Abstract
Background
Metoclopramide is frequently prescribed as an adjuvant for the postpyloric placement of nasoenteric tubes (NETs). However, a recent meta‐analysis showed that metoclopramide was not beneficial in adults. Thus, this study aimed to reevaluate the effect of metoclopramide on the postpyloric placement of NETs.
Methods
A systematic search of PubMed, Embase, the Cochrane Library, Web of Science, Chinese National Knowledge Infrastructure (CNKI), and Wanfang data was conducted up to August 2020 for randomized controlled trials (RCTs) comparing metoclopramide with placebo or no intervention. Trial sequential analysis (TSA) was used for the primary outcomes (the success rate of the postpyloric placement of NETs).
Results
Seven eligible RCTs that included 520 participants were identified. The results of the pooled effect sizes showed that metoclopramide significantly facilitated the postpyloric placement of NETs (relative risk [RR], 1.48; 95% CI, 1.11–1.97; P = .007; I 2 = 37%). However, the risk‐of‐bias assessment and the TSA results indicated that the qualities of the RCTs and the sample sizes were insufficient to confirm the efficacy of metoclopramide. Further subgroup analysis revealed that successful postpyloric placement was more pronounced in studies in which spiral NETs were employed (RR, 1.85; 95% CI, 1.41–2.43; P < .001; I 2 = 0%). Additionally, overall adverse events were minimal.
Conclusions
The evidence accumulated so far was not strong enough to demonstrate metoclopramide's beneficial effects on the postpyloric placement of NETs. Further high‐quality, large‐sample RCTs are required to elucidate the effects of metoclopramide.
Keywords: enteral nutrition, meta‐analysis, metoclopramide, nasoenteric tube, postpyloric placement
BACKGROUND
Early enteral nutrition (EN) by a feeding tube is the preferred method for hospitalized patients to maintain nutrition support if oral intake is not possible. 1 , 2 The advocated EN route for those who are intractably intolerant of gastric feeding or deemed to be at high risk of aspiration is postpyloric feeding, which can favorably mitigate gastric retention, gastroesophageal reflux, and aspiration pneumonia. 3 , 4 , 5
The establishment of postpyloric feeding access to the duodenum or jejunum is usually attempted by the bedside placement of a nasoenteric tube (NET), especially in intensive care units. However, successful postpyloric placement of NETs remains a tough challenge for physicians when there is limited access to equipment resources (eg, endoscopic or fluoroscopic guidance). Thus, many studies have been dedicated to improving the success rate of postpyloric NET placement independent of special device assistance. 6 , 7 , 8 , 9 Of these studies, a critical theme that has intrigued physicians is the effect of prokinetic agents as an adjuvant for the postpyloric placement of NETs. 10 , 11 , 12 , 13 , 14 , 15 , 16
One of the popular prokinetic agents—namely, metoclopramide, which is a specific antagonist of D2 (dopamine) receptors—has been frequently introduced in procedures of postpyloric placement. However, controversy exists regarding the effect of metoclopramide. The latest meta‐analysis of randomized controlled trials (RCTs) performed by da Silva et al concluded that metoclopramide was not beneficial in the postpyloric placement of NETs in adults. 17 Conversely, a recent large‐sample RCT conducted by Hu et al strongly suggested that metoclopramide could improve the success rate of postpyloric placement. 16 Although da Silva's meta‐analysis was well designed and strictly performed, the authors did not include Hu's study, which was published several months after their work was published. Eventually, only four RCTs 10 , 11 , 12 , 13 published in the last century were included in their meta‐analysis. Additionally, the overall quality of evidence given by da Silva's findings was very low, which indicated that the true effect of metoclopramide was likely to be substantially different from the estimate of the effect. There remains a possibility that other nonincluded studies may also be eligible for the meta‐analysis.
Therefore, an updated meta‐analysis was performed to reevaluate the effect of metoclopramide on the postpyloric placement of NETs by formulating novel search strategies and using the updated databases to find additional potential studies.
METHODS
The present study adhered to the Preferred Reporting Items of Systematic Reviews and Meta‐Analyses (PRISMA) statement guideline for performing and reporting. 18 The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42019123424).
Search strategy
We systematically retrieved studies from the establishment of the database to August 2020 from electronic databases, including PubMed, Embase, the Cochrane Library, Web of Science, Chinese National Knowledge Infrastructure (CNKI) (www.cnki.net), and Wanfang data (www.wanfangdata.com.cn), to identify RCTs that examined the effect of metoclopramide on the postpyloric placement of NETs. No language or publication date limitations were made to be as sensitive as possible. A hand search of the reference lists from trials located by electronic searches was performed to identify other potentially eligible studies. Details of the search strategy are available in Table S1.
