ABSTRACT
Osteoderms are mineralised structures consisting mainly of calcium phosphate and collagen. They form directly within the skin, with or without physical contact with the skeleton. Osteoderms, in some form, may be primitive for tetrapods as a whole, and are found in representatives of most major living lineages including turtles, crocodilians, lizards, armadillos, and some frogs, as well as extinct taxa ranging from early tetrapods to dinosaurs. However, their distribution in time and space raises questions about their evolution and homology in individual groups. Among lizards and their relatives, osteoderms may be completely absent; present only on the head or dorsum; or present all over the body in one of several arrangements, including non‐overlapping mineralised clusters, a continuous covering of overlapping plates, or as spicular mineralisations that thicken with age. This diversity makes lizards an excellent focal group in which to study osteoderm structure, function, development and evolution. In the past, the focus of researchers was primarily on the histological structure and/or the gross anatomy of individual osteoderms in a limited sample of taxa. Those studies demonstrated that lizard osteoderms are sometimes two‐layered structures, with a vitreous, avascular layer just below the epidermis and a deeper internal layer with abundant collagen within the deep dermis. However, there is considerable variation on this model, in terms of the arrangement of collagen fibres, presence of extra tissues, and/or a cancellous bone core bordered by cortices. Moreover, there is a lack of consensus on the contribution, if any, of osteoblasts in osteoderm development, despite research describing patterns of resorption and replacement that would suggest both osteoclast and osteoblast involvement. Key to this is information on development, but our understanding of the genetic and skeletogenic processes involved in osteoderm development and patterning remains minimal. The most common proposition for the presence of osteoderms is that they provide a protective armour. However, the large morphological and distributional diversity in lizard osteoderms raises the possibility that they may have other roles such as biomechanical reinforcement in response to ecological or functional constraints. If lizard osteoderms are primarily for defence, whether against predators or conspecifics, then this ‘bony armour’ might be predicted to have different structural and/or mechanical properties compared to other hard tissues (generally intended for support and locomotion). The cellular and biomineralisation mechanisms by which osteoderms are formed could also be different from those of other hard tissues, as reflected in their material composition and nanostructure. Material properties, especially the combination of malleability and resistance to impact, are of interest to the biomimetics and bioinspired material communities in the development of protective clothing and body armour. Currently, the literature on osteoderms is patchy and is distributed across a wide range of journals. Herein we present a synthesis of current knowledge on lizard osteoderm evolution and distribution, micro‐ and macrostructure, development, and function, with a view to stimulating further work.
Keywords: osteoderms, dermal skeleton, lizard, Squamata, development, function, evolution, armour
I. INTRODUCTION
Vertebrate skin is a large organ that supports and protects the body, and provides the interface between an animal and its environment. The skin consists of two layers – the epidermis (derived from ectoderm) and the underlying dermis (originating from mesoderm and the neural crest), separated by a fibrous basement membrane. In amniotes (reptiles, including birds, and mammals), cells of the epidermis are invested with intermediate filament keratins (formerly α‐keratin) across all taxa and additionally corneous beta‐protein (CBP, formerly β‐keratin) in reptiles and birds (Greenwold et al., 2014; Holthaus et al., 2018). The outermost layer of the epidermis or stratum corneum, consists of anucleated keratinocytes with a cornified lipid envelope in place of the plasma membrane. The underlying dermis is bilaminar, and organised into superficial (stratum superficiale or papillary) and deep (stratum compactum or reticular) layers. The dermis includes various sense organs, nerves and blood vessels, and variable amounts of glandular and fatty tissue. These structures are supported, especially in amniotes, by a meshwork of collagen and elastin fibres forming a loose flexible connective tissue. However, in many vertebrates, plates or nodules of rigid tissue develop within the dermis, composed of varying amounts of organic (e.g. collagen) and inorganic (hydroxyapatite) material.
Among ‘fish’ (including cyclostomes, actinopterygians, chondrichthyans, and non‐tetrapodan sarcopterygians), the components of the dermal skeleton are termed scales and range from thick mineralised plates (e.g. extant sturgeons and gars), through thinner elasmoid scales (e.g. living lungfish), to the thin, weakly mineralised scales of many modern bony (actinopterygian) fish, and the tooth‐like placoid scales of sharks and rays (e.g. Sire, Donoghue & Vickaryous, 2009; Witzmann, 2011; Schultze, 2016; Mondejar‐Fernandez, 2018). In most fish, the scale is made up of two primary components. The more superficial part is a hard protective hypermineralised layer (cell poor, collagen poor, avascular) of variable thickness and composition (ganoine, hyaloine, enameloid) that forms close to the epidermal–dermal interface (Zylberberg et al., 1992). The formation of this tissue is thought to involve interactions between dermis and epidermis (ectoderm–mesoderm) (e.g. Reif, 1982; Sire et al., 2009), possibly with epidermal cell contributions (but see Mongera & Nüsslein‐Volhard, 2013). Underlying the hypermineralised layer are deeper layers of less dense, collagen‐rich, vascularised tissue. Depending on the thickness and arrangement of these two tissue layers, fish scales may be termed elasmoid (most living fish), ganoid (living birchirs and gars), cosmoid (extinct lungfish and their relatives), or placoid (sharks, rays and related groups).
Scales were retained in stem‐ and early tetrapods (e.g. Tiktaalik, Acanthostega), particularly on the ventral surface of the body (gastral scales) but often also dorsally (Witzmann, 2009, 2011). Whether these integumentary structures are termed scales or osteoderms (ODs) in tetrapods, and the nature of the distinction between the two, is something of a moot point (Castanet et al., 2003; Vickaryous & Sire, 2009), but there is a general agreement that, in tetrapods, integumentary structures generally lack the hypermineralised layer found in most fish. Nonetheless, the tendency to form mineralisations within the skin is evidently an ancient trait, although it is variably expressed in terrestrial vertebrates.
In crown‐group tetrapods, ODs vary in size, shape, and distribution, often within a single individual. They make up a significant yet somewhat understudied area of the evolutionary history of the tetrapod skeleton, and generally provide the only evidence of the skin structure in fossil taxa. ODs have been lost and regained in different tetrapod lineages through geological time (Hill, 2005). They were retained in many extinct groups of amphibians [lepospondyls, non‐lissamphibian temnospondyls (e.g. Witzmann & Soler‐Gijón, 2010; Witzmann, 2011; Buchwitz et al., 2012)] and although they have been lost in most thin‐skinned living amphibians, they are expressed in some frogs (Ruibal & Shoemaker, 1984). Mineralisations are also found in the skin of some caecilians (Wake, 1975; Zylberberg, Castanet & de Ricqlès, 1980; Zylberberg & Wake, 1990), although it remains unclear if these elements are homologous with ODs (Vickaryous & Sire, 2009).
ODs are more common in amniotes (Hill, 2005; Vickaryous & Sire, 2009) and stem‐amniotes (e.g. Chroniosuchidae; Buchwitz et al., 2012). They have been recorded in representative species of many reptilian lineages including parareptiles [e.g. parieasaurs, procolophonids (Cisneros, 2008; Scheyer & Sander, 2009)], turtles (e.g. Barrett et al., 2002; Clarac et al., 2020), crocodylians (e.g. Seidel, 1979; Frey, 1988; Vickaryous & Hall, 2008; Hill, 2010; Dubansky & Dubansky, 2018), non‐avian dinosaurs (e.g. de Buffrénil, Farlow & de Ricqlès, 1986; Dodson et al., 1998; de Ricqlès et al., 2001; Scheyer & Sander, 2004; Main et al., 2005; D'Emic, Wilson & Chatterjee, 2009; Rogers et al., 2011; Burns & Currie, 2014; Brown, 2017; Vidal et al., 2017), other archosaurs (e.g. Cerda & Desojo, 2010; Cerda et al., 2013, 2015; Scheyer, Desojo & Cerda, 2014; Cerda, Desojo & Scheyer, 2018), the sauropterygian placodonts (e.g. Scheyer, 2007), and lepidosaurs (discussed in Section II.1). They are also recorded in some mammals [e.g. armadillos, glyptodonts, mylodontid sloths (Hill, 2006; Vickaryous & Hall, 2006; McDonald, 2018)] and in early non‐mammalian synapsids [e.g. Elliotsmithia longiceps (Reisz, Dilkes & Berman, 1998); see also Botha‐Brink & Modesto, 2007].
Squamates (lizards, including amphisbaenians, and snakes) are a large and successful reptilian group with more than 10,000 species and a near‐global distribution. They show a diversity of body size and morphology, occupy multiple ecological niches, and have a long evolutionary history (over 250 million years). ODs have been lost in snakes and amphisbaenians, but are present in many clades of lizards including Scincidae, Cordylidae, Gerrhosauridae, Anguidae, Lacertidae, Helodermatidae, and Varanidae (Fig. 1). By far the greatest diversity of tetrapod ODs, in terms of shape, distribution, and expression, is found in lizards (Vickaryous & Sire, 2009), making them an ideal group in which to study OD evolution, development, and function. Our aim in this review is to collate current knowledge of squamate ODs, as a foundation and stimulus for further work.
Fig. 1.
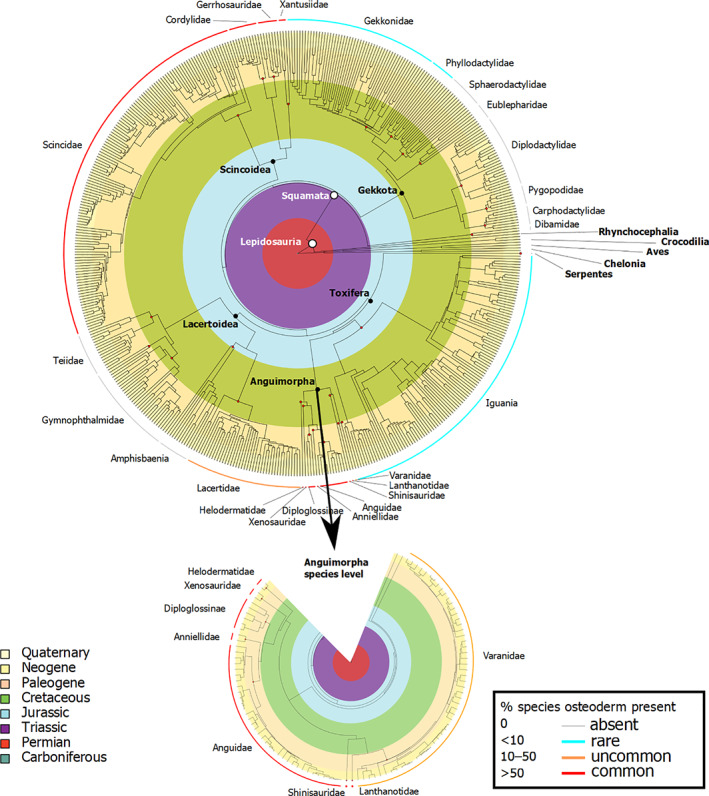
A time‐calibrated phylogeny, modified from Zheng & Wiens (2016) showing osteoderm (OD) abundance across extant lizard genera as taken from the literature, plotted over geological era, with outgroups. An expanded species‐level phylogeny is shown for Anguimorpha. Abundance is estimated as the proportion (% of species) within a clade that develop ODs: common, >50%; uncommon, 10–50%; rare, <10%. Prepared in R using packages phytools (Revell, 2012), Geiger (Pennell et al., 2014), ape (Paradis & Schliep, 2019), and plotrix (Lemon, 2013).
II. EVOLUTIONARY HISTORY AND DISTRIBUTION IN SQUAMATES
(1). Evolutionary history
The reptilian group Lepidosauria comprises Rhynchocephalia and Squamata, sister clades that separated from one another at least 230 million years ago (e.g. Jones et al., 2013). Lepidosauromorpha (Lepidosauria and its stem taxa) is the sister group of Archelosauria, encompassing turtles and archosaurs (Crawford et al., 2015) within neodiapsid reptiles. Today, Rhynchocephalia is represented by a single species, Sphenodon punctatus, the Tuatara of New Zealand, whereas Squamata consists of more than 10,000 species of lizards, snakes, and amphisbaenians grouped, on molecular data (e.g. Pyron, Burbrink & Wiens, 2013; Zheng & Wiens, 2016; Burbrink et al., 2020; but contra Losos, Hillis & Greene, 2012), into Gekkota, Dibamidae, Scincoidea (Scincidae, Cordyliformes, Xantusiidae), Lacertoidea (Teiioidea, Lacertidae, Amphisbaenia), and Toxicofera (Iguania, Anguimorpha, Serpentes) (Fig. 1). There remains uncertainty as to whether Gekkota, Dibamidae, or both, represent the first major branches from the squamate crown (e.g. Pyron et al., 2013; Burbrink et al., 2020).
The early fossil record of rhynchocephalians is relatively good (Jones et al., 2013), but ODs have only been recorded once, in the Early Cretaceous (~120 million years ago) species Pamizinsaurus tlayuaensis from the Tlayúa Formation in Puebla, Mexico (Reynoso, 1997). The generic name Pamizinsaurus stems from a Nahuatl word pamizintl, meaning corn, which refers to the bead‐like ODs covering the body and giving the appearance of a corn‐cob (Reynoso, 1997). However, no other rhynchocephalian has been found with any trace of ODs, despite several complete skeletons. Moreover, ODs have not been recorded in any of the species currently placed on the lepidosaurian stem, although this may be an artefact of a poor fossil record and incomplete specimens. Given the widespread occurrence of ODs in Archelosauria, it seems likely that OD development was suppressed in stem‐lepidosaurs, with re‐expression occurring rarely in rhynchocephalians and more widely in squamates.
