Abstract
Background
The Florin model is the commonly accepted theory of coniferous seed scale evolution. It describes the derivation of extant seed scale morphology from the morphology of fossil conifers via the reduction of complex to simple axillary structures. In this framework the seed scale is composed of a reduced lateral shoot with fertile and sterile appendages which are interpreted as leaf homologues.
Scope
The Florin model has three crucial problems that we address here: (1) the original derivation series does not take the ontogeny of extant conifers into account, (2) it cannot explain the morphology of all extant conifers and (3) Taxaceae were originally excluded. Examination of seed cones of extant conifers shows that ovules occur in three different positions in the cone: in an axillary position, replacing a leaf or terminating the cone axis. By interpreting the fertile appendage or seed-bearing structure as a leaf, not all positions are possible. The exclusion of Taxaceae from conifers is in stark contrast to recent molecular phylogenetic studies, which include Taxaceae in conifers as sister to Cupressaceae. Therefore, the Florin model does not offer an adequate explanation for taxaceous morphology.
Conclusion
We conclude that the seed-bearing structure of conifers cannot be interpreted as homologous to a leaf. In the interpretation we present here, the seed-bearing structure is the modified funiculus of the ovule, multiples of which laterally fuse to form the seed scale. The seed scales of all extant conifers can be derived from a Cunninghamia-like morphology via fusion and reduction of individual funiculi.
Keywords: Conifer, Florin model, seed-cone morphology, character evolution
INTRODUCTION
The interpretation of the coniferous seed cone and seed scale by Rudolf Florin (1931, 1951) and the model named after him are still the basis for interpreting coniferous seed cones today. In the Florin model the seed scale of extant and fossil conifers comprises up to three fused parts, i.e. a stalked ovule, sterile appendages and a rudimentary axis. In the time since its inception the Florin model has been criticized and revised by several authors. For example, Schweitzer (1963) criticized the presence of stalked ovules and presented a re-interpretation without them, whereas Clement-Westerhof (1988) revised the attachment of the ovule to the fertile appendage. However, the core of the Florin model, a continued fusion of the constituent parts and the presence of a rudimentary lateral axis and sporophylls, has always remained despite revisions.
In this viewpoint we present a new interpretation of the coniferous seed scale that challenges these core principles of the Florin model. To achieve this, we incorporate insights gained from palaeobotany, and ontogenetic and phylogenetic studies into a new model of conifer seed scale evolution.
In the past the term seed scale has been used for a variety of parts in the seed cone. To avoid confusion and misinterpretation we will use the following terms for structures (Supplementary Data Fig. S1):
Cone unit: repeating structure on a coniferous seed cone comprising a single bract and all axillary structures
Seed scale: a single or multiple structures axillary to a bract, the entirety of all seed-bearing structures, which may be fused to various degrees
Seed-bearing structure: a single structure supporting the ovule/seed, part of the seed scale, which may be conspicuous or reduced to only an ovule
THE FLORIN MODEL AND ITS PROBLEMS
In our view the Florin model deals with two separate parts of the evolution of the coniferous seed scale: (1) the morphological interpretation of the constituent parts of the seed scale, and (2) the mechanisms by which those parts evolved from the Carboniferous onwards (Florin, 1951, 1954). Morphologically the coniferous seed scale is currently interpreted as a lateral short shoot in the axil of the bract. This short shoot can have multiple appendages that are currently widely interpreted as leaves and sporophylls (Clement-Westerhof, 1988; Rothwell et al., 2011). In extant conifers all appendages and the short shoot are fused together or reduced so that only the sporophylls are visible. For example, the seed scale of Pinaceae in the Florin model comprises at least two sporophylls (one for each ovule) and a short shoot, which are completely fused together to form the typical scale-like form (Mundry, 2000). The other extreme is the cone units of, for example, Cupressus L., in which the short shoot is reduced so drastically that only the individual ovules remain visible (Jagel and Dörken, 2015). From a mechanistic perspective our current understanding of seed scale evolution has not changed since the descriptions by Florin. He inferred that the seed scale of extant conifers can be derived from the seed scale of Palaeozoic conifers via fusion and reduction (Florin, 1951). Although the specifics of fossils and possible derivation series have been debated, the mechanism of fusion and reduction from the complex seed scale of Palaeozoic conifers to the simple seed scale of extant conifers has never been doubted (Clement-Westerhof, 1988; Meyen, 1997; Rothwell et al., 2011; Escapa et al., 2013; Leslie et al., 2018).
In our view the Florin model has three problems. The first arises from the genus Cryptomeria (Thunb. ex L. f.) D.Don, a cupressaceous conifer. In his derivation series from Palaeozoic to extant conifers, Florin (1951) interpreted the morphology of the seed scale of Cryptomeria as the most basal one in conifers. He inferred that the ‘teeth’ that close the cone of Cryptomeria are the seed-bearing structure and are homologous to the appendages (leaf homologues) found in the seed scale of the Permian conifer Pseudovoltzia Florin and the seed-bearing structures of all other extant conifers (Florin, 1951). Ontogenetic studies of coniferous seed scales have shown that in all examined taxa with a conspicuous seed-bearing structure, the ovule develops after and on the former (e.g. Takaso and Tomlinson, 1991; Tomlinson, 1992; Herting and Stützel, 2020). However, the development of Cryptomerias’ ‘teeth’ only starts after the ovules are well developed, and the ‘teeth’ are initiated abaxial to ovules, as seen from the cone axis (Farjon and Garcia, 2003). The position and time of initiation of the ‘teeth’ suggest that they are best interpreted as a sterile second row of ovules. Additionally, seed scales with multiple rows of ovules can be found in taxa that are both more basal and more derived within Cupressaceae s.l., such as Sequoia (Luerss.) Quinn (Takaso and Tomlinson, 1992) and Cupressus (Jagel and Dörken, 2015), respectively. Thus, the ‘teeth’ of Taxodioideae [Cryptomeria, Glyptostrobus (Staunton ex D.Don) K.Koch, Taxodium L.C.Rich.] are not homologous to the seed-bearing structures of the other extant conifers, and the derivation series originally proposed by Florin (1951) is not suitable to homologize the seed scales of fossil and extant conifers.
