Abstract
Background
Treatment with glucagon‐like peptide‐2 (GLP‐2) analogs improve intestinal adaptation in patients with short bowel syndrome–associated intestinal failure (SBS‐IF) and may reduce parenteral support requirements. Apraglutide is a novel, long‐acting GLP‐2 analog designed for once‐weekly dosing. This trial investigated the safety and efficacy of apraglutide in patients with SBS‐IF.
Methods
In this placebo‐controlled, double‐blind, randomized, crossover phase 2 trial, eight adults with SBS‐IF were treated with once‐weekly 5‐mg apraglutide doses and placebo for 4 weeks, followed by once‐weekly 10‐mg apraglutide doses for 4 weeks, with a washout period of 6–10 weeks between treatments. Safety was the primary end point. Secondary end points included changes from baseline in urine volume output compared with placebo, collected for 48 h before and after each treatment period.
Results
Common treatment‐related adverse events (AEs) were mild to moderate and included polyuria, decreased stoma output, stoma complications, decreased thirst, and edema. No serious AEs were considered to be related to apraglutide treatment. The safety profile was comparable for the lower and higher doses. Treatment with once‐weekly 5‐ and 10‐mg apraglutide doses significantly increased urine volume output by an adjusted mean of 714 ml/day (95% CI, 490–939; P < .05) and 795 ml/day (95% CI, 195–1394; P < .05), respectively, compared with placebo, with no significant differences between doses.
Conclusions
Once‐weekly apraglutide was well tolerated at both tested doses and significantly increased urine volume output, providing evidence for increased intestinal fluid absorption. A phase 3 trial is underway in adults with SBS‐IF.
Keywords: glucagon‐like peptide‐2, intestinal adaptation, intestinal failure, parenteral support, short bowel syndrome
CLINICAL RELEVANCY STATEMENT
There are few evidence‐based treatments that enhance intestinal adaptation in patients with short bowel syndrome–associated intestinal failure (SBS‐IF). Teduglutide is currently the only approved glucagon‐like peptide‐2 (GLP‐2) analog for the treatment of SBS‐IF, and it requires daily subcutaneous injection through multiple reconstitution steps. Results from this trial showed that once‐weekly dosing with apraglutide, a next generation, long‐acting GLP‐2 analog, increases fluid absorption in patients with SBS‐IF. Apraglutide is expected to reduce long‐term parenteral support requirements and positively contribute to patient care and compliance by enabling once‐weekly administrations, which in turn may improve quality of life.
INTRODUCTION
Short bowel syndrome (SBS) is a state of malabsorption caused by extensive surgical resection of the small bowel, resulting in decreased intestinal length and compromised gut function. 1 SBS‐associated intestinal failure (SBS‐IF) occurs when a patient's gut function is reduced below the minimum function necessary to maintain health and/or growth such that parenteral support (PS), including intravenous supplementation of fluids, electrolytes, or nutrients, is needed. 2 SBS‐IF is an often neglected form of organ failure with limited treatment possibilities. 3 Identifying new treatments could improve morbidity and mortality, alleviate debilitating symptoms, and reduce patient treatment burden and healthcare costs.
The pathophysiological traits of SBS‐IF are often caused by disturbances in the neuroendocrine feedback mechanisms that regulate fluid and nutrient absorption. 4 This includes decreased postprandial secretion of glucagon‐like peptide 2 (GLP‐2), produced by intestinal L‐cells predominantly located in the terminal ileum and proximal colon. Exogenous GLP‐2 administration in patients with SBS‐IF promotes intestinal adaptation. 5 Mechanisms may include delaying an accelerated gastric emptying, 6 , 7 decreasing gastrointestinal (gastric, biliary, and pancreatic) hypersecretion, 8 promoting intestinal growth, 9 enhancing intestinal barrier function, 10 , 11 and increasing intestinal blood flow. 12
The GLP‐2 analog, teduglutide, was approved for use in patients with SBS in 2012. It differs from native GLP‐2 by one amino acid substitution, which prevents cleavage by the proteolytic enzyme dipeptidyl peptidase‐IV, thereby extending its half‐life to approximately 3–5 h, compared with 7 min for native GLP‐2. 13 , 14 Once‐daily, subcutaneous (SC) treatment with teduglutide is associated with significant improvements in intestinal function and PS reductions in patients with SBS. 15 , 16 However, its relatively short half‐life necessitates daily SC injection through multiple reconstitution steps. Apraglutide is a synthetic GLP‐2 analog designed to provide long‐lasting, constant GLP‐2 exposure. It differs from native GLP‐2 by four amino acid substitutions, 17 and it has a longer elimination half‐life (72 h) 18 than both native GLP‐2 and teduglutide because of low clearance resulting from DPP‐IV resistance and high plasma protein binding. 19 Therefore, apraglutide has the potential for less frequent injections than teduglutide and is a candidate for a once‐weekly dosing regimen.
Until now, phase 2 trials of GLP‐2 analogs have used laborious and expensive inpatient metabolic balance studies to evaluate functional improvements in intestinal absorption. 9 , 20 However, the hydration status in patients with SBS‐IF can be monitored by measuring urine volume output and urinary sodium excretion. This knowledge has been applied in phase 3 trials, in which PS reductions have been made based on increases in urine production. 15 , 16 This trial investigated the safety and efficacy of once‐weekly treatment with apraglutide in patients with SBS‐IF using a novel approach whereby urine volume and urinary sodium excretion were included as markers of functional intestinal rehabilitation.
