Abstract
The research describes for the first time a possible case of pituitary gigantism in fossil mammals, precisely in deer. The pathology was detected in 2 long bones (tibia and metatarsus) belonging to an individual of an unusual large size found at the Bate cave (Rethymnon, Northern Crete). It formed the basis of Candiacervus major, the largest among the endemic deer species recorded in the Pleistocene‐Early Holocene of Crete. Radiological and histomorphological examinations highlighted a reduction in cortical bone thickness and the presence of wide lacunae inside of the bone tissue. The pathological conditions suggest a pituitary gigantism diagnosis also supported by some morphological evidence, such as the extremely elongated distal part of the metatarsal diaphysis, the proportionally small proximal epiphysis, and some bone gracility. The diagnosis of a case of pituitary gigantism as presumed responsible for the extraordinary elongation of the tibia and the metatarsal bone is intriguing as they are, respectively, the paratype and the holotype of the C. major. The species represents a case of a deviation from the “island rule” in Pleistocene large mammals. The new evidence recommends a taxonomic and nomenclatural revision of this species. The main outcomes of this research are as follows: (i) a case of pituitary gigantism is described for the first time in an extinct mammal; (ii) it is underlined that paleohistology may provide interesting clues for disentangling taxonomic and nomenclatural issues; (iii) one of the very few cases of gigantism in insular mammals is being questioned.
Keywords: Candiacervus major, Cretan deer, island rule, paleohistology, pituitary gigantism
Morphological, radiological, and histomorphometric analyses conducted on the bones that are the paratype and holotype of Candiacervus major suggest that this giant Cretan deer would have been affected by pituitary gigantism. This implies that:
1) One case of pituitary gigantism is described in an extinct animal for the first time
2) A well‐known exception to the island rule ceases to exist
3) Paleohistology can make a contribution to solving taxonomic problems
INTRODUCTION
During the last couple of decades, studies in the fields of paleohistology and paleopathology have been increasing in relevance given their importance for better understanding the life history, paleoecology, behavior, paleohabitat, traumatology, and diseases in extinct and extant vertebrate species (see among several others, Castanet 2006; Waldron 2008; Canoville & Laurin 2010; Stein 2010; García‐Martínez et al. 2011; Jordana & Köhler 2011; Jordana et al. 2011; Thomas 2012; Marín‐Moratalla et al. 2013; Stein & Werner 2013; Amson et al. 2014; Martínez‐Maza et al. 2014; Kolb et al. 2015a,b; Bartosiewicz 2016, 2018; Lyras et al. 2016, 2019; Palombo & Zedda 2016; Olivier et al. 2017; Veitschegger 2017; Orlandi‐Oliveras et al. 2018; Veitschegger et al. 2018; Zoboli et al. 2018; Labarca & Pacheco 2019; Miszkiewicz et al. 2019; Woolley et al. 2019; de Souza Barbosa et al. 2020; Xing et al. 2020; Zedda et al. 2020). Interesting results about the growth rate and the seasonality of death are also achieved by the histological approach (Castanet et al. 2004; Sander & Andrassy 2006; Köhler & Moyà‐Solà 2009; Köhler et al. 2012; Kolb et al. 2015a).
Several histological studies of long bone tissue in vertebrates deal with the growth dynamics (Waskow et al. 2014; Kolb et al. 2015b; Veitschegger et al. 2018; Lyras et al. 2019). However, no histological analyses have been performed to date to scrutinize whether the particular large size of some extinct mammals may or may not be related to the pituitary gland activity.
This research aims to scrutinize the reliability of 2 alternative hypotheses: (i) the extraordinary large size shown by some bones of an endemic deer (Candiacervus major) found in the Late Pleistocene deposit of the Bate cave (Rethymnon, Crete) depends on an unusual increase in size due to the adaptive radiation process characterizing the evolution of the endemic Cretan deer (de Vos 2000, 2006); the excessive large size depends on pathological conditions. To investigate the consistency of the latter hypothesis, we analyzed the microstructure and the histological features of the bone tissue in the tibia and the metatarsal bone, which are respectively the paratype and the holotype of the Candiacervus major species (Capasso Barbato & Petronio 1986).
Pituitary gigantism
The phenomenon of an excessive and prolonged growth due to chronic overactivity of the pituitary gland is a rare syndrome, sporadically recorded in nature and not described in depth in ancient remains. The cases reported to date in ancient historical time are very few. To date, only 3 human individuals show bones denoting an anomalous growth due to gigantism. One was found in a necropolis of Giza (Egypt), dated back to the Fifth Dynasty (Mulhern 2005; Galassi et al. 2017), the second in an Imperial Roman necropolis (3rd century) near Rome (Italy) (Minozzi et al. 2012, 2015), and the third in a necropolis near to Cordoba (Spain), dated back to the VIII–XII centuries (Viciano et al. 2015).
It is well known (Kronenberg 2003; Rossellò‐Diez & Joyner 2015; Tritos & Klibanski 2016) that under normal physiological conditions, the growth in length of long bones at a young age is due to the stimulation of chondroblast cells in the metaphyseal cartilage by the growth hormone (GH) secreted by the pituitary gland. During the growing period, in case of GH hypersecretion (frequently caused by a benign pituitary adenoma), an over stimulation of the growth plates occurs, leading to an excessive bone lengthening (Mazziotti et al. 2018, 2019). Such endocrine disorder is named pituitary gigantism that produces an excessive increase in stature (Eugster & Pescovitz 1999; Schmidt et al. 2007). If the GH hypersecretion conversely occurs in adulthood, the bones change their shape due to the excessive periosteal apposition of bone tissue. This condition is named acromegaly (Scacchi & Cavagnini 2006; Chanson & Salenave 2008). Sometimes, the GH hypersecretion continues from a young age to adulthood so that the morphological traits due to gigantism and acromegaly are both present (de Herder 2009; Melmed 2009).
Although the macroscopic features of long bones (e.g. marked slenderness and fragility) have been frequently described in humans affected by gigantism (Mazziotti et al. 2018, 2019), less attention has been devoted to the microscopic characteristics of the bone tissue and its modifications. Evidence demonstrated that the GH excess may induce bone resorption resulting in a reduction of trabecular bone volume, trabecular thickness, and leads to bone tissue porosity (Ueland et al. 2002; Ueland 2005; Dalle Carbonare et al. 2018; Mazziotti et al. 2019). These histomorphological alterations are caused by the stimulation of osteoclast activity, leading to bone demineralization and can increase the risk of fractures related to bone fragility (Andreassen & Hoxlund 2001; Kužma et al. 2019). In sum, the characteristic features of long bones affected by this endocrine disorder mainly depend on the imbalance between the overstimulation of growth plates, leading to a progressive increase in bone tissue formation at the level of the metaphyseal zone, and increase in bone resorption in at the level of the epiphyseal and diaphyseal zones. As a result, the long bones are slender, thin, and fragile.
Cretan Late Pleistocene fauna and its deer
During the Late Pleistocene, the island of Crete was inhabited by an ecologically unbalanced mammalian fauna (Mus catreus and Mus minotaurus faunal complexes) dominated by endemic deer and including a dwarf elephant Palaeoloxodon creutzburgi, an otter Lutrogale cretensis, a shrew Crocidura zimmermanni, and a giant mice Mus minotaurus (e.g., Lax 1996; Palombo 2009; Van der Geer et al. 2010 with bibliography).
For decades, issues related to the systematics, taxonomy, and evolutionary patterns of the Cretan deer have been attracting the attention of researches who extensively analyzed also their differences in size, feeding behavior, and locomotion. The Cretan deer phylogenetic relationships remain, however, a matter of major debate (Kotsakis et al. 1976; de Vos 1979, 1996, 2000, 2006; Capasso Barbato & Petronio 1986; Capasso Barbato 1989, 1990, 1992, 1995; Caloi & Palombo 1995, 1996; Van der Geer et al. 2006, 2013; Iliopoulos et al. 2010; Van der Geer 2014, 2018; Altamura 2016).
Some authors, for instance, considered the small Cretan deer as a monophyletic group phylogenetically related to Megacerini (e.g., Sondaar & Boekschoten 1967; Kurtèn 1968; Malatesta 1980) or to a still unidentified ancestor (de Vos 1996, 2000, 2006). Others hypothesized that the Cretan deer originated from 2 dispersal phases (e.g., de Vos 1979, 1984; de Vos & Dermitzakis 1986; Caloi & Palombo 1996). Others (e.g., Capasso Barbato 1990) believed that the Cretan deer is a polyphyletic group, stemming from 2 different continental deer lineages (Praemegaceros and Cervus).
Authors also disagree about the number of deer species present on Crete. de Vos, for instance, firstly distinguished 6 monospecific size‐groups (de Vos 1979), based on the limb bone biometrical data from the localities known at that time. Later de Vos (1984) split the size‐group 2 in 3 species. Consequently, the author considered present on Crete at least 8 taxonomic units (Candiacervus ropalophorus, Candiacervus sp. IIa, IIb, IIc, Candiacervus cretensis, Candiacervus rethymnensis, Candiacervus sp. V, Candiacervus sp. VI), based on the skull morphology and proportion (4 morphotypes of skull, 5 types of antlers, 3 morphotypes of P2, and 3 size groups in the teeth), as well on long bone length.
Conversely, Capasso Barbato (1989, 1990, 1992) recognized on Crete 5 Cervus endemic species and 2 subgenus. She ascribed to the new subgenus Leptocervus the species Cervus (Leptocervus) rethymnensis, Cervus (Leptocervus) dorothensis, and Cervus (Leptocervus) major. Accordingly, the Cretan deer would constitute a polyphyletic group, whose ancestors entered Crete at different times.
To date, the debate is still open even if the majority of researchers consider the Cretan deer as a monophyletic group (including not less than 5 and not more than 8 species, belonging to 6 size‐groups [see below]), stemming from a single continental, still debated ancestor. It probably reached the island by swimming during a Middle Pleistocene glacio‐eustatic low‐sea‐level stand (e.g. de Vos 1996, 2000, 2006; van der Geer et al. 2010, 2013; van der Geer 2018; Palombo 2018; Heckeberg 2020).
When the mainland ancestor of the Cretan deer arrived on the island, many habitats and empty niches were likely available due the absence of other middle‐sized herbivores, deer competitors on the mainland (Palombo 2009, p. 349, Fig. 7). This fact allowed deer to undergo an adaptive radiation, typically documented by the presence of taxa of different size in the same area at the same time (e.g. de Vos 2000, 2006).
Figure 7.
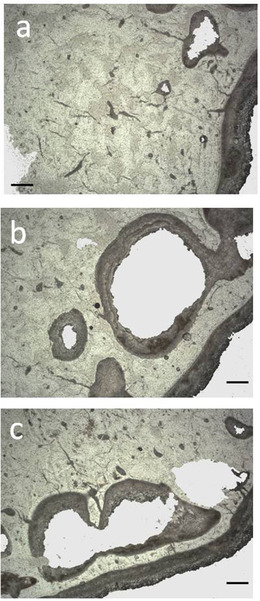
Histological pictures from the diaphysis of the Candiacervus major from Bate cave: tibia (a,b), metatarsus (c). Wide multiple cavities inside of bone wall can be noted in the subperiosteal zone (a,c) and subendosteal zone (b). Bar = 80 μm. (transmitted light microscope; (a) PSC1854; (b) PSC1857; (c) PSC1865).
The genus Candiacervus includes several coexisting species, each showing not only different size but also peculiar cranial, dental, and long bone morphological traits, suggesting they were adapted to different habitats and occupied a diverse set of niches (see e.g. de Vos 1984, 1996; Caloi & Palombo 1995, 1996; Palombo 2009; Altamura 2016; van der Geer 2018 and references therein).
