ehrlich M, Shani E, Jander G, Chamovitz DA. 2015. The glucosinolate breakdown product indole‐3‐carbinol acts as an auxin antagonist in roots of Arabidopsis thaliana . The Plant Journal 82: 547–555. [DOI] [PubMed] [Google Scholar]
- Kavitha A, Prabhakar P, Vijayalakshmi M, Venkateswarlu Y. 2010. Purification and biological evaluation of the metabolites produced by Streptomyces sp. TK‐VL_333. Research in Microbiology 161: 335–345. [DOI] [PubMed] [Google Scholar]
- Kettles GJ, Drurey C, Schoonbeek H‐J, Maule AJ, Hogenhout SA. 2013. Resistance of Arabidopsis thaliana to the green peach aphid, Myzus persicae, involves camalexin and is regulated by microRNAs. New Phytologist 198: 1178–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare D, Choi H, Huh SU, Bassin B, Kim J, Martinoia E, Sohn KH, Paek K‐H, Lee Y. 2017. Arabidopsis ABCG34 contributes to defense against necrotrophic pathogens by mediating the secretion of camalexin. Proceedings of the National Academy of Sciences, USA 114: E5712–E5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee BW, Schroeder FC, Jander G. 2008. Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid). The Plant Journal 54: 1015–1026. [DOI] [PubMed] [Google Scholar]
- Kliebenstein DJ, Rowe HC, Denby KJ. 2005. Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. The Plant Journal 44: 25–36. [DOI] [PubMed] [Google Scholar]
- Little D, Gouhier‐Darimont C, Bruessow F, Reymond P. 2007. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiology 143: 784–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Kracher B, Ziegler J, Birkenbihl RP, Somssich IE. 2015. Negative regulation of ABA signaling by WRKY33 is critical for Arabidopsis immunity towards Botrytis cinerea 2100. eLife 4: e07295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sun T, Sun Y, Zhang Y, Radojičić A, Ding Y, Tian H, Huang X, Lan J, Chen S et al. 2020. Diverse roles of the salicylic acid receptors NPR1 and NPR3/NPR4 in plant immunity. Plant Cell 32: 4002–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortzing T, Kunze SA, Hilker M, Lortzing V. 2020. Arabidopsis, tobacco, nightshade and elm take insect eggs as herbivore alarm and show similar transcriptomic alarm responses. Scientific Reports 10: 16281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortzing V, Oberländer J, Lortzing T, Tohge T, Steppuhn A, Kunze R, Hilker M. 2019. Insect egg deposition renders plant defence against hatching larvae more effective in a salicylic acid‐dependent manner. Plant, Cell & Environment 42: 1019–1032. [DOI] [PubMed] [Google Scholar]
- Maier BA, Kiefer P, Field CM, Hemmerle L, Bortfeld‐Miller M, Emmenegger B, Schäfer M, Pfeilmeier S, Sunagawa S, Vogel CM et al. 2021. A general non‐self response as part of plant immunity. Nature Plants 7: 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mert‐Türk F, Bennett MH, Mansfield JW, Holub E. 2003. Camalexin accumulation in Arabidopsis thaliana following abiotic elicitation or inoculation with virulent or avirulent Hyaloperonospora parasitica . Physiological and Molecular Plant Pathology 62: 137–145. [Google Scholar]
- Mucha S, Heinzlmeir S, Kriechbaumer V, Strickland B, Kirchhelle C, Choudhary M, Kowalski N, Eichmann R, Hueckelhoven R, Grill E et al. 2019. The formation of a camalexin biosynthetic metabolon. Plant Cell 31: 2697–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Návarová H, Bernsdorff F, Döring A‐C, Zeier J. 2012. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24: 5123–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Métraux JP. 1999. Salicylic acid induction‐deficient mutants of Arabidopsis express PR‐2 and PR‐5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11: 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie P, Li X, Wang S, Guo J, Zhao H, Niu D. 2017. Induced systemic resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET‐ and NPR1‐dependent signaling pathway and activates PAMP‐triggered immunity in Arabidopsis. Frontiers in Plant Science 8: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oome S, Raaymakers TM, Cabral A, Samwel S, Böhm H, Albert I, Nürnberger T, Van den Ackerveken G. 2014. Nep1‐like proteins from three kingdoms of life act as a microbe‐associated molecular pattern in Arabidopsis. Proceedings of the National Academy of Sciences, USA 111: 16955–16960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovskis Z, Reymond P. 2020. Pieris brassicae eggs trigger interplant systemic acquired resistance against a foliar pathogen in Arabidopsis. New Phytologist 228: 1652–1661. [DOI] [PubMed] [Google Scholar]