Study selection
Studies with the following criteria were included1: RCTs comparing metoclopramide with placebo or no intervention for the placement of NETs, irrespective of publication status, language, or anonymizing2; RCTs with parallel design3; age ≥18 years; and studies4 that provided sufficient data on baseline and final measures of the success rate of postpyloric placement in the metoclopramide and control groups. The exclusion criteria were as follows1: duplicated data2; studies examining metoclopramide in combination with other prokinetic agents3; enteral metoclopramide for the introduction of NETs; and studies4 that lacked the necessary information for the methodology.
Data extraction
Two reviewers independently extracted data from each study to obtain the following information: first author's name, publication date, study location, mean age and gender of participants, the sample size in each group, study design, the timing and dosage of metoclopramide, the type of NETs, confirmation of postpyloric feeding tube location, follow‐up period after initial placement, the success rate of placement, and adverse events in the metoclopramide and control groups. If we encountered a multiarmed study, we planned to use data only from the metoclopramide arm vs the placebo or no‐intervention arms. All discrepancies were rechecked, and a consensus was achieved by discussion with a third author.
Types of outcome measures
The primary outcome of this study was the success rate of the postpyloric placement of NETs, which was defined as the migration of the tube tip through the pylorus and into the duodenum or jejunum, as confirmed by abdominal or chest radiography. An overall and comprehensive evaluation of the success rate of post‐D1 (reaching the second portion of the duodenum or beyond), post‐D2 (reaching the third portion of the duodenum or beyond), post‐D3 (reaching the fourth portion of the duodenum or beyond), and proximal jejunum placement was analyzed as a secondary outcome. Adverse events involving drug side effects and tube insertion complications were also analyzed.
Quality assessment
The methodological quality of the included studies was independently evaluated by two reviewers using the Cochrane Risk of Bias tool, which consists of seven criteria to assess the risk of bias: random sequence generation and allocation concealment (selection bias), anonymizing of participants and personnel (performance bias), anonymizing of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. Each aforementioned item could be graded “high risk,” “low risk,” or “unclear.”
Statistical analysis
The analysis was performed based on the intention‐to‐treat method. All studies were pooled in reporting the same primary outcomes, secondary outcomes, or adverse events together. Given that the outcomes we targeted were presented as dichotomous data, we calculated the relative risk (RR) and 95% CI for each outcome with the random‐effects model. Statistical analyses were performed using Review Manager 5.3 Software (The Cochrane Collaboration, Copenhagen, Denmark) and Stata 12.0 software (Stata Corp, College Station, Texas). A P‐value <.05 was considered statistically significant.
We assessed the clinical heterogeneity by exploring the clinical and methodological characteristics of the included studies. The statistical heterogeneity between studies was quantified by the chi‐squared test, with α = .05 used for statistical significance and I 2. I 2 values <30, 30–59, 60–75, and >75% were classified as low, moderate, substantial, and considerable heterogeneity, respectively. 19 Prespecified subgroup analyses for metoclopramide dosage (10 vs 20 mg), the timing of medication (prior to insertion vs after insertion), the type of feeding tube tip (spiral vs straight), and the number of centers (single center vs multicenter) were used to identify the possible influence of covariates on our primary outcomes. Additionally, we also performed sensitivity analyses to examine the robustness of primary outcomes by changing to a fixed‐effect model and excluding studies that had a high risk of bias, were published in the last century, involved <20 patients, or had a weight of <2%. We visually inspected the potential publication bias with graphical (Begg funnel plot) and statistical tests (Egger test).
Trial sequential analysis
Trial sequential analysis (TSA) is a method combining an a priori information size calculation for a meta‐analysis with a threshold of statistical significance to evaluate the accumulated evidence. 20 We performed a TSA of metoclopramide vs placebo or no intervention for primary outcomes to control for the risks of type І (false positive) and type Ⅱ (false negative) errors due to sparse data and repetitive testing of accumulating data. If the cumulative z‐curve crosses the threshold boundaries, the evidence obtained in this meta‐analysis is sufficient for the proof of metoclopramide's beneficial effects, and no further RCTs are required. However, the evidence is insufficient to reach a conclusion if the cumulative z‐curve does not cross any boundary. The TSA software (version 0.9.5.9 Beta; Copenhagen Trial Unit, Copenhagen, Denmark) was applied to evaluate the reliability of the meta‐analyses and to determine whether the current meta‐analysis sample size was sufficiently large with the following assumptions: the control event proportion calculated from the included studies; an RR reduction of −20% for primary outcomes; α = .05 (two‐sided); and a power (1 − β) of 80%.
Grading quality of evidence
Two investigators independently used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Guideline Development Tool (GRADEpro; McMaster University 2014, Hamilton, Canada) to evaluate the quality of evidence for each outcome. The quality of evidence was stratified into four grades—namely, high, moderate, low, or very low—according to risk of bias, inconsistency, indirectness, imprecision, and publication bias.
