Abstract
Background and Aims
Fruit traits and their inter-relationships can affect foraging choices by frugivores, and hence the probability of mutualistic interactions. Certain combinations of fruit traits that determine the interaction with specific seed dispersers are known as dispersal syndromes. The dispersal syndrome hypothesis (DSH) states that seed dispersers influence the combination of fruit traits found in fruits. Therefore, fruit traits can predict the type of dispersers with which plant species interact. Here, we analysed whether relationships of fruit traits can be explained by the DSH. To do so, we estimated the inter-relationships between morphological, chemical and display groups of fruit traits. In addition, we tested the importance of each trait group defining seed dispersal syndromes.
Methods
Using phylogenetically corrected fruit trait data and fruit–seed disperser networks, we tested the relationships among morphological, chemical and display fruit traits with Pearson’s correlations and phenotypic integration indices. Then, we used perMANOVA to test if the fruit traits involved in the analysis supported the functional types of seed dispersers.
Key Results
Morphological traits showed strong intragroup relationships, in contrast to chemical and display traits whose intragroup trait relationships were weak or null. Accordingly, only the morphological group of traits supported three broad seed disperser functional types (birds, terrestrial mammals and bats), consistent with the DSH.
Conclusions
Altogether, our results give some support to the DSH. Here, the three groups of traits interacted in different ways with seed disperser biology. Broad functional types of seed dispersers would adjust fruit consumption to anatomical limitations imposed by fruit morphology. Once this anatomical filter is sovercome, seed dispersers use almost all the range of variation in chemical and display fruit traits. This suggests that the effect of seed dispersers on fruit traits is modulated by hierarchical decisions. First, morphological constraints define which interactions can actually occur; subsequently, display and composition determine fruit preferences.
Keywords: Fleshy fruits, fruit chemical composition, endozoochory, seed dispersal syndromes, frugivory, fruit traits, fruit colour, trait matching
INTRODUCTION
Fleshy fruit traits fulfil a variety of functions in plants that ultimately can affect seed fate and plant reproduction (Eriksson, 2008; Niederhauser and Matlack, 2015; Rosin and Poulsen, 2018).Fleshy fruits are a conspicuous and accessible source of energy and nutrients to the heterotrophs co-occurring in the environment (Cazetta et al., 2008; Fleming and Kress, 2013). Consequently, their traits may allow plant species to attract seed dispersers, while repelling predators (Schaefer et al., 2003). In addition, some traits such as seed size can affect post-dispersal survival rates (Rosin and Poulsen, 2018). Therefore, fruit traits are subjected to an array of evolutionary forces imposed by mutualistic and agonistic interactions (Jordano, 1995; Cipollini and Levey, 1997; Mack, 2000). Fruit trait combinations that have emerged from complex evolutionary pathways currently affect the ability of seed dispersers to interact with them (González-Castro et al., 2015; Blendinger et al., 2016; Dehling et al., 2016). In addition, foraging preferences of dispersers according to their handling skills or digestive capabilities can lead to a differential use of fruit trait combinations (Valenta and Nevo, 2020; Rojas et al., 2021). If seed dispersers are an important evolutionary force, the combination of fruit traits could be used as a base to predict potential seed dispersers (i.e. fruit dispersal syndrome; van der Pijl, 1982; Valenta and Nevo, 2020). Thus, fruit syndromes could be defined as fruit trait combinations that determine the identity of seed dispersers limiting or promoting fruit usage through preference and the ability to manipulate and digest fruits (Fischer and Chapman, 1993; Fleming and Kress, 2013; Valenta and Nevo, 2020).
From the time when fruit syndromes were first proposed (van der Pijl, 1982), the bibliography of fruit dispersal syndromes refers to certain combinations of fruit traits that determine or filter the most probable and/or effective seed disperser. Dispersal syndromes have been supported for some fruit trait groups, such as colour, odour and fruit morphology (Gautier-Hion et al., 1985; Lomáscolo et al., 2010; Sinnott-Armstrong et al., 2018; Valenta and Nevo, 2020). Nevertheless, in addition to morphology and display (e.g. colour or odour), chemical composition could play a role in determining fruit dispersal syndromes, due to physiological differences in animal preferences, and capabilities to process macronutrients and to tolerate or metabolize toxic compounds (Levey and Martínez del Rio, 2001; Karasov and Martínez del Rio, 2007; Rojas et al., 2021). On the other hand, the dispersal syndrome tries to explain how specific fruit trait combinations result in a fruit syndrome, particularly through the influence of seed dispersers on the fruit trait combination (van der Pijl, 1982; Wing and Tiffney, 1987). The dispersal syndrome hypothesis (DSH; Valenta and Nevo, 2020) implies that fruit traits evolved in response to mutualistic interactions with seed dispersers, in which case correlation between two or more fruit traits (i.e. integration) should reflect the effects of seed dispersers to some extent (van der Pijl, 1982; Wing and Tiffney, 1987; Valido et al., 2011).
The DSH proposes that fruit traits are the result of a two-way interaction between plants and dispersers. A certain combination of traits determines which dispersers can consume fruits; and differential dispersal effectiveness of seeds can favour certain traits over others. Thus, it determines how traits are related and integrated in a dispersal syndrome. As said previously, fruit traits functioning allow plants to overcome other complementary processes that directly affect the reproductive success of plants (e.g. fruit, seed, and seedling survival; Mack, 2000; Wang and Smith, 2002). Altogether, the processes faced by fruits and seeds lead us to visualize a gradient of seed disperser effects on fruit traits. In one extreme, seed dispersers are the main force shaping the relationships of fruit traits (i.e. pure DSH). Thus, fruit traits that are more linked with mutualistic interactions are expected to be more related and integrated (Wing and Tiffney, 1987; Valenta and Nevo, 2020). Accordingly, it should be possible to accurately predict seed dispersers through a combination of fruit display (colour and odour), morphology (size and shape) and chemical content (macronutrients and secondary metabolites). In the opposite extreme of the gradient, fruit traits are the result of multiple selective forces whose combination is independent of, or weakly explained by, the behaviour of seed dispersers (Mack, 2000; Eriksson, 2016). In this case, fruit traits would not allow us to predict seed dispersers, but the mutualism will be affected mainly through filtering or limiting the interactions (Olesen et al., 2011; Dehling et al., 2016). Consequently, fruit display, morphology and chemical content would be loosely related and not integrated.