The taxa of small body size (deer of the size group 1 and 2 of de Vos (1979, 1984), recently ascribed to 4 species, Candiacervus ropalophorous de Vos, 1984, Candiacervus devosi van der Geer, 2018, Candiacervus listeri van der Geer, 2018, Candiacervus reumeri van der Geer, 2018 (van der Geer 2018)) are recorded by a plentiful fossil record in several localities, mainly caves, opening along the island northern coastline (Iliopoulos et al. 2010). The 2 moderately larger species (size groups 3, Candiacervus cretensis (Simonelli, 1907), and 4, Candiacervus rethymnensis Kuss, 1975 (de Vos 1979, 1984)) are also present in some localities with a number of specimens. Conversely, only a few remains of the large species Candiacervus dorothensis (Capasso Barbato, 1992) (size groups 5 of de Vos (1979, 1984)) are reported from the Bate cave, while a small number of bones and fragments of the largest deer Candiacervus major (Capasso Barbato & Petronio, 1986) (size groups 6 of de Vos (1979, 1984)) were found at the Bate and Liko cave (Fig. 1). The latter species, which at the Bate cave is represented by a few remains belonging to a single individual, deserves particular interest.
Figure 1.
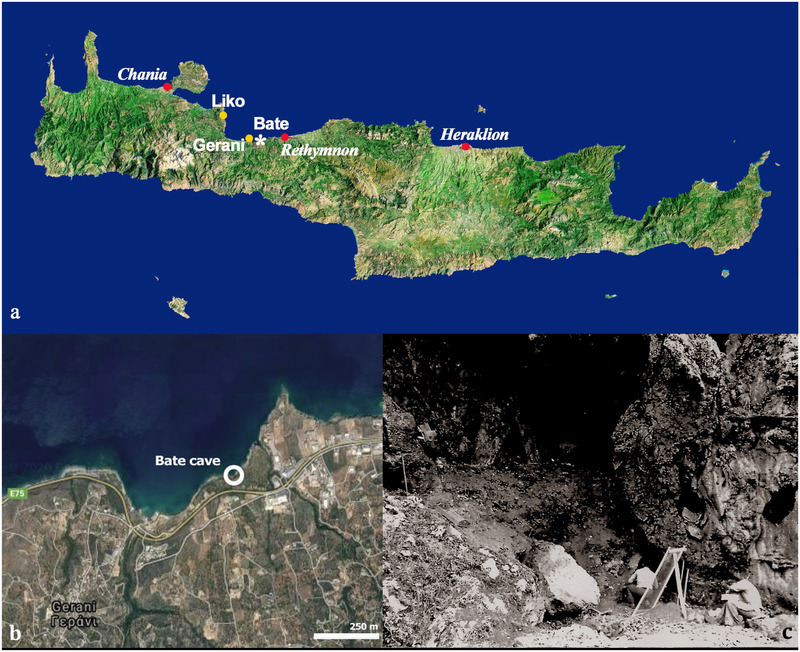
Bate cave (Rethymnon): (a,b) localization of the Bate cave within the isle of Crete; (c) Bate cave in 1975 during a geological survey made by a team of the Sapienza University of Rome (Italy).
Bate cave and its faunal record
The Bate cave, located on the Northern Cretan coast, not far from the Zourida gorge, in the cliffs below the Rethymnon‐Chania National Road (Fig. 1), was discovered in 1975 during a geological survey made by a team of the Sapienza University of Rome (Italy) led by Alberto Malatesta (Kotsakis et al. 1976). The fossil assemblage of the Bate cave belongs to the youngest Pleistocene Cretan fauna complex, the so‐called Mus minotaurus “sub‐zone” (Mayhew 1977). The age of the fossiliferous deposit is not firmly established because of the inconsistency of data obtained by different authors/methodologies (see Lax 1996). Belluomini and Delitala (1983, 1988) indicated an age from 63 500 ± 20% to 69 000 ± 20% years (Amino Acid Racemization, AAR, dating on deer bones). Reese et al. (1996) warned that AAR dating done on bones are “very much less reliable than tooth enamel.” The authors’ AAR dating on deer teeth are indeed significantly older: 152 000 ± 20% to 105 000 ± 20%, suggesting a late Middle to early Late Pleistocene age for the deposits of the Bate cave (Rees et al. 1996).
The vertebrate fauna retrieved from poorly stratified fossiliferous layers filling the Bate cave includes, among others, a rich sample of deer, belonging to 5 size‐groups (size‐group 1, 2, 3, 5, and 6 of de Vos 1979). The record of the newly created species Candiacervus major (size‐group 6) counts few bones (6 vertebrae, including the epistropheus, and the third and fourth cervical vertebrae, 2 incomplete humeri, 1 radius‐ulna, 3 pelvis fragments, 1 incomplete femur, 1 complete tibia, 1 complete metatarsus and 2 fragments, 1 phalanx). The complete/nearly complete bones likely belonging to a single individual as suggested by their dimensions (cfr. Capasso Barbato & Petronio 1986). According to the diagnosis provided by Capasso Barbato and Petronio (1986), the species is characterized by very long and slender limb bones.
MATERIALS AND METHODS
Materials
The Candiacervus major tibia (specimen number 28 in Capasso Barbato & Petronio 1986) and metatarsal bone (specimen number 30 in Capasso Barbato & Petronio 1986) herein studied are part of the rich fossil sample collected in the 70s at the Bate cave and currently stored in the University Museum of Earth Science (MUST, previously Museum of Paleontology), Department of Earth Sciences of the Sapienza University (Rome, Italy).
We compared the dimensions and proportions of the C. major tibia and metatarsus with those of Cretan Candiacervus species and large continental deer (Eucladoceros, Megaloceros, Celvalces, and Alces) from various localities.
The histomorphological features shown by the bones of this species have been compared with those of a left metatarsus (MPUR/V coll. Bate, n 16b in Capasso Barbato 1989) belonging to the group of the smallest deer found in the cave. The left metatarsal bone could be identified as C. ropalophorus (and so called here from now on), though the hypothesis that it could represent an individual belonging to one or another of the species of size‐group 2 (Candiacervus sp. II, de Vos 1979, 1984) cannot be excluded.
Methods
Bone measurements were taken using a digital caliper accurate to 0.01 mm. Multivariate statistical analysis have been performed by means of PAST software (Hammer et al. 2001).
The bones were submitted to radiological analysis. The digital radiographic images of the tibia and metatarsus were captured exposing the bones at a distance of 120 cm from the X‐ray tube programmed with 100 kV, 10 mA, 5 s of time. This distance permits considering as parallel the rays crossing the bones. The radiological images corresponding to the central part of the shaft enable us to measure the dimension of the medullary cavity and the thickness of the cortical bone (Croker et al. 2016). The parameter R/t (the ratio between the radius of the whole bone section and the cortical thickness) has been calculated in a tibia and metatarsus of C. major and Eucladoceros giulii from Untersmassfeld (Germany) (tibia num. IQW 1980/16 501 (Mei. 16 022) metatarsus num. IQW 1980/16 500 (Mei. 16 021); Senckenberg Research Station of Quaternary Palaeontology Weimar, collection) following Currey and Alexander (1985). The obtained parameters have been compared to the Cervidae R/t parameters available in literature (Laurin et al. 2011; Amson & Kolb 2016).
The radiotransparency of the bone tissue was estimated by subjecting to a colorimetric analysis a square of 3 mm per side of the diaphysis radiological images, using the Scion image software (Scion Corporation, Frederick, MD, USA). The reference values were 0 (white, corresponding to maximum radiopacity) and 255 (black, corresponding to maximum radiotransparency) (Mura et al. 2013). A metatarsus of an extant Cervus elaphus was used as reference comparison (mean value 78).
A sample approximately 0.5 cm thick was removed from the tibia and metatarsal bone at the level of the diaphysis by means of a steel cutting disk mounted in a mini‐portable electrical screwdriver (Bosch). The small bone blocks were embedded with a bicomponent epoxy resin (Araldite 2020, Huntsman, Basel, Switzerland), using a degassing vacuum chamber (Ablaze1, Ablaze, Hong Kong) to avoid bubbles. It was cut and ground by means of a thin section cutting and grinding machine (Geoform102, Metkon Instruments, Bursa, Turkey) and an abrasive cutting machine (Metacut302, Metkon Instruments) by using a diamond disk (Dimos Ø 250, Metkon Instruments). Sections with final thickness of 80 μm were mounted onto glass slides with Eukitt (Merck, Darmstadt, Germany) and covered. Sections were observed and photographed using a Zeiss Axiophot microscope at 2.5×, 10×, and 20× magnifications under transmitted light and polarized light. Images were digitalized and the measurements have been taken using the Scion Image software. The bone tissue types have been described following the classification proposed by Francillon‐Vieillot et al. (1990). The same protocol and histomorphometric procedures were followed in order to compare morphological and histomorphological features of the C. ropalophorus metatarsal bone (Palombo & Zedda 2016).
The analyzed samples and the slides are deposited in the paleontological slide collection (PSC) at the Section of Anatomy of the Department of Veterinary Medicine of the University of Sassari (Italy) and are available for repeatability. The slide catalog numbers are the following: C. major tibia from PSC1844 to PSC1859, metatarsus from PSC1860 to PSC1868; C. ropalophorus metatarsus from PSC1869 to PSC1875.
RESULTS
Main morphological features and biometrical peculiarities
The tibia and metatarsus of C. major belong to an adult individual. The epiphyses and the diaphysis are fully fused as a single ossified structure, so that the growth plate cartilage (or “metaphysis”) is completely ossified (see Calderòn et al. 2019 as regards to the fusion time in deer).
The main features of both long bones are their remarkable length and the extreme gracility of the medio‐distal part of the tibia and the medio‐proximal part of the metatarsus. In particular, the metatarsus has a narrow proximal epiphysis, a massive and progressively enlarged distal portion of the diaphysis (with a high positioned foramen), and a very short distal epiphysis (Fig. 2, Table 1) (for further details refer to Capasso Barbato & Petronio 1986, comparison tables, pp. 72–73, 76–77). The C. major metatarsal length is about 334%, 293%, 225%, 168%, and 134% than those of, respectively, C. ropalophoros, Candiacervus spp.II, C. cretensis, C. rethymnensis, and C. dorothensis (Table 2). The estimation of the shoulder height, based on metapodial length (Godynicky 1965), confirms high stature of C. major (Table 3), which was about 3.4, 2.9, 2.6, 1.8 and 1.3 times taller than C. ropalophoros, Candiacervus spp.II, C. cretensis, C. rethymnensis, and C. dorothensis (size‐group 5), respectively.
Figure 2.
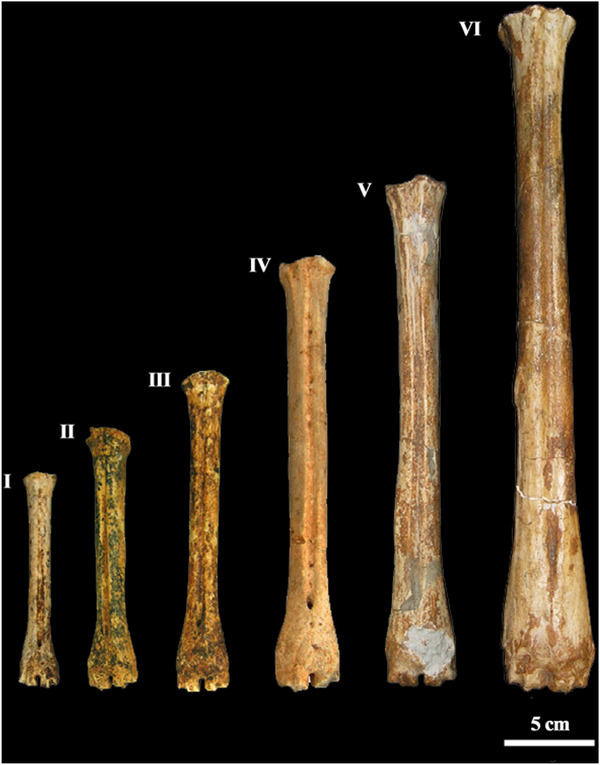
Metapodial bones of the Cretan endemic deer numbered (from the left to the right) according to the size‐groups defined by de Vos (1979, 1984): Candiacervus ropalophorous de Vos 1984 (=size‐group I), Candiacervus spp. II (=size‐group II), Candiacervus cretensis (Simonelli 1907) (=size‐group III), Candiacervus rethymnensis Kuss 1975 (=size‐group IV), Candiacervus dorothensis (=size‐group V) (Capasso Barbato 1992), and the metatarsus from Bate cave, holotype of the species Candiacervus “major” (=size‐group VI) (Capasso Barbato & Petronio 1986).
