Abstract
Background and Aims
Macro- and micromorphology of seeds are diagnostic characteristics of importance in delimiting taxa in Allium (Amaryllidaceae). However, there is no consensus on the phylogenetic significance of testa cell characteristics and whether they reflect the different evolutionary levels recognized in Allium.
Methods
Seeds of 95 species (98 samples) representing 14 subgenera and 58 sections of Allium were examined using scanning electron microscopy (SEM) for such traits as periclinal wall surface area of ten testa cells, distance between testa cells (macromorphology), testa cell shapes, and arrangement and structure of anticlinal and periclinal walls (micromorphology). The data matrix was subjected to cladistic analysis. The produced phylogenetic tree was examined against the molecular tree obtained from publically available ITS sequences.
Key Results
The periclinal wall surface area of ten testa cells and the distance between them, examined for the first time, were found useful for delimitation of species in Allium. Based on seed macro- and micromorphology, we present a taxonomic key and a hypothetical reconstruction of the migration routes during the early stages of evolution of Allium.
Conclusions
The ancestors of Allium originated in an area bounded by the Caucasus, Central Asia and Iran. The seed testa morphology-based evolutionary state of a species is determined by two parameters: the shape of the periclinal walls and curvature of the anticlinal walls.
Keywords: Seed macromorphology, seed micromorphology, phylogenetic analysis, SEM, testa cell, Allium taxonomy
INTRODUCTION
Allium (Amaryllidaceae), one of the largest genera in the family (Friesen et al., 2006; Li et al., 2010), comprises more than 1000 species classified into 15 subgenera and 85 sections (Friesen et al., 2006; Fritsch et al., 2010). The genus ranges throughout the northern hemisphere with the main centre of diversity in southwest and central Asia (Khassanov, 2018). Species of Allium are easily recognized by their membranous or fibrous bulb tunic, free or nearly free tepals, subgynobasic style and distinct odour and taste (Friesen et al., 2006). The genus is economically significant since it contains many essential vegetable crops and ornamental and medicinal plants (Fritsch and Friesen, 2002).
Studies of the seed morphology of Allium have shown that not only the shape and size of the seeds but also the sculpturing of the testa are diverse among but not within species and are taxonomically important characteristics (von Bothmer, 1974; Pastor, 1981; Kruse, 1984, 198, 1988, 1994; Ilarslan and Koyuncu, 1997; Fritsch et al., 2006; Neshati and Fritsch, 2009; Choi and Cota-Sanchez, 2010; Bednorz et al., 2011; Celep et al., 2012; Lin and Tan, 2017; Veiskarami et al., 2018; Baasanmunkh et al., 2021). Kruse (1984, 1986, 1988, 1994) showed that many seed testa character combinations in Allium are section- and species-specific and Fritsch et al. (2006) suggested that verrucae and anticlinal curvature type of the epidermal part of seeds may indicate a stage of evolutionary development. Later, Celep et al. (2012) in their study of micromorphological seed characteristics, such as the shape of the cells of the testa and the sculpturing of the periclinal walls, concluded that the seed coat patterns in Allium in general reflect phylogenetic trends, but cannot tell apart the basal and advanced evolutionary levels within the genus. In contrast, Lin and Tan (2017) distinguished three evolutionary developmental stages of testa cell characteristics (primitive, intermediate and advanced) and accordingly divided Allium into six distinct groups (Tuberosum, Mongolicum, Strictum, Atrosanguineum, Platyspathum and Delicatulum). Both the above studies (Celep et al., 2012; Lin and Tan, 2017), however, had a very limited geographic coverage (Turkey and Xinjiang Provinces of China, respectively), and limited coverage of taxa within Allium (9 and 19 sections, respectively). The limited geographic and taxonomic scope of the above studies seriously affected the generality of their findings. This narrow geographic and taxonomic focus is common for all the Allium seed micromorphology studies conducted to date (Table 1). A study based on a much more comprehensive coverage of the genus taxonomy and geography is needed to resolve the question of the phylogenetic significance of testa cell characteristics and whether they reflect the different evolutionary levels recognized in Allium. In an attempt to fill the existing gaps, we conducted the widest to date coverage of Allium, comprising 95 species (98 samples; the choice of species was made to ensure that all taxa within the genus were represented) representing 58 sections and 14 subgenera.
Table 1.
List of the seed coat studies with the number of investigated taxa and the geographic range covered
| No. | Author | Subgenus | Section | Species | Locality |
|---|---|---|---|---|---|
| 1 | Kruse (1984) | 7 | 10 | 35 | Worldwide |
| 2 | Kruse (1986) | 10 | 19 | 70 | Worldwide |
| 3 | Kruse (1988) | 8 | 15 | 53 | Worldwide |
| 4 | Kruse (1994) | 8 | 23 | 105 | Worldwide |
| 5 | Fritsch et al. (2006) | 1 | 15 | 88 | Worldwide |
| 6 | Neshati and Fritsch (2009) | 4 | 11 | 20 | Iran |
| 7 | Choi and Cota-Sanchez (2010) | 2 | 3 | 5 | Canada |
| 8 | Bednorz et al. (2011) | 5 | 5 | 8 | Poland |
| 9 | Choi et al. (2012) | 9 | 16 | 35 | Asia and North America |
| 10 | Celep et al. (2012) | 4 | 9 | 62 | Turkey |
| 11 | Lin and Tan (2017) | 7 | 19 | 38 | China |
| 12 | Duman et al. (2017) | 1 | 1 | 6 | Turkey |
| 13 | Veiskarami et al. (2018) | 2 | 6 | 23 | Worldwide |
| 14 | Khorasani et al. (2020) | 1 | 5 | 13 | Central Asia |
| 15 | Baasanmunkh et al. (2020) | 7 | 24 | 48 | Central Asia |
| 16 | Baasanmunkh et al. (2021) | 5 | 13 | 24 | Worldwide |
| 17 | Present study | 14 | 58 | 95 | Worldwide |
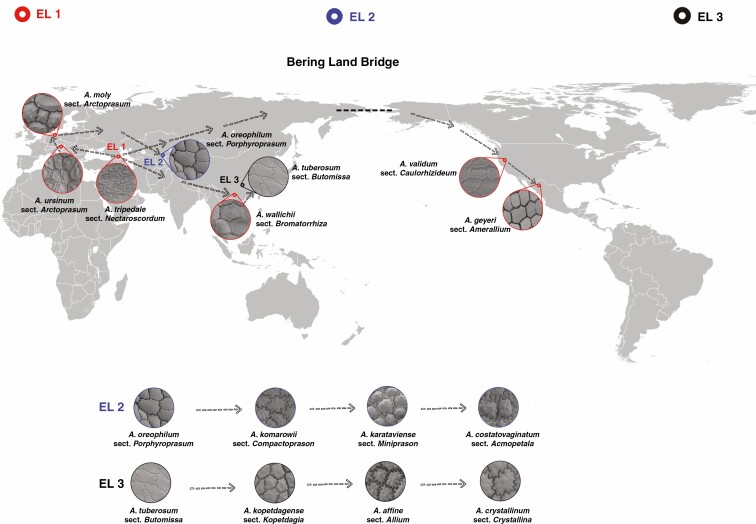
For the past two decades, DNA markers (plastid DNA and nuclear ribosomal DNA) have been utilized to reveal evolutionary processes and taxonomic relationships within the entire genus Allium (Dubouzet and Shinoda, 1999; Fritsch and Friesen, 2002; Friesen et al., 2006; Li et al., 2010) or groups within it, such as subgenus Amerallium (Samoylov et al., 1995, 1999), subgenus Melanocrommyum (Dubouzet and Shinoda, 1998; Gurushidze et al., 2008; Fritsch et al., 2010) and section Cepa (Miller) Prokhanov (Yusupov et al., 2019, 2021; Liu et al., 2020). In our study, we used publicly available (deposited in NCBI) sequences of the ITS region to construct a phylogenetic dendrogram of 72 species, 56 sections and 14 subgenera of Allium (the species were chosen from a list of 95 species available from the NCBI) representing three evolutionary lineages (EL1, EL2, EL3) (Friesen et al., 2006; Li et al., 2010; Xie et al., 2020) to determine how closely the molecular- and seed morphology-based Allium phylogenies correspond.
Our goal was to describe seed macro- and micromorphology and adequately evaluate the diagnostic value of testa morphological characteristics in the entire genus Allium and to compare the results with existing hypotheses on the evolution and biogeography of the genus (Li et al., 2010, 2016; Xie et al., 2020; Hauenschild et al., 2017). More specific questions included the following: (1) Are various types of testa cell ornamentations indicative of the stage of evolutionary process in Allium? (2) Which factors resulted in different testa cell characteristics? (3) Do any previously uninvestigated testa cell characteristics have taxonomic value in Allium? To answer the last question, we studied two seed surface ultrastructure traits for the first time: periclinal wall surface area of ten testa cells and the distance between two adjacent testa cells.
MATERIALS AND METHODS
Taxon sampling and specimen examination
Ninety-five species representing 14 subgenera and 58 sections of Allium L. were investigated in this study. Information on taxonomic affiliation, collection data and deposition of voucher specimens are provided in Table 2. Specimens of Allium were examined from the National Herbarium of Uzbekistan, the Institute of Botany in Uzbekistan Academy of Sciences (TASH), the herbarium of Kunming Institute of Botany, Chinese Academy of Sciences (KUN), the herbarium of the Institute of Botany, Chinese Academy of Sciences (PE) and the herbarium of the Botanical Institute of the National Herbarium of Georgia (TBI).
Table 2.
Seed testa micromorphology of 95 species (98 samples) of Allium (Amaryllidaceae)
| No. | Taxon | Seed length and width (mm average measurement) | L/W ratio (mm) | Seed shape | Area of 10 periclinal wall testa surface (mm2 average measurement) | Distance between testa cells (mm) | Testa cells: shape, arrangement | Dominant testa cell shapes | Anticlinal wall: curvature type | Periclinal wall: shape and micromorphology | Herbarium data | Figure: seed; cell |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
Subg. Nectaroscordum (Lindl.) Asch. & Graebn Sect. Nectaroscordum (Lindl.) Gren. & Godr., A. tripedale Trautv. |
3.22–1.8 | 1.79 | (Broadly) ovoid (shrivelled) | 0.012 | 0.007–0.01 | 4–7 edged, close. | 5 edged | Straight to arched | Gradually concave from edge to centre, many intermediate verrucae on edge | Azerbaijan, Karabakh, TBI1034308 | 1A; 8A |
| 2 |
Subg. Amerallium Traub
Sect. Arctoprasum Kirschl. (Ophioscorodon (Wallr.) Endl.) A. ursinum L. |
2.46–2.54 | 0.97 | Broadly ovoid | 0.012 | 0.003–0.005 | 4–7 edged, loose with reticulate tissue | 5 edged | Straight to arched | Gradually concave from edge to centre, one large verruca in centre | Niederosterreich, Austria (PE 00156365) |
1B; 8B |
| 3 | Sect. Bromatorrhiza Ekberg. A. wallichii Kunth |
3.04–1.78 | 1.71 | Ovoid (shrivelled) | 0.022 | 0.001–0.002 | 4–8 edged, loose with reticulate tissue. | 6 edged | Straight to arched | Flat to slightly concave from edge to centre, many small verrucae | Yunnan, China (KUN 0359135) |
1C; 8C |
| 4 | Sect. Caulorhizideum Traub A. validum S. Watson |
4.73–1.81 | 2.61 | Narrowly ovoid | 0.019 | 0.01–0.013 | 4–8 edged, close | 6 edged | Straight to arched | Flat, many small verrucae | Washington, USA (PE00156367) |
1D; 8D |
| 5 | Sect. Amerallium Traub A. geyeri S. Watson |
2.13–1.45 | 1.47 | Ovoid | 0.011 | 0.002–0.007 | 5–7 edged, loose with clear meshes of reticulate tissue and with narrow connecting thread. | 6 edged | Straight to arched | Flat to slightly convex, many small verrucae | Riverton, USA (PE 00114968) |
1E; 8E |
| 6 | Sect. Molium G. Don ex Koch A. moly L. |
2.86–2.32 | 1.22 | Broadly ovoid (shrivelled) | 0.022 | 0.007–0.017 | 4 to many edged, loose with reticulate tissue. | 6 edged | Arched to S-type | Gradually convex, 1–4 intermediate verrucae on central area of epidermis | Tubingen, Germany (KUN 0358576) |
1F; 8F |
| 7 |
Subg. Caloscordum (Herb.) R.M. Fritsch
Sect. Caloscordum (Herb.) Baker. A. neriniflorum (Herb.) Baker. |
1.71–1.63 | 1.05 | Broadly ovoid shrivelled) | 0.012 | 0.0009–0.004 | 4–7 edged, loose with reticulate tissue. | 6 edged | Straight to arched | Slightly convex, many small verrucae | Heilongjiang Province, China (PE 00115792) |
1G; 8G |
| 8 |
Subg. Anguinum (G. Don ex Koch) N. Friesen
Sect. Anguinum G. Don ex Koch A. prattii C.H. Wright |
2.20–1.66 | 1.32 | Broadly ovoid | 0.007 | 0.005–0.01 | 77 | 6 edged | Straight to Arched | Gradually concave from edge to centre, intermediate to large verrucae | Yunnan, China (KUN 0358650) |
1H; 8H |
| 9 |
Subg. Porphyroprason (Ekberg) R.M. Fritsch
Sect. Porphyroprasum Ekberg. A. oreophilum C. A. Mey. |
2.39–1.95 | 1.23 | Broadly ovoid | 0.015 | 0.0009–0.004 | Many edged to oblong, loose with inserted pattern | Oblong | S-type | Convex, many small verrucae | Osh, Kyrgyzstan (TASH 65) | 1I; 8I |
| 10 |
Subg. Vvedenskya (Kamelin) R.M. Fritsch
Sect. Vvedenskya Kamelin A. kujukense Vved. |
2.05–1.56 | 1.31 | Broadly ovoid | 0.01 | 0.002–0.005 | Many edged to oblong, loose with inserted pattern. | Oblong | Arched to S-type | Flat to convex, plane | Shymkent, Kazakhstan (TASH 2) | 1J; 8J |
| 11 |
Subg. Melanocrommyum (Webb et Berth.) Rouy.
Sect. Acanthoprason Wendelbo A. akaka S.G. Gmel. ex Schult. & Schult.f. |
2.69–1.92 | 1.40 | (Broadly) ovoid (shrivelled) | 0.016 | 0.0002–0.00068 | Orbicular to oblong, loose with inserted pattern | Oblong | U- to Ω-type | Gradually convex, many intermediate on central area | Azerbaijan, TBI1032951 | 1K; 8K |
| 12 | Sect. Megaloprason Webb et Berth. A. insufficiens Vved. |
1.66–1.16 | 1.43 | (Broadly) ovoid (shrivelled) | 0.02 | 0.001–0.002 | Variably ovoid to elliptic, close with inserted pattern | Oblong | S- to U-type | Slightly convex, many small and intermediate verrucae | Kurgan Tyube, Tajikistan (TASH 1) |
1L; 8L |
| 13 | A. sarawschanicum Regel | 2.42–1.93 | 1.25 | (Broadly) ovoid (shrivelled) | 0.016 | 0.0005–0.001 | Orbicular to elliptic, close with inserted pattern | Orbicular | U-type | Globular convex, 3–8 intermediate verrucae | Kashkadarya, Uzbekistan (TASH 110) |
1M; 8M |
| 14 | Sect. Miniprason R.M. Fritsch A. karataviense Regel |
3.78–2.68 | 1.41 | (Broadly) ovoid (shrivelled) | 0.014 | 0.001–0.004 | Orbicular to elliptic, close with inserted pattern | Orbicular | U- to Ω-type | Globular convex, 1–5 intermediate verrucae on central area | Pskem, Tashkent, Uzbekistan (TASH) 10820151) TASH | 1N; 8N |
| 15 | Sect. Popovia F.O. Khassanov et R.M. Fritsch A. gypsaceum Popov et Vved. |
2.26–1.65 | 1.37 | (Broadly) ovoid (shrivelled) | 0.011 | 0.004–0.009 | Oblong, close with inserted pattern | Oblong | Ω-type | Flat to convex, many intermediate and large verrucae, granulose | Surkhandarya, Uzbekistan (TASH 33 (1)) |
1O; 8O |
| 16 | Sect. Verticillata Kamelin A. verticillatum Regel |
1.93–1.42 | 1.36 | Flattened ovoid (shrivelled) |
0.012 | 0 | Oblong, close with inserted pattern | Oblong | Arched to S-type | Convex or concave, many small verrucae | Chatlkal Range, Tashkent, Uzbekistan (TASH 146) |
2A; 9A |
| 17 | Sect. Acmopetala R.M. Fritsch A. costatovaginatum Kamelin et Levichev |
2.45–1.46 | 1.68 | (Broadly) ovoid (shrivelled) | 0.009 | 0.00064–0.00097 | Ovoid to oblong, close with inserted pattern | Oblong | Ω-type | Gradually convex, many intermediate verrucae | Bashkysylsai, Tashkent, Uzbekistan (TASH 21 Isotypus) |
2B; 9B |
| 18 |
A. tschimganicum
B. Fedtsch. |
2.76–1.77 | 1.56 | (Broadly) ovoid (shrivelled) | 0.015 | 0.003–0.005 | Elliptic to oblong, close with inserted pattern | Elliptic | S- to U-type | Convex with granules, one large verruca on central area, intermediate verrucae on edge | Parkent, Tashkent, Uzbekistan (TASH 40) |
2C; 9C |
| 19 | A. tashkenticum F.O. Khass. & R.M. Fritsch ex F.O. Khass | 3.01–1.56 | 1.93 | Flattened ovoid | 0.01 | 0 | Elliptic to triangular, close with inserted pattern | Elliptic | Ω-type | Gradually convex, 1–4 intermediate verrucae on central area and many small verrucae on edge | Nurekatasay, Tashkent, Uzbekistan (TASH 972) |
2D; 9D |
| 20 | A. zergericum F. Khass. et R.M. Fritsch | 2.81–2.00 | 1.405 | Broadly ovoid (shrivelled) | 0.02 | 0 | Suborbicular to elliptic, close with inserted pattern | Elliptic | Ω-type | Convex or concave, many small verrucae | Fergana mountain, Uzbekistan (TASH Typus) |
2E; 9E |
| 21 | Sect. Aroidea F.O. Khass. et R.M. Fritsch A. aroides Vved. et Popov |
3.21–1.63 | 1.97 | Flattened ovoid | 0.014 | 0–0.00048 | 4 to many edged, close | 6 edged | Arched to S-type | Slightly concave or convex from edge to centre, many small verrucae | Samarkand, Uzbekistan (TASH) |
2F; 9F |
| 22 | Sect. Asteroprason R.М. Fritsch A. cristophii Trautv. |
3.08–2.45 | 1.26 | Broadly ovoid (shrivelled) | 0.02 | 0–0.002 | Oblong to suborbicular, close with inserted pattern | Oblong | U- to Ω-type | Convex, many intermediate verrucae, granulose | Babadurmaz, Turkmenistan (TASH 24) |
2G; 9G |
| 23 | Sect. Compactoprason R.M. Fritsch A. giganteum Regel |
2.48–1.77 | 1.4 | (Broadly) ovoid (shrivelled) | 0.016 | 0.002–0.005 | Elliptic to oblong, loose with inserted pattern | Oblong | U- to Ω-type | Convex, many intermediate verrucae, granulose | Tashkent, Uzbekistan (TASH) | 2H; 9H |
| 24 | A. komarowii Lipsky | 3.80–2.31 | 1.65 | Broadly ovoid | 0.019 | 0–0.002 | Ovoid to irregular, loose with inserted pattern | Oblong | U- to Ω-type | Convex, many intermediate verrucae | Jizakh, Uzbekistan (TASH) | 2I; 9I |
| 25 | Sect. Tulipifolia R.M. Fritsch & N. Friesen A. robustum Kar. & Kir. |
2.54–1.52 | 1.67 | Ovoid | 0.012 | 0.002–0.003 | 4–5 edged to oblong, close with inserted pattern | Rectangular | S-type | Convex with granulate, one large verruca in centre, intermediate verrucae on edge | Dzungarian Alatau , Kazakhstan (TASH) |
2J; 9J |
| 26 | Sect. Kaloprason C. Koch A. alexeianum Regel |
2.14–1.57 | 1.36 | Broadly ovoid (shrivelled) | 0.015 | 0.0006–0.001 | Orbicular to oblong, loose with inserted pattern | Oblong | U- to Ω-type | Gradually convex, many intermediate verrucae on central area, many small verrucae on edge, granulose | Jizakh, Uzbekistan (TASH 956) |
2K; 9K |
| 27 | A. baissunense Lipsky | 3.13–1.83 | 1.71 | (Broadly) ovoid (shrivelled) | 0.009 | 0–0.002 | Orbicular to oblong, loose with inserted pattern | Oblong | U- to Ω-type | Globular convex, many intermediate verrucae on central area, granulose, many small verrucae on edge | Surkhandarya, Uzbekistan (TASH 538) |
2L; 9L |
| 28 | A. protensum Wendelbo. | 2.4–1.4 | 1.71 | Flattened ovoid | 0.012 | 0–0.002 | Orbicular to oblong, loose with inserted pattern | Oblong | U- to Ω-type | Gradually convex, many intermediate verrucae on central area, many small verrucae on edge, granulose | Tashkent, Uzbekistan (TASH) | 2M; 9M |
| 29 | A. rhodanthum Vved. | 3.1–2.1 | 1.48 | (Broadly) flat ovoid | 0.017 | 0–0.002 | Suborbicular to elliptic, close with inserted pattern | Elliptic | U- to Ω-type | Slightly convex, many intermediate verrucae on central area, many small verrucae on edge, granulose | Surkhandarya Uzbekistan (TASH 21) |
2N; 9N |
| 30 | Sect. Procerallium R.M. Fritsch A. stipitatum Regel |
3.43–2.57 | 1.33 | Broadly ovoid | 0.03 | 0.00086–0.004 | Elliptic to triangular, loose with inserted pattern | Elliptic | U- to Ω-type | Convex with granules, 1–5 intermediate verrucae on central area and many small verrucae on edge | Jizzakh, Uzbekistan (TASH 308) |
2O; 9O |
| 31 | Sect. Regeloprason C. Koch A. regelii Trautv. |
4.31–2.85 | 1.51 | (Broadly) ovoid | 0.027 | 0 | Orbicular to elliptic, close with inserted pattern | Orbicular | U- to Ω-type | Slightly concave, many small verrucae | Mary, Turkmenistan (TASH) |
3A; 10A |
| 32 | A. cupuliferum Regel | 2.71–2.25 | 1.2 | (Broadly) ovoid (shrivelled) | 0.013 | 0–0.001 | Orbicular to elliptic, close with inserted pattern | Orbicular | U- to Ω-type | Gradually convex, many intermediate verrucae on central area of shrivelled epidermis | Forish, Jizzakh, Uzbekistan (TASH) |
3B; 10B |
| 33 | A. isakulii R.M. Fritsch & F.O. Khass. | 2.69–1.95 | 1.38 | (Broadly) ovoid (shrivelled) | 0.013 | 0–0.003 | Orbicular to elliptic, loose with inserted pattern | Orbicular | U- to Ω-type | Gradually convex, 1–4 intermediate verrucae on central area, many small verrucae | Forish, Jizzakh, Uzbekistan (TASH) | 3C; 10C |
| 34 | Sect. Stellata (F.O. Khass. & R.M. Fritsch) R.M. Fritsch A. taeniopetalum Popov & Vved. |
2.29–1.71 | 1.34 | (Broadly) ovoid | 0.02 | 0–0.001 | Orbicular to elliptic, loose with inserted pattern | Orbicular | Ω-type | Gradually convex, one large verruca central area, many intermediate and many small verrucae on edge | Forish, Jizzakh, Uzbekistan (TASH) |
3D; 10D |
| 35 | Sect. Melanocrommyum Webb & Berth. A. cardiostemon Fisch. & C.A. Mey. |
2.52–1.37 | 1.84 | Flattened ovoid (shrivelled) | 0.008 | 0–0.003 | Orbicular to oblong, loose with inserted pattern | Oblong | Ω-type | Gradually convex, many intermediate verrucae on central area, many small verrucae | Armenia, TBI1033229 | 3E; 10E |
| 36 | A. woronowii Miscz. ex Grossh. | 2.02–1.52 | 1.33 | Broadly ovoid | 0.014 | 0.002–0.008 | Orbicular to elliptic, close with inserted pattern | Orbicular | S- to U-type | Gradually convex, many intermediate and small verrucae | Azerbaijan, Nachitshevan, Shachbuz TBI1034296 |
3F; 10F |
| 37 |
Subg. Butomissa (Salisb.) N. Friesen
Sect. Austromontana N. Friesen A. oreoprasum Schrenk. |
3.03–1.87 | 1.62 | Ovoid | 0.02 | 0.003–0.009 | Elliptic to triangular, loose with reticulate tissue and inserted pattern | Elliptic | S- to U-type | Concave or convex, many intermediate and small verrucae, granulose | Naryn, Kyrgyzstan (TASH 453) |
3G; 10G |
| 38 | Sect.Butomissa Webb et Berth. A. ramosum L. |
