Abstract
Aims
We report population pharmacokinetic (popPK) and exposure–response (E–R) analyses for efficacy (induced amenorrhoea [IA]) and safety (unbound oestradiol [E2] concentrations) of the selective progesterone receptor modulator vilaprisan. Results were used to inform the dose for the Phase 3 programme in patients with uterine fibroids.
Methods
A popPK model was developed using data from Phase 1 and 2 studies (including ASTEROID 1 and 2). The relationship between vilaprisan exposure (steady‐state AUC) and IA after oral administration of 0.5, 1, 2 or 4 mg/day over 3 months was analysed in ASTEROID 1 using logistic regression and qualified in ASTEROID 2 by comparing simulated and observed probability for IA after 2 mg/day. The exposure–E2 relationship was analysed visually.
Results
Vilaprisan clearance was 22.7% lower in obese vs non‐obese patients. The E–R relationship for IA in ASTEROID 1 was steep and consistent with ASTEROID 2, with a maximum probability (P max) of 59% (95% CI: 49–68%). The exposure at which 50% of P max is obtained was 36.9 μg*h/L (95% CI: 27.7–48.7 μg*h/L). Simulations showed that 36% of the patients will be below 90% of P max for IA after 1 mg/day compared to 2% after 2 mg/day. E2 levels tended to decrease with increasing exposure. While E2 levels remained largely within the physiologic follicular phase range, the clinical relevance of this decrease will be evaluated in long‐term studies.
Conclusions
A 2 mg/day dose was selected for Phase 3 as E–R analyses show this dose results in a close to maximum probability for IA, without any safety concerns noted.
Keywords: modelling and simulation, NONMEM, pharmacodynamics, pharmacometrics, population analysis
What is already known about this subject
Vilaprisan is a novel selective progesterone receptor modulator for the treatment of symptomatic uterine fibroids and endometriosis.
Linear pharmacokinetics up to a dose of 30 mg after daily oral administration for 28 consecutive days was shown.
Dose‐dependent induced amenorrhoea in patients was demonstrated.
What this study adds
A dose of 2 mg/day of vilaprisan resulted in a close to maximum probability for induced amenorrhoea.
This dose was well tolerated, with a moderate and transient decrease in unbound serum oestradiol levels.
This dose was selected for further evaluation in the Phase 3 programme in patients with uterine fibroids.
1. INTRODUCTION
Uterine fibroids (UFs, also called leiomyomas or myomas) are common benign tumours of the myometrium that occur in approximately 70% of women of reproductive age, 1 , 2 and are often associated with heavy menstrual bleeding, pelvic pain and/or bulk‐related symptoms depending on their number, size and location. Selective progesterone receptor modulators (SPRM) decrease the size of fibroids and related symptoms. 3 Vilaprisan is a novel SPRM eliciting strong antagonistic activity at the progesterone receptor for the treatment of symptomatic UFs and endometriosis. 4 Several clinical pharmacology studies have been conducted to characterize the safety, pharmacokinetics (PK) 5 and pharmacodynamics (PD). 6 Vilaprisan showed linear PK up to a dose of 30 mg after daily oral administration for 28 consecutive days. Vilaprisan is almost completely eliminated from plasma by hepatic metabolism. CYP3A4/5 have been identified as the main enzymes involved in the metabolism of vilaprisan. Biotransformation occurs mainly by oxidation reactions at the steroid skeleton, as well as reductions of the 3‐keto group, leading to a complex metabolite pattern with rather low exposure (<10%) to single metabolites in plasma that are not pharmacologically active. Vilaprisan has demonstrated dose‐dependent induction of amenorrhoea (IA) in healthy women in a Proof of Concept study (PoC) 7 and in patients with UFs in the placebo‐controlled dose range finding study ASTEROID 1 8 and ASTEROID 2. 9 Vilaprisan is currently in Phase 3 of clinical development for the treatment of patients with UFs. The objectives of the present study were to evaluate the vilaprisan E–R relationship for IA (as efficacy parameter) and unbound serum oestradiol (E2) concentrations (as safety parameter) to inform dose selection for Phase 3.
2. METHODS
2.1. Bioanalytical methods
A validated high‐performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) method was used to determine levels of vilaprisan in plasma samples (see the Supporting Information for further details and an overview of the performance of this bioanalytical assay). E2 concentrations were measured by an immunoassay using the Cobas Estradiol III/Elecsys estradiol III assay. 10
2.2. Study population
The present popPK and E–R analyses used data from two Phase 1 and two Phase 2 clinical trials, with a total of 414 subjects resulting in 2717 samples for PK analysis and 354 subjects for E–R analyses (Table 1).
TABLE 1.