MATERIALS AND METHODS
Trial design and participants
This phase 2 trial (NCT03415594) comprised a double‐blind, crossover, randomized, placebo‐controlled period (part A) followed by an open‐label extension period (part B) (Figure 1). Eight adult patients (aged ≥18 to ≤80 years) with SBS‐IF were enrolled in the trial. The main inclusion criteria were SBS‐IF secondary to surgical resection of the small bowel with a jejuno‐ or ileostomy, ≥6 months since the last bowel resection, a fecal output ≥1500 g/day as recorded within the last 18 months, and PS infusions ≥3 times per week for ≥12 months according to the patient's medical record. Thus, remnant bowel function was based on PS dependency and not on the anatomical definition of <200 cm of remnant bowel. 2 Exclusion criteria included clinical signs of inflammatory bowel disease; a history of cancer within 5 years; an inadequate hepatic, kidney, or heart function; hospitalization within 1 month of the screening visit; and treatment with native GLP‐2 or a GLP‐2 analog within the last 3 months. Additional inclusion and exclusion criteria are described in the Supplementary Material.
FIGURE 1.
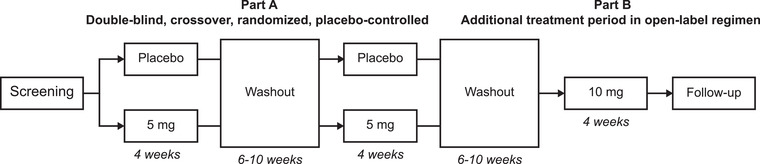
Trial design. Screening was performed up to 25 days before the baseline visit. Follow‐up was performed 4–6 weeks after the last dose. The washout period was 6–10 weeks after the last dose in the treatment period
In part A, patients were treated with 5‐mg apraglutide or placebo once‐weekly for 4 weeks, followed by a washout period of 6–10 weeks, and then the alternate treatment for a further 4 weeks. At the end of part A, patients underwent a second washout period of 6–10 weeks before entering part B. Part B was an open‐label dosing regimen with 10‐mg apraglutide given once‐weekly for 4 weeks. The additional open‐label treatment period was included to explore the safety and tolerability of 10 mg. The trial was performed at the Department of Intestinal Failure and Liver Diseases, Rigshospitalet, Copenhagen, Denmark and was approved by the Danish Medicines Agency and the Regional Committee on Health Research Ethics (project ID H‐17037606). Procedures were carried out in accordance with the ethical standards of the Helsinki Declaration, and the trial was overseen by Larix, Copenhagen, Denmark. A randomization list was provided by an unblinded statistician from Larix who was not otherwise involved in the trial (further described in the Supplementary Material).
Procedures
The trial drug was provided as a freeze‐dried powder for reconstitution and was administered as SC injections in the abdominal area. The first two injections were performed at the hospital, and subsequent injections were performed at the hospital or were self‐administered by the patient, depending on the patient's preferences. Safety assessments (described in Table S1) included observation for injection site reactions, vital signs, blood samples including antiapraglutide antibodies, electrocardiogram, urinalysis, and body weight.
Efficacy assessments were identical for each treatment period of 29 days. Patients performed home, 48‐h urine collections at baseline (days −2 to 1) and at the end of the treatment period (days 27–29, initiated 5 days after the fourth/last administration of the trial drug). Weekly PS and daily dietary fluid intake were kept constant during this period, allowing urine volume output to be used as a sign of increased fluid absorption. Patients were required to create a 24‐h drinking menu based on their habitual dietary fluid intake at the baseline visit (day −3) and to adhere to their menu during each urine collection. Dietary fluid intake was recorded in a paper diary. Urine was collected by the patient using a container with volume markings. Patients recorded urine volumes in their diary. After completing the collection, patients transferred approximately 100 ml of urine from the container to a separate sample collection container, which was submitted at the next trial visit and used for measuring urinary sodium excretion. Urine sodium was measured with a COBAS 8000 modular analyzer using an ion‐specific electrode system (Roche Diagnostics, Indianapolis, IN, US) with the lower limit of quantification of 10 mmol/L. Samples with sodium levels below the lower limit of quantification were analyzed by flame photometry. The amount of sodium excreted per 48 h was calculated by multiplying the concentration of sodium by the 48‐h urine volume.
Patients were required to record their 48‐h spontaneous dietary fluid intake for days 20–22 to investigate whether increases in intestinal absorption were associated with decreases in spontaneous dietary fluid intake. Because of the short treatment period of 4 weeks, PS volume reductions were allowed between days 4 and 24 if judged clinically necessary by the investigator because of clinical signs of fluid retention, such as edema, excessive unintended weight gain, and/or polyuria. Clinical signs could be observed by the investigator during trial visits or reported by the patient during telephone visits throughout the trial. PS volume reductions were assessed during days 20–22. Patients returned to their baseline PS volume and content during the end of the treatment urine collection (days 27–29). Concomitant medications, including proton‐pump inhibitors, loperamide, and opiates, were kept unchanged and stable throughout the trial. If necessary, adjustments could be made in the drinking menu and prescribed PS volume and content between treatment periods.