Table 1.
Measurements of tibia and metatarsus of Candiacervus major
| Tibia | Metatarsus | ||
|---|---|---|---|
| Measurements (mm) | Bate cave | Bate cave | Liko † |
| Total length | 483.6 | 406.2 | — |
| Mediolateral width of the proximal epiphysis | 80.2 | 45.9 | — |
| Antero‐posterior depth of the proximal epiphysis | 79.8 | 46.5 | — |
| Mediolateral width of the diaphysis | 28.5 | 27.1 | — |
| Mediolateral width of the distal diaphysis portion | 50.3 | — | — |
| Mediolateral width of the distal epiphysis | 48.8 | 46.2 | 50.6 |
| Antero‐posterior depth of the distal epiphysis | 30.7 | 31.9 | 33.6 |
† Data from de Vos (1979).
Table 2.
Comparison among the length of the tibia and metatarsus of all Cretan Candiacervus species
| Tibia | Metatarsus | |||||
|---|---|---|---|---|---|---|
| Taxa | Min–Max | M | N | Min–Max | M | N |
| Candiacervus major | 483.6 | 483.6 | 1 | 406.2 | 406.2 | 1 |
| Candiacervus from Gerani IV † (size‐group 1) | 145.3–175.5 | 161.5 | 68 | 110.0–131.0 | 121.64 | 66 |
| Candiacervus from Liko † (size‐group 2) | 169.9–205.2 | 186.5 | 21 | 123.2–152.5 | 138.4 | 45 |
| Candiacervus size‐group 3 †∧ | — | — | — | 166.0–180.0 | 174.0 | 3 |
| Candiacervus size‐group 4 † | — | — | — | 217.5–241.8 | 229.65 | 2 |
| Candiacervus cretensis ∧ | 248.0 | 248.0 | 1 | — | — | — |
| Candiacervus cretensis § | — | — | — | 110.0–145.0 | — | — |
| Candiacervus cretensis° | — | — | — | 123.0–132.0 | 128.4 | 5 |
| Candiacervus size‐group 4 † | 313.6–314.9 | 314.25 | 2 | 217.5–241.8 | 229.65 | 2 |
| Candiacervus ropalophorus/Candiacervus spp.II ∧ | 148.3–201.0 | 171.42 | 75 | 99.0–148.5 | 127.54 | 128 |
| (Candiacervus ropalophorus) ∧∧ | 148.3–169.0 | 163.4 | 33 | 99.0–123.8 | 114.70 | 32 |
| (Candiacervus ropalophorus vel C. spp.II) ∧∧ | — | — | — | 124.0–131.0 | 129.51 | 55 |
| (Candiacervus spp.II) ∧∧ | 170.0–201.0 | 177.72 | 42 | 131.5–148.5 | 138.29 | 42 |
| Candiacervus dorothensis ∧ | 370.0 | 370.0 | 1 | 301.0–301.4 | 301.2 | 2 |
Measurements (in mm) from: †de Vos (1979), §Kuss (1975), °Malatesta (1980), ∧Capasso Barbato (1989) (= C. ropalophorus), ∧∧tentative separation of the specimens identified as Candiacervus ropalophorus by Capasso Barbato (1995) in 2 groups (C. ropalophorus = size‐group 1 and Candiacervus spp.II = size‐group 2) according to the dimensional range given by de Vos (1979) for metatarsal bones. M, mean value; N, number of bones.
Table 3.
Comparison among the shoulder height of all Cretan deer of the genus Candiacervus on the basis of the index of Godynicky (1965)
| Shoulder height | |||
|---|---|---|---|
| Taxa | Min–Max | M | N |
| Candiacervus major | — | 1848 | 1 |
| Candiacervus size‐group 1† | 501–596 | 553 | 66 |
| Candiacervus size‐group 2† | 561–594 | 630 | 45 |
| Candiacervus size‐group 3†∧ | 755–819 | 792 | 3 |
| Candiacervus size‐group 4† | 990–1100 | 1045 | 2 |
| Candiacervus cretensis § | 501–660 | — | — |
| Candiacervus cretensis° | 560–601 | 584 | 5 |
| Candiacervus ropalophorus/Candiacervus spp. II∧ | 450–676 | 580 | 128 |
| (?Candiacervus ropalophorus)∧∧ | 450–589 | 552 | 70 |
| (?Candiacervus spp. II)∧∧ | 592–676 | 614 | 58 |
| Candiacervus dorothensis ∧ | 1370–1371 | 1370 | 2 |
The peculiar proportions of the C. major metatarsus are highlighted by the results of Principal Component Analysis (PCA) (Fig. 3). In the PCA graph, the metatarsus sets apart because the proximal and distal epiphysis are, respectively, mediolaterally narrower and larger than in other mainland and continental deer, even similar in size to C. major.
Figure 3.
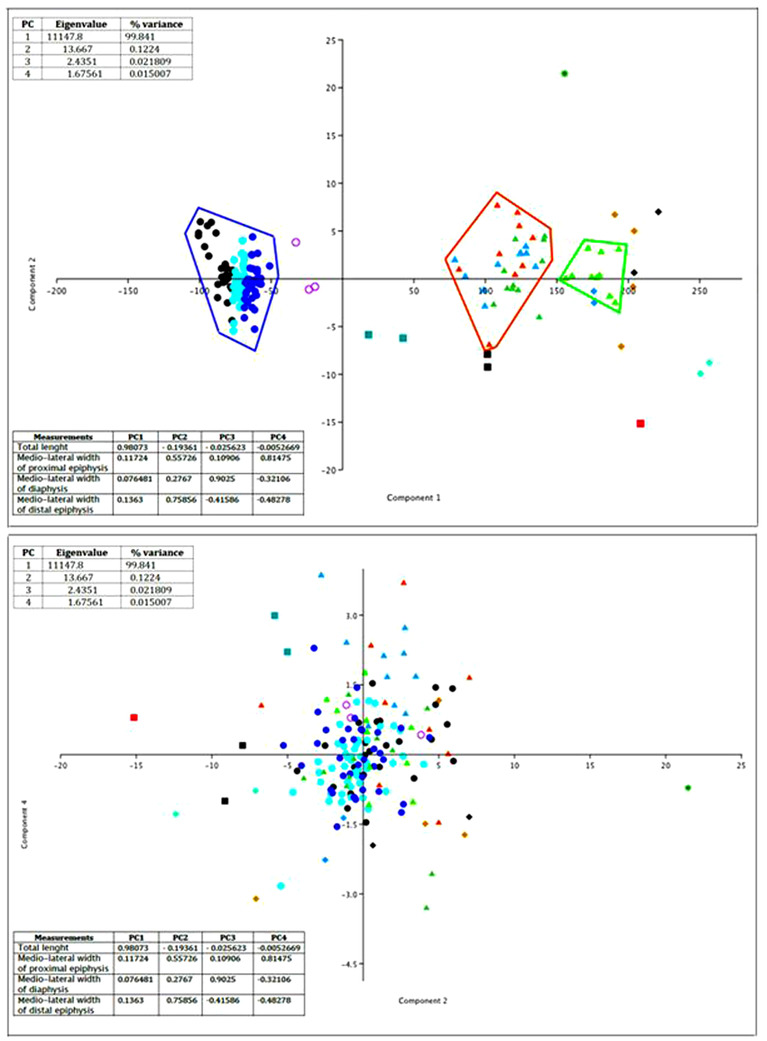
Diagrams resulting from the principal components analysis (PCA) computed by using as variables the total length and the mediolateral width at the proximal and distal epiphysis and diaphysis of metatarsal bones of Cretan deer and slender large continental deer (cases). Above: PCA graph based on the first component, in which the total length is the most loading variable, and the second component, in which the mediolateral width of the proximal epiphysis is the most loading variable. Below: PCA graph based on the second component and the fourth component, in which the mediolateral width of the distal epiphysis is the most loading variable. Symbols— Candiacervus : Black dots, C. ropalophorus from Bate cave (1); blue dots, C. spp.2 Bate (1); aqua dots, C. ropalophorus/C. spp.2 from Bate; black filled squares, C. dorothensis Bate (1); red filled square, C. major from Bate cave; violet circles, C. cretensis from Liko (2); dark cyan filled squares, C. rethymnensis from Liko and Mavro Mouri (2). Eucladoceros : Light green filled triangles, E. giuli from Untermassfeld (Germany) (3); light brown filled triangles, E. dicranios from Olivola (Italy); orange filled triangles, E. dicranios vel E. ctenoides from Vardarno (Italy); dark green filled triangles, E. ctenoides ( = E. senezensis vireti) from Saint Vallier (France) (6); doger blue filled triangles, E. ctenoides ( = E. senezensis senezensis) from Senèze France (6). Megaceros : dark green star, M. giganteus from Villereversure (France) (6). Cervalces : azur filled diamonds, Cervalces gallicus from Senèze (France) (6); aquamarine filled diamonds, Cervalces latifrons from Tiraspol (Moldova) and Tunguska river (Siberia) (5). Alces alces: black filled diamonds, A. alces from North America (Alaska, Isle Royale, and other mainland US countries) (average value) (4); light brown, A. alces from Norge (6). (1) Capasso Barbato (1989); (2) Kuss (1975); (3) Kahlke (1997); (4) A. Valli unpublished; (5) Sher (1971); (6) Silva et al. (2014).
The slenderness index (SI, the ratio percentage of the minimum breadth, measured near the middle of the shaft and the maximum length), of the tibia (SIt = 0.066) and the metatarsal bone (SIm = 0.073), denotes the gracility of the diaphysis in C. major metatarsus with respect to other Cretan deer from Bate cave (C. ropalophorous, C. ropalophorous vel C. spp.2, Candiacervus spp. II, C. dorothensis), C. cretensis from Liko, C. rethymnensis from Liko and Mavro Mouri, and slender large continental deer such as Epivillafranchian Eucladoceros giulii from Untermassfeld (Germany), Villafranchian Eucladoceros dicranios vel E. ctenoides from Italy, Villafranchian Eucladoceros ctenoides (=E. senezensis) from France, Cervalces gallicus from Senèze (SIm = 0.082, 0.085), Cervalces latifrons from Moldova and Siberia), and extant Alces alces from Norge and North America) (Table 4; source of data and references as in Fig. 3).
Table 4.