Although molecular divergence estimates (e.g. Jones et al., 2013) place the origin of squamates into the early‐mid Triassic (supported by records of middle Triassic rhynchocephalians), there are no unequivocal records of squamates until the Middle Jurassic (UK, Russia, China, Central Asia). Most of these early lizard specimens are represented by isolated elements, making it difficult to be certain whether ODs were present. However, Changetisaurus estesi (Middle Jurassic, Kyrgyzstan; Fedorov & Nessov, 1992), reportedly has a cover of rectangular ODs, as does an as‐yet unnamed lizard specimen from the Middle Jurassic of China (Conrad et al., 2013). The phylogenetic position of neither lizard is certain, but they may be related to Paramacellodidae, a fossil group better known from Jurassic and Early Cretaceous deposits across northern continents (North America, Europe, Asia; Evans & Chure, 1998). Paramacellodids are typically placed on the stem of Scincoidea, and like many living scincoids they bore a complete covering of imbricating rectangular ODs [e.g. Sharovisaurus karatauensis, Upper Jurassic, Kazakhstan (Hecht & Hecht, 1984; see also Richter, 1994)]. Hongshanxi xiei (Mid‐Upper Jurassic, China; Dong et al., 2019) also has ODs, but they are restricted to the temporal region of the skull and vary in their size and shape [as also seen in some (unrelated) modern lacertids]. ODs have been reported in several other Cretaceous lizards including the Early Cretaceous Scandensia ciervensis [Spain (Bolet & Evans, 2011), cycloid ODs], and Yabeinosaurus robustus [China (Dong, Wang & Evans, 2017), small, scattered ODs], and several Late Cretaceous taxa including: the scincoid Parmeosaurus scutatus [Late Cretaceous, China (Dong et al., 2018), complete cover of imbricate rectangular ODs single dorsally, and binary (= two ODs joined into a compound structure) ventrally], the cordyliform Konkasaurus mahalana [Madagascar (Krause, Evans & Gao, 2003), rectangular ODs]; and several monstersaurs [Gobiderma pulchrum, Mongolia (Borsuk‐Białynicka, 1984; Conrad et al., 2011); Chianghsia nankangensis, China (Mo, Xu & Evans, 2012)]. Post Cretaceous–Paleogene (K–P) boundary, there are numerous records of Paleogene and Neogene squamate taxa with ODs, notably among Anguimorpha [e.g. glyptosaurines (de Buffrénil, Sire & Rage, 2010; de Buffrénil et al., 2011); anguids and anniellids (Estes, 1983); helodermatids (Mead et al., 2012); ‘necrosaurs’ (Estes, 1983; Smith & Habersetzer, 2021)] and Scincoidea (e.g. Gerrhosauridae; Estes, 1983).
(2). Distribution of osteoderms in living lizard taxa
Amongst extant lizard taxa, ODs have been reported for many taxa (up to 25% of species; see Table 1), although their presence is not necessarily universal within even a single genus (e.g. Varanus; Erickson et al., 2003). ODs are common among scincids (Camp, 1923; Camaiti et al., 2019), gerrhosaurids (Camp, 1923; Nance, 2007), cordylids (Broeckhoven et al., 2015; Stanley, 2016; Broeckhoven, du Plessis & Hui, 2017b; Broeckhoven, De Kock & Le Fras Nortier Mouton, 2017a ; Broeckhoven et al., 2018a ), xenosaurids (Gao & Norell, 1998; Bhullar, 2011), helodermatids (Moss, 1969; Maisano et al., 2019; Kirby et al., 2020; Iacoviello et al., 2020), anguids (Hoffstetter, 1962; Strahm & Schwartz, 1977; Zylberberg & Castanet, 1985; Levrat‐Calviac et al., 1986; Bochaton et al., 2015), anniellids (Bhullar & Bell, 2008), shinisaurids (Bever et al., 2005; Conrad et al., 2014), and lanthanotids (McDowell & Bogert, 1954; Maisano et al., 2002, 2019). ODs are also found in some (but not all) species of varanids (Erickson et al., 2003; Maisano et al., 2019; Kirby et al., 2020) and lacertids (Estes et al., 1988; Borsuk‐Białynicka et al., 1999; Constantini & Dell'Omo, 2010). ODs are virtually absent from iguanians with two published exceptions, a species of leaf chameleon (Brookesia perarmata) and some individual specimens of the marine iguana (Amblyrhynchus cristatus) (de Queiroz, 1987; Schucht et al., 2020). ODs are rare in gekkotans, but have been reported from a small number of species across two different clades: Gekkonidae and Phyllodactylidae (Schmidt, 1912a ; Levrat‐Calviac, 1986; Levrat‐Calviac & Zylberberg, 1986; Vickaryous et al., 2015; Paluh et al., 2017; Scherz et al., 2017; Laver et al., 2020). It is worth noting that small, superficially OD‐like elements have been described for two genera of sphaerodactylid geckos (Aristelliger and Teratoscincus) (Bauer & Russell, 1989; Griffing et al., 2018). These irregularly shaped elements, known as parafrontal bones, form within a layer of connective tissue above the orbit but deep to the dermis. Hence, they are considered to be separate ossifications (Bauer & Russell, 1989; Griffing et al., 2018). ODs are absent from snakes and the specialised burrowing groups Amphisbaenia and Dibamidae.
Table 1.
Osteoderms reported in the literature for extant lizards
| Taxon | Number of extant species a | Taxonomic distribution of osteoderms within the clade b | Key references | |
|---|---|---|---|---|
| Dibamidae | 25 | Absent | ||
| Gekkota |
Eublepharidae Gekkonidae Carphodactylidae Diplodactylidae Phyllodactylidae Sphaerodactylidae Pygopodidae |
40 1356 32 155 151 228 46 |
Absent Rare (3 spp.; Gekko gecko, G. reevesii, Geckolepis maculata) Absent Absent Rare (at least 6 spp. of Tarentola) Absent Absent |
Levrat‐Calviac & Zylberberg ( 1986 ); Levrat‐Calviac, Castanet & Zylberberg ( 1986 ); Vickaryous, Meldrum & Russell ( 2015 ); Paluh, Griffing & Bauer ( 2017 ); Scherz et al. ( 2017 ); Laver et al. ( 2020 ). |
| Scincoidea | Scincidae | 1709 | Common | Camp ( 1923 ); Oliver ( 1951 ); Paluh & Bauer ( 2017 ); Camaiti et al. ( 2019 ) |
| Gerrhosauridae | 37 | Common | Camp ( 1923 ); Nance ( 2007 ) | |
| Cordylidae | 70 | Common | Broeckhoven, Diedericks & Le Fras Nortier Mouton ( 2015 ); Broeckhoven et al. ( 2018a )); Stanley ( 2016 ) | |
| Xantusiidae | 35 | Common | Strahm & Schwartz ( 1977 ) | |
| Lacertoidea | Lacertidae | 350 | Uncommon | Estes, De Queiroz & Gauthier ( 1988 ); Arnold ( 1989 ); Arnold, Arribas & Carranza ( 2007 ); Barahona & Barbadillo ( 1998 ); Borsuk‐Białynicka, Lubka & Böhme ( 1999 ); Constantini & Dell'Omo ( 2010 ) |
| Amphisbaenia | 201 | Absent | ||
| Teiidae | 170 | Absent | ||
| Alopoglossidae | 28 | Absent | ||
| Gymnophthalmidae | 267 | Absent | ||
| Toxifera | Iguania | 1968 | Rare (reported for only 2 spp.; Brookesia perarmata and Amblyrhynchus cristatus) | de Queiroz ( 1987 ); Schucht et al. ( 2020 ) |
| Helodermatidae | 5 | Common | Moss ( 1969 ); Maisano et al. ( 2019 ); Kirby et al. ( 2020 ); Iacoviello et al. ( 2020 ) | |
|
Xenosauridae Diploglossidae |
12 51 |
Common Common |
Hoffstetter ( 1962 ); Strahm & Schwartz ( 1977 ); Zylberberg & Castanet ( 1985 ); Good & Schwenk ( 1985 ); Bochaton et al. ( 2015 ) |
|
|
Anniellidae Anguidae Shinisauridae Lanthanotidae Varanidae |
6 85 1 1 83 |
Common Common Common Common Uncommon to rare |
Bhullar & Bell ( 2008 ) Gao & Norell ( 1998 ); Bhullar ( 2011 ) Bever, Bell & Maisano ( 2005 ); Conrad, Head & Carrano ( 2014 ) McDowell & Bogert ( 1954 ); Maisano et al. ( 2002 , 2019 ) Erickson et al. ( 2003 ); Maisano et al. ( 2019 ); Kirby et al. ( 2020 ) |
|
| Serpentes | 3789 | Absent |
III. OSTEODERM MACRO‐ AND MICROSTRUCTURE
(1). Macrostructure
Until recently, most reports of lizard OD morphology (i.e. macrostructure) were derived from the study of dried skeletal elements or diaphonised skin stained with Alizarin red (e.g. Strahm & Schwartz, 1977). However, since the early 2000s computed tomography (CT) scanning has become the leading tool for the study of the size, shape, and in situ distribution of ODs. CT scanning is non‐invasive, able to generate high‐resolution two‐dimensional and three‐dimensional data rapidly, and can be used to map the distribution of ODs across body regions or the entire lizard (e.g. Maisano et al., 2002; Bever et al., 2005; Paluh et al., 2017; Maisano et al., 2019; Laver et al., 2020; Iacoviello et al., 2020), as reviewed in Broeckhoven & du Plessis (2018). Further, there are several open‐source repositories of CT data archiving OD‐bearing lizards, including Digimorph (digimorph.org) and Morpho Source (morphosource.org). The potential for integrating the spatial morphological information with that provided by classical histology and functional modelling is currently being explored in several groups (Iacoviello et al., 2020)
Lizard ODs vary in size, shape, and body‐wide distribution (Fig. 2). For example, whereas ODs are typically confined to the head of some lacertids (Fig. 2F), their distribution is more pervasive in many scincids, anguids, and cordyliforms (Fig. 2A–C). In these groups, ODs may form a comprehensive body‐wide covering of imbricating or interlocking rectangular or cycloid plates, or may be confined to specific regions due to differing selective pressures (Camp, 1923; Richter, 1994; Stanley, 2013, 2016; Broeckhoven et al., 2015, 2017a , 2017b ; Broeckhoven et al., 2018a ) (Figs 2 and 3). In some species, individual ODs may fuse together, creating compound mosaics (Fig. 3C), tessellations (Fig. 2E) or mats of bone (e.g. skeletally mature gekkotans and Varanus komodoensis; Paluh et al., 2017; Maisano et al., 2019), or even fuse with the underlying cranial bones [e.g. cordylids, gerrhosaurids, xenosaurids, lacertids, some scincids, and helodermatids (Bhullar, 2011; Dubke, Hipsley & Müller, 2018; Maisano et al., 2019)]. Variation also occurs across ontogenetic series, as outlined in Section IV.
Fig. 2.
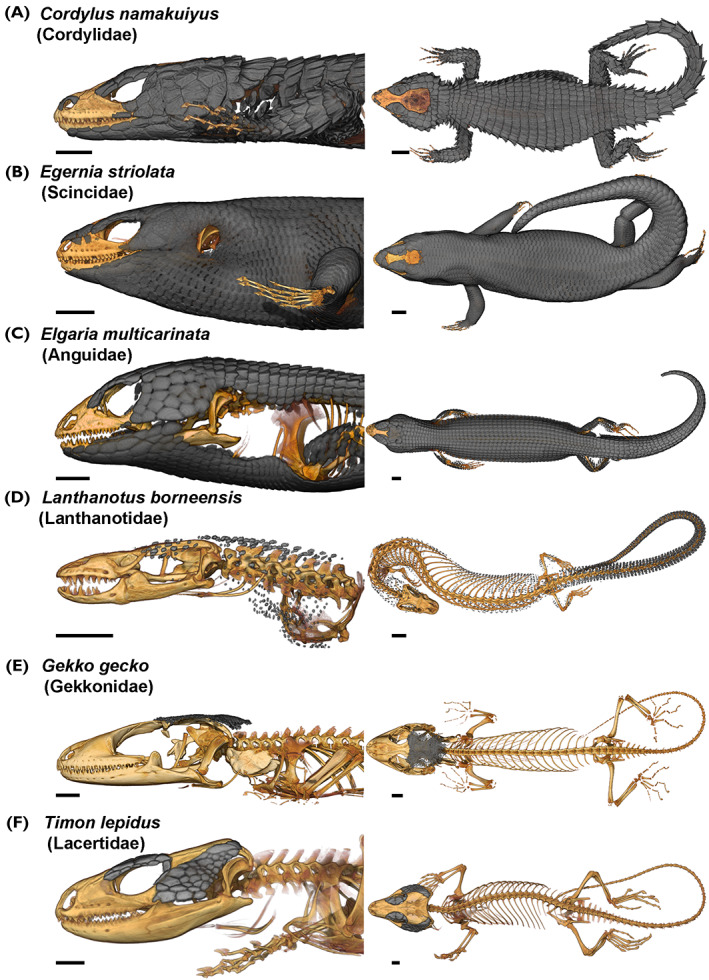
Osteoderm shape and location patterns in six lizard species from different families. Computed tomography (CT) reconstructions of the bones (orange) and osteoderms (grey) in (A) Cordylus namakuiyus (Cordylidae), (B) Egernia striolata (Scincidae), (C) Elgaria multicarinata (Anguidae), (D) Lanthanotus borneensis (Lanthanotidae), (E) Gekko gecko (Gekkonidae), and (F) Timon lepidus (Lacertidae). Left lateral view of the cranium and the most rostral part of the body (left) and dorsal view of the whole body (right). Note that the specimen of T. lepidus presented here had no post‐cranial osteoderms; osteoderm covering of the body is susceptible to ontogenetic changes in every species. Osteoderms covering of the frontal and parietal bones were only represented in grey when they were distinct (not fused) from the bone in the X‐ray pictures to avoid overinterpretation of the data. CT‐scan data were downloaded from MorphoSource. Scale bars: 5 mm.
Fig. 3.
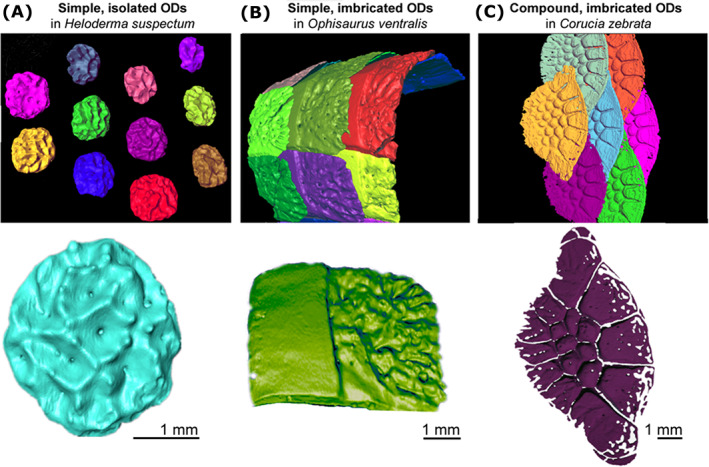
Shapes and distribution pattern of some dorsal skin osteoderms (ODs) in three species of lizards. Dorsal views of the distribution of neighbouring osteoderms (top) and the morphology of isolated osteoderms (bottom) in (A) Heloderma suspectum (Helodermatidae), (B) Ophisaurus ventralis (Anguidae), and (C) Corucia zebrata (Scincidae).
As a class of skeletal element, ODs are highly polymorphic, even within the same species or individual (Erickson et al., 2003; Vickaryous et al., 2015; Maisano et al., 2019). For example, in V. komodoensis there are four distinct OD morphotypes across the head alone: rosette, platy, dendritic, and vermiform (Maisano et al., 2019). Similarly, across the body of Cordylus namakuiyus, ODs vary in overall shape (rectangular to circular), and in the presence and sharpness of their keel (Stanley, 2016) (Fig. 2A). Other OD morphologies include simple granular bones (e.g. postcranial ODs in Tarentola spp.; Vickaryous et al., 2015; Fig. 4), keeled plates (e.g. fossil varanoid ‘necrosaurs’; Estes, 1983), spherical or globose studs [e.g. Heloderma (Mead et al., 2012; Iacoviello et al., 2020; Kirby et al., 2020); Figs 3A and 4), interlocking tesserae [cranial ODs in Gekko gecko, Fig. 2E (Vickaryous et al., 2015; Laver et al., 2020)], cycloid [Geckolepis (Schmidt, 1912a ; Paluh et al., 2017)] or palmate shapes (Anniella spp.; Bhullar & Bell, 2008), and compound elements consisting of multiple plates joined together [e.g. scincids (Otto, 1909; Camp, 1923; Estes et al., 1988); Fig. 3C]. The terminology of individual OD element morphology has also been variable across individual publications and may benefit from a systematic approach.
