Abstract
End‐stage heart failure (ESHF) in pediatric age is an ongoing challenge. Heart transplantation is the final option, but its long‐term outcomes are still suboptimal in children. An alternative patient‐tailored surgical protocol to manage ESHF in children is described. Retrospective, single‐center analysis of pediatric patients admitted to our institution between April 2004 and February 2021 for ESHF. Our current protocol is as follows: (a) Patients <1 year with isolated left ventricular dysfunction due to dilated cardiomyopathy underwent pulmonary artery banding (PAB). (b) Patients <10 years and <20 kg, who did not meet previous criteria were managed with Berlin Heart EXCOR. (c) Patients >10 years or >20 kg, underwent placement of intracorporeal Heartware. Primary outcomes were survival, transplant incidence, and postoperative adverse events. A total of 24 patients (mean age 5.3 ± 5.9 years) underwent 26 procedures: PAB in 6 patients, Berlin Heart in 11, and Heartware in 7. Two patients shifted from PAB to Berlin Heart. Overall survival at 1‐year follow‐up and 5‐year follow‐up was 78.7% (95%CI = 62%‐95.4%) and 74.1% (95%CI = 56.1%‐92.1%), respectively. Berlin Heart was adopted in higher‐risk settings showing inferior outcomes, whereas a PAB enabled 67% of patients to avoid transplantation, with no mortality. An integrated, patient‐tailored surgical strategy, comprehensive of PAB and different types of ventricular assist devices, can provide satisfactory medium‐term results for bridging to transplant or recovery. The early postoperative period is critical and requires strict clinical vigilance. Selected infants can benefit from PAB that has demonstrated to be a safe bridge to recovery.
Keywords: heart transplantation, mechanical circulatory support, pediatric heart failure, pulmonary artery banding, ventricular rehabilitation
An integrated and patient‐tailored surgical protocol for end‐stage heart failure in children, comprehensive of pulmonary artery banding for left ventricular rehabilitation and long‐term mechanical circulatory support devices, can provide very good early and medium‐term survival.
Abbreviations
- AE
adverse event
- AKI
acute kidney injury
- BH
Berlin Heart EXCOR
- CHD
congenital heart disease
- CI
confidence interval
- DCM
dilated cardiomyopathy
- EF
ejection fraction
- ESHF
end‐stage heart failure
- HR
hazard ratio
- HT
heart transplantation
- HVAD
Heartware
- IQR
interquartile range
- LV
left ventricle
- MCS
mechanical circulatory support
- MOF
multiorgan failure
- PAB
pulmonary artery banding
- RV
right ventricle
- SD
standard deviation
- VAD
ventricular assist device
1. INTRODUCTION
Despite important therapeutic advances that have been achieved for adults with end‐stage heart failure (ESHF), its management in children refractory to maximal anticongestive medical therapy is an ongoing challenge. 1 , 2 , 3 , 4 Heart transplantation (HT) is the ultimate treatment for these patients, but its long‐term outcomes in children are still suboptimal. In fact, 25‐year survival is <40%, 5 worsened by cumulative morbidities related to immunosuppressive therapy, graft rejection, and social issues. 4 , 5 , 6 All these factors, together with the constant lack of adequate donors, contribute to make HT a not appealing option, especially in infants.
To improve outcomes, or even avoid HT, alternative surgical strategies have been developed. Mechanical circulatory support (MCS) with a ventricular assist device (VAD) is an effective strategy in children as a bridge to HT, recovery, or rarely destination therapy. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 The extracorporeal Berlin Heart EXCOR (BH) can currently provide effective support even in neonates and infants, 17 , 18 , 20 , 21 whereas intracorporeal VAD is reported to have excellent long‐term results in older children or teenagers. 22 However, the incidence of major and disabling complications after MCS is not negligible, whereas the length of hospitalization and social costs are a serious burden. 23 , 24
Recently, Schranz et al 25 proposed an innovative application of the traditional pulmonary artery banding (PAB) as a treatment of ESHF in dilated cardiomyopathy (DCM) in infants with preserved right ventricular (RV) function, aimed at promoting myocardial recovery. 30 A recent multicenter study has reported encouraging short and midterm results on a larger cohort. 26 , 31
Since 2004, we have gradually developed a surgical program for the management of children with ESHF, which has evolved across the years, to provide our patients with the most effective and state‐of‐the‐art alternatives to HT. As new evidences were emerging, we have adopted the most innovative surgical strategies for ESHF, which currently include MCS, and PAB for left ventricular (LV) rehabilitation, as a bridge to HT, or to favor myocardial remodeling and recovery. We report our 15 years’ institutional experience with this evolving approach for ESHF surgical treatment, focusing on indications, outcomes, and limitations of each technique.
2. MATERIALS AND METHODS
This is a retrospective, single‐center analysis of pediatric patients admitted to our institution for ESHF between April 2004 and February 2021. The study was approved by our institutional Ethics Committee (protocol 59004), and the patients’ informed consent was obtained. Patients were included in the study regardless of ESHF etiology, and indications to surgery were bridge to transplantability, HT, or destination therapy. Patients who underwent ECMO implantation for acute onset of cardiac failure (ie, cardiogenic shock) were excluded from this study. After admission, all patients were evaluated by our interdisciplinary team, to optimize medical therapy and clinical conditions. If maximal medical therapy (including inotropic infusion, mechanical ventilation, NO inhalation) was ineffective, patients were considered for surgical treatment. In cases of acute myocarditis, immunomodulatory therapy with steroids or intravenous immunoglobulin was administered, if applicable. When myocardial dysfunction was proven to be unresponsive to maximal medical therapy, and ECMO was not considered a suitable option, these patients were enrolled in the ESHF surgical protocol.
