Abstract
Background
Electromagnetic muscle stimulation (EMMS) is a non‐invasive body contouring technology for strengthening, firming, and toning the abdomen, buttocks, and thighs that is associated with high patient satisfaction.
Aims
To gain a greater understanding of factors contributing to patient satisfaction with EMMS.
Methods
This was a retrospective, non‐comparative study of patient information and questionnaires regarding EMMS treatments to abdomen and/or buttocks collected July 1 to December 1, 2019 from clinical practices in the United States. Questionnaires collected and included for study analysis were the Subject Experience Questionnaire (SEQ), the Body Satisfaction Questionnaire (BSQ), and the Subject‐rated Global Aesthetic Improvement Scale (SGAIS).
Results
Responses from 146 treated patients were analyzed (abdomen only: n = 94; buttocks only, n = 30; abdomen and buttocks: n = 22). Patients were 79% female with mean age of 41.3 years (range: 19–73). Frequently cited reasons for seeking EMMS treatment were a desire to appear more toned (89%) or slimmer (42%), and to feel stronger (38%). BSQ and SGAIS scores were improved 4 weeks after treatment. On post‐treatment SEQ, most patients reported being “satisfied” or “very satisfied” with abdomen (83.2%; n = 89/107) or buttocks (57.4%; n = 27/47) treatment. Most patients reported feeling stronger after abdomen treatment, and across both body areas, patients were more confident, happier with their overall appearance, and motivated to work out and maintain treatment results.
Conclusion
This retrospective study of patient questionnaires provides important information on aesthetic and functional factors that can contribute to high patient satisfaction following EMMS treatment of the abdomen and/or buttocks.
Keywords: electromagnetic muscle stimulation, non‐invasive body contouring, patient satisfaction
1. INTRODUCTION
Non‐invasive body contouring procedures are increasingly popular. Recent consumer surveys indicate that body sculpting is the most frequently considered aesthetic treatment. 1 Further, the American Society of Plastic Surgeons' 2019 “Plastic Surgery Report” listed body contouring as one of the fastest growing minimally invasive procedures. 2 While many methods of body contouring, such as cryolipolysis, abdominal etching, or ultrasound, laser, or radiofrequency devices target reduction of adipose tissue, 2 , 3 contouring and aesthetic improvements can also be accomplished by targeting and toning the underlying muscles via muscle stimulation devices.
Nonvolitional electrical or electromagnetic muscle stimulation (EMMS) has been used for decades in therapeutic applications for injury recovery, to prevent muscle atrophy, and to strengthen skeletal muscles. 4 , 5 , 6 , 7 Indeed, neuromuscular electrical stimulation has been shown to increase the strength of athletes by 30%–40% when used as an adjunct to traditional exercise. 6 , 8 , 9 Only recently have these muscle stimulation technologies been applied for aesthetic use. However, most muscle stimulation devices available to consumers for non‐therapeutic use rely on electrode‐based stimulation and are limited by the amount of current that can be delivered to elicit muscle contractions strong enough to produce clinically significant changes due to potential for pain or burns. 8 To overcome these issues, EMMS devices were introduced in the clinic setting. Recently, CoolTone®, an EMMS (or MMS) device, was FDA‐cleared for strengthening, toning, and firming the muscles of the abdomen, buttocks, and thighs. With EMMS, a magnetic field induces a current at the muscle level, leading to sustained, nonvolitional contractions. 8 , 9 , 10 Due to the weak conductive properties of skin, fat, and bone, the electromagnetic field induces a current selective for the muscle layer.
Multiple clinical studies have demonstrated the safety and effectiveness of EMMS devices for improving muscle tone in the abdomen and buttocks. 11 , 12 , 13 , 14 , 15 Patient satisfaction and potential impacts on quality of life are increasingly important measures of the effectiveness of aesthetic treatments, including non‐invasive body contouring. Recent studies of EMMS treatment of abdomen and buttocks have reported high (>85%) subject‐rated satisfaction. 12 , 16 However, these findings provide only a general overview of patient experience.
Thus, the goal of this study was to retrospectively analyze records of patient‐reported outcomes following EMMS (aka MMS) treatment with a CoolTone® (Allergan plc) prototype to the abdomen and/or buttocks. The current study evaluated multiple questionnaires measuring overall satisfaction with treatment, both aesthetic and functional factors contributing to general treatment satisfaction, treatment area perception, and reasons for seeking EMMS treatment.