The second problem of the Florin model is that it cannot explain the morphology of all extant conifer seed scales. The most prominent example are the cones of Juniperus L. Ontogenetic and morphological studies of Juniperus cones show that the ovules can occur in three principal positions in the cone: (1) axillary to a bract, (2) continuing cone phyllotaxis instead of a bract, and (3) terminal on the cone axis (Schulz et al., 2003). Additionally, ovules in all three positions may occur mixed within the same cone. If mixed, those in the axil of a bract are basal within the cone to those that take the position of a cone scale. Schulz et al (2003) suggested a terminal brachyblast (short shoot) consisting of lateral ovules and also or exclusively an ovule terminating the cone axis. Most importantly, the terminal position of an ovule is not reconcilable with the basic assumption in the Florin model that seed-bearing structures of conifers are sporophylls, because a leaf can never terminate an axis (Marilaun, 1890). Therefore, not all existing seed cone morphologies can be explained within the framework of the Florin model.
The third problem for the Florin model is the family Taxaceae. Taxaceae are problematic for the Florin model for two reasons. First, similar to Juniperus the ovules of taxaceous taxa are found in a terminal position (Harris, 1976; Röwekamp and Stützel, 1999). Second, in the presentation of his model Florin (1951) himself noted that some Taxaceae do not fit within it. Consequently, Florin (1954) separated Cephalotaxus as a family of its own, excluded taxads from Coniferales and placed them in their own order Taxales. This separation has been disproven and Taxaceae have been included in the conifers since at least the molecular phylogenetic studies of the past 20 years (e.g. Stefanovic et al., 1998; Leslie et al., 2012). This means that the model used for describing conifer seed scale evolution must also explain Taxaceae morphology. Leslie et al. (2018) extended the Florin model by proposing a disassociation between the ovule and the supporting bract, and shift of the ovule into a terminal position. However, the underlying problem is not resolved because, as stated above, in the framework of the Florin model the ovule is situated on a sporophyll. Thus, even if the sporophyll is inconspicuous, it can never be found in terminal position as a leaf homologue.
In summary, ontogenetic and phylogenetic studies have revealed that the Florin model cannot explain the diversity of extant conifer seed scale morphology. The seed-bearing structure of extant conifers must fulfil the following criteria: (1) may take axillary, leafor terminal position; (2) and interpretation should be applicable over the course of the whole ontogeny.
THE NEW INTERPRETATION
Keeping the aforementioned criteria in mind, we interpret the coniferous seed-bearing structure as the ovule and its stalk – the funiculus (see text box). Consequently, the seed scale is either just the funiculus, when there is only one ovule (e.g. Araucaria Juss.), or the fusion of at least as many funiculi as there are ovules (e.g. Pinus L.). The ovule is a stalked nucellus mantled by the integument, with the nucellus being homologous to the megasporangium of ferns. Thus, the ovule is essentially a stalked sporangium, as found in ferns or taxa that pre-date the development of the cormus, i.e. the plant body is differentiated in root, shoot and leaves. In the telome theory, each sporangium is formed by a single fertile telome, an undifferentiated axial structure (Zimmermann, 1930). Reproductive structures are exposed to environmental selection pressure only a rather short phase of the life cycle. Additionally, their precise function is essential for the survival of the species and these structures tend to be very conservative during evolution. Therefore, we interpret the seed-bearing structure, i.e. ovule and funiculus, as a single fertile telome. Accordingly, the seed scale, which is formed by one to many seed-bearing structures, comprises one to many fertile telomes. Being neither shoot nor leaf, it is unrestricted by the principles of phyllotaxis, and may occur in all positions outlined above. This means that in the most common case of an axillary position of the seed-bearing structures, they insert directly axillary without a lateral short shoot.
In contrast to the Florin model in its current iteration, our interpretation of the seed-bearing structure meets both criteria set above. First, the retention of its telome characteristics allows the funiculus to occur in all observable positions of the ovule in extant conifers, even if they occur mixed [e.g. Juniperus phoenicia L. (Jagel, 2001)]. Second, ontogenetically the funiculus always precedes the ovule. It may be very short and grow in length only later, but as the part attaching the ovule to the plant it is present earlier than all structures characterizing the ovule (e.g. Tomlinson, 1992; Farjon and Garcia, 2003; Herting and Stützel, 2020).