After several refinements through the years, our current protocol (Figure 1) includes the following modalities:
Patients <1 year with LV dysfunction due to DCM and preserved RV function were firstly considered for PAB according to Giessen protocol. 27 Exclusion criteria were as follows: biventricular failure, ≥moderate tricuspid regurgitation, pulmonary hypertension (mean pulmonary pressure >25 mm Hg), and associated major congenital heart disease (CHD). The PAB was tightened until we obtained right ventricular pressure increased to 70% of systemic pressure, or until echocardiographic shift of interventricular septum, or TAPSE reduction, or increasing tricuspid regurgitation (as described elsewhere 27 , 31 ).
Patients <10 years and <20 kg, who did not meet PAB inclusion criteria, were managed with either left or biventricular BH.
Patients >10 years or ≥20 kg with isolated LV failure underwent placement of intracorporeal Heartware (HVAD).
FIGURE 1.
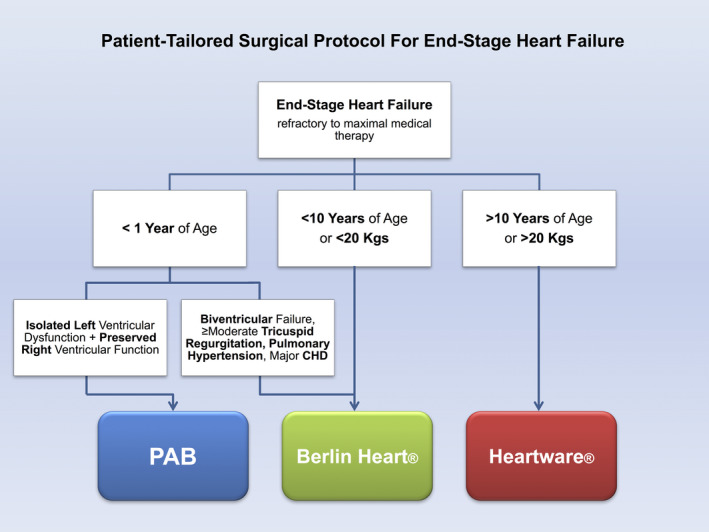
Patient‐tailored protocol for end‐stage heart failure [Color figure can be viewed at wileyonlinelibrary.com]
Patients who underwent VAD implantation were managed according to our institutional antithrombotic protocol for MCS, which has been refined across the years. Figure 2 shows our current protocol. Primary outcomes were survival and incidence of HT; secondary outcomes were onset of adverse event (AE) defined acute kidney injury (AKI) when creatinine level was >110 μmol/L and urine output was <2 cc/kg/hr, or need of renal replacement therapy; neurological injury or cerebrovascular accident; bacteremia or infection requiring prolonged antibiotic therapy (>7 days); multiorgan failure (MOF), defined as the significant deterioration of ≥2 organs’ function; other.
FIGURE 2.
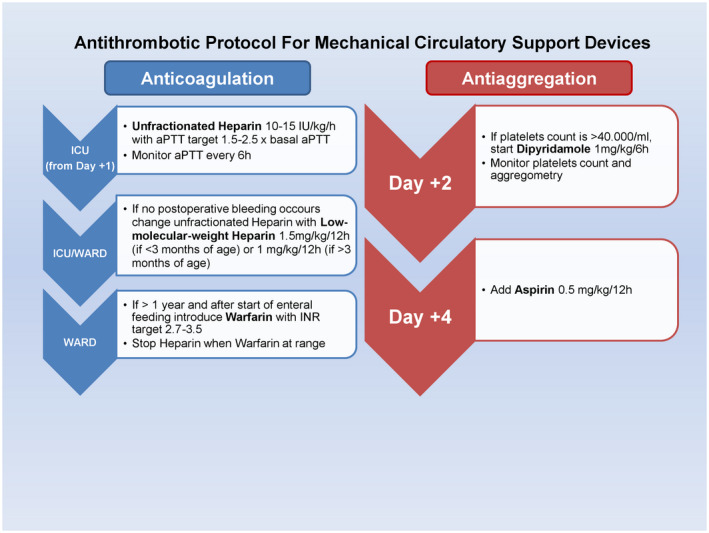
Antithrombotic protocol for mechanical circulatory support devices [Color figure can be viewed at wileyonlinelibrary.com]
2.1. Statistical analysis
Data are summarized as mean (SD) or median (IQR) in case of quantitative variables, as counts and percentages in case of categorical variables. Normality of quantitative variables was checked with Shapiro–Wilk test. Quantitative variables were compared across groups with Kruskal–Wallis test and categorical variables with Fisher's exact test. Survival free from death was estimated with Kaplan–Meier method along with 95% confidence interval (CI) at 1, 2, and 5 years of follow‐up considering age at admission. Comparison between groups was made with log‐rank test. Univariable/multivariable analysis of mortality risk factors was not performed due to the small number and selection bias of patients. Statistical significance was set at P value <.05. Analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC).
3. RESULTS
3.1. Baseline characteristics
We enrolled 24 children (mean age 5.3 ± 5.9 years; male 71%) who required surgical management for ESHF not responsive to maximal medical therapy. Twenty‐six surgical procedures were performed (two patients underwent two different main procedures). The initial surgical strategy was PAB in 6 patients (25%), BH in 11 (46%), and HVAD in 7 (29%).