- Pashalidou FG, Fatouros NE, van Loon JJA, Dicke M, Gols R. 2015. Plant‐mediated effects of butterfly egg deposition on subsequent caterpillar and pupal development, across different species of wild Brassicaceae. Ecological Entomology 40: 444–450. [Google Scholar]
- Pastorczyk M, Kosaka A, Piślewska‐Bednarek M, López G, Frerigmann H, Kułak K, Glawischnig E, Molina A, Takano Y, Bednarek P. 2020. The role of CYP71A12 monooxygenase in pathogen‐triggered tryptophan metabolism and Arabidopsis immunity. New Phytologist 225: 400–412. [DOI] [PubMed] [Google Scholar]
- Pedras MSC, Hossain S. 2011. Interaction of cruciferous phytoanticipins with plant fungal pathogens: indole glucosinolates are not metabolized but the corresponding desulfo‐derivatives and nitriles are. Phytochemistry 72: 2308–2316. [DOI] [PubMed] [Google Scholar]
- Pedras MSC, Hossain S, Snitynsky RB. 2011. Detoxification of cruciferous phytoalexins in Botrytis cinerea: spontaneous dimerization of a camalexin metabolite. Phytochemistry 72: 199–206. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Van der Does D, Zamioudis C, Leon‐Reyes A, Van Wees SCM. 2012. Hormonal modulation of plant immunity. Annual Review of Cell and Developmental biology 28: 489–521. [DOI] [PubMed] [Google Scholar]
- Rajniak J, Barco B, Clay NK, Sattely ES. 2015. A new cyanogenic metabolite in Arabidopsis required for inducible pathogen defence. Nature 525: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P. 2013. Perception, signaling and molecular basis of oviposition‐mediated plant responses. Planta 238: 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE. 2000. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietz S, Stamm A, Malonek S, Wagner S, Becker D, Medina‐Escobar N, Vlot AC, Feys BJ, Niefind K, Parker JE. 2011. Different roles of Enhanced Disease Susceptibility1 (EDS1) bound to and dissociated from Phytoalexin Deficient4 (PAD4) in Arabidopsis immunity. New Phytologist 191: 107–119. [DOI] [PubMed] [Google Scholar]
- Sanchez L, Courteaux B, Hubert J, Kauffmann S, Renault J‐H, Clément C, Baillieul F, Dorey S. 2012. Rhamnolipids elicit defense responses and induce disease resistance against biotrophic, hemibiotrophic, and necrotrophic pathogens that require different signaling pathways in Arabidopsis and highlight a central role for salicylic acid. Plant Physiology 160: 1630–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Vallet A, Ramos B, Bednarek P, López G, Piślewska‐Bednarek M, Schulze‐Lefert P, Molina A. 2010. Tryptophan‐derived secondary metabolites in Arabidopsis thaliana confer non‐host resistance to necrotrophic Plectosphaerella cucumerina fungi. The Plant Journal 63: 115–127. [DOI] [PubMed] [Google Scholar]
- Schuhegger R, Nafisi M, Mansourova M, Petersen BL, Olsen CE, Svatos A, Halkier BA, Glawischnig E. 2006. CYP71B15 (PAD3) catalyzes the final step in camalexin biosynthesis. Plant Physiology 141: 1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J, Zeier J. 2013. Long‐distance communication and signal amplification in systemic acquired resistance. Frontiers in Plant Science 4: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl E, Bellwon P, Huber S, Schlaeppi K, Bernsdorff F, Vallat‐Michel A, Mauch F, Zeier J. 2016. Regulatory and functional aspects of indolic metabolism in plant systemic acquired resistance. Molecular Plant 9: 662–681. [DOI] [PubMed] [Google Scholar]
- Stahl E, Brillatz T, Ferreira Queiroz E, Marcourt L, Schmiesing A, Hilfiker O, Riezman I, Riezman H, Wolfender J‐L, Reymond P. 2020. Phosphatidylcholines from Pieris brassicae eggs activate an immune response in Arabidopsis. eLife 9: e60293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanato F, Abou‐Mansour E, Buchala A, Kretschmer M, Mosbach A, Hahn M, Bochet CG, Métraux J‐P, Schoonbeek H. 2009. The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana . The Plant Journal 58: 499–510. [DOI] [PubMed] [Google Scholar]
- Sticher L, Mauch‐Mani B, Métraux JP. 1997. Systemic acquired resistance. Annual Review of Phytopathology 35: 235–270. [DOI] [PubMed] [Google Scholar]