3.93–2.34 | 1.68 | Flattened ovoid | 0.018 | 0.002–0.008 | Long to narrowly 4–7 edged to elliptic, loose with reticulate tissue | Elliptic | Arched to S-type | Plane to slightly concave, many small verrucae | Gorno-Altaisk, Russia (PE 00138973) |
3H; 10H |
| 39 | A. ramosum L. | 3.97–2.33 | 1.7 | (Broadly) ovoid (shrivelled) | 0.02 m | 0.001–0.003 | 4–7 edged, close with irregularly inserted pattern | 6 edged | Arched to S-type | Flat to slightly concave or convex, many small verrucae | Kibray, Tashkent, Uzbekistan (TASH) |
3I; 10I |
| 40 | A. tuberosum Rottler ex Spreng. | 3.45–2.37 | 1.45 | (Broadly) ovoid (shrivelled) | 0.04 | 0.002–0.005 | Long to narrowly 4–7 edged to elliptic, loose with reticulate tissue | Elliptic | Arched to S-type | Plane to slightly concave or convex, many small verrucae | Guizhou, China (KUN 0358965) |
3J; 10J |
| 41 | A. aff. tuberosum Rottler ex Spreng. | 2.79–1.60 | 1.74 | Flattened ovoid | 0.021 | 0.003–0.006 | 5–7 edged, loose with unclear meshes of reticulate tissue | 6 edged | Straight to arched | Gradually concave or convex from edge to centre, small to intermediate verrucae, granulose | Jalal–Abad, Kyrgyzstan (TASH 284) |
3K; 10K |
| 42 |
Subg. Cyathophora (R.M. Fritsch) R.M. Fritsch
Sect. Coleoblastus Ekberg. A. mairei Levl. |
2.10–1.55 | 1.35 | Broadly ovoid | 0.013 | 0.005–0.008 | 5–7 edged, loose with clear meshes of reticulate tissue | 6 edged | Straight to arched | Gradually concave from thickened edge to centre, many small verrucae in central area of epidermis | Yunnan, China (KUN 0358567) |
3L; 10L |
| 43 | Sect. Cyathophora R.M. Fritsch A. cyathophorum Bureau & Franch. |
2.64–1.32 | 2 | Flattened ovoid | 0.015 | 0.004–0.010 | 5–6 edged, loose with reticulate tissue and inserted pattern | 6 edged | Straight to arched | Gradually convex from edge to centre, small to intermediate verrucae, granulose | Yunnan, China (KUN 0358252) |
3M; 10M |
| 44 |
Subg. Rhizirideum (G. Don ex Koch) Wendelbo
Sect. Caespitosoprason N. Friesen A. subangulatum Regel |
2.10–1.30 | 1.61 | Flattened ovoid | 0.007 | 0.0008–0.002 | 5–6 edged, loose with reticulate tissue and inserted pattern | 6 edged | S-type | Slightly convex, many small verrucae | Qinghai, China (PE 01570803) |
3N; 10N |
| 45 | Sect. Eduardia N. Friesen A. przewalskianum Regel |
2.70–1.50 | 1.8 | Ovoid | 0.014 | 0.005–0.008 | Long to narrowly 4–7 edged to elliptic, loose with clear meshes of reticulate tissue with indented connecting thread | Elliptic | Straight to arched | Gradually concave from edge to centre, marginal bulge, many small verrucae | Tibet, China (KUN 0358856) |
3O; 10O |
| 46 | Sect. Tenuissima (Tzagolova) Hanelt. A. tenuissimum L. |
2.22–1.40 | 1.58 | (Broadly) ovoid (shrivelled) | 0.016 | 0–0.003 | 5–7 edged, loose with reticulate tissue and inserted pattern | 6 edged | S-type | Slightly concave or convex, many small verrucae | Jilin, China (PE 00139786) |
4A; 11A |
| 47 | Sect. Rhizirideum G. Don ex Koch s.s. A. denudatum Redouté |
1.77–1.31 | 1.29 | Broadly ovoid | 0.007 | 0.002–0.011 | 5–7 edged, loose with inserted pattern and with clear meshes of reticulate tissue and indented connecting thread | 6 edged | Straight to arched | Gradually concave from edge to centre, marginal bulge, many small verrucae | Russia, Dagestan, TBI1033015 | 4B; 11B |
| 48 |
Subg. Allium
Sect. Brevispatha Valsecchi A. margaritae B. Fedtsch. |
2.50–1.30 | 1.92 | Ovoid | 0.007 | 0.001–0.003 | 4–6 edged, loose with meshes of reticulate tissue | 5 edged | S-type | Slightly convex, many small verrucae | Taraz, Jambyl, Kazakhstan (TASH 544 (16)) |
4C; 11C |
| 49 | Sect. Codonoprassum Rchb. A. lenkoranicum Miscz. ex Grossh. |
3.60–1.90 | 1.89 | Ovoid | 0.01 | 0.003–0.005 | Triangular to 5 edged, loose with unclear meshes of reticulate tissue and inserted pattern | 5 edged | S-type | Convex, many intermediate verrucae, granulose | Akhal, Turkmenistan (TASH 2) |
4D; 11D |
| 50 | Sect. Crystallina F.O. Khass. et Yengalycheva A. crystallinum Vved. |
3.31–1.87 | 1.77 | Flattened ovoid | 0.022 | 0.002–0.005 | Orbicular to elliptic, loose with inserted pattern | Elliptic | S- to U-type | Globular convex, 1–5 intermediate and many small verrucae | Kashkadarya, Uzbekistan (TASH 143) |
4E; 11E |
| 51 | Sect. Eremoprasum (Kamelin) F.O. Khassanov, R.M. Fritsch et N. Friesen A. popovii Vved. |
2.03–1.48 | 1.37 | (Broadly) ovoid | 0.006 | 0.001–0.004 | Orbicular to oblong, loose with inserted pattern | Oblong | S-type | Convex, many small verrucae | Navoi, Uzbekistan (TASH 45) |
4F; 11F |
| 52 | A. sabulosum Stev. | 2.72–1.66 | 1.62 | Flattened ovoid | 0.019 | 0.002–0.004 | Orbicular to elliptic, loose with inserted pattern | Elliptic | U- to Ω-type | Slightly concave or convex, many small verrucae | Tomdi, Navoi, Uzbekistan (TASH 177) |
4G; 11G |
| 53 | Sect. Kopetdagia F.O. Khass. A. kopetdagense Vved. |
2.75–2.16 | 1.27 | (Broadly) ovoid | 0.01 | 0.001–0.005 | 5–7 edged, loose with inserted unclear pattern | 5 edged | Arched to S-type | Gradually convex from edge to centre, one large verruca on central area and many small verrucae on edge, granulose | Karakala, Turkmenistan (TASH) |
4H; 11H |
| 54 | Sect. Minuta F.O. Khass. A. anisotepalum Vved. |
2.41–1.01 | 2.39 | Narrowly ovoid, flattened ovoid | 0.006 | 0.001–0.002 | Orbicular to elliptic, loose with inserted pattern | Elliptic | U- to Ω-type | Slightly convex, many small verrucae | Uzbek-Gava, Jalal-Abad, Kyrgyzstan (TASH) |
4I; 11I |
| 55 | A. minutum Vved. | 2.35–1.02 | 2.3 | (Narrowly) ovoid | 0.012 | 0.002.–0.005 | Orbicular to elliptic, loose with clear meshes of reticulate tissue with indented connecting thread | Elliptic | U- to Ω-type | Slightly convex, many small and granulose intermediate verrucae | Zaalaisky Range, Kyrgyzstan (TASH 65) |
4J; 11J |
| 56 | Sect. Allium A. affine Ledeb. |
2.54–1.17 | 2.017 | Flattened ovoid | 0.018 | 0.004–0.06 | Orbicular to oblong, loose with inserted pattern and with indented connecting thread | Oblong | U-type | Convex, many intermediate verrucae | Georgia, TBI1032955 | 4K; 11K |
| 57 | A. atroviolaceum Boiss. | 3.26–1.58 | 2.06 | (Narrowly) flattened ovoid | 0.02 | 0.001–0.003 | Orbicular to elliptic, loose with inserted pattern | Elliptic | U-type | Convex, one large verruca in centre, many intermediate verrucae on edge, granulose | Zangiata, Tashkent, Uzbekistan (TASH) |
4L; 11L |
| 58 | A. aucheri Boiss. | 3.11–1.42 | 2.19 | Narrowly ovoid | 0.022 | 0.002–0.006 | Oblong to elliptic, loose with reticulate tissue and inserted pattern | Elliptic | S- to U-type | Concave, many intermediate and small verrucae, granulose | Armenia, Semenovskii Pass, TBI10332208 |
4M; 11M |
| 59 | A. dictyoscordum Vved. | 3.15–2.20 | 1.43 | Broadly ovoid | 0.024 | 0.003–0.008 | Orbicular to oblong, loose with inserted pattern and with indented connecting thread | Oblong | S- to U-type | Convex, many intermediate verrucae | Akhal, Turkmenistan (TASH 53) | 4N; 11N |
| 60 | A. filidens Regel | 3.23–2.14 | 1.51 | (Broadly) ovoid | 0.012 | 0.002–0.004 | Orbicular to elliptic, loose with inserted pattern | Elliptic | U- to Ω-type | Convex, 1–3 large verrucae in centre, many intermediate verrucae on edge, granulose | Tashkent, Uzbekistan (TASH 35546) |
4O; 11O |
| 61 | A. guttatum Steven | 2.46–1.15 | 2.14 | (Narrowly) flattened ovoid (shrivelled) | 0.011 | 0.002–0.006 | Orbicular to elliptic, loose with inserted pattern and with unclear meshes of reticulate tissue with indented connecting thread | Elliptic | U- to Ω-type | Convex, 5–7 intermediate verrucae, granulose | Bessarabia, Eastern Europe (PE 00114993) |
5A; 12A |
| 62 | A. ugami (Vved.) R.M. Fritsch & F.O. Khass. | 3.02–1.88 | 1.6 | (Broadly) ovoid | 0.016 | 0.001–0.005 | Orbicular to elliptic and triangular, loose with inserted pattern | Elliptic | U- to Ω-type | Convex, one or two large granulose verrucae in centre, many intermediate granulose verrucae on edge | Tashkent, Uzbekistan (TASH 173 Syntypus) |
5B; 12B |
| 63 | A. vineale L. | 2.77–1.21 | 2.29 | (Narrowly) ovoid (shrivelled) | 0.016 | 0.002–0.011 | Orbicular to oblong, loose with inserted pattern | Oblong | U- to Ω-type | Gradually convex from edge to centre, marginal bulge or not, many intermediate and small verrucae | Armenia, TBI1034279 | 5C;12C |
| 64 | Sect. Avulsea F.O. Khass. A. fibrosum Regel |
2.55–1.27 | 2 | Flattened ovoid | 0.013 | 0.002–0.004 | Orbicular to elliptic, loose with inserted pattern | Elliptic | S- to U-type | Convex, one or two large verrucae in centre, many intermediate verrucae on edge, granulose | Ashgabat, Turkmenistan (TASH25) |
5D; 12D |
| 65 | A. griffithianum Boiss. | 2.14–1.15 | 1.86 | Ovoid | 0.008 | 0.0009–0.004 | Orbicular to elliptic and triangular, loose with inserted pattern | Elliptic | U-type | Gradually convex, large verrucae in centre, many small and intermediate verrucae on edge, granulose | Zaaminsu, Jizzakh, Uzbekistan (TASH) |
5E; 12E |
| 66 | A. pamiricum Wendelbo | 3.31–1.48 | 2.24 | Flattened ovoid | 0.009 | 0–0.003 | Orbicular to elliptic, close with inserted pattern | Elliptic | U-type | Concave or convex, 1–4 intermediate verrucae in centre, many small verrucae on edge, granulose | Khorog, Tajikistan (TASH) |
5F; 12F |
| 67 | Sect. Brevidentia F.O. Khass. et Yengalycheva A. brevidens Vved. |
2.40–1.33 | 1.8 | Ovoid | 0.01 | 0.002–0.005 | Orbicular to elliptic and triangular, loose with inserted pattern and with unclear meshes of reticulate tissue with indented connecting thread | Elliptic | U-type | Convex, 1–3 intermediate granulose verrucae on central area and many small verrucae | Babatag range, Tajikistan (TASH 546) |
5G; 12G |
| 68 | A. ophiophyllum Vved. | 2.12–1.21 | 1.75 | Ovoid (shrivelled) | 0.012 | 0.002–0.007 | Orbicular to oblong, loose with inserted pattern | Oblong | S- to U-type | Globular convex, many intermediate granulose verrucae | Kashkadarya, Uzbekistan (TASH 482) |
5H; 12H |
| 69 | Sect. Coerulea (Omelczuk) F.O. Khass. A. caesioides Drobow ex Vved. |
1.70–0.91 | 1.87 | Ovoid | 0.01 | 0.003–0.008 | Orbicular to oblong, loose with inserted pattern | Oblong | S- to U-type | Gradually concave or convex from edge to centre, marginal bulge or not, many small verrucae | Jalal–Abad, Kyrgyzstan (TASH) |
5I; 12I |
| 70 | A. elegans Drobow. | 2.29–1.53 | 1.5 | (Broadly) ovoid (shrivelled) | 0.01 | 0.003–0.01 | Orbicular to elliptic, loose with inserted pattern and with unclear meshes of reticulate tissue with indented connecting thread | Elliptic | S-type | Convex, 1–5 intermediate granulose verrucae and many small verrucae | Sugd, Tajikistan (TASH 87) | 5J; 12J |
| 71 | A. caesium Schrenk. | 2.16–1.15 | 1.88 | Ovoid (shrivelled) | 0.012 | 0.002–0.007 | Orbicular to oblong and elliptic, loose with inserted pattern and with unclear meshes of reticulate tissue with indented connecting thread | Oblong | S- to U-type | Convex, one or two large granulose verrucae and many small verrucae | Namangan, Uzbekistan (TASH) |
5K; 12K |
| 72 | A. delicatulum Siev. ex Schult. & Schult. f. | 2.32–1.08 | 2.15 | Ovoid | 0.008 | 0.001–0.004 | Orbicular to oblong and elliptic, loose with inserted pattern | Oblong | U- to Ω-type | Gradually concave from edge to centre, marginal bulge or not, small verrucae | Tarangul lake, Severo-Kazakhstan, Kazakhstan (TASH 1111) |
5L; 12L |
| 73 | A. caeruleum Pall. | 2.13–1.40 | 1.52 | (Broadly) ovoid | 0.012 | 0.001–0.003 | Triangular to elliptic, loose with inserted pattern | Elliptic | Ω-type | Gradually concave from edge to centre, marginal bulge or not, small verrucae | Suusamyr, Kyrgyzstan (TASH 1115) |
5M; 12M |
| 74 | Sect. Mediasia F.O. Khass., S.C. Yengalycheva et N. Friesen A. turkestanicum Regel |
2.93–1.89 | 1.55 | (Broadly) ovoid | 0.011 | 0.001–0.007 | 5–7 edged, loose with inserted pattern and with large clear meshes of reticulate tissue with broadly connecting thread | 6 edged | Arched to S-type | Convex, many small verrucae | Jizzakh, Uzbekistan (TASH) |
5N; 12N |
| 75 | Sect. Multicaulia F.O. Khass. et Yengalycheva. A. ferganicum Vved. |
2.42–1.19 | 2.03 | Ovoid | 0.015 | 0.001–0.005 | Orbicular to elliptic and oblong with inserted pattern and with large clear meshes of reticulate tissue with broadly connecting thread | Oblong | U-type | Convex, 1–3 intermediate verrucae and many small verrucae | Namangan, Uzbekistan (TASH 1099) |
5O; 12O |
| 76 | A. borszczowii Regel | 3.58–2.51 | 1.43 | (Broadly) ovoid | 0.023 | 0.0008–0.003 | Triangular to elliptic, loose with inserted pattern | Elliptic | U-type | Slightly convex, many small verrucae | Karakalpakstan, Uzbekistan (TASH 18) |
6A; 13A |
| 77 | A. borszczowii Regel | 3.06–1.37 | 2.23 | Flattened ovoid | 0.018 | 0.002–0.004 | Triangular to elliptic and oblong, loose with inserted pattern | Oblong | U- to Ω-type | Convex, many small verrucae | Badhyz, Turkmenistan (TASH 1172) |
6B; 13B |
| 78 | Sect. Haneltia F.O. Khass. A. haneltii F.O. Khass. & R.M. Fritsch |
2.41–1.22 | 1.97 | Flattened ovoid | 0.019 | 0–0.003 | Triangular to elliptic and oblong, loose with inserted pattern | Elliptic | U-type | Slightly convex, 1–4 intermediate granulose verrucae and many small verrucae | Pop, Namangan, Uzbekistan (TASH 107) |
6C; 13C |
| 79 | Sect. Pallasia (Tzagolova) F.O. Khass., R.M. Fritsch et N. Friesen A. pallasii Murr. |
2.72–1.39 | 1.96 | Ovoid (shrivelled) | 0.011 | 0.002–0.009 | Oblong to elliptic, loose with inserted pattern and with small clear meshes of reticulate tissue with narrowly connecting thread | Oblong | Arched to S-type | Flat to concave, many small verrucae | Xinjiang, China (KUN 0358637) |
6D; 13D |
| 80 | A. tanguticum Regel | 2.45–1.39 | 1.76 | Flattened ovoid | 0.008 | 0.007–0.021 | 4–7 edged, loose with clear meshes of reticulate tissue and indented connecting thread | 6 edged | Straight to arched | Gradually concave from edge to centre, marginal bulge, small verrucae | Gansu, China (PE 00139762) |
6E; 13E |
| 81 |
Subg. Reticulatobulbosa (Kamelin) N. Friesen
Sect. Nigrimontana N. Friesen A. drobovii Vved. |
3.97–2.31 | 1.72 | Flattened ovoid | 0.025 | 0.008–0.012 | 4–7 edged, loose with inserted pattern and with reticulate tissue | 6 edged | Straight to arched | Gradually concave from thickened edge to centre, one large granulose verruca in centre, many small verrucae on edge | Pskem, Tashkent, Uzbekistan (TASH 10820151) |
6F; 13F |
| 82 | Sect. Reticulatobulbosa Kamelin A. lineare L. |
3.00–1.54 | 1.95 | Flattened ovoid | 0.02 | 0.006–0.15 | 4–7 edged, loose with inserted pattern and with clear meshes of reticulate tissue and indented connecting thread | 6 edged | Straight to arched | Gradually concave from thickened edge to centre, one large granulose verruca in centre, many small verrucae on edge | Kazakhstan (TASH 805) | 6G; 13G |
| 83 | Sect. Campanulata Kamelin A. barsczewskii Lipsky |
3.83–2.05 | 1.87 | Ovoid | 0.01 | 0.005–0.009 | 5–7 edged, loose with clear meshes of reticulate tissue and indented connecting thread | 6 edged | Straight to arched | Gradually convex from thickened edge to centre, many granulose verrucae in centre, many small verrucae on edge | Parkent, Tashkent, Uzbekistan (TASH) | 6H; 13H |
| 84 | Sect. Scabriscapa (Tscholok.) N. Friesen A. eriocoleum Vved. |
2.85–2.07 | 1.37 | (Broadly) ovoid (shrivelled) | 0.01 | 0.006–0.008 | 4–7 edged, loose with clear meshes of reticulate tissue and broadly connecting thread | 6 edged | Straight to arched | Gradually convex from thickened edge to centre, one large granulose verruca in centre, many small verrucae on edge | Tashkent, Uzbekistan (TASH) | 6I; 13I |
| 85 | A. sulphureum Vved. | 2.84–2.07 | 1.37 | Broadly ovoid | 0.012 | 0.003–0.006 | Orbicular to elliptic, loose with inserted pattern and with unclear meshes of reticulate tissue with indented connecting thread | Elliptic | U- to Ω-type | Convex, one or two large granulose verrucae in centre, many intermediate granulose verrucae on edge | Surkhandarya, Uzbekistan (TASH 736) |
6J; 13J |
| 86 |
Subg. Polyprason Radic
Sect. Daghestanica (Tscholok.) N. Friesen A. albovianum C.H. Wright |
3.13–1.48 | 2.11 | Ovoid (shrivelled) | 0.005 | 0.003–0.011 | 4–many edged, loose with inserted pattern and with clear meshes of reticulate tissue and indented connecting thread | 6 edged | Arched to S-type | Gradually concave from edge to centre, intermediate to large verrucae | Georgia, TBI1038711 | 6K; 13K |
| 87 | Sect. Falcatifolia A. korolkowii Regel |
2.17–1.16 | 1.87 | Flattened ovoid | 0.008 | 0.003–0.005 | 5–6 edged to oblong, loose with clear meshes of reticulate tissue and with indented connecting thread | Oblong | S-type | Convex, many small verrucae in centre, intermediate verrucae on edge | Almaty, Kazakhstan (TASH 5739) |
6L; 13L |
| 88 | A. carolinianum DC. | 3.24–1.80 | 1.3 | Ovoid (shrivelled) | 0.01 | 0.005–0.009 | 4–7 edged, loose with reticulate tissue | 6 edged | Straight to arched | Slightly concave, many small verrucae | Surkhandarya, Uzbekistan (TASH) |
6M; 13M |
| 89 | Sect. Oreiprason F. Herm. A. talassicum Regel |
2.77–1.62 | 1.71 | Flattened ovoid | 0.006 | 0.002–0.006 | 5–7 edged, loose with inserted pattern and with clear meshes of reticulate tissue | 6 edged | Straight to arched | Convex, many small verrucae | Aflatun river, Jalal–Abad, Kyrgyzstan (TASH) |
6N; 13N |
| 90 |
Subg. Cepa(Mill.) Radic
Sect. Cepa (Mill.) Prokh. |
3.52–2.60 | 1.35 | Broadly ovoid | 0.008 | 0.002–0.007 | 4–7 edged, loose with unclear meshes of reticulate tissue | 6 edged | Straight to arched | Convex, many small verrucae | Khujand, Tajikistan (TASH 264) |
6O; 13O |
| 91 | A. praemixtum Vved. | 3.30–2.42 | 1.36 | (Broadly) ovoid (shrivelled) | 0.009 | 0.002–0.006 | 5–7 edged, loose with reticulate tissue | 6 edged | Straight to arched | Globular convex, many small verrucae | Khujand, Tajikistan (TASH 265) |
7A; 14A |
| 92 | A. altaicum Pall. | 3.58–1.98 | 1.8 | (Broadly) ovoid (shrivelled) | 0.01 | 0.005–0.014 | 4–7 edged, loose with reticulate tissue and with narrow connecting thread | 6 edged | Straight to arched | Slightly convex, many small verrucae | Almaty, Kazakhstan (TASH 1174) |
7B; 14B |
| 93 | A. galanthum Kar. & Kir. | 3.39–2.18 | 1.55 | (Broadly) ovoid (shrivelled) | 0.008 | 0.003–0.009 | 4–7 edged, loose with reticulate tissue and with narrow connecting thread | 6 edged | Straight to arched | Convex, many small verrucae | Naryn, Kyrgyzstan (TASH 1280) |
7C; 14C |
| 94 | A. pskemense B. Fedtsch. | 3.86–2.85 | 1.35 | (Broadly) ovoid (shrivelled) | 0.012 | 0.006–0.014 | 4–7 edged, loose with reticulate tissue and with narrow connecting thread | 6 edged | Straight to arched | Convex, many small verrucae | Pskem, Tashkent, Uzbekistan (TASH) |
7D; 14D |
| 95 | A. oschaninii O. Fedtsch. | 3.47–2.32 | 1.5 | (Broadly) ovoid | 0.01 | 0.004–0.008 | 5–7 edged, loose with reticulate tissue and with narrow connecting thread | 6 edged | Straight to arched | Gradually convex, many small to intermediate verrucae | Malguzar, Jizzakh, Uzbekistan (TASH 214) |
7E; 14E |
| 96 | Sect. Schoenoprasum Dumort. A. karelinii Poljak. |
2.73–1.42 | 1.92 | Flattened ovoid | 0.004 | 0.002–0.006 | 4–7 edged, loose with clear meshes of reticulate tissue and with narrow connecting thread | 6 edged | Straight to arched | Flat to slightly convex, many small verrucae | Almaty, Kazakhstan (TASH3916) |
7F; 14F |
| 97 | Sect. Annuloprason Egorova A. fedtschenkoanum Regel |
3.57–1.74 | 2.05 | Flattened ovoid | 0.009 | 0.002–0.006 | 4–7 edged to oblong, close | 6 edged | Straight to arched | Globular convex, many small verrucae on surface | Surkhandarya, Uzbekistan (TASH 580) |
7G; 14G |
| 98 | Sect. Condensatum N. Friesen A. condensatum Turcz. |
3.05–1.64 | 1.86 | Flattened ovoid | 0.011 | 0.003–0.011 | Long 4–7 edged, close with unclear meshes | 6 edged | Straight to arched | Flat to slightly convex, many small verrucae | Hebei, China (KUN 0358234) |
7H; 14H |
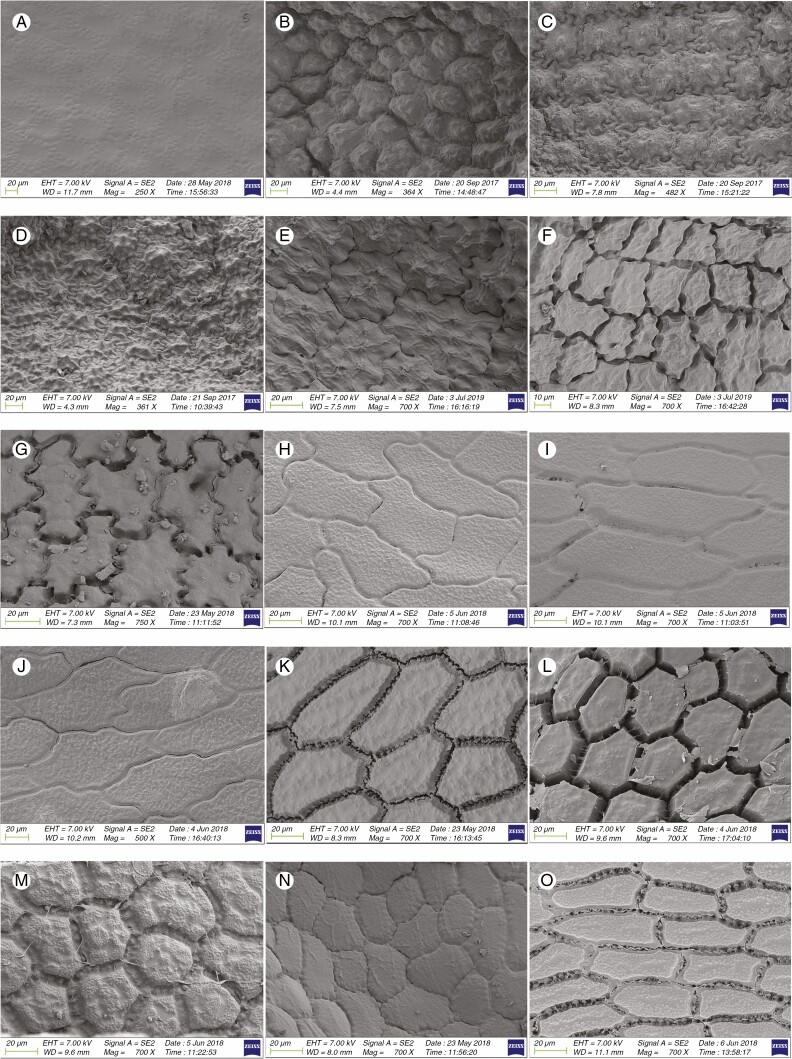
The seeds were examined under a Zeiss Sigma 300 (Zeiss, Oberkochen, Germany) scanning electron microscope (SEM) at 7 kV at Kunming Institute of Botany, Chinese Academy of Sciences, to determine that they were typical in size and maturity. Dried seed samples were then affixed to specimen tabs and then coated with platinum in a sputter coater. The seeds were then examined for morphometric measurement and observation using the SEM. For the average morphometric measurements, three to five seeds for each sample, depending on availability, were measured. The terminology of seed micromorphology follows Barthlott and Ehler (1977) and Celep et al. (2012) for explaining the seed surface elements. The seed measurements employed ImageJ software (Schneider et al., 2012). The distance between testa cells and ten testa cells of the periclinal surface area was measured using the special plugin 3D viewer of the software ImageJ. There were ten repetitions of each measurement type per species.