Body weight (determined using a leveled platform scale) and body composition (determined at the same time of day, relative to PS administration, by dual energy x‐ray absorptiometry [DXA; Norland XR‐36 DXA densitometer, Ford Atkinson, WI, US]) were measured at baseline and at the end of treatment. Blood samples for fasting plasma citrulline, a suggested marker of enterocyte mass, 21 were collected at baseline; during the first, second, and final treatment visits; 4 days after the first treatment; and 4–6 weeks after the last treatment. Plasma citrulline levels were determined as described in the Supplementary Material.
Outcomes
The primary end point was safety. An adverse event (AE) was defined as any untoward medical occurrence (sign, symptom, or disease) not necessarily causally related to treatment. A serious AE (SAE) was defined as an AE that resulted in death, was life‐threatening, required hospitalization, prolonged exciting hospitalization, resulted in persistent or significant disability or incapacity, caused a congenital anomaly or birth defect, or was a medically important event. Secondary objectives related to efficacy included changes from baseline to the end of treatment in urine volume and urinary sodium excretion; changes from baseline to near end of treatment in PS volume and spontaneous dietary fluid intake; and changes from baseline to the end of treatment in body weight, lean body mass, fat mass, bone mineral content, and plasma citrulline.
Statistics
No formal sample size calculation was performed for this trial. The sample size was based on the observed magnitude of effects in previous GLP‐2 analog phase 2 trials, 9 , 20 taking into account practical and logistic considerations and the number of eligible patients. Safety was assessed in all patients who had received at least one dose of trial drug (active or placebo). Efficacy was assessed following a modified intent‐to‐treat principle including all randomized patients with at least one valid postbaseline efficacy measurement (full analysis set). All efficacy analyses were considered exploratory, even when statistical analyses were employed. Two different statistical analyses were conducted: one for part A and another for parts A and B combined. Analysis for part A was based on a 2 × 2 crossover design, whereas analysis for parts A + B assumed no period effect in order to estimate the differences between the three different treatments (placebo and apraglutide 5 mg and 10 mg). We only included the results from part A in the scope of this paper to demonstrate the effects of 5 mg compared with the placebo. An analysis of covariance was used to assess the effects of apraglutide, adjusted for period‐specific baseline measurements for the outcome variable, dietary fluid intake, and PS volume. All statistical tests were performed using a two‐sided test at a 5% significance level. Estimates were presented with approximate 95% CIs and P‐values. SAS version 9.4 was used for the analysis.
RESULTS
Of the 12 patients screened between May 8, 2018, and April 23, 2019, eight were randomized to treatment. The remaining four were excluded because of inadequate hepatic or renal function (three patients) and a catheter‐related bloodstream infection (CRBSI) that occurred after the screening visit (one patient). All eight patients completed part A of the trial and entered part B. One patient discontinued part B after the first drug administration because of exhaustion from the trial procedures and a perceived lack of effect. Consequently, eight patients comprised the safety analysis set and full analysis set. Demographics and baseline characteristics of the patients are presented in Table 1. All patients had been stable on PS and did not have a reconstructable GI tract. Six patients had a jejunostomy and remnant small bowel lengths of 30, 50, 50, 80, 120, and 200 cm, respectively. Two patients had an ileostomy with remnant small bowel lengths of 260 and 300 cm, respectively. No patients had a colon in continuity. Three patients had previously been treated with a GLP‐2 analog in clinical trials (≥6 months ago). Individual patient plots illustrated that all effects reverted to baseline after the washout period; thus, no carryover effect was observed. This was also confirmed by the lack of significant P‐values from a linear model (Figure S1).
TABLE 1.
Demographics and baseline characteristics
| Total (n = 8) | |
|---|---|
| Age, years | 58 (36–78) |
| Sex | |
| Female | 4 |
| Weight at baseline, kg | 79.6 (54.0–95.5) |
| Body mass index, kg/m2 | 24.5 (20.2–31.0) |
| Race, White | 8 |
| PS volume, ml/day | 3315 (425–6616) |
| PS energy, kJ/day | 4419 (0–10,317) |
| Days of PS per week | 6.5 (3–7) |
| Urine volume output, ml/day | 2395 (392–3575) |
| Dietary fluid intake, ml/day | 2605 (1300–5475) |
| Urinary sodium excretion, mmol/day | 38 (4–265) |
| Plasma citrulline levels, μmol/L | 4.2 (1.7–17.9) |
| Cause of resection | |
| Crohn's disease | 2 |
| Mesenteric vascular disease | 3 |
| Surgical complications to ulcerative colitis | 1 |
| Surgical complications | 2 |
| Disease characteristics | |
| Small bowel length, cm | 100 (30–300) |
| Jejunostomy | 6 |
| Ileostomy | 2 |
| Colon in continuity | 0 |
| Concomitant medication | |
| Proton‐pump inhibitor | 7 |
| Opioids or opioid agonists | 4 |
| Loperamide | 3 |
Note: Data represent median (range) or number of patients. PS is scheduled PS at trial entry based on weekly average.
Abbreviation: PS, parenteral support.