Fig. 4.
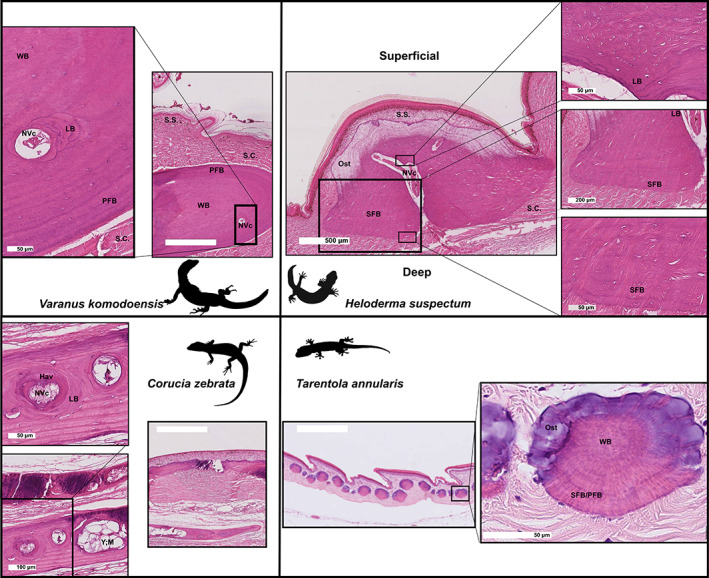
Histological overview of osteoderms from Varanus komodoensis, Heloderma suspectum, Tarentola annularis, and Corucia zebrata, demonstrating diversity of osteoderm size, tissue characteristics and bone fibre patterning. All images stained with haematoxylin and eosin. Hav, Haversian structure; LB, lamellar bone; NVc, neurovascular canal; Ost, osteodermine; PFB, parallel‐fibred bone; S.C., stratum compactum; SFB, Sharpey‐fibre bone; S.S., stratum superficiale; WB, woven bone; Y; M, yellow marrow. Scale bars: main images all 200 μm; higher magnifications as indicated. Varanus komodoensis and Heloderma suspectum from the same specimens as Kirby et al. (2020).
While ODs are firmly anchored within the dermis (discussed in Section IV.2), in at least two species of gecko, they can also be shed. Geckolepis is an arboreal genus of fish‐scaled gecko capable of regional integumentary loss (Paluh et al., 2017; Scherz et al., 2017). Regional integumentary loss is a dramatic antipredation mechanism where the skin can be avulsed to escape predation (Bauer, Russell & Shadwick, 1989, 1992; Paluh et al., 2017). Similar to tail autotomy, regional integumentary loss is associated with pre‐existing planes of weakness that partially divide the dermis (Bauer et al., 1989, 1992). When the gecko is grasped, it can slough a portion of its epidermis and superficial dermis, along with any associated ODs.
(2). Microstructure
At the level of histology, ODs are typically heterogeneous in their composition, including bone, mineralised and unmineralised collagen fibre bundles, blood vessels, nerves, and yellow marrow (Fig. 4) (Moss, 1969; Strahm & Schwartz, 1977; Zylberberg & Castanet, 1985; Vickaryous & Sire, 2009; de Buffrénil et al., 2010; Bochaton et al., 2015; Broeckhoven et al., 2017b ; Iacoviello et al., 2020; Kirby et al., 2020). Moreover, the bone tissue and its fibrillary matrix often varies, even within a single OD. This diversity includes both cancellous and compact bone, spatially organised into woven‐fibred, parallel‐fibred and lamellar matrices (Moss, 1969; Scheyer, 2007; de Buffrénil et al., 2010; Vickaryous & Sire, 2009). The cortical borders of ODs may also become enriched with Sharpey's fibres, giving rise to Sharpey‐fibred bone, an ossified matrix heavily invested with extrinsic collagenous fibres (Moss, 1969; Strahm & Schwartz, 1977; de Buffrénil et al., 2011; Vickaryous et al., 2015; Kirby et al., 2020).
Until recently, details of lizard OD bone histology were known for only three species, the anguimorphs Heloderma suspectum and Anguis fragilis, and the gekkotan Tarentola mauritanica (Schmidt, 1912a , 1912b , 1914a ; Moss, 1969; Zylberberg & Castanet, 1985; Levrat‐Calviac et al., 1986; Levrat‐Calviac & Zylberberg, 1986). However, renewed interest in OD microstructure over the last decade is expanding this taxonomic list to include a much broader representation of lizards, including several other species of gekkotans, various scincids and cordylids, a varanid, and even a chamaeleonid (Vickaryous et al., 2015; Broeckhoven et al., 2017b ; Paluh et al., 2017; Canei & Nonclercq, 2020; Iacoviello et al., 2020; Kirby et al., 2020; Schucht et al., 2020). As a result, a better understanding of how OD histology varies across taxa is beginning to emerge. For example, in the anguid A. fragilis, ODs are composed of a superficial layer of woven‐fibred bone and a deeper layer or plate of lamellar bone (Zylberberg & Castanet, 1985). By contrast, the bony contribution of Heloderma spp. ODs is primarily Sharpey‐fibred bone, with lamellar bone only deposited around neurovascular bundles, thus forming secondary osteons (Haversian systems) (Moss, 1969; Iacoviello et al., 2020; Kirby et al., 2020). In the cordylids Smaug giganteus and Ouroborus cataphractus, each OD consists of an outer cortex of parallel‐fibred bone, with a deeper layer of woven‐fibred bone and a cancellous core lined with lamellar bone (Broeckhoven et al., 2017b ). A similar arrangement, with a parallel‐fibred bone cortex and a cancellous core lined with lamellar bone was recently described for the chameleon Brookesia perarmata (Schucht et al., 2020). The histology of regenerated ODs typically resembles that of the original (e.g. in the geckos T. annularis and T. mauritanica; Vickaryous et al., 2015). However, in some diploglossine anguids regenerated ODs develop a tissue (Sharpey‐fibre bone) not present in the prototypic form (Bochaton et al., 2015).
The large investment of bone in lizard ODs has raised the possibility of using these elements for skeletochronological studies. Although ODs sampled from across the body may hold some utility in this regard (at least in anguids), regenerated ODs are entirely unreliable indices of age (Bochaton et al., 2015; see also Guarino, Mezzasalma & Odierna, 2016). Among crocodylians, for which the use of ODs in skeletochronology has been more intensively studied, the emerging consensus is that the age estimates from the elements should be viewed with caution (Klein, Scheyer & Tütken, 2009). Although cyclical growth marks may be present, age estimation is complicated by remodelling (e.g. by reproductive‐aged females or pathological calcium deficiencies) and/or close spacing of annual bone deposits (Tucker, 1997; Klein et al., 2009).
Among some species, the superficial surface (closest to the epidermis) of the OD is covered by an enigmatic capping tissue (Moss, 1969; de Buffrénil et al., 2011; Vickaryous et al., 2015; Iacoviello et al., 2020; Kirby et al., 2020). This tissue, termed osteodermine (de Buffrénil et al., 2011), is a dense, avascular, cell‐poor, hypermineralised enamel‐like layer that lacks intrinsic collagen (de Buffrénil et al., 2010, 2011; Vickaryous et al., 2015). To date, osteodermine has only been reported for a few distantly related squamate species, including the anguimorphan Heloderma suspectum, the geckos T. annularis and T. mauritanica, and at least one unnamed fossil glyptosaurine anguid (Moss, 1969; de Buffrénil et al., 2011; Vickaryous et al., 2015; Kirby et al., 2020). A comparable capping tissue has also been reported for the scincids Scincus and Eumeces schneideri (Canei & Nonclercq, 2020). Available evidence reveals that even among closely related taxa, the expression of osteodermine is irregular. For example, although osteodermine is present in two species of Tarentola geckos (T. annularis and T. mauritanica), it is absent from four others (T. americana, T. crombiei, T. chazaliae, and T. neglecta; Levrat‐Calviac, 1986; Vickaryous et al., 2015). At the level of histology osteodermine is characterised by a series of concentrically organised periodic growth lines. Unlike most of the osteodermine matrix, these growth lines stain with various connective tissue dyes (Vickaryous et al., 2015). Based on its structural similarity to other hypermineralised tissues (including enamel, ganoine, and hyaloine), osteodermine is predicted to share a comparable mode of development (de Buffrénil et al., 2011). Of these, osteodermine appears to resemble hyaloine most closely, in that both tissues develop at a short distance from the epidermis (Sire, 1993; Sire et al., 2009). Accordingly, osteodermine deposition may involve a dynamic or inductive interaction between the dermis and epidermis (Levrat‐Calviac & Zylberberg, 1986; de Buffrénil et al., 2011; Vickaryous et al., 2015; Kirby et al., 2020). Keratin, independent from that of the superficial keratinous scute, has also been recorded in the basal part of the OD in Heloderma (Iacoviello et al., 2020).
In addition to routine serial histology, a number of other microscopic and analytical strategies have been employed to study OD structure and composition. These include transmission electron microscopy, scanning electron microscopy, and microradiography (Zylberberg & Castanet, 1985; Levrat‐Calviac, 1986; Levrat‐Calviac & Zylberberg, 1986), as well as multi‐rotation polarised light microscopy (Kirby et al., 2020), atomic force microscopy and finite element analyses (Iacoviello et al., 2020). To date these methods have only been used to investigate a handful of species (Anguis fragilis, Tarentola mauritanica, and Heloderma suspectum). A more comprehensive survey of OD microstructure across a more diverse sample of lizards is needed to provide a broader picture of OD microscopic diversity, and to provide a firmer basis for an understanding of form–function relationship.
IV. DEVELOPMENT
(1). General observations
As for all vertebrates, the epidermis of lizard skin is dominated by keratinocytes, while the underlying dermis is a fibrous mesh of connective tissues invested with blood vessels, and nerves. ODs develop within the dermis, either within the more loosely organised superficial dermis (stratum superficiale) or adjacent to the boundary between the superficial and deep dermis (stratum compactum) (Vickaryous & Sire, 2009; Vickaryous et al., 2015). While the epidermis does not appear to contribute directly to the ossified portion of the OD, it may participate in the formation of the superficial capping tissues (see Section IV.2).
Although their formation is relatively delayed compared to the rest of the skeleton, ODs have been documented among comparatively small, skeletally immature lizards (Schmidt, 1912a , 1912b , 1914a ; Vickaryous et al., 2015; Stanley, 2016; Laver et al., 2020). Early OD development takes place asynchronously across the body, and appears to be associated with maturation of the epidermis and dermis (Vickaryous & Sire, 2009). Interestingly, the pattern in which ODs first appear across the body varies among taxa. For example, while OD‐bearing geckos and the anguimorph Heloderma first develop ODs across the head and cervical regions (Moss, 1969; Vickaryous et al., 2015; Laver et al., 2020), in cordylids they first appear across the tail and along the caudal margins of the head (Stanley, 2016).
(2). Osteoderm development in lizards
OD development is best understood from the study of Heloderma (Schmidt, 1912b ; Moss, 1969; see also Kirby et al., 2020), Anguis (Schmidt, 1914a ) and the gekkotans Tarentola and Gekko (Schmidt, 1912a ; Vickaryous et al., 2015; see also Laver et al., 2020). At the level of histology, ODs begin within the dermis as a concentration of dense irregular connective tissue. While cells are present both within and adjacent to these connective tissue primordia, they do not form any obvious aggregations. Further, there is no sign of a placode or any direct involvement of the epidermis. Initially, OD primordia are weakly defined and difficult to distinguish from the fibrous collagen of the surrounding dermis. Primarily, they appear to be variably shaped concentrations of dermis with various associated mineralised collagen fibres (e.g. Schmidt, 1914a , 1912a , 1912b ; Vickaryous et al., 2015). As they grow in size, ODs begin to ossify. Although this early mode of ossification is often characterised as direct osseous metaplasia, that is the direct transformation of the pre‐existing soft tissue (here the dermis) into bone (sensu Haines & Mohuiddin, 1968; Beresford, 1981), detailed investigations of this initial stage of deposition are lacking. Alternatively, lizard ODs may employ a form of intramembranous ossification as was recently reported for the crocodylian Alligator mississippiensis (Dubansky & Dubansky, 2018). Regardless, ODs in lizards develop without a cartilaginous precursor and routinely incorporate large collagen fibres from the surrounding dermis into the bony matrix. These perforating, or Sharpey's, fibres then anchor each OD within the dermis, and even with other adjacent ODs (Moss, 1969; Strahm & Schwartz, 1977; de Buffrénil et al., 2011; Vickaryous et al., 2015).
As growth continues, ODs become increasingly well‐defined within the dermis and the matrix becomes dominated by bone of various collagenous organisations. Large extrinsic bundles of unmineralised collagen may also be present passing through the matrix. At this stage, OD development does involve intramembranous ossification, characterised by an enveloping seam of osteoid and osteoblasts. Although the origin of these osteoblasts remains unclear, they are hypothesised to originate from the neural crest (Smith & Hall, 1990; see also Sire et al., 2009; Vickaryous & Sire, 2009) or resident populations of latent osteoprogenitors (or possibly their mesenchymal stem cell precursors; Vickaryous et al., 2015). More recent hypotheses in crocodylians, where an endothelial‐to‐mesenchymal transition of osteoblasts has been posited, have yet to be tested in squamates (Dubansky & Dubansky, 2018).
Osteodermine deposition (when it occurs), begins with a thin layer of vitreous tissue capping the bony OD. At this early stage, osteodermine is associated with a population of fibroblast‐like cells of uncertain identity. With continued growth, the number of associated cells diminishes and the accumulating tissue begins to incorporate perpendicularly radiating Sharpey's fibres (Vickaryous et al., 2015).