Clinical trials of vilaprisan included in the analysis
| Study | Phase | Treatment | Population | Subjects valid for PK (N) | Samples valid for PK (N) | Subjects valid for ER (N) |
|---|---|---|---|---|---|---|
| PoC 7 | 1 | 0.1, 0.5, 1, 2, or 5 mg/d, and placebo | Healthy tubal‐ligated young women | 57 | 1051 | NA |
| PD 6 | 1 | 0.5, 1, 2, or 4 mg/d | Healthy young women | 67 | 819 | NA |
| ASTEROID 1 8 | 2b | 0.5, 1, 2, or 4 mg/d and placebo | UF patients | 217 | 600 | 275 |
| ASTEROID 2 9 | 2 | Vilaprisan 2 mg/d, Ulipristal 5 mg/d and placebo | UF patients | 73 | 247 | 79 |
| Total | 414 | 2717 | 354 |
UF: uterine fibroids; PK: population PK model; PoC: Proof of Concept; ER: exposure–response model; PD: pharmacodynamic study; NA: not applicable.
In the Phase 1 PoC study, 73 tubal‐ligated women aged 18–45 years were randomized equally to placebo tablets or either 0.1, 0.5, 1, 2 or 5 mg/d vilaprisan for 12 weeks, and followed up until the second menstrual bleeding after end of treatment. 7 In ASTEROID 1, 309 women with heavy menstrual bleeding due to UFs were randomized equally to oral placebo or either 0.5, 1, 2 or 4 mg/day vilaprisan for 12 weeks, with a 24 week followup. 8 In the Phase 1 PD study, the effect of vilaprisan on ovarian function was assessed in 70 healthy young women that were randomized to either 0.5, 1, 2 or 4 mg/day vilaprisan for 12 weeks. 6 ASTEROID 2 was a multi‐arm, randomized, parallel‐group, placebo‐ and active comparator (ulipristal acetate 5 mg)‐controlled Phase 2 study carried out in 70 centres in 16 European countries. 9 A dose of 2 mg/day vilaprisan was administered either continuously for 24 weeks, or intermittently for two 12‐week courses separated by a break to allow one menstruation. Details of the study design are provided in Table S2 in the Supporting Information.
The studies were conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. Study protocols and amendments were approved by independent ethics committees in all participating countries. All participants provided written informed consent prior to study enrolment.
2.3. Population pharmacokinetic and exposure–response analyses
Densely sampled PK data from the two Phase 1 studies and sparsely sampled PK from the two Phase 2 studies (see Table 1) were used to evaluate the variability in the PK including a covariate analysis and to estimate the individual area under the concentration–time curve at steady‐state (AUC(0–24)ss)) for the Phase 2 patient population using a popPK approach (for details, see the Supplementary Methods section in the Supporting Information). Covariates were pre‐selected based on prior knowledge/biological plausibility and are listed in Table S3 in the Supporting Information.
Based on an exposure–response analysis conducted in rabbits using the McPhail index AUC(0–24)ss was identified as being associated with efficacy (data on file). This was subsequently confirmed in a PoC study 7 and PD study, 6 showing a good and consistent relationship of AUC(0–24)ss to the main efficacy parameter, i.e. induction of amenorrhoea, and was therefore selected for further exposure–response analysis. The relationship between vilaprisan exposure and the probability for IA, which was defined as no scheduled or unscheduled bleeding/spotting after the end of the initial bleeding episode until the end of treatment, was analysed using logistic regression.
The probability for IA is described by Equation 1:
| (1) |
in which P 0 is the probability for IA after placebo treatment, P max is the maximum probability for IA, EAUC50 is the AUC(0–24)ss resulting in 50% of P max and γ defines the steepness of the E–R relationship. Further details can be found in the Supplementary Methods section in the Supporting Information.
The E–R parameters were estimated using the ASTEROID 1 data only, since this was a dose‐range finding study covering a broad vilaprisan exposure range. Consequently, the E–R relationship for ASTEROID 2 (placebo or 2 mg/day for 12 weeks) was simulated using the ASTEROID 1 parameters and compared with the observed probabilities from ASTEROID 2: (i) after the first 12‐week treatment (period 1) with vilaprisan (treatment groups A1 and B1); (ii) after the second 12‐week treatment (period 2) with vilaprisan (A2) = patients that were treated with placebo for 12 weeks and then 2 mg vilaprisan for 12 weeks; and (iii) after the first 12‐week treatment (period 1) with placebo (A2, B2, C2) = patients that were treated with placebo for 12 weeks. Patients treated with ulipristal acetate were not considered (treatment groups C1 period 1 and 2, C2 period 2 and C3 period 1 and 2) (see Table S2 in the Supporting Information).