Safety results
Most patients had at least one treatment‐related AE (TRAE) (Table 2), with no obvious difference between apraglutide doses. All TRAEs were mild to moderate in severity and none resulted in treatment reductions or discontinuations. Eight SAEs were reported by five patients, none of which were considered to be related to the trial drug. SAEs were distributed equally between placebo and treatment periods and included three cases of mechanical complications related to the tunneled central venous catheter for PS administration, requiring hospitalization for catheter replacement and five cases of CRBSI. One patient had four recurrent events of CRBSI: two occurred during the washout period after treatment with 5‐mg apraglutide (treatment period 1), one occurred during washout after treatment with placebo (treatment period 2), and one occurred between treatment with 10‐mg (treatment period 3) and the end‐of‐trial visit. The last CRBSI was reported by one patient during washout after placebo treatment (treatment period 1).
TABLE 2.
Commonly reported treatment‐related adverse events
| Placebo (n = 8) | 5 mg (n = 8) | 10 mg (n = 8) | Total (n = 8) | |
|---|---|---|---|---|
| Any related adverse events | 8 | 8 | 8 | 8 |
| Polyuria | 1 | 4 | 6 | 7 |
| Gastrointestinal stoma output decreased | 0 | 3 | 6 | 6 |
| Stoma complication | 0 | 6 | 6 | 6 |
| Gastrointestinal stoma complication | 0 | 5 | 5 | 5 |
| Gastrointestinal stoma output abnormal | 0 | 4 | 4 | 5 |
| Thirst decreased | 0 | 3 | 4 | 5 |
| Edema | 0 | 2 | 2 | 4 |
| Increased weight | 0 | 1 | 2 | 3 |
| Decreased appetite | 0 | 1 | 2 | 3 |
| Injection site reaction | 0 | 1 | 3 | 3 |
Note: Data represent the number of patients. Adverse events, according to the Medical Dictionary for Regulatory Activities (MedDRA), are preferred terms occurring in at least two patients. Data are from a crossover trial; each patient received each treatment. The MedDRA‐preferred term "stoma complications" included the reported terms: “increased stoma diameter” and “slower passage through stoma.” The preferred term "gastrointestinal stoma output abnormal" included the reported terms: “more solid stoma output” and “increased smell of stoma output.” The preferred term "gastrointestinal stoma complication" included the reported term “increased stoma protrusion.”
All patients were negative for antiapraglutide antibodies at screening. Three patients developed antiapraglutide antibodies during the trial: two at the end of the 5‐mg treatment period and one at the end of the 10‐mg treatment period. One of the three patients who developed antiapraglutide antibodies during the trial had previously been treated with a GLP‐2 analog in 2016. The same patient reported 10 injection site reactions. The other two antiapraglutide‐positive patients did not report any injection site reactions. No effects of antiapraglutide antibodies were detected in the pharmacodynamic response to apraglutide or in the number or duration of AEs.
Efficacy results
Urine volume output and urinary sodium excretion
The individual changes from baseline to the end of each treatment in urine volume output and urinary sodium excretion are plotted in Figures 2 and 3. Compared with the placebo, 5‐ and 10‐mg apraglutide significantly increased urine volume output by an adjusted mean of 714 and 795 ml/day, respectively, corresponding to a significant daily increase of 49% with 5‐mg apraglutide and a relative change of 34% with 10 mg that trended towards statistical significance (Table 3). Apraglutide 5 mg did not significantly change urinary sodium excretion compared with the placebo, whereas 10‐mg apraglutide significantly increased urinary sodium excretion by an adjusted mean of 88 mmol/day (Table 3).
FIGURE 2.
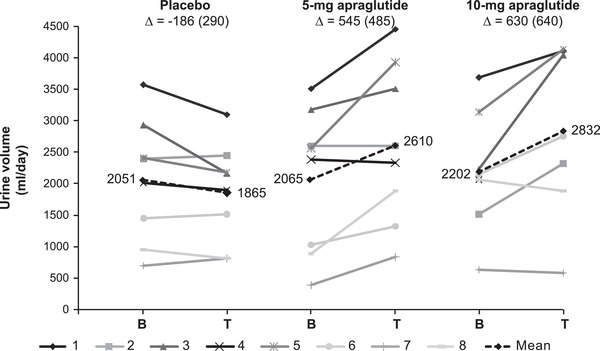
Individual and mean changes from baseline to the end of treatment in urine volume output. The dashed line represents the mean, and ∆ represents the mean change from baseline (SD). One patient discontinued after the first dose of 10 mg (data excluded graphically). The difference in grayscale shows the individual patients. B, baseline; T, treatment
FIGURE 3.
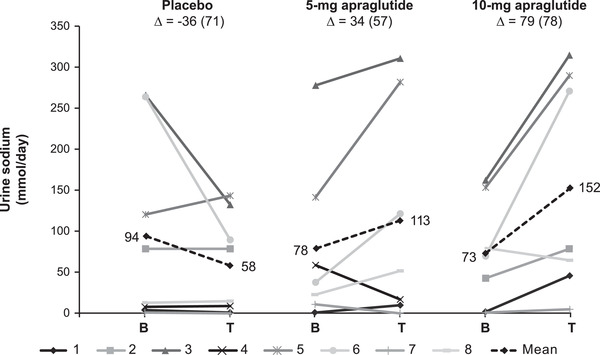
Individual and mean changes from baseline to the end of treatment in urinary sodium excretion. The dashed line represents the mean, and ∆ represents the mean change from baseline (SD). One patient discontinued after the first dose of 10 mg, and one patient did not provide a baseline sample for 5 mg (data excluded graphically). The difference in grayscale shows individual patients. B, baseline; T, treatment
TABLE 3.