The taxa investigated in this study were classified in accordance with Friesen et al. (2006), Li et al. (2010), Choi et al. (2012); Fritsch and Abbasi (2013) (subgenus Melanocrommyum), Fritsch (2016) (subgenus Melanocrommyum), Fritsch et al. (2010) (subgenus Melanocrommyum), Sinitsyna et al., (2016), Khassanov (2018) (subgenus Allium), Baasanmunkh et al. (2021) and Friesen et al. (2021) (Supplementary Data Table S1). The orthography of taxonomic names was adopted from the World Checklist of Selected Plant Families (WCSP, 2022).
Phylogenetic analyses
To investigate the phylogenetic significance of seed testa cell micromorphology, DNA sequences of 72 species, 56 sections and 14 subgenera representing three major lineages of Allium were downloaded from NCBI (see Appendix) and the phylogenetic tree based on ITS sequences of Allium was constructed. Furthermore, the correlation of testa cell characteristics with evolutionary trends was examined and discussed. The species were compared through five seed morphological parameters [cell arrangement, anticlinal wall undulation, periclinal wall (shape), periclinal wall (verrucae), seed shape]. The sequences were aligned using MEGA 7.0 (Hall, 1999). The best-fitting substitution models for Bayesian inference were selected using MrModeltest 2.3 (Nylander et al., 2004). Bayesian inference employed MrBayes v.3.2.6 with a Metropolis-coupled Markov chain Monte Carlo approach and maximum likelihood employed RAxML v.8.2.10 in the GTRGAMMA substitution model at the Cipres Portal (https://www.phylo.org/portal2). Phylogenetic analyses were also performed with the maximum parsimony method using PAUP* 4.0a169 (current). The maximum parsimony bootstrap analysis was performed with heuristic search, TBR (tree bisection–reconnection) branch-swapping, 1000 bootstrap replicates, random addition sequence with ten replicates, and a maximum of 1000 trees saved per round.
RESULTS AND DISCUSSION
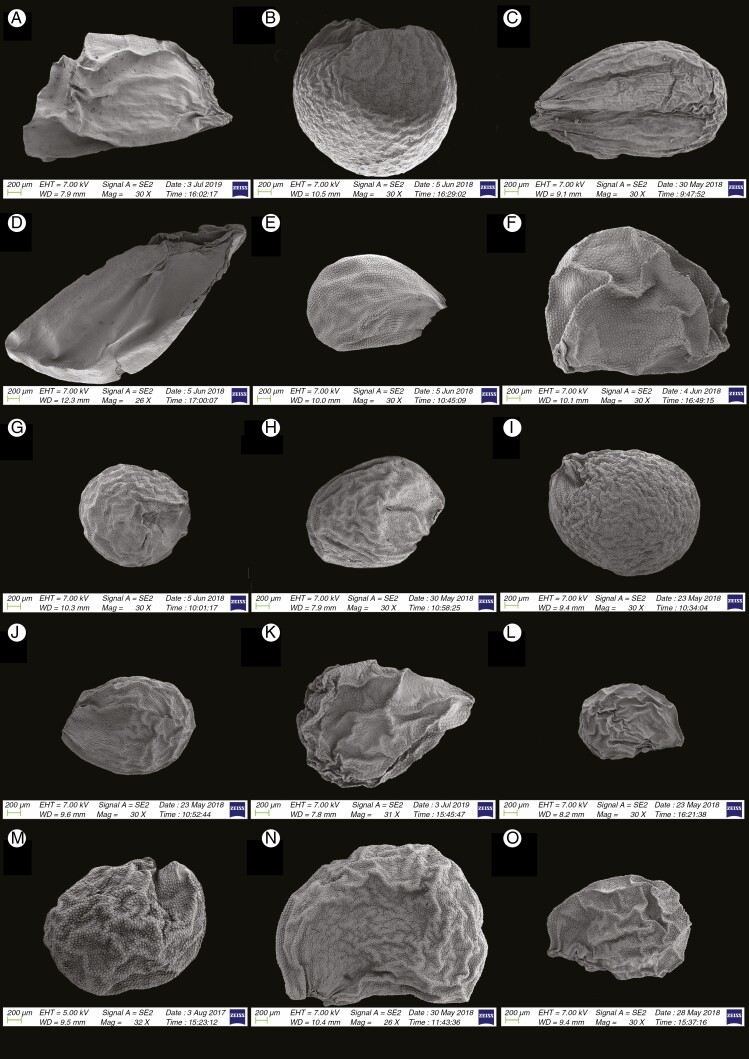
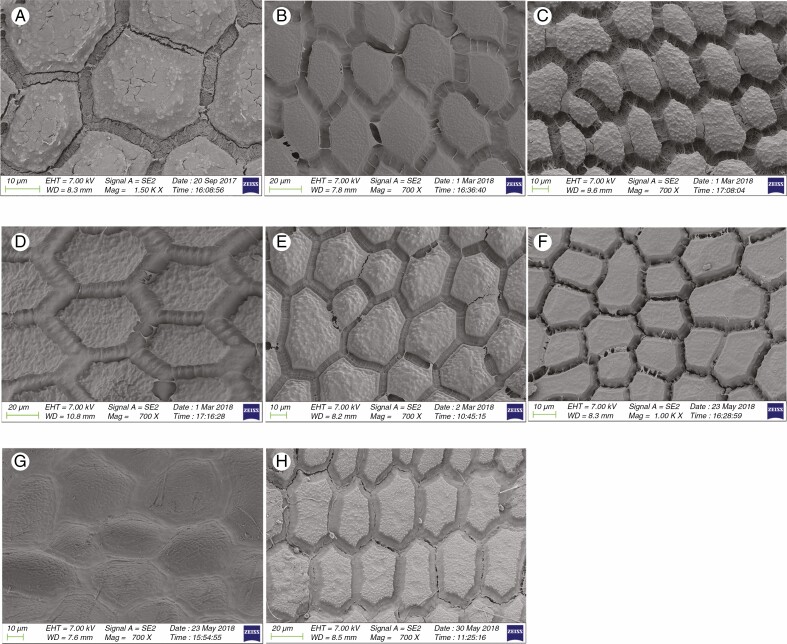
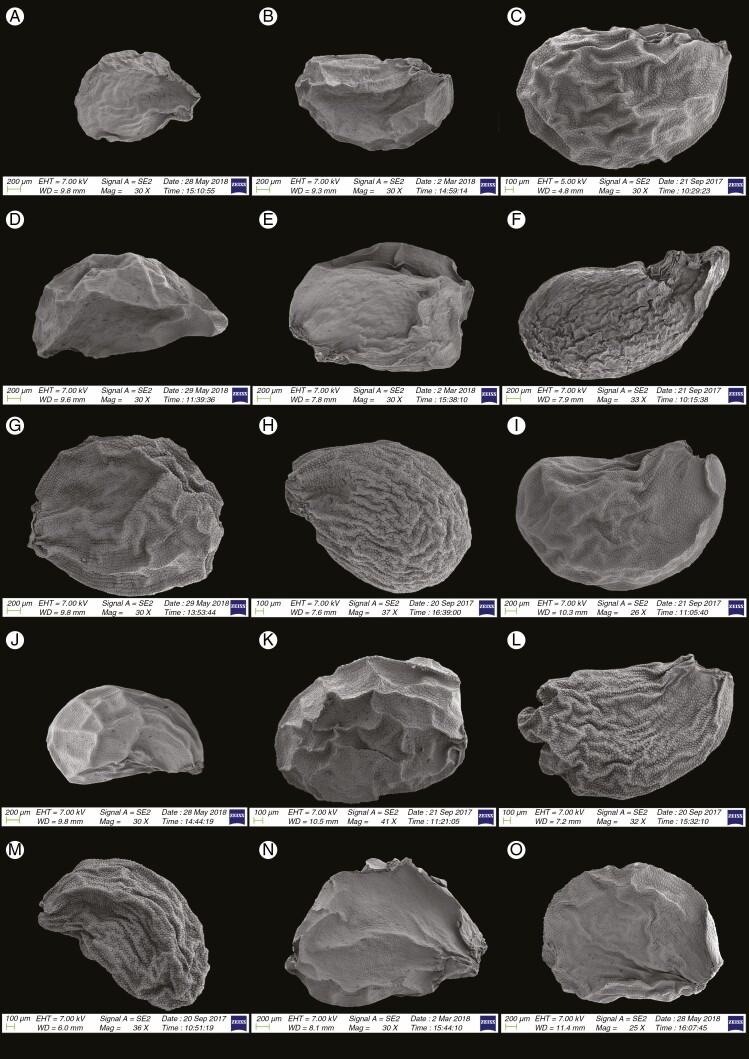
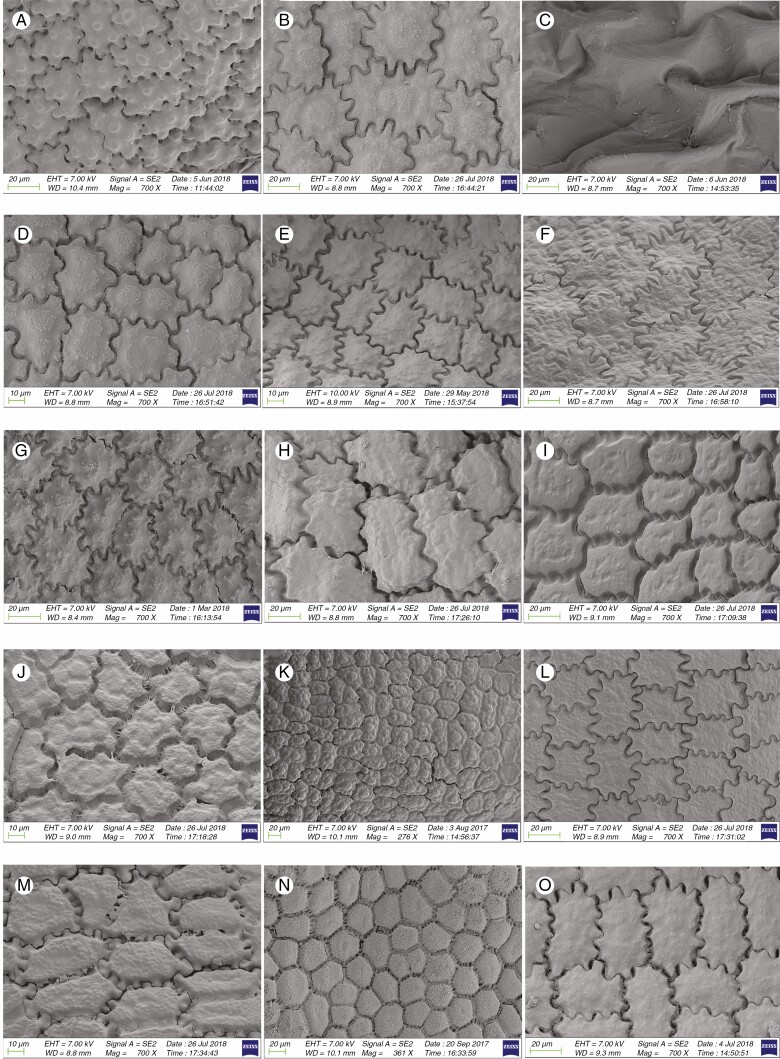
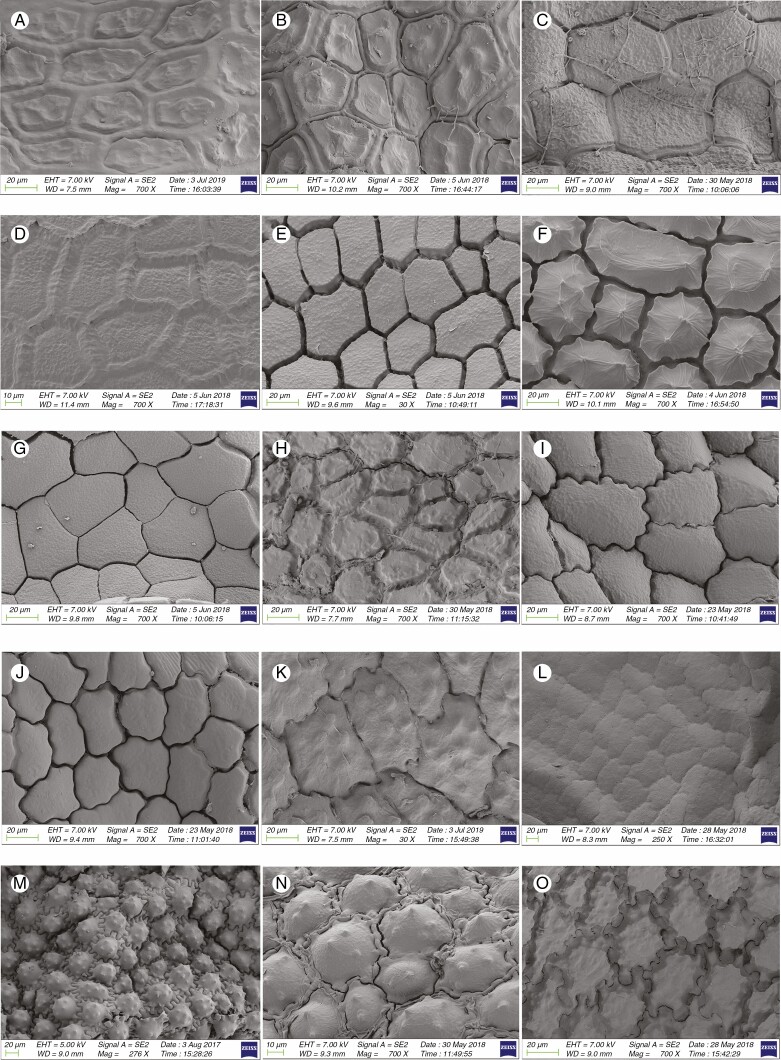
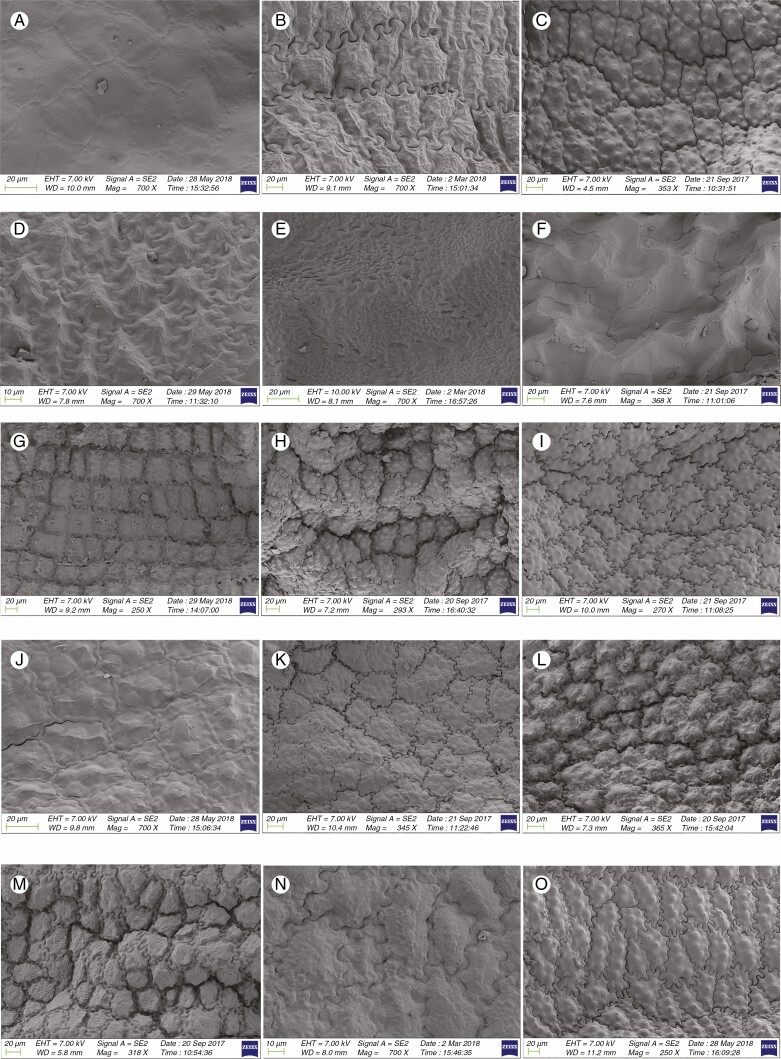
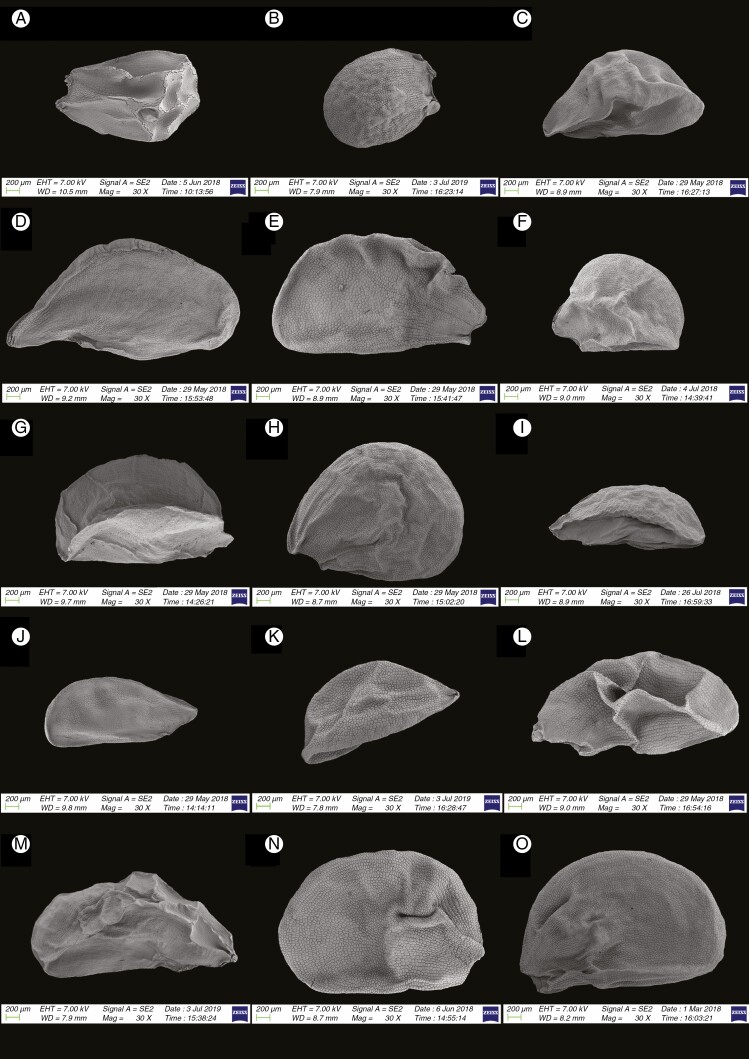
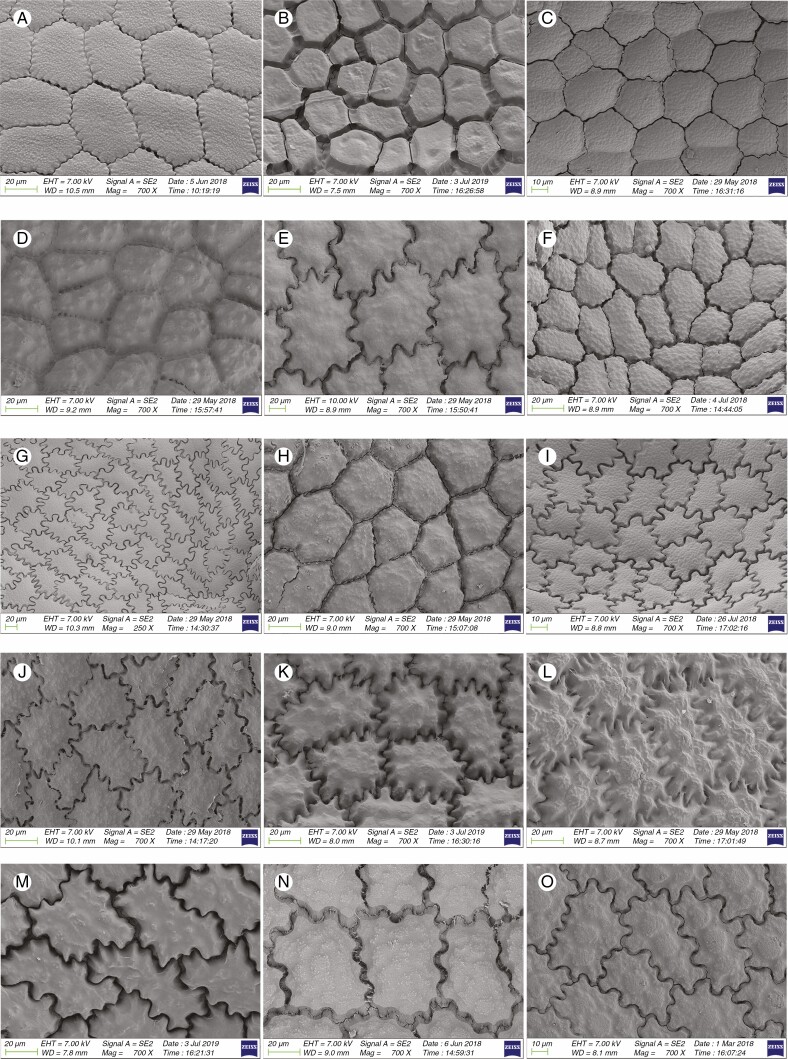
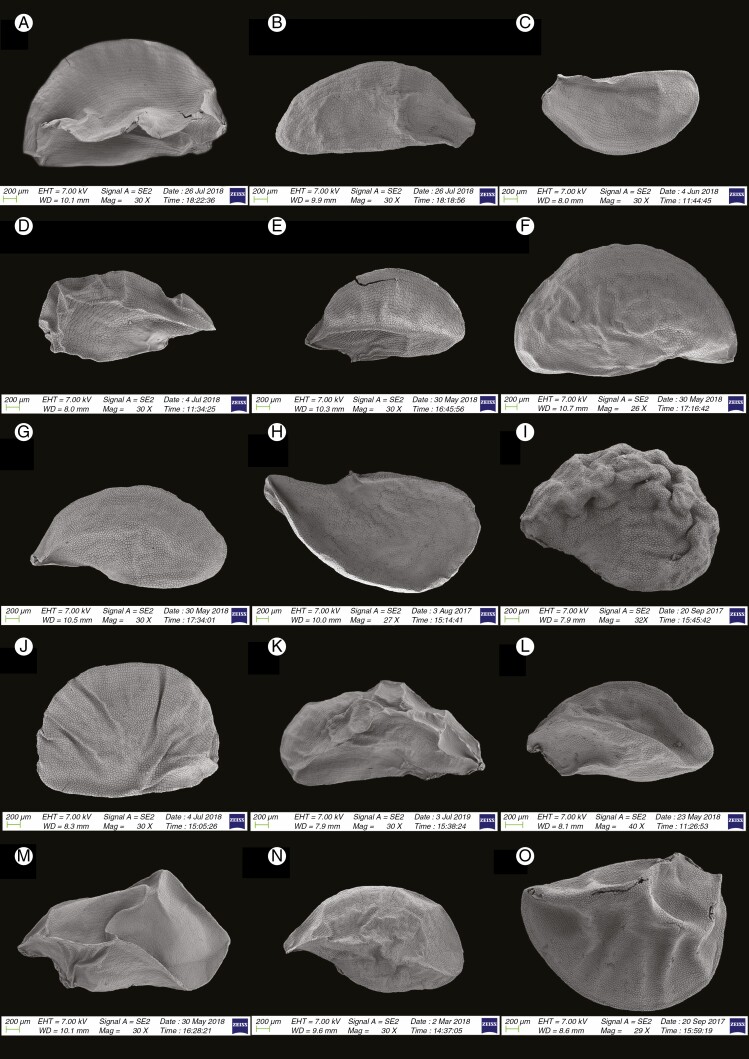
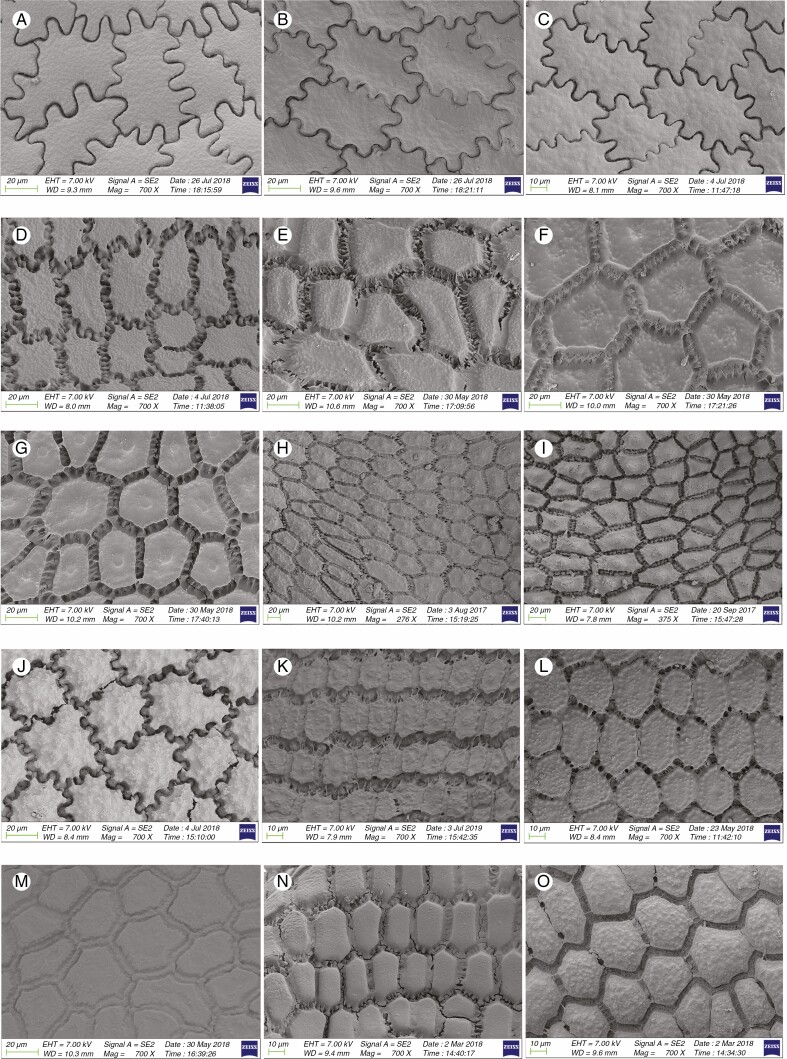
Information on taxonomic affiliation, vouchers, measurements of seed macro- and micromorphology of 95 species (98 samples) of Allium are provided in Table 2. SEM photographs of seeds of all taxa studied are presented in Figs 1–14.