It was further investigated whether the E–R relationship is different in the Black or African American population compared to the rest of the ASTEROID 1 study population of mainly Caucasian patients.
The relationship between vilaprisan exposure in terms of AUC(0–24)ss and the moving median of the individual unbound E2 concentration, which was calculated on the basis of the total E2, sex hormone binding globulin and albumin concentrations in plasma, was evaluated visually for trends (see Supplementary Methods section in the Supporting Information).
2.4. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20.11
3. RESULTS
3.1. Study population characteristics
A total of 414 subjects with 2717 vilaprisan plasma concentrations were included in the popPK analysis. A summary of the demographics of these subjects is presented in Table S4 in the Supporting Information. Subjects in the analysis population were predominantly Caucasians (70.8%), followed by Asians (13.8%), Blacks or African Americans (12.6%, hereafter referred to as “Blacks”), Hispanics (2.4%) and others (0.5%). The majority of Blacks, Asians and Hispanics was included in ASTEROID 1. Regarding body size parameters, the study populations were similar for median body weight and median fat‐free mass (FFM). The Phase 1 studies had slightly lower values for both parameters, while ASTEROID 1 had the highest variability, including subjects with very high values for body weight and FFM.
The distribution of the body size parameters differed between racial subgroups. Blacks and Hispanics had higher body weight and body mass index (BMI) values. However, within the North American population, the body size parameters were similar among the different subgroups (see Figure S3 in the Supporting Information).
In the E–R analyses, 275 subjects from ASTEROID 1 and 79 subjects from ASTEROID 2 were included. A summary of the demographics of these subjects is presented in Table S5 in the Supporting Information.
3.2. PopPK model
The PK of vilaprisan was described by a linear two‐compartment model with first‐order elimination from the central compartment and first‐order absorption kinetics including a lag time.
Inter‐individual variability was described for the apparent clearance (CL/F) and apparent central volume of distribution (V2/F). Residual variability was described by a proportional error model with a significantly lower variability for the Phase 1 PoC study than for the other studies.
This was consistent with the procedural differences between the Phase 1 PoC study and the other studies. The Phase 1 PoC study was conducted under highly controlled conditions, as all subjects received their vilaprisan dose under supervision in the morning and under fasting conditions. In the other studies, drug administration and food intake were less controlled.
Body weight was identified as a significant covariate for V2/F. V2/F was scaled by body weight using an allometric model with the commonly accepted coefficient of 1.
BMI was identified as significant covariate for CL/F, yielding a CL/F estimate of 13.4 L/h for non‐obese subjects with BMI values <30 kg/m2. For obese subjects with BMI values ≥30 kg/m2, the CL/F was estimated to be 77.3% of the non‐obese value.
A detailed description of the popPK model development including the covariate analysis is given in the Supplementary Methods section in the Supporting Information. The parameter estimates of the base model, i.e. the popPK model without covariate effects, and the final popPK model are shown in Table S6 in the Supporting Information. The PK parameter estimates obtained from the final model were 13.4 L/h for CL/F, 92.1 L for V2/F (for a subject with a body weight of 67 kg), 472 L for the apparent volume of distribution of peripheral compartment V3/F, 38.7 L/h for the apparent intercompartmental clearance between central and peripheral compartment Q1/F, 0.496 L/h for the absorption rate constant k a, and 0.134 h for the absorption lag‐time ALAG1. All parameters of the final model were estimated with acceptable standard errors (relative standard errors <31%). The body weight and BMI effect explained 1.2% and 4.4% of the V2/F and CL/F variability (%CV), respectively, while the residual variability did not change compared to the base model.
3.3. Exposure–response analysis for induced amenorrhoea in ASTEROID 1
The E–R relationship for IA in 275 patients in ASTEROID 1 was steep with a maximum probability P max of 59% (95% confidence interval [CI]: 49–68%). The exposure at which 50% of P max is obtained was 36.9 μg*h/L (95% CI: 27.7–48.7 μg*h/L) (Figure 1). The parameters were estimated with sufficient precision (Table S7 in the Supporting Information).
FIGURE 1.