The etiology of ESHF was DCM in eight patients (33%), CHD in seven (29%), myocarditis in seven (29%), other (LV‐noncompaction and lipid‐storage cardiomyopathy) in two patients (8%). Among those children with CHD, three patients had a functional single‐ventricle anomaly (hypoplastic left heart syndrome in two, unbalanced double outlet right ventricle with pulmonary atresia in one neonate); two had Shone's complex; one had tetralogy of Fallot with subaortic stenosis; one had a congenitally corrected transposition of great arteries. All of them had already undergone previous radical or palliative surgery. Complete demographic characteristics are summarized in Table 1.
TABLE 1.
Preoperative demographic characteristics, overall and according to the initial treatment
| Total (n = 24) | PAB (n = 6) | Berlin Heart (n = 11) | Heartware (n = 7) | P value* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Age (years) | 5.3 (5.9) | 1.3 (0.7‐10.7) | 1.1 (1.2) | 0.7 (0.5‐1.6) | 2.5 (3.4) | 1.1 (0.6‐2.4) | 13.3 (2.1) | 14.3 (10.7‐14.7) | .001 |
| Dilated cardiomyopathy | 10.4 (6.1) | 12.5 (3.5‐14.7) | |||||||
| Congenital heart disease | 4.7 (5.4) | 1 (0.4‐10.7) | |||||||
| Myocarditis | 1.2 (1.1) | 0.8 (0.5‐1.4) | |||||||
| Others | 1.6 (1.2) | 1.6 | |||||||
| Body weight (kg) | 19.6 (21) | 8 (6.6‐27) | 7.6 (2.5) | 7.4 (5.6‐9) | 9.1 (6.5) | 7 (4.6‐10.5) | 46.4 (20.9) | 47 (28‐65) | .001 |
| Ejection fraction (%) | 17.3 (8.8) | 15 (10‐25) | 14.5 (5.5) | 12.5 (12.5‐19.5) | 16.5 (10.2) | 15 (5‐25) | 20.9 (8.8) | 24 (11‐30) | .42 |
| INTERMACS | 2.3 (0.9) | 2.5 (1‐2) | 3 (0) | 3 (3‐3) | 1.4 (0.5) | 1 (1‐2) | 3 (0.6) | 3 (3‐3) | .001 |
| n | % | n | % | n | % | n | % | ||
|---|---|---|---|---|---|---|---|---|---|
| Male | 17 | 71 | 4 | 67 | 7 | 64 | 6 | 86 | .73 |
| Heart failure etiology | .001 | ||||||||
| Dilated cardiomyopathy | 8 | 33 | 0 | 2 | 18 | 6 | 86 | ||
| Congenital heart disease | 7 | 29 | 0 | 6 | 55 | 1 | 14 | ||
| Myocarditis | 7 | 29 | 5 | 83 | 2 | 18 | 0 | ||
| Other | 2 | 8 | 1 | 17 | 1 | 9 | 0 | ||
| ≥2 Inotropes | 19 | 79 | 3 | 50 | 10 | 91 | 6 | 86 | .14 |
| Mechanical support | 8 | 33 | 0 | 7 | 64 | 1 | 14 | .016 | |
| Intubation | 13 | 54 | 4 | 67 | 8 | 73 | 1 | 14 | .058 |
| Neurological compromise | 4 | 17 | 2 | 33 | 0 | 2 | 29 | .088 | |
| Acute kidney injury | 4 | 17 | 1 | 17 | 3 | 27 | 0 | .33 | |
| Indication to surgery | .27 | ||||||||
| Bridge to transplant | 19 | 79 | 6 | 100 | 9 | 82 | 4 | 57 | |
| Bridge to transplantability | 4 | 17 | 0 | 2 | 18 | 2 | 29 | ||
| Destination therapy | 1 | 4 | 0 | 0 | 1 | 14 |
Abbreviations: IQR, interquartile range; PAB, pulmonary artery banding; SD, standard deviation.
P value refers to comparisons of variables between the three groups.
Bold values are to outline the variables.
Overall, surgery for ESHF resulted in early mortality in three patients (12.5%). At a median follow‐up of 4.4 (0.8‐8.4) years, 16 patients (67%) underwent HT after a median time of 3.1 (0.9‐8) months from surgery, and 3 of them died after it. Seventeen patients survived (71%). Overall survival at 1‐year follow‐up and 5‐year follow‐up was 78.7% (95%CI = 62%‐95.4%) and 74.1% (95%CI = 56.1%‐92.1%), respectively (Figure 3). Treatment flowchart is shown in Figure 4. Major neurological events occurred in eight patients (33%), and led to death for severe hemorrhage in three patients, whereas they caused residual motor or cognitive deficits in two patients (12% among survivors at last follow‐up).
FIGURE 3.