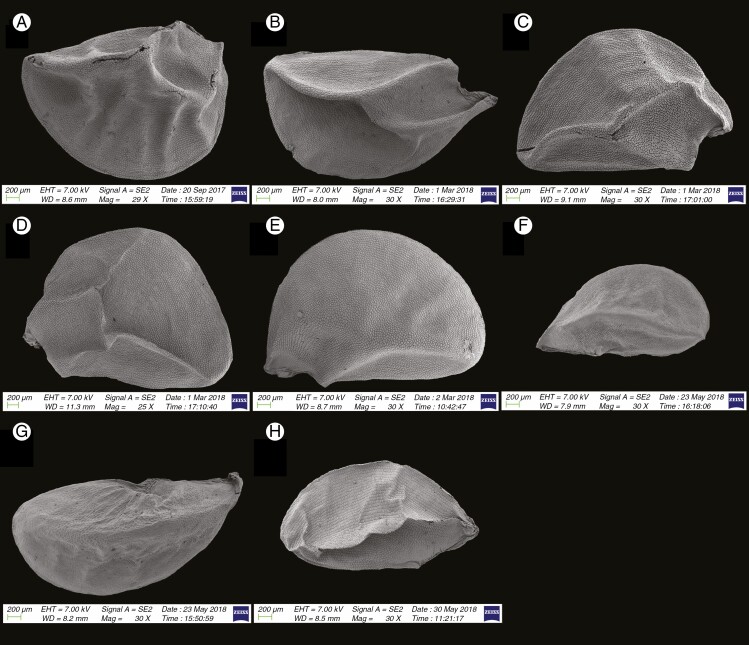
Fig. 1.
SEM micrographs of seeds of 15 Allium species. (A) Subg. Nectaroscordum: A. tripedale (sect. Nectaroscordum). (B–F) Subg. Amerallium: (B) A. ursinum (sect. Arctoprasum); (C) A. wallichii (sect. Bromatorrhiza); (D) A. validum (sect. Caulorhizideum); (E) A. geyeri (sect. Amerallium); (F) A. moly (sect. Molium). (G) Subg. Caloscordum: A. neriniflorum (sect. Caloscordum). (H) Subg. Anguinum: A. prattii (sect. Anguinum). (I) Subg. Porphyroprason: A. oreophilum (sect. Porphyroprasum). (J) Subg. Vvedenskya: A. kujukense (sect. Vvedenskya). (K–O) Subg. Melanocrommyum: (K) A. akaka (sect. Acanthoprason); (L) A. insufficiens (sect. Megaloprason); (M) A. sarawschanicum (sect. Megaloprason); (N) A. karataviense (sect. Miniprason); (O) A. gypsaceum (sect. Popovia).
Fig. 14.
SEM micrographs of seeds of eight Allium species in subg. Cepa: (A) A. praemixtum (sect. Cepa); (B) A. altaicum (sect. Cepa); (C) A. galanthum (sect. Cepa); (D) A. pskemense (sect. Cepa); (E) A. oschaninii (sect. Cepa); (F) A. karelinii (sect. Schoenoprasum); (G) A. fedschenkoanum (sect. Annuloprason); (H) A. condensatum (sect. Condensatum).
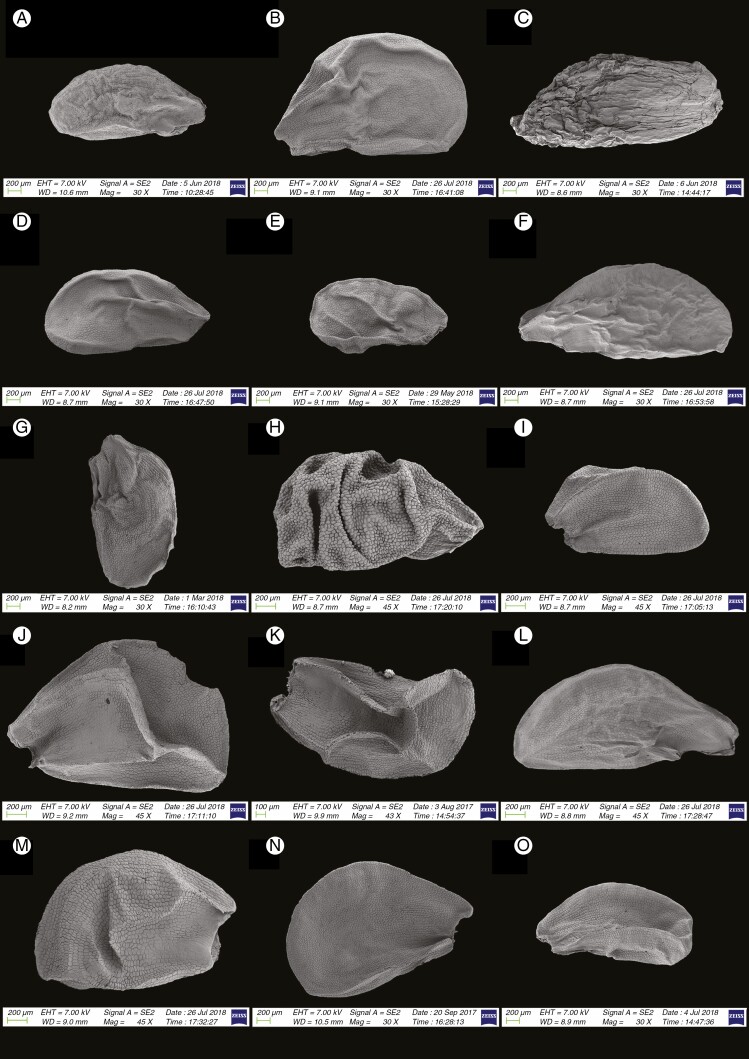
Fig. 2.
SEM micrographs of seeds of 15 Allium species in subg. Melanocrommyum: (A) A. verticillatum (sect. Verticillata); (B) A. costatovaginatum (sect. Acmopetala); (C) A. tschimganicum (sect. Acmopetala); (D) A. tashkenticum (sect. Acmopetala); (E) A. zergericum (sect. Acmopetala); (F) A. aroides (sect. Aroidea); (G) A. cristophii (sect. Asteroprason); (H) A. giganteum (sect. Compactoprason); (I) A. komarowii (sect. Compactoprason); (J) A. robustum (sect. Tulipifolia); (K) A. alexeianum (sect. Kaloprason); (L) A. baissunense (sect. Kaloprason); (M) A. protensum (sect. Kaloprason); (N) A. rhodanthum (sect. Kaloprason); (O) A. stipitatum (sect. Procerallium).
Fig. 12.
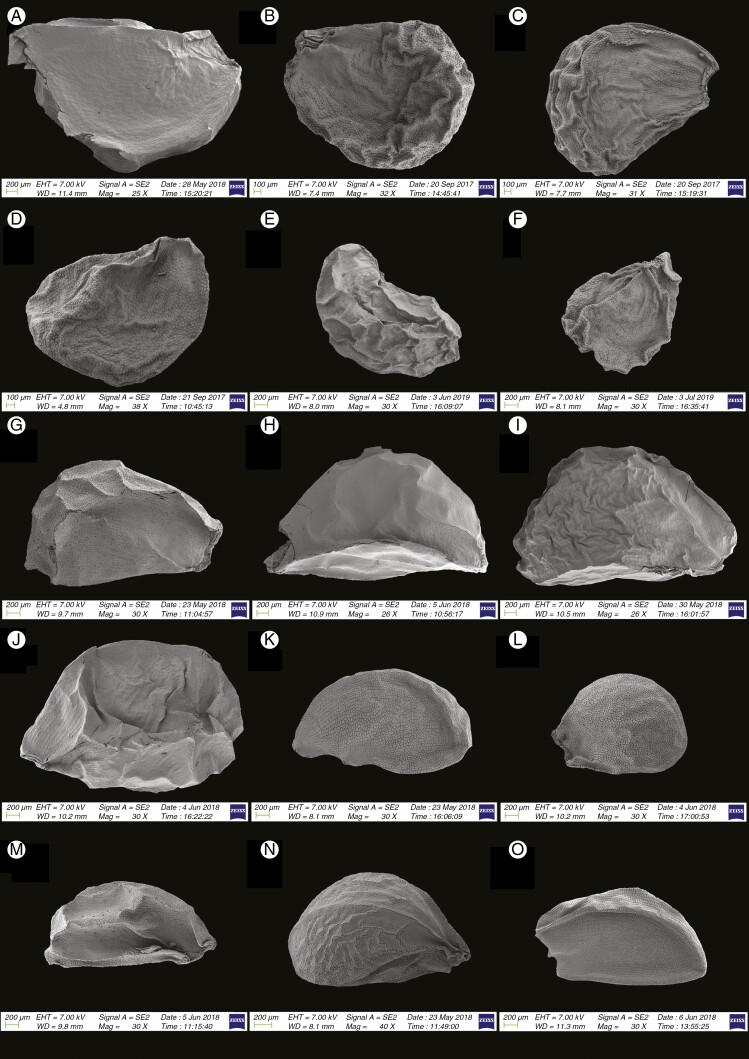
SEM micrographs of seeds of 15 Allium species in subg. Allium: (A) A. guttatum (sect. Allium); (B) A. ugami (sect. Allium); (C) A. vineale (sect. Allium); (D) A. fibrosum (sect. Avulsea); (E) A. griffithianum (sect. Avulsea); (F) A. pamiricum (sect. Avulsea); (G) A. brevidens (sect. Brevidentia); (H) A. ophiophyllum (sect. Brevidentia); (I) A. caesioides (sect. Coerulea); (J) A. elegans (sect. Coerulea); (K) A. caesium (sect. Coerulea) (L) A. delicatulum (sect. Coerulea); (M) A. caeruleum (sect. Coerulea); (N) A. turkestanicum (sect. Mediasia); (O) A. ferganicum (sect. Multicaulia).
We recognized the following categories of seed shape: ovoid, broadly ovoid, narrowly ovoid, ovoid shrivelled and flattened ovoid (Table 2). Figures 1–7 show the seed shapes of the species of Allium organized according to their taxonomic position. In our morphometric measurements, A. caesioides Wendelbo [subgenus Allium, section Coerulea (Omelczuk) F.O. Khass (Fig. 5I)] had the shortest (1.70 mm) and narrowest (0.91 mm) seeds; A. validum S. Watson (subgenus Amerallium, section Caulorhizideum Traub, Fig. 1D) had the longest (4.73 mm). The widest seeds (2.85 mm) were observed in A. regelii Trautv. [subgenus Melanocrommyum (Webb et Berth.) Rouy, section Regeloprason C. Koch (Fig. 3A)]. Allium ursinum L. (subgenus Amerallium, section Arctoprasum Kirschl., Fig. 1B) had the smallest length/width (L/W) ratio (0.97); A. validum (subgenus Amerallium, section Caulorhizideum, Fig. 1D) had the highest L/W ratio (2.61). The greatest periclinal wall surface area (0.04 mm2) of ten testa cells was in A. tuberosum Rottler ex Spreng. [subgenus Butomissa (Salisb.) N. Friesen, section Butomissa Webb et Berth. Fig. 10J)]. The least periclinal wall surface area (0.004 mm2) of 10 testa cells was in A. karelinii Poljak. (subgenus Cepa, section Schoenoprasum Dumort., Fig. 14F). The greatest distance between two testa cells was in A. validum (subgenus Amerallium, section Caulorhizideum, Fig. 8D); the shortest distance between two testa cells was in several species of subgenus Melanocrommyum (A. verticillatum Regel, A. zergericum F.O. Khass. & R.M. Fritsch, A. tashkenticum F.O. Khass. & R.M. Fritsch, A. regelii Trautv.) (Figs 9A, D, N and 10A)
Fig. 7.
SEM micrographs of seeds of eight Allium species in subg. Cepa: (A) A. praemixtum (sect. Cepa); (B) A. altaicum (sect. Cepa); (C) A. galanthum (sect. Cepa); (D) A. pskemense (sect. Cepa); (E) A. oschaninii (sect. Cepa); (F) A. karelinii (sect. Schoenoprasum); (G) A. fedschenkoanum (sect. Annuloprason); (H) A. condensatum (sect. Condensatum).
Fig. 5.
SEM micrographs of seeds of 15 Allium species in subg. Allium: (A) A. guttatum (sect. Allium); (B) A. ugami (sect. Allium); (C) A. vineale (sect. Allium); (D) A. fibrosum (sect. Avulsea); (E) A. griffithianum (sect. Avulsea); (F) A. pamiricum (sect. Avulsea); (G) A. brevidens (sect. Brevidentia); (H) A. ophiophyllum (sect. Brevidentia); (I) A. caesioides (sect. Coerulea); (J) A. elegans (sect. Coerulea); (K) A. caesium (sect. Coerulea) (L) A. delicatulum (sect. Coerulea); (M) A. caeruleum (sect. Coerulea); (N) A. turkestanicum (sect. Mediasia); (O) A. ferganicum (sect. Multicaulia).
Fig. 3.
SEM micrographs of seeds of 15 Allium species. (A–F) Subg. Melanocrommyum: (A) A. regelii (sect. Regeloprason); (B) A. cupuliferum (sect. Regeloprason); (C) A. isakulii (sect. Regeloprason); (D) A. taeniopetalum (sect. Stellata); (E) A. cardiostemon (sect. Melanocrommyum); (F) A. woronowii (sect. Melanocrommyum). (G–K) Subg. Butomissa: (G) A. oreoprasum (sect. Austromontana); (H) A. ramosum (sect. Butomissa); (I) A. ramosum (sect. Butomissa); (J) A. tuberosum (sect. Butomissa); (K) A. aff. tuberosum. (L, M) Subg. Cyathophora: (L) A. mairei (sect. Coleoblastus); (M) A. cyathophorum (sect. Cyathophora). (N, O) Subg. Rhizirideum: (N) A. subangulatum (sect. Rhizomatosa); (O) A. przewalskianum (sect. Eduardia).
Fig. 10.
SEM micrographs of seeds of 15 Allium species. (A–F) Subg. Melanocrommyum: (A) A. regelii (sect. Regeloprason); (B) A. cupuliferum (sect. Regeloprason); (C) A. isakulii (sect. Regeloprason); (D) A. taeniopetalum (sect. Stellata); (E) A. cardiostemon (sect. Melanocrommyum); (F) A. woronowii (sect. Melanocrommyum). (G–K) Subg. Butomissa: (G) A. oreoprasum (sect. Austromontana); (H) A. ramosum (sect. Butomissa); (I) A. ramosum (sect. Butomissa); (J) A. tuberosum (sect. Butomissa); (K) A. aff. tuberosum. (L, M) Subg. Cyathophora: (L) A. mairei (sect. Coleoblastus); (M) A. cyathophorum (sect. Cyathophora). (N, O) Subg. Rhizirideum: (N) A. subangulatum (sect. Rhizomatosa); (O) A. przewalskianum (sect. Eduardia).
Fig. 8.
SEM micrographs of seeds of 15 Allium species. (A) Subg. Nectaroscordum: A. tripedale (sect. Nectaroscordum). (B–F) Subg. Amerallium: (B) A. ursinum (sect. Arctoprasum); (C) A. wallichii (sect. Bromatorrhiza); (D) A. validum (sect. Caulorhizideum); (E) A. geyeri (sect. Amerallium); (F) A. moly (sect. Molium). (G) Subg. Caloscordum: A. neriniflorum (sect. Caloscordum). (H) Subg. Anguinum: A. prattii (sect. Anguinum). (I) Subg. Porphyroprason: A. oreophilum (sect. Porphyroprasum). (J) Subg. Vvedenskya A. kujukense (sect. Vvedenskya). (K–O) Subg. Melanocrommyum: (K) A. akaka (sect. Acanthoprason); (L) A. insufficiens (sect. Megaloprason); (M) A. sarawschanicum (sect. Megaloprason); (N) A. karataviense (sect. Miniprason); (O) A. gypsaceum (sect. Popovia).
Fig. 9.
SEM micrographs of seeds of 15 Allium species in subg. Melanocrommyum: (A) A. verticillatum (sect. Verticillata); (B) A. costatovaginatum (sect. Acmopetala); (C) A. tschimganicum (sect. Acmopetala); (D) A. tashkenticum (sect. Acmopetala); (E) A. zergericum (sect. Acmopetala); (F) A. aroides (sect. Aroidea); (G) A. cristophii (sect. Asteroprason); (H) A. giganteum (sect. Compactoprason); (I) A. komarowii (sect. Compactoprason); (J) A. robustum (sect. Tulipifolia); (K) A. alexeianum (sect. Kaloprason); (L) A. baissunense (sect. Kaloprason); (M) A. protensum (sect. Kaloprason); (N) A. rhodanthum (sect. Kaloprason); (O) A. stipitatum (sect. Procerallium).
Among 95 species (98 samples), the most important differences were in the shape and arrangement of epidermal cells of the seed testa, particularly in the shape and micromorphology of the anticlinal and periclinal walls. We used the following categories of shape of the epidermal cells: oblong, four- to eight-edged, many edged, orbicular, elliptic, rectangular, triangular, and suborbicular. The types of cellular arrangement were loose or close and with reticulate tissue or with an inserted pattern (Table 2). Nearly all the seeds of all species were verrucate, except in one species, A. kujukense Vved. (subgenus Vvedenskya, section Vvedenskya, Fig. 8J). The types of representative features and dominant shapes of the seed testa cells are provided in Table 3 to convey the features of the testa cells more effectively.
Table 3.