Comparison among the slenderness index (the ratio percentage of the minimum breadth, measured near the middle of the shaft and the maximum length) in the metatarsal bones of Candiacervus major, the other Cretan Candiacervus species, and extinct and extant continental large deer (data source and references as in Fig. 3)
| Metatarsus | |
|---|---|
| Taxa | Slenderness index (SIm) |
| Candiacervus major (Bate cave) | 0.073 |
| Candiacervus ropalophorous (Bate cave) | 0.086–0.141; M 0.134 |
| Candiacervus ropalophorous vel C. spp. II (Bate cave) | 0.084–0.119; M 0.101 |
| Candiacervus spp. II (Bate cave) | 0.083–0.124; M 0.101 |
| Candiacervus cretensis (Liko cave) | 0.097–0.104; M 0.098 |
| Candiacervus rethymnensis (Liko cave) | 0.084 |
| Candiacervus dorothensis (Bate cave) | 0.079, 0.078 |
| Eucladoceros dicranios vel E. ctenoides (Italy) | 0.078–0.104; M 0.092 |
| Eucladoceros ctenoides (France) | 0.078–0.11; M 0.090 |
| Eucladoceros giulii (Untermassfeld, Germany) | 0.079–0.089; M 0.083 |
| Cervalces gallicus (Senèze, France) | 0.082, 0.085 |
| Cervalces latifrons (Moldova) | 0.1 |
| Cervalces latifrons (Siberia, Russia) | 0.084 |
| Alces alces (Norge) | 0.079–0.089; M 0.084 |
| Alces alces (North America) | 0.080–0.085, M 0.083 |
Radiology
The radiological analysis of the tibia and metatarsus of C. major from the Bate cave confirms the achievement of adulthood due to the absence of the metaphyseal lines and shows a remarkable enlargement of the medullary cavity due to the thickness reduction of the cortical bone along the whole diaphysis (Fig. 4). The cortical bone tissue at level of mid‐diaphysis (measured in the mediolateral radiographic view) is 4.7 mm thick in the tibia and 4.5 mm thick in the metatarsus. At the same level, the diameter of the medullary cavity is 19.1 mm in the tibia and 18.1 mm in the metatarsus, with R/t of 3.10 and 3.04, respectively (Table 5). The diaphyseal bone tissue is proportionally thicker (3.9 mm; R/t = 1.67) in the metatarsus of C. ropalophorus (Fig. 5).
Figure 4.
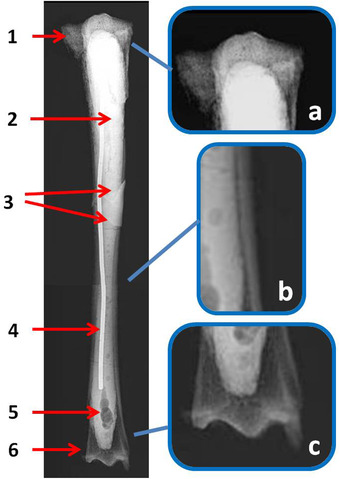
Candiacervus major, Bate cave (Rethymnon), tibia (specimen number 28 in Capasso Barbato & Petronio 1986). Radiological analysis in craniocaudal view. Wide bone reabsorption area in the proximal (1) and distal (6) epiphyses. The entire medullary cavity has been filled with a sealing material (2) showing air bubbles (5) and incorporating a metal pin (4) in order to strengthen the bone. This suffered a post mortem fracture whose breaking lines can be seen (3). On the right, some enlarged details of the epiphyses (a, c) and diaphysis wall (b) show the rarefied status of the bone tissue.
Table 5.
Comparison between morphometrical data of the tibia and metatarsus of Candiacervus major and Eucladoceros giulii
| Mediolateral width of the diaphysis | Radius of the whole section (R) | Medullary cavity width | Cortical bone thickness (t) | R/t | ||
|---|---|---|---|---|---|---|
| Candiacervus major | Tibia | 28.5 | 14.25 | 19.10 | 4.7 | 3.10 |
| Metatarsus | 27.1 | 13.55 | 18.10 | 4.5 | 3.04 | |
| Eucladoceros giulii | Tibia | 40.6 | 20.3 | 20.1 | 10.25 | 1.98 |
| Metatarsus | 29.2 | 14.6 | 15.1 | 7.05 | 2.07 | |
The measurements of C. major are taken from the mediolateral view of the radiological images at level of the central part of the shaft and those of E. giulii from Fig. 10.
Figure 5.
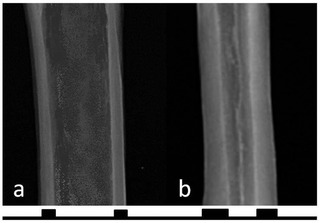
Radiological comparison of the central part of the metatarsal diaphysis between Candiacervus major (a) and Candiacervus ropalophorus (b). A reduction in cortical bone thickness in the diaphysis leads to a remarkable enlargement of the medullary cavity in C. major.
The radiotransparency of the diaphyseal and epiphyseal bone tissue is higher than in normal bone tissue, indicating a widespread bone porosity in both bones.
The radiotransparency values were 191 in the tibia epiphyses (mean), 185 in the tibia diaphysis, 188 in the metacarpus epiphyses (mean), and 179 in the metacarpus diaphysis. The high radiotransparency of C. major bones is confirmed by the mean value (184) that is more than twice the reference value of C. elaphus.
The radiological examination enables us to reconstruct some events that occurred to the tibia after its finding. Due to the bone fragility, the tibia broke in 2 pieces during the excavation and was restored. During the restoration works, not affecting the cortical compact tissue, the spongy tissue was partially removed. The cavity was filled with mastic and the bone was reinforced by introducing an iron cylinder in the medullary cavity (Fig. 4).
Histomorphology
Light microscope observations reveal the presence of the plexiform, irregular Haversian tissue, and sometimes dense Haversian bone tissue. In the osteons, the external cement line is not always identifiable, but the position of the osteocyte lacunae, circularly arranged, indicates the presence of the bone lamellae surrounding the Haversian canal (Fig. 6). The use of a polarized light microscope confirmed this arrangement.
Figure 6.
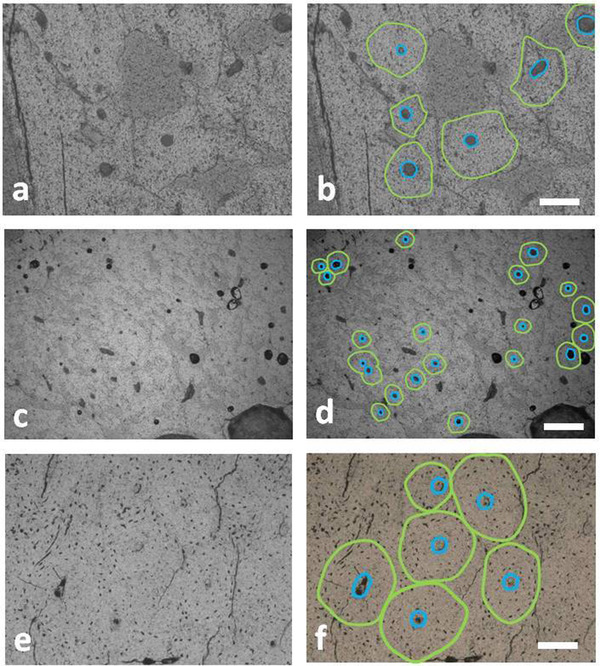
Secondary osteons in the diaphyseal bone of the tibia (a,b) and metatarsal bone (c,d) of Candiacervus major and metatarsal bone of Candiacervus ropalophorus (e,f). The green line identifies the bone lamella delimiting the osteon area and the blue line indicates the perimeter of the Haversian canal. Bar = 80 μm. (transmitted light microscope; (a) PSC 1848; (c) PSC1867; (e) PSC1873).
The mean diameter of the C. major osteons (obtained by measuring 43 secondary osteons) is 64 μm and that of Haversian canals is 18 μm. The osteons are almost circular as indicated by their very low eccentricity (e = 0.16). The tibial osteon size and their Haversian canals are 25% bigger than that of metatarsal ones. In the metatarsal bone of C. ropalophorus, the size of secondary osteons (n = 18) are 13% bigger (mean diameter 72 μm) than those of C. major, whereas the size of the Haversian canals is nearly the same. The secondary osteons of C. ropalophorus are moderately elliptical (e = 0.43).
One of the main peculiarities of the bone tissue of C. major is related to the presence of wide cavities inside of diaphyseal bone wall in both the tibia and metatarsus (Fig. 7). The empty spaces in the bone tissue are elongated parallelly to the periosteal or endosteal surfaces and seem often interconnected to each other. Their size can reach to a few hundred microns. A thin layer of dark, concreted sediment, sometimes more than 70 μm thick, covers the surface of these cavities. Sometimes, the dark sediment creeps into the cracks present in the bone wall. These wide cavities make the compact diaphyseal bone very porous and irregular. The area occupied by these empty spaces is about 18% of the diaphyseal bone wall. The bone tissue of the C. ropalophorus lacks cavities and looks compact and homogeneous.
Skeletochronology and growth marks study
In the outer zone of the diaphyseal bone wall of C. major tibia and metatarsus, a few dark lines parallel to the periosteal surface are detectable somewhere in the histological slides. Following some studies dealing with the significance of these lines (Castanet et al. 2004; Köhler et al. 2012; Kolb 2016; Nacarino‐Meneses et al. 2016a,b; Calderòn et al. 2019), the lines could be regarded as lines of arrested growth (LAGs), which are often associated with ectothermic animals, but also present in large mammals such as some deer (Woodward Ballard et al. 2013). Their analysis permits inferring not only the approximate age of death but also the growth rate during the life of the analyzed individual. Each line corresponds to a period in which a low periosteal activity occurs, while the bone tissue between 2 lines, named growth zone, corresponds to the period with high activity. In the C. major tibia and metatarsus, at least 5 LAGs can be detected (Fig. 8), suggesting that the deer might be about 6 years old at death. In the tibia, the first 3 growth zones (from the deepest part to the periosteal surface) measure about 40 μm, while the last 2 growth zones only 15 μm. The estimated growth rate (i.e. the bone tissue formed each day in the growth period, Kolb 2016) is 0.16 μm/d during the 2nd, 3rd, and 4th year of life—at the time that the first 3 growth zones formed—and of 0.05 μm/d during the 5th and 6th year of life, when the last 2 growth zones formed. The metatarsus of C. major shows the same skeletochronology pattern. These data further confirm that the C. major bones found at the Bate cave belong to a single individual.
Figure 8.
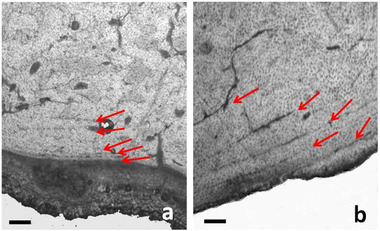
Lines of arrested growth (LAGs) in the tibia of Candiacervus major (a) and metatarsus of Candiacervus ropalophorus (b). The arrows indicate the last 5 subperiosteal lines suggesting an age of death more than 6 years. For skeletochronology considerations, see the text. Bar = 80 μm. (transmitted light microscope; (a) PSC1854; (b) PSC1871).
Conversely, the growth zones of the C. ropalophorus metatarsus (Fig. 8), the first that is 150 μm thick, while the other show the constant thickness of 70 μm. The estimated growth rate is 0.6 μm/d during the 2nd year of life (corresponding to the first growth zone) and 0.3 μm/d from the 3rd year of life onward. The absence of metaphyseal lines between the epiphyses and the diaphysis confirms that the individual was adult.