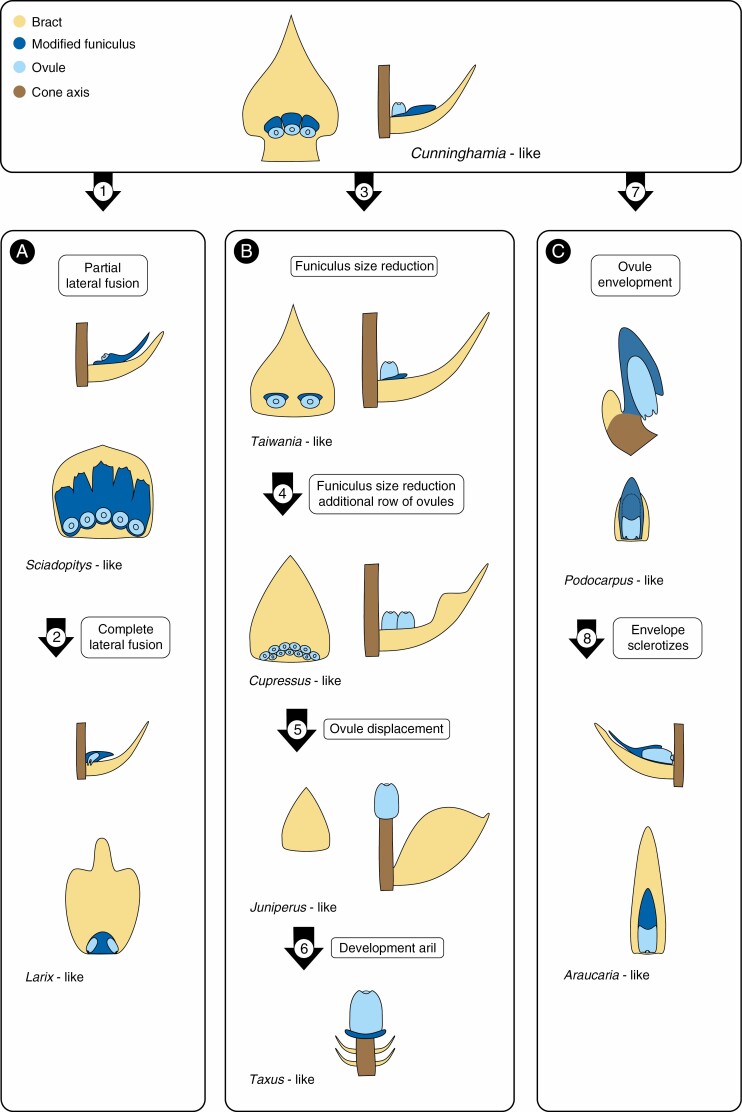
In our 2020 paper on the ontogeny of the seed scale of Araucaria araucana (Molina) K.Koch, we briefly showed that the seed scales of Araucariaceae, Pinaceae, Podocarpaceae and Sciadopityaceae can be derived from a Cunninghamia R.Br. ex A.Rich.-like morphology if the funiculus is assumed to be the seed-bearing structure (Fig. 1) (Herting and Stützel, 2020). Note in the following that we shorten terms such as Cunninghamia-like morphology to just Cunninghamia for readability purposes. We use extant taxa for a comparative analysis of the ontogeny and morphology of the cone units. The outcome is then used to infer a possible development of structures from the fossil record. In other words, the morphology and ontogeny of extant taxa used in our derivation series serves as a stand-in for possible fossil forms. Therefore, the order of taxa in our derivation series is not representative of our understanding of conifer phylogeny. We consider the morphology of the cone unit of Cunninghamia, a phylogenetically derived taxon (e.g. Spencer et al., 2015; Leslie et al., 2018), as the most basal in extant conifers. Cunninghamia and many Permian Voltzian Voltziales (e.g. Florin, 1951; Schweitzer, 1963) share crucial morphological features of the cone unit: (1) a one to one relationship of ovules and seed-bearing structures, (2) unfused seed-bearing structures and (3) inverted seeds. Therefore, we chose the morphology of Cunninghamia as the basal morphology in our analyses. This can be risky, however, because heterobathmic effects should be considered. We agree with Spencer et al. (2015) that the ontogeny is crucial information for ascertaining homology. Thus, we use extant taxa instead of fossils because this approach allows us to incorporate the ontogeny of the cone units into the analyses. Ontogenetic data is rarely available in the fossil record, especially concerning the time frame in the ontogeny we focus on, which is from bud break to pollination. Ontogenetic stages found in the fossil record are usually from later stages of development, for example development of the seed in Manifera talaris Looy et Stevenson (Looy and Stevenson 2014). We use ontogenetic data because they have seldom been used in studies presenting derivation series, which focused of deriving extant from fossil taxa (e.g. Florin, 1951; Rothwell et al., 2011).
Fig. 1.
Expanded derivation series of extant conifer seed scale morphology. The morphology of all extant conifers can be derived from a Cunninghamia-like form in one of the three pathways shown. The Pinaceae-like form (represented here by Larix) (A), with a continuous scale supporting more than one ovule, can be derived via: first the postgenital partly fusion of funiculi (1) to form a Sciadopitys-like scale; and second the congenital complete lateral fusion of funiculi to form a continuous scale (2). The Taxaceae-like form (represented here by Taxus) (B), with a single terminal ovule, can be derived via size reduction of the funiculus first to a ridge abaxial of the ovule (3) as in Taiwania; second to inconspicuousness (4), in a Cupressus-like form; thirdly, via the reduction to a single ovule and the displacement of this ovule to the apex of the cone axis, as found in some species of Juniperus (5); and lastly, through the development of an aril and size reduction of the bract (6). The Araucariaceae-like form (represented here by Araucaria) (C), with a scale enveloping a single ovule, can be derived via: first the reduction of the number of ovules to one per cone unit and envelopment of that ovule by its funiculus (7), resembling a Podocarpus-like morphology; and second the enveloping tissue becoming sclerenchymatic (8). Yellow: bract; light blue: ovule; dark blue: funiculus and outgrowths of the funiculus; brown: cone axis. Figure expanded from Herting and Stützel (2020).
The seed scale of Pinaceae may be derived from a Cunninghamia-like morphology through Sciadopitys Sieb. & Zucc., via lateral fusion of the funiculi and a heterochronic shift (‘Zeitkorrelationsänderung’ sensuIhlenfeldt, 1971), resulting eventually in the lateral congenital fusion of the funiculi (Fig. 1A) (Herting and Stützel, 2020). On the other hand, Araucariaceae are derived from Cunninghamia through Podocarpaceae, via reduction of the number of ovules per cone unit and the envelopment of the ovule by an adaxial outgrowth of the funiculus (Fig. 1C) (Herting and Stützel, 2020).