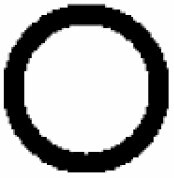
Dominant curvature types of anticlinal wall and dominant testa cell shapes
| A. Anticlinal wall: dominant curvature type | B. Dominant testa cell shape | ||||
|---|---|---|---|---|---|

|
Straight to arched | 7 |
|
Orbicular | 1 |

|
S-type | 6 |

|
Elliptic | 2 |

|
Arched to S-type | 5 |

|
Oblong | 3 |
|
U-type | 4 |
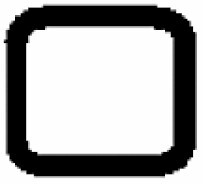
|
Rectangular | 4 |
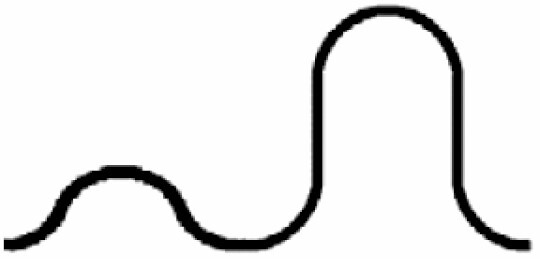
|
S- to U-type | .3 |
|
5 edged | 5 |
|
Ω-type | 2 |

|
6 edged | 6 |
|
U- to Ω-type | 1 | |||
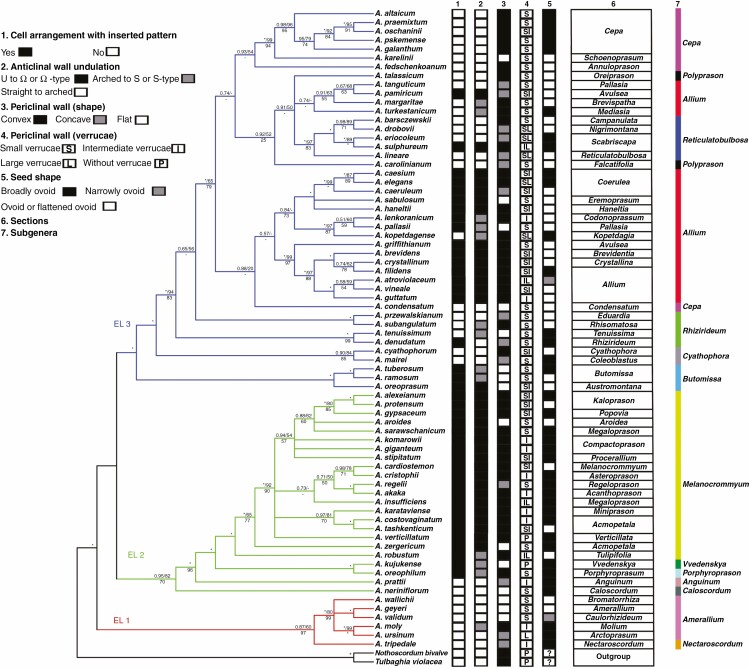
Morphological characteristics of five selected traits in the phylogenetic tree
The five morphological characters mapped onto the parsimony strict consensus tree can be seen in Fig. 15. The first character (1, Fig. 15), cell arrangement, has two states: with inserted pattern and without inserted pattern. Mostly, the second evolutionary lineage and the middle parts of the phylogenetic tree represent cell arrangement with inserted pattern. The anticlinal wall undulation type (2, Fig. 15) had three states: (1) straight to arched; (2) arched to S or S-type; and (3) U- to Ω- type and Ω-type. The members of the first evolutionary lineages and upper part of the third evolutionary lineages show mostly straight to arched anticlinal walls. Periclinal wall shape (3, Fig. 15) includes three different types (convex, flat, concave). The second characteristic of periclinal walls (verrucae on the periclinal wall) (4, Fig. 15) was grouped according to the height of verrucae on the periclinal wall (without, small, intermediate and large). The last studied characteristic of seed morphology (5, Fig. 15) is its shape. Although seed shape includes various types, the traits were assigned to three categories (broadly ovoid, narrowly ovoid, and ovoid or flattened ovoid).
Fig. 15.
The trees were constructed with Bayesian inference posterior probability/maximum parsimony, which are given on each branch; maximum likelihood is below branches. *Maximum support in all three analyses or maximum support value for only one or two methods. Morphological characteristics of five selected features were used to evaluate the three evolutionary lineages. The testa characteristics of species selected as outgroup [Nothoscordum bivalve, Tulbaghia violacea were taken from Kruse (1986)].
Previous studies suggested that undulating anticlinal walls with a close surface and distinctly convex periclinal walls with prominent verrucae were the advanced characteristics, while straight or arched anticlinal walls with flat, smooth or evenly granulose periclinal walls were ancient characteristics (von Bothmer, 1974; Pastor, 1981; Kruse, 1984, 1986, 1988, 1994; Ilarslan and Koyuncu, 1997; Fritsch et al., 2006; Neshati and Fritsch, 2009; Lin and Tan, 2017). Our study suggested that such characteristics as loose cellular arrangement, small verrucae or not verrucate, concave periclinal wall, straight to arched anticlinal wall and broadly ovoid seed reflected a basal evolutionary trend. At the same time, reticulate tissue or a mesh-like cellular arrangement, intermediate verrucae, a flat periclinal wall, arched to S or S-type and narrowly ovoid seeds were intermediate characteristics. Closely arranged cells, large verrucae, convex periclinal walls, U- to Ω-type and Ω-type and ovoid or flattened ovoid seeds reflected an advanced evolutionary trend. Members of the first evolutionary lineage along with the representatives of the outgroup [testa characteristics of outgroup members were derived from Kruse (1986)] in the phylogenetic tree showed more primitive characteristics. However, most traits possessed by the members of the second evolutionary lineage were advanced, but they mostly had broadly ovoid seeds (ancient character). Members of the third evolutionary lineage showed mostly primitive testa characteristics (except in subgenus Allium, which exhibited advanced characteristics), but with mostly convex periclinal walls, which is an advanced characteristic. The detailed characteristics of the subgenera and sections examined in this study are provided below.
Subgenus Nectaroscordum (Lindl.) Asch. & Graebn.
Subgenus Nectaroscordum is represented by A. tripedale Trautv. from section Nectaroscordum (Lindl.) Gren. & Godr. (Table 2). The seed morphology of this species is described here for the first time. The seeds were ovoid (shrivelled, somewhat broadly), seed size was 3.22–1.8 mm, and the L/W ratio was 1.79 (Fig. 1A). The distance between testa cells was 0.007–0.01 mm, close arrangement, periclinal wall surface area of ten cells was 0.012 mm2, cells of anticlinal wall undulation type were straight to arched, cells of periclinal wall type were gradually concave from edge to centre, there were many intermediate verrucae on edge, and the dominant testa shape was five-edged (Fig. 8A).
In 1986, Kruse (1986) studied the testa cell sculpture of A. siculum Ucria as a representative of the subgenus Nectaroscordum, and the testa cell structure of this species was very similar to that of A. ursinum of the subgenus Amerallium (section Arctoprasum). In comparing testa cell sculpturing of A. tripedale (Fig. 8A) and A. ursinum (Fig. 8B), we saw that they were to some extent similar. Our observations support Kruse’s findings (1986). For example, undulation type (straight to arched), periclinal wall shape (gradually concave from edge to centre) and periclinal wall surface area of ten testa cells (0.012 mm2) were the same, but these two species differed from each other in seed size, shape, cell arrangement and verrucae. This connection, to a certain extent, indicates taxonomic relationship. According to the molecular-based phylogenetic tree of Li et al. (2010), both of them belong to the first evolutionary lineage of Allium.
Allium tripedale (section Nectaroscordum) belongs to the first evolutionary lineage in the phylogenetic tree (Fig. 15) and shows mostly group-specific traits for the first evolutionary lineage (cell arrangement without inserted pattern, straight to arched anticlinal wall, concave periclinal wall, broadly ovoid seeds). However, the verrucae on the periclinal wall were intermediate in size rather than being small in this species.
Subgenus Amerallium Traub
The five species examined varied in seed shape from broadly ovoid (A. moly L., A. ursinum) to ovoid (A. wallichii Kunth, A. geyeri S. Watson) and narrowly ovoid (A. validum S. Watson). The L/W ratio ranged from 0.97 to 2.61 (Fig. 1B–F). The distance between testa cells was 0.001–0.013 mm, close, loose or loose with reticulate tissue arrangement, and the periclinal wall surface area of ten cells was 0.012–0.022 mm2 (Table 2). The cells of anticlinal wall undulation type were mostly straight to arched, except for A. moly (section Molium) having arched to S-type (Fig. 8F). In most sections the periclinal wall type cells were flat with many small verrucae (sections Caulorhizideum, Amerallium Traub, Bromatorrhiza Ekberg) and gradually convex with several intermediate verrucae (section Molium) or gradually concave with large verrucae in the centre (section Arctoprasum). The dominant testa shape was five- to six-edged (Fig. 8B–F).
The species of section Molium were described by Kruse (1988) as having wide depressed channel-like anticlinal walls and specific verrucate testa patterns with a distinctly raised central verruca surrounded by small granules. However, Bednorz et al. (2011) found that A. moly had anticlinal walls typical for section Molium but periclinal walls were flatter and non-granulate although having similar verrucate sculpturing and S-like undulation. Our observations confirmed the findings of Bednorz et al. (2011) for the same species (A. moly).
All species of subgenus Amerallium analysed in the phylogenetic analysis were representatives of the first evolutionary lineage (Fig. 15). The majority showed traits of the first evolutionary lineage. Most characteristics of A. ursinum (section Arctoprasum) are primitive traits. However, A. ursinum shows advancement in the verrucae on the periclinal wall (large). Allium moly (section Molium) shows primitive characteristics (cell arrangement without inserted pattern, broadly ovoid seeds), intermediate characteristics (arched to S-type anticlinal walls, intermediate verrucae) and advanced characteristics in the convex periclinal wall. Most characteristics in A. wallichii (section Bromatorrhiza) are primitive and match the first evolutionary linage (except for ovoid or flattened ovoid seeds, which are an advanced characteristic). Allium validum (section Caulorhizideum) mostly reflects primitive characteristics. However, in terms of seed shape and periclinal wall it was intermediate (flat periclinal wall, narrowly ovoid). Most characteristics shown by A. geyeri (section Amerallium) are primitive with the exception of the flat periclinal wall (intermediate trait) and ovoid or flattened ovoid seeds (advanced character) (Fig. 15).
Subgenus Caloscordum (Herb.) R.M. Fritsch
Only A. neriniflorum (Herb.) Baker was investigated in section Caloscordum (Herb.) R.M. Fritsch (Table 2). The seeds of A. neriniflorum were broadly ovoid and shrivelled, were 1.71–1.63 mm long and had an L/W ratio of 1.05 (Fig. 1G). The distance between testa cells was 0.0009–0.004 mm and the reticulate tissue was loosely arranged. The surface area of ten testa cells was 0.012 mm2, seed testa cell anticlinal wall undulation type was straight to arched, the periclinal wall was slightly convex with many small verrucae, and the dominant testa shape was six-edged (Fig. 8G).
According to Choi et al. (2012) four types of moderately flat periclinal walls can be distinguished in Allium (smooth, minutely roughened, granulate and verrucate). Allium neriniflorum exhibits the smooth type and lacks micro relief. Our observations confirmed this finding. In addition, we observed verrucae on slightly convex periclinal walls in this species.
Allium neriniflorum (section Caloscordum) shows all characteristics of the first evolutionary line (cell arrangement without inserted pattern, straight to arched anticlinal walls, flat periclinal wall, broadly ovoid seeds). Therefore, its position is in the basal part of the second evolutionary line (Fig. 15).
Subgenus Anguinum (G. Don ex Koch) N. Friesen
Only A. prattii C.H. Wright was investigated in section Anguinum (Table 2). It is native to Eastern Asia. The seeds of A. prattii were broadly ovoid and 2.20–1.66 mm long, and L/W ratio was 1.32 (Fig. 1G). The distance between testa cells was 0.005–0.01 mm and close with an inserted pattern, surface area of ten testa cells was 0.007 mm2, seed testa cell anticlinal wall undulation was straight to arched, the periclinal wall was gradually concave from edge to centre, there were intermediate to large verrucae, and the dominant testa shape was six-edged (Fig. 8H).
Choi et al. (2012) examined three species (A. microdictyon Prokh., A. ochotense Prokh., A. tricoccum Aiton) representing subgenus Anguinum. The micromorphology of the seeds of these species were similar and showed nearly smooth periclinal walls and straight anticlinal walls with a convex channel. Our observations were partly congruent with these reports as our results showed intermediate to large verrucae on gradually concave periclinal walls and straight to arched anticlinal walls for A. prattii.
Allium prattii (section Anguinum) shows characteristics of the first evolutionary lineage (cell arrangement without inserted pattern, straight to arched anticlinal wall, flat periclinal wall, broadly ovoid seeds). Therefore, its position in the phylogenetic tree is in the basal part of the second evolutionary lineage (Fig. 15). However, in terms of verrucae on the periclinal walls, A. prattii has intermediate type verrucae.
Subgenus Porphyroprason (Ekberg) R.M. Fritsch
Allium oreophilum was investigated from section Porphyroprason (Ekberg) R.M. Fritsch (Table 2). Allium oreophilum is native to Asia. The seeds of A. oreophilum were broadly ovoid, the seeds were 2.39–1.95 mm long, and the L/W ratio was 1.23 (Fig. 1I). The distance between testa cells was 0.0009–0.004 mm and loose with inserted pattern, surface area of ten testa cells was 0.015 mm2, seed testa cell anticlinal wall undulation type was S-type, the periclinal wall was convex with many small verrucae, and the dominant testa shape was oblong (Fig. 8I).
Lin and Tan (2017) examined two sections (Oreiprason F. Herm., section Falcatifolia N. Friesen) of subgenus Porphyroprason and found them to be characterized by having straight to arched anticlinal walls and concave periclinal walls with different sized verrucae. Baasanmunkh et al. (2021) also investigated these sections and reported that all the species examined possessed almost the same characteristics: straight anticlinal walls and densely granulate periclinal walls. However, A. hymenorrhizum Ledeb. showed one central verruca and small marginal verrucae. Our observation is partly congruent with the above-mentioned studies as A. oreophilum showed many small verrucae on convex periclinal walls and S-type anticlinal walls.
Allium oreophilum (section Porphyroprasum) shows the characteristics that belong to the second and third evolutionary lineages in the phylogenetic tree (Fig. 15). However, in terms of periclinal wall verrucae type and seed shape the species shows group-specific characters for the first evolutionary lineage (small verruca type, broadly ovoid seeds).
Subgenus Vvedenskya (Kamelin) R.M. Fritsch
Only A. kujukense was investigated from section Vvedenskya (Table 2), which is native to Kazakhstan. The seeds of A. kujukense were broadly ovoid and 2.05–1.56 mm long, with an L/W ratio of 1.31 (Fig. 1J). The distance between testa cells was 0.002–0.005 mm and loose with inserted pattern, surface area of ten testa cells was 0.01 mm2, seed testa cell anticlinal wall undulation type was arched to S-type, the periclinal wall was flat to convex without verrucae, and the dominant testa shape was oblong (Fig. 8J).
Our study was the first to investigate A. kujukense. The testa cell structure was similar to that of A. oreophilum in our study, with a loose inserted pattern, oblong testa cells dominating. However, there were differences in the periclinal walls with many small verrucae (plane for A. kujukense) and undulation type of anticlinal wall (S-type) in A. oreophilum.
Allium kujukense differs from A. oreophilum by having a flat periclinal wall without verrcuae (Fig. 15).
Subgenus Melanocrommyum (Webb & Berth.) Rouy
Twenty-six species from 15 sections of subgenus Melanocrommyum were investigated (Table 2). The seeds were broadly ovoid or ovoid shrivelled (A. akaka S. G. Gmelin ex Schult. & Schult. f., A. insufficiens Vved., A. sarawschanicum Regel, A. karataviense Regel, A. gypsaceum Popov & Vved., A. costatovaginatum Kamelin & Levichev, A. tschimganicum B. Fedtsch., A. zergericum F.O. Khass. & R.M. Fritsch, A. cristophii Trautv., A. giganteum Regel, A. komarowii Lipsky, A. alexeianum Regel, A. baissunense (Lipsky) F.O. Khass. & R.M. Fritsch, A. stipitatum Regel, A. regelii Trautv., A. cupuliferum Regel, A. isakulii R.M. Fritsch & F.O. Khass., A. taeniopetalum Popov & Vved., A. woronowii Miscz. ex Grossh.), flattened ovoid (shrivelled) (A. verticillatum Regel, A. aroides Popov & Vved., A. protensum Wendelbo, A. tashkenticum, A. cardiostemon Fisch. & C.A. Mey.) and ovoid (A. robustum Kar. & Kir.) (Figs 1K–3F) with an L/W ratio of 1.2–1.97 (Table 1). The distance between testa cells was 0–0.009 mm and close or loose with inserted pattern, and the area of ten testa cells was 0.008–0.03 mm2. The seed testa cell anticlinal wall undulation type was mostly U- to Ω-type (A. akaka, A. karataviense, A. cristophii, A. giganteum, A. alexeianum, A. rhodanthum, A. protensum, A. stipitatum, A. regelii, A. cupuliferum, A. isakulii) and Ω type (A. gypsaceum, A. costatovaginatum, A. zergericum, A. tashkenticum, A. cardiostemon), rarely S- to U-type (A. insufficiens, A. tschimganicum, A. woronowii) and S-type (A. robustum). The periclinal walls were mostly convex with one to eight intermediate verrucae. The dominant testa shape was elliptic to oblong (Figs 8K–10F).
According to previous studies (Fritsch et al., 2006; Neshati and Fritsch, 2009; Choi and Cota-Sanchez, 2010; Bednorz et al., 2011; Celep et al., 2012; Lin and Tan, 2017) convex periclinal walls with several large verrucate sculptures and combined S- to Ω-type undulate anticlinal walls are the most characteristic of this subgenus. We examined 15 sections in this subgenus. Our observations confirmed previous findings. Earlier papers (Kruse, 1994; Fritsch et al., 2006) suggested that among species of subgenus Melanocrommyum, A. aroides (section Aroidea) and A. verticillatum (section Verticillata) had more or less flat and granulose periclinal cell walls without verrucae. Our findings matched these characteristics for the same species.
Nineteen species of subgenus Melanocrommyum were used in the phylogenetic analysis. Most species showed characteristics of the third evolutionary lineages in the phylogenetic tree (Fig. 15). Allium robustum (section Tulipifolia R.M. Fritsch & N. Friesen) mostly showed advanced characteristics (cellular arrangement with inserted pattern, convex periclinal wall with large and small verrucae, ovoid or flattened ovoid seeds). However, in terms of anticlinal wall type A. robustum exhibited the arched to S or S undulation type. Characteristics of A. zergericum and A. costatovaginatum (section Acmopetala R.M. Fritsch) are primitive (small verrucae, broadly ovoid seeds for A. zergericum; broadly ovoid seeds for A. costatovaginatum) the remaining traits as advanced characteristics. Two species of section Compactoprason R.M. Fritsch (A. komarowii A. giganteum) were used in the phylogenetic analysis. Those two species shared the same advanced seed characteristics (cell arrangement with inserted pattern, U- to Ω-type or Ω-type anticlinal wall, convex periclinal wall), intermediate characteristics (intermediate verrucae) and primitive characteristics (broadly ovoid seeds). Two species of section Megaloprason Webb et Berth. (A. insufficiens, A. sarawschanicum) were used in the phylogenetic analysis. These two species show the same seed characteristics of a primitive character (broadly ovoid seeds) and other advanced characteristics. The seeds of A. gypsaceum (section Popovia F.O. Khass. & R.M. Fritsch) show primitive characteristics (broadly ovoid seeds), intermediate characteristics (small and intermediate verrucae) and other characteristics as advanced traits. Allium aroides (section Aroidea) showed primitive characteristics (small verrucae) and intermediate characteristics (flat periclinal wall), with the remaining characteristics advanced. Most characteristics shown by A. stipitatum (section Procerallium R.M. Fritsch) were advanced. However, in the type of verrucae (small and intermediate) and seed shape (broadly ovoid) the species reflects primitive characteristics. Two species of section Kaloprason C. Koch (A. alexeianum, A. protensum) were used in the phylogenetic analysis. These two species have almost the same primitive seed characteristics (small and intermediate verrucae), and other advanced characteristics. However, A. alexeianum reflected primitive seed characteristics (broadly ovoid). Allium karataviense (section Miniprason R.M. Fritsch) showed mostly advanced and primitive characteristics (broadly ovoid seeds), intermediate (intermediate and small verrucae). Almost all the characteristics of Allium tashkenticum and A. costatovaginatum (section Acmopetala R.M. Fritsch) are advanced. Most characteristics reflected by A. cristophii (section Asteroprason) are advanced characteristics. However, in terms of verruca type this species shows advanced traits (small and intermediate verrucae). Allium cardiostemon (section Melanocrommyum) shows the characteristics that belong to the third evolutionary lineage (advanced). However, regarding verrucae type the species shows primitive traits (small and intermediate verrucae). Allium akaka (section Acanthoprason) shows characteristics that are primitive (broadly ovoid seeds), intermediate (intermediate verrucae type) and advanced (remaining traits). Most characteristics shown by A. verticillatum (section Verticillata) are advanced characteristics. But in terms of seed shape and verrucae type the taxon has primitive characteristics (broadly ovoid, without verrucae) (Fig. 15).
Subgenus Butomissa (Salisb.) N. Friesen
In this subgenus three species (five samples) from two sections were investigated (Table 2). The seeds of this subgenus were ovoid and L/W ratio was 1.45–1.74 (Fig. 3G–K). The distance between testa cells was 0.002–0.009 mm and loose with reticulate tissue, and the area of ten testa cells was 0.018–0.04 mm2. The seed testa cell anticlinal wall undulation type was mostly arched to S-type (section Butomissa) or S- to U-type (section Austromontana N. Friesen). The periclinal walls of this subgenus were mostly flat (somewhat slightly convex or concave) with many small verrucae. The dominant testa shape was elliptic (Fig. 10G–K).
According to previous papers (Kruse, 1984, 1986, 1988, 1994; Ilarslan and Koyuncu, 1997; Fritsch et al., 2006), dominant sculpture patterns in the subgenus Butomissa were straight anticlinal walls and granulous sculptures of the periclinal walls. After a few years Choi et al. (2012) also found irregularly curved anticlinal wall boundaries covered with granulate periclinal walls in two species: A. ramosum L. and A. tuberosum (section Butomissa). But afterwards Lin and Tan (2017) examined three species (A. tuberosum, A. ramosum, A. oreoprasum Schrenk) and described more diverse sculpture patterns in terms of periclinal and anticlinal walls. In addition to sculptural characteristics described previously, we found convex and concave periclinal walls, a close cellular arrangement dominating an arched to S-type anticlinal wall in A. oreoprasum. Baasanmunkh et al. (2021) distinguished the two sections (Butomissa and Austromontana) on the basis of seed shape. In our research the seed shapes of the two studied sections were also easily distinguished. We examined the two samples of A. ramosum from Russia and Uzbekistan. Although the samples were from different places the testa cell ornamentation of samples has nearly the same pattern.
Three species of this subgenus were used in the phylogenetic analysis (Fig. 15). Two species, A. ramosum and A. tuberosum (section Butomissa) show nearly the same characters of small verrucae (broadly ovoid for A. tuberosum) as primitive characteristics, a flat periclinal wall, arched to S or S-type anticlinal wall as intermediate characteristics, and remaining traits as advanced. Allium oreoprasum (section Austromontana) shows both primitive (cell arrangement with inserted pattern, small and intermediate verrucae) and advanced traits (convex periclinal wall, U- to Ω-type or Ω-type, ovoid or flattened ovoid seed shape).
Subgenus Cyathophora (R.M. Fritsch) R.M. Fritsch
In this subgenus two species from two sections were investigated (Table 2). The seeds were flattened ovoid or broadly ovoid, and the L/W ratio was 1.35–2 (Fig. 3L, M). The distance between testa cells was 0.004–0.01 mm and loose with reticulate tissue, and the area of ten testa cells was 0.013–0.015 mm2. The seed testa cell anticlinal wall undulation type was strongly straight to arched. The periclinal walls were gradually convex or gradually concave with one large verruca in the centre or small to intermediate verrucae, but always granulose verrucae. The dominant testa shape was six-edged (Fig. 10L, M).
Two species, A. mairei H. Lév. and A. cyathophorum Bur. et Franch., belonging to sections Coleoblastus Ekberg. and Cyathophora R.M. Fritsch, were examined in this research. The seed coat patterns of the two sections are rather similar. The differences between the two sections were only in the shapes of periclinal walls. When compared with other related subgenera the testa cell characteristics of this subgenus were rather similar to those of subgenus Reticulatobulbosa as the subgenus had straight to arched anticlinal walls and gradually concave or convex periclinal walls from edge to centre. However, each species of subgenus Reticulatobulbosa showed one large granulose verruca in centre.
All examined species of this subgenus were used in the phylogenetic analysis (Fig. 15). Most species show the characteristics belonging to the first and second evolutionary lineages despite their place in the third evolutionary lineage. Allkum mairei (section Coleoblastus) has only primitive characteristics (cell arrangement without inserted pattern, straight to arched anticlinal wall, small verrucae, broadly ovoid seeds, concave periclinal wall), whereas A. cyathophorum (section Cyathophora) shows primitive characteristics (cell arrangement without inserted pattern, straight to arched anticlinal wall, small and intermediate verrucae) and advanced characteristics (convex periclinal wall, ovoid or flattened ovoid seed shape).