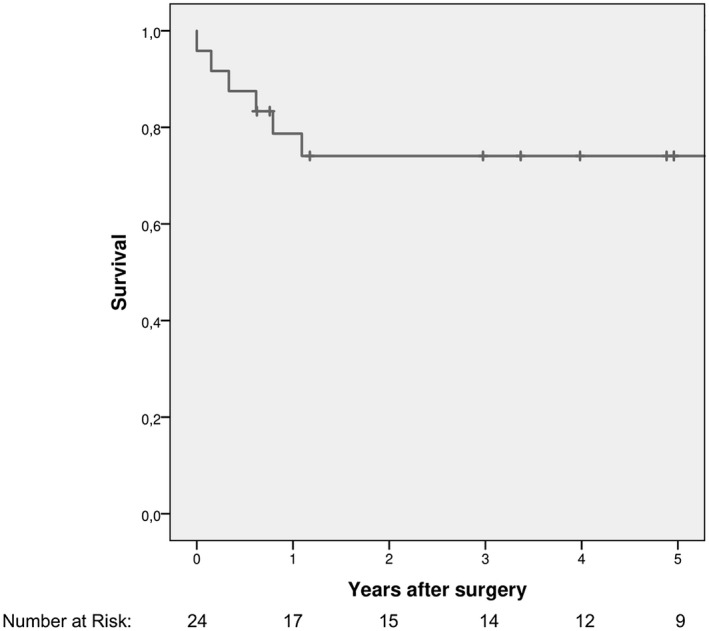
Kaplan–Meier plot of overall survival rate (n = 24)
FIGURE 4.
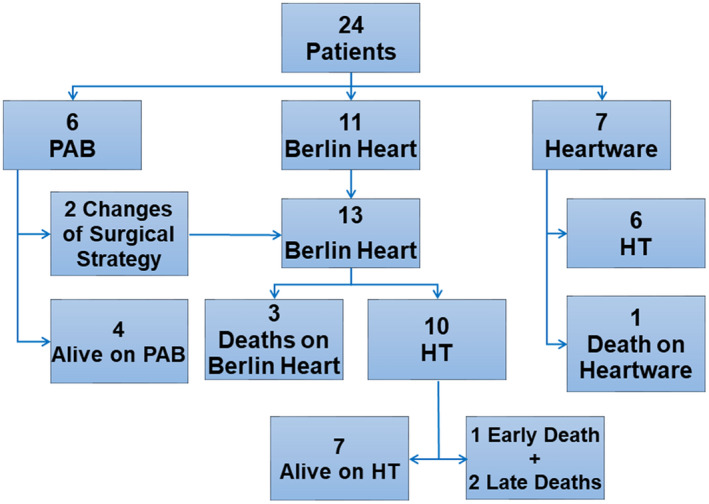
Treatment flow‐chart (n = 24) [Color figure can be viewed at wileyonlinelibrary.com]
3.1.1. PAB strategy
The most common ESHF etiology was myocarditis (83%), and all patients underwent PAB as bridge to HT. Two patients (33%) underwent an additional tightening of the PAB within 48 hours from the initial surgery. Five patients improved their hemodynamic conditions and could be discharged home (Table 2), after a mean hospital stay of 48.6 ± 27.4 days.
TABLE 2.
Postoperative course, overall and according to initial treatment
| Total (n = 24) | PAB (n = 6) | Berlin Heart (n = 11) | Heartware (n = 7) | P value* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Follow‐up (years) | 4.5 (3.7) | 4.4 (0.8‐8.4) | 3.6 (1.7) | 3.7 (2.4‐5.1) | 3.8 (4.5) | 1.1 (0.6‐9.3) | 6.3 (3.2) | 7.1 (4.9‐8.8) | .32 |
| Time to transplantation (months) | 6.7 (11.1) | 3.1 (0.9‐8) | 8.6 (6.9) | 8.6 | 3.9 (3.7) | 3.4 (0.4‐6.6) | 9.8 (17.8) | 1.5 (0.9‐17.7) | .64 |
| n | % | n | % | n | % | n | % | ||
|---|---|---|---|---|---|---|---|---|---|
| Prolonged mechanical ventilation | 16 | 67 | 5 | 83 | 9 | 82 | 2 | 29 | .058 |
| Major infection | 9 | 38 | 3 | 50 | 3 | 27 | 3 | 43 | .65 |
| Surgical revision for bleeding | 9 | 38 | 0 | 6 | 55 | 3 | 43 | .090 | |
| Neurological event | 8 | 33 | 1 | 17 | 6 | 55 | 1 | 14 | .21 |
| Acute kidney injury | 6 | 25 | 1 | 17 | 4 | 36 | 1 | 14 | .59 |
| Multiorgan failure | 4 | 17 | 0 | 4 | 36 | 0 | .12 | ||
| Other complication | 2 | 8 | 1 | 17 | 0 | 1 | 14 | .28 | |
| Reintervention | 8 | 33 | 3 | 50 | 3 | 27 | 2 | 29 | .74 |
| Discharged home on PAB/mechanical support | 8 | 33 | 5 | 83 | 0 | 3 | 43 | .001 | |
| Early death | 4 | 17 | 0 | 3 | 27 | 0 | .30 |
Abbreviations: IQR, interquartile range; PAB, pulmonary artery banding; SD, standard deviation.
P value refers to comparisons of variables between the three groups.
Bold values are to outline the variables.
Two patients after PAB (one early and one late) did not respond to this therapy. One 3.5‐years‐old child affected by acute lymphocytic myocarditis with a preoperative EF of 12% did not improve LV function, and required elective BH implantation 33 days after PAB, followed by HT after 13 months of uncomplicated MCS. The other one, with a diagnosis of associated LV‐noncompaction, 3 months after discharge home developed a severe RV failure after acute pneumonia, requiring MCS support (emergent ECMO, followed by BH) and underwent a successful HT 3 days later. Both these patients have been discharged home in good clinical conditions after HT and are currently doing well.