Here we expand on that derivation series and show that the coniferous taxa without a conspicuous seed scale can be derived from Cunninghamia. These taxa are most of Cupressaceae s.l. and Taxaceae. Cunninghamia has one lobe-like modified funiculus per ovule and the ovules are turned inwards after pollination (Farjon and Garcia, 2003). These lobe-like modified funiculi are successively reduced until they are no longer detectable in taxa such as Cupressus, where the ovules emerge directly in the axil of the bract and are not turned in after pollination (Fig. 1B). The most extreme reduction can be observed in the cones of Juniperus squamata Buch.-Ham. ex D.Don, which at maturity comprise a single whorl of bracts and a single ovule terminal on the cone axis (Jagel and Dörken, 2015). An intermediate form is found in Taiwania Hayata; here the funiculi are reduced compared to Cunninghamia, but are still observable (Farjon and Garcia, 2003). The reduction of the conspicuous modified funiculus can be explained by a shift of timing in the ontogenetic sequence. The emergence of the funiculus and the ovule on it are moved closer together until they become indistinguishable. This is a prime example of a ‘basal abbreviation of the ontogenetic sequence’ (‘basale ontogenetische Abbreviation’ sensuIhlenfeldt, 1971), which means that steps early in the ontogeny are skipped.
In Taxaceae the ovule emerges terminally on a second- or third-order branch and is preceded by one or two whorls of leaves (Röwekamp and Stützel, 1999). Thus, we consider this second- or third-order branch with the terminal ovule to be the cone of Taxaceae and use it for comparison with conifer cones. The cone morphology of Taxaceae is very similar to that of some species of Juniperus (e.g. J. squamata), which comprise a terminal ovule and, in J. squamata, one whorl of bracts. Therefore, the fertile structure of Taxaceae may be derived from Cunninghamia similarly to Juniperus (Fig. 1B). The strongly reduced cones with terminal ovules of Taxaceae and Juniperus are an example of parallel evolution in conifers. The aril of Taxaceae is a further indication of the presence of the funiculus because the aril is defined as an outgrowth of the funiculus (Marilaun, 1890). This definition leads to an interesting observation concerning Podocarpaceae (except Phyllocladus L.C.Rich & A.Rich) and Araucariaceae. In both families an adaxial outgrowth distal to the ovule envelops or envelops and turns the ovule; the only difference to the aril of Taxaceae is the sequence of emergence in the ontogeny and it appears ‘one-sided’ due to its position (Tomlinson, 1992; Herting and Stützel, 2020). In Podocarpaceae (except Phyllocladus) and Araucariaceae, the outgrowth emerges before pollination, whereas in Taxaceae and Phyllocladus the aril emerges after pollination (Tomlinson et al., 1989; Röwekamp and Stützel, 1999). This again can be explained by a heterochronic shift during the ontogeny; thus, we conclude that the aril of Taxaceae and Phyllocladus and the enveloping tissue in Podocarpaceae and Araucariaceae evolved in parallel from the funiculus.
In this new framework, we expect certain trait combinations to occur in crown conifers, while some other combinations should not occur. As outlined above, the funiculus is functionally linked to the turning of the ovule. Thus, we expect that a conspicuous funiculus is always paired with an inward facing ovule, e.g. in Pinaceae or Podocarpaceae, which is also suggested by ancestral state reconstructions of crown conifers (Herting et al., 2020). Consequently, an inconspicuous funiculus should always occur together with an outward facing ovule, e.g. in Cupressaceae s.s. Other combinations, such as a conspicuous funiculus with an outward-facing ovule, should not occur in this framework of interpretation.
In summary, we have shown that the seed-bearing structure is best interpreted as a modified funiculus. Hence, the seed scale is best interpreted as the fusion product of at least as many funiculi as there are ovules. Based on this interpretation, the seed scales of all extant conifers can be derived from a morphology similar to that of Cunninghamia and that the aril of Taxaceae and the enveloping tissues of podocarps and Araucariaceae are homologous. The structures termed seed scale in Cryptomeria, Taxodium and Glyptostrobus are not homologous to the seed scale of other conifers discussed here.
THE MODIFIED FUNICULUS
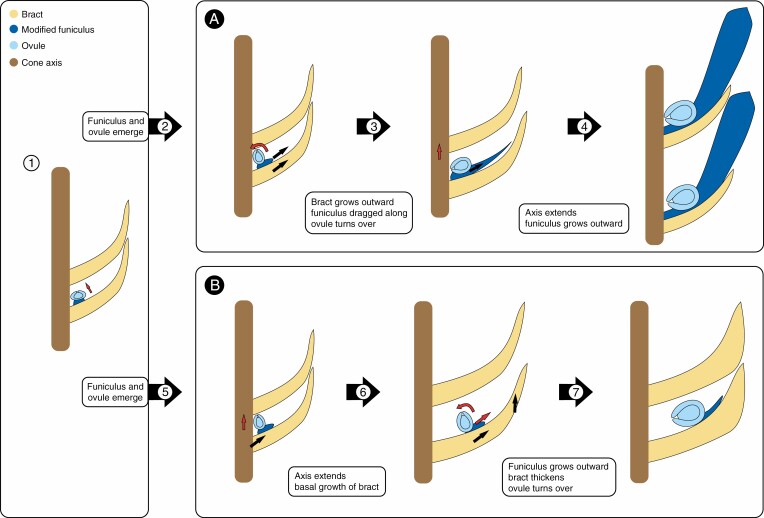
During the ontogeny of taxa with conspicuous funiculi, the mechanisms of modification of the funiculi are revealed. A notable difference in such taxa is the position relative to the cone axis of the ovule on its funiculus. Although the ovule is always adaxially attached, the offset from the cone axis varies, e.g. directly axillary in Pinaceae, or recaulescently shifted on the bract in Cunninghamia (Mundry, 2000; Farjon and Garcia, 2003). Additionally, a conspicuous modified funiculus always occurs together with ovules or seeds that are turned inwards (Tomlinson, 1992; Mundry, 2000; Jagel and Dörken, 2014; Herting et al., 2020; Herting and Stützel, 2020). A factor in the modification of the funiculus is the available space in the bud, especially in taxa with cones comprising many cone units. The growth restriction enforced by this confined space results in friction between emerging ovules and the superadjacent bracts (Fig. 2B). Continued intercalary growth of the bracts then leads to turning over of the ovule and elongation of the funiculus into a scale-like form (Fig. 2C). The influence of spatial constraint within the bud on the morphology of emerging organs has been shown for angiosperm leaves, e.g. Acer pseudoplatanus (Kobayashi et al., 1998; Couturier et al., 2009, 2011, 2012; Edwards et al., 2016). Couturier et al. (2011), in particular, show in their ‘kirigami theory’ that the shape of leaves is determined by packing in the bud and contact between successive leaves. A similar influence of friction can be observed on proximal leaves of the inflorescence of Drimia maritima (L.) Stearn; through friction with the bulb during inflorescence emergence the proximal leaves are distinctly shaped like an upward pointing hook, while leaves that develop after emergence are linear to lanceolate shaped, with intermediate forms between (Fig. 3). This comparison is not intended to infer homology between the funiculus of conifers and the leaves, but to illustrate that friction within buds can influence the morphology of the emerging plant organ. Depending on when the cone axis extends, breaking contact between the ovule and the superadjacent bract, the ovule is either facing outwards or inwards during pollination. The resulting gap between the bracts must subsequently be closed to protect the developing seed, and in some conifers this is done by an enlarging and sclerotizing funiculus (e.g. Pinaceae) or a thickening sclerotizing bract (e.g. Cunninghamia) (Figs. 2A, B). If the funiculus remains short, closure of the cone after pollination may be achieved by a ventral thickening or bulge of the cone scale (Cupressus, Sequoiadendron J.Buchholz). In the latter case the micropyle may be directed to the cone axis (Sequioadendron) or to the periphery, depending on whether the intercalary growth of the cone scale takes place basal (Sequoiadendron) or distal to the insertion of the funiculi. This is an example of the same function being performed by different morphological structures. Another example is the turning over of the ovule in Podocarpaceae. Here the ovule is turned by an outgrowth of the funiculus, not by friction within the bud. The funicular outgrowth subsequently envelops the developing ovule and ensures protection of the seed during endozoochorous dispersal (Tomlinson, 1992). These examples show that the morphology of the funiculus is highly variable and that its modifications are tightly linked either to pollination or to seed dispersal. The disparity observed between the seed cones and the modified funiculi probably stems from multiple functional demands on a seed cone (summarized in Leslie and Losada, 2019).
Fig. 2.
Possible ways of modification of the funiculus in taxa with a morphology like Pinaceae (A) and Cunninghamia (B). In both cases the ovule and the funiculus emerge and grow upwards towards the next bract until the ovule hits the next bract (1, red arrow). In the Pinaceae-like morphology (A) growth in the part of the bract distal from the funiculus drags along the funiculus extending it towards the tip of the bract (2, black arrows). The funiculus is dragged along by friction and the ovule is turned by friction with the next bract (2, red arrow). After the bud breaks, the cone axis extends for pollination (3, red arrow) and the funiculus expands into the opening space (3, black arrow). The funiculus then expands, exceeding the bract and closing the cone by thickening and sclerotizing (4). In the Cunninghamia-like morphology (B), the ovule and funiculus are moved towards the tip of the bract by growth of the bract basal from the funiculus (5, black arrow). Additionally, the cone opens via elongation of the cone axis (5, red arrow). The opening space is closed after pollination by thickening and elongation of the bract (6, black arrows). The elongation additionally repositions the ovule and funiculus distally onto the bract. The funiculus extends slightly and the ovule turned via this growth and friction with the next bract (6, red arrows). As a result, the cone is closed by the bracts and the modified funiculus remains comparatively small (7). Yellow: bract; light blue: ovule; dark blue: funiculus and outgrowths of the funiculus; brown: cone axis.
Fig. 3.
Photograph of the proximal part of the inflorescence of Drimia maritima. Arrow a: hook-like proximal leaf with a typical fusion of the adaxial sides, which are the result of friction with the bud during emergence of the inflorescence. Arrow b: leaf further apical in the inflorescence; the upward pointing tip is still present, but there is no further adaxial fusion.
IMPACT OF THE INTERPRETATION
Early-divergent fossil conifers are usually split into Walchian Voltziales and Voltzian Voltziales (Taylor et al., 2009) (Supplementary Data Fig. S2). The former occur exclusively in the Palaeozoic, whereas the latter have been described from the Permian to the Cretaceous. According to the Florin model the seed scale of Voltzian Voltziales comprises an axillary dwarf shoot with fertile and sterile leafy appendages. However, in phylogenetic reconstructions, Voltzian Voltziales and extant conifers form a clade (Rothwell et al., 2005; Herrera et al., 2020). Thus, it is necessary to re-investigate whether the inference of an axillary dwarf shoot is necessary to explain the morphology of Voltzian Voltziales. Voltzian Voltziales and extant conifers share the adaxial (relative to the cone axis) and subterminal attachment of the ovule on the seed-bearing structure. Additionally, there are always at least as many appendages as ovules in Voltzian Voltziales, similar to the one-to-one of ovule to funiculus in Cunninghamia (Schweitzer, 1963; Looy and Stevenson, 2014; Herrera et al., 2015). We infer that the fertile appendages of Voltzian Voltziales are the funiculi of the ovules and are homologous to the funiculi of extant conifers. Thus, the seed-bearing structure of Voltzian Voltziales and extant conifers is only the funiculus. Similar to extant conifers, the funiculi of Voltzian Voltziales can be observed in various stages of fusion in different taxa, from partly fused in Pseudovoltzia (Schweitzer, 1963), to almost completely fused in Majonica Clement-Westerhof (Clement-Westerhof, 1987), and to completely fused in Compsostrobus Delevoryas & Hope (Delevoryas and Hope, 1987). Additionally, the ovules of Voltzian Voltziales are found in a similar position on the funiculi as in extant conifers, e.g. near the apex in Pseudovoltzia and Cunninghamia (Schweitzer, 1963; Farjon and Garcia, 2003), or near the base in Krassilovia Herrera, Shi, Leslie, Knopf, Ichinnorov, Takahashi, Crane et Herendeen and in Pinaceae (Mundry, 2000; Herrera et al., 2015).