Subgenus Rhizirideum G. Don f. ex Koch
In this subgenus four species from four sections were investigated (Table 2). The seeds were flattened ovoid or (broadly) ovoid, and the L/W ratio was 1.58–1.87 (Figs 3N–O and 4A, B). The distance between testa cells was 0–0.008 mm and loose with reticulate tissue, and the area of ten testa cells was 0.007–0.016 mm2. The seed testa cell anticlinal wall undulation type was variable, showing transitions from straight to arched and to S-type forms, always with a marginal bulge. The periclinal walls were flat to convex (except in A. przewalskianum Regel, where they were concave at the centre) with many intermediate verrucae, which gave rise to a marginal bulge on the edge. The dominant testa shape was six-edged (Figs 10N–O and 11A, B).
Fig. 4.
SEM micrographs of seeds of 15 Allium species. (A, B) Subg. Rhizirideum: (A) A. tenuissimum (sect. Tenuissima); (B) A. denudatum (sect. Rhizirideum). (C–O) Subg. Allium: (C) A. margaritae (sect. Brevispatha); (D) A. lenkoranicum (sect. Codonoprasum); (E) A. crystallinum (sect. Crystallina); (F) A. popovii (sect. Eremoprasum); (G) A. sabulosum (sect. Eremoprasum); (H) A. kopetdagense (sect. Kopetdagia); (I) A. anisotepalum (sect. Minuta); (J) A. minutum (sect. Minuta); (K) A. affine (sect. Allium); (L) A. atroviolaceum (sect. Allium); (M) A. aucheri (sect. Allium); (N) A. dictyoscordum (sect. Allium); (O) A. filidens (sect. Allium).
Fig. 11.
SEM micrographs of seeds of 15 Allium species. (A, B) Subg. Rhizirideum: (A) A. tenuissimum (sect. Tenuissima); (B) A. denudatum (sect. Rhizirideum). (C–O) Subg. Allium: (C) A. margaritae (sect. Brevispatha); (D) A. lenkoranicum (sect. Codonoprasum); (E) A. crystallinum (sect. Crystallina); (F) A. popovii (sect. Eremoprasum); (G) A. sabulosum (sect. Eremoprasum); (H) A. kopetdagense (sect. Kopetdagia); (I) A. anisotepalum (sect. Minuta); (J) A. minutum (sect. Minuta); (K) A. affine (sect. Allium); (L) A. atroviolaceum (sect. Allium); (M) A. aucheri (sect. Allium); (N) A. dictyoscordum (sect. Allium); (O) A. filidens (sect. Allium).
The species A. subangulatum Regel, A. przewalskianum, A. tenuissimum L. and A. denudatum Redouté, belonging to sects Rhizirideum Traub, Rhizomatosa Egorova, Eduardia N. Friesen and Tenuissima (Tzagolova) Hanelt., were examined. Bednorz et al. (2011) examined A. nutans L. (section Rhizirideum) and found that the species had hollowly depressed, straight, strip-like anticlinal walls and periclinal walls that were convex and verrucate with central verruca periclinal walls. Lin and Tan (2017) examined two species of section Rhizirideum (A. nutans, A. senescens L.) and found that the species possesses small to intermediate verrucae on flat or gradually concave periclinal walls and straight to arched anticlinal walls. Our results support these previous authors’ findings. Based on phylogenetic molecular research (Friesen et al., 2020), sections Caespitosoprason and Rhizomatosa are the same sections or synonyms. According to previous reports (Kruse, 1988; Choi et al., 2012; Lin and Tan, 2017; Baasanmunkh et al., 2021), most species in section Rhizirideum were characterized by having straight anticlinal walls and convex periclinal walls with intermediate verrucae or granules. Our observations support these findings, but A. subangulatum possesses an S-type anticlinal wall. Lin and Tan (2017) examined A. przewalskianum (section Eduardia) and found straight to arched anticlinal walls and gradually concave periclinal walls, which is similar to our findings. S-type anticlinal walls were reported for A. anisopodium Ledeb. and A. tenuissimum (Kruse, 1988; Choi et al., 2012), and for A. anisopodium Ledeb., A. tenuissimum and A. vodopjanovae N. Friesen (Baasanmunkh et al., 2021). We examined the type species of section Tenuissima (A. tenuissimum) and our findings were congruent with all of the above-mentioned findings.
The four species of subgenus Rhizirideum were used in the phylogenetic analysis (Fig. 15). Most species showed characteristics of the first and second evolutionary lineages despite their place in the third evolutionary lineage. Allium tenuissimum (section Tenuissima) showed primitive (cell arrangement without inserted pattern, small verrucae, broadly ovoid seeds) and intermediate characteristics (arched to S or S-type anticlinal wall, flat periclinal wall). Almost all of the characteristics of A. przewalskianum (section Eduardia) were primitive except for the seed shape (ovoid or flattened ovoid). Allium subangulatum (section Rhizomatosa Egorova) showed primitive (cell arrangement without inserted pattern, small verrucae), intermediate (arched to S or S-type anticlinal wall) and advanced characteristics.
Subgenus Allium
Thirty-two species from 14 sections of subgenus Allium were investigated (Table 2). The seeds were (broadly) ovoid, except for species of section Minuta F.O. Khass. and some species of section Allium, which were narrowly ovoid. The L/W ratio was 1.27–2.39 (Figs 4C–6E). The distance between testa cells was 0–0.06 mm and loose with inserted pattern and with reticulate tissue, and the area of ten testa cells was 0.006–0.024 mm2. The seed testa cell anticlinal wall undulation in subgenus Allium was known to vary from arched to S-, U- and Ω-type, but in this study the species never exhibited the straight to arched type. The periclinal walls were convex to concave with many small, intermediate and large verrucae. The dominant testa shape was oblong to elliptic (Figs 11C–13E).
Fig. 6.
SEM micrographs of seeds of 15 Allium species. (A–E) Subg. Allium: (A) A. borszczowii (sect. Multicaulia); (B) A. borszczowii (sect. Multicaulia); (C) A. haneltii (sect. Haneltia); (D) A. pallasii (sect. Pallasia); (E) A. tanguticum (sect. Pallasia). (F–J) Subg. Reticulatobulbosa: (F) A. drobovii (sect. Nigrimontana); (G) A. lineare (sect. Reticulatobulbosa); (H) A. barsczewskii (sect. Campanulata); (I) A. eriocoleum (sect. Scabriscapa); (J) A. sulphureum (sect. Scabriscapa). (K–N) Subg. Polyprason: (K) A. albovianum (sect. Oreoprason); (L) A. korolkowii (sect. Falcatifolia); (M) A. carolinianum (sect. Falcatifolia); (N) A. talassicum (sect. Oreiprason). (O) Subg. Cepa: A. praemixtum (sect. Cepa).
Fig. 13.
SEM micrographs of seeds of 15 Allium species. (A–E) Subg. Allium: (A) A. borszczowii (sect. Multicaulia); (B) A. borszczowii (sect. Multicaulia); (C) A. haneltii (sect. Haneltia); (D) A. pallasii (sect. Pallasia); (E) A. tanguticum (sect. Pallasia). (F–J) Subg. Reticulatobulbosa: (F) A. drobovii (sect. Nigrimontana); (G) A. lineare (sect. Reticulatobulbosa); (H) A. barsczewskii (sect. Campanulata); (I) A. eriocoleum (sect. Scabriscapa); (J) A. sulphureum (sect. Scabriscapa). (K–N) Subg. Polyprason: (K) A. albovianum (sect. Oreoprason); (L) A. korolkowii (sect. Falcatifolia); (M) A. carolinianum (sect. Falcatifolia); (N) A. talassicum (sect. Oreiprason). (O) Subg. Cepa: A. praemixtum (sect. Cepa).
Due to the diversity and richness of subgenus Allium among other subgenera, a perfect phylogenetic classification of this complex subgenus has not been perfectly developed. According to previous papers (Fritsch et al., 2006; Neshati and Fritsch, 2009; Choi and Cota-Sanchez, 2010; Bednorz et al., 2011; Celep et al., 2012; Lin and Tan, 2017; Veiskarami et al., 2018; Baasanmunkh et al., 2021) macromorphologies and testa cell sculpture were highly variable in subgenus Allium. In addition, Celep et al. (2012), Lin and Tan (2017), Veiskarami et al. (2018) and Baasanmunkh et al. (2021) reported that the testa cells of the seeds of most species of the subgenera Allium and Melanocrommyum were rather similar. They both showed convex periclinal walls with several large verrucae and S- to Ω-type anticlinal walls. We examined sections Brevispatha Valsecchi, Codonoprasum Rchb., Crystallina F.O. Khassanov et S.C. Yengalycheva, Eremoprasum (Kamelin) F.O. Khass., R.M. Fritsch et N. Friesen, Kopetdagia F.O. Khassanov, Minuta, Allium Wendelbo, Avulsea F.O. Khassanov, Brevidentia F.O. Khass. et Yengalycheva, Coerulea, Mediasia F.O. Khass., S.C. Yengalycheva et N. Friesen, Multicaulia F.O. Khass. et S.C. Yengalycheva, Haneltia and Pallasia (Tzagolova) F.O. Khass., R.M. Fritsch et N. Friesen of subgenus Allium.
Cesmedziev and Terzijski (1997) found convex periclinal walls that were concave in the centre in section Brevispatha, but Celep et al. (2012) examined three members of section Brevispatha and found that they had flat, granulous periclinal walls with flat verrucae and straight to curved anticlinal walls. Our observations of A. margaritae B. Fedtsch. confirmed their findings, but the species we examined did not have a concavity in the centre. Kruse (1994) reported that section Brevispatha showed a general similarity to the seed surface pattern of section Codonoprasum. In our study we examined A. lenkoranicum Miscz. ex Grossh., representing section Codonoprasum and found that this species was similar to A. margaritae; these two sections share the same group (the second clade) of subgenus Allium according to a previous molecular phylogenetic study (Friesen et al., 2006). Veiskarami et al. (2018) also examined representatives of the first and the second groups of this subgenus and suggested that potential applicability of anticlinal wall traits present synapomorphy for certain clades. In our research the sections belonging to the second group of subgenus Allium showed more ancient characteristics and at the same time the first group showed the advanced character state. In our research, a majority of the sections of subgenus Allium supported the evolutionary clades within subgenus Allium suggested in the molecular phylogenetic study by Friesen et al. (2006). Two samples of A. borszczowii from different places were examined. Although the samples were collected from different places (Uzbekistan and Turkmenistan) the testa cell pattern has nearly the same ornamentation.
Nineteen species of subgenus Allium were used in the phylogenetic analysis. Most species showed characteristics of the third evolutionary lineages in the phylogenetic tree (Fig. 15). Characteristics of A. margaritae (section Brevispatha) belonged to three evolutionary lines (cell arrangement without inserted pattern and small verrucae as primitive characteristics, arched to S or S-type as intermediate character, convex periclinal wall and ovoid or flattened ovoid seeds as advanced characteristics). Seed testa characteristics of A. turkestanicum Regel (section Mediasia) were similar to those of A. margaritae (section Brevispatha). However, the only difference was in seed shape (broadly ovoid for A. turkestanicum). All characteristics shown by A. crystallinum Vved. (section Crystallina) are advanced (except for small and intermediate verrucae). Allium filidens Regel, A. atroviolaceum Boiss., A. guttatum Steven and A. vineale L. are displayed in the phylogenetic tree as representatives of section Allium. Those species show the same characteristics in cell arrangement (with inserted pattern), anticlinal wall (U- to Ω-type or Ω-type) and periclinal wall (convex) as advanced characteristics. Seed testa characteristics of A. brevidens Vved. (section Brevidentia) were the same as those of A. filidens (section Allium) except for seed shape characteristics (ovoid or flattened ovoid). Allium caeruleum Pall., A. elegans Drobow and A. caesium Schrenk are displayed in the phylogenetic tree as representatives of section Coerulea. The three species show the same characteristics regarding cell arrangement (with inserted pattern) and anticlinal wall (U- to Ω-type or Ω-type) as advanced characteristics. However, while A. caeruleum has concave periclinal walls A. elegans and A. caesium have convex periclinal walls. There is also a difference in seed shape between these species. Testa characteristics of A. haneltii F.O. Khass. & R.M. Fritsch (section Haneltia) are the same as those of A. brevidens (section Brevidentia). Allium pallasii Murr. (section Pallasia) had a cell arrangement with inserted pattern, ovoid or flattened ovoid seed shape as advanced characteristics and arched to S or S-type, flat periclinal wall as intermediate characteristics. Allium kopetdagense Vved. (section Kopetdagia) showed three different characteristics (cell arrangement without inserted pattern, broadly ovoid seeds as primitive characteristic; arched to S or S-type anticlinal wall as intermediate characteristics; and convex periclinal wall as an advanced characteristic (Fig. 15).
Subgenus Reticulatobulbos (Kamelin) N. Friesen
Five species from four sections of subgenus Reticulatobulbosa were investigated (Table 2). The seeds were flattened ovoid or (broadly) ovoid and the L/W ratio was 1.37–1.95 (Fig. 6F–J). The distance between testa cells was 0.005–0.015 mm and loose with a clear reticulate mesh and indented connecting thread, and the area of ten testa cells was 0.01–0.025 mm2. The seed testa cell anticlinal wall undulation type was straight to arched. The periclinal walls were convex with one large verruca at the centre (mostly granulose) and many small verrucae on the edge. The dominant testa shape was six-edged (Fig. 13F–J).
Allium drobovii Vved., A. lineare L., A. barsczewskii Lipsky, A. eriocoleum Vved. and A. sulphureum Vved. in subgenus Reticulatobulbosa were analysed. According to previous findings (Kruse, 1984, 1986, 1988, 1994; Ilarslan and Koyuncu, 1997; Fritsch et al., 2006), straight anticlinal walls and granulous sculptures of the periclinal walls dominate in the subgenus. Lin and Tan (2017) examined two species, A. strictum Schrad. and A. flavidum Ledeb., of section Reticulatobulbosa. In our research A. lineare represented this section. Many previous studies (Choi et al., 2012; Lin and Tan, 2017; Baasanmunkh et al., 2021) reported concave periclinal walls with one large central verruca and straight to arched anticlinal walls for section Reticulatobulbosa. Our observations confirmed these findings for A. lineare.Kruse (1988) found that A. barsczewskii (section Campanulata Kamelin) had U-type undulate anticlinal walls with verrucate periclinal walls. However, in our research A. barsczewskii (section Campanulata) had convex periclinal walls with many granulose verrucae in the centre and straight to arched anticlinal walls. The seed surface ultrastructure of section Scabriscapa (Tscholok.) N. Friesen was analysed by Neshati and Fritsch (2009) and Baasanmunkh et al. (2021). According to the former authors A. scabriscapum Boiss. had convex periclinal walls with central verrucae and strip-like anticlinal walls. The latter researchers reported periclinal walls with one central verruca, marginal small verrucae and straight anticlinal walls for A. trachyscordum Vved. Our observations were similar to those of both authors, but A. eriocoleum had straight to arched anticlinal walls. Allium drobovii (section Nigrimontana N. Friesen) was analysed here for the first time. Its testa sculpture was similar to other representatives of the genus.
All species of subgenus Reticulatobulbosa examined in our study were used in the phylogenetic analysis as representative of the third evolutionary lineage (Fig. 15). Allium drobovii (section Nigrimontana) and A. lineare (section Reticulatobulbosa) showed the same seed testa characteristics: cell arrangement without inserted pattern, straight to arched anticlinal wall type, concave periclinal wall as a primitive character state and small and large verrucae on the periclinal wall, and ovoid or flattened ovoid seeds as an advanced character. The testa cells of A. barsczewskii (section Campanulata) were similar to the above-mentioned sections. However, the periclinal walls of A. barsczewskii were convex. Most characteristics shown by these species, such as cell arrangement without inserted pattern, straight to arched anticlinal walls and intermediate and small verrucae, and concave periclinal wall, belong to the first evolutionary lineages. However, A. barsczewskii showed convex periclinal walls. Allium eriocoleum (section Scabriscapa) had primitive testa characteristics such as those of sections Nigrimontana and Reticulatobulbosa. However, in the broadly ovoid seeds the species is primitive (Fig. 15).
Subgenus Polyprason Radic
We investigated four species from three sections of subgenus Polyprason (A. albovianum C.H. Wright, A. korolkowii Regel A. carolinianum, A. talassicum Regel; Table 2). The seeds were flattened ovoid or (broadly) ovoid with an L/W ratio of 1.3–2.11 (Fig. 6K–N). The distance between testa cells was 0.001–0.021 mm and loose with a clear reticulate mesh and indented connecting thread, and the area of ten testa cells was 0.005–0.02 mm2. The anticlinal walls of seed testa cells of most species were straight to arched. The periclinal walls were gradually concave or gradually convex with many small verrucae on the edge. The dominant testa shape was six-edged and oblong (Fig. 13K–N).
In subgenus Polyprason, several species of sections Falcatifolia and Oreiprason were studied by Lin and Tan (2017) and Baasanmunkh et al. (2021). The reports in both studies showed that the two sections have similar seed testa patterns. However, we found some differences between the sections in anticlinal and periclinal wall traits. The former section (section Falcatifolia: A. carolinianum) matched with the results presented by the above-mentioned authors regarding anticlinal wall traits. The testa characteristics of section Oreiprason (A. albovianum) did not support the findings reported by Lin and Tan (2017) and Baasanmunkh et al. (2021) since the species had arched to S-type anticlinal walls. According to Baasanmunkh et al. (2021) subgenera Polyprason and Reticulatobulbosa were similar in seed shape and seed testa features in the species they examined. Our observations were congruent with their findings.
Two species (A. talassicum, A. carolinianum) representing sections Oreiprason and Falcatifolia were used in the phylogenetic analysis as representatives of subgenus Polyprason (Fig. 15). Most characteristics of these species belong to the first evolutionary lineage (cell arrangement without inserted pattern, straight to arched anticlinal wall type, small verrucae on the periclinal wall). However, in terms of periclinal wall shape and seed shape the species showed advanced characteristics (convex periclinal wall, ovoid or flattened ovoid seed shape) (Fig. 15).
Subgenus Cepa (Mill.) Radic
Eight species from four sections of subgenus Cepa were investigated (Table 2). The seeds of subgenus Cepa were flattened ovoid, except in section Cepa (broadly ovoid) and the L/W ratio was 1.86–2.05 (Figs 6O and 7). The distance between testa cells was 0.002–0.014 mm and loose with clear reticulate mesh tissue and indented connecting threads, and the area of ten testa cells was 0.004–0.011 mm2. The seed testa cell anticlinal wall undulation type was straight to arched. The periclinal walls were convex with many small verrucae. The dominant testa shape was six-edged (Figs 13O and 14).
We studied two samples of each of A. praemixtum Vved. and A. altaicum Vved. species and one sample of each of A. galanthum Kar. & Kir., A. pskemense B. Fedtsch., A. praemixtum, A. oschaninii O. Fedtsch., A. karelinii Poljal., A. fedschenkoanum Regel and A. condensatum Turcz. species in subgenus Cepa. Kruse (1988, 1994) and Bednorz et al. (2011) observed species of Cepa and found that the seed testa sculpturing of the periclinal walls of most species was densely granulate. Lin and Tan (2017) also examined seven members of subgenus Cepa; the testa characteristics of these species were very similar to that of the species Kruse (1988, 1994) and Bednorz et al. (2011) examined. Our research also showed the same results as those of Lin and Tan (2017). We found that all of the species we examined in subgenus Cepa had straight to arched anticlinal walls. According to Lin and Tan (2017)A. galanthum is characterized by having straight to arched anticlinal walls and periclinal walls with intermediate verrucae. Our observations were congruent with their findings. Baasanmunkh et al. (2021) examined A. oschaninii (section Cepa) and supported the reports of Veiskarami et al. (2018) that A. oschaninii showed straight anticlinal walls and periclinal walls with large verrucae. In contrast, we found straight to arched anticlinal walls and gradually convex periclinal walls with small to intermediate verrucae for the same species. Species in section Cepa share the same testa sculpture pattern except that one species, A. praemixtum, had globular convex periclinal walls with many small verrucae. Lin and Tan (2017) examined A. atrosanguineum Schrenk from section Annuloprason Egorova. It was found that this species had S-type anticlinal walls and periclinal walls with tuberculate or many intermediate verrucae. Baasanmunkh et al. (2021) examined A. fedschenkoanum of section Annuloprason and found similarity between the testa sculpturing of A. atrosanguineum and A. fedschenkoanum. They found that only the inflorescence colour and habitat differed between them. We also examined A. fedtschenkoanum and agree with their findings. From previous findings of the above-mentioned authors, a group-specific characteristic for section Annuloprason is many intermediate or small verrucae on globular periclinal walls. However, according to Lin and Tan (2017), A. weschniakowii Regel (section Annuloprason) did not reflect these characteristics. Further study is needed to study testa characteristics of section Annuloprason. The two samples of A. praemixtum were examined and turned out to have nearly the same seed testa pattern.
Allium altaicum, A. galanthum, A. pskemense, A. fedschenkoanum, A. praemixtum, A. oschaninii, A. karelinii and A. condensatum, representing sects Cepa, Annuloprason, Schoenoprasum and Condensatum of subgenus Cepa, were used in the phylogenetic analysis (Fig. 15). Allium altaicum, A. pskemense and A. galanthum (sect. Cepa) show the same character states of the testa cells, such as cell arrangement (without inserted pattern), anticlinal wall undulation (straight to arched), verrucae on the periclinal wall (small) and seed shape (broadly ovoid) in the phylogenetic tree. All of these characteristics are primitive (except for the convex periclinal wall) despite their location in the third evolutionary lineage. Testa cell characteristics of A. fedschenkoanum (section Annuloprason) are similar to those of members of section Cepa. However, in seed shape A. fedschenkoanum has ovoid or flattened ovoid seeds.
Taxonomic, evolutionary and biogeographical significance of seed macro- and micromorphology in the genus Allium
The results of our study are in line with the common view that seed macromorphology (size and shape) and micromorphology (testa cell shape, shape and sculpturing of periclinal walls, curvature of anticlinal walls) in Allium are the key taxonomic characteristics at species rank (von Bothmer, 1974; Pastor, 1981; Kruse, 1984, 1986, 1988, 1994; Ilarslan and Koyuncu, 1997; Fritsch et al., 2006; Neshati and Fritsch, 2009; Choi and Cota-Sanchez, 2010; Bednorz et al., 2011; Celep et al., 2012; Lin and Tan, 2017; Veiskarami et al., 2018; Baasanmunkh et al., 2021). In addition, our seed coat micromorphology-based classification supported the molecular phylogenetic trees by Friesen et al. (2006) and Li et al. (2010), confirming the findings of previous investigators (Celep et al., 2012; Lin and Tan, 2017).
Fritsch et al. (2006) recognized two seed testa characters, verrucae types on periclinal walls and the shape of anticlinal walls, as directly reflecting the primitive versus advanced evolutionary developmental stages in Allium. Celep et al. (2012) disagreed with this view, stating that ‘Seed coat patterns appear to mark different evolutionary levels inside of many taxonomic groups and variation of the testa characters is sufficient to distinguish taxa at sectional level. However, seed coat patterns do not directly indicate basal or advance evolutionary levels.’ The latter view was challenged by Lin and Tan (2017), who claimed that the seed testa characteristics directly correspond to the primitive, intermediate and advanced developmental stages. Our results support this claim and also suggest that the micromorphology (as well as macromorphology) of seeds of species of Allium reflect evolutionary levels. Such traits as loose cellular arrangement, verrucae small or absent, concave periclinal walls, straight to arched anticlinal wall, and broadly ovoid seeds appear to be ancient traits in Allium. The reticulate mesh-like cellular arrangement, intermediate verrucae, flat periclinal wall, arched to S or S-type, and narrowly ovoid seeds reflect the intermediate developmental stage. Close cellular arrangement, large verrucae, convex periclinal wall, U- to Ω- type and Ω-type, ovoid or flattened ovoid seeds appear to be the most advanced traits. Members of the first evolutionary lineage along with the species selected as the outgroup species, Nothoscordum bivalve, Tulbaghia violacea [testa characteristics of outgroup species were derived from Kruse (1986)] show more primitive characteristics (Fig. 15). Members of the second evolutionary lineage possess intermediate characteristics, except for members of subgenus Melanocrommyum, which show advanced characteristics (Table 2). In the third evolutionary lineage, the taxa retained mostly primitive testa characteristics (except in subgenus Allium, in which the species have mostly convex periclinal wall and undulated anticlinal walls, which are advanced characteristics) (Fig. 15). All members of the subgenera Allium and Melanocrommyum had advanced testa cell features, confirming previous findings (Fritsch et al., 2006; Neshati and Fritsch, 2009; Choi and Cota-Sanchez, 2010; Bednorz et al., 2011). According to Hanelt et al. (1992) bulb-forming species evolved from rhizomatous species and subgenera Allium and Melanocrommyum are derived from subgenus Rhizirideum. Adaptive evolution of morphological characteristics or complex hybridization are two possible reasons for the advanced testa characteristics reflected in Melanocrommyum and also for less advanced characters of the subgenera in the third evolutionary lineage.
