Abstract
Purpose
The vitreous proteome might provide an attractive gateway to discriminate between various uveitis aetiologies and gain novel insights into the underlying pathophysiological processes. Here, we investigated 180 vitreous proteins to discover novel biomarkers and broaden disease insights by comparing (1). primary vitreoretinal lymphoma ((P)VRL) versus other aetiologies, (2). sarcoid uveitis versus tuberculosis (TB)‐associated uveitis and (3). granulomatous (sarcoid and TB) uveitis versus other aetiologies.
Methods
Vitreous protein levels were determined by proximity extension assay in 47 patients with intraocular inflammation and a prestudy diagnosis (cohort 1; training) and 22 patients with a blinded diagnosis (cohort 2; validation). Differentially expressed proteins identified by t‐tests on cohort 1 were used to calculate Youden’s indices. Pathway and network analysis was performed by ingenuity pathway analysis. A random forest classifier was trained to predict the diagnosis of blinded patients.
Results
For (P)VRL stratification, the previously reported combined diagnostic value of IL‐10 and IL‐6 was confirmed. Additionally, CD70 was identified as potential novel marker for (P)VRL. However, the classifier trained on the entire cohort (cohort 1 and 2) relied primarily on the interleukin score for intraocular lymphoma diagnosis (ISOLD) or IL‐10/IL‐6 ratio and only showed a supportive role for CD70. Furthermore, sarcoid uveitis displayed increased levels of vitreous CCL17 as compared to TB‐associated uveitis.
Conclusion
We underline the previously reported value of the ISOLD and the IL‐10/IL‐6 ratio for (P)VRL identification and present CD70 as a potentially valuable target for (P)VRL stratification. Finally, we also show that increased CCL17 levels might help to distinguish sarcoid uveitis from TB‐associated uveitis.
Keywords: proteomics, proximity extension assay, intraocular, vitreous, (P)VRL, sarcoidosis, tuberculosis, uveitis
Introduction
Uveitis is an important cause of vision loss. In developed nations, vision loss is estimated to occur in 5–10% of all uveitis cases (London et al. 2010). In stark contrast, 25% of all sight loss in developing countries is attributed to uveitis (Emmett T Cunningham & Sivakumar 2010; London et al. 2010). Based on anatomical localization, uveitis can be classified as anterior, intermediate, posterior or pan‐uveitis (Seve et al. 2017). Aetiologically uveitis can roughly be classified into infectious uveitis and non‐infectious uveitis, while masquerade syndrome displays highly overlapping clinical features (Krishna et al. 2017). Uveitis classification and characterization, including genetic features (e.g. human leukocyte antigen (HLA)‐B27/A29/B51) and intraocular cellular and cytokine measurement (e.g. interleukin (IL)‐6 and IL‐10), are vital for diagnostic, prognostic and therapeutic purposes (Seve et al. 2017; Hou et al. 2020). Yet, swift and appropriate diagnosis of the uveitis entity can still be troublesome. This is, at least partly, due to substantial overlap in anatomical localization and clinical symptoms between different uveitis aetiologies, as well as difficulties to further stratify based on potential infectious cause (Krishna et al. 2017; Seve et al. 2017). These challenges often delay diagnosis and consequently initiation of optimal treatment, potentially resulting in an increased risk of vision loss (Krishna et al. 2017).
The intraocular proteome provides an attractive gateway to unravel differences in pathophysiological pathways that underlie different uveitis entities (Velez et al. 2016; Kasudhan et al. 2018; Dao et al. 2020; Mohanty et al. 2020). Importantly, a proteomics approach may identify protein markers with the potential to improve diagnosis, monitor disease activity or that represent potential treatment targets.
Primary vitreoretinal lymphoma ((P)VRL) often masquerades intermediate and/or posterior uveitis, which severely hampers reaching diagnosis. However, high vitreous levels of IL‐10 and a vitreous IL‐10/IL‐6 ratio >1 can be of help to distinguish (P)VRL from infectious and non‐infectious uveitis (Read et al. 2002; Sagoo et al. 2014; Carbonell et al. 2021). In line with other studies (Cassoux et al. 2007; Saleh et al. 2012; Fisson et al. 2013; Caraballo et al. 2014; Kuo et al. 2020), we also observed the discriminative value of a vitreous IL‐10/IL‐6 ratio >1 to identify (P)VRL cases, which in our study yielded a sensitivity and specificity of 100% (de Hoog et al. 2019). Despite the IL‐10/IL‐6 ratio being a robust marker for (P)VRL, several studies have reported a minority of (P)VRL patients presenting with IL‐10/IL‐6 ratios <1 (Costopoulos et al. 2016; Kuo et al. 2020), indicating that additional diagnostic tools are required. The interleukin score for intraocular lymphoma diagnosis (ISOLD) proposed by Costopoulos et al. in 2016 provides a quantification of the probability of (P)VRL instead of a cut‐off value based on the IL‐10/IL‐6 ratio, thereby slightly improving diagnostic performance (Costopoulos et al. 2016; Kuo et al. 2020). Notably, integration of cellular analysis (e.g. B‐lymphocytes, SmIgκ or SmIgλ, CD4+ T‐lymphocytes, CD8+ T‐lymphocytes, monocytes, natural killer cells) with 27 measured soluble parameters and the IL‐10/IL‐6 ratio in our previous study did not further improve discrimination between (P)VRL and other uveitis entities (de Hoog et al. 2019). Although several clusters could be identified among the non‐(P)VRL uveitis cases based on soluble parameters, none of these clusters represented a single uveitis aetiology (de Hoog et al. 2019). Improved classification of such cases may thus require additional, or more uveitis type‐specific, soluble markers.
In this study, we used a targeted proteomics approach to measure 180 proteins related to immunity, oncology and neurology in vitreous from 69 patients, who presented with intraocular inflammation. The specific aim was to identify biomarkers or combinations of biomarkers to discriminate between various underlying aetiologies.
Material and methods
Patients and sample collection
Vitreous samples were collected by pars plana vitrectomy with a 3cc syringe connected to the aspiration line of the vitrectome (Constellation; Alcon, Fort Worth, TX, USA). To avoid intraocular fluid infusion during vitreous collection, eye pressure was maintained by indentation or temporal air infusion. In total, 1.5–2.0 ml vitreous was collected. Within 30 minutes postcollection, the samples were put on ice at the Laboratory Medical Immunology, Erasmus MC, University Medical Center, Rotterdam, the Netherlands. Subsequently (within two hours), samples were centrifuged for five minutes at 500g, supernatant was collected and directly stored at −80°C until further use.
In total, 69 patients presenting with intraocular inflammation who visited the outpatient clinics of the departments of Ophthalmology and Clinical Immunology of the Erasmus MC, University Medical Center, Rotterdam, the Netherlands, or the Rotterdam Eye Hospital, Rotterdam, the Netherlands, were included. In all these patients, vitreous collection was performed as part of the diagnostic workup and prior to treatment for the underlying condition. During the present study, the final diagnosis was known for 47 included patients (training cohort, cohort 1) while work‐up results were initially blinded for the remaining 22 cases (validation cohort, cohort 2). In addition to these 69 patients, two (P)VRL patients treated with rituximab and/or methotrexate were included.
All included patients underwent full ophthalmologic examination. Based on the clinical manifestations, a diagnostic workup according to the Dutch national uveitis guideline was performed to elucidate the cause of intraocular inflammation. This workup included erythrocyte sedimentation rate, blood count, serum angiotensin converting enzyme levels, QuantiFERON‐TB Gold test (QFT), syphilis serum screening, HLA‐B27 determination in case of anterior uveitis or other relevant radiological examinations. Vitreous specimens were routinely analysed for cytology and screened for infectious agents through microbiological culture, polymerase chain reaction and Goldmann‐Witmer coefficient (GWC) in routine clinical/diagnostic laboratories of the Erasmus MC. The final clinical diagnosis was established by the ophthalmologist based on the combinations of clinical characteristics, imaging and laboratory tests. Of note, IL‐10 and IL‐6 measurements were not part of the diagnostic workup. Patient group characteristics are presented in Table 1, while an overview of individual patient characteristics is provided in Table S1.