In this study, we aim to test the importance both of seed dispersers in fruit trait relationships, and of fruit traits in determining seed dispersers (i.e. in defining the dispersal syndromes as a fact). To do so, we (1) test the expectations raised by pure DSH vs. no DSH by analysing the correlation pattern and the integration of three groups of fruit traits (morphology, display and chemical). In addition, (2) we assess how well the combination of fruit traits defines the dispersal syndrome by analysing whether fruits dispersed by the same rough group of seed dispersers share fruit trait combinations. We expect that fruits in a community should be somewhere in the middle of the gradient delimited by pure DSH and no DSH, due to the complex combination of eco-evolutionary processes that determine plant reproduction.
MATERIALS AND METHODS
Study area and study system
We sampled native fruit species of the sub-tropical Andean cloud forests known as Austral Yungas, in Tucumán province (26°03′–27°40′S, 64°55′–65°57′W), Northwest Argentina. Altitude in the study area ranges from approx. 500 to 1900 m a.s.l. The climate is sub-tropical, with dry winters (May to September) and wet summers (November to March) (Brown et al., 2001). Average annual rainfall varies between 1100 and 1500 mm throughout the mountain range, with approx. 80 % of rainfall occurring in summer. Average annual temperature is 19 °C (Hunzinger, 1997). The native plant–frugivore network includes at least 58 seed disperser species, belonging to 13 bird families, and seven mammalian families, who feed regularly on fleshy fruits of around 240 plant species belonging to 61 families.
Fruit sampling and trait measurement
We grouped 15 fruit traits into three categories, which respond to the way traits interact with the disperser. (1) We considered that morphological traits were those interacting with the anatomy of the animal (fruit mass and equatorial diameter, total and one seed mass and number of seeds). (2) Chemical traits were those that interacted with animal digestive physiology (non-structural carbohydrates, lipids, proteins, phenolics, tannins, alkaloids, carotenoids and water content). (3) Display traits were those related to fruit detectability (colour components: hue, chroma and brightness).
From 2013 to 2017, we collected fresh fruits of native plants throughout the year. We collected fruits randomly from different plants of each species (from eight to 30 depending on the species). We selected only ripe fruits without blemishes or damage, and cleaned each fruit with distilled water. To estimate the ‘morphological group’ of traits, we used approx. 20 ripe fruits from different plants of each species and measured maximum fruit equatorial diameter with a caliper to the nearest 0.1 mm. We weighted the mass of the entire fresh fruit with a digital scale to the nearest 0.1 mg, and the mass of individual seeds with a precision lab scale to the nearest 0.01 mg. We then counted the number of seeds per fruit. With the raw data, we estimated the mean values of these variables and total seed mass (i.e. the mass considering all seeds) per fruit.
We used a minimum of 50 g of fruits per species collected from 8–30 individuals (up to 500 g or ten fruits of different individuals for large fruits) to measure chemical traits. For this, we first freeze-dried them and removed the seeds from the pulp with forceps and needles. We stored samples of freeze-dried seedless fruits which had been ground into a pulp at −20 °C until analysed. In these samples, we measured non-structural carbohydrates with the phenol–sulfuric acid method (DuBois et al., 1956), proteins with the Kjeldahl technique (Bradstreet, 1954), and lipids and carotenoids with a soxhlet and colour measurement (AOCS, 1999; Rodriguez, 2001). We estimated total phenolic concentration with the Folin–Cicolteau method (Singleton et al., 1999), and condensed tannins (hereafter tannins) with the dimethyl-amino-cinnamaldehyde method (Prior et al., 2010). Additional details are provided in Supplementary data Material 1.
We measured the reflectance of approx. 20 ripe fruits collected from 8–30 individual plants (see Ordano et al., 2017 for additional details). We used an Ocean Optics USB-2000 spectrometer with a PX-2 pulsed xenon light source to measure reflectance as the proportion of a standard white reference tile (WS-1-SS; Ocean Optics, Duiven, The Netherlands). We fixed the illumination and reflection angle at 45°. We used a coaxial fibre cable (QR-400-7-UV-VIS-BX; Ocean Optics) for all measurements, and kept a constant distance between the fruit sample and the measuring probe. We processed spectral data with SPECTRASUITE software (version 10.4.11; Ocean Optics) and calculated them in 5 nm wide spectral intervals over a 300–700 nm range, to incorporate the entire range of UV that is visible to frugivores. To avoid bias due to assigning seed disperser functional types a priori (see below), we used the coloration data as three raw components (bright, chroma and hue) instead, as taxa-specific vision models.