Unusual for a skeletal element, ODs in at least some lizards are also capable of regenerating. Regenerated ODs have been reported for the anguids A. fragilis (Schmidt, 1914a ; Bryant & Bellairs, 1967), Diploglossus monotropis, D. plei, Celestus bivittatus, C. occiduus, and Ophiodes striatus (Bochaton et al., 2015), and the geckos T. annularis and T. mauritanica (Vickaryous et al., 2015). OD regeneration is also predicted to occur in Geckolepis maculata, an OD‐bearing gecko species capable of regional integumentary skin loss (Paluh et al., 2017). Similar to the original elements, regenerated ODs form within the (regenerated) superficial dermis adjacent to the contact with the deep dermis. In Tarentola, regenerated ODs also develop a capping layer of osteodermine, which is associated with a monolayer of fibroblast‐like cells (Vickaryous et al., 2015). However, regenerated ODs are not identical replacements. For example, in A. fragilis, T. annularis and T. mauritanica, regenerated ODs differ from their original counterparts in that they are smaller and demonstrate a different overall pattern of distribution across the tail (Bryant & Bellairs, 1967; Vickaryous et al., 2015). In Diploglossus and C. occiduus, regenerated ODs have a pit‐like superficial ornamentation and bevelled edges not present in the original organs (Bochaton et al., 2015). As a result of these additional articulations, the regenerated tail is reportedly less flexible than the original. Further, the histology of regenerated ODs is also different in D. monotropis and C. occiduus: whereas the largest regenerated ODs develop Sharpey‐fibred bone (along the lateral margins), this tissue does not form in original ODs (Bochaton et al., 2015).
(3). Osteoderm development in other amniotes
Outside of lizards, OD development has been investigated in the crocodylians Caiman crocodilus (Schmidt, 1914b ) and Alligator mississippiensis (Vickaryous & Hall, 2008; Dubansky & Dubansky, 2018) and the armadillo Dasypus novemcinctus (Vickaryous & Hall, 2006). As in lizards, OD formation in both these taxa occurs within well‐differentiated dermis, asynchronously across the body. Although OD formation is relatively delayed compared with most of the skeleton, it does vary: in crocodylians, ODs develop 9–12 months after hatching (Vickaryous & Hall, 2008; Dubansky & Dubansky, 2018), while in armadillos they first appear in the late‐stage fetus (Vickaryous & Hall, 2006). Whereas OD development in crocodylians has been reported to occur via direct osseous metaplasia in mainly paravertebral ODs (Vickaryous & Hall, 2008), emerging evidence from nuchal ODs points towards intramembranous ossification (Dubansky & Dubansky, 2018). While this may partially reflect the difference in underlying soft tissue architecture with the paravertebral ODs co‐opted for bracing (Salisbury & Frey, 2001), the more recent study by Dubansky & Dubansky (2018) found that while OD development initially involved the formation of a fibrous concentration within the dermis, this primordium was soon populated by an aggregation of mesenchymal and osteoblastic cells. With continued growth, osteoid is deposited and mineralised to yield bone. Intriguingly, the authors found that some of the osteoblasts associated with osteoid expressed the endothelial marker TIE‐1 (tyrosine kinase with immunoglobulin‐like and EGF‐like domains‐1). By way of explanation, it was proposed that at least some of the osteoprogenitors contributing to OD formation are derived from endothelial cell populations, raising the possibility of an endothelial‐to‐mesenchymal transition (Dubansky & Dubansky, 2018). In this scenario, endothelial cells from small‐calibre blood vessels separate from the existing vasculature, become mesenchymal and then migrate towards presumptive ODs. Once invested in the presumptive OD, these mesenchymal cells differentiate into osteoblasts and contribute to the formation of osteogenic cell condensations (Dubansky & Dubansky, 2018). Armadillo ODs also develop from a condensation of osteoblasts via intramembranous ossification (Vickaryous & Hall, 2006). However, the role (if any) of endothelial cells in the osteogenic population remains unknown. It is also worth noting that unlike some lizards, ODs in crocodylians and armadillos do not regenerate (Pressinotti et al., 2013; see also Vickaryous et al., 2015).
(4). Future targets
As noted above, many aspects of OD development are poorly understood. In lizards, some of the key questions include: what is/are the cellular origin(s) of the osteoblasts involved in OD development? Which cell type is responsible for osteodermine/capping tissue deposition? Does the epidermis participate? Is there a role for endothelial‐to‐mesenchymal transition in lizard ODs? To understand better the developmental mechanisms underlying OD formation in lizards, future studies will need first to dissect cellular behaviours of the relevant cell populations including proliferation and differentiation, using histological markers and gene expression studies. Such step‐by‐step analyses sampling both phylogenetic diversity and multiple developmental stages hold promise to uncover the basic principles of OD biogenesis from the perspective of cellular events. Comparative analyses of ODs of different shape and structure aimed at investigating cell behaviour during their development will help determine how alterations in cellular biology affect OD morphogenesis in different species. Once morphogenesis is understood at the cellular level, we can begin to explain how OD shape diversity and distribution pattern are generated by changes in the underlying molecular and genetic processes.
Specific molecular targets for analysis of lizard OD development can be drawn from the literature on other species forming mineralised dermal structures, and their relationship with epidermal elements such as the chelonian carapace and plastron, and crocodilian ODs. Intratendinous metaplasia (the transition from mature dense connective tissue to mineralised tissue), as described above for lizards, is also found in portions of other reptilian and dinosaur ODs and turtle shells (see also Scheyer & Sander, 2004; Scheyer, 2007; Horner, Woodward & Bailleul, 2016). In the formation of turtle shells intramembranous ossification is associated with the sequential and overlapping Hedgehog and canonical Wnt (segment polarity pathway wingless' vertebrate homolog) signalling which are proposed to direct osteochondrogenic cells to choose an osteogenic path and prevent them from transdifferentiating into chondrocytes (Rice et al., 2016). Additionally, similar to alligator ODs, ossification centres in the dermis are seen in the plastron and carapace of the turtle shell (Cherepanov, 1997; Cebra‐Thomas et al., 2005, 2007; Vickaryous & Hall, 2008; Hirasawa, Nagashima & Kuratani, 2013). These findings suggest testable predictions and provide a platform allowing further dissection of OD development in lizards at the cellular, molecular and genetic levels.
Additional molecular targets may be derived from the literature on odontoblasts and cranial dermal bone osteoblasts, the cells responsible for bone and dentine formation which are derived from neural crest mesenchymal cells (Sire & Kawasaki, 2012). Their differentiation and resulting tissue morphogenesis are known to be regulated by skeletogenic signalling molecules and transcription factors. Mesenchymal (possibly neural crest cell‐derived) condensations give rise to skeletogenic cells, such as scleroblasts, and are also thought to be involved in synthesis of type‐I collagen and acidic secretory calcium‐binding phosphoproteins (SCPPs) (Kawasaki, Suzuki & Weiss, 2004). These acidic SCPPs are encoded by the gene SPARCL1 and help regulate crystallisation of calcium phosphate‐based mineral in bone and dentine in vertebrates. SCPPs have been identified in the genomes of humans, lizards, chickens, frogs and zebrafish (Danio rerio). Specifically, dentine sialophosphoprotein 1 and 2 (DSPP1, DSPP2), dentine matrix acidic phosphoprotein 1 (DMP1), integrin‐binding sialoprotein (IBSP), matrix extracellular phosphoglycoprotein (MEPE) and secreted phosphoprotein 1 (SPP1) have been identified on chromosome 8 in lizards (Sire & Kawasaki, 2012). Thus, future studies will need to investigate expression patterns of multiple developmental genes and their combinations to define more precisely relevant developmental processes and to distinguish among various alternative interpretations.
V. BIOMECHANICS, BIOMIMETICS AND BIOINSPIRATIONS
(1). Biomechanics
Classical engineering methods and techniques are promising tools to advance our fundamental understanding of the selective advantages of different OD morphologies and arrangements from a biomechanical point of view. Material characterisation using compressive or tensile forces applied to a single or sheets of ODs enables us to estimate their inherent mechanical properties. On the other hand, computational modelling techniques such as finite element (FE) methods are powerful tools allowing us to model ODs virtually, and to alter their structure and morphologies in hypothetical scenarios to understand possible correlations between their structure, form, and function (Fig. 5).
Fig. 5.
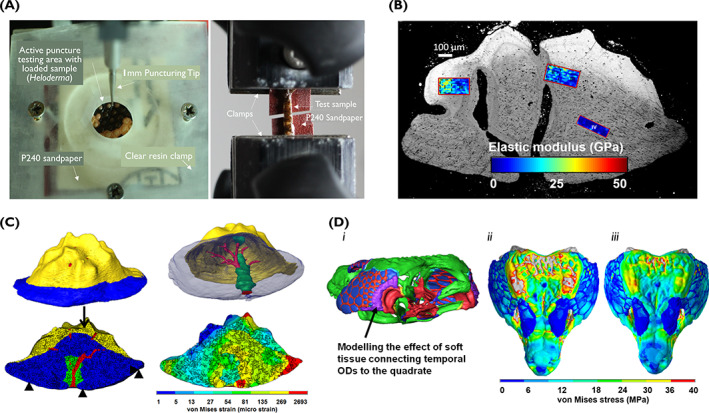
Biomechanical characterisation of Heloderma suspectum osteoderm (OD). (A) Illustration of material characterisation (tensile and puncture testing) of sheets of skin (unpublished data). (B) Atomic force microscopy of a single OD. (C) Finite element simulation of a single OD under compression (adopted and modified from Iacoviello et al., 2020). (D) (i) Three‐dimensional reconstruction of the skull of the lizard Timon lepida; green, cranial bone; blue, free ODs; purple, temporal‐ODs and quadrate attachment. (ii, iii) Modelling anterior biting. Von Mises stress due to bilateral bite of 170 N (based on published data), with temporal ODs not connected (ii) or connected (iii) to the quadrate.
A relatively large body of research has characterised the mechanical properties of fish scales (e.g. Song, Ortiz & Boyce, 2011; Allison et al., 2013; Yang et al., 2013, 2014; Ebenstein et al., 2015; Arola et al., 2018) and ODs in a range of tetrapod species (Chen et al., 2011; Rhee, Horstemeyer & Ramsay, 2011; Damiens et al., 2012; Chen, Yang & Meyers, 2014, 2015) but very few studies have applied these techniques to lizard ODs (Broeckhoven et al., 2015, 2017a , 2017b ; Iacoviello et al., 2020). This is unfortunate given that lizards show the greatest diversity in OD macro‐ and microstructure of any tetrapod group. Table 2 summarises the range of mechanical properties that have been measured in ODs in only three studies in lizards. These data show a large variability in results for the same species. This could be due to the implemented technique, the treatment of the sample before characterisation, or the anatomical region from which the sample was collected.
Table 2.
Summary of mechanical testing carried out on lizard osteoderms (ODs) and comparison species, to quantify their elastic modulus and load to failure under puncture testing. *Compression puncture test was carried out using the upper jaw of various mongoose species and load to failure (unit N) was reported.
| Specimen | E (GPa) | Testing method | Reference | |
|---|---|---|---|---|
| Turtle shell | ||||
| Terrapene carolinensis | 18.3–24.8 | Nanoindentation | Rhee et al. ( 2009 ) | |
| Chelydra serpentina | 0.5–22.1 | Nanoindentation | Balani et al. ( 2011 ) | |
| Terrapene carolina | 0.4–1.01 | Compression | Damiens et al. ( 2012 ) | |
| Trachemys scripta | 0.9–1.7 | Compression | Zhang et al. ( 2012 ) | |
| Trachemys scripta | 0.7–16.5 | Nanoindentation | Achrai & Wagner ( 2013 ) | |
| Dermochelys coriacea | 0.8–1.8 | Compression | Chen et al. ( 2015 ) | |
| Centrochelys sulcata | 3.2–5.5 | Nanoindentation | Jongpairojcosit & Jearanaisilawong ( 2017 ) | |
| Armadillo | 0.1– 0.4 | Tensile | Chen et al. ( 2011 ) | |
| Dasypus novemcinctus | 1.3–4.6 | Microindentation | Rhee et al. ( 2011 ) | |
| Alligator | 0.9–1.2 | Compression | Sun & Chen ( 2013 ) | |
| Alligator mississipiensis | 1.4–20 | Nanoindentation | ||
| 2.2–5.7 | Compression | Chen et al. ( 2014 ) | ||
| Cordylidae* | ||||
| Ouroborus cataphractus | 63.7–79.5 (N) | |||
| Karusasaurus polyzonus | 18.7–25.6 (N) | Compression on sheet of ODs | Broeckhoven et al. ( 2015 ) | |
| Namazonurus peersi | 14.4–19.0 (N) | |||
| Cordylus macropholis | 18.7–27.0 (N) | |||
| Cordylus cordylus | 14.8–21.9 (N) | |||
| Cordylidae | ||||
| Ouroborus* cataphractus | 75.7 ± 23.5 (N) | Compression | Broeckhoven et al. ( 2017b ) | |
| Smaug* giganteus | 110.4 ± 12.4 (N) | |||
| Heloderma suspectum | 2.7–19.1 | Atomic force microscopy on single OD | Iacoviello et al. ( 2020 ) | |
Given that ODs are not homogenous, variation would be expected in the inherent mechanical properties of different regions of a single OD. The study of Iacoviello et al. (2020) is, to the best of our knowledge, the only example highlighting the variation in the mechanical properties of tissues within a single OD in Heloderma (Fig. 5A–C). They found that the capping tissue (osteodermine) had the highest elastic modulus (ca. 20 GPa), followed by a mineralised region displaying cells and a bone‐like morphology (surrounding the blood vessel channels in the OD; ca. 10 GPa), and then by a relatively softer deep region composed of cells with regularly interspaced fibre bundles that weave into a mineralised matrix (ca. 3 GPa). Considering sheets of ODs, given the morphological variations within and among species, it is likely that the overall stiffness and toughness of ODs could vary and that they would deform differently depending on the way that they are loaded (mimicking different predators; Broeckhoven et al., 2015, 2017b ). In this respect, more work needs to be carried out across a wider range of lizards to map out the mechanical properties of ODs and to correlate their properties to other factors, such as their macroscopic structure and ecological differences.
A number of other studies have used the FE method to investigate the role and function of ODs in a range of tetrapod species (Rivera & Stayton, 2011; du Plessis et al., 2018). However, very few studies have applied this technique to lizard ODs (Clarac et al., 2017, 2019; Iacoviello et al., 2020). From a thermal perspective, the FE results of Clarac et al. (2017) suggested that although the mineralised parts of ODs may not themselves play a role in cutaneous heat conduction, the network of blood vessels within the ODs could contribute to heat exchange between the lizard and its environment. From a structural perspective, it seems that, depending on how loosely or rigidly ODs are attached to the skeleton, they may contribute to the overall loading that the skeleton undertakes (Fig. 5D; Xue et al., 2017). On the other hand, ODs may be optimised and adapted to the mechanical environment that they experience. The FE studies of Clarac et al. (2019) suggested that the dorsal keel on crocodylomorph ODs might play a role in reducing the overall stress across the OD. Similarly, the FE studies of Iacoviello et al. (2020) suggested that the structural heterogeneity within a single OD could represent an adaptation to enhance its resistance to the external load. Nonetheless, given the morphological and structural diversity of lizard ODs, further work in a wider range of species is needed to describe the biomechanical spectrum of these structures.