Table 1.
Patient group characteristics.
| Uveitis aetiology | Mean age (SD) | Male/Female |
|---|---|---|
| All intraocular inflammation (N = 69) | 59 (17) | 29/40 |
| Annotated intraocular inflammation (n = 47) | 59 (16) | 17/30 |
| Blind tested intraocular inflammation (n = 22) | 59 (20) | 12/10 |
| Cohort 1 (N = 47; annotated) | ||
| Idiopathic Uveitis (n = 8) | 64 (12) | 2/6 |
| (P)VRL Uveitis (n = 7) | 57 (21) | 5/2 |
| Granulomatous Uveitis (N = 21) | 61 (15) | 4/17 |
|
56 (14) | 3/3 |
|
63 (13) | 1/14 |
| Uveitis associated with systemic disease (n = 2)* | 62 (21) | 0/2 |
| Non‐TB Infectious Uveitis (n = 6) | 46 (21) | 6/0 |
| Other (n = 3)** | 64 (8) | 0/3 |
| Cohort 2 (N = 22; Blind tested) | ||
| Idiopathic Uveitis (n = 6) | 63 (14) | 3/3 |
| (P)VRL Uveitis (n = 5) | 73 (14) | 4/1 |
| Uveitis associated with systemic disease (n = 2)*** | 40 (6) | 1/1 |
| Non‐TB Infectious Uveitis (n = 5) | 59 (20) | 2/3 |
| Sarcoid Uveitis (n = 2) | 32 (16) | 1/1 |
| Leukemia (n = 1) | 85 (n/a) | 1/0 |
| Other (n = 1)**** | 77 (n/a) | 0/1 |
| Treated (P)VRL Uveitis (n = 2) | 67 (12) | 1/1 |
(P)VRL = primary vitreoretinal lymphoma, SD = standard deviation, TB = tuberculosis.
One multiple sclerosis patient, one colitis ulcerosa patient.
One vasoproliferative tumour patient, one patients with floaters and one retinal detachment patient.
Two multiple sclerosis patients.
One patients with a complicated cataract surgery.
Targeted proteomics assay
Vitreous levels of 180 proteins, associated with immunity, oncology and neurology, were measured in close collaboration with Olink using their proximity extension immunoassay (PEA; Olink Proteomics, Uppsala Sweden) with the immune‐oncology and neurology panels (Table S2). In short, 1 µl of vitreous was used for each panel and incubated with pairs of antibodies coupled to cDNA strands. Upon binding of the target protein by the antibody pair, the close proximity of the antibodies allows dimerization of the complementary DNA strands and amplification by polymerase chain reaction (PCR). Detected PCR products were normalized, and a protein expression value (NPX), serving as a surrogate marker for protein concentration, was calculated (Solier & Langen 2014).
Validation of PEA results by Luminex and ELISA
Vitreous levels of C‐C motif chemokine ligand (CCL)2, CCL17, CD40, C‐X‐C motif chemokine ligand (CXCL)13, FASL, IL‐6 and IL‐10 were measured by Luminex magnetic bead‐based assays (R&D Systems Europe, Abingdon, United Kingdom). Ezrin (EZR) was measured by ELISA (LSBio, Seattle, WA, USA). The assays were conducted according to the manufacturer’s instructions.
Analysis and statistics
Proteins detected with PEA in more than 25% of all vitreous samples were included in the final data analysis. This cut‐off value resulted in the inclusion of 76 and 88 out of the 92 proteins from the immune‐oncology and neurology panels, respectively. Next to these proteins, the IL‐10/IL‐6 ratio and ISOLD [(−12.208 + 4.648 × log(IL‐10 + 1) − 1.669 × log(IL‐6 + 1))] were calculated. Individual students’ t‐tests were performed for each included protein followed by multiple testing correction using false discovery rate. The seven proteins that were further validated by ELISA or bead‐based assay were compared by Mann–Whitney U. A corrected p < 0.05 was considered significantly different. Initial assessment of the diagnostic potential of the identified significantly differentially expressed proteins plus the IL‐10/IL‐6 ratio and the ISOLD was performed by calculation of Youden’s indices on cohort 1 (training cohort). To access coherent predictive value of the top five discriminative (highest Youden’s indices) biomarkers stratifying (P)VRL from other uveitis aetiologies within cohort 1, Youden’s indices for these biomarkers were reanalysed on cohort 2 (validation cohort).
Disease pathway and network identification was done by loading the proteins that were differently expressed between (P)VRL and the other uveitis aetiologies and the proteins differentially expressed between granulomatous uveitis (sarcoid and TB) and the other aetiologies into ingenuity pathway analysis, which identifies pathways that encompass the provided proteins. Extracted pathways were filtered for human experimentally observed results.
Cohort 1 was used to train a random forest classifier for (P)VRL. Cohort 2 (initially blinded) was classified and subsequently compared to the final clinical diagnosis. The model was cross‐validated using 10 unique partitions. The final model consisted of 500 trees and considered 165 (all) features at each split, with an error rate of 0%. Classification by machine learning was conducted in R using the caret package (Max Kuhn et al. 2020; Team 2020). Figures were produced using the package ggplot2 (Wickham, 2016). Performance of prediction was evaluated by comparing predictions to clinical diagnosis using a confusion matrix. Feature permutation importance was calculated to evaluate the relative contribution of individual protein markers to the predictive capacity of the classifier. To avoid misconceptions due to overfitting on the relatively limited training cohort, both cohorts were combined into one large master cohort. A new random forest classifier was trained based on this expanded cohort to study the relative shifts in feature permutation importance for the included markers upon expansion of the cohort.
Results
Patients characteristics
In this study, vitreous samples from patients with ocular inflammation caused by various underlying aetiologies were included during the diagnostic workup and divided over two cohorts. Cohort 1 contained vitreous samples from 47 patients selected from a prospective cohort study that we conducted from 2012 to 2015 (de Hoog et al. 2019). This cohort consisted of patients with the following diagnoses: 15 sarcoid uveitis, 7 (P)VRL, 6 TB‐associated uveitis, 6 non‐TB infectious uveitis, 2 uveitis associated with systemic disease, 8 idiopathic uveitis and 3 patients with masquerade syndrome other than (P)VRL (Table S1). Cohort 2 consisted of vitreous samples from 22 patients with intraocular inflammation for whom further work‐up results and the final clinical diagnosis were blinded. Retrospectively, this cohort consisted of 2 sarcoid uveitis, 5 (P)VRL, 5 non‐TB infectious uveitis, 2 uveitis associated with systemic disease, 6 idiopathic uveitis and two patients with masquerade syndrome other than (P)VRL (Table S1). This cohort was used to validate the findings from cohort 1. To examine the effect of treatment on vitreous soluble parameters in (P)VRL, two patients treated with rituximab and/or methotrexate were included. An overview of group characteristics is presented in Table 1.