- Stuttmann J, Hubberten H‐M, Rietz S, Kaur J, Muskett P, Guerois R, Bednarek P, Hoefgen R, Parker JE. 2011. Perturbation of Arabidopsis amino acid metabolism causes incompatibility with the adapted biotrophic pathogen Hyaloperonospora arabidopsidis . Plant Cell 23: 2788–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji J, Jackson EP, Gage DA, Hammerschmidt R, Somerville SC. 1992. Phytoalexin accumulation in Arabidopsis thaliana during the hypersensitive reaction to Pseudomonas syringae pv syringae . Plant Physiology 98: 1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsamakis G, Bittner N, Fatouros NE, Kunze R, Hilker M, Lortzing V. 2020. Priming by timing: a rabidopsis thaliana adjusts its priming response to Lepidoptera eggs to the time of larval hatching. Frontiers in Plant Science 11: 619589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kan JAL, Shaw MW, Grant‐Downton RT. 2014. Botrytis species: relentless necrotrophic thugs or endophytes gone rogue? Molecular Plant Pathology 15: 957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsa P, Sanchez L, Clément C, Baillieul F, Dorey S. 2010. Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. International Journal of Molecular Sciences 11: 5095–5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso J, van Kan JAL. 2018. Many shades of grey in Botrytis‐host plant interactions. Trends in Plant Science 23: 613–622. [DOI] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. 2009. Salicylic acid, a multifaceted hormone to combat disease. Annual Review of Phytopathology 47: 177–206. [DOI] [PubMed] [Google Scholar]
- Wu J, Baldwin IT. 2010. New insights into plant responses to the attack from insect herbivores. Annual Review of Genetics 44: 1–24. [DOI] [PubMed] [Google Scholar]
- Xu J, Meng J, Meng X, Zhao Y, Liu J, Sun T, Liu Y, Wang Q, Zhang S. 2016. Pathogen‐responsive MPK3 and MPK6 reprogram the biosynthesis of indole glucosinolates and their derivatives in Arabidopsis immunity. Plant Cell 28: 1144–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier J. 2021. Metabolic regulation of systemic acquired resistance. Current Opinion in Plant Biology 62: 102050. [DOI] [PubMed] [Google Scholar]
- Zhang W, Corwin JA, Copeland D, Feusier J, Eshbaugh R, Chen F, Atwell S, Kliebenstein DJ. 2017. Plastic transcriptomes stabilize immunity to pathogen diversity: the jasmonic acid and salicylic acid networks within the Arabidopsis/Botrytis pathosystem. Plant Cell 29: 2727–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso JM, Ecker JR, Normanly J, Chory J, Celenza JL. 2002. Trp‐dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes & Development 16: 3100–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang X, He Y, Sang T, Wang P, Dai S, Zhang S, Meng X. 2020. Differential phosphorylation of the transcription factor WRKY33 by the protein kinases CPK5/CPK6 and MPK3/MPK6 cooperatively regulates camalexin biosynthesis in Arabidopsis. Plant Cell 32: 2621–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J‐M, Zhang Y. 2020. Plant immunity: danger perception and signaling. Cell 181: 978–989. [DOI] [PubMed] [Google Scholar]
- Zimmerli L, Métraux JP, Mauch‐Mani B. 2001. β‐Aminobutyric acid‐induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinerea . Plant Physiology 126: 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Replicate numbers and summary statistics.
Fig. S1 Egg extract (EE) treatment reduces Botrytis cinerea growth in distal leaves.
Fig. S2 Time course of EE‐induced reduction of B. cinerea infection.
Fig. S3 Salicylic acid (SA) quantification in SA biosynthesis mutants.
Fig. S4 Exogenous SA infiltration does not trigger EE‐induced systemic acquired resistance (SAR).
Fig. S5 Glucosinolates concentrations in EE‐ and B. cinerea‐treated plants.
Fig. S6 Indole‐3‐carboxylic acid (ICA) accumulates in response to B. cinerea infection.
Fig. S7 ICA conjugates accumulate in response to EE treatment.
Fig. S8 EE induces camalexin accumulation in local leaves.
Fig. S9 Early time course of camalexin accumulation.
Fig. S10 Camalexin concentrations in various indolic mutants.
Methods S1 Lines used in this study.
Methods S2 Measurement of B. cinerea growth by quantitative polymerase chain reaction (qPCR).
Methods S3 Metabolite analyses.
Table S2 Time‐course of single glucosinolate species accumulation.
Table S3 Single glucosinolate species in Col‐0 and the tmyb mutant.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