2. MATERIALS AND METHODS
2.1. Study design and subjects
This was a retrospective, non‐comparative review of basic patient information (ie, age, gender, body mass index [BMI]) and patient questionnaires from adult patients who received EMMS treatment to the abdomen and/or buttocks between July 1 and December 1, 2019.
Completed survey data from 146 patients were collected from 16 aesthetic clinical practices in the United States, including dermatology and plastic surgery practices, that had received a CoolTone® prototype EMMS device for evaluation as part of routine clinical practice and in accordance with the manufacturer's standard instructions for use. Criteria for inclusion into the retrospective analysis were that patients must have received EMMS treatment to the abdomen and/or buttocks with the CoolTone® prototype and completed surveys and information forms on EMMS experience between July 1, 2019, and December 1, 2019. No medical device intervention occurred as part of this study, and no safety data were collected since this was a retrospective analysis of pre‐existing data. Of the 146 patients whose survey data were included in this analysis, n = 72 received the treatment for free, while n = 40 paid for the treatment, and no information was available on payment for n = 34 patients.
2.2. Treatment
Surveys reviewed in this retrospective analysis were completed by patients who underwent treatment in accordance with the current accepted practice of administering four EMMS treatment sessions to the abdomen and/or buttocks over a 2‐week period, with no consecutive treatment days, and returned for follow‐up visits 4 weeks after the final treatment. Each treatment session consisted of one 30‐min treatment cycle on the abdomen and/or two 30‐min treatment cycles on the buttocks, one on each buttock.
2.3. Patient‐reported outcomes
The primary endpoint of this retrospective study was to evaluate overall patient experience with EMMS treatment as measured by patient questionnaires. A Subject Experience Questionnaire (SEQ) collected at baseline before treatment assessed patients' reasons for seeking EMMS treatment and was completed once per patient, regardless of body area(s) to be treated. The measure included eight phrases (eg, “I want to appear more toned”) and patients selected all statements that aligned with their reason(s) for seeking treatment. Patient SEQs from 4 weeks after the final treatment were also collected to inform overall satisfaction and treatment experience. All questionnaires, except the baseline SEQ, were specific to the treated body area (ie, abdomen or buttocks). Satisfaction was analyzed via a single question on the SEQ with responses on a 5‐point Likert scale from “very dissatisfied” to “very satisfied”. Treatment experience was analyzed by level of agreement on a 5‐point Likert scale from “strongly disagree” to “strongly agree” with 6–8 phrases relating to experience with treatment results (eg, “I feel stronger”, “I feel motivated to work out and maintain these results”).
Body Satisfaction Questionnaires (BSQs) for abdomen and/or buttocks from baseline, immediately after the 4th treatment, and 4 weeks after the final treatment were also collected for analysis. The BSQ measures patients' perceptions of the shape and appearance of the treated body area using a set of ten dichotomous items (eg, firm vs wobbly, clothes are too tight vs clothes fit well). 8 , 9 , 14 The items are rated on a 5‐point semantic differential scale from one (most negative) to five (most positive). Total score range is from 10 to 50, with an increase in score reflecting a patients' perceived improvement in appearance.
Perceived change in the appearance of abdomen and/or buttocks after EMMS treatment was reflected through subject‐rated Global Aesthetic Improvement Scale (SGAIS). Patients rated the change in appearance of their abdomen and/or buttocks using a 7‐point Likert scale from “very much worse” to “very much improved”. SGAIS responses from 4 weeks after final treatment were collected, and scores were analyzed separately for each treatment area.
2.4. Statistical analyses
Demographics, SEQ, SGAIS, and overall satisfaction are summarized descriptively. BSQ responses from immediately after 4th treatment and 4 weeks after final treatment were compared to baseline responses using paired two‐tailed t tests. Note that, due to the retrospective nature of this study, missing information on patient information parameters (eg, gender, age, BMI) and across questionnaires led to differing sample sizes for select parameters, time points, and questions.
3. RESULTS
3.1. Subject demographics and baseline characteristics
Per study inclusion criteria, basic patient information and questionnaire data from a total of 146 patients were included in this retrospective analysis; however, the sample size for each questionnaire and demographic measure differ due to missing data/availability of information (Table 1). Of these, n = 94 patients received EMMS treatment to abdomen only, n = 30 to buttocks only, and n = 22 to abdomen and buttocks. Subject surveys regarding experience for each area treated were collected, representing n = 116 for abdomen and n = 52 for buttocks.