(2). Biomimetics and bioinspiration
Despite a relatively large body of research on the development of biomimetic and bioinspired structures, based on what we know from fish scales [e.g. Chintapalli et al., 2014; Rudykh, Ortiz & Boyce, 2015; Martini, Balit & Barthelat, 2017; see also Connors et al., 2019 on chiton scales] and ODs from a range of tetrapod species (see review by Yang et al., 2013), to the best of our knowledge no study has used lizard ODs as a model system. The existing literature in this area has highlighted the potential applications of scales/ODs and the concept of tessellation, for body armour with increased fracture resistance (Fratzl et al., 2016; Naleway et al., 2016). However, our understanding of the effect of different OD morphologies in enhancing, for example, the fracture resistance or shock absorption capacity of body armour is still limited. Building on a greater knowledge of lizard OD patterning and morphology, future studies can use advanced computational and experimental techniques to characterise the biomechanics of ODs in different lizards. Such studies can naturally set the foundation for future biomimetic and bioinspiration research focused on lizard ODs.
VI. FUNCTION
The formation of skeletal elements within the skin has high costs in terms of nutrients and energy (Giles, 1983; Spence, 2012), therefore OD function must warrant these costs. In tetrapods generally, ODs are most commonly hypothesised to function as defensive armour, whether against predators, conspecifics, or aggressive prey (Moss, 1972; Seidel, 1979; Albertson et al., 2009; Vickaryous & Sire, 2009; Hayashi et al., 2010; Yang et al., 2012; Vickaryous et al., 2015; Broeckhoven et al., 2018a ; Laver et al., 2020), so much so that the terms ‘osteoderms’ and ‘armour’ are sometimes used as synonyms (Yang et al., 2012; Laver et al., 2020). Among lizards, only the OD‐rich skin of five cordylid species has been tested for its toughness (Broeckhoven et al., 2015). Skin toughness was found to be correlated with OD coverage and thickness but not with the thickness of the epidermal armour (i.e. β‐keratin). However, with the exception of Ouroborus cataphractus, which could withstand simulated bites from several mongoose species, the skin of those lizards tested failed to endure the simulated bites of their mammalian predators. The early diversification of ODs in cordylids was suggested to be coupled with rapid defensive trait diversification (Broeckhoven et al., 2016). However, that diversification of defensive traits appeared unrelated to predation risk associated with microhabitat use (Broeckhoven et al., 2016, 2018a ). Therefore, although the primary role of ODs is likely to reinforce the structural rigidity and mechanical toughness of the skin, it would be simplistic to imply that this is their only function.
Several additional or alternative hypotheses of OD function have been proposed across tetrapods, including thermoregulation due to their vascularisation (Seidel, 1979; de Buffrénil et al., 1986; Farlow, Hayashi & Tattersall, 2010; Hayashi et al., 2010; Broeckhoven et al., 2015, 2017b ; Clarac et al., 2017, 2020; see Section V.1), structural support of the vertebral column (Frey, 1988; Losos et al., 2002; Buchwitz et al., 2012), social display/recognition (Main et al., 2005; Hayashi et al., 2010; Saitta, 2015), metabolic or mineral regulation, lactate sequestration, and calcium reserve (Moss, 1972; Seidel, 1979; Jackson, Andrade & Abe, 2003; Marinho, 2007; Warren & Jackson, 2008; Farlow et al., 2010; Janis et al., 2012; Dacke et al., 2015; Paluh et al., 2017; Vidal et al., 2017), water retention (Khalil & Abdel‐Messeih, 1962; Witzmann, 2009), as exoskeletal attachment for tendons (Seidel, 1979), or as camouflage (Albertson et al., 2009; Schucht et al., 2020). Yet, to date, many of these hypotheses remain untested, and for those that have (e.g. lactate sequestration, locomotor support, thermoregulation), the work has mainly focused on crocodiles or turtles, rather than terrestrial squamates.
In squamates, Camp (1923) associated the absence of OD expression (e.g. in amphisbaenians, dibamids) with burrowing. Coe & Kunkel (1906) similarly observed that the ODs of the Anniellidae (Anguimorpha) are greatly reduced, apparently by vacuolisation, and concluded that this was due to their subterranean life. However, caecilians (Amphibia) burrow and have skeletal elements within their skin, albeit with a potentially more flexible structure (Zylberberg & Wake, 1990). ODs have also been suggested to reduce flexibility during locomotion (Losos et al., 2002), offering an alternative/additional hypothesis for their loss or reduction in long‐bodied limb‐reduced squamates, but many elongated anguids (e.g. Ophisaurus, Anguis) retain an extensive covering of ODs apparently without compromising their mobility. Moreover, there is a substantial OD cover in fossorial scincids and Heloderma, also a habitual burrow‐dweller (Rieppel, 1981; Miralles et al., 2015; Iacoviello et al., 2020; Kirby et al., 2020), albeit one that is fully limbed.
The observation that the X‐ray density of ODs in Alligator spp. was greater in females with ripe ovarian follicles compared to those that had recently laid eggs (Dacke et al., 2015) led to the suggestion that lizard ODs may sometimes play a role in calcium storage (Paluh et al., 2017). However, Laver et al. (2020) argued that the enlarged calcium‐dense endolymphatic sacs in the small number of OD‐bearing gekkotans (Gekko gecko, G. reevesii, Geckolepis spp., Tarentola spp.), in comparison with the majority of OD‐less species, were evidence that, at least in gekkotans, ODs are structures that require rather than provide calcium resources, their importance being in other roles, notably in reinforcement of the skin.
Cordylids and their sister group gerrhosaurids form a small clade of scincoid lizards from sub‐Saharan Africa that show striking variation in OD coverage from a full‐body covering (e.g. Broadleysaurus) to almost complete absence (e.g. Platysaurus) (Stanley, 2013, 2016; Broeckhoven et al., 2015, 2017a , 2017b ; Broeckhoven et al., 2018a ). This renders cordylids an interesting study group in which to explore OD function. This series of papers emphasised the multi‐functionality of ODs (e.g. protection versus thermoregulation, as high vascularity may weaken ODs; Broeckhoven et al., 2017b ), and the possible trade‐offs between these different roles (Losos et al., 2002). They found that ODs could be sexually dimorphic (Broeckhoven et al., 2017a ), developing on the trunks of male lizards around the time of sexual maturity when agonistic intraspecific encounters occur where males bite each other's bodies. In this case, the role of ODs is likely protective.
Thus, the presence, absence, and degree of OD cover for individual species (or individual animals within each species) likely depends on the interplay of, and trade‐offs between, a combination of factors, including environmental factors (e.g. aridity versus humidity, prevalence of shelter and ground cover), the likelihood and type of predator encounters, the ability to capture agile prey, and the danger from conspecific agonists or aggressive prey (Broeckhoven et al., 2015, 2017a ; Broeckhoven et al., 2018a ; Broeckhoven, Le Fras Nortier Mouton & Hui, 2018b ). The selective pressures on ODs expressed in different anatomical regions may also vary, leading to differential regional expression, for example in the cranium (Lacertididae) or the tail [Platysaurus (Cordylidae)] while lost elsewhere.
While lizard natural ‘body armour’ might serve as bioinspiration for the development of artificial protective materials, it would be important to focus on those species in which ODs serve a likely protective function, whether from predators or conspecific aggression, although other functions cannot be excluded. Therefore, data on the function(s) of ODs are important for reverse engineered applications.
VII. CONCLUSIONS
The ability to express integumentary mineralisations is a trait common to vertebrates and is almost certainly an ancestral trait.
Osteoderms (or their homologues) were present in the earliest tetrapods and have been lost or retained across different tetrapod lineages from amphibians through reptiles and into mammals.
There is no single function that can be ascribed to osteoderms. When present, they may be protective, but there will be trade‐offs in terms of skin permeability, water retention, body mass, flexibility, and calcium costs. However, hypotheses of function remain rarely tested, and purported trade‐offs need to be re‐evaluated in the light of a proper examination of the phylogenetic history of the clade and detailed information on the ecology of the species under study. Very little is currently known of the biomechanical properties of squamate osteoderms.
Osteoderms do not seem to have been expressed in stem‐lepidosaurs, perhaps reflecting their generally small size, and are found in only one extinct representative of Rhynchocephalia. They are, however, common in lizards and have been found in fossil squamates dating back to at least the Middle Jurassic (China).
The superficial covering of hypermineralised capping tissue (osteodermine or similar) on the osteoderms of many squamates seems to be a derived trait for Squamata, although it may represent a re‐activation of ancient ancestral pathways like those found in many fish.
Developmental studies are needed to establish the cellular population (s) from which squamate osteoderms develop, and particularly whether there is an ectodermal component to the formation of the capping tissue.
REFERENCES
- Achrai, B. & Wagner, H. D. (2013). Micro‐structure and mechanical properties of the turtle carapace as a biological composite shield. Acta Biomaterialia 9(4), 5890–5902. [DOI] [PubMed] [Google Scholar]
- Albertson, R. C. , Cresko, W. , Detrich, W. & Postlethwait, J. H. (2009). Evolutionary mutant models for human disease. Trends in Genetics 25(2), 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison, P. G. , Chandler, M. Q. , Rodriguez, R. I. , Williams, B. A. , Moser, R. D. , Weiss, C. J. Jr. , Poda, A. R. , Lafferty, B. J. , Kennedy, A. J. , Seiter, J. M. , Hodo, W. D. & Cook, R. F. (2013). Mechanical properties and structure of the biological multilayered material system, Atractosteus spatula scales. Acta Biomaterialia 9(2), 5289–5296. [DOI] [PubMed] [Google Scholar]
- Arnold, E. N. (1989). Towards a phylogeny and biogeography of the Lacertidae: relationships within an Old World family of lizards derived from morphology. Bulletin of the British Museum of Natural History 55, 209–257. [Google Scholar]
- Arnold, E. N. , Arribas, O. & Carranza, S. (2007). Systematics of the Palaearctic and oriental lizard tribe Lacertini (Squamata: Lacertidae: Lacertinae), with descriptions of eight new genera. Zootaxa 1430, 1–86. [Google Scholar]
- Arola, D. , Murcia, S. , Stossel, M. , Pahuja, R. , Linley, T. , Devaraj, A. , Ramulu, M. , Ossa, E. A. & Wang, J. (2018). The limiting layer of fish scales: structure and properties. Acta Biomaterialia 67, 319–330. [DOI] [PubMed] [Google Scholar]
- Balani, K. , Patel, R. R. , Keshri, A. K. , Lahiri, D. & Agarwal, A. (2011). Multi‐scale hierarchy of Chelydra serpentina: microstructure and mechanical properties of turtle shell. Journal of the Mechanical Behavior of Biomedical Materials 4(7), 1440–1451. [DOI] [PubMed] [Google Scholar]
- Barahona, F. & Barbadillo, L. J. (1998). Inter‐ and intraspecific variation in the post‐natal skull of some lacertid lizards. Journal of Zoology 245, 393–405. [Google Scholar]
- Barrett, P. M. , Clarke, J. B. , Brinkman, D. B. , Chapman, S. D. & Ensom, P. C. (2002). Morphology, histology and identification of the ‘granicones’ from the Purbeck Limestone Formation (Lower Cretaceous: Berriasian) of Dorset, southern England. Cretaceous Research 23(2), 279–295. [Google Scholar]
- Bauer, A. M. & Russell, A. P. (1989). Supraorbital ossifications in geckos (Reptilia: Gekkonidae). Canadian Journal of Zoology 67, 678–684. [Google Scholar]
- Bauer, A. M. , Russell, A. P. & Shadwick, R. E. (1989). Mechanical properties and morphological correlates of fragile skin in gekkonid lizards. Journal of Experimental Biology 145, 79–102. [Google Scholar]
- Bauer, A. M. , Russell, A. P. & Shadwick, R. E. (1992). Skin mechanics and morphology in Sphaerodactylus roosevelti (Reptilia: Gekkonidae). Herpetologica 48, 124–133. [Google Scholar]
- Beresford, W. A. (1981). Chondroid bone, secondary cartilage and metaplasia. Urban and Schwarzenberg, Baltimore. [Google Scholar]
- Bever, G. S. , Bell, C. J. & Maisano, J. (2005). The ossified braincase and cephalic osteoderms of Shinisaurus crocodilurus (Squamata, Shinisauridae). Palaeontologia Electronica 8.1(4A), 1–36. [Google Scholar]
- Bhullar, B. A. S. (2011). The power and utility of morphological characters in systematic: a fully resolved phylogeny of Xenosaurus and its fossil relatives (Squamata: Anguimorpha). Bulletin of the Museum of Comparative Zoology 160, 65–181. [Google Scholar]
- Bhullar, B. A. S. & Bell, C. J. (2008). Osteoderms of the California legless lizard Anniella (Squamata: Anguidae) and their relevance for considerations of miniaturization. Copeia 4, 785–793. [Google Scholar]
- Bochaton, C. , De Buffrénil, V. , Lemoine, M. , Bailon, S. & Ineich, I. (2015). Body location and tail regeneration effects on osteoderms morphology – are they useful tools for systematic, paleontology, and skeletochronology in diploglossine lizards (Squamata, Anguidae)? Journal of Morphology 276, 1333–1344. [DOI] [PubMed] [Google Scholar]
- Bolet, A. & Evans, S. E. (2011). New material of the enigmatic Scandensia, an Early Cretaceous lizard from the Iberian Peninsula. Special Papers in Palaeontology 86, 99–108. [Google Scholar]
- Borsuk‐Białynicka, M. (1984). Anguimorphans and related lizards from the Late Cretaceous of the Gobi Desert, Mongolia. Palaeontologia Polonica 46, 5–105. [Google Scholar]
- Borsuk‐Białynicka, M. , Lubka, M. & Böhme, W. (1999). A lizard from Baltic amber (Eocene) and the ancestry of the crown group lacertids. Acta Palaeontologica Polonica 44(4), 349–382. [Google Scholar]