Uveitis aetiologies are characterized by differential expression of vitreous proteins
In order to evaluate whether the vitreous proteome could distinguish between different uveitis entities and (P)VRL, a total of 180 proteins were measured using PEA. This revealed 11 significantly more abundant proteins in (P)VRL as compared to all other uveitis aetiologies, of which EZR (p = 3.5E‐10), CCL8 (p = 9.5E‐04) and CCL2 (p = 7.6E‐03) were most significantly differentially expressed. Moreover, the calculated IL‐10/IL‐6 ratio (p = 5.1E‐3) and ISOLD (p = 1.2E‐7) value were significantly increased in (P)VRL patients as compared to all other uveitis cases (Fig. 1A; Table 2; Table S3). Comparison between sarcoid uveitis and TB‐associated uveitis revealed significantly higher levels of CCL17 (p = 1.7E‐3) and CXCL13 (p = 1.3E‐2) in sarcoid uveitis (Fig. 1B; Table 2; Table S3). Comparison of granulomatous uveitis (sarcoidosis and TB) with all other uveitis aetiologies revealed 16 proteins, of which EZR (p = 2.4E‐2), CD8A (p = 2.4E‐2) and GZMB (p = 2.4E‐2) were most significantly under expressed in vitreous of granulomatous uveitis (Fig. 1C; Table 2; Table S3).
Fig. 1.
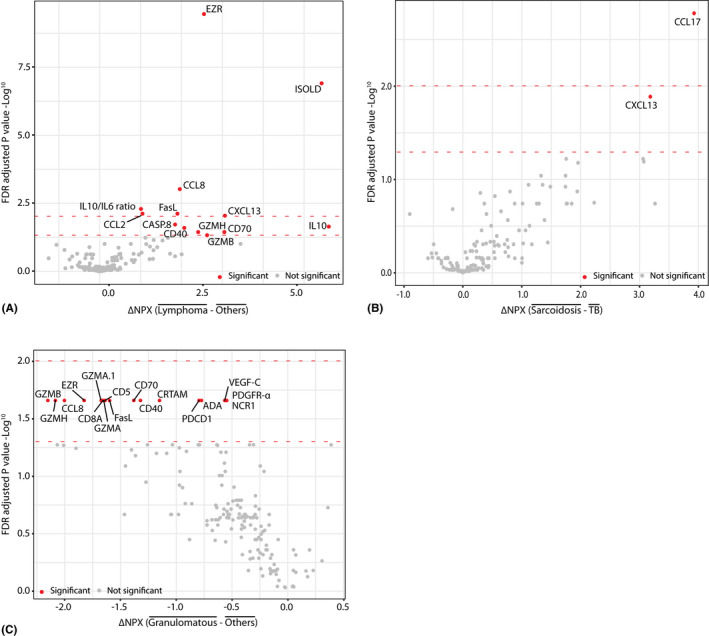
Differential expression of vitreous proteins and calculated biomarkers obtained from proximity extension assay (PEA) results within cohort 1, comparing (A) primary vitreoretinal lymphoma ((P)VRL) versus all other aetiologies (B) (suspected) sarcoid uveitis versus TB‐associated uveitis cases and (C) granulomatous (sarcoid and TB) uveitis versus all other uveitis aetiologies. Statistical analysis was performed in R, biomarkers were analysed by individual students' t‐tests followed by false discovery rate (FDR) multiple testing correction. The lower red dotted line represents the FDR corrected p‐value of 0.05, and upper red dotted line represents the FDR corrected p‐value of 0.01. NPX = normalized protein expression.
Table 2.
Top five significantly differentially expressed proteins, based on cohort 1, detected by PEA, comparing (P)VRL with all other aetiologies, sarcoid uveitis with TB‐associated uveitis and granulomatous (sarcoid and TB) uveitis with all other aetiologies
| Protein | p‐value | FDR adjusted p‐value | ∆ NPX | Fold change |
|---|---|---|---|---|
| (P)VRL versus other aetiologies | ||||
| EZR | 2.1E‐12 | 3.5E‐10 | 3.2 | 1.9 |
| ISOLD | 1.5E‐09 | 1.2E‐07 | n/a | n/a |
| CCL8 | 1.7E‐05 | 9.5E‐04 | 2.5 | 1.5 |
| IL‐10/IL‐6.ratio | 1.2E‐04 | 5.1E‐03 | n/a | n/a |
| CCL2 | 2.6E‐04 | 7.6E‐03 | 1.3 | 1.1 |
| Sarcoidosis versus TB‐associated | ||||
| CCL17 | 9.9E‐06 | 1.7E‐03 | 3.9 | 4.2 |
| CXCL13 | 1.6E‐04 | 1.3E‐02 | 3.2 | 1.7 |
| Granulomatous (sarcoidosis and TB‐associated) versus other aetiologies | ||||
| EZR | 1.5E‐04 | 2.4E‐02 | −1.8 | 0.6 |
| CD8A | 4.5E‐04 | 2.4E‐02 | −1.7 | 0.4 |
| GZMB | 5.4E‐04 | 2.4E‐02 | −2.1 | 0.4 |
| GZMH | 6.8E‐04 | 2.4E‐02 | −2.1 | 0.5 |
| CRTAM | 9.9E‐04 | 2.4E‐02 | −1.2 | 0.1 |
(P)VRL = primary vitreoretinal lymphoma, FDR = false discovery rate, NPX = normalized protein expression, PEA = proximity extension assay, TB = tuberculosis.
To determine the diagnostic potential for the differentially expressed proteins, IL‐10/IL‐6 ratio and ISOLD, optimal sensitivity and specificity was determined by calculated Youden’s indices based on cohort 1. For this, the following comparisons were made: (1). (P)VRL versus all other uveitis aetiologies, (2). sarcoid uveitis versus TB‐associated uveitis and (3). granulomatous (sarcoid and TB) uveitis versus all other uveitis aetiologies (Table 3 and Table 4). Based on Youden’s indices, the markers previously described as diagnostic markers for (P)VRL, namely IL‐10/IL‐6 ratio, ISOLD and high IL‐10, showed a sensitivity and specificity of 100%. Interestingly, CD70, the ligand for CD27, also distinguished (P)VRL cases from other uveitis aetiologies with a sensitivity and specificity of 100%. Furthermore, EZR showed a similar aptitude (sensitivity 100%, specificity 98%) to discriminate (P)VRL cases from other uveitis aetiologies. Albeit with a lower sensitivity (86%) and specificity (80%), our current findings also underline CCL2 as a discriminating factor for (P)VRL, in line with our previous report (de Hoog et al. 2019).
Table 3.
Optimal sensitivity and specificity as determined by Youden’s index calculation, based on cohort 1, comparing (P)VRL with all other aetiologies, sarcoid uveitis with TB‐associated uveitis and granulomatous with all other aetiologies.