TABLE 1.
Demographics and baseline characteristics
| Parameters | N a | % | Mean (range) |
|---|---|---|---|
| Sex | 136 | ||
| Female | 107 | 78.7% | – |
| Male | 29 | 21.3% | – |
| Age | 134 | – | 41.3 (19–73) |
| Weight | 77 | – | 142.8 (103.0–220.2) |
| BMI (kg/m2) | 106 | – | 23.0 (16.7–34.0) |
| Treatment area | 146 | – | – |
| Total abdomen | 116 | 79.4% | – |
| Total buttocks | 52 | 35.6% | – |
| Abdomen only | 94 | 64.4% | – |
| Buttocks only | 30 | 20.5% | – |
| Abdomen and buttocks | 22 | 15.1% | – |
Sample sizes for demographics parameters may not mirror total number of surveyed patients (n = 146) because sex, age, weight, BMI were optional entries on the patient information form.
Of the 146 total patients, n = 136 provided information on their gender; of these, the majority were female (n = 107, 78.7%; male, n = 29, 21.31%) with a mean age of 41.3 years (range: 19–73, n = 134). Mean weight of patients was 142.8 lbs (range: 103.0–220.2, n = 77), and mean BMI was 23.0 kg/m2 (range: 16.7–34.0, n = 106).
3.2. Subject Experience Questionnaire
3.2.1. Reason for seeking EMMS treatment
The most frequently cited reasons by patients (n = 145) for seeking EMMS treatment (Figure 1) were a desire to appear more toned (89%) or slimmer (42%) and to feel stronger (38%). Other patient reasons for seeking treatment included wanting to look better in clothes (35%) and wanting to improve athletic performance (30%).
FIGURE 1.
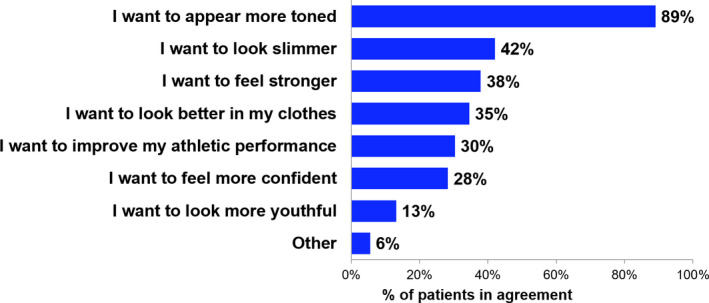
Subject Experience Questionnaire (SEQ) at Baseline—Reasons for Seeking Electromagnetic Muscle Stimulation (EMMS) Treatment. Percentage of patients in agreement with each statement as a reason for seeking EMMS treatment from the baseline SEQ. Note that patients could have selected multiple statements, and patients completed this questionnaire once, regardless of treated body area. n = 145 for all statements; one patient was excluded from analysis due to missing data
3.2.2. Overall satisfaction
At 4 weeks after the final treatment, the majority of patients (83.2%; n = 89/107) reported being “satisfied” or “very satisfied” with EMMS treatment of the abdomen, and 57.4% (n = 27/47) reported being “satisfied” or “very satisfied” with buttocks treatment (Table 2). An additional post hoc analysis of overall satisfaction among patients who paid for the procedure (abdomen: 80.0% satisfaction rate, n = 25; buttocks: 55.6% satisfaction rate, n = 18) or received free treatment (abdomen: 81.1% satisfaction rate, n = 53; buttocks: 57.1% satisfaction rate, n = 21) showed no significant difference in overall satisfaction. Data related to treatment costs or payment were not available for 29 patients who received abdominal treatment and were not available for eight patients who received buttocks treatment.
TABLE 2.
Subject Experience Questionnaire 4 weeks after treatment–overall satisfaction
| Abdomen | Buttocks | |||
|---|---|---|---|---|
| N | % | N | % | |
| Very satisfied | 41 | 38.3% | 10 | 21.3% |
| Satisfied | 48 | 44.9% | 17 | 36.2% |
| Not sure | 13 | 12.1% | 13 | 27.7% |
| Dissatisfied | 3 | 2.8% | 4 | 8.5% |
| Very dissatisfied | 2 | 1.9% | 3 | 6.4% |
| Total responses | 107 a | – | 47 b | – |
Nine patients were excluded from analysis due to missing data.
Five patients were excluded from analysis due to missing data.