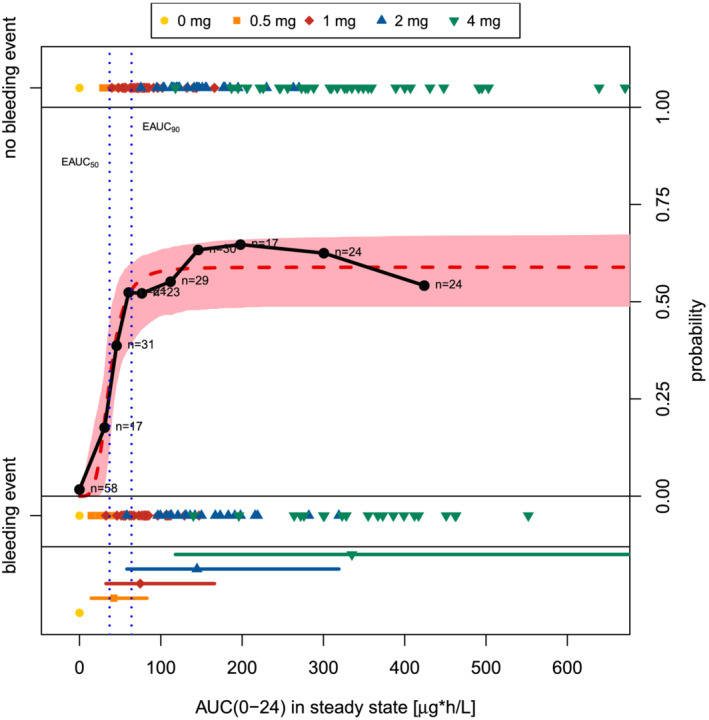
Predicted and observed probability of induced amenorrhoea (no bleeding event) vs AUC(0–24)ss after multiple dosing of placebo and vilaprisan in ASTEROID 1. Red area: 95% CI of the predicted probability of non‐bleeding; red dashed line: predicted probability of non‐bleeding by the final categorical PK/PD model; black circles with solid line: observed probability in distinct AUC(0–24)ss intervals; observed probability = number of subjects with induced amenorrhoea divided by number of subjects within AUC(0–24)ss bin; n: number of binned individuals. Coloured symbols with lines: median, minimum and maximum of AUC(0–24)ss of the respective dose (note that for scaling reasons, the highest AUC(0–24)ss value in the 4 mg dose group is not shown). Coloured symbols without lines: Individual AUC(0–24)ss indicating occurrence/absence of a bleeding event; note that for scaling reasons the highest AUC(0–24)ss value in the 4 mg dose group is not shown. Vertical blue dashed lines: vilaprisan exposure at 50% or 90% of the maximum probability for IA (EAUC50 / 90 )
Simulations revealed that the exposure resulting in 90% of P max for IA (EAUC90 ) will be reached by 64% of the patients after a once daily dose of 1 mg. This proportion will increase to 98% after a once daily dose of 2 mg (Table S8 in the Supporting Information).
The covariate analysis yielded no statistically significant difference in the E–R relationship of the different racial subgroups and the overall population. While P max for IA tended to be lower for Black or African Americans than for the other subgroups, the E–R relationship of each subgroup falls within the 95% CI of the overall population (Figure 2).
FIGURE 2.
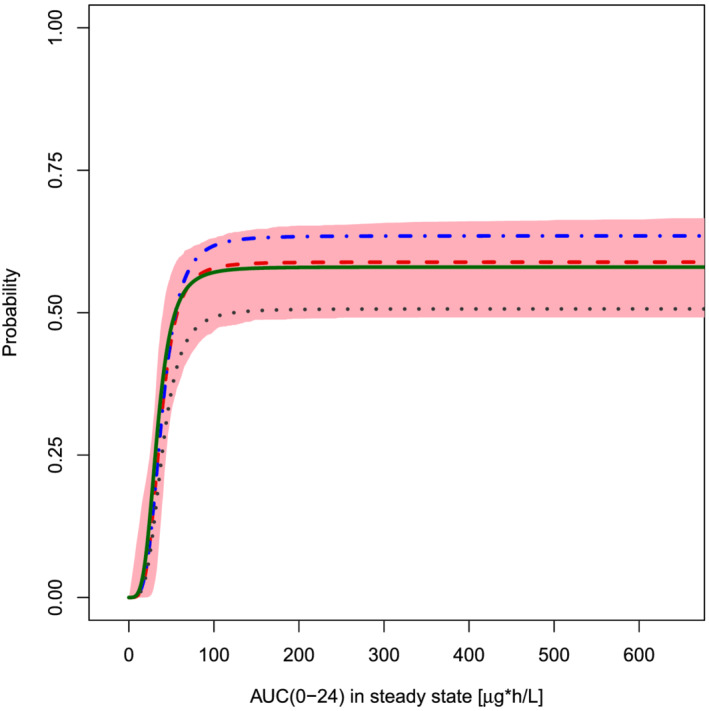
Predicted probability of induced amenorrhoea (no bleeding event) vs AUC(0–24)ss in all racial subgroups in ASTEROID 1. Red area: 95% CI of the predicted probability of non‐bleeding; predicted probability of non‐bleeding for all categories combined (red dashed line, n = 275), for Black or African Americans (black dotted line, n = 59), for Caucasian, Hispanic and other/not specified (blue dotted/dashed line, n = 146), and for Asian (green solid line, n = 70)
3.4. Exposure–response analysis for induced amenorrhoea in ASTEROID 2
The E–R model for ASTEROID 1 was applied to simulate the IA efficacy data of ASTEROID 2 and compared to the observed probability from ASTEROID 2 (see Table 2 and Figure S9a‐c in the Supporting Information).