- Botha‐Brink, J. & Modesto, S. P. (2007). A mixed‐age classed ‘pelycosaur’ aggregation from South Africa: earliest evidence of parental care in amniotes? Proceedings of the Royal Society B 274, 2829–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckhoven, C. , De Kock, C. & Le Fras Nortier Mouton, P. (2017a). Sexual dimorphism in osteoderm expression and the role of male intrasexual aggression. Biological Journal of the Linnean Society 122(2), 329–339. [Google Scholar]
- Broeckhoven, C. , Diedericks, G. , Hui, C. , Makhubo, B. G. & Le Fras Nortier Mouton, P. (2016). Enemy at the gates: rapid defensive trait diversification in an adaptive radiation of lizards. Evolution 70(11), 2647–2656. [DOI] [PubMed] [Google Scholar]
- Broeckhoven, C. , Diedericks, G. & Le Fras Nortier Mouton, P. (2015). What doesn't kill you might make you stronger: functional basis for variation in body armour. Journal of Animal Ecology 84(5), 1213–1221. [DOI] [PubMed] [Google Scholar]
- Broeckhoven, C. & du Plessis, A. (2018). X‐ray microtomography in herpetological research: a review. Amphibia‐Reptilia 39(4), 377–401. [Google Scholar]
- Broeckhoven, C. , du Plessis, A. & Hui, C. (2017b). Functional trade‐off between strength and thermal capacity of dermal armor: insights from girdled lizards. Journal of Mechanical Behaviour of Biomedical Materials 74, 189–194. [DOI] [PubMed] [Google Scholar]
- Broeckhoven, C. , El Adak, Y. , Hui, C. , Van Damme, R. & Stankowich, T. (2018a). On dangerous ground: the evolution of body armour in cordyline lizards. Proceedings of the Royal Society B 285, 20180513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckhoven, C. , Le Fras Nortier Mouton, P. & Hui, C. (2018b). Proximate causes of variation in dermal armour: insights from armadillo lizards. Oikos 127, 1449–1458. [Google Scholar]
- Brown, C. M. (2017). An exceptionally preserved armoured dinosaur reveals the morphology and allometry of osteoderms and their horny epidermal coverings. Peer J 5, e4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, S. V. & Bellairs, F. L. S. A. A. (1967). Tail regeneration in the lizards Anguis fragilis and Lacerta dugesii . Zoological Journal of the Linnean Society 46, 297–305. [Google Scholar]
- Buchwitz, M. , Witzmann, F. , Voigt, S. & Golubev, V. (2012). Osteoderm microstructure indicates the presence of a crocodylian‐like trunk bracing system in a group of armoured basal tetrapods. Acta Zoologica 93(3), 260–280. [Google Scholar]
- de Buffrénil, V. , Dauphin, Y. , Rage, J. C. & Sire, J.‐Y. (2011). An enamel‐like tissue, osteodermine, on the osteoderms of a fossil anguid (Glyptosaurinae) lizard. Comptes Rendus Palevol 10, 427–437. [Google Scholar]
- de Buffrénil, V. , Farlow, J. O. & de Ricqlès, A. (1986). Growth and function of Stegosaurus plates: evidence from bone histology. Paleobiology 12(4), 459–473. [Google Scholar]
- de Buffrénil, V. , Sire, J.‐Y. & Rage, J.‐C. (2010). The histological structure of glyptosaurine osteoderms (Squamata: Anguidae), and the problem of osteoderm development in squamates. Journal of Morphology 271, 729–737. [DOI] [PubMed] [Google Scholar]
- Burbrink, F. T. , Grazziotin, F. G. , Pyron, R. A. , Cundall, D. , Donnellan, S. , Irish, F. , Keogh, J. S. , Kraus, F. , Murphy, R. W. , Noonan, B. , Raxworthy, C. J. , Ruane, S. , Lemmon, A. R. , Lemmon, E. M. & Zaher, H. (2020). Interrogating genomic‐scale data for Squamata (lizards, snakes, and amphisbaenians) shows no support for key traditional morphological relationships. Systematic Biology 69, 502–520. [DOI] [PubMed] [Google Scholar]
- Burns, M. E. & Currie, P. J. (2014). External and internal structure of ankylosaur (Dinosauria, Ornithischia) osteoderms and their systematic relevance. Journal of Vertebrate Paleontology 34, 835–851. [Google Scholar]
- Camaiti, M. , Villa, A. , Wencker, L. C. M. , Bauer, A. M. , Stanley, E. L. & Delfino, M. (2019). Descriptive osteology and patterns of limb loss of the European limbless skink Ophiomorus punctatissimus (Squamata, Scincidae). Journal of Anatomy 235, 313–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp, C. L. (1923). Classification of the Lizards. Bulletin of the American Museum of Natural History 48, 289–481. [Google Scholar]
- Canei, J. & Nonclercq, D. (2020). Morphological study of integument and corporal skeletal muscles of two psammophilous members of Scincidae (Scincus scincus and Eumeces schneideri). Journal of Morphology 282, 230–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanet, J. , Francillon‐Vieillot, H. , de Ricqlès, A. & Zylberberg, L. (2003). The skeletal histology of the Amphibia. In Amphibian Biology, vol. 5: Osteology (eds Heatwole H. and Davies M.), pp. 1598–1683. Surrey Beatty, Chipping Norton. [Google Scholar]
- Cebra‐Thomas, J. A. , Betters, E. , Yin, M. , Plafkin, C. , McDow, K. & Gilbert, S. F. (2007). Evidence that a late‐emerging population of trunk neural crest cells forms the plastron bones in the turtle Trachemys scripta . Evolution and Development. 9, 267–277. [DOI] [PubMed] [Google Scholar]
- Cebra‐Thomas, J. , Tan, F. , Sistla, S. , Estes, E. , Bender, G. , Kim, C. , Riccio, P. & Gilbert, S. F. (2005). How the turtle forms its shell: a paracrine hypothesis of carapace formation. Journal of Experimental Zoology Part B Molecular and Developmental Evolution 304, 558–569. [DOI] [PubMed] [Google Scholar]
- Cerda, I. A. & Desojo, J. B. (2010). Dermal armour histology of aetosaurs (Archosauria: Pseudosuchia), from the Upper Triassic of Argentina and Brazil. Lethaia 44, 417–428. [Google Scholar]
- Cerda, I. A. , Desojo, J. B. & Scheyer, T. M. (2018). Novel data on aetosaur (Archosauria, Pseudosuchia) osteoderm microanatomy and histology: palaeobiological implications. Palaeontology 61, 721–745. [Google Scholar]
- Cerda, I. A. , Desojo, J. B. , Scheyer, T. M. & Schultz, C. L. (2013). Osteoderm microstructure of “rauisuchian” archosaurs from South America. Geobios 46, 273–283. [Google Scholar]
- Cerda, I. A. , Desojo, J. B. , Trotteyn, M. J. & Scheyer, T. M. (2015). Osteoderm histology of Proterochampsia and Doswellidae (Reptilia; Archosauriformes) and their evolutionary and paleobiological implications. Journal of Morphology 276, 385–402. [DOI] [PubMed] [Google Scholar]
- Chen, I. H. , Kiang, J. H. , Correa, V. , Lopez, M. I. , Chen, P.‐Y. , McKittrick, J. & Meyers, M. A. (2011). Armadillo armor: mechanical testing and micro‐structural evaluation. Journal of the Mechanical Behaviour of Biomedical Materials 4(5), 713–722. [DOI] [PubMed] [Google Scholar]
- Chen, I. H. , Yang, W. & Meyers, M. A. (2014). Alligator osteoderms: mechanical behavior and hierarchical structure. Materials Science and Engineering C. 35(1), 441–448. [DOI] [PubMed] [Google Scholar]
- Chen, I. H. , Yang, W. & Meyers, M. A. (2015). Leatherback sea turtle shell: a tough and flexible biological design. Acta Biomaterialia 28, 2–12. [DOI] [PubMed] [Google Scholar]
- Cherepanov, G. (1997). The origins of the bony shell of turtles as a unique evolutionary model in reptiles. Russian Journal of Herpetology 4(2), 155–162. [Google Scholar]
- Chintapalli, R. K. , Mirkhalaf, M. , Dastjerdi, A. K. & Barthelat, F. (2014). Fabrication, testing and modeling of a new flexible armor inspired from natural fish scales and osteoderms. Bioinspiration and Biomimetics 9, 36005–36014. [DOI] [PubMed] [Google Scholar]
- Cisneros, J. C. (2008). Phylogenetic relationships of procolophonid parareptiles with remarks on their geological record. Journal of Systematic Palaeontology 6, 345–366. [Google Scholar]
- Clarac, F. , Goussard, F. , Buffrénil, V. & Sansalone, V. (2019). The function(s) of bone ornamentation in the crocodylomorph osteoderms: a biomechanical model based on a finite element analysis. Paleobiology 45(1), 182–200. [Google Scholar]
- Clarac, F. , Goussard, F. , Teresi, L. , Buffrénil, V. & Sansalone, V. (2017). Do the ornamented osteoderms influence the heat conduction through the skin? A finite element analysis in Crocodylomorpha. Journal of Thermal Biology 69, 39–53. [DOI] [PubMed] [Google Scholar]
- Clarac, F. , Scheyer, T. M. , Desojo, J. B. , Cerda, I. A. & Sanchez, S. (2020). The evolution of dermal shield vascularisation in Testudinata and Pseudosuchia: phylogenetic constraints versus ecophysiological adaptations. Philosophical Transactions of the Royal Society B 375, 20190132. 10.1098/rstb.2019.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe, W. R. & Kunkel, B. W. (1906). Studies on the California limbless lizard, Anniella . Transactions of the Kansas Academy of Science 12, 349–403. [Google Scholar]
- Connors, M. , Yang, T. , Hosny, A. , Deng, Z. , Yazdandoost, F. , Massaadi, H. , Eernisse, D. , Mirzaeifar, R. , Dean, M. N. , Weaver, J. C. , Ortiz, C. & Li, L. (2019). Bioinspired design of flexible armor based on chiton scales. Nature Communications 10, 5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, J. L. , Head, J. J. & Carrano, M. T. (2014). Unusual soft‐tissue preservation of a crocodile lizard (Squamata, Shinisauria) from the Green River Formation (Eocene) and shinisaur relationships. Anatomical Record 297, 545–559. [DOI] [PubMed] [Google Scholar]
- Conrad, J. L. , Rieppel, O. , Gauthier, J. A. & Norell, M. A. (2011). Osteology of Gobiderma pulchrum (Monstersauria, Lepidosauria, Reptilia). Bulletin of the American Museum of Natural History 362, 1–88. [Google Scholar]
- Conrad, J. L. , Wang, Y. , Xu, X. , Pyron, A. & Clark, J. (2013). Skeleton of a heavily armoured and long‐legged Middle Jurassic lizard (Squamata, Reptilia). Journal of Vertebrate Paleontology 73, 108. [Google Scholar]
- Constantini, D. & Dell'Omo, G. (2010). Sex‐specific predation on two lizard species by kestrels. Russian Journal of Ecology 41, 99–101. [Google Scholar]
- Crawford, N. G. , Parham, J. F. , Sellas, A. B. , Faircloth, B. C. , Glenn, T. C. , Papenfuss, T. J. , Henderson, J. B. , Hansen, M. H. & Simison, W. B. (2015). A phylogenomic analysis of turtles. Molecular Phylogenetics and Evolution 83, 250–257. [DOI] [PubMed] [Google Scholar]
- Dacke, C. G. , Elsey, R. M. , Trosclair, P. L. , Sugiyama, T. , Nevarez, J. G. & Schweitzer, M. H. (2015). Alligator osteoderms as a source of labile calcium for eggshell formation. Journal of Zoology 297(4), 255–264. [Google Scholar]
- Damiens, R. , Rhee, H. , Hwang, Y. , Park, S. J. , Hammi, Y. , Lim, H. & Horstemeyer, M. F. (2012). Compressive behavior of a turtle's shell: experiment, modeling, and simulation. Journal of the Mechanical Behavior of Biomedical Materials 6, 106–112. [DOI] [PubMed] [Google Scholar]
- D'Emic, M. D. , Wilson, J. A. & Chatterjee, S. (2009). The titanosaur (Dinosauria: Sauropoda) osteoderm record: review and first definitive specimen from India. Journal of Vertebrate Paleontology 29(1), 165–177. [Google Scholar]
- Dodson, P. , Krause, D. W. , Forster, C. A. , Sampson, S. D. & Ravoavy, F. (1998). Titanosaurid (Sauropoda) osteoderms from the Late Cretaceous of Madagascar. Journal of Vertebrate Paleontology 18, 563–568. [Google Scholar]
- Dong, L. P. , Wang, Y. & Evans, S. E. (2017). A new lizard (Reptilia: Squamata) from the Early Cretaceous Yixian Formation of China, with a taxonomic revision of Yabeinosaurus . Cretaceous Research 72, 161–171. [Google Scholar]
- Dong, L. , Wang, Y. , Mou, L. , Zhang, G. & Evans, S. E. (2019). A new Jurassic lizard from China. Geodiversitas 41, 623–641. [Google Scholar]
- Dong, L. , Xing, X. , Wang, Y. & Evans, S. E. (2018). The lizard genera Bainguis and Parmeosaurus from the Upper Cretaceous of China and Mongolia. Cretaceous Research 85, 95–108. [Google Scholar]
- Dubansky, B. H. & Dubansky, B. D. (2018). Natural development of dermal ectopic bone in the American Alligator (Alligator mississippiensis) resembles heterotopic ossification disorders in humans. Anatomical Record 301(1), 56–76. [DOI] [PubMed] [Google Scholar]
- Dubke, M. , Hipsley, C. A. & Müller, J. (2018). Comparative skull osteology and preliminary systematic revision of the African lizard genus Heliobolus (Squamata: Lacertidae). African Journal of Herpetology 67, 160–197. [Google Scholar]
- Ebenstein, D. , Calderon, C. , Troncoso, O. P. & Torres, F. G. (2015). Characterization of dermal plates from armored catfish Pterygoplichthys pardalis reveals sandwich‐like nanocomposite structure. Journal of the Mechanical Behavior of Biomedical Materials 45, 175–182. [DOI] [PubMed] [Google Scholar]
- Erickson, G. M. , de Ricqlès, A. , de Buffrénil, V. , Molnar, R. E. & Bayless, M. K. (2003). Vermiform bones and the evolution of gigantism in Megalania — How a reptilian fox became a lion. Journal of Vertebrate Paleontology 23, 966–970. [Google Scholar]
- Estes, R. (1983). Sauria terrestrial, Amphisbaenia. In Encyclopedia of Paleoherpetology (Volume 10A, ed. Wellnhofer P.), pp. 1–249. Gustav Fischer Verlag, Stuttgart. [Google Scholar]
- Estes, R. , De Queiroz, K. & Gauthier, J. (1988). Phylogenetic relationships within Squamata. In Phylogenetic Relationships of the Lizard Families: Essays Commemorating Charles L. Camp (eds Estes R. and Pregill G.), pp. 119–291. Stanford University Press, Stanford. [Google Scholar]
- Evans, S. E. & Chure, D. C. (1998). Paramacellodid lizard skulls from the Jurassic Morrison Formation at Dinosaur National Monument, Utah. Journal of Vertebrate Paleontology 18, 99–114. [Google Scholar]
- Farlow, J. O. , Hayashi, S. & Tattersall, G. J. (2010). Internal vascularity of the dermal plates of Stegosaurus (Ornithischia, Thyreophora). Swiss Journal of Geosciences 103(2), 173–185. [Google Scholar]
- Fedorov, P. V. & Nessov, L. A. (1992). A lizard from the boundary of the Middle and Late Jurassic of north‐east Fergana. Bulletin of St. Petersburg University . Geology and Geography 3, 9–14 [In Russian]. [Google Scholar]
- Fratzl, P. , Kolednik, O. , Fischer, F. D. & Dean, M. N. (2016). The mechanics of tessellations – bioinspired strategies for fracture resistance. Chemical Society Reviews 45, 252–267. [DOI] [PubMed] [Google Scholar]
- Frey, E. (1988). Das Tragsystem der Krokodile ‐ eine biomechanische und phylogenetische Analyse. Stuttgarter Beiträge zur Naturkunde A 426, 1–60. [Google Scholar]
- Gao, K. & Norell, M. A. (1998). Taxonomic revision of Carusia (Reptilia: Squamata) from the Late Cretaceous of the Gobi Desert and phylogenetic relationships of anguinomorphan lizards. American Museum Novitates 3230, 1–55.