3.2.3. Overall treatment experience
Additional responses from SEQ reflected patients' level of agreement with 6–8 phrases describing aesthetic and functional perceptions surrounding treatment outcomes (Table 3). The survey review demonstrated that, following abdominal EMMS treatment, the majority of patients reported feeling more confident (77.1%; n = 84/109) and happier with their overall appearance (78.7%; n = 85/108). Most patients (69.7%; n = 76/109) also reported that they feel and look better in their clothes. These perceived aesthetic changes were accompanied by functional changes, with most patients reporting improved athletic performance (66.1%; n = 72/109) and that they felt stronger (83.5%; n = 91/109). Most patients (79.6%; n = 86/108) felt motivated to follow‐up with additional EMMS treatments in order to maintain results, and 97.2% (n = 105/108) of patients agreed that they were motivated to work out and maintain treatment results.
TABLE 3.
Subject Experience Questionnaire 4 weeks after treatment–overall treatment experience
| Question | Treatment area | |||
|---|---|---|---|---|
| Abdomen | Buttocks | |||
| N | % | N | % | |
| I feel stronger | 91/109 | 83.5 | – | – |
| My athletic performance has improved | 72/109 | 66.1 | – | – |
| I feel more energetic | 43/108 | 39.8 | – | – |
| I feel more confident | 84/109 | 77.1 | 24/47 | 51.1 |
| I am happier with my overall appearance | 85/108 | 78.7 | 29/47 | 61.7 |
| My clothes feel and look better | 76/109 | 69.7 | 20/47 | 42.6 |
| My buttocks feel lifted and toned | – | – | 27/47 | 57.4 |
| I feel motivated to follow‐up with additional treatment to maintain these treatment results | 86/108 | 79.6 | 29/47 | 61.7 |
| I feel motivated to work out and maintain these results | 105/108 | 97.2 | 43/47 | 91.5 |
Table lists the number and percentage of patients choosing agree or strongly agree with each question. Group sizes vary per question due to varying responses and missing data per individual patients.
Survey review also showed that patients who received EMMS treatment to buttocks (Table 3) reported feeling more confident (51.1%; n = 24/47) and happier with their overall appearance (61.7%; n = 29/47). A majority of patients agreed that their buttocks felt lifted and toned (57.4%; n = 27/47), and that they felt motivated to receive additional EMMS treatments (61.7%; n = 29/47) and workout to maintain results (91.5%; n = 43/47).
3.3. Body Satisfaction Questionnaire
At baseline prior to treatment, the mean BSQ score was 32.1 for patients that had received treatment to the abdomen (n = 111) and 28.3 for patients that had received treatment to the buttocks (n = 49; Figure 2). Scores were significantly higher for both areas immediately after the 4th EMMS treatment, with mean scores of 37.6 (change from baseline: +5.5 points; n = 102; p < 0.05) for patients that received treatment to the abdomen and 32.8 (change from baseline: +4.5 points, n = 46; p < 0.05) for patients who received treatment to the buttocks. BSQ scores remained higher than baseline 4 weeks after the final treatment for patients who received treatment to the abdomen (change from baseline: +5.8; n = 104; p < 0.05) and buttocks (change from baseline: +3.5; n = 41, p < 0.05), and were not significantly different from the scores immediately after the 4th treatment.
FIGURE 2.
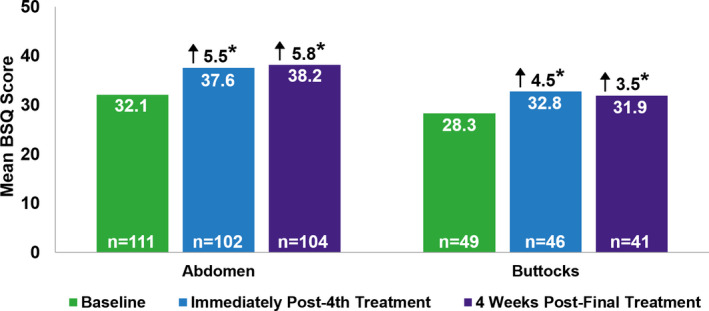
Body Satisfaction Questionnaire (BSQ). Mean BSQ scores for abdomen and buttocks at baseline, immediately after the 4th treatment, and 4 weeks after the final treatment. Possible scores range from 10 to 50, with an increase in score reflecting a patients' perceived improvement in appearance. Arrows represent the mean change from baseline in BSQ score. *, p<0.05 vs baseline BSQ scores. Due to missing data, patients were excluded from analysis of BSQ scores as follows: abdomen (baseline: n = 5; immediately after the 4th treatment: n = 14; and 4 weeks after the final treatment: n = 12) and buttocks (baseline: n = 3; immediately after the 4th treatment: n = 6; and 4 weeks after the final treatment: n = 11)
3.4. Subject‐rated Global Aesthetic Improvement Scale
At 4 weeks post‐final treatment, the mean SGAIS score was 1.5 for patients who received treatment to the abdomen, with 89.8% (n = 97/108) of patients reporting at least a 1‐point improvement in abdominal appearance (Table 4). The mean SGAIS score for patients who received treatment to the buttocks was 0.9 at the same timepoint, with 70.2% (n = 33/47) of patients reporting at least a 1‐point improvement in the appearance of buttocks. A subset of patients (abdomen, n = 10; buttocks, n = 14) reported no change in appearance and one patient reported worse appearance after abdominal EMMS treatment.