The remaining four patients are alive and well, after a median follow‐up time of 3.7 (2.4‐5.1) years. Levosimendan intravenous infusion at 0.2 mcg/kg/min for 48 hours was administered in all at least once (range 1‐3), after PAB. All but one (the most recent) underwent an elective percutaneous procedure for PAB balloon dilatation, after a median of 11 (7‐15) months from surgery, due to progressive RV hypertension and increased (≥moderate) tricuspid regurgitation. Procedures were well tolerated and patients gradually recovered from LV dysfunction, allowing delisting from HT in three cases, without recurrence of heart failure at last follow‐up, as we described elsewhere. 31
Analyzing the outcomes of this group, despite freedom from HT was 66.6% at last follow‐up, because PAB strategy failed in two cases, requiring VAD support, survival was not affected since it was 100% at 1‐year follow‐up and 5‐year follow‐up (Figure 5). Noticeably, those patients who lastly required HT were the first patients of our experience.
FIGURE 5.
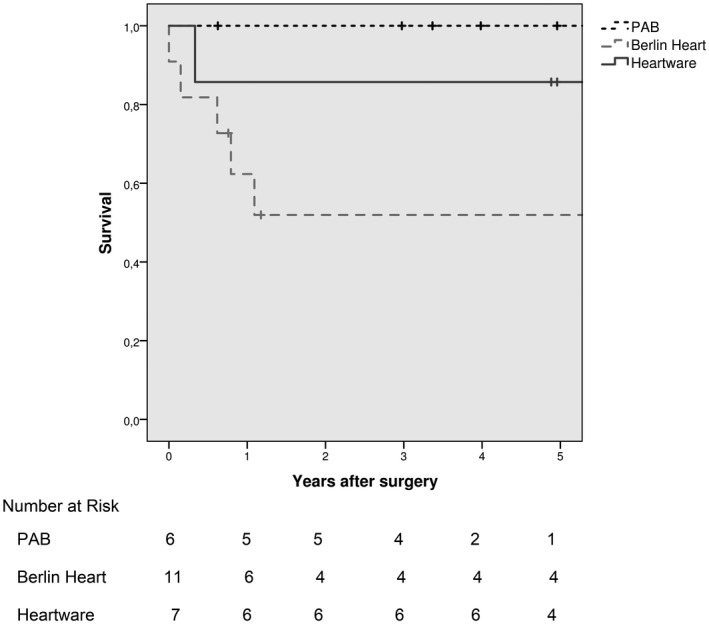
Kaplan–Meier plot of survival rate according to initial treatment (n = 24). *Of note that, although survival rate for PAB was 100%, this strategy failed in two over six patients, and these two patients survived by conventional VAD therapy
3.1.2. VAD strategy
Berlin heart EXCOR
Eleven patients had a BH implantation for ESHF caused mostly by complex CHD, requiring VAD support as bridge to HT or transplantability (to assess reversibility of pulmonary hypertension) (Table 1). Four of them were <1 year of age. Their preoperative clinical conditions were worse than the other two groups, as depicted by the higher need for ECMO support (64%, P = 0.014) and worse INTERMACS score (1.4 ± 0.5, P = .001; Table 1).
Berlin Heart was used as biventricular VAD in four patients (36%), left (systemic) VAD in the remaining seven; one of the latter required an extracorporeal right VAD (Biomedicus) for 5 days. In patients with univentricular circulation BH worked as “systemic” VAD (ventricular apex‐ascending aorta or neo‐aorta cannulation), and the indication was single‐ventricle end‐stage dysfunction. Lastly, two patients underwent associated procedures during VAD placement (atrial septal defect closure and aortic valve closure).
The postoperative course was characterized by a higher (but not statistically significant) rate of severe complications, such as mediastinal bleeding (55%), neurological events (55%), AKI (36%), and MOF (36%; Table 2). During support, the artificial ventricle was replaced for clotting in six cases (mean of 1.3 ± 1.6 changes/patient), whereas two patients underwent urgent surgical procedures for semilunar valves regurgitation. No patient on BH support was discharged home.
Eight patients (73%) survived to HT after a median time of 3.4 (0.4‐6.6) months, whereas three died due to progressive MOF. After HT, one child died early because of sepsis. At a median follow‐up of 1.1 (0.6‐9.3) years, there are five survivors in good hemodynamic status. Two patients died late because of systemic nodular lymphoma and acute pneumonia 1 and 10 years after HT, respectively. Survival rate was lower, although not statistically significant, than the other groups (62.3% [95%CI = 32.9%‐91.7%] and 51.9% [95%CI = 21.1%‐82.7%] at 1‐year follow‐up and 5‐year follow‐up, respectively; P = .097; Figure 5).
Heartware HVAD
Intrathoracic HVAD was used in seven patients (mean age 13.3 ± 2.1 years), as bridge to HT or transplantability in six patients, and destination therapy in one patient with Duchenne syndrome. Two patients needed a temporary right VAD for 3 and 4 days, respectively.
Concomitant procedures were performed in two patients: aortic valve closure in one, interatrial septal stent removal, and defect closure in the other.
The postoperative course presented fewer complications than the other groups (although not statistically relevant, Table 2). It is noticeable that the three older patients (43%) were discharged home on MCS support.