Seed dispersers
We used a database of 10 243 fruit–disperser interactions compiled by P.G.B. from different sources. The heterogeneous characteristic of the data sources (systematic observations and faeces from different surveys; see Ordano et al., 2017) hindered the direct use of the frequencies of the observed interactions between plant and animal species to define functional groups of seed dispersers. Consequently, we merged the sources of information previously calculating the proportion in which each plant species interacted with each disperser species. Next, we categorized seed dispersers into five functional types based on similarities in the handling and treatment by the animals of the fruit and seeds (Gautier-Hion et al., 1985; Valenta and Nevo, 2020). The functional types were: small masher birds (<100 g, i.e. birds that chew the fruit before ingesting it), small gulper birds (<100 g, i.e. birds that swallow the entire fruit), large birds (>100 g), bats and terrestrial mammals. Then, we averaged each category and obtained a heterogeneous quantitative matrix in which fruit species had different proportions of interaction with the different seed disperser functional types. Then, fruits were assigned to a seed disperser category based on the functional type that interacted proportionally more with that fruit species. Finally, we obtained a rough categorical classification of fruit species belonging to disperser functional types, i.e. we proposed seed dispersal syndromes for fruit species.
Statistical analysis
Current species-specific traits are the consequence of a long evolutionary process, and hence there may be lack of independence among species (Revell et al., 2008; Paradis, 2012). To account for phylogenetic correlations, we used phylogenetic independent contrast (PIC) from the ‘ape’ package (Paradis and Schliep, 2018), i.e. we corrected the autocorrelation derived from phylogenetic relationships and obtained a fruit trait matrix without the phylogenetic effect. For that, the phylogeny of our assemblage of plants was needed, which we derived from a megaphylogeny of vascular plants using the ‘V.phylomaker’ package (Jin and Qian, 2019). Once we obtained the tree, we resolved the remaining polytomies with the Mesquite software (Maddison and Maddison, 2019). We solved polytomies in the Myrtaceae family based on Nadra et al. (2018) and Mazine et al. (2018) for the genus Myrcianthes and on Särkinen et al. (2013) and Chiarini et al. (2018) for polytomies in the genus Solanum. For the remaining unresolved polytomies (six species), we used the function ‘multi2di’ from the ‘ape’ package (Paradis and Schliep, 2018). The resulting fruit species phylogenetic tree is shown in Supplementary data Fig. S1.
Using the PICs of the grouped fruit traits, we performed Pearson’s correlation analyses with all traits to understand the trait by trait relationships in fruits. Subsequently, we calculated the phenotypic integration index corrected for small sample size (PIc) based on the variance of the eigenvalues of the correlation matrix of phenotypic traits (Wagner, 1984). To estimate intertrait relationships, we calculated PIc for each group of traits separately and with the full set of traits. Then, we compared the observed and predicted PIc of random association among traits following a homogeneous correlation pattern (i.e. all values with the same chance of association) with the ‘PHENIX’ package (Torices and Muñoz-Pajares, 2015). Finally, we presented a network of correlations to visualize the plotted correlations with the ‘qgraph’ package (Epskamp et al., 2012). This allowed us to visually understand fruit trait relationships, and helped us elucidate the way in which different group of traits interact with each other.
To test whether fruit traits allowed us to predict the more frequent functional types of seed dispersers (i.e. whether there was a combination of traits defining fruit dispersal syndromes), we performed perMANOVA (permutational multivariate analysis of variance) and post-hoc comparisons. We first performed perMANOVAs with Euclidean trait distances among fruits as response variables and the five frugivore types (masher, gulper and large-bodied birds, mammals and bats) as fixed effects. Subsequently, we performed post-hoc tests for differences between groups (e.g. bird mashers vs. bats). PerMANOVAs were run for the full set of traits and for each group separately. Then, we visually represented the relationships between fruit species in the multivariate space of fruit traits using non-metric multidimensional scaling (NMDS) both for the full set of fruit traits and for each group of traits. In the multivariate spaces, we plotted the ellipsoids representing the functional types of dispersers to visually discern the relationships between fruit traits and their dispersers. To test the relationship between traits and the ordination, we estimated the correlation between each trait and the NMDS axes. Thus, combinations of fruit traits supporting functional types of dispersers would imply a dispersal syndrome in fruits (i.e. dispersal syndrome as a fact). We performed perMANOVA, post-hoc test and the NMDS test with the ‘vegan’ package (Oksanen et al., 2017). We modelled all variables in a lognormal distribution, and we transformed z to reach comparable scales. All comparisons were Bonferroni corrected to reduce type I error.
RESULTS
Seed disperser functional types
We measured fruit traits of 134 fleshy fruit species belonging to 47 plant families, and obtained seed dispersal data for 94 species distributed in five frugivore functional types. Twenty-seven of them were mostly consumed by bird mashers, 30 by bird gulpers, ten by large-bodied birds, 12 by terrestrial mammals and 15 by bats (Supplementary data Fig. S1).
Fruit trait relationships
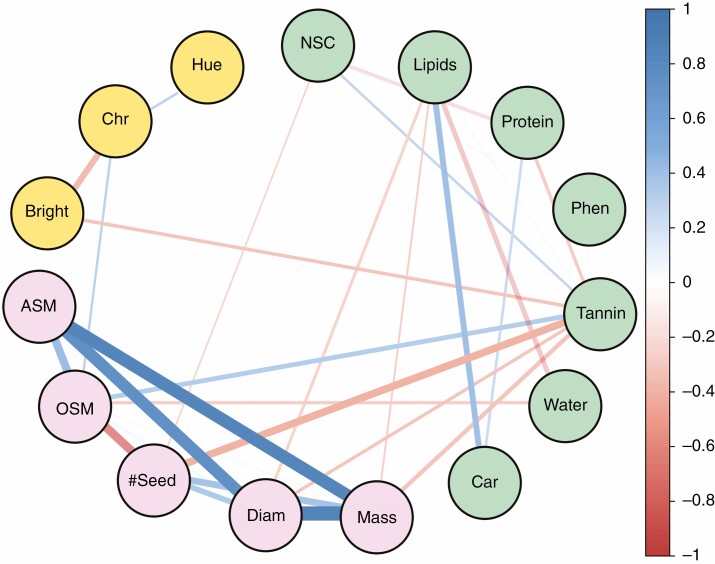
With the exception of the morphological traits, correlations among other fruit traits showed a weak association (absolute values of Pearson’s correlation coefficient r < 0.4; Fig. 1). Among them, three showed r values above 0.25: brightness and chroma; lipids and carotenoids; and tannins and seed number. Morphological traits showed stronger intragroup correlations than the other trait groups (Fig. 1). Fruit mass and total seed mass correlated strongly and positively (r = 0.95). The same occurred with fruit mass and diameter (r = 0.86), and diameter and total seed mass (r = 0.83; Fig. 1). Individual seed mass and number of seeds correlated negatively (r = –0.63). Accordingly, PIc for morphological traits differed from predicted random values (Table 1). In contrast, PIc of the full set of traits, as well as chemical and display groups did not differ from the null model (Table 1).