In contrast to Voltzian Voltziales, Walchian Voltziales have a clearly distinguishable axillary dwarf shoot, which has spirally arranged leaves and stalked ovules interspersed with the leaves (summarized in Taylor et al., 2009). In our new framework of interpretation, the stalk of the ovule of Walchian Voltziales is the funiculus. The funiculus of Walchian Voltziales is recurved towards the cone axis in most taxa, so that the ovule points inwards, e.g. Ortiseia Clement-Westerhof (Clement-Westerhof, 1984). Superficially this seems reminiscent of Voltzian Voltziales or extant conifers. However, the funiculus of Walchian Voltziales is recurved towards its abaxial side relative to its supporting axis (axillary dwarf shoot), rather than the adaxial side in Voltzian Voltziales and extant conifers. The three groups of conifers have in common that their ovules are attached subterminally on the funiculus and that the ovule faces towards the cone axis. Additionally, the funiculus of Walchian Voltziales can show severe modifications in the chalazal regions, such as horn-like outgrowths in Ortiseia (Clement-Westerhof, 1984); this demonstrates the modifiability of the funiculus.
In addition to fossil conifers, Gnetophytes should be considered in this new framework because some molecular phylogenetic studies infer a close relationship between conifers and Gnetophytes (Magallón and Sanderson, 2002; Ran et al., 2010; Zhong et al., 2010; Burleigh et al., 2012). For this we follow the interpretation of the gnetalean ‘flower’ by Mundry and Stützel (2004), which infers that the ‘flower’ sits terminally on an almost completely reduced lateral axis and is preceded by one (Ephedra L.) or two (Gnetum L., Welwitschia Hook f.) pairs of decussate leaves (Takaso and Bouman, 1986; Mundry and Stützel, 2004; Yang, 2004). Multiples of these ‘flowers’ form the cone-like inflorescence of Gnetophytes. The morphology of the gnetalean ‘flower’ is strikingly similar to that of J. squamata or Taxaceae; both have a terminal ovule that is preceded by one (Juniperus) or two (Taxaceae) whorls of leaves. In contrast to Taxaceae, Gnetophytes lack an arillus and thus an implicit sign of the funiculus. Nonetheless, the Gnetophytes may be derived from a Cunninghamia-like morphology in a similar fashion to Taxaceae, as outlined above (Fig. 1). That means that one gnetalean ‘flower’ would be homologous to a single coniferous seed cone. Therefore, from a purely morphological standpoint there is merit in the inclusion of Gnetophytes into conifers. However, this complex is best resolved in a dedicated study of the comparative morphology of Gnetophytes and conifers.
CONCLUSION
Interpreting the coniferous seed scale as the fused funiculi remedies the discrepancies between the interpretation of the morphology of coniferous female cone units and the reconstructed phylogenies. Previous interpretations of coniferous reproductive morphology carried with them erroneous assumptions dating back decades. These assumptions have not been addressed, such as the initial exclusion of Taxaceae from conifers. In the framework presented above, all female cone units are homologous, as they may all be derived from a common ancestral morphology. Interpreting the seed-bearing structure as the funiculus opens new perspectives from which to study conifer evolution and possibly taxa such as Gnetophytes or Gingko. We strongly advocate re-investigation of extant and fossil conifers in the framework provided here, to further uncover mechanisms of the modification of the funiculus.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Fig. S1: Explanatory drawings of the constituent parts of the coniferous seed scale. Fig. S2: Example comparison between the cone units of Walchian and Voltzian Voltziales.
Text Box.
In our model of seed scale evolution, the seed scale of crown conifers evolved from multiple free ovule + seed-bearing structure units like those found in Cunninghamia and Permian Voltzian Voltziales, e.g. Pseudovoltzia. We see the ovule + seed-bearing structure unit as the ovule + funiculus. The unit inserts directly in the axil of the bract without a lateral short shoot, instead of a bract, or terminally on the cone axis. Together, ovule + funiculus represent a single fertile telome, an undifferentiated axial structure that is neither shoot nor leaf. The morphology of extant conifers can be derived by modification of the funiculus from a lobe-like ancestral form, e.g. as found in Cunninghamia and Permian Voltzian Voltziales. The modifications include, for example, lateral fusion of the recurved funicle to form a continuous scale (Pinaceae), reduction of the number of ovule + funiculus units and envelopment of the ovule by the aril-like funiculus (Podocarpaceae). A size reduction of the funiculus with or without a shift of the ovule onto the cone scale leads to complete lack of a scale-like seed scale (Cupressaceae s.s.). Sterile ovules may form scale-like units which are also termed the seed scale (Cryptomeria, Taxodium, Glyptostrobus), but these are not homologous to the seed scales addressed here.
ACKNOWLEDGMENTS
We thank the Handling Editor, Karolina Heyduk, and Review Editor, Trude Schwarzacher, for managing our manuscript and giving helpful suggestions that improved it. We also thank two anonymous reviewers for their comments and suggestions. J.H. additionally thanks N. van Aaken for proof-reading drafts.
Contributor Information
Julian Herting, Ruhr-Universität Bochum, Fakultät für Biologie und Biotechnologie, Evolution und Biodiversität der Pflanzen, Universitätsstraße 150, 44801 Bochum, Germany.