RESULTS
Study characteristics
A total of 771 articles were screened, 29 were identified for full‐text review, and seven met the prespecified eligibility criteria and were included (Figure 1, Table S2). These studies were conducted in the US 10 , 11 , 12 , 13 , 14 and China 15 , 16 between 1984 and 2015, of which five studies were published in the last century. 10 , 11 , 12 , 13 , 14 In total, 520 participants were enrolled in these studies, of whom 267 were randomly assigned to the metoclopramide group and 253 to the control group. The method of metoclopramide administration was intravenously or intramuscularly given at a dose of 10–20 mg prior to or after insertion. Straight NETs were employed in five studies, 10 , 11 , 12 , 13 , 14 and spiral NETs were introduced in another two studies 15 , 16 (Table 1).
Figure 1.
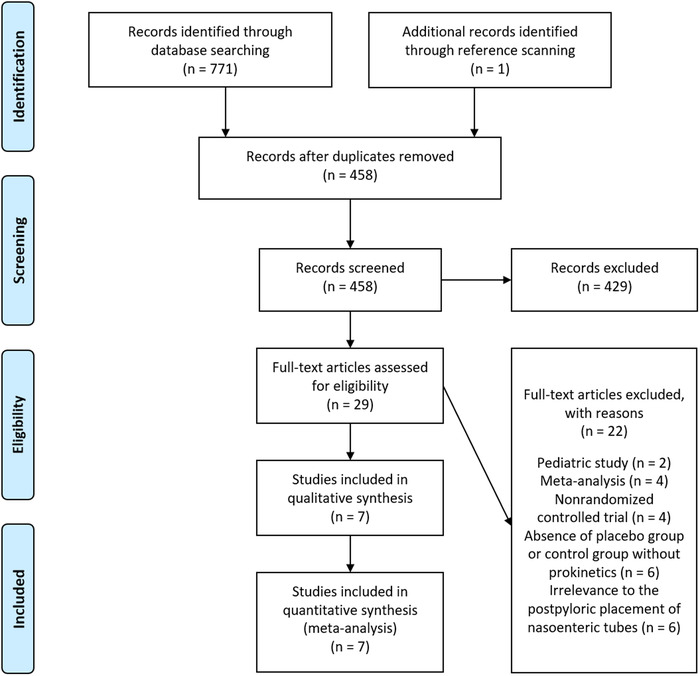
Preferred Reporting Items of Systematic Reviews and Meta‐Analyses flow diagram of study selection
Table 1.
Characteristics of included studies
| Author | Country | Participants | Mean age, years | Tube tip | Metoclopramide dose and administration | Methods for confirming postpyloric tube location | Primary outcomes | No. of participants, M:C | No. of successes (%), M:C |
|---|---|---|---|---|---|---|---|---|---|
| Whatley, 1984 | USA | Adult | 46 | Straight | Metoclopramide 20 mg IV over 10 min before insertion | Abdominal x‐ray within 4 h of metoclopramide administration or insertion | Successful postpyloric placement | 5:5 | 4 (80.0):0 |
| Seifert, 1987 | USA | Adult | NR | Straight | Metoclopramide 20 mg in 4‐ml volume IV 15 min before insertion | Abdominal radiography 4 h after insertion | Successful postpyloric placement | 9:10 | 1 (11.1):2 (20.0) |
| Kittinger, 1987 | USA | Adult | 64.8 | Straight | Metoclopramide 10 mg in 2‐ml volume IM or IV after insertion | Plain abdominal film 1 h after insertion | Successful postpyloric placement | 35:35 | 21 (60.0):17 (48.6) |
| Heiselman, 1995 | USA | Adult | NR | Straight | Metoclopramide 10 mg IV over 10 min before insertion | Auscultation of the right upper abdominal quadrant and abdominal radiography 45 min after tube advancement | Successful postpyloric placement | 59:46 | 32 (54.2):21 (45.7) |
| Paz, 1996 | USA | Adult | 63.1 | Straight | Metoclopramide 10 mg IV over 15 min before insertion | Chest radiograph 30 min after insertion and repeated 24 h later | Successful postpyloric placement and attempt | 20:16 | 0:2 (12.5) |
| Chen, 2009 | China | Adult | 67.6 | Spiral | Metoclopramide 10 mg IV before insertion and again after 12 h | Abdominal x‐ray scan 24 h after insertion | Successful postpyloric placement | 39:42 | 28 (71.8):18 (42.8) |
| Hu, 2015 | China | Adult | 61.7 | Spiral | Metoclopramide 20 mg IV over 10 min before insertion | Abdominal x‐ray scan 24 h after insertion | Successful postpyloric placement | 100:99 | 55 (55.0):27 (27.3) |
Abbreviations: C, control group; IM, intramuscularly; IV, intravenously; M, metoclopramide group; NR, not reported.