Urine volume output, urinary sodium excretion, PS volume and dietary fluid intake
| Analysis part A | Analysis parts A + B | |||||
|---|---|---|---|---|---|---|
| Secondary end point | 5 mg vs placebo (n = 8) | P‐value | 10 mg vs placebo (n = 8) | P‐value | 5 mg vs 10 mg (n = 8) | P‐value |
| Absolute urine volume, ml/day | 714 (490–939) | .002 | 795 (195–1394) | .014 | 84 (−514 to 682) | .761 |
| Relative urine volume, % | 49 (4–94) | .041 | 34 (−4 to 71) | .072 | −14 (−51 to 23) | .420 |
| Urine sodium excretion, mmol/day | 66 (−69 to 201) | .171 | 88 (20–156) | .017 | 32 (−37 to 101) | .325 |
| Urine sodium excretion, % | 189 (−350 to 729) | .270 | 432 (−87 to 951) | .092 | 266 (−266 to 798) | .287 |
| PS volume, ml/day | −94 (−344 to 156) | .356 | −469 (−941 to 4) | .052 | −380 (−851 to 91) | .103 |
| Relative PS volume, % | −13 (−41 to 15) | .276 | −28 (−51 to −4) | .025 | −15 (−38 to 9) | .195 |
| Dietary fluid intake, ml/day | −242 (−560 to 76) | .103 | −363 (−641 to −86) | .015 | −119 (−396 to 157) | .362 |
| Relative dietary fluid intake, % | −9 (−18 to 1) | .068 | −15 (−25 to −5) | .006 | −7 (−17 to 3) | .169 |
| Plasma citrulline, μmol/L | 17.7 (−6.3 to 41.7) | .100 | 14.0 (−2.3 to 30.3) | .084 | −3.6 (−19.8 to 12.6) | .632 |
| Relative plasma citrulline, % | 66 (−13 to 145) | .077 | 42 (−10 to 94) | .100 | −23 (−74 to 29) | .344 |
Note: Data represent mean (95% CI), adjusted for the period‐specific baseline value of each end point, PS, and dietary fluid intake in a two‐sided analysis of covariance model. Part A was also adjusted for period, whereas parts A + B assumed no period effect.
Abbreviation: PS, parenteral support.
Dietary fluid intake
At days 20–22, 10‐mg apraglutide significantly decreased absolute and relative dietary fluid intake by an adjusted mean of 363 ml/day and −15%, respectively (Table 3). No significant changes were found for 5‐mg apraglutide compared with the placebo.
PS volume
At days 20–22, 10‐mg apraglutide significantly decreased the relative daily PS volume by −28% compared with the placebo (Table 3). A similar trend was seen for the absolute change in daily PS volume, which decreased by 469 ml/day (Table 3). No significant changes were found for 5‐mg apraglutide compared with the placebo.
Plasma citrulline
Plasma citrulline tended to increase by an adjusted mean of 17.7 μmol/L with 5‐mg apraglutide, corresponding to a relative increase of 66% (Table 3). The changes from baseline for the 10‐mg dose vs the placebo were numerically smaller and not significant.
Body weight and body composition
During the 5‐mg treatment period, all patients had an increase in fat mass with either a decrease or increase in lean body mass. During the 10‐mg treatment period, both increases and decreases in lean body mass and fat body mass were observed. A statistical trend towards increased fat mass was found for 5‐mg apraglutide compared with the placebo (Table S2). There was no significant change in lean body mass, body weight, or bone mineral content compared with the placebo. Apraglutide 10 mg did not significantly change any parameter of body composition or body weight (Table S2).
Apraglutide 5 mg vs 10 mg
No statistically significant differences were found between the lower and higher doses of apraglutide for any efficacy end point (Table 3, Table S2).
DISCUSSION
This first ever single‐center, double‐blind, crossover, randomized, placebo‐controlled phase 2 trial of a once‐weekly next generation GLP‐2 analog in patients with SBS‐IF found that apraglutide was safe and well tolerated and had significant benefits for intestinal fluid absorption, which was illustrated by increases in urine volume output compared with the placebo.
The safety of apraglutide was generally comparable to treatment with native GLP‐2 and other GLP‐2 analogs. 4 , 9 , 20 Common TRAEs were mild to moderate in severity, were consistent with the physiological effect of GLP‐2, and were not dose dependent. Injection site reactions were rare, reflecting the once‐weekly dosing regimen. Some AEs, such as edema and polyuria, would likely have resolved with adequate PS reductions. However, because of the short treatment period of 4 weeks, PS volume was only reduced if patients had clinical signs of fluid retention. According to the study protocol, AEs were defined as “any untoward medical occurrence.” Consequently, decreased stoma output was reported as an AE despite being a beneficial outcome.