TABLE 2.
Observed and predicted probability for induced amenorrhoea in ASTEROID 2
| Selected population | Treatment period | Bin a | Subjects (n) | Median AUC(0–24)ss within bin (μg*h/L) [minimum, maximum] | Observed probability b | Predicted probability [95% CI] | Corresponding figure |
|---|---|---|---|---|---|---|---|
| Treatment groups A1, B1 and placebo groups A2, B2, C2 in period 1 | 1 | 1 | 18 | 0.00 [0.00, 0.00] | 0.00 | 0.00 [0.00, 0.00] | Figure S9a |
| 2 | 20 | 122 [86.4, 140] | 0.60 | 0.58 [0.48, 0.65] | |||
| 3 | 21 | 192 [141, 177] | 0.71 | 0.59 [0.49, 0.66] | |||
| 4 | 20 | 243 [193, 379] | 0.60 | 0.59 [0.49, 0.66] | |||
| Treatment group A1 in period 1 | 1 | 1 | 14 | 125 [86.4, 166] | 0.57 | 0.58 [0.48, 0.65] | Figure S9b |
| 2 | 14 | 212 [177, 379] | 0.64 | 0.59 [0.49, 0.66] | |||
| Treatment group A1 in period 2 of patients who achieved IA in period 1 | 2 | 1 | 8 | 166 [86.4, 121] | 0.63 | 0.59 [0.49, 0.66] | Figure S9c |
| 2 | 9 | 210 [182, 379] | 0.44 | 0.59 [0.49, 0.66] |
CI: Confidence interval.
Bin: Patients were grouped by individual AUC(0–24)ss in four respectively two bins with similar number of subjects within each bin, i.e. the first bin includes the subjects with the lowest exposure values.
Observed probability: number of subjects with induced amenorrhoea divided by number of subjects within each bin.
The probability for IA in ASTEROID 2 was consistent with the simulated E–R relationship based on ASTEROID 1, as the majority of the per bin observed probabilities in ASTEROID 2 were within the CI predicted for ASTEROID 1. Furthermore, there was no obvious difference in the probability for IA after period 1 (first 12‐week treatment, Figures S9a and S9b in the Supporting Information) compared to period 2 (second 12‐week treatment, Figure S9c in the Supporting Information).
3.5. Exposure–response analysis on oestradiol concentrations
A trend of lower unbound E2 concentrations with increasing vilaprisan exposure was observed with the lowest unbound E2 concentrations after 4 mg/day (Figure 3).
FIGURE 3.
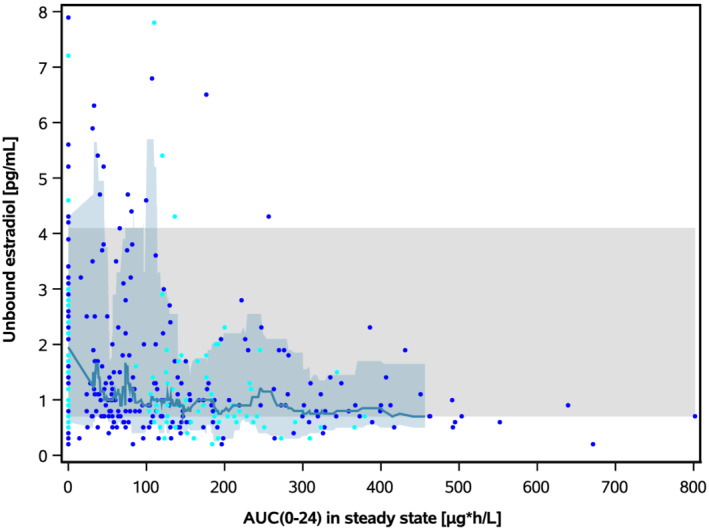
Unbound oestradiol concentration vs AUC(0–24)ss after multiple dosing of placebo and vilaprisan in ASTEROID 1 and ASTEROID 2. One observation per subject following 3 months of treatment with vilaprisan or placebo is shown for the exposure–response population (27 subjects did not contribute to this assessment, i.e. 7.6%, due to missing E2 data). ASTEROID 2: observations from period 1 following placebo treatment are shown for groups A2, B2 and C2, observations from period 1 following verum treatment are shown for groups A1 and B1 and period 2 for groups A2 and B2. Blue symbols: ASTEROID 1. Cyan symbols: ASTEROID 2. Solid line: moving median of the observations. Blue band: 10th to 90th percentile of the observations. Grey band: 10th to 90th percentile of the baseline observations (individual observations not shown) from all subjects in ASTEROID 1 and ASTEROID 2
4. DISCUSSION
In this analysis, the relationship between vilaprisan dose, vilaprisan plasma exposure and induced amenorrhoea or serum oestradiol concentrations as efficacy or safety parameters, respectively, was evaluated to inform the dose for the Phase 3 programme in patients with uterine fibroids.