Fig. 1.
Network of correlations among fruit traits. Lines connecting traits indicate P-values <0.05. Absolute Pearson’s correlation coefficient r was shown to be above 0.20. Line thickness and colour show r-values; thickness represents the strength of the correlation, and blue and red lines show positive and negative values, respectively. The chemical groups of traits are presented in green, morphological traits in pink and display traits in orange. A strong positive correlation among morphological traits is observed. Display group is vaguely connected with the rest of the traits. Chemical traits show a heterogeneous pattern of correlations among intra and extra groups of traits. NSC, non-structural carbohydrates; Phen, phenolics; Car, carotenoids; Mass, fruit mass; Diam, diameter; #Seed, seed number; OSM, individual seed mass; ASM, all seed mass; Chr, chroma; Hue, hue.
Table 1.
Phenotypic integration index corrected for small samples (PIc) estimated for the full set of fruit traits and for the groups of morphological, chemical and display fruit traits
| PIc | Simulation mean PIc | P-value | |
|---|---|---|---|
| Full set of traits | 0.805 | 0.771 | 0.377 |
| Morphological | 1.481 | 0.257 | <0.001 |
| Chemical | 0.227 | 0.335 | 0.825 |
| Colour | 0.196 | 0.120 | 0.200 |
Observed PIc values of the full set of traits, chemical and display traits did not differ from simulated mean, following a uniform distribution of correlation between traits (i.e. correlated by chance). The morphological group of traits was more integrated than expected by chance. The results suggest that fruit traits follow an integration pattern that is unlikely to be predicted by seed disperser effects.
Dispersal syndrome support
The perMANOVA results supported the existence of groups (i.e. fruit dispersal syndromes proposed) with the full set of fruit traits (R2 = 0.212, F = 3.301, P = 0.001). The post-hoc comparison supported three seed disperser functional types (Table 2; Supplementary data Table S1). Bird mashers, bird gulpers and large-bodied birds failed to be detected as different groups; thus, from hereon, we treat all three bird functional types merged as birds. Bats, terrestrial mammals and birds occupied different areas of the fruit trait space (Table 2). The perMANOVA of morphological traits showed a similar but stronger pattern than the full set of traits (R2 = 0.413, F = 15.075, P = 0.0003; Table 2). The chemical and display groups of fruit traits failed to support the proposed seed disperser types (chemical: R2 = 0.081, F = 1.122, P = 0.322; display: R2 = 0.048, F = 0.929, P = 0.474).
Table 2.
Seed dispersal syndromes differentiated with perMANOVA post-hoc comparisons with the full set and morphological fruit traits
| Seed dispersal syndrome | Masher birds | Gulper birds | Large birds | Bats | Terrestrial mammals |
|---|---|---|---|---|---|
| Masher birds | |||||
| Gulper birds | – | ||||
| Large birds | – | – | |||
| Bats | * | * | * | ||
| Terrestrial mammals | * | * | * | * |
The lower diagonal of the matrix shows the results of post-hoc comparisons between pairs of seed dispersal syndromes. A dash indicates no difference between compared groups, while asterisks indicate significant differences between compared groups. Post-hoc comparison did not support the existence of the three bird syndromes but showed that these differed from those of bats and terrestrial mammals. As all seed dispersal syndromes followed the same pattern, we used one table. Detailed information about post-hoc comparisons can be found in Supplementary data Table S1.
Fruit traits involved in dispersal syndromes
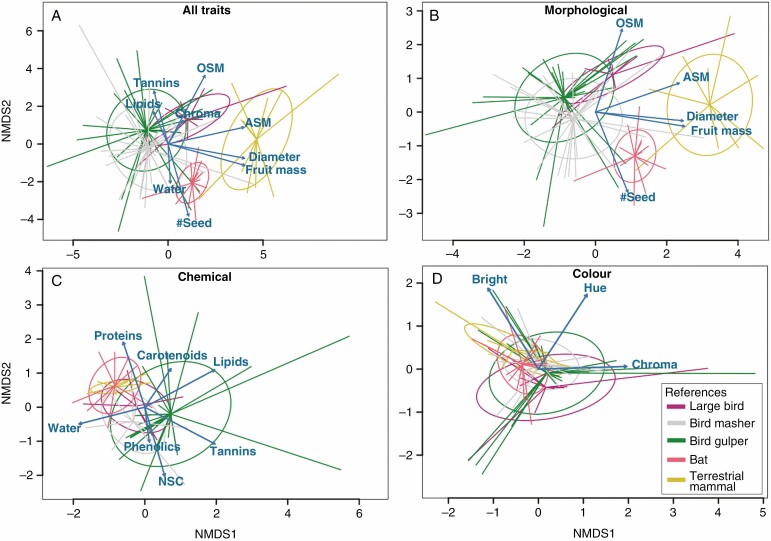
The NMDS ordinations helped to visualize the seed disperser functional types, and their relationships with fruit traits (Fig. 2). For the full set of fruit traits (stress = 0.14 with two axes; Fig. 2A), we found three different functional types of seed dispersers (birds, bats and terrestrial mammals) as supported by perMANOVA. The variables that contributed the most to the ordination were total seed mass, fruit mass and fruit diameter for the first axis; and individual seed mass, number of seeds, chroma, water, lipids and tannins for the second axis. Birds occupied a wide space in the NMDS, making it difficult to properly designate further fruit trait associations. In general, they were associated with decreasing values of total seed mass, fruit mass and fruit diameter. Bats were associated with increases in seed number and water; and decreasing values of individual seed mass, chroma, lipids and tannins. Terrestrial mammals were associated with increasing values of total seed mass, fruit mass and equatorial diameter.