Risk‐of‐bias assessment
The risk of bias of the included studies is shown in Figure S1, Figure S2, and Table S3. All studies were rated as having a low or unclear risk of selection bias. In this domain, Hu's study generated the allocation sequence by computer and three studies 10 , 11 , 14 by a random number table. The others did not describe the allocation methods used and simply indicated that the participants were randomized. None described the method of allocation concealment except Hu's study, which performed this by telephone verification with the randomization center. For anonymizing, three studies 10 , 12 , 16 were rated as having a high risk of performance bias because they did not have a double‐anonymized design, and two studies 10 , 13 were rated as having a high risk of detection bias, considering that the outcome assessors were not anonymized to the treatment protocol. Of these studies, Kittinger's study was described as double‐anonymized; however, the full details of how this was achieved were not given in the published report. Additionally, Chen's study did not provide detailed information on whether the study was designed as double‐anonymized. Therefore, it is unclear whether participants, personnel, and outcome assessors were adequately anonymized in Kittinger's and Chen's studies. Hu's study was an open‐label trial; however, to minimize potential bias, the tube tip position, confirmed by abdominal radiography, was reviewed by an expert group of intensivists and radiologists without identifying information about the study participants. Thus, we rated a high risk on performance bias but a low risk on detection bias for Hu's study. All studies were considered at low risk of attrition bias and reporting bias, and five studies 10 , 11 , 12 , 14 , 15 had an unclear risk of other potential bias owing to a lack of sample size calculations.
Primary outcome
Data from seven studies comparing the success rate of the postpyloric placement of NETs between the metoclopramide and control groups with a total of 520 participants were available. When we pooled the data together, the metoclopramide group showed a significant increase in the success rate of the postpyloric placement of the NETs compared with that of the control group (RR, 1.48; 95% CI, 1.11–1.97; P = .007). The I 2 value of 37% indicated moderate statistical heterogeneity (Figure 2).
Figure 2.
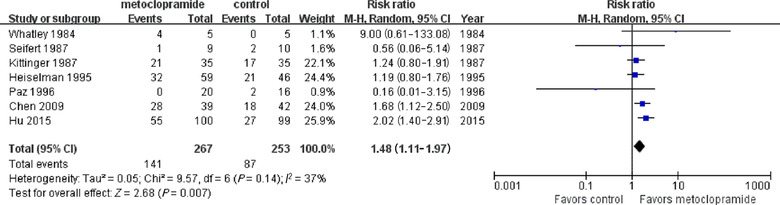
The effect of metoclopramide on the postpyloric placement of nasoenteric tubes
The results were robust to multiple sensitivity analyses, including changing to a fixed‐effect model and excluding studies that had a high risk of bias, were published in the last century, involved <20 patients, or had a weight of <2% (Figure S3). Visual inspection of the funnel plot indicated no evidence of asymmetry in the effect of metoclopramide on postpyloric placement (Figure S4), as confirmed by Egger regression test (P = .605).
However, the TSA provided the required information size of 3319 for the primary outcomes in this meta‐analysis (information deficit 2799) (Figure 3). Additionally, the cumulative z‐curve only crossed the conventional boundary, which indicated that the evidence was insufficient to reach a conclusion that metoclopramide is beneficial for the postpyloric placement of NETs, and further high‐quality, large‐sample RCTs are required.