Six patients (86%) underwent HT after a median time of 1.5 (0.9‐17.7) months from implantation, and they are all alive at a median follow‐up of 7.1 (4.9‐8.8) years. After a multidisciplinary evaluation, the patient with Duchenne syndrome, given the unexpected mild course of the extra‐cardiac manifestations of his condition, was considered suitable for an HT, and he was finally transplanted after 4 years of VAD support. One last patient with complex CHD S/P multiple procedures experienced a cerebral hemorrhage 15 days after HVAD implant, and MCS was discontinued 4 months later because of nonreversible brain injury and coma state. Survival rate in this group was 85.7% [95%CI = 59.2%‐99.1%] at 1‐year follow‐up and 5‐year follow‐up (Figure 5).
4. DISCUSSION
We describe a patient‐tailored strategy for the surgical management of ESHF in infants and children, which has been designed according to the patient's age, size, and cause of ESHF (Figure 1). With the aim of delaying or, if possible, avoiding the need for HT, we propose a novel, integrated surgical program, comprising PAB for LV rehabilitation and long‐term MCS. Our experience shows that selected patients can benefit from such a patient‐tailored protocol, which can provide satisfactory early and medium‐term results, with an overall 5‐year survival rate of 74.1% (Figure 3), which is similar to the international standard of care. 5 , 6 , 7 , 8 , 9 , 10 , 11 Noticeably, PAB strategy permitted survival without MCS and myocardial recovery avoiding HT in selected infants.
To date, long‐term MCS is the standard of care for children with ESHF, as bridge to HT or, rarely, to recovery, 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 whose effectiveness relies on the unloading of the failing ventricle, maintaining organ perfusion. In our series, outcomes in such patients are substantially comparable with what reported in current literature, 12 , 17 , 18 , 20 , 21 , 22 , 23 , 24 in terms of survival and complication rates, which are still a significant drawback of MCS (Table 2). In particular, infections and mediastinal bleeding, although similar to studies including infants <10 kg, 24 affected up to 27% and 55% of patients supported by BH, respectively. Significant neurological AEs (both thrombotic and ischemic) occurred in 33% of our patients, with an incidence similar to what reported elsewhere, 12 , 17 , 18 , 20 , 21 , 22 , 23 , 24 but have been lethal in about one‐third of cases. Of note, most of these patients had experienced sudden major cardiac events (ie, cardiac arrest or ECMO) before VAD implantation, which may have increased the risk of postoperative neurological morbidity. Therefore, a proactive VAD implant approach is recommended to avoid hemodynamic instability that can cause these fearsome AEs. 8 , 9 , 10 , 11 Lastly, complex CHD was the most frequent cause of MCS with BH, which in our series was associated with worse survival (Figure 5). Although small numbers did not allow to perform univariate/multivariable analysis, CHD still represents a more challenging subgroup and carries an intrinsic higher risk of AE, because of previous multiple surgeries, reinterventions, and peculiar anatomies, as described elsewhere. 9 , 15 , 16 , 17 , 18 , 20 , 21 In fact, in these patients, we found a significantly greater need for preoperative ECMO before BH implant (64%) and worse INTERMACS class at admission (median 1; Table 1).
In contrast, those patients who underwent HVAD implantation experienced fewer postoperative complications, even if not absent, with a 5‐year survival of 85.7%, similar to what described elsewhere. 8 , 10 , 13 , 15 , 19 , 22 The more favorable outcome in this subgroup may be due to the better preoperative INTERMACS score (median 3) and to the fact that the main ESHF etiology was DCM, which is a well‐known protective factor in listed patients. 5 , 16
Noteworthy, outcomes of MCS in neonates and infants present with consistent morbidity 18 and mortality as high as 40% confirmed also in multicenter registries. 9 , 17 , 18 , 20 , 21 In addition, long‐term results of HT in infants are far than optimal. 5 Based on previous experience derived from retraining of the subpulmonary ventricle in transposition of great arteries, the role of PAB has been reinvented for LV remodeling in DCM with preserved RV function. 25 , 26 , 27 The acute RV afterload increase caused by PAB induces changes of ventricular chamber geometry and optimization of LV preload, 26 , 27 , 28 which enhance LV rehabilitation. Multicenter experience with PAB in children has been reported, 26 with encouraging results, demonstrating that PAB in selected patients has a realistic potential for myocardial recovery. 30 , 32 Our experience is consistent with published data, 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 with a 5‐year survival of 100% (Figure 5) and 67% freedom from HT, permitting the survival of selected children with ESHF without the use of MCS. Of note, PAB was early successful in all but one older patient (3.5 years old) who underwent this treatment in our early experience, and did not respond to PAB, requiring BH implantation and subsequent HT. Later, it was demonstrated that cardiomyocyte proliferation tends to be greater in infancy, with a subsequent decline with aging. 32 This might be the reason for PAB’s failure in our older patient, in whom a reduced cardiac regenerative potential could be hypothesized. This assumption may be supported by the fact that LV function improved substantially, enabling delisting in three‐fourth of the remaining patients <1 year, who were strictly followed up with rigorous and full anticongestive therapy. These findings would be in line with the well‐established capacity of the neonatal heart to regenerate after an insult, which is observed in experimental conditions in other mammals. From this experience, our indications for PAB have been refined in an empiric way, lowering the age threshold to 1 year, which has enabled us to improve clinical outcomes. However, the intrinsic molecular mechanism for this to occur need to be thoroughly investigated.