TABLE 4.
Subject‐reported Global Aesthetic Improvement Scale 4 weeks after treatment
|
Abdomen a (n = 108) |
Buttocks b (n = 47) |
|||||
|---|---|---|---|---|---|---|
| % | Average age, years | Average BMI (kg/m2) | % | Average age, years | Average BMI (kg/m2) | |
| Very much improved |
14.8% (n = 16) |
39.9 (n = 14) |
24.5 (n = 11) |
2.1% (n = 1) |
54.0 (n = 1) |
24.1 (n = 1) |
| Much improved |
33.3% (n = 36) |
40.3 (n = 32) |
23.2 (n = 25) |
19.1% (n = 9) |
38.9 (n = 8) |
23.1 (n = 7) |
| Improved |
41.7% (n = 45) |
41.4 (n = 37) |
23.2 (n = 33) |
48.9% (n = 23) |
44.1 (n = 17) |
23.1 (n = 17) |
| No change |
9.3% (n = 10) |
43.3 (n = 10) |
21.0 (n = 8) |
29.8% (n = 14) |
45.0 (n = 13) |
22.8 (n = 10) |
| Worse |
0.9% (n = 1) |
50.0 (n = 1) |
18.2 (n = 1) |
0% (n = 0) |
– | – |
| Much worse |
0% (n = 0) |
– | – |
0% (n = 0) |
– | – |
| Very much worse |
0% (n = 0) |
– | – |
0% (n = 0) |
– | – |
Group sizes for age and BMI vary, as age and BMI information were not available or provided by all patients.
Eight patients were excluded from analysis due to missing data
Five were excluded from analysis due to missing data.
4. DISCUSSION
This retrospective, non‐comparative study of patient‐reported outcome measures supports prior findings of improvement of body satisfaction following EMMS treatments to the abdomen and/or buttocks, 14 , 15 and confirms significant improvements in self‐rated aesthetic appearance. Comprehensive subject experience questionnaires suggest that, in addition to aesthetic changes, functional improvements may be seen following EMMS treatment to the abdomen. This study is also the first to provide perspectives on why patients seek EMMS treatment, which can help clinicians better understand patient motivators for treatment. Commonly cited reasons for seeking EMMS treatment include a desire to appear more toned, look slimmer, and feel stronger. The more aesthetically motivated desire to look slimmer lends way for EMMS to be used as a standalone treatment or as part of a multimodal plan comprised of surgical and non‐surgical procedures.
Electromagnetic muscle stimulation treatment improved abdominal and buttock appearance, as reflected by significant increases in patient‐rated BSQ scores and the majority of patients reporting at least a 1‐grade improvement in SGAIS scores 4 weeks after the final treatment. A majority of patients also reported being “satisfied” or “very satisfied” with the appearance of their abdomen and/or buttocks following treatment. These findings are in line with recent prospective trials of EMMS treatment of the abdomen and/or buttocks that reported improvements in BSQ and SGAIS scores at both 4 and 12 weeks after treatment. 14 , 15 Additional long‐term studies assessing patient satisfaction with EMMS treatments, as well as need for ongoing maintenance treatments, are warranted.
Comparisons between SEQ responses at baseline and 4 weeks after the final treatment session demonstrate that treatment with EMMS was in line with patients' treatment goals, as indicated by concordance between the top reason for seeking treatment (ie, a desire to appear more toned) and patient reports following buttock treatment that their buttocks felt lifted and toned (57.4% agreement). Similarly, the third most commonly cited reason to seek body contouring was to feel stronger, and 83.5% of patients agreed that they felt stronger after abdominal toning with EMMS.