Fig. 2.
NMDS in two dimensions for the full set of traits (A) the morphological group (B), the chemical group (C) and the display group of traits (D). Blue vectors represent statistically important traits related to the ordination. The five functional seed disperser types are represented by a spider (centroid to data), with an ellipse showing the standard deviation. Large-bodied birds are presented in purple, gulper birds (GU) in green, masher birds (MA) in grey, terrestrial mammals (TM) in orange and bats (BA) in pink.
The morphological group of traits (stress = 0.03 with two axes; Fig. 2B) showed almost the same pattern as the full trait set, highlighting the importance of morphological traits in fruit ordination, and its relationship with disperser functional types. In the NMDSs of chemical (stress= 0.10) and display (stress= 0.06) groups of traits, it was not possible to clearly separate seed disperser functional types (Fig. 2C and D, respectively). Altogether, these results suggest that the most likely functional type could be predicted only with morphological traits rather than by a complex array of morphological, chemical and display traits.
Discussion
According to our results, fleshy fruit traits were weakly inter-related, except for morphological traits that showed tight relationships between each morphological trait and as a group of traits. On the other hand, intra- and inter-relationships of display and chemical traits were weak. This suggests that morphological traits, but not the other types of traits, follow the dispersal syndrome hypothesis (DSH). Therefore, interactions with seed dispersers can be an evolutionary path that structures morphological trait relationships, as proposed by the DSH. Fruit species that shared functionally similar seed dispersers had similar fruit traits, i.e. dispersal syndromes were supported and defined mainly by morphological fruit traits, which allows discrimination between three dispersal syndromes (birds, bats and terrestrial mammals) using specific traits describing seed size and load. Altogether, these results suggest that the analysed community lay somewhere in between the pure DSH and the no-DSH, consistent with our initial expectations. In addition, they highlight that morphological matching between fruits and dispersers is a strong determinant of which interactions can occur (Olesen et al., 2011).
Overall, the group of morphological traits itself was enough to establish the functional types of dispersers that are more related to fruits sharing similar morphological traits, giving support to the DSH. Morphological traits describing fruit size and seed load (seed size and number) were strongly related. In line with previous research, fruit morphological traits act as a filter that limits the interaction with seed dispersers at the community level (Olesen et al., 2011; Burns, 2013; Dehling et al., 2016; Bender et al., 2018). Fruit size and seed load are key traits that determine fruit handling time and the ability of ingestion (Levey, 1987). Consequently, fruit morphology directly affects the probability of occurrence of seed dispersal interactions with animals. Thus, seed dispersers could be a significant selective force integrating morphological traits. However, neglecting the influence of other selective forces could be misleading, even when the different extant mechanisms are not mutually exclusive. Traits related to fruit and seed size are governed by isometric scaling, i.e. an increase in one of them will lead to increases of the same magnitude in the others (Wagner, 1984). This constitutes the main explanation around the observed strong positive relationships among morphological fruit traits. In addition, the negative association between seed number and individual seed mass highlights the importance of post-dispersal processes for a plant’s fitness (Eriksson, 2016; Rosin and Poulsen, 2018). Thus, plant reproductive success is directly affected by seed load, influencing seed dispersal as stated before, but also seedling survival and establishment (Wang and Smith, 2002); i.e. large seed survive better while having a small seed increases the chance to establish in favourable sites (Fleming and Kress, 2013). Although it is likely that fruit morphological traits emerge independently from mutualistic interactions (Eriksson, 2016), limitations imposed by fruit size and anatomical matching produce differential interactions of certain fruit species with specific types of dispersers (Olesen et al., 2011; Dehling et al., 2016). This could reinforce the relationship found in the morphological group of fruit traits. Thus, the influence of dispersers on fruits proposed by the DSH could occur, at least when it comes to the morphological group of fruit traits.
The display group of fruit traits was not important in defining fruit seed dispersal syndromes in the sub-tropical Andean forests. This is not in line with the expectations under the pure DSH, in which display plays a key role delimiting syndromes (Valenta and Nevo, 2020). The usefulness of utilizing colour to delimit seed dispersal syndromes could be a particular feature of the assemblage, making it difficult to extrapolate to conclusions based on different communities (Poisot et al., 2014). In addition, the role of display in fruits could be accomplished by multiple traits, beyond colour (Ordano et al., 2017; Valenta and Nevo 2020). Fruit odours are a key feature attracting potential dispersers, specially mammals, that are particularly sensitive to this signal (Kalko and Condon, 1998; Korine and Kalko, 2005; Lomáscolo et al., 2010). Moreover, fruit display traits attract not only mutualists, but also antagonists (Schaefer et al., 2007). Consequently, non-disperser fruit consumers could be another important evolutionary force shaping fruit display as an aposematic signal; nevertheless, this is yet to be explored. Future work should include other untested display fruit traits (odour, plant structure, crop size, etc.) that could be useful in delimiting dispersal syndromes together with morphological traits.