| Protein | Youden’s index | Sensitivity | Specificity | AUC | Cut‐off value |
|---|---|---|---|---|---|
| (P)VRL versus other aetiologies | |||||
| EZR | 0.98 | 1.00 | 0.98 | 0.99 | 5.90 |
| ISOLD | 1.00 | 1.00 | 1.00 | 1.00 | −10.07 |
| CCL8 | 0.68 | 0.86 | 0.83 | 0.82 | 6.79 |
| IL‐10/IL‐6 ratio | 1.00 | 1.00 | 1.00 | 1.00 | 0.79 |
| CCL2 | 0.66 | 0.86 | 0.80 | 0.84 | 12.88 |
| FasL | 0.70 | 1.00 | 0.70 | 0.90 | 4.45 |
| CXCL13 | 0.78 | 0.86 | 0.93 | 0.90 | 10.74 |
| CASP.8 | 0.78 | 1.00 | 0.78 | 0.88 | 6.84 |
| IL‐10 | 1.00 | 1.00 | 1.00 | 1.00 | 4.88 |
| CD40 | 0.88 | 1.00 | 0.88 | 0.94 | 10.11 |
| GZMH | 0.63 | 0.86 | 0.78 | 0.87 | 4.28 |
| CD70 | 1.00 | 1.00 | 1.00 | 1.00 | 3.52 |
| GZMB | 0.63 | 0.86 | 0.78 | 0.87 | 3.85 |
| Sarcoidosis versus TB‐associated | |||||
| CCL17 | 0.45 | 0.67 | 0.78 | 0.70 | 5.22 |
| CXCL13 | 0.18 | 0.87 | 0.31 | 0.47 | 5.77 |
| Granulomatous (sarcoidosis and TB‐associated) versus other aetiologies | |||||
| EZR | 0.55 | 0.89 | 0.67 | 0.80 | 3.04 |
| CD8A | 0.47 | 0.62 | 0.86 | 0.76 | 1.57 |
| GZMB | 0.47 | 0.62 | 0.86 | 0.77 | 3.47 |
| GZMH | 0.49 | 0.73 | 0.76 | 0.75 | 3.07 |
| CRTAM | 0.51 | 0.65 | 0.86 | 0.79 | 1.28 |
| PDGFR‐α | 0.54 | 0.54 | 1.00 | 0.73 | 1.89 |
| CD5 | 0.44 | 0.54 | 0.91 | 0.75 | 3.21 |
| FasL | 0.46 | 0.65 | 0.81 | 0.76 | 4.30 |
| VEGFC | 0.52 | 0.62 | 0.91 | 0.74 | −0.49 |
| PDCD1 | 0.48 | 0.58 | 0.91 | 0.77 | 1.50 |
| ADA | 0.46 | 0.65 | 0.81 | 0.76 | −0.15 |
| CD40 | 0.49 | 0.54 | 0.95 | 0.75 | 9.71 |
| CCL8 | 0.44 | 0.54 | 0.91 | 0.76 | 6.39 |
| CD70 | 0.58 | 0.77 | 0.81 | 0.82 | 1.08 |
| GZMA | 0.45 | 0.69 | 0.76 | 0.75 | 4.41 |
| NCR1 | 0.40 | 0.54 | 0.86 | 0.69 | 1.33 |
(P)VRL = primary vitreoretinal lymphoma, AUC = area under the curve, TB = tuberculosis.
Vitreous CCL17 levels were found to distinguish sarcoid uveitis from TB‐associated uveitis, with a sensitivity of 67% and a specificity of 78% (Table 3).
Proteins differentially expressed between granulomatous uveitis and all other aetiologies yielded either poor sensitivity or specificity, limiting their potential as a diagnostic biomarker (Table 3).
Table 4 Optimal sensitivity and specificity as determined by Youden’s index calculation, based on cohort 2, comparing untreated (P)VRL with all other aetiologies.
| Protein | Youden’s index | Sensitivity | Specificity | AUC | Cut‐off value |
|---|---|---|---|---|---|
| (P)VRL versus other aetiologies | |||||
| IL‐10/IL‐6 ratio | 1.00 | 1.00 | 1.00 | 1.00 | 0.69 |
| ISOLD | 1.00 | 1.00 | 1.00 | 1.00 | ‐9.88 |
| IL‐10 | 1.00 | 1.00 | 1.00 | 1.00 | 6.13 |
| CD70 | 0.60 | 0.60 | 1.00 | 0.79 | 3.58 |
| EZR | 0.60 | 0.60 | 1.00 | 0.78 | 6.21 |
Top five discriminative biomarkers based on Youden’s index calculations on cohort 1.
(P)VRL = primary vitreoretinal lymphoma, AUC = area under the curve.
Functional pathway and network analysis
To identify pathways and networks associated with disease, proteins differentially expressed between (P)VRL and all the other aetiologies and proteins differentially expressed between granulomatous uveitis and all the other aetiologies were used.
Pathway analysis revealed ‘autoimmune thyroid disease signalling’ and ‘allograft rejection signalling’ as the top two significant identified pathways comparing (P)VRL versus other aetiologies (Table S4). Comparison between granulomatous uveitis and the other aetiologies revealed ‘systemic lupus erythematous in B‐cell signalling’ as the most significant pathway related to granulomatous uveitis, with ‘autoimmune thyroid disease signalling’ as second most significant pathway (Table S5).
Further detailed analysis of (P)VRL versus other aetiologies or granulomatous uveitis versus other aetiologies identified three networks for both. The top ranked network with differentially expressed proteins from (P)VRL analysis contained 9 of the 11 (P)VRL associated proteins. The top ranked network with differentially expressed proteins from granulomatous uveitis analysis contained 14 of the 16 identified proteins associated with granulomatous uveitis. Both of these networks were associated with ‘cell death and survival’, ‘cell‐to‐cell signalling’ and ‘interaction and haematological system development and function’ (Fig. 2A,B).
Fig. 2.
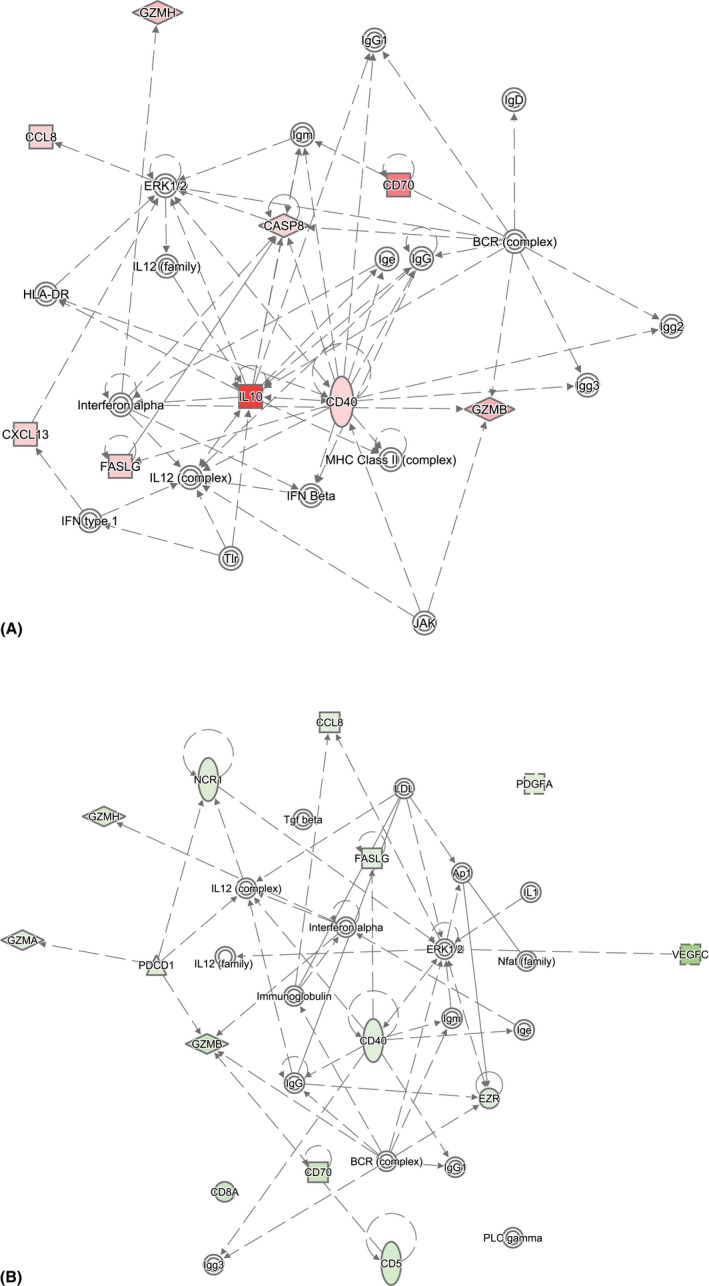
Network analysis based on differentially expressed vitreous proteins comparing (A) (P)VRL versus all other aetiologies and (B) granulomatous versus all other aetiologies. Differentially upregulated proteins are depicted in red, while downregulated proteins are depicted in green. A solid line represents a direct protein–protein interaction, while a dotted line indicates an indirect relationship. Networks were filtered to only include human experimentally observed data.