Figure 3.
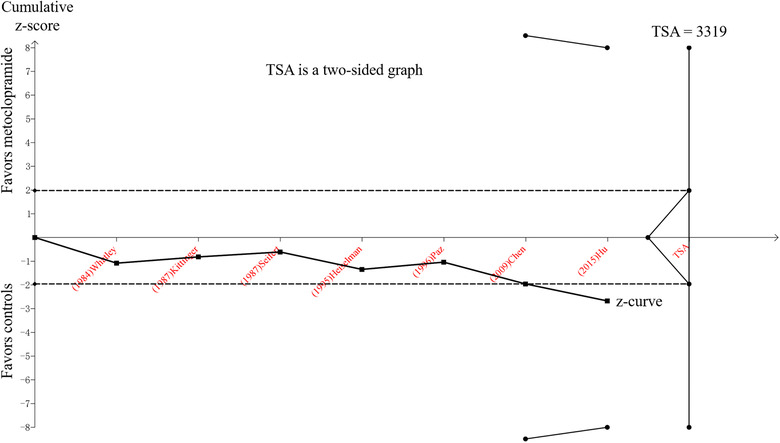
TSA for comparison of the success rate of postpyloric placement of nasoenteric tubes between the metoclopramide group and control group. The line of black dots represents the trial sequential monitoring boundaries for the benefit and the futility boundaries. The line of black squares is the cumulative z‐curve. The black dotted lines are conventional P = .05 lines. The required sample size for a conclusive result was 3319. TSA, trial sequential analysis
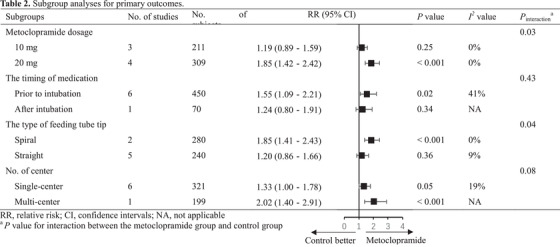
Further subgroup analysis was performed to determine whether there were important subgroup differences, in light of moderate statistical heterogeneity in the primary outcomes. The results showed significant effect sizes for studies administering 20 mg metoclopramide (RR, 1.85; 95% CI, 1.42–2.42; P < .001; I 2 = 0%) vs those administering 10 mg metoclopramide (RR, 1.19; 95% CI, 0.89–1.59; P = .25; I 2 = 0%), studies administering metoclopramide prior to insertion (RR, 1.55; 95% CI, 1.09–2.21; P = .02; I 2 = 41%) vs those administering metoclopramide after insertion (RR, 1.24; 95% CI, 0.80–1.91; P = .34), studies employing a spiral tube (RR, 1.85; 95% CI, 1.41–2.43; P < .001; I 2 = 0%) vs those employing a straight tube (RR, 1.20; 95% CI, 0.86–1.66; P = .36; I 2 = 9%), and studies with multiple centers (RR, 2.02; 95% CI, 1.40–2.91; P < .001) vs those with a single center (RR, 1.33; 95% CI, 1.00–1.78; P = .05; I 2 = 19%). There were significant interactions between subgroups regarding metoclopramide dosage (P interaction = .03) and the type of feeding tube tip (P interaction = .04) (Table 2, Figure S5).
Table 2.
Subgroup analyses for primary outcomes
|
Abbreviations: NA, not applicable; RR, relative risk.
P‐value for interaction between the metoclopramide group and control group.
Secondary outcomes
Two studies 15 , 16 reported the accurate locations of the feeding tube tip in the duodenum or jejunum. In particular, the feeding tubes used in their studies were all spiral NETs. The pooled results revealed a significant effect of metoclopramide on the post‐D1, post‐D2, and post‐D3 placement of spiral NETs except for proximal jejunum placement (Figure 4).
Figure 4.
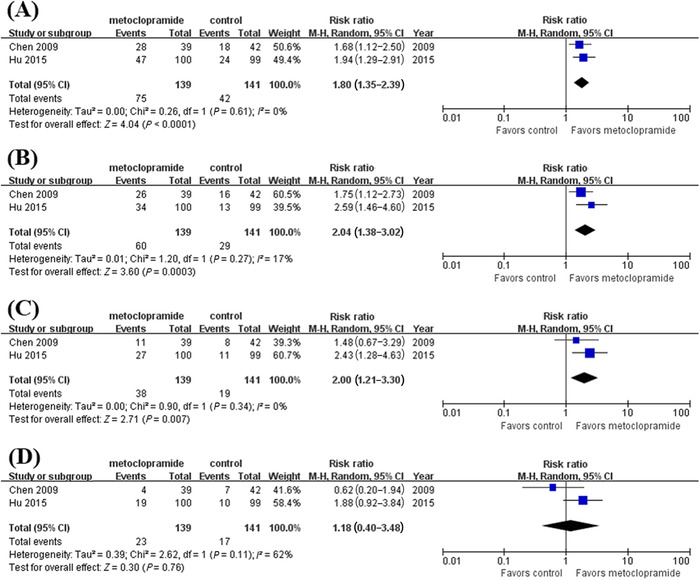
The effect of metoclopramide on post‐D1 (A), post‐D2 (B), post‐D3 (C), and proximal jejunum placement of nasoenteric tube (D). post‐D1, reaching the second portion of the duodenum or beyond; post‐D2, reaching the third portion of the duodenum or beyond; post‐D3, reaching the fourth portion of the duodenum or beyond
Summary of adverse events
Adverse events are summarized in Figure 5 according to four studies 10 , 12 , 15 , 16 with adverse event data. Of those, five patients encountered drug side effects, including lethargy, dysphoria, or amyostasia, and 32 patients encountered tube insertion complications, including nasal mucosa bleeding, airway misplacement, pain, nausea, or vomiting. These adverse events were considered mild and resolved quickly without causing any severe consequences. The pooled results revealed the safety profile of metoclopramide for the postpyloric placement of NETs (RR, 1.61; 95% CI, 0.87–2.99; P = .13; I 2 = 0%).
Figure 5.