DISCUSSION
The diagnosis of pituitary gigantism: histomorphological and biomechanical considerations
The results obtained from the macro–microscopical investigations of the paratype and holotype of the Candiacervus major species on the one hand show the presence of the plexiform, irregular Haversian, and dense Haversian bone tissue matching the typical bone histology of extant (Ruddle 1997; Gomez et al. 2013) and extinct deer species (Amson et al. 2015; Kolb et al. 2015b; Lyras et al. 2019). On the other hand, various anomalous peculiarities suggest that the Bate giant individual was affected by a generalized bone disorder. The main features highlighting this disorder are listed, discussed, and referenced in Table 6. The sum of evidence suggests a case of pituitary gigantism as the possible cause of the high stature and the peculiarity of long bone of C. major. We are aware that each single datum is not per se a proof, but all the results obtained together support this diagnosis as the most plausible. The main clinical evidence of a pituitary gigantism is the lengthening of long bones, associated with a reduction of the thickness of the diaphyseal bone wall (Mazziotti et al. 2018, 2019). The bone slenderness was the result of a fast, anomalous growing in length of the diaphysis due to the hyperstimulation of the growth plates located between each epiphysis and the diaphysis. Indeed, pituitary gigantism depends on GH excess that occurs during a young age when epiphyseal plates can excessively grow in length. This hormonal disorder can coexist during life with acromegaly, a phenomenon due to the same cause but which occurs in adulthood (Eugster & Pescovitz 1999; de Herder 2009; Melmed 2009). Moreover, the thinness of the diaphyseal bone wall in the long bones of the giant Cretan deer evidences a pathological condition, strengthening the diagnosis of a pituitary gigantism. Conversely, the diaphyseal bone walls of the small‐ and middle‐sized representatives of the genus Candiacervus are the thickest among those of the representatives of 24 genus of extinct and extant Cervidae (Amson & Kolb 2016). Amson and Kolb (2016) explained the peculiar thickness of the diaphyseal bone wall of the humerus, radius, and tibia of C. ropalophorus and Candiacervus spp. II as due to the phylogenetic affinity of these species with the giant deer of the Megacerini tribe (Megaloceros and Sinomegaceros sensu Vislobokova 2013). The value of R/t more than 3.0 both in tibia and in metatarsus of C. major is higher than those reported by Amson and Kolb (2016) ranging in the Cervidae the tibia from 1.5 R/t (Puda puda) to 2.9 (Alces alces), definitely higher than the R/t value of C. ropalophorus (1.53) and in Candiacervus spp. II (1.64), and higher even than the R/t in the tibia (1.98) metatarsus (2.07) of E. giulii, one of the continental deer more similar in size to C. major (Fig. 9 and Table 5).
Table 6.
List of evidences at different observation levels supporting the diagnosis of pituitary gigantism
| Observation level | List of evidences | Description | References |
|---|---|---|---|
| External morphology and morphometry | Extremely length of long bones | This is the main evidence suggesting the diagnosis of pituitary gigantism. It is due to an overstimulation of the chondroblasts present in the growth plates of long bones. Such condition is a typical effect of a great presence of growth hormone (GH) | Kronenberg (2003); Rossellò‐Diez & Joyner (2015); Tritos & Klibanski (2016) |
| Slenderness index (SI = 0.066 in tibia and 0.074 in metatarsal bone) | The length of long bones is very disproportionate with respect to the transverse diameter of diaphysis | de Herder (2009); Melmed (2009) | |
| Articular surfaces very short | The size of the epiphyses of long bones do not follow the development of the diaphysis, so that the whole long bone appears disproportionate | Killinger et al. (2012) | |
| Bone fragility and fracture predisposition | The risk of fractures is very increased because the bones lose robustness and become fragile. The causes of this condition are attributable to an excess of GH that induces bone demineralization and bone reabsorption | Andreassen & Hoxlund (2001); Kužma et al. (2019) | |
| Radiology | Extreme thinning of the diaphyseal bone wall (and consequently medullary cavity enlargement) | The thickness of the diaphyseal bone wall is the result of a process of enlargement of the medullary cavity due to reabsorption of subendosteal bone and a process of subperiosteal bone apposition. During the life, in physiological condition, these 2 processes are balanced and tend to gradually thicken the wall. In case of excess of GH, the enlargement of the medullary cavity prevails over the subperiosteal bone apposition, as confirmed by the growth zones delimited by LAGs | Eugster & Pescovitz (1999); Schmidt et al. (2007); Mazziotti et al. (2018, 2019) |
| Bone radiotransparency | One of the effects of the GH on bones is a process of reabsorption of bone tissue stimulating the activity of osteoclasts. In case of overactivity of GH, as in case of pituitary gigantism, this process can be vigorous leading to a lowering of radiopacity typical of bone tissue (radiotransparency), and formation of bone pores within the compact bone tissue (bone porosity) which can give rise to large cavities hundreds of μm long | Ueland et al. (2002); Ueland (2005); Marotti et al. (2004); Dalle Carbonare et al. (2018) | |
| Bone porosity | |||
| Histology | Presence of wide cavities in diaphyseal bone wall | ||
| Secondary osteons almost circular | Such information suggests that the bone tissue is subjected almost all to compression loads and not to mechanical stress in craniocaudal or mediolateral direction. It seems that the limb long bones were engaged in supporting the body weight than in participating in fast gaits | Zedda et al. (2015, 2019, 2020) | |
| Restricted growth zone between LAGs | This suggests an unbalance between the elongation of long bones and enlargement of their diaphysis, in favor of the former. This is the histological base of the bone slenderness | ||
| Other indirect supports | Absence of carnivores predators | This condition allows the survival of individuals affected by pathologies restricting movements | Palombo & Zedda (2016); Lyras et al. (2019) |
| Unique individual | The number of LAGs is the same in the tibia and metatarsal bone suggesting that both bones belonged to a 6‐ to 7‐year‐ old deer |
Figure 9.

Tibia and metatarsus of Eucladoceros giulii from Untermassfeld (Germany): natural transversal section at about the mid‐point of the diaphysis (above); distal portions in frontal view (below). (a,b) Tibia dex. IQW 1980/16 501 (Mei. 16 022); (c,d) metatarsus dex. IQW 1980/16 500 (Mei. 16 021), barescale = 3 cm. Images credit Prof. Ralf‐Dietrich Kahlke (Senckenberg Research Station of Quaternary Palaeontology Weimar, Germany).
The thickness of the cortical diaphyseal bone is a strong genetic character so that it has been used as phylogenetic signal for evolutionary studies (Cubo et al. 2005; de Ricqlès et al. 2016; Mitchell et al. 2017). Therefore, the peculiar R/t value of C. major further supports the hypothesis of a pathological status of the Bate cave individual.
The bone reabsorption due to the overactivity of osteoclasts is another typical signal of pituitary gigantism (Andreassen & Hoxlund 2001). The reabsorption induces bone porosity in the epiphyses and the diaphysis causing the radiotransparency observed during radiological examination (Ueland et al. 2002; Ueland 2005; Dalle Carbonare et al. 2018). The bone radiotransparency of C. major (mean value 184) quantified by the colorimetric analysis is more than twice higher than that observed in the healthy bone tissue of C. elaphus (mean value 78).
The pathological process of bone remodeling and reabsorption causing radiotransparency was vigorous in the bone limbs of the giant Cretan deer, as documented by the presence of wide cavities in the diaphyseal bone. The cavities are very wide, reaching some hundreds of μm, and are definitely wider than the cavities (wide few tens of μm) present in herbivores during stress periods of their life such as pregnancy, lactation, or antler formation (Hillier & Bell 2007; Amson et al. 2015). Abnormal cavities inside the cortical diaphyseal bone, caused by an active osteoclastic bone resorption similar to those observed in C. major, have been described in a human metatarsus affected by segmental gigantism of upper and lower limbs (Marotti et al. 2004, p. 415, Fig. 4).
It might be speculated that the peculiar conformation of long bones may have made the large deer found at the Bate cave somehow vulnerable, affecting its life. It is challenging to say whether or not the propensity to fractures of long bones limited its movements to a fairly restricted range, hampering the chance to long distance movements de facto, potentially reducing the animal home range. However, the LAGs are very close to each other, indicating an extremely reduced subperiosteal bone formation activity. This means that the growth of the bones occurred almost all in length rather than in width. The growth rate of the subperiosteal diaphyseal bone in the giant deer (0.16 μm/d in the first 4 years of life and only 0.05 μm/d in the last 2 years before death) is lower than that estimated for C. ropalophorus by Kolb et al. (2015a), and in this study. Furthermore, since osteons showing an elliptical shape have been supposed typical of animal species with pronounced locomotor activity (e.g. mouflon compared to sheep (Giua et al. 2014), goat compared to sheep (Zedda et al. 2017), wild boar compared to domestic pigs (Zedda et al. 2019), and wild horse compared to domestic horse (Zedda et al. 2020)), the almost circular shape of the Bate individual osteons could suggest poor performance during fast and agile movements (Zedda et al. 2008, 2015; Zedda & Babosova 2021; Skedros et al. 2013), as already inferred by the morphological features shown by the autopodium bones (Caloi & Palombo 1995, 1996).
The island rule
Following the observations on extant insular mammals by Foster (1964), the island rule describes the pattern of change in body size in insular vertebrates, predicting that large species tend to reduce their size while small species tend to increase their size (Van Valen 1973; Lomolino 1985, 2005; Lister 1989; Whittaker & Fernández‐Palacios 2007; Lokatis & Jeschke 2018). The generality of the island rule has been however questioned by various authors (see e.g. among others Meiri et al. 2004, 2006, 2008; Itescu et al. 2014). Differences in body size among the mainland ancestors and insular taxa, island geological history, time of isolation and insular ecosystem functioning may influence the speed and magnitude of body size changes (see e.g., Lomolino et al. 2012, 2013; Faurby & Svenning 2016). The processes related to ecological displacement may be a major force driving diversification in body size in the case of adaptive radiation (see e.g., Lomolino et al. 2013; Mazza et al. 2015; Palombo 2018). However, some departures from the predictions of this rule are recorded in extant large mammals getting larger than their mainland ancestor (e.g. the Kodiak bear Ursus arctos middendorffi and the Arctic fox Alopex lagopus from Mednyi Island [Bering Sea] (McNab 2001, 2010)) and in some small mammals getting smaller (e.g. Mastomys huberti (Ganem et al. 1995), Sundamys muelleri (Nor 1996), Parantechinus apicalis (Mills et al. 2004)).
The giant Cretan deer has been regarded for a long time as the most prominent departure from the island rule recorded among the fossil Pleistocene large mammals. The long bones of the giant deer from Bate cave, ascribed to C. major, are indeed longer than those of most of the roughly contemporaneous deer known on Greek mainland (cf. Capasso Barbato & Petronio 1986). The metatarsus, for instance, nearly reaches the size of C. latifrons metatarsal bones, is slightly larger than the largest known specimens of Megaloceros giganteus (length of the largest metatarsus = 391 mm) (Kazakhstan, Shpansky 2014), and is similar to the length of the largest specimens of extant Alces alces from Alaska (414.3 mm) (Silvia et al. 2014).
The oldest case of gigantism known to date in fossil large mammals is that of the Miocene cervoid Hoplitomeryx sp. from Scontrone (Abruzzi, central Italy) (Mazza et al. 2015). Hoplitomeryx sp. (consisting of few largely incomplete long bones and one astragalus larger but proportionally equivalent to the other astragali, Mazza et al. 2015, Fig. 4) is the largest among the 7 species of different sizes that lived contemporaneously on the palaeoisland. Mazza et al. (2015) explained its presence suggesting that the Hoplitomeryx “could freely develop new adaptive solutions, radiating from a stem species into a diverse set of niches and originating a set of coexisting species of different sizes, one of them giant” as already suggested for Cretan deer (Mazza et al. 2015, p. 276).
Consequently, the peculiar evolutionary radiation characterizing the Cretan deer could account for the unusually large size of C. major. Assuming as true that the ancestor of Cretan deer was a large or a medium‐size deer, the 4 species of size group 1 and 2 (C. ropalophorous, C. devosi, C. listeri, C. reumeri) (van der Geer 2018) significantly reduced their size (withers height about 50 cm), 2 (C. cretensis and C. rethymnesis) were moderately larger, while the stature of C. dorothensis falls in the range of modern red deer. In the Cretan predator‐free scenario, where the only other herbivore, Palaeoloxodon creutzburgi, could hardly be considered a competitor, these Candiacervus species differentiated each other in habitat and behavior, likely occupying, if available, all the niches typical of mainland deer.
The high stature and the poor limb maneuverability shown by C. major might be related to the advantage to avoid competition with other deer species in a low productivity environment by having access to a band of vegetation represented by sparse high trees, So that the gigantism hypothesis would be reasonable. Nevertheless, the amount of evidence resulting from this research highly questions the soundness of this hypothesis.