Validation of PEA‐identified differentially expressed proteins
Next, we wanted to validate discriminating proteins identified by PEA. Therefore, CCL2, CCL17, CD40, CXCL13, FASL, IL‐10 and EZR were analysed using other types of immunoassays. This confirmed the significantly higher levels of FASL, CCL2, CXCL13 and IL‐10 in (P)VRL vitreous samples (Fig. S1A–D). Furthermore, (P)VRL patients displayed an increased IL‐10/IL‐6 ratio and increased ISOLD (Fig. S1E, F). In contrast, the (P)VRL associated increase of CD40 and EZR, as identified by PEA, could not be confirmed (Fig. S1G, H). Although the average CCL17 concentration did not differ significantly between vitreous of sarcoid uveitis and TB‐associated uveitis, CCL17 was detected in four out of eight (histologically confirmed) sarcoid uveitis patients, but in none of the TB‐associated uveitis samples (n = 6; Fig. S1I).
Validation of previously identified top 5 (P)VRL discriminating proteins by Youden’s indices
To validate the diagnostic potential of the individual top 5 (P)VRL discriminating biomarkers identified by Youden’s indices calculations on cohort 1, we re‐evaluated these biomarkers in cohort 2. Youden’s indices confirmed a sensitivity and specificity of 100% for the IL‐10/IL‐6 ratio, ISOLD and IL‐10 levels. However, the sensitivity of both CD70 and EZR dropped from 100% observed in cohort 1 to 60% in cohort 2 (Table 6). Notably, based on the cut‐off value obtained from Youden’s index calculation, all five biomarkers classified the two (P)VRL cases that had been treated as non‐(P)VRL (data not shown).
Table 6.
Included patients with an idiopathic uveitis diagnosis and the follow‐up conclusion.
| Patient number | Date initial diagnosis | Year last follow‐up | Final diagnosis | Action after last follow‐up |
|---|---|---|---|---|
| 29 | 13‐08‐2012 | 2013 | Idiopathic uveitis | Referred to own ophthalmologist |
| 30 | 09‐11‐2012 | 2013 | Idiopathic uveitis | Referred to own ophthalmologist |
| 31 | 28‐11‐2012 | 2021 | Idiopathic uveitis | Still under observation |
| 32 | 03‐12‐2012 | 2017 | Idiopathic uveitis | Discharged |
| 33 | 07‐01‐2013 | 2019 | Idiopathic uveitis | Discharged |
| 34 | 13‐12‐2013 | 2014 | Idiopathic uveitis | Referred to own ophthalmologist |
| 35 | 03‐01‐2014 | 2021 | Idiopathic uveitis | Still under observation |
| 36 | 24‐10‐2014 | 2016 | Idiopathic uveitis | Referred to own ophthalmologist |
| 60 | 19‐11‐2012 | 2021 | Multiple organ lymphoma with secondary uveitis | Still under observation |
| 61 | 11‐12‐2015 | 2021 | Idiopathic uveitis | Still under observation |
| 62 | 26‐02‐2016 | 2019 | Idiopathic uveitis | Discharged |
| 63 | 29‐07‐2016 | 2018 | Idiopathic uveitis | Referred to own ophthalmologist |
| 64 | 04‐11‐2016 | 2017 | Idiopathic uveitis | Referred to own ophthalmologist |
| 65 | 02‐12‐2016 | 2017 | Idiopathic uveitis | Referred to own ophthalmologist |
Prediction of (P)VRL diagnosis of blinded samples using a random forest classifier
To study the diagnostic value of assessing multiple biomarkers, we further employed a machine learning approach. Cohort 1 was used to train a random forest classifier for the various uveitis aetiologies. To validate the model, vitreous samples from cohort 2 were classified and subsequently compared to the final clinical diagnosis retrieved by the ophthalmologist from the patient’s medical file. The aim was to train a classifier able to identify patients with untreated uveitis related to TB, sarcoidosis and (P)VRL and separate them from other uveitis aetiologies. However, retrospective confirmation of the clinical diagnosis revealed that cohort 2 only contained two patients with sarcoid uveitis and no patients with TB‐associated uveitis. As this would result in unreliable results for these aetiologies, we shifted our focus to the stratification of (P)VRL against all other aetiologies (Table 1). The classifier correctly identified five out of five pretreatment (P)VRL patients in cohort 2 (Table 5). Sixteen other patients were correctly classified as having a uveitis aetiology unrelated to (P)VRL. One patient with a varicella‐zoster virus (VZV) infection was erroneously classified as (P)VRL. This gave the classifier a sensitivity of 100% and specificity of 94% for pretreatment (P)VRL‐related uveitis in the validation set. Notably, the two follow‐up samples from treated (P)VRL patients were not recognized as (P)VRL by the classifier, most probably due to altered vitreous proteome composition as a result of treatment.
Table 5.
Classifier identification.
| Classifier 1 | Clinical diagnosis | Classifier 2 | Clinical diagnosis | ||
|---|---|---|---|---|---|
| (P)VRL | Other | (P)VRL | Other | ||
| (P)VRL | 5 | 1 | (P)VRL | 12 | 0 |
| Other | 0 | 16 | Other | 0 | 57 |
(P)VRL = primary vitreoretinal lymphoma.
Prediction of (P)VRL diagnosis of blinded samples by individual vitreous proteins
As using a set of 165 markers is not feasible in routine diagnostics, we then calculated the feature permutation importance for each of the markers to assess their relative contribution to the predictive capacity of the classifier (Fig. 3A). Four major contributing markers were identified: the previously reported ISOLD, and IL‐10 and the novel markers CD70 and EZR, with all other markers lagging behind.
Fig. 3.
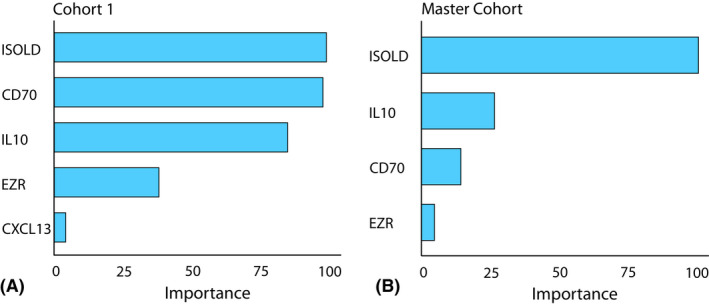
Feature permutation importance based on 165 included biomarkers. (A) Top five important proteins to stratify (P)VRL cases from all other uveitis aetiologies based on cohort 1. (B) Top four important proteins to stratify (P)VRL cases from all other uveitis aetiologies based on the entire cohort (cohort 1 and 2).
However, the classifier was trained to identify (P)VRL based only on the 7 (P)VRL cases in the original dataset while the variance in CD70 and EZR levels in vitreous among (P)VRL cases in the validation cohort 2 increased significantly (Fig. 4). To increase the sample size and reduce overfitting on the original 7 (P)VRL cases, we then combined cohort 1 and 2 into a master cohort. A new random forest classifier was trained based on this expanded cohort to study the relative shifts in feature permutation importance for the included markers upon expansion of the cohort. All 12 (P)VRL‐related uveitis patients and all 57 patients with a diagnosis unrelated to (P)VRL were identified correctly in the cross‐validated model. The same four major markers were identified once again (Fig. 3B), but the relative weight of the markers shifted significantly. The ISOLD maintained its strong impact, but the relative importance of elevated IL‐10 and CD70 levels diminished. The relative importance of EZR was reduced strongly and provided little additional predictive value in the larger cohort.
Fig. 4.