Lastly, we learnt that these patients require strict and proactive surveillance, in which MCS can be readily available if PAB results ineffective. This seems to suggest that LV remodeling, based on a hypothesized biological crosstalk between the two ventricles, 30 necessitates sufficient time to allow for adequate circulatory stabilization. A possibility is that PAB induces cardiac remodeling by modifications of the intracardiac pressure parameters, and the existence of a mechanosensing apparatus (ie, signaling to the cardiomyocyte consequent to wall stress) is strongly supported by the notion that the master activator of cardiomyocyte proliferation, the YAP transcriptional cofactor, 33 is regulated by signals in the Hippo pathway that senses mechanical clues at the cardiomyocyte sarcolemma. 34 , 35 Thus, these patients go through a critical period for progressive RV adaptation after PAB and LV remodeling. This calls for extreme care in the immediate postoperative months, to intervene whenever required if cardiac failure occurs as a response to acute stress like a common disease (such as pneumonia), as we experienced in our series.
4.1. Limitations
The retrospective, single‐center nature of our study and the limited number of patients imply a careful interpretation of data. Different inclusion criteria were arbitrary, even if supported by literature and experience data. This and the heterogeneity of patients’ clinical conditions and ESHF etiologies should dictate attention in comparing outcomes. Besides the small dimensions of cohorts, comparisons were made not to demonstrate a superiority of a strategy over another, but to evaluate the effectiveness of a patient‐tailored surgical protocol, defined by clinical characteristics of patients, whose critical analysis is mandatory to better understand the different outcomes of study groups. Survival curves may overestimate the prognosis of the whole pediatric population with ESHF, due to patients’ inclusion in analysis after hospital admission.
5. CONCLUSIONS
We propose an innovative, patient‐tailored surgical strategy for the treatment of ESHF in infants and children, comprehensive of PAB for LV rehabilitation, and long‐term MCS as a bridge to HT. In our hands, it has provided good early and medium‐term results, with a 5‐year survival of 74.1%. Such an approach may delay if not avoid MCS or HT in selected infants. A strict, postoperative clinical vigilance is advocated to contain complications related to MCS or to slow myocardial remodeling after PAB.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceived and wrote the article: Ponzoni, Padalino
Performed statistical analyses: Ponzoni, Frigo
Contributed to the final version of the manuscript: Castaldi, Cerutti, Di Salvo, Vida
ACKNOWLEDGEMENTS
Open access funding enabled by Universita degli Studi di Padova.
Ponzoni M, Frigo AC, Castaldi B, Cerutti A, Di Salvo G, Vida VL, et al. Surgical strategies for the management of end‐stage heart failure in infants and children: A 15‐year experience with a patient‐tailored approach. Artif. Organs. 2021;45:1543–1553. 10.1111/aor.14057
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
REFERENCES
- 1. Razzouk AJ, Bailey LL. Heart transplantation in children for end‐stage congenital heart disease. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2014;17:69–76. [DOI] [PubMed] [Google Scholar]
- 2. Schranz D, Voelkel NF. “Nihilism” of chronic heart failure therapy in children and why effective therapy is withheld. Eur J Pediatr. 2016;175:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Recla S, Schmidt D, Logeswaran T, Esmaeili A, Schranz D. Pediatric heart failure therapy: why β1‐receptor blocker, tissue ACE‐I and mineralocorticoid‐receptor‐blocker? Transl Pediatr. 2019;8:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zangwill S. Five decades of pediatric heart transplantation: challenges overcome, challenges remaining. Curr Opin Cardiol 2017;32:69–77. [DOI] [PubMed] [Google Scholar]
- 5. Dipchand AI. Current state of pediatric cardiac transplantation. Ann Cardiothorac Surg. 2018;7:31–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen JM, Canter CE, Hsu DT, Kindel SJ, Law YM, McKeever JE, et al. Current topics and controversies in pediatric heart transplantation: proceedings of the pediatric heart transplantation summit 2017. World J Pediatr Congenit Heart Surg. 2018;9:575–81. [DOI] [PubMed] [Google Scholar]
- 7. Miller JR, Epstein DJ, Henn MC, Guthrie T, Schuessler RB, Simpson KE, et al. Early biventricular assist device use in children: a single‐center review of 31 patients. ASAIO J. 2015;61:688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kindel SJ, Everitt MD. A contemporary review of paediatric heart transplantation and mechanical circulatory support. Cardiol Young. 2016;26:851–9. [DOI] [PubMed] [Google Scholar]
- 9. Law SP, Oron AP, Kemna MS, Albers EL, McMullan DM, Chen JM, et al. Comparison of transplant waitlist outcomes for pediatric candidates supported by ventricular assist devices versus medical therapy. Pediatr Crit Care Med. 2018;19:442–50. [DOI] [PubMed] [Google Scholar]
- 10. Hetzer R, Javier MFDM, Delmo Walter EM. Role of paediatric assist device in bridge to transplant. Ann Cardiothorac Surg. 2018;7:82–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeewa A, Imamura M, Canter C, Niebler RA, VanderPluym C, Rosenthal DN, et al. Long‐term outcomes after transplantation after support with a pulsatile pediatric ventricular assist device. J Heart Lung Transplant. 2019;38:449–55. [DOI] [PubMed] [Google Scholar]
