Abstract
Aims
In heart failure with reduced ejection fraction (HFrEF), there is an ‘obesity paradox’, where survival is better in patients with a higher body mass index (BMI) and weight loss is associated with worse outcomes. We examined the effect of a sodium–glucose co‐transporter 2 inhibitor according to baseline BMI in the Dapagliflozin And Prevention of Adverse‐outcomes in Heart Failure trial (DAPA‐HF).
Methods and results
Body mass index was examined using standard categories, i.e. underweight (<18.5 kg/m2); normal weight (18.5–24.9 kg/m2); overweight (25.0–29.9 kg/m2); obesity class I (30.0–34.9 kg/m2); obesity class II (35.0–39.9 kg/m2); and obesity class III (≥40 kg/m2). The primary outcome in DAPA‐HF was the composite of worsening heart failure or cardiovascular death. Overall, 1348 patients (28.4%) were under/normal‐weight, 1722 (36.3%) overweight, 1013 (21.4%) obesity class I and 659 (13.9%) obesity class II/III. The unadjusted hazard ratio (95% confidence interval) for the primary outcome with obesity class 1, the lowest risk group, as reference was: under/normal‐weight 1.41 (1.16–1.71), overweight 1.18 (0.97–1.42), obesity class II/III 1.37 (1.10–1.72). Patients with class I obesity were also at lowest risk of death. The effect of dapagliflozin on the primary outcome and other outcomes did not vary by baseline BMI, e.g. hazard ratio for primary outcome: under/normal‐weight 0.74 (0.58–0.94), overweight 0.81 (0.65–1.02), obesity class I 0.68 (0.50–0.92), obesity class II/III 0.71 (0.51–1.00) (P‐value for interaction = 0.79). The mean decrease in weight at 8 months with dapagliflozin was 0.9 (0.7–1.1) kg (P < 0.001).
Conclusion
We confirmed an ‘obesity survival paradox’ in HFrEF. We showed that dapagliflozin was beneficial across the wide range of BMI studied.
Clinical Trial Registration: ClinicalTrials.gov NCT03036124.
Keywords: Heart failure, Dapagliflozin, SGLT2 inhibitor, Obesity, Body mass index, Adiposity
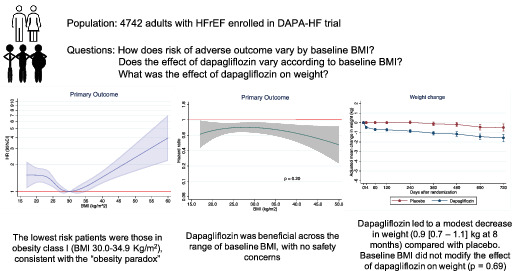
Key findings from the analyses of body mass index in the DAPA‐HF trial. BMI, body mass index; HFrEF, heart failure with reduced ejection fraction.
Introduction
The potential interaction between body mass index (BMI) and the effect of sodium–glucose co‐transporter 2 (SGLT2) inhibitors in patients with heart failure (HF) and reduced ejection fraction (HFrEF) is of special interest given the ‘obesity survival paradox’ described in this syndrome and the fact that SGLT2 inhibitors, unlike other effective therapies, cause a modest weight reduction. Multiple studies have confirmed that survival in overweight and obese patients is better than in non‐obese patients, although the explanation for this finding is uncertain and disputed. 1 , 2 , 3 , 4 , 5 , 6 Other studies have shown that low body weight is associated with poorer outcome in HFrEF and weight loss (whether intentional or unintentional) is linked to worse survival, independently of other risk factors. 7 , 8 , 9 , 10 , 11 Conversely, at least two of the major classes of drugs improving survival in HF, renin–angiotensin system (RAS) blockers and beta‐blockers, either prevent weight loss or lead to weight gain. 7 , 11 , 12 , 13 The potential explanations for this are likely multiple and complex and may include reduction in direct and indirect cachectic effects of neurohumoral activation and associated inflammation, as well as improved haemodynamics, nutrition and physical activity leading to gain in adipose tissue and muscle mass. Although mineralocorticoid receptor antagonists (MRA) do not change weight in patients with HFrEF, a significant quantitative interaction between the effect of eplerenone and adiposity has been described, whereby the benefit of this treatment was greater in patients with larger waist circumference. 14 Consequently, we examined the effect of a SGLT2 inhibitor, a treatment anticipated to cause modest weight loss, 15 , 16 according to baseline BMI in the Dapagliflozin and Prevention of Adverse‐outcomes in Heart Failure trial (DAPA‐HF). Analysis by BMI category <30 kg/m2 compared with ≥30 kg/m2 was a pre‐specified subgroup analysis in DAPA‐HF but we provide more granularity about the effect of dapagliflozin according to BMI, analysed by World Health Organization obesity class and by using BMI as a continuous variable.
Methods
Patients and study design
The design and primary results of the DAPA‐HF trial are published. 17 , 18 , 19 , 20 The trial was approved by an ethics committee at each participating centre and all patients gave written informed consent.
Inclusion criteria included age of at least 18 years, New York Heart Association (NYHA) functional classes II to IV, left ventricular ejection fraction (LVEF) ≤40%, an elevated N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), and standard HF drug and device therapy.
Key exclusion criteria included symptoms of hypotension or a systolic blood pressure <95 mmHg, an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 and type 1 diabetes. There were no exclusion criteria related to BMI.
Patients were randomized to receive either dapagliflozin (10 mg once daily) or matching placebo in a 1:1 ratio. Randomization was stratified based on diabetes status.
Following randomization, follow‐up visits took place after 14 days and at 2, 4, 8 and 12 months, and every 4 months thereafter. Weight was measured at each study visit without shoes and in light clothing. Patient height was measured at visit 1, without shoes.
Baseline body mass index categories
Body mass index was calculated using measurements of height and weight at randomization (weight in kilograms divided by height in meters squared). In this analysis, patients were divided into BMI groups according to World Health Organization categories, namely: underweight (<18.5 kg/m2); normal weight (18.5–24.9 kg/m2); overweight (25.0–29.9 kg/m2); obesity class I (30.0–34.9 kg/m2); obesity class II (35.0–39.9 kg/m2) and obesity class III (≥40 kg/m2). 21
Outcomes
The primary outcome was a composite of cardiovascular death or a worsening HF event (an unplanned hospitalization for HF or an urgent visit for worsening HF requiring intravenous therapy), whichever occurred first. Secondary outcomes included hospitalization for HF or cardiovascular death (not reported here as essentially the same as the primary outcome); all HF hospitalizations (first and recurrent) and cardiovascular death; change from baseline to 8 months in the total symptom score of the Kansas City Cardiomyopathy Questionnaire (KCCQ‐TSS); the incidence of a composite worsening renal function endpoint (because of the small number of renal events overall, this endpoint was not examined in these subgroups) and all‐cause death. 22 Change in body weight from baseline was a pre‐specified exploratory endpoint.
Pre‐specified safety analyses included any serious adverse event, adverse events leading to discontinuation of trial treatment, adverse events of interest (i.e. volume depletion, renal events, major hypoglycaemic events, bone fractures, diabetic ketoacidosis, amputation) and any diagnosis of Fournier's gangrene, as well as laboratory findings of note. Diabetic ketoacidosis and Fournier's gangrene were not examined here because of small numbers.