Just like display, the chemical group of traits showed weak relationships with seed dispersal syndromes of fruits. Again, fruits interact with more than mutualists (Cipollini and Levey, 1997). The trade-off between attraction and deterrence could explain the lack of dependence between chemical traits, and the low performance found when using this group of traits to delimit seed dispersal syndromes. In addition, other mechanisms such as diet complementation could explain the unclustered pattern of fruit species in the chemical fruit traits’ space, and the high overlap of functional types of seed dispersers in the same multivariate space (Murphy, 1994; Raubenheimer et al., 2009). Diet complementarity proposes that items in animal diets are a mixture of chemicals. Thus, mixing fruits with different chemical traits can allow various species to obtain a more balanced diet (Murphy, 1994) or avoid the accumulation of high doses of specific toxic compounds (Raubenheimer and Jones, 2006). If diet complementation promotes fruit mixing, then diffuse associations between the chemical composition of fruits and their dispersers would be expected.
The weak relationships among morphological, chemical and display groups of traits suggest that each group of traits interacts differentially with the environment (Valido et al., 2011). In addition, the uneven relationship each group had with seed dispersers is in line with a hierarchical structure in fruit selection (Sallabanks, 1993; Poisot et al., 2014). Again, fruit morphology and disperser anatomy matching modulates which interactions are able to occur (Olesen et al., 2011). Once the anatomical filter imposed by fruit morphology is overcome, animals have to incorporate distinct chemical traits (i.e. different fruit species); seed dispersers perceive the differences in the display traits of different fruit species. Incorporation of different traits depends on the characteristics of the assemblage of fruiting species (Poisot et al., 2014). Thus, seed dispersers could accumulate a diversity of display and chemical fruit traits, hindering the expectation that particular traits from these groups are closely related to different seed disperser functional types. In addition, fruit interactions with non-mutualist agents such as pathogens and predators could drive the array of traits found in fruits. As a result, the relationships between seed dispersers, chemical and display traits become diffuse.
In this study, we found that the DSH mechanism is unlikely to occur in a univariate way. Instead, the influence of seed dispersers could be multivariate and hierarchically structured among fruit trait groups. Fruit morphology includes the most important traits involved in the delimitation of rough dispersal syndromes. Fruit size and seed load work as the main filter for seed dispersers, which must select among the available fruit species those that they are anatomically able to manipulate and ingest. Other chemical and display fruit traits involved as cues or signals in the communication with animals (Schaefer and Ruxton, 2011) are not tightly integrated in the form of dispersal syndromes. Thus, once morphological match barriers are overcome, the relationship between fruit traits and their dispersers become much weaker. From the animal perspective, fruits more easily detected or preferred may depend on the environmental setting in which plant–animal encounters occur (Poisot et al., 2014). From the plant perspective, interactions with other organisms (e.g. predators) can modulate these traits (Schaefer et al., 2003). This allows a decoupling between morphology, which has been shown to be useful in delimiting dispersal syndromes, display traits and chemical composition of the fruit pulp. Finally, our findings propose that seed dispersers could balance what they ingest by mixing different fruit traits in their diet (Felton et al., 2009). As suggested by Valenta and Nevo (2020), further questions should be answered to understand the effect of seed dispersers on fruit trait evolution. On this path, we found no evidence that seed dispersers exert strong selective pressure on an integrated set of fruit traits (that interact with different animal senses), which would at best be restricted to a few inter-related fruit and seed size traits.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Material 1: measurement of fruit chemical traits. Figure S1: phylogram of fleshy fruited plant species used in the analysis. Table S1: perMANOVA post-hoc comparisons to test which functional types of seed dispersers are supported by fruit traits.
ACKNOWLEDGEMENTS
We are grateful to Miguel Verdú for helpful advice on the construction of the phylogeny and comparative analysis. We would also like to thank Mariano Ordano and David Vergara-Tabares for useful comments about the ideas that gave rise to this work. Finally, we thank Teresa Morán-López for critically reviewing final drafts of the manuscript. This study complies with the current laws of Argentina.
Contributor Information
Tobias Nicolas Rojas, Instituto de Ecología Regional, Universidad Nacional de Tucumán & CONICET, CC 34, 4107 Yerba Buena, Tucumán, Argentina.
Iris Catiana Zampini, Instituto de Bioprospección y Fisiología Vegetal, Universidad Nacional de Tucumán & CONICET, San Lorenzo 1469, 4000 San Miguel de Tucumán, Tucumán, Argentina; Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Miguel Lillo 2005, 4000 San Miguel de Tucumán, Tucumán, Argentina.
María Inés Isla, Instituto de Bioprospección y Fisiología Vegetal, Universidad Nacional de Tucumán & CONICET, San Lorenzo 1469, 4000 San Miguel de Tucumán, Tucumán, Argentina; Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Miguel Lillo 2005, 4000 San Miguel de Tucumán, Tucumán, Argentina.
Pedro G Blendinger, Instituto de Ecología Regional, Universidad Nacional de Tucumán & CONICET, CC 34, 4107 Yerba Buena, Tucumán, Argentina; Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Miguel Lillo 2005, 4000 San Miguel de Tucumán, Tucumán, Argentina.
FUNDING
T.N.R. was supported by doctoral fellowships from the National Fund for Scientific and Technological Research (FONCyT), and the National Scientific and Technical Research Council (CONICET), Argentina. The study was partially funded by CONICET (PIP 2014–592) and FONCyT (PICT 2013-1280) grants awarded to P.G.B.