Thomas Stützel, Ruhr-Universität Bochum, Fakultät für Biologie und Biotechnologie, Evolution und Biodiversität der Pflanzen, Universitätsstraße 150, 44801 Bochum, Germany.
LITERATURE CITED
- Burleigh JG, Barbazuk WB, Davis JM, Morse AM, Soltis PS. 2012. Exploring diversification and genome size evolution in extant gymnosperms through phylogenetic synthesis. Journal of Botany 2012: 1–6. [Google Scholar]
- Clement-Westerhof JA. 1984. Aspects of Permian palaeobotany and palynology. IV. The conifer Ortiseia FLORIN from the Val Gardena formation of the Dolomites and the Vicentinian alps (Italy) with special reference to a revised concept of the Walchiaceae (GÖPPERT) SCHIMPER. Review of Palaeobotany and Palynology 41: 51–166. [Google Scholar]
- Clement-Westerhof JA. 1987. Aspects of Permian palaeobotany and palynology, VII. The Majonicaceae, a new family of Late Permian conifers. Review of Palaeobotany and Palynology 52: 375–402. [Google Scholar]
- Clement-Westerhof JA. 1988. Morphology and phylogeny of paleozoic conifers. In: Beck CB, ed. Origin and evolution of gymnosperms. New York: Columbia University Press, 298–337. [Google Scholar]
- Couturier E, Brunel N, Douady S, Nakayama N. 2012. Abaxial growth and steric constraints guide leaf folding and shape in Acer pseudoplatanus. American Journal of Botany 99: 1289–1299. [DOI] [PubMed] [Google Scholar]
- Couturier E, Courrech du Pont S, Douady S. 2009. A global regulation inducing the shape of growing folded leaves. PLoS One 4: e7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier E, du Pont SC, Douady S. 2011. The filling law: a general framework for leaf folding and its consequences on leaf shape diversity. Journal of Theoretical Biology 289: 47–64. [DOI] [PubMed] [Google Scholar]
- Delevoryas T, Hope RC. 1987. Further observations on the late Triassic conifers Compsostrobus neotericus and Voltzia andrewsii. Review of Palaeobotany and Palynology 51: 59–64. [Google Scholar]
- Edwards EJ, Spriggs EL, Chatelet DS, Donoghue MJ. 2016. Unpacking a century-old mystery: winter buds and the latitudinal gradient in leaf form. American Journal of Botany 103: 975–978. [DOI] [PubMed] [Google Scholar]
- Escapa IH, Cúneo NR, Rothwell GW, Stockey RA. 2013. Pararaucaria delfueyoi sp. nov. from the late Jurassic Cañadón Calcáreo formation, Chubut, Argentina: insights into the evolution of the Cheirolepidiaceae. International Journal of Plant Sciences 174: 458– 470. [Google Scholar]
- Farjon A, Ortiz Garcia S. 2003. Cone and ovule development in Cunninghamia and Taiwania (Cupressaceae sensu lato) and its significance for conifer evolution. American Journal of Botany 90: 8–16. [DOI] [PubMed] [Google Scholar]
- Florin R. 1931. Untersuchungen zur Stammesgeschichte der Coniferales und Cordaitales, 1st edn. Stockholm: Almquist & Wiksells Boktryckeri. [Google Scholar]
- Florin R. 1951. Evolution in cordaites and conifers. Acta Horti Bergiani 15: 285-388. [Google Scholar]
- Florin R. 1954. The female reproductive organs of conifers and taxads. Biological Reviews 29: 367–387. [Google Scholar]
- Harris TM. 1976. The Mesozoic gymnosperms. Review of Palaeobotany and Palynology 21: 119–134. [Google Scholar]
- Herrera F, Shi G, Leslie AB, et al. 2015. A new Voltzian seed cone from the early Cretaceous of Mongolia and its implications for the evolution of ancient conifers. International Journal of Plant Sciences 176: 791–809. [Google Scholar]
- Herrera F, Shi G, Mays C, et al. 2020. Reconstructing Krassilovia mongolica supports recognition of a new and unusual group of Mesozoic conifers. PLoS One 15: e0226779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting J, Stützel T. 2020. Morphogenesis of the seed cone of Araucaria araucana (Molina) K. Koch and the evolution of the coniferous seed scale. Flora 273: 151719. [Google Scholar]
- Herting J, Stützel T, Klaus KV. 2020. The ancestral conifer cone: what did it look like? a modern trait-evolution approach. International Journal of Plant Sciences 181: 871–886. [Google Scholar]
- Ihlenfeldt H-D. 1971. Über ontogenetische Abbreviationen und Zeitkorrelationsänderungen und ihre Bedeutung für Morphologie und Systematik. Berichte der Deutschen Botanischen Gesellschaft 84: 91–107. [Google Scholar]
- Jagel A. 2001. Morphologische und morphogenetische Untersuchungen zur Systematik und Evolution der Cupressaceae s. l. (Zypressengewächse). Dissertation, Ruhr-Universität Bochum,Bochum. [Google Scholar]
- Jagel A, Dörken VM. 2014. Morphology and morphogenesis of the seed cones of the Cupressaceae - part I: Cunninghamioideae, Arthrotaxoideae, Taiwanoideae, Sequoioideae Taxodioideae. Bulletin of the Cupressus Conservation Project 3: 117–136. [Google Scholar]
- Jagel A, Dörken VM. 2015. Morphology and morphogenesis of the seed cones of the Cupressaceae - part II: Cupressoideae. Bulletin of the Cupressus Conservation Project 4: 51–78. [Google Scholar]
- Kobayashi H, Kresling B, Vincent JFV. 1998. The geometry of unfolding tree leaves. Proceedings of the Royal Society of London. Series B 265: 147– 154. [Google Scholar]