4.1. Pharmacokinetics
The pharmacokinetics of vilaprisan were adequately described by a two‐compartment model with first‐order elimination and absorption kinetics. This analysis focused on the effect of typical demographical covariates. Of these, body size was selected on the basis that vilaprisan is a highly lipophilic compound showing a pronounced distribution into tissues. 11
Body weight was identified as a significant covariate for V2/F, which was scaled using an allometric model with the commonly accepted coefficient of 1. BMI was identified as a significant covariate for CL/F, yielding a higher CL/F estimate for non‐obese than for obese subjects. This BMI effect is consistent with the covariate findings for other CYP3A4 substrates. 12 As stated by Brill et al., obesity is associated with a lower CYP3A4 activity. However, the underlying mechanism is unclear. 12
Although statistically significant, the identified covariates could only explain small parts of the observed variability (<5%). Furthermore, the overall sample size was still considered to be too limited to perform an extensive covariate analysis to identify intrinsic and extrinsic factors that could explain (part of) the observed variability in vilaprisan PK. Such an analysis would require additional data from Phase 3 studies.
4.2. Exposure–response for efficacy
The E–R analysis of IA in ASTEROID 1 and 2 shows that patients receiving a vilaprisan dose ≥2 mg/day have a close to maximum probability for IA, whereas, due to the steep E–R relationship, lower doses will result in reduced efficacy on IA. The dose–exposure response relationship deviated from the respective dose–response relationship, while showing statistically similar maximum mean effects at doses ≥1 mg/day. 8 This can be rationalized as follows. While the average exposure after 1 mg/day was sufficient for efficacy, there were patients with a lower individual exposure due to, for example, variability in CYP3A4 metabolic activity as discussed previously, which was not expected to result in maximum efficacy. We showed that 36% of the patients had exposure levels that are considered insufficient to reach efficacy after 1 mg/day. After a 2 mg/day dose, this was the case for only 2% of the patients, which indicates that a 2 mg/day dose would be the optimal dose to achieve maximum efficacy in most patients and thus avoiding the risk of less than full treatment effect in some patients. In summary, by considering the inter‐individual variability in exposure, the need for a higher dose to achieve maximum efficacy in the majority of patients becomes apparent. In addition to rapid induction of amenorrhoea, treatment with vilaprisan resulted in significant dose‐dependent reduction of fibroid volume. 8
Evaluating the impact of racial categories on the E–R relationship of IA showed that there was no statistically significant difference in IA between the Black or African American, Asian and rest of the world population (Caucasian, Hispanics and others). The absolute number of Black or African American patients (n = 59) in ASTEROID 1 seems low for a robust assessment, but the proportion of Black or African American women in ASTEROID 1 was even higher than the proportion of Black or African American women in the general US population (22% vs ~18%). Although it cannot be excluded that results may be affected by the low absolute number of Black or African American women—for example, due to an unbalanced distribution of other PK‐relevant covariates such as body weight—we consider the available exposure–response data supportive to suggest a similar exposure–response relationship in this patient population. A higher number of Black or African American women is expected to be included in Phase 3 to allow for a robust exposure–response analysis to further evaluate potential differences between the Black or African American and other populations. In addition, these data will also allow the further characterization of the potential effect of extrinsic factors such as the influence of comedications on vilaprisan clearance due to induction or inhibition of CYP3A4 activity.
Of note, for ulipristal, another SPRM, an observational cohort study found that White women treated with ulipristal experienced higher amenorrhoea rates than Black or African American women. 13 Black or African American women were more dissatisfied with ulipristal acetate compared to White women, despite similar improvement in symptom severity on the Uterine Fibroid Symptom and Quality of Life questionnaire. Since no information on the exposure levels of ulipristal acetate in the two patient populations was provided in the publication, it is not clear whether these findings contrast with the findings described here for vilaprisan, reflecting a difference between molecules. Different definitions of amenorrhoea, different analysis methods and different sample sizes further complicate a comparison.