Statistical analysis
Because of the small number of patients in the underweight category, this category was combined with the normal weight category, and obesity class II was combined with obesity class III for the same reason, in the main analysis.
Baseline characteristics are reported for each BMI category as means ± standard deviation (SD), median with interquartile range (Q1–Q3) and proportions, as appropriate. A non‐parametric test for trend across groups, an extension of the Wilcoxon rank sum test, was used to examine for variation in baseline characteristics across groups of increasing BMI.
The effect of dapagliflozin compared to placebo on each outcome across BMI categories was examined using Cox regression (this and all other models described below were stratified by diabetes status). Event rates per 100 person‐years and hazard ratios (HRs) adjusted for previous HF hospitalization (except for all‐cause death) are reported for each BMI category. Likelihood ratio tests are reported to examine for any interaction between BMI category and treatment effect. A semi‐parametric proportional‐rates model was used to estimate the effect on recurrent HF hospitalizations. 23 For analysis of change in KCCQ‐TSS the proportion of patients who had a clinically significant (5 point) improvement or deterioration in KCCQ‐TSS was calculated and the odds ratio between patients receiving dapagliflozin vs. placebo calculated in each BMI category. Missing KCCQ data were imputed using multiple imputation method previously described. 24 The effect of dapagliflozin compared with placebo on each of the time to first event endpoints over BMI as a continuous variable was modelled as a fractional polynomial.
The relationship between BMI as a continuous variable, adjusted for treatment and history of HF hospitalization (apart from all‐cause death) with stratification by diabetes status, and the risk of each major clinical outcome was examined as a restricted cubic spline. This was repeated with additional adjustment for clinical variables (including sex, age, race, region, systolic blood pressure, eGFR, heart rate, hypertension, atrial fibrillation, ischaemic aetiology, LVEF, NYHA class, myocardial infarction, coronary artery bypass graft and stroke) and NT‐proBNP (log).
Body mass index was also considered as a categorical variable and the HR for each outcome was examined using Cox regression with obesity category 1 as the referent with the same adjustment as for the restricted cubic spline analysis.
As an exploratory analysis we examined the association between a 2% reduction in BMI at 8 months and subsequent cardiovascular death or HF hospitalization and subsequent death from any cause.
A mixed model for repeated measurement was used to examine change in weight, systolic blood pressure, glycated haemoglobin (HbA1c), creatinine, haematocrit and heart rate over time (adjusted for baseline values, randomized treatment, and interaction of treatment and visit, with a random intercept and slope per patient). Change in NT‐proBNP was examined using a linear regression model adjusted for baseline NT‐proBNP. The interaction between BMI group and treatment on the occurrence of pre‐specified adverse events was tested using logistic regression with an interaction between treatment and BMI group.
All analyses were conducted using Stata version 16 (Stata Corp., College Station, TX, USA) or SAS version 9.4 (SAS Institute, Cary, NC, USA). A P‐value of <0.05 was considered statistically significant.
Results
Of the 4744 patients randomized, two did not have a recorded height and were not included in this analysis. Of the 4742 people included, BMI ranged from 14 to 77 kg/m2 with a median value of 27 (Q1–Q3, 24–31) kg/m2 and a mean of 28.2 (SD ± 6.0) kg/m2. Overall, 87 patients (1.8%) were underweight and 1261 (26.6%) normal weight, giving a total of 1348 patients (28.4%) who were normal or underweight. Among the remaining patients, 1722 (36.3%) were overweight, and 1672 (35.3%) were obese; 1013 (21.4%) were in obesity class I, 441 (9.3%) in obesity class II and 218 (4.6%) in obesity class III, i.e. 659 (13.9%) were in obesity class II or III.
Patient characteristics
Compared to individuals with a lower BMI, those in the highest BMI category were younger, more likely to be female, and mainly white (Table 1 ). Patients in the highest BMI category were more likely to come from North America and less likely to come from Asia.
Table 1.
Baseline characteristics by body mass index category
| Under/normal‐weight (BMI < 24.9 kg/m2) | Overweight (BMI 25.0–29.9 kg/m2) | Obesity class I (BMI 30.0–34.9 kg/m2) | Obesity class II/III (BMI ≥ 35.0 kg/m2) | P‐value (for trend) | |
|---|---|---|---|---|---|
| Patients, n | 1348 | 1722 | 1013 | 659 | |
| BMI (kg/m2), median (IQR) | 22 (21–24) | 27 (26–28) | 31 (30–33) | 38 (36–41) | |
| Female sex, n (%) | 334 (24.8) | 346 (20.1) | 227 (22.4) | 202 (30.7) | 0.02 |
| Age at randomization (years) | 67 ± 12 | 67 ± 10 | 66 ± 10 | 63 ± 11 | <0.001 |
| Race, n (%) a | |||||
| White | 621 (46.1) | 1283 (74.5) | 854 (84.3) | 573 (86.9) | <0.001 |
| Black or African | 47 (3.5) | 69 (4.0) | 58 (5.7) | 52 (7.9) | |
| Asian | 656 (48.7) | 349 (20.3) | 87 (8.6) | 24 (3.6) | |
| Other | 24 (1.8) | 21 (1.2) | 14 (1.4) | 10 (1.5) | |
| Region, n (%) | |||||
| North America | 107 (7.9) | 238 (13.8) | 173 (17.1) | 158 (24.0) | <0.001 |
| South America | 200 (14.8) | 307 (17.8) | 182 (18.0) | 127 (19.3) | |
| Europe | 397 (29.5) | 833 (48.4) | 574 (56.7) | 350 (53.1) | |
| Asia/Pacific | 644 (47.8) | 344 (20.0) | 84 (8.3) | 24 (3.6) | |
| Systolic blood pressure (mmHg) | 118 ± 16 | 122 ± 16 | 125 ± 16 | 125 ± 16 | <0.001 |
| Diastolic blood pressure (mmHg) | 71 ± 10 | 74 ± 10 | 75 ± 10 | 76 ± 10 | <0.001 |
| Heart rate (bpm) | 72 ± 12 | 71 ± 12 | 71 ± 11 | 73 ± 12 | 0.25 |
| Creatinine (mg/dL) | 1.13 ± 0.34 | 1.18 ± 0.33 | 1.23 ± 0.36 | 1.20 ± 0.36 | <0.001 |