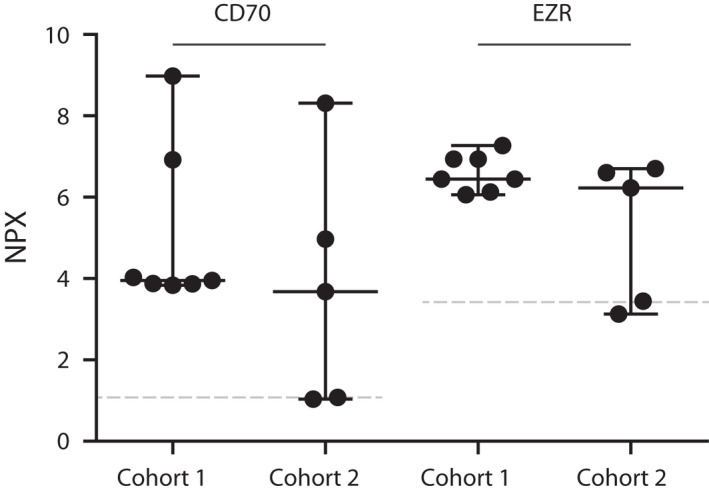
PEA results for CD70 and Ezrin (EZR) in vitreous of (P)VRL patients comparing cohort 1 with cohort 2, indicating increased variance in cohort 2. The grey dotted lines represent the median of all non‐(P)VRL (n = 57) included cases (cohort 1 and 2).
Discussion
In this study, we show the potential use of extensive PEA proteomics analysis to identify vitreous biomarkers to discriminate between diverse uveitis aetiologies. Most PEA‐identified differentially expressed proteins (FASL, CCL2, CXCL13 and IL‐10) could be validated by ELISA or Luminex bead‐based assays, underlining the validity of the PEA. Furthermore, a trained classifier was able to identify pretreatment (P)VRL cases with a sensitivity of 100% and specificity of 94%. The classifier highlighted 3 markers with high predictive value, underlining previous reports describing the diagnostic value of the ISOLD and IL‐10, and introduced CD70 as a novel marker with potential diagnostic value. Should the added value of CD70 for (P)VRL hold true after validation in novel, larger cohorts, CD70 may serve to increase confidence in (P)VRL diagnosis in the rare cases where the IL‐10/IL‐6 ratio or ISOLD are not definitive. Future studies will have to reveal whether in such rare events CD70 can indeed fill the diagnostic gap to identify these rare (P)VRL cases.
Furthermore, higher CCL17 vitreous levels were specifically associated with sarcoid uveitis when compared to TB‐associated uveitis. Most likely, this finding was due to decreased CCL17 vitreous levels in TB‐associated uveitis, as these were significantly lower than other uveitis cases (sarcoid uveitis excluded). While CCL17 levels did not differ between sarcoid uveitis and non‐TB related uveitis (data not shown), vitreous CCL17 may thus represent a novel biomarker to distinguish sarcoid uveitis from granulomatous TB‐associated uveitis. This could be of special interest for improved stratification of QFT‐positive uveitis cases without further clinical signs of TB (Ness & Virchow 2001; Varma et al. 2006; Schrijver et al. 2020).
In contrast to our previous study, in which vitreous IL‐6 levels were significantly lower in (P)VRL cases as compared to other uveitis aetiologies, IL‐6 was not indicated (although included in the immune‐oncology panel) as significantly differentially expressed by PEA and validation analysis. Nonetheless, a negative trend in vitreous IL‐6 levels could be observed in (P)VRL as compared to other aetiologies (data not shown). Furthermore, the calculated IL‐10/IL‐6 ratio and ISOLD resulted in a smaller P‐value compared to IL‐10 measurement alone (Table S3). These findings support the notion of malignant B‐cell infiltrates producing high amounts of IL‐10 in the absence of increased IL‐6 levels that are typically present under inflammatory conditions (Venkatesh et al. 2019). Additionally, the observed increase of CXCL13 in vitreous from (P)VRL underlines the earlier described malignant B‐cell migration to the intraocular space through CXCR4 and CXCR5 interaction (Kalogeropoulos et al. 2019). Increased CCL2 in vitreous from (P)VRL confirms data from our previous study (de Hoog et al. 2019), while increased expression of CD70, the cellular ligand for CD27, has been observed on diffuse large B‐cell lymphoma and mantle cell lymphoma cells (Phillips et al. 2019). Interestingly, various mutations in the CD70 gene have been described to occur in a substantial percentage of large diffuse B‐cell lymphoma (DLBCL) (Bertrand et al. 2013; de Miranda et al. 2014). Moreover, it was suggested that anti‐CD70 antibodies might be a possible treatment for CD70 positive lymphomas as normal lymphocytes have low CD70 expression (Israel et al. 2005). These findings underline the importance of future studies that explore the pathophysiological meaning of elevated CD70 in (P)VRL and potential therapeutic options related to this molecule.
A recent paper by Park et al. showed that methotrexate treatment results in a reduction in the aqueous humour levels of IL‐10, which also affected the IL‐10/IL‐6 ratio (Park et al. 2020). In our current study, the vitreous of two (P)VRL patients both treated with methotrexate, of which one in combination with rituximab, showed lower IL‐10 levels and consequently decreased IL‐10/IL‐6 ratios and ISOLDs. Notably, the (P)VRL classifier and top five discriminating biomarkers calculated by Youden’s indices did not classify these patients as (P)VRL. Though our sample size is limited, these results suggest that the observed decrease of IL‐10 levels in the aqueous humour may be reflected by a similar decrease in the vitreous. Indeed, Kuiper et al. previously described a good correlation between IL‐10 and IL‐6 in paired aqueous humour and vitreous samples of (P)VRL patients (Kuiper et al. 2015).
CCL17 vitreous levels, as determined by PEA, were significantly higher (~fourfold) in sarcoid uveitis patients as compared to TB‐associated uveitis patients. Validation of these results with another type of immunoassay displayed measurable CCL17 vitreous levels in 50% of the histologically confirmed sarcoid uveitis patients, while none of the vitreous from TB‐associated uveitis patients contained measurable levels. CCL17, also known as TARC, is a chemotactic factor for T‐helper (Th)2 lymphocytes (Ritz et al. 2004). In sarcoidosis, with disease progression, a shift occurs from the initial inflammatory Th1 response to a more Th2 dominated response that is also associated with fibrosis (Moller 2003; Nguyen et al. 2018). Our data may therefore suggest that a subgroup of sarcoid uveitis cases in our study did progress to an intraocular environment in favour of a Th2 dominated response. (Moller 2003; Tsunemi et al. 2006; Nguyen et al. 2018). Conversely, a well‐established Th1 response is essential for long‐term control of latent TB infection (LTBI) (da Silva et al. 2015). Although the exact biological meaning of the difference in vitreous CCL17 levels requires further study, it is tempting to propose CCL17 as a marker to distinguish sarcoid uveitis cases from LTBI‐associated uveitis. This will be subject of future studies.
Lower levels of the CD8+ T‐lymphocyte associated proteins CD8A, granzyme H (GZMH), granzyme B (GZMB) and cytotoxic and regulatory T‐cell molecule (CRTAM) were detected in granulomatous uveitis cases. This suggests a less prominent role for CD8+ T‐lymphocytes in granulomatous uveitis as compared to the other uveitis cases in our study. IPA linked reduction of these proteins to a state of inflammation associated with several conditions characterized by fibrosis (e.g. hepatic fibrosis, chronic obstructive pulmonary disease, allograft rejection) (Friedman 2004; Li & Yang 2009; Wang et al. 2020). Indeed, fibrosis is associated with chronic sarcoidosis and healed tuberculosis lesions (Grunewald et al. 2019; Evans et al. 2020). Despite being of pathophysiological relevance, all proteins significantly differentially expressed in granulomatous uveitis versus all the other uveitis aetiologies displayed poor Youden’s indices with either poor sensitivity and/or specificity rendering them unsuitable as potential biomarkers.