- Giles, N. (1983). The possible role of environmental calcium levels during the evolution of phenotypic diversity in Outer Hebridean populations of the Three‐spined stickleback, Gasterosteus aculeatus . Journal of Zoology 199(4), 535–544. [Google Scholar]
- Good, D. A. & Schwenk, K. (1985). A new species of Abronia (Lacertilia: Anguidae) from Oaxaca, Mexico. Copeia 1985, 135–141. [Google Scholar]
- Greenwold, M. J. , Bao, W. , Jarvis, E. D. , Hu, H. , Li, C. , Gilbert, M. T. P. , Zhang, G. & Sawyer, R. H. (2014). Dynamic evolution of the alpha (α) and beta (β) keratins has accompanied integument diversification and the adaptation of birds into novel lifestyles. BMC Evolutionary Biology 14, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffing, A. H. , Daza, J. D. , Deboer, J. C. & Bauer, A. M. (2018). Developmental osteology of the parafrontal bones of the Sphaerodactylidae. Anatomical Record 301, 581–606. [DOI] [PubMed] [Google Scholar]
- Guarino, F. M. , Mezzasalma, M. & Odierna, G. (2016). Usefulness of postpygal vertebrae and osteoderms for skeletochronology in the limbless lizard Anguis veronensis Pollinii, 1818 (Squamata: Sauria: Anguidae). Herpetozoa 29, 69–75. [Google Scholar]
- Haines, R. W. & Mohuiddin, A. (1968). Metaplastic bone. Journal of Anatomy 103, 527–538. [PMC free article] [PubMed] [Google Scholar]
- Hayashi, S. , Carpenter, K. , Scheyer, T. M. , Watabe, M. & Suzuk, I. D. (2010). Function and evolution of ankylosaur dermal armor. Acta Palaeontologica Polonica 55(2), 213–228. [Google Scholar]
- Hecht, M. K. & Hecht, B. M. (1984). A new lizard from Jurassic deposits of Middle Asia. Paleontological Journal 18, 133–136. [Google Scholar]
- Hill, R. V. (2005). Integration of morphological data sets for phylogenetic analysis of Amniota: the importance of integumentary characters and increased taxonomic sampling. Systematic Biology 54(4), 530–547. [DOI] [PubMed] [Google Scholar]
- Hill, R. V. (2006). Comparative anatomy and histology of xenarthran osteoderms. Journal of Morphology 267, 1441–1460. [DOI] [PubMed] [Google Scholar]
- Hill, R. V. (2010). Osteoderms of Simosuchus clarki (Crocodyliformes: Notosuchia) from the Late Cretaceous of Madagascar. Journal of Vertebrate Paleontology 30(Suppl. 1), 154–176. [Google Scholar]
- Hirasawa, T. , Nagashima, H. & Kuratani, S. (2013). The endoskeletal origin of the turtle carapace. Nature Communications 4, 2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstetter, R. (1962). Observations sur les ostéodermes et la classification des anguidés actuels et fossils (Reptiles, Sauriens). Bulletin du Museum national d'Histoire naturelle 34, 149–157. [Google Scholar]
- Holthaus, K. B. , Eckhart, L. , Dalla Valle, L. & Alibardi, L. (2018). Review: evolution and diversification of corneous beta‐proteins, the characteristic epidermal proteins of reptiles and birds. Journal of Experimental Zoology Part B Molecular Development and Evolution. 330, 438–453. [DOI] [PubMed] [Google Scholar]
- Horner, J. R. , Woodward, H. N. & Bailleul, A. M. (2016). Mineralized tissues in dinosaurs interpreted as having formed through metaplasia: a preliminary evaluation. Comptes Rendus ‐ Palevol, Academie des Sciences 15(1–2), 183–203. [Google Scholar]
- Iacoviello, F. , Moeendarbary, E. , Kirby, A. , Javanmardi, Y. , Shabanli, M. , Tsolaki, E. , Sharp, A. , Hayes, M. , Keevend, K. , Brett, D. , Shearing, P. , Olivo, A. , Herrmann, I. K. , Evans, S. E. , Moazen, M. & Bertazzo, S. (2020). The multiscale hierarchical structure of osteoderms defines their distinct mechanical properties and functions. Acta BioMaterialia 107, 194–203. [DOI] [PubMed] [Google Scholar]
- Jackson, D. C. , Andrade, D. V. & Abe, A. S. (2003). Lactate sequestration by osteoderms of the broad‐nose caiman, Caiman latirostris, following capture and forced submergence. Journal of Experimental Biology 206, 3601–3606. [DOI] [PubMed] [Google Scholar]
- Janis, C. M. , Devlin, K. , Warren, D. E. & Witzmann, F. (2012). Dermal bone in early tetrapods: a palaeophysiological hypothesis of adaptation for terrestrial acidosis. Proceedings of the Royal Society B: Biological Sciences 279(1740), 3035–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M. E. H. , Anderson, C. L. , Hipsley, C. A. , Muller, J. , Evans, S. E. & Schoch, R. (2013). Integration of molecules and new fossils supports a Triassic origin for Lepidosauria (lizards, snakes, and tuatara). BMC Evolutionary Biology (Section: Phylogenetics and Phylogeography) 13, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongpairojcosit, N. & Jearanaisilawong, P. (2017). Mechanical properties and numerical simulation of Sulcata tortoise carapace. Journal of the Mechanical Behavior of Biomedical Materials 72, 261–267. [DOI] [PubMed] [Google Scholar]
- Kawasaki, K. , Suzuki, T. & Weiss, K. M. (2004). Genetic basis for the evolution of vertebrate mineralized tissue. Proceedings of the National Academy of Sciences 101(31), 11356–11361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil, F. & Abdel‐Messeih, G. (1962). Tissue constituents of reptiles in relation to their mode of life. 1. Water content. Comparative Biochemistry and Physiology 5, 327–330. [DOI] [PubMed] [Google Scholar]
- Kirby, A. , Vickaryous, M. , Boyde, A. , Olivo, A. , Moazen, M. , Bertazzo, S. & Evans, S. (2020). A comparative histological study of the osteoderms in the lizards Heloderma suspectum (Squamata: Helodermatidae) and Varanus komodoensis (Squamata: Varanidae). Journal of Anatomy 236, 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, N. , Scheyer, T. & Tütken, T. (2009). Skeletochronology and isotopic analysis of a captive individual of Alligator mississippiensis Daudin, 1802. Fossil Record 12, 121–131. [Google Scholar]
- Krause, D. , Evans, S. E. & Gao, K. (2003). First definitive record of a Mesozoic lizard from Madagascar. Journal of Vertebrate Paleontology 23(4), 842–856. [Google Scholar]
- Laver, R. J. , Morales, C. H. , Heinicke, M. P. , Gamble, T. , Longoria, K. , Bauer, A. M. & Daza, J. D. (2020). The development of cephalic armor in the Tokay gecko (Squamata: Gekkonidae: Gekko gecko). Journal of Morphology 281, 213–228. [DOI] [PubMed] [Google Scholar]
- Lemon, J. (2013). Plotrix: a package in the red light district of R. R‐News 6, 8–12. [Google Scholar]
- Levrat‐Calviac, V. (1986). Étude comparée des ostéodermes de Tarentola mauritanica et de T. neglecta (Gekkonidae, Squamata). Archives d'anatomie microscopique et de morphologie expérimentale 75, 29–43. [Google Scholar]
- Levrat‐Calviac, V. , Castanet, J. & Zylberberg, L. (1986). The structure of the osteoderms in two lizards: Tarentola mauritanica and Anguis fragilis . In Studies in Herpetology: Proceedings of the European Herpetological Meeting (ed. Rocek Z.), pp. 341–344. Charles University, Prague. [Google Scholar]
- Levrat‐Calviac, V. & Zylberberg, L. (1986). The structure of the osteoderms in the gekko: Tarentola mauritanica . American Journal of Anatomy 446, 437–446. [DOI] [PubMed] [Google Scholar]
- Losos, J. B. , Hillis, D. M. & Greene, H. W. (2012). Who speaks with a forked tongue? Science 338(6113), 1428–1429. [DOI] [PubMed] [Google Scholar]
- Losos, J. B. , Mouton, P. L. F. N. , Bickel, R. , Cornelius, I. & Ruddock, L. (2002). The effect of body armature on escape behaviour in cordylid lizards. Animal Behaviour 64(2), 313–321. [Google Scholar]
- Main, R. P. , de Ricqlès, A. , Horner, J. R. & Padian, K. (2005). The evolution and function of thyreophoran dinosaur scutes: implications for plate function in stegosaurs. Paleobiology 31, 291–314. [Google Scholar]
- Maisano, J. A. , Bell, C. J. , Gauthier, J. A. & Rowe, T. (2002). The osteoderms and palpebral in Lanthanotus borneensis (Squamata: Anguimorpha). Journal of Herpetology 36, 678–682. [Google Scholar]
- Maisano, J. A. , Laduc, T. J. , Bell, C. J. & Barber, D. (2019). The cephalic osteoderms of Varanus komodoensis as revealed by high‐resolution X‐ray computed tomography. The Anatomical Record 302, 1675–1680. [DOI] [PubMed] [Google Scholar]
- Marinho, T. (2007). Functional aspects of titanosaur osteoderms. Anuario do Instituto de Geociencias 30, 251. [Google Scholar]
- Martini, R. , Balit, Y. & Barthelat, F. (2017). A comparative study of bio‐inspired protective scales using 3D printing and mechanical testing. Acta Biomaterialia 55, 360–372. [DOI] [PubMed] [Google Scholar]
- McDonald, H. G. (2018). An overview of the presence of osteoderms in sloths: implications for osteoderms as a plesiomorphic character of the Xenarthra. Journal of Mammalian Evolution 25, 485–493. [Google Scholar]
- McDowell, S. B. & Bogert, C. M. (1954). The systematic position of Lanthanotus and the affinities of the anguinomorphan lizards. Bulletin of the American Museum of Natural History 105, 1–142. [Google Scholar]
- Mead, J. I. , Schubert, B. W. , Wallace, S. C. & Swift, S. L. (2012). Helodermatid lizard from the Mio‐Pliocene Oak‐Hickory Forest of Tennessee, Eastern USA, and a review of monstersaurian osteoderms. Acta Palaeontologica Polonica 57(1), 111–121. [Google Scholar]
- Miralles, A. , Hipsley, C. A. , Erens, J. , Gehara, M. , Rakotoarison, A. , Glaw, F. , Müller, J. & Vences, M. (2015). Distinct patterns of desynchronized limb regression in Malagasy scincine lizards (Squamata, Scincidae). PLoS ONE 10(6), e0126074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo, J. Y. , Xu, X. & Evans, S. E. (2012). A large predatory lizard (Platynota, Squamata) from the Late Cretaceous of South China. Journal of Systematic Palaeontology 10, 333–339. [Google Scholar]
- Mondejar‐Fernandez, J. (2018). On cosmine: its origins, biology, and implications for sarcopterygian interrelationships. Cybium 42, 41–65. [Google Scholar]
- Mongera, A. & Nüsslein‐Volhard, C. (2013). Scales of fish arise from mesoderm. Current Biology 23(9), R338–R339. 10.1016/j.cub.2013.02.056. [DOI] [PubMed] [Google Scholar]
- Moss, M. L. (1969). Comparative histology of dermal sclerifications in reptiles. Acta Anatomica 73(4), 510–533. [DOI] [PubMed] [Google Scholar]
- Moss, M. L. (1972). The vertebrate dermis and the integumental skeleton. American Zoologist 12(1), 27–34. [Google Scholar]
- Naleway, S. E. , Taylor, J. R. A. , Porter, M. M. , Meyers, M. A. & McKittrick, J. (2016). Structure and mechanical properties of selected protective systems in marine organisms. Materials Science and Engineering C 59, 1143–1167. [DOI] [PubMed] [Google Scholar]
- Nance, H. A. (2007). Cranial osteology of the African gerrhosaurid Angolosaurus skoogi (Squamata: Gerrhosauridae). African Journal of Herpetology 56, 39–75. [Google Scholar]
- Oliver, J. A. (1951). Ontogenetic changes in osteodermal ornamentation in skinks. Copeia 1951(2), 127–130. [Google Scholar]
- Otto, H. (1909). Die Beschuppung de Brevilinguier und Ascalaboten. Jenaische Zeitschrift für Naturwissenschaft 44, 193–252. [Google Scholar]
- Paluh, D. J. & Bauer, A. M. (2017). Comparative skull anatomy of terrestrial and crevice‐dwelling Trachylepis skinks (Squamata: Scincidae) with a survey of resources in scincid cranial osteology. PLoS ONE 12(9), e0184414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh, D. J. , Griffing, A. H. & Bauer, A. M. (2017). Sheddable armour: identification of osteoderms in the integument of Geckolepis maculate . African Journal of Herpetology 66, 12–24. [Google Scholar]
- Paradis, E. & Schliep, K. (2019). Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528. [DOI] [PubMed] [Google Scholar]
- Pennell, M. W. , Eastman, J. M. , Slater, G. J. , Brown, J. W. , Uyeda, J. C. , Fitzjohn, R. G. , Alfaro, M. E. & Harmon, L. J. (2014). Geiger v2.0: an expanded suite of methods for fitting macroevolutionary models to phylogenetic trees. Bioinformatics 30, 2216–2218. [DOI] [PubMed] [Google Scholar]
- du Plessis, A. , Broeckhoven, C. , Yadroitsev, I. , Yadroitsava, I. & le Roux, S. G. (2018). Analyzing nature's protective design: the glyptodont body armor. Journal of the Mechanical Behavior of Biomedical Materials 82, 218–223. [DOI] [PubMed] [Google Scholar]
- Pressinotti, L. N. , Borges, R. M. , de Lima, A. P. A. , Aleixo, V. M. , Iunes, R. S. , Borges, J. C. S. , Cogliati, B. & da Silva, J. R. M. C. (2013). Low temperatures reduce skin healing in the Jacaré do Pantanal (Caiman yacare, Daudin 1802). Biology Open 16, 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyron, R. A. , Burbrink, F. T. & Wiens, J. J. (2013). A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology 13, 93. 10.1186/1471-2148-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Queiroz, K. (1987). Phylogenetic Systematics of Iguanine Lizards. A Comparative Osteological Study, Edition (Volume 118), pp. 1–203. Berkeley and Los Angeles: University of California Publications in Zoology. [Google Scholar]
- Reif, W. E. (1982). Evolution of dermal skeleton and dentition in vertebrates The odontode regulation theory. Evolutionary Biology 15, 287–368. [Google Scholar]