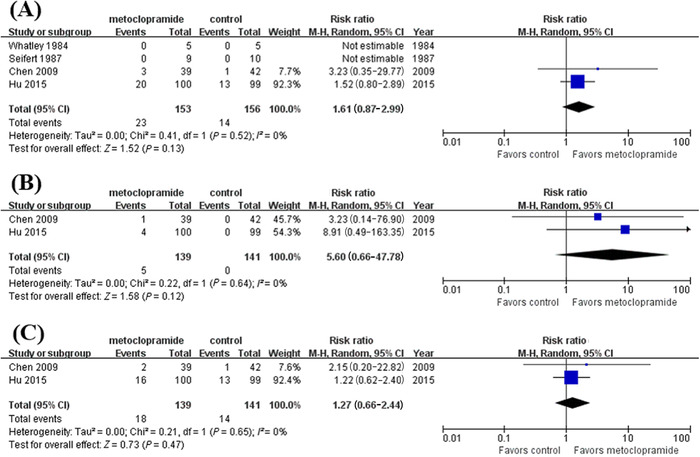
The comparison for any adverse events (A), drug side effects (B), and tube insertion complications (C) between the metoclopramide group and control group
Summary of findings
Table S4 shows a summary of the findings with the GRADE assessments of the overall quality of the evidence for the effect of metoclopramide on the postpyloric placement of NETs. The evidence was graded as low for postpyloric placement owing to a downgrade for serious risk of bias and a small number of trials with relatively few participants or events; low for post‐D1 and post‐D3 placement owing to a downgrade for serious risk of bias and insufficient study number to assess publication bias; moderate for post‐D2 placement owing to a large effect; and moderate for adverse events owing to a downgrade for serious risk of bias.
DISCUSSION
In the present meta‐analysis, we included seven RCTs in the final analysis. Our results revealed that metoclopramide could improve the success rate of the postpyloric placement of NETs; however, the evidence accumulated so far was insufficient. Furthermore, metoclopramide was found to facilitate the postpyloric, post‐D1, post‐D2, and post‐D3 placement of spiral NETs. Using metoclopramide in the short term for postpyloric placement of NETs did not significantly increase the risk of adverse events.
As a conventional prokinetic agent, metoclopramide has a significant effect on the increase in antral contraction amplitude and the improvement of GI peristalsis. 11 Therefore, in theory, metoclopramide should be beneficial for the passage of NETs through the stomach and into the duodenum or jejunum. Unfortunately, neither da Silva's meta‐analysis nor our results demonstrated the efficacy of metoclopramide, even though three RCTs were newly included in this updated meta‐analysis. Several clinical heterogeneities deriving from participants (eg, age, comorbidities, and concomitant medication), 21 , 22 , 23 operators (eg, years of training, professional status, and educational degree of operators), 13 , 24 intervention (eg, the timing of medication, the dosage of metoclopramide, and the type of feeding tube), 10 , 16 outcome assessment (eg, the follow‐up period and the assessment personnel), 10 , 11 , 12 , 13 , 14 , 15 , 16 and the like, as well as insufficient sample sizes, may be liable for failing to establish metoclopramide's beneficial effects.
In further subgroup analyses, we found that the administration of 20 mg metoclopramide had a significantly higher success rate of postpyloric placement than did the administration of 10 mg metoclopramide. Although both 10 and 20 mg metoclopramide are plausible according to drug instruction, there has been a suggestion that metoclopramide displays disposition dose dependency and obeys linear kinetics in individuals with intravenous or oral doses from 5 to 20 mg. 25 , 26 Additionally, we found that the administration of metoclopramide prior to insertion could facilitate postpyloric placement, whereas the interaction between subgroups did not reach statistical significance. Active gastric peristalsis at the time of feeding tube insertion is a key factor in achieving postpyloric placement with metoclopramide. 10 The insignificant interaction may be due to only one study that administered metoclopramide after insertion. With respect to the number of centers, the multicenter RCT with a large sample size may provide a more representational result, and the insignificant interaction may be due to only one study being designated as multicenter.
Recently, a novel NET with a spiral tip for postpyloric placement with the assistance of prokinetic agents has emerged as a promising approach, 16 , 27 , 28 , 29 as demonstrated by the subgroup results stratified by spiral or straight tube tip. In previous studies, Lai et al demonstrated that a spiral NET in conjunction with metoclopramide is preferable to a straight NET for postpyloric placement. 30 Additionally, a recent RCT also demonstrated the efficacy and safety of metoclopramide for the postpyloric placement of spiral NETs. 28 The spiral design may contribute to taking full advantage of gastrointestinal peristalsis to pass the tip through the pylorus and into the duodenum and jejunum. 31 Furthermore, metoclopramide was found to facilitate the post‐D1, post‐D2, and post‐D3 placement of spiral NETs. Thus, spiral feeding tubes may be more appropriate for postpyloric placement if available. However, we failed to show a beneficial effect of metoclopramide on proximal jejunum placement, and there was significant between‐study heterogeneity (I 2 = 62%). This may be attributed to the relatively few cases in which tube tips could spontaneously migrate to the proximal jejunum even with the aid of metoclopramide and the impaired gastrointestinal function of the critically ill patients enrolled in their studies. However, given that few studies included in this meta‐analysis focused on spiral NETs, the beneficial effects of metoclopramide on postpyloric, post‐D1, post‐D2, post‐D3, and proximal jejunum placement warrant further investigation.