In a recent study by Fuglsang et al, patients with SBS‐IF reported (on average) 2.5 hospital admissions per year, with CRBSIs accounting for 19% of admissions. 22 In our trial, five CRBSIs were observed in two patients during the 42‐week study period. None of the CRBSIs were assessed as treatment‐related, and events mainly occurred during the washout periods. This rate appears higher than previously reported in the literature because of the small sample size and was driven by one patient having four recurrent CRBSI events. This may be explained by several factors. First, there was concern during the trial that the patient did not adhere to the recommended central venous catheter care procedures. Secondly, CRBSI was defined as clinical symptoms of infection, elevation of biochemical blood tests indicating infection, and/or a positive blood culture. Consequently, the frequent trial visits may have increased the likelihood of detecting CRBSIs.
In this study, treatment with once‐weekly 5‐ and 10‐mg apraglutide significantly increased urine volume output compared with the placebo. Apraglutide 10 mg significantly increased urinary sodium excretion at the end of treatment and reduced spontaneous dietary fluid intake and PS volume near the end of treatment. Overall, no significant differences between the lower and higher doses of apraglutide were found. Collectively, the increases in urine volume output and urinary sodium excretion reflect increases in intestinal fluid and sodium absorption. This is important for patients with SBS‐IF, who are at high risk of dehydration, sodium depletion, and renal impairment, 23 , 24 and for whom fluid and electrolyte abnormalities are a major cause of morbidity and hospitalizations. 22 , 25
During long‐term apraglutide treatment, increases in urine volume output may enable further reductions in PS volume. Treatment with 5‐mg apraglutide increased the relative urine volume output in six of eight patients compared with the placebo. The changes were clinically relevant because they exceeded the 10% increase that would have triggered a reduction in PS volume in previous teduglutide clinical trials. 15 , 16 Some patients who initially had an increase in urine volume production on 5 mg had a diminished effect on 10 mg. These patients had a reduction in their PS volume, and/or spontaneous dietary fluid intake, in the period leading up to the end of treatment urine collection. Thus, individual patients illustrate that the effects on fluid absorption are dynamic and complex and that all treatment effects should be considered.
The effects of apraglutide on intestinal absorption of fluid and sodium were similar to those previously reported for the GLP‐2 analogs teduglutide and glepaglutide. 19 , 20 Teduglutide and glepaglutide significantly decreased fecal wet weight output and increased wet weight absorption. 7 , 18 Consistent with the improvements in wet weight absorption, teduglutide and 10‐mg glepaglutide significantly increased urine volume output by an adjusted mean of 555 and 368 g/day, respectively. Both GLP‐2 analogs significantly improved sodium absorption and consequently increased urinary sodium excretion by an adjusted mean of 53 and 34 mmol/day, respectively.
Intestinal biopsies were not collected in this trial. Although intestinal biopsies would provide evidence for the intestinotrophic effect, it is well established that GLP‐2 analogs increase villus height and crypt depth. 9 , 20 The morphological changes are believed to increase the absorptive surface. The plasma concentration of citrulline increased during both active treatment periods. This suggests an increased enterocyte mass and supports the expected intestinotrophic effects of apraglutide on the intestinal epithelium.
Large intraindividual and interindividual variability was observed for apraglutide's effect on body composition and body weight. Both increases and decreases in lean body mass and fat body mass were seen during active treatments. A statistical trend towards increased fat mass was found for 5‐mg apraglutide compared with the placebo. Short‐term treatment with native GLP‐2 (teduglutide and glepaglutide) has been associated with increases in lean body mass, 16 , 20 with either a decrease 5 or no change in fat mass. 16 , 20 The increase in lean body mass has been attributed to transient fluid retention at the start of GLP‐2 treatment prior to adjustment to the new equilibrium. 26 Fat mass estimation errors can occur because of variation in soft tissue hydration. 27 We believe that the effects on body composition may vary depending on the characteristics of the patient population, their hydration, and their nutrition status. Furthermore, the tendency to retain fluid may depend on sodium homeostasis. 28
Only a few studies have addressed what to expect when treatment with GLP‐2 or analogs is stopped. 26 , 29 This study showed that the treatment effect was not sustained and that parameters of absorption and citrulline concentrations reverted to baseline levels after a washout period of 4–6 weeks.
This study has a number of potential limitations. Because of the small sample size, no strong conclusions can be made for secondary end point, despite P‐values. In addition, 10‐mg apraglutide was compared with the placebo in the statistical analysis, even though the 10‐mg dose was administered in an open‐label regimen. Another limitation is that although urinary sodium excretion is considered an effective marker of fluid and sodium depletion, 24 , 25 , 30 , 31 high intraindividual variability in urinary sodium excretion has been described even among patients adhering to a fixed diet. 32 Consequently, a larger patient population is likely needed to demonstrate the full effect of apraglutide on urinary sodium excretion. Finally, the study included patients with previous exposure to GLP‐2 or other GLP‐2 analogs. This is because, although teduglutide has been approved for the treatment of SBS‐IF, patients in Denmark are currently not treated with teduglutide, except in clinical trials, because of its high cost. Carryover effects were eliminated by excluding patients who received GLP‐2 or GLP‐2 analogs within the 3 months prior to enrollment.