In addition to aesthetic improvements, this study suggests that EMMS treatment can lead to functional improvements and may impact well‐being. Most patients agreed that abdominal toning improved their strength and athletic performance, and that treatment of the buttocks or abdomen raised confidence and happiness with their overall appearance. Of particular note, almost all patients reported feeling motivated to work out to maintain their results (abdomen: 97.2%; buttocks: 91.5%), which suggests that a desired effect was not only achieved by treatment, but worth maintaining. These functional improvements following abdomen treatment may be due to a myriad of effects not measured in the current study, including decreased lower back pain 17 , 18 or better balance and posture observed with volitional strengthening of the abdomen 19 or the broader core. 20 , 21 , 22 EMMS treatment may also mimic the positive physiological and psychological effects of volitional exercise. 23 , 24 , 25 However, it is not currently known whether nonvolitional muscle stimulation elicits the same effects, and future studies are needed. In addition, patient lifestyle changes related to diet and exercise made between the initial treatment and the 4‐week follow‐up were not captured in this study, and it is possible that patients' increased motivation to work out translated to more physical activity and additional muscle strengthening and toning.
Overall, the majority of patients reported perceived improvements in their overall appearance after EMMS treatment. Almost 90% of patients who received abdominal EMMS treatment, and 70.2% of patients who received buttocks treatment, had at least a 1‐grade improvement on the SGAIS. A small subset of patients reported no change in the appearance of the treated body area on the SGAIS; these data may be explained by a myriad of potential factors, including patient selection, patients' expectations and ability to objectively analyze their appearance, treatment parameters (eg, intensity, number of treatments, timing between treatment and assessment, etc. Furthermore, aesthetic improvements might not have been seen if other concerns, such as skin laxity, dimpling, or cellulite predominated, and tailored treatment plans taking into consideration all factors are likely to have improved SGAIS ratings. Another potential explanation is that ones' buttocks is not easily visible day‐to‐day, and frontal view mirrors cannot provide a view for patients to fully appreciate any treatment‐related changes in their buttocks. However, the majority of patients still reported significant positive changes in measures of aesthetic and non‐aesthetic factors for both treated areas. Indeed, 4 weeks after treatment, the majority of patients who received abdominal treatment reported feeling stronger, while patients who received abdominal and/or buttocks treatment reported feeling more confident and motivated to follow‐up with additional EMMS treatments. Furthermore, 97.2% and 91.5% of patients who had their abdomen or buttocks treated, respectively, reported feeling motivated to work out and maintain their results. This suggests that, even if visible, subjectively assessed effects are not as apparent, EMMS treatment is associated with a positive patient experience.
As EMMS treatment is relatively new, prospective trials will be essential to help us gain a better understanding of optimal treatment parameters (eg, intensity, number of treatments), patient selection (eg, BMI, subcutaneous fat thickness), etc., and their interplay with one another. However, this non‐comparative, retrospective analysis of patient questionnaire data provides important insight into patient motivators for treatment and the aesthetic and functional improvements that can be experienced after EMMS treatment of the abdomen and buttocks.
4.1. Limitations
This study has some limitations. Since this was a retrospective study, not all demographic data and/or complete survey responses were available. Baseline SEQ data may have been biased due to predefined answer options. In addition, there were differences in sample size of responses for abdomen vs buttocks, and there may have been variability in description and explanations of treatment procedures and outcomes provided by physicians and treating staff across different sites, as well as patients' preconceived notions regarding EMMS treatment. Another potential limitation is that data on whether EMMS treatment was free or required payment were not available for all patients. A post hoc analysis of available data showed no significant differences in overall satisfaction for either body area between patients who paid vs those who received free treatment. However, these data were not available for n = 29 and n = 8 patients who received abdominal or buttocks treatment, respectively. As the deidentified data do not include information to link patients to investigational site, it is not possible to obtain the missing payment data for these patients.
5. CONCLUSIONS
Taken together, the data from this retrospective, non‐comparative study highlight the effectiveness of EMMS treatments for body contouring of the abdomen and buttocks from the perspective of patients. Treatment met patients' expectations on a variety of measures, and high overall satisfaction was accompanied by patients' perception of aesthetic and functional improvements. Overall, these findings are indicative of the evolution of the aesthetics field, with greater interest and acknowledgement of how treatments affect the overall well‐being of patients rather than purely visual aesthetic improvements measured with clinically validated scales.