| Creatinine (µmol/L) | 100.4 ± 29.7 | 104.7 ± 29.3 | 108.4 ± 31.8 | 106.0 ± 31.5 | <0.001 |
| eGFR (mL/min/1.73 m2) | 68.0 ± 20.2 | 65.3 ± 19.0 | 63.6 ± 18.8 | 65.8 ± 19.4 | <0.001 |
| NT‐proBNP (pg/mL), median (IQR) | 1736 (986–3318) | 1423 (854–2668) | 1285 (799–2271) | 1248 (782–2153) | <0.001 |
| ECG AF/flutter | 2206 (1399–3661) | 2167 (1317–3445) | 1845 (1188–2826) | 1619 (1120–2482) | <0.001 |
| ECG not AF/flutter | 1572 (874–3176) | 1271 (760–2320) | 1139 (709–1985) | 1109 (658–2010) | <0.001 |
| Ischaemic aetiology, n (%) | 695 (51.6) | 1030 (59.8) | 630 (62.2) | 318 (48.3) | 0.62 |
| Time from HF diagnosis, n (%) | 0.07 | ||||
| 0–3 months | 44 (3.3) | 49 (2.8) | 33 (3.3) | 24 (3.6) | |
| >3–6 months | 138 (10.2) | 131 (7.6) | 80 (7.9) | 44 (6.7) | |
| >6–12 months | 182 (13.5) | 194 (11.3) | 112 (11.1) | 67 (10.2) | |
| >1–2 years | 196 (14.5) | 246 (14.3) | 155 (15.3) | 89 (13.5) | |
| >2–5 years | 304 (22.6) | 402 (23.3) | 242 (23.9) | 157 (23.8) | |
| >5 years | 484 (35.9) | 700 (40.7) | 391 (38.6) | 278 (42.2) | |
| LVEF (%) | 30 ± 7 | 31 ± 7 | 32 ± 7 | 31 ± 7 | <0.001 |
| Baseline HbA1c (%) | 6.30 ± 1.26 | 6.44 ± 1.31 | 6.64 ± 1.43 | 6.82 ± 1.39 | <0.001 |
| Baseline HbA1c in patients with T2DM (%) | 7.37 ± 1.60 | 7.34 ± 1.56 | 7.44 ± 1.55 | 7.46 ± 1.41 | 0.01 |
| Baseline HbA1c in patients without T2DM (%) | 5.72 ± 0.38 | 5.77 ± 0.40 | 5.77 ± 0.41 | 5.81 ± 0.38 | 0.008 |
| NYHA class, n (%) | <0.001 | ||||
| II | 960 (71.2) | 1194 (69.3) | 651 (64.3) | 396 (60.1) | |
| III | 374 (27.7) | 510 (29.6) | 355 (35.0) | 259 (39.3) | |
| IV | 14 (1.0) | 18 (1.0) | 7 (0.7) | 4 (0.6) | |
| KCCQ‐TSS at baseline, median (IQR) | 83 (66–96) | 79 (61–92) | 75 (56–92) | 66 (47–83) | <0.001 |
| Past medical history, n (%) | |||||
| Hypertension | 835 (61.9) | 1277 (74.2) | 836 (82.5) | 572 (86.8) | <0.001 |
| Prior diagnosis of T2DM | 431 (32.0) | 665 (38.6) | 500 (49.4) | 387 (58.7) | <0.001 |
| Atrial fibrillation | 435 (32.3) | 672 (39.0) | 406 (40.1) | 305 (46.3) | <0.001 |
| Hospitalization for HF | 656 (48.7) | 827 (48.0) | 462 (45.6) | 305 (46.3) | 0.15 |
| Myocardial infarction | 549 (40.7) | 827 (48.0) | 482 (47.6) | 234 (35.5) | 0.34 |
| Stroke | 139 (10.3) | 170 (9.9) | 99 (9.8) | 58 (8.8) | 0.32 |
| COPD | 169 (12.5) | 197 (11.4) | 123 (12.1) | 96 (14.6) | 0.28 |
| PCI | 408 (30.3) | 657 (38.2) | 372 (36.7) | 187 (28.4) | 0.97 |
| CABG | 184 (13.6) | 313 (18.2) | 209 (20.6) | 93 (14.1) | 0.08 |
| Treatments, n (%) | |||||
| ACE inhibitor | 766 (56.8) | 968 (56.2) | 571 (56.4) | 356 (53.9) | 0.29 |
| ARB | 368 (27.3) | 479 (27.8) | 273 (26.9) | 186 (28.2) | 0.84 |
| ARNI | 93 (6.9) | 191 (11.1) | 129 (12.7) | 94 (14.3) | <0.001 |
| Diuretic | 1234 (91.5) | 1597 (92.7) | 964 (95.2) | 637 (96.7) | <0.001 |
| Digoxin | 292 (21.7) | 324 (18.8) | 159 (15.7) | 112 (17.0) | 0.001 |
| Beta‐blocker | 1254 (93.0) | 1663 (96.6) | 987 (97.4) | 652 (98.9) | <0.001 |
| MRA | 961 (71.3) | 1209 (70.2) | 735 (72.6) | 465 (70.6) | 0.86 |
| Oral anticoagulant | 482 (35.8) | 736 (42.7) | 429 (42.3) | 321 (48.7) | <0.001 |
| Antiplatelet | 733 (54.4) | 982 (57.0) | 568 (56.1) | 309 (46.9) | 0.01 |
| Statin | 830 (61.6) | 1194 (69.3) | 715 (70.6) | 437 (66.3) | 0.002 |
| Implantable cardioverter‐defibrillator b | 261 (19.4) | 467 (27.1) | 302 (29.8) | 210 (31.9) | <0.001 |
| Cardiac resynchronization therapy | 88 (6.5) | 129 (7.5) | 84 (8.3) | 52 (7.9) | 0.14 |
| Glucose‐lowering medication (in patients with history of diabetes at baseline), n/N (%) | |||||
| Biguanide | 206/431 (46.4) | 329/665 (49.5) | 274/500 (54.8) | 207/387 (53.5) | 0.03 |
| Sulfonylurea | 95/431 (22.0) | 156/665 (23.5) | 105/500 (21.0) | 82/387 (21.2) | 0.52 |
| DPP‐4 inhibitor | 102/431 (23.7) | 107/665 (16.1) | 46/500 (9.2) | 55/387 (14.2) | <0.001 |
| GLP‐1 agonist | 3/431 (0.7) | 5/665 (0.8) | 5/500 (1) | 8/387 (2.1) | 0.05 |
| Insulin | 87/431 (20.2) | 165/665 (24.8) | 156/500 (31.2) | 132/387 (34.1) | <0.001 |
Plus‐minus values are means ± standard deviation. Percentages may not total 100 due to rounding.
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; DPP‐4, dipeptidyl peptidase 4; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide 1; HbA1c, glycated haemoglobin; HF, heart failure; IQR, interquartile range; KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; T2DM, type 2 diabetes mellitus.
To convert NT‐proBNP from pg/mL to ng/L, multiply by 1.
Race was reported by the investigators.
This category includes either an implantable cardioverter‐defibrillator or cardiac resynchronization therapy with a defibrillator.
Those in the highest BMI category had a higher NYHA class and worse KCCQ‐TSS compared with the lowest BMI category, although there was no difference in history of prior HF hospitalization (46.3% vs. 48.7%).
Patients with higher BMI had a higher prevalence of history of atrial fibrillation (46.3% in the highest compared with 32.3% in lowest BMI category) but a lower NT‐proBNP level [median 1248 (Q1–Q3, 782–2153) pg/mL vs. 1736 (986–3318) pg/mL] (to convert to ng/L, multiply by 1). They were more likely to have a history of hypertension and higher baseline blood pressure. Obese participants were more likely to have type 2 diabetes (58.7% vs. 32.0%), a higher HbA1c level and lower eGFR. The frequency of coronary heart disease was not greater in patients with a higher BMI.
Each of a diuretic, a beta‐blocker and sacubitril/valsartan were used more often in patients with a higher BMI, as was a defibrillating device. Conversely, digoxin was used less frequently, despite patients with a higher BMI having a greater prevalence of atrial fibrillation. Among patients with type 2 diabetes at baseline, there was greater use of insulin in patients with higher BMI (34.1% in the highest compared with 20.2% in the lowest BMI category) but less use of dipeptidyl peptidase‐4 inhibitors (14.2% vs. 23.7%).