With regard to safety, concern about the use of metoclopramide has been expressed because of its potential role in causing adverse events. 16 Some investigators felt an increase in dose to 20 mg intravenously would run the risk of an increased incidence of side effects. However, the adverse events that participants encountered were minimal and mild with no need for special treatment. Additionally, our results showed that there was no significant difference between the metoclopramide and control groups either in drug side effects or tube insertion complications. Therefore, metoclopramide may be safe in a regular dose of no more than 20 mg in the short term for the postpyloric placement of NETs when more attention has been paid to its contraindications (eg, patients in epilepsy and renal or liver dysfunction) to avoid severe adverse events. However, we also should realize that adverse events in an investigator‐initiated trial often underreport adverse events compared with trials performed for drug registration with a regulatory agency.
Additionally, there were several limitations worth noting. First, some studies were at high risk of bias, and moderate statistical heterogeneity was present in the primary outcomes, which influenced the quality of evidence and the interpretation of findings. Second, the follow‐up period in the included studies was not consistent, ranging from 30 min to 24 h after insertion. Third, the results of secondary outcomes and subgroup analyses might only serve as a useful hint for metoclopramide's beneficial effects on the postpyloric placement of spiral NETs because relatively few studies provided accurate locations of the feeding tube tip. Finally, the overall quality of the evidence was low. Thus, the negative results of metoclopramide for postpyloric placement should be considered with caution.
CONCLUSIONS
In conclusion, the present meta‐analysis indicated that the evidence accumulated so far was not strong enough to demonstrate metoclopramide's beneficial effects on the postpyloric placement of NETs. The findings may provide better insights into the effect of metoclopramide and help develop an alternative approach for postpyloric placement. In the future, to elucidate the effects of metoclopramide, it is necessary to perform high‐quality, large‐sample RCTs.
CONFLICT OF INTEREST
None declared.
FUNDING INFORMATION
This work was supported by the Major Program of Summit Project, Guangdong Province High‐level Hospital Construction Project of Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences (DFJH2020028 to Chunbo Chen), the Science and Technology Planning Project of Guangzhou, China (201803010058 to Chunbo Chen), and the Phase | Clinical Research Training Course “Seed Funding” of Guangdong Provincial People's Hospital (2018lcpx03 to Bei Hu).
AUTHOR CONTRIBUTIONS
Chunbo Chen and Yiyu Deng equally contributed to the conception and design of the research; Xin Ouyang contributed to the design of the research; Rong Qu and Bei Hu contributed to the acquisition and analysis of the data; Yifan Wang and Fen Yao contributed to the acquisition, analysis, and interpretation of the data. Bo Lv and Cheng Sun drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Supporting information
Supporting Information
Ouyang X, Qu R, Hu B, et al. Is metoclopramide beneficial for the postpyloric placement of nasoenteric tubes? A systematic review and meta‐analysis of randomized controlled trials. Nutr Clin Pract. 2022;37:316–327. 10.1002/ncp.10725
Contributor Information
Yiyu Deng, Email: yiyudeng666@163.com.
Chunbo Chen, Email: gghccm@163.com.
REFERENCES
- 1. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48‐79. [DOI] [PubMed] [Google Scholar]
- 2. McClave SA, Taylor BE, Martindale RG, et al; Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159‐211. [DOI] [PubMed] [Google Scholar]
- 3. Alkhawaja S, Martin C, Butler RJ, Gwadry‐Sridhar F. Post‐pyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database Syst Rev. 2015(8):CD008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jiyong J, Tiancha H, Huiqin W, Jingfen J. Effect of gastric versus post‐pyloric feeding on the incidence of pneumonia in critically ill patients: observations from traditional and bayesian random‐effects meta‐analysis. Clin Nutr. 2013;32(1):8‐15. [DOI] [PubMed] [Google Scholar]
- 5. Deane AM, Dhaliwal R, Day AG, Ridley EJ, Davies AR, Heyland DK. Comparisons between intragastric and small intestinal delivery of enteral nutrition in the critically ill: a systematic review and meta‐analysis. Crit Care. 2013;17(3):R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang QJ, Jiang CF, Chen QT, Shi J, Shi B. Erythromycin for promoting the postpyloric placement of feeding tubes: a systematic review and meta‐analysis. Gastroenterol Res Pract. 2018;2018:1671483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis K, Alqahtani Z, McIntyre L, et al. The efficacy and safety of prokinetic agents in critically ill patients receiving enteral nutrition: a systematic review and meta‐analysis of randomized trials. Crit Care. 2016;20(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tiancha H, Jiyong J, Min Y. How to promote bedside placement of the postpyloric feeding tube: a network meta‐analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr. 2015;39(5):521‐530. [DOI] [PubMed] [Google Scholar]