4.3. Exposure–response for safety
While the exposure–unbound E2 relationship showed a trend of lower E2 with increasing vilaprisan exposure, the E2 concentrations mostly remained within the physiologic range for the follicular phase and E2 levels returned to baseline for all patients in both ASTEROID 1 8 and ASTEROID 2. 9 Although a decrease in E2 levels will contribute to ovulation inhibition and consequently to induction of amenorrhoea, 6 the potential effect of a decrease in E2 levels on long‐term safety is considered to be of higher clinical relevance. This clinical relevance cannot be assessed based on the available vilaprisan short‐term Phase 2 data and will be further evaluated in long‐term safety studies. As both Phase 2 studies showed that a daily treatment with vilaprisan for 12 weeks was well tolerated without safety concerns, no further E–R evaluations other than unbound E2 were considered relevant to select a safe and efficacious vilaprisan dose for the treatment of UFs.
While both efficacy and safety (change in unbound E2) are related to AUC, the identified difference in exposure between non‐obese and obese subjects is not considered clinically relevant for a 2 mg/day dose. Almost all subjects will be above 90% of the maximum effect and the (transient) decrease in unbound E2 was observed to be consistent over the exposure range after a 2 mg dose.
In conclusion, a dose of 2 mg/day of vilaprisan results in a close to maximum probability for IA and is well tolerated, with a moderate and transient decrease in E2 levels. These data informed the selection of the 2 mg/day dose for further evaluation in the Phase 3 programme in patients with UFs.
COMPETING INTERESTS
All authors are employees of Bayer AG and may own stock/options in the company. No author received an honorarium or other form of financial support related to the development of this manuscript.
CONTRIBUTORS
K.P. and C.S. were responsible for the planning, conduct and evaluation of the Phase 2 studies. G.S., M.F., B.A.P. and M.S.‐M. participated in data analyses. All authors participated in data interpretation and collaborated in the preparation of the manuscript, supported by a medical writer (funded by Bayer AG), and critically reviewed and provided revisions to the manuscript. All authors provided final approval of the manuscript. Medical writing and editorial support were provided by Maximilian Becker (Bayer AG).
CLINICAL TRIAL REGISTRATION
Supporting information
FIGURE S1 Distribution of individual η estimates for V2/F (ETA2)
FIGURE S2 Prediction‐corrected visual predictive check for vilaprisan plasma concentrations vs time after dose, final popPK model, 2 mg dose group
FIGURE S3 Distribution of body weight and body mass index stratified by study and racial category
FIGURE S4 Individual η estimates for CL/F (ETA1) vs baseline covariates obtained from the base model
FIGURE S5 Individual η estimates for V2/F (ETA2) vs baseline covariates obtained from the base model
FIGURE S6 Individual η estimates for CL/F (ETA1) vs baseline covariates obtained from the final popPK model
FIGURE S7 Individual η estimates for V2/F (ETA2) vs baseline covariates obtained from the final popPK model
FIGURE S8 Goodness‐of‐fit plots obtained from the final popPK model
FIGURE S9 Predicted and observed probability of induced amenorrhoea (no bleeding event) vs AUC(0–24)ss after multiple dosing in ASTEROID 2
TABLE S1 Accuracy and precision of vilaprisan determination in plasma
TABLE S2 Treatment groups of ASTEROID 2
TABLE S3 Pre‐selected covariates
TABLE S4 Summary of demographics of PK population (excluding subjects treated with placebo)
TABLE S5 Summary of demographics of the exposure–response population (including subjects treated with placebo)
TABLE S6 Parameter estimates obtained from the base model and the final popPK model
TABLE S7 Estimated parameters of the exposure–response model for induced amenorrhoea
TABLE S8 Percentage of patients who reach a probability of the maximum induced amenorrhoea rate after multiple dose administration of vilaprisan
ACKNOWLEDGEMENTS
The authors acknowledge Alexander Solms (Bayer AG) for analysing and providing input on model development. Barbara Schuett was responsible for the planning and conduct of the Phase 1 studies. This study was funded by Bayer AG.
Sutter G, Frei M, Schultze‐Mosgau M‐H, Petersdorf K, Seitz C, Ploeger BA. Assessment of the safe and efficacious dose of the selective progesterone receptor modulator vilaprisan for the treatment of patients with uterine fibroids by exposure–response modelling and simulation. Br J Clin Pharmacol. 2022;88(2):734-741. 10.1111/bcp.15014
The authors confirm that the Principal Investigators for this paper are Barbara Schuett, Corinna Draeger, Lina Bradley and Kristina Gemzell‐Danielsson, and that they had direct clinical responsibility for patients.
Funding information Bayer AG
DATA AVAILABILITY STATEMENT
The authors have chosen not to share the data.