Relationship between baseline body mass index and hospitalization and mortality outcomes
The relationship between BMI modelled as a continuous variable and the primary outcome, was a non‐linear relationship, with the lowest rate of the primary outcome in patients of BMI around 30 kg/m2 (Figure 1 ). A similar relationship was observed for the other outcomes of interest, including all‐cause mortality, although the relationship between higher BMI and either type of death was less steep than for the primary outcome or worsening HF. These relationships between BMI and outcomes persisted in models adjusted for other clinical variables and NT‐proBNP, although, the excess risk in the lowest BMI category was attenuated and that in the highest BMI category accentuated.
Figure 1.
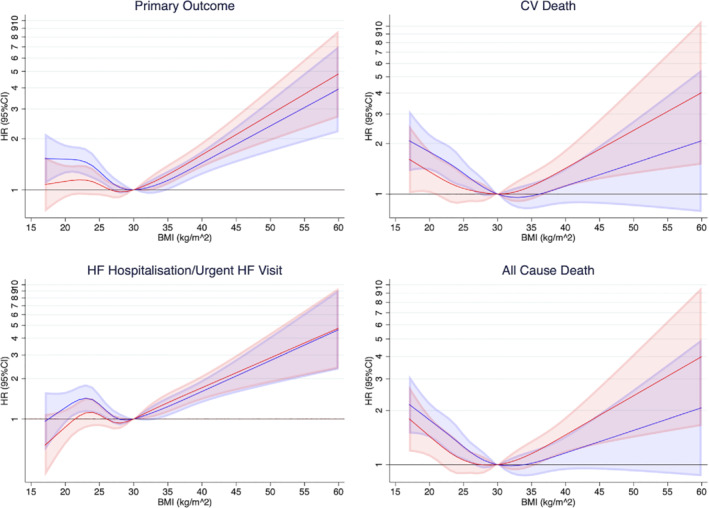
Risk of outcomes according to body mass index (BMI). These restricted cubic splines demonstrate the risk of each outcome modelling BMI as a continuous variable. The baseline spline in blue is adjusted for history of heart failure (HF) hospitalization (apart from for all‐cause death), randomized treatment and stratified by diabetes status. The red spline has additional adjustment for age, sex, race, region, systolic blood pressure, estimated glomerular filtration rate, heart rate, hypertension, atrial fibrillation, ischaemic aetiology, left ventricular ejection fraction, New York Heart Association class, myocardial infarction, coronary artery bypass graft, stroke, and N‐terminal pro‐B‐type natriuretic peptide. The reference point is BMI 30 kg/m2. The shaded areas represent 95% confidence intervals (CI). The lowest risk of the primary outcome is around 30 kg/m2. There is evidence of a U‐shaped relationship seen with higher risk with both low and very high BMI. A similar pattern is seen for the other outcomes of interest. The adjusted models show the excess risk in the lowest BMI category is attenuated while the risk in the highest BMI categories is accentuated. CV, cardiovascular; HR, hazard ratio.
Examination of event rates using BMI categories (rather than BMI as a continuous variable) showed the same pattern, with the lowest rate of primary outcome in patients in obesity class I with a rate of 13.8 (11.3–16.8) per 100 person‐years in the placebo group, compared with a rate of 17.4 (14.8–20.4) per 100 person‐years in the normal/underweight category and a rate of 19.2 (15.5–23.7) person‐years in obesity class II/III category. A similar pattern was seen for cardiovascular and all‐cause death. The pattern for HF hospitalization or an urgent HF visit was slightly different, with the lowest rate in the overweight category, rather than the obesity class I category (Table 2 ). The same picture was seen including all patients in each BMI category with adjustment for randomized treatment and considering underweight and obesity class III as separate groups (online supplementary Table S1 ). With additional adjustment for clinical variables and NT‐proBNP, the difference between BMI groups was attenuated. Underweight and obesity class III remained at highest risk for mortality outcomes, while underweight had lower rates of hospitalization for HF.
Table 2.
Effect of randomized treatment on outcomes according to body mass index category
| Outcome | Under/normal‐weight (n = 1348) | Overweight (n = 1722) | Obesity class I (n = 1013) | Obesity class II/III (n = 659) | P‐value for interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 676) | Dapagliflozin (n = 672) | Placebo (n = 857) | Dapagliflozin (n = 865) | Placebo (n = 501) | Dapagliflozin (n = 512) | Placebo (n = 337) | Dapagliflozin (n = 322) | ||
| CV death or HF hospitalization/urgent HF visit | |||||||||
| No (%) | 151 (22.3) | 118 (17.6) | 169 (19.7) | 141 (16.3) | 96 (19.2) | 68 (13.3) | 86 (25.5) | 59 (18.3) | |
| Rate (95% CI) | 17.4 (14.8–20.4) | 13.0 (10.9–15.6) | 14.5 (12.5–16.9) | 11.7 (9.9–13.8) | 13.8 (11.3–16.8) | 9.3 (7.4–11.8) | 19.2 (15.5–23.7) | 13.4 (10.4–17.3) | |
| Hazard ratio a (95% CI) | 0.74 (0.58–0.94) | 0.81 (0.65–1.02) | 0.68 (0.50–0.92) | 0.71 (0.51–1.00) | 0.79 | ||||
| CV death | |||||||||
| No (%) | 88 (13.0) | 76 (11.3) | 93 (10.9) | 88 (10.2) | 52 (10.4) | 37 (7.2) | 40 (11.9) | 26 (8.1) | |
| Rate (95% CI) | 9.4 (7.7–11.6) | 8.1 (6.5–10.1) | 7.5 (6.1–9.2) | 7.0 (5.6–8.6) | 7.0 (5.4–9.2) | 4.9 (3.5–6.7) | 8.2 (6.0–11.1) | 5.5 (3.7–8.0) | |
| Hazard ratio a (95% CI) | 0.85 (0.63–1.16) | 0.94 (0.70–1.25) | 0.70 (0.46–1.07) | 0.67 (0.41–1.10) | 0.58 | ||||
| HF hospitalization/urgent HF visit | |||||||||
| No (%) | 95 (14.1) | 61 (9.1) | 106 (12.4) | 87 (10.1) | 65 (13.0) | 48 (9.4) | 60 (17.8) | 41 (12.7) | |
| Rate (95% CI) | 10.9 (8.9–13.4) | 6.7 (5.2–8.7) | 9.1 (7.5–11.0) | 7.2 (5.8–8.9) | 9.3 (7.3–11.9) | 6.6 (5.0–8.7) | 13.4 (10.4–17.2) | 9.3 (6.8–12.6) | |
| Hazard ratioa (95% CI) | 0.60 (0.44–0.83) | 0.80 (0.60–1.06) | 0.71 (0.49–1.03) | 0.72 (0.48–1.07) | 0.67 | ||||
| All‐cause death | |||||||||
| No (%) | 109 (16.1) | 89 (13.2) | 109 (12.7) | 103 (11.9) | 62 (12.4) | 48 (9.4) | 49 (14.5) | 36 (11.2) | |
| Rate (95% CI) | 11.7 (9.7–14.1) | 9.5 (7.7–11.7) | 8.8 (7.3–10.6) | 8.1 (6.7–9.9) | 8.4 (6.5–10.8) | 6.3 (4.8–8.4) | 10.0 (7.6–13.2) | 7.6 (5.5–10.5) | |