DISCLOSURE
S. Fabi and S. Shridharani serve as consultants, researchers, and advisory board members for Allergan Aesthetics, an AbbVie company. M.P. Goldman and A. Moradi have received research support from and consulted for Allergan Aesthetics, an AbbVie company. D. Rapaport serves as consultant, speakers bureau, and advisory board member for Allergan Aesthetics, an AbbVie company. F. Tsai Fu is an employee of Allergan Aesthetics, an AbbVie company and may hold stock/stock options in the company.
AUTHOR CONTRIBUTIONS
F. Tsai Fu participated in study design and data analysis. A. Moradi, D. Rapaport, and S. Shridharani participated in data acquisition. All authors contributed to interpretation of the data and critical revision of the manuscript for intellectual content. All authors approved the final manuscript.
ETHICS APPROVAL
The study protocol conformed to the ethical principles of the 1975 Declaration of Helsinki. The Salus institutional review board approved the study protocol with full waiver of informed consent. All patient data were deidentified by an independent third party before being provided to the investigator for analyses.
ACKNOWLEDGEMENTS
This study was sponsored by Allergan plc, Dublin, Ireland, prior to its acquisition by AbbVie Inc. Writing and editorial assistance were provided to the authors by Sarah J. Cross, PhD, of AbbVie Inc, and funded by AbbVie Inc. We thank Alda Karic, PharmD, of AbbVie Inc for her invaluable input on the manuscript. All authors meet the ICMJE criteria for authorship, and neither honoraria nor other forms of payment were made for authorship.
Moradi A, Fabi S, Rapaport D, Shridharani S, Goldman MP, Fu FT. Electromagnetic muscle stimulation: A retrospective study of patient experience. J Cosmet Dermatol. 2022;21:271–278. 10.1111/jocd.14401
Funding information
This study was sponsored by Allergan plc, Dublin, Ireland prior to its acquisition by AbbVie Inc
DATA AVAILABILITY STATEMENT
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our‐science/clinical‐trials/clinical‐trials‐data‐and‐information‐sharing/data‐and‐information‐sharing‐with‐qualified‐researchers.html.
REFERENCES
- 1. 2019 Consumer Survey on Cosmetic Dermatology Procedures [press release]. American Society for Dermatology Surgery (ASDS), 2019. [Google Scholar]
- 2. Shridharani SM, Broyles JM, Matarasso A. Liposuction devices: technology update. Med Devices. 2014;7:241‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mazzoni D, Lin MJ, Dubin DP, Khorasani H. Review of non‐invasive body contouring devices for fat reduction, skin tightening and muscle definition. Australas J Dermatol. 2019;60(4):278‐283. [DOI] [PubMed] [Google Scholar]
- 4. Shimada Y, Sakuraba T, Matsunaga T, Misawa A, Kawatani M, Itoi E. Effects of therapeutic magnetic stimulation on acute muscle atrophy in rats after hindlimb suspension. Biomed Res. 2006;27(1):23‐27. [DOI] [PubMed] [Google Scholar]
- 5. Samuels JB, Pezzella A, Berenholz J, Alinsod R. Safety and efficacy of a non‐invasive high‐intensity focused electromagnetic field (HIFEM) device for treatment of urinary incontinence and enhancement of quality of life. Lasers Surg Med. 2019;51(9):760‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Porcari JP, McLean KP, Foster C, Kernozek T, Crenshaw B, Swenson C. Effects of electrical muscle stimulation on body composition, muscle strength, and physical appearance. J Strength Cond Res. 2002;16(2):165‐172. [PubMed] [Google Scholar]
- 7. Alamer A, Melese H, Nigussie F. Effectiveness of neuromuscular electrical stimulation on post‐stroke dysphagia: a systematic review of randomized controlled trials. Clin Interv Aging. 2020;15:1521‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Porcari J, Ryskey A, Foster C. The effects of high intensity neuromuscular electrical stimulation on abdominal strength and endurance, core strength, abdominal girth, and perceived body shape and satisfaction. Int J of Kinesiol Sports Sci. 2018;6(1):19. [Google Scholar]
- 9. Porcari JP, Miller J, Cornwell K, et al. The effects of neuromuscular electrical stimulation training on abdominal strength, endurance, and selected anthropometric measures. J Sports Sci Med. 2005;4(1):66‐75. [PMC free article] [PubMed] [Google Scholar]