In conclusion, once‐weekly 5‐ and 10‐mg apraglutide dosing for 4 weeks was well tolerated and generally safe. This trial was the first to demonstrate the clinical effects of a GLP‐2 analog with weekly dosing; previous trials used daily dosing. 5 , 9 , 15 , 20 Once‐weekly treatment with apraglutide increased urine volume output and improved other markers of intestinal rehabilitation in patients with SBS‐IF at both tested doses. No additional benefit appeared to be gained by increasing the dose. The currently available GLP‐2 analog, teduglutide, is administered daily and requires multiple reconstitution steps prior to each injection. 33 Apraglutide is expected to positively contribute to patient care and compliance by enabling once‐weekly (instead of daily) administration, which in turn may improve quality of life. 34 The reduced injection frequency may increase patient acceptability and decrease the risk of injection site reactions. A multicenter, multinational phase 3 trial has been initiated to confirm the safety and efficacy of apraglutide and explore potential additional benefits linked to its longer half‐life.
FUNDING INFORMATION
Funding for this study was provided by VectivBio AG
CONFLICT OF INTERESTS
Palle B. Jeppesen has served as a consultant and speaker for VectivBio AG and was the principal investigator in this study. Johanna Eliasson has served as a consultant for VectivBio AG and was a study investigator. Mark K. Hvistendahl and Nanna Freund were study investigators. Federico Bolognani and Christian Meyer are employees of VectivBio AG.
AUTHOR CONTRIBUTIONS
Johanna Eliasson, Mark K. Hvistendahl, Federico Bolognani, Christian Meyer, and Palle B. Jeppesen designed the research. Johanna Eliasson, Nanna Freund, and Palle B. Jeppesen conducted the research. Johanna Eliasson and Palle B. Jeppesen analyzed the data. Johanna Eliasson wrote the paper. Mark K. Hvistendahl, Nanna Freund, Federico Bolognani, Christian Meyer,and Palle B. Jeppesen provided a constructive review of the manuscript. Johanna Eliasson had primary responsibility for the final paper. All authors read and approved the final manuscript.
Supporting information
Supplementary Material
Additional supplementary material may be found online in the Supplementary Material section at the end of the article.
Eliasson J, Hvistendahl MK, Freund N, Bolognani F, Meyer C, Jeppesen PB. Apraglutide, a novel glucagon‐like peptide 2 analog, improves fluid absorption in patients with short bowel syndrome intestinal failure: Findings from a placebo‐controlled, randomized phase 2 trial. JPEN J Parenter Enteral Nutr. 2022;46:896–904. 10.1002/jpen.2223
REFERENCES
- 1. Jeppesen PB. Spectrum of short bowel syndrome in adults: intestinal insufficiency to intestinal failure. JPEN J Parenter Enteral Nutr. 2014;38(Suppl 1):8S‐13S. [DOI] [PubMed] [Google Scholar]
- 2. Pironi L, Arends J, Baxter J, et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34(2):171‐180. [DOI] [PubMed] [Google Scholar]
- 3. Jeppesen PB. The long road to the development of effective therapies for the short gut syndrome: a personal perspective. Dig Dis Sci. 2019;64(10):2717‐2735. [DOI] [PubMed] [Google Scholar]
- 4. Jeppesen PB, Hartmann B, Hansen BS, et al. Impaired meal stimulated glucagon‐like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut. 1999;45(4):559‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon‐like peptide 2 improves nutrient absorption and nutritional status in short‐bowel patients with no colon. Gastroenterology. 2001;120(4):806‐815. [DOI] [PubMed] [Google Scholar]
- 6. Wojdemann M, Wettergren A, Hartmann B, Holst JJ. Glucagon‐like peptide‐2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol. 1998;33(8):828‐832. [DOI] [PubMed] [Google Scholar]
- 7. Hvistendahl MK, Naimi RM, Enevoldsen LH, Madsen JL, Fuglsang S, Jeppesen PB. Effect of glepaglutide, a long‐acting glucagon‐like peptide‐2 analog, on gastrointestinal transit time and motility in patients with short bowel syndrome: findings from a randomized trial. JPEN J Parenter Enteral Nutr. 2020;44(8):1535‐1544. [DOI] [PubMed] [Google Scholar]
- 8. Wojdemann M, Wettergren A, Hartmann B, Hilsted L, Holst JJ. Inhibition of sham feeding‐stimulated human gastric acid secretion by glucagon‐like peptide‐2. J Clin Endocrinol Metab. 1999;84(7):2513‐2517. [DOI] [PubMed] [Google Scholar]
- 9. Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX‐0600), a dipeptidyl peptidase IV resistant glucagon‐like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54(9):1224‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP‐2‐driven improvement of gut permeability. Gut. 2009;58(8):1091‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benjamin MA, McKay DM, Yang PC, Cameron H, Perdue MH. Glucagon‐like peptide‐2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47(1):112‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bremholm L, Hornum M, Andersen UB, Hartmann B, Holst JJ, Jeppesen PB. The effect of glucagon‐like peptide‐2 on mesenteric blood flow and cardiac parameters in end‐jejunostomy short bowel patients. Regul Pept. 2011;168(1‐3):32‐38. [DOI] [PubMed] [Google Scholar]