Taxonomical and nomenclatural open issues
The metatarsus and tibia herein described are, respectively, the holotype and the paratype (together with the radius‐ulna, which share with the other long bones the same elongated, slender shape) of Candiacervus major (Fig. 10), a species that is possibly represented by a single pathological individual at the Bate cave and by a couple of fragments at Liko. Therefore, a taxonomic and a nomenclatural review of large‐sized Cretan deer would be desirable in view of new diagnosis and selection of the types of the species. It seems rational to suppose that the “giant” deer affected by pituitary gigantism could be a pathological individual belonging to C. dorothensis that is the closest in size and the most similar as regards to long limb morphology and proportion, and this would imply that C. major is a subjective synonym of C. dorothensis. Further studies will clarify whether the giant deer from the Bate cave actually could be a pathological individual belonging to the C. dorothensis species or not. In both cases, a complex nomenclatural issue arises, whose discussion is beyond the purpose of this research.
Figure 10.
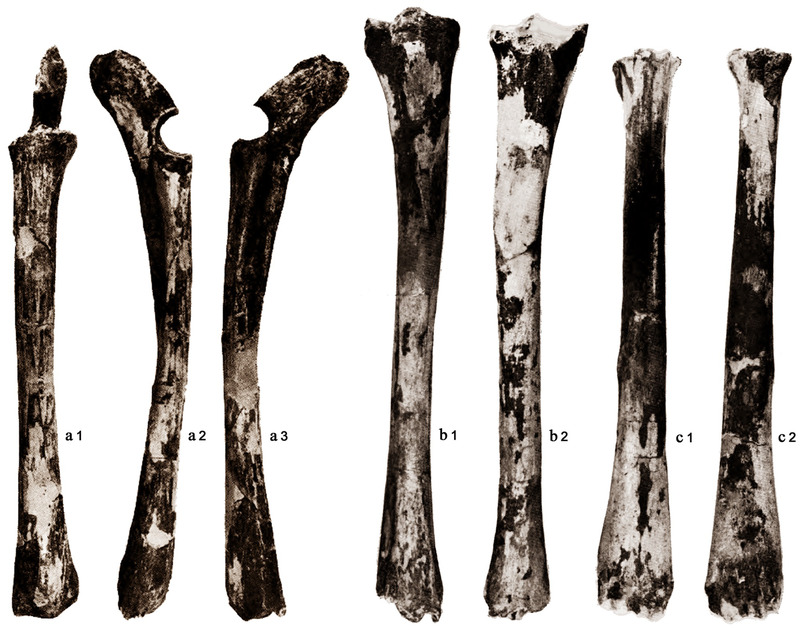
Holotype and paratypes of the species Candiacervus major (Bate cave, Rethymnon, Crete). (a) Radius and ulna (paratype) in anterior (a1), medial (a2), and lateral (a3) view; (b) tibia (paratype) in anterior (b1) and posterior (b2) view; c) metatarsus (holotype) in anterior (c1) and posterior (c2) view. Modified from Capasso Barbato and Petronio (1986, Tav I‐II).
Concluding remarks
Macroscopical observations, osteometrical analyses, radiological and histological examinations of the tibia and metatarsus of the giant Cretan deer from the Bate cave support the hypothesis that the examined bones belong to an individual affected by pituitary gigantism. The results obtained deserve attention and further discussion since they describe for the first time a case of pituitary gigantism in an extinct mammal. The gigantism case presented here may significantly contribute to a better recognition of a syndrome rare in nature, poorly described in ancient remains and almost unknown in fossil mammals, and it could further validate the generality of the island rule. This highlights the potential that paleohistological investigations may have in paleontological studies to unravel paleoecological, evolutionary taxonomic, and nomenclatural issues.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors thank the Integrative Zoology Editor J. Buckeridge for their valuable comments to the previous version of the manuscript. The authors are grateful to Prof. Ralf‐Dietrich Kahlke for providing the unpublished images of the Eucladoceros giulii tibia and metatarsus from Untermassfield stored at the Senckenberg Research Station of Quaternary Palaeontology in Weimar (Germany).
Palombo MR, Zedda M (2022). The intriguing giant deer from the Bate cave (Crete): could paleohistological evidence question its taxonomy and nomenclature? Integrative Zoology 17, 54–77.
M.R.P. and M.Z. participated equally in the work
REFERENCES
- Altamura S (2016). Dietary behaviour of the Pleistocene Cretan dwarf deer: Preliminary clues from mesowear analysis of ex gr. Candiacervus ropalophorus from Bate cave. Alpine and Mediterranean Quaternary 29, 77–89. [Google Scholar]
- Amson E, Muizon CD, Laurin M et al. (2014). Gradual adaptation of bone structure to aquatic lifestyle in extinct sloths from Peru. Proceedings of the Royal Society of London, Series B 281, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amson E, Kolb C, Scheyer TM et al. (2015). Growth and life history of Middle Miocene deer (Mammalia, Cervidae) based on bone histology. Comptes Rendus Palevol 14, 637–45. [Google Scholar]
- Amson E, Kolb C (2016). Scaling effect on the mid‐diaphysis properties of long bones. The case of the Cervidae (deer). Science of Nature 103, 1–10. [DOI] [PubMed] [Google Scholar]
- Andreassen TT, Hoxlund H (2001). The effect of growth hormone on cortical and cancellous bone. Journal of Musculoskeletal and Neuron Interactions 2, 49–58. [PubMed] [Google Scholar]
- Bartosiewicz L (2016). The palaeopathology of wild mammals in archaeology [Vadon élő emlősállatok betegségei a régészetben]. Archeometriai Műhely 13, 19–30. [Google Scholar]
- Bartosiewicz L (2018). Taphonomy and disease prevalence in animal palaeopathology: The proverbial “Veterinary Horse”. In: Bartosiewicz L, Gál E, eds. Care or Neglect? Evidence of Animal Disease in Archaeology. Oxbow Books, Budapest, Oxford, p. 185. [Google Scholar]
- Belluomini G, Delitala L (1983). Datazione di resti ossei e denti del Pleistocene superiore e dell'Olocene dell'area mediterranea con il metodo della racemizzazione degli aminoacidi. Geografia Fisica e Dinamica Quaternaria 6, 21–30. [Google Scholar]
- Belluomini G, Delitala L (1988). Amino acid racemization dating of quaternary deposits of Central and Southern Italy. Organic Geochemistry 13, 735–40. [Google Scholar]
- Böhmer C, Rössner GE (2018). Dental paleopathology in fossil rhinoceroses: Etiology and implications. Journal of Zoology 304, 3–12. [Google Scholar]
- Calderòn T, De Miguel D, Arnold W et al. (2019). Calibration of life history traits with epiphyseal closure, dental eruption and bone histology in captive and wild red deer. Journal of Anatomy 235, 205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caloi L, Palombo MR (1995). Functional aspects and ecological implications in Pleistocene endemic cervids of Sardinia, Sicily and Crete. Geobios 28, 247–58. [Google Scholar]
- Caloi L, Palombo MR (1996). Functional aspects and ecological implications in Hippopotami and Cervids of Crete. In: Reese DS, ed. Pleistocene and Holocene Fauna of Crete and Its First settlers. Monographs in World Archaeology 28. Prehistory Press of Madison, Madison, WI, pp. 125–52. [Google Scholar]
- Canoville A, Laurin M (2010). Evolution of humeral microanatomy and lifestyle in amniotes, and some comments on paleobiological inferences. Biological Journal of the Linnean Society 100, 384–406. [Google Scholar]
- Capasso Barbato L (1989). Cervidi Endemici del Pleistocene di Creta (PhD thesis). Università Consorziate, Modena, Italy. [Google Scholar]
- Capasso Barbato L (1990). Les Cervidés endémiques de Crètes. Quaternaire 3–4, 265–70. [Google Scholar]
- Capasso Barbato L (1992). Nuova specie di cervide del Pleistocene di Creta. Atti Accademia Nazionale dei Lincei, Memorie Lincee, Scienze Fisiche e Naturali (Serie 9) 1, 183–220. [Google Scholar]
- Capasso Barbato L (1995). Un tentativo di analisi cladistica applicata ad un cervide endemico del Pleistocene di Creta. Geologica Romana 31, 243–8. [Google Scholar]
- Capasso Barbato L, Petronio C (1986). Cervus major n. sp. of Bate Cave (Rethymnon, Crete). Atti Accademia Nazionale dei Lincei, Memorie Lincee, Scienze Fisiche, Matematiche e Naturali (Serie 8, 2a) 18, 59–100. [Google Scholar]
- Castanet J (2006). Time recording in bone microstructures of endothermic animals; functional relationships. Comptes Rendus Palevol 5, 629–36. [Google Scholar]
- Castanet J, Croci S, Aujard F et al. (2004). Lines of arrested growth in bone and age estimation in a small primate: Microcebus murinus . Journal of Zoology 263, 31–9. [Google Scholar]
- Chanson P, Salenave S (2008). Acromegaly. Orphanet Journal of Rare Diseases 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker SL, Reed W, Donlon D (2016). Comparative cortical bone thickness between the long bones of humans and five common non‐human mammal taxa. Forensic Science International 260, 104.e1–e17. [DOI] [PubMed] [Google Scholar]
- Cubo J, Ponton F, Laurin M et al. (2005). Phylogenetic signal in bone microstructure of sauropsids. Systematic Biology 54, 562–74. [DOI] [PubMed] [Google Scholar]
- Currey JD, Alexander RM (1985). The thickness of the walls of tubular bones. Journal of Zoology 206, 453–68. [Google Scholar]
- Dalle Carbonare L, Micheletti V, Cosaro E et al. (2018). Bone histomorphometry in acromegaly patients with fragility vertebral fractures. Pituitary 21, 56–64. [DOI] [PubMed] [Google Scholar]
- de Herder WW (2009). Acromegaly and gigantism in the medical literature. Case descriptions in the era before and the early years after the initial publication of Pierre Marie (1886). Pituitary 12, 236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ricqlès A, Bourdon E, Legendre LJ et al. (2016). Preliminary assessment of bone histology in the extinct elephant bird Aepyornis (Aves, Palaeognathae) from Madagascar. Comptes Rendus Palevol 15, 205–16. [Google Scholar]
- de Souza Barbosa FH, da Silva LHM, de Araújo‐Júnior HI (2020). Differentiating taphonomic and paleopathological features in Vertebrate Paleontology: a study case with Quaternary mammals. PalZ, Palaeontologische Zeitschrift 94, 595–601. [Google Scholar]
- de Vos J (1996). Taxonomy, ancestry and speciation of the endemic Pleistocene deer of Crete compared with the taxonomy, ancestry and speciation of Darwin's finches. In: Reese DS, ed. Pleistocene and Holocene Fauna of Crete and its First settlers. Monographs in World Archaeology 28. Prehistory Press of Madison, Madison, WI, pp. 111–9. [Google Scholar]
- de Vos J (2000). Pleistocene deer fauna in Crete: its adaptive radiation and extinction. Tropics 10, 125–34. [Google Scholar]
- de Vos J (1979). The endemic Pleistocene deer of Crete. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen B 82, 59–90. [Google Scholar]
- de Vos J (1984). The endemic Pleistocene deer of Crete. Verhandelingen der Koninklijke Akademie van Wetenschappen, afd. Natuurkunde, Eerste Reeks 31, 1–100. [Google Scholar]
- de Vos J (2006). Notes about parallels in the evolution of the Pleistocene cervids from Greece (Crete, Kassos and Karpathos), Japan (the Ryukyu‐islands) and Philippines (Masbate). Hellenic Journal of Geosciences 41, 127–40. [Google Scholar]
- de Vos J, Dermitzakis MD (1986). Models of the development of Pleistocene deer on Crete (Greece). Modern Geology 10, 243–8. [Google Scholar]
- Eugster EA, Pescovitz OH (1999). Gigantism. The Journal of Clinical Endocrinology & Metabolism 84, 4379–84. [DOI] [PubMed] [Google Scholar]
- Faurby S, Svenning JC (2016). Resurrection of the island rule: Human‐driven extinctions have obscured a basic evolutionary pattern. The American Naturalist 187, 812–20. [DOI] [PubMed] [Google Scholar]
- Foster JB (1964). Evolution of mammals on islands. Nature 202, 234–5. [Google Scholar]
- Francillon‐Vieillot H, de Buffrénil V, Castanet J et al. (1990). Microstructure and mineralization of skeletal vertebral tissues. In: Carter JG, ed. Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends. American Geophysical Union, Washington, pp. 471–530. [Google Scholar]
- Galassi FM, Henneberg M, de Herder W et al. (2017). Oldest case of gigantism? Assessment of the alleged remains of Sa‐Nakht, king of ancient Egypt. The Lancet Diabetes & Endocrinology 5, 580–1. [DOI] [PubMed] [Google Scholar]
- Ganem G, Granjon L, Ba K et al. (1995). Body size variability and water balance: A comparison between mainland and island populations of Mastomys huberti (Rodentia: Muridae) in Senegal. Experientia 51, 402–10. [DOI] [PubMed] [Google Scholar]
- García‐Martínez R, Marín‐Moratalla N, Jordana X et al. (2011). The ontogeny of bone growth in two species of dormice: reconstructing life history traits. Comptes Rendus Palevol 10, 489–98. [Google Scholar]
- Giua S, Farina V, Cacchioli A et al. (2014). Comparative histology of the femur between mouflon (Ovis aries musimon) and sheep (Ovis aries aries). Journal of Biological Research 87, 74–7. [Google Scholar]
- Godynicky S (1965). Determination of deer height on the basis of metacarpal and metatarsal bones. Roczniki Wyzszej Szkoly Rolnieczej w Poznaniu 25, 39–51. [Google Scholar]
- Gomez S, Garcia AJ, Luna S et al. (2013). Labeling studies on cortical bone formation in the antlers of red deer (Cervus elaphus). Bone 52, 506–15. [DOI] [PubMed] [Google Scholar]
- Hammer Ø, Harper DA, Ryan PD (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4, 1–9. [Google Scholar]
- Heaney LR (1978). Island area and body size of insular mammals: Evidence from the tricolored squirrel (Callosciurus prevosti) of Southeast Asia. Evolution 32, 29–44. [DOI] [PubMed] [Google Scholar]
- Heckeberg NS (2020). The systematics of the Cervidae: A total evidence approach. PeerJ 8, e8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier ML, Bell LS (2007). Differentiating human bone from animal bone: A review of histological methods. Journal of Forensic Sciences 52, 249–63. [DOI] [PubMed] [Google Scholar]
- Iliopoulos G, Eikamp H, Fassoulas C (2010). A new Late Pleistocene mammal locality from western Crete. In: Proceedings of the 12th International Congress; 2010, Patras, Greece. Bulletin of the Geological Society of Greece, vol. 43.