- 9. Lenart S, Polissar NL. Comparison of two methods for postpyloric placement of enteral feeding tubes. Am J Crit Care. 2003;12(4):357‐360. [PubMed] [Google Scholar]
- 10. Whatley K, Turner WW Jr, Dey M, Leonard J, Guthrie M. When does metoclopramide facilitate transpyloric intubation? JPEN J Parenter Enteral Nutr. 1984;8(6):679‐681. [DOI] [PubMed] [Google Scholar]
- 11. Kittinger JW, Sandler RS, Heizer WD. Efficacy of metoclopramide as an adjunct to duodenal placement of small‐bore feeding tubes: a randomized, placebo‐controlled, double‐blind study. JPEN J Parenter Enteral Nutr. 1987;11(1):33‐37. [DOI] [PubMed] [Google Scholar]
- 12. Seifert CF, Cuddy PG, Pemberton LB, Lyman EL, Schmitt P. A randomized trial of metoclopramide's effects on the transpyloric intubation of weighted feeding tubes. Nutr Supp Serv. 1987;11(7):11‐13. [Google Scholar]
- 13. Heiselman DE, Hofer T, Vidovich RR. Enteral feeding tube placement success with intravenous metoclopramide administration in ICU patients. Chest. 1995;107(6):1686‐1688. [DOI] [PubMed] [Google Scholar]
- 14. Paz HL, Weinar M, Sherman MS. Motility agents for the placement of weighted and unweighted feeding tubes in critically ill patients. Intensive Care Med. 1996;22(4):301‐304. [DOI] [PubMed] [Google Scholar]
- 15. Chen C, Zeng H, Wu Y, et al. Erythromycin and metoclopramide improves the success rate of post‐pyloric placement of the spiral distal end nasal‐enteral feeding tubes. Chin J Pract Intern Med. 2009;29(1):39‐41. [Google Scholar]
- 16. Hu B, Ye H, Sun C, et al. Metoclopramide or domperidone improves post‐pyloric placement of spiral nasojejunal tubes in critically ill patients: a prospective, multicenter, open‐label, randomized, controlled clinical trial. Crit Care. 2015;19(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Silva CCR, Bennett C, Saconato H, Atallah ÁN. Metoclopramide for post‐pyloric placement of naso‐enteral feeding tubes. Cochrane Database Syst Rev. 2015;1(1):CD003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. W64. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses? Int J Epidemiol. 2009;38(1):276‐286. [DOI] [PubMed] [Google Scholar]
- 21. Berger MM, Bollmann MD, Revelly JP, et al. Progression rate of self‐propelled feeding tubes in critically ill patients. Intensive Care Med. 2002;28(12):1768‐1774. [DOI] [PubMed] [Google Scholar]
- 22. Chen W, Sun C, Wei R, et al. Establishing decision trees for predicting successful postpyloric nasoenteric tube placement in critically ill patients. JPEN J Parenter Enteral Nutr. 2018;42(1):132‐138. [DOI] [PubMed] [Google Scholar]
- 23. Hu L, Nie Z, Zhang Y, et al. Development and validation of a nomogram for predicting self‐propelled postpyloric placement of spiral nasoenteric tube in the critically ill: mixed retrospective and prospective cohort study. Clin Nutr. 2019;38(6):2799‐2805. [DOI] [PubMed] [Google Scholar]
- 24. Sun C, Lv B, Zheng W, et al. The learning curve in blind bedside postpyloric placement of spiral tubes: data from a multicentre, prospective observational study. J Int Med Res. 2019;47(5):1884‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bateman DN. Clinical pharmacokinetics of metoclopramide. Clin Pharmacokinet. 1983;8(6):523‐529. [DOI] [PubMed] [Google Scholar]
- 26. Wright MR, Axelson JE, Rurak DW, et al. Linearity of metoclopramide kinetics at doses of 5–20 mg. Br J Clin Pharmacol. 1988;26(4):469‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lv B, Hu L, Chen L, et al. Blind bedside postpyloric placement of spiral tube as rescue therapy in critically ill patients: a prospective, tricentric, observational study. Crit Care. 2017;21(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu B, Ouyang X, Lei L, et al. Erythromycin versus metoclopramide for post‐pyloric spiral naso‐enteric tube placement: a randomized non‐inferiority trial. Intensive Care Med. 2018;44(12):2174‐2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiao Y, He Z, Long Y, et al. Simo decoction versus domperidone suspension for post‐pyloric spiral nasoenteric tube placement: a multicenter, randomized, non‐inferiority trial. Clin Nutr. 2020;39(8):2406‐2412. [DOI] [PubMed] [Google Scholar]
- 30. Lai CW, Barlow R, Barnes M, Hawthorne AB. Bedside placement of nasojejunal tubes: a randomised‐controlled trial of spiral‐ versus straight‐ended tubes. Clin Nutr. 2003;22(3):267‐270. [DOI] [PubMed] [Google Scholar]
- 31. Bengmark S. Progress in perioperative enteral tube feeding. Clin Nutr. 1998;17(4):145‐152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