- Reisz, R. R. , Dilkes, D. W. & Berman, D. S. (1998). Anatomy and relationships of Elliotsmithia longiceps Broom, a small synapsid (Eupelycosauria: Varanopseidae) from the Late Permian of South Africa. Journal of Vertebrate Palaeontology 18(3), 602–611. [Google Scholar]
- Revell, L. J. (2012). phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3, 217–223. [Google Scholar]
- Reynoso, V. H. (1997). A "beaded" sphenodontian (Diapsida: Lepidosauria) from the Early Cretaceous of central Mexico. Journal of Vertebrate Paleontology 17(1), 52–59. [Google Scholar]
- Rhee, H. , Horstemeyer, M. F. , Hwang, Y. , Lim, H. , El Kadiri, H. & Trim, W. (2009). A study on the structure and mechanical behavior of the Terrapene carolina carapace: a pathway to design bio‐inspired synthetic composites. Materials Science and Engineering C 29(8), 2333–2339. [Google Scholar]
- Rhee, H. , Horstemeyer, M. F. & Ramsay, A. (2011). A study on the structure and mechanical behavior of the Dasypus novemcinctus shell. Materials Science and Engineering C. 31(2), 363–369. [Google Scholar]
- Rice, R. , Kallonen, A. , Cebra‐Thomas, J. & Gilbert, S. F. (2016). Development of the turtle plastron, the order‐defining skeletal structure. Proceedings of the National A cademy of Sciences of the USA 113, 5317–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, A. (1994). Lacertilia aus der Unteren Kreide von Uña und Galve (Spanien) und Anoual (Marokko). Berliner geowissenschaftliche Abhandlungen 14, 1–147. [Google Scholar]
- de Ricqlès, A. , Pereda‐Suberbiola, X. , Gasparini, Z. & Olivero, E. B. (2001). Histology of dermal ossifications in an ankylosaurian dinosaur from the Late Cretaceous of Antarctica. Asociación Paleontológica Argentina, Publicación Especial 7, 171–174. [Google Scholar]
- Rieppel, O. (1981). The skull and the jaw adductor muscles in some burrowing scincomorph lizards of the genus Acontias, Typhlosaurus, and Feylinia . Journal of Zoology, London 195, 493–528. [Google Scholar]
- Rivera, G. & Stayton, C. T. (2011). Finite element modeling of shell shape in the freshwater turtle Pseudemys concinna reveals a trade‐off between mechanical strength and hydrodynamic efficiency. Journal of Morphology 272(10), 1192–1203. [DOI] [PubMed] [Google Scholar]
- Rogers, K. C. , D'Emic, M. , Rogers, R. , Vickaryous, M. & Cagan, A. (2011). Sauropod dinosaur osteoderms from the Late Cretaceous of Madagascar. Nature Communications 2, 564. 10.1038/ncomm1578. [DOI] [PubMed] [Google Scholar]
- Rudykh, S. , Ortiz, C. & Boyce, M. C. (2015). Flexibility and protection by design: imbricated hybrid microstructures of bio‐inspired armor. Soft Matter 11, 2547. [DOI] [PubMed] [Google Scholar]
- Ruibal, R. & Shoemaker, V. (1984). Osteoderms in frogs. Journal of Herpetology 18, 313–328. [Google Scholar]
- Saitta, E. T. (2015). Evidence for sexual dimorphism in the plated dinosaur Stegosaurus mjosi (Ornithischia, Stegosauria) from the Morrison Formation (Upper Jurassic) of western USA. PLoS ONE 10(4), e0123503. 10.1371/journal.pone.0123503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury, S. W. & Frey, E. (2001). A biomechanical transformation model for the evolution of semi‐spheroidal articulations between adjoining vertebral bodies in crocodilians. In Crocodilian Biology and Evolution (eds Grigg G. C., Seebacher F. and Franklin C. F.), pp. 85–134. Surrey Beatty and Sons, Chipping Norton. [Google Scholar]
- Scherz, M. D. , Daza, J. D. , Kohler, J. , Vences, M. & Glaw, F. (2017). Off the scale: a new species of fish‐scale gecko (Squamata: Gekkonidae: Geckolepis) with exceptionally large scales. PeerJ 5, e2955. 10.7717/peerj.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer, T. M. (2007). Skeletal histology of the dermal armor of Placodontia: the occurrence of ‘postcranial fibro‐cartilaginous bone’ and its developmental implications. Journal of Anatomy 211(6), 737–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer, T. M. , Desojo, J. B. & Cerda, I. A. (2014). Bone histology of phytosaur, aetosaur, and other archosauriform osteoderms (Eureptilia, Archosauromorpha). Anatomical Record 297, 240–260. [DOI] [PubMed] [Google Scholar]
- Scheyer, T. M. & Sander, P. M. (2004). Histology of ankylosaur osteoderms: implications for systematics and function. Journal of Vertebrate Paleontology 24, 874–893. [Google Scholar]
- Scheyer, T. M. & Sander, P. M. (2009). Bone microstructures and mode of skeletogenesis in osteoderms of three pareiasaur taxa from the Permian of South Africa. Journal of Evolutionary Biology 22, 1153–1162. [DOI] [PubMed] [Google Scholar]
- Schmidt, W. J. (1912a). Studien am Integument der Reptilien. I. Die Haut der Geckoniden. Zeitschrift für Wissenschaftliche Zoologie 101, 138–258. [Google Scholar]
- Schmidt, W. J. (1912b). Studien am Integument der Reptilien. II. Die Hautverknöcherungen von Heloderma . In Festschrift zum 60 Geburtstage des Herrn Geheimen Hofrats Prof. Dr. J. W. Spengel. Part 2 (eds Brauer A., Döderlein L., Dollo L., Ludwig H., Mark E. L., Weber M. and Weismann A. ), pp. 219–228. Verlag Gustav Fischer, Jena. Zoologische Jahrbücher.Supplement 15 [Google Scholar]
- Schmidt, W. J. (1914a). Studien am Integument der Reptilien. V. Anguiden. Zoologische Jahrbücher Abteilung fur Anatomie und Ontogenie der Tiere 38, 1–102. [Google Scholar]
- Schmidt, W. J. (1914b). Studien am Integument der Reptilien. VI. Über die Knochenschuppen der Crocodile. Zoologische Jahrbücher 38, 643–666. [Google Scholar]
- Schucht, P. J. , Ruehr, P. T. , Geier, B. , Glaw, F. & Lambertz, M. (2020). Armored with skin and bone: the integumentary morphology of the Antsingy Leaf Chameleon Brookesia perarmata (Iguania: Chamaeleonidae). Journal of Morphology 280, 754–764. [DOI] [PubMed] [Google Scholar]
- Schultze, H. P. (2016). Scales, enamel, cosmine, ganoine, and early osteichthyans. Comptes Rendus Palevol 15(1–2), 83–102. [Google Scholar]
- Seidel, M. R. (1979). The osteoderms of the American Alligator and their functional significance. Herpetologica 35(4), 375–380. [Google Scholar]
- Sire, J.‐Y. (1993). Development and fine structure of the bony scutes in Corydoras arcuatus (Siluriformes, Callichthyidae). Journal of Morphology 215, 225–244. [DOI] [PubMed] [Google Scholar]
- Sire, J.‐Y. , Donoghue, P. C. J. & Vickaryous, M. K. (2009). Origin and evolution of the integumentary skeleton in non‐tetrapod vertebrates. Journal of Anatomy 214(4), 409–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sire, J. Y. & Kawasaki, K. (2012). Origin and evolution of bone and dentin and of acidic secretory calcium‐binding phosphoproteins. In Phosphorylated extracellular matrix proteins of bone and dentin, volume 2: PEM Proteins of Bone and Dentin (ed. Goldberg M.), pp. 3–60. Bentham eBooks, Paris. [Google Scholar]
- Smith, K. T. & Habersetzer, J. (2021). The anatomy, phylogenetic relationships, and autecology of the carnivorous lizard “Saniwa” feisti Stritzke, 1983 from the Eocene of Messel, Germany. Comptes Rendus Palevol 20(23), 441–506. [Google Scholar]
- Smith, M. M. & Hall, B. K. (1990). Developmental and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biological Reviews 65, 277–374. [DOI] [PubMed] [Google Scholar]
- Song, J. , Ortiz, C. & Boyce, M. C. (2011). Threat‐protection mechanics of an armored fish. Journal of the Mechanical Behavior of Biomedical Materials 4(5), 699–712. [DOI] [PubMed] [Google Scholar]
- Spence, R. (2012). Calcium and salinity as selective factors in plate morph evolution of the three‐spined stickleback (Gasterosteus aculeatus). Journal of Evolutionary Biology 25(10), 1965–1974. [DOI] [PubMed] [Google Scholar]
- Stanley, E.L. (2013). Systematics and morphological diversification of the Cordylidae (Squamata). Unpublished Thesis. The Richard Gilder Graduate School, New York.
- Stanley, E. L. (2016). A review of Cordylus machadoi (Squamata: Cordylidae) in southwestern Angola, with the description of a new species from the Pro‐Namib desert. Zootaxa 4061, 201–226. [DOI] [PubMed] [Google Scholar]
- Strahm, M. H. & Schwartz, A. (1977). Osteoderms in the lizard subfamily Diploglossinae and their taxonomic importance. Biotropica 9, 58–72. [Google Scholar]
- Sun, C.‐Y. & Chen, P.‐Y. (2013). Structural design and mechanical behavior of alligator (Alligator mississippiensis) osteoderms. Acta Biomaterialia 9(11), 9049–9064. [DOI] [PubMed] [Google Scholar]
- Tucker, A. D. (1997). Validation of skeletochronology to determine age of freshwater crocodiles (Crocodylus johnsoni). Marine and Freshwater Research 48, 343–351. [Google Scholar]
- Uetz, P. , Freed, P. & Hošek, J. (2020). The Reptile Database. Electronic file available at http://www.reptile-database.org. Accessed 9.2020
- Vickaryous, M. K. & Hall, B. K. (2006). Osteoderm morphology and development in the nine‐banded armadillo, Dasypus novemcinctus (Mammalia, Xenarthra, Cingulata). Journal of Morphology 267, 1273–1283. [DOI] [PubMed] [Google Scholar]
- Vickaryous, M. K. & Hall, B. K. (2008). Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. Journal of Morphology 269, 398–422. [DOI] [PubMed] [Google Scholar]
- Vickaryous, M. K. , Meldrum, G. & Russell, A. P. (2015). Armored geckos: a histological investigation of osteoderm development in Tarentola (Phyllodactylidae) and Gekko (Gekkonidae) with comments on their regeneration and inferred function. Journal of Morphology 276(11), 1345–1357. [DOI] [PubMed] [Google Scholar]
- Vickaryous, M. K. & Sire, J.‐Y. (2009). The integumentary skeleton of tetrapods: origin, evolution, and development. Journal of Anatomy 214(4), 441–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal, D. , Ortega, F. , Gasco, F. , Serrano‐Martinez, A. & Sanz, J. L. (2017). The internal anatomy of titanosaur osteoderms from the Upper Cretaceous of Spain is compatible with a role on oogenesis. Scientific Reports 7, 42035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake, M. (1975). Another scaled caecilian (Gymnophiona: Typhlonectidae). Herpetologica 31, 134–136. [Google Scholar]
- Warren, D. E. & Jackson, D. C. (2008). Lactate metabolism in anoxic turtles: an integrative review. Journal of Comparative Physiology B 178, 133–148. [DOI] [PubMed] [Google Scholar]
- Witzmann, F. (2009). Comparative histology of sculptured dermal bones in basal tetrapods, and the implications for the soft tissue dermis. Palaeodiversity 2, 233–270. [Google Scholar]
- Witzmann, F. (2011). Morphological and histological changes of dermal scales during the fish‐to‐tetrapod transition. Acta Zoologica 92(3), 281–302. [Google Scholar]
- Witzmann, F. & Soler‐Gijón, R. (2010). The bone histology of osteoderms in temnospondyl amphibians and in the chroniosuchian Bystrowiella . Acta Zoologica 91, 96–114. [Google Scholar]
- Xue, J. , Marghoub, A. , Bertazzo, S. , Evans, S. E. & Moazen, M. (2017). Biomechanics of osteoderms in a lizard skull–a preliminary finite element study. Journal of Anatomy 2016, B18. 10.1111/joa.12637. [DOI] [Google Scholar]
- Yang, W. , Chen, I. H. , Gludovatz, B. , Zimmermann, E. A. , Ritchie, R. O. & Meyers, M. A. (2013). Natural flexible dermal armor. Advanced Materials 25, 31–48. [DOI] [PubMed] [Google Scholar]
- Yang, W. , Chen, I. , Mckittrick, J. & Meyers, M. A. (2012). Flexible dermal armor in nature. Journal of the Minerals, Metals and Materials Society 64(4), 475–485. [Google Scholar]
- Yang, W. , Sherman, V. R. , Gludovatzb, B. , Mackey, M. , Zimmermann, E. A. , Chang, E. H. , Schaible, E. , Qin, Z. , Markus, J. , Buehler, M. J. , Ritchie, R. O. & Meyers, M. A. (2014). Protective role of Arapaima gigas fish scales: structure and mechanical behavior. Acta Biomaterialia. 10(8), 3599–3614. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Wu, C. , Zhang, C. & Chen, Z. (2012). Microstructure and mechanical property of turtle shell. Theoretical and Applied Mechanics Letters 2(1), 014009. [Google Scholar]
- Zheng, Y. & Wiens, J. J. (2016). Combining phylogenomic and supermatrix approaches, and a time‐calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Molecular Phylogenetics & Evolution 94, 537–547. [DOI] [PubMed] [Google Scholar]
- Zylberberg, L. & Castanet, J. (1985). New data on the structure and the growth of the osteoderms in the reptile Anguis fragilis (Anguidae, Squamata). Journal of Morphology 186, 327–342. [DOI] [PubMed] [Google Scholar]
- Zylberberg, L. , Castanet, J. & de Ricqlès, A. (1980). Structure of the dermal scales in Gymnophiona (Amphibia). Journal of Morphology 165, 41–54. [DOI] [PubMed] [Google Scholar]
- Zylberberg, L. , Geraudie, J. , Meunier, F. & Sire, J.‐Y. (1992). Biomineralization in the integumental skeleton of the living lower vertebrates. In Bone: Bone Metabolism and Mineralization (Volume 4, ed. Hall B. K.), pp. 171–224. CRC Press, Boca Raton. [Google Scholar]
- Zylberberg, L. & Wake, M. H. (1990). Structure of the scales of Dermophis and Microcaecilia (Amphibia: Gymnophiona) and a comparison to dermal ossifications of other vertebrates. Journal of Morphology 206, 25–43. [DOI] [PubMed] [Google Scholar]