- Itescu Y, Karraker NE, Raia P et al. (2014). Is the island rule general? Turtles disagree. Global Ecology Biogeography 23, 689–700. [Google Scholar]
- Jordana X, Köhler M (2011). Enamel microstructure in the fossil bovid Myotragus balearicus (Majorca, Spain): Implications for life‐history evolution of dwarf mammals in insular ecosystems. Palaeogeography Palaeoclimatology, Palaeoecology 300, 59–66. [Google Scholar]
- Jordana X, Galtés I, Isidro A et al. (2011). Estudio macroscópico, radiológico y histológico de una fractura tibial de Myotragus balearicus . In: Gonzàlez MA et al., eds. Paleopatología: Ciencia Multidisciplinar. Universidad Autónoma de Madrid: Universidad Complutense de Madrid, pp. 435–9. [Google Scholar]
- Kahlke HD (1997). Die Cerviden‐Reste aus dem unterpleistozän von Untermassfeld. In: Kahlke RD, ed. Das Pleistozän von Untermaßfeld bei Meiningen (Thüringen),1. Monograph des Röm German Zentralmus, Mainz, pp. 181–276. [Google Scholar]
- Killinger Z, Kuzma M, Sterančàkovà L et al. (2012). Osteoarticular Changes in Acromegaly. International Journal of Endocrinology 2012, 839282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M, Marín‐Moratalla N, Jordana X et al. (2012). Seasonal bone growth and physiology in endotherms shed light on dinosaur physiology. Nature 487, 358–61. [DOI] [PubMed] [Google Scholar]
- Köhler M, Moyà‐Solà S (2009). Slow life history and physiological plasticity: Survival strategies of a large mammal in a resource‐poor environment. PNAS 106, 20354–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb C (2016). Bone and tooth microstructure in extinct and extant mammals and implications for growth and life history evolution, with an emphasis on cervids as a case study (Doctoral dissertation). University of Zurich, Zurich. [Google Scholar]
- Kolb C, Scheyer TM, Lister AM et al. (2015a). Growth in fossil and extant deer and implications for body size and life history evolution. BMC Evolutionary Biology 15, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb C, Scheyer TM, Veitschegger K et al. (2015b). Mammalian bone palaeohistology: A survey and new data with emphasis on island forms. PeerJ 3, e1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsakis T, Melentis J, Sirna G (1976). Seconda spedizione paleontologica Lincea nell'isola di Creta (1975). Accademia Nazionale dei Lincei, Quaderno 223, 3–10. [Google Scholar]
- Kronenberg HM (2003). Developmental regulation of the growth plate. Nature 423, 332–6. [DOI] [PubMed] [Google Scholar]
- Kurtén B (1968). Pleistocene Mammals of Europe. Weidenfeld & Nicolson, London, UK, pp. 317. [Google Scholar]
- Kuss SE (1968). Pleistozane Muriden der Insel Kreta. Neues Jahrbuch fur Mineralogie, Geologie und Palaontologie: Abhandlung 132, 55–69. [Google Scholar]
- Kuss SE (1975). Die pleistozänen Hirsche der ostmediterranen Inseln Kreta, Kasos, Karpatos und Rhodos (Griechenland). Berichte der Naturforschenden Gesellschaft zu Freiburg im Breisgau 65, 25–79. [Google Scholar]
- Kužma M, Killinger Z, Jackuliak P et al. (2019). Pathophysiology of growth hormone secretion disorders and their impact on bone microstructure as measured by trabecular bone score. Physiological Research 68, 121–9. [DOI] [PubMed] [Google Scholar]
- Labarca R, Pacheco A (2019). Palaeopathological analysis of a Chilean gomphothere (Proboscidea: Gomphotheriidae). International Journal of Paleopathology 26, 14–21. [DOI] [PubMed] [Google Scholar]
- Laurin M, Canoville A, Germain D (2011). Bone microanatomy and lifestyle: a descriptive approach. Comptes Rendus Palevol 10, 381–402. [Google Scholar]
- Lax EM (1996). A Gazetteer of Cretan paleontological localities. In: Reese DS, ed. Pleistocene and Holocene Fauna of Crete and Its First Settlers. Monography in World Archaeology, vol. 28. Prehistory Press, Madison, Wisconsin, pp. 1–132. [Google Scholar]
- Lister AM (1989). Rapid dwarfing of red deer on Jersey in the last interglacial. Nature 342, 539–42. [DOI] [PubMed] [Google Scholar]
- Lokatis S, Jeschke JM (2018). The island rule: An assessment of biases and research trends. Journal of Biogeography 45, 289–303. [Google Scholar]
- Lomolino MV (1985). Body size of mammals on islands: The island rule rexamined. The American Naturalist 125, 310–16. [Google Scholar]
- Lomolino MV (2005). Body size evolution in insular vertebrates: Generality of the island rule. Journal of Biogeography 32, 1683–99. [Google Scholar]
- Lomolino MV, Sax DF, Palombo MR et al. (2012). Of mice and mammoths: Evaluations of causal explanations for body size evolution in insular mammals. Journal of Biogeography 39, 842–54. [Google Scholar]
- Lomolino MV, Van der Geer AA, Lyras GA et al. (2013). Of mice and mammoths: Generality and antiquity of the island rule. Journal of Biogeography 40, 1427–39. [Google Scholar]
- Lyras GA, Giannakopoulou A, Lillis T et al. (2019). Paradise lost: Evidence for a devastating metabolic bone disease in an insular Pleistocene deer. International Journal of Paleopathology 24, 213–26. [DOI] [PubMed] [Google Scholar]
- Lyras GA, Giannakopoulou A, Lillis T et al. (2016). Bone lesions in a Late Pleistocene assemblage of the insular deer Candiacervus sp. II from Liko cave (Crete, Greece). International Journal of Paleopathology 14, 36–45. [DOI] [PubMed] [Google Scholar]
- Malatesta A (1980). Dwarf deer and other Late Pleistocene fauna of the Simonelli Cave in Crete. Atti della Accademia Nazionale dei Lincei 249, 3–97. [Google Scholar]
- Marín‐Moratalla N, Jordana X, Köhler M (2013). Bone histology as an approach to providing data on certain key life history traits in mammals: Implications for conservation biology. Mammalian Biology 78, 422–9. [Google Scholar]
- Marotti F, Bertolani MF, Palumbo C (2004). Bone growth, modeling and remodeling in a supernumerary metatarsal bone associated with segmental gigantism in cutis marmorata telangiectatica congenita. Histology and Histopathology 19, 413–20. [DOI] [PubMed] [Google Scholar]
- Martínez‐Maza C, Alberdi MT, Nieto‐Diaz M et al. (2014). Life history traits of the Miocene Hipparion concudense (Spain) inferred from bone histological structure. PLoS ONE 9, e103708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew DF (1977). The endemic Pleistocene murids of Crete. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen 80, 182–214. [Google Scholar]
- Mazza PPA, Rossi MA, Agostini S (2015). Hoplitomerycidae (Late Miocene, Italy), an example of giantism in insular ruminants. Journal of Mammalian Evolution 22, 271–7. [Google Scholar]
- Mazziotti G, Frara S, Giustina A (2018). Pituitary diseases and bone. Endocrine Reviews 39, 440–88. [DOI] [PubMed] [Google Scholar]
- Mazziotti G, Lania AGA, Canalis E (2019). Bone disorders associated with acromegaly: Mechanisms and treatment. European Journal of Endocrinology 181, 45–56. [DOI] [PubMed] [Google Scholar]
- McNab BK (2001). Functional adaptations to island life. In: Woods CA, Sergile FE, eds. The West Indies. Biogeography of the West Indies: Patterns and Perspectives. CRC Press, Boca Raton, FL, pp. 191–210. [Google Scholar]
- McNab BK (2010). Geographic and temporal correlations of mammalian size reconsidered: A resource rule. Oecologia 164, 13–23. [DOI] [PubMed] [Google Scholar]
- Meiri S, Dayan T, Simberloff D (2004). Body size of insular carnivores: Little support for the island rule. The American Naturalist 163, 469–79. [DOI] [PubMed] [Google Scholar]
- Meiri S, Dayan T, Simberloff D (2006). The generality of the island rule reexamined. Journal of Biogeography 33, 1571–7. [Google Scholar]
- Meiri S, Cooper N, Purvis A (2008). The island rule: made to be broken? Proceedings of the Royal Society B: Biological Sciences 275, 141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed S (2009). Acromegaly pathogenesis and treatment. The Journal of Clinical Investigation 119, 3189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills HR, Moro D, Spencer PBS (2004). Conservation significance of island versus mainland populations: A case study of dibblers (Parantechinus apicalis) in Western Australia. Animal Conservation 7, 387–95. [Google Scholar]
- Minozzi S, Pantano W, Di Gennaro F et al. (2012). Pituitary disease from the past: A rare case of gigantism in skeletal remains from the Roman Imperial age. The Journal of Clinical Endocrinology & Metabolism 97, 4302–3. [DOI] [PubMed] [Google Scholar]
- Minozzi S, Pantano W, Catalano P et al. (2015). The Roman giant: Overgrowth syndrome in skeletal remains from the Imperial age. International Journal of Osteoarchaeology 25, 574–84. [Google Scholar]
- Miszkiewicz JJ, Louys J, Beck R et al. (2019). Island rule and bone metabolism in fossil murines from Timor. Biological Journal of Linnean Society 129, 570–86. [Google Scholar]
- Mitchell J, Legendre LJ, Lefèvre C et al. (2017). Bone histological correlates of soaring and high‐frequency flapping flight in the furculae of birds. Zoology 122, 90–9. [DOI] [PubMed] [Google Scholar]
- Mulhern DM (2005). A probable case of gigantism in a Fifthy Dynasty skeleton from the Western cemetery at Giza, Egypt. International Journal of Osteoarchaeology 15, 261–75. [Google Scholar]
- Mura A, Gadau S, Zedda M (2013). Morphological and morphometrical features of Sardinian wild cat (Felis lybica sarda). Journal of Biological Research 86, 35–7. [Google Scholar]
- Nacarino‐Meneses C, Jordana X, Köhler M (2016a). First approach to bone histology and skeletochronology of Equus hemionus . Comptes Rendus Palevol 15, 267–77. [Google Scholar]
- Nacarino‐Meneses C, Jordana X, Köhler M (2016b). Histological variability in the limb bones of the Asiatic wild ass and its significance for life history inferences. PeerJ 4, e2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nor SM (1996). The mammalian fauna on the islands at the northern tip of Sabah, Borneo. Fieldiana Zoology 83,1–51. [Google Scholar]