- 12. Hetzer R, Kaufmann F, Delmo Walter EM. Paediatric mechanical circulatory support with Berlin Heart EXCOR: development and outcome of a 23‐year experience. Eur J Cardiothorac Surg. 2016;50:203–10. [DOI] [PubMed] [Google Scholar]
- 13. Miller JR, Eghtesady P. Ventricular assist device use in congenital heart disease with a comparison to heart transplant. J Comp Eff Res. 2014;3:533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bryant R, Rizwan R, Villa CR, Zafar F, Wells D, Chin C, et al. Transplant outcomes for congenital heart disease patients bridged with a ventricular assist device. Ann Thorac Surg. 2018;106:588–94. [DOI] [PubMed] [Google Scholar]
- 15. Santamaria RL, Jeewa A, Cedars A, Buchholz H, Conway J. Mechanical circulatory support in pediatric and adult congenital heart disease. Can J Cardiol. 2020;36:223–33. [DOI] [PubMed] [Google Scholar]
- 16. Peng DM, Koehl DA, Cantor RS, McMillan KN, Barnes AP, McConnell PI, et al. Outcomes of children with congenital heart disease implanted with ventricular assist devices: an analysis of the Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs). J Heart Lung Transplant. 2019;38:420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Molfetta A, Gandolfo F, Filippelli S, Perri G, Di Chiara L, Iacobelli R, et al. The use of Berlin Heart EXCOR VAD in children less than 10 kg: a single center experience. Front Physiol. 2016;6:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rohde S, Antonides CFJ, Dalinghaus M, Muslem R, Bogers AJJC. Clinical outcomes of paediatric patients supported by the Berlin Heart EXCOR: a systematic review. Eur J Cardiothorac Surg. 2019;56:830–9. [DOI] [PubMed] [Google Scholar]
- 19. Miller JR, Lancaster TS, Epstein DJ, DuPont NC, Simpson KE, Castleberry C, et al. Outcomes and trends of ventricular assist device selection in children with end‐stage heart failure. ASAIO J. 2017;63:464–9. [DOI] [PubMed] [Google Scholar]
- 20. de By TMMH, Schweiger M, Waheed H, Berger F, Hübler M, Özbaran M, et al. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS): first EUROMACS Paediatric (Paedi‐EUROMACS) report. Eur J Cardiothorac Surg. 2018;54:800–8. [DOI] [PubMed] [Google Scholar]
- 21. de By TMMH, Antonides CFJ, Schweiger M, Sliwka J, Davies B, Berger F, et al. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS): second EUROMACS Paediatric (Paedi‐EUROMACS) report. Eur J Cardiothorac Surg. 2020;57:1038–50. [DOI] [PubMed] [Google Scholar]
- 22. Padalino MA, Bottio T, Tarzia V, Bortolussi G, Cerutti A, Vida VL, et al. HeartWare ventricular assist device as bridge to transplant in children and adolescents. Artif Organs. 2014;38:418–22. [DOI] [PubMed] [Google Scholar]
- 23. Almond CS, Morales DL, Blackstone EH, Turrentine MW, Imamura M, Massicotte MP, et al. Berlin Heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US children. Circulation. 2013;127:1702–11. [DOI] [PubMed] [Google Scholar]
- 24. Conway J, St Louis J, Morales DL, Law S, Tjossem C, Humpl T. Delineating survival outcomes in children <10 kg bridged to transplant or recovery with the Berlin Heart EXCOR Ventricular Assist Device. JACC Heart Fail. 2015;3:70–7. [DOI] [PubMed] [Google Scholar]
- 25. Schranz D, Veldman A, Bartram U, Michel‐Behnke I, Bauer J, Akintürk H. Pulmonary artery banding for idiopathic dilative cardiomyopathy: a novel therapeutic strategy using an old surgical procedure. J Thorac Cardiovasc Surg. 2007;134:796–7. [DOI] [PubMed] [Google Scholar]
- 26. Schranz D, Akintuerk H, Bailey L, Miera O, Danne F, Kavarana MN, et al. Pulmonary artery banding for functional regeneration of end‐stage dilated cardiomyopathy in young children: world network report. Circulation. 2018;137:1410–2. [DOI] [PubMed] [Google Scholar]
- 27. Schranz D, Rupp S, Müller M, Schmidt D, Bauer A, Valeske K, et al. Pulmonary artery banding in infants and young children with left ventricular dilated cardiomyopathy: a novel therapeutic strategy before heart transplantation. J Heart Lung Transplant. 2013;32:475–81. [DOI] [PubMed] [Google Scholar]
- 28. Latus H, Hachmann P, Gummel K, Recla S, Voges I, Mueller M, et al. Biventricular response to pulmonary artery banding in children with dilated cardiomyopathy. J Heart Lung Transplant. 2016;35:934–8. [DOI] [PubMed] [Google Scholar]
- 29. Schranz D, Recla S, Malcic I, Kerst G, Mini N, Akintuerk H. Pulmonary artery banding in dilative cardiomyopathy of young children: review and protocol based on the current knowledge. Transl Pediatr. 2019;8:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rupp S, Schranz D. Cardiac regeneration in children. Pediatr Cardiol. 2015;36:713–8. [DOI] [PubMed] [Google Scholar]
- 31. Di Candia A, Castaldi B, Bordin G, Cerutti A, Reffo E, Biffanti R, et al. Pulmonary artery banding for ventricular rehabilitation in infants with dilated cardiomyopathy: early results in a single‐center experience. Front Pediatr. 2020;16:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park S‐Y, et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A. 2013;110:1446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morikawa Y, Heallen T, Leach J, Xiao Y, Martin JF. Dystrophin‐glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature. 2017;547:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chakraborty S, Njah K, Pobbati AV, Lim YB, Raju A, Lakshmanan M, et al. Agrin as a mechanotransduction signal regulating YAP through the hippo pathway. Cell Rep. 2017;18:2464–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.