- 13. Marier JF, Beliveau M, Mouksassi MS, et al. Pharmacokinetics, safety, and tolerability of teduglutide, a glucagon‐like peptide‐2 (GLP‐2) analog, following multiple ascending subcutaneous administrations in healthy subjects. J Clin Pharmacol. 2008;48(11):1289‐1299. [DOI] [PubMed] [Google Scholar]
- 14. Marier JF, Mouksassi MS, Gosselin NH, Beliveau M, Cyran J, Wallens J. Population pharmacokinetics of teduglutide following repeated subcutaneous administrations in healthy participants and in patients with short bowel syndrome and Crohn's disease. J Clin Pharmacol. 2010;50(1):36‐49. [DOI] [PubMed] [Google Scholar]
- 15. Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143(6):1473‐1481. [DOI] [PubMed] [Google Scholar]
- 16. Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, SJ O'Keefe. Randomised placebo‐controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60(7):902‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wisniewski K, Sueiras‐Diaz J, Jiang G, et al. Synthesis and pharmacological characterization of novel glucagon‐like peptide‐2 (GLP‐2) analogues with low systemic clearance. J Med Chem. 2016;59(7):3129‐3139. [DOI] [PubMed] [Google Scholar]
- 18. Bolognani F, Gal P, Moerland M, et al. The pharmakokinetic and pharmacodynamic relationship between apraglutide and citrulline: a randomized, placebo‐controlled, double‐blind study in healthy volunteers. Clin Nutr ESPEN. 2020;40:413‐414. [Google Scholar]
- 19. Hargrove DM, Alagarsamy S, Croston G, et al. Pharmacological characterization of apraglutide, a novel long‐acting peptidic glucagon‐like peptide‐2 agonist, for the treatment of short bowel syndrome. J Pharmacol Exp Ther. 2020;373(2):193‐203. [DOI] [PubMed] [Google Scholar]
- 20. Naimi RM, Hvistendahl M, Enevoldsen LH, et al. Glepaglutide, a novel long‐acting glucagon‐like peptide‐2 analogue, for patients with short bowel syndrome: a randomised phase 2 trial. Lancet Gastroenterol Hepatol. 2019;4(5):354‐363. [DOI] [PubMed] [Google Scholar]
- 21. Crenn P, Coudray‐Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology. 2000;119(6):1496‐1505. [DOI] [PubMed] [Google Scholar]
- 22. Fuglsang KA, Brandt CF, Scheike T, Jeppesen PB. Hospitalizations in patients with nonmalignant short‐bowel syndrome receiving home parenteral support. Nutr Clin Pract. 2020;35(5):894‐902. [DOI] [PubMed] [Google Scholar]
- 23. Lauverjat M, Hadj Aissa A, Vanhems P, Bouletreau P, Fouque D, Chambrier C. Chronic dehydration may impair renal function in patients with chronic intestinal failure on long‐term parenteral nutrition. Clin Nutr. 2006;25(1):75‐81. [DOI] [PubMed] [Google Scholar]
- 24. Ladefoged K, Olgaard K. Sodium homeostasis after small‐bowel resection. Scand J Gastroenterol. 1985;20(3):361‐369. [DOI] [PubMed] [Google Scholar]
- 25. Messaris E, Sehgal R, Deiling S, et al. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rectum. 2012;55(2):175‐180. [DOI] [PubMed] [Google Scholar]
- 26. Jeppesen PB, Lund P, Gottschalck IB, et al. Short bowel patients treated for two years with glucagon‐like peptide 2: effects on intestinal morphology and absorption, renal function, bone and body composition, and muscle function. Gastroenterol Res Pract. 2009;2009:616054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pietrobelli A, Wang Z, Formica C, Heymsfield SB. Dual‐energy X‐ray absorptiometry: fat estimation errors due to variation in soft tissue hydration. Am J Physiol. 1998;274(5):E808‐E816. [DOI] [PubMed] [Google Scholar]
- 28. Paller MS, Schrier RW. Pathogenesis of sodium and water retention in edematous disorders. Am J Kidney Dis. 1982;2(2):241‐254. [DOI] [PubMed] [Google Scholar]
- 29. Compher C, Gilroy R, Pertkiewicz M, et al. Maintenance of parenteral nutrition volume reduction, without weight loss, after stopping teduglutide in a subset of patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2011;35(5):603‐609. [DOI] [PubMed] [Google Scholar]
- 30. Sterns RH. Disorders of plasma sodium–causes, consequences, and correction. N Engl J Med. 2015;372(1):55‐65. [DOI] [PubMed] [Google Scholar]
- 31. Armstrong LE. Assessing hydration status: the elusive gold standard. J Am Coll Nutr. 2007;26(Suppl 5):575S‐84S. [DOI] [PubMed] [Google Scholar]
- 32. Weaver CM, Martin BR, McCabe GP, et al. Individual variation in urinary sodium excretion among adolescent girls on a fixed intake. J Hypertens. 2016;34(7):1290‐1297. [DOI] [PubMed] [Google Scholar]
- 33. Shire. Product information. GATTEX® (teduglutide) instructions for use, revised 05/2019. https://www.shirecontent.com/PI/PDFS/Gattex_USA_ENG.pdf (accessed Aug 19, 2021)
- 34. Chen K, Mu F, Xie J, et al. Impact of teduglutide on quality of life among patients with short bowel syndrome and intestinal failure. JPEN J Parenter Enteral Nutr. 2020;44(1):119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Additional supplementary material may be found online in the Supplementary Material section at the end of the article.