- Olivier C, Houssaye A, Jalil NE et al. (2017). First palaeohistological inference of resting metabolic rate in an extinct synapsid, Moghreberia nmachouensis (Therapsida: Anomodontia). Biological Journal of Linnean Society 121, 409–19. [Google Scholar]
- Orlandi‐Oliveras G, Nacarino‐Meneses C, Koufos GD et al. (2018). Bone histology provides insights into the life history mechanisms underlying dwarfing in hipparionins. Scientific Reports 8, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo MR, Köhler M, Sola SM et al. (2008). Brain versus body mass in endemic ruminant artiodactyls: A case studied of Myotragus balearicus and smallest Candiacervus species from Mediterranean Islands. Quaternary International 182, 160–83. [Google Scholar]
- Palombo MR (2009). Body size structure of Pleistocene mammalian communities: What support is there for the “island rule”? Integrative Zoology 4, 341–56. [DOI] [PubMed] [Google Scholar]
- Palombo MR (2018). Insular mammalian fauna dynamics and paleogeography: A lesson from the Western Mediterranean islands. Integrative Zoology 13, 2–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo MR, Zedda M (2016). Surviving in a predator‐free environment: Hints from a bone remodelling process in a dwarf Pleistocene deer from Crete. Comptes Rendus Palevol 15, 245–54. [Google Scholar]
- Reese DS, Belluomini G, Ikeya M (1996). Absolute dates for the Pleistocene fauna of Crete. In: Reese DS, ed. Pleistocene and Holocene Fauna of Crete and Its first Settlers. Monography in World Archaeology 28. Prehistory Press, Madison, WI, pp. 47–51. [Google Scholar]
- Roselló‐Díez A, Joyner AL (2015). Regulation of long bone growth in vertebrates; it is time to catch up. Endocrinology Review 36, 646–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle JL (1997). An investigation of bone histology as a potential age indicator in roe deer (PhD thesis). University College, London. [Google Scholar]
- Sander PM, Andrassy P (2006). Lines of arrested growth and long bone histology in Pleistocene large mammals from Germany: What do they tell us about dinosaur physiology? Palaeontographica Abteilung A 277, 143–59. [Google Scholar]
- Scacchi M, Cavagnini F (2006). Acromegaly. Pituitary 9, 297–303. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Kammer B, Grasser M et al. (2007). Endochondral gigantism: A newly recognized skeletal dysplasia with pre and postnatal overgrowth and endocrine abnormalities. American Journal of Medical Genetics A 143A, 1868–75. [DOI] [PubMed] [Google Scholar]
- Sher AV (1971). Mammals and stratigraphy of the Pleistocene of the extreme Northeast of the USSR and North America. Nauka, Moscow (in Russian); In English: Pleistocene mammals and stratigraphy of the Far Northeast USSR and North America; Intern. Geology Review 16 (7–10), 1974, pp. 1–284.
- Shpansky AV (2014). Skeleton of the giant deer Megaloceros giganteus giganteus (Blumenbach, 1803) (Mammalia, Artiodactyla) from the Irtysh Region near Pavlodar. Paleontology Journal 48, 534–50. [Google Scholar]
- Silvia WJ, Peterson RO, Vucetich JA et al. (2014). Variation in metatarsal morphology among subgroups of North American moose (Alces alces). Alces: a Journal Devoted to the Biology and Managment of Moose 50, 159–70. [Google Scholar]
- Simonelli V (1907). Mammiferi quaternari dell'isola di Candia I. Memorie, Accademia delle Scienze. Istituto di Bologna , Classe di Scienze Fisiche 6, 455–71. [Google Scholar]
- Skedros JG, Knight AN, Clark GC et al. (2013). Scaling of Haversian canal surface area to secondary osteon bone volume in ribs and limb bones. American Journal of Physical Anthropology 151, 230–44. [DOI] [PubMed] [Google Scholar]
- Sondaar PY, Boekschoten GJ (1967). Quaternary MAMMALS in the South Aegean island arc; with notes on other fossil Mammals from the coastal regions of the Mediterranean. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen 70, 556–76. [Google Scholar]
- Stein K (2010). Long bone histology of basalmost and derived Sauropodomorpha: the convergence of fibrolamellar bone and the evolution of giantism and nanism (dissertation). Rheinischen Friedrich‐Wilhelms‐Universität Bonn, Bonn, Germany, pp. 213. [Google Scholar]
- Stein KW, Werner J (2013). Preliminary analysis of osteocyte lacunar density in long bones of tetrapods: All measures are bigger in sauropod dinosaurs. PLoS ONE 8, e77109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R (2012). Nonhuman paleopathology. In: Buikstra JE, Roberts C, eds. The Global History of Paleopathology: Pioneers and Prospects. OUP, US, pp. 652–66. [Google Scholar]
- Tritos NA, Klibanski A (2016). Effects of growth hormone on bones. Progress in Molecular Biology and Translational Science 138, 193–211. [DOI] [PubMed] [Google Scholar]
- Ueland T (2005). GH/IGF‐I and bone resorption in vivo and in vitro. European Journal of Endocrinology 152, 327–32. [DOI] [PubMed] [Google Scholar]
- Ueland T, Ebbesen EN, Thomsen JS et al. (2002). Decreased trabecular bone biomechanical competence, apparent density, IGF‐II and IGFBP‐5 content in acromegaly. European Journal of Clinical Investigation 32, 122–8. [DOI] [PubMed] [Google Scholar]
- Van der Geer A, Lyras G, De Vos J et al. (2010). Evolution of Island Mammals: Adaptation and Extinction of Placental Mammals on Islands. Wiley‐Blackwell, London, pp. 479. [Google Scholar]
- Van der Geer AA (2014). Parallel patterns and trends in functional structures in extinct island mammals. Integrative Zoology 9, 167–82. [DOI] [PubMed] [Google Scholar]
- Van der Geer AA (2018). Uniformity in variety: Antler morphology and evolution in a predator‐free environment. Palaeontologia Electronica 21, 1–31. [Google Scholar]
- Van der Geer AA, Lyras G, Vos JD et al. (2013). Morphology of articular surfaces can solve a phylogenetic issue: One instead of two ancestors for Candiacervus (Mammalia: Cervoidea). Zitteliana B31, 33–4. [Google Scholar]
- Van der Geer AA, de Vos J, Lyras G et al. (2006). New data on the Pleistocene Cretan deer Candiacervus sp. II (Mammalia, Cervinae). Courier Forschungsinstitut Senckenberg 256, 131–7. [Google Scholar]
- Van Valen L (1973). A new evolutionary law. Evolutionary Theory 1, 1–33. [Google Scholar]
- Veitschegger K (2017). Life history evolution in extant and extinct Laurasiatheria–case studies elucidating the junctions among selective forces, disparity, and trait evolution (Doctoral dissertation). University of Zurich, Zurich, Switzerland. [Google Scholar]
- Veitschegger K, Kolb C, Amson E et al. (2018). Palaeohistology and life history evolution in cave bears, Ursus spelaeus sensu lato. PLoS ONE 13, e0206791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viciano J, De Luca S, Lopez‐Lazaro S et al. (2015). A probable case of gigantism/acromegaly in skeletal remains from the Jewish necropolis of “Ronda Sur” (Lucena, Cordoba, Spain; VIII–XII centuries CE). Anthropologischer Anzeiger, Journal of Biological Clinical Anthropology 72, 67–87. [DOI] [PubMed] [Google Scholar]
- Vislobokova IA (2013). Morphology, taxonomy, and phylogeny of megacerines (Megacerini, Cervidae, Artiodactyla). Paleontology Journal 47, 833–950. [Google Scholar]
- Waldron T (2008). Palaeopathology. Cambridge University Press, Cambridge. [Google Scholar]
- Waskow K, Sander PM (2014). Growth record and histological variation in the dorsal ribs of Camarasaurus sp. (Sauropoda). Journal of Vertebrate Paleontology 34, 852–69. [Google Scholar]
- Whittaker RJ, Fernández‐Palacios JM (2007). Island Biogeography: Ecology, Evolution, and Conservation. Oxford University Press, Oxford. [Google Scholar]
- Woodward Ballard H, Padian K, Lee AH (2013). Skeletochronology. In: Bone Histology of Fossil Tetrapods: Advancing Methods, Analysis, and Interpretation. University of California Press, California, pp. 195–215. [Google Scholar]
- Woolley MR, Chinsamy A, Govender R, Bester MN (2019). Microanatomy and histology of bone pathologies of extant and extinct phocid seals. Historical Biology, 1–16, 10.1080/08912963.2019.1689238. [DOI] [Google Scholar]
- Xing L, Niu K, Wang D, Rothschild BM, Evans SE (2020). Palaeopathology in a Cretaceous terrestrial lizard from China. Historical Biology, 1–5, 10.1080/08912963.2020.1733550. [DOI] [Google Scholar]
- Zedda M, Babosova R (2021). Does the osteon morphology depend on the body mass? A scaling study on macroscopic and histomorphometric differences between cow (Bos taurus) and sheep (Ovis aries). Zoomorphology 140, 169–81. [Google Scholar]
- Zedda M, Brits D, Giua S et al. (2019). Distinguishing domestic pig femora and tibiae from wild boar through microscopic analyses. Zoomorphology 138, 159–70. [Google Scholar]
- Zedda M, Lepore G, Manca P et al. (2008). Comparative bone histology of adult horses (Equus caballus) and cows (Bos taurus). Anatomy Histology Embryology 37, 442–5. [DOI] [PubMed] [Google Scholar]
- Zedda M, Lepore G, Biggio GP et al. (2015). Morphology, morphometry and spatial distribution of secondary osteons in equine femur. Anatomy Histology Embryology 44, 328–32. [DOI] [PubMed] [Google Scholar]
- Zedda M, Palombo MR, Brits D et al. (2017). Differences in femoral morphology between sheep (Ovis aries) and goat (Capra hircus): Macroscopic and microscopic observations. Zoomorphology 136, 145–58. [Google Scholar]
- Zedda M, Sathé V, Chakraborty P et al. (2020). A first comparison of bone histomorphometry in extant domestic horses (Equus caballus Linnaeus, 1758) and a Pleistocene Indian wild horse (Equus namadicus Falconer & Cautley, 1849). Integrative Zoology 15, 448–60. [DOI] [PubMed] [Google Scholar]
- Zoboli D, Zedda M, Pillola GL et al. (2018). Does a relationship exist between palaeopathologies and insularity? A case study of some bones of Prolagus sardus (Wagner, 1829) from Sardinia (Italy). Alpine and Mediterranean Quaternary 31, 75–86. [Google Scholar]