Although this study clearly confirms the diagnostic potential of IL‐10 and IL‐6 measurement to stratify (P)VRL from other uveitis causes, interpretation of obtained results should be made with caution. This is exemplified in our study by one (P)VRL case displaying an IL‐10/IL‐6 ratio below 1. Although, in line with other studies (Costopoulos et al. 2016; Kuo et al. 2020), we demonstrated that in such a case calculation of the ISOLD can be of value. Furthermore, our classifier misclassified one VZV patient as (P)VRL, as a result of exceptionally high EZR, IL‐10 and CD70 values. Thus, the false‐positive event resulting from inclusion of CD70 suggests that the clinical utility of CD70 may be limited in the presence of a marker such as ISOLD. Fourteen patients in our study were classified as idiopathic uveitis, and at the moment of study, the exact underlying inflammatory cause in these patients was unknown. Therefore, we reviewed patients’ files for follow‐up within Erasmus MC or Rotterdam Eye Hospital (Table 6). This revealed that 13 of these 14 patients were still classified as idiopathic uveitis. The remaining patient was recently diagnosed and treated for lymphoma with multiple organ involvement which was associated with reduced uveal inflammation.
Of note, our study design came with a number of inherent limitations, as the inclusion of a cohort of undiagnosed cases presenting with uveitis‐like symptoms resulted in small sample sizes for some uveitis aetiologies in cohort 2. As a result, only (P)VRL was sufficiently represented in cohort 2 to validate our findings from cohort 1. Additionally, identification of false‐positive biomarkers or overestimation of biomarker importance can more readily occur with limited sample size. However, coherent results from analyses of two independently collected cohorts ((P)VRL versus other aetiologies) underline the validity of the here presented results. Furthermore, due to the lack of long‐term follow‐up data of included patients, we cannot formally exclude the possibility of diagnostic misclassification of the idiopathic patients, or of a future (P)VRL diagnosis, which would then affect the calculated sensitivities and specificities in our present study.
In conclusion, our results underline the previously reported value of the ISOLD and the IL‐10/IL‐6 ratio for diagnosing (P)VRL. Moreover, our data show the potential of the PEA as a biomarker discovery tool in vitreous samples by highlighting CD70 as a novel marker with potential diagnostic value for (P)VRL. Additionally, intravitreal CCL17 was identified as a potential biomarker to discriminate between granulomatous uveitis related to sarcoidosis or TB. However, the added benefit of these markers must be thoroughly validated in larger cohort studies before introduction to the clinic can be considered.
Conflict of interest
Benjamin Schrijver, P. Martijn Kolijn, Josianne C.E.M. ten Berge, Nicole M.A. Nagtzaam, Angelique L.C.T. van Rijswijk, Sigrid M.A. Swagemakers, Peter J. van der Spek, Tom O.A.R. Missotten, Mirjam E.J. van Velthoven, Joeri de Hoog, Martin P. van Hagen, Anton W. Langerak and Willem A. Dik report no conflict of interest.
Supporting information
Fig. S1. Validation of six, PEA identified, significant differentially expressed vitreous proteins in cohort 1 (A) FasL, (B) CCL2, (C) CXCL13, (D) IL‐10, (G) CD40 and (H) EZR) comparing (P)VRL versus all other uveitis aetiologies, (E) the calculated IL‐10/IL‐6 ratio, (F) interleukin score for intraocular lymphoma diagnosis (ISOLD) and the most significantly differently expressed protein (I) CCL17 comparing (suspected) sarcoid uveitis versus TB‐associated uveitis. Statistical analysis was performed in GraphPad Prism 5.0, and a Mann–Whitney U test was used to compare groups. ** = p < 0.01, *** = p < 0.001.
Table S1. Patient characteristics.
Table S2. Overview off all antibody pair targets within the Olink immuno‐oncology and neurology panel.
Table S3. All significant differently expressed proteins, based on cohort 1, detected by PEA, comparing (P)VRL with all other etiologies, sarcoid uveitis with TB‐associated uveitis and granulomatous (sarcoid uveitis and TB‐associated uveitis) with all other etiologies.
Table S4. Pathways, associated diseases and functions related to differentially expressed proteins comparing (P)VRL versus all other etiologies extracted with IPA.
Table S5. Pathways, associated diseases and functions related to differentially expressed proteins comparing Granulomatous versus all other etiologies extracted with IPA.
References
- Bertrand P, Maingonnat C, Penther D et al. (2013): The costimulatory molecule CD70 is regulated by distinct molecular mechanisms and is associated with overall survival in diffuse large B‐cell lymphoma. Genes Chromosomes Cancer 52: 764–774. [DOI] [PubMed] [Google Scholar]
- Caraballo JN, Snyder MR, Johnston PB, Bp ON, Raja H, Balsanek JG, Peters BE & Pulido JS (2014): Vitreoretinal lymphoma versus uveitis: cytokine profile and correlations. Ocul Immunol Inflamm 22: 34–41. [DOI] [PubMed] [Google Scholar]
- Carbonell D, Mahajan S, Chee SP et al. (2021): Consensus recommendations for the diagnosis of vitreoretinal lymphoma. Ocul Immunol Inflamm 1–14. [DOI] [PubMed] [Google Scholar]
- Cassoux N, Giron A, Bodaghi B et al. (2007): IL‐10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci 48: 3253–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costopoulos M, Touitou V, Golmard JL et al. (2016): ISOLD: A new highly sensitive interleukin score for intraocular lymphoma diagnosis. Ophthalmology 123: 1626–1628. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, London NJS & Rathinam SR (2010): Uveitis: a global view. Expert Rev Ophthalmol 5: 113–114. [Google Scholar]
- da Silva MV, Tiburcio MG, Machado JR, Silva DA, Rodrigues DB, Rodrigues V & Oliveira CJ (2015): Complexity and controversies over the cytokine profiles of t helper cell subpopulations in tuberculosis. J Immunol Res 2015: 639107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao D, Caplash S, Gangaputra S, Vitale S, Jabs DA, McCoy JP & Sen HN & MR Group (2020): Hierarchical cluster analysis of serum and vitreous cytokines in intermediate, posterior and panuveitis. Invest Ophthalmol Visual Sci 61: 3655. [Google Scholar]
- de Hoog J, Dik WA, Lu L et al. (2019): Combined cellular and soluble mediator analysis for improved diagnosis of vitreoretinal lymphoma. Acta Ophthalmol 97: 626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miranda NF, Georgiou K, Chen L et al. (2014): Exome sequencing reveals novel mutation targets in diffuse large B‐cell lymphomas derived from Chinese patients. Blood 124: 2544–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S, Butler JR, Mattila JT & Kirschner DE (2020): Systems biology predicts that fibrosis in tuberculous granulomas may arise through macrophage‐to‐myofibroblast transformation. PLOS Comput Biol 16: e1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisson S, Ouakrim H, Touitou V et al. (2013): Cytokine profile in human eyes: contribution of a new cytokine combination for differential diagnosis between intraocular lymphoma or uveitis. PLoS One 8: e52385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL (2004): Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 1: 98–105. [DOI] [PubMed] [Google Scholar]
- Grunewald J, Grutters JC, Arkema EV, Saketkoo LA, Moller DR & Müller‐Quernheim J (2019): Sarcoidosis (Primer). Nat Rev Dis Primers 5(1): 45. [DOI] [PubMed] [Google Scholar]
- Hou S, Li N, Liao X, Kijlstra A & Yang P (2020): Uveitis genetics. Exp Eye Res 190: 107853. [DOI] [PubMed] [Google Scholar]
- Israel BF, Gulley M, Elmore S, Ferrini S, Feng WH & Kenney SC (2005): Anti‐CD70 antibodies: a potential treatment for EBV+ CD70‐expressing lymphomas. Mol Cancer Ther 4: 2037–2044. [DOI] [PubMed] [Google Scholar]
- Kalogeropoulos D, Vartholomatos G, Mitra A et al. (2019): Primary vitreoretinal lymphoma. Saudi J Ophthalmol 33: 66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasudhan KS, Sarkar S, Gupta V, Gupta A & Chakraborti A (2018): Identification of unique proteins in vitreous fluid of patients with noninfectious uveitis. Acta Ophthalmol 96: e989–e1003. [DOI] [PubMed] [Google Scholar]
- Krishna U, Ajanaku D, Denniston AK & Gkika T (2017): Uveitis: a sight‐threatening disease which can impact all systems. Postgrad Med J 93: 766–773. [DOI] [PubMed] [Google Scholar]
- Kuiper J, ten Dam‐van Loon N, Domanian A, Schellekens P, Nierkens S, Radstake T & de Boer J (2015): Correlation between measurement of IL‐10 and IL‐6 in paired aqueous humour and vitreous fluid in primary vitreoretinal lymphoma. Acta Ophthalmol 93: e680–e681. [DOI] [PubMed] [Google Scholar]
- Kuo DE, Wei MM, Knickelbein JE, Armbrust KR, Yeung IYL, Lee AY, Chan CC & Sen HN (2020): Logistic regression classification of primary vitreoretinal lymphoma versus uveitis by interleukin 6 and interleukin 10 levels. Ophthalmology 127: 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C & Yang CW (2009): The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol 5: 513–519. [DOI] [PubMed] [Google Scholar]
- London NJ, Rathinam SR & Cunningham ET Jr (2010): The epidemiology of uveitis in developing countries. Int Ophthalmol Clin 50: 1–17. [DOI] [PubMed] [Google Scholar]
- Max Kuhn JW, Weston S, Williams A et al. (2020): Classification and Regression Training. R package version 6.0‐86.