- 10. Roth BJ, Basser PJ. A model of the stimulation of a nerve fiber by electromagnetic induction. IEEE Trans Biomed Eng. 1990;37(6):588‐597. [DOI] [PubMed] [Google Scholar]
- 11. Jacob C, Kinney B, Busso M, et al. High intensity focused electro‐magnetic technology (hifem) for non‐invasive buttock lifting and toning of gluteal muscles: a multi‐center efficacy and safety study. J Drugs Dermatol. 2018;17(11):1229‐1232. [PubMed] [Google Scholar]
- 12. Jacob CI, Paskova K. Safety and efficacy of a novel high‐intensity focused electromagnetic technology device for noninvasive abdominal body shaping. J Cosmet Dermatol. 2018;17(5):783‐787. [DOI] [PubMed] [Google Scholar]
- 13. Kinney BM, Kent DE. MRI and CT assessment of abdominal tissue composition in patients after high‐intensity focused electromagnetic therapy treatments: one‐year follow‐up. Aesthet Surg J. 2020;40(12):NP686‐NP693. [DOI] [PubMed] [Google Scholar]
- 14. Kilmer SL, Cox SE, Zelickson BD, et al. Feasibility study of electromagnetic muscle stimulation and cryolipolysis for abdominal contouring. Dermatol Surg. 2020;46(Suppl 1):S14‐S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fabi S, Dover JS, Tanzi E, Bowes LE, Tsai FUF, Odusan A. A 12‐week, prospective, non‐comparative, non‐randomized study of magnetic muscle stimulation for improvement of body satisfaction with the abdomen and buttocks. Lasers Surg Med. 2020;53(1):79‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katz B, Bard R, Goldfarb R, Shiloh A, Kenolova D. Ultrasound assessment of subcutaneous abdominal fat thickness after treatments with a high‐intensity focused electromagnetic field device: a multicenter study. Dermatol Surg. 2019;45(12):1542‐1548. [DOI] [PubMed] [Google Scholar]
- 17. Lima LV, Abner TSS, Sluka KA. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J Physiol. 2017;595(13):4141‐4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verbrugghe J, Agten A, Stevens S, et al. High intensity training to treat chronic nonspecific low back pain: effectiveness of various exercise modes. J Clin Med. 2020;9(8):2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haruyama K, Kawakami M, Otsuka T. Effect of core stability training on trunk function, standing balance, and mobility in stroke patients. Neurorehabil Neural Repair. 2017;31(3):240‐249. [DOI] [PubMed] [Google Scholar]
- 20. Carpes FP, Reinehr FB, Mota CB. Effects of a program for trunk strength and stability on pain, low back and pelvis kinematics, and body balance: a pilot study. J Bodyw Mov Ther. 2008;12(1):22‐30. [DOI] [PubMed] [Google Scholar]
- 21. Granacher U, Lacroix A, Muehlbauer T, Roettger K, Gollhofer A. Effects of core instability strength training on trunk muscle strength, spinal mobility, dynamic balance and functional mobility in older adults. Gerontology. 2013;59(2):105‐113. [DOI] [PubMed] [Google Scholar]
- 22. Motealleh A, Mohamadi M, Moghadam MB, Nejati N, Arjang N, Ebrahimi N. Effects of core neuromuscular training on pain, balance, and functional performance in women with patellofemoral pain syndrome: a clinical trial. J Chiropr Med. 2019;18(1):9‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwarz L, Kindermann W. Changes in beta‐endorphin levels in response to aerobic and anaerobic exercise. Sports Med. 1992;13(1):25‐36. [DOI] [PubMed] [Google Scholar]
- 24. Paungmali A, Joseph LH, Punturee K, Sitilertpisan P, Pirunsan U, Uthaikhup S. Immediate effects of core stabilization exercise on β‐endorphin and cortisol levels among patients with chronic nonspecific low back pain: a randomized crossover design. J Manipulative Physiol Ther. 2018;41(3):181‐188. [DOI] [PubMed] [Google Scholar]
- 25. Hicks SD, Jacob P, Perez O, Baffuto M, Gagnon Z, Middleton FA. The transcriptional signature of a runner's high. Med Sci Sports Exerc. 2019;51(5):970‐978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our‐science/clinical‐trials/clinical‐trials‐data‐and‐information‐sharing/data‐and‐information‐sharing‐with‐qualified‐researchers.html.