| Hazard ratioa (95% CI) | 0.81 (0.61–1.07) | 0.93 (0.71–1.21) | 0.76 (0.52–1.10) | 0.76 (0.49–1.17) | 0.77 | ||||
| Total hospitalizations for HF and CV death (recurrent events) | |||||||||
| No. events | 217 | 155 | 253 | 220 | 141 | 108 | 131 | 84 | |
| Rate (95% CI) | 23.4 (20.5–26.8) | 16.6 (14.2–19.4) | 20.5 (18.1–23.2) | 17.4 (15.3–19.9) | 19.1 (16.2–22.5) | 14.3 (11.9–17.3) | 26.8 (22.6–31.8) | 17.8 (14.3–22.0) | |
| Rate ratio a , b (95% CI) | 0.70 (0.54–0.91) | 0.86 (0.67–1.11) | 0.75 (0.53–1.06) | 0.67 (0.46–0.98) | 0.63 | ||||
| Change in KCCQ‐TSS at 8 months | |||||||||
| Change in KCCQ at 8 months (mean ± SD) | 2.5 ± 18.5 | 4.7 ± 16.9 | 3.3 ± 18.9 | 5.5 ± 18.7 | 2.9 ± 18.6 | 6.5 ± 18.9 | 5.3 ± 22.3 | 9.8 ± 20.7 | 0.40 |
| Proportion improved (≥5 points) at 8 months (%) | 50.6 (46.5–54.6) | 58.0 (54.0–62.1) | 52.2 (48.6–55.8) | 59.0 (55.6–62.4) | 51.1 (46.7–55.6) | 57.6 (53.2, 61.9) | 48.2 (42.7–53.7) | 57.9 (51.9–63.8) | |
| Odds ratio for improvement at 8 months (95% CI) | 1.16 (1.03–1.30) | 1.14 (1.03–1.26) | 1.12 (0.98–1.27) | 1.22 (1.03–1.44) | 0.81 | ||||
| Proportion deteriorated (≥5 points) at 8 months (%) | 33.8 (29.8–37.7) | 26.9 (23.3–30.5) | 31.0 (27.7–34.3) | 25.4 (22.2–28.6) | 33.2 (28.9–37.4) | 24.4 (20.6–28.2) | 35.5 (30.1–40.9) | 23.5 (18.7–28.4) | |
| Odds ratio for deterioration at 8 months (95% CI) | 0.85 (0.75–0.97) | 0.88 (0.78–0.98) | 0.82 (0.71–0.94) | 0.75 (0.63–0.89) | 0.21 | ||||
CI, confidence interval; CV, cardiovascular; HF, heart failure; KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; SD, standard deviation.
Adjusted for history of HF hospitalization (apart from all‐cause death) and stratified by diabetes status.
The total number of hospitalizations for HF and CV deaths was analysed by means of the semiparametric proportional‐rates model, in which the treatment effect is reported as a rate ratio.
At 8 months, a reduction in BMI of >2% was associated with a higher risk of cardiovascular death or HF hospitalization, with a HR of 1.36 (95% CI 1.12–1.67) in the overall population. In the placebo group, the HR was 1.50 (95% CI 1.14–1.97) and in the dapagliflozin group it was 1.22 (95% CI 0.91–1.65) (P‐value for interaction = 0.42). For the outcome of all‐cause mortality, the HR was 1.55 (95% CI 1.21–1.99) in the overall population, 1.98 (95% CI 1.40–2.78) in the placebo group and 1.18 (0.82–1.69) in the dapagliflozin group (P‐value for interaction = 0.07).
Change in weight with dapagliflozin across body mass index categories
Change in weight from baseline, according to baseline BMI category, is shown in Figure 2 and online supplementary Table S2 . Overall change in weight by randomized treatment is shown in online supplementary Figure S1 . The mean overall placebo‐corrected decrease in weight at 8 months with dapagliflozin was 0.9 (0.7–1.1) kg (P < 0.001). BMI did not modify the effect of dapagliflozin on weight (P‐value for interaction = 0.69) (Graphical Abstract).
Figure 2.
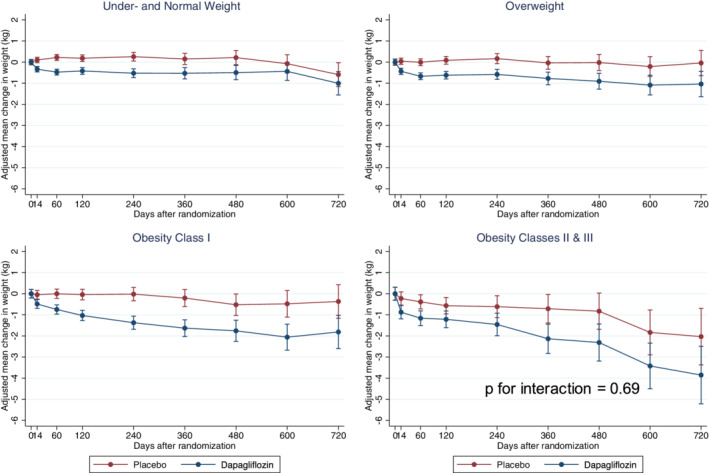
Change in weight over time by body mass index (BMI) category. Change in weight in patients randomized to dapagliflozin and placebo over time within each BMI category demonstrates modest weight loss regardless of baseline BMI in patients randomized to dapagliflozin. There was no significant interaction between baseline BMI and randomized treatment on change in weight.
Change in systolic blood pressure, heart rate, glycated haemoglobin, creatinine, haematocrit and NT‐proBNP with dapagliflozin across body mass index categories
Changes in systolic blood pressure, HbA1c, creatinine, haematocrit and heart rate from baseline, according to baseline BMI category and treatment assignment, are shown in online supplementary Table S2 and online supplementary Figure S2 . There was no significant interaction between BMI and the effect of treatment on systolic blood pressure, haematocrit or heart rate. Dapagliflozin reduced HbA1c more in patients with higher BMI due to the larger proportion of obese patients with diabetes (P‐value for interaction = 0.004). Obese patients had a smaller rise in creatinine than less obese patients (P‐value for interaction = 0.005) (online supplementary Figure S2 ). The mean overall placebo‐corrected decrease in NT‐proBNP at 8 months with dapagliflozin was 303 (150–457) pg/mL (P ≤ 0.001). BMI did not modify the effect of dapagliflozin on NT‐proBNP (online supplementary Table S2 ).
Effect of dapagliflozin, compared with placebo, on outcomes, according to baseline body mass index
The effect of treatment according to baseline BMI analysed as a categorical variable is shown in Table 2 and analysed as a continuous variable in online supplementary Figure S3 .
Dapagliflozin reduced the risk of the primary outcome to a similar extent across all BMI categories: HR (95% CI) 0.74 (0.58–0.94) for normal/underweight; 0.81 (0.65–1.02) for overweight; 0.68 (0.50–0.92) for obesity class I and 0.71 (0.51–1.00) for obesity class II/III category (P‐value for interaction = 0.79). This was also true for the other outcomes examined (Table 2 ). There was no interaction between BMI and the effect of dapagliflozin.