- Mohanty V, Pinto SM, Subbannayya Y, Najar MA, Murthy KB, Prasad TSK & Murthy KR (2020): Digging deeper for the eye proteome in vitreous substructures: a high‐resolution proteome map of the normal human vitreous base. OMICS J Integr Biol 24: 379–389. [DOI] [PubMed] [Google Scholar]
- Moller DR (2003): Treatment of sarcoidosis – from a basic science point of view. J Intern Med 253: 31–40. [DOI] [PubMed] [Google Scholar]
- Ness T & Virchow JC. (2001): [Posterior uveitis: sarcoidosis or tuberculosis] Posteriore Uveitis: Sarkoidose oder Tuberkulose. Ophthalmologe 98: 207–211. [DOI] [PubMed] [Google Scholar]
- Nguyen CTH, Kambe N, Ueda‐Hayakawa I, Kishimoto I, Ly NTM, Mizuno K & Okamoto H (2018): TARC expression in the circulation and cutaneous granulomas correlates with disease severity and indicates Th2‐mediated progression in patients with sarcoidosis. Allergol Int 67: 487–495. [DOI] [PubMed] [Google Scholar]
- Park YG, Park WK, Kim RY, Kim M & Park YH (2020): Serial changes in the aqueous IL‐10 level after intravitreal methotrexate injection as an indicator of primary vitreoretinal lymphoma recurrence. Sci Rep 10: 15992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T, Barr PM, Park SI et al. (2019): A phase 1 trial of SGN‐CD70A in patients with CD70‐positive diffuse large B cell lymphoma and mantle cell lymphoma. Invest New Drugs 37: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read RW, Zamir E & Rao NA (2002): Neoplastic masquerade syndromes. Surv Ophthalmol 47: 81–124. [DOI] [PubMed] [Google Scholar]
- Ritz SA, Cundall MJ, Gajewska BU et al. (2004): The lung cytokine microenvironment influences molecular events in the lymph nodes during Th1 and Th2 respiratory mucosal sensitization to antigen in vivo. Clin Exp Immunol 138: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoo MS, Mehta H, Swampillai AJ, Cohen VM, Amin SZ, Plowman PN & Lightman S (2014): Primary intraocular lymphoma. Surv Ophthalmol 59: 503–516. [DOI] [PubMed] [Google Scholar]
- Saleh M, Nikolitch K, Bourcier T, Speeg C & Gaucher D (2012): Repeated IL‐10 measurement in aqueous humor and OCT imaging are valuable tools to monitor intraocular lymphoma treated with intravitreal injections of methotrexate. Graefes Arch Clin Exp Ophthalmol 250: 761–764. [DOI] [PubMed] [Google Scholar]
- Schrijver B, Hardjosantoso H, Ten Berge J, Schreurs MWJ, Van Hagen PM, Brooimans RA, Rothova A & Dik WA (2020): No Evidence for circulating retina specific autoreactive T‐cells in latent tuberculosis‐associated uveitis and sarcoid uveitis. Ocul Immunol Inflamm 1–7. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Seve P, Cacoub P, Bodaghi B et al. (2017): Uveitis: Diagnostic work‐up. A literature review and recommendations from an expert committee. Autoimmun Rev 16: 1254–1264. [DOI] [PubMed] [Google Scholar]
- Solier C & Langen H (2014): Antibody‐based proteomics and biomarker research ‐ current status and limitations. Proteomics 14: 774–783. [DOI] [PubMed] [Google Scholar]
- Team RC (2020): A language and environment for statistical computing.
- Tsunemi Y, Saeki H, Nakamura K et al. (2006): CCL17 transgenic mice show an enhanced Th2‐type response to both allergic and non‐allergic stimuli. Eur J Immunol 36: 2116–2127. [DOI] [PubMed] [Google Scholar]
- Varma D, Anand S, Reddy AR, Das A, Watson JP, Currie DC, Sutcliffe I & Backhouse OC (2006): Tuberculosis: an under‐diagnosed aetiological agent in uveitis with an effective treatment. Eye (Lond) 20: 1068–1073. [DOI] [PubMed] [Google Scholar]
- Velez G, Roybal CN, Colgan D, Tsang SH, Bassuk AG & Mahajan VB (2016): Precision medicine: personalized proteomics for the diagnosis and treatment of idiopathic inflammatory disease. JAMA Ophthalmol 134: 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh R, Bavaharan B, Mahendradas P & Yadav NK (2019): Primary vitreoretinal lymphoma: prevalence, impact, and management challenges. Clin Ophthalmol 13: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhou J, Wang J, Li S, Fukunaga A, Yodoi J & Tian H (2020): Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct Target Ther 5: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016): ggplot2: Elegant Graphics for Data Analysis. New York: Springer‐Verlag. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Validation of six, PEA identified, significant differentially expressed vitreous proteins in cohort 1 (A) FasL, (B) CCL2, (C) CXCL13, (D) IL‐10, (G) CD40 and (H) EZR) comparing (P)VRL versus all other uveitis aetiologies, (E) the calculated IL‐10/IL‐6 ratio, (F) interleukin score for intraocular lymphoma diagnosis (ISOLD) and the most significantly differently expressed protein (I) CCL17 comparing (suspected) sarcoid uveitis versus TB‐associated uveitis. Statistical analysis was performed in GraphPad Prism 5.0, and a Mann–Whitney U test was used to compare groups. ** = p < 0.01, *** = p < 0.001.
Table S1. Patient characteristics.
Table S2. Overview off all antibody pair targets within the Olink immuno‐oncology and neurology panel.
Table S3. All significant differently expressed proteins, based on cohort 1, detected by PEA, comparing (P)VRL with all other etiologies, sarcoid uveitis with TB‐associated uveitis and granulomatous (sarcoid uveitis and TB‐associated uveitis) with all other etiologies.
Table S4. Pathways, associated diseases and functions related to differentially expressed proteins comparing (P)VRL versus all other etiologies extracted with IPA.
Table S5. Pathways, associated diseases and functions related to differentially expressed proteins comparing Granulomatous versus all other etiologies extracted with IPA.