- Leslie AB, Beaulieu J, Holman G, et al. 2018. An overview of extant conifer evolution from the perspective of the fossil record. American Journal of Botany 105: 1531–1544. [DOI] [PubMed] [Google Scholar]
- Leslie AB, Beaulieu JM, Rai HS, Crane PR, Donoghue MJ, Mathews S. 2012. Hemisphere-scale differences in conifer evolutionary dynamics. Proceedings of the National Academy of Sciences of the United States of America 109: 16217–16221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie AB, Losada JM. 2019. Reproductive ontogeny and the evolution of morphological diversity in conifers and other plants. Integrative and Comparative Biology 59: 548–558. [DOI] [PubMed] [Google Scholar]
- Looy CV, Stevenson RA. 2014. Earliest occurrence of autorotating seeds in conifers: the Permian (Kungurian-Roadian) Manifera talaris gen. et sp. nov. International Journal of Plant Sciences 175: 841–854. [Google Scholar]
- Magallón S, Sanderson MJ. 2002. Relationships among seed plants inferred from highly conserved genes: sorting conflicting phylogenetic signals among ancient lineages. American Journal of Botany 89: 1991–2006. [DOI] [PubMed] [Google Scholar]
- von Marilaun AK. 1890. Pflanzenleben. Leipzig und Wien: Verlag des Bibliographischen Instituts. [Google Scholar]
- Meyen SV. 1997. Permian conifers of Western Angaraland. Review of Palaeobotany and Palynology 96: 351–447. [Google Scholar]
- Mundry I. 2000. Morphologische und morphogenetische Untersuchungen zur Evolution der Gymnospermen. Stuttgart: Schweizerbart. [Google Scholar]
- Mundry M, Stützel T. 2004. Morphogenesis of the reproductive shoots of Welwitschia mirabilis and Ephedra distachya(Gnetales), and its evolutionary implications. Organisms Diversity & Evolution 4: 91–108. [Google Scholar]
- Ran JH, Gao H, Wang XQ. 2010. Fast evolution of the retroprocessed mitochondrial rps3 gene in Conifer II and further evidence for the phylogeny of gymnosperms. Molecular Phylogenetics and Evolution 54: 136–149. [DOI] [PubMed] [Google Scholar]
- Rothwell GW, Mapes G, Hernandez-Castillo GR. 2005. Hanskerpia gen. nov. and phylogenetic relationships among the most ancient conifers (Voltziales). Taxon 54: 733. [Google Scholar]
- Rothwell GW, Stockey RA, Mapes G, Hilton J. 2011. Structure and relationships of the Jurassic conifer seed cone Hughmillerites juddii gen. et comb. nov: implications for the origin and evolution of Cupressaceae. Review of Palaeobotany and Palynology 164: 45–59. [Google Scholar]
- Röwekamp I, Stützel T. 1999. Female reproductive structures in Taxales. Flora 194: 145–157. [Google Scholar]
- Schulz C, Jagel A, Stützel T. 2003. Cone morphology in Juniperus in the light of cone evolution in Cupressaceae s.l. Flora 198: 161–177. [Google Scholar]
- Schweitzer H-J. 1963. Der weibliche Zapfen von Pseudovoltzia liebeana und seine Bedeutung für die Phylogenie der Koniferen. Palaeontographica Abteilung B: Palaeophytologie 113: 1– 29. [Google Scholar]
- Spencer AR, Mapes G, Bateman RM, Hilton J, Rothwell GW. 2015. Middle Jurassic evidence for the origin of Cupressaceae: a paleobotanical context for the roles of regulatory genetics and development in the evolution of conifer seed cones. American Journal of Botany 102: 942–961. [DOI] [PubMed] [Google Scholar]
- Stefanoviac S, Jager M, Deutsch J, Broutin J, Masselot M. 1998. Phylogenetic relationships of conifers inferred from partial 28S rRNA gene sequences. American Journal of Botany 85: 688. [PubMed] [Google Scholar]
- Takaso T, Bouman F. 1986. Ovule and seed ontogeny in Gnetum gnemon L. The Botanical Magazin Tokyo 99: 241–266. [Google Scholar]
- Takaso T, Tomlinson PB. 1991. Cone and ovule development in Sciadopitys. American Journal of Botany 78: 417–428. [Google Scholar]
- Takaso T, Tomlinson PB. 1992. Seed cone and ovule ontogeny in Metasequoia, Sequoia and Sequoiadendron (Taxodiaceae-Coniferales). Botanical Journal of the Linnean Society 109: 15– 37. [Google Scholar]
- Taylor TN, Taylor EL, Krings M. 2009. Paleobotany: The biology and evolution of fossil plants, 2nd edn. London: Academic Press. [Google Scholar]
- Tomlinson PB. 1992. Aspects of cone morphology and development in Podocarpaceae (Coniferales). International Journal of Plant Sciences 153: 572– 588. [Google Scholar]
- Tomlinson PB, Takaso T, Rattenbury JA. 1989. Cone and ovule ontogeny in Phyllocladus (Podocarpaceae). Botanical Journal of the Linnean Society 99: 209–221. [Google Scholar]
- Yang Y. 2004. Ontogeny of triovulate cones of Ephedra intermedia and origin of the outer envelope of ovules of Ephedraceae. American Journal of Botany 91: 361–368. [DOI] [PubMed] [Google Scholar]
- Zhong B, Yonezawa T, Zhong Y, Hasegawa M. 2010. The position of gnetales among seed plants: overcoming pitfalls of chloroplast phylogenomics. Molecular Biology and Evolution 27: 2855–2863. [DOI] [PubMed] [Google Scholar]
- Zimmermann W. 1930. Die Phylogenie der Pflanzen: ein Überblick über Tatsachen und Probleme. Jena: G. Fischer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.