Dapagliflozin increased (improved) mean KCCQ‐TSS as compared to placebo between baseline and 8 months, with a consistent effect across BMI categories. Overall, the proportion of patients with a clinically meaningful (≥5‐point) improvement was larger, and the proportion with a ≥5‐point deterioration smaller, in the dapagliflozin group, compared with the placebo group. This pattern was consistent across BMI categories (Table 2 ).
Pre‐specified adverse events according to baseline body mass index category
None of the pre‐specified adverse events of interest were common and none occurred at a significantly different frequency across BMI categories or by randomized therapy in each category (Table 3 ).
Table 3.
Adverse events by body mass index category and randomized treatment
| Adverse event | Under/normal‐ weight | Overweight | Obesity class I | Obesity class II/III | P‐value for interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | Dapagliflozin | Placebo | Dapagliflozin | Placebo | Dapagliflozin | Placebo | Dapagliflozin | ||
| No | 676 | 668 | 855 | 865 | 500 | 511 | 337 | 322 | |
| Discontinuation due to adverse event, n (%) | 36 (5.3) | 36 (5.4) | 33 (3.9) | 46 (5.3) | 25 (5.0) | 15 (2.9) | 22 (6.5) | 14 (4.3) | 0.09 |
| Volume depletion, n (%) | 53 (7.8) | 56 (8.4) | 53 (6.2) | 62 (7.2) | 40 (8.0) | 39 (7.6) | 16 (4.7) | 21 (6.5) | 0.80 |
| Renal adverse event, n (%) | 55 (8.1) | 36 (5.4) | 53 (6.2) | 56 (6.5) | 35 (7.0) | 32 (6.3) | 27 (8.0) | 29 (9.0) | 0.29 |
| Bone fracture, n (%) | 14 (2.1) | 19 (2.8) | 16 (1.9) | 18 (2.1) | 11 (2.2) | 5 (1.0) | 9 (2.7) | 7 (2.2) | 0.31 |
| Amputation, n (%) | 0 (0.0) | 0 (0.0) | 6 (0.7) | 4 (0.5) | 5 (1.0) | 5 (1.0) | 1 (0.3) | 4 (1.2) | 0.23 |
| Major hypoglycaemia, n (%) | 2 (0.3) | 1 (0.1) | 2 (0.2) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.6) | n/a |
Discussion
The key findings of this study were that an ‘obesity survival paradox’ persists in patients with HFrEF and that dapagliflozin was beneficial across the wide range of BMI studied, including patients with a normal weight or who were underweight.
In keeping with prior studies, we found that there was a U‐shaped relationship between BMI and outcomes in patients with HFrEF with the nadir in mortality rates in the BMI range 30.0–34.9 kg/m2 and the highest risk of death in patients who were normal or underweight. The U‐shaped relationship between BMI and outcome was attenuated but not eliminated after adjustment for known prognostic variables including NT‐proBNP. However, the possibility of unmeasured confounding remains.
Although in many ways it is counterintuitive that obesity could be advantageous for survival in HFrEF, it is also the case that weight loss, whether voluntary or involuntary, is associated with worse outcomes and two key treatments (RAS blockers and beta‐blockers) that improve survival prevent weight loss or lead to gain in weight. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 These observations have resulted in caution in HF guidelines about treating obesity, even extending to dietary intervention. For example, the European Society of Cardiology guidelines on management of HF state that in patients with ‘moderate obesity’ (BMI <35 kg/m2), weight loss cannot be recommended. 25 , 26 , 27 In DAPA‐HF we anticipated that dapagliflozin would lead to a modest reduction in weight, reflecting increased urinary excretion of glucose and loss of calories. 15 , 16 However, the average weight reduction observed by 8 months was small (<1 kg), in keeping with the findings of prior trials using agents in this class, although the absolute reduction was greater in patients with a higher BMI treated for longer (approximately 1.5 kg by 16 months in those with class II/III obesity). Despite this, we found the magnitude of the improvement with dapagliflozin in clinical outcomes was consistent across the spectrum of BMI studied. Specifically, there was no evidence of diminished benefit in patients in the lowest BMI category, although we had few underweight patients. Similarly, we did not observe a larger benefit in obese patients, which is also relevant, in view of the hypothesis that the ‘obesity survival paradox’ might be explained by a greater effect of treatment of patients with a higher BMI, a suggestion given some credence by the finding of more benefit from eplerenone in obese patients in EMPHASIS‐HF. 14
While we did find some support for the ‘obesity survival paradox’ in DAPA‐HF, it is also very clear that high BMI is associated with a greater risk of non‐fatal outcomes, and, to a lesser degree, death as well. For the primary composite outcome, the event rate was highest in patients with class II/III obesity, as was the case for worsening HF events. Moreover, in DAPA‐HF, patients with class II/III obesity had worse symptoms, with average KCCQ‐TSS a huge 17 points lower than patients in the normal/underweight category. As well as having worse symptoms and a higher rate of hospitalization for worsening HF, HFrEF patients with obesity also have an increased risk of developing diabetes and other problems including obstructive sleep apnoea and atrial fibrillation, compared with non‐obese patients. 27 , 28 , 29 A BMI of >35 kg/m2 is generally considered a contraindication to heart transplantation. 30 , 31 Consequently, while scientifically interesting, the ‘obesity survival paradox’ is clinically less important that the adverse associations of obesity, given how common obesity is compared with low BMI. In DAPA‐HF, 35% of patients were obese while only 1.8% were underweight. This emphasizes the importance of finding safe approaches to reducing weight in patients with HF. 26 , 32
Study limitations
There are some limitations to our report. BMI is only one measure of adiposity and does not distinguish well between lean and fat mass or measure fat distribution. Conventional definitions of obesity, based on BMI, may not accurately reflect geographic variation as the association between BMI, percentage body fat and body fat distribution may differ across populations, although we adjusted for both race and region in our models. 33 Waist/hip circumference and skinfold thickness, which would give more information on body fat distribution, were not measured in DAPA‐HF. We had only 87 patients in the ‘underweight’ BMI category and clearly our findings cannot be extrapolated to patients with a low BMI. We did not have data on physical activity or physical fitness to relate to BMI or the effects of treatment. 34 , 35 , 36 Some of the weight loss seen with dapagliflozin might be attributable to diuresis, although calorie loss and diuresis are mechanistically intertwined with SGLT2 inhibitors. Moreover, while the absolute reduction in weight was larger in more obese patients, an opposite pattern was seen in reduction in NT‐proBNP, and there was no difference in change in haematocrit by BMI class, providing some indirect evidence against a strong association between diuresis and weight loss.
Conclusion
In conclusion, the benefit of dapagliflozin on clinical outcomes was consistent across the spectrum of BMI. Treatment with dapagliflozin led to a small reduction in weight across the BMI categories examined, without any safety concern.
Funding
The DAPA‐HF trial was funded by AstraZeneca. C.A., M.C.P. and J.J.V.M. are supported by a British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217.