REFERENCES
- 1. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100‐107. [DOI] [PubMed] [Google Scholar]
- 2. Stewart EA, Cookson CL, Gandolfo RA, Schulze‐Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124(10):1501‐1512. [DOI] [PubMed] [Google Scholar]
- 3. Spitz IM. Mifepristone: where do we come from and where are we going? Clinical development over a quarter of a century. Contraception. 2010;82(5):442‐452. [DOI] [PubMed] [Google Scholar]
- 4. Wagenfeld A, Bone W, Schwede W, Fritsch M, Fischer OM, Moeller C. BAY 1002670: a novel, highly potent and selective progesterone receptor modulator for gynaecological therapies. Hum Reprod. 2013;28(8):2253‐2264. [DOI] [PubMed] [Google Scholar]
- 5. Schultze‐Mosgau MH, Schuett B, Hafner FT, et al. Pharmacokinetics and safety of the selective progesterone receptor modulator vilaprisan in healthy postmenopausal women. Int J Clin Pharmacol Ther. 2017;55(1):16‐24. [DOI] [PubMed] [Google Scholar]
- 6. Schutt B, Schultze‐Mosgau MH, Draeger C, et al. Effect of the novel selective progesterone receptor modulator vilaprisan on ovarian activity in healthy women. J Clin Pharmacol. 2018;58(2):228‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schutt B, Kaiser A, Schultze‐Mosgau MH, et al. Pharmacodynamics and safety of the novel selective progesterone receptor modulator vilaprisan: a double‐blind, randomized, placebo‐controlled phase 1 trial in healthy women. Hum Reprod. 2016;31(8):1703‐1712. [DOI] [PubMed] [Google Scholar]
- 8. Bradley LD, Singh SS, Simon J, et al. Vilaprisan in women with uterine fibroids: the randomized phase 2b ASTEROID 1 study. Fertil Steril. 2019;111(2):240‐248. [DOI] [PubMed] [Google Scholar]
- 9. Gemzell‐Danielsson K, Heikinheimo O, Zatik J, et al. Efficacy and safety of vilaprisan in women with uterine fibroids: data from the phase 2b randomized controlled trial ASTEROID 2. Eur J Obstet Gynecol Reprod Biol. 2020;252:7‐14. [DOI] [PubMed] [Google Scholar]
- 10. Roche . Elecsys Estradiol III. Available from: https://www.rochecanada.com/content/dam/rochexx/roche-ca/products/docs/package_inserts/Can%20PI%20Estradiol%20III-06656021190-V6-En.pdf. Accessed on 27 May 2021.
- 11. Schultze‐Mosgau MH, Hochel J, Prien O, et al. Characterization of the pharmacokinetics of vilaprisan: bioavailability, excretion, biotransformation, and drug–drug interaction potential. Clin Pharmacokinet. 2018;57(8):1001‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277‐304. [DOI] [PubMed] [Google Scholar]
- 13. Murji A, Crosier R, Chow T, Ye XY, Shirreff L. Role of ethnicity in treating uterine fibroids with ulipristal acetate. Fertil Steril. 2016;106(5):1165‐1169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Distribution of individual η estimates for V2/F (ETA2)
FIGURE S2 Prediction‐corrected visual predictive check for vilaprisan plasma concentrations vs time after dose, final popPK model, 2 mg dose group
FIGURE S3 Distribution of body weight and body mass index stratified by study and racial category
FIGURE S4 Individual η estimates for CL/F (ETA1) vs baseline covariates obtained from the base model
FIGURE S5 Individual η estimates for V2/F (ETA2) vs baseline covariates obtained from the base model
FIGURE S6 Individual η estimates for CL/F (ETA1) vs baseline covariates obtained from the final popPK model
FIGURE S7 Individual η estimates for V2/F (ETA2) vs baseline covariates obtained from the final popPK model
FIGURE S8 Goodness‐of‐fit plots obtained from the final popPK model
FIGURE S9 Predicted and observed probability of induced amenorrhoea (no bleeding event) vs AUC(0–24)ss after multiple dosing in ASTEROID 2
TABLE S1 Accuracy and precision of vilaprisan determination in plasma
TABLE S2 Treatment groups of ASTEROID 2
TABLE S3 Pre‐selected covariates
TABLE S4 Summary of demographics of PK population (excluding subjects treated with placebo)
TABLE S5 Summary of demographics of the exposure–response population (including subjects treated with placebo)
TABLE S6 Parameter estimates obtained from the base model and the final popPK model
TABLE S7 Estimated parameters of the exposure–response model for induced amenorrhoea
TABLE S8 Percentage of patients who reach a probability of the maximum induced amenorrhoea rate after multiple dose administration of vilaprisan
Data Availability Statement
The authors have chosen not to share the data.