LITERATURE CITED
- AOCS . 1999. Official methods and recommended practices of the AOCS, 5th edn. Champaign, IL: AOCS. [Google Scholar]
- Bender IMA, Kissling WD, Blendinger PG, et al. 2018. Morphological trait matching shapes plant–frugivore networks across the Andes. Ecography 41: 1910–1919. [Google Scholar]
- Blendinger PG, Martin E, Osinaga Acosta O, Ruggera RA, Aráoz E. 2016. Fruit selection by Andean forest birds: influence of fruit functional traits and their temporal variation. Biotropica 48: 677–686. [Google Scholar]
- Bradstreet RB. 1954. Kjeldahl method for organic nitrogen. Analytical Chemistry 26: 185–187. [Google Scholar]
- Brown A, Kappelle M. 2001. Argentina. In: Kappelle M, Brown A. eds. Bosques Nublados del Neotrópico. Costa Rica: IMBIO, 623–659. [Google Scholar]
- Burns KC. 2013. What causes size coupling in fruit–frugivore interaction webs? Ecology 94: 295–300. [DOI] [PubMed] [Google Scholar]
- Cazetta E, Schaefer HM, Galetti M. 2008. Does attraction to frugivores or defense against pathogens shape fruit pulp composition? Oecologia 155: 277–286. [DOI] [PubMed] [Google Scholar]
- Chiarini F, Sazatornil F, Bernardello G, Chiarini F, Sazatornil F, Bernardello G. 2018. Data reassessment in a phylogenetic context gives insight into chromosome evolution in the giant genus Solanum (Solanaceae). Systematics and Biodiversity 16: 397–416. [Google Scholar]
- Cipollini ML, Levey DJ. 1997. Secondary metabolites of fleshy vertebrate-dispersed fruits: adaptive hypotheses and implications for seed dispersal. The American Naturalist 150: 346–372. [DOI] [PubMed] [Google Scholar]
- Dehling DM, Jordano P, Schaefer HM, Böhning-Gaese K, Schleuning M. 2016. Morphology predicts species’ functional roles and their degree of specialization in plant–frugivore interactions. Proceedings of the Royal Society B: Biological Sciences 283: 20152444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28: 350–356. [Google Scholar]
- Epskamp S, Cramer AOJ, Waldorp LJ, Schmittmann VD, Borsboom D. 2012. qgraph: network visualizations of relationships in psychometric data. Journal of Statistical Software 48: 1–18. [Google Scholar]
- Eriksson O. 2008. Evolution of seed size and biotic seed dispersal in angiosperms: paleoecological and neoecological evidence. International Journal of Plant Sciences 169: 863–870. [Google Scholar]
- Eriksson O. 2016. Evolution of angiosperm seed disperser mutualisms: the timing of origins and their consequences for coevolutionary interactions between angiosperms and frugivores. Biological Reviews 91: 168–186. [DOI] [PubMed] [Google Scholar]
- Felton AM, Felton A, Wood JT, et al. 2009. Nutritional ecology of Ateles chamek in lowland Bolivia: how macronutrient balancing influences food choices. International Journal of Primatology 30: 675–696. [Google Scholar]
- Fischer K, Chapman CA. 1993. Frugivores and fruit syndromes: differences in patterns at the genus and species level. Oikos 66: 472–482. [Google Scholar]
- Fleming TH, Kress WJ. 2013. The ornaments of life: coevolution and conservation in the tropics. Chicago, IL: University of Chicago Press. [Google Scholar]
- Gautier-Hion A, Duplantier J-M, Quris R, et al. 1985. Fruit characters as a basis of fruit choice and seed dispersal in a tropical forest vertebrate community. Oecologia 65: 324–337. [DOI] [PubMed] [Google Scholar]
- González-Castro A, Yang S, Nogales M, Carlo TA. 2015. Relative importance of phenotypic trait matching and species’ abundances in determining plant–avian seed dispersal interactions in a small insular community. AoB PLANTS 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunzinger H. 1997. Hydrology of montane forest in the sierra de San javier, Tucumán, Argentina. Mountain Research and Development 17: 299e308. [Google Scholar]
- Jin Y, Qian H. 2019. V.PhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography 42: 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordano P. 1995. Angiosperm fleshy fruits and seed dispersers: a comparative analysis of adaptation and constraints in plant animal interactions. American Naturalist 145: 163–191. [Google Scholar]
- Kalko EKV, Condon MA. 1998. Echolocation, olfaction and fruit display: how bats find fruit of flagellichorus cucurbits. Functional Ecology 12: 364–372. [Google Scholar]
- Karasov WH, Martínez del Rio C. 2007. Ecological physiology. Princeton, NJ: Princeton University Press. [Google Scholar]
- Korine C, Kalko EKV. 2005. Fruit detection and discrimination by small fruit-eating bats (Phyllostomidae): echolocation call design and olfaction. Behavioral Ecology and Sociobiology 59: 12–23. [Google Scholar]
- Levey DJ. 1987. Sugar-tasting ability and fruit selection in tropical fruit-eating birds. The Auk 104: 173–179. [Google Scholar]
- Levey DJ, Martínez del Rio C. 2001. It takes guts (and more) to eat fruit: lessons from avian nutritional ecology. The Auk 118: 819–831. [Google Scholar]
- Lomáscolo SB, Levey DJ, Kimball RT, Bolker BM, Alborn HT. 2010. Dispersers shape fruit diversity in Ficus (Moraceae). Proceedings of the National Academy of Sciences, USA 107: 14668–14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack AL. 2000. Did fleshy fruit pulp evolve as a defence against seed loss rather than as a dispersal mechanism? Journal of Biosciences 25: 93–97. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. 2019. Mesquite: a modular system for evolutionary analysis. http://www.mesquiteproject.org