Conflict of interest: P.S.J. reported his employer being paid by AstraZeneca for his time working on the study and receiving personal fees from and his employer being paid by Novartis; grants and personal fees from Boehringer Ingelheim; personal fees from Cytokinetics and Vifor Pharma outside the submitted work; and being the director of Global Clinical Trials Partners Ltd. K.F.D. reported his employer, the University of Glasgow, being paid by AstraZeneca (sponsor of DAPA‐HF) for his involvement in the DAPA‐HF trial and receiving personal fees from Eli Lilly outside the submitted work. J.B. reported receiving personal fees from AstraZeneca during the conduct of the study and grants from the Ministry of Health/Grant Agency for Health Research of the Czech Republic and personal fees from Novartis, Boehringer Ingelheim, Amgen, Medpace, and Pfizer outside the submitted work. C.E.C. reported receiving honorarium for lectures from AstraZeneca, Boehringer Ingelheim, Daiichi‐Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, and Sanofi. M.D. reported receiving personal fees from AstraZeneca during the conduct of the study. J.H. reported receiving grants and personal fees from AstraZeneca Canada and Boerhinger Ingelheim/Eli Lilly during the conduct of the study and grants and personal fees from Servier Canada, Novartis, Pfizer, and Bayer; personal fees from Otsuka, Alnylam, and Akcea; grants from Medtronic; and serving on the medical advisory board for Caridiol outside the submitted work. C.E.A.L. reported receiving personal fees and financial reimbursement to the institution from AstraZeneca during the conduct of the study and personal fees from Novartis and Pfizer outside the submitted work. M.C.P. reported receiving lecture fees from AstraZeneca and Eli Lilly during the conduct of the study and personal fees from Novo Nordisk, AstraZeneca, NAPP Pharmaceuticals, Takeda Pharmaceutical, Alnylam, Bayer, Resverlogix, and Cardiorentis and grants and personal fees from Boehringer Ingelheim and Novartis outside the submitted work. M.S. reported receiving personal fees and nonfinancial support from AstraZeneca and personal fees from Novo Nordisk and Bohringer Ingelheim outside the submitted work. S.E.I. reported receiving personal fees from AstraZeneca during the conduct of the study and personal fees from AstraZeneca, Boehringer Ingelheim, Merck, VTV Therapeutics, Sanofi/Lexicon, and Novo Nordisk outside the submitted work. L.K. reported receiving grants from AstraZeneca to the institution for participation in Dapa‐HF steering committee during the conduct of the study and personal fees from speakers honorarium from AstraZeneca and Novartis outside the submitted work. M.N.K. reported receiving grants and personal fees from AstraZeneca and Boehringer Ingelheim and personal fees from Sanofi, Amgen, Novo Nordisk, Merck, Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia, Novartis, Applied Therapeutics, Amarin, and Eli Lilly outside the submitted work. F.A.M. reported receiving personal fees from AstraZeneca during the conduct of the study. P.P. reported receiving personal fees and fees to his institution from participation as an investigator in clinical trials from AstraZeneca during the conduct of the study and from Boehringer Ingelheim, Servier, Novartis, Berlin‐Chemie, Bayer, Renal Guard Solutions, Pfizer, Respicardia, Cardiorentis, and Cibiem; grants, personal fees, and fees to his institution from Impulse Dynamics; and fees to his institution from Vifor, Corvia, and Revamp Medical outside the submitted work. M.S.S. reported receiving grants and personal fees from AstraZeneca during the conduct of the study; grants and personal fees from Amgen, Intarcia, Janssen Research and Development, Medicines Company, MedImmune, Merck, and Novartis; personal fees from Anthos Therapeutics, Bristol‐Myers Squibb, CVS Caremark, DalCor, Dyrnamix, Esperion, IFM Therapeutics, Ionis; and grants from Daiichi‐Sankyo, Bayer, Pfizer, Poxel, Eisai, GlaxoSmithKline, Quark Pharmaceuticals, and Takeda outside the submitted work; and is a member of the TIMI Study Group, which has also received institutional research grant support through Brigham and Women's Hospital from Abbott, Aralez, Roche, and Zora Biosciences. S.D.S. reported receiving grants from AstraZeneca during the conduct of the study and grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, Bristol‐Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, GlaxoSmithKline, Ionis, Lone Star Heart, Mesoblast, MyoKardia, National Institutes of Health/National Heart, Lung, and Blood Institute, Novartis, Sanofi Pasteur, and Theracos and personal fees from Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Bristol‐Myers Squibb, Cardior, Corvia, Cytokinetics, Daiichi‐Sankyo, Gilead, GlaxoSmithKline, Ironwood, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, and Tenaya outside the submitted work. O.B. reported receiving personal fees from AstraZeneca outside the submitted work. A.M.L. reported receiving being a full‐time employee of and shareholder in AstraZeneca during the conduct of the study. D.L. is an employee of AstraZeneca. M.S. reported receiving personal fees from and being a full‐time employee and shareholder of AstraZeneca outside the submitted work. J.J.V.M. reported receiving grants and his employer being paid by AstraZeneca, Theracos, and GlaxoSmithKline during the conduct of the study and grants and his employer being paid by Novartis, Amgen, Bristol‐Myers Squibb, Bayer, Abbvie, Dal‐Cor, Kidney Research UK, and Cardurion and grants from British Heart Foundation outside the submitted work. No other disclosures were reported.
Supporting information
Table S1. Outcomes by body mass index (BMI) category: (A) by four groups of BMI, (B) by six groups of BMI.
Table S2. Change in weight, NT‐proBNP, systolic blood pressure, creatinine, haematocrit and heart rate at 8 months by body mass index group and randomized treatment.
Figure S1. Absolute and relative change in weight by randomized treatment.
Figure S2. Change in systolic blood pressure, glycated haemoglobin, creatinine, haematocrit and heart rate over time by body mass index category.
Figure S3. Treatment effect of dapagliflozin on outcomes according to body mass index as a continuous variable.