If the seed macro- and micromorphology is phylogenetically informative, we can try to infer, using the testa cell characteristics, the possible migration routes of Allium during its early evolution. Most probably, Allium originated in the early Eocene (Xie et al., 2020). The place of origin is difficult to infer from the currently available data, but most probably it was an area confined to the Caucasus, Central Asia and Iran. From there, the taxa comprising the first evolutionary lineage migrated either to the west (towards southern Europe and the eastern Mediterranean) or to the east (towards eastern Asia and the Far East). For example, subgenus Nectaroscordum apparently took the western route. In our study, A. tripedale, representing subgenus Nectaroscordum shows the most primitive testa cell characteristics (straight to arched anticlinal walls, concave periclinal walls). The sister to Nectaroscordum, subgenus Amerallium, apparently took both routes, and as a result its representatives are now in North America, the Mediterranean and eastern Asia. Some researchers (Hanelt et al., 1992; Li et al., 2010) suggested that Amerallium originated in Asia and migrated to North America via the Bering Land Bridge. Biogeographers have long studied the relationships between eastern Asian and western North American biotas (Li, 1952; Thorne, 1972; Zhengyi, 1983; Hong, 1993; Wen, 1999; Nie et al., 2005; Zhou et al., 2012). The migrations of Asian and American biota have been hypothesized to be via the Bering Land Bridge across the northern Pacific (Hopkins, 1967) during most of the Tertiary. This bridge remained accessible for floristic exchanges until the Pliocene (Hamilton, 1983). For example, Nie et al. (2005) examined two closely related species of Kelloggia, K. chinensis and K. galioides (Rubiaceae), distributed in East Asia and North America, respectively, and found Asia to be the place of origin. Sanmartin et al. (2001) recognized three distinct phases of trans-Beringian migrations: Bering Land Bridges I, II and III. The first migration between the two continents (Bering Land Bridge I) took place from the late Cretaceous to the Palaeogene around 70–20 million years ago. This period embraces the time when species of Allium arose and the three evolutionary lineages emerged (Xie et al., 2020). Our seed testa morphological study of sections Arctoprasum, Molium and Bromatorrhiza from the Old World and sections Amerallium and Caulorhizideum from the New World support this hypothesized migration. European species of Amerallium (A. ursinum) have concave periclinal walls but North American Amerallium (A. validum, A. geyeri) and the Chinese species (A. wallichii) have flat or flat to slightly convex periclinal walls and straight to arched anticlinal walls (Table 2). We hypothesize from this that the ancestral species of Allium had concave periclinal and straight to arched anticlinal walls. The ancestral species apparently was similar to A. tripedale of section Nectaroscorum (Fig. 16). Further evolution of the testa cell structure was towards flat periclinal and arched to S, U anticlinal walls (second evolutionary lineage), and then to convex periclinal and U, U- to Ω-type, Ω-type anticlinal walls (second and third evolutionary lineages). Primitive testa cell characteristics in the sections from the third evolutionary lineage, such as the eastern Asian section Butomissa (A. tuberosum), suggest that these characteristics were apparently inherited from ancient ancestors of the first evolutionary line, while some primitive characteristics could have also been acquired through hybridization (Fig. 16). European section Molium (A. moly), with less primitive seed testa traits (convex periclinal wall and arched to S-type anticlinal wall), could be one of the ancestors of the subgenera in the second and third evolutionary lineages that have more advanced testa cell characteristics. Whether species bear primitive or advanced traits mostly determines two seed characteristics: the shape of the periclinal wall and the type of undulation of the anticlinal wall. The proposed evolution of the seed testa in the biogeographical context is presented in Figure 16.
Fig. 16.
Distribution of Allium throughout the world based on testa cell characteristics of seeds. Locating and branching of species on the map was carried out according to herbarium data (Table 2) and position in the ITS-based phylogenetic tree (Fig. 15), respectively.
CONCLUSIONS
This study provided detailed information on the seed micro- and macromorphological characteristics of the genus Allium (Amaryllidaceae). Our results showed that testa cell characteristics are useful for identifying species of Allium: the anticlinal versus periclinal walls at the sectional level and the verrucae on the periclinal walls within sections. Two new characteristics of the testa cells, surface area of periclinal walls and distance between two adjacent cells, examined for the first time in this study, were found to be taxonomically important. We hypothesize, based on the observed testa cell diversity patterns, that the ancestors of Allium originated in a region bounded by the Caucasus, Central Asia and Iran. The seed testa morphology-based evolutionary state of a species is determined by two parameters: the shape of the periclinal walls and curvature of the anticlinal walls.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: list of examined species used in the analyses with their correct taxonomic positions based on existing reliable publications.
ACKNOWLEDGEMENTS
The authors are indebted to Drs David E. Boufford from Harvard University Herbaria (USA) and Jacob B Landis from Cornell University for editing the English. We also thank the curators of the herbaria TASH, KUN, TBI and PE for the use of their herbarium collections. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. H.S., T.D. and K.T. conceived and designed the research. Z.Y., D.M. and K.T. collected the material. S.V., K.T., F.K., T.D., H.S. and I.E. discussed the results and revised the manuscript. D.D., T.D. and H.S. funded the research. All of the authors read and approved the manuscript.
APPENDIX
Previously published ITS accessions obtained from GenBank. ITS accessions of Allium and outgroups obtained from GenBank. 1Friesen et al. (2000); 2Friesen et al. (2006); 3Gurushidze et al. (2008); 4Nguyen et al. (2008); 5Li et al. (2010); 6Abugalieva et al. (2017); 7Friesen et al. (2001, Botanical Garden of the University of Osnabrueck, Germany, unpubl. res.); 8Wheeler et al. (2013); 9Khedim et al. (2017, Université des Sciences et de la Technologie Houari Boumediene, Algeria, unpubl. res); 10Bagheri et al. (2020); 11Friesen et al. (2003, Botanical Garden of the University of Osnabrueck, Germany, unpubl. res.); 12Veiskarami et al. (2019, University of Tehran, Iran, unpubl. res.); 13Stafford et al. (2016).
Allium: A. akaka S.G. Gmel. ex Schult. & Schult.f. FM1772423; A. albidum Fisch. ex M. Bieb. AJ4118412; A. alexeianum Regel FM1772473; A. altaicum Pall. AJ4119282; A. aroides Popov & Vved. AJ4119152; A. atroviolaceum Boiss. AJ4118842; A. barsczewskii Lipsky MG2820116; A. brevidens Vved. AJ4127217; A. caeruleum Pall. AJ4119032; A. caesium Schrenk AJ4127317; A. cardiostemon Fisch. & C.A.Mey. AJ4119712; A. carolinianum DC. AJ2502901; A. condensatum Turcz. AJ4127527; A. costatovaginatum Kamelin & Levichev FM1772863; A. christophii Trautv. AJ4119662; A. crystallinum Vved. AJ4127247; A. cyathophorum Bureau & Franch. GQ1810935; A. drobovii Vved. AJ4118952; A. elegans Drobow AJ4127307; A. eriocoleum Vved. MG2820156; A. fedschenkoanum Regel AJ4118442; A. filidens Regel AJ4127237; A. galanthum Kar. & Kir. AJ4119052; A. geyeri S.Watson KC1196598; A. giganteum Regel FM1773203; A. griffithianum Boiss. AJ4118622; A. guttatum Steven MG5468199; A. gypsaceum Popov & Vved. AJ4119692; A. haneltii F.O.Khass. & R.M.Fritsch AJ4127257; A. insufficiens Vved. FM1773343; A. karataviense Regel AJ4119222; A. karelinii Poljak AJ4118762; A. komarowii Lipsky AJ4119672; A. kopetdagense Vved. AJ4119502; A. kujukense Vved. AJ4119472; A. lenkoranicum Miscz. ex Grossh. MT30316210; A. lineare L. AJ4119512; A. mairei H.Lév. AJ2502981; A. margaritae B.Fedtsch. AJ4127327; A. moly L. AJ4127437; A. neriniflorum (Herb.) G.Don AJ4119132; A. oreophilum C.A.Mey. AJ4119312; A. oreoprasum Schrenk AJ4118672; A. oschaninii O.Fedtsch. AJ4119402; A. pallasii Murray GQ1810775; A. pamiricum Wendelbo AJ4127367; A. praemixtum Vved. AJ4118732; A. prattii C.H.Wright GQ1810875; A. protensum Wendelbo FM1773803; A. przewalskianum Regel AJ4118522; A. pskemense B.Fedtsch. AJ4119072; A. ramosum L. AJ2502951; A. regelii Trautv. AJ4119722; A. robustum Kar. & Kir. FM1773913; A. sabulosum Steven ex Bunge MG2820246; A. sarawschanicum Regel AJ4119352; A. stipitatum Regel AJ4119112; A. subangulatum Regel AJ4118702; A. sulphureum Vved. AJ4127597; A. talassicum Regel AJ4118652; A. tanguticum Regel AJ4118932; A. tashkenticum F.O.Khass. & R.M.Fritsch; FM1774343; A. tenuissimum L. AJ4118462; A. tripedale Trautv. HF93435011; A. tuberosum Rottler ex Spreng. AJ4119142; A. turkestanicum Regel AJ4119682; A. ursinum L. AJ4127447; A. validum S.Watson EU0961884; A. verticillatum Regel AJ4119102; A. vineale L. MK77688712; A wallichii Kunth AJ2502941; A. zergericum F.O.Khass. & R.M.Fritsch FM1774563.
Outgroup: Nothoscordum bivalve (L.) Britton AJ2503011; Tulbaghia violacea Harv. KU69219013.
KEY TO THE 95 SPECIES OF ALLIUM USED IN THIS STUDY BASED ON SEED MACRO- AND MICROMORPHOLOGY
| 1a. Anticlinal walls straight to arched, arched to S-type, S-type. |
| 2a. Seed shape (broadly) ovoid (shrivelled). |
| 3a. Periclinal wall shapes concave. |
| 4a. Cell arrangement close. |
| 5a. Seed length and width 3.22–1.8 mm, dominant testa cell shape 5-edged, periclinal walls with many intermediate verrucae on edge, testa shapes 4- to 7-edged……………………………………………………….……………………………..........................................……….……………........….A. tripedale |
| 5b. Seed length and width 2.20–1.66 mm, dominant testa cell shape 6-edged, periclinal walls with intermediate to large verrucae, testa shapes 4- to many-edged ……………………………………………………………………………………………………………….……….................……………………… A. prattii |
| 4b. Cell arrangement loose. |
| 6a. Periclinal walls with one large verruca in centre or intermediate to large verrucae. |
| 7a. Anticlinal wall straight to arched, seed length and width 2.46–2.54 mm, area of 10 periclinal wall testa surface cells 0.012 mm2, seed shape broadly ovoid, periclinal wall with one large verruca in centre ……………………………………………………….…………………………...........................… A. ursinum |
| 7b. Anticlinal wall arched to S-type, seed length and width 3.13–1.48 mm, area of 10 periclinal wall testa surface cells 0.005 mm2, seed shape ovoid (shrivelled), periclinal wall with intermediate to large verrucae ……………………………………………………….………………….......................………… A. albovianum |
| 6b. Periclinal walls with many small verrucae (in central area of epidermis). |
| 8a. Dominant testa cell shape elliptic or oblong. |
| 9a. Periclinal wall arched to S-type, plane to slightly concave. |
| 10a. Seed length and width 3.45–2.37 mm, area of 10 periclinal wall testa surface cells 0.04 mm2, dominant testa cell shape elliptic. ...................... A. tuberosum |
| 10b. Seed length and width 2.72–1.39 mm, area of 10 periclinal wall testa surface cells 0.011 mm2, dominant testa cell shape oblong. ...........................A. pallasii |
| 9b. Periclinal wall straight to arched, with marginal bulge, gradually concave from edge to centre .................................................................... A. przewalskianum |
| 8b. Dominant testa cell shape 6-edged. |
| 11a. Seed shape broadly ovoid, periclinal wall gradually concave, cell arrangement with clear meshes of reticulate tissue. |
| 12a. Seed length and width 2.10–1.55 mm, area of 10 periclinal wall testa surface cells 0.013 mm2, cell arrangement without inserted pattern and indented connecting thread, periclinal wall with many small verrucae in central area of epidermis. ........................................................................................... A. mairei |
| 12b. Seed length and width 1.77–1.31 mm, area of 10 periclinal wall testa surface cells 0.007 mm2, cell arrangement with inserted pattern and indented connecting thread, periclinal wall with marginal bulge, many small verrucae. ...................................................................................................... A. denudatum |
| 11b. Seed shape ovoid (shrivelled), periclinal wall (flat to) slightly concave, cell arrangement with clear meshes of reticulate tissue. |
| 13a. Periclinal wall with inserted pattern, anticlinal wall S-type. ................................................................................................................................. A. tenuissimum |
| 13b. Periclinal wall without inserted pattern, anticlinal wall straight to arched. |
| 14a. Area of 10 periclinal wall testa surface 0.022 mm2, distance between testa cells 0.001–0.002 mm, periclinal wall flat to slightly concave from edge to centre. ..................................................................................................................................................................................................................................... A. wallichii |
| 14b. Area of 10 periclinal wall testa surface 0.01 mm2, distance between testa cells 0.005–0.009 mm, periclinal wall slightly concave. ................ A. carolinianum |
| 3b. Periclinal wall shape convex. |
| 15a. Anticlinal wall straight to arched, arched to S-type. |
| 16a. Cell arrangement with connecting thread. |
| 17a. Cell arrangement with narrow connecting thread. |
| 18a. Periclinal wall convex. |
| 19a. Seed length and width 3.39–2.18 mm, area of 10 periclinal wall testa surface cells 0.008 mm2, distance between testa cells 0.003–0.009 mm. .....A. galanthum |
| 19b. Seed length and width 3.86–2.85 mm, area of 10 periclinal wall testa surface cells 0.012 mm2, distance between testa cells 0.006–0.014 mm. ........ A. pskemense |
| 18b. Periclinal wall gradually, flat to slightly or slightly convex. |
| 20a. Periclinal wall with many small verrucae, flat to (slightly) convex periclinal wall. |
| 21a. Seed length and width 2.13–1.45 mm, cell arrangement with clear meshes of reticulate tissue, distance between testa cells 0.002–0.007 mm. ......... A. geyeri |
| 21b. Seed length and width 3.58–1.98 mm, cell arrangement with reticulate tissue, distance between testa cells 0.005–0.014 mm. ............................... A. altaicum |
| 20b. Periclinal wall with many small to intermediate verrucae, gradually convex periclinal wall. ................................................................................. A. oschaninii |
| 17b. Cell arrangement with (broadly) indented connecting thread. |
| 22a. Anticlinal wall straight to arched, periclinal wall gradually convex from thickened edge to centre, without inserted pattern. |
| 23a. Seed length and width 3.83–2.05 mm, periclinal wall with many granulose verrucae in centre, many small verrucae on edge. ........................ A. barsczewskii |
| 23b. Seed length and width 2.85–2.07 mm, periclinal wall with one large granulose verruca in centre, many small verrucae on edge. ...................... A. eriocoleum |
| 22b. Anticlinal wall straight to arched, periclinal wall convex, with inserted pattern. ............................................................................................... A. turkestanicum |
| 16b. Cell arrangement without connecting thread. |
| 24a. Periclinal walls straight to arched. |
| 25a. Seed length and width 1.71–1.63 mm, distance between testa cells 0.0009–0.004 mm, periclinal wall slightly convex. .................................... A. neriniflorum |
| 25b. Seed length and width 1.71–1.63 mm, distance between testa cells 0.002–0.007 mm, periclinal globular convex. ............................................. A. praemixtum |
| 24b. Periclinal walls arched to S-type. |
| 26a. Cell arrangement without inserted pattern, dominant testa cell shape 6-edged. ............................................................................................................... A. moly |
| 26b. Cell arrangement with inserted pattern, dominant testa cell shape 5-edged, oblong. |
| 27a. Seed length and width 2.05–1.56 mm, periclinal wall flat to convex, plane. ............................................................................................................ A. kujukense |
| 27b. Seed length and width 2.75–2.16 mm, periclinal wall gradually convex from edge to centre, one large verruca on central area and many small verrucae on edge, granulose. ................................................................................................................................................................................................... A. kopetdagense |
| 15b. Anticlinal wall S-type. |
| 28a. Dominant testa cell shape 5-edged, rectangular. |
| 29a. Periclinal wall shape convex with large and/or intermediate verrucae. |
| 30a. Seed length and width 2.54–1.52 mm, cell arrangement close, periclinal wall with one large verruca in centre, intermediate verrucae on edge. ...... A. robustum |
| 30b. Seed length and width 3.60–1.90 mm, cell arrangement loose, periclinal wall with many intermediate verrucae. ........................................... A. lenkoranicum |
| 29b. Periclinal wall shape slightly convex with many small verrucae. ........................................................................................................................... A. margaritae |
| 28b. Dominant testa cell shape oblong, elliptic. |
| 31a. Cell arrangement with indented connecting thread. ...................................................................................................................................................... A. elegans |
| 31b. Cell arrangement without indented connecting thread. |
| 32a. Seed length and width 2.39–1.95 mm, area of 10 periclinal wall testa surface cells 0.015 mm2, testa cell shape many-edged to oblong. ........... A. oreophilum |
| 32b. Seed length and width 2.03–1.48 mm, area of 10 periclinal wall testa surface cells 0.006 mm2, testa cell shape orbicular to oblong. ...................... A. popovii |
| 2b. Seed shape narrowly (flattened) ovoid. |
| 33a. Cell arrangement close. |
| 34a. Anticlinal wall straight to arched. |
| 35a. Periclinal wall globular convex. ..................................................................................................................................................................... A. fedtschenkoanum |
| 35b. Periclinal wall flat, flat to slightly convex. |
| 36a. Seed length and width 3.05–1.64 mm, cell arrangement with unclear meshes, periclinal wall flat to slightly convex. ...................................... A. condensatum |
| 36b. Seed length and width 4.73–1.81 mm, cell arrangement without unclear meshes, periclinal wall flat. ................................................................... ...A. validum |
| 34b. Anticlinal wall arched to S-type. |
| 37a. Seed length and width 1.93–1.42 mm, cell arrangement with inserted pattern, testa cell shape oblong. ............................................................ A. verticillatum |
| 37b. Seed length and width 3.21–1.63 mm, cell arrangement without inserted pattern, testa cell shape 4- to many-edged. ............................................... A. aroides |
| 33b. Cell arrangement loose. |
| 38a. Cell arrangement with connecting thread. |
| 39a. Periclinal wall gradually concave. |
| 40a. Area of 10 periclinal wall testa surface cells 0.008 mm2, periclinal wall with marginal bulge, small verrucae. ................................................... .A. tanguticum |
| 40b. Area of 10 periclinal wall testa surface cells 0.02 mm2, periclinal wall with one large granulose verruca in centre, many small verrucae on edge. ...... A. lineare |
| 39b. Periclinal wall flat to slightly convex or convex. |
| 41a. Seed length and width 2.17–1.16 mm, dominant testa cell shape oblong, anticlinal wall S-type, periclinal wall with many small verrucae in centre, intermediate verrucae on edge. ................................................................................................................................................................................. A. korolkowii |
| 41b. Seed length and width 2.73–1.42 mm, dominant testa cell shape 6-edged, anticlinal wall straight to arched, periclinal wall with many small verrucae. .... .A. karelinii |
| 38b. Cell arrangement without connecting thread. |
| 42a. Periclinal wall plane to slightly, gradually concave. |
| 43a. Periclinal wall straight to arched. |
| 44a. Seed length and width 2.79–1.60 mm, distance between testa cells 0.003–0.006 mm, periclinal walls with small to intermediate verrucae. ... A. aff. tuberosum |
| 44b. Seed length and width 3.97–2.31 mm, distance between testa cells 0.008–0.012 mm, periclinal walls with one large granulose verruca in centre, many small verrucae on edge. ......................................................................................................................................................................................................... A. drobovii |
| 43b. Periclinal wall arched to S-type. ................................................................................................................................................................................ A. ramosum |
| 42b. Periclinal wall (gradually) (slightly) convex. |
| 45a. Periclinal wall straight to arched. |
| 46a. Area of 10 periclinal wall testa surface cells 0.015 mm2, gradually convex periclinal walls with small to intermediate verrucae. .................. A. cyathophorum |
| 46b. Area of 10 periclinal wall testa surface cells 0.006 mm2, convex periclinal walls with many small verrucae. ....................................................... A. talassicum |
| 45b. Periclinal wall S-type. ........................................................................................................................................................................................ A. subangulatum |
| 1b. Anticlinal walls S- to U-type, U-type, U- to Ω-type, Ω-type. |
| 47a. Loose cell arrangement. |
| 48a. Seed shape (broadly) ovoid (shrivelled). |
| 49a. Periclinal wall shape concave. |
| 50a. Dominant testa cell shape elliptic. |
| 51a. Seed length and width 3.03–1.87 mm, anticlinal wall S- to U-type, periclinal wall with many intermediate and small verrucae. ...................... A. oreoprasum |
| 51b. Seed length and width 2.13–1.40 mm, anticlinal wall Ω-type, periclinal wall with marginal bulge or not, small verrucae. .................................. A. caeruleum |
| 50b. Dominant testa cell shape oblong. |
| 52a. Distance between testa cells 0.003–0.008 mm, anticlinal wall S- to U-type. ........................................................................................................... A. caesioides |
| 52b. Distance between testa cells 0.001–0.004 mm, anticlinal wall U- to Ω-type. ....................................................................................................... A. delicatulum |
| 49b. Periclinal wall shape convex. |
| 53a. Cell arrangement with connecting thread. |
| 54a. Dominant testa cell shape oblong. |
| 55a. Anticlinal wall S- to U-type. |
| 56a. Convex periclinal wall. |
| 57a. Seed length and width 3.15–2.20 mm, area of 10 periclinal wall testa surface cells 0.024 mm2, periclinal wall with many intermediate verrucae. ................................................................................................................................................................................................................................................A. dictyoscordum |
| 57b. Seed length and width 2.16–1.15 mm, area of 10 periclinal wall testa surface cells 0.012 mm2, periclinal wall with 1 or 2 large granulose verrucae and many small verrucae. ............................................................................................................................................................................................................. A. caesium |
| 56b. Globular convex periclinal wall. .......................................................................................................................................................................... A. ophiophyllum |
| 55b. Anticlinal wall U-type. ............................................................................................................................................................................................ A. ferganicum |
| 54b. Dominant testa cell shape elliptic. |
| 58a. Anticlinal wall U-type, periclinal wall with 1–3 intermediate granulose verrucae on central area and many small verrucae. ................................. A. brevidens |
| 58b. Anticlinal wall U- to Ω-type, periclinal wall with 1 or 2 large granulose verrucae in centre, many intermediate granulose verrucae on edge. ... A. sulphureum |
| 53b. Cell arrangement without connecting thread. |
| 59a. Globular, gradually convex periclinal walls. |
| 60a. Dominant testa cell shape oblong. |
| 61a. Periclinal wall many intermediate verrucae. |
| 62a. Distance between testa cells 0.0002–0.00068 mm, anticlinal wall U- to Ω-type. ........................................................................................................... A. akaka |
| 62b. Distance between testa cells 0.002–0.007 mm, anticlinal wall S- to U-type. ..................................................................................................... A. ophiophyllum |
| 61b. Periclinal wall many intermediate verrucae and many small verrucae on edge. |
| 63a. Seed length and width 2.14–1.57 mm, area of 10 periclinal wall testa surface cells 0.015 mm2, periclinal wall gradually convex....................... A. alexeianum |
| 63b. Seed length and width 3.13–1.83 mm, area of 10 periclinal wall testa surface cells 0.009 mm2, periclinal wall globular convex. ...................... A. baissunense |
| 60b. Dominant testa cell orbicular, elliptic. |
| 64a. Large verruca or verrucae on central area of periclinal wall. |
| 65a. Area of 10 periclinal wall testa surface cells 0.02 mm2, anticlinal wall Ω-type. ................................................................................................ A. taeniopetalum |
| 65b. Area of 10 periclinal wall testa surface cells 0.008 mm2, anticlinal wall U-type. ................................................................................................ A. griffithianum |
| 64b. One to four intermediate verrucae on central area of periclinal wall. ........................................................................................................................... A. isakulii |
| 59b. Convex periclinal wall. |
| 66a. Dominant testa cell shape oblong. |
| 67a. Seed length and width 2.48–1.77 mm, testa cell shapes elliptic to oblong. ............................................................................................................. A. giganteum |
| 67b. Seed length and width 3.80–2.31 mm, testa cell shapes ovoid to irregular. ............................................................................................................. A. komarowii |
| 66b. Dominant testa cell shape elliptic. |
| 68a. Intermediate verrucae on central area of periclinal wall. ......................................................................................................................................... A. stipitatum |
| 68b. Large verrucae on central area of periclinal wall. |
| 69a. Seed length and width 3.23–2.14 mm, area of 10 periclinal wall testa surface cells 0.012 mm2. ................................................................................ A. filidens |
| 69b. Seed length and width 3.02–1.88 mm, area of 10 periclinal wall testa surface cells 0.016 mm2. ................................................................................. A. ugami |
| 48b. Seed shape flattened ovoid, narrowly ovoid. |
| 70a. Dominant testa cell shape oblong. |
| 71a. Anticlinal wall U- to Ω-type. |
| 72a. Seed length and width 2.4–1.4 mm, distance between testa cells 0–0.002 mm. ..................................................................................................... A. protensum |
| 72b. Seed length and width 2.77–1.21 mm, distance between testa cells 0.002–0.011 mm. ............................................................................................... A. vineale |
| 71b. Anticlinal wall Ω-type, U-type. |
| 73a. Area of 10 periclinal wall testa surface cells 0.008 mm2, distance between testa cells 0–0.003 mm, anticlinal wall Ω-type, cell arrangement without indented connecting thread. ............................................................................................................................................................................................... A. cardiostemon |
| 73b. Area of 10 periclinal wall testa surface 0.018 mm2, distance between testa cells 0.004–0.06 mm, anticlinal wall Ω-type, cell arrangement with indented connecting thread. ............................................................................................................................................................................................................ A. affine |
| 70b. Dominant testa cell shape elliptic. |
| 74a. Cell arrangement with connecting thread. |
| 75a. Periclinal walls slightly convex, many small and granulose intermediate verrucae. .................................................................................................. A. minutum |
| 75b. Periclinal walls convex, five to seven intermediate verrucae, granulose. .................................................................................................................. A. guttatum |
| 74b. Cell arrangement without connecting thread. |
| 76a. Periclinal wall globular, slightly convex. |
| 77a. Testa cell shapes orbicular to elliptic. |
| 78a. Globular, slightly convex periclinal wall. |
| 79a. Seed length and width 3.31–1.87 mm, area of 10 periclinal wall testa surface cells 0.022 mm2, anticlinal wall S- to U-type periclinal wall with 1–5 intermediate and many small verrucae. ................................................................................................................................................................ A. crystallinum |
| 79b. Seed length and width 2.41–1.01 mm, area of 10 periclinal wall testa surface cells 0.022 mm2, anticlinal wall U- to Ω-type, periclinal wall with many small verrucae. .............................................................................................................................................................................................................. A. anisotepalum |
| 78b. Convex periclinal wall. |
| 80a. Seed length and width 3.26–1.58 mm, anticlinal wall U-type. ......................................................................................................................... A. atroviolaceum |
| 80b. Seed length and width 2.55–1.27 mm, anticlinal wall S- to U-type. ......................................................................................................................... A. fibrosum |
| 77b. Testa cell shapes triangular to elliptic and oblong. |
| 81a. Periclinal wall with many small verrucae. ............................................................................................................................................................ A. borszczowii |
| 81b. Periclinal wall with 1–4 intermediate granulose verrucae and many small verrucae. ................................................................................................. A. haneltii |
| 76b. Periclinal wall globular, slightly concave. |
| 82a. Seed shape flattened ovoid, anticlinal wall U- to Ω-type, periclinal wall with many small verrucae. ................................................................... A. sabulosum |
| 82b. Seed shape narrowly ovoid, anticlinal wall S- to U-type, periclinal wall with many intermediate and small verrucae. ..............................................A. aucheri |
| 47b. Close cell arrangement. |
| 83a. Dominant testa cell shape orbicular. |
| 84a. Anticlinal wall U- to Ω-type or U-type. |
| 85a. Globular convex periclinal wall. |
| 86a. Seed length and width 2.42–1.93 mm, distance between testa cells 0.0005–0.001 mm, anticlinal wall U-type. ......................................... A. sarawschanicum |
| 86b. Seed length and width 3.78–2.68 mm, distance between testa cells 0.001–0.004 mm, anticlinal wall U- to Ω-type. ........................................ A. karataviense |
| 85b. Slightly concave, gradually convex periclinal wall. |
| 87a. Periclinal wall slightly concave with many small verrucae. .......................................................................................................................................... A. regelii |
| 87b. Periclinal wall gradually convex with many intermediate verrucae on central area of shrivelled epidermis. ...................................................... A. cupuliferum |
| 84b. Anticlinal wall S- to U-type. .................................................................................................................................................................................... A. woronowii |
| 83b. Dominant testa cell shape elliptic, oblong. |
| 88a. Seed shape broadly ovoid (shrivelled). |
| 89a. Dominant testa cell shape elliptic. |
| 90a. Anticlinal wall S- to U-type, periclinal wall convex and granulate, one large verruca on central area, intermediate verrucae on edge. ........ A. tschimganicum |
| 90b. Anticlinal wall Ω-type, periclinal wall convex or concave, many small verrucae. ................................................................................................. A. zergericum |
| 89b. Dominant testa cell shape oblong. |
| 91a. Anticlinal wall Ω-type. |
| 92a. Distance between testa cells 0.004–0.009 mm, periclinal wall flat to convex with many intermediate and large verrucae. .................................. A. gypsaceum |
| 92b. Distance between testa cells 0.00064–0.00097 mm, periclinal wall gradually convex with many intermediate verrucae. ......................... A. costatovaginatum |
| 91b. Anticlinal wall S- to U-type, U- to Ω-type. |
| 93a. Seed length and width 1.66–1.16 mm, anticlinal wall S- to U-type, periclinal wall slightly convex, many small and intermediate verrucae. .... A. insufficiens |
| 93b. Seed length and width 3.08–2.45 mm, anticlinal wall U- to Ω-type, periclinal wall convex, many intermediate verrucae. ................................... A. cristophii |
| 88b. Seed shape flattened ovoid. |
| 94a. Test cell shapes orbicular or suborbicular to elliptic. |
| 95a. Area of 10 periclinal wall testa surface 0.017 mm2, anticlinal wall U- to Ω-type. .............................................................................................. A. rhodanthum |
| 95b. Area of 10 periclinal wall testa surface 0.009 mm2, anticlinal wall U-type. ........................................................................................................... A. pamiricum |
| 94b. Test cell shapes elliptic to triangular. .................................................................................................................................................................. A. tashkenticum |
Contributor Information
Ziyoviddin Yusupov, CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; International Joint Lab for Molecular Phylogeny and Biogeography, Institute of Botany, Academy of Sciences of Uzbekistan, Tashkent 100125, Uzbekistan; Yunnan International Joint Laboratory for Biodiversity of Central Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China.