- Mazine FF, Eustáquio J, Faria Q, et al. 2018. Phylogeny and biogeography of the hyper-diverse genus Eugenia (Myrtaceae : Myrteae), with emphasis on E. sect. Umbellatae, the most unmanageable clade. Taxon 67: 752–769. [Google Scholar]
- Murphy ME. 1994. Dietary complementation by wild birds: considerations for field studies. Journal of Biosciences 19: 355–368. [Google Scholar]
- Nadra MG, Giannini NP, Acosta JM. 2018. Evolution of pollination by frugivorous birds in Neotropical Myrtaceae. PeerJ: 6:e5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhauser EC, Matlack GR. 2015. All frugivores are not equal: exploitation competition determines seed survival and germination in a fleshy-fruited forest herb. Plant Ecology 216: 1203–1211. [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, et al. 2017. vegan: community ecology package. https://cran.r-project.org/web/packages/vegan/index.html [Google Scholar]
- Olesen JM, Bascompte J, Dupont YL, Elberling H, Rasmussen C, Jordano P. 2011. Missing and forbidden links in mutualistic networks. Proceedings of the Royal Society B: Biological Sciences 278: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordano M, Blendinger PG, Lomáscolo SB, et al. 2017. The role of trait combination in the conspicuousness of fruit display among bird-dispersed plants. Functional Ecology 31: 1718–1727. [Google Scholar]
- Paradis E. 2012. Analysis of Phylogenetics and Evolution with R. Baltimore, MD: Springer. [Google Scholar]
- Paradis E, Schliep K. 2018. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35: 526–528. [DOI] [PubMed] [Google Scholar]
- van der Pijl L. 1982. Principles of dispersal in higher plants. Cham: Springer Nature. [Google Scholar]
- Poisot T, Stouffer DB, Gravel D. 2014. Beyond species: why ecological interaction networks vary through space and time. Oikos 124: 243–251. [Google Scholar]
- Prior RL, Fan E, Ji H, et al. 2010. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. Journal of the Science of Food and Agriculture 90: 1473–1478. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D, Jones SA. 2006. Nutritional imbalance in an extreme generalist omnivore: tolerance and recovery through complementary food selection. Animal Behaviour 71: 1253–1262. [Google Scholar]
- Raubenheimer D, Simpson SJ, Mayntz D. 2009. Nutrition, ecology and nutritional ecology: toward an integrated framework. Functional Ecology 23: 4–16. [Google Scholar]
- Revell LJ, Harmon LJ, Collar DC. 2008. Phylogenetic signal, evolutionary process, and rate. Systematic Biology 57: 591–601. [DOI] [PubMed] [Google Scholar]
- Rodriguez D. 2001. A guide to carotenoid analysis in foods. Washington, DC: ILSI Press. [Google Scholar]
- Rojas TN, Bruzzone OA, Zampini IC, Isla MI, Blendinger PG. 2021. A combination of rules govern fruit trait preference by frugivorous bat and bird species: nutrients, defence and size. Animal Behaviour 176: 111–123. [Google Scholar]
- Rosin C, Poulsen JR. 2018. Seed traits, not density or distance from parent, determine seed predation and establishment in an Afrotropical forest. Biotropica 50: 881–888. [Google Scholar]
- Sallabanks R. 1993. Hierarchical mecanisms of fruit selection by an avian frugivore. Ecology 74: 1326–1336. [Google Scholar]
- Särkinen T, Bohs L, Olmstead RG, Knapp S. 2013. A phylogenetic framework for evolutionary study of the nightshades (Solanaceae): a dated 1000-tip tree. BMC Evolutionary Biology 13: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer HM, Ruxton GD. 2011. Plant–animal communication. Oxford: Oxford University Press. [Google Scholar]
- Schaefer HM, Schmidt V, Winkler H. 2003. Testing the defence trade-off hypothesis: how contents of nutrients and secondary compounds affect fruit removal. Oikos 102: 318–328. [Google Scholar]
- Schaefer HM, Schaefer V, Vorobyev M. 2007. Are fruit colors adapted to consumer vision and birds equally efficient in detecting colorful signals? The American Naturalist 169: S159–S169. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods in Enzymology 299: 152–178. [Google Scholar]
- Sinnott-Armstrong MA, Downie AE, Federman S, Valido A, Jordano P, Donoghue MJ. 2018. Global geographic patterns in the colours and sizes of animal-dispersed fruits. Global Ecology and Biogeography 27: 1–13. [Google Scholar]
- Torices R, Muñoz-Pajares AJ. 2015. PHENIX: an R package to estimate a size-controlled phenotypic integration index. Applications in Plant Sciences 3: 1400104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta K, Nevo O. 2020. The dispersal syndrome hypothesis: how animals shaped fruit traits, and how they did not. Functional Ecology 34: 1158–1169. [Google Scholar]
- Valido A, Schaefer HM, Jordano P. 2011. Colour, design and reward: phenotypic integration of fleshy fruit displays. Journal of Evolutionary Biology 24: 751–760. [DOI] [PubMed] [Google Scholar]
- Wagner G. 1984. On the eigenvalue distribution of genetic and phenotypic dispersion matrices: evidence for a nonrandom organization of quantitative character variation. Journal of Mathematical Biology 21: 77–95. [Google Scholar]
- Wang BC, Smith TB. 2002. Closing the seed dispersal loop. Trends in Ecology and Evolution 17: 379–386. [Google Scholar]
- Wing SL, Tiffney BH. 1987. The reciprocal interaction of angiosperm evolution. Review of Palaeobotany and Palynology 50: 179–210. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.