References
- 1. Oga EA, Eseyin OR. The obesity paradox and heart failure: a systematic review of a decade of evidence. J Obes 2016;2016:9040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oreopoulos A, Padwal R, Kalantar‐Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta‐analysis. Am Heart J 2008;156:13–22. [DOI] [PubMed] [Google Scholar]
- 3. Mahajan R, Stokes M, Elliott A, Munawar DA, Khokhar KB, Thiyagarajah A, Hendriks J, Linz D, Gallagher C, Kaye D, Lau D, Sanders P. Complex interaction of obesity, intentional weight loss and heart failure: a systematic review and meta‐analysis. Heart 2019;106:58–68. [DOI] [PubMed] [Google Scholar]
- 4. Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis 2018;61:151–156. [DOI] [PubMed] [Google Scholar]
- 5. Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez‐Jimenez F, Arbab‐Zadeh A, Mukherjee D, Lazar JM. Meta‐analysis of the relation of body mass index to all‐cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol 2015;115:1428–1434. [DOI] [PubMed] [Google Scholar]
- 6. Zhang J, Begley A, Jackson R, Harrison M, Pellicori P, Clark AL, Cleland JGF. Body mass index and all‐cause mortality in heart failure patients with normal and reduced ventricular ejection fraction: a dose–response meta‐analysis. Clin Res Cardiol 2019;108:119–132. [DOI] [PubMed] [Google Scholar]
- 7. Anker SD, Negassa A, Coats AJS, Afzal R, Poole‐Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin‐converting‐enzyme inhibitors: an observational study. Lancet 2003;361:1077–1083. [DOI] [PubMed] [Google Scholar]
- 8. Rossignol P, Masson S, Barlera S, Girerd N, Castelnovo A, Zannad F, Clemenza F, Tognoni G, Anand IS, Cohn JN, Anker SD, Tavazzi L, Latini R. Loss in body weight is an independent prognostic factor for mortality in chronic heart failure: insights from the GISSI‐HF and Val‐HeFT trials. Eur J Heart Fail 2015;17:424–433. [DOI] [PubMed] [Google Scholar]
- 9. Zamora E, Díez‐López C, Lupón J, de Antonio M, Domingo M, Santesmases J, Troya MI, Díez‐Quevedo C, Altimir S, Bayes‐Genis A. Weight loss in obese patients with heart failure. J Am Heart Assoc 2016;5:e002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okuhara Y, Asakura M, Orihara Y, Naito Y, Tsujino T, Ishihara M, Masuyama T. Effects of weight loss in outpatients with mild chronic heart failure: findings from the J‐MELODIC study. J Card Fail 2019;25:44–50. [DOI] [PubMed] [Google Scholar]
- 11. Pocock SJ, McMurray JJV, Dobson J, Yusuf S, Granger CB, Michelson EL, Pfeffer MA, Solomon SD, Anker SD. Weight loss and mortality risk in patients with chronic heart failure in the Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J 2008;29:2641–2650. [DOI] [PubMed] [Google Scholar]
- 12. Clark AL, Coats AJS, Krum H, Katus HA, Mohacsi P, Salekin D, Schultz MK, Packer M, Anker SD. Effect of beta‐adrenergic blockade with carvedilol on cachexia in severe chronic heart failure: results from the COPERNICUS trial. J Cachexia Sarcopenia Muscle 2017;8:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rössner S, Taylor CL, Byington RP, Furberg CD. Long term propranolol treatment and changes in body weight after myocardial infarction. Br Med J 1990;300:902–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olivier A, Pitt B, Girerd N, Lamiral Z, Machu JL, McMurray JJV, Swedberg K, van Veldhuisen DJ, Collier TJ, Pocock SJ, Rossignol P, Zannad F, Pizard A. Effect of eplerenone in patients with heart failure and reduced ejection fraction: potential effect modification by abdominal obesity. Insight from the EMPHASIS‐HF trial. Eur J Heart Fail 2017;19:1186–1197. [DOI] [PubMed] [Google Scholar]
- 15. Cai X, Ji L, Chen Y, Yang W, Zhou L, Han X, Zhang S, Ji L. Comparisons of weight changes between sodium‐glucose cotransporter 2 inhibitors treatment and glucagon‐like peptide‐1 analogs treatment in type 2 diabetes patients: a meta‐analysis. J Diabetes Investig 2017;8:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee PC, Ganguly S, Goh SY. Weight loss associated with sodium‐glucose cotransporter‐2 inhibition: a review of evidence and underlying mechanisms. Obes Rev 2018;19:1630–1641. [DOI] [PubMed] [Google Scholar]
- 17. McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD; DAPA‐HF Committees and Investigators . A trial to evaluate the effect of the sodium‐glucose co‐transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA‐HF). Eur J Heart Fail 2019;21:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD. The Dapagliflozin And Prevention of Adverse‐outcomes in Heart Failure (DAPA‐HF) trial: baseline characteristics. Eur J Heart Fail 2019;21:1402–1411. [DOI] [PubMed] [Google Scholar]
- 19. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 20. Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett J, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Vinh PN, Schou M, Tereshchenko S, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD, Johanson P, Greasley PJ, Boulton D, Bengtsson O, Jhund PS, McMurray JJV. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020;323:1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Heath Organisation . Obesity: Preventing and managing the global epidemic. Report of a WHO Consultation (WHO Technical Report Series 894); 2000. [PubMed]
- 22. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City cardiomyopathy questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 23. Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Ser B Stat Methodol 2000;62:711–730. [Google Scholar]
- 24. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, Demets DL, Inzucchi SE, Køber L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjöstrand M, Langkilde AM, McMurray JJV. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA‐HF trial. Circulation 2020;141:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 26. Vest AR, Chan M, Deswal A, Givertz MM, Lekavich C, Lennie T, Litwin SE, Parsly L, Rodgers JE, Rich MW, Schulze PC, Slader A, Desai A. Nutrition, obesity, and cachexia in patients with heart failure: a consensus statement from the Heart Failure Society of America Scientific Statements Committee. J Card Fail 2019;25:380–400. [DOI] [PubMed] [Google Scholar]
- 27. Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, Jessup M, Kosiborod M, Pritchett AM, Ramasubbu K, Rosendorff C, Yancy C. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation 2016;134:e535–e578. [DOI] [PubMed] [Google Scholar]
- 28. Chung MK, Eckhardt LL, Chen LY, Ahmed HM, Gopinathannair R, Joglar JA, Noseworthy PA, Pack QR, Sanders P, Trulock KM. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation 2020;141:E750–E772. [DOI] [PubMed] [Google Scholar]
- 29. Javed S, Gupta D, Lip GYH. Obesity and atrial fibrillation: making inroads through fat. Eur Heart J Cardiovasc Pharmacother 2021;7:59–67. [DOI] [PubMed] [Google Scholar]
- 30. Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger‐Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EAM, Zuckermann A. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10‐year update. J Heart Lung Transplant 2016;35:1–23. [DOI] [PubMed] [Google Scholar]
- 31. Foroutan F, Doumouras BS, Ross H, Alba AC. Impact of pretransplant recipient body mass index on post heart transplant mortality: a systematic review and meta‐analysis. Clin Transplant 2018;32:e13348. [DOI] [PubMed] [Google Scholar]
- 32. McDowell K, Petrie MC, Raihan NA, Logue J. Effects of intentional weight loss in patients with obesity and heart failure: a systematic review. Obes Rev 2018;19:1189–1204. [DOI] [PubMed] [Google Scholar]
- 33. Chandramouli C, Tay WT, Bamadhaj NS, Tromp J, Teng THK, Yap JJL, MacDonald MR, Hung CL, Streng K, Naik A, Wander GS, Sawhney J, Ling LH, Richards AM, Anand I, Voors AA, Lam CSP. Association of obesity with heart failure outcomes in 11 Asian regions: a cohort study. PLoS Med 2019;16:e1002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res 2019;124:799–815. [DOI] [PubMed] [Google Scholar]
- 35. Lavie CJ, Carbone S, Kachur S, O'Keefe EL, Elagizi A. Effects of physical activity, exercise, and fitness on obesity‐related morbidity and mortality. Curr Sports Med Rep 2019;18:292–298. [DOI] [PubMed] [Google Scholar]
- 36. Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Healthy weight and obesity prevention: JACC Health Promotion Series. J Am Coll Cardiol 2018;72:1506–1531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Outcomes by body mass index (BMI) category: (A) by four groups of BMI, (B) by six groups of BMI.
Table S2. Change in weight, NT‐proBNP, systolic blood pressure, creatinine, haematocrit and heart rate at 8 months by body mass index group and randomized treatment.
Figure S1. Absolute and relative change in weight by randomized treatment.
Figure S2. Change in systolic blood pressure, glycated haemoglobin, creatinine, haematocrit and heart rate over time by body mass index category.
Figure S3. Treatment effect of dapagliflozin on outcomes according to body mass index as a continuous variable.