Ibrokhimjon Ergashov, International Joint Lab for Molecular Phylogeny and Biogeography, Institute of Botany, Academy of Sciences of Uzbekistan, Tashkent 100125, Uzbekistan; Yunnan International Joint Laboratory for Biodiversity of Central Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; University of Chinese Academy of Sciences, Beijing, China.
Sergei Volis, International Joint Lab for Molecular Phylogeny and Biogeography, Institute of Botany, Academy of Sciences of Uzbekistan, Tashkent 100125, Uzbekistan; Yunnan International Joint Laboratory for Biodiversity of Central Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China.
Dilmurod Makhmudjanov, CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; International Joint Lab for Molecular Phylogeny and Biogeography, Institute of Botany, Academy of Sciences of Uzbekistan, Tashkent 100125, Uzbekistan; Yunnan International Joint Laboratory for Biodiversity of Central Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China.
Davron Dekhkonov, International Joint Lab for Molecular Phylogeny and Biogeography, Institute of Botany, Academy of Sciences of Uzbekistan, Tashkent 100125, Uzbekistan.
Furkat Khassanov, International Joint Lab for Molecular Phylogeny and Biogeography, Institute of Botany, Academy of Sciences of Uzbekistan, Tashkent 100125, Uzbekistan; Yunnan International Joint Laboratory for Biodiversity of Central Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China.
Komiljon Tojibaev, International Joint Lab for Molecular Phylogeny and Biogeography, Institute of Botany, Academy of Sciences of Uzbekistan, Tashkent 100125, Uzbekistan; Yunnan International Joint Laboratory for Biodiversity of Central Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China.
Tao Deng, CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; Yunnan International Joint Laboratory for Biodiversity of Central Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China.
Hang Sun, CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; Yunnan International Joint Laboratory for Biodiversity of Central Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China.
FUNDING
This study was supported by the International Partnership Program of Chinese Academy of Sciences (151853KYSB20180009), the Belt and Road Project of West Light Foundation of the Chinese Academy of Sciences, the ‘Tree of life: monocots of Uzbekistan’ State Program, the Foundation of the State Research Project (FZ-20200929321), the Second Tibetan Plateau Scientific Expedition and Research (STEP) Program (2019QZKK0502), the Strategic Priority Research Program of Chinese Academy of Sciences (XDA20050203), the Key Projects of the Joint Fund of the National Natural Science Foundation of China (U1802232), the Youth Innovation Promotion Association of Chinese Academy of Sciences (2019382) and the Yunnan Young & Elite Talents Project (YNWR-QNBJ-2019-033).
LITERATURE CITED
- Abugalieva S, Volkova L, Genievskaya Y, et al. 2017. Taxonomic assessment of Allium species from Kazakhstan based on ITS and matK markers. BMC Plant Biology 17: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baasanmunkh S, Lee JK, Jang JE, et al. 2020. Studies in the Aegean flora. XXI. Biosystematic studies in the Allium ampeloprasum complex. S eed morphology of Allium L. (Amaryllidaceae) from central Asian countries and its taxonomic implications. Plants 9: 1239. doi: 10.3390/plants9091239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baasanmunkh S, Choi H, Oyuntsetseg B, Friesen N. 2021. Seed testa sculpture of species of Allium L. (Amaryllidaceae) and its taxonomic implications. Turczaninowia 24: 154–161. [Google Scholar]
- Bagheri A, Blattner FR, Fritsch RM. 2020. Allium gilanense, a new species of Allium sect. Codonoprasum (Amaryllidaceae) from Iran: evidence from morphological and molecular data. Phytotaxa 474: 283–292. doi: 10.11646/phytotaxa.474.3.7. [DOI] [Google Scholar]
- Barthlott W, Ehler N. 1977. Raster-elektronenmikroskopie der Epidermis-Oberflächen von Spermatophyten. Mainz: Akademie der Wissenchaften und der Literatur. [Google Scholar]
- Bednorz L, Krzyminska A, Czarna A. 2011. Seed morphology and testa sculptures of some Allium L. species (Alliaceae). Acta Agrobotanica 64: 33–38. [Google Scholar]
- Von Bothmer R. 1974. Studies in the Aegean flora. XXI. Biosystematic studies in the Allium ampeloprasum complex. Sweden: Opera Botanica, 1–104. [Google Scholar]
- Celep F, Koyuncu M, Fritsch RM, Kahraman A, Doğan M. 2012. Taxonomic importance of seed morphology in Allium (Amaryllidaceae). Systematic Botany 37: 893–912. doi: 10.1600/036364412x656563. [DOI] [Google Scholar]
- Cesmedziev I, Terzijski D. 1997. A scanning electron microscopic study of the spermoderm in Allium subg. Codonoprasum (Alliaceae). Bocconea 5: 755–758. [Google Scholar]
- Choi HJ, Cota-Sanchez JH. 2010. A taxonomic revision of Allium (Alliaceae) in the Canadian prairie provinces. Botany 88: 787–809. doi: 10.1139/b10-056. [DOI] [Google Scholar]
- Choi HJ, Giussani LM, Jang CG, Oh BU, Cota-Sánchez JH. 2012. Systematics of disjunct northeastern Asian and northern north American Allium (Amaryllidaceae). Botany 90: 491–508. doi: 10.1139/b2012-031. [DOI] [Google Scholar]
- Dubouzet J, Shinoda K. 1998. Phylogeny of Allium L. subg. Melanocrommyum (Webb et Berth.) Rouy based on DNA sequence analysis of the internal transcribed spacer region of nrDNA. Theoretical and Applied Genetics 97: 541–549. [Google Scholar]
- Dubouzet J, Shinoda K. 1999. Relationships among Old and New World Alliums according to ITS DNA sequence analysis. Theoretical and Applied Genetics 98: 422–433. [Google Scholar]
- Duman H, Ekşi G, Özbek F. 2017. Two new species of Allium L. sect. Allium (Amaryllidaceae) from Turkey. Plant Systematics and Evolution 303: 1271–1291. doi: 10.1007/s00606-017-1437-4. [DOI] [Google Scholar]
- Friesen N, Fritsch RM, Pollner S, Blattner FR. 2000. Molecular and morphological evidence for an origin of the aberrant genus Milula within Himalayan species of Allium (Alliacae). Molecular Phylogenetics and Evolution 17: 209–218. doi: 10.1006/mpev.2000.0844. [DOI] [PubMed] [Google Scholar]
- Friesen N, Fritsch RM, Blattner FR. 2006. Phylogeny and new intrageneric classification of Allium (Alliaceae) based on nuclear ribosomal DNA ITS sequences. Aliso 22: 372–395. [Google Scholar]
- Friesen N, Smirnov SV, Shmakov AI, Herden T, Oyuntsetseg B, Hurka H. 2020. Allium species of section Rhizomatosa, early members of the Central Asian steppe vegetation. Flora 263: 151536. doi: 10.1016/j.flora.2019.151536. [DOI] [Google Scholar]
- Friesen N, Smirnov SV, Leweke M, Seregin AP, Fritsch RM. 2021. Taxonomy and phylogenetics of Allium section Decipientia (Amaryllidaceae): morphological characters do not reflect the evolutionary history revealed by molecular markers. Botanical Journal of the Linnean Society 197: 190–228. [Google Scholar]
- Fritsch RM. 2016. A preliminary review of Allium subg. Melanocrommyum in Central Asia. Gatersleben: Leibniz-Institut für Pflanzengenetik und Kulturpflanzenforschung Gatersleben. [Google Scholar]
- Fritsch R, Abbasi M. 2013. A taxonomic review of Allium subg. Melanocrommyum in Iran. Gatersleben: Leibniz-Institut für Pflanzengenetik und Kulturpflanzenforschung Gatersleben. [Google Scholar]
- Fritsch R, Friesen N. 2002. Evolution, domestication and taxonomy. In: Rabinowitch HD, Currah L, eds. Allium crop science: recent advances. Wallingford:CABI, 5–30. [Google Scholar]
- Fritsch R, Kruse J, Adler K, Rutten T. 2006. Testa sculptures in Allium L. subg. Melanocrommyum (Webb & Berth.) Rouy (Alliaceae). Feddes Repertorium 117: 250–263. [Google Scholar]
- Fritsch RM, Blattner FR, Gurushidze M. 2010. New classification of Allium L. subg. Melanocrommyum (Webb & Berthel.) Rouy (Alliaceae) based on molecular and morphological characters. Phyton 49: 145–220. [Google Scholar]
- Gurushidze M, Fritsch RM, Blattner FR. 2008. Phylogenetic analysis of Allium subg. Melanocrommyum infers cryptic species and demands a new sectional classification. Molecular Phylogenetics and Evolution 49: 997–1007. doi: 10.1016/j.ympev.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Hall T. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95.– . [Google Scholar]
- Hamilton W. 1983. Cretaceous and Cenozoic history of the northern continents. Annals of the Missouri Botanical Garden 70: 440–458. doi: 10.2307/2992082. [DOI] [Google Scholar]
- Hanelt P, Schultze-Motel J, Fritsch R, Kruse J, Maass H, Ohle H, Pistrick K. 1992. Infrageneric grouping of Allium – the Gatersleben approach. Germany: Institute of Plant Genetics and Crop Plant Research. [Google Scholar]
- Hauenschild F, Favre A, Schnitzler J, Michalak I, Freiberg M, Muellner-Riehl AN. 2017. Spatio-temporal evolution of Allium L. in the Qinghai-Tibet-Plateau region: immigration and in situ radiation. Plant Diversity 39: 167–179. doi: 10.1016/j.pld.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D-Y. 1993. Eastern Asian–North American disjunctions and their biological significance. Cathaya 5: 1–39. [Google Scholar]
- Hopkins DM. 1967. The Bering Land Bridge. Redwood City, CA: Stanford University Press. [Google Scholar]
- Ilarslan H, Koyuncu M. 1997. Türkiye’de yetisen bazi endemik Allium (sogan) türlerinin tohum morfolojileri. Ot Sistematik Botanik Dergisi 4: 99–116. [Google Scholar]
- Khassanov FO. 2018. Taxonomical and ethnobotanical aspects of Allium species from Middle Asia with particular reference to subgenus Allium.In: Shigyo M, Khar A, Abdelrahman M, eds. The Allium genomes. Cham: Springer, 11–21. [Google Scholar]
- Khorasani M, Mehrvarz SS, Zarre S. 2020. Seed morphology and testa ultrastructure in Allium stipitatum complex (Amaryllidaceae; Allioideae) and their systematic significance. Turkish Journal of Botany 44: 618–632. doi: 10.3906/bot-2004-25. [DOI] [Google Scholar]
- Kruse J. 1984. Rasterelektronenmikroskopische Untersuchungen an Samen der Gattung Allium L. Die Kulturpflanze 32: 89–101. [Google Scholar]
- Kruse J. 1986. Rasterelektronenmikroskopische Untersuchungen an Samen der Gattung Allium L. II. Die Kulturpflanze 34: 207–228. [Google Scholar]
- Kruse J. 1988. Rasterelektronenmikroskopische Untersuchungen an Samen der Gattung Allium L. III. Die Kulturpflanze 36: 355–368. [Google Scholar]
- Kruse J. 1994. Rasterelektronenmikroskopische Untersuchungen an Samen der Gattung Allium L. IV. Feddes Repertorium 105: 457–471. doi: 10.1002/fedr.19941050711. [DOI] [Google Scholar]
- Li H-L. 1952. Floristic relationships between eastern Asia and eastern North America. Transactions of the American Philosophical Society 42: 371–429. doi: 10.2307/1005654. [DOI] [Google Scholar]
- Li Q-Q, Zhou S-D, He X-J, Yu Y, Zhang Y-C, Wei X-Q. 2010. Phylogeny and biogeography of Allium (Amaryllidaceae: Allieae) based on nuclear ribosomal internal transcribed spacer and chloroplast rps16 sequences, focusing on the inclusion of species endemic to China. Annals of Botany 106: 709–733. doi: 10.1093/aob/mcq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q-Q, Zhou S-D, Huang D-Q, He X-J, Wei X-Q. 2016. Molecular phylogeny, divergence time estimates and historical biogeography within one of the world’s largest monocot genera. AoB Plants 8: plw041. doi: 10.1093/aobpla/plw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-Y, Tan D-Y. 2017. Seed testa micromorphology of thirty-eight species of Allium (Amaryllidaceae) from Central Asia, and its taxonomic implications. Nordic Journal of Botany 35: 189–200. doi: 10.1111/njb.01259. [DOI] [Google Scholar]
- Liu L-F, Yusupov Z, Suyunkulov H, Jiang Z-L. 2020. The complete chloroplast genome of Allium ferganicum . Mitochondrial DNA Part B 5: 2772–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neshati F, Fritsch RM. 2009. Seed characters and testa sculptures of some Iranian Allium L. species (Alliaceae). Feddes Repertorium 120: 322–332. doi: 10.1002/fedr.200911112. [DOI] [Google Scholar]
- Nguyen NH, Driscoll HE, Specht CD. 2008. A molecular phylogeny of the wild onions (Allium; Alliaceae) with a focus on the western North American center of diversity. Molecular Phylogenetics and Evolution 47: 1157–1172. doi: 10.1016/j.ympev.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Nie Z-L, Wen J, Sun H, Bartholomew B. 2005. Monophyly of Kelloggia Torrey ex Benth. (Rubiaceae) and evolution of its intercontinental disjunction between western North America and eastern Asia. American Journal of Botany 92: 642–652. doi: 10.3732/ajb.92.4.642. [DOI] [PubMed] [Google Scholar]
- Nylander JA, Ronquist F, Huelsenbeck JP, Nieves-Aldrey J. 2004. Bayesian phylogenetic analysis of combined data. Systematic Biology 53: 47–67. [DOI] [PubMed] [Google Scholar]
- Pastor J. 1981. Estudio palinológico del género Allium en la Península Ibérica y Baleares. Botanica Macaronesica 8: 189–214. [Google Scholar]
- Samoylov A, Klaas M, Hanelt P. 1995. Use of chloroplast DNA polymorphisms for the phylogenetic study of the subgenera Amerallium and Bromatorrhiza (genus Allium). Feddes Repertorium 106: 161–167. doi: 10.1002/fedr.19951060306. [DOI] [Google Scholar]
- Samoylov A, Friesen N, Pollner S, Hanelt P. 1999. Use of chloroplast DNA polymorphisms for the phylogenetic study of Allium subgenus Amerallium and subgenus Bromatorrhiza (Alliaceae) II. Feddes Repertorium 110: 103–109. doi: 10.1002/fedr.19991100118. [DOI] [Google Scholar]
- Sanmartín I, Enghoff H, Ronquist F. 2001. Patterns of animal dispersal, vicariance and diversification in the Holarctic. Biological Journal of the Linnean Society 73: 345–390. [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinitsyna TA, Herden T, Friesen N. 2016. Dated phylogeny and biogeography of the Eurasian Allium section Rhizirideum (Amaryllidaceae). Plant Systematics and Evolution 302: 1311–1328. doi: 10.1007/s00606-016-1333-3. [DOI] [Google Scholar]
- Stafford GI, Wikkelsø MJ, Nancke L, Jäger AK, Möller M, Rønsted N. 2016. The first phylogenetic hypothesis for the southern African endemic genus Tulbaghia (Amaryllidaceae, Allioideae) based on plastid and nuclear DNA sequences. Botanical Journal of the Linnean Society 181: 156–170. doi: 10.1111/boj.12417. [DOI] [Google Scholar]
- Thorne RF. 1972. Major disjunctions in the geographic ranges of seed plants. Quarterly Review of Biology 47: 365–411. doi: 10.1086/407399. [DOI] [Google Scholar]
- Veiskarami G, Khodayari H, Heubl G, Zarre S. 2018. Seed surface ultrastructure as an efficient tool for species delimitation in the Allium ampeloprasum L. alliance (Amaryllidaceae, Allioideae). Microscopy Research and Technique 81: 1275–1285. doi: 10.1002/jemt.23134. [DOI] [PubMed] [Google Scholar]
- WCSP. 2022. World checklist of selected plant families.Facilitated by the Royal Botanic Gardens, Kew. http://wcsp.science.kew.org/ (22 February 2022, date last accessed). [Google Scholar]
- Wen J. 1999. Evolution of eastern Asian and eastern North American disjunct distributions in flowering plants. Annual Review of Ecology and Systematics 30: 421–455. doi: 10.1146/annurev.ecolsys.30.1.421. [DOI] [Google Scholar]
- Wheeler EJ, Mashayekhi S, McNeal DW, Columbus JT, Pires JC. 2013. Molecular systematics of Allium subgenus Amerallium (Amaryllidaceae) in North America. American Journal of Botany 100: 701–711. doi: 10.3732/ajb.1200641. [DOI] [PubMed] [Google Scholar]
- Xie D-F, Tan J-B, Yu Y, et al. 2020. Insights into phylogeny, age and evolution of Allium (Amaryllidaceae) based on the whole plastome sequences. Annals of Botany 125: 1039–1055. doi: 10.1093/aob/mcaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusupov Z, Deng T, Liu C, Lin N, Tojibaev K, Sun H. 2019. The complete chloroplast genome of Allium fistulosum. Mitochondrial DNA Part B 4: 489–490. doi: 10.1080/23802359.2018.1545532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusupov Z, Deng T, Volis S, et al. 2021. Phylogenomics of Allium section Cepa (Amaryllidaceae) provides new insights on domestication of onion. Plant Diversity 43: 102–110. doi: 10.1016/j.pld.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhengyi W. 1983. On the significance of Pacific intercontinental discontinuity. Annals of the Missouri Botanical Garden 70: 577–590. doi: 10.2307/2398977. [DOI] [Google Scholar]
- Zhou Z, Wen J, Li G, Sun H. 2012. Phylogenetic assessment and biogeographic analyses of tribe Peracarpeae (Campanulaceae). Plant Systematics and Evolution 298: 323–336. